Published online Jan 21, 2023. doi: 10.3748/wjg.v29.i3.425
Peer-review started: September 13, 2022
First decision: October 30, 2022
Revised: November 15, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 21, 2023
Processing time: 120 Days and 19.7 Hours
The coronavirus disease 2019 (COVID-19) represents a global health and econ
Core Tip: The association between coronavirus disease-19 and hepatic injury is demonstrated by determining the viral tropism and its different pathological implications. A better understanding of the diversity and risk factors of severe acute respiratory syndrome coronavirus-2-induced hepatic injury provides a fundamental approach to overcoming adverse effects. Moreover, vaccination can influence assessment and evaluation.
- Citation: Ali FEM, Abd El-Aziz MK, Ali MM, Ghogar OM, Bakr AG. COVID-19 and hepatic injury: cellular and molecular mechanisms in diverse liver cells. World J Gastroenterol 2023; 29(3): 425-449
- URL: https://www.wjgnet.com/1007-9327/full/v29/i3/425.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i3.425
Coronavirus (CoV) is derived from the Latin word "corona," which means "crown"[1]. It can cause va
In COVID-19, viral tropism is responsible for spreading infection outside the respiratory tract and predisposing it to systemic symptoms, aggravating pre-existing disorders, and multiorgan damage in the kidney, heart, nervous system, liver, and gastrointestinal tract[12,13]. However, the available data indicate the second multiorgan dysfunction inherent to the immune discrepancy or cytokine storm, developing hypoxic or ischemic injury and drug-induced injury[14,15]. Although viral tropism should be considered to understand the SARS-CoV-2 infection, the S protein of the virus mediates SARS-CoV-2 cell entrance, which represents a high affinity for cells expressing angiotensin-converting enzyme 2 receptors (ACE2)[16]. Furthermore, the affinity of the S protein to ACE2 receptors increases when SARS-CoV-2 is proteolytically activated[17]. In an in vitro study by Letko et al[18], the S protein of lineage B beta-coronaviruses such as SARS-CoV and the recent SARS-CoV-2 significantly improved its affinity for its receptor when it was pre-incubated with proteolytically activated trypsin. Trypsin is expressed by liver epithelial cells[19]. Additionally, the protein of the SARS-CoV-2 contains a furin-like proteolytic site that has never been observed in other coronaviruses[20]. It is worth mentioning that furin is expressed in organs such as the salivary glands, liver, kidney, and pancreas involved in SARS-CoV-2 infection[21]. As a result, to determine tropism for a particular tissue, ACE2 should be present at the host cell surface[22]. Consequently, ACE2 expression is considered a mirror of viral load[23]. Controversially, the highest levels of ACE2 are detected in the small intestine, testis, heart, colon, and thyroid gland[24]. Nevertheless, respiratory system symptoms are dominant in COVID-19 because the nasal ciliated cells are the primary targets for SARS-CoV-2 replication in the early stages of infection[25]. Besides, ACE2 is abundantly expressed in more than 80% of alveolar lung cells, consequently affecting all respiratory functions[23].
With increasing COVID-19 prevalence and mortality rates, as of 14 August 2022, the WHO reported that over 587 million people were infected with SARS-COV-2, including over 6 million deaths[26]. Therefore, the nation’s healthcare systems face overwhelming psychological and economic burdens. Consequently, the most efficient method to prevent infection is to separate symptomatic persons, quarantine others, and manage concomitants while increasing immunization rates.
The molecular test is the most practical method to confirm the diagnosis of COVID-19, using the reverse transcription polymerase chain reaction (RT-PCR) to detect viral genetic materials in different sample swabs from the nasal cavity, mouth, sputum, and feces[27,28]. This molecular test provides high sensitivity and specificity; however, it has several drawbacks, such as requiring trained technicians, being time-consuming, high cost, shortages in test kit supplies, and false negative thresholds[29]. Therefore, it is critical to develop new quick, reliable, and affordable diagnostic techniques.
Patients with fever, cough, and chest pain with breathing problems or pneumonia are usually diagnosed by imaging tests, such as chest X-ray or computed tomography (CT)[30]. Imaging tests are predominantly available worldwide, and the scanning process is relatively simple and rapid, enabling a large population’s screening[31]. In a study based on chest X-ray findings and severity scores, a chest X-ray is a limited tool because it has an abnormality observed at a specific point[32]. In the same context, Borghesi A. and R. Maroldi mentioned that chest X-ray is an insensitive diagnostic tool for the early detection of lung abnormalities. In contrast, it is a valuable tool for monitoring (day after day) the rapid progression of lung abnormalities in infected patients, particularly in intensive care units[33]. Despite its limited sensitivity, the appearance of a local or bilateral patchy shadow infiltrating a chest X-ray is the most typical radiological presentation[34].
Currently, CT plays a pivotal role and is the main technique for diagnosing and following patients with COVID-19[35]. The CT finding is more sensitive than the chest x-ray, particularly in the initial assessment[32,36]. CT findings may be present early, even before the onset of the symptoms[36]. Addi
Moreover, clinical pathologists have a significant role in monitoring inflammatory markers, including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and white blood cells (WBCs). The most significant markers during SARS-COV-2 infection and highly associated with COVID-19 progression were lymphocytopenia, elevated CRP, and alternation in the ESR levels[40-42]. Data obtained from 452 patients with COVID-19 revealed that lymphocytopenia, high WBCs, a high neutrophil-lymphocyte ratio, and lower percentages of monocytes, eosinophils, and basophils were mainly observed in severe cases[43]. Similar findings were demonstrated by Henry et al[44] (2020) in their meta-analysis of 21 studies that included 3377 individuals who tested positive for COVID-19. They found that patients with severe and fatal diseases had more dramatic leukocytosis and lymphocytopenia and thrombocytopenia than mild to moderate diseased and survivor patients. The study by Mardani et al[41] (2022) attempted to explain the association between the inflammatory markers and COVID-19 progression and found that elevated CRP was correlated with the severity of COVID-19; furthermore, high ESR levels were observed in the severe cases only. Additionally, interleukin (IL)-7, IL-8, IL-9, IL-10, granulocyte-colony stimulating factor, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-alpha (TNF-α), and vascular endothelial growth factor A (VEGFA) were all found at high blood levels in COVID-19 patients[45].
In contrast, children show inconsistency and require further investigation. According to Del Valle et al[46] (2022) children with SARS-CoV-2-associated community-acquired pneumonia have low CRP levels. Additionally, a systematic review by Patel NA (2020) describes 2914 pediatric patients with COVID-19, the lab results for these children indicate stable WBC, lymphocyte count, and CRP levels[47]. Even though pneumonia causes an elevated CRP level, pneumonia with COVID-19 causes a drastic increase in CRP. This was revealed in a retrospective comparative study by analysis of the laboratory markers among children affected with pneumonia in the presence or absence of SARS-CoV-2 infection[48]. A meta-analysis study covers 20 eligible studies to identify the laboratory abnormalities among 1810 pediatric patients including Leukopenia, lymphopenia and elevated CRP[49]. Furthermore, the major conclusion of a retrospective cohort study by Graff et al[50] (2021), which included 454 patients, was that elevated CRP is a predictor of severe COVID-19 in children. All the previous studies show defects in the number of people involved in the studies. Hence, we recommend further investigation into many children.
Numerous investigations have demonstrated that liver damage occurred in SARS patients. This damage primarily took the form of mild to moderate elevations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) in the early stages of the illness. Some individuals’ blood albumin levels dropped as their bilirubin levels increased[51]. Compared to moderate cases, patients were more likely to have severe hepatic damage[52]. According to recent investigations into COVID-19, liver damage can occur in between 14.8% and 53% of cases, with aberrant ALT/AST values and slightly increased bilirubin levels serving as the significant indicators[53]. Severe cases reduced albumin (26.3-30.9 g/L)[54]. In recent research, including 1100 Chinese patients, Guan et al[34] found that 56% of patients with a severe COVID-19 infection and about 18% of patients with a non-severe COVID-19 disease had increased blood AST levels. Additionally, it was shown that patients with a non-severe COVID-19 illness accounted for 20% of patients with increased blood levels of ALT. In contrast, patients with severe COVID disease constituted 28% of patients. In COVID-19 fatality cases, liver damage occurred between 58.06% and 78% of the time[55]. A study showed that a patient with severe COVID-19 had blood ALT and AST values of 7590 and 1445 U/L, respectively[54].
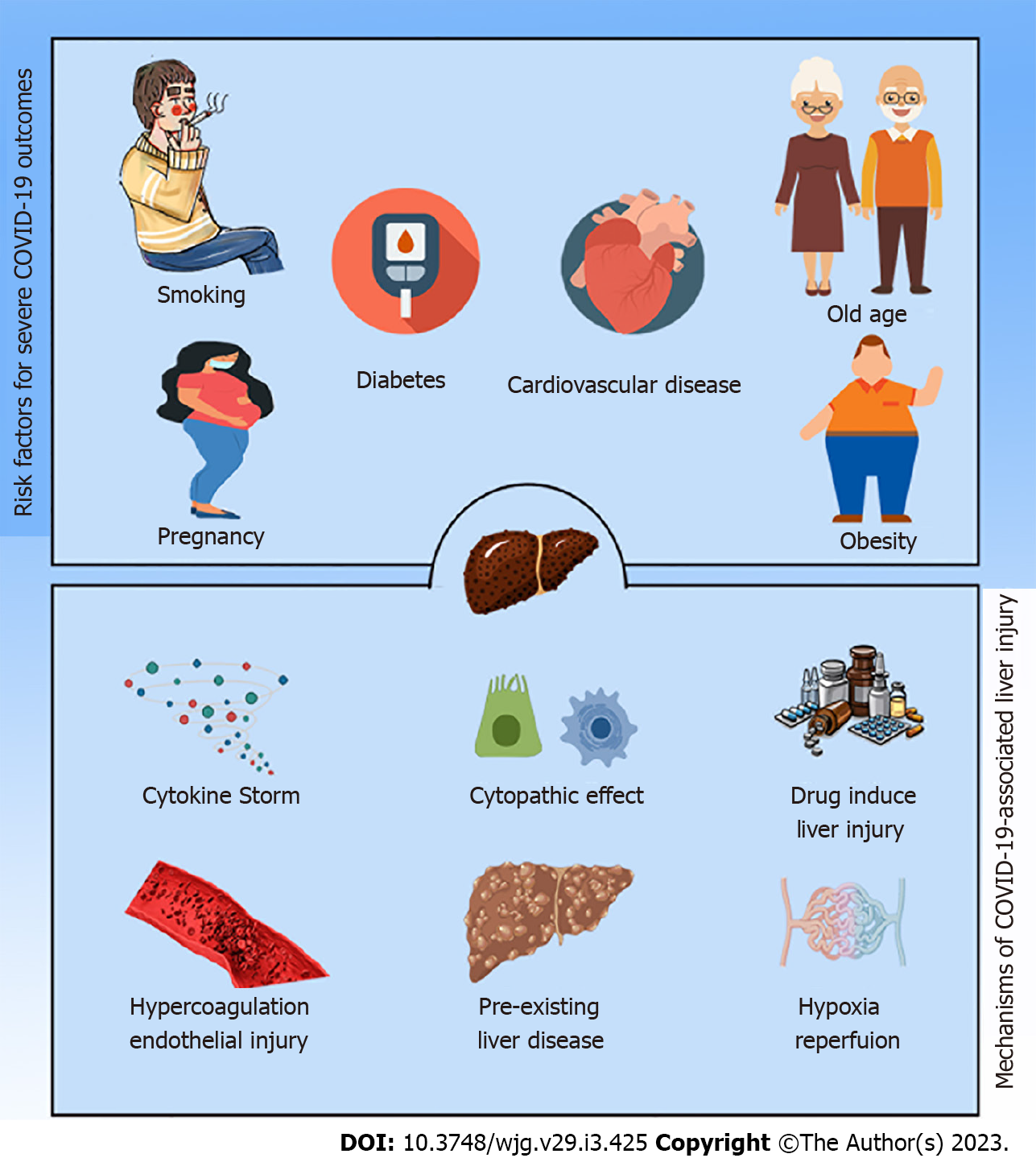
Intriguingly, lifestyle characteristics such as smoking, a high body mass index (BMI), male gender, postmenopausal status, and higher age in females were cited as the most significant risk factors for SARS-CoV-2 infection, regardless of comorbidities[56-58]. According to some studies, the age for an elevated risk is > 64 or > 65 years old. With six records, hypertension[59] and diabetes[60] are the most prevalent pre-existing comorbidities, followed by cardiovascular disease with three records. On rare occasions, associations were found between severity and TB, chronic renal illness, chronic obstructive pulmonary disease, or cerebrovascular disease. Significant effects on disease severity were reported for eight comorbidities that emerged because of COVID-19 infection[61]. Among them are organ failure, immune dysfunction, acute liver damage, hypoproteinemia, acute RDS, severe pneumonia, an uncontrolled inflammatory response, and hypercoagulable conditions[58,61].
Because their host defenses are compromised, patients with pre-existing liver diseases, such as cirrhosis of the liver and hepatocellular carcinoma (HCC), are more susceptible to infections and sepsis in general. Chronic liver diseases (CLDs) were present in 0.6% to 1.4% of hospitalized COVID-19 patients[62,63]. These individuals were more likely to experience severe illness (up to 60%) and increased death (up to 18%)[64]. Additionally, SARS-CoV-2 infection worsened the clinical prognosis and exacerbated liver damage in persons with CLDs resulting in decompensation in 20% of cirrhotic patients and worsening the clinical outcomes of people who were unstable[65].
The relationship between metabolically associated fatty liver disease (NAFLD) and COVID-19, among instances of chronic liver disorders and COVID-19, has received full attention. According to two investigations by Qian et al[66] and Ji et al[67], Patients with NAFLD have a longer viral shedding period and are more likely to have abnormal liver functions from the time of admission until discharge. Moreover, other investigations reported the same findings, with more significant mortality in patients with NAFLD, obesity, and those over 60 years old[68].
Additionally, the chance of rapid SARS-CoV-2 infection and developing COVID-19 complications appear with immunomodulatory and immunosuppressive drugs mainly used in autoimmune liver diseases. Therefore, patients with autoimmune hepatitis receiving immunosuppressive therapy should be viewed as having a high risk of developing severe COVID-19[69]. In contrast, the incidence of SARS-CoV-2 infection in patients with autoimmune hepatitis was like the general population, and the prevalence of severe COVID-19 was low[70]. Hence, we recommended further studies on patients with autoimmune hepatitis receiving immunosuppressive therapy.
Finally, according to preliminary findings on coinfection with SARS-CoV-2 and other viruses, it seems to cause severe progression, poor outcomes, or vial reactivation as in the case of the hepatitis C virus (HCV) and Hepatitis B virus (HBV) coinfection[71-73].
Recent research shows that the frequent symptoms of fever and cough coincide with the beginning of COVID-19 infection. Other clinical characteristics, such as diarrhea, nausea, vomiting, and lack of appetite, represent at least a digestive system symptom[34]. CoV infection has been linked to liver damage in SARS and Middle East respiratory disease patients[74]. In cases with COVID-19, abnormal liver function was observed, shown as isolated elevations in blood transaminase and lactate dehydrogenase (LDH) levels[75]. Alkaline phosphatase (ALP), LDH, ALT, AST, and prothrombin time levels gradually increased during the hospitalization of the first COVID-19 case in the United States[76]. According to a study from Jin Yin-tan Hospital, out of the 99 patients with COVID-19, 43 had ALT or AST levels above the normal range, 75 had elevated LDH levels, and one had a severe disruption in liver function[54]. With 3.75% of all cases in Jiangsu province being imported and cases outside Wuhan, liver damage was said to be less common in these patients[77]. In an analysis of liver function among patients outside intensive care units, males were more likely to experience liver impairment than females[78]. In pediatric instances, liver damage was discovered in 22% of kids, most often between 2 and 18 d after admission[79]. In Wuhan, liver injury is a common factor among patients who are admitted to the ICU and non-survivors hospitalized patients. This reflects the relationship between liver injury and the severity of COVID-19[80]. Fifty-two patients who required mechanical breathing or had at least 60% inspired oxygen been included in a study of critically ill individuals. Twenty-nine percent of patients with critical conditions had liver damage. Fifteen percent had acute renal disease, and fifteen percent had cardiac injury[81]. In a multicenter study involving 1099 patients and 552 hospitals, abnormal liver function was generally detected in critically ill participants, whereas jaundice was less frequently observed in COVID-19 patients. In harmony with the elevation of total bilirubin levels in 10% of patients, the percentage was increased in severe cases up to 20.5%[34]. Furthermore, a multicenter retrospective cohort study including 5771 patients in Hubei province suggested that upregulation in liver injury markers, particularly AST, is closely correlated with the probability of death during COVID-19[75]. Therefore, the dynamic patterns of liver injury markers and their putative risk variables may provide a significant explanation for the liver damage linked to COVID-19. Additionally, all studies indicated that liver injury parameters should be monitored during hospitalization.
ACE2 expression aroused the curiosity of researchers and scientists due to unusual ACE2 hepatic distribution and unexpected outcomes. Chai and colleagues assumed the hepatic abnormalities during COVID-19 were ascribed to cholangiocytes dysfunction, not due to hepatocytes damage (Figure 1). Their investigation using single-cell RNA-seq revealed that the primary target for SARS-CoV-2 in the liver was cholangiocytes. The ACE2 expression in hepatocytes is 20 times less than observed in cholangiocytes. Despite this, clinical data from COVID-19 patients showed rising ALT, AST, and LDH levels, while ALP and gamma-glutamyl transferase, which describe bile duct injury, did not significantly increase[82]. At the same time, histological and immunohistochemistry assessments of Kupffer cells and T and B lymphocytes did not express ACE2[83], even though COVID-19-infected patients’ livers frequently showed Kupffer cell activation and proliferation[84,85]. Additionally, systemic inflammation typically results in Kupffer cell activation and proliferation[84]. Although Kupffer cells do not express, ACE2 may have a crucial role in the propagation of inflammation that results in SARS-CoV-2-mediated liver damage. It is noteworthy that prediction of SARS-CoV-2 consecutive signaling, and outcome is challenging because the expression of ACE2 level is regulated by many factors and conditions, for example, liver fibrosis, liver cirrhosis, hypertension, diabetes, chronic pulmonary diseases, hypoxia, old age, and smoking, which represent factors for COVID-19[17,86,87].
SARS-CoV-2 uses ACE2 receptors to invade host cells and utilizes other molecules to facilitate infection, such as furin, transmembrane serine protease 11A (TMPRSS11a), and neuropilin-1[88,89].
Neuropilin-1 is embedded in the liver, causing physiological and pathological conditions. Activation of the neuropilin-1 cascade triggers angiogenesis process via controlling cell proliferation, cell survival, and cell migration[90]. Regardless of the cause of hepatic injury and conditions resulting from a viral infection, the elevation of neuropilin-1 is the defense mechanism. Consequently, neuropilin-1 may influence liver damage induced by SARS-CoV-2[89]. Neuropilin-1 has been reported to be found and expressed in liver sinusoidal endothelial cells and hepatic stellate cells[91]. Meanwhile, hepatic stellate cells’ activation is postulated to be the primary cause of liver disease and fibrosis[92,93]. In different conditions, the hepatic stellate increases proinflammatory and profibrotic cytokines[94]. One of those cytokines is IL-6, produced when SARS-CoV-2 activates the immune system in COVID-19 patients and is associated with altered liver enzyme levels[95]. Therefore, propagation of neuropilin-1 expression with activation of hepatic stellate cells promotes signaling transcription and stimulates the release of growth factors such as transforming growth factor -β and VEGF, elucidating their role in the progression of liver damage during SARA-CoV-2 infection[96].
All the data mentioned above are consistent with a detailed histological examination clarifying the possible mechanisms of hepatic injury. Wang et al[84] uncovered the presence of intact SARS-CoV-2 viral particles in the cytoplasm of hepatocyte samples obtained from 156 dead COVID-19 patients. Further observations revealed conspicuous mitochondrial swelling, endoplasmic reticulum dilatation, glycogen granule decrease, fibrin deposition, granulomas, massive central necrosis, and apoptosis. Another study by Fiel et al[97], using in situ hybridization and electron microscopy, reported that SARS-CoV-2 directly invades liver cells and induces histological changes such as apoptosis, especially in cholangiocytes, abundant mitoses, mixed inflammatory infiltrates in portal tracts, endothelins, and severe bile duct damage. In a case study by Melquist et al[98], the direct SARS-CoV-2 cytopathic effect caused a rapid progression of acute hepatitis to fulminant liver failure with a mild increase in transaminase levels without developing respiratory symptoms. Data from the international study involving 130 centers in 29 countries revealed that the stage of liver disease is closely correlated with COVID-19 mortality. The highest rates of hepatic decompensation and mortality were observed in patients with advanced liver cirrhosis and those with alcoholic liver disease[99].
SARS-CoV-2 induces immune dysregulation associated with the unspecified release of proinflammatory cytokines and coagulation enzymes. The massive release of cytokines is known as a cytokine storm or cytokine release syndrome and is characterized by the magnitude of the release of interferons, TNFs, ILs, and chemokines[100]. Hence, uncontrolled systemic proinflammatory cytokine release represents unfavorable clinicopathological conditions in COVID-19 patients, for instance, progressive liver damage and liver failure.
IL-6 is the most significant cytokine in liver hepatocytes and is a crucial inducer of the acute phase response and infection defense[101]. IL-6 stimulates hepatocytes during the initial phase of inflammation to upregulate CRP, fibrinogen, haptoglobin, alpha-antitrypsin, and serum amyloid-A which induce acute inflammatory phase[101]. Additionally, prolonged inflammation stimulates IL-6, targeting monocyte chemotaxis toward tissue-destructive injury[102]. Furthermore, IL-6 induces multiple effects during the storm via the activation of different transduction signaling pathways, e.g., nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), janus kinase (JAK)/ signal transducer and activator of transcription (STAT), and the Akt/Phosphatidylinositol-3-kinase pathway[71,103].
Similarly, attention must be paid to the crucial roles of the ACE/Ang II/ angiotensin II receptor type 1 pathways. Ang II can directly activate the NF-κB pathway, increasing the secretion of IL-6, IL-1β, TNF-α, and IL-10[104]. Moreover, Ang II has been reported to induce mitogen-activated protein kinases act
A case series study by Li et al[106], revealed elevated serum transaminase levels attributed to systemic inflammation, cytokine storm syndrome, and hepatocyte damage. Darif et al[95] reported that hepatic injury in patients with COVID-19 was attributed to systemic inflammation. Therefore, significant elevations in CRP, TNFα, and IL-6, concomitant with a significant elevation in aminotransaminase, describe hepatic injury associated with SARS-CoV-2 infection[107,108]. All these data confirm the relationship between inflammation during COVID-19 and hepatic injury.
One of the most common complications of COVID-19 is acute respiratory distress syndrome requiring a high level of management[81,109,110]. COVID-19 is associated with impaired respiration, an insult to blood flow, and hypotension, which are clues to hypoxic hepatitis, and might exacerbate liver damage or even lead to liver failure[11,106]. Ischemia induces profoundly detrimental cellular effects and results in metabolic abnormalities, for example, disturbances in lipid metabolism as well as lack of oxygen supply initiate hepatocellular death[112]. Furthermore, rapid recovery of blood flow with reoxygenation of hepatocytes results in metabolic abnormalities, the generation of reactive oxygen species, an inflammatory response, and cellular death[113]. Hence, hepatic ischemia deteriorates hepatic status via destructive cellular reactions concomitant with immune stimulation[112-114]. Hypoxia has been determined as the primary pathway to regulating ACE2 expression in hepatic cells[115]. These phenomena rapidly progress with a conspicuous elevation of transaminase levels, accompanied by LDH elevation[116]. A retrospective study by Huang et al[117] revealed that hypoxic hepatitis is apparent in intensive care units and is often associated with a drastic elevation in ALT levels, multiorgan damage, and high mortality risk. Additionally, patients with COVID-19 and hypoxic hepatitis are sometimes comorbid with respiratory failure, septic shock, or heart failure[53,80,118]. All these findings suggest an association between hepatic ischemia/hypoxia-reperfusion injury and liver injury during the SARS-CoV-2 infection.
SARS-CoV-2 induces hypercoagulation, with the incidence of pulmonary embolism associated with complications aggravating heart failure and liver congestion[119]. Hypercoagulation and clotting disorders might occur through direct infection of platelets or a cytokine storm[120]. As mentioned above, patients with COVID-19 reported a change in platelet count and prothrombin time with an elevation in D-dimer and fibrinogen concentrations[80,121-123]. A multicenter, retrospective cohort study found that patients who died from COVID-19 were more likely to have severe hematological (lymphopenia, ferritin, and elevated D-dimer) and cardiogenic factors (troponin and lactate dehydrogenase), providing support for this hypothesis[80]. Goshua et al[123] reported that patients with COVID-19 showed a disturbance in epitheliopathy and platelet activation markers, particularly von Willebrand factor (vWF) antigen, P-selectin, and soluble thrombomodulin, anticipating a poor outcome or death. Furthermore, a case report study by Antunes de Brito et al[124] observed hepatic artery thr
SARS-CoV-2 infection induces a pathological thromboinflammation response, including platelet hyperreactivity, hypercoagulability, and hypofibrinolysis[127]. SARS-CoV-2 binds to ACE2 receptors on the surface of endothelial cells and subsequently induces endothelial injury[127]. Additionally, SARS-CoV-2 invades megakaryocytes and platelets[128]. Endothelial cell activation and injury were confirmed by elevation of several blood hemostatic factors including vWF, thrombomodulin, and factor VIII[122,123]. Collectively, they trigger a platelet plug activation[129,130]. A procoagulant molecule and platelet tissue factor, produced by hepatocytes and endothelial cells, attach, and activate factor VII, a procoagulant molecule that circulates in the blood. Activated factor VII activates factor X, which subsequently resulted in thrombin formation. Thrombin promotes a series of coagulation processes to produce fibrin which build a substantial fibrin mesh, in addition to platelet activation and aggregation[131,132]. Furthermore, Ang II increases plasminogen activator inhibitor-1 (PAI-1) expression in endothelial cells, which inhibits fibrinolysis and induces a hypercoagulable state[133].
Furthermore, hypoxia promotes coagulation through multiple pathways, such as hypercoagulation and inflammation. Hypoxia attenuates endothelial cells’ anticoagulant function by suppressing thrombomodulin with increased PAI-1 upregulation. It promotes NF-κB and toll-like receptor 4 signaling pathways in macrophages and neutrophils, stimulating the release of IL-6 and TNFα[134-136].
Excessive inflammatory cytokines, particularly IL-6, facilitate SARS-CoV-2 and mediate coa
Several medications can induce liver dysfunction and hepatocellular damage. Some are used as over-the-counter medications, for example, paracetamol, and others are used with precautions such as antibiotics, including azithromycin[140]. Although drug-induced liver damage is rare, it can im
Several antiviral medications, supportive care, and trials of complementary therapies are among the therapeutic options being investigated against SARS-CoV-2. Hepatotoxicity from nucleoside analogs and protease inhibitors, which are used to manage COVID-19, can occur because the liver is involved in the metabolism of many medications. In a case study from Wuhan, after receiving lopinavir and ritonavir, the patient developed liver damage[143]. A recent randomized controlled study compared the elevation of AST, ALT, and total bilirubin in COVID-19 patients associated with lopinavir and ritonavir[144]. In a retrospective analysis of COVID-19, Fan et al[145] found that significantly elevated liver enzymes and liver abnormalities were in harmony with receiving combination therapy. In this study, 47.3% of the released patients had increased liver function tests (LFTs) at baseline, and 23.7% experienced abnormalities during hospitalization, which might be due to treatments or the disease.
It was discovered that many COVID-19 patients had previously used antipyretics and analgesics, most frequently paracetamol, whose overdose is recognized as a cause of liver injury with a significant elevation of serum aminotransferases[146]. Additionally, hepatic injury worsens in critical illnesses and patients with preexisting CLDs[147]. Therefore, healthcare providers should be aware of over-the-counter medications used to control common COVID-19 symptoms such as fever and pain. Physicians play a role in monitoring abnormalities in LFTs as they can indicate unknown drug hepatotoxicity.
Hydroxychloroquine is one of the drugs suggested for COVID-19 therapy regimens, an anti-malarial medicine that relies on scant data in limited clinical settings[148]. Based on clinical data, hydroxychloroquine hepatotoxicity during COVID-19 is rare[149]. A few incidences of significant increases in aminotransferases brought on by hydroxychloroquine have never been documented[150]. Therefore, patients with liver disorders should use hydroxychloroquine cautiously since it can accumulate[151].
Azithromycin is an antibacterial drug belongs to macrolide antibiotic. It was used to treat bacterial infections before treating COVID-19 alone or in combination with hydroxychloroquine[152]. Hence, the hepatic injury should be considered due to the high use of these medications. Liver damage may rarely occur within the first two to three weeks of starting azithromycin. Most patients fully recover from it, and it is predominately a hepatocellular pattern[153].
Remdesivir belongs to an adenosine analog with antiviral action[154]. A multicenter, randomized, double-blind, placebo-controlled trial by Wang et al[155] revealed that 10% of the remdesivir group had high blood bilirubin and 5% had increased aminotransferases. Additionally, Remdesivir was used to treat COVID-19 in a case series (n = 53), and 23% of the patients experienced elevated liver enzyme levels that required early treatment termination[156]. However, clinical data implied that the relation between remdesivir and hepatic injury during COVID-19 treatment needs more explanation[154].
To conclude, medications that reduce inflammation and preserve the liver should be given to individuals who are expected to experience liver damage, regardless of the drug, dosage, or dose[140].
All studies regarding possible mechanisms of COVID-19-associated liver injury were summarized in Table 1.
| Cause of liver injury | The main finding of the study | Ref. |
| SARS-CoV-2 directly invades the liver and displays hepatic impairment characterized by liver enzyme abnormalities | Wang et al[84], 2020 | |
| SARS-CoV-2 tropism | Intrahepatic SARS-CoV-2 contributes to liver inflammation, endothelium, and bile duct damage | Fiel et al[97], 2021 |
| SARS-CoV-2 cytopathic effect involved in the rapid progression of acute liver injury to acute liver failure | Melquist et al[98], 2020 | |
| Cytokine storm | Elevation of liver enzymes in COVID-19 is mainly related to immune dysregulation caused by cytokine storm and hepatic damage | Li et al[106], 2020 |
| Systemic inflammation is the fuel for hepatic injury in COVID-19 patients | Effenberger et al[244], 2021 | |
| Hypoxic liver injury | Hypoxic hepatitis is not a rare condition in COVID-19 patients admitted to the intensive care unit and is dramatically associated with elevated liver enzymes | Huang et al[117], 2020 |
| Hepatic artery thrombosis is highly associated with hepatic injury and abdominal pain during COVID-19 | Antunes de Brito et al[124],2021 | |
| SARS-CoV-2 induces severe disruption of the intrahepatic blood vessel and also affects the endothelial layer of blood vessels | Sonzogni et al[125], 2020 | |
| Endothelial cells and liver injury | Hepatic injury is attributed to platelet-fibrin microthrombi in the hepatic sinusoids along with some portal vein platelet aggregates | Rapkiewicz et al[126], 2020 |
| SARS-CoV-2 activates IL-6/JAK/STAT pathway consequently, stimulating coagulopathy and hepatic epitheliopathy | McConnell et al[139], 2021 |
HBV is a double-stranded DNA virus, a member of the Hepadnaviridae family. In contrast, HCV is a single-stranded RNA virus belonging to the Flaviviridae family[100]. Recently, several studies have indicated that the coinfection of COVID-19 and HCV is a predictor of acute-on-chronic liver failure and a high potential for ICU admission. A cohort study indicated that HCV patients with SARS-CoV-2 coinfection were more likely to be hospitalized. However, the mortality rate did not change[157].
In a retrospective cohort study that included 242 patients with hepatitis C cirrhosis, 46 patients were coinfected with SARS-CoV-2 and HCV and had high levels of ferritin, creatinine, blood urea nitrogen, prothrombin time, and HCV viral load, anticipating the development of acute-on-chronic liver failure and the potential for ICU admission[158]. An observational study by Toma et al[159] among patients with SARS-CoV-2, active HCV, and cure HCV in a control group showed the highest serum concentrations of ALT, AST, CRP, and ferritin. Moreover, serum and fecal calprotectin were detected in a patient with SARS-CoV-2 infection. In a serological study by León et al[160], patients with both HCV coinfection demonstrated a considerable elevation in IL-6 and IL-17, with lower TNF-α levels when compared with patients infected with HCV or SARS-CoV-2.
On the other hand, a nationwide population-based study has reported that patients infected with HBV were predisposed to have severe symptoms of SARS-CoV-2, a high probability of ICU admission, and more organ failures than patients without HBV infections, especially in older patients[161].
In addition, severe monocytopenia, lymphopenia, hypoalbuminemia, and lipid metabolism de
Acute HBV infection during pregnancy is not a risk factor for fetal death or teratogenicity. However, many complications in HBV-infected pregnant women may be associated with an increased risk of gestational diabetes, postpartum hemorrhage, premature birth, and low birth weight[169]. Furthermore, in a prospective cohort study, Rajan et al[170] indicated that pregnant women with both HBV and SARS-CoV-2 coinfection had a high proportion of preterm deliveries and a low mean birth weight. In rare cases, the coinfection of both viruses has led to intrahepatic cholestasis in pregnancy and acute fatty liver disease of pregnancy (AFLP)[171]. Nevertheless, there is some indication that HBV and COVID-19 coinfection does not lead to worse results[170,172]. On the other hand, some studies have provided evidence that treatment regimens including antivirals, hepatoprotective, and low-dose dexamethasone drugs might be recommended in cases of pregnant women with HBV and COVID-19 coinfection, besides coagulation function monitoring as part of the management process[171,173].
Similarly, pregnant women with HCV infection are more likely to have infants born prematurely, stillborn infants, newborns with low birth weight, or infants with birth abnormalities[174,175]. Furthermore, from an epidemiological point of view, the worldwide hepatitis elimination program has been affected due to COVID-19 spreading, and this may require new policies and strategies for hepatitis elimination[176-178].
Ahmed et al[179] reported a case-report study in which a 26-year-old Asian female pregnant patient was affected by a sudden onset of severe preeclampsia complicated by AFLP and acute kidney injury (AKI) following SARS-CoV-2 infection. Besides, the comorbidities of SARS-CoV-2 and preeclampsia in pregnancy can lead to AFLP and AKI. This comorbidity can cause calcifications of the bowel and gallbladder of the fetus[180,181], besides a liver parenchymal disease associated with liver rupture[182], liver coagulation, liver impairment, and preterm delivery[183]. Furthermore, a pregnant woman with SARS-CoV-2 infection at 28 wk with a low-lying placenta was complicated by obstetric cholestasis and several episodes of minor antepartum hemorrhage[184]. Moreover, placental insufficiency and subsequent fetal hypoxia may occur[185].
Recently, pregnant patients who were coinfected with SARS-CoV-2 showed a higher risk of developing complications than those who were not pregnant. Studies have shown that pregnant women with SARS-CoV-2 infection increased the probability of developing preeclampsia compared to individuals who did not have SARS-CoV-2 infection during pregnancy[186]. Nevertheless, symptomatic patients were more likely to have preeclampsia than asymptomatic ones[186,187].
On the other hand, several hypotheses may illustrate the high rate of preeclampsia associated with SARS-CoV-2 infection. A direct cytopathic effect with dysregulation of the RAAS system induces a change in the placenta’s function[188-191] because it controls the proliferation of trophoblasts, angiogenesis, and placental blood supply. Thus, the interaction between SARS-CoV-2 and ACE2 receptors described in RAS system down-regulation and reduction of vasodilatory angiotensin 1 to 7 results in continuous vasoconstriction and pro-inflammatory effects of angiotensin II, which finally lead to a pathophysiological mechanism of preeclampsia[192-196]. A study conducted by Verma et al[197] suggested that the infected placenta had a reduction in ACE2 receptor expression, proangiogenic factors, and an increase in the production of soluble FMS-like tyrosine kinase-1 (sFlt-1), which are biomarkers for preeclampsia. An in-silico study by Seethy et al[198] concluded that interactions between SARS-CoV-2 and the placenta are regulated through trophoblast invasion, migration, proliferation, and differentiation processes by the milk fat globule-EGF factor 8 protein, plasminogen activator, and protease-activated receptor 2 proteins.
In parallel, pregnant women might be able to develop a pre-eclampsia-like syndrome characterized by proteinuria, hypertension, thrombocytopenia, the elevation of liver enzymes, an abnormal uterine artery pulsatility index, and increased sFlt-1/placental growth factor[199], besides preeclampsia, coagulopathy, and the HELLP (hemolysis, elevated liver enzymes, low platelet count)[200].
Recently, it has been hypothesized that patients with a hepatic illness have a higher mortality rate after SARS-CoV-2 infection. Non-invasive indices, including the Fibrosis-4 index (FIB-4), the NAFLD fibrosis score, and the AST to platelet ratio index, have been developed to determine the severity of fibrosis, which plays a crucial role in assessing liver fibrosis[201]. In a multicenter observational study, Kim et al[202] reported that patients with diabetes mellitus (DM) showed a higher FIB-4 index, serious complications such as severe respiratory failure, venous thromboembolism, hepatic injury, and a high mortality rate compared to patients without DM. Meanwhile, the FIB-4 index might be used to assess the risk of progression to hepatic illness in middle-aged patients with COVID-19[203]. An association was observed between liver fibrosis scores and poor outcomes, and these findings were consistent with previous research that found worse outcomes in COVID-19 individuals with pre-existing chronic liver disorders, including a high proportion of ICU admission and the need for mechanical ventilation[204,205]. An explanation for liver injury could be the presence of high levels of lymphocytes and natural killer cells inside the hepatic tissue[206].
On the other hand, An et al[207] conducted a STROBE observational study and reported that patients with liver cirrhosis and COVID-19 were frequently admitted to the hospital more than those with liver cirrhosis only. Unlikely, in the same study, cirrhotic patients who lacked COVID-19 experienced more severe liver cirrhosis-related consequences and needed immediate treatment. In a multicenter cohort study, Bajaj et al[208] illustrated that those with cirrhosis alone or with COVID-19 had equal death rates, while patients with COVID-19 alone had a greater mortality rate.
As discussed above, having an infection makes pregnant women more susceptible to developing more severe symptoms. Biomarkers such as ALT, AST, ALP, elevated D-dimer levels, fibrin degradation, and prolonged prothrombin time lead to liver injury, liver fibrosis, and liver cirrhosis; hence, increasing the possibility of preeclampsia with HELLP syndrome[179].
HCC is the third most important cause of cancer-related mortality and the sixth most frequent cancer in the world. SARS-CoV-2 virus infection has recently been considered a risk factor for cancer patients because SARS-CoV-2 might aggravate liver damage in HCC patients[209]. Furthermore, a US multi-center study by Kim et al[210] reported that having HCC indicates a greater mortality rate in individuals with HCC infected by SARS-CoV-2 than COVID-19 alone, especially in patients with obesity, DM, hypertension, hyperlipidemia, older patients (≥ 65 years), and Hispanic ethnicity. Also, in China, patients with HCC and COVID-19 were shown to be more susceptible to a higher risk of death and admission to the ICU[211]. In parallel, Leo et al[212] retrospectively analyzed 119 patients with HCC and COVID-19 infection. They found that about one-third of patients required hospital admission. Two-thirds had an elevation of transaminases, particularly ALP, which was independently linked to a high mortality rate, higher CRP levels, and more severe respiratory failure upon admission to the hospital.
According to the American Society of Transplantation, there has yet to be an agreement on the ideal timing of liver transplantation (LT) in patients infected with SARS-CoV-2. However, it is recommended that before transplantation, recipients should have a negative SARS-CoV-2 test[213]. Nevertheless, Martinez-Reviejo et al[214] determined that, regardless of symptoms at the time of infection, using LT from SARS-CoV-2 positive donors appears to be a safe technique with a low risk of transmission. Furthermore, a multicenter network study by Mansoor et al[215] found that LT patients with COVID-19 had a substantially larger possibility of hospitalization but not mortality, thrombosis, or ICU admission when compared to those without LT and COVID-19. In contrast, a case-control study by Shafiq et al[216] stated that regarding death and hospitalization rates, there was no significant difference between the case and control groups in liver enzyme ratios, and both had a normalized value at the time of discharge. In addition, the only difference in the patient’s pathological characteristics is the type of liver graft, alkaline phosphatase levels, and lymphovascular invasion[217]. A case-report study indicated that some LT could be successful in active SARS-CoV-2 patients without developing post-operative COVID-19 symptoms[213]. Furthermore, an Italian multicenter series by Romagnoli et al[218] found that liver transplantation from COVID-19-positive donors to informed recipients with SARS-CoV-2 immunity might help increase the safety of the donor pool. Rela et al[219] reported a successful LT in patients with severe liver failure due to cholestasis with good graft function and recovering function in the native liver remnant.
Collectively, the effect of comorbid hepatic disorders with SARS-CoV-2 infection was summarized in Table 2.
| Hepatic disorders | Main finding | Ref. |
| SARS-CoV-2 comorbidity with HCV shows a high percentage of ferritin, white blood cell count, prothrombin time, lymphocyte count, and hypoglycemia | Cerbu et al[159], 2022 | |
| SARS-CoV-2 and HCV coinfection reported higher levels of IL-6 and IL-17, and TNF-α when compared with HCV and COVID-19 alone | León et al[161], 2022 | |
| HCV | The Serum levels of ALT, AST, CRP and ferritin, and calprotectin were significantly elevated in patients with COVID-19 infection than in patients with active HCV and patients with cured HCV infection | Toma et al[160], 2022 |
| HCV patients with SARS-CoV-2 infection are more likely to be hospitalized with a high possibility of liver fibrosis and mortality | Butt et al[158], 2021 | |
| Individuals with HCV and SARS-CoV-2 co-infection are more vulnerable to developing liver cirrhosis | Afifyet al[245], 2021 | |
| Patients with a history of HBV are anticipated to have a worse outcome with a high probability of ICU admission, and more organ failures | Choe et al[162], 2022 | |
| S SARS-CoV-2 and chronic HBV showed severe monocytopenia, lymphopenia, thrombocytopenia, hypoalbuminemia, and lipid metabolism deficiency in the liver | Zou et al[164], 2021 | |
| HBV | Patients with HBV and SARS-CoV-2 coinfection died from severe liver disease and haptic sclerosis | Chen et al[163], 2020 |
| Patients with HBV who have COVID-19 were more likely to develop devastating illnesses and/or death. Additionally, the elevation of LDH, and D-dimer, with decreased albumin, and albumin/globulin ratio is helpful for early clinical surveillance | Wang et al[165], 2022 | |
| Patients with DM with advanced liver fibrosis infected by SARS-CoV-2 are assumed to have a 10-time risk of mortality when compared with patients without comorbidities | Kim et al[203], 2021 | |
| The high proportion of ICU admission, and the need for mechanical ventilation | Hassnine et al[206], 2022 | |
| Liver cirrhosis | Patients with liver cirrhosis and COVID-19 were admitted to the hospital than liver cirrhosis alone | An et al[208], 2021 |
| Those with cirrhosis alone or cirrhosis with COVID-19 had equal death rates, while patients with COVID-19 alone had a greater mortality rate | Bajaj et al[209], 2021 | |
| HCC predicts a greater mortality rate in individuals with HCC infected by SARS-CoV-2 than COVID-19 alone, especially in patients with obesity, diabetes mellitus, hypertension, and hyperlipidemia, older patients ≥65 years, and Hispanic ethnicity | Kim et al[211], 2021 | |
| HCC | HCC and COVID-19 were shown to be more susceptible to have a higher risk of death and admitted to the ICU | Liang et al[212], 2020 |
| Patients with HCC-COVID-19 coinfection found that about one-third of patients need hospital admission, and two-thirds of patients have an elevation of transaminases. Alkaline phosphatase which independently linked to a high mortality rate, higher C reactive protein levels, and more severe respiratory failure upon admission to the hospital | Leo et al[213], 2022 | |
| LT patients with COVID-19 had a considerably increased risk of hospitalization but not a significantly higher risk of mortality, thrombosis, or need for ICU admission | Mansoor et al[216], 2021 | |
| High alkaline phosphatase levels, and lymphovascular invasion | Shafiq et al[217], 2022 | |
| LT | LT cases could be successful in active SARS-CoV-2 patients without developing post-operative COVID-19 symptoms | Mouch et al[214], 2022 |
| Found that liver transplantation from COVID-19-positive donors to informed recipients who have SARS-CoV-2 immunity may help to increase the donor pool safely | Romagnoli et al[219], 2021 | |
| Successful LT In patients with severe liver failure due to cholestasis with a good graft function and recovering function in the native liver remnant | Rela et al[220], 2022 |
Recombinant DNA, mRNA, and adenovirus vector-based technologies were the three main methods of vaccine development that demonstrated immediate success. All have been shown to help prevent infections, especially in severe diseases, because breakthrough infections are typically asymptomatic or mild-to-moderate. BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) were emergently approved in the United States as the first mRNA vaccines[220]. Following that, an Emergency Use Authorization (EUA) license was granted for the two most effective adenovirus-based vaccinations in the United States (Ad26.COV2.S) (Janssen-Johnson & Johnson) and Europe (ChAdOx1.nCoV-19; Oxford-Astra Zeneca). Adenovirus-vectored vaccines have demonstrated effectiveness in China (Ad5-vectored COVID-19 vaccine) and countries that produce traditional, inactivated viral vaccines. The most frequently used COVID-19 vaccinations are intramuscular injections, and a first dose is recommended to be followed by a second dose within three to four weeks. Currently, a booster dose is recommended administrated after six months of the initial immunization. Individuals 18 years of age and older may get a booster dose of the Johnson & Johnson COVID-19 vaccination 2 mo following the initial single dose[221,222].
An intramuscular mRNA vaccine called BNT 162b2 is administered in two doses (30 µg per dose) at 21-d intervals. The vaccine is accessible in multidose vials and must be refrigerated at a temperature between 60 °C and 80 °C[223], which might present a logistical challenge in developing nations. According to phase I/II/III, randomized, placebo-controlled trials published in December 2020, the Food and Drug Administration (FDA) approved it for emergency use[224]. In the study, 43448 volunteers were randomly assigned in a 1:1 ratio to the vaccination arm and the placebo arm. Compared to the placebo, the vaccination showed a 95% efficiency in preventing COVID-19, and this efficacy was maintained for subgroups based on age, sex, BMI, ethnicity, and comorbidities. Local site responses were the most prevalent adverse effects. Young patients were more likely to experience systemic symptoms such as fever, joint discomfort, and chills, which increased following the second dosage[225]. Just three individuals with moderate or severe liver disease were included in the trials, with 214 participants having mild liver disease. The virological status and disease severity of patients with HBV and HCV infections were included; however, it was unknown how severe their conditions were. Furthermore, immunosuppressive drug users were excluded. Hence, more information is required concerning people with liver illnesses[226,227].
The mRNA-1273 is another mRNA vaccination given in two doses of 100 µg each, separated by 28 d. Based on phase III randomized placebo-controlled trial published in December 2020, in which 30420 participants were randomly allocated to the immunization and placebo groups in a 1:1 ratio, the FDA approved the vaccine. The effectiveness of the vaccination in preventing COVID-19 was 94.1%. Only the placebo group experienced severe COVID-19, resulting in one participant’s death. Serious, unanticipated adverse reactions to vaccinations were more frequent in the vaccine group, but none were fatal or forced to be completed until the research’s end. After the second dose and in younger people, the unwanted local and systemic responses were more prevalent[228,229]. Although the liver condition was not specified, the study included 196 individuals with liver disease (divided equally between the vaccination and placebo groups). Participants in the experiment who were on systemic immunosuppressive medication were not allowed. For individuals with hepatic illness, no independent efficacy and safety data were available[228].
ChAdOx1 nCoV-19 vaccine (AZD1222) was created by the University of Oxford, which uses a replication-deficient chimpanzee adenovirus as a vector containing the gene encoding for the SARS-CoV-2 spike glycoprotein. Storage conditions may be kept between 2 and 8 °C and are less strict than mRNA vaccines. AstraZeneca and Serum Institute of India produce it (SII). In December 2020, the UK granted emergency use authorization for the vaccine produced by AstraZeneca. The vaccine, produced by SII under the brand name COVISHIELDTM®, was approved for use in India by the Drug Controller General of India[229]. Two intramuscular vaccine doses, each containing 0.5 mL, were given over a 4–6 wk interval. In patients who got a single dose, antibody responses peaked on day 28, and in individuals who received a booster dose four weeks later, they peaked on day 56[228,229]. A pooled intermediate analysis of four randomized controlled trials by Voysey et al[230] conducted in Brazil, South Africa, and the UK, which included 23848 people, was used to support the authorization. Of these, 11636 patients were included in the interim study. The experiment showed total vaccination effectiveness of 70.1%. After 21 d following immunization, 10 COVID cases were recorded; all were in the control group and included two cases of severe COVID and one case of death. In addition, only three of the 175 cases with adverse effects might have been caused by vaccination. Individuals with hepatic disorders were mostly excluded from the 4 studies described above. Patients with severe liver diseases were not included in the trials in the UK and Brazil, although the severity standards were unclear. Furthermore, individuals using immunosuppressive drugs and those with alcohol dependence were excluded. Abnormal LFTs, Australian antigen-positive status, CLDs, and alcohol misuse were listed as exclusion criteria in a South African study. Only two individuals (one from each vaccination and control group) had abnormal liver function[231].
This full-length SARS-CoV-2 S protein-containing non-replicating human adenovirus type 26 triggers an immune response to the SARS-CoV-2 infection. The SARS-CoV-2 virus is prevented from invading type 2 alveolar cells in the lungs by an antibody directed against the S protein, lessening the severity and morbidity of the infection[232]. Adjuvant properties, scalability, and broad tissue tropism are benefits of adenoviral vectors[233,234]. Since these labs need biosafety level 2 certification, vaccine production will likely go more slowly during this pandemic. Additionally, a person with immunity to viral vectors would reduce the vaccine’s efficacy. Employing the chimpanzee adenovirus (ChAdOx1), which serves as an alternative to the human Ad vector and does not confer any immunity on humans, Oxford/AstraZeneca could overcome this drawback[235,236].
Moreover, Sadoff et al[231] revealed that a single-shot Janssen vaccination prevents severe SARS-CoV-2 infections. A total of 43783 seronegative volunteers participated in this study, and they were separated into two age groups: Group 1 (18-59 years old) and group 2 (≥ 60 years old). These participants were randomly divided into two groups of like-minded individuals in a 1:1 ratio, one receiving the placebo and the other the vaccination. The study group collected 468 confirmed cases after receiving the vaccination for 14 d. A total of 464 cases, including 116 from the vaccination group and 348 from the placebo group, were mild to moderate in severity, indicating an effectiveness of 66.9%. More than 66 moderates to severe-critical cases were confirmed to belong to the vaccine group after 28 d of follow-up, compared to 193 cases that belonged to the placebo group. Moreover, less severe-critical cases were observed among older patients than younger patients, suggesting possible early protection from the vaccine, especially in the elderly. The effectiveness of the immunization was equal across all age groups after 28 d[233].
At least five distinct COVID-19 vaccines, including conventional inactivated viral vaccines and vaccines based on an adenovirus vector, have been created and given the go-ahead for use in China. The safety and efficacy of the majority have not been extensively reported. As part of its international COVID-19 immunization global project known as COVAX®, the WHO has authorized two vaccines, the Sinopharm, Beijing, and Sinovac Corona Vac vaccines, both traditional inactivated viral vaccines, are essential to China’s ambition to immunize most of its inhabitants by 2022[237]. After two dosages, the efficacy rates in clinical trials examining their safety and effectiveness from various regions of the world range from 50% to 91%. Other nations use these vaccinations, including Russia, Turkey, Brazil, Chile, Argentina, Peru, Mexico, Egypt, the UAE, Jordan, Morocco, Indonesia, and Pakistan. Although the range and incidence of adverse effects following the Sinopharm and Sinovac COVID-19 vaccinations are not documented, the methods of manufacture would imply that these vaccines are generally safe and unlikely to cause hepatocellular damage[222,238,239].
Patients with CLDs are particularly susceptible groups to increase the risk of death and more severe types of COVID-19. Many procedures or treatments for this demographic were postponed due to hospital overcrowding or to avoid putting patients at further risk. This population requires specific attention due to their underlying condition. Therefore, for these patients, immunization should also be a top priority. Interestingly, vaccination appears to be safe in stable CLDs[224]. Additionally, immunization priority was given to the high-risk liver disease such decompensated cirrhosis, liver cancer, and liver transplant recipients. They should receive the vaccination faster when their scores are higher. Indeed, the severity of the immune response induced by vaccine in these participants is unknown, and it is anticipated that it will be insufficient given their underlying illnesses and treatments. The mRNA COVID-19 vaccines are especially remarkable since they are expected to have favorable, safe, and effective characteristics in these individuals[240]. Accordingly, to get COVID-19 vaccinations, patients with CLDs receiving medical care do not need to cease their medication. Besides, patients with HCC receiving systemic or locoregional therapy can get the vaccine without interrupting their medical care. Nevertheless, immunization should be postponed until the situation is stabilized in recent disease or fever cases. Intriguingly, immune-related adverse events are a potential outcome of vaccination interactions with immune checkpoint inhibitors, which raises concerns about their usage in patients with certain liver disorders (such as HCC) and calls for more research[241]. Influenza and pneumococcal vaccines are recommended for patients with advanced liver disorders to avoid lower immunogenicity in liver disease patients[242,243].
Despite the lack of long-term safety evidence about liver diseases patients vaccinated by SARS-CoV-2 vaccines, it is crucial to balance the potential benefits of vaccination against any possible risks, especially considering the catastrophic implications of SARS-CoV-2 infection in at-risk groups. When new vaccines are introduced, evaluation of safety and immunological response to immunization in individuals with liver disease should be conducted[244]. National and international perspective registries should start as quickly as possible, ideally without governmental obstacles. Individuals at risk should prioritize SARS-CoV-2 infection prevention by vaccination, given the promising short-term safety results of the recently approved vaccines[245].
Hepatic injuries have been approved to be associated with SARS-CoV-2 infection. Indeed, several factors have been embedded in the pathological forms of SARS-CoV-2 hepatic injuries, including viral tropism, cytokine storm, hypoxia, endothelial cells, and even some drugs that treat COVID-19. In addition, previous studies have proved that pregnant women and neonates with hepatic illness are risky for COVID-19 complications. Due to the fast spread of new SARS-CoV-2 strains, vaccines were administered and developed accordingly. In the present review, we believe that patients with CLDs especially those have severe cirrhosis, liver decompensation, and hepatobiliary cancer should be given a priority to get SARS-CoV-2 immunization. Since it is unknown whether vaccination gives sterilizing immunity and inhibits transmission from asymptomatic patients, preventative measures, such as wearing masks, proper hand washing, and social seclusion, remain of utmost relevance.
| 1. | Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69:635-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 920] [Cited by in RCA: 784] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 2. | Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health? Lancet. 2020;395:542-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 588] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 3. | Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1674] [Cited by in RCA: 1611] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 4. | Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3747] [Cited by in RCA: 3408] [Article Influence: 486.9] [Reference Citation Analysis (1)] |
| 5. | Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 6. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14346] [Article Influence: 2391.0] [Reference Citation Analysis (10)] |
| 7. | Fisher D, Heymann D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | El Zowalaty ME, Järhult JD. From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans - Call for a One Health approach. One Health. 2020;9:100124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. Coronavirus disease 2019 (COVID-19): situation report, 70. [Internet] [accessed 2020]. Available from: https://apps.who.int/iris/handle/10665/331683. |
| 10. | Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3581] [Cited by in RCA: 2894] [Article Influence: 482.3] [Reference Citation Analysis (0)] |
| 11. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17899] [Article Influence: 2983.2] [Reference Citation Analysis (2)] |
| 12. | Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245-e253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 13. | Wong NA, Saier MH Jr. The SARS-Coronavirus Infection Cycle: A Survey of Viral Membrane Proteins, Their Functional Interactions and Pathogenesis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 14. | Marjot T, Eberhardt CS, Boettler T, Belli LS, Berenguer M, Buti M, Jalan R, Mondelli MU, Moreau R, Shouval D, Berg T, Cornberg M. Impact of COVID-19 on the liver and on the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: An updated EASL position paper. J Hepatol. 2022;77:1161-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Hsu RJ, Yu WC, Peng GR, Ye CH, Hu S, Chong PCT, Yap KY, Lee JYC, Lin WC, Yu SH. The Role of Cytokines and Chemokines in Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Front Immunol. 2022;13:832394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 16. | Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M, Castaldo G, Bianco A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung. 2020;198:867-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 352] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 17. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 18. | Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1933] [Cited by in RCA: 2254] [Article Influence: 375.7] [Reference Citation Analysis (1)] |
| 19. | Koshikawa N, Hasegawa S, Nagashima Y, Mitsuhashi K, Tsubota Y, Miyata S, Miyagi Y, Yasumitsu H, Miyazaki K. Expression of trypsin by epithelial cells of various tissues, leukocytes, and neurons in human and mouse. Am J Pathol. 1998;153:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1142] [Cited by in RCA: 1248] [Article Influence: 208.0] [Reference Citation Analysis (0)] |
| 21. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Conceicao C, Thakur N, Human S, Kelly JT, Logan L, Bialy D, Bhat S, Stevenson-Leggett P, Zagrajek AK, Hollinghurst P, Varga M, Tsirigoti C, Tully M, Chiu C, Moffat K, Silesian AP, Hammond JA, Maier HJ, Bickerton E, Shelton H, Dietrich I, Graham SC, Bailey D. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS Biol. 2020;18:e3001016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 23. | Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd, Kato T, Lee RE, Yount BL, Mascenik TM, Chen G, Olivier KN, Ghio A, Tse LV, Leist SR, Gralinski LE, Schäfer A, Dang H, Gilmore R, Nakano S, Sun L, Fulcher ML, Livraghi-Butrico A, Nicely NI, Cameron M, Cameron C, Kelvin DJ, de Silva A, Margolis DM, Markmann A, Bartelt L, Zumwalt R, Martinez FJ, Salvatore SP, Borczuk A, Tata PR, Sontake V, Kimple A, Jaspers I, O'Neal WK, Randell SH, Boucher RC, Baric RS. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182:429-446.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1265] [Cited by in RCA: 1201] [Article Influence: 200.2] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Wang Y, Luo W, Huang L, Xiao J, Li F, Qin S, Song X, Wu Y, Zeng Q, Jin F. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci. 2020;17:1522-1531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 25. | Ahn JH, Kim J, Hong SP, Choi SY, Yang MJ, Ju YS, Kim YT, Kim HM, Rahman MDT, Chung MK, Hong SD, Bae H, Lee CS, Koh GY. Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 26. | World Health Organization. Weekly epidemiological update on COVID-19. [Internet] [accessed 17 August 2022]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. |
| 27. | Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323:1502-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 832] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 28. | Russo A, Minichini C, Starace M, Astorri R, Calò F, Coppola N; Vanvitelli COVID-19 group. Current Status of Laboratory Diagnosis for COVID-19: A Narrative Review. Infect Drug Resist. 2020;13:2657-2665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172:577-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4286] [Cited by in RCA: 3391] [Article Influence: 565.2] [Reference Citation Analysis (2)] |
| 30. | Landete P, Quezada Loaiza CA, Aldave-Orzaiz B, Muñiz SH, Maldonado A, Zamora E, Sam Cerna AC, Del Cerro E, Alonso RC, Couñago F. Clinical features and radiological manifestations of COVID-19 disease. World J Radiol. 2020;12:247-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Tung-Chen Y, Martí de Gracia M, Díez-Tascón A, Alonso-González R, Agudo-Fernández S, Parra-Gordo ML, Ossaba-Vélez S, Rodríguez-Fuertes P, Llamas-Fuentes R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med Biol. 2020;46:2918-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 32. | Yasin R, Gouda W. Chest X-ray findings monitoring COVID-19 disease course and severity. Egyptian Journal of Radiology and Nuclear Medicine. 2020;51:193. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. La radiologia medica. 2020;125:509-513. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 34. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19022] [Article Influence: 3170.3] [Reference Citation Analysis (9)] |
| 35. | Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296:E15-E25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1106] [Cited by in RCA: 954] [Article Influence: 159.0] [Reference Citation Analysis (2)] |
| 36. | Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang XH; , for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team, Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 881] [Cited by in RCA: 1141] [Article Influence: 190.2] [Reference Citation Analysis (1)] |
| 37. | Li Y, Xia L. Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management. AJR Am J Roentgenol. 2020;214:1280-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 654] [Article Influence: 109.0] [Reference Citation Analysis (1)] |
| 38. | Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 914] [Cited by in RCA: 796] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 39. | Alsharif W, Qurashi A. Effectiveness of COVID-19 diagnosis and management tools: A review. Radiography (Lond). 2021;27:682-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 40. | Chu H, Zhou J, Wong BH, Li C, Chan JF, Cheng ZS, Yang D, Wang D, Lee AC, Yeung ML, Cai JP, Chan IH, Ho WK, To KK, Zheng BJ, Yao Y, Qin C, Yuen KY. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J Infect Dis. 2016;213:904-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 41. | Mardani R, Namavar M, Ghorbi E, Shoja Z, Zali F, Kaghazian H, Aghasadeghi MR, Sadeghi SA, Sabeti S, Darazam IA, Ahmadi N, Mousavi-Nasab SD. Association between serum inflammatory parameters and the disease severity in COVID-19 patients. J Clin Lab Anal. 2022;36:e24162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Pu SL, Zhang XY, Liu DS, Ye BN, Li JQ. Unexplained elevation of erythrocyte sedimentation rate in a patient recovering from COVID-19: A case report. World J Clin Cases. 2021;9:1394-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3380] [Article Influence: 563.3] [Reference Citation Analysis (1)] |
| 44. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1184] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 45. | Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 600] [Reference Citation Analysis (0)] |
| 46. | Del Valle R, Ballesteros Á, Calvo C, Sainz T, Mendez A, Grasa C, Molina PR, Mellado MJ, Sanz-Santaeufemia FJ, Herrero B, Calleja L, Soriano-Arandes A, Melendo S, Rincón-López E, Hernánz A, Epalza C, García-Baeza C, Rupérez-García E, Berzosa A, Ocaña A, Villarroya-Villalba A, Barrios A, Otheo E, Galán JC, Rodríguez MJ, Mesa JM, Domínguez-Rodríguez S, Moraleda C, Tagarro A. Comparison of pneumonia features in children caused by SARS-CoV-2 and other viral respiratory pathogens. Pediatr Pulmonol. 2022;57:2374-2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Patel NA. Pediatric COVID-19: Systematic review of the literature. Am J Otolaryngol. 2020;41:102573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 48. | Zhamankulov A, Rozenson R, Morenko M, Akhmetova U, Tyo A, Poddighe D. Comparison between SARS-CoV-2 positive and negative pneumonia in children: A retrospective analysis at the beginning of the pandemic. World J Exp Med. 2022;12:26-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Badal S, Thapa Bajgain K, Badal S, Thapa R, Bajgain BB, Santana MJ. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: A systematic review and meta-analysis. J Clin Virol. 2021;135:104715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 50. | Graff K, Smith C, Silveira L, Jung S, Curran-Hays S, Jarjour J, Carpenter L, Pickard K, Mattiucci M, Fresia J, McFarland EJ, Dominguez SR, Abuogi L. Risk Factors for Severe COVID-19 in Children. Pediatr Infect Dis J. 2021;40:e137-e145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 51. | Zeng QL, Yu ZJ, Ji F, Li GM, Zhang GF, Xu JH, Lin WB, Zhang GQ, Li GT, Cui GL, Wang FS. Dynamic changes in liver function parameters in patients with coronavirus disease 2019: a multicentre, retrospective study. BMC Infect Dis. 2021;21:818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Chang HL, Chen KT, Lai SK, Kuo HW, Su IJ, Lin RS, Sung FC. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105:439-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30479] [Article Influence: 5079.8] [Reference Citation Analysis (13)] |
| 54. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13063] [Article Influence: 2177.2] [Reference Citation Analysis (4)] |
| 55. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2565] [Article Influence: 427.5] [Reference Citation Analysis (2)] |
| 56. | Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36:e3319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 884] [Cited by in RCA: 914] [Article Influence: 152.3] [Reference Citation Analysis (1)] |
| 57. | Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Liu S, Yang JK. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front Public Health. 2020;8:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1408] [Cited by in RCA: 1354] [Article Influence: 225.7] [Reference Citation Analysis (0)] |
| 58. | Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49:15-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 59. | Rubin SJS, Falkson SR, Degner NR, Blish C. Clinical characteristics associated with COVID-19 severity in California. J Clin Transl Sci. 2020;5:e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Hu L, Chen S, Fu Y, Gao Z, Long H, Ren HW, Zuo Y, Wang J, Li H, Xu QB, Yu WX, Liu J, Shao C, Hao JJ, Wang CZ, Ma Y, Wang Z, Yanagihara R, Deng Y. Risk Factors Associated With Clinical Outcomes in 323 Coronavirus Disease 2019 (COVID-19) Hospitalized Patients in Wuhan, China. Clin Infect Dis. 2020;71:2089-2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 61. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14869] [Article Influence: 2478.2] [Reference Citation Analysis (1)] |
| 62. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6575] [Article Influence: 1095.8] [Reference Citation Analysis (0)] |
| 63. | Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin YF, Duan Q, Luo G, Fan S, Lu Y, Feng A, Zhan Y, Liang B, Cai W, Zhang L, Du X, Li L, Shu Y, Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80:656-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 709] [Cited by in RCA: 646] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 64. | Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 65. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 66. | Qian ZP, Mei X, Zhang YY, Zou Y, Zhang ZG, Zhu H, Guo HY, Liu Y, Ling Y, Zhang XY, Wang JF, Lu HZ. [Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 67. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (2)] |
| 68. | Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jiang C, Zhou Y, Liu S, Ye C, Zhang P, Xing Y, Guo H, Tang W. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1186] [Cited by in RCA: 1482] [Article Influence: 247.0] [Reference Citation Analysis (0)] |
| 69. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 70. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 71. | Ali FEM, Mohammedsaleh ZM, Ali MM, Ghogar OM. Impact of cytokine storm and systemic inflammation on liver impairment patients infected by SARS-CoV-2: Prospective therapeutic challenges. World J Gastroenterol. 2021;27:1531-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (3)] |
| 72. | Alqahtani SA, Buti M. COVID-19 and hepatitis B infection. Antivir Ther. 2020;25:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Shokri S, Mahmoudvand S. The possibility of hepatitis C reactivation in COVID-19 patients treated with corticosteroids. Ann Hepatol. 2022;27:100704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Lee JY, Kim YJ, Chung EH, Kim DW, Jeong I, Kim Y, Yun MR, Kim SS, Kim G, Joh JS. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect Dis. 2017;17:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 76. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3853] [Article Influence: 642.2] [Reference Citation Analysis (2)] |
| 77. | Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, Xu W, Zhang C, Yu J, Jiang B, Cao H, Li L. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis. 2020;71:706-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (2)] |
| 78. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 79. | Wang D, Ju XL, Xie F, Lu Y, Li FY, Huang HH, Fang XL, Li YJ, Wang JY, Yi B, Yue JX, Wang J, Wang LX, Li B, Wang Y, Qiu BP, Zhou ZY, Li KL, Sun JH, Liu XG, Li GD, Wang YJ, Cao AH, Chen YN. [Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China]. Zhonghua Er Ke Za Zhi. 2020;58:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 80. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18396] [Article Influence: 3066.0] [Reference Citation Analysis (13)] |
| 81. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6709] [Article Influence: 1118.2] [Reference Citation Analysis (1)] |
| 82. | Stevens JP, Kolachala VL, Joshi GN, Nagpal S, Gibson G, Gupta NA. Angiotensin-converting Enzyme-2 (ACE2) Expression in Pediatric Liver Disease. Appl Immunohistochem Mol Morphol. 2022;30:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 83. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4194] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 84. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 85. | Lax SF, Skok K, Zechner PM, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome. Ann Intern Med. 2021;174:139-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Cai Y, Ye LP, Song YQ, Mao XL, Wang L, Jiang YZ, Que WT, Li SW. Liver injury in COVID-19: Detection, pathogenesis, and treatment. World J Gastroenterol. 2021;27:3022-3036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Xu Y, Cao Z, Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 722] [Article Influence: 120.3] [Reference Citation Analysis (1)] |
| 88. | Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1386] [Cited by in RCA: 1640] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 89. | Benedicto A, García-Kamiruaga I, Arteta B. Neuropilin-1: A feasible link between liver pathologies and COVID-19. World J Gastroenterol. 2021;27:3516-3529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 90. | Kim YM, Jung CH, Seo M, Kim EK, Park JM, Bae SS, Kim DH. mTORC1 phosphorylates UVRAG to negatively regulate autophagosome and endosome maturation. Mol Cell. 2015;57:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 91. | Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, Hannan RD. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23:8862-8877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 361] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 92. | Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (2)] |
| 93. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 94. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 752] [Article Influence: 50.1] [Reference Citation Analysis (1)] |
| 95. | Darif D, Hammi I, Kihel A, El Idrissi Saik I, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb Pathog. 2021;153:104799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 96. | Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E, Charlton M, Watts RJ, Mukhopadhyay D, Shah VH. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J Clin Invest. 2010;120:2379-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 97. | Fiel MI, El Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, Advani R, Kilaru S, Pourmand K, Ward S, Thung SN, Schiano T. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;11:763-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 98. | Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, Bassett J. COVID-19 presenting as fulminant hepatic failure: A case report. Medicine (Baltimore). 2020;99:e22818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 99. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 389] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 100. | Auriti C, De Rose DU, Santisi A, Martini L, Piersigilli F, Bersani I, Ronchetti MP, Caforio L. Pregnancy and viral infections: Mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 101. | Idalsoaga F, Ayares G, Arab JP, Díaz LA. COVID-19 and Indirect Liver Injury: A Narrative Synthesis of the Evidence. J Clin Transl Hepatol. 2021;9:760-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Napodano C, Pocino K, Stefanile A, Marino M, Miele L, Gulli F, Basile V, Pandolfi F, Gasbarrini A, Rapaccini GL, Basile U. COVID-19 and hepatic involvement: The liver as a main actor of the pandemic novel. Scand J Immunol. 2021;93:e12977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Khezri MR, Varzandeh R, Ghasemnejad-Berenji M. The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS-CoV-2 induced coagulopathy. Cell Mol Biol Lett. 2022;27:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 104. | Okamoto H, Ichikawa N. The pivotal role of the angiotensin-II-NF-κB axis in the development of COVID-19 pathophysiology. Hypertens Res. 2021;44:126-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 105. | Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: The anger of inflammation. Cytokine. 2020;133:155151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 106. | Li X, Zhang ZC, Zhang PL. Severe COVID-19 patients with liver injury: a seven-case series. Eur Rev Med Pharmacol Sci. 2020;24:7855-7860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 107. | Amiri-Dashatan N, Koushki M, Ghorbani F, Naderi N. Increased inflammatory markers correlate with liver damage and predict severe COVID-19: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13:282-291. [PubMed] |
| 108. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 109. | Qiu H, Tong Z, Ma P, Hu M, Peng Z, Wu W, Du B; China Critical Care Clinical Trials Group (CCCCTG). Intensive care during the coronavirus epidemic. Intensive Care Med. 2020;46:576-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 110. | Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 111. | Dunn GD, Hayes P, Breen KJ, Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. 1973;265:174-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 147] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 112. | Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39:788-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 113. | Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 709] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 115. | Hu Y, Liu L, Lu X. Regulation of Angiotensin-Converting Enzyme 2: A Potential Target to Prevent COVID-19? Front Endocrinol (Lausanne). 2021;12:725967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 116. | Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 117. | Huang H, Li H, Chen S, Zhou X, Dai X, Wu J, Zhang J, Shao L, Yan R, Wang M, Wang J, Tu Y, Ge M. Prevalence and Characteristics of Hypoxic Hepatitis in COVID-19 Patients in the Intensive Care Unit: A First Retrospective Study. Front Med (Lausanne). 2020;7:607206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-associated liver injury: from bedside to bench. J Gastroenterol. 2021;56:218-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 119. | Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected with Pulmonary CT Angiography. Radiology. 2020;296:E186-E188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 391] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 120. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 1198] [Article Influence: 199.7] [Reference Citation Analysis (0)] |
| 121. | Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1669] [Cited by in RCA: 2076] [Article Influence: 346.0] [Reference Citation Analysis (14)] |
| 122. | Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA. 2020;324:799-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 123. | Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, Dela Cruz CS, Dumont A, Halene S, Hwa J, Koff J, Menninger H, Neparidze N, Price C, Siner JM, Tormey C, Rinder HM, Chun HJ, Lee AI. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575-e582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 824] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 124. | Antunes de Brito CA, de Oliveira Filho JRB, Marques DT, Lencastre MDC, de Almeida JR, Lopes EP. COVID-19 and Hepatic Artery Thrombosis: A Case Report. Am J Case Rep. 2021;22:e932531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 125. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 126. | Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 127. | Lippi G, Sanchis-Gomar F, Henry BM. Coronavirus disease 2019 (COVID-19): the portrait of a perfect storm. Ann Transl Med. 2020;8:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 128. | Barrett TJ, Bilaloglu S, Cornwell M, Burgess HM, Virginio VW, Drenkova K, Ibrahim H, Yuriditsky E, Aphinyanaphongs Y, Lifshitz M, Xia Liang F, Alejo J, Smith G, Pittaluga S, Rapkiewicz AV, Wang J, Iancu-Rubin C, Mohr I, Ruggles K, Stapleford KA, Hochman J, Berger JS. Platelets contribute to disease severity in COVID-19. J Thromb Haemost. 2021;19:3139-3153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 129. | Hadid T, Kafri Z, Al-Katib A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2021;47:100761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 130. | Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 131. | Page EM, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. 2021;200:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 132. | Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 133. | Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (8)] |
| 134. | McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and Liver Injury: Role of Inflammatory Endotheliopathy, Platelet Dysfunction, and Thrombosis. Hepatol Commun. 2022;6:255-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 135. | Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 484] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 136. | Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020;27:3209-3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 137. | Kichloo A, Dettloff K, Aljadah M, Albosta M, Jamal S, Singh J, Wani F, Kumar A, Vallabhaneni S, Khan MZ. COVID-19 and Hypercoagulability: A Review. Clin Appl Thromb Hemost. 2020;26:1076029620962853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 138. | D'Ardes D, Boccatonda A, Cocco G, Fabiani S, Rossi I, Bucci M, Guagnano MT, Schiavone C, Cipollone F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J Gastroenterol. 2022;28:1102-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 139. | McConnell MJ, Kawaguchi N, Kondo R, Sonzogni A, Licini L, Valle C, Bonaffini PA, Sironi S, Alessio MG, Previtali G, Seghezzi M, Zhang X, Lee AI, Pine AB, Chun HJ, Fernandez-Hernando C, Qing H, Wang A, Price C, Sun Z, Utsumi T, Hwa J, Strazzabosco M, Iwakiri Y. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol. 2021;75:647-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 140. | Vitiello A, La Porta R, D'Aiuto V, Ferrara F. The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt Liver J. 2021;11:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 141. | Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291-13295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2135] [Article Influence: 125.6] [Reference Citation Analysis (9)] |
| 142. | Garcia-Cortes M, Robles-Diaz M, Stephens C, Ortega-Alonso A, Lucena MI, Andrade RJ. Drug induced liver injury: an update. Arch Toxicol. 2020;94:3381-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 143. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 331] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 144. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3643] [Article Influence: 607.2] [Reference Citation Analysis (7)] |
| 145. | Wu H, Liu S, Luo H, Chen M. Progress in the Clinical Features and Pathogenesis of Abnormal Liver Enzymes in Coronavirus Disease 2019. J Clin Transl Hepatol. 2021;9:239-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 146. | Ortiz GX, Lenhart G, Becker MW, Schwambach KH, Tovo CV, Blatt CR. Drug-induced liver injury and COVID-19: A review for clinical practice. World J Hepatol. 2021;13:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 147. | LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012 . [PubMed] |
| 148. | Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 149. | Makin AJ, Wendon J, Fitt S, Portmann BC, Williams R. Fulminant hepatic failure secondary to hydroxychloroquine. Gut. 1994;35:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 150. | Cheema B, Triplett D, Krishnamurthy P. 2306 Hydroxychloroquine-Induced Acute Liver Injury. The American Journal of Gastroenterology. 2019;114: S1286. [DOI] [Full Text] |
| 151. | Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB; AGA Institute. Electronic address: ewilson@gastro.org. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159:320-334.e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (1)] |
| 152. | Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, Wurtz N, Rolain JM, Colson P, La Scola B, Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145:104228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 153. | Martinez MA, Vuppalanchi R, Fontana RJ, Stolz A, Kleiner DE, Hayashi PH, Gu J, Hoofnagle JH, Chalasani N. Clinical and histologic features of azithromycin-induced liver injury. Clin Gastroenterol Hepatol. 2015;13:369-376.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 154. | Zhai G, Li M, Wang Y, Wu J. Drug-Induced Liver Disturbance During the Treatment of COVID-19. Front Pharmacol. 2021;12:719308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 155. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2511] [Article Influence: 418.5] [Reference Citation Analysis (0)] |
| 156. | Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1926] [Cited by in RCA: 1899] [Article Influence: 316.5] [Reference Citation Analysis (0)] |
| 157. | Butt AA, Yan P, Chotani RA, Shaikh OS. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int. 2021;41:1824-1831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 158. | Cerbu B, Grigoras ML, Bratosin F, Bogdan I, Citu C, Bota AV, Timircan M, Bratu ML, Levai MC, Marincu I. Laboratory Profile of COVID-19 Patients with Hepatitis C-Related Liver Cirrhosis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 159. | Toma L, Dodot M, Zgura A, Bacalbasa N, Silaghi A, Simu R, Isac T, Mercan-Stanciu A. Calprotectin in viral systemic infections-COVID-19 vs hepatitis C virus. Clin Exp Med. 2022;22:311-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 160. | León FJF, da Silva LL, Santos AC, Duarte da Costa V, Miguel JC, Marques JT, Nascimento GP, Ferreira da Silva E, Lewis-Ximenez LL, Villar LM, de Paula VS. Immunological and virological aspects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and hepatitis C virus. J Med Virol. 2022;94:2296-2301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 161. | Choe JW, Jung YK, Yim HJ, Seo GH. Clinical Effect of Hepatitis B Virus on COVID-19 Infected Patients: A Nationwide Population-Based Study Using the Health Insurance Review & Assessment Service Database. J Korean Med Sci. 2022;37:e29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 162. | Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 163. | Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu, Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (5)] |
| 164. | Wang J, Lu Z, Jin M, Wang Y, Tian K, Xiao J, Cai Y, Zhang X, Chen T, Yao Z, Yang C, Deng R, Zhong Q, Deng X, Chen X, Yang XP, Wei G, Wang Z, Tian J, Chen XP. Clinical characteristics and risk factors of COVID-19 patients with chronic hepatitis B: a multi-center retrospective cohort study. Front Med. 2022;16:111-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 165. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1308] [Article Influence: 218.0] [Reference Citation Analysis (8)] |
| 166. | Librero Jiménez M, López Garrido MÁ, Fernández Cano MC. Letter to the editor: Reactivation of HBV triggered by SARS-CoV-2 in a patient with cirrhosis. Hepatology. 2022;75:765-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 167. | Jindal A. Letter to the Editor: Outcomes in chronic hepatitis B infection and COVID-19-Not always benign! Hepatology. 2022;75:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 168. | Sagnelli C, Montella L, Grimaldi P, Pisaturo M, Alessio L, De Pascalis S, Sagnelli E, Coppola N. COVID-19 as Another Trigger for HBV Reactivation: Clinical Case and Review of Literature. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 169. | Cryer AM, Imperial JC. Hepatitis B in Pregnant Women and their Infants. Clin Liver Dis. 2019;23:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 170. | Rajan M, Sachan S, Abhinay A, Yadav DP, Verma B. Maternal and neonatal outcomes of COVID-19 co-infection in pregnant women with chronic hepatitis B virus infection: A prospective cohort study. Int J Gynaecol Obstet. 2022;158:221-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 171. | Li QY, An ZY, Li C, Zu M, Chen L, Zhang JN, Zhao YY, Shen N, Ge QG. Chronic Active Hepatitis B with COVID-19 in Pregnancy: A Case Report. J Clin Transl Hepatol. 2021;9:133-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 172. | Cui AM, Cheng XY, Shao JG, Li HB, Wang XL, Shen Y, Mao LJ, Zhang S, Liu HY, Zhang L, Qin G. Maternal hepatitis B virus carrier status and pregnancy outcomes: a prospective cohort study. BMC Pregnancy Childbirth. 2016;16:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 173. | Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438-e440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 1083] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 174. | Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 175. | Piffer S, Mazza A, Dell'Anna L. Serological screening for hepatitis C during pregnancy: Seroprevalence and maternal and neonatal outcomes in 45,000 pregnant women. Eur J Obstet Gynecol Reprod Biol. 2020;254:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 176. | Mandel E, Peci A, Cronin K, Capraru CI, Shah H, Janssen HLA, Tran V, Biondi MJ, Feld JJ. The impact of the first, second and third waves of covid-19 on hepatitis B and C testing in Ontario, Canada. J Viral Hepat. 2022;29:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 177. | Wingrove C, James C, Wang S. The impact of COVID-19 on hepatitis services and civil society organisations. Lancet Gastroenterol Hepatol. 2021;6:682-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 178. | Gamkrelidze A, Handanagic S, Shadaker S, Turdziladze A, Tsereteli M, Getia V, Aslanikashvili A, Surguladze S, Gvinjilia L, Kuchuloria T, Tskhomelidze I, Armstrong PA. The impact of COVID-19 pandemic on the 2020 hepatitis C cascade of care in the Republic of Georgia. Public Health. 2022;205:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 179. | Ahmed I, Eltaweel N, Antoun L, Rehal A. Severe pre-eclampsia complicated by acute fatty liver disease of pregnancy, HELLP syndrome and acute kidney injury following SARS-CoV-2 infection. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 180. | Choudhary A, Singh V, Bharadwaj M, Barik A. Pregnancy With SARS-CoV-2 Infection Complicated by Preeclampsia and Acute Fatty Liver of Pregnancy. Cureus. 2021;13:e15645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 181. | Sileo FG, Tramontano AL, Leone C, Meacci M, Gennari W, Ternelli G, LA Marca A, Lugli L, Berardi A, Facchinetti F, Bertucci E. Pregnant woman infected by Coronavirus disease (COVID-19) and calcifications of the fetal bowel and gallbladder. Minerva Obstet Gynecol. 2021;73:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 182. | Ambrož R, Stašek M, Molnár J, Špička P, Klos D, Hambálek J, Skanderová D. Spontaneous liver rupture following SARS-CoV-2 infection in late pregnancy: A case report. World J Clin Cases. 2022;10:5042-5050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (2)] |
| 183. | Ronnje L, Länsberg JK, Vikhareva O, Hansson SR, Herbst A, Zaigham M. Complicated COVID-19 in pregnancy: a case report with severe liver and coagulation dysfunction promptly improved by delivery. BMC Pregnancy Childbirth. 2020;20:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 184. | Anness A, Siddiqui F. COVID-19 complicated by hepatic dysfunction in a 28-week pregnant woman. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 185. | Rabiei M, Soori T, Abiri A, Farsi Z, Shizarpour A, Pirjani R. Maternal and fetal effects of COVID-19 virus on a complicated triplet pregnancy: a case report. J Med Case Rep. 2021;15:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 186. | Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68-89.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 181] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 187. | Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, Manuck TA, Miodovnik M, Sowles A, Clark K, Gyamfi-Bannerman C, Mendez-Figueroa H, Sehdev HM, Rouse DJ, Tita ATN, Bailit J, Costantine MM, Simhan HN, Macones GA; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Disease Severity and Perinatal Outcomes of Pregnant Patients With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol. 2021;137:571-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 188. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14580] [Article Influence: 2430.0] [Reference Citation Analysis (3)] |
| 189. | Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894-904.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2090] [Cited by in RCA: 2258] [Article Influence: 376.3] [Reference Citation Analysis (0)] |
| 190. | Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3079] [Cited by in RCA: 2824] [Article Influence: 470.7] [Reference Citation Analysis (11)] |
| 191. | Lumbers ER, Delforce SJ, Arthurs AL, Pringle KG. Causes and Consequences of the Dysregulated Maternal Renin-Angiotensin System in Preeclampsia. Front Endocrinol (Lausanne). 2019;10:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 192. | Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental Pathology in COVID-19. Am J Clin Pathol. 2020;154:23-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 448] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 193. | Jaiswal N, Puri M, Agarwal K, Singh S, Yadav R, Tiwary N, Tayal P, Vats B. COVID-19 as an independent risk factor for subclinical placental dysfunction. Eur J Obstet Gynecol Reprod Biol. 2021;259:7-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 194. | Bloise E, Zhang J, Nakpu J, Hamada H, Dunk CE, Li S, Imperio GE, Nadeem L, Kibschull M, Lye P, Matthews SG, Lye SJ. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol. 2021;224:298.e1-298.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 195. | Todros T, Masturzo B, De Francia S. COVID-19 infection: ACE2, pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2020;253:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 196. | Tamanna S, Clifton VL, Rae K, van Helden DF, Lumbers ER, Pringle KG. Angiotensin Converting Enzyme 2 (ACE2) in Pregnancy: Preeclampsia and Small for Gestational Age. Front Physiol. 2020;11:590787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 197. | Verma S, Joshi CS, Silverstein RB, He M, Carter EB, Mysorekar IU. SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system. Med (N Y). 2021;2:575-590.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 198. | Seethy AA, Singh S, Mukherjee I, Pethusamy K, Purkayastha K, Sharma JB, Sharma RS, Dhar R, Karmakar S. Potential SARS-CoV-2 interactions with proteins involved in trophoblast functions - An in-silico study. Placenta. 2021;103:141-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 199. | Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, Lopez-Martinez RM, Balcells J, Fernandez-Hidalgo N, Carreras E, Suy A. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 200. | Vlachodimitropoulou Koumoutsea E, Vivanti AJ, Shehata N, Benachi A, Le Gouez A, Desconclois C, Whittle W, Snelgrove J, Malinowski AK. COVID-19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020;18:1648-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 201. | Zhang J, Liu F, Song T, Li Z, Xia P, Tang X, Xu M, Shen Y, Ma J, Liu X, Yu P. Liver Fibrosis Scores and Clinical Outcomes in Patients With COVID-19. Front Med (Lausanne). 2022;9:829423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 202. | Kim SW, Jeon JH, Moon JS, Kim MK. High Fibrosis-4 Index Is Related with Worse Clinical Outcome in Patients with Coronavirus Disease 2019 and Diabetes Mellitus: A Multicenter Observational Study. Endocrinol Metab (Seoul). 2021;36:800-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 203. | Ibáñez-Samaniego L, Bighelli F, Usón C, Caravaca C, Fernández Carrillo C, Romero M, Barreales M, Perelló C, Madejón A, Marcos AC, Albillos A, Fernández I, García-Samaniego J, Calleja JL, Bañares R. Elevation of Liver Fibrosis Index FIB-4 Is Associated With Poor Clinical Outcomes in Patients With COVID-19. J Infect Dis. 2020;222:726-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 204. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a Pooled Analysis. SN Compr Clin Med. 2020;2:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 205. | Hassnine AA, Elsayed AM. COVID-19 in Cirrhotic Patients: Is Portal Vein Thrombosis a Potential Complication? Can J Gastroenterol Hepatol. 2022;2022:5900468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 206. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 896] [Article Influence: 74.7] [Reference Citation Analysis (1)] |
| 207. | An Y, Ma Z, Guo X, Tang Y, Meng H, Yu H, Peng C, Chu G, Wang X, Teng Y, Zhang Q, Zhu T, Wang B, Tong Z, Zhao H, Lu H, Qi X. Comparison of liver biochemical abnormality between COVID-19 patients with liver cirrhosis versus COVID-19 alone and liver cirrhosis alone: A STROBE observational study. Medicine (Baltimore). 2021;100:e25497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 208. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 209. | Luo M, Ballester MP, Soffientini U, Jalan R, Mehta G. SARS-CoV-2 infection and liver involvement. Hepatol Int. 2022;16:755-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 210. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (2)] |
| 211. | Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3332] [Cited by in RCA: 3154] [Article Influence: 525.7] [Reference Citation Analysis (0)] |
| 212. | Leo M, Galante A, Pagnamenta A, Ruinelli L, Ponziani FR, Gasbarrini A, De Gottardi A. Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points. Dig Liver Dis. 2022;54:565-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 213. | Mouch CA, Alexopoulos SP, LaRue RW, Kim HP. Successful liver transplantation in patients with active SARS-CoV-2 infection. Am J Transplant. 2022;22:2694-2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 214. | Martinez-Reviejo R, Tejada S, Cipriano A, Karakoc HN, Manuel O, Rello J. Solid organ transplantation from donors with recent or current SARS-CoV-2 infection: A systematic review. Anaesth Crit Care Pain Med. 2022;41:101098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 215. | Mansoor E, Perez A, Abou-Saleh M, Sclair SN, Cohen S, Cooper GS, Mills A, Schlick K, Khan A. Clinical Characteristics, Hospitalization, and Mortality Rates of Coronavirus Disease 2019 Among Liver Transplant Patients in the United States: A Multicenter Research Network Study. Gastroenterology. 2021;160:459-462.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 216. | Shafiq M, Gibson C. Clinical outcomes of coronavirus disease 2019 in liver transplant recipients. World J Hepatol. 2022;14:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 217. | Akbulut S, Sahin TT, Ince V, Yilmaz S. Impact of COVID-19 pandemic on clinicopathological features of transplant recipients with hepatocellular carcinoma: A case-control study. World J Clin Cases. 2022;10:4785-4798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 218. | Romagnoli R, Gruttadauria S, Tisone G, Maria Ettorre G, De Carlis L, Martini S, Tandoi F, Trapani S, Saracco M, Luca A, Manzia TM, Visco Comandini U, De Carlis R, Ghisetti V, Cavallo R, Cardillo M, Grossi PA. Liver transplantation from active COVID-19 donors: A lifesaving opportunity worth grasping? Am J Transplant. 2021;21:3919-3925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 219. | Rela M, Rajakannu M, Veerankutty FH, Vij M, Rammohan A. First report of auxiliary liver transplantation for severe cholangiopathy after SARS-CoV-2 respiratory infection. Am J Transplant. 2022;22:3143-3145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 220. | Lamb YN. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs. 2021;81:495-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 221. | Wibawa T. COVID-19 vaccine research and development: ethical issues. Trop Med Int Health. 2021;26:14-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 222. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 223. | Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 184] [Reference Citation Analysis (0)] |
| 224. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 11149] [Article Influence: 1858.2] [Reference Citation Analysis (1)] |
| 225. | FDA authorizes Pfizer-BioNTech COVID-19 vaccine. Med Lett Drugs Ther. 2021;63:1-2. [PubMed] |
| 226. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7937] [Article Influence: 1587.4] [Reference Citation Analysis (1)] |
| 227. | Luxi N, Giovanazzi A, Capuano A, Crisafulli S, Cutroneo PM, Fantini MP, Ferrajolo C, Moretti U, Poluzzi E, Raschi E, Ravaldi C, Reno C, Tuccori M, Vannacci A, Zanoni G, Trifirò G; Ilmiovaccino COVID19 collaborating group. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021;44:1247-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 228. | Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1802] [Article Influence: 300.3] [Reference Citation Analysis (0)] |
| 229. | Wu Q, Dudley MZ, Chen X, Bai X, Dong K, Zhuang T, Salmon D, Yu H. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021;19:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 230. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3551] [Cited by in RCA: 3502] [Article Influence: 700.4] [Reference Citation Analysis (0)] |
| 231. | Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, Offergeld K, Scheper G, Taylor KL, Robb ML, Treanor J, Barouch DH, Stoddard J, Ryser MF, Marovich MA, Neuzil KM, Corey L, Cauwenberghs N, Tanner T, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187-2201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2024] [Cited by in RCA: 1876] [Article Influence: 375.2] [Reference Citation Analysis (0)] |
| 232. | Umakanthan S, Chattu VK, Ranade AV, Das D, Basavarajegowda A, Bukelo M. A rapid review of recent advances in diagnosis, treatment and vaccination for COVID-19. AIMS Public Health. 2021;8:137-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 233. | Belete TM. A review on Promising vaccine development progress for COVID-19 disease. Vacunas. 2020;21:121-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 234. | Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: The current state of play. Paediatr Respir Rev. 2020;35:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 235. | Awadasseid A, Wu Y, Tanaka Y, Zhang W. Current advances in the development of SARS-CoV-2 vaccines. Int J Biol Sci. 2021;17:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 236. | COVID-19 Vaccines. 2022 Nov 30. In: Drugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006 . [PubMed] |
| 237. | Doroftei B, Ciobica A, Ilie OD, Maftei R, Ilea C. Mini-Review Discussing the Reliability and Efficiency of COVID-19 Vaccines. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 238. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (2)] |
| 239. | Leise MD, Talwalkar JA. Immunizations in chronic liver disease: what should be done and what is the evidence. Curr Gastroenterol Rep. 2013;15:300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 240. | Härmälä S, Parisinos CA, Shallcross L, O'Brien A, Hayward A. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta-analysis. BMJ Open. 2019;9:e031070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 241. | Sharma A, Patnaik I, Kumar A, Gupta R. COVID-19 Vaccines in Patients With Chronic Liver Disease. J Clin Exp Hepatol. 2021;11:720-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 242. | Ekpanyapong S, Bunchorntavakul C, Reddy KR. COVID-19 and the Liver: Lessons Learnt from the EAST and the WEST, A Year Later. J Viral Hepat. 2022;29:4-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 243. | Moss P, Berenbaum F, Curigliano G, Grupper A, Berg T, Pather S. Benefit-risk evaluation of COVID-19 vaccination in special population groups of interest. Vaccine. 2022;40:4348-4360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 244. | Effenberger M, Grander C, Grabherr F, Griesmacher A, Ploner T, Hartig F, Bellmann-Weiler R, Joannidis M, Zoller H, Weiss G, Adolph TE, Tilg H. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2021;53:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (2)] |
| 245. | Afify S, Eysa B, Hamid FA, Abo-Elazm OM, Edris MA, Maher R, Abdelhalim A, Abdel Ghaffar MM, Omran DA, Shousha HI. Survival and outcomes for co-infection of chronic hepatitis C with and without cirrhosis and COVID-19: A multicenter retrospective study. World J Gastroenterol. 2021;27:7362-7375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (7)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poddighe D, Kazakhstan; Suravajhala PN, India S-Editor: Liu GL L-Editor: A P-Editor: Liu GL