Published online Sep 7, 2023. doi: 10.3748/wjg.v29.i33.4942
Peer-review started: May 29, 2023
First decision: June 20, 2023
Revised: July 22, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: September 7, 2023
Processing time: 94 Days and 23.5 Hours
Hepatitis due to hepatitis B virus (HBV) reactivation can be serious and poten
Core Tip: Patients with chronic or past resolved hepatitis B virus (HBV) infection are at risk of reactivation of the virus when they receive chemotherapy or immunosuppressive therapy. Therefore, before treatment, patients should be screened for HBV markers, specifically hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen. Prophylactic antiviral therapy is important for HBsAg-positive patients, and is a reasonable option for patients with resolved HBV infection who are scheduled to receive high-risk therapy such as anti-CD20 monoclonal antibodies, anti-CD79 monoclonal antibodies, bispecific antibodies, chimeric antigen receptor-T cell therapy, or hematopoietic stem cell transplantation. For other patients with resolved HBV infection, pre-emptive antiviral therapy guided by serial monitoring of HBV DNA is a reasonable option.
- Citation: Mak JWY, Law AWH, Law KWT, Ho R, Cheung CKM, Law MF. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies in the targeted therapy era. World J Gastroenterol 2023; 29(33): 4942-4961
- URL: https://www.wjgnet.com/1007-9327/full/v29/i33/4942.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i33.4942
Patients with chronic or resolved hepatitis B virus (HBV) infection are at risk of viral reactivation during chemotherapy or immunosuppressive therapy, commonly in patients receiving anti-cancer therapy for hematological malignancies or hematopoietic stem cell transplantation (HSCT). The earliest reports of HBV reactivation were in patients with lymphoma[1], and the highest risk of HBV reactivation is in patients receiving potent anti-CD20 monoclonal antibodies such as rituximab or obinutuzumab, which result in profound B-cell depletion.
There have been major advances in the development of new targeted therapy in the treatment of hematological malignancies in the past two decades. Bruton’s tyrosine kinase (BTK) inhibitors are increasingly used in chronic lym
Chimeric antigen receptor (CAR)-T cell therapy is a promising intervention which can be applied to lymphoid mali
This article will review the current published data on the clinical course and risk factors for HBV reactivation when using these novel therapies in patients with hematological malignancies. The recommended choice and duration of antiviral prophylaxis together with monitoring after stopping antiviral prophylaxis will also be discussed.
Antibody to hepatitis B core (anti-HBc) is a good marker of current and past HBV infection as it persists even after hepatitis B surface antigen (HBsAg) is no longer detectable, while anti-HBs can be present due to successful hepatitis B vaccination or previous infection.
HBV reactivation is defined as exacerbation of chronic hepatitis B (CHB) or reactivation of past resolved hepatitis B infection. In general, reactivation is characterized by an increase from baseline in the HBV DNA level in patients with CHB, but it can also be defined as reverse HBsAg seroconversion, or the appearance of HBV DNA in serum when there is absence of HBsAg. The definition of HBV reactivation varies among different international guidelines and the infor
When a patient has been infected with HBV, the virus enters the hepatocytes where the viral genome is released and transported into the nucleus. Once inside the nucleus, the viral genome is then converted into plasmid-like covalently closed circular DNA (cccDNA), which can persist in the hepatocytes in a latent and stable state[8].
HBV reactivation may occur at any time during or after chemotherapy. There are five stages in the course of HBV reactivation[9]. The first stage includes an asymptomatic elevation in markers of viral replication, with detectable HBV DNA levels in patients who are HBsAg-positive or -negative, or the reappearance of HBsAg in previously HBsAg-negative patients. In the second phase, serum HBV DNA levels continue to raise, and serum ALT and aspartate amino
In patients receiving immunosuppressive therapy, the loss of immune control may result in viral replication inside the hepatocytes without any increase in ALT levels. Nevertheless, upon immune reconstitution, sometimes during immuno
Male sex and older age were identified to be risk factors for HBV reactivation[11-13]. A study in 626 HBsAg-positive patients who were undergoing chemotherapy for a variety of malignancies showed that there was almost a 3-fold increase of the incidence of HBV reactivation in men but the exact mechanism was not clear[13]. Chen et al[14] analyzed the risk of HBV reactivation among 1962 patients with hematological malignancy in Taiwan. The presence of hepatocellular carcinoma (HCC) and absence of antiviral prophylaxis were independent risk factors for HBV reactivation in HBV carriers. Among patients who were HBsAg negative at diagnosis, liver cirrhosis, diabetes mellitus, allogeneic stem cell transplantation, and low anti-HBs titers were independent risk factors for HBV reactivation[14]. Lymphoma is also associated with a higher risk of HBV reactivation[15]. Both the underlying disease and the anti-cancer therapy may con
The identified virologic risk factors for HBV reactivation include the presence of intrahepatic cccDNA and detectable HBV DNA levels[16-18]. Signs of increased viral replication, such as HBsAg or hepatitis B e antigen (HBeAg) positivity and detectable baseline HBV DNA, before treatment, are predictive of the patient meeting the criteria for HBV reac
HBV genotype is also related to treatment response and disease severity and progression[7,9]. For example, it was found that HBV genotype B is associated with HBeAg seroconversion at an earlier age, less active hepatic necroinflammation, more prolonged remission after HBeAg seroconversion, a slower rate of cirrhotic progression, and a reduced rate of HCC development compared with genotype C[7].
Salpini et al[23] identified mutations in HBsAg as being risk factors for reactivation. Using population-based and ultradeep sequencing, they analyzed the genetic diversity of HBsAg in 29 patients and found that 75.9% of HBV-reactivated patients carried mutations localized in immune-active HBsAg regions compared with only 3.1% of control patients (P < 0.001)[23]. The majority of these mutations resided in the B-cell epitopes of the HBs antigenic loop. Some of the mutations are known to hamper HBsAg recognition by humoral response, which may explain the frequent reac
Chemotherapy: Anthracycline chemotherapy (e.g., doxorubicin, daunorubicin, and idarubicin) is a common form of treatment for hematological cancers such as lymphoma and acute myeloid leukemia (AML). The risk of HBV reactivation is significant in patients receiving doxorubicin as part of the chemotherapeutic regimen[6].
Chen et al[24] found that there was an increase in p21 expression during treatment with doxorubicin. The increase in p21 expression promotes the expression of CCAAT/enhancer-binding protein α (C/EBPα), which helps to activate HBV replication by enhancing the binding of C/EBPα to the HBV promoter. Kostyusheva et al[25] studied the effects of DNA-damaging compounds such as doxorubicin and hydrogen peroxide on the replication or reactivation of HBV and found that both doxorubicin and hydrogen peroxide dose-dependently activated HBV replication[25]. If doxorubicin is planned, anti-HBV prophylaxis is recommended for patients who are receiving doxorubicin if they have either CHB or a past resol
Steroids: Steroids are commonly combined with chemotherapy or immunomodulatory drugs in the treatment of many hematological malignancies such as lymphoid malignancies and MM. Steroids can increase the HBV replication through two mechanisms. First, they can prevent T and B cell proliferation by suppressing cell-mediated immunity through the inhibition of interleukins[26]. Second, they exert a direct suppressive effect on T cell-mediated immunity through the sti
Cheng et al[28] randomized 50 lymphoma patients who were HBsAg-positive and receiving the same chemotherapeutic regimen with or without the addition of corticosteroids, and compared the rate of HBV reactivation. The cumulative incidence of HBV reactivation was significantly higher in the corticosteroid group at 9 mo (73% vs 38%, respectively, P = 0.03). In a separate prospective cohort study with 6 years of follow-up, HBV reactivation occurred at 4 to 32 mo (median 10 mo) after the administration of steroids[29]. Most patients had malignancies or rheumatologic diseases.
The risk of HBV reactivation is further increased in patients who receive high-dose steroids (> 20 mg/d of pred
Tyrosine kinase inhibitors: Currently, treatment with tyrosine kinase inhibitors (TKIs), e.g., imatinib, nilotinib, dasatinib, and ponatinib, is a standard therapy for chronic myeloid leukemia (CML). The exact mechanism for HBV reactivation with TKIs is not known but it may be related to immune restoration. There are some published data on the risk of HBV reactivation in patients receiving TKIs. One hundred and forty-two adult Taiwanese CML patients were enrolled in a study to assess the rate of HBV reactivation during TKI therapy, including imatinib (n = 43, 30.3%), dasatinib (n = 48, 33.8%), nilotinib (n = 37, 26.1%), ponatinib (n = 1, 0.7%), and two or more TKIs (n = 13, 9.2%)[31]. Nineteen patients were HBV carriers and the rate of HBV reactivation was 26.3%; HBV reactivation was detected between 3 and 51 mo after the use of TKIs. Three patients experienced HBV-related hepatitis with an increase in ALT of more than 100 U/L[29]. One of the patients with HBV reactivation had received antiviral prophylaxis with entecavir; he was then given tenofovir after HBV reactivation.
A Korean study involved 69 patients with CHB being assessed for HBV reactivation[30]. Forty-six patients did not receive antiviral prophylaxis and the rate of HBV reactivation was 26% in this group of patients[32]. HBV reactivation was detected in seven patients who received imatinib, two patients receiving dasatinib, one nilotinib recipient, and one patient treated with radotinib therapy.
We would recommend prophylactic antiviral therapy to HBV carriers, and monitor HBV DNA and liver enzymes every 1 to 3 mo in patients with past resolved HBV infection, during TKI treatment. If the HBV DNA level rises, pre-emptive treatment with antiviral agents should be given. TKIs may also be used in combination with chemotherapy in the treatment of Philadelphia-positive ALL[33,34]. The combination with chemotherapy will likely lead to a deeper immuno
Anti-CD20 monoclonal antibodies: Treatment for a number of different hematological malignancies, including CLL and B-cell lymphoma, often includes B cell-depleting agents such as anti-CD20 monoclonal antibodies. Rituximab, obinu
Both HBsAg-positive patients and those with resolved HBV infection are susceptible to HBV reactivation when they receive rituximab[38-41]. The incidence varies from 8.3% to 25% in patients with resolved HBV infection receiving rituximab-based chemotherapy[42-46]. Rituximab is a significant risk factor for HBV reactivation.
Obinutuzumab is a second-generation anti-CD20 monoclonal antibody. It has an engineered fragment crystallizable portion and a modified elbow hinge region[47]. Obinutuzumab has shown better efficacy than rituximab in several types of lymphoid diseases, by inducing direct cell death and enhancing antibody-dependent cellular cytotoxicity[48,49]. It can potentially cause more profound suppression of CD20 than rituximab[48,49]. It is used in patients with CLL and follicular lymphoma with promising results[48,49].
Kusumoto et al[50] performed a prospective study in 326 B-cell lymphoma patients with past resolved HBV infection who received obinutuzumab- (n = 155) or rituximab-containing immunochemotherapy (n = 171) in the phase 3 GALLIUM[48] and GOYA[51] studies. Of the 326 patients with resolved HBV infection, 119 (36.5%) received nucleos(t)ide analog treatment (NAT). Among these 119 patients, 94 received prophylactic NAT and 25 received pre-emptive NAT. The rate of HBV reactivation was 10.8% without antiviral prophylaxis, whereas only two of the 94 patients who received prophylactic NAT (2.1%) had HBV reactivation[48]. It was shown that the baseline detectable HBV DNA was strongly associated with an increased risk of reactivation while prophylactic NAT significantly decreased the risk on multivariate Cox ana
The reactivation rate in patients receiving obinutuzumab- and rituximab-based chemotherapy was 13.2% and 6.1%, respectively[50]. Although no significant difference in the risk of HBV reactivation between these two different immunochemotherapy regimens was demonstrated in the multivariate analysis, it might be due to confounding factors including imbalance of baseline risk factors. Anti-HBV prophylaxis is recommended in patients with either CHB or past resolved infection receiving anti-CD20 monoclonal antibody.
Polatuzumab vedotin: Polatuzumab vedotin is an antibody-drug conjugate targeting CD79b, which is universally expressed on the surface of malignant B cells. CD79b is a signaling component of the B-cell receptor which is located on the surface of normal B cells as well as most of the mature B-cell tumors and 95% of DLBCL[52]. Polatuzumab vedotin was found to be useful in combination with bendamustine and rituximab (pola-BR) for patients with relapsed or refrac
To date, there are no published data or reported cases of HBV reactivation in patients receiving polatuzumab vedotin. In view of the profound B-cell suppression that occurs when these regimens are used to treat lymphoma, we would recommend antiviral prophylaxis for patients receiving polatuzumab vedotin if they have either CHB or past resolved HBV infection.
Inotuzumab ozogamicin: Inotuzumab ozogamicin is an antibody conjugate in which a humanized monoclonal antibody against CD22 is conjugated to the cytotoxic antibiotic calicheamicin[55]. After binding to CD22 on the leukemic cell surface, the CD22-conjugate complex is rapidly internalized, releasing the calicheamicin. Once released, the cytotoxic portion of the conjugate binds to the minor groove of DNA in these leukemic cells, and induces double-strand cleavage and subsequent apoptosis.
More than 90% of patients with B-cell ALL express CD22 and this cell-surface glycoprotein is not shed into the extra
Blinatumomab: Blinatumomab is a bispecific T-cell engager, with two binding sites: One for CD3-positive cytotoxic T cells and the other for CD19-positive B cells. By drawing the two types of immune cells together, blinatumomab facilitates the recognition and destruction of CD19-positive ALL blasts by the patient’s own endogenous T cells[57]. It has been used with success in the treatment of relapsed/refractory ALL or as consolidation therapy[58,59].
We did not identify any published cases of HBV reactivation in patients with CHB or past resolved infection receiving blinatumomab. HBV prophylaxis is recommended for both chronic and resolved HBV infection in patients receiving blinatumomab, in view of profound B-cell depletion seen with this agent.
Daratumumab and isatuximab: Daratumumab is a human immunoglobulin G1 monoclonal antibody which targets CD38-expressing cells. Several of the combination regimens containing daratumumab have shown promising results in the treatment of newly diagnosed and refractory/relapsed MM[60-63]. It also has an emerging role in the treatment of amy
The principal mechanism of daratumumab in MM is to induce death of CD38-expressing myeloma cells via antibody-dependent and complement-dependent cytotoxicity, as well as via antibody-dependent cellular phagocytosis[66]. Dara
There was a report of HBV reactivation occurring on day 15 of the third course of a daratumumab-containing regimen in a MM patient with resolved HBV infection[68]. Lee et al[69] also conducted a retrospective study of 93 patients with resolved HBV infection who had been treated with daratumumab and found that the risk of HBV reactivation was 6.5% at a median follow-up period of 8.7 mo. One patient later died of hepatic failure despite treatment with tenofovir. These reports highlight the risk of reactivation of resolved HBV infection after daratumumab treatment in MM patients.
Isatuximab, another anti-CD38 monoclonal antibody, has demonstrated benefits in the treatment of patients with relapsed/refractory and high-risk MM[70-73]. We would recommend anti-HBV prophylaxis in both HBV carriers or those with resolved HBV infection during treatment with either daratumumab or isatuximab in view of the similar mecha
CAR-T cell therapy: CAR-T cell therapy is a promising immunotherapy with curative intent for several types of hematological malignancies including NHL[74-77], ALL[78], and MM[79,80]. CAR-T cell therapy involves removal of the patient’s own T cells, reprogramming these cells with a CAR construct, and then returning them to the patient’s blood
The issue of HBV reactivation in patients receiving CAR-T cell therapy remains unexplored, and the data on HBV reactivation in these patients are limited. CAR-T cells may predispose HBV immune patients to reactivation due to its cytotoxicity against B cells. The proper prevention strategy and duration of antiviral prophylaxis in patients receiving CAR-T cell therapy are still unclear and should be further investigated. There has been a fatal case of HBV reactivation after CAR-T cell therapy[2]. HBV reactivation can be a significant complication in CAR-T cell treatment and clinicians should be cautious about this complication particularly in areas where HBV is still prevalent.
Patients can also have late HBV reactivation occurring more than 1 year after CAR-T cell therapy. CAR-T cells can persist in the blood for a long time, resulting in prolonged B-cell aplasia and a persistent reduction in immunoglobulin production, thus contributing to late reactivation[82].
Table 1 summarizes the published data on the HBV reactivation in patients receiving CAR-T cell therapy[83-89]. The rate of HBV reactivation ranged from 0% to 20% for CHB patients[83-89]. We recommend that clinicians administer anti-HBV prophylaxis during the CAR-T cell therapy and for at least 1 year afterwards in patients who had CHB or past resol
| Ref. | Indication for CAR-T | N | CHB, n | Past resolved HBV infection, n | Antiviral prophylaxis, % patients | Definition of HBV reactivation | Rate of HBV reactivation | HBV-related death |
| Prospective studies | ||||||||
| Liu et al[87], 2020 | B-cell lymphoma | 17 | 6 | 11 | 100% for CHB, and 45.5% for past infection (entecavir) | Elevation of HBV DNA levels to > 1000 IU/mL and/or HBsAg reverse seroconversion in HBsAg-negative patients | 0 | 0 |
| Yang et al[89], 2020 | DLBCL | 15 | 15 | 0 | 100% (lamivudine, entecavir, tenofovir, or adefovir dipivoxil) | Positive follow-up HBV-DNA test if the baseline HBV-DNA is undetectable/negative or > 10-fold increase from baseline | 20% | 0 |
| Li et al[86], 2021 | ALL, B-cell lymphoma | 30 | 0 | 30 | No prophylaxis | Elevation of HBV DNA ≥ 100 IU/mL for two consecutive measurements | 6.6% | 0 |
| Wang et al[88], 2020 | ALL, B-cell lymphoma, PCM | 70 | 12 | 29 | 100% for CHB (entecavir, tenofovir disoproxil, or lamivudine). Nil for patients with past HBV infection | > 1 log increase in HBV DNA, HBV DNA-positive when previously negative, HBV DNA > 2000 IU/mL if no baseline level was available, or reverse sero-conversion from HBsAg-negative to positive | 16.7% with chronic infection and 34.4 % with past infection | 0 |
| Retrospective studies | ||||||||
| Cao et al[83], 2020 | ALL, NHL | 89 | 19 | 37 | 100% for chronic infection, and 5.4% for past infection | 100-fold increase in HBV DNA when compared with baseline or HBV DNA ≥ 103 IU/mL in a patient with a previously undetectable level or reverse seroconversion from HBsAg negative to HBsAg positive | 5.3% for CHB | 0 |
| Han et al[85], 2020 | Multiple myeloma | 9 | 1 | 8 | 100% for CHB, 25% for past infection (lamivudine/entecavir) | HBsAg seroconversion or increase in HBV DNA levels by at least 10-fold or 1 × 109 copies/mL | 12.5% for past infection | 0 |
| Cui et al[84], 2021 | DLBCL, B-ALL | 20 | 5 | 15 | 100% for CHB (entecavir or tenofovir), 13.3% for past HBV infection (entecavir) | For CHB: (1) ≥ 2 log increase in HBV DNA compared to the baseline level; (2) HBV DNA ≥ 3 log IU/mL in a patient with previously undetectable level; and (3) HBV DNA ≥ 4 log IU/mL if the baseline level is not available. For resolved HBV infection: HBV DNA is detectable; reverse HBsAg seroconversion | 6.2% for past infection | 0 |
Bispecific antibodies: The development of bispecific antibodies has been an important advance in the treatment of relapsed or refractory B-cell lymphomas[90-92], including DLBCL[93-95] and follicular lymphoma[96]. Bispecific anti
Since there are a lack of prospective or retrospective studies on the risk of HBV reactivation in patients receiving bispecific antibodies, the real incidence of HBV reactivation is unclear. However, bispecific antibodies will profoundly suppress B-cell activity. These drugs are highly potent and the effect on B-cell depletion is expected to be significant. Therefore, we recommend antiviral prophylaxis against HBV in patients with either CHB and past resolved HBV infec
BTK inhibitors: BTK inhibitors such as ibrutinib, acalabrutinib, and zanubrutinib have shown success in the treatment of many lymphoid malignancies including CLL[97-102], mantle cell lymphoma[103,104], marginal zone B-cell lymphoma[105], and Waldenström’s macroglobulinemia[106]. Ibrutinib blocks B-cell antigen receptor signaling, thus reducing mali
The effect of ibrutinib or other BTK inhibitors on HBV reactivation has not been extensively studied, and there are no guidelines on the prophylaxis and management of HBV reactivation during treatment with ibrutinib. Table 2 summarizes the current data on the risk of HBV reactivation and their outcomes in patients receiving BTK inhibitors[108-111]. Most existing data are from retrospective studies. The rate of HBV reactivation ranged from 1.9% to 8.3% for past resolved infec
| Ref. | Disease type | Therapy | N | CHB, n | Past resolved HBV infection, n | Antiviral prophyl-axis, % patients | Definition of HBV reactivation | Rate of HBV reactivation, % patients | HBV-related death | ||||||
| Hammond et al[108], 2018 | CLL, MCL, LPL | Ibrutinib | 21 | 0 | 21 | 4.8% | HBV DNA > 100 IU/mL on 2 consecutive measurements ± reappearance of HBsAg | 9.5% | 0 | ||||||
| Innocenti et al[109], 2019 | CLL | Ibrutinib | 34 | 0 | 12 | 42% for past infection (lamivudine) | Increase in serum ALT and HBV DNA in HBsAg-positive patients or elevation of HBV DNA ± HBsAg recurrence in anti-HBc-positive patients | 8.3% | 0 | ||||||
| Innocenti et al[110], 2022 | CLL | Ibrutinib | 108 | 0 | 108 | 67.6% (lamivudine) | HBsAg seroconversion and/or an increase of serum HBV DNA by ≥ 1 log above the LLD of the assay | 1.9% | 0 | ||||||
| Ni et al[111], 2022 | DLBCL | Ibrutinib or zanu-brutinib | 55 | 4 | 26 | 100% for CHB and 34.6% for past infection (entecavir) | > 1 log increase in HBV DNA, HBV DNA-positive when previously negative, HBV DNA > 2000 IU/mL if no baseline level was available, or reverse seroconversion from HBsAg-negative to -positive | 7.69% for past infection | 0 | ||||||
B cell lymphoma-2 inhibitors: The B cell lymphoma (BCL)-2 inhibitor venetoclax is commonly used in the treatment of CLL[112,113] and in combination with azacitidine or low-dose cytarabine in AML patients who are not fit for intensive chemotherapy[114,115]. Venetoclax is a potent inhibitor of the antiapoptotic BCL-2 protein. AML stem cells express BCL-2 and depend on BCL-2 for survival. Venetoclax has synergistic effects when used in combination with azacitidine.
There is a lack of large retrospective or prospective studies on the incidence of HBV reactivation in patients receiving venetoclax, so the risk is unclear. Because of its mechanism of action, venetoclax will profoundly suppress B-cell activity. Thus, the same prophylactic or pre-emptive antiviral management approach used for patients receiving BTK inhibitors should be applied in patients receiving venetoclax.
Proteasome inhibitors: The proteasome inhibitors have become the backbone treatment for MM. They include borte
Ataca Atilla et al[117] conducted a retrospective study in 178 MM patients who had received lenalidomide and/or bortezomib. They found that the rate of HBV reactivation was 3% after bortezomib and 8% after bortezomib and lenalidomide[117]. Lee et al[118] reported HBV reactivation in 5.2% of 230 MM patients with past resolved HBV infection after a median follow-up of 2.4 years. One hundred and thirty-three patients (58%) had received bortezomib-based the
Mya et al[116] reported an HBV reactivation incidence of 5.5% in 273 relapsed or refractory MM patients who had received bortezomib and dexamethasone therapy. Li et al[119] conducted a retrospective study of HBV reactivation in patients receiving regimens containing bortezomib. Twenty-seven of the 139 patients were HBsAg positive and 22 of them were given antiviral prophylaxis with lamivudine or entecavir. HBV reactivation occurred in six HBsAg-positive and two HBsAg-negative/anti-HBc-positive cases from a total of 139 patients[119]. Antiviral prophylaxis is recom
Immune checkpoint inhibitors: Immune checkpoint inhibitors (ICI) are effective in the treatment of solid tumors, and have also shown efficacy in the treatment of lymphoma[120-124]. ICIs can block the localization and traffic of activated lymphocytes, thus inhibiting the inflammatory response associated with immune-mediated diseases[125]. They may also reduce the local immune control of HBV replication in the liver, predisposing patients to HBV reactivation.
Table 3 summarizes the data on HBV reactivation in patients receiving ICIs[126-129]. The rate of HBV reactivation among HBsAg-positive cancer patients is 0.5% to 5.3% during ICI therapy. Prophylactic antiviral treatment is recom
| Ref. | Disease type | Therapy | N | CHB, n | Past resolved HBV infection, n | Antiviral prophylaxis, % patients | Definition of HBV reactivation | Rate of HBV reactivation | HBV-related death |
| Zhang et al[129], 2019 | Solid tumors, lymphoma (7%) | PD-1/PD-L1 inhibitors (pembrolizumab, nivolumab, toripalimab, camrelizumab, sintilimab, atezolizumab) | 114 | 114 | 0 | 74.6% received prophylaxis (entecavir, tenofovir, lamivudine, telbivudine, adefovir) | AASLD 2018 guidelines | 6 (5.3%) | 0 |
| Wong et al[127], 2021 | Solid tumors | PD-1 inhibitors (nivolumab, pembrolizumab, spartalizumab), PD-L1 inhibitors (atezolizumab, avelumab, durvalumab), CTLA-4 inhibitors (ipilimumab, tremelimumab) | 990 | 397 | 225 | 100% for CHB, and 11.3% for past HBV infection (entecavir, TAF, TDF, lamivudine, telbivudine, ADV) | AASLD 2018 guidelines | 2/397 (0.5%); none in the resolved HBV group | 0 |
| Yoo et al[128], 2022 | Solid tumors, lymphoma (1.8%) | PD-1 inhibitors (nivolumab, pembrolizumab), PD-L1 inhibitors (atezolizumab, avelumab), CTLA-4 inhibitors (ipilimumab, tremelimumab) | 3465 | 511 | 564 | 90.8% for CHB, 1.1% for HBsAg negative patients (entecavir, tenofovir, lamivudine, telbivudine, adefovir, clevudine) | AASLD 2018 guidelines | 1% for chronic HBV infection, 0% for past HBV infection | 0 |
| Lasagna et al[126], 2023 | Solid tumors | Pembrolizumab, nivolumab, atezolizumab | 150 | 0 | 150 | Nil | AASLD 2018 guidelines | 0% | 0 |
Existing guidelines on the drug classes and the corresponding risk of HBV reactivation are summarized in Table 4, while Table 5 summarizes the international guidelines on the management of patients with HBV infection receiving chemo
| Drug class | Drug or dose | Risk of HBV reactivation | |
| For HBsAg-positive patients | For HBsAg-negative/anti-HBc-positive patients | ||
| Anti-CD20 monoclonal antibodies | Rituximab, obinutuzumab, ofatumumab | High (30%-60%) | High (> 10%) |
| Anthracycline chemotherapy | Doxorubicin, daunorubicin, epirubicin | High (15%-30%) | High (> 10%) |
| Steroids | Moderate/high dose ≥ 4 wk | High (> 10%) | Moderate (1%-10%) |
| Low dose ≥ 4 wk | Moderate (1%-10%) | Low (< 1%) | |
| Low dose ≤ 1 wk | Low (< 1%) | Low (< 1%) | |
| Tyrosine kinase inhibitors | Imatinib, nilotinib, dasatinib | High to moderate | Low (< 1%) |
| Immune checkpoint inhibitors | Nivolumab, pembrolizumab | High (> 10%) | Uncertain |
| Proteasome inhibitor | Bortezomib | Moderate (1%-10%) | Moderate (1%-10%) |
| Guideline | HBV screening | Screening tests | HBsAg-positive patients | HBsAg-negative, anti-HBc-positive patients | Choice of antiviral agent | Duration of antiviral therapy | Monitoring after prophylaxis | Ref. |
| American Gastroenterological Association 2015 guideline | High risk of HBV reactivation (> 10%) and moderate risk of HBV reactivation (1%-10%). Routine screening not recommended for low risk of HBV reactivation (< 1%) | HBsAg, anti-HBc, HBV DNA if serology positive | Prophylactic antiviral therapy | Antiviral prophylaxis over monitoring for patients if the chemotherapy is associated with high or moderate risk of HBV reactivation | Drug with high barrier to resistance is favored over LMV | 6 mo after discontinuation of therapy and at least 12 mo for B-cell depleting agents | Not defined | [6] |
| European Association for the Study of the Liver 2017 | All candidates for CT or IST | HBsAg, anti-HBc, and anti-HBs | Anti-HBV prophylaxis | Anti-HBV prophylaxis if they are at high risk of HBV reactivation. Pre-emptive therapy for moderate (10%) or low (1%) risk of HBV reactivation, and monitor HBsAg and/or HBV DNA every 1-3 mo during and after IST | ETV or TDF or TAF | At least 12 mo (18 mo for high-risk therapy) after the last course of therapy | LFT and HBV DNA every 3 to 6 mo during prophylaxis and for ≥ 12 mo after NA withdrawal | [3] |
| American Association for the Study of Liver Diseases 2018 | All patients for CT and IST | HBsAg and anti-HBc | Anti-HBV prophylaxis | On-demand therapy except for patients receiving anti-CD20 antibody therapy or SCT (monitor ALT, HBV DNA, HBsAg every 1-3 mo) | ETV or TDF or TAF | At least 6 mo after discontinuation of IST. At least 12 mo for B cell-depleting agents | For up to 12 mo after cessation of anti-HBV therapy | [7] |
| American Society of Clinical Oncology 2020 update | All candidates for CT or IST | HBsAg, anti-HBc, and anti-HBs | Anti-HBV prophylaxis | High risk, e.g., anti-CD20 antibody therapy or stem cell transplantation: Prophylaxis. Others: On-demend therapy (monitor HBsAg and HBV DNA every 3 mo) | ETV, TDF, TAF | At least 12 mo after cessation of IST | High risk: Monthly for the first 3 mo after NA withdrawal and then every 3 mo (duration not specified). Resolved HBV and not high risk: Not necessary | [4] |
| The Asian Pacific Association for the Study of the Liver 2021 | All patients planned to receive IST | HBsAg, anti-HBs and anti-HBc, quanti-tative HBV DNA for HBsAg-positive patients | Anti-HBV prophylaxis in high and moderate-risk groups, and low-risk group with advanced liver fibrosis or cirrhosis. Pre-emptive treatment in low-risk group without advanced liver fibrosis or cirrhosis | Anti-HBV prophylaxis in high-risk group and moderate-risk group with advanced liver fibrosis or cirrhosis. Pre-emptive treatment in low-risk group without advanced liver fibrosis or cirrhosis | ETV, TDF or TAF | 6 mo after the completion of IST for HBsAg-positive patients, without advanced liver fibrosis or cirrhosis and with low level of HBV DNA | HBV DNA every 3 mo | [5] |
| Therapy | Chronic HBV infection | Past resolved HBV infection |
| CAR-T (e.g., axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel) | Antiviral prophylaxis | Antiviral prophylaxis |
| Bispecific antibodies (e.g., glofitamab, mosunetuzumab) | Antiviral prophylaxis | Antiviral prophylaxis |
| BTK inhibitors (e.g., ibrutinib, acalabrutinib, zanubrutinib) | Antiviral prophylaxis | Antiviral prophylaxis or monitoring and pre-emptive therapy1 |
| BCL-2 inhibitors (venetoclax) | Antiviral prophylaxis | Antiviral prophylaxis or monitoring and pre-emptive therapy1 |
| Anti-CD19 monoclonal antibody (blinatumumab) | Antiviral prophylaxis | Antiviral prophylaxis |
| Anti-CD22 monoclonal antibody (inotuzumab) | Antiviral prophylaxis | Antiviral prophylaxis |
| Anti-CD79 monoclonal antibody (polatuzumab) | Antiviral prophylaxis | Antiviral prophylaxis |
| Anti-CD38 monoclonal antibody (daratumumab) | Antiviral prophylaxis | Antiviral prophylaxis |
In order to prevent HBV reactivation among patients with hematological malignancies, it is essential to identify those with HBV infection before starting chemotherapy or immunotherapy. This starts with screening for the presence of HBsAg and anti-HBc in blood. The commercial immunoassays usually capture HbsAg, having specificity for epitopes present on the antigenic α determinant. The enzyme-linked immunosorbent assay method used in HBsAg detection has a sensitivity and specificity of both about 80%, compared with more than 90% using the immunochromatographic test[130]. Complete loss of anti-HBc with chronic and high viremic HBV infection after allogeneic stem cell transplantation has been reported[131]. However, there might be some rare scenarios where HBsAg or anti-HBc is falsely negative. For example, mutations within the α determinant may affect the conformation of the surface epitope such that it is unrecognizable to the test, or mutations in other parts of the viral genome may affect HbsAg secretion or expression, resulting in diagnostic escape[132]. Moreover, there has been a case report describing complete loss of anti-HBc after allogeneic stem cell transplantation in a patient with resolved HBV infection who previously had positive anti-HBs and anti-HBc prior to the stem cell transplant[131]. Isolated anti-HBs without anti-HBc may be present in pretreated patients without previous hepatitis B vaccination[133,134]. Thus, a more sensitive combined screening strategy is advisable, including serological testing for HBsAg, anti-HBc, and anti-HBs and a sensitive test for HBV DNA.
A preventive strategy is more effective than a pre-emptive strategy in HBsAg-positive patients[135,136]. We recommend giving NAT for prophylaxis in all HBsAg-positive candidates prior to immunosuppressive therapy irrespective of their HBV DNA status because the risk of HBV reactivation is high in this group of patients. This approach is highly effective, with a number needed to treat to prevent one episode of HBV reactivation of three[135].
It is not always possible to prevent the development of hepatitis or hepatitis flares if antiviral therapy is started after the onset of HBV reactivation[42], since it will take some weeks or even months for the antiviral therapy to reduce viral loads, and the inflammation and necrosis of the liver will be ongoing during this period[137].
The risk of HBV reactivation in this group varies considerably, depending on the level of viremia and the immunosuppressive regimens administered. In general, if HBV DNA is detectable, the patient would be given anti-HBV prophylaxis and treated similarly to HBsAg-positive patients. If HBV DNA is undetectable, then the risk of reactivation associated with the immunosuppressive regimen will be assessed. High-risk groups such as those receiving anti-CD20 monoclonal antibodies should receive antiviral prophylaxis with NAT. Pre-emptive treatment is recommended for moderate- and low-risk groups, with HBV DNA monitoring every 1-3 mo.
Huang et al[43] compared pre-emptive with prophylactic entecavir therapy during R-CHOP chemotherapy in patients with lymphoma and resolved hepatitis B. Prophylactic entecavir treatment significantly reduced the risk of HBV reac
HBV vaccination can be considered in patients who are HBsAg-negative, anti-HBc-negative, and anti-HBs-negative[3]. Anti-HBs potentially provide a protective effect against HBV reactivation[45,138-141]. The results of a meta-analysis showed that, among patients not receiving antiviral prophylaxis, the reactivation risk was 14% in the 388 patients who had anti-HBc only vs 5.0% in 1284 patients with concomitant anti-HBs. The pooled odds ratio of HBV reactivation was 0.21 (95% confidence interval: 0.14-0.32) in those with anti-HBs compared with anti-HBc only[141].
It is rare to have HBV reactivation in patients with isolated anti-HBs, but there have been occasional reports of HBV reactivation in patients with only anti-HBs seropositivity[133,134]. In one report, a patient with follicular lymphoma, who had not been vaccinated for hepatitis B, was positive for anti-HBs but negative for anti-HBc prior to starting chemo
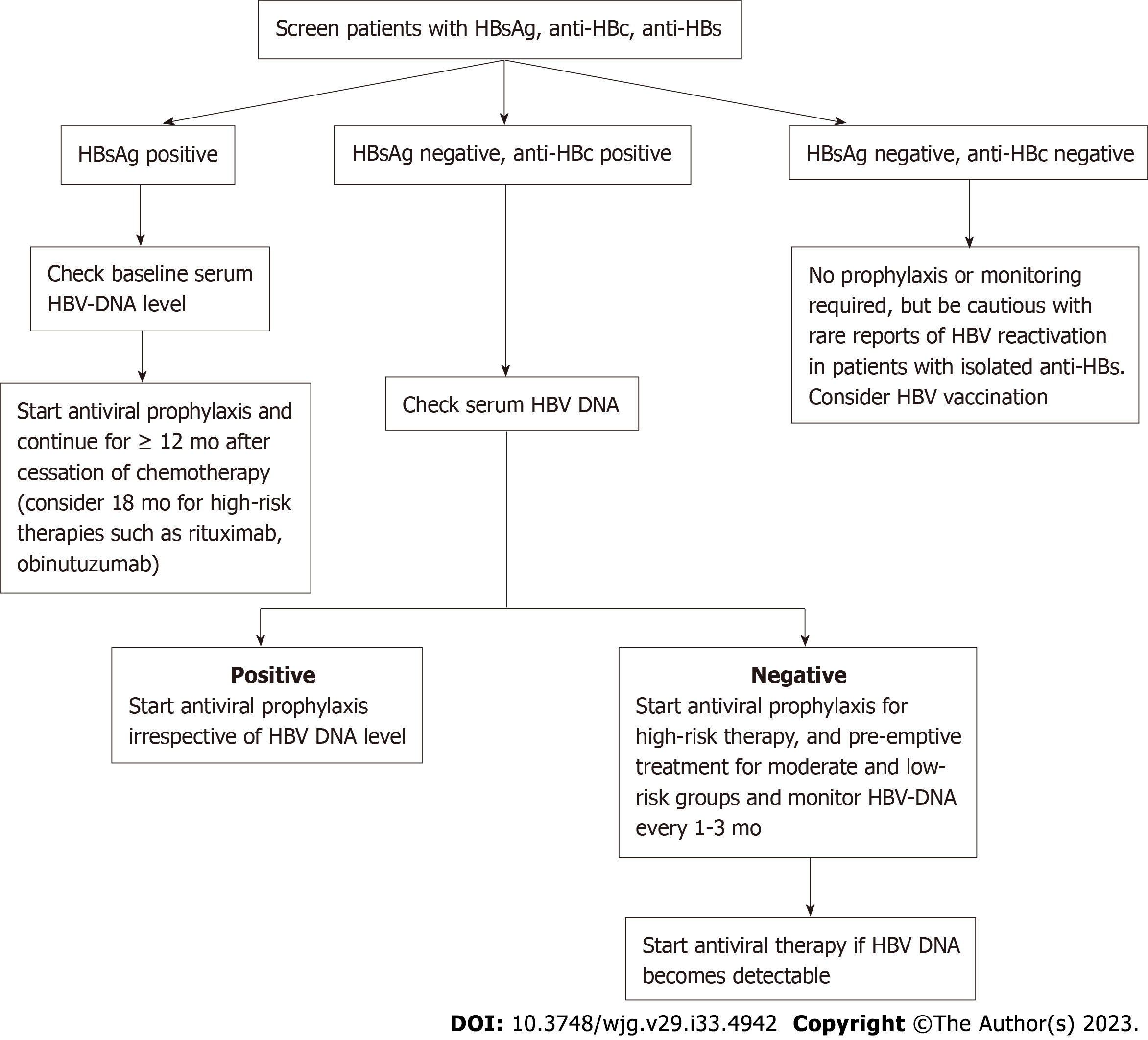
In a separate report, a patient with DLBCL (also without a record of hepatitis B vaccination) had a pre-chemotherapy HBV profile that was positive for anti-HBs (127 IU/mL) but negative for HBsAg and anti-HBc. She developed HBV reactivation after completing rituximab-based chemotherapy. Antiviral treatment with entecavir was started after HBV reactivation was detected. Despite that, she had clinical deterioration with development of hepatic encephalopathy and died of liver failure finally[134]. Figure 1 shows a suggested algorithm for HBV testing and management of patients with hematological malignancies receiving anticancer therapy.
For the treatment of chronic HBV infection, entecavir and tenofovir are the preferred antiviral agents because they have high genetic barriers to resistance compared with lamivudine. Huang et al[142] performed a prospective randomized study in 121 HBsAg-positive patients with untreated DLBCL. Sixty patients received lamivudine prophylaxis and 61 received entecavir prophylaxis. Various endpoints occurred at a significantly lower rate in the entecavir than the lami
A meta-analysis of 770 patients with lymphoma showed that, in patients with CHB, the risk of HBV reactivation was significantly higher in those receiving prophylactic lamivudine compared with entecavir (P < 0.001)[143]. The superior prophylactic efficacy of entecavir is supported by studies in allogenic HSCT recipients and solid tumor patients, which showed a lower rate of HBV reactivation with entecavir compared with lamivudine[20,144]. Meta-analyses have also shown that tenofovir and entecavir are the most effective antiviral agents for the prevention of HBV reactivation[145,146]. Entecavir treatment of HBV patients with lamivudine-resistant viral strains is usually unsuccessful due to the rapid selection of additional mutants[147], highlighting the importance in choosing an effective initial anti-viral therapy.
Most guidelines recommend continuing antiviral therapy for 1 year after the cessation of anti-cancer therapy, and some guidelines recommend extending antiviral treatment for up to 18 mo after the last dose of cancer therapy (Table 6)[3,4,148]. Delayed HBV reactivation has been reported in patients who received anti-CD20 antibody therapy such as ritu
HBV reactivation can develop after cessation of NAT[149,151,152], so monitoring for HBV reactivation is recommended after stopping anti-HBV prophylaxis (Table 6). In general, liver function tests and HBV DNA are monitored every 3 mo for a minimum of 12 mo after discontinuation of antiviral agents[3,4,7]. Monitoring for more than 12 mo is recommended for patients who received anti-CD20 monoclonal antibody therapy.
Prophylaxis is better than treatment because fatal outcomes may still occur in patients with HBV reactivation even with antiviral treatment[153]. Foont and Schiff[154] performed a systematic review on the use of lamivudine for the pro
The purpose of the treatment is prevention of severe hepatitis and also hepatic failure, which are potentially fatal. It is important to closely monitor the patient’s liver enzymes, clotting profile, and bilirubin levels. Patients can still progress to hepatic failure despite therapy with nucleoside analogs[42], especially when there is already a marked increase in liver enzymes or jaundice. Liver transplantation is an option for patients with liver failure and there have been reported cases of successful transplantation in patients with chemotherapy-induced HBV reactivation[156-160]. Benten et al[161] found a low recurrence of pre-existing extrahepatic malignancies after liver transplantation.
Many novel therapies have emerged for the treatment of hematological malignancies in the past two decades and the results are promising. The issue of prevention of HBV reactivation is an important part of the management. Hepatitis due to HBV reactivation is a potentially fatal complication of cancer chemotherapy in patients with hematological malig
Entecavir and tenofovir are the preferred choices for prophylactic therapy. Preventative antiviral therapy should be continued for at least 12 mo after the cessation of chemotherapy; longer durations are recommended for patients who received rituximab or those who had high levels of serum HBV DNA before starting chemotherapy. Checking the HBV DNA before the cessation of antiviral therapy is recommended. We would also recommend monitoring liver function and HBV DNA levels for at least 12 mo after the cessation of antiviral prophylaxis.
| 1. | Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 618] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 2. | Wei J, Zhu X, Mao X, Huang L, Meng F, Zhou J. Severe early hepatitis B reactivation in a patient receiving anti-CD19 and anti-CD22 CAR T cells for the treatment of diffuse large B-cell lymphoma. J Immunother Cancer. 2019;7:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4005] [Article Influence: 445.0] [Reference Citation Analysis (1)] |
| 4. | Hwang JP, Feld JJ, Hammond SP, Wang SH, Alston-Johnson DE, Cryer DR, Hershman DL, Loehrer AP, Sabichi AL, Symington BE, Terrault N, Wong ML, Somerfield MR, Artz AS. Hepatitis B Virus Screening and Management for Patients With Cancer Prior to Therapy: ASCO Provisional Clinical Opinion Update. J Clin Oncol. 2020;38:3698-3715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 5. | Lau G, Yu ML, Wong G, Thompson A, Ghazinian H, Hou JL, Piratvisuth T, Jia JD, Mizokami M, Cheng G, Chen GF, Liu ZW, Baatarkhuu O, Cheng AL, Ng WL, Lau P, Mok T, Chang JM, Hamid S, Dokmeci AK, Gani RA, Payawal DA, Chow P, Park JW, Strasser SI, Mohamed R, Win KM, Tawesak T, Sarin SK, Omata M. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol Int. 2021;15:1031-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221-244.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (1)] |
| 7. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3080] [Article Influence: 385.0] [Reference Citation Analysis (1)] |
| 8. | Chang Y, Jeong SW, Jang JY. Hepatitis B Virus Reactivation Associated With Therapeutic Interventions. Front Med (Lausanne). 2021;8:770124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 9. | Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 476] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 10. | Bessone F, Dirchwolf M. Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations. World J Hepatol. 2016;8:385-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Tohme RA, Bulkow L, Homan CE, Negus S, McMahon BJ. Rates and risk factors for hepatitis B reactivation in a cohort of persons in the inactive phase of chronic hepatitis B-Alaska, 2001-2010. J Clin Virol. 2013;58:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Wang B, Mufti G, Agarwal K. Reactivation of hepatitis B virus infection in patients with hematologic disorders. Haematologica. 2019;104:435-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, Hui P, Leung NW, Zee B, Johnson PJ. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 14. | Chen CY, Tien FM, Cheng A, Huang SY, Chou WC, Yao M, Tang JL, Tien HF, Sheng WH. Hepatitis B reactivation among 1962 patients with hematological malignancy in Taiwan. BMC Gastroenterol. 2018;18:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Law MF, Ho R, Cheung CK, Tam LH, Ma K, So KC, Ip B, So J, Lai J, Ng J, Tam TH. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies treated with anticancer therapy. World J Gastroenterol. 2016;22:6484-6500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, Chan AT, Mok TS, Lee JJ, Leung TW, Zhong S, Johnson PJ. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004;22:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Yeo W, Chan PK, Hui P, Ho WM, Lam KC, Kwan WH, Zhong S, Johnson PJ. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 368] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013;33:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Chen WC, Cheng JS, Chiang PH, Tsay FW, Chan HH, Chang HW, Yu HC, Tsai WL, Lai KH, Hsu PI. A Comparison of Entecavir and Lamivudine for the Prophylaxis of Hepatitis B Virus Reactivation in Solid Tumor Patients Undergoing Systemic Cytotoxic Chemotherapy. PLoS One. 2015;10:e0131545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, Hou JL, Wen YM, Nanj A, Liang R. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99:2324-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 175] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, Lam KC, Johnson PJ. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 248] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 23. | Salpini R, Colagrossi L, Bellocchi MC, Surdo M, Becker C, Alteri C, Aragri M, Ricciardi A, Armenia D, Pollicita M, Di Santo F, Carioti L, Louzoun Y, Mastroianni CM, Lichtner M, Paoloni M, Esposito M, D'Amore C, Marrone A, Marignani M, Sarrecchia C, Sarmati L, Andreoni M, Angelico M, Verheyen J, Perno CF, Svicher V. Hepatitis B surface antigen genetic elements critical for immune escape correlate with hepatitis B virus reactivation upon immunosuppression. Hepatology. 2015;61:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 24. | Chen YF, Chong CL, Wu YC, Wang YL, Tsai KN, Kuo TM, Hong MH, Hu CP, Chen ML, Chou YC, Chang C. Doxorubicin Activates Hepatitis B Virus Replication by Elevation of p21 (Waf1/Cip1) and C/EBPα Expression. PLoS One. 2015;10:e0131743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Kostyusheva A, Brezgin S, Bayurova E, Gordeychuk I, Isaguliants M, Goptar I, Urusov F, Nikiforova A, Volchkova E, Kostyushev D, Chulanov V. ATM and ATR Expression Potentiates HBV Replication and Contributes to Reactivation of HBV Infection upon DNA Damage. Viruses. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Kelling M, Sokol L, Dalia S. Hepatitis B Reactivation in the Treatment of Non-Hodgkin Lymphoma. Cancer Control. 2018;25:1073274818767879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Cao X, Wang Y, Li P, Huang W, Lu X, Lu H. HBV Reactivation During the Treatment of Non-Hodgkin Lymphoma and Management Strategies. Front Oncol. 2021;11:685706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, Kao WY, Uen WC, Hsu CH, Tien HF, Chao TY, Chen LT, Whang-Peng J; Lymphoma Committee of Taiwan Cooperative Oncology Group. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 232] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Notsumata K, Nomura Y, Tanaka A, Ueda T, Sanada T, Watanabe H, Toya D. Efficient Prophylactic Management of HBV Reactivation by an Information Technology Encoding System: Results of a 6-year Prospective Cohort Study. Intern Med. 2020;59:2457-2464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Zhong Z, Liao W, Dai L, Feng X, Su G, Gao Y, Wu Q, Yang P. Average corticosteroid dose and risk for HBV reactivation and hepatitis flare in patients with resolved hepatitis B infection. Ann Rheum Dis. 2022;81:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Wang YH, Liang JD, Sheng WH, Tien FM, Chen CY, Tien HF. Hepatitis B reactivation during treatment of tyrosine kinase inhibitors-Experience in 142 adult patients with chronic myeloid leukemia. Leuk Res. 2019;81:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Uhm J, Kim SH, Oh S, Zang DY, Do YR, Lee WS, Chang MH, Lee SE, Kim DW. High incidence of hepatitis B viral reactivation in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Blood. 2018;132:3010. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Ribera JM, García-Calduch O, Ribera J, Montesinos P, Cano-Ferri I, Martínez P, Esteve J, Esteban D, García-Fortes M, Alonso N, González-Campos J, Bermúdez A, Torrent A, Genescà E, Mercadal S, Martínez-Lopez J, García-Sanz R. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6:5395-5402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Sugiura I, Doki N, Hata T, Cho R, Ito T, Suehiro Y, Tanaka M, Kako S, Matsuda M, Yokoyama H, Ishikawa Y, Taniguchi Y, Hagihara M, Ozawa Y, Ueda Y, Hirano D, Sakura T, Tsuji M, Kamae T, Fujita H, Hiramoto N, Onoda M, Fujisawa S, Hatta Y, Dobashi N, Nishiwaki S, Atsuta Y, Kobayashi Y, Hayakawa F, Ohtake S, Naoe T, Miyazaki Y. Dasatinib-based 2-step induction for adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6:624-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Lazdina U, Alheim M, Nyström J, Hultgren C, Borisova G, Sominskaya I, Pumpens P, Peterson DL, Milich DR, Sällberg M. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J Gen Virol. 2003;84:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Xu X, Shang Q, Chen X, Nie W, Zou Z, Huang A, Meng M, Jin L, Xu R, Zhang JY, Fu J, Wang L, Tang Z, Xie Y, Yang X, Zhang Z, Wang FS. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol Immunol. 2015;12:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, Dai MS, Chiu BC, Fintel B, Cheng Y, Chuang SS, Lee MY, Chen TY, Lin SF, Kuo CY. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 38. | Guo YF, Pan JX, Zhuang WH. Concurrent and reactivation of hepatitis B virus infection in diffuse large B-cell lymphoma: risk factors and survival outcome. Infect Agent Cancer. 2018;13:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Oh MJ, Lee HJ. A study of hepatitis B virus reactivation associated with rituximab therapy in real-world clinical practice: a single-center experience. Clin Mol Hepatol. 2013;19:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Pei SN, Chen CH, Lee CM, Wang MC, Ma MC, Hu TH, Kuo CY. Reactivation of hepatitis B virus following rituximab-based regimens: a serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol. 2010;89:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | Tsai YF, Yang CI, Du JS, Lin MH, Tang SH, Wang HC, Cho SF, Liu YC, Su YC, Dai CY, Hsiao HH. Rituximab increases the risk of hepatitis B virus reactivation in non-Hodgkin lymphoma patients who are hepatitis B surface antigen-positive or have resolved hepatitis B virus infection in a real-world setting: a retrospective study. PeerJ. 2019;7:e7481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, Kao WY, Chiu CF, Lin SF, Lin J, Chang CS, Tien HF, Liu TW, Chen PJ, Cheng AL; Taiwan Cooperative Oncology Group. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 43. | Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, Yang MH, Tzeng CH, Lee PC, Lin HC, Lee SD. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 44. | Kusumoto S, Tanaka Y, Suzuki R, Watanabe T, Nakata M, Takasaki H, Fukushima N, Fukushima T, Moriuchi Y, Itoh K, Nosaka K, Choi I, Sawa M, Okamoto R, Tsujimura H, Uchida T, Suzuki S, Okamoto M, Takahashi T, Sugiura I, Onishi Y, Kohri M, Yoshida S, Sakai R, Kojima M, Takahashi H, Tomita A, Maruyama D, Atsuta Y, Tanaka E, Suzuki T, Kinoshita T, Ogura M, Mizokami M, Ueda R. Monitoring of Hepatitis B Virus (HBV) DNA and Risk of HBV Reactivation in B-Cell Lymphoma: A Prospective Observational Study. Clin Infect Dis. 2015;61:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 45. | Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, Gill H, Lam YF, Lie AK, Lai CL, Kwong YL, Yuen MF. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3736-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 46. | Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, Chan HL, Hui EP, Lei KI, Mok TS, Chan PK. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 506] [Article Influence: 29.8] [Reference Citation Analysis (1)] |
| 47. | Owen C, Stewart DA. Obinutuzumab for the treatment of lymphoproliferative disorders. Expert Opin Biol Ther. 2012;12:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T, Opat S, Owen CJ, Samoylova O, Kreuzer KA, Stilgenbauer S, Döhner H, Langerak AW, Ritgen M, Kneba M, Asikanius E, Humphrey K, Wenger M, Hallek M. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1166] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 49. | Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trněný M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med. 2017;377:1331-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 594] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 50. | Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, Peters MG, Tanaka Y, Zelenetz AD, Kuriki H, Fingerle-Rowson G, Nielsen T, Ueda E, Piper-Lepoutre H, Sellam G, Tobinai K. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood. 2019;133:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Vitolo U, Trněný M, Belada D, Burke JM, Carella AM, Chua N, Abrisqueta P, Demeter J, Flinn I, Hong X, Kim WS, Pinto A, Shi YK, Tatsumi Y, Oestergaard MZ, Wenger M, Fingerle-Rowson G, Catalani O, Nielsen T, Martelli M, Sehn LH. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2017;35:3529-3537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 52. | Pfeifer M, Zheng B, Erdmann T, Koeppen H, McCord R, Grau M, Staiger A, Chai A, Sandmann T, Madle H, Dörken B, Chu YW, Chen AI, Lebovic D, Salles GA, Czuczman MS, Palanca-Wessels MC, Press OW, Advani R, Morschhauser F, Cheson BD, Lenz P, Ott G, Polson AG, Mundt KE, Lenz G. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia. 2015;29:1578-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 53. | Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M, Hirata J, Penuel E, Paulson JN, Cheng J, Ku G, Matasar MJ. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2020;38:155-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 600] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 54. | Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trněný M, Sharman JP, Herbaux C, Burke JM, Matasar M, Rai S, Izutsu K, Mehta-Shah N, Oberic L, Chauchet A, Jurczak W, Song Y, Greil R, Mykhalska L, Bergua-Burgués JM, Cheung MC, Pinto A, Shin HJ, Hapgood G, Munhoz E, Abrisqueta P, Gau JP, Hirata J, Jiang Y, Yan M, Lee C, Flowers CR, Salles G. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N Engl J Med. 2022;386:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 610] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 55. | Shor B, Gerber HP, Sapra P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol Immunol. 2015;67:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 56. | Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, Gökbuget N, O'Brien S, Wang K, Wang T, Paccagnella ML, Sleight B, Vandendries E, Advani AS. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375:740-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1069] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 57. | Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, Itin C, Prang N, Baeuerle PA. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 58. | Foà R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, Canichella M, Viero P, Ferrara F, Lunghi M, Fabbiano F, Bonifacio M, Fracchiolla N, Di Bartolomeo P, Mancino A, De Propris MS, Vignetti M, Guarini A, Rambaldi A, Chiaretti S; GIMEMA Investigators. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383:1613-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 59. | Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foà R, Bassan R, Arslan Ö, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Brüggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z, Topp MS. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med. 2017;376:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1172] [Cited by in RCA: 1641] [Article Influence: 182.3] [Reference Citation Analysis (0)] |
| 60. | Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K, Yang H, Klippel Z, Zahlten-Kumeli A, Usmani SZ. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 328] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 61. | Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon SS, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O'Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P; POLLUX Investigators. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:1319-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1166] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 62. | Facon T, Kumar S, Plesner T, Orlowski RZ, Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, Goldschmidt H, O'Dwyer M, Perrot A, Venner CP, Weisel K, Mace JR, Raje N, Attal M, Tiab M, Macro M, Frenzel L, Leleu X, Ahmadi T, Chiu C, Wang J, Van Rampelbergh R, Uhlar CM, Kobos R, Qi M, Usmani SZ; MAIA Trial Investigators. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med. 2019;380:2104-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 783] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 63. | Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P; CASTOR Investigators. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375:754-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1200] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 64. | Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Sanchorawala V, Gibbs S, Mollee P, Venner CP, Lu J, Schönland S, Gatt ME, Suzuki K, Kim K, Cibeira MT, Beksac M, Libby E, Valent J, Hungria V, Wong SW, Rosenzweig M, Bumma N, Huart A, Dimopoulos MA, Bhutani D, Waxman AJ, Goodman SA, Zonder JA, Lam S, Song K, Hansen T, Manier S, Roeloffzen W, Jamroziak K, Kwok F, Shimazaki C, Kim JS, Crusoe E, Ahmadi T, Tran N, Qin X, Vasey SY, Tromp B, Schecter JM, Weiss BM, Zhuang SH, Vermeulen J, Merlini G, Comenzo RL; ANDROMEDA Trial Investigators. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N Engl J Med. 2021;385:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 439] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 65. | Palladini G, Kastritis E, Maurer MS, Zonder J, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Bumma N, Kaufman JL, Medvedova E, Kovacsovics T, Rosenzweig M, Sanchorawala V, Qin X, Vasey SY, Weiss BM, Vermeulen J, Merlini G, Comenzo RL. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 66. | van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 67. | Zhang S, Zhao J, Zhang Z. Humoral immunity, the underestimated player in hepatitis B. Cell Mol Immunol. 2018;15:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Kikuchi T, Kusumoto S, Tanaka Y, Oshima Y, Fujinami H, Suzuki T, Totani H, Kinoshita S, Asao Y, Narita T, Ito A, Ri M, Komatsu H, Iida S. Hepatitis B virus reactivation in a myeloma patient with resolved infection who received daratumumab-containing salvage chemotherapy. J Clin Exp Hematop. 2020;60:51-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Lee SK, Sung PS, Park SS, Min CK, Nam H, Jang JW, Choi JY, Yoon SK. Reactivation of Resolved Hepatitis B After Daratumumab for Multiple Myeloma. Clin Infect Dis. 2021;73:e1372-e1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, Leleu X, Schjesvold F, Moreau P, Dimopoulos MA, Huang JS, Minarik J, Cavo M, Prince HM, Macé S, Corzo KP, Campana F, Le-Guennec S, Dubin F, Anderson KC; ICARIA-MM study group. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 71. | Leypoldt LB, Besemer B, Asemissen AM, Hänel M, Blau IW, Görner M, Ko YD, Reinhardt HC, Staib P, Mann C, Lutz R, Munder M, Graeven U, Peceny R, Salwender H, Jauch A, Zago M, Benner A, Tichy D, Bokemeyer C, Goldschmidt H, Weisel KC. Isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: interim analysis of the GMMG-CONCEPT trial. Leukemia. 2022;36:885-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 72. | Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, Hajek R, Špička I, Baker R, Kim K, Martinez G, Min CK, Pour L, Leleu X, Oriol A, Koh Y, Suzuki K, Risse ML, Asset G, Macé S, Martin T; IKEMA study group. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. 2021;397:2361-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 73. | Spicka I, Moreau P, Martin TG, Facon T, Martinez G, Oriol A, Koh Y, Lim A, Mikala G, Rosiñol L, Yağci M, Cavo M, Risse ML, Asset G, Macé S, van de Velde H, Yong K. Isatuximab plus carfilzomib and dexamethasone in relapsed multiple myeloma patients with high-risk cytogenetics: IKEMA subgroup analysis. Eur J Haematol. 2022;109:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 74. | Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2882] [Cited by in RCA: 4302] [Article Influence: 537.8] [Reference Citation Analysis (0)] |
| 75. | Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3408] [Cited by in RCA: 4576] [Article Influence: 508.4] [Reference Citation Analysis (0)] |
| 76. | Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, Fleury I, Bachanova V, Foley SR, Ho PJ, Mielke S, Magenau JM, Holte H, Pantano S, Pacaud LB, Awasthi R, Chu J, Anak Ö, Salles G, Maziarz RT; JULIET Investigators. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1919] [Cited by in RCA: 3077] [Article Influence: 439.6] [Reference Citation Analysis (0)] |
| 77. | Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, McSweeney PA, Miklos DB, Pagel JM, Kersten MJ, Milpied N, Fung H, Topp MS, Houot R, Beitinjaneh A, Peng W, Zheng L, Rossi JM, Jain RK, Rao AV, Reagan PM. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382:1331-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1387] [Article Influence: 231.2] [Reference Citation Analysis (0)] |
| 78. | Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, Sauter C, Wang Y, Santomasso B, Mead E, Roshal M, Maslak P, Davila M, Brentjens RJ, Sadelain M. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 2131] [Article Influence: 266.4] [Reference Citation Analysis (0)] |
| 79. | Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, Russo D, Auclair R, Fitzgerald L, Cadzin B, Wang X, Sikder D, Senechal B, Bermudez VP, Purdon TJ, Hosszu K, McAvoy DP, Farzana T, Mead E, Wilcox JA, Santomasso BD, Shah GL, Shah UA, Korde N, Lesokhin A, Tan CR, Hultcrantz M, Hassoun H, Roshal M, Sen F, Dogan A, Landgren O, Giralt SA, Park JH, Usmani SZ, Rivière I, Brentjens RJ, Smith EL. GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med. 2022;387:1196-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 322] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 80. | Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A, Lam LP, Morgan RA, Friedman K, Massaro M, Wang J, Russotti G, Yang Z, Campbell T, Hege K, Petrocca F, Quigley MT, Munshi N, Kochenderfer JN. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380:1726-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 1336] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 81. | Haslauer T, Greil R, Zaborsky N, Geisberger R. CAR T-Cell Therapy in Hematological Malignancies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 82. | Wang Y, Li H, Song X, Qi K, Cheng H, Cao J, Shi M, Yan Z, Jing G, Pan B, Sang W, Wang X, Zhao K, Chen C, Chen W, Zheng J, Li Z, Xu K. Kinetics of immune reconstitution after anti-CD19 chimeric antigen receptor T cell therapy in relapsed or refractory acute lymphoblastic leukemia patients. Int J Lab Hematol. 2021;43:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Cao W, Wei J, Wang N, Xu H, Xiao M, Huang L, Cao Y, Li C, Xiao Y, Gu C, Zhang S, Li D, Zhang Y, Zhang T, Zhou J. Entecavir prophylaxis for hepatitis B virus reactivation in patients with CAR T-cell therapy. Blood. 2020;136:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Cui R, Lyu C, Li Q, Jiang Y, Mou N, Yang Z, Liu X, Deng Q, Li L. Humanized anti-CD19 chimeric antigen receptor-T cell therapy is safe and effective in lymphoma and leukemia patients with chronic and resolved hepatitis B virus infection. Hematol Oncol. 2021;39:75-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Han L, Zhou J, Zhou K, Zhu X, Zhao L, Fang B, Yin Q, Wei X, Zhou H, Li L, Xu B, Zhang J, Song Y, Gao Q. Safety and efficacy of CAR-T cell targeting BCMA in patients with multiple myeloma coinfected with chronic hepatitis B virus. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 86. | Li P, Zhou L, Ye S, Zhang W, Wang J, Tang X, Liu J, Xu Y, Qian W, Liang A. Risk of HBV Reactivation in Patients With Resolved HBV Infection Receiving Anti-CD19 Chimeric Antigen Receptor T Cell Therapy Without Antiviral Prophylaxis. Front Immunol. 2021;12:638678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Liu W, Huang W, Wang M, Lv R, Li J, Wang Y, Deng S, Yi S, Liu H, Rao Q, Xu Y, Lv L, Qiu L, Zou D, Wang J. Risk of hepatitis B reactivation is controllable in patients with B-cell lymphoma receiving anti-CD19 CAR T cell therapy. Br J Haematol. 2020;191:126-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Wang Y, Liu Y, Tan X, Pan B, Ge J, Qi K, Cheng H, Cao J, Shi M, Yan Z, Qiao J, Jing G, Wang X, Sang W, Xia R, Zhang X, Li Z, Gale RP, Zheng J, Zhu F, Xu K. Safety and efficacy of chimeric antigen receptor (CAR)-T-cell therapy in persons with advanced B-cell cancers and hepatitis B virus-infection. Leukemia. 2020;34:2704-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Yang C, Xie M, Zhang K, Liu H, Liang A, Young KH, Qian W. Risk of HBV reactivation post CD19-CAR-T cell therapy in DLBCL patients with concomitant chronic HBV infection. Leukemia. 2020;34:3055-3059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, Barnes JA, O'Brien SM, Chávez JC, Duell J, Rosenwald A, Crombie JL, Ufkin M, Li J, Zhu M, Ambati SR, Chaudhry A, Lowy I, Topp MS. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9:e327-e339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 91. | Minson A, Dickinson M. Glofitamab CD20-TCB bispecific antibody. Leuk Lymphoma. 2021;62:3098-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 92. | van der Horst HJ, de Jonge AV, Hiemstra IH, Gelderloos AT, Berry DRAI, Hijmering NJ, van Essen HF, de Jong D, Chamuleau MED, Zweegman S, Breij ECW, Roemer MGM, Mutis T. Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment. Blood Cancer J. 2021;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 93. | Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, Khan C, Wróbel T, Offner F, Trněný M, Wu SJ, Cartron G, Hertzberg M, Sureda A, Perez-Callejo D, Lundberg L, Relf J, Dixon M, Clark E, Humphrey K, Hutchings M. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2022;387:2220-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 493] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 94. | Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, Salles G, Martínez-Lopez J, Crump M, Thomas DN, Morcos PN, Ferlini C, Bröske AE, Belousov A, Bacac M, Dimier N, Carlile DJ, Lundberg L, Perez-Callejo D, Umaña P, Moore T, Weisser M, Dickinson MJ. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J Clin Oncol. 2021;39:1959-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 340] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 95. | Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, Do YR, Feldman T, Gasiorowski R, Jurczak W, Kim TM, Lewis DJ, van der Poel M, Poon ML, Cota Stirner M, Kilavuz N, Chiu C, Chen M, Sacchi M, Elliott B, Ahmadi T, Hutchings M, Lugtenburg PJ. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol. 2023;41:2238-2247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 408] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 96. | Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, Kuruvilla J, Canales M, Dietrich S, Fay K, Ku M, Nastoupil L, Cheah CY, Wei MC, Yin S, Li CC, Huang H, Kwan A, Penuel E, Bartlett NL. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 97. | Brown JR, Eichhorst B, Hillmen P, Jurczak W, Kaźmierczak M, Lamanna N, O'Brien SM, Tam CS, Qiu L, Zhou K, Simkovic M, Mayer J, Gillespie-Twardy A, Ferrajoli A, Ganly PS, Weinkove R, Grosicki S, Mital A, Robak T, Osterborg A, Yimer HA, Salmi T, Wang MD, Fu L, Li J, Wu K, Cohen A, Shadman M. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2023;388:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 336] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 98. | Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J, Simpson D, Grosicki S, Devereux S, McCarthy H, Coutre S, Quach H, Gaidano G, Maslyak Z, Stevens DA, Janssens A, Offner F, Mayer J, O'Dwyer M, Hellmann A, Schuh A, Siddiqi T, Polliack A, Tam CS, Suri D, Cheng M, Clow F, Styles L, James DF, Kipps TJ; RESONATE-2 Investigators. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373:2425-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1276] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 99. | Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR, Hillmen P, Stephens DM, Ghia P, Barrientos JC, Pagel JM, Woyach J, Johnson D, Huang J, Wang X, Kaptein A, Lannutti BJ, Covey T, Fardis M, McGreivy J, Hamdy A, Rothbaum W, Izumi R, Diacovo TG, Johnson AJ, Furman RR. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 738] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 100. | Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan-Khan A, Furman RR, O'Brien S, Yenerel MN, Illés A, Kay N, Garcia-Marco JA, Mato A, Pinilla-Ibarz J, Seymour JF, Lepretre S, Stilgenbauer S, Robak T, Rothbaum W, Izumi R, Hamdy A, Patel P, Higgins K, Sohoni S, Jurczak W. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J Clin Oncol. 2021;39:3441-3452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 530] [Cited by in RCA: 464] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 101. | Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, Kamdar M, Munir T, Walewska R, Corbett G, Fogliatto LM, Herishanu Y, Banerji V, Coutre S, Follows G, Walker P, Karlsson K, Ghia P, Janssens A, Cymbalista F, Woyach JA, Salles G, Wierda WG, Izumi R, Munugalavadla V, Patel P, Wang MH, Wong S, Byrd JC. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 491] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 102. | Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, Parikh SA, Coutre S, Hurria A, Brown JR, Lozanski G, Blachly JS, Ozer HG, Major-Elechi B, Fruth B, Nattam S, Larson RA, Erba H, Litzow M, Owen C, Kuzma C, Abramson JS, Little RF, Smith SE, Stone RM, Mandrekar SJ, Byrd JC. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N Engl J Med. 2018;379:2517-2528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 741] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 103. | Tam CS, Anderson MA, Pott C, Agarwal R, Handunnetti S, Hicks RJ, Burbury K, Turner G, Di Iulio J, Bressel M, Westerman D, Lade S, Dreyling M, Dawson SJ, Dawson MA, Seymour JF, Roberts AW. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med. 2018;378:1211-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 104. | Wang ML, Jurczak W, Jerkeman M, Trotman J, Zinzani PL, Belada D, Boccomini C, Flinn IW, Giri P, Goy A, Hamlin PA, Hermine O, Hernández-Rivas JÁ, Hong X, Kim SJ, Lewis D, Mishima Y, Özcan M, Perini GF, Pocock C, Song Y, Spurgeon SE, Storring JM, Walewski J, Zhu J, Qin R, Henninger T, Deshpande S, Howes A, Le Gouill S, Dreyling M; SHINE Investigators. Ibrutinib plus Bendamustine and Rituximab in Untreated Mantle-Cell Lymphoma. N Engl J Med. 2022;386:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 105. | Strati P, Coleman M, Champion R, Ma S, Patti C, Levy MY, Lossos IS, Geethakumari PR, Lam S, Calvo R, Higgins K, Budde LE. A phase 2, multicentre, open-label trial (ACE-LY-003) of acalabrutinib in patients with relapsed or refractory marginal zone lymphoma. Br J Haematol. 2022;199:76-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 106. | Dimopoulos MA, Tedeschi A, Trotman J, García-Sanz R, Macdonald D, Leblond V, Mahe B, Herbaux C, Tam C, Orsucci L, Palomba ML, Matous JV, Shustik C, Kastritis E, Treon SP, Li J, Salman Z, Graef T, Buske C; iNNOVATE Study Group and the European Consortium for Waldenström’s Macroglobulinemia. Phase 3 Trial of Ibrutinib plus Rituximab in Waldenström's Macroglobulinemia. N Engl J Med. 2018;378:2399-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 107. | Cameron F, Sanford M. Ibrutinib: first global approval. Drugs. 2014;74:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 108. | Hammond SP, Chen K, Pandit A, Davids MS, Issa NC, Marty FM. Risk of hepatitis B virus reactivation in patients treated with ibrutinib. Blood. 2018;131:1987-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Innocenti I, Morelli F, Autore F, Corbingi A, Pasquale R, Sorà F, Pompili M, Laurenti L. HBV reactivation in CLL patients with occult HBV infection treated with ibrutinib without viral prophylaxis. Leuk Lymphoma. 2019;60:1340-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | Innocenti I, Reda G, Visentin A, Coscia M, Motta M, Murru R, Moia R, Gentile M, Pennese E, Quaglia FM, Albano F, Cassin R, Deodato M, Ielo C, Frustaci AM, Piciocchi A, Rughini A, Arena V, Di Sevo D, Tomasso A, Autore F, Del Poeta G, Scarfò L, Mauro FR, Tedeschi A, Trentin L, Pompili M, Foà R, Ghia P, Cuneo A, Laurenti L. Risk of hepatitis B virus reactivation in chronic lymphocytic leukemia patients receiving ibrutinib with or without antiviral prophylaxis. A retrospective multicentric GIMEMA study. Haematologica. 2022;107:1470-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 111. | Ni Y, Gao L, Lu Y, Ye S, Zhou L, Qian W, Liang A, Li P. Risk of HBV reactivation in relapsed or refractory diffuse large B-cell lymphoma patients receiving Bruton tyrosine kinase inhibitors therapy. Front Immunol. 2022;13:982346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 112. | Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, Samoylova O, Liberati AM, Pinilla-Ibarz J, Opat S, Sivcheva L, Le Dû K, Fogliatto LM, Niemann CU, Weinkove R, Robinson S, Kipps TJ, Tausch E, Schary W, Ritgen M, Wendtner CM, Kreuzer KA, Eichhorst B, Stilgenbauer S, Hallek M, Fischer K. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 113. | Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, Robrecht S, Warburton S, Humphrey K, Samoylova O, Liberati AM, Pinilla-Ibarz J, Opat S, Sivcheva L, Le Dû K, Fogliatto LM, Niemann CU, Weinkove R, Robinson S, Kipps TJ, Boettcher S, Tausch E, Humerickhouse R, Eichhorst B, Wendtner CM, Langerak AW, Kreuzer KA, Ritgen M, Goede V, Stilgenbauer S, Mobasher M, Hallek M. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N Engl J Med. 2019;380:2225-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 653] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 114. | DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, Koller E, Havelange V, Leber B, Esteve J, Wang J, Pejsa V, Hájek R, Porkka K, Illés Á, Lavie D, Lemoli RM, Yamamoto K, Yoon SS, Jang JH, Yeh SP, Turgut M, Hong WJ, Zhou Y, Potluri J, Pratz KW. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 2002] [Article Influence: 333.7] [Reference Citation Analysis (0)] |
| 115. | Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, Kim I, Stevens DA, Fiedler W, Pagoni M, Samoilova O, Hu Y, Anagnostopoulos A, Bergeron J, Hou JZ, Murthy V, Yamauchi T, McDonald A, Chyla B, Gopalakrishnan S, Jiang Q, Mendes W, Hayslip J, Panayiotidis P. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 586] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 116. | Mya DH, Han ST, Linn YC, Hwang WY, Goh YT, Tan DC. Risk of hepatitis B reactivation and the role of novel agents and stem-cell transplantation in multiple myeloma patients with hepatitis B virus (HBV) infection. Ann Oncol. 2012;23:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 117. | Ataca Atilla P, Yalçıner M, Atilla E, İdilman R, Beksaç M. Hepatitis B Reactivation Rate and Fate Among Multiple Myeloma Patients Receiving Regimens Containing Lenalidomide and/or Bortezomib. Turk J Haematol. 2019;36:266-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Lee JY, Lim SH, Lee MY, Kim H, Sinn DH, Gwak GY, Choi MS, Lee JH, Jung CW, Jang JH, Kim WS, Kim SJ, Kim K. Hepatitis B reactivation in multiple myeloma patients with resolved hepatitis B undergoing chemotherapy. Liver Int. 2015;35:2363-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Li J, Huang B, Li Y, Zheng D, Zhou Z, Liu J. Hepatitis B virus reactivation in patients with multiple myeloma receiving bortezomib-containing regimens followed by autologous stem cell transplant. Leuk Lymphoma. 2015;56:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 120. | Armengol M, Santos JC, Fernández-Serrano M, Profitós-Pelejà N, Ribeiro ML, Roué G. Immune-Checkpoint Inhibitors in B-Cell Lymphoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 121. | Chan TSY, Li J, Loong F, Khong PL, Tse E, Kwong YL. PD1 blockade with low-dose nivolumab in NK/T cell lymphoma failing L-asparaginase: efficacy and safety. Ann Hematol. 2018;97:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | Kim SJ, Lim JQ, Laurensia Y, Cho J, Yoon SE, Lee JY, Ryu KJ, Ko YH, Koh Y, Cho D, Lim ST, Enemark MB, D'Amore F, Bjerre M, Ong CK, Kim WS. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020;136:2754-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 123. | Meti N, Esfahani K, Johnson NA. The Role of Immune Checkpoint Inhibitors in Classical Hodgkin Lymphoma. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 124. | Tao R, Fan L, Song Y, Hu Y, Zhang W, Wang Y, Xu W, Li J. Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: a multicenter, single-arm, phase 2 trial (ORIENT-4). Signal Transduct Target Ther. 2021;6:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 125. | Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol. 2009;27:339-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 126. | Lasagna A, Albi G, Maserati R, Zuccarini A, Quaccini M, Baldanti F, Sacchi P, Bruno R, Pedrazzoli P. Occult hepatitis B in patients with cancer during immunotherapy with or without chemotherapy: A real-life retrospective single-center cohort study. Front Oncol. 2023;13:1044098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 127. | Wong GL, Wong VW, Hui VW, Yip TC, Tse YK, Liang LY, Lui RN, Mok TS, Chan HL, Chan SL. Hepatitis Flare During Immunotherapy in Patients With Current or Past Hepatitis B Virus Infection. Am J Gastroenterol. 2021;116:1274-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 128. | Yoo S, Lee D, Shim JH, Kim KM, Lim YS, Lee HC, Yoo C, Ryoo BY, Choi J. Risk of Hepatitis B Virus Reactivation in Patients Treated With Immunotherapy for Anti-cancer Treatment. Clin Gastroenterol Hepatol. 2022;20:898-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 129. | Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, Zhao M, Zhang B, Jiang W, Lin Z, Ma Y, Yang Y, Huang Y, Zhao H, Xu R, Hong S, Zhang L. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. 2019;7:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 130. | Navvabi N, Khadem Ansari MH, Navvabi A, Chalipa HR, Zitricky F. Comparative assessment of immunochromatography and ELISA diagnostic tests for HBsAg detection in PCR-confirmed HBV infection. Rev Gastroenterol Mex (Engl Ed). 2022;87:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 131. | Gärtner BC, Jung W, Welsch C, Fischinger J, Schubert J, Zeuzem S, Mueller-Lantzsch N, Wend UC, Gerlich WH. Permanent loss of anti-HBc after reactivation of hepatitis B virus infection in an anti-HBs and anti-HBc-positive patient after allogeneic stem cell transplantation. J Clin Virol. 2007;38:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 132. | Osiowy C. Detection of HBsAg mutants. J Med Virol. 2006;78 Suppl 1:S48-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 133. | Awerkiew S, Däumer M, Reiser M, Wend UC, Pfister H, Kaiser R, Willems WR, Gerlich WH. Reactivation of an occult hepatitis B virus escape mutant in an anti-HBs positive, anti-HBc negative lymphoma patient. J Clin Virol. 2007;38:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 134. | Ferreira R, Carvalheiro J, Torres J, Fernandes A, Giestas S, Mendes S, Agostinho C, Campos MJ. Fatal hepatitis B reactivation treated with entecavir in an isolated anti-HBs positive lymphoma patient: a case report and literature review. Saudi J Gastroenterol. 2012;18:277-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 135. | Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, Lin TH, Hsiao HH, Young JH, Chang MC, Liao YM, Li CC, Wu HB, Tien HF, Chao TY, Liu TW, Cheng AL, Chen PJ. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology. 2008;47:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 246] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 136. | Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, Zhang HY, Lie A, Ngan R, Liang R. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 137. | Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 138. | Cho Y, Yu SJ, Cho EJ, Lee JH, Kim TM, Heo DS, Kim YJ, Yoon JH. High titers of anti-HBs prevent rituximab-related viral reactivation in resolved hepatitis B patient with non-Hodgkin's lymphoma. J Med Virol. 2016;88:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 139. | Koo YX, Tay M, Teh YE, Teng D, Tan DS, Tan IB, Tai DW, Quek R, Tao M, Lim ST. Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis B core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol. 2011;90:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Matsubara T, Nishida T, Shimoda A, Shimakoshi H, Amano T, Sugimoto A, Takahashi K, Mukai K, Yamamoto M, Hayashi S, Nakajima S, Fukui K, Inada M. The combination of anti-HBc and anti-HBs levels is a useful predictor of the development of chemotherapy-induced reactivation in lymphoma patients with resolved HBV infection. Oncol Lett. 2017;14:6543-6552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 141. | Paul S, Dickstein A, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology. 2017;66:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (1)] |
| 142. | Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, Wu X, Peng J, Xu B, Lin Y, Cao Y, Li H, Lin S, Liu Q, Lin T. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 143. | Yu S, Luo H, Pan M, Luis AP, Xiong Z, Shuai P, Zhang Z. Comparison of entecavir and lamivudine in preventing HBV reactivation in lymphoma patients undergoing chemotherapy: a meta-analysis. Int J Clin Pharm. 2016;38:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | Shang J, Wang H, Sun J, Fan Z, Huang F, Zhang Y, Jiang Q, Dai M, Xu N, Lin R, Liu Q. A comparison of lamivudine vs entecavir for prophylaxis of hepatitis B virus reactivation in allogeneic hematopoietic stem cell transplantation recipients: a single-institutional experience. Bone Marrow Transplant. 2016;51:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Zhang MY, Zhu GQ, Shi KQ, Zheng JN, Cheng Z, Zou ZL, Huang HH, Chen FY, Zheng MH. Systematic review with network meta-analysis: Comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642-30658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 146. | Zhang MY, Zhu GQ, Zheng JN, Cheng Z, Van Poucke S, Shi KQ, Huang HH, Chen FY, Zheng MH. Nucleos(t)ide analogues for preventing HBV reactivation in immunosuppressed patients with hematological malignancies: a network meta-analysis. Expert Rev Anti Infect Ther. 2017;15:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 147. | Geipel A, Seiz PL, Niekamp H, Neumann-Fraune M, Zhang K, Kaiser R, Protzer U, Gerlich WH, Glebe D; HOPE Consortium. Entecavir allows an unexpectedly high residual replication of HBV mutants resistant to lamivudine. Antivir Ther. 2015;20:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 148. | Arora A, Anand AC, Kumar A, Singh SP, Aggarwal R, Dhiman RK, Aggarwal S, Alam S, Bhaumik P, Dixit VK, Goel A, Goswami B, Kumar M, Madan K, Murugan N, Nagral A, Puri AS, Rao PN, Saraf N, Saraswat VA, Sehgal S, Sharma P, Shenoy KT, Wadhawan M; Members of the INASL taskforce on Hepatitis B. INASL Guidelines on Management of Hepatitis B Virus Infection in Patients receiving Chemotherapy, Biologicals, Immunosupressants, or Corticosteroids. J Clin Exp Hepatol. 2018;8:403-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 149. | Ceccarelli L, Salpini R, Sarmati L, Svicher V, Bertoli A, Sordillo P, Ricciardi A, Perno CF, Andreoni M, Sarrecchia C. Late hepatitis B virus reactivation after lamivudine prophylaxis interruption in an anti-HBs-positive and anti-HBc-negative patient treated with rituximab-containing therapy. J Infect. 2012;65:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | Lee IC, Huang YH, Chu CJ, Lee PC, Lin HC, Lee SD. Hepatitis B virus reactivation after 23 months of rituximab-based chemotherapy in an HBsAg-negative, anti-HBs-positive patient with follicular lymphoma. J Chin Med Assoc. 2010;73:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 151. | Hara T, Oka K, Iwai N, Inada Y, Tsuji T, Okuda T, Nagata A, Komaki T, Kagawa K. Hepatitis B Virus Reactivation 55 Months Following Chemotherapy Including Rituximab and Autologous Peripheral Blood Stem Cell Transplantation for Malignant Lymphoma. Intern Med. 2021;60:417-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 152. | Law MF, Lai HK, Chan HN, Ha CY, Ng C, Yeung YM, Yip SF. The impact of hepatitis B virus (HBV) infection on clinical outcomes of patients with diffuse large B-cell lymphoma. Eur J Cancer Care (Engl). 2015;24:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 153. | Ifuku H, Kusumoto S, Tanaka Y, Totani H, Ishida T, Okada M, Murakami S, Mizokami M, Ueda R, Iida S. Fatal reactivation of hepatitis B virus infection in a patient with adult T-cell leukemia-lymphoma receiving the anti-CC chemokine receptor 4 antibody mogamulizumab. Hepatol Res. 2015;45:1363-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 154. | Foont JA, Schiff ER. Avoid the tragedy of hepatitis B reactivation in immunosuppressed patients. Nat Clin Pract Gastroenterol Hepatol. 2007;4:128-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 155. | An J, Shim JH, Kim SO, Choi J, Kim SW, Lee D, Kim KM, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. Comprehensive outcomes of on- and off-antiviral prophylaxis in hepatitis B patients undergoing cancer chemotherapy: A competing risks analysis. J Med Virol. 2016;88:1576-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 156. | Hung CM, Jeng LB, Lee WC, Yu MC, Kuo LM, Chen MF. Fulminant hepatic failure caused by hepatitis B virus activation after chemotherapy for breast cancer treated with liver transplantation: a case report. Transplant Proc. 2003;35:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 157. | Kim SG, Chun JM, Jin R, Kim JY, Won DI, Hwang YJ. Living donor liver transplantation for acute hepatic failure caused by reactivation of hepatitis B virus infection after chemotherapy for hematologic malignancy: case reports. Transplant Proc. 2010;42:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 158. | Noterdaeme T, Longrée L, Bataille C, Deroover A, Lamproye A, Delwaide J, Beguin Y, Honoré P, Detry O. Liver transplantation for acute hepatic failure due to chemotherapy-induced HBV reactivation in lymphoma patients. World J Gastroenterol. 2011;17:3069-3072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 159. | Sperl J, Frankova S, Kieslichova E, Oliverius M, Janousek L, Honsova E, Trunecka P, Spicak J. Urgent liver transplantation for chemotherapy-induced HBV reactivation: a suitable option in patients recently treated for malignant lymphoma. Transplant Proc. 2013;45:2834-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 160. | Stange MA, Tutarel O, Pischke S, Schneider A, Strassburg CP, Becker T, Barg-Hock H, Bastürk M, Wursthorn K, Cornberg M, Ott M, Greten TF, Manns MP, Wedemeyer H. Fulminant hepatic failure due to chemotherapy-induced hepatitis B reactivation: role of rituximab. Z Gastroenterol. 2010;48:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 161. | Benten D, Sterneck M, Panse J, Rogiers X, Lohse AW. Low recurrence of preexisting extrahepatic malignancies after liver transplantation. Liver Transpl. 2008;14:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gerlich W, Germany; Krygier R, Poland S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ