Published online Sep 7, 2023. doi: 10.3748/wjg.v29.i33.5005
Peer-review started: June 14, 2023
First decision: August 5, 2023
Revised: August 7, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: September 7, 2023
Processing time: 78 Days and 17.8 Hours
Although endoscope-assisted magnetic compression anastomosis has already been reported for colonic anastomosis, there is no report on a single-approach operation using the natural orifice.
To design a deformable self-assembled magnetic anastomosis ring (DSAMAR) for colonic anastomosis for use in single-approach operation and evaluate its feasibility and safety through animal experiments.
The animal model for colonic stenosis was prepared by partial colonic ligation in eight beagles. The magnetic compression anastomosis of their colonic stricture was performed by endoscopically assisted transanal implantation of the DSAMAR. The anastomotic specimen, obtained 2 wk after the operation, was observed by both the naked eye and a light microscope.
The DSAMAR was successfully inserted into the proximal end of colon stenosis through the anus. The DSAMAR of seven dogs was successfully transformed into rings, while that of the remaining dog was removed after the first deformation failed. The rings were successfully retransformed after optimization. All animals underwent colonic anastomosis using the DSAMAR. No device-related or procedure-related adverse events were observed. The colostomy specimens of the experimental dogs were obtained 2 wk after the operation. Both gross and histological observations showed good anastomotic healing.
The DSAMAR is a safe and feasible option for the treatment of colon stenosis. Its specific deformation and self-assembly capability maximize the applicability of the minimally invasive treatment.
Core Tip: By combining magnetic compression anastomosis with endoscopic technology, we could design a deformable self-assembled magnetic anastomosis ring (DSAMAR) to perform magnetic compression anastomosis with only a single channel. We then verified the feasibility of magnetic compression anastomosis for the recanalization of colonic stenosis through animal experiments. The results showed that minimally invasive treatment of colonic stenosis can be achieved using the DSAMAR.
- Citation: Zhang MM, Zhao GB, Zhang HZ, Xu SQ, Shi AH, Mao JQ, Gai JC, Zhang YH, Ma J, Li Y, Lyu Y, Yan XP. Novel deformable self-assembled magnetic anastomosis ring for endoscopic treatment of colonic stenosis via natural orifice. World J Gastroenterol 2023; 29(33): 5005-5013
- URL: https://www.wjgnet.com/1007-9327/full/v29/i33/5005.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i33.5005
Benign colonic stenosis is a disease with various causes, including post-colectomy anastomotic stenosis[1], transcatheter arterial embolization[2], and acute pancreatitis[3]. Benign colonic stenosis is generally treated by endoscopic balloon dilatation (EBD)[4] and surgery[5]. EBD is widely performed in clinics because of its effectiveness, technical safety, and ease of process. However, in some cases, sufficient dilatation is not achieved because of the formation of stiff fibrosis tissue at the stenosis site, which is often resistant to balloon pressure[6]. Open surgery, laparoscopic stenosis resection, and bypass anastomosis are the traditional treatment strategies for benign colonic stenosis. Although a good therapeutic effect can be obtained through surgery, it is no longer the first choice of clinicians because of trauma and various perioperative adverse events.
Magnetic compression anastomosis or magnamosis, a novel technique, can be used for managing digestive tract anastomosis[7-9], vascular anastomosis[10,11], pathologic fistula[12], and therapeutic fistula[13]. The use of magnamosis along with endoscopy for treating gastrointestinal stenosis has been reported[14]. The cylinder or circle is the most commonly used shape of the magnet in such cases. In endoscopic magnetic compression anastomosis, the daughter and parent magnets are placed at the two opposite ends of the narrow digestive duct. The patient must have two channels: A natural orifice and the digestive tract fistula[14,15]. However, some patients only have a natural orifice, making magnetic compression anastomosis very difficult to perform. Therefore, for successful magnamosis, the magnet must be designed in a way that allows efficient optimization of the operation path. The design of the magnet must meet two conditions: first, the magnet should be able to pass through the narrow digestive cavity; and second, the magnet should be able to create wide anastomoses.
This study aims to design a novel deformable self-assembled magnetic anastomosis ring (DSAMAR) that can be placed at the natural orifice of the body. It also explores the feasibility of the DSAMAR in digestive tract anastomosis using dogs as experimental animals.
The experimental protocol was approved by the Committee for Ethics of Animal Experiments of Xi’an Jiaotong University (license No. 2022-1451). Eight beagles (male = 4, female = 4) were acquired from the Laboratory Animal Center of the Xi’an Jiaotong University (Xi’an, China) as experimental animals. The research protocol and all the experimental procedures were conducted strictly in accordance with the Guidelines for the Care and Use of Experimental Animals issued by the Xi’an Jiaotong University Medical Center.
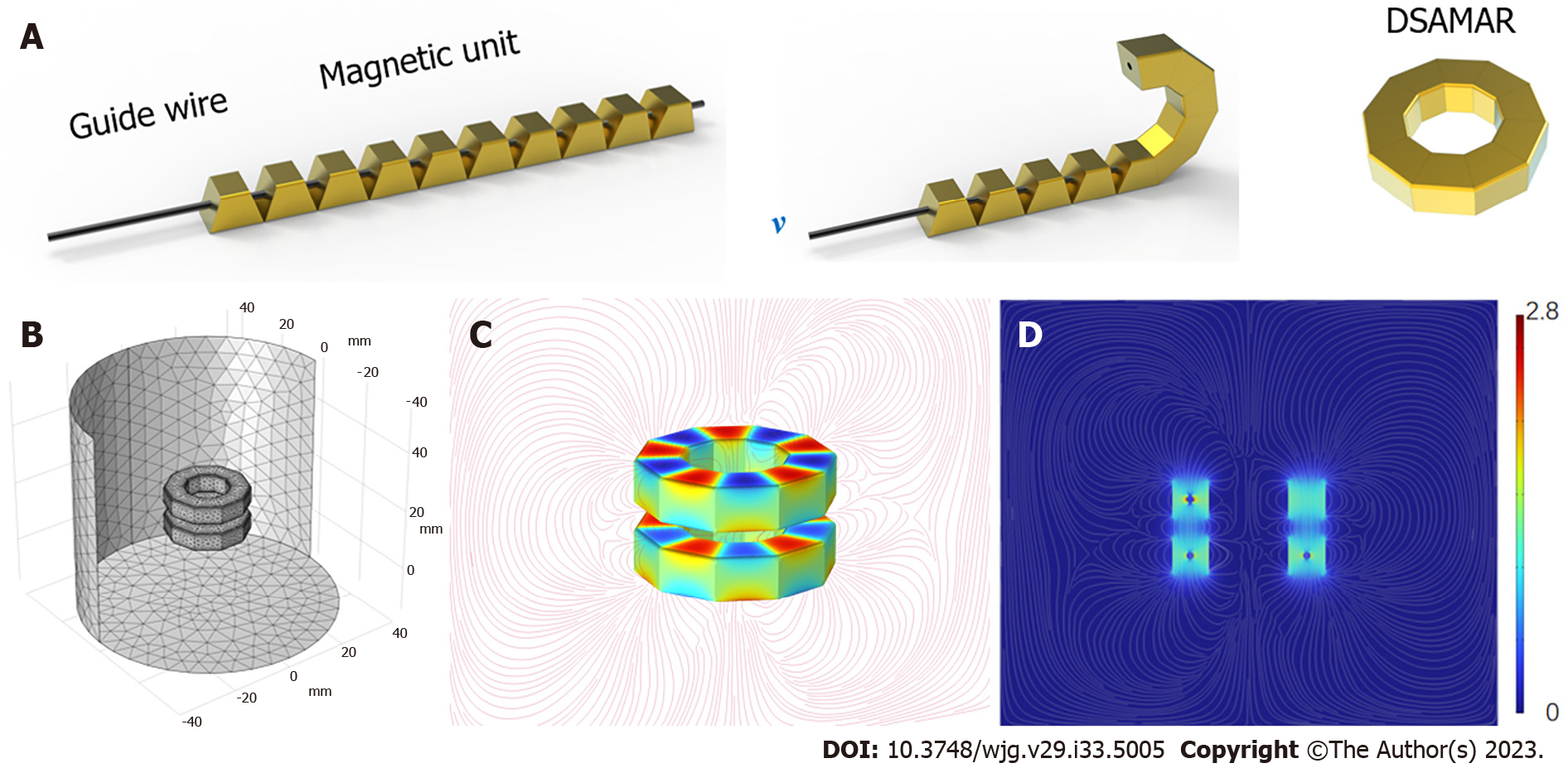
The DSAMAR consists of 10 trapezoidal magnetic units assembled in the opposite order of the N and S poles of the adjacent magnetic units. The long-axis direction of each magnetic unit contains a 1-mm diameter round hole that allows the stainless steel guide wire to pass through. Owing to the opposite N–S polarity of each magnetic unit, passing 10 sequentially arranged trapezoidal magnetic units through a hard guide wire and forming a linear arrangement is possible. When the guide wire is slowly drawn out, the inclined planes of the adjacent magnetic units attract each other and are sequentially arranged and assembled into a DSAMAR (Figure 1A) (Video 1). The magnetic unit is processed by N50-sintered neodymium-iron-boron, and its surface is coated with titanium nitride. Each magnetic unit of a DSAMAR weighs 1.25 g, the maximal magnetic field intensity at its compression surfaces is 480 mT, and the suction of two DSAMARs at zero distance can reach 184 N. The magnetic flux density can be calculated by computer simulations (Figure 1B-D).
This study aimed to verify the feasibility of the DSAMAR, an innovative surgical method. Hence, no control group was used. Eight beagles (age: > 1 year; weight: 12–15 kg) were used as experimental animals. Their operation time, postoperative adverse events, survival rate, and magnetic-ring-expel time were recorded. One month after the operation, the anastomosis was achieved and its healing was observed both by the naked eye and a light microscope.
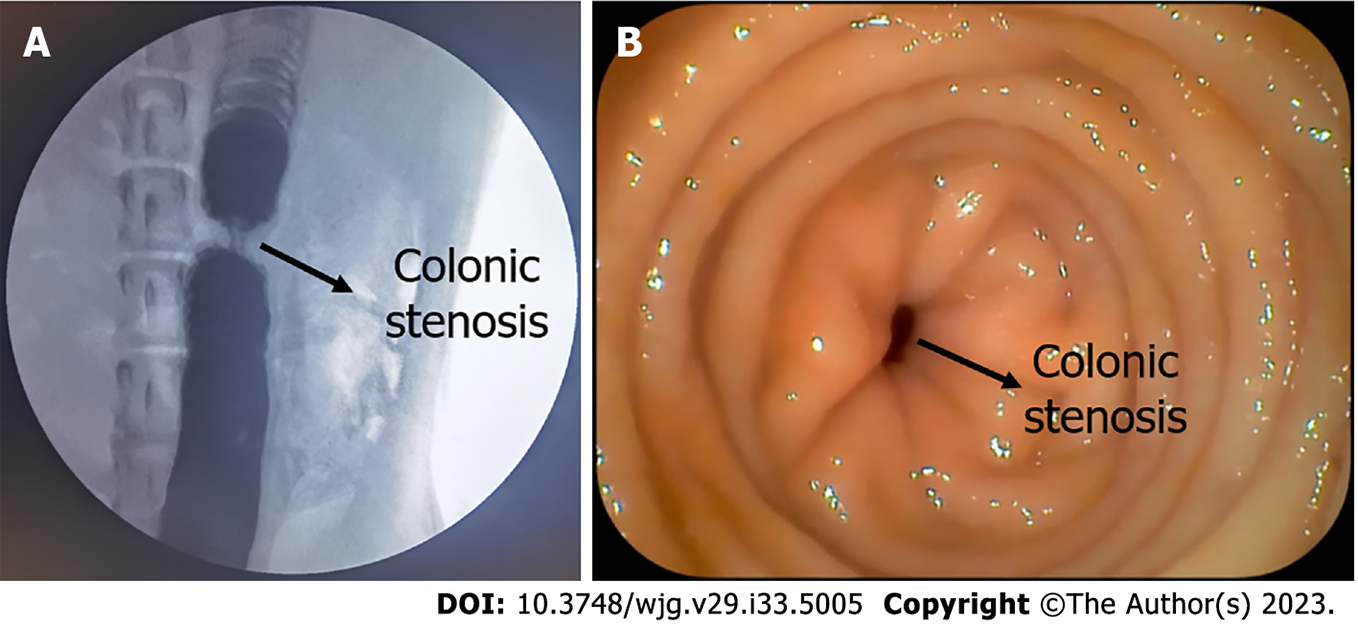
The beagles were given a slag-free liquid diet 2 d before surgery. In addition, they were fasted and their water intake was restricted 6 h before surgery. They were anesthetized by an intravenous injection of 3% pentobarbital sodium (1 mL/kg) and fixed in the supine position. Repeat enemas with soapy water were conducted to clean the intestines. A 14Fr gastric tube was inserted into the colon through the anus. An approximately 10-cm-long incision in the middle of the lower abdomen was made into the abdominal cavity. The descending colon was exposed and ligated on the 14Fr gastric tube with a 1-0 silk thread in the middle part of the descending colon. The gastric tube was removed and a colon stenosis model was formed. The diameter of the colon in the narrow segment was approximately 4.5 mm. The abdomen was then closed layer by layer. The colon stenosis model was evaluated both by colonoscopy and colonography (Figure 2).
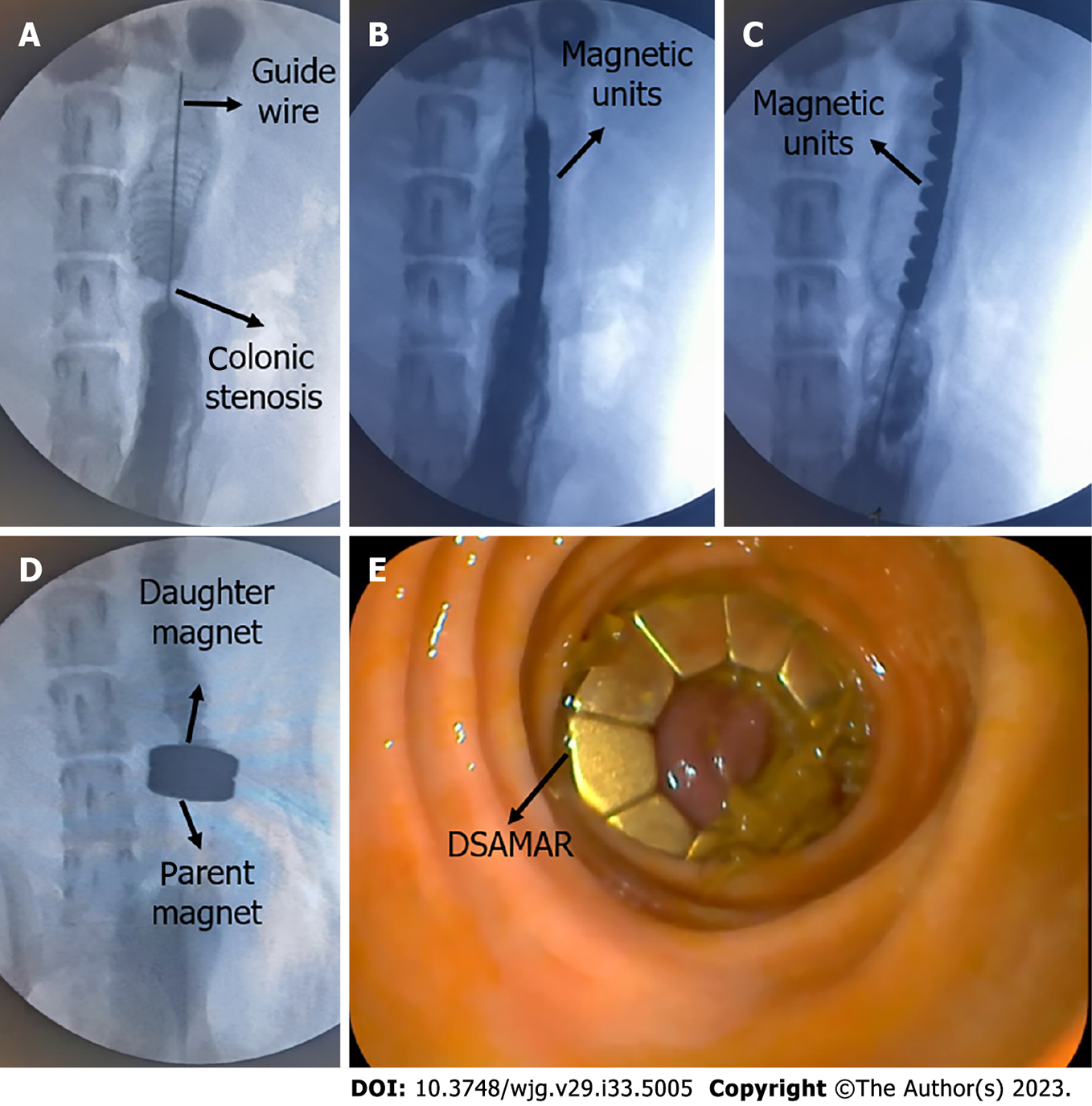
First, transanal colonoscopy was performed to determine the location and extent of colon stenosis. A guide wire was inserted through the colonoscopy biopsy hole. Under X-ray, the head end of the guide wire was passed through the colon stenosis segment. The position of the head end of the guide wire was maintained, and then the colonoscope was withdrawn (Figure 3A). Through the other end of the guide wire, 10 trapezoidal magnetic units were successively inserted in the opposite order of the N and S poles of the adjacent magnetic units. Again, under X-ray, a 5Fr push tube was used to push the magnetic units along the guide wire to make them enter the proximal end of the stenosis colon (Figure 3B and C). The magnetic units were then slowly pushed out of the guide wire one by one and transformed into a ring in the colon according to a predetermined plan. Then, the guide wire and the push tube were removed. Another DSAMAR, self-assembled in vitro, was inserted through the anus. Under X-ray, a colonoscope was used to push the magnet to the distal end of the stenosis colon. At this time, the two DSAMARs could automatically attract and compress the colon stenosis (Figure 3D and E). The two DSAMARs were then placed in the colon.
All dogs were managed in a single cage after emergence from anesthesia. Pethidine hydrochloride (1 mg/kg) was intramuscularly injected every 12 h for 3 d after the operation for analgesia. The dogs were given intravenous nutritional support until the DSAMAR detached from the anus. The time that the magnets took to get expelled from all dogs was recorded. The dogs resumed oral feeding after the DSAMAR was expelled. Colonoscopy and colonography were performed to evaluate the patency of the colon.
All dogs were euthanized 2 wk after the operation, and their colonic anastomosis specimens were obtained. The healing of anastomosis was observed by the naked eye. The anastomotic specimen was then soaked overnight in 10% formalin for fixation. Then, the specimen was embedded in paraffin and a 4-μm-thick section from the anastomosis was prepared. The sections were stained with hematoxylin and eosin (H&E) and Masson trichrome and examined under a bright-field microscope.
SPSS statistical 20.0 software was used for data analysis. The quantitative data of normal distribution were described by mean ± SD, while those of non-normal distribution were described by the median. Differences between the groups were compared by an independent sample t-test or a nonparametric test. P < 0.05 indicated a significant difference.
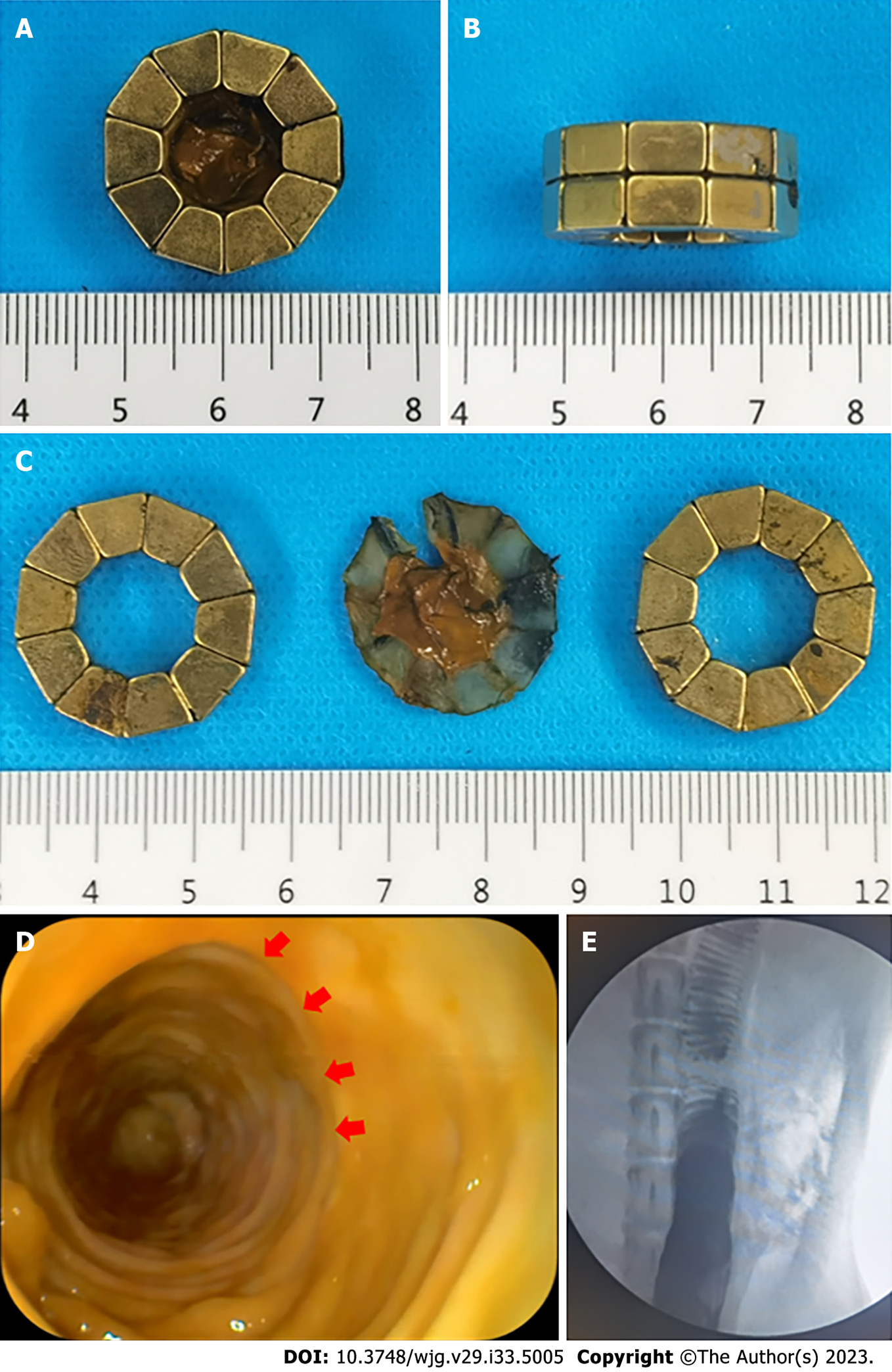
The colonic stenosis model was successfully prepared in all dogs (success rate = 100%). In seven of the eight beagles, the DSAMAR was successfully deformed only once after implantation in the proximal colon. In the remaining dog, the DSAMAR did not form during deformation, but it was successfully deformed after the removal of the magnetic ring and its re-implantation. The distal colon magnet was placed without any issue, and the DSAMARs at both ends of the stenosis were automatically attracted to each other. The endoscopic procedures (except for the model-preparation time) took 20.88 ± 5.69 min to complete, which was within the acceptable range (15–32 min). The average expulsion time of the magnets was 6.13 ± 0.99 d, again within the accepted range (5–8 d). The data related to animal experiments are listed in Table 1. The discharged DSAMARs are shown in Figure 4A-C. Colonoscopy and colonography were performed after the discharge of the magnet and a good patency of the colon was observed (Figure 4D and E).
The survival rate of all dogs was 100% in 2 wk after surgery. The dogs were generally in good condition after surgery, with no intestinal bleeding, perforation, obstruction, and other adverse events. After the dogs resumed normal feeding, they all excreted formed feces.
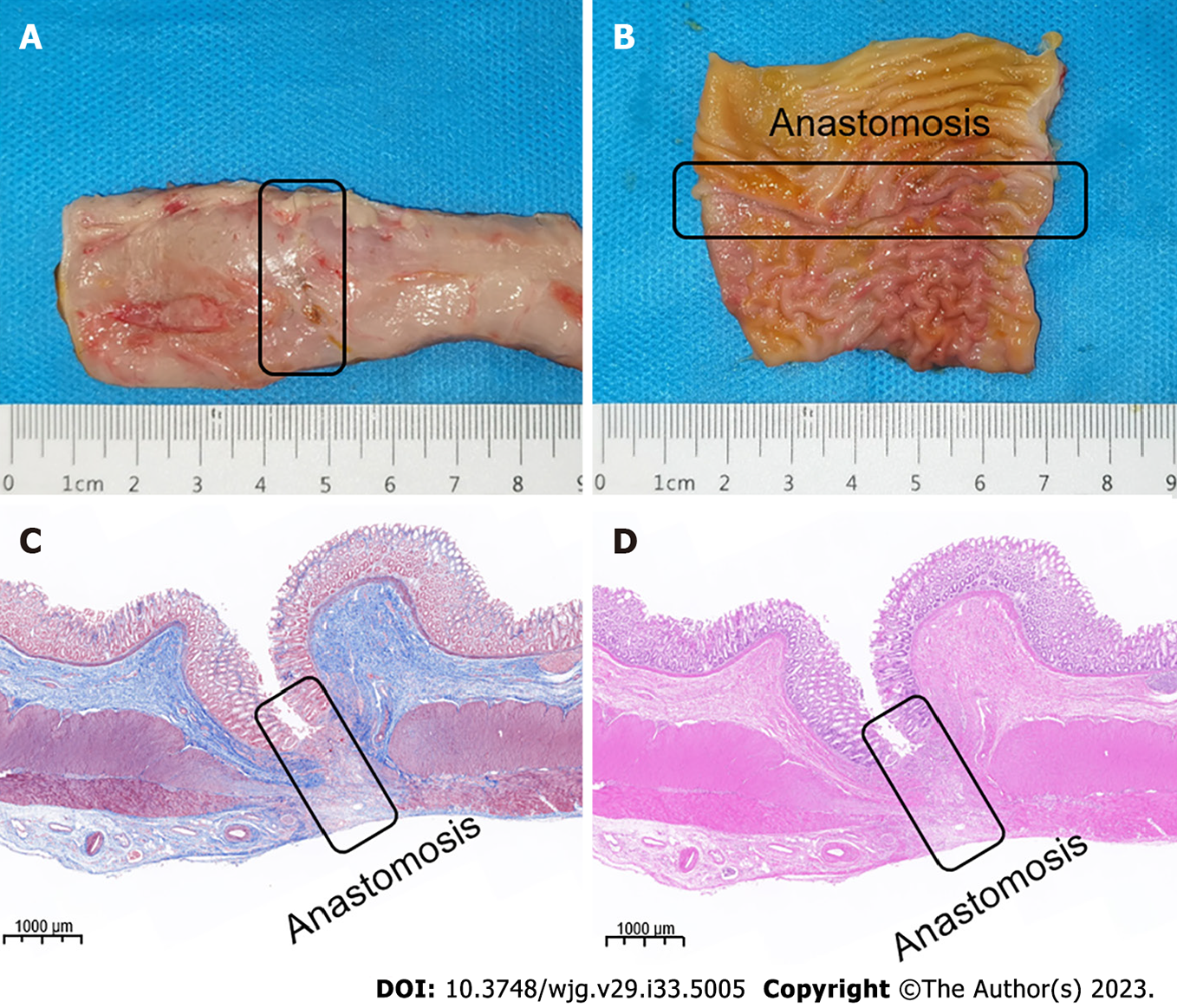
The experimental dogs were euthanized 2 wk after surgery, and their colonic anastomosis gross specimens were obtained by laparotomy. The colonic stenosis was not observed by the naked eye. The mucosa of the magnetic compression anastomosis was smooth and flat (Figure 5A and B). H&E and Masson staining showed good continuity and mucosal healing of the anastomotic mucosa (Figure 5C and D).
The DSAMAR along with endoscopy is a feasible treatment for gastrointestinal stenosis. Magnetic compression anastomosis is a safe technique for digestive tract anastomosis[7-9]. Non-surgical digestive tract anastomosis can be achieved when magnetic compression anastomosis is combined with endoscopy. Although most of the cases have been reported in clinical settings[14-16], the safety and feasibility of magnetic compression anastomosis are beyond doubt. To the best of our knowledge, the DSAMAR is the only magnet that can enter the narrow intestine through the natural orifice and perform the self-assembly anastomosis, which greatly increases the potential application of the magnamosis technology.
The special deformation mode of the DSAMAR allows the magnet to pass through the narrow section of the digestive tract with a minimal cross-section. The DSAMAR then deforms and self-assembles into a ring, with a slimming deformation ratio of up to 1:16.35. This creates suitable conditions for the implantation of a magnet ring through the single channel of the natural orifice. Approximately 100% success rate of the deformation self-assembly of the DSAMAR in vitro can be achieved. Only one of the eight dogs had an unplanned deformation of the DSAMAR when failed to form a ring. This happened because the colon was not inflated enough to provide sufficient space for the DSAMAR to deform. In this case, the DSAMAR was re-inserted into the proximal colon and inflated by a colonoscope to fully expand the colon, which helped successfully complete the deformable self-assembly.
The result of animal experiments provides the best proof of the potential clinical transformation of the DSAMAR. The gross specimen and histological observation of the colonic anastomosis in dogs showed that the DSAMAR established a good anastomosis, which was not significantly different from that achieved using the conventional magnetic ring of digestive tract anastomosis reported previously. Different degrees of the narrowing of the human digestive tract after magnetic compression anastomosis have been reported, consistent with the results of previous studies[17-18]. There may be still some differences in the long-term anastomosis effect of magnetic compression anastomosis in humans and animals. This phenomenon needs to be further investigated through clinical studies with larger samples. Nevertheless, our study not only confirms the advantages of magnamosis, but also fully affirms the ingenious design of the DSAMAR. Compared with the currently available magnamosis rings, the DSAMAR can further optimize the magnetic compression anastomosis of colorectal anastomosis stenosis in postoperative patients with esophageal stenosis (non-atresia) and no fistula. This is because the DSAMAR uses only the natural orifice of a patient to successfully place the magnet.
This study has certain limitations also. The animal experiments only verified the application of the DSAMAR for the treatment of colonic stenosis. The animal sample size was small and the observation time of anastomosis was also quite short. We plan to verify the feasibility of the application of the DSAMAR in esophageal and duodenal stenoses in future studies and further observe the long-term patency of magnetic compression anastomosis. In previous studies on animal experiments[19], the colonic stenosis model was prepared by the partial ligation of silk thread, which was different from that used clinically. It may lead to differences in the anastomosis effect of the clinical application compared to that observed with animal experiments.
This study demonstrated the feasibility and safety of the DSAMAR for the treatment of digestive tract stenosis through the natural orifice by conducting animal experiments. Because of its unique deformable self-assembly, the DSAMAR can be used to handle complex gastrointestinal stenosis cases. In our next studies, we plan to further optimize the design of the DSAMAR and improve the endoscopic operation process through more animal experiments. It is expected to be used in clinical practice in the near future.
Magnetic compression anastomosis, or magnamosis, has been widely used for anastomotic recanalization of gastrointestinal stenosis. At present, the magnets applied to the digestive tract lumen anastomosis are usually cylindrical or circular.
In order to achieve magnetic anastomosis recanalization through a single-access natural orifice, we designed a deformable self-assembled magnetic anastomosis ring (DSAMAR). It is expected to provide a more minimally invasive treatment method for patients with colonic stenosis.
To verify the feasibility and safety of using DSAMAR to recanalize colonic stenosis through the single-approach natural orifice.
Colonic stricture models were established in 8 beagle dogs. DSAMAR was used to achieve magnamosis recanalization of colonic stenosis and evaluate the formation of colonic anastomosis.
The results showed that the use of DSAMAR for recanalization of colonic stenosis was feasible and the anastomosis formation was good.
The feasibility and safety of using DSAMAR to achieve colonic stenosis recanalization through a single-approach natural orifice were verified through animal experiments.
The design of DSAMAR is ingenious. In addition to colon stenosis, it can also be used for minimally invasive treatment of digestive tract stenosis such as esophageal stricture and duodenal obstruction. It could provide a more minimally invasive treatment for patients with gastrointestinal stenosis.
| 1. | Zhou W, Xia L, Wang Z, Cao G, Chen L, Chen E, Zhang W, Song Z. Transanal Minimally Invasive Surgery for Rectal Anastomotic Stenosis After Colorectal Cancer Surgery. Dis Colon Rectum. 2022;65:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Murata M, Watanabe Y, Miyamoto S. Colonic Stenosis Caused by Transcatheter Arterial Embolization. Clin Gastroenterol Hepatol. 2022;20:e645-e646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Mandal AK, Kafle P, Puri P, Chaulagai B, Hassan M, Bhattarai B, Kanth R, Gayam V. Acute Pancreatitis Causing Descending Colonic Stricture: A Rare Sequelae. J Investig Med High Impact Case Rep. 2019;7:2324709619834594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Hirai F. Current status of endoscopic balloon dilation for Crohn's disease. Intest Res. 2017;15:166-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Bemelman WA, Allez M. The surgical intervention: earlier or never? Best Pract Res Clin Gastroenterol. 2014;28:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Moroi R, Shiga H, Nochioka K, Chiba H, Shimoyama Y, Kuroha M, Tosa M, Kakuta Y, Kayaba S, Takahashi S, Kinouchi Y, Masamune A. Endoscopic radial incision and cutting for benign stenosis of the lower gastrointestinal tract: An investigation of novel endoscopic treatment in multicenter trial. J Gastroenterol Hepatol. 2022;37:1554-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Gagner M, Krinke T, Lapointe-Gagner M, Buchwald JN. Side-to-side duodeno-ileal magnetic compression anastomosis: design and feasibility of a novel device in a porcine model. Surg Endosc. 2023;37:6197-6207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 8. | Hornok Z, Kubiak R, Csukas D, Ferencz A, Cserni T. Esophageal Magnetic Anastomosis Device (EMAD) to Simplify and Improve Outcome of Thoracoscopic Repair for Esophageal Atresia with Tracheoesophageal Fistula: A Proof of Concept Study. J Pediatr Surg. 2023;58:1489-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 9. | Muensterer OJ, Sterlin A, Oetzmann von Sochaczewski C, Lindner A, Heimann A, Balus A, Dickmann J, Nuber M, Patel VH, Manfredi MA, Jennings RW, Smithers CJ, Fauza DO, Harrison MR. An experimental study on magnetic esophageal compression anastomosis in piglets. J Pediatr Surg. 2020;55:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Obora Y, Tamaki N, Matsumoto S. Nonsuture microvascular anastomosis using magnet rings: preliminary report. Surg Neurol. 1978;9:117-120. [PubMed] |
| 11. | Zhang M, Ma J, An Y, Lyu Y, Yan X. Construction of an intrahepatic portosystemic shunt using the magnetic compression technique: preliminary experiments in a canine model. Hepatobiliary Surg Nutr. 2022;11:611-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Gao Y, Wu RQ, Lv Y, Yan XP. Novel magnetic compression technique for establishment of a canine model of tracheoesophageal fistula. World J Gastroenterol. 2019;25:4213-4221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 13. | Uygun I, Okur MH, Cimen H, Keles A, Yalcin O, Ozturk H, Otcu S. Magnetic compression ostomy as new cystostomy technique in the rat: magnacystostomy. Urology. 2012;79:738-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Kamada T, Ohdaira H, Hoshimoto S, Narihiro S, Suzuki N, Marukuchi R, Takeuchi H, Yoshida M, Yamanouchi E, Suzuki Y. Magnetic compression anastomosis with atypical anastomosis for anastomotic stenosis of the sigmoid colon: a case report. Surg Case Rep. 2020;6:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Russell KW, Rollins MD, Feola GP, Scaife ER. Magnamosis: a novel technique for the management of rectal atresia. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Kamada T, Ohdaira H, Takeuchi H, Takahashi J, Ito E, Suzuki N, Narihiro S, Yoshida M, Yamanouchi E, Suzuki Y. New Technique for Magnetic Compression Anastomosis Without Incision for Gastrointestinal Obstruction. J Am Coll Surg. 2021;232:170-177.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Lu G, Li J, Ren M, Ma F, Sun X, Lv Y, He S. Endoscopy-assisted magnetic compression anastomosis for rectal anastomotic atresia. Endoscopy. 2021;53:E437-E439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Ellebaek MBB, Qvist N, Rasmussen L. Magnetic Compression Anastomosis in Long-Gap Esophageal Atresia Gross Type A: A Case Report. European J Pediatr Surg Rep. 2018;6:e37-e39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Hiratsuka T, Inomata M. A novel animal model of colonic stenosis to aid the development of new stents for colon strictures. Surg Endosc. 2022;36:3152-3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martino A, Italy;