Published online Jul 28, 2023. doi: 10.3748/wjg.v29.i28.4416
Peer-review started: February 23, 2023
First decision: May 16, 2023
Revised: June 5, 2023
Accepted: July 5, 2023
Article in press: July 5, 2023
Published online: July 28, 2023
Processing time: 153 Days and 3.4 Hours
The association between diabetes mellitus (DM) and the increased risk and progression of cholangiocarcinoma (CCA) has been reported with unclear underlying mechanisms. Previous studies showed that γ-aminobutyric acid (GABA) B2 receptor (GABBR2) was upregulated in CCA cells cultured in high glucose (HG) conditions. Roles of GABA receptors in CCA progression have also been studied, but their association with DM and hyperglycemia in CCA remains unclarified.
To investigate the effects of hyperglycemia on GABBR2 expression and the potential use of GABBR2 as a CCA therapeutic target.
CCA cells, KKU-055 and KKU-213A, were cultured in Dulbecco Modified Eagle’s Medium supplemented with 5.6 mmol/L (normal glucose, NG) or 25 mmol/L (HG) glucose and assigned as NG and HG cells, respectively. GABBR2 expression in NG and HG cells was investigated using real-time quantitative polymerase chain reaction and western blot. Expression and localization of GABBR2 in CCA cells were determined using immunocytofluorescence. GABBR2 expression in tumor tissues from CCA patients with and without DM was studied using immunohistochemistry, and the correlations of GABBR2 with the clinicopathological characteristics of patients were analyzed using univariate analysis. Effects of baclofen, a GABA-B receptor agonist, on CCA cell proliferation and clonogenicity were tested using the MTT and clonogenic assays. Phospho-kinases arrays were used to screen the affected signaling pathways after baclofen treatment, and the candidate signaling molecules were validated using the public transcriptomic data and western blot.
GABBR2 expression in CCA cells was induced by HG in a dose- and time-dependent manner. CCA tissues from patients with DM and hyperglycemia also showed a significantly higher GABBR2 expression compared with tumor tissues from those with euglycemia (P < 0.01). High GABBR2 expression was significantly associated with a poorer non-papillary histological subtype but with smaller sizes of CCA tumors (P < 0.05). HG cells of both tested CCA cell lines were more sensitive to baclofen treatment. Baclofen significantly suppressed the proliferation and clonogenicity of CCA cells in both NG and HG conditions (P < 0.05). Phospho-kinase arrays suggested glycogen synthase kinase 3 (GSK3), β-catenin, and the signal transducer and activator of transcription 3 (STAT3) as candidate signaling molecules under the regulation of GABBR2, which were verified in NG and HG cells of the individual CCA cell lines. Cyclin D1 and c-Myc, the common downstream targets of GSK3/β-catenin and STAT3 involving cell proliferation, were accordingly downregulated after baclofen treatment.
GABBR2 is upregulated by HG and holds a promising role as a therapeutic target for CCA regardless of the glucose condition.
Core Tip: Diabetes mellitus is associated with an increased risk and progression of cholangiocarcinoma (CCA). The γ-aminobutyric acid (GABA) B2 receptor (GABBR2) was upregulated in CCA cells cultured in high glucose and in CCA tissues from patients with hyperglycemia. High GABBR2 expressions were significantly correlated with a non-papillary histotype and smaller sizes of CCA tumors. The treatment of baclofen, a GABA-B receptor agonist, significantly suppressed CCA cell proliferation and clonogenicity, suggesting that GABBR2 is a potential target for CCA treatment. Baclofen inhibited multiple kinases and signal transducers in CCA, resulting in downregulated downstream target proteins involving cell proliferation and suppression of CCA cell growth.
- Citation: Saengboonmee C, Sorin S, Sangkhamanon S, Chomphoo S, Indramanee S, Seubwai W, Thithuan K, Chiu CF, Okada S, Gingras MC, Wongkham S. γ-aminobutyric acid B2 receptor: A potential therapeutic target for cholangiocarcinoma in patients with diabetes mellitus. World J Gastroenterol 2023; 29(28): 4416-4432
- URL: https://www.wjgnet.com/1007-9327/full/v29/i28/4416.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i28.4416
Diabetes mellitus (DM) is a global public health problem, and its incidence is increasing every year[1]. The associations between DM and cancers have been recognized for decades from epidemiological observations[2,3]; however, the molecular mechanisms underlying the linkage are not fully elucidated. Liver cancers, including hepatocellular carcinoma and cholangiocarcinoma (CCA), are among the high-risk malignancies developing in patients with DM[4-6]. DM is not only associated with an increased risk of CCA but also associated with a poor survival of patients who have a poorly controlled blood glucose level[7]. Individuals with DM infected with Opisthorchis viverrine (O. viverrini), a carcinogenic liver fluke, are at a greater risk of developing CCA compared to those either with liver fluke infection or having DM alone[8]. The hamster CCA carcinogenesis model also showed that hamsters with DM and O. viverrini infection had more hepatic morbidities compared with those infected with O. viverrini or having DM alone[9]. CCA once developed, itself, has a poor prognosis, and the 5-year survival rate of all types is approximately 10%[10]. The attempts to investigate novel medications, e.g., targeted therapy[11] or immunotherapy[12-14], reported limited efficacy or benefit for a particular population. Other underlying factors of patients have been reported to influence the progression of CCA. In line with this, the study in patients with CCA who have DM also reported that DM is associated with a shorter overall survival and is an independent prognostic factor for patients with CCA[7].
Previous studies by the current authors found that diabetogenic glucose concentration is a promoting factor for CCA aggressiveness[15,16]. High glucose (HG) promotes CCA proliferation and metastatic potential by regulating several signaling pathways, such as the signal transducer and activator of transcription 3 (STAT3)[15,17] and the nuclear factor-kappa B pathways[18]. HG also promotes cell cycle progression by enhancing cell cycle machinery proteins, namely, cyclin D1, cyclin E, cyclin A, and cyclin-dependent kinase 2, suggesting potential targets for CCA treatment in patients with DM[19]. Transcriptomic analysis of CCA cells cultured in HG vs normal glucose (NG) also showed several potential targets for CCA under diabetogenic glucose conditions. Among the top 5 upregulated genes, γ-aminobutyric acid (GABA) B2 receptor (GABBR2) is one of the promising therapeutic targets that are striking[18]. Given that GABBR2 is upregulated in CCA cells cultured in HG, and both agonist and antagonist of this receptor are clinically available, GABBR2 is thus a great potential target for drug repurposing for CCA treatment.
GABBR2 is a G-protein coupled receptor for GABA, an inhibitory neurotransmitter abundantly found in the central nervous system[20-22]. GABBR2 is also expressed in the gastrointestinal tract and is speculated to be important for the differentiation of several gastrointestinal epithelial cells[23,24], including cholangiocytes[25]. The roles of GABBR2 outside the central nervous system, however, are not fully understood. As a G-protein coupled receptor, GABBR2 can interact with various adaptor proteins and then signal the different downstream pathways[23]. Previous studies in CCA tissues showed that only the GABA-B receptor was differentially expressed between CCA cells and adjacent normal cholangiocytes among all subtypes of receptors (GABA-A, GABA-B, and GABA-C receptors)[26], suggesting its potential as a therapeutic target. Treatment of CCA cells with GABA showed the suppression of growth and invasive ability[26-28]. Which GABA-B receptor subtypes are responsible for the suppression of CCA aggressiveness, however, remains unclear. In contrast, in some cancers, GABA exerted pro-tumor effects on cancer cells[29], and activating the GABA-B receptor can promote the migration of cancer cells[30]. These controversial findings and the finding that HG enhanced the expression of GABBR2 in CCA cells have led to the investigation of the roles of GABBR2 in CCA under diabetic conditions. Discovering the significance of GABBR2 in CCA, especially in patients with DM, might suggest the opportunities to improve the therapeutic outcome of CCA by repurposing GABA-B receptor agonists available in clinical practice.
CCA tissues were obtained from patients who underwent surgical resection at Srinagarind Hospital, Faculty of Medicine, and archived at the Cholangiocarcinoma Research Institute, Khon Kaen University. Clinical and pathological data, including preoperative fasting blood glucose (FBG) and diabetes status, were retrieved from the medical records of Srinagarind Hospital. Patients with DM and preoperative FBG ≥ 126 mg/dL were defined as having DM, while those who had FBG < 126 mg/dL were defined as having no DM[31]. Written informed consent was obtained from all participants. The protocol of the study has been reviewed and approved by the Khon Kaen University Ethics Committee for Human Research (approval No. HE641441), based on the Declaration of Helsinki and the ICH-Good Clinical Practice Guidelines.
CCA cell lines, KKU-055 and KKU-213A, were established from Thai CCA patients[32] and obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). Cells were cultured in Dulbecco Modified Eagle’s Medium (DMEM, Gibco, Carlsbad, CA), supplemented with 10% fetal bovine serum (Gibco) and 1% antibiotic-antimycotic (Gibco). Cells were cultured in DMEM containing 5.6 mmol/L glucose (NG) and 25 mmol/L (HG) for at least 5 passages and assigned as NG and HG cells, respectively[15,18]. Cells were subcultured when the confluence reached 80%. HG-induced GABBR2 expression was done by sequentially culturing NG cells in DMEM with 5.6, 15, and 25 mmol/L glucose for at least 5 passages, and cell lysates were collected timely for the experiments.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to determine GABBR2 mRNA expression in CCA cells. Confluent CCA cells were lysed using TRIzol (ThermoFisher Scientific, Waltham, MA), and RNA extraction was performed according to the manufacturer recommendations. Total RNA was reverse-transcribed using the MultiScribe Reverse Transcriptase Kit (ThermoFisher Scientific) and qPCR was performed using LightCycler® 480 System (Roche Diagnostics, Rotkreuz, Switzerland). Primers for GABBR2 are: Forward, 5’-TGGAGGCGTCTGTCCATCCGT-3’ and reverse, 5’-GTCTTGCGTCAGCGTGCCCA-3’. β2-microglobulin was used as the internal control, and the primer sequences are: Forward, 5’-AAGATGAGTATGCCTGCCG-3’ and reverse, 5’-CGGCATCTTCAAACCTCC-3’.
Primary antibodies used to detect the proteins by western blot were: GABBR2 (1:500, Proteintech, Rosemont, IL), pSTAT3 (Y705) (1:500, Cell Signaling Technology, Danvers, MA), pSTAT3 (S727) (1:500, Cell Signaling Technology), STAT3 (1:1000, Cell Signaling Technology), p-glycogen synthase kinase 3 (GSK3)α/β (1:1000, Cell Signaling Technology), GSK3α/β (1:1000, Cell Signaling Technology), β-catenin (1:1000, Cell Signaling Technology), cyclin D1 (1:1000, Cell Signaling Technology), c-Myc (1:500, Santa Cruz Biotechnology, Dallas, TX), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:10000, Millipore Sigma, Burlington, MA).
Cell lysates were collected in radioimmunoprecipitation assay (RIPA) lysis buffer containing protease inhibitor (Sigma Aldrich, St. Louis, MO) and phosphatase inhibitor (Sigma Aldrich, St. Louis, MO). NG and HG cells of both cell lines were lysed after culture in their respective media at 72 h after incubation for determination of GABBR2 expression. Cells treated with baclofen (Sigma) were collected in the lysates at 24, 48, and 72 h after incubation with the drug. Total proteins (20 μg/well) were electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Amersham-Hybond® polyvinylidene fluoride membrane (GE Healthcare, Buckinghamshire, United Kingdom). After blocking with 5% skim milk in Tris-buffered saline with 0.1% Tween-20 for 1 h at room temperature, the membranes were then incubated with the primary antibodies at 4 °C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Enhanced Chemiluminescence kit (Millipore Sigma, Burlington, MA) was applied to develop the signals, which were detected using ImageQuant® LAS 400 (GE Healthcare, Uppsala, Sweden). Band intensities of proteins were quantified using Image J (National Institute of Health, Bethesda, MD), in which the intensities of GAPDH were used as the loading controls.
CCA (NG and HG) cells were seeded into 48 well plates at a density of 5000 cells/well and incubated for 72 h. After removing media and washing with phosphate-buffered saline, cells were fixed using 4% paraformaldehyde for 30 min at room temperature, and non-specific antigens were blocked using 5% skim milk. Anti-GABBR2 antibody (1:200) was then incubated at 4 °C overnight, and Alexa-Fluo-488 conjugated secondary antibody (Invitrogen, Waltham, MA) was applied and incubated at room temperature for 1 h. Fluorescent signals were detected using a fluorescent microscope (Nikon Ti-U Inverted Fluorescence Microscope, 20X) using NIS-Elements imaging software. Mean fluorescent intensities were calculated using Image J (National Institute of Health).
Immunohistochemical staining for GABBR2 was performed according to the standard immunohistochemistry protocol. Briefly, paraffin-embedded formalin-fixed CCA tissues (6 μm thickness) were rehydrated using serial-graded ethanol. Antigens were retrieved by heating the sample in 0.1 M citrate buffer (pH = 6.0) in a pressure cooker for 5 min. Endogenous peroxidase was blocked using 3% H2O2 in methanol, and non-specific antigens were blocked using 1% fetal bovine serum (Gibco). CCA tissues were then incubated with anti-GABBR2 antibody (1:100, Abcam, Cambridge, MA) at room temperature overnight in a moisture chamber. In the DAKO EnVision™ + System, horseradish peroxidase-conjugated secondary antibody (Dako, Carpinteria, Denmark) was then applied and further incubated at room temperature for 1 h. The signals were developed using 3,3’-diaminobenzidine (DAB, Sigma, St. Louise, MO) and graded as the IHC index (intensity × frequency; where intensity: No staining = 0, mild = 1, moderate = 2, strong =3; and frequency: 0% = 0, 0%-25% = 1+, 25%-50% = 2+, > 50% = 3+), by two researchers (CS and SaS). Microscopic photographs were obtained using Nikon NIS-Elements software (Nikon, Tokyo, Japan). High and low expression of GABBR2 in CCA tissues was classified by using a median score of IHC index as a cut-off point.
CCA (NG and HG) cells (1 x 103 cells/well) were seeded into 96 well plates and incubated overnight, and then cells were incubated with complete media containing varied concentrations of baclofen (Sigma), a GABA-B receptor agonist, for 72 h. Then, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Invitrogen, Carlsbad, CA) was added at a final concentration of 0.5 mg/mL and further incubated for 4 h in a 37 °C incubator. Formazan crystals were dissolved in dimethyl sulfoxide, and OD540 was measured using a microplate reader (Tecan, Männedorf, Switzerland).
To determine the possible time-dependent effect of the GABA-B receptor agonist, NG and HG cells of KKU-055 were incubated with 1000 μM baclofen, and KKU-213A cells were incubated with 800 μM baclofen for 24, 48, and 72 h. MTT assay was then performed at each time point to determine the effect of baclofen on CCA cell growth in different glucose conditions.
NG and HG cells of KKU-055 and KKU-213A in control media or media containing 800 μM baclofen were seeded into a 24-well plate at a density of 200 cells/well. Cells were allowed to grow and form the colonies for 7 d before being fixed with 4% paraformaldehyde. The colonies were stained with 0.5% crystal violet and counted under a light microscope. Only a cluster with > 50 cells was counted as a colony.
The Proteome Profiler Human Phospho-Kinase Array Kit (#ARY003B, R&D system, Minneapolis, MN) was used to screen the signaling pathways affected by baclofen. HG cells (5 × 105 cells) of KKU-213A were seeded into 6-cm dishes and allowed to adhere overnight. Then, cells were incubated with 800 μM baclofen for 24 h. Cell lysates (control and treatment) were collected using RIPA buffer containing protease and phosphatase inhibitors. Then, 200 μg of total proteins for each group were incubated with the membrane arrays overnight following the recommendation of the manufacturer. The membranes were then incubated with a cocktail-detection antibody and streptavidin-horseradish peroxidase. The signals were detected with Chemireagent provided in the same kit and quantified using an ImageQuant® LAS 400 (GE Healthcare). The candidate pathways were then selected and verified in individual cell lines cultured in different glucose conditions by western blot.
The GEO datasets were retrieved using GEO2R[33]. The GSE89749 (n = 120)[34] dataset of Thai CCA cases was used for the analysis of the correlation between the expression levels of GABBR2, STAT3, GSK3B, CTNNB1, MYC, and CCND1 using Pearson’s correlation coefficient.
All quantitative data, presented as the mean ± SD, were analyzed using student’s t-test, one-way ANOVA, or two-way ANOVA with Tukey’s multiple comparisons using IBM SPSS Statistics for Windows, version 28.0 (IBM, Armonk, NY). Univariate analysis was performed using the Chi-square test or Fisher’s Exact test regarding the assumption of each model. Data visualization was done using GraphPad Prism 9 (Dotmatics, San Diego, CA). Statistical significance was considered at P < 0.05.
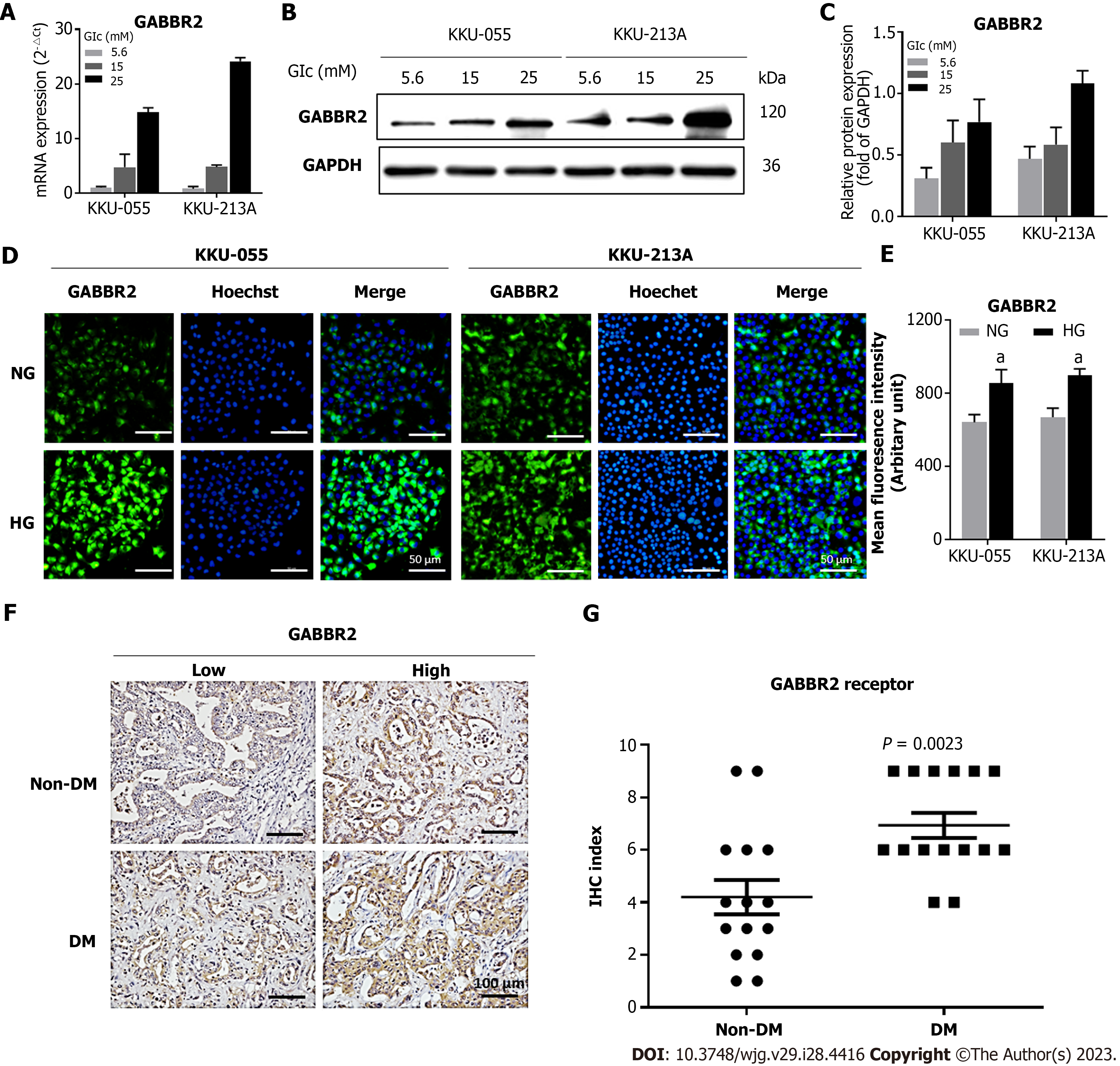
From the previous comparative transcriptomic analysis, GABBR2 is one of the top 5 upregulated genes in KKU-213A cells cultured in HG compared to those cultured in NG conditions[18]. To verify the results from the transcriptomics, determination of GABBR2 expression in different CCA cell lines was performed. GABBR2 expression was dose-dependently upregulated by various glucose concentrations ranging from 5.6 mmol/L to 25 mmol/L at both mRNA (Figure 1A) and protein levels (Figures 1B and C) in both KKU-055 and KKU-213A cells. As the expression of GABBR2 in both CCA cell lines was significantly different between those cultured in 5.6 mmol/L and 25 mmol/L (P < 0.05), these two glucose conditions were included in a further study and assigned as NG and HG cells, respectively.
Immunocytofluorescent staining for GABBR2 in both cell lines showed that GABBR2 was localized at membranous and cytoplasmic compartments of CCA cells (Figure 1D). Mean fluorescent intensities of GABBR2 in HG cells of both cell lines were significantly higher than those in NG cells (P < 0.05) (Figure 1E) and were concordant with the expression levels determined by RT-qPCR and western blot.
To affirm that HG-induced GABBR2 expression in CCA cell lines is translatable to patients with CCA, the tumor tissues from patients with pre-operative hyperglycemia (FBG ≥ 126 mg/dL) and euglycemia (FBG < 126 mg/dL) were studied. Immunohistochemistry of CCA tissues revealed that GABBR2 was significantly upregulated in tumor tissues from patients with DM who had hyperglycemia compared to those without DM (P < 0.01) (Figures 1F-G). Cytoplasmic localization of GABBR2 was observed similarly to that found in CCA cell lines.
To investigate the clinical significance of GABBR2 expression, the associations between GABBR2 expression in CCA tissues and the clinicopathological characteristics of patients were analyzed by univariate analysis. High GABBR2 expression was significantly associated with a non-papillary histological type of CCA (P < 0.05). In contrast, high GABBR2 expression was significantly associated with CCA tumor sizes smaller than 7 cm in the longest diameter (P < 0.05) (Table 1), suggesting that GABBR2 is a potential target for CCA treatment.
| Clinicopathological data | GABBR2 expression | P value | |
| High (IHC index ≥ 8) | Low (IHC index < 8) | ||
| Diabetic status | |||
| DM | 13 | 2 | 0.109 |
| Non-DM | 8 | 7 | |
| Sex | |||
| Male | 10 | 6 | 0.580 |
| Female | 8 | 3 | |
| Survival | |||
| ≥ 5 mo | 14 | 6 | 1.000 |
| < 5 mo | 7 | 3 | |
| Histological type | |||
| Papillary | 4 | 6 | 0.030a |
| Non-papillary | 17 | 3 | |
| Histological grading | |||
| Well differentiated | 13 | 7 | 0.672 |
| Moderately differentiated | 5 | 1 | |
| Poorly differentiated | 3 | 1 | |
| Tumor size (longest diameter) | |||
| > 7 cm | 6 | 7 | 0.020a |
| ≤ 7 cm | 15 | 2 | |
| Regional lymph node involvement | |||
| Yes | 10 | 8 | 0.543 |
| No | 8 | 4 | |
| Distant metastasis | |||
| Metastasis | 14 | 4 | 0.418 |
| Non-metastasis | 7 | 5 | |
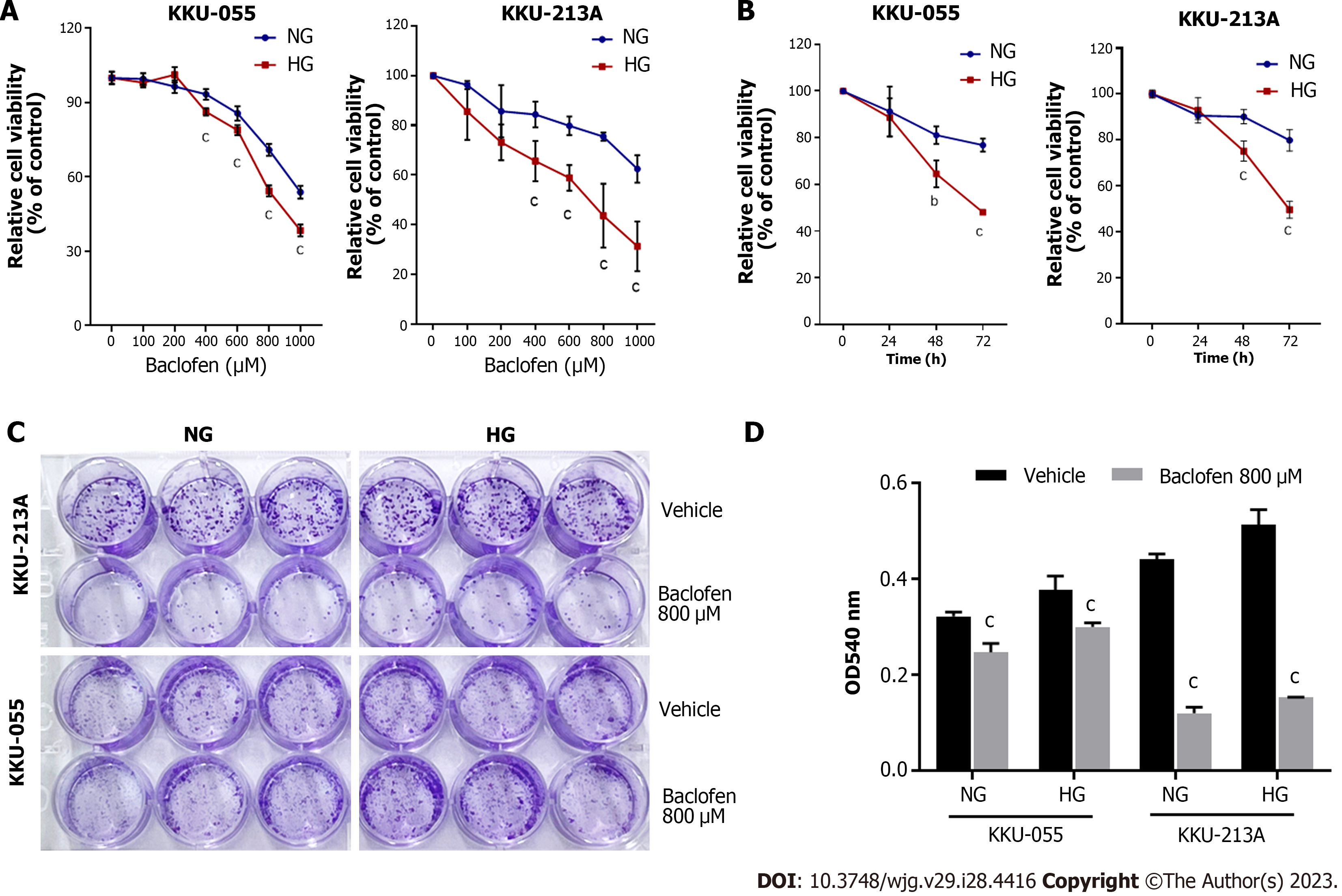
As GABBR2 was upregulated in HG cells and CCA tissues from patients with hyperglycemia, the therapeutic potential of using GABBR2 as a target was investigated. Baclofen, a GABA-B receptor agonist, was applied in NG and HG cells of both CCA cell lines with various concentrations and time points. The treatment of baclofen did not alter the expression levels of GABBR2 in both NG and HG cells (data not shown). The proliferation of both NG and HG cells of KKU-055 and KKU-213A was significantly suppressed in a dose- and time-dependent manner, compared with the control group (Figures 2A and B). HG cells of both cell lines showed a significantly higher sensitivity to baclofen treatment at every dosage of baclofen and at 48 and 72 h of the incubation (Figures 2A and B).
Baclofen treatment not only suppressed the proliferation of CCA cells, but the ability to form colonies was also inhibited. Clonogenicity of both NG and HG cells of KKU-055 and KKU-213A was significantly inhibited by baclofen treatment compared with the control group (Figures 2C and D).
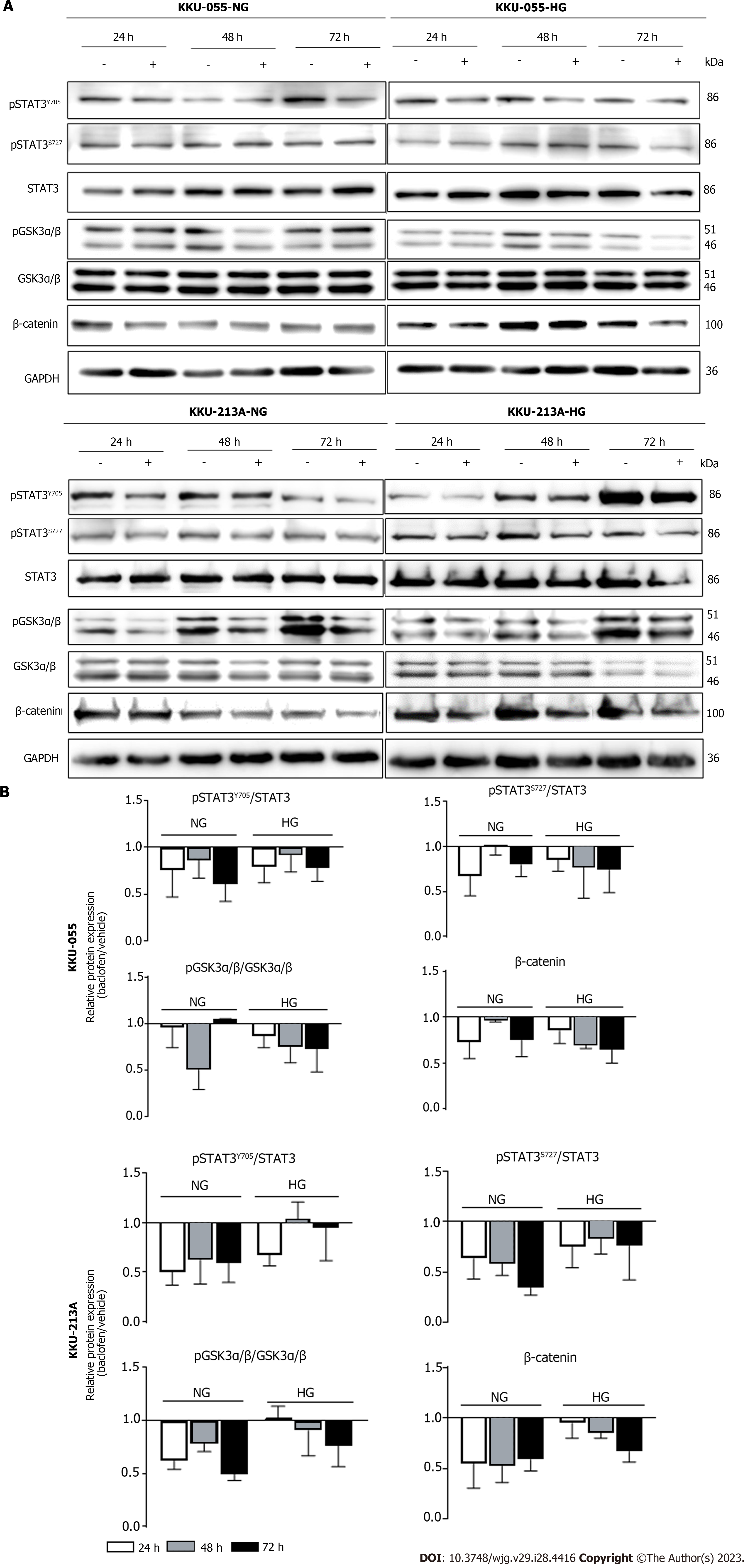
To unveil the underlying mechanisms in which baclofen inhibits the proliferation of CCA cells, the phospho-kinase arrays were used to screen HG cells of KKU-213A with and without baclofen treatment. Phosphorylation of several kinases and signal transducers was suppressed in KKU-213A-HG after being treated with baclofen (Figures 3A and B). The kinases and signal transducers belonging to the same pathways and reported for their roles in CCA progression were prioritized and included for further verification in downstream experiments. By these criteria, GSK3α/β, β-catenin, and STAT3 were then included for validation in the individual CCA cell lines in different glucose conditions.
The associations between GABBR2 and its signal transducers suggested by the phospho-kinase arrays were first verified for their mRNA expression using public transcriptomic databases. The expression of GABBR2 at the RNA level was significantly correlated with the expression of STAT3 in a transcriptomic analysis of clinical CCA samples from Thai patients (P < 0.05) (Figure 3C).
The effects of baclofen on the STAT3 and GSK3α/β/β-catenin signaling pathways were then validated in the individual CCA cell lines, both in NG and HG cells. Baclofen suppressed STAT3 phosphorylation at both Y705 and S272 positions and NG cells were affected the most in both cell lines. Phosphorylation of GSK3α/β was also decreased time-dependently in HG cells, and the levels of β-catenin were then accordingly decreased in both cell lines. The GSK3α/β/β-catenin signaling pathway was also inhibited in NG cells of both cell lines as shown by the decreased phosphorylation levels of GSK3α/β. Total β-catenin proteins, the GSK3α/β downstream target, were also decreased starting 24 h after treatment (Figures 4A and B).
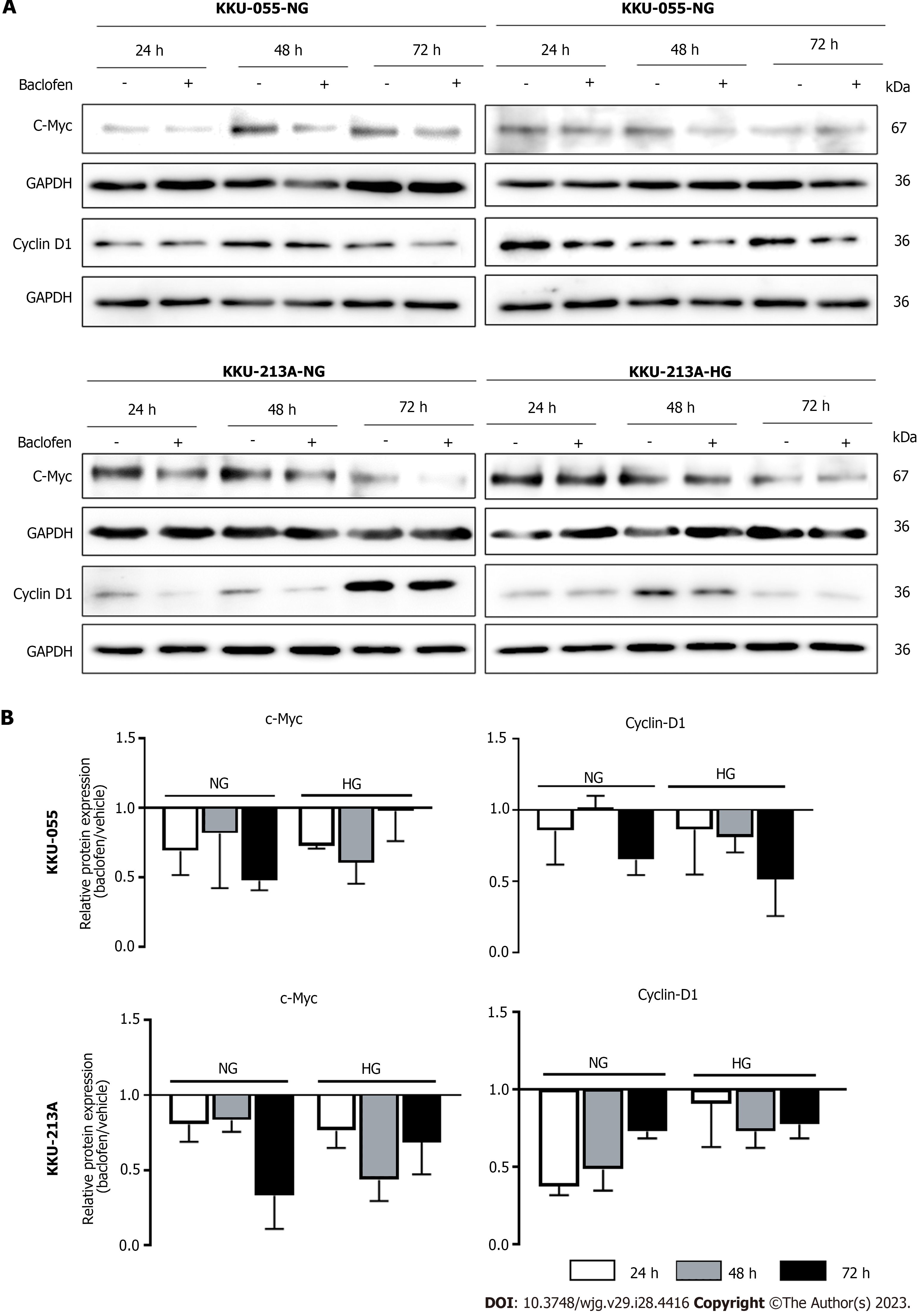
According to the antiproliferative effects of baclofen via the suppression of GSK3α/β/β-catenin and STAT3 pathways, the common downstream targets of both pathways functioning in cell proliferation were then explored. Expression of c-Myc, a transcription factor involved in cell proliferation, and cyclin D1, a cell cycle regulatory protein, was examined for their correlation with GABBR2 at the mRNA and protein levels after baclofen treatment. Analyzing a public transcriptomic dataset, mRNA expression levels of MYC (encoding for c-Myc) and CCND1 (encoding for cyclin D1) were not correlated with mRNA level of GABBR2 (Supplementary Figure 1). However, both c-Myc and cyclin D1 expression at the protein level were suppressed at every time point after baclofen treatment in NG and HG cells of both CCA cell lines. Suppression of c-Myc was greater affected at the early time point in which cyclin D1 was gradually affected time-dependently in HG cells. The time-dependent suppression of c-Myc in NG cells after baclofen treatment was also observed (Figures 5A and B).
HG and hyperglycemia-induced aggressiveness of CCA cells has been reported with partially understood mechanisms[15,16,18,19]. The present study not only revealed the mechanisms by which HG activates the STAT3 and GSK3/β-catenin pathways, but also identified GABBR2 as the upstream receptor that is promising for CCA treatment, especially in patients with DM. Roles of GABA and its receptor have been studied in several types of cancer, including non-liver fluke-associated CCA[26-28,35]. The known mechanisms of actions of GABA and its receptor on CCA are by regulating several signaling pathways, e.g., STAT3 and protein kinase A. This study, however, determined for the first time the association between hyperglycemia and the expression of GABBR2, and also identified GSK3/β-catenin signaling, an uncovered downstream pathway, that is modulated by GABBR2 in CCA. Roles of GABBR2 in the progression of CCA in both in vitro models and in the tumor tissues of CCA patients were also reported.
These current investigations first identified that GABBR2, a subtype of GABA-B receptor, was upregulated in CCA cells cultured in HG. This is of particular importance since approximately 60% of patients with CCA in Thailand had their blood glucose levels in a range of pre-DM and DM[36,37]. Targeting GABBR2 would then be beneficial to a substantial group of CCA patients. Univariate analysis revealed that high expression of GABBR2 in tumor tissues was associated with a poorer histological subtype of CCA. High GABBR2 expression was associated with smaller tumor sizes compared with the group with low GABBR2 expression. These findings are in agreement with previous reports demonstrating that both high GABA-A or GABA-B receptors are associated with better prognostic outcomes for CCA patients[26,38]. The upregulated GABBR2 in CCA tissues of patients thus suggested a therapeutic strategy by using a natural GABA or even GABA receptor agonists. Patients with DM who have upregulated GABBR2 would, therefore, potentially benefit from targeting GABBR2 with available GABA or GABA analogs. The present study also found that HG cells of CCA with upregulated GABBR2 were more sensitive to baclofen, a GABA-B receptor agonist. This affirms the advantage of using GABA-B agonist as an add-on therapy for CCA treatment in patients with DM. As shown by western blot, the investigated signaling molecules were markedly suppressed in both NG and HG cells. This might be the result of the screening and recruitment of candidate pathways that were only from the HG cells whereas the signaling molecules in NG cells affected by baclofen were not screened and compared. Regardless of the glucose condition of the cell cultures, these results still verified that baclofen is effective for CCA treatment and is probably useful for CCA treatment in both patients with and without DM. As the available standard and developing treatment for CCA is of limited efficacy and needs more investigation[10-13], the findings in the present work may add value for alternative treatment of CCA. Especially, baclofen is a known drug in clinical practice in which its toxicity and therapeutic windows are known[39]. This medication is, therefore, highly promising for a repurposing aim. Of note, this study showed for the first time that a GABA receptor agonist is an alternative strategy to natural GABA treatment for CCA.
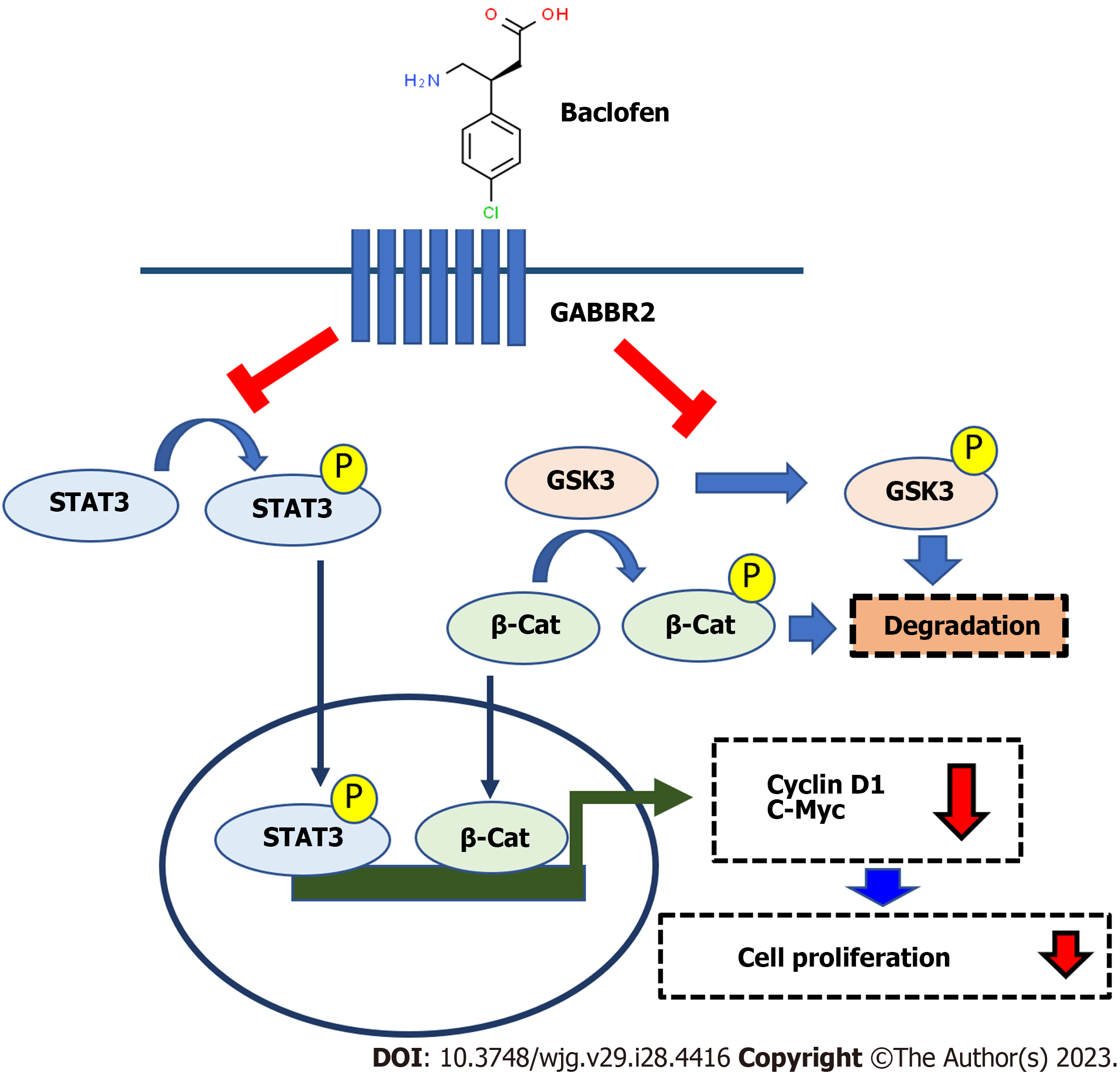
Suppression of GSK3/β-catenin, a new intracellular signaling mode under the regulation of GABBR2 in CCA, is shown in addition to the known affected STAT3 pathway[26]. Both GSK3/β-catenin and STAT3 are reported as crucial signaling pathways for CCA carcinogenesis and progression[40,41]. GSK3 is a kinase phosphorylating β-catenin for proteasomal degradation. The functions of GSK3 are inhibited by phosphorylation by its kinase regulators[42], hence decreased phosphorylated GSK3α/β in CCA cells after the baclofen treatment indicated the active state of GSK3. This is also evidenced by the decreased β-catenin, a GSK3 downstream target which is a known transcription factor for cell proliferation. Moreover, STAT3 phosphorylation was also inhibited after baclofen treatment, which is consistent with the previous findings in CCA cells treated with GABA. STAT3 has also been reported as an alternative downstream target of GSK3 phosphorylation[43]. The decreased levels of β-catenin and STAT3 phosphorylation in the present report might thus be a consequence of GSK3 inhibition by a GABBR2 agonist. As β-catenin and STAT3 control cell proliferation via the expression of c-Myc and cyclin D[44,45], these two common effector-proteins were also down-regulated after baclofen treatment. These suggested that the activation of GABBR2 protein by its natural or synthetic ligands might be a crucial step for the regulation of downstream signaling, although there is no correlation among GABBR2, MYC, and CCND1 at the mRNA level. These findings not only add to the understanding of the molecular linkages among DM, hyperglycemia, and CCA progression but also imply the therapeutic strategies of the combined targeting multiple signaling pathways to improve CCA treatment outcomes. The schematic summary of the findings from the current study is depicted in Figure 6.
GABA is an inhibitory neurotransmitter mostly found in the central nervous system. It is a derivative of the non-essential amino acid glutamate and can be synthesized by converting glutamine as a precursor[29]. GABA functions by binding to its receptors which have three subtypes (GABA-A, GABA-B, and GABA-C receptors). Different GABA receptors are responsible for various physiological functions both within and outside the nervous system. While the GABA-A receptors are found to be important for hepatocytes[46], GABA-B receptors are also needed for the differentiation of biliary epithelial cells[25]. In addition to physiological roles, GABA and its receptors are also reported for both pro-tumor[29,30] and anti-tumor effects[28,47], depending on the cancer type. The previous study showed that all subtypes of GABA receptors were expressed in CCA cells[28]. Still, only GABA-B receptor was differentially expressed between CCA cells and normal adjacent cholangiocytes in tumor tissues from patients[26]. The GABA-B receptor then holds a high potential for targeting in CCA treatment. Treatment of GABA in CCA cells exerted antiproliferative[28] and anti-invasive effects[35] on CCA cells by inhibiting multiple pathways, i.e., cyclic AMP–dependent regulation of the protein kinase A/extracellular signal-regulated kinase (PKA/ERK)[27,28] and STAT3[26,38] pathways. Low GABA-A and GABAB-B receptor expression was also associated with a poor prognosis in CCA patients[38]. The roles of GABA receptors under hyperglycemia in CCA patients with DM are for the first time reported in the present study. The study of a GABA-B receptor agonist, instead of using natural GABA, is also reiterated and suggested for a potential translation to a clinical study.
This study, nevertheless, has some limitations. First, all CCA tissues and CCA cell lines used in the study were derived from O. viverrini-associated CCA patients. As different molecular backgrounds of CCA in different areas have been noted[48,49], the current findings may be needed to verify in CCA from the non-endemic areas of liver fluke. Noteworthy, the correlations between GABBR2 and STAT3, GSK3B, and CTNNB1, were not observed in the analysis using a dataset from The Cancer Genome Atlas which included non-liver fluke-associated CCA[50].
Second, although the in vivo effect of natural GABA on CCA growth has been reported in several studies, the treatment of GABA receptor agonists in the animal model remains limited. A further in vivo study of GABA-B receptor agonists at an optimal therapeutic dosage in animal models should be certified. Third, the association of GABBR2 expression and nuclear localization of its downstream transcription factor, namely, β-catenin, needs further proof in clinical samples using appropriate techniques. Lastly, a combination of several modalities as the therapeutic regimen for CCA is recommended. The study of combining GABA-B receptor agonists with another standard or alternative modality will help translate these findings to clinical practice, especially in the treatment of CCA patients with DM who are likely suffering from a poor prognosis.
GABBR2 is upregulated by HG in both CCA cell lines and tumor tissues from patients. Targeting GABBR2 with GABA-B receptor agonists shows the potential of using GABBR2 as a therapeutic target and repurposing GABA-B receptor agonists for CCA treatment, especially for those patients with DM and hyperglycemia.
The association between diabetes mellitus (DM) and cholangiocarcinoma (CCA) progression has been established with unclear mechanisms. Our previous study showed that γ-aminobutyric acid B2 receptor (GABBR2) is among the top 5 upregulated genes in CCA cells cultured in high glucose (HG). Thus, GABBR2 is highly potential for a repurposing aim in CCA treatment.
Approximately 60% of Thai patients with CCA had fasting blood glucose in a range of pre-diabetes or DM. Targeting the molecules underlying hyperglycemia-induced aggressiveness of CCA cells might improve the prognosis of CCA patients with DM.
This study aimed to investigate the effects of hyperglycemia on GABBR2 expression and the potential use of GABBR2 as a CCA therapeutic target.
CCA cells cultured in normal glucose or HG conditions were used as models of in vitro euglycemia and hyperglycemia, respectively. Baclofen, a GABBR2 agonist, was used to study the functional roles of CCA cells. Western blot, immunocytofluorescence, and immunohistochemistry were used to study molecular mechanisms.
HG induced GABBR2 expression in both cell lines and in patients’ CCA tissues. Baclofen treatment significantly suppressed CCA cell growth, while cells cultured in HG showed a significantly higher sensitivity. The effects of baclofen on CCA cell growth were achieved by the suppression of the signal transducer and activator of transcription 3 and glycogen synthase kinase 3/β-catenin pathways.
The expression of GABBR2 in CCA is induced in hyperglycemic conditions. Baclofen significantly suppresses the growth of CCA cells and thus holds a high promise as a repurposing drug for CCA treatment.
Investigating baclofen’s effects at an optimal therapeutic dosage in in vivo models would verify the present work and facilitate the translation for clinical study in CCA cases.
We would like to thank Mr. Chitsakul Phuyao, Cholangiocarcinoma Research Institute, Khon Kaen University, for his technical help in histological sections. This manuscript has been edited for English presentation by Prof. James A. Will, University of Wisconsin, USA, via KKU Publication Clinic, Khon Kaen University.
| 1. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1867] [Article Influence: 373.4] [Reference Citation Analysis (2)] |
| 2. | Zhu B, Qu S. The Relationship Between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front Endocrinol (Lausanne). 2022;13:800995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 3. | Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1291] [Cited by in RCA: 1508] [Article Influence: 94.3] [Reference Citation Analysis (2)] |
| 4. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 393] [Article Influence: 65.5] [Reference Citation Analysis (1)] |
| 5. | Mantovani A, Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5:270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Saengboonmee C, Seubwai W, Lert-Itthiporn W, Sanlung T, Wongkham S. Association of Diabetes Mellitus and Cholangiocarcinoma: Update of Evidence and the Effects of Antidiabetic Medication. Can J Diabetes. 2021;45:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Fu K, Yang X, Wu H, Gong J, Li X. Diabetes and PKM2 affect prognosis in patients with intrahepatic cholangiocarcinoma. Oncol Lett. 2020;20:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Thinkhamrop K, Khuntikeo N, Laohasiriwong W, Chupanit P, Kelly M, Suwannatrai AT. Association of comorbidity between Opisthorchis viverrini infection and diabetes mellitus in the development of cholangiocarcinoma among a high-risk population, northeastern Thailand. PLoS Negl Trop Dis. 2021;15:e0009741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Chaidee A, Onsurathum S, Intuyod K, Pannangpetch P, Pongchaiyakul C, Pinlaor P, Pairojkul C, Ittiprasert W, Cochran CJ, Mann VH, Brindley PJ, Pinlaor S. Co-occurrence of opisthorchiasis and diabetes exacerbates morbidity of the hepatobiliary tract disease. PLoS Negl Trop Dis. 2018;12:e0006611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Rizzo A, Brandi G. First-line Chemotherapy in Advanced Biliary Tract Cancer Ten Years After the ABC-02 Trial: "And Yet It Moves!". Cancer Treat Res Commun. 2021;27:100335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Goyal L, Kongpetch S, Crolley VE, Bridgewater J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021;95:102170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 12. | Rizzo A, Ricci AD, Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin Investig Drugs. 2021;30:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Ricci AD, Rizzo A, Brandi G. Immunotherapy in Biliary Tract Cancer: Worthy of a Second Look. Cancer Control. 2020;27:1073274820948047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Viscardi G, Tralongo AC, Massari F, Lambertini M, Mollica V, Rizzo A, Comito F, Di Liello R, Alfieri S, Imbimbo M, Della Corte CM, Morgillo F, Simeon V, Lo Russo G, Proto C, Prelaj A, De Toma A, Galli G, Signorelli D, Ciardiello F, Remon J, Chaput N, Besse B, de Braud F, Garassino MC, Torri V, Cinquini M, Ferrara R. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 133] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 15. | Saengboonmee C, Seubwai W, Pairojkul C, Wongkham S. High glucose enhances progression of cholangiocarcinoma cells via STAT3 activation. Sci Rep. 2016;6:18995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Phoomak C, Vaeteewoottacharn K, Silsirivanit A, Saengboonmee C, Seubwai W, Sawanyawisuth K, Wongkham C, Wongkham S. High glucose levels boost the aggressiveness of highly metastatic cholangiocarcinoma cells via O-GlcNAcylation. Sci Rep. 2017;7:43842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Detarya M, Thaenkaew S, Seubwai W, Indramanee S, Phoomak C, Saengboonmee C, Wongkham S, Wongkham C. High glucose upregulates FOXM1 expression via EGFR/STAT3 dependent activation to promote progression of cholangiocarcinoma. Life Sci. 2021;271:119114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Saengboonmee C, Phoomak C, Supabphol S, Covington KR, Hampton O, Wongkham C, Gibbs RA, Umezawa K, Seubwai W, Gingras MC, Wongkham S. NF-κB and STAT3 co-operation enhances high glucose induced aggressiveness of cholangiocarcinoma cells. Life Sci. 2020;262:118548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Saengboonmee C, Detarya M, Sangkhamanon S, Sawanyawisuth K, Seubwai W, Wongkham S. High Glucose Induced Upregulation of Cyclin a Associating with a Short Survival of Patients with Cholangiocarcinoma: A Potential Target for Treatment of Patients with Diabetes Mellitus. Nutr Cancer. 2022;74:1734-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Smart TG, Stephenson FA. A half century of γ-aminobutyric acid. Brain Neurosci Adv. 2019;3:2398212819858249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Shaye H, Stauch B, Gati C, Cherezov V. Molecular mechanisms of metabotropic GABA(B) receptor function. Sci Adv. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Evenseth LSM, Gabrielsen M, Sylte I. The GABA(B) Receptor-Structure, Ligand Binding and Drug Development. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Auteri M, Zizzo MG, Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res. 2015;93:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 24. | Hyland NP, Cryan JF. A Gut Feeling about GABA: Focus on GABA(B) Receptors. Front Pharmacol. 2010;1:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 25. | Mancinelli R, Franchitto A, Glaser S, Meng F, Onori P, Demorrow S, Francis H, Venter J, Carpino G, Baker K, Han Y, Ueno Y, Gaudio E, Alpini G. GABA induces the differentiation of small into large cholangiocytes by activation of Ca(2+) /CaMK I-dependent adenylyl cyclase 8. Hepatology. 2013;58:251-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Huang Q, Zhu CL, Liu CH, Xie F, Zhu K, Hu SY. Gamma-aminobutyric acid binds to GABAb receptor to inhibit cholangiocarcinoma cells growth via the JAK/STAT3 pathway. Dig Dis Sci. 2013;58:734-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Wang C, Zhu CL, Huang Z, Wang G, Huang Q, Liu CH, Xie F, Wang W. γ-aminobutyric acid inhibits the growth of cholangiocarcinoma via cAMP / PKA signal pathway. Int J Clin Exp Med. 2016;9:9992-9998. |
| 28. | Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T, Taffetani S, Marzioni M, Summers R, Reichenbach R, Alpini G. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437-11446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Huang D, Wang Y, Thompson JW, Yin T, Alexander PB, Qin D, Mudgal P, Wu H, Liang Y, Tan L, Pan C, Yuan L, Wan Y, Li QJ, Wang XF. Cancer-cell-derived GABA promotes β-catenin-mediated tumour growth and immunosuppression. Nat Cell Biol. 2022;24:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 30. | Xia S, He C, Zhu Y, Wang S, Li H, Zhang Z, Jiang X, Liu J. GABA(B)R-Induced EGFR Transactivation Promotes Migration of Human Prostate Cancer Cells. Mol Pharmacol. 2017;92:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1604] [Article Influence: 534.7] [Reference Citation Analysis (70)] |
| 32. | Sripa B, Seubwai W, Vaeteewoottacharn K, Sawanyawisuth K, Silsirivanit A, Kaewkong W, Muisuk K, Dana P, Phoomak C, Lert-Itthiporn W, Luvira V, Pairojkul C, Teh BT, Wongkham S, Okada S, Chamgramol Y. Functional and genetic characterization of three cell lines derived from a single tumor of an Opisthorchis viverrini-associated cholangiocarcinoma patient. Hum Cell. 2020;33:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991-D995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4527] [Cited by in RCA: 7229] [Article Influence: 516.4] [Reference Citation Analysis (0)] |
| 34. | Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, Choo SP, Myint SS, Thanan R, Nagarajan S, Lim WK, Ng CCY, Boot A, Liu M, Ong CK, Rajasegaran V, Lie S, Lim AST, Lim TH, Tan J, Loh JL, McPherson JR, Khuntikeo N, Bhudhisawasdi V, Yongvanit P, Wongkham S, Totoki Y, Nakamura H, Arai Y, Yamasaki S, Chow PK, Chung AYF, Ooi LLPJ, Lim KH, Dima S, Duda DG, Popescu I, Broet P, Hsieh SY, Yu MC, Scarpa A, Lai J, Luo DX, Carvalho AL, Vettore AL, Rhee H, Park YN, Alexandrov LB, Gordân R, Rozen SG, Shibata T, Pairojkul C, Teh BT, Tan P. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017;7:1116-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 723] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 35. | Huang Q, Liu C, Wang C, Hu Y, Qiu L, Xu P. Neurotransmitter γ-aminobutyric acid-mediated inhibition of the invasive ability of cholangiocarcinoma cells. Oncol Lett. 2011;2:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Saengboonmee C, Seubwai W, Wongkham C, Wongkham S. Diabetes mellitus: Possible risk and promoting factors of cholangiocarcinoma: Association of diabetes mellitus and cholangiocarcinoma. Cancer Epidemiol. 2015;39:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Thonsri U, Wongkham S, Wongkham C, Hino S, Nakao M, Roytrakul S, Koga T, Seubwai W. High glucose-ROS conditions enhance the progression in cholangiocarcinoma via upregulation of MAN2A2 and CHD8. Cancer Sci. 2021;112:254-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Kawasaki K, Kuboki S, Nojima H, Yoshitomi H, Furukawa K, Takayashiki T, Miyazaki M, Ohtsuka M. GABA signaling inhibits tumor progression by suppressing STAT3-mediated EMT in human intrahepatic cholangiocarcinoma. HPB. 2018;20:S694. [DOI] [Full Text] |
| 39. | Romito JW, Turner ER, Rosener JA, Coldiron L, Udipi A, Nohrn L, Tausiani J, Romito BT. Baclofen therapeutics, toxicity, and withdrawal: A narrative review. SAGE Open Med. 2021;9:20503121211022197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 40. | You L, Lin J, Yu Z, Qian Y, Bi Y, Wang F, Zhang L, Zheng C, Zhang J, Li W, Cai Y, Gao Y, Kong X, Sun X. Nobiletin suppresses cholangiocarcinoma proliferation via inhibiting GSK3β. Int J Biol Sci. 2022;18:5698-5712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Dokduang H, Techasen A, Namwat N, Khuntikeo N, Pairojkul C, Murakami Y, Loilome W, Yongvanit P. STATs profiling reveals predominantly-activated STAT3 in cholangiocarcinoma genesis and progression. J Hepatobiliary Pancreat Sci. 2014;21:767-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 42. | Glibo M, Serman A, Karin-Kujundzic V, Bekavac Vlatkovic I, Miskovic B, Vranic S, Serman L. The role of glycogen synthase kinase 3 (GSK3) in cancer with emphasis on ovarian cancer development and progression: A comprehensive review. Bosn J Basic Med Sci. 2021;21:5-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem. 2008;283:21934-21944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Lecarpentier Y, Schussler O, Hébert JL, Vallée A. Multiple Targets of the Canonical WNT/β-Catenin Signaling in Cancers. Front Oncol. 2019;9:1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 45. | Carpenter RL, Lo HW. STAT3 Target Genes Relevant to Human Cancers. Cancers (Basel). 2014;6:897-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 406] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 46. | Erlitzki R, Gong Y, Zhang M, Minuk G. Identification of gamma-aminobutyric acid receptor subunit types in human and rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G733-G739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Chen ZA, Bao MY, Xu YF, Zha RP, Shi HB, Chen TY, He XH. Suppression of Human Liver Cancer Cell Migration and Invasion via the GABAA Receptor. Cancer Biol Med. 2012;9:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 48. | Chan-On W, Nairismägi ML, Ong CK, Lim WK, Dima S, Pairojkul C, Lim KH, McPherson JR, Cutcutache I, Heng HL, Ooi L, Chung A, Chow P, Cheow PC, Lee SY, Choo SP, Tan IB, Duda D, Nastase A, Myint SS, Wong BH, Gan A, Rajasegaran V, Ng CC, Nagarajan S, Jusakul A, Zhang S, Vohra P, Yu W, Huang D, Sithithaworn P, Yongvanit P, Wongkham S, Khuntikeo N, Bhudhisawasdi V, Popescu I, Rozen SG, Tan P, Teh BT. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45:1474-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 400] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 49. | Jinawath N, Chamgramol Y, Furukawa Y, Obama K, Tsunoda T, Sripa B, Pairojkul C, Nakamura Y. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology. 2006;44:1025-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Tang Z, Li C, Kang B, Gao G, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98-W102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5550] [Cited by in RCA: 7449] [Article Influence: 827.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liao Z, Singapore; Rizzo A, Italy; Zhao W, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Cai YX