INTRODUCTION
Primary biliary cholangitis (PBC) is a chronic cholestatic progressive liver disease of autoimmune etiology, characterized by the destruction, necrosis, and apoptosis of small bile duct epithelial cells, in the terminal stage of which cirrhosis develops[1-3]. PBC development is preceded by a long asymptomatic period[1,2], during which time there are no physical signs of the disease. The earliest and most common symptoms are fatigue, weakness, and malaise. Detection of serum antimitochondrial autoantibodies (AMAs) at a titer ≥ 1: 40 during this period serves as a pathognomonic marker of PBC development. AMAs are detectable months to years before PBC manifests clinically, indicating their primary immunopathogenetic role rather than a secondary phenomenon occurring as a consequence of cholestasis[4,5]. However, AMAs titer does not correlate with disease activity or duration[4-7]. Identifying the causes and mechanisms of AMAs formation may contribute to understanding the pathogenesis of the development of clinical, morphologic, biochemical, and immunologic signs of PBC.
AMAs are not strictly specific for PBC[4]. They are classified as immunoglobulin (Ig) M which reacts with multiple antigens in mitochondria, designated M1-M9[8]. The highly sensitive and most frequent (> 95%) AMAs found in PBC are anti-M2 IgM[8]. In patients with classical PBC, the antigenic components of AMAs are related to the dihydrolipoyl transacetylase (E2 subunit) of the pyruvate dehydrogenase (PDC) complex (E2 PDC), which localizes to the inner mitochondrial membrane[8]. Immunization of laboratory animals with E2 PDC recombinant polypeptide leads to AMAs formation but not cholangiocyte damage[9], indicating that AMAs do not trigger the destruction of biliary epithelial cells [(BECs), cholangiocytes].
It is critical to understand how the E2 PDC antigen of BECs, located on the inner membrane of mitochondria in small and medium-sized bile ducts, can be a target of immune effector mechanisms[4]. The theory of antigenic mimicry is discussed below.
AMAs AND THE THEORY OF ANTIGENIC MIMICRY
The PDC in prokaryotes is structurally similar to that of eukaryotes[10]. Antibodies from the serum of patients with PBC have been shown to react with yeast and bacterial proteins[11,12]. Therefore, it has been suggested that AMAs in PBC arise due to cross-reactivity to exogenous bacterial antigens (antigenic mimicry)[13,14] and that the disease may have a bacterial origin[15]. However, there is no clear evidence of an infectious agent[4]. In classic bacterial antigenic exposure, IgM is the first antibody secreted by the adaptive immune system, and after 3-4 wk, IgG is produced. IgM can persist in the blood for up to 3 mo, followed by its decline. However, in PBC, the level of IgM-related AMAs does not decline over the course of the disease, which does not align with the bacterial nature of antigens triggering the production of AMAs. With continuous exposure to thymus-independent antigens and decreased immune tolerance to them, IgM synthesis can become stable[16]. However, there is low likelihood that bacterial antigen is constantly present in patients with PBC and triggers the production of AMAs[10]. It is more logical to assume that the antigen is from the patients’ own tissues, namely the epithelium of the biliary tract. The production of AMAs in patients with PBC is initiated once E2 PDC becomes an immunomodified antigen, leaves the mitochondria, exits the cholangiocyte, enters the blood, and meets immunocompetent T and B lymphocytes which recognize it as a foreign antigen. To date, the triggers and mechanisms that initiate these processes in cholangiocytes remain unknown.
In the last decade, scientific data have shown that the “protective umbrella” of bicarbonate (HCO3-) protects cholangiocytes from the toxic effects of bile acids. In PBC, the production of HCO3- decreases, which leads to increased bile acid intake into cholangiocytes (theory of defective “biliary HCO3- umbrella”). Based on these data, we hypothesize that the gradual accumulation of bile acids in BECs may serve as a trigger mechanism for AMAs formation, ductulopenia development, and one of the early clinical signs of PBC, namely weakness in the asymptomatic stage.
AGGRESSIVE AND DEFENSE FACTORS OF CHOLANGIOCYTES
Bile is an aggressive medium for cholangiocytes, which are epithelial cells lining the intrahepatic and extrahepatic bile ducts. The presence of bile acids in bile, which have potent detergent properties, can cause damage to the cell membranes of cholangiocytes. Hydrophobic bile acids are cytotoxic to many cell types[17]. However, BECs under physiological conditions are exposed to very high (millimolar) concentrations of hydrophobic bile acids without signs of cytotoxicity[18], indicating the presence of mechanisms protecting cholangiocytes from the toxic effects of bile acids.
The conjugation of bile acids and formation of mixed micelles with cholesterol and phospholipids are considered defense mechanisms at the levels of hepatocytes, bile capillaries, and Hering’s canals[18]. Defense factors that enter the bile during its passage through bile ducts include the production and secretion of mucin and HCO3-[19]. Under physiological conditions, the main function of cholangiocytes is biliary secretion of HCO3-[20]. HCO3- is produced by cholangiocytes lining the biliary tree. Mucin glycoprotein is produced by peribiliary glands (PBGs)[21], which are located in the wall of large intrahepatic and extrahepatic bile ducts and are directly connected with the bile duct lumen. Experimental evidence indicates that the glycocalyx, which covers the apical surface of large cholangiocyte membranes with glycosylated mucins and other glycan-containing membrane glycoproteins, stabilizes the biliary HCO3- umbrella, thereby helping to protect human large cholangiocytes from bile acid toxicity[22]. In addition, the mucin produced by PBGs protects the cholangiocytes of only large bile ducts[19]. Thus, the cholangiocytes of large intrahepatic and extrahepatic bile ducts have dual protection: The mucin produced by PBGs and HCO3-. Intralobular, interlobular, and septal bile ducts do not contain PBGs, which is accompanied by the absence of mucin in them[21]. As a result, only HCO3- serves as a factor of BEC defense at the levels of intralobular, interlobular, and septal ducts. Under physiological conditions, there is a balance between aggressive factors (bile acids) and defense factors (HCO3- and/or mucin secretion).
CHOLANGIOCYTE DEFENSE MECHANISMS
Cholangiocytes are polarized epithelial cells that line the intrahepatic and extrahepatic bile ducts and are responsible for regulating bile volume, modifying bile, and maintaining bile pH (alkalinity)[23,24]. They play an important role in modifying the composition of primary bile by secreting water, chloride (Cl-), and HCO3-[25], and by absorbing bile acid salts, amino acids, and glucose. Small and large cholangiocytes are distinguished depending on their size and location in small and large bile ducts[26]. They are differently involved in the processes of secretion and absorption[27]. The secretion of HCO3- with human bile accounts for 25%-40% of the total volume of secreted bile and maintains physiologic pH in the lumen of bile ducts[17,28,29].
In the process of bile formation, predominantly conjugated bile acids and a minimal amount of unconjugated bile acids enter the bile capillary. Under physiological conditions, both conjugated and unconjugated bile acids are secreted into bile by hepatocytes in anionic (deprotonated, ionized, negatively charged) form[30]. HCO3-, which is secreted by cholangiocytes into the lumen of the bile duct, creates a slightly alkaline pH of hepatic bile due to its buffering properties, keeping bile acids in a deprotonated state. The ionized form of bile acids are impermeable to BECs due to the presence of negatively charged HCO3- molecules on the apical surface of the cytoplasmic membrane of cholangiocytes[30]. Thus, the secretion of HCO3- ions protects cholangiocytes from uncontrolled transmembrane bile acid entrance and their cytotoxicity. This protective mechanism, which preserves cholangiocytes and normal bile flow along the biliary tree, is called the biliary HCO3- umbrella.
MAIN REGULATORS OF HCO3- PRODUCTION AND SECRETION BY CHOLANGIOCYTES
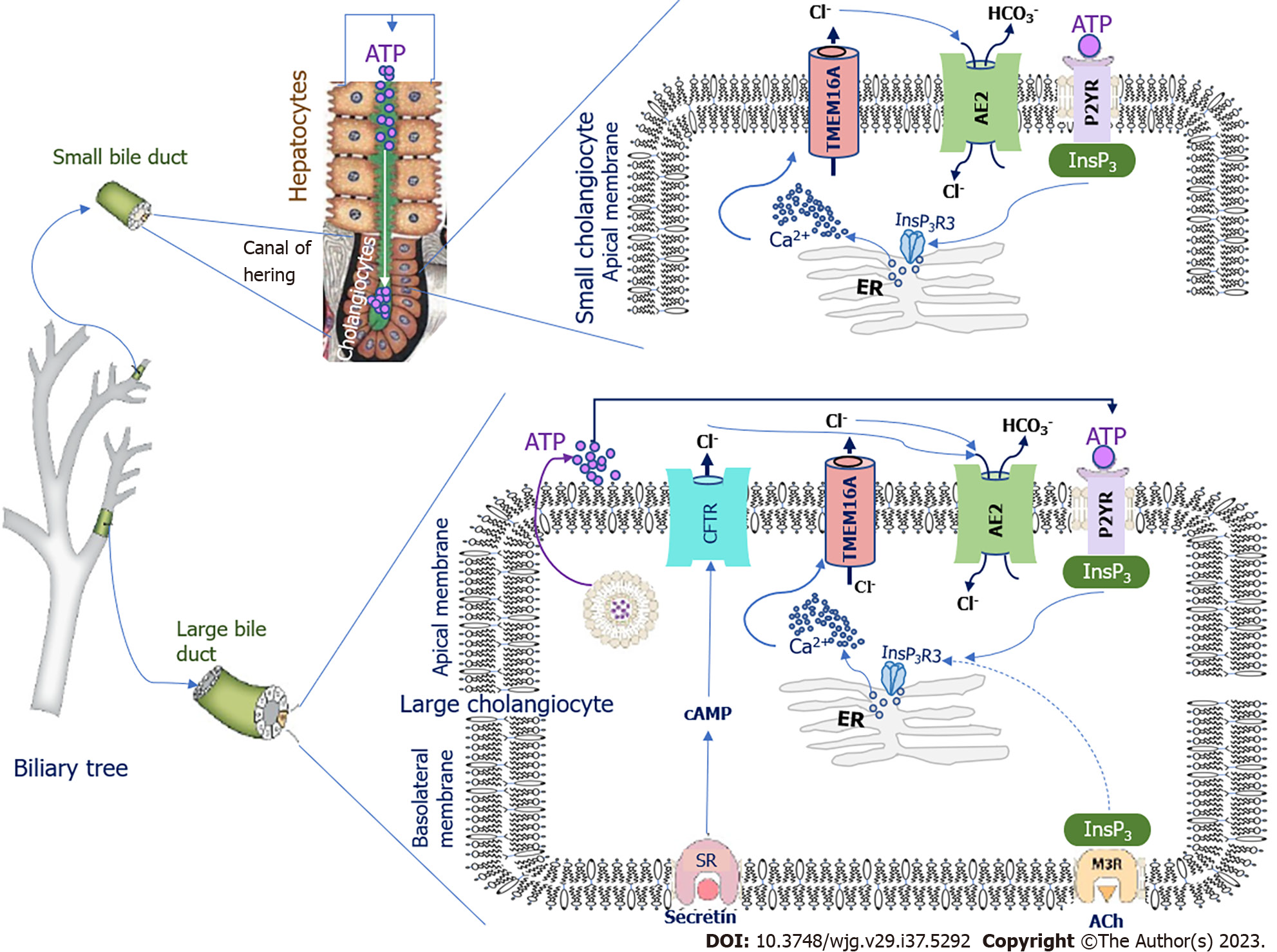
The pH fluctuation in bile ducts depends on the rate of HCO3- production by cholangiocytes. The signaling pathways regulating HCO3- secretion differ in large and small cholangiocytes[31]. In small cholangiocytes, activation of HCO3- secretion is due to biliary adenosine triphosphate (ATP) secreted from upstream hepatocytes of Hering’s canals (Figure 1). Cholangiocytes express apical membrane proteins of the purinergic receptor (P2YR) family, which are stimulated by ATP[32]. Luminal ATP binds to P2YR, stimulating intracellular calcium (Ca2+) release via inositol-1,4,5-trisphosphate receptor type 3 (InsP3R3)[33]. In cholangiocytes, InsP3R3 is the major receptor isoform localized in the apical region[31,34]; it is involved in InsP3R3-mediated cell signaling and Ca2+ secretion[35]. InsP3R3 is the only receptor that promotes the opening of intracellular Ca2+ channels and release of Ca2+ ions[34]. Ca2+ is one of the second messengers in cholangiocytes that modulates and regulates diverse cellular functions such as ion channel activation, secretion, cell proliferation, and apoptosis[34,36]. Ca2+ release from subapical stores in the endoplasmic reticulum triggers and locally activates transmembrane 16A Cl- channels (TMEM16A) on the apical membrane of cholangiocytes (Figure 1)[36-38]. The appearing Cl- concentration gradient on the apical membrane activates the Cl-/HCO3- anion exchanger 2 (AE2), also Slc4A2, which leads to the secretion of HCO3- into the lumen of the bile duct.
Figure 1 Schematic of bicarbonate secretion by small and large cholangiocytes.
AE2: Chloride/bicarbonate anion exchanger 2; TMEM16A: Transmembrane 16A chloride channels; ATP: Adenosine triphosphate; P2YR: Purinergic receptor family; InsP3: Inositol-1,4,5-trisphosphate; InsP3R3: Inositol-1,4,5-trisphosphate receptor type 3; ER: Endoplasmic reticulum; SR: Secretin receptor; cAMP: Cyclic adenosine monophosphate; Ach: Acetylcholine; CFTR: Cystic fibrosis transmembrane conductance regulator; M3R: Muscarinic acetylcholine M3 receptor; HCO3-: Bicarbonate; Cl-: Chloride; Ca2+: Calcium.
In large cholangiocytes, with the exception of the Ca2+-dependent pathway of HCO3- secretion, there is an additional mechanism involving the hormones secretin and somatostatin[39] (Figure 1). Secretin is produced by the S cells of the duodenal mucosa, and stimulates the production of HCO3- by the intestinal mucosa itself as well as by cholangiocytes and pancreatic ductular epithelial cells[39]. Secretin regulates the secretion of HCO3- and Cl- into bile by large cholangiocytes through interaction with secretin receptors (SRs) located on the basolateral membrane of BECs[39-42] (Figure 1).
As a result of this interaction, the formation of cyclic adenosine monophosphate (cAMP) is stimulated via G proteins. cAMP activates the cystic fibrosis transmembrane conductance regulator (CFTR) through adenylate cyclase, resulting in the secretion of Cl- ions into the bile duct[28]. The appearing Cl- concentration gradient on the apical membrane of cholangiocytes activates AE2, which leads to the secretion of HCO3- into the bile duct lumen in exchange for the intracellular entry of Cl- ions into the cholangiocytes[20,24,26,43]. In parallel, ATP from cholangiocytes enters the bile duct lumen by exocytosis, which stimulates the secretion of HCO3-via a Ca2+-dependent mechanism[44]. SRs and CFTR are not found in small cholangiocytes; therefore, secretin is unable to stimulate the secretion and entry of HCO3- and Cl- into bile in small cholangiocytes[24]. However, a Ca2+-dependent mechanism of HCO3- secretion is present in both small and large cholangiocytes (Figure 1)[20,24]. Somatostatin, which binds to the somatostatin receptor, counteracts the stimulating effect of secretin, inhibits fluid secretion, and slows the production and entry of HCO3- from cholangiocytes into the lumen of the bile duct[45].
MECHANISMS OF BILE ACID PROTONATION-DEPROTONATION AND THEIR ENTRY INTO CHOLANGIOCYTES
Uncontrolled, carrier-independent, passive diffusion of unconjugated primary bile acids into BECs is determined by their polarity and degree of protonation[18,46,47]. Protonation of bile acids is an exponential function of pH. When the pH of hepatic bile acidifies, bile acids may undergo protonation. The degree of protonation of bile acids depends on both their dissociation constant (pKa) and the pH of the bile. The pKa values for unconjugated primary bile acids are 5-6[48-50]. Conjugation of primary bile acids with amino acids reduces pKa values to 4-5 for conjugates with glycine and to 1-2 for conjugates with taurine, which improves their solubility in water and reduces their lipophilicity[48-50]. The low pKa values of the taurine conjugates of primary bile acids indicate that they are stronger acids than glycine conjugates. Therefore, taurine-conjugated bile acids are in a dissociated (deprotonated) form even at acidic bile pH values. Whereas glycine conjugates, with higher pKa values, are weak acids and will quickly change to a protonated state at the slightest acidification of bile[50].
Ionized (deprotonated, negatively charged) bile acids are unable to overcome the biliary HCO3- umbrella on the outer hemi leaflet of the apical cytoplasmic membrane of cholangiocytes[18,47]. Under normal physiological conditions, a small amount of unconjugated protonated primary bile acids is taken up by cholangiocytes. The neutral intracellular pH further promotes the transport of unconjugated protonated primary bile acids into the peribiliary vascular plexus with subsequent return to hepatocytes and re-release into biliary capillaries[51]. Such a biliary-hepatic shunt aims to prevent the accumulation of toxic bile acids with strong detergent properties in cholangiocytes[18,47].
Conjugated bile acids can be transported through the apical and basolateral membranes of cholangiocytes with the aid of specific transporters[26,52-56]. Conjugates of bile acids with glycine in human hepatic bile account for three-quarters of all conjugated bile acids and have a pKa close to 4[57]. At physiologic pH (approximately 7.4), glycine conjugates of primary bile acids, as relatively weak acids, are partially protonated (nonpolar), which promotes their absorption by cholangiocytes in micromolar amounts. Small shifts in local pH to an acidic region in biliary ducts lead to an increase in protonated glycine conjugates of primary bile acids. A significant increase in the ratio of protonated:deprotonated glycine-conjugated bile acids will lead to increased absorption by cholangiocytes.
Bile acid conjugates with taurine in hepatic bile account for one-quarter of all conjugated bile acids. They are stronger acids and have a pKa of 1-2[57,58]. Therefore, changes in biliary pH have little effect on their protonation. Most of the taurine conjugates of bile acids are in anionic form and thus are not able to enter cholangiocytes. Therefore, taurine conjugates of primary bile acids are less toxic to cholangiocytes.
Active functioning of bile acid transporters in the basolateral membrane of cholangiocytes, as a rule, leads to the rapid removal of hydrophobic bile acids from the intracellular space and their delivery back into hepatocytes[59]. Therefore, the accumulation of toxic bile acids with detergent properties in cholangiocytes does not occur under normal conditions.
THEORY OF DEFECTIVE BILIARY HCO3- UMBRELLA IN PBC
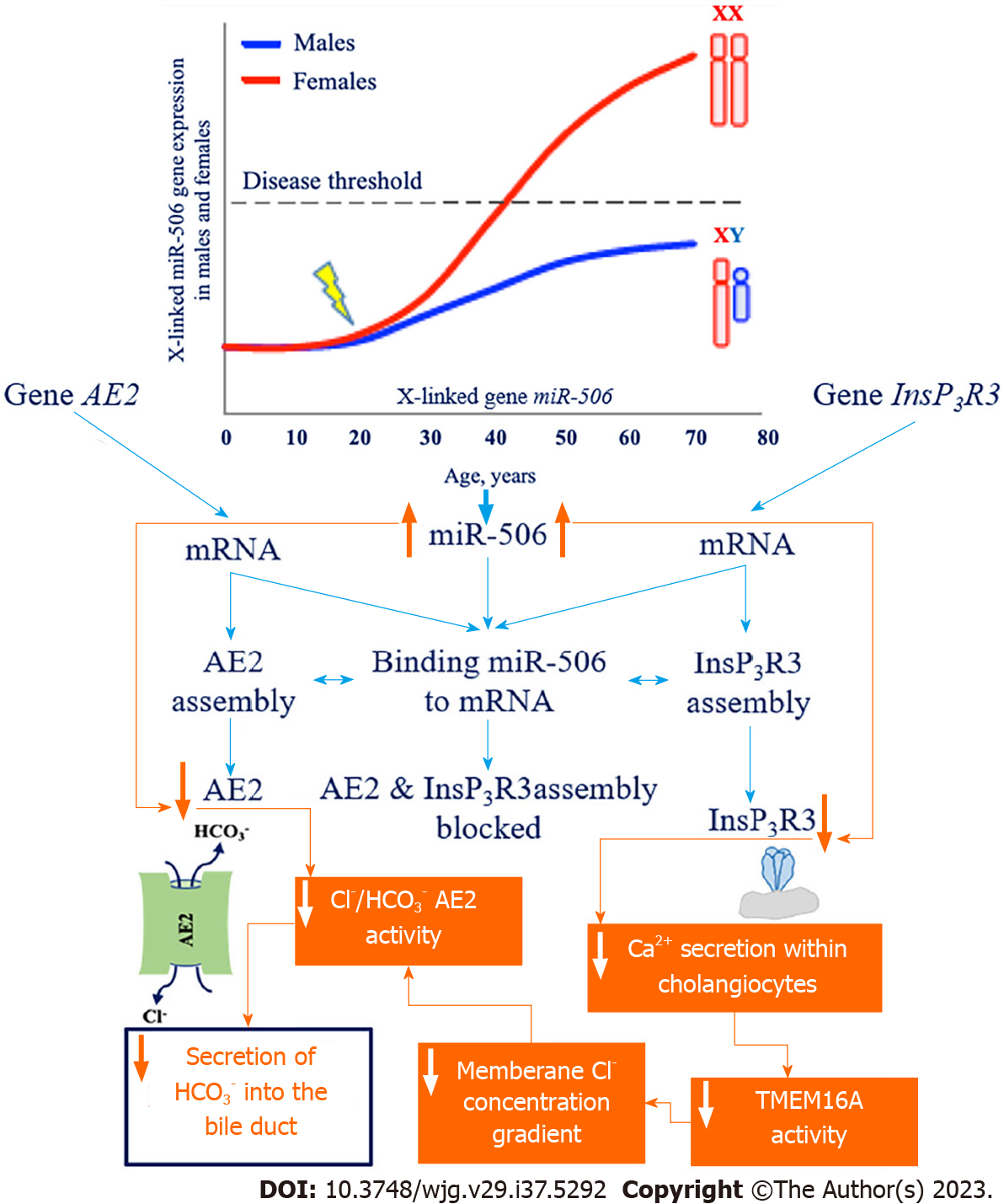
In PBC, there is a reduction in the protective role of HCO3- for cholangiocytes. The theory of defective “biliary HCO3- umbrella” has been extensively discussed[1,17,22]. This theory is based on a number of clinical and experimental works showing insufficient HCO3- supply to the bile ducts in PBC, which leads to a shift in the pH of intraductal (hepatic) bile to the slightly acidic region and an increase of pH in cholangiocytes to the slightly alkaline region. The reasons for the insufficient production of HCO3- by cholangiocytes are unknown. The involvement of InsP3R3 and AE2 in this process is discussed. The expression of InsP3R3 and AE2 genes is reduced in the liver biopsy specimens and blood mononuclear cells of patients with PBC, indicating their dysfunction and involvement in the pathogenesis of this disease[60,61]. The decreased expression and activity of InsP3R3 and AE2 is associated with increased microRNA 506 (miR-506) expression in cholangiocytes[62]. MiRNAs are small noncoding RNAs 22-23 nucleotides long that inhibit gene expression by full or partial pairing with initial sequences located in the 3’-untranslated region (3’-UTR) of mRNA[62].
The 3’-UTR region of the mRNA of InsP3R3[62] and the 3’-UTR region of the mRNA of AE2[63] contain binding sites for miR-506. MiR-506 binding to the 3’-UTR regions of the mRNA of InsP3R3 and AE2 prevents the translation of these proteins. In this way, miR-506 is a regulator of InsP3R3 and AE2 expression (Figure 2). The expression of miR-506 likely undergoes epigenetic regulation and can vary by individual as a result of polymorphisms in nuclear factor kappa B[30].
Figure 2 Mechanism of inositol-1,4,5-trisphosphate receptor type 3 and chloride/carbonate (chloride/bicarbonate) anion exchanger 2 gene expression reduction due to the increase in the amount of micro-RNA 506 and its activity.
InsP3R3: Inositol-1,4,5-trisphosphate receptor type 3; AE2: Chloride/bicarbonate anion exchanger 2; miR-506: Micro-RNA 506; TMEM16A: Transmembrane 16A chloride channels; HCO3-: Bicarbonate; Cl-: Chloride.
An increase in the amount and activity of X-linked miR-506 has been reported in the cholangiocytes of patients with PBC[63], which leads to the decreased expression and activity of InsP3R3 and AE2, potentially explaining the prevalence of this disease in women[30] (Figure 2).
Decreased expression and activity of InsP3R3 in cholangiocytes in PBC[64] impairs intracellular Ca2+ secretion and its use as a messenger in signaling to the transmembrane Cl- channel TMEM16A[44]. Impaired Ca2+ signaling in cholangiocytes in PBC is evidenced by the absence of ATP stimulation of P2YRs on the apical membrane[36]. Decreased Ca2+-dependent activity of TMEM16A on the apical membrane of cholangiocytes leads to the decreased secretion of Cl- ions into the lumen of bile ducts, which is accompanied by decreased activity of the chlorine/HCO3- anion exchanger and impaired secretion of biliary HCO3-. In models of cholangiocytes expressing miR-506, InsP3R3-mediated reduction in intracellular Ca2+ release and decreased fluid and HCO3- secretion into bile ducts has been shown[36,44]. Binding of miR-506 to the 3’-UTR of AE2 mRNA also contributes to decreased Cl-/HCO3- anion exchanger activity and decreased HCO3- secretion by cholangiocytes (Figure 2). Human cholangiocytes isolated from biopsy specimens of patients with PBC show decreased AE2 activity[65]. Thus, the homeostasis of intracellular pH (pHi) in cholangiocytes and bile duct pH in patients with PBC may undergo changes[30]. Changes in intra- and extracellular pH in PBC associated with the loss of InsP3R3 and decreased activity of AE2 promote the protonation of bile acids, their entry into cholangiocytes, and the development of damage to the latter[44].
Destruction of biliary epithelium of small intrahepatic bile ducts during the early asymptomatic stage of PBC is most likely related to the imbalance between the aggressive factors (bile acids) and defense factors (biliary HCO3- umbrella) of cholangiocytes. Because intralobular, interlobular, and septal bile ducts, which are damaged in PBC, do not contain PBGs producing mucin glycoproteins (mucin supraepithelial layer)[21], this defense mechanism for small cholangiocytes most likely does not play a pathogenetic role in the development of PBС.
MECHANISM OF CHOLANGIOCYTE DAMAGE AND DESTABILIZATION OF BILIARY HCO3- UMBRELLA
Decrease of HCO3- supply to bile ducts, due to a decrease in InsP3R3 and AE2 activity, will shift pH in the bile duct lumen to an acidic region[66]. Simultaneously, due to the retention and accumulation of HCO3- in the cytosol of cholangiocytes, there is gradual alkalinization of intracellular pHi in patients with PBC[30,65,67]. Complete AE2 deficiency will lead to intracellular alkalosis of cholangiocytes[61]. However, the reduced (rather than absent) expression of InsP3R3 and AE2 genes has been observed in patients with PBC[61].
A shift of pH to a slightly acidic region in the lumen of bile ducts will increase the amount of protonated unconjugated and glycine-conjugated primary bile acids. This will lead to their increased entry into the small cholangiocytes (intralobular, interlobular, septal) of the bile ducts. Once in the slightly alkaline pHi within the cholangiocytes, the protonated bile acids will undergo deprotonation. Alkalinization of pHi and ionization of glycine-conjugated and unconjugated primary bile acids within cholangiocytes reduces the process of their difundation from intracellular to peribiliary space. As a result, there is a delayed and gradual accumulation of glycine-conjugated and unconjugated primary bile acids in small cholangiocytes. The theory of defective biliary HCO3- umbrella helps to explain the intracellular uncontrolled increased entry and accumulation of bile acids in small BECs.
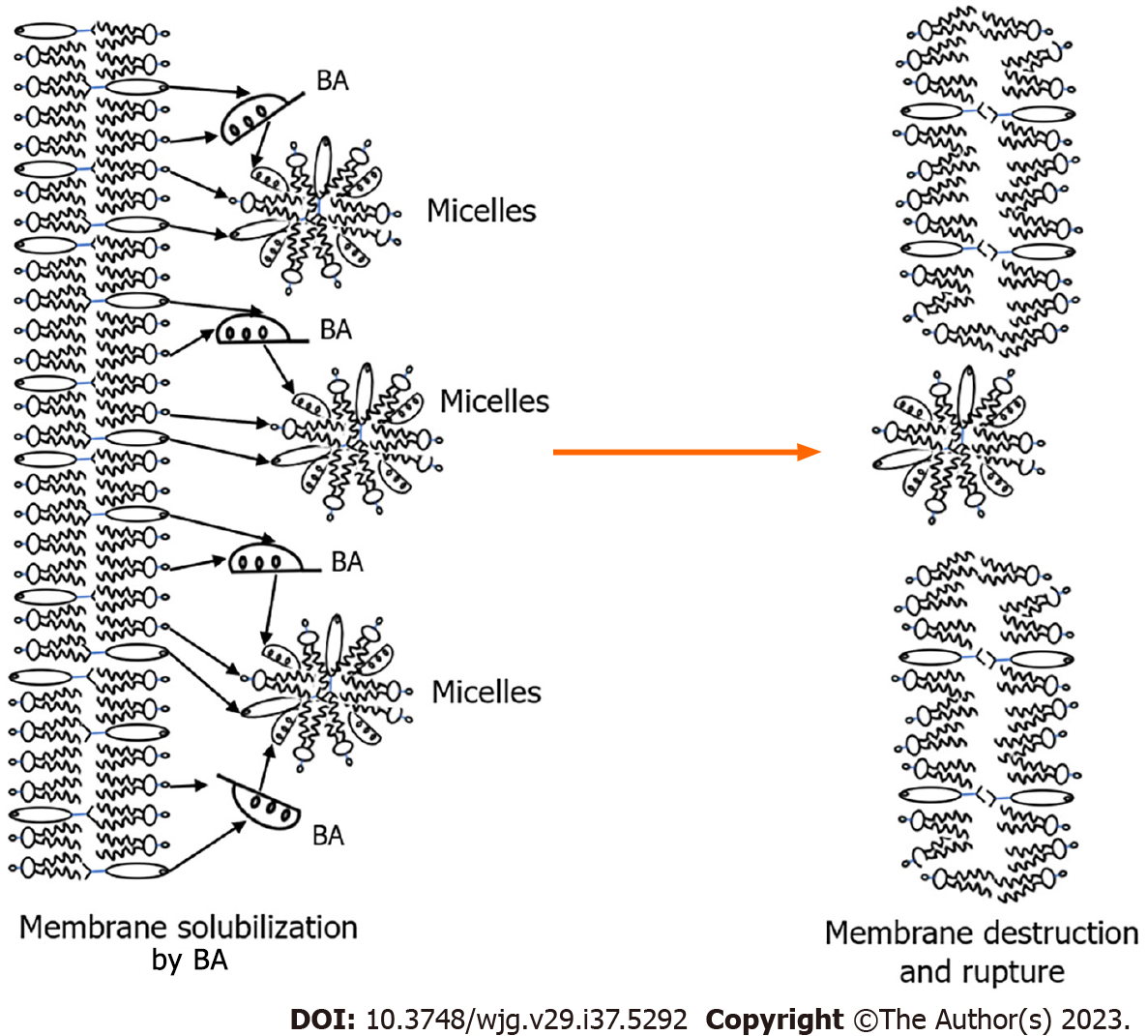
The presence of the mucin-containing glycocalyx layer on the apical surface of large cholangiocytes protects them from penetration and the damaging effect of protonated conjugated and unconjugated bile acids. Intracellular accumulation of hydrophobic bile acids is a prerequisite for their cytotoxic effects[68]. As strong detergents, they are able to solubilize phospholipids and cholesterol from membrane structures of cholangiocytes, which leads to damage and destruction of cytoplasmic membrane and membranes of cell organelles (Figure 3). Thus, the entry and accumulation of unconjugated bile acids with stronger detergent properties in small cholangiocytes is more toxic for the cell than the accumulation of conjugated bile acids.
Figure 3 Solubilization of phospholipids and cholesterol from membrane structures by bile acids.
BA: Bile acids.
The chronic damaging effect of bile acids on membrane structures triggers accelerated senescence, necrosis, and/or apoptosis of BECs[69]. Bile acids destroy the membranes of cell organelles and nuclear membrane of cholangiocytes with the release of apoptogenic factors. The barrier function of the biliary epithelium is impaired, resulting in concomitant damage, inflammation, and oxidative stress. Cytokines, chemokines, and pro-inflammatory mediators released by cholangiocytes probably stimulate apoptotic and proliferative responses as well as activate fibrogenesis[70]. Bile acids also mediate their toxic, apoptotic effects through specific signaling pathways at the intracellular level. The intrinsic apoptotic pathway is activated, including mitochondrial translocation of B-cell lymphoma 2-associated X protein, release of cytochrome C from mitochondria, activation of caspase 3, cleavage of poly (ADP-ribose) polymerase, and DNA fragmentation[71]. There is evidence that miR-506 activates the apoptosis pathway upon stimulation with toxic bile acids[66].
Proinflammatory cytokines additionally increase miR-506 expression[30]. A vicious circle develops that supports senescence, apoptosis, and proliferation of cholangiocytes. Ultimately, ductulopenia develops[30]. Together, this reflects the direct effects of bile acids on cholangiocytes rather than the nonspecific effects resulting from periportal inflammation[31].
From a pathophysiologic point of view, common to all cholangiopathies is the coexistence of cholangiocyte death and proliferation, as well as various degrees of portal inflammation and fibrosis[70]. Cell death induces the activation of inflammatory and profibrogenic pathways that trigger the development and progression of fibrosis, which gradually leads to small bile duct ductulopenia[72]. The conceptual mechanisms of these processes have been described in reviews[69,72].
Disruption of apoptosis is considered a trigger of PBC and already in the asymptomatic stage leads to the development of small bile duct ductulopenia, one of the early morphologic signs of the disease[73]. Apoptosis depends on mitochondrial permeabilization associated with excessive intracellular accumulation of bile acids[74-76]. In addition, bile acids and incomplete apoptosis of BECs diverted to necrosis can lead to pathogenic effects on intracellular components with subsequent generation of AMAs[77].
MECHANISM OF MITOCHONDRIAL PERMEABILIZATION AND AMAs FORMATION
Solubilization of phospholipids and cholesterol from the outer membrane of mitochondria by bile acids leads to their permeabilization[78]. There is an increase in the permeability of the mitochondrial outer membrane to ions and solutes[71,78]. There is leakage of the contents of the intermembrane space into the cytosol and loss of membrane potential. Mitochondria swell, their outer membrane swells, and the release of apoptogenic factors occurs[78]. The inner mitochondrial membrane, the main target for AMA formation, is opened. Further solubilization by bile acids of phospholipids and cholesterol from the inner membrane and destruction of mitochondria can lead to the release and degradation of PDC. The latter includes three enzymes: E1 PDC, E2 PDC, and E3 PDC[73]. Each of these enzymes, in addition to the protein part, has cofactors: E1 PDC contains thiamine pyrophosphate as a cofactor; E2 PDC contains lipoic acid and coenzyme A; and E3 PDC contains flavin adenine dinucleotide and nicotinamide adenine dinucleotide. E1 and E3 PDC are protein complexes that do not contain lipid components. Therefore, they are unlikely to be affected by bile acids accumulated in cholangiocytes, since they have an effect on lipid components. Sera from PBC patients do not show serologically detectable reactivity against the E1 and E3 components of PDC[79].
E2 PDC is a lipoprotein with two lipoic acid binding sites[8]. E2 PDCs contain an essential lysine residue in the lipoyl domain to which lipoic acid is covalently attached[8]. The lipoic-lysine bond at position 173 is highly conserved across species and is essential for antigen recognition[80]. AMAs target immunodominant epitopes containing lipoic acid.
The importance of chemical xenobiotics capable of modifying lipoic acid in E2 PDC has been previously shown for the appearance of serologic reactivity of this complex[81,82]. Alteration of the conformational structure of the lipoyl domain of E2 PDC, due to chemical modification of lipoic acid may contribute to the loss of immune tolerance[83,84]. Most likely, such chemical modifiers in PBC are bile acids accumulating in cholangiocytes upon loss of the protective properties of the biliary HCO3- umbrella. Bile acids can interact with the lipoic acid of the antigen-recognized E2 PDC. The result of such an interaction may be immunomodification of the E2 PDC complex with acquisition of autoantigenic properties and loss of immune tolerance[66]. This assumption is supported by a number of studies performed at the end of the last century. In these works, it was shown that the main immunogenic region on E2 PDC recognized by sera from patients with PBC is localized in the lipoyl-containing domain[85-87]. The lipoic acid content of E2 PDC is thought to play a role as a potent adjuvant[88]. The presentation of immunomodified E2 PDC complex to lymphocytes can lead to stimulation of the T-cell subpopulation and specific production of AMAs[66,88,89].
A defective biliary HCO3- umbrella triggers a continuous and endless process of accumulation and detergent action of bile acids on small cholangiocytes with the formation of AMAs. Since the disruption of HCO3- entry into the lumen of the bile duct is constant, the production of AMAs will be continuous. As a result, an elevated level of IgM (M2) will be constantly maintained in the plasma of PBC patients. The appearance of AMAs in serum is another early immunologic pathognomonic sign of PBC, which occurs in the asymptomatic stage of the disease.
DYSFUNCTION OF THE PDC COMPLEX AND THE FIRST CLINICAL SIGNS OF THE ASYMPTOMATIC STAGE OF PBC
AMAs detection in the asymptomatic stage of the disease is accompanied by the appearance of the first subjective clinical signs, namely weakness, malaise, fatigue, and decreased performance[90]. Fatigue is the most common symptom of PBC in the asymptomatic and early stage of the disease[91-93], occurring in about 40%-80% of patients[94,95]. However, there is no correlation between fatigue and the severity or duration of the disease[95-98].
The mechanism underlying fatigue development is closely related to gradually progressive energy deficiency[99]. The latter is most likely related to the involvement of the PDC in the pathologic process of PBC development. The PDC is a very important metabolic enzyme. PDC functions in every cell and is required for the conversion of pyruvate to acetyl-CoA, which is incorporated into the Krebs cycle and is essential for the body to obtain energy in the form of ATP[73]. As mitochondria in cholangiocytes permeabilize and PDC becomes involved in AMAs production, there is a gradual decrease in ATP synthesis. This leads to the development of local energy deficiency, which in turn, enhances the senescence and apoptosis processes of small BECs initiated by bile acids. A vicious cycle occurs, contributing to the progression of ductulopenia and AMAs formation. In this case, AMAs are able to react with polypeptides including E2 PDC in the mitochondria of almost any cell. It has been shown that antibodies to PBC cross-react with polypeptides in the mitochondria of beef heart, presumably related to E2 PDC[100]. ATP production decreases and energy deficiency develops throughout the organism.
The development of energy deficiency is accompanied by an increase in glycogenolysis and a decrease in glycogenogenesis. The study by Green et al[101] showed that in the initial stages of PBC, there was a gradual decrease in glycogen stores in the liver associated with increased glycogenolysis and decreased glycogenogenesis. The authors also demonstrated that glucokinase activity significantly dropped (down to zero) in patients with PBC, indicating a decrease in glycogen formation in the liver[101]. At the same time, hexokinase (phosphorylates hexoses), which is responsible for glycogen synthesis mainly in muscles, was significantly increased in patients with PBC during this period compared to healthy individuals[101]. As a result of the developing energy deficiency in the asymptomatic stage of the disease, the first clinical signs of expressed weakness, rapid fatigue, decreased performance, functional status, and quality of life appear in patients with PBC[90,102-104].
CONCLUSION
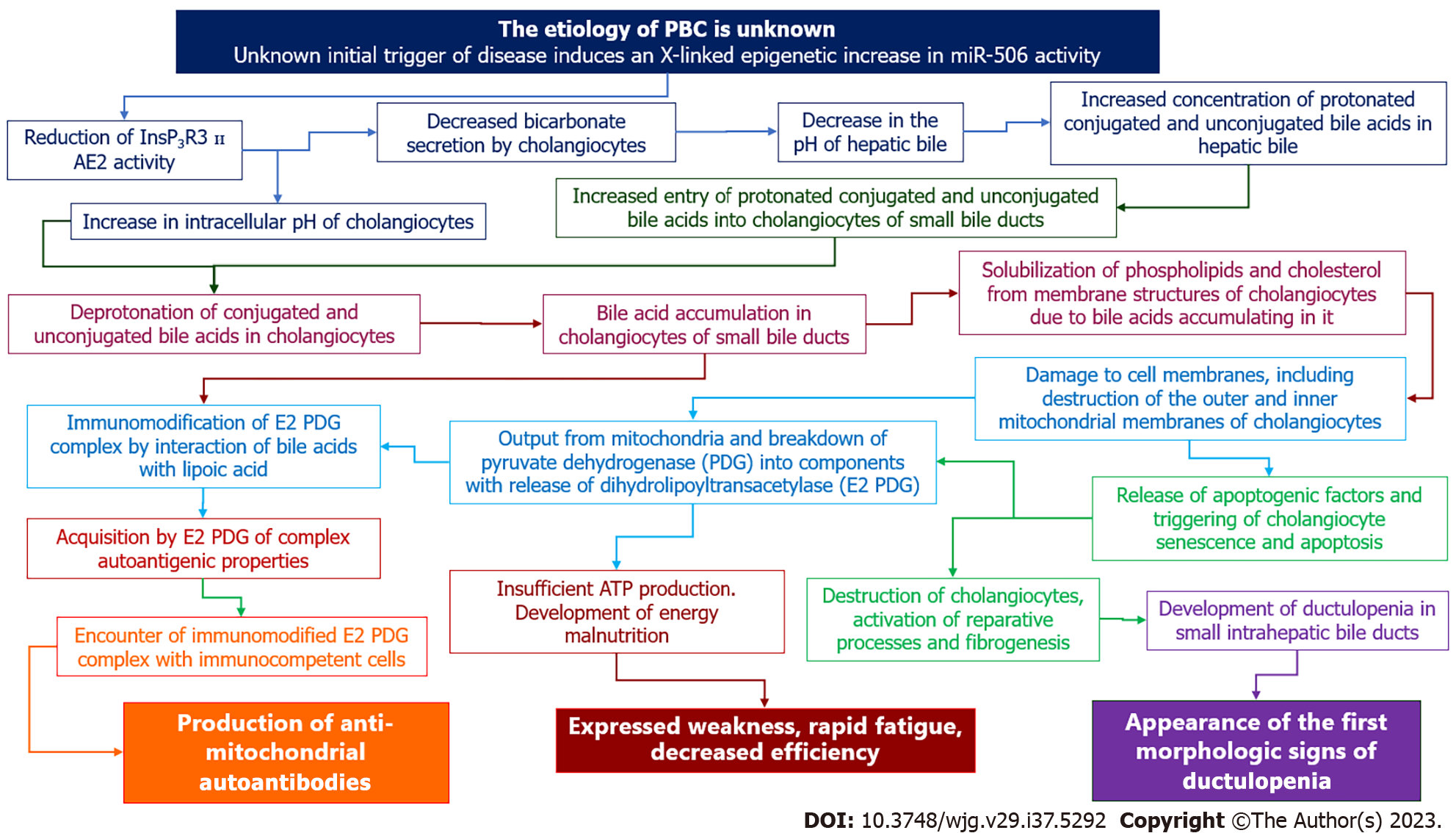
Based on the growing body of knowledge on the molecular mechanisms underlying the development of cholangiocyte damage in patients with PBC, we proposed a hypothesis to explain the pathogenesis of the initial morphologic (ductulopenia), immunologic (AMAs) and clinical (weakness, malaise, rapid fatigue) signs of the disease in the asymptomatic stage (Figure 4).
Figure 4 Mechanism of anti-mitochondrial antibody formation, development of ductulopenia, weakness, fatigue and malaise in the asymptomatic stage of primary biliary cholangitis: hypothesis.
InsP3R3: Inositol-1,4,5-trisphosphate receptor type 3; AE2: Chloride/bicarbonate anion exchanger 2; PDG: Pyruvate dehydrogenase; ATP: Adenosine triphosphate.
Evidence suggests that in susceptible individuals, an unknown initial trigger causes an X-linked epigenetic change that leads to gene reactivation and increased expression of miR-506[30]. The increased synthesis and activation of miR-506 leads to inhibition of InsP3R3 and AE2 translation[105]. As a result, HCO3- entry into the bile duct lumen is reduced and HCO3- accumulation in the cytosol of cholangiocytes occurs[30]. Changes in extra- and intracellular pH alter the protonation (in the lumen of the bile duct) and deprotonation (intracholangiocyte) of bile acids, leading to an increase in the uncontrolled entry and accumulation of unconjugated and glycine-conjugated bile acids into the BECs.
The detergent properties of bile acids trigger cell membrane disruption, senescence and apoptosis of cholangiocytes, mitochondrial permeabilization, destruction and immunomodification of E2 PDC, followed by AMAs formation. Senescence, apoptosis, and proliferation of cholangiocytes lead to the gradual development of ductulopenia. The involvement of PDC in the pathological process contributes to insufficient ATP synthesis, development of energy deficiency, and occurrence of the nonspecific clinical sign of fatigue. The development of ductulopenia is accompanied by the development of intrahepatic cholestasis. Cholangiocytes are the main target in the initial stage of PBC. However, as soon as cholestasis develops, hepatocytes are also involved in the pathological process, which leads to their damage[30].
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Tsoulfas G, Greece S-Editor: Qu XL L-Editor: Webster JR P-Editor: Yu HG