Published online Sep 21, 2023. doi: 10.3748/wjg.v29.i35.5154
Peer-review started: July 5, 2023
First decision: August 10, 2023
Revised: August 23, 2023
Accepted: September 5, 2023
Article in press: September 5, 2023
Published online: September 21, 2023
Processing time: 71 Days and 9.3 Hours
Patients with sepsis are at high risk for acute gastrointestinal injury (AGI), but the diagnosis and treatment of AGI due to sepsis are unsatisfactory. Heparanase (HPA) plays an important role in septic AGI (S-AGI), but its specific mechanism is not completely understood, and few clinical reports are available.
To explore the effect and mechanism of HPA inhibition in S-AGI patients.
In our prospective clinical trial, 48 patients with S-AGI were randomly assigned to a control group to receive conventional treatment, whereas 47 patients were randomly assigned to an intervention group to receive conventional treatment combined with low molecular weight heparin. AGI grade, sequential organ failure assessment score, acute physiology and chronic health evaluation II score, D-dimer, activated partial thromboplastin time (APTT), anti-Xa factor, inter
Serum HPA and SCD-1 levels were significantly reduced in the intervention group compared with the control group (P < 0.05). In addition, intestinal fatty acid-binding protein, D-lactate, AGI grade, motilin, and gastrin levels and sequential organ failure assessment score were significantly decreased (P < 0.05) in the intervention group. However, LC3B, APTT, anti-Xa factor, and CD4/CD8 were significantly increased (P < 0.05) in the intervention group. No significant differences in interleukin-6, tumour necrosis factor-α, d-dimer, acute physiology and chronic health evaluation II score, length of ICU stay, length of hospital stay, or 28-d survival were noted between the two groups (P > 0.05). Correlation analysis revealed a significant negative correlation between HPA and LC3B and a significant positive correlation between HPA and AGI grade. ROC curve analysis showed that HPA had higher specificity and sensitivity in diagnosis of S-AGI.
HPA has great potential as a diagnostic marker for S-AGI. Inhibition of HPA activity reduces SDC-1 shedding and alleviates S-AGI symptoms. The inhibitory effect of HPA in gastrointestinal protection may be achieved by enhanced autophagy.
Core Tip: Heparanase (HPA) plays an important role in the occurrence and development of septic acute gastrointestinal injury (S-AGI). Our experimental results show that HPA has great potential as a diagnostic marker for S-AGI. Inhibition of HPA activity reduces syndecan-1 shedding, reduces inflammatory response, improves coagulation and immune function, and alleviates S-AGI symptoms. The inhibitory effect of HPA on gastrointestinal protection may be achieved by increasing the level of autophagy.
- Citation: Chen TT, Lv JJ, Chen L, Li M, Liu LP. Heparanase inhibition leads to improvement in patients with acute gastrointestinal injuries induced by sepsis. World J Gastroenterol 2023; 29(35): 5154-5165
- URL: https://www.wjgnet.com/1007-9327/full/v29/i35/5154.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i35.5154
Sepsis, a life-threatening condition caused by the host’s dysfunctional response to infection, is a common condition in the intensive care unit (ICU) and is associated with acute organ dysfunction and a high risk of death[1]. Sepsis has become an important public health problem worldwide due to its extremely high prevalence and mortality[2-4]. The intestine is one of the organs most vulnerable to dysfunction caused by sepsis[5]. It has been reported that sepsis causes acute gastrointestinal injury (AGI) in more than 90% of patients[6] and that gastrointestinal function is an important determinant of outcome in ICU patients[7]. Thus, AGI is the central link of sepsis. During sepsis, increased cytokine levels lead to increased intestinal mucosal permeability, in which activated myosin light streptokinase increases paracellular permeability and leads to contraction or opening of tight junctions in the apical region. Increased intestinal permeability subsequently leads to increased systemic inflammation through a positive feedback loop, forming a vicious cycle[8,9]. Treatment of septic AGI (S-AGI) currently consists mainly of prevention and correction of intestinal flora disorders, administration of intestinal mototropic agents, and early restoration of intestinal nutrition. However, these treatments do not necessarily have satisfactory therapeutic results[10]. Therefore, it is of great significance to explore treatment for S-AGI.
Heparanase (HPA) is the only enzyme in the body that can degrade heparin/heparin sulfate. HPA exists in lysosomes in the form of protonase and is widely activated in the context of tumours, inflammation, injury, hypertrophic lesions and immune reactions[11,12]. HPA degrades the heparin sulfate side chain of heparan sulfate proteoglycan (HSPG) and destroys the extracellular matrix and basement membrane, thereby damaging the structural integrity of cells[13]. In addition, HPA exhibits nonenzymatic functions, including cell signaling, adhesion, and differentiation[14]. HPA plays an important role in sepsis. A recent study demonstrated that HPA expression increases during sepsis and is associated with mortality[15]. In our previous review, we reasonably hypothesized that HPA is involved in the occurrence and development of S-AGI[16]. However, the mechanism is unclear, especially in clinical practice, and needs further investigation.
Low molecular weight heparin (LMWH) derived from common heparin is widely used due to its excellent efficacy, good predictability, low risk of bleeding, and reduced number of side effects[17]. With deepening of research, LMWH has been used in other applications in addition to anticoagulation as an anti-inflammatory, anti-fibrosis, antitumour, or antiviral agent[18-20]. These actions are all achieved by inhibiting HPA. As an inhibitor of HPA, LMWH is widely used in sepsis and inflammatory bowel disease[21,22]. Therefore, LMWH was selected as the intervention drug for the intervention group. In this study, we aimed to explore whether the gastrointestinal symptoms of S-AGI patients improve after HPA suppression and whether indicators of inflammation, coagulation, immunity, and survival status improve. The possible mechanism was also explored.
This study was a prospective double-blind randomized controlled trial approved by the Ethics Committee of the First Hospital of Lanzhou University. The ethics number is LDYYLL2022-270. S-AGI patients in the ICU of the First Hospital of Lanzhou University were selected from March 2022 to February 2023. The flow chart is presented in Figure 1, and 95 patients were finally included in the study.
Inclusion criteria: (1) Age ≥ 18 years old, sex unrestricted; (2) Patient meets the diagnostic criteria for sepsis 3.0 [positive or suspected infection with Sequential Organ Failure Assessment (SOFA) ≥ 2 points][1]; (3) Patient meets the AGI diagnostic criteria [(ESICM) 2012 recommendation AGI severity rating][6]; and (4) Informed consent signed by the patient or his or her family.
Exclusion criteria: (1) Combined with underlying gastrointestinal diseases (tumour, tuberculosis, inflammatory diseases, etc.); (2) Gastrointestinal surgery; (3) Patients with terminal disease expected to die within 24 h; (4) Patients with neurogenic shock, cerebrovascular accident, or craniocerebral trauma; and (5) Patients with definite haemorrhagic disease.
Patients who met the inclusion criteria were randomly assigned to the control group or the intervention group by hierarchical randomization generated by SAS statistical software. A letter for each random number was prepared in duplicate in a blind manner and sealed. At the time of statistical analysis, the blinding was exposed twice, the first blinding involved dividing the patients into groups, and the specific drugs in each group were determined at the second blinding. However, if the patient’s condition recurred or haemodynamic instability affected the patient’s prognosis during the study, it was terminated, and the blinding was urgently removed.
The control group included 48 patients who received conventional treatment; 47 patients in the intervention group were treated with LMWH in addition to conventional treatment. The control group received special intensive care as needed, including oxygen or mechanical ventilation, antimicrobial therapy, vasopressor administration, fluid resuscitation, blood glucose control, nutritional support, analgesia, sedation, or renal replacement therapy. The control group did not receive heparin as the standard of care for S-AGI patients. In the intervention group, patients were administered LMWH sodium (4000 U qd, subcutaneous injection) for 7 consecutive days in addition to receiving standard treatment as described above. The control group was given the same dose of saline (subcutaneous injection) for 7 consecutive days.
Baseline data, such as age, sex, body mass index, source of infection, indicators of infection, AGI grade, SOFA score, and Acute Physiology And Chronic Health Evaluation II (APACHE II) score, of all patients were collected at admission. Gastrointestinal functional status was observed at 1, 3 and 7 d after treatment. Specifically, AGI grading assessment, SOFA score, APACHE II score, D-dimer, activated partial thromboplastin time (APTT), and anti-Xa factor coagulation index data were collected. Serum interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), HPA, syndecan-1 (SDC-1), LC3B, intestinal fatty acid binding protein (IFABP), D-lactate, motilin and gastrin levels were measured by enzyme-linked immunosorbent assay (ELISA). CD4 and CD8 T cells were detected by flow cytometry. The length of ICU stay and length of hospital stay were assessed, as was survival status at 28 d of all patients.
Serum samples were diluted at an appropriate ratio, and the standard working solution was configured according to the kit instructions (Elabscience, Shanghai, China). Standard, blank and sample wells were established. Then, 100 μL of standard, standard and sample diluent and serum samples to be tested were added and incubated at 37 °C for 90 min. The biotinylated antibody working solution, enzyme binding working solution, substrate solution and termination solution were added successively. After the reaction was terminated, the optical density (OD value) of each well was immediately measured based on an enzyme label at 450 nm.
FITC-labelled (the reagents were purchased from Boster, Wuhan, China) mouse anti-human CD3 antibody (2 μL), APC-labelled mouse anti-human CD4 antibody (1 μL), and PerCP/Cy5.5 mouse anti-CD8B monoclonal antibody (1 μL) were placed into flow cytometry test tubes. One hundred microlitres of whole peripheral blood was obtained and incubated at room temperature for 15 min after shaking and mixing. Then, 500 μL of haemolysin, 200 μL of phosphate buffered saline and 100 μL of fully mixed microspheres were added, and the specimens were assessed by flow cytometry. Cells were analysed by Kaluza Analysis software to obtain CD4 and CD8 T-cell counts.
Normally distributed data are expressed as the mean ± standard deviation (SD) and were compared with a t test. Nonnormally distributed data are expressed as the median (interquartile range) and were compared using the Mann-Whitney U test. Counting data were tested using χ2 tests. The Kolmogorov-Smirnov test was used to test the normal distribution of data. To take into account the repeated nature of the variables, analysis of variance for repeated measurements of the general linear model was implemented. Correlations were analysed using the Pearson method. The Kaplan-Meier method was used to generate a survival curve within 28 d after inclusion. The diagnostic value of HPA was evaluated by receiver operator characteristic (ROC) curve analysis. Graphs were generated using GraphPad Prism 8.0.2 software (SYSTAT, United States), and P < 0.05 was considered statistically significant.
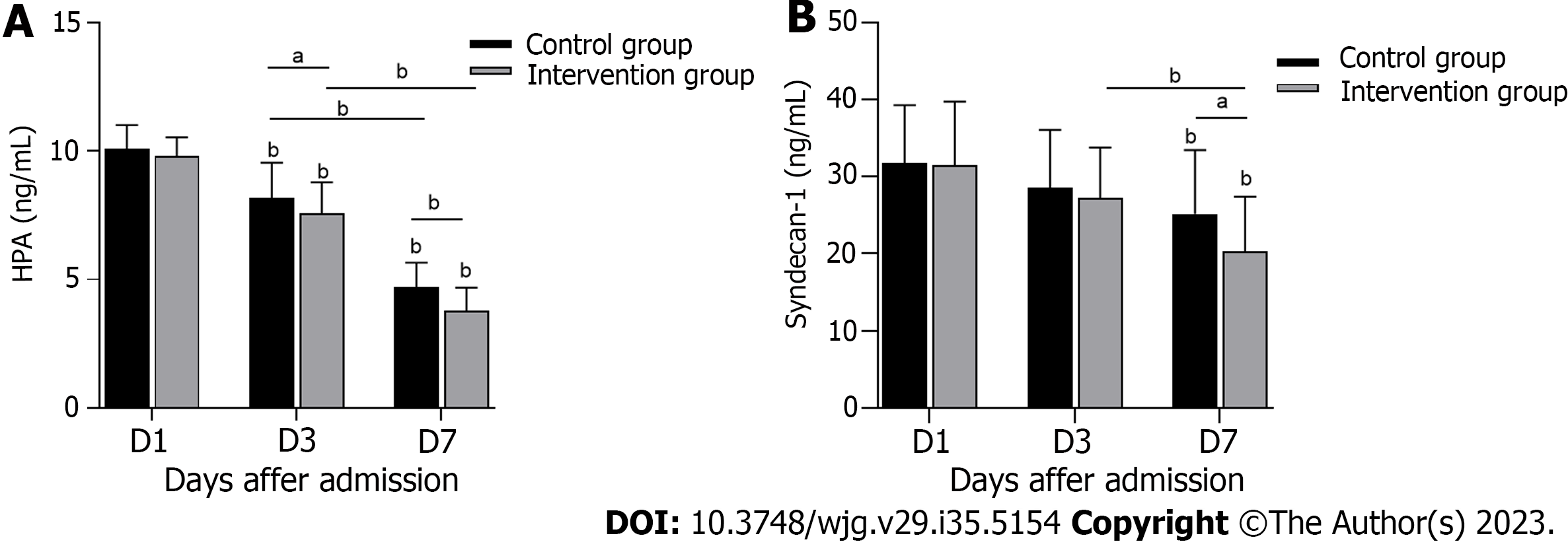
A total of 130 patients were screened during the trial (Figure 1). Regarding loss to follow-up, 7 patients were transferred to hospitals for treatment or contact was lost after discharge and could not be followed up. In total, 95 patients with S-AGI were finally included. Of these patients, 48 were randomly assigned to the control group and 47 to the intervention group. The baseline data and clinical parameters of the patients at admission are presented in Table 1. The mean age of the control group was 59.90 ± 18.81 years old, and 68.75% were male. The mean age of patients in the intervention group was 60.98 ± 14.10 years old, and 70.21% were male. In the control group, 9 patients (18.75%) were classified as having AGI grade I, 13 patients (27.08%) as having AGI grade II, 20 patients (41.67%) as having AGI grade III, and 6 patients (12.50%) as having AGI grade IV. In the intervention group, 8 cases (17.02%), 10 cases (21.28%), 22 cases (46.81%) and 7 cases (14.89%) were classified as AGI grades I, II, III and IV, respectively. No significant differences in serum white blood cell counts or procalcitonin, HPA and SDC-1 levels were noted between the two groups (P > 0.05). Overall, the two groups were well balanced in terms of baseline characteristics.
| Variable | Control group (n = 48) | Intervention group (n = 47) | P value |
| Age, mean (SD), yr | 59.90 (18.81) | 60.98 (14.10) | 0.752 |
| Sex, male, n (%) | 33 (68.75) | 33 (70.21) | 0.877 |
| BMI, mean (SD), kg/m2 | 22.62 (4.08) | 23.89 (5.10) | 0.788 |
| MODS, n (%) | 33 (68.75) | 34 (72.34) | 0.701 |
| Septic shock, n (%) | 32 (66.67) | 31 (65.96) | 0.942 |
| APACHE II score, median (IQR) | 22 (19, 29) | 23 (19, 35) | 0.966 |
| SOFA score, median (IQR) | 9 (7,10.75) | 9 (7, 13) | 0.871 |
| Infection score, n (%) | |||
| Lung | 10 (20.83) | 16 (34.04) | 0.149 |
| Urinary tract | 2 (4.17) | 1 (2.13) | 0.57 |
| Intra-abdominal | 14 (29.17) | 16 (34.04) | 0.609 |
| Central nervous system | 13 (27.08) | 7 (14.89) | 0.145 |
| Blood/vascular access | 3 (6.25) | 4 (8.51) | 0.673 |
| Other | 5 (10.42) | 2 (4.26) | 0.25 |
| Confirmed unknown | 1 (2.08) | 1 (2.13) | 0.988 |
| Initial AGI grade, n (%) | |||
| I | 9 (18.75) | 8 (17.02) | 0.826 |
| II | 13 (27.08) | 10 (21.28) | 0.509 |
| III | 20 (41.67) | 22 (46.81) | 0.614 |
| IV | 6 (12.50) | 7 (14.89) | 0.734 |
| WBC, mean (SD), (109/L) | 19.20 (9.91) | 15.92 (9.65) | 0.424 |
| PCT, mean (SD), (ng/mL) | 10.77 (21.64) | 11.19 (17.58) | 0.919 |
| HPA, mean (SD), (ng/mL) | 10.10 (0.91) | 9.81 (0.72) | 0.095 |
| Syndecan-1, mean (SD), (ng/mL) | 31.77 (7.49) | 31.45 (8.29) | 0.845 |
The serum HPA concentration in the control group was significantly higher than that in the intervention group on the 3rd and 7th d of treatment (Figure 2A) (P < 0.05). Serum SDC-1 also showed a difference between the two groups on the 7th d of treatment (Figure 2B) (P < 0.05). The above data indicate that serum HPA and SDC-1 levels were effectively inhibited in S-AGI patients in the intervention group.
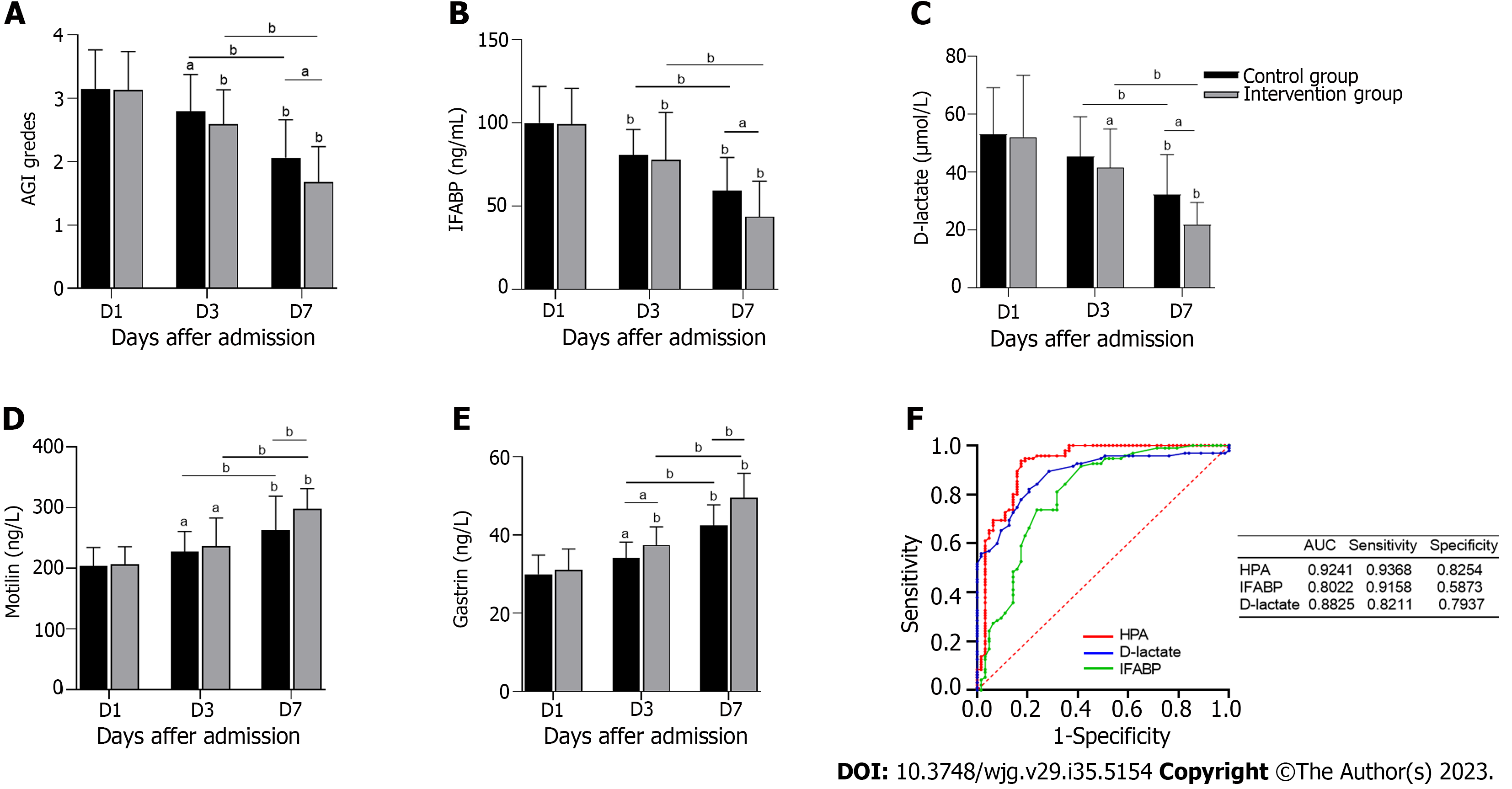
AGI ratings were assessed on the 1st, 3rd and 7th d after treatment (Figure 3A). The AGI grades of both groups decreased and were significantly lower in the intervention group than in the control group on the 7th d (P < 0.05). As shown in Table 2, the number of AGI II, III and IV patients in the intervention group was significantly lower after 7 d of treatment than after 1 and 3 d of treatment. In addition, the number of AGI II, III and IV patients were significantly lower in the intervention group than in the control group. IFABP and D-lactate are intestinal barrier biomarkers. Figures 3B and C shows that serum IFABP and D-lactate concentrations on the 7th d were significantly lower than those on the 1st d, with the concentrations in the intervention group being significantly lower than those in the control group (P < 0.05). Motilin and gastrin are indicators of gastrointestinal motility. As shown in Figures 3D and E, motilin and gastrin levels increased significantly in the intervention group after 7 d of treatment (P < 0.05). All the above data indicate that inhibition of HPA significantly improved gastrointestinal function, the intestinal barrier and gastrointestinal dynamics in S-AGI patients.
| AGI I, n (%) | AGI II, n (%) | AGI III, n (%) | AGI IV, n (%) | |||||
| Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | |
| Day 1 | 0 (0) | 0 (0) | 6 (12.50) | 6 (12.77) | 29 (60.42) | 29 (61.70) | 13 (27.08) | 12 (25.53) |
| Day 3 | 0 (0) | 0 (0) | 14 (29.17) | 20 (42.55) | 30 (62.50) | 26 (55.32) | 4 (8.33) | 1 (2.13) |
| Day 7 | 7 (14.58) | 17 (36.17) | 31 (64.58) | 28 (59.57) | 10 (20.83) | 2 (4.26) | 0 (0) | 0 (0) |
As shown in Figure 3F, we plotted ROC curves for HPA, IFABP and D-lactate and calculated their AUC values. IFABP and D-lactate are biomarkers of septic AGI, but the AUC for HPA of 0.9241 (95% confidence interval: 0.8690-0.9707) was the largest of the three. The sensitivity and specificity of HPA were 93.68% and 82.54%, respectively, and compared with the sensitivity of D-lactate (82.11% and 79.37%) and the sensitivity of IFABP (91.58% and 58.73%), HPA was still highest. These results indicate that HPA has better diagnostic efficacy in S-AGI. Overall, HPA exhibits great potential as a biomarker for S-AGI.
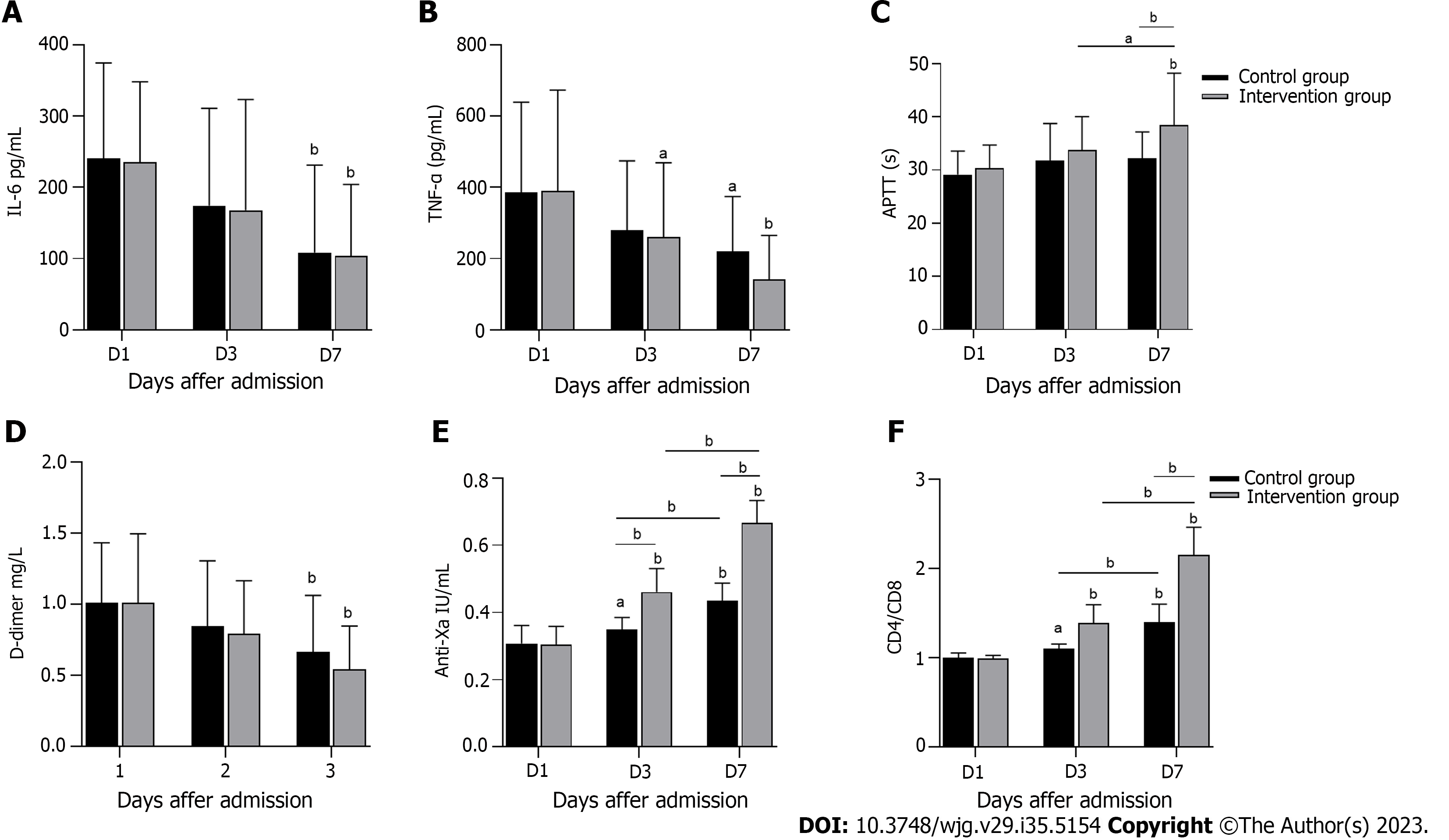
Figure 4 shows the inflammation, coagulation and immune indices of the two groups after treatment. As illustrated in Figures 4A and B, IL-6 and TNF-α serum levels decreased significantly on the 7th d of treatment compared with on the 1st d (P < 0.05). Despite the lack of a significant difference between the two groups, levels of inflammatory cytokines in the intervention group were reduced. After 7 d of treatment, APTT and anti-Xa factor levels in the two groups increased significantly compared with those on the 1st d of treatment (P < 0.05), whereas D-dimer levels decreased significantly (P < 0.05). APTT and anti-Xa factor levels increased significantly in the intervention group compared with the control group (P < 0.05) (Figures 4C-E). The anticoagulation effect in the intervention group was better than that in the control group. As shown in Figure 4F, the intervention group exhibited significantly more CD4/CD8 cells than the control group (P < 0.05). In conclusion, compared with the control group, the intervention group exhibited better anticoagulant effects and immune enhancement effects.
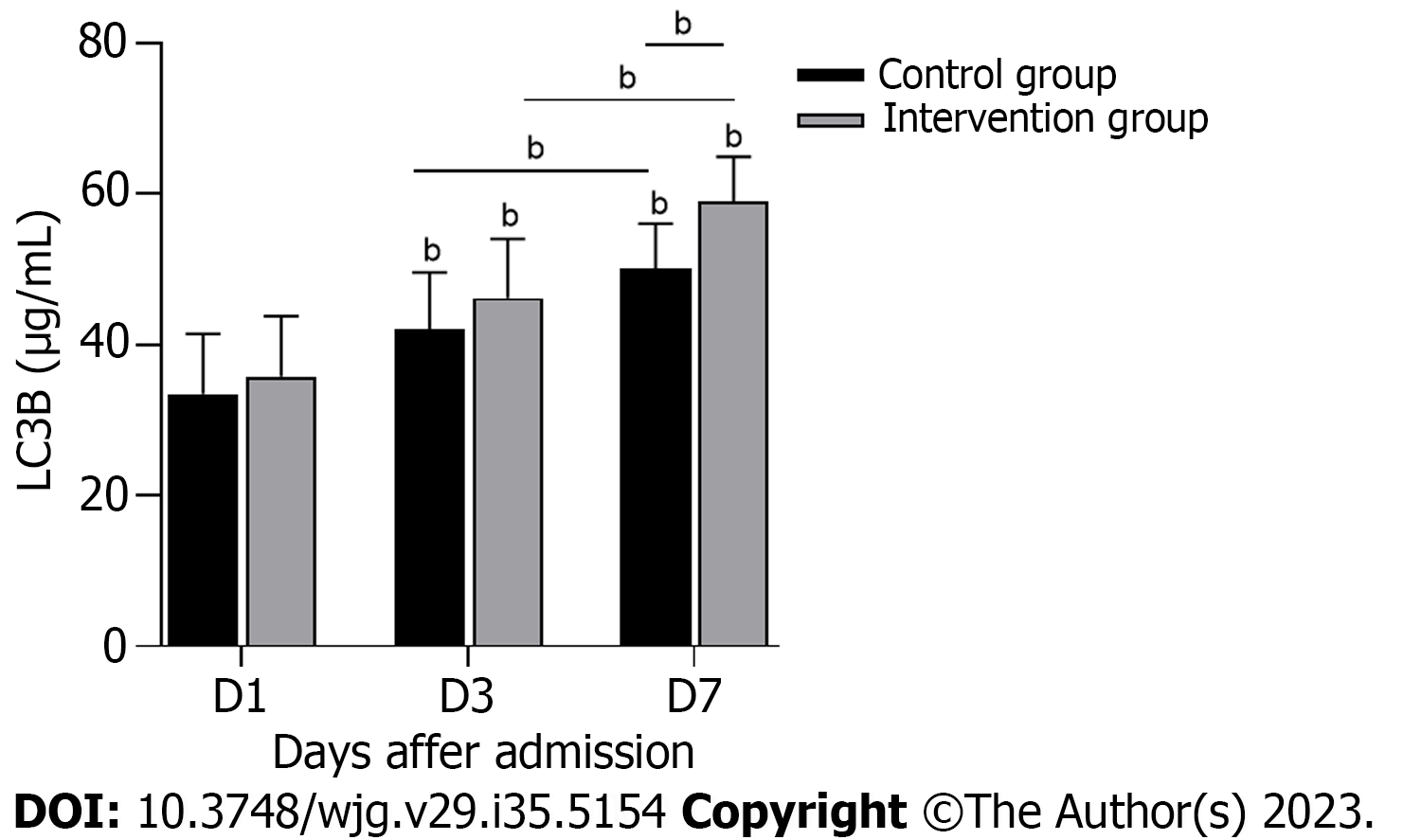
To explore the possible mechanism by which HPA inhibition improves gastrointestinal symptoms in S-AGI patients, autophagy was assessed (Figure 5). The LC3B level of the intervention group was significantly higher than that of the control group (P < 0.05). As shown in Table 3, a significant negative correlation was noted between HPA and LC3B and a significant positive correlation between HPA and AGI grade. Thus, the decrease in serum HPA and SDC-1 is critical for S-AGI patients, and HPA correlates significantly with autophagy and gastrointestinal functional status.
| Control group | Intervention group | ||||||||
| LC3B (μg/mL) | AGI grade | LC3B (μg/mL) | AGI grade | ||||||
| HPA (ng/mL) | Day 1 | r = -0.8394 | P < 0.001 | r = 0.8441 | P < 0.001 | r = -0.8456 | P < 0.001 | r = 0.7106 | P < 0.001 |
| Day 3 | r = -0.9545 | P < 0.001 | r = 0.7670 | P < 0.001 | r = -0.8882 | P < 0.001 | r = 0.8135 | P < 0.001 | |
| Day 7 | r = -0.8258 | P < 0.001 | r =0.7657 | P < 0.001 | r = -0.8724 | P < 0.001 | r = 0.7839 | P < 0.001 | |
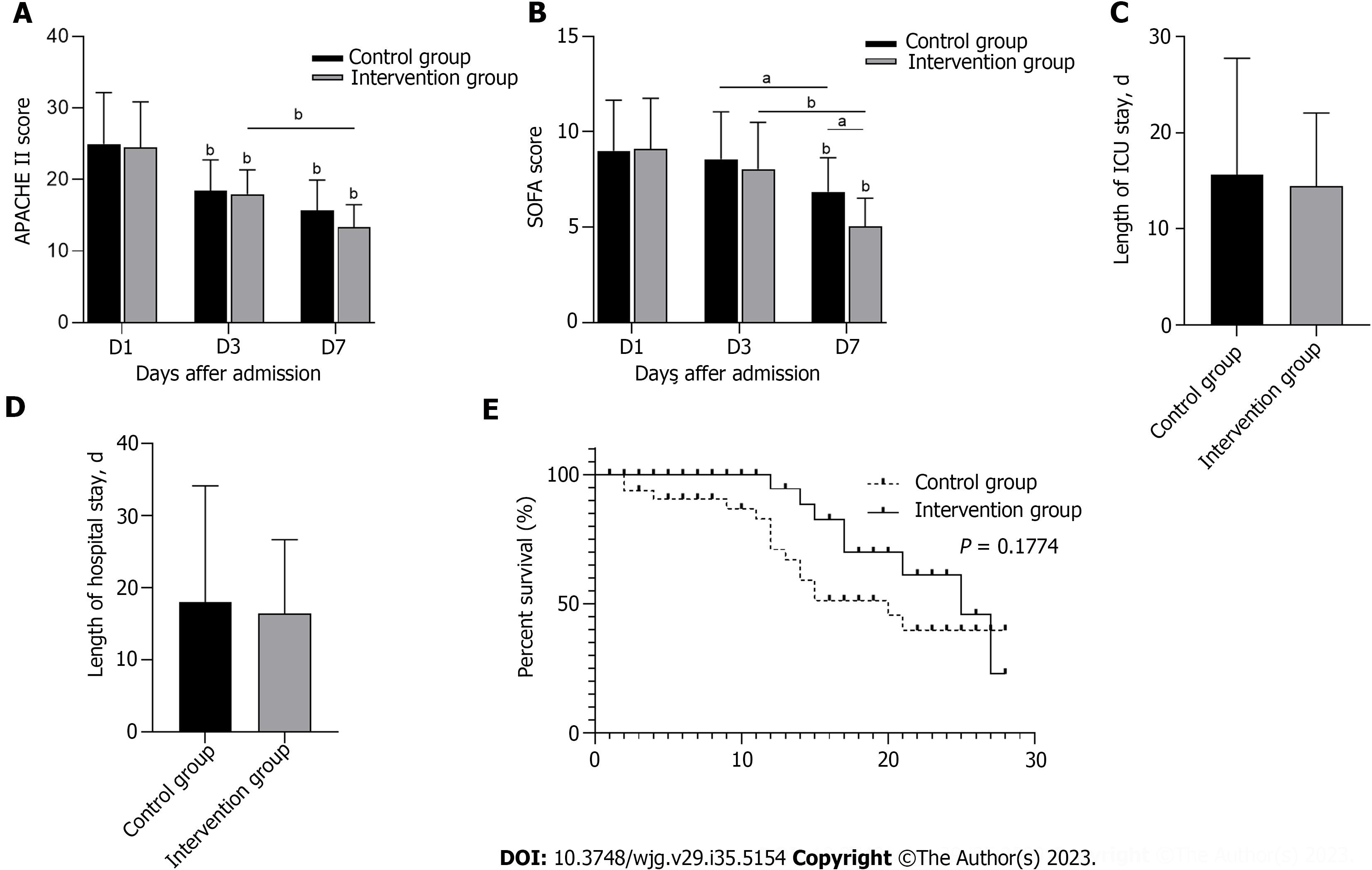
Within 7 d of ICU treatment, the APACHE II score and SOFA score of the two groups had significantly decreased compared to those before ICU treatment (P < 0.05), and the SOFA score of the intervention group was significantly lower than that of the control group on the 7th d (P < 0.05). However, APACHE II scores did not significantly differ between the two groups (Figures 6A and B). Figures 6C and D shows the length of ICU stay and the length of hospital stay. Although no significant difference was noted between the control group and the intervention group, both stays were shorter in the intervention group. The 28-d survival curve presented in Figure 6E demonstrates no significant difference between the two groups (P > 0.05). These results indicated that HPA inhibition improves the clinical severity score of patients but does not significantly improve the length of hospital stay or survival rate.
S-AGI is easily missed clinically. Complex assessment of AGI grading is not based on specific symptoms but rather includes subjective assessment of the overall development of the patient’s disease. The ideal approach is to replace this grading system with one or two biomarkers[23]. Therefore, it is important to explore potential biomarkers and effective therapeutic agents for S-AGI. In this study, we selected LMWH as an intervention drug to reduce HPA levels (Figure 2). Our results indicate that HPA inhibition significantly improved the gastrointestinal functional status of S-AGI patients, reduced the AGI score, improved the intestinal mucosal barrier and gastrointestinal dynamics of patients (Figure 3 and Table 2), and contributed to their early recovery. Regarding the specific mechanism of LMWH in treatment of S-AGI, we hypothesized that LMWH inhibits HPA, protects the glycocalyx, and alleviates damage to the intestinal barrier, thus improving symptoms. This activity is not related to the direct anticoagulant properties of LMWH. Similarly, Tang et al[24] reported that heparin prevents caspase-11-dependent coagulation activation and reduces mortality in sepsis, regardless of its direct anticoagulant properties.
The glycocalyx is a complex, negatively charged gel layer on one side of the lumen of endothelial cells. During sepsis, the glycocalyx becomes degraded through activation of various enzymes and/or release of reactive oxygen species[25,26]. A degraded glycocalyx induces white blood cell binding and extravasation as well as platelet recruitment, resulting in increased inflammation and increased risk of thrombosis. In addition, loss of calyx can lead to capillary leakage, which leads to oedema and reduced blood volume throughout the body. Together with thrombosis, these effects lead to tissue hydroperitoneum and organ failure[27,28]. Thus, protection of glycocalyx integrity and the intestinal barrier is essential for treatment of S-AGI. SDC-1 is a biomarker for the glycocalyx and is a transmembrane HSPG that is expressed primarily by intestinal epithelial cells; this protein is strongly associated with inflammatory processes and the integrity of the intestinal mucosa[18]. A recent meta-analysis showed that SDC-1 levels may be a useful predictor of sepsis-related complications and mortality[29]. Therefore, SDC-1 plays a crucial role in S-AGI. HPA is closely related to SDC-1, which degrades the heparin sulfate side chain of HSPG[13], accelerates shedding of SDC-1 from endothelial cells, and increases serum SDC-1 concentrations. LMWH inhibits HPA activity and prevents endothelial cell injury[28]. Therefore, our intervention results also revealed high HPA and SDC-1 levels in the context of decreased S-AGI after treatment. As HPA was significantly inhibited after conventional treatment combined with LMWH treatment, the concentrations of HPA and SDC-1 decreased more significantly (Figures 2A and B). This finding is consistent with previously reported conclusions[15,30].
Our correlation analysis revealed a significant positive correlation between HPA and AGI levels, with AGI levels decreasing significantly after LMWH inhibited HPA (Tables 2 and 3). Additionally, ROC curve analysis suggested that HPA may serve as a biomarker for S-AGI given that HPA is more specific and sensitive than IFABP and D-lactate (Figure 3F). In conclusion, our results indicate that the gastrointestinal symptoms of S-AGI patients are improved and AGI scores are reduced after HPA inhibition. HPA is expected to serve as a diagnostic biomarker for S-AGI.
In sepsis, extensive cross-talk occurs between inflammatory and clotting pathways, accompanied by overactivity and immunosuppression of the inflammatory and clotting responses, which interferes with microcirculation perfusion and leads to organ failure[31,32]. Patients with S-AGI also exhibit excessive inflammation, hypercoagulability, and immunosuppression, and these conditions improve after treatment, as shown in Figure 4. Unfortunately, there was no significant difference in inflammation between the two groups. HPA activates macrophages, leading to secretion of monocyte chemoattractant protein-1, TNF-α, and IL-1β, independent of heparin sulfate degradation activity[33], and these cytokines appear to be elevated in coronavirus disease 2019 patients[34]. It is worth mentioning that LMWH targets factor Xa to play an anticoagulant role and exhibits high anti-Xa activity[35]; hence, the anticoagulant effect in the intervention group was significantly better than that in the control group. In addition, according to the LMWH dose in our treatment plan, no associated bleeding risk was noted during patient treatment, indicating that LMWH is safe and effective. In this study, we found that CD4/CD8 levels in the intervention group were significantly increased. Therefore, HPA inhibition inhibits hypercoagulability and improves immune function in S-AGI patients.
To further investigate the possible mechanism by which HPA is reduced to improve S-AGI, we measured changes in serum LC3B levels in patients during treatment. The intervention results showed that the LC3B level was increased in the intervention group after treatment, with a significant negative correlation noted between HPA and LC3B (Figure 5, Table 3). LC3B is a marker of autophagy. Autophagy is the process by which bacteria and viruses that have escaped from phagosomes or damaged mitochondria are enclosed in vesicles, which fuse with lysosomes to form autophagosomes, followed by degradation of the contents[36]. In the early stage of sepsis, autophagy occurs in the heart, brain, lung, liver, kidney and other important organs and plays a protective role in the body. With the progression of sepsis, the body enters a period of continuous immunosuppression, and autophagy activity decreases[37]. This finding is consistent with our results. However, the results for LC3B are only indirect evidence and cannot directly show that HPA correlates completely with autophagy. Therefore, we hypothesize that HPA might aggravate S-AGI by inhibiting autophagy, and we are performing further basic experiments to test this hypothesis. LMWH inhibits HPA, thus enhancing the level of autophagy and playing a protective role in the gastrointestinal tract.
Although HPA inhibition offers many advantages, it did not significantly reduce the length of hospital stay or increase the 28-d survival rate of S-AGI patients (Figure 6). We hypothesize that the reason may be the complex aetiology of ICU patients, critical conditions, mixed interference factors during treatment, and/or the small study sample. Thus, the intervention group did not achieve our expected effect.
Finally, our experiment has some limitations: (1) Given our single-centre design and small sample size, the results may not be generalizable, and the conclusion needs to be confirmed by large-scale clinical prospective trials; (2) LMWH is not a specific HPA inhibitor, but a safe and effective specific HPA inhibitor is currently not available in clinical practice. Therefore, further development of new drugs is needed; and (3) Inhibition of HPA may enhance the level of autophagy and thus protect the gastrointestinal tract in sepsis, and this mechanism needs to be verified by basic experiments.
Our intervention results showed that LMWH inhibits HPA activity in S-AGI, reduces SDC-1 shedding, prevents endothelial cell damage, maintains intestinal epithelial cell integrity and barrier function, actively exerts anticoagulant effects, improves patients’ immune function and gastrointestinal symptoms, and reduces SOFA scores. Mechanistically, HPA inhibition may play a protective role in the gastrointestinal tract by enhancing the level of autophagy. HPA represents a potential biomarker of S-AGI, and HPA inhibitors may also serve as drugs for treatment of S-AGI.
Patients with sepsis are at high risk for acute gastrointestinal injury (AGI), heparanase (HPA) plays an important role in septic AGI (S-AGI), but its specific mechanism is not completely understood, and few clinical reports are available.
This study is to explore the effect and mechanism of HPA inhibition in S-AGI patients.
To prove the role of HPA in S-AGI and search for effective biomarkers and therapeutic targets for the diagnosis of S-AGI.
The therapeutic effect of S-AGI patients in control group and low molecular weight heparin group was compared by a prospective double-blind randomized controlled trial. To evaluate the feasibility of HPA as a diagnostic biomarker for S-AGI.
HPA inhibitors can significantly improve AGI score, gastrointestinal function, coagulation function and immune function in S-AGI patients. The inhibitory effect of HPA in gastrointestinal protection may be achieved by enhanced autophagy.
HPA has great potential as a diagnostic marker for S-AGI. Inhibition of HPA activity reduces syndecan-1 shedding and alleviates S-AGI symptoms. The inhibitory effect of HPA in gastrointestinal protection may be achieved by enhanced autophagy.
HPA has great potential as a diagnostic biomarker for S-AGI, and its inhibitor is a good therapeutic drug choice in clinical practice.
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 18845] [Article Influence: 1884.5] [Reference Citation Analysis (4)] |
| 2. | Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5802] [Cited by in RCA: 6046] [Article Influence: 241.8] [Reference Citation Analysis (0)] |
| 3. | Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 716] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 4. | Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 1588] [Article Influence: 198.5] [Reference Citation Analysis (0)] |
| 5. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4952] [Article Influence: 825.3] [Reference Citation Analysis (7)] |
| 6. | Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (2)] |
| 7. | Hu B, Sun R, Wu A, Ni Y, Liu J, Guo F, Ying L, Ge G, Ding A, Shi Y, Liu C, Xu L, Jiang R, Lu J, Lin R, Zhu Y, Wu W, Xie B. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: a multicenter, prospective, observational study. Crit Care. 2017;21:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Sun J, Zhang J, Wang X, Ji F, Ronco C, Tian J, Yin Y. Gut-liver crosstalk in sepsis-induced liver injury. Crit Care. 2020;24:614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 9. | Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML, Coopersmith CM. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock. 2016;46:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 10. | Chen FQ, Xu WZ, Gao HY, Wu LJ, Zhang H, Cheng L, Mei JQ. Clinical effect of Changweishu on gastrointestinal dysfunction in patients with sepsis. J Int Med Res. 2020;48:300060520919579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999;5:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 624] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 12. | Lerner I, Hermano E, Zcharia E, Rodkin D, Bulvik R, Doviner V, Rubinstein AM, Ishai-Michaeli R, Atzmon R, Sherman Y, Meirovitz A, Peretz T, Vlodavsky I, Elkin M. Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J Clin Invest. 2011;121:1709-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Fernandes CL, Escouto GB, Verli H. Structural glycobiology of heparinase II from Pedobacter heparinus. J Biomol Struct Dyn. 2014;32:1092-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Masola V, Bellin G, Gambaro G, Onisto M. Heparanase: A Multitasking Protein Involved in Extracellular Matrix (ECM) Remodeling and Intracellular Events. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Eustes AS, Campbell RA, Middleton EA, Tolley ND, Manne BK, Montenont E, Rowley JW, Krauel K, Blair A, Guo L, Kosaka Y, Medeiros-de-Moraes IM, Lacerda M, Hottz ED, Neto HCF, Zimmerman GA, Weyrich AS, Petrey A, Rondina MT. Heparanase expression and activity are increased in platelets during clinical sepsis. J Thromb Haemost. 2021;19:1319-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Chen TT, Lv JJ, Chen L, Gao YW, Liu LP. Role of heparinase in the gastrointestinal dysfunction of sepsis (Review). Exp Ther Med. 2022;23:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Kaczor-Kamińska M, Stalińska K, Kamiński K, Pisarek A, Maziarz U, Feldman A, Wróbel M. Murine cellular model of mucopolysaccharidosis, type IIIB (MPS IIIB) - A preliminary study with particular emphasis on the non-oxidative l-cysteine metabolism. Biochimie. 2020;174:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Yan Y, Ji Y, Su N, Mei X, Wang Y, Du S, Zhu W, Zhang C, Lu Y, Xing XH. Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: A review. Carbohydr Polym. 2017;160:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Du S, Yu Y, Xu C, Xiong H, Yang S, Yao J. LMWH and its derivatives represent new rational for cancer therapy: construction strategies and combination therapy. Drug Discov Today. 2019;24:2096-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Kwon PS, Oh H, Kwon SJ, Jin W, Zhang F, Fraser K, Hong JJ, Linhardt RJ, Dordick JS. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020;6:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 238] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 21. | Huang Y, Kong C. Low-molecular-weight heparin alleviates sepsis-induced renal inflammatory response and improves kidney function. Minerva Med. 2020;111:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ahmad A, Vaghasiya K, Kumar A, Alam P, Raza SS, Verma RK, Khan R. Enema based therapy using liposomal formulation of low molecular weight heparin for treatment of active ulcerative colitis: New adjunct therapeutic opportunity. Mater Sci Eng C Mater Biol Appl. 2021;121:111851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Reintam Blaser A, Padar M, Mändul M, Elke G, Engel C, Fischer K, Giabicani M, Gold T, Hess B, Hiesmayr M, Jakob SM, Loudet CI, Meesters DM, Mongkolpun W, Paugam-Burtz C, Poeze M, Preiser JC, Renberg M, Rooijackers O, Tamme K, Wernerman J, Starkopf J. Development of the Gastrointestinal Dysfunction Score (GIDS) for critically ill patients - A prospective multicenter observational study (iSOFA study). Clin Nutr. 2021;40:4932-4940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 24. | Tang Y, Wang X, Li Z, He Z, Yang X, Cheng X, Peng Y, Xue Q, Bai Y, Zhang R, Zhao K, Liang F, Xiao X, Andersson U, Wang H, Billiar TR, Lu B. Heparin prevents caspase-11-dependent septic lethality independent of anticoagulant properties. Immunity. 2021;54:454-467.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 25. | Belousoviene E, Kiudulaite I, Pilvinis V, Pranskunas A. Links between Endothelial Glycocalyx Changes and Microcirculatory Parameters in Septic Patients. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Patterson EK, Cepinskas G, Fraser DD. Endothelial Glycocalyx Degradation in Critical Illness and Injury. Front Med (Lausanne). 2022;9:898592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 28. | Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2019;17:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 29. | Sun T, Wang Y, Wu X, Cai Y, Zhai T, Zhan Q. Prognostic Value of Syndecan-1 in the Prediction of Sepsis-Related Complications and Mortality: A Meta-Analysis. Front Public Health. 2022;10:870065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Anand D, Ray S, Srivastava LM, Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin Biochem. 2016;49:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Juffermans NP, van den Brom CE, Kleinveld DJB. Targeting Endothelial Dysfunction in Acute Critical Illness to Reduce Organ Failure. Anesth Analg. 2020;131:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54:2450-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 623] [Article Influence: 124.6] [Reference Citation Analysis (0)] |
| 33. | Blich M, Golan A, Arvatz G, Sebbag A, Shafat I, Sabo E, Cohen-Kaplan V, Petcherski S, Avniel-Polak S, Eitan A, Hammerman H, Aronson D, Axelman E, Ilan N, Nussbaum G, Vlodavsky I. Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscler Thromb Vasc Biol. 2013;33:e56-e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 955] [Cited by in RCA: 959] [Article Influence: 159.8] [Reference Citation Analysis (0)] |
| 35. | Park J, Byun Y. Recent advances in anticoagulant drug delivery. Expert Opin Drug Deliv. 2016;13:421-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong WT, Leung CC, Choi G, Wang MH, Gin T, Chan MT, Wu WK. Autophagy in sepsis: Degradation into exhaustion? Autophagy. 2016;12:1073-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 37. | Wen X, Xie B, Yuan S, Zhang J. The "Self-Sacrifice" of ImmuneCells in Sepsis. Front Immunol. 2022;13:833479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal; Leowattana W, Thailand S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX