Published online Aug 21, 2023. doi: 10.3748/wjg.v29.i31.4763
Peer-review started: June 3, 2023
First decision: July 10, 2023
Revised: July 20, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: August 21, 2023
Processing time: 76 Days and 9 Hours
Gastric cancer (GC) incidence based on the endoscopic Kyoto classification of gastritis has not been systematically investigated using time-to-event analysis.
To examine GC incidence in an endoscopic surveillance cohort.
This study was retrospectively conducted at the Toyoshima Endoscopy Clinic. Patients who underwent two or more esophagogastroduodenoscopies were en
A total of 6718 patients were enrolled (median age 54.0 years; men 44.2%). During the follow-up period (max 5.02 years; median 2.56 years), GC developed in 34 patients. The average frequency of GCs per year was 0.19%. Kyoto atrophy scores 1 [HR with score 0 as reference: 3.66, 95% confidence interval (CI): 1.06 to 12.61], 2 (11.60, 3.82-35.27), IM score 2 (9.92, 4.37-22.54), EF score 1 (4.03, 1.63-9.96), DR scores 1 (6.22, 2.65-14.56), and 2 (10.01, 3.73-26.86) were associated with GC incidence, whereas nodularity scores were not. The total Kyoto scores of 4 (HR with total Kyoto scores 0-1 as reference: 6.23, 95%CI: 1.93 to 20.13, P = 0.002) and 5-8 (16.45, 6.29-43.03, P < 0.001) were more likely to develop GC, whereas the total Kyoto scores 2-3 were not. The HR of the total Kyoto score for developing GC per 1 rank was 1.75 (95%CI: 1.46 to 2.09, P < 0.001).
A high total Kyoto score (≥ 4) was associated with GC incidence. The endoscopy-based diagnosis of gastritis can stratify GC risk.
Core Tip: A high total Kyoto score (≥ 4) was associated with gastric cancer (GC) incidence. Adjusted hazard ratios (HRs) for the total Kyoto scores of 4 and 5-8 were high at 6.23 and 16.45, respectively, compared to the total Kyoto scores of 0-1. The HR of the total Kyoto score for developing GC per 1 rank was 1.75.
- Citation: Toyoshima O, Nishizawa T, Yoshida S, Matsuno T, Fujisawa G, Toyoshima A, Ebinuma H, Fujishiro M, Saito Y, Suzuki H. Gastric cancer incidence based on endoscopic Kyoto classification of gastritis. World J Gastroenterol 2023; 29(31): 4763-4773
- URL: https://www.wjgnet.com/1007-9327/full/v29/i31/4763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i31.4763
Gastric cancer (GC) is a global health problem and the third most common cause of cancer-related deaths worldwide[1]. Helicobacter pylori (H. pylori) infection is estimated to account for 89% of GC cases, and H. pylori-related gastritis is a precursor of GC[2-6]. Evaluation of H. pylori-related gastritis is clinically important because it allows for the risk stratification of GC[2,7-9]. Endoscopy detects early GCs and precisely diagnoses gastritis. Periodic endoscopic screening can reduce deaths from GC[10]. Recent advances in endoscopic technology allow for more accurate endoscopic diagnosis of gastritis[11,12].
The endoscopy-based Kyoto classification of gastritis was advocated by the Japan Society for Gastrointestinal Endoscopy in 2013. This classification aims to ensure that the endoscopic diagnosis of gastritis is unified and matched with the histopathology[13]. Recently, the Kyoto classification has been widely used in clinical practice and vigorously studied worldwide[14,15]. To assess GC risk, the Kyoto classification individually scores five H. pylori-related gastritis findings, such as atrophy, intestinal metaplasia (IM), enlarged fold (EF), nodularity, and diffuse redness (DR), and defines their sum as the total Kyoto score.
Several investigations have clarified that not only individual Kyoto scores but also the total Kyoto score are associated with H. pylori infection and GC risks[14]. For example, the total Kyoto score was associated with H. pylori infection, presence of GC[16-19], and GC risk indicators, such as serum pepsinogen titer, serum H. pylori antibody titer, histological distribution of neutrophil infiltration in the gastric mucosa, and genotype of the single nucleotide polymorphism. Collectively, total Kyoto scores of 0, ≥ 2, and ≥ 4 indicated a normal stomach, H. pylori-infected gastritis, and gastritis at risk for GC, respectively[14].
To date, GC incidence based on the Kyoto classification scores has not been systematically investigated using time-to-event analysis. Therefore, we examined GC incidence according to the five individual Kyoto scores and the total Kyoto score in the endoscopic surveillance cohort and verified the GC risks of endoscopic gastritis in Japan, which is a high GC morbidity area.
This cohort study was retrospectively conducted at the Toyoshima Endoscopy Clinic. We obtained data from the Toyoshima Endoscopy Clinic Database. This study was approved by the institutional review board of the Yoyogi Mental Clinic (approval no. RKK227). Written informed consent was obtained from patients at the time of esophagogastroduodenoscopy (EGD) to use their data for research purposes. The study’s protocol was published on our institute’s website (www.ichou.com) so that patients could opt out of the study. All clinical investigations were conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
This study enrolled patients who underwent two or more EGDs at the Toyoshima Endoscopy Clinic, an urban area in Tokyo, Japan, between April 2017 and April 2022. We excluded patients with a previous surgical gastrectomy at baseline and those who underwent the last EGD within one month after the index EGD. The indications for the index EGD included screening, surveillance for gastritis or other upper gastrointestinal diseases, and examination for symptoms or abnormal findings on other tests. H. pylori status was determined using serum anti-H. pylori antibodies, urea breath test, histology, and/or endoscopy.
In the Kyoto classification, the total Kyoto score was developed as a GC risk score[14]. The total Kyoto score is calculated as the sum of the following five Kyoto scores: Atrophy, IM, EF, nodularity, and DR, and ranges from 0 to 8 (Supple
EGDs were performed by gastrointestinal endoscopists using the Olympus’ (Tokyo, Japan) endoscopic system (EVIS X1 or LUCERA ELITE) and endoscope (GIF-XZ1200, GIF-1200N, GIF-HQ290, GIF-H290Z, or GIF-XP290N). The T-File System (STS-MEDIC Inc., Tokyo, Japan) was used to file endoscopic images and document endoscopic findings.
The Kyoto classification scores were assessed using white light imaging without magnification. The endoscopists diagnosed the Kyoto classification scores on-site during EGD, and it was retrospectively reviewed by experienced endoscopists. Endoscopists performed EGDs after learning from textbooks and journal articles on the Kyoto classification of gastritis[14,21].
GC was histologically diagnosed based on biopsy or resected specimens. GC morphology and histology were classified based on the Japanese classification of gastric carcinoma[22] and Lauren’s classification[23], respectively.
This cohort study evaluated GC incidence based on patients’ age, sex, H. pylori status, and endoscopic Kyoto classification scores of gastritis, such as atrophy, IM, EF, nodularity, DR, and total Kyoto scores. The primary outcome was GC incidence according to the total Kyoto score. We performed a time-to-event analysis with the start time as the date of index EGD. Data were censored on the date of the last EGD. The effects of Kyoto classification scores on GC development were estimated. The total Kyoto scores were categorized into 4 (i.e., 0-1, 2-3, 4, and 5-8) based on the frequency of GC increasing stepwise with the total Kyoto scores of 0-1, 2-3, and ≥ 4 in a cross-sectional study[24] and the number of patients in this study. The secondary outcomes were GC incidence according to the Kyoto atrophy, IM, EF, nodularity, and DR scores.
Baseline characteristics were compared between GC and non-GC groups using binomial logistic regression model. Kaplan-Meier curves were constructed according to patient age, sex, H. pylori status, and Kyoto classification scores (i.e., atrophy, IM, EF, nodularity, DR, and the total Kyoto scores). Statistical differences were estimated using the log-rank tests. The average frequency of GCs per year was calculated by dividing the number of events by the total person-years of observation. Hazard ratios (HRs) with 95% confidence intervals (CIs) for GC development were estimated using the Cox proportional hazards regression model. In multivariate analysis, HRs were adjusted for patient age and sex. A P value < 0.05 (two-sided) was defined as statistically significant. Statistical analysis was conducted using BellCurve for Excel version 4.03 (Social Survey Research Information Co., Ltd., Tokyo, Japan).
A total of 30585 EGDs in 16969 patients were performed during the study period. Six thousand seven hundred forty-four patients underwent two or more EGDs. Of these, 19 patients with a previous surgical gastrectomy and 7 with a follow-up period of less than 1 mo were excluded. A total of 6718 patients were enrolled.
The baseline patient characteristics are shown in Table 1. The median age of the patients was 54 years with the interquartile range (IQR) of 46-64 years. Men accounted for 44.2% of the cases. The proportion of patients with H. pylori status of uninfected, eradicated, and currently infected was 55.9%, 33.7%, and 10.4%, respectively. Patients were followed up for up to 5.02 years (median 2.56 years, IQR 1.74-3.64 years). The median (IQR) of Kyoto classification scores were 0 (0-1) for atrophy; 0 (0-0) for IM, EF, nodularity, and DR; and 0 (0-2) for total Kyoto. Supplementary Table 2 is shown with the mean and standard deviation. During the follow-up period, 37 GCs occurred in 34 patients. The characteristics of GCs are presented in Table 2. All GCs were superficial and within the submucosal depth. Lauren’s intestinal type made up 89.1% of GCs.
| All | Gastric cancer | Non-gastric cancer | P value1 | |
| No. | 6718 | 34 | 6684 | |
| Age, median (IQR), yr | 54 (46-64) | 69.5 (57.8-73.8) | 54 (46-64) | < 0.001 |
| Male sex, No. (%) | 2969 (44.2) | 17 (50.0) | 2952 (44.2) | 0.495 |
| H. pylori status, No. (%) | < 0.001 | |||
| Uninfected | 3754 (55.9) | 5 (14.7) | 3749 (56.1) | |
| Eradicated | 2264 (33.7) | 20 (58.8) | 2244 (33.6) | |
| Currently infected | 700 (10.4) | 9 (26.5) | 691 (10.3) | |
| Duration of follow up, median (IQR), yr | 2.56 (1.74-3.64) | 1.03 (0.85-1.78) | 2.57 (1.76-3.64) | < 0.001 |
| No. EGD per patient, median (IQR) | 2 (2-4) | 2 (2-3) | 2 (2-4) | 0.652 |
| Kyoto classification score, median (IQR) | ||||
| Atrophy | 0 (0-1) | 2 (1-2) | 0 (0-1) | < 0.001 |
| Intestinal metaplasia | 0 (0-0) | 2 (0-2) | 0 (0-0) | < 0.001 |
| Enlarged folds | 0 (0-0) | 0 (0-0) | 0 (0-0) | < 0.001 |
| Nodularity | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0.916 |
| Diffuse redness | 0 (0-0) | 1 (1-1) | 0 (0-0) | < 0.001 |
| Total Kyoto | 0 (0-2) | 5 (4-5) | 0 (0-2) | < 0.001 |
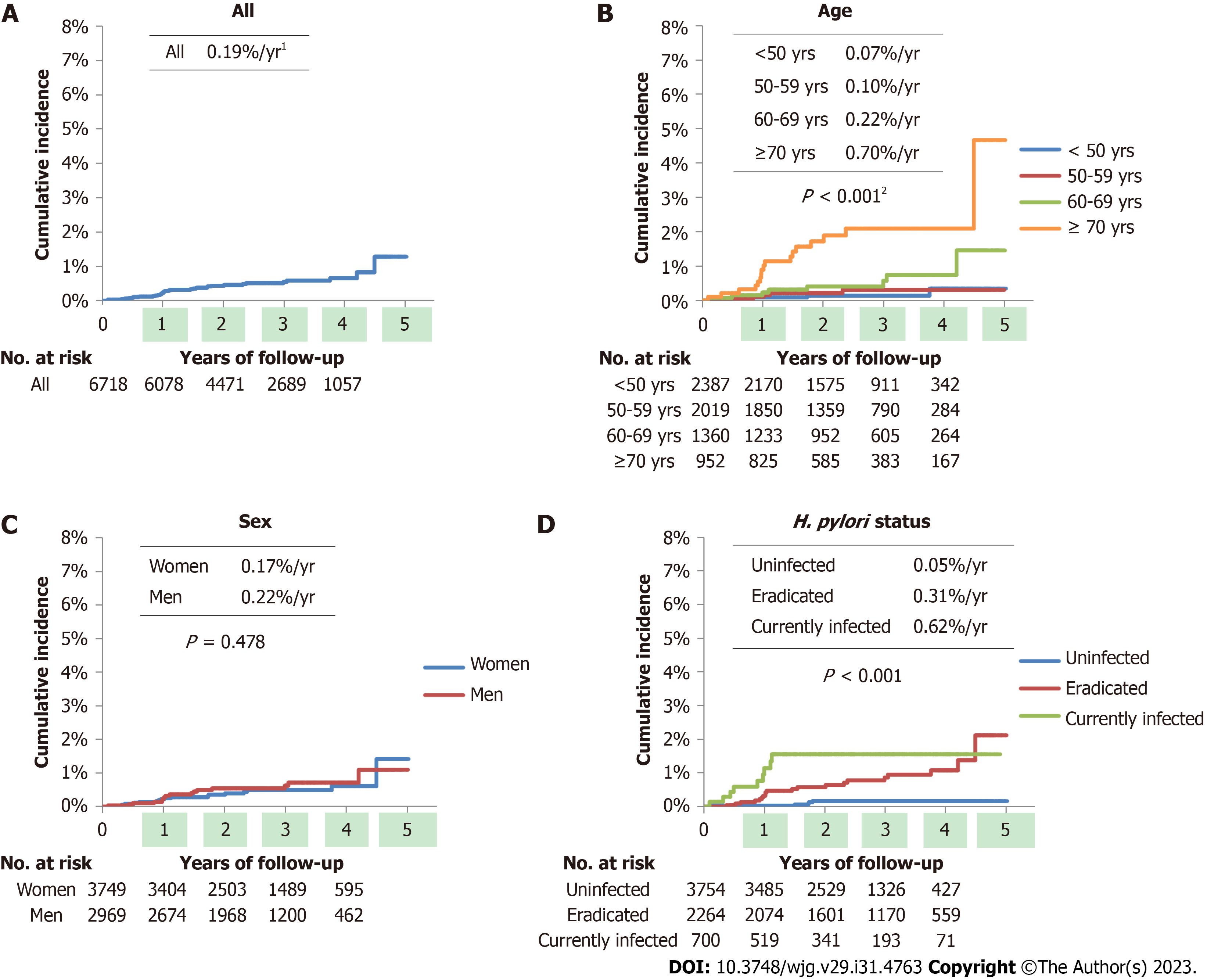
Kaplan-Meier curves of GC development according to patient demographic characteristics are shown in Figure 1. In this study, the average frequency of GCs was 0.19%/year. Age and H. pylori status were associated with GC incidence (both P < 0.001), whereas sex was not.
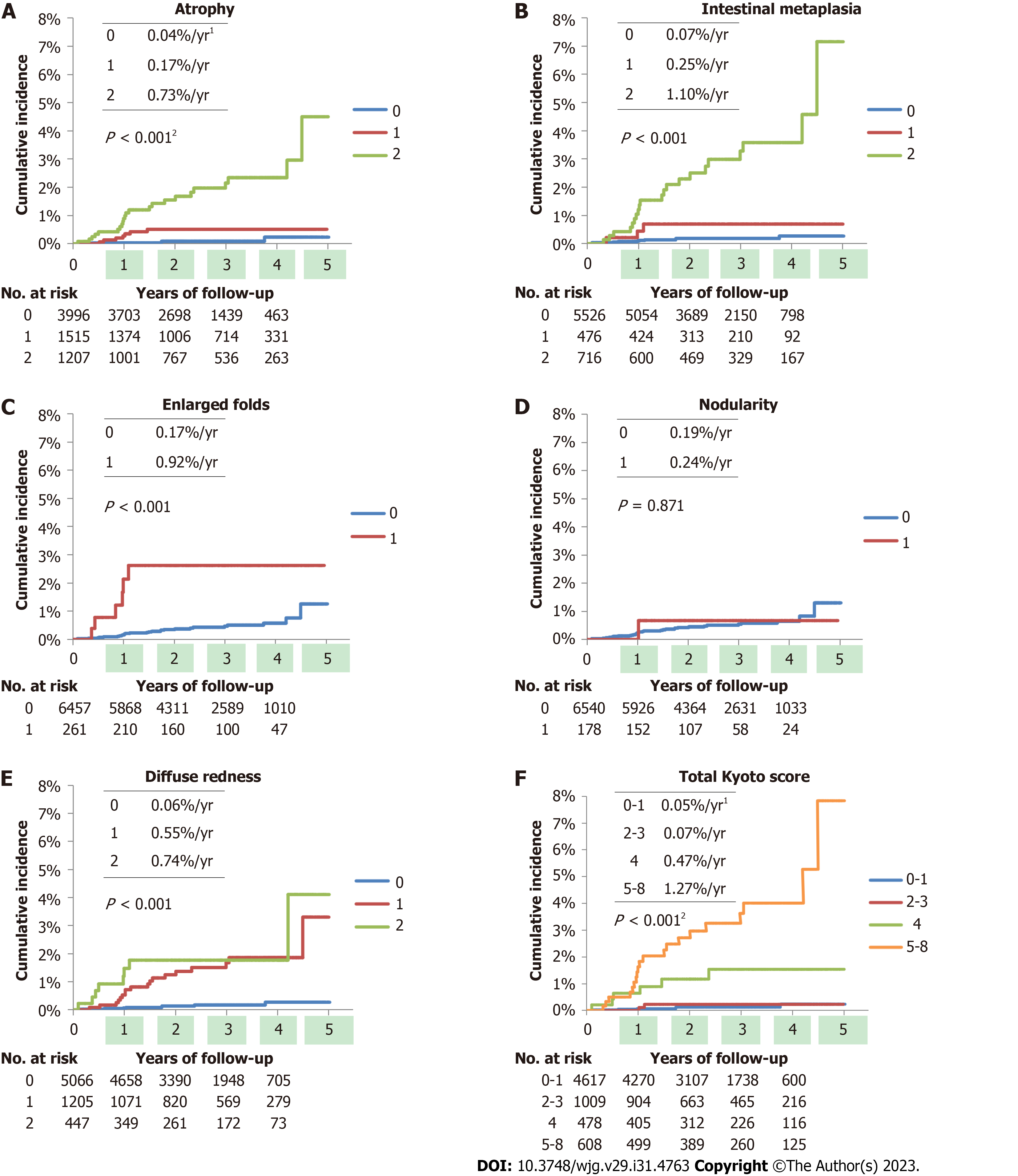
Figures 2A-E show the cumulative incidence using the Kaplan-Meier method for each Kyoto classification score. Atrophy, IM, EF, and DR scores were associated with GC development (all P < 0.001), whereas nodularity was not. The average frequencies of GCs per year were 0.04%, 0.17%, and 0.73% for atrophy scores of 0, 1, and 2; 0.07%, 0.25%, and 1.10% for IM scores of 0, 1, and 2; 0.17% and 0.92% for EF scores of 0 and 1; and 0.06%, 0.55%, and 0.74% for DR scores of 0, 1, and 2, respectively. The total Kyoto score was associated with GC development (P < 0.001), as shown in Figure 2F. The average frequencies of GCs were 0.05, 0.07, 0.47, and 1.27% per year for the total Kyoto scores of 0-1, 2-3, 4, and 5-8, respectively.
Table 3 provides the crude and adjusted HRs for GC development according to Kyoto classification scores. Multivariate analysis showed that atrophy scores 1 (adjusted HR with score 0 as reference: 3.66, 95%CI: 1.06 to 12.61), 2 (11.60, 3.82-35.27), IM score 2 (9.92, 4.37-22.54), EF score 1 (4.03, 1.63-9.96), DR scores 1 (6.22, 2.65-14.56), and 2 (10.01, 3.73-26.86) were significantly associated with GC incidence, independent of patient age and sex, whereas nodularity score was not.
| GC patients, No. | Non-GC patients, No. | Crude HR1 | 95%CI | P value | Adjusted HR1,2 | 95%CI | P value | |
| Age (yr) | ||||||||
| < 50 | 4 | 2383 | Reference | |||||
| 50-59 | 5 | 2014 | 1.48 | 0.40-5.50 | 0.562 | |||
| 60-69 | 8 | 1352 | 3.37 | 1.01-11.19 | 0.047 | |||
| ≥ 70 | 17 | 935 | 10.75 | 3.62-31.96 | < 0.001 | |||
| Sex | ||||||||
| Women | 17 | 3732 | Reference | |||||
| Men | 17 | 2952 | 1.27 | 0.65-2.48 | 0.488 | |||
| H. pylori status | ||||||||
| Uninfected | 5 | 3749 | Reference | Reference | ||||
| Eradicated | 20 | 2244 | 6.00 | 2.25-16.05 | < 0.001 | 3.81 | 1.40-10.38 | 0.009 |
| Currently infected | 9 | 691 | 11.60 | 3.88-34.64 | < 0.001 | 9.53 | 3.17-28.65 | < 0.001 |
| Atrophy | ||||||||
| 0 | 4 | 3992 | Reference | Reference | ||||
| 1 | 7 | 1508 | 4.40 | 1.29-15.06 | 0.018 | 3.66 | 1.06-12.61 | 0.040 |
| 2 | 23 | 1184 | 19.02 | 6.56-55.12 | < 0.001 | 11.60 | 3.82-35.27 | < 0.001 |
| Intestinal metaplasia | ||||||||
| 0 | 10 | 5516 | Reference | Reference | ||||
| 1 | 3 | 473 | 3.42 | 0.94-12.44 | 0.062 | 2.25 | 0.60-8.43 | 0.228 |
| 2 | 21 | 695 | 16.01 | 7.52-34.05 | < 0.001 | 9.92 | 4.37-22.54 | < 0.001 |
| Enlarged folds | ||||||||
| 0 | 28 | 6429 | Reference | Reference | ||||
| 1 | 6 | 255 | 5.41 | 2.23-13.10 | < 0.001 | 4.03 | 1.63-9.96 | 0.003 |
| Nodularity | ||||||||
| 0 | 33 | 6507 | Reference | Reference | ||||
| 1 | 1 | 177 | 1.18 | 0.16-8.62 | 0.872 | 2.51 | 0.33-18.86 | 0.372 |
| Diffuse redness | ||||||||
| 0 | 8 | 5058 | Reference | Reference | ||||
| 1 | 18 | 1187 | 9.05 | 3.92-20.85 | < 0.001 | 6.22 | 2.65-14.56 | < 0.001 |
| 2 | 8 | 439 | 12.26 | 4.60-32.68 | < 0.001 | 10.01 | 3.73-26.86 | < 0.001 |
| Total Kyoto | ||||||||
| 0-1 | 6 | 4615 | Reference | Reference | ||||
| 2-3 | 2 | 1008 | 1.48 | 0.30-7.34 | 0.631 | 1.12 | 0.22-5.63 | 0.887 |
| 4 | 6 | 473 | 9.54 | 3.07-29.62 | < 0.001 | 6.23 | 1.93-20.13 | 0.002 |
| 5-8 | 20 | 588 | 25.58 | 10.25-63.84 | < 0.001 | 16.45 | 6.29-43.03 | < 0.001 |
The total Kyoto scores of 4 (adjusted HR with total Kyoto scores 0-1 as reference: 6.23, 95%CI: 1.93 to 20.13, P = 0.002) and 5-8 (16.45, 6.29-43.03, P < 0.001) were more likely to develop GC, whereas the total Kyoto scores of 2-3 were not. In per 1 rank analysis, an adjusted HR of the total Kyoto score for developing GC was 1.75 (95%CI: 1.46 to 2.09, P < 0.001).
We found that high total Kyoto scores, especially ≥ 4, were associated with GC incidence. The adjusted HR of the total Kyoto score for GC development was 1.75 per 1 rank analysis. Additionally, adjusted HRs for the total Kyoto scores of 4 and 5-8 were high at 6.23 and 16.45, respectively, compared to the total Kyoto scores of 0-1. The total Kyoto score was associated with the cumulative incidence of GC (P < 0.001). The average frequencies of GCs per year increased with the total Kyoto score (0.05%, 0.07%, 0.47%, and 1.27% for the total Kyoto scores of 0-1, 2-3, 4, and 5-8, respectively). This is the first report that shows that high total Kyoto scores represent GC risks in a time-to-event analysis. This finding is consistent with those of several previous studies. In cross-sectional studies, we and Lin et al[19] reported that the odds ratios of the total Kyoto score for GC were 1.6 and 1.5 per 1 rank, respectively[18]. Liu et al[24] indicated an increased trend of GC frequency in patients with total Kyoto scores of 0-1, 2-3, and ≥ 4. Some investigators showed that the mean total Kyoto scores of the patients with GC, H. pylori-infected GC, and H. pylori-eradicated GC were 4.0-4.6, 4.8-5.6, and 4.2, respectively[18,25,26]. Taken together, a total Kyoto score ≥ 4 was available for determining GC risks.
This cohort study demonstrated that patients with endoscopy-based atrophy, IM, EF, and DR are more likely to develop GC. In contrast, nodularity was not associated with GC incidence. Endoscopic atrophy has been shown to be a predictor of GC development. Shichijo et al[27] described a significantly higher adjusted HR of severe atrophy for developing GC as 9.3, while we identified significantly higher adjusted HRs of Kyoto atrophy scores 1 and 2 as 3.7 and 11.6, respectively. Several cohort studies have shown that severe endoscopic atrophy is associated with a high incidence of GC, especially in patients who have undergone H. pylori eradication[9,27,28]. These studies revealed that the average frequencies of GCs per year for non-to-mild, moderate, and severe atrophy were 0.06%-0.15%, 0.12%-0.34%, and 0.31%-1.60%, respectively. Similarly, our study showed that the average frequencies of GCs per year for total Kyoto scores of 0, 1, and 2 were 0.04%, 0.17%, and 0.73%, respectively. Since more than half of the study patients were uninfected with H. pylori, our study may present a lower GC incidence in patients with a Kyoto atrophy score of 0. Two meta-analyses also showed that a high Kyoto atrophy score provided a high-risk ratio of 2.8-8.0[29,30]. Thus, our study results are in line with those of previous studies.
Although the risk of GC in histological IM has been well studied[2,8,27,31,32], few studies have examined GC risks associated with endoscopic IM. A high Kyoto IM score has been identified as a risk factor for GC, especially multiple GC[14,18]. This study revealed that endoscopic corpus IM was associated with GC development (adjusted HR: 9.92), which is supported by Sakitani et al[33], who clarified histological corpus IM as a predictor of GC. The consistency of IM between endoscopy and histology has been demonstrated[13]; we successfully verified endoscopic IM as a risk factor for GC using event history analysis.
Watanabe et al[34] provided an adjusted HR of EF for GC development as high as 43.3, whereas our study’s adjusted HR was 4.03. These results are similar; however, the difference in HRs between them may be attributed to the inclusion of many H. pylori-uninfected individuals in the study population. However, whether nodularity is a risk factor for GC remains controversial. Nodularity has been reported to be a juvenile and histologically diffuse-type GC risk[35,36], whereas we have shown in a cross-sectional study that the odds ratio for GC of nodularity is low at 0.5[18]. After adjusting for age and sex, no association between nodularity and GC was observed. Although nodularity decreases with age[37], the risk of intestinal-type GC increases with age. Furthermore, intestinal-type cancers are more common than diffuse-type cancers. Therefore, age offsets the association of nodularity with GC, especially in older generations, although nodularity is associated with diffuse-type GC in the young generation[35]. As the association between nodularity and the risk of developing GC is still debated, nodularity might be listed separately. For example, the total Kyoto classification score for atrophy 1, IM 0, EF 1, nodularity 1, and DR 1 might be reported as 3 + 1 instead of 4.
Several studies have reported that DR is strongly associated with H. pylori infection[15,38-40], but little is known about the association between DR and GC incidence. This study identified Kyoto DR scores of 1 and 2 as indicators of GC incidence (adjusted HRs: 6.22 and 10.01, respectively). Since DR presents inflammatory cell infiltration caused by H. pylori[13] and H. pylori infection is a definite risk factor for GC[2,3], DR is expressed as a GC risk. Additionally, since the Kyoto DR score includes RAC as a negative factor and RAC is inversely associated with GC development[41], a high Kyoto DR score may be associated with high GC incidence.
This study has some limitations. First, this was a single-center, retrospective cohort study. Although the endoscopy data were well-organized, multi-center, prospective studies are warranted. Second, this study was conducted only in areas with a high GC prevalence in Asia. Therefore, studies in Western countries are warranted. Third, the total Kyoto score has shortcomings in the scoring design, which simply adds five individual Kyoto scores[14]. IM is associated with a high risk of intestinal-type GC but a low risk of diffuse-type GC[18]. In addition, EF and nodularity are high risks for diffuse-type GC but low risks for intestinal-type GC. Therefore, the evaluation of GC risk using a scoring system that individually predicts the risks of intestinal- and diffuse-type GCs is needed.
In conclusion, a high total Kyoto score of ≥ 4 was associated with GC incidence in a cohort study. The endoscopy-based diagnosis of gastritis can stratify GC risk.
The Japan Gastroenterological Endoscopy Society advocated the Kyoto classification, a new grading system for endo
Although a high Kyoto score is believed to reflect an increased gastric cancer (GC) risk, it has not been systematically investigated using time-to-event analysis.
We examined GC incidence according to the total Kyoto score in the endoscopic surveillance cohort and verified the GC risks of endoscopic gastritis.
Patients who underwent two or more esophagogastroduodenoscopies were enrolled. GC incidence was based on Kyoto classification scores. Hazard ratios (HRs) adjusted for age and sex were calculated using a Cox hazard model.
A total of 6718 patients were enrolled. The annual incidence rate of GC was 0.19%. The total Kyoto scores of 4 [HR with total Kyoto scores 0-1 as reference: 6.23, 95% confidence interval (CI): 1.93 to 20.13, P = 0.002] and 5-8 (16.45, 6.29-43.03, P < 0.001) were more likely to develop GC, whereas the total Kyoto scores 2-3 were not. The HR of the total Kyoto score for developing GC per 1 rank was 1.75 (95%CI: 1.46 to 2.09, P < 0.001).
A total Kyoto score ≥ 4 could predict GC risk. The endoscopic Kyoto classification of gastritis can stratify GC risk.
This was a single-center, retrospective cohort study, and multi-center, prospective studies are warranted.
We would like to thank Shido Inc. (www.Shido.co.jp) for consulting the statistical analysis. We would like to thank clinical laboratory engineer Tadahiro Yamakawa for managing the endoscopy database of the Toyoshima Endoscopy Clinic.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56694] [Article Influence: 7086.8] [Reference Citation Analysis (135)] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3249] [Article Influence: 130.0] [Reference Citation Analysis (1)] |
| 3. | Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 732] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 4. | Sugano K. Effect of Helicobacter pylori eradication on the incidence of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2019;22:435-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Choi Y, Kim N, Yun CY, Choi YJ, Yoon H, Shin CM, Park YS, Ahn SH, Joong Park D, Lee HS, Kim JW, Lee KW, Chang W, Park JH, Lee YJ, Lee KH, Kim YH, Lee DH, Kim HH. Effect of Helicobacter pylori eradication after subtotal gastrectomy on the survival rate of patients with gastric cancer: follow-up for up to 15 years. Gastric Cancer. 2020;23:1051-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Takahashi Y, Yamamichi N, Kubota D, Shimamoto T, Nagao S, Sakuma N, Sakaguchi Y, Yakabi S, Tsuji Y, Wada R, Mitsushima T, Ichinose M, Fujishiro M. Risk factors for gastric cancer in Japan in the 2010s: a large, long-term observational study. Gastric Cancer. 2022;25:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Song H, Ekheden IG, Zheng Z, Ericsson J, Nyrén O, Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 721] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 9. | Take S, Mizuno M, Ishiki K, Kusumoto C, Imada T, Hamada F, Yoshida T, Yokota K, Mitsuhashi T, Okada H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. 2020;55:281-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Hamashima C, Shabana M, Okada K, Okamoto M, Osaki Y. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci. 2015;106:1744-1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (3)] |
| 12. | Takeda T, Asaoka D, Nojiri S, Nishiyama M, Ikeda A, Yatagai N, Ishizuka K, Hiromoto T, Okubo S, Suzuki M, Nakajima A, Nakatsu Y, Komori H, Akazawa Y, Nakagawa Y, Izumi K, Matsumoto K, Ueyama H, Sasaki H, Shimada Y, Osada T, Hojo M, Kato M, Nagahara A. Linked Color Imaging and the Kyoto Classification of Gastritis: Evaluation of Visibility and Inter-Rater Reliability. Digestion. 2020;101:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Toyoshima O, Nishizawa T, Yoshida S, Matsuno T, Odawara N, Toyoshima A, Sakitani K, Watanabe H, Fujishiro M, Suzuki H. Consistency between the endoscopic Kyoto classification and pathological updated Sydney system for gastritis: A cross-sectional study. J Gastroenterol Hepatol. 2022;37:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Toyoshima O, Nishizawa T. Kyoto classification of gastritis: Advances and future perspectives in endoscopic diagnosis of gastritis. World J Gastroenterol. 2022;28:6078-6089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (4)] |
| 15. | Wang K, Zhao J, Jin H, Meng L, Fan Y, Zhou Y, Ye C, Li M, Ma P, Zhu L, Ye Y, Lyu B. Establishment of a modified Kyoto classification scoring model and its significance in the diagnosis of Helicobacter pylori current infection. Gastrointest Endosc. 2023;97:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017;32:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Toyoshima O, Nishizawa T, Yoshida S, Aoki T, Nagura F, Sakitani K, Tsuji Y, Nakagawa H, Suzuki H, Koike K. Comparison of endoscopic gastritis based on Kyoto classification between diffuse and intestinal gastric cancer. World J Gastrointest Endosc. 2021;13:125-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Lin J, Su H, Zhou Q, Pan J, Zhou L. Predictive value of nomogram based on Kyoto classification of gastritis to diagnosis of gastric cancer. Scand J Gastroenterol. 2022;57:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 780] [Article Influence: 43.3] [Reference Citation Analysis (5)] |
| 21. | Haruma K, Kato M, Inoue K, Murakami K, Kamada T. Kyoto Classification of Gastritis. 1st Edition. Tokyo Japan: Nihon Medical Center, 2017. |
| 22. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2952] [Article Influence: 196.8] [Reference Citation Analysis (1)] |
| 23. | Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4391] [Article Influence: 146.4] [Reference Citation Analysis (1)] |
| 24. | Liu XM, Ma XY, Liu F, Liu ZL, Tang XY, Ji MZ, Zheng JX. Gastric Cancer Screening Methods: A Comparative Study of the Chinese New Gastric Cancer Screening Score and Kyoto Classification of Gastritis. Gastroenterol Res Pract. 2022;2022:7639968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Ohno A, Miyoshi J, Kato A, Miyamoto N, Yatagai T, Hada Y, Kusuhara M, Jimbo Y, Ida Y, Tokunaga K, Okamoto S, Hisamatsu T. Endoscopic severe mucosal atrophy indicates the presence of gastric cancer after Helicobacter pylori eradication -analysis based on the Kyoto classification. BMC Gastroenterol. 2020;20:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Sugimoto M, Kawai Y, Morino Y, Hamada M, Iwata E, Niikura R, Nagata N, Koyama Y, Fukuzawa M, Itoi T, Kawai T. Efficacy of high-vision transnasal endoscopy using texture and colour enhancement imaging and narrow-band imaging to evaluate gastritis: a randomized controlled trial. Ann Med. 2022;54:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, Fukayama M, Koike K. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc. 2016;84:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 28. | Kaji K, Hashiba A, Uotani C, Yamaguchi Y, Ueno T, Ohno K, Takabatake I, Wakabayashi T, Doyama H, Ninomiya I, Kiriyama M, Ohyama S, Yoneshima M, Koyama N, Takeda Y, Yasuda K. Grading of Atrophic Gastritis is Useful for Risk Stratification in Endoscopic Screening for Gastric Cancer. Am J Gastroenterol. 2019;114:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Sui Z, Chen J, Li P, Shao L, Ye J, Lu X, Cai J. Risk for gastric cancer in patients with gastric atrophy: a systematic review and meta-analysis. Transl Cancer Res. 2020;9:1618-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Xiao S, Fan Y, Yin Z, Zhou L. Endoscopic grading of gastric atrophy on risk assessment of gastric neoplasia: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018;21:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 32. | Na YS, Kim SG, Cho SJ. Risk assessment of metachronous gastric cancer development using OLGA and OLGIM systems after endoscopic submucosal dissection for early gastric cancer: a long-term follow-up study. Gastric Cancer. 2023;26:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 33. | Sakitani K, Hirata Y, Watabe H, Yamada A, Sugimoto T, Yamaji Y, Yoshida H, Maeda S, Omata M, Koike K. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol. 2011;26:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Watanabe M, Kato J, Inoue I, Yoshimura N, Yoshida T, Mukoubayashi C, Deguchi H, Enomoto S, Ueda K, Maekita T, Iguchi M, Tamai H, Utsunomiya H, Yamamichi N, Fujishiro M, Iwane M, Tekeshita T, Mohara O, Ushijima T, Ichinose M. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131:2632-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Kamada T, Tanaka A, Yamanaka Y, Manabe N, Kusunoki H, Miyamoto M, Tanaka S, Hata J, Chayama K, Haruma K. Nodular gastritis with helicobacter pylori infection is strongly associated with diffuse-type gastric cancer in young patients. Dig Endosc. 2007;19:180-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Nishikawa I, Kato J, Terasoma S, Matsutani H, Tamaki H, Tamaki T, Kuwashima F, Nakata H, Tomeki T, Matsunaka H, Ibata Y, Yamashita Y, Maekita T, Higashi K, Ichinose M. Nodular gastritis in association with gastric cancer development before and after Helicobacter pylori eradication. JGH Open. 2018;2:80-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Nishizawa T, Sakitani K, Suzuki H, Yoshida S, Kataoka Y, Nakai Y, Ebinuma H, Kanai T, Toyoshima O, Koike K. Clinical features of cardiac nodularity-like appearance induced by Helicobacter pylori infection. World J Gastroenterol. 2020;26:5354-5361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Yoshii S, Mabe K, Watano K, Ohno M, Matsumoto M, Ono S, Kudo T, Nojima M, Kato M, Sakamoto N. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 39. | Zhao J, Xu S, Gao Y, Lei Y, Zou B, Zhou M, Chang D, Dong L, Qin B. Accuracy of Endoscopic Diagnosis of Helicobacter pylori Based on the Kyoto Classification of Gastritis: A Multicenter Study. Front Oncol. 2020;10:599218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Fiuza F, Maluf-Filho F, Ide E, Furuya CK Jr, Fylyk SN, Ruas JN, Stabach L, Araujo GA, Matuguma SE, Uemura RS, Sakai CM, Yamazaki K, Ueda SS, Sakai P, Martins BC. Association between mucosal surface pattern under near focus technology and Helicobacter pylori infection. World J Gastrointest Endosc. 2021;13:518-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 41. | Kawamura M, Uedo N, Koike T, Kanesaka T, Hatta W, Ogata Y, Oikawa T, Iwai W, Yokosawa S, Honda J, Asonuma S, Okata H, Ohyauchi M, Ito H, Abe Y, Ara N, Kayaba S, Shinkai H, Shimokawa T. Kyoto classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan. Dig Endosc. 2022;34:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma L, China; Senchukova M, Russia S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH