Published online Jul 28, 2023. doi: 10.3748/wjg.v29.i28.4405
Peer-review started: June 1, 2023
First decision: June 16, 2023
Revised: June 22, 2023
Accepted: July 11, 2023
Article in press: July 11, 2023
Published online: July 28, 2023
Processing time: 54 Days and 18.1 Hours
Post-acute pancreatitis diabetes (PAPD) is the second most common type of diabetes below type 2 diabetes mellitus. Due to the boom in research on this entity carried out during the last decade, its recognition has increased. However, much of the medical community still does not recognize it as a medium and long-term complication of acute pancreatitis (AP). Recent prospective cohort studies show that its incidence is about 23% globally and 34.5% in patients with severe AP. With the overall increase in the incidence of AP this complication will be certainly seen more frequently. Due to its high morbidity, mortality and difficult control, early detection and treatment are essential. However, its risk factors and pathophysiological mechanisms are not clearly defined. Its diagnosis should be made excluding pre-existing diabetes and applying the criteria of the American Diabetes Association after 90 d of resolution of one or more AP episodes. This review will show the evidence published so far on the incidence and prevalence, risk factors, possible pathophysiological mechanisms, clinical outcomes, clinical characteristics and preventive and corrective management of PAPD. Some important gaps needing to be clarified in forthcoming studies will also be discussed.
Core Tip: Post-acute pancreatitis diabetes (PAPD) is the second most common type of diabetes below type II diabetes mellitus. Its incidence is about 23% globally and 34.5% in severe acute pancreatitis (AP). With the overall increase in the incidence of AP this complication will also increase. Due to its high mortality, early detection and treatment are essential. Diagnosis should be made excluding pre-existing diabetes and applying the criteria of the American Diabetes Association after 90 d of resolution of AP episodes. This review will show published evidence on the incidence, risk factors, pathophysiology, clinical outcomes, clinical characteristics and preventive and corrective management of PAPD.
- Citation: García-Compeán D, Jiménez-Rodríguez AR, Muñoz-Ayala JM, González-González JA, Maldonado-Garza HJ, Villarreal-Pérez JZ. Post-acute pancreatitis diabetes: A complication waiting for more recognition and understanding. World J Gastroenterol 2023; 29(28): 4405-4415
- URL: https://www.wjgnet.com/1007-9327/full/v29/i28/4405.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i28.4405
Diabetes of the exocrine pancreas (DEP) derives from the dysfunction of the exocrine component of the pancreas. It is the second most common type of diabetes after type 2 diabetes mellitus (T2DM)[1]. Its incidence has tripled in the last decade, reaching annual incidence increase of 2.8%[2]. It is associated with higher mortality compared to T2DM[3].
Despite the fact that the relationship between diabetes and diseases of the exocrine pancreas has been known for long time, there have been few advances in the knowledge of the epidemiology and pathophysiological mechanisms of the DEP, which has made it difficult to establish a clear classification of this entity[4].
Terms such as pancreatic, pancreatoprive, pancreatogenic, postpancreatectomy diabetes, and others have been used for this condition. The term “type 3c diabetes” has been attributed to the American Diabetes Association (ADA). The truth is that this nomenclature was not assigned as such to the DEP, but adopted this name because it appeared within the group “other specific types of diabetes” in subsection c of group 3 in the 1998 publication of the ADA on the classification of diabetes. In addition, the use of the term “type 3c diabetes” was not promoted in 2019 guidelines of the ADA[5]. In order to avoid more confusion, the term “diabetes of the exocrine pancreas” has been used more frequently since 2017[6-8]. There is evidence that an increase in the accumulation of intrapancreatic fat around the pancreatic islets of Langerhans may have pathophysiological role in three of the most frequent types of DEP: Post-pancreatitis diabetes (PPD), pancreatic cancer-related diabetes, and cystic fibrosis-related diabetes, but not on T2DM and T1DM[9,10]. However, each of this type of diabetes has different pathophysiology, so it is appropriate to address them separately.
On the other side, the pancreatitis giving rise to diabetes can be acute or chronic, so it is reasonable to make distinction between both: Post-acute pancreatitis diabetes (PAPD) and post-chronic pancreatitis diabetes. A simplified core classification of DEP is shown in Table 1.
| Type of diabetes | Definition |
| Post pancreatitis diabetes | Diabetes identified in a patient with pancreatitis without previous detection of diabetes |
| Post-acute pancreatitis diabetes | Diabetes identified after one or more episodes of acute pancreatitis |
| Post-chronic pancreatitis diabetes | Diabetes identified in a patient with diagnosis of chronic pancreatitis |
| Pancreatic cancer related diabetes | Diabetes associated to pancreatic cancer in a patient without history of diabetes |
| Cystic fibrosis related diabetes | Diabetes associated to cystic fibrosis in a patient without history of diabetes |
Acute pancreatitis (AP) is one of the most common gastrointestinal causes of hospital admissions worldwide, accounting for more than 275000 cases per year. The global incidence rate of AP is increasing[11]. Because AP is the most common disease of the exocrine pancreas nowadays, it is probably the most common cause of DEP. However, this complication has been ignored by most physicians who care for patients recovering from AP[1]. In the last decade, epidemiological, clinical, and translational research on PAPD has boomed, and some important aspects of this pathology are now more clearly known[12,13].
In this review, the evidences published so far on the incidence, risk factors, possible pathophysiology, clinical outcomes, clinical characteristics, and management of PAPD will be discussed. However, the knowledge of a large part of these aspects is still incomplete, so some important gaps will be pointed out which will have to be clarified in future research studies.
AP is an inflammatory disease of the exocrine pancreas whose global incidence is 34/100000 inhabitants per year, with some geographical differences[14]. This condition has mortality rate from 1 to 2/100000 person-years[15]. Biliary lithiasis, alcohol abuse, endoscopic retrograde cholangiopancreatography, hypertriglyceridemia, and some drugs are the most common causes. On the other hand, 80% of patients have mild pancreatitis associated with few complications and short hospital stay. However, up to 20% of patients may have severe or necrotizing pancreatitis, giving rise to local and systemic complications, increased mortality and long hospital stay[16].
Studies in general population have shown that an episode of AP confers at least twice the risk of subsequent diabetes compared with controls[1]. Two meta-analyses published in 2014 and 2019[17,18], (with 31 studies and 13894 adult patients with no history of DM or prediabetes), evaluated the prevalence of diabetes after one or more episodes of AP. Only 3 were case-control and 28 were non-comparative prospective cohort studies. The cumulative pooled incidence for diabetes was 23% (95%CI: 16%-31%). The diabetes incidence was higher in the populations that had severe AP than in those with mild AP (39% vs 14%). The case-control studies and 12 cohort studies had significant methodological shortcomings (few patients, short follow-up or deficient methods for defining diabetes). In the 16 remaining best-quality studies, an overall incidence of PAPD of 27.8% (range 8% to 54%), and of 38.4% (range 16%-54%), only in the severe forms, was found. The cumulative incidence of diabetes reached up to 41% in studies with at least 5 years of follow-up[19-34] (Table 2). The wide range in diabetes incidence of these studies may be due to differences in methodological design, patient selection, and diabetes diagnostic methods.
| Ref. | N | Follow-up, mo | AP grade, % | Definition of diabetes | Prediabetes, % | Diabetes, % |
| Johansen and Ornsholt[20], 1972 | 22 | 24 | NS | OGTT | 0 | 18 |
| Olszewski et al[19], 1978 | 25 | 12 | NS | OGTT, serum insulin | ND | 28 |
| Eriksson et al[21], 1992 | 36 | 74 | S:56; M:44 | OGTT | 11 | 53 |
| Angelini et al[22], 1993 | 118 | 53 | S:70; M:30 | OGTT | ND | 8 |
| Malecka et al[23], 2002 | 82 | 56 | S:34; M:66 | OGTT, serum insulin | 2 | 16 |
| Kaya et al[24], 2007 | 112 | 12 | S:32; M:68 | OGTT, fasting glucose, c-pep | 24 | 21 |
| Andersson et al[25], 2010 | 39 | 45 | S:35; M:65 | Serum insulin, OGTT | 33 | 23 |
| Garip et al[26], 2013 | 96 | 32 | S:36; M:64 | Fasting glucose, OGTT | ND | 34 |
| Vujasinovic et al[27], 2014 | 100 | 32 | NS | Fasting glucose, OGTT | ND | 14 |
| Winter Gasparoto et al[28], 2015 | 16 | 38.4 | NS | OGTT, HOMA | ND | 31.2 |
| Severe AP | ||||||
| Doepel et al[29], 1993 | 37 | 74 | S:100 | Fasting glucose, C- pep, A1cHb, OGTT | ND | 54 |
| Yasuda et al[30], 2008 | 41 | 56 | S:100 | Fasting glucose | ND | 39 |
| Gupta et al[31], 2009 | 30 | 31 | S:100 | Fasting and postprandial glucose, OGTT, C-pep | 20 | 20 |
| Uomo et al[32], 2010 | 38 | 179 | S:100 | OGTT | ND | 16 |
| Chandrasekaran et al[33], 2015 | 35 | 26.2 | S:100 | OGTT | ND | 48.5 |
| Tu et al[34], 2017 | 113 | 42.9 | S:90; M:10 | OGTT A1cHb | ND | 30 |
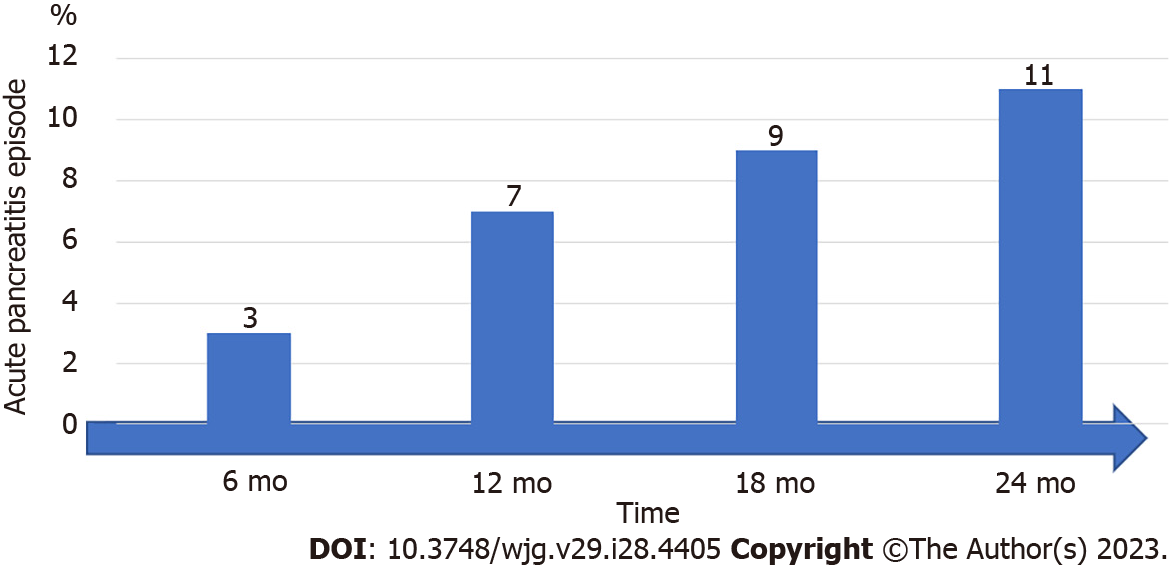
The time at which diabetes appears after AP is unknown[35]. A recent prospective study, which assessed the course of glycemia over months, reported that the proportion of patients who developed diabetes after an AP episode was 3% at 6 mo, 7% at 12 mo, 9% at 18 mo, and 11% at 24 mo[36] (Figure 1).
Some authors have not found association between the severity of AP and the incidence of PAPD[37-39], while others have found strong relationship[28,40-43]. A higher prevalence of PAPD was found in patients with severe AP than with mild AP in the most recent meta-analysis[18]. In another study, intensive care stay during the AP episode was associated with higher risk of developing diabetes in the 2 years after discharge[44]. The differences in the prevalence of PAPD in the severe forms shown in these studies could be explained, by the different definitions of severity and the diversity of scales used for assessing severity, such as Ranson, APACHE II, BISAP, or the Atlanta classification[45]. It seems that the strongest risk factors in the development of PAPD are pancreatic necrosis and recurrent episodes of AP[40,46]. For recurrent AP, one study evaluated computed tomography evidence of pancreatic volume loss in patients with a single episode of AP compared with recurrent pancreatitis. The investigators found that total pancreatic volume was significantly reduced in those with recurrent AP and these patients also had a strong association with endocrine and exocrine insufficiency[46]. Diabetes in patients with severe and recurrent AP may be due to structural damage of the β cells of the pancreatic islets. Nevertheless, it is important to highlight that the increased risk of diabetes also in patients with mild AP (without necrosis) suggests that there could be other mechanisms involved in its pathophysiology.
Other studies have shown that advanced age and male gender are significant risk factors[47]. Additionally, the alcoholic etiology of pancreatitis seems to increase this incidence[18]. On the other hand, some parameters of metabolic dysfunction, such as obesity and dyslipidemia, could be important risk factors, including genetic factors, particularly in patients with family history of DM.
In total, PAPD risk factors have been poorly or incompletely studied, mostly in retrospective studies with unclear defining parameters. However, the definition of risk factors is important to predict the incidence of diabetes in order to adopt an effective screening strategy of diabetes in patients who recover from AP, particularly in the mild form which is the most frequently seen. Specially designed prospective studies are required in order to clear this issue.
In the other side, some biological markers with the aim of predicting the development of PAPD have been investigated. One study found that elevated plasma levels of interleukin (IL)-1β and interferon γ in individuals with AP and normal glycemia may predict the onset of de novo diabetes during follow-up[48]. Another study found that elevated basal insulin and glucagon plasma levels were associated with de novo diabetes post AP (OR: 1.99 and 3.44 respectively)[49]. Another prospective, longitudinal cohort study found that the variability of glucose plasma levels in the early stages of AP may predict the development of diabetes at 2-year follow-up[50]. Although the results of these studies may appear promising, the research on this field is still very limited and these findings need to be validated before being used in clinical practice.
The pathophysiologic relationship between AP and diabetes seems to be bidirectional[51]. On the one hand, patients with T1DM and T2DM have higher risk of developing AP, as demonstrated in a meta-analysis with 5.7 million participants and 14124 cases. Patients with diabetes had higher risk of AP than individuals without diabetes (HR: 1.74)[52]. Likewise, other studies have reported that patients with diabetes develop more severe AP[42].
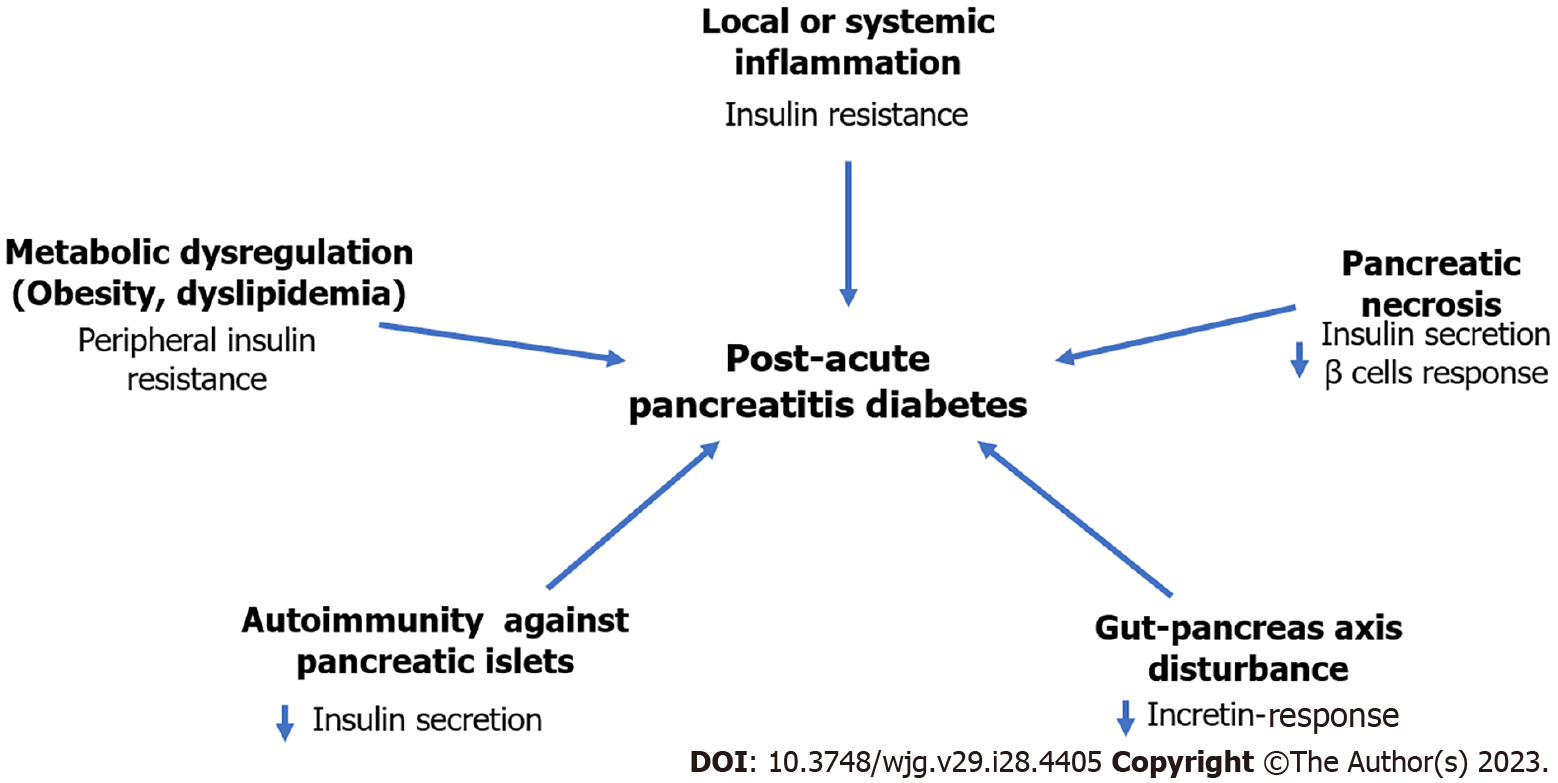
Despite it is accepted that AP can give rise to diabetes, the pathophysiological mechanisms are still unknown. In necrotizing AP, diabetes may be attributed to structural damage of pancreatic parenchyma. However, in the mild AP the involved mechanisms are less clear. Overall, it has been hypothesized that the pathophysiology of PAPD may be multifactorial, involving diverse mechanisms that could have effect at different levels of the glucose metabolism regulation pathways. It may be possible that one or more of these mechanisms may predominate in the different diabetes phenotypes. According to some evidence collected so far, some of the possible mechanisms are discussed in the following sections (Figure 2).
This complication of AP has already been discussed previously in the above sections[28,40-42,53,54]. In a recent meta-analysis, patients who displayed pancreatic necrosis during the AP attack(s) had a higher frequency of diabetes than those without necrosis (37% vs 11%)[18]. In other series, the incidence exceeds 50% of the cases[29]. Notwithstanding, the relation between diabetes incidence and the extension and site of necrosis has not been completely defined. In a recent study with 109 patients with AP the incidence of de novo diabetes in patients with pancreatic necrosis demonstrated by contrasted computed tomography scan was higher (66.6%) than in those without necrosis (27.8%). However, no relationship was found between diabetes incidence and necrosis rate or site of necrosis (head, body or tail of pancreas)[26]. This may be explained because diabetes may be due, in addition to destruction of β cells of the pancreas, to insulin resistance. And also, because β cells are located homogeneously in the different segments of the pancreas.
Pancreatic necrosis may also induce exocrine pancreas insufficiency (EPI). From 15 to 30% of patients who have an episode of AP may have chronic pancreatitis after 3 years of follow-up[47].
It has been speculated that the local and systemic inflammatory response occurring in AP patients may result in post-translational modifications of endogenous islet cell proteins, such as insulin, nucleic acids, and other proteins. Such modified neoepitopes may act as autoantigens, inducing an autoimmune response against components of the Langerhans islet[55]. To date, the frequency of autoimmunity during and after an AP episode has not been evaluated, particularly in patients who develop de novo diabetes.
Obesity and hypertriglyceridemia are risk factors for the development of both T2DM and AP[56,57]. Their presence prior to AP may result in greater risk of developing diabetes and may accelerate the onset of this condition. Both factors are independently associated with increased risk of clinical severity of AP, which could explain the risk of diabetes[58,59]. In fact, hypertriglyceridemia is one of the most frequent causes of AP, only below biliary and alcoholic etiology. The impact that visceral obesity may have is unknown. However, there is evidence that increased accumulation of intrapancreatic fat around the islets of Langerhans may have pathophysiological role in acute and chronic PPD[9,10]. Insulin resistance may be the mechanism in these patients[60].
During the course of AP, serum IL-6 levels increase as a consequence of the local and systemic inflammatory response. It has been hypothesized that this and other cytokines could favor the development of chronic hyperglycemia[61]. Multiple studies have found that the role of IL-6 on impaired glucose metabolism is primarily through insulin resistance[62,63].
It has been suggested that of some of the pancreatic and intestine functional interconnections involved in the digestion, absorption, and utilization of nutrients which regulate glucose homeostasis may be disturbed in PAPD[53]. This assertion is based on the fact that 15% to 30% of patients who have AP show chronic pancreatitis 3 years after the acute episode[47,64]. The most common endocrine dysfunction results from decreased levels of insulin, glucagon, and pancreatic polypeptide[65]. Impaired secretion of enteral glucoregulatory hormones (incretins secreted by intestinal epithelium cells), such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) may also be observed. These peptides are associated with increased insulin secretion, glucagon secretion modulation and reduction of hepatic and peripheral insulin resistance[66]. It is important to underline that the reduction of glucagon secretion is the cause of severe hypoglycemia due to the loss of its counter-regulatory effect[67]. Therefore, the combined hormonal abnormalities that occur in PAPD result in a severely disrupted endocrine environment that is different from the pattern of abnormalities seen in T1DM or T2DM. Hence the need to understand and recognize these entities separately.
In summary, it is essential to determine the relative contribution of each of these pathophysiological mechanisms involved in PAPD, as well as their interrelationship with the possible genetic predispositions. In order to clarify this important issue, both clinical and basic studies in experimental models of PAPD are needed due to the difficulty in obtaining tissue samples from human beings.
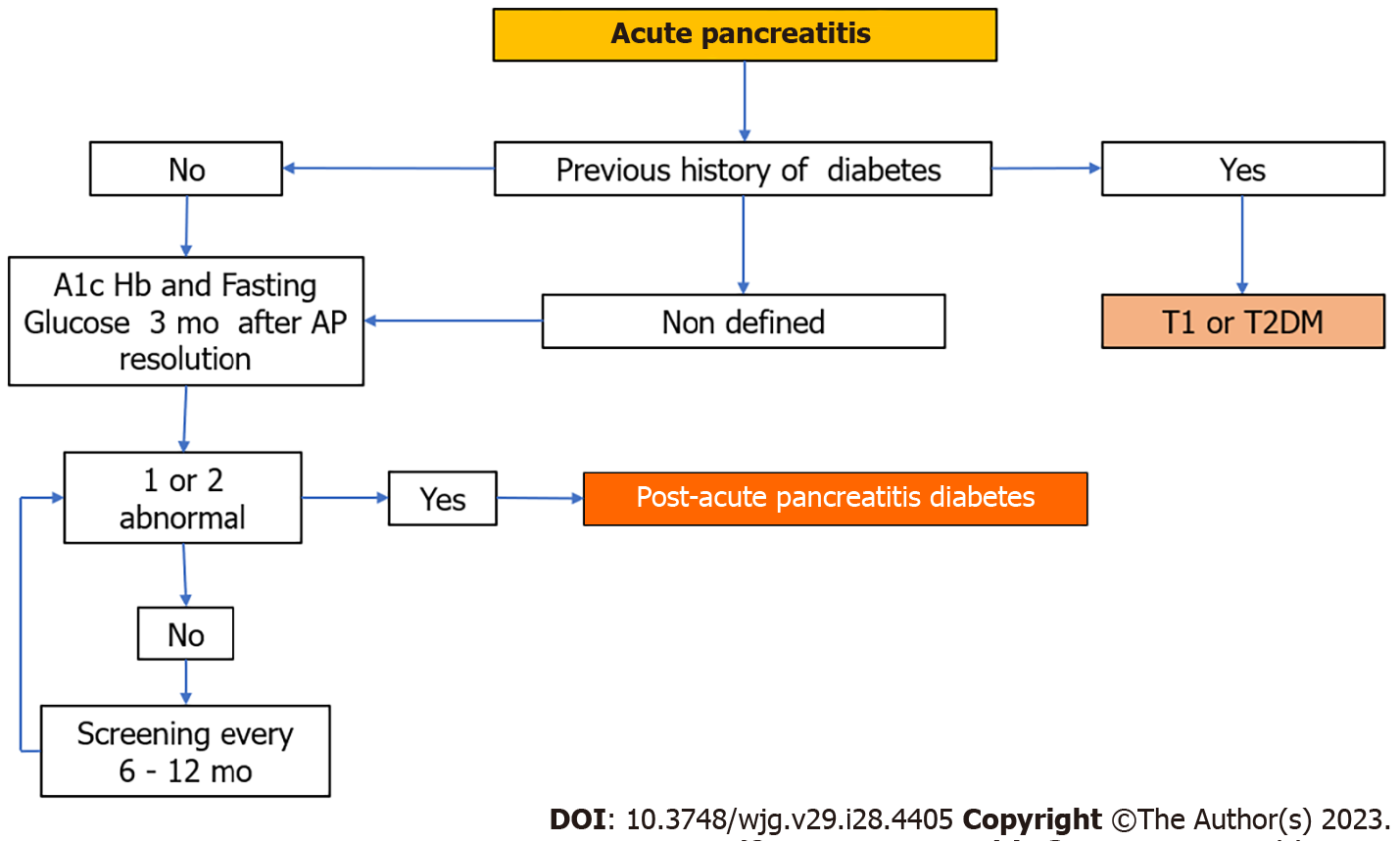
For stablishing the diagnosis of PAPD it is important to rule out preexisting diabetes (mainly T2DM) which sometimes is difficult. The diagnostic criteria for PAPD proposed some years ago that included the demonstration of EPI as well as the absence of autoantibodies directed against β cells of the pancreas are not currently valid[68,69] because only 30% of patients show evidence of EPI 3 years after AP[64,70]. In addition, patients who develop EPI have increased risk of diabetes[71], so it should be considered as a risk factor and not as its defining characteristic. On the other hand, early and intermediate stages of EPI are difficult to demonstrate as functional tests and sophisticated imaging techniques are often not available in real life practice and are costly. In total, the term PAPD should be reserved specifically to de novo diabetes in individuals after AP with or without morphological or functional evidence of chronic pancreatitis and without the need to demonstrate the absence of anti-β cell antibodies.
The diagnosis of diabetes should be established based on the criteria recommended by the ADA: Glycosylated hemoglobin (HbA1c) ≥ 48 mm/moL or 6.5% and/or fasting glucose > 7 mmmol/L or 126 mg/dL[72], which must be performed more than 90 d after AP resolution. This is important because HbA1c levels reflect the mean plasma glucose concentration in the previous 8-12 wk and also due to the stress hyperglycemia that can occur before this period. It has been shown that the oral glucose tolerance test is better for detecting early-stage diabetes as it is not affected by stress and does not require 90 d to give reliable results.
Some clinical characteristics and biochemical markers can help to differentiate PAPD from T2DM. PAPD has greater glycemic variability and more difficult control, showing frequent hypoglycemic episodes and more insulin requirements[73]. From the biochemical point of view, PAPD patients have lower baseline and stimulated levels of insulin, glucagon and C-peptide[1]. Besides, pre and postprandial serum levels of oxyntomodulin (an intestinal peptide derived from proglucagon that participates in the regulation of the pancreatic exocrine function), have been found to be significantly higher in patients with PAPD compared to T2DM and healthy controls. This opens the possibility of being used as a specific biomarker[74]. For some, the presence of diagnostic autoimmune markers for T1DM (i.e., islet cell antibodies or antibodies against glutamic acid decarboxylase, insulin, tyrosine phosphatase-like proteins, or zinc transporter)[75], rules out the diagnosis of PAPD. However, as already mentioned, an autoimmune component triggered by neoepitopes induced by the systemic inflammatory response of AP at the level of the β cells has not been ruled out as patho-physiological mechanism of PAPD in some patients[55]. Finally, it is also important to identify overlapping causes of DEP such as pancreatic surgery, cystic fibrosis, toxic pancreatic medications, hemochromatosis, and pancreatic cancer.
In summary, the diagnosis of PAPD should be based on the exclusion of any type of preexisting diabetes before AP and on identification of diabetes ninety days after AP based on the ADA criteria. For now, screening for PAPD should be performed in all patients who have had at least one episode of AP. The knowledge of the risk factors will contribute to selecting patients for screening in the future. Although there is no consensus on the frequency of screening, it is recommended to be carried out every 6 mo the first year and every year thereafter. Figure 3 shows a simplified PAPD diagnostic algorithm.
It has been demonstrated that PAPD has higher short- and long-term morbidity and mortality than T2DM[3]. A recent population-based study of 139843 individuals showed that those with PAPD had significantly higher risk of pancreatic cancer than those with T2DM or those with no history of pancreatitis (adjusted RR: 6.94; P < 0.05)[76]. It is important to underline that in patients recovering from AP, diabetes may be the clinical manifestation of pancreatic cancer, so early detection strategies for these neoplasms should be applied. Another recent study with 10549 individuals showed that patients with PAPD, compared to T2DM, had higher all-cause mortality (RR: 1.13), cancer (RR: 1.14), infections (RR: 2.52), and gastrointestinal disease (RR: 2.56). Likewise, the risk of rehospitalizations was significantly higher, which represented greater economic burden[77].
The data so far available regarding the treatment of PAPD are very scarce, however, some rationale may be useful to guide treatment decisions with the understanding that refinement will be required based on the results of well-conducted future therapeutic studies. The management of PAPD should ideally be preventive and corrective.
Preventive management aims to reduce the incidence of diabetes which would be achieved if the risk factors and predictive clinical and biochemical markers were clearly known. It seems that pancreatic necrosis and recurrent episodes of AP are the most solid risk factors. Possibly in patients with these complications a more aggressive and earlier management of AP, the performance of early cholecystectomy in biliary AP and stopping alcohol consumption could have some beneficial impact. In this context, well-conducted studies are required in order to demonstrate this issue.
For corrective management it is suggested to apply the ADA recommendations for the treatment of T2DM and T1DM with some nuances[78]. It is important to be aware of the fragile stability of glycemia of these patients. This leads that a large part of patients be treated with insulin. In a large population-based study, higher proportion of patients with PAPD were already on insulin therapy within 5 years compared to T2DM (20.9% vs 4.1% respectively), and had poorer glycemic control (defined as HBA1c ≥ 7%)[1].
It is important to remember that about 30% of patients with AP develop EPI[70]. At this point, one study reported increased postprandial responses of GLP-1 and GIP in patients with chronic pancreatitis and EPI following pancreatic enzyme substitution (PES). Concurrently, both plasma insulin, plasma C-peptide, and total insulin secretion increased after PES. These results suggest that secretion of GLP-1 and GIP is under influence of the digestion and absorption of nutrients in the small intestine and that PES increased insulin secretion[66]. Concomitant improvement of glycemic control was not assessed in diabetic patients from this study. As a result of these findings, the assessment of therapeutic effects of PES from the early stages of diabetes development may be warranted.
Finally, the management of PAPD is complex and requires a common approach, preferably by a medical team that includes gastroenterologists, endocrinologists, primary care physicians, nutritionists, and behavioral health specialists.
PAPD is currently the second most common type of diabetes. It is increasingly known as a result of the recently published research around this entity. However, much of the medical community still ignores its existence. With the increasing global incidence of AP, the frequency of this type of diabetes will certainly increase.
Due to its high morbidity, mortality and difficult treatment, its recognition as a complication of AP is of paramount importance. Pancreatic necrosis and recurrence seem to be the strongest risk factors. Its pathophysiological mechanisms and other risk factors are not yet clearly known. The diagnosis should be based on the exclusion of any type of preexisting diabetes and on identification of diabetes ninety days after AP based on the ADA criteria. Screening for diabetes should be performed in all patients who have had at least one episode of AP. Management is not yet standardized.
It was recently announced the launch of a multicenter clinical study designed to understand the frequency and phenotypes of this type of diabetes. This study has been called Diabetes RElated to Acute Pancreatitis and its Mechanisms and is supported by The National Institute of Diabetes and Digestive and Kidney Diseases[79]. In this research project, it is planned to study risk factors and some of the mechanisms possibly involved in the pathophysiology of PAPD[80-82].
Certainly, the results of this and other similar forthcoming studies will contribute to the clarification of some important gaps that still persist in the knowledge of PAPD making possible a more effective screening and better preventive and corrective management.
| 1. | Woodmansey C, McGovern AP, McCullough KA, Whyte MB, Munro NM, Correa AC, Gatenby PAC, Jones SA, de Lusignan S. Incidence, Demographics, and Clinical Characteristics of Diabetes of the Exocrine Pancreas (Type 3c): A Retrospective Cohort Study. Diabetes Care. 2017;40:1486-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 2. | Pendharkar SA, Mathew J, Petrov MS. Age- and sex-specific prevalence of diabetes associated with diseases of the exocrine pancreas: A population-based study. Dig Liver Dis. 2017;49:540-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Shivaprasad C, Aiswarya Y, Kejal S, Sridevi A, Anupam B, Ramdas B, Gautham K, Aarudhra P. Comparison of CGM-Derived Measures of Glycemic Variability Between Pancreatogenic Diabetes and Type 2 Diabetes Mellitus. J Diabetes Sci Technol. 2021;15:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M; HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5:153-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 449] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 5. | Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1635] [Cited by in RCA: 2138] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 6. | Wynne K, Devereaux B, Dornhorst A. Diabetes of the exocrine pancreas. J Gastroenterol Hepatol. 2019;34:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Duggan SN, O'Connor DB, Antanaitis A, Campion JR, Lawal O, Ahmed M, Tisdall AR, Sherlock M, Boran G, le Roux C, Gibney J, Conlon KC. Metabolic dysfunction and diabetes mellitus during long-term follow-up of severe acute pancreatitis: A case-matched study. Pancreatology. 2020;20:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Ramalho GX, Dytz MG. Diabetes of the Exocrine Pancreas Related to Hereditary Pancreatitis, an Update. Curr Diab Rep. 2020;20:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (2)] |
| 9. | Singh RG, Cervantes A, Kim JU, Nguyen NN, DeSouza SV, Dokpuang D, Lu J, Petrov MS. Intrapancreatic fat deposition and visceral fat volume are associated with the presence of diabetes after acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2019;316:G806-G815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Singh RG, Nguyen NN, DeSouza SV, Pendharkar SA, Petrov MS. Comprehensive analysis of body composition and insulin traits associated with intra-pancreatic fat deposition in healthy individuals and people with new-onset prediabetes/diabetes after acute pancreatitis. Diabetes Obes Metab. 2019;21:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Yu B, Li N, Li J, Wan J, He W, Zhu Y, Lu N. The Clinical Characteristics of Acute Pancreatitis in Gerontal Patients: A Retrospective Study. Clin Interv Aging. 2020;15:1541-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Petrov MS. Diagnosis of endocrine disease: Post-pancreatitis diabetes mellitus: prime time for secondary disease. Eur J Endocrinol. 2021;184:R137-R149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 13. | Bendor CD, Bardugo A, Zucker I, Cukierman-Yaffe T, Lutski M, Derazne E, Shohat T, Mosenzon O, Tzur D, Sapir A, Pinhas-Hamiel O, Kibbey RG, Raz I, Afek A, Gerstein HC, Tirosh A, Twig G. Childhood Pancreatitis and Risk for Incident Diabetes in Adulthood. Diabetes Care. 2020;43:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Baeza-Zapata AA, García-Compeán D, Jaquez-Quintana JO; Collaborators. Acute Pancreatitis in Elderly Patients. Gastroenterology. 2021;161:1736-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 528] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 16. | González-González JA, Castañeda-Sepúlveda R, Martínez-Vázquez MA, García-Compean D, Flores-Rendón AR, Maldonado-Garza HJ, Bosques-Padilla F, Garza-Galindo AA. [Clinical characteristics of acute pancreatitis in Mexico]. Rev Gastroenterol Mex. 2012;77:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Das SL, Singh PP, Phillips AR, Murphy R, Windsor JA, Petrov MS. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 18. | Zhi M, Zhu X, Lugea A, Waldron RT, Pandol SJ, Li L. Incidence of New Onset Diabetes Mellitus Secondary to Acute Pancreatitis: A Systematic Review and Meta-Analysis. Front Physiol. 2019;10:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Olszewski S, Kinalska I, Długosz J, Stasiewicz J, Gabryelewicz A. The glucose tolerance, insulin response and pancreatic exocrine function in patients after acute pancreatitis. Endokrinologie. 1978;71:183-191. [PubMed] |
| 20. | Johansen K, Ornsholt J. Frequency of diabetes after acute pancreatitis. Metabolism. 1972;21:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Eriksson J, Doepel M, Widén E, Halme L, Ekstrand A, Groop L, Höckerstedt K. Pancreatic surgery, not pancreatitis, is the primary cause of diabetes after acute fulminant pancreatitis. Gut. 1992;33:843-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Angelini G, Cavallini G, Pederzoli P, Bovo P, Bassi C, Di Francesco V, Frulloni L, Sgarbi D, Talamini G, Castagnini A. Long-term outcome of acute pancreatitis: a prospective study with 118 patients. Digestion. 1993;54:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Malecka-Panas E, Gasiorowska A, Kropiwnicka A, Zlobinska A, Drzewoski J. Endocrine pancreatic function in patients after acute pancreatitis. Hepatogastroenterology. 2002;49:1707-1712. [PubMed] |
| 24. | Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World J Gastroenterol. 2007;13:3090-3094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Andersson B, Pendse ML, Andersson R. Pancreatic function, quality of life and costs at long-term follow-up after acute pancreatitis. World J Gastroenterol. 2010;16:4944-4951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Garip G, Sarandöl E, Kaya E. Effects of disease severity and necrosis on pancreatic dysfunction after acute pancreatitis. World J Gastroenterol. 2013;19:8065-8070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Vujasinovic M, Tepes B, Makuc J, Rudolf S, Zaletel J, Vidmar T, Seruga M, Birsa B. Pancreatic exocrine insufficiency, diabetes mellitus and serum nutritional markers after acute pancreatitis. World J Gastroenterol. 2014;20:18432-18438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 28. | Winter Gasparoto RC, Racy Mde C, De Campos T. Long-term outcomes after acute necrotizing pancreatitis: what happens to the pancreas and to the patient? JOP. 2015;16:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Doepel M, Eriksson J, Halme L, Kumpulainen T, Höckerstedt K. Good long-term results in patients surviving severe acute pancreatitis. Br J Surg. 1993;80:1583-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Long-term outcome of severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Gupta R, Wig JD, Bhasin DK, Singh P, Suri S, Kang M, Rana SS, Rana S. Severe acute pancreatitis: the life after. J Gastrointest Surg. 2009;13:1328-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Uomo G, Gallucci F, Madrid E, Miraglia S, Manes G, Rabitti PG. Pancreatic functional impairment following acute necrotizing pancreatitis: long-term outcome of a non-surgically treated series. Dig Liver Dis. 2010;42:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Chandrasekaran P, Gupta R, Shenvi S, Kang M, Rana SS, Singh R, Bhasin DK. Prospective comparison of long term outcomes in patients with severe acute pancreatitis managed by operative and non operative measures. Pancreatology. 2015;15:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Tu J, Zhang J, Ke L, Yang Y, Yang Q, Lu G, Li B, Tong Z, Li W, Li J. Endocrine and exocrine pancreatic insufficiency after acute pancreatitis: long-term follow-up study. BMC Gastroenterol. 2017;17:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Shen HN, Yang CC, Chang YH, Lu CL, Li CY. Risk of Diabetes Mellitus after First-Attack Acute Pancreatitis: A National Population-Based Study. Am J Gastroenterol. 2015;110:1698-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Bharmal SH, Cho J, Alarcon Ramos GC, Ko J, Stuart CE, Modesto AE, Singh RG, Petrov MS. Trajectories of glycaemia following acute pancreatitis: a prospective longitudinal cohort study with 24 months follow-up. J Gastroenterol. 2020;55:775-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Ho TW, Wu JM, Kuo TC, Yang CY, Lai HS, Hsieh SH, Lai F, Tien YW. Change of Both Endocrine and Exocrine Insufficiencies After Acute Pancreatitis in Non-Diabetic Patients: A Nationwide Population-Based Study. Medicine (Baltimore). 2015;94:e1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Nikkola J, Laukkarinen J, Lahtela J, Seppänen H, Järvinen S, Nordback I, Sand J. The Long-term Prospective Follow-up of Pancreatic Function After the First Episode of Acute Alcoholic Pancreatitis: Recurrence Predisposes One to Pancreatic Dysfunction and Pancreatogenic Diabetes. J Clin Gastroenterol. 2017;51:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Ibars EP, Sánchez de Rojas EA, Quereda LA, Ramis RF, Sanjuan VM, Peris RT. Pancreatic function after acute biliary pancreatitis: does it change? World J Surg. 2002;26:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Umapathy C, Raina A, Saligram S, Tang G, Papachristou GI, Rabinovitz M, Chennat J, Zeh H, Zureikat AH, Hogg ME, Lee KK, Saul MI, Whitcomb DC, Slivka A, Yadav D. Natural History After Acute Necrotizing Pancreatitis: a Large US Tertiary Care Experience. J Gastrointest Surg. 2016;20:1844-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Tu J, Yang Y, Zhang J, Yang Q, Lu G, Li B, Tong Z, Ke L, Li W, Li J. Effect of the disease severity on the risk of developing new-onset diabetes after acute pancreatitis. Medicine (Baltimore). 2018;97:e10713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Xiao B, Xu HB, Jiang ZQ, Hu JX, Yang GD. Acute Pancreatitis in Patients With a Medical History of Type 2 Diabetes Mellitus: Clinical Findings and Magnetic Resonance Imaging Characteristics. Pancreas. 2020;49:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Vipperla K, Papachristou GI, Slivka A, Whitcomb DC, Yadav D. Risk of New-Onset Diabetes Is Determined by Severity of Acute Pancreatitis. Pancreas. 2016;45:e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Walker A, O'Kelly J, Graham C, Nowell S, Kidd D, Mole DJ. Increased risk of type 3c diabetes mellitus after acute pancreatitis warrants a personalized approach including diabetes screening. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Richardson A, Park WG. Acute pancreatitis and diabetes mellitus: a review. Korean J Intern Med. 2021;36:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Avanesov M, Löser A, Smagarynska A, Keller S, Guerreiro H, Tahir E, Karul M, Adam G, Yamamura J. Clinico-radiological comparison and short-term prognosis of single acute pancreatitis and recurrent acute pancreatitis including pancreatic volumetry. PLoS One. 2018;13:e0206062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Patra PS, Das K. Longer-term outcome of acute pancreatitis: 5 years follow-up. JGH Open. 2021;5:1323-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Bharmal SH, Kimita W, Ko J, Petrov MS. Cytokine signature for predicting new-onset prediabetes after acute pancreatitis: A prospective longitudinal cohort study. Cytokine. 2022;150:155768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 49. | Bharmal SH, Kimita W, Ko J, Petrov MS. Pancreatic and gut hormones as predictors of new-onset prediabetes after non-necrotising acute pancreatitis: a prospective longitudinal cohort study. Endocr Connect. 2021;10:715-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Bharmal SH, Cho J, Ko J, Petrov MS. Glucose variability during the early course of acute pancreatitis predicts two-year probability of new-onset diabetes: A prospective longitudinal cohort study. United European Gastroenterol J. 2022;10:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Lee YK, Huang MY, Hsu CY, Su YC. Bidirectional Relationship Between Diabetes and Acute Pancreatitis: A Population-Based Cohort Study in Taiwan. Medicine (Baltimore). 2016;95:e2448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Aune D, Mahamat-Saleh Y, Norat T, Riboli E. Diabetes mellitus and the risk of pancreatitis: A systematic review and meta-analysis of cohort studies. Pancreatology. 2020;20:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Hart PA, Bradley D, Conwell DL, Dungan K, Krishna SG, Wyne K, Bellin MD, Yadav D, Andersen DK, Serrano J, Papachristou GI. Diabetes following acute pancreatitis. Lancet Gastroenterol Hepatol. 2021;6:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 54. | Burkhart RA, Gerber SM, Tholey RM, Lamb KM, Somasundaram A, McIntyre CA, Fradkin EC, Ashok AP, Felte RF, Mehta JM, Rosato EL, Lavu H, Jabbour SA, Yeo CJ, Winter JM. Incidence and severity of pancreatogenic diabetes after pancreatic resection. J Gastrointest Surg. 2015;19:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 55. | Kahara T, Takamura T, Otoda T, Ishikura K, Matsushita E. Transient anti-GAD antibody positivity and acute pancreatitis with pancreas tail swelling in a patient with susceptible haplotype for type 1 diabetes mellitus. Intern Med. 2009;48:1897-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Ma JH, Yuan YJ, Lin SH, Pan JY. Nomogram for predicting diabetes mellitus after the first attack of acute pancreatitis. Eur J Gastroenterol Hepatol. 2019;31:323-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Wu D, Xu Y, Zeng Y, Wang X. Endocrine pancreatic function changes after acute pancreatitis. Pancreas. 2011;40:1006-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Smeets XJNM, Knoester I, Grooteman KV, Singh VK, Banks PA, Papachristou GI, Duarte-Rojo A, Robles-Diaz G, Kievit W, Besselink MGH, Verdonk RC, Van Santvoort HC, Drenth JPH, Belias M, Van Geenen EJM; Dutch Pancreatitis Study Group. The association between obesity and outcomes in acute pancreatitis: an individual patient data meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 60. | Pendharkar SA, Singh RG, Bharmal SH, Drury M, Petrov MS. Pancreatic Hormone Responses to Mixed Meal Test in New-onset Prediabetes/Diabetes After Non-necrotizing Acute Pancreatitis. J Clin Gastroenterol. 2020;54:e11-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Petrov MS. Panorama of mediators in postpancreatitis diabetes mellitus. Curr Opin Gastroenterol. 2020;36:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 62. | Gillies N, Pendharkar SA, Asrani VM, Mathew J, Windsor JA, Petrov MS. Interleukin-6 is associated with chronic hyperglycemia and insulin resistance in patients after acute pancreatitis. Pancreatology. 2016;16:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 63. | Xu E, Pereira MMA, Karakasilioti I, Theurich S, Al-Maarri M, Rappl G, Waisman A, Wunderlich FT, Brüning JC. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat Commun. 2017;8:14803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Hollemans RA, Hallensleben NDL, Mager DJ, Kelder JC, Besselink MG, Bruno MJ, Verdonk RC, van Santvoort HC; Dutch Pancreatitis Study Group. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology. 2018;18:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 65. | Dite P, Bojkova M, Belobradkova J, Zak P, Kianicka B. Chronic Pancreatitis and Diabetes of Exocrine Pancreas / Type 3c Diabetes Mellitus / Post-pancreatitis Diabetes Mellitus. J Gastrointestin Liver Dis. 2022;31:371-374. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Knop FK, Vilsbøll T, Larsen S, Højberg PV, Vølund A, Madsbad S, Holst JJ, Krarup T. Increased postprandial responses of GLP-1 and GIP in patients with chronic pancreatitis and steatorrhea following pancreatic enzyme substitution. Am J Physiol Endocrinol Metab. 2007;292:E324-E330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Donowitz M, Hendler R, Spiro HM, Binder HJ, Felig P. Glucagon secretion in acute and chronic pancreatitis. Ann Intern Med. 1975;83:778-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 65] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19:7276-7281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (3)] |
| 69. | Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)--are we neglecting an important disease? Eur J Intern Med. 2013;24:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 70. | Huang W, de la Iglesia-García D, Baston-Rey I, Calviño-Suarez C, Lariño-Noia J, Iglesias-Garcia J, Shi N, Zhang X, Cai W, Deng L, Moore D, Singh VK, Xia Q, Windsor JA, Domínguez-Muñoz JE, Sutton R. Exocrine Pancreatic Insufficiency Following Acute Pancreatitis: Systematic Review and Meta-Analysis. Dig Dis Sci. 2019;64:1985-2005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 71. | Cho J, Scragg R, Pandol SJ, Petrov MS. Exocrine Pancreatic Dysfunction Increases the Risk of New-Onset Diabetes Mellitus: Results of a Nationwide Cohort Study. Clin Transl Sci. 2021;14:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2231] [Cited by in RCA: 2324] [Article Influence: 145.3] [Reference Citation Analysis (1)] |
| 73. | Singh A, Aggarwal M, Garg R, Stevens T, Chahal P. Post-pancreatitis diabetes mellitus: insight on optimal management with nutrition and lifestyle approaches. Ann Med. 2022;54:1776-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Bharmal SH, Cho J, Stuart CE, Alarcon Ramos GC, Ko J, Petrov MS. Oxyntomodulin May Distinguish New-Onset Diabetes After Acute Pancreatitis From Type 2 Diabetes. Clin Transl Gastroenterol. 2020;11:e00132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 75. | Petrov MS, Basina M. DIAGNOSIS OF ENDOCRINE DISEASE: Diagnosing and classifying diabetes in diseases of the exocrine pancreas. Eur J Endocrinol. 2021;184:R151-R163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 76. | Cho J, Scragg R, Petrov MS. Postpancreatitis Diabetes Confers Higher Risk for Pancreatic Cancer Than Type 2 Diabetes: Results From a Nationwide Cancer Registry. Diabetes Care. 2020;43:2106-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 77. | Cho J, Scragg R, Petrov MS. Risk of Mortality and Hospitalization After Post-Pancreatitis Diabetes Mellitus vs Type 2 Diabetes Mellitus: A Population-Based Matched Cohort Study. Am J Gastroenterol. 2019;114:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Cusi K, Das SR, Gibbons CH, Giurini JM, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Kosiborod M, Leon J, Lyons SK, Murdock L, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Sun JK, Woodward CC, Young-Hyman D, Gabbay RA; on behalf of the American Diabetes Association. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S1-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 184] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 79. | Serrano J, Laughlin MR, Bellin MD, Yadav D, Chinchilli VM, Andersen DK; Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Type 1 Diabetes in Acute Pancreatitis Consortium: From Concept to Reality. Pancreas. 2022;51:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Casu A, Grippo PJ, Wasserfall C, Sun Z, Linsley PS, Hamerman JA, Fife BT, Lacy-Hulbert A, Toledo FGS, Hart PA, Papachristou GI, Bellin MD, Yadav D, Laughlin MR, Goodarzi MO, Speake C; Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Evaluating the Immunopathogenesis of Diabetes After Acute Pancreatitis in the Diabetes RElated to Acute Pancreatitis and Its Mechanisms Study: From the Type 1 Diabetes in Acute Pancreatitis Consortium. Pancreas. 2022;51:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 81. | Dungan KM, Hart PA, Andersen DK, Basina M, Chinchilli VM, Danielson KK, Evans-Molina C, Goodarzi MO, Greenbaum CJ, Kalyani RR, Laughlin MR, Pichardo-Lowden A, Pratley RE, Serrano J, Sims EK, Speake C, Yadav D, Bellin MD, Toledo FGS; Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Assessing the Pathophysiology of Hyperglycemia in the Diabetes RElated to Acute Pancreatitis and Its Mechanisms Study: From the Type 1 Diabetes in Acute Pancreatitis Consortium. Pancreas. 2022;51:575-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Tirkes T, Chinchilli VM, Bagci U, Parker JG, Zhao X, Dasyam AK, Feranec N, Grajo JR, Shah ZK, Poullos PD, Spilseth B, Zaheer A, Xie KL, Wachsman AM, Campbell-Thompson M, Conwell DL, Fogel EL, Forsmark CE, Hart PA, Pandol SJ, Park WG, Pratley RE, Yazici C, Laughlin MR, Andersen DK, Serrano J, Bellin MD, Yadav D; Type 1 Diabetes in Acute Pancreatitis Consortium (T1DAPC). Design and Rationale for the Use of Magnetic Resonance Imaging Biomarkers to Predict Diabetes After Acute Pancreatitis in the Diabetes RElated to Acute Pancreatitis and Its Mechanisms Study: From the Type 1 Diabetes in Acute Pancreatitis Consortium. Pancreas. 2022;51:586-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Liu C, China S-Editor: Fan JR L-Editor: A P-Editor: Yu HG