Published online Jul 21, 2023. doi: 10.3748/wjg.v29.i27.4334
Peer-review started: March 27, 2023
First decision: May 18, 2023
Revised: June 4, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: July 21, 2023
Processing time: 107 Days and 17.8 Hours
There is no consensus on the recommended duration of and optimal time to stop azathioprine (AZA) therapy in inflammatory bowel disease (IBD). Determining the optimal duration and cessation time can help to balance the risks of long-term intake with the possibility of relapse after cessation.
To describe the events following AZA cessation.
Retrospective analysis was performed to examine data from adult patients affected by IBD who were followed at the University of Padua and had started but then discontinued AZA between 1995 and 2022. Data on therapy duration, reasons for cessation, and type of relapse after cessation were collected. Cox regression models were used to estimate the risk of relapse in different sub
A total of 133 ulcerative colitis patients and 141 Crohn’s disease patients were included. Therapy with AZA was stopped in the 1st year in approximately 34% of patients but was continued for more than 10 years in approximately 10% of cases. AZA discontinuation was due to primary failure or disease relapse in 30% of patients and due to disease remission in 25.2% of patients. Most of the remaining cases stopped AZA therapy due to side effects (primarily clinical intolerance, cytopenia, and pancreatic disease). Patients who stopped AZA for clinical remission had an 83% lower risk of relapse during the observation time than other groups, with a relapse-free rate of 89% after 1 year and 79% after 2 years.
AZA administration is effective and safe, but it requires careful monitoring for potential minor and major side effects. Only 10% of patients who achieved remission with AZA needed a new treatment within 1 year of drug interruption.
Core Tip: Prolonged use of azathioprine (AZA) remains controversial, and the time of interruption is uncertain. This retrospective study analyzed our single-center data of patients affected by inflammatory bowel disease who had started and then discontinued AZA between 1995 and 2022. AZA administration was effective and safe, and only 10% of patients who achieved remission with AZA needed a new treatment within 1 year of drug interruption.
- Citation: Crepaldi M, Maniero D, Massano A, Pavanato M, Barberio B, Savarino EV, Zingone F. Azathioprine monotherapy withdrawal in inflammatory bowel diseases: A retrospective mono-centric study. World J Gastroenterol 2023; 29(27): 4334-4343
- URL: https://www.wjgnet.com/1007-9327/full/v29/i27/4334.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i27.4334
The immunosuppressant drug azathioprine (AZA) has been used in the treatment of inflammatory bowel diseases (IBDs) since the 1950s, and it represented one of the main treatments for these disorders until the introduction of biological drugs[1]. AZA is primarily effective as a steroid-sparing therapy for obtaining steroid-free long-term remission in Crohn's disease (CD) and ulcerative colitis (UC), but its delayed onset of action precludes its use in the induction phase[2-6]. AZA indications are well defined, but controversy exists regarding the risk-benefit ratio associated with AZA suspension[5,6]. The risk of relapse after withdrawal of AZA in patients with CD and UC who reached sustained remission was lower than 50% at 5 years[7]. Other studies found that sustained remission with AZA was associated with a 1-year moderate-to-severe relapse rate of approximately 23% to 41%[8-10]. Hawthorne and co-workers showed that patients who achieved disease remission with AZA experienced a doubling of the relapse rate after AZA withdrawal[11]. Mantzaris[1] suggested that drug withdrawal should be better considered after at least 4 years of “depth” of remission (clinical, serological, endoscopic and histological for UC), and close monitoring using biological markers of inflammation after AZA withdrawal was essential[1]. In contrast, Vilien et al[10] observed that patients with CD who were in remission after more than 2 years of continuous AZA treatment benefit from further continued treatment[10].
The occurrence of adverse effects plays a crucial role in the decision to discontinue AZA treatment. These effects are classified as "dose-dependent" (e.g., myelotoxicity, hepatitis, opportunistic infections and cancer) and "dose-independent", which includes allergic reactions, such as rash or fever. There are also idiosyncratic reactions, such as pancreatitis, that are frequently associated with AZA use[12]. The prevalence of these adverse effects among patients ranges from 6% to 30%[13]. The most critical and potentially life-threatening adverse event of AZA is myelosuppression, which occurs more frequently during the 1st months and commonly manifests as leukopenia. The overall incidence rate of myelotoxicity in IBD patients receiving AZA is approximately 3% per patient and year of treatment, while severe myelotoxicity occurs in fewer than 1% of patients, and the risk of mortality during the year of treatment is lower than 0.1%[14]. The side effects of hepatotoxicity are related to the TPMT genotype, which was reported in approximately 5% of patients with AZA/mercaptopurine therapy[15]. Discontinuation of AZA is considered when severe cholestatic jaundice develops but also with a persistent increase in liver function values despite a 50% reduction in drug dose[16]. Pancreatitis is a much less frequent adverse effect of AZA than hepatotoxicity, and it occurs within the 1st month of AZA treatment. Acute pancreatitis (AP) is diagnosed when two of three findings appear, including abdominal pain suggestive of pancreatitis, serum amylase and/or lipase levels at least three times the average level, and characteristic findings on imaging[15]. AZA treatment is associated with an increased risk of developing several types of cancer, including non-melanoma skin cancers, urinary tract cancers, and hematological malignancies, such as non-Hodgkin's lymphoma, hepatosplenic T-cell lymphoma or gastrointestinal lymphomas. The risk of hematological neoplasia correlates with the duration of AZA treatment, and it is higher in patients with leukopenia that lasts longer than 20 d[17]. Therefore, the duration of AZA therapy should balance the risks associated with long-term intake with the risks of relapse upon withdrawal/suspension.
The primary aim of our study was to examine the events following AZA suspension. Moreover, we also described reasons for cessation and side effects of AZA treatment.
We performed a longitudinal observational retrospective study on IBD patients included in the registry "The Paduan Gastrointestinal Disease Natural History Registry”: A longitudinal, retrospective and prospective study" (CESC code: 5370/AO/22), which was approved by the Ethical Committees of the Padova University Hospital. The registry collected data on the diagnosis and follow-up of IBD patients followed at our center. We selected IBD patients aged ≥ 18 years who started and then discontinued AZA monotherapy between January 1995 and January 2022. The exclusion criteria were a combination therapy of AZA and biological drugs in naïve patients, the continuation of AZA therapy, the presence of a pouch after colectomy for CU, indeterminate IBD and refusal to sign the informed consent for inclusion in the registry.
All data were retrieved manually from the registry and collected in a specific database. The following information was considered: Type of disease, date of diagnosis, location and phenotype of diseases, familiarity with IBD, and comorbidities. The following data on AZA therapy were included: The reason for initiation of AZA therapy, duration (in months) of AZA therapy, dose of the drug and any changes during its administration, concomitant therapies with systemic or topical corticosteroids and/or oral or topical mesalazine, reason for discontinuation of AZA and any disease relapse events after AZA discontinuation. Disease relapse was considered when one of the following events after AZA suspension occurred: Disease-related hospitalizations and/or surgeries, initiation of biological drugs or resumption of AZA therapy.
All patients were assessed every 6 mo in our outpatients’ clinic or earlier when needed using clinical and laboratory parameters.
Clinical activity was evaluated using the Harvey Bradshaw index for CD patients and the partial Mayo score for UC patients[18,19]. C-reactive protein (CRP) levels (positive when > 0.5 mg/dL) and fecal calprotectin values were also evaluated at each follow-up examination. Endoscopy was performed according to current guidelines[5,6]. The endoscopic activity was evaluated using the Simple Endoscopic Score or Rutgeers score for CD patients and the Mayo endoscopic score for UC patients[20-22].
The results are summarized as frequencies and percentages (categorical variables) and means with SD (continuous variables). Categorical variables were compared using the χ2 test, and continuous variables were compared using Student’s t test. The time of observation after AZA interruption was calculated using the date of AZA discontinuation as the start date. In contrast, the study end date was considered the earliest date of surgery, date of hospitalization, date of initiation of a new therapy (biologic therapy or AZA), or date of the last follow-up (January 31, 2022).
We calculated overall rates of disease relapse per 10 person-years. We used a Cox regression model to estimate the hazard ratios (HRs) of disease relapse in patients according to AZA suspicion. All HRs were adjusted for sex, age, and type of disease. A P value < 0.05 was considered statistically significant for all statistical tests. Data were analyzed using STATA11 software (Stata Corp., College Station, TX, United States).
The initial data collection identified 2006 IBD patients included in our registry. A total of 366 of these patients had started AZA as monotherapy during the study period, and 274 (133 UC and 141 CD) patients were ultimately included based on our inclusion and exclusion criteria. Of the included patients, 57% were male. The average age at diagnosis for CD patients was 33.2 ± 14.2 years and 28.8 ± 14.4 years for UC patients, with a statistically significant difference between the two diseases (P = 0.01). Table 1 summarizes the main demographic and clinical characteristics of our patients at the time of enrollment. The main reason for AZA prescription was the maintenance of remission in steroid-dependent patients (> 90% in both diseases). No patient had a history of neoplasia before AZA administration.
| Characteristic | Crohn's disease | Ulcerative colitis | P value |
| Included patients | 141 | 133 | |
| Sex, % males | 54.6% | 59.4% | 0.42 |
| Mean age at IBD diagnosis in yr | 33.2 ± 14.2 | 28.8 ± 14.4 | 0.01 |
| Disease location | |||
| Upper GI-L4 | 5.7% | ||
| Small bowel-L1 | 17.8% | ||
| Ileum + colon-L3 | 61% | ||
| Pancolitis-L2 | 15.6% | 61% | |
| Left-sided colitis | 37.5% | ||
| Proctosigmoiditis | 1.5% | ||
| Perianal | 7.8% | ||
| Phenotype | |||
| Inflammatory | 56.7% | ||
| Stenosing | 24.8% | ||
| Fistulizing | 7.8% | ||
| Fistulizing + Stenosing | 5.7% | ||
| Inflammatory + Fistulizing | 2.2% | ||
| Inflammatory + Stenosing | 2.8% | ||
| Reasons for AZA introduction | 0.23 | ||
| Steroid-dependent | 95.8% | 90.2% | |
| Steroid-refractory | 2.1% | 5.3% | |
| Steroid intolerance | 0.7% | 3% | |
| Other reasons | 1.4% | 1.5% | |
| Comorbidities | |||
| PSC | 0% | 2.3% | 0.07 |
| Autoimmune rheumatic diseases | 7.8% | 3.0% | 0.08 |
| Cardiac involvement | 4.3% | 7.5% | 0.25 |
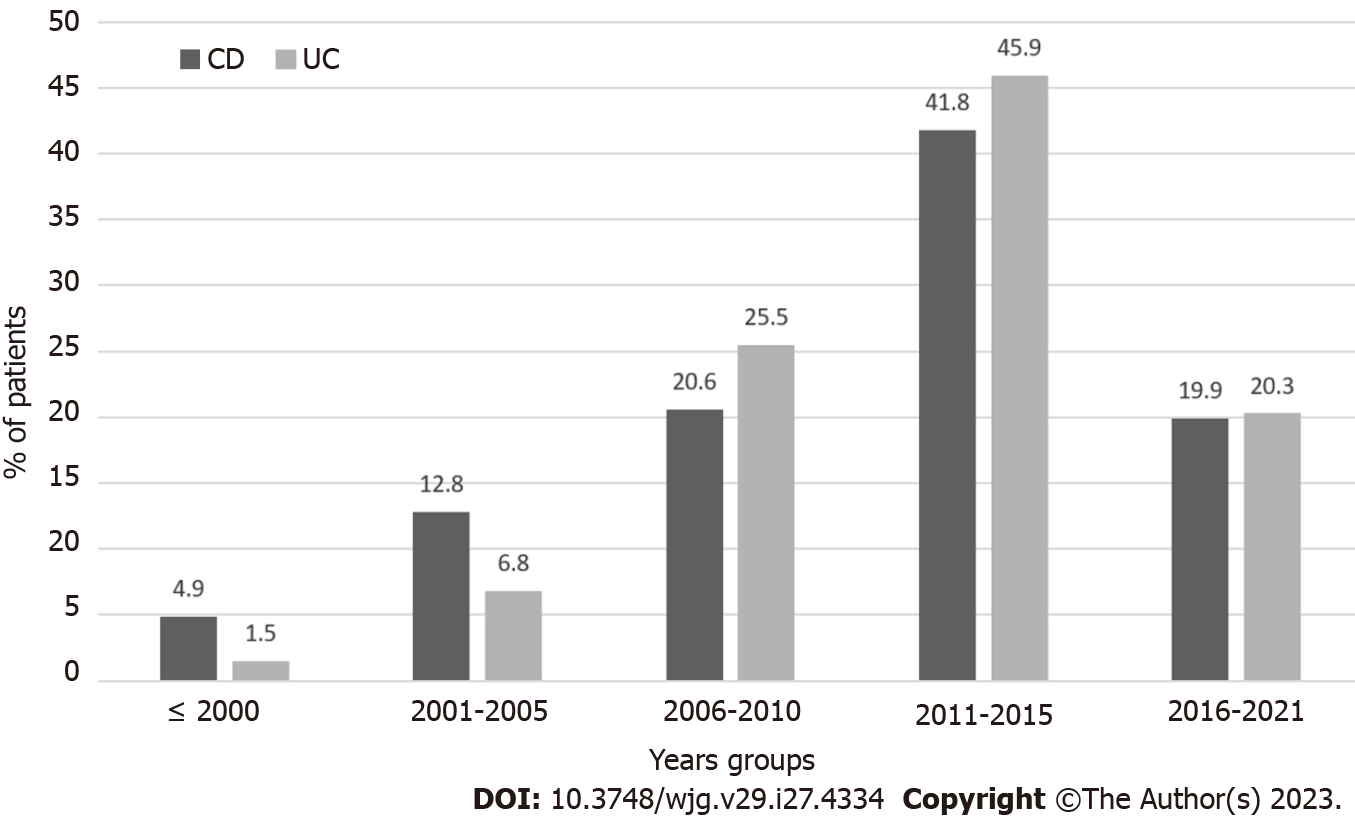
We evaluated the temporal trend of starting AZA in UC and CD patients as monotherapy during the study period. Figure 1 shows a peak in use in 2011-2015. AZA was started in 45.9% (61/133) of UC patients and 41.8% (59/141) of CD patients included in the study (Figure 1). Considering the time between the IBD diagnosis and the introduction of AZA therapy, 26.9% of CD patients started treatment with AZA within the 1st year of diagnosis, 29.8% within the 5th year, and 43.3% after the 5th year. In contrast, 12.8% of UC patients took AZA within the 1st year, 46.6% within 5 years, and 40.6% after the 5th year (P = 0.002).
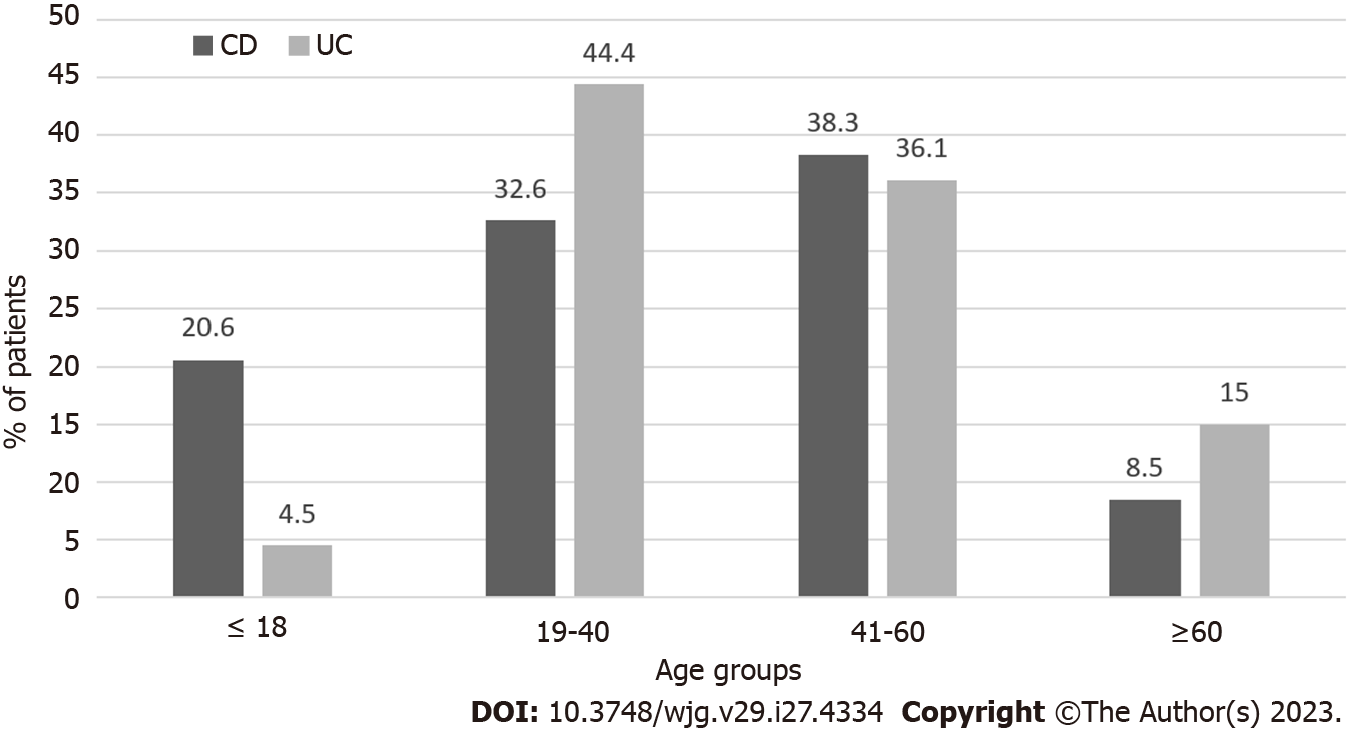
We found a different age distribution for the introduction of AZA (P = 0.001). A higher percentage of CD (20.6%) patients started taking AZA before age 18 compared to UC (4.5%) patients. Conversely, a higher percentage of UC patients (44.4%) started AZA in the age group 19-40 years compared to CD patients (32.6%) (Figure 2).
At the introduction of AZA therapy, 13 of the patients with UC (9.6%) and 17 with CD (11.6%) had never received corticosteroid therapy, and 98.5% of patients with UC and 100% of patients with CD were naïve to biologic drugs.
The duration of AZA therapy was less than 1 mo for 2 patients with UC (1.5%) and 7 patients with CD (5%) due to intolerance, and it was discontinued within 1 year in 43 patients with UC (32.3%) and 47 patients with CD (33.3%). AZA treatment was continued for 1 year to 5 years in 28.6% of UC patients and 31.9% of CD patients and continued for 5-10 years in 35 patients with UC (26.3%) and 27 patients with CD (19.1%). Therapy was administered for longer than 10 years in 15 patients with UC (11.3%) and 15 patients with CD (10.7%) (P = 0.266). Of the 274 patients undergoing treatment with AZA, 96.4% received combined therapy with oral 5-ASA. None of these patients received chronic steroid therapy.
Overall, the onset of recurrent infections was observed in 13 patients: 6 patients experienced cytomegalovirus infections; 2 patients experienced varicella-zoster virus; 1 patient contracted herpes simplex; 1 patient had candida; and 1 patient had Staphylococcus. For 2 patients, the infections had an unknown etiology. Eleven of these 13 infections (84.6%) occurred in the 1st 5 years of treatment. Two AZA-treated patients developed cancers and discontinued the treatment within 12 mo. The first cessation was due to the onset of melanoma, and the second discontinuation was due to anal squamous cell carcinoma. Another patient developed basal cell carcinoma after 108 mo of AZA therapy, which was then discontinued. The reasons for the cessation of AZA therapy are summarized in Table 2. Forty-four patients (16.1%) discontinued therapy due to inefficacy, thirty-seven patients (13.5%) discontinued therapy due to disease relapse, sixty-nine patients (25.2%) discontinued therapy due to disease remission [average time of sustained remission: 5 years and 4 mo (SD: 3.2)], and 41% discontinued therapy due to side effects.
| Reason for discontinuation | Patients, n | % of 274 total |
| Inefficacy | 44 | 16.1 |
| Relapse | 37 | 13.5 |
| Remission | 69 | 25.2 |
| Pancreatitis or isolated raised in amylase/lipase or ALT > 2×ULN | 48 | 17.5 |
| Leukopenia | 23 | 8.4 |
| Infections | 13 | 4.5 |
| Unspecified intolerance | 9 | 3.3 |
| Nausea/vomiting | 15 | 5.5 |
| Neoplasia | 3 | 1.1 |
| Asthenia | 2 | 0.7 |
| Pregnancy desire | 4 | 1.4 |
| Surgery not associated with IBD | 2 | 0.7 |
| Unspecific patient voluntary | 5 | 2.1 |
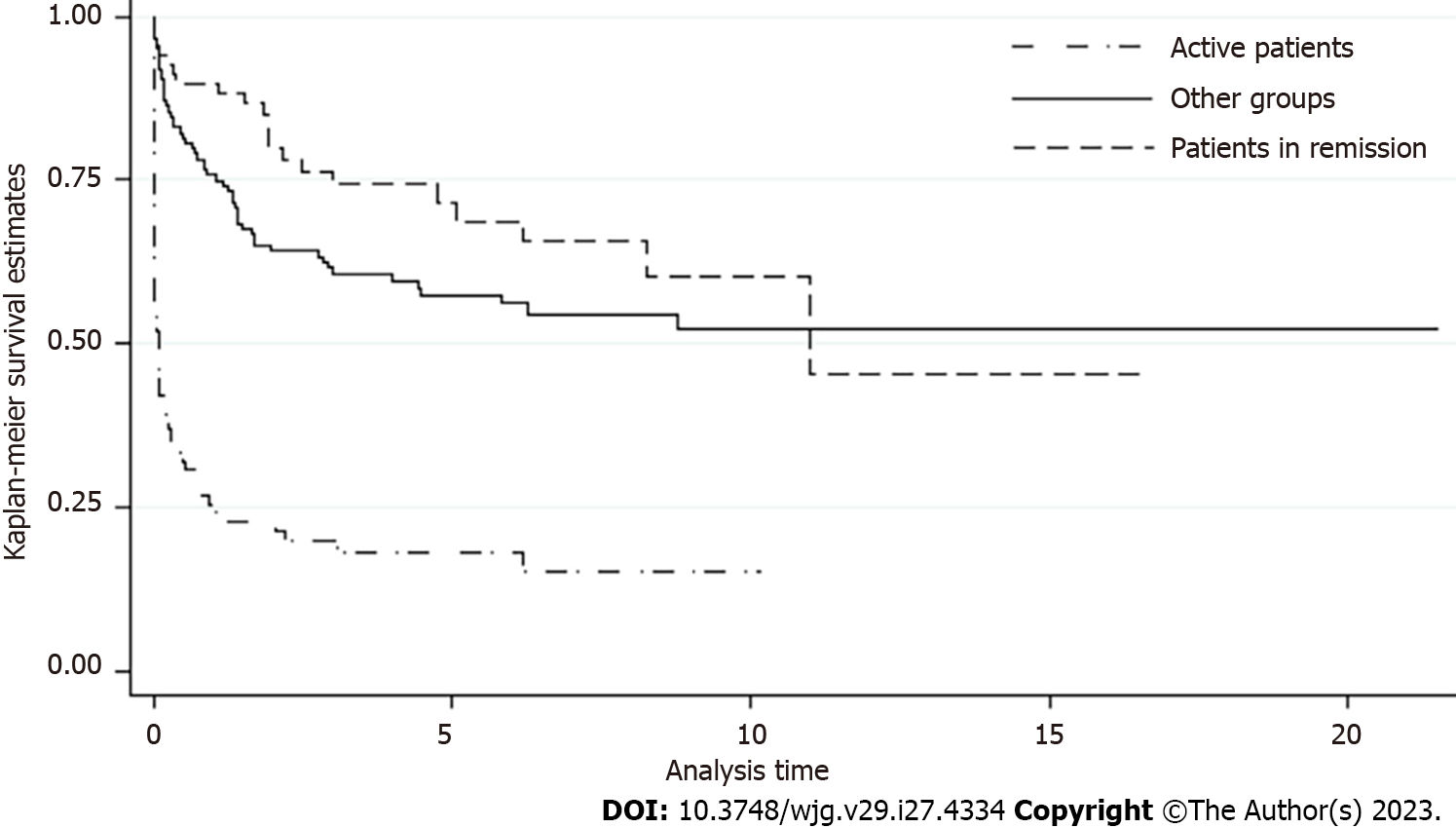
The median follow-up duration after cessation of AZA treatment was 3.5 ± 4 years. The incidence rate of disease relapse was 1.43 per 10 person-years, with no differences between sex or type of disease (Supplementary Table 1). Patients older than 35 years had a lower rate of disease relapse after AZA discontinuation than younger patients (Table 3). The highest rate of disease relapse occurred in patients who were active at the time of AZA suspension (due to inefficacy or relapse), with a rate of 7 per 10 person-years. Otherwise, patients who stopped AZA for clinical remission had an 83% lower risk of relapse during the observation time than active patients (HR: 0.17, confidence interval: 0.01-0.03). This lower risk was also confirmed after adjustment for age. Figure 3 shows a survival of 89% after 1 year and 79% at 2 years in remission patients.
| Group or subgroup | Events | Rate per 10 person-yr (95%CI) | HR (95%CI) | Adjusted HR (95%CI) |
| Entire population | 141 | 1.4 (1.2-1.7) | ||
| UC | 64 | 1.4 (1.1-1.7) | 1 | |
| CD | 77 | 1.5 (1.2-1.8) | 1.16 (0.8-1.6) | |
| Males | 85 | 1.7 (1.4-2.1) | 1 | |
| Females | 56 | 1.2 (0.9-1.5) | 0.76 (0.5-1.1) | |
| Age ≤ 35 yr | 62 | 1.9 (1.4-2.4) | 1 | |
| Age > 35 yr | 79 | 1.2 (0.9-1.5) | 0.69 (0.5-0.9) | |
| Active patients | 66 | 7.0 (5.5-8.9) | 1 | 1 |
| Patients in remission | 21 | 0.7 (0.4-1.0) | 0.17 (0.1-0.3) | 0.17 (0.1-0.3) |
| Other groups | 54 | 0.9 (0.7-1.2) | 0.25 (0.2-0.4) | 0.26 (0.1-0.4) |
IBD is an inflammatory disease of the gastrointestinal tract with a chronic intermittent course. Although biologic and small molecule therapies have become treatment mainstays, AZA remains an immunomodulating agent that is used to sustain corticosteroid-free remission or in cases of corticosteroid dependence/resistance. There is no consensus on the recommended duration, optimal dose, or cessation of AZA therapy[23]. The present study investigated events following AZA cessation.
The disease characteristics of our patients, including location and disease behavior, are consistent with published data regarding the efficacy and safety of AZA therapy in IBD[24-26]. However, we found a higher percentage of CD patients who started AZA before 18 years of age than UC patients. This significant difference may be explained by the earlier onset of CD and the different therapeutic management of pediatric UC and CD based on ECCO/ESPGHAN guidelines[25,27,28]. The frequency of initiating AZA therapy within 1 year after diagnosis was significantly higher in CD patients, which confirmed the different modes of treatment approach in the two diseases[5,6,29]. AZA was prescribed primarily between 2011 and 2015 (Figure 1). After this period, AZA prescriptions decreased with the parallel introduction of new biologics on the market, which suggests that the role of AZA in the management of IBD patients was progressively replaced by new, more potent and safer drugs, such as anti-tumor necrosis factor-α, vedolizumab or ustekinumab[5,9].
Regarding the optimal time for drug interruption, Holtmann et al[30] assessed the flare incidences and steroid doses before, during treatment and after discontinuation of AZA. They concluded that AZA continuation beyond 4 years seemed beneficial, but its discontinuation may be considered after 3-4 years in CD patients in complete remission without steroid requirement[30]. Our study showed the safety of drug continuation in half of our patients. A total of 28.6% of UC patients and 31.9% of CD patients continued AZA treatment beyond the 5th year, and 26.3% of UC patients and 19.1% of CD patients continued AZA treatment beyond the 10th year, with no difference between the two diseases. We reported 2 patients who developed cancer (melanoma and anal squamous cell carcinoma) and discontinued the treatment within 12 mo, and we reported 1 patient with basal cell carcinoma after 108 mo of AZA therapy. However, 85% of the infections required drug suspicion in the 1st 5 years of treatment.
The most common adverse events that led to AZA suspension were pancreatic and hepatic disorders, including AP, isolated raised in serum lipase and amylase or in liver enzymes, which were detected in 17.5% of patients[31]. This percentage is higher than large retrospective and prospective treatment trials, such as the SONIC trial[32]. However, only AP was considered in this study. We reported leukopenia as the second most frequent adverse effect (8.4%), which is consistent with the literature. Myelosuppression most commonly manifests as leukopenia, and the frequency in the literature varies from 2.2% to 15% of patients. The risk of recurrent infections (4.5%) was lower than the literature (7.4%-14.1%)[15]. Connell et al[33] did not observe an increased risk of cancer over the expected risk in a population of 755 patients treated with AZA for 30 years[33]. However, different studies showed the carcinogenic potential of AZA, particularly for the development of lymphoma, urinary tract cancer and skin cancer[34,35]. We did not report any case of tuberculosis, but AZA has recently been shown to be associated with this disease[36].
A recent Italian multicenter observational retrospective study investigated the relapse risk after withdrawal of AZA in UC and found that it occurred in one-third of patients beyond the 1st year after AZA withdrawal and in half of patients after 2 years of withdrawal. A higher risk of relapse disease was identified in patients with extensive colitis, those with a lack of sustained remission during AZA, and those with discontinuation due to toxicity[37]. A multicenter retrospective cohort study on 237 patients with sustained clinical remission (≥ 3 years) described moderate/severe relapse in 23% of CD patients and 12% of UC patients at 12 mo and 39% of CD patients and 26% of UC patients at 24 mo. Elevated CRP at withdrawal was associated with higher relapse rates at 12 mo for CD patients, and an elevated white cell count was predictive at 12 mo for UC patients[8]. A 2018 systematic review[38] described four randomized controlled trials with a total of 215 participants providing data on the rate of clinical relapse following the discontinuation of AZA monotherapy in CD patients[9,10,39,40]. The follow-up period was 12 mo for two studies[9,10], 18 mo for one study[39] and 24 mo for one study[40]. A total of 32.4% (36/111) of participants assigned to AZA withdrawal experienced clinical relapse compared to 13.5% (14/104) of patients assigned to therapeutic continuation. Our study revealed a lower rate of disease relapse than these previous studies and reported a risk of 10% at 1-year follow-up among the 69 patients who had interrupted AZA due to remission. In this subgroup, the suspicion occurred after an average time of 5 years and 4 mo (SD: 3.2) of sustained remission, and there were no differences between sex or type of diseases. In line with the recommendations provided by Holtmann et al[30], we suggest discontinuation of AZA after 5 years of complete remission[30].
The main strengths of our study were the inclusion of a homogeneous population followed at the same tertiary center and the very low relapse rate in the cohort. The main limitation was the small size. Moreover, our data were collected retrospectively, which is associated with the risks of missing data and recall bias. Future prospective research could examine outcomes after the withdrawal of AZA and after the long-term remission of IBD.
Our study confirmed the decreasing use of AZA in recent years due to the introduction of biological drugs and the risk of related adverse events. The present study found that the highest rate of disease relapse was observed among patients who were active at the time of AZA cessation (due to inefficacy or relapse) and patients younger than 35 years of age. Only 10% of patients who achieved a sustained remission with AZA needed a new treatment within 1 year of drug interruption.
Before the advent of biological drugs, azathioprine (AZA) was used worldwide to treat inflammatory bowel disease (IBD) patients and is still used. It is recognized that this immunomodulating agent could induce and sustain steroid-free long-term remission. However, clinicians cannot ignore the possible adverse effects of long-term AZA treatment and the risk of relapses after its discontinuation. In this retrospective study, we want to share the experience of our tertiary center with IBD patients treated with AZA.
Determining the optimal duration and cessation time helps balance the risks of long-term intake with the possibility of relapse after cessation.
In this study, we analyzed IBD patients who started and discontinued AZA. We have focused on patients' demographic and clinical characteristics, reasons for cessation, side effects, and disease incidence rate after AZA withdrawal.
We conducted a retrospective study, including IBD patients older than 18 who had started AZA between 1995 and 2022 and then discontinued for any reason and were followed at our IBD clinic. For categorical variables, we have used the χ2 test and Student's t-test for continuous variables. We have estimated disease relapse hazard ratios using the Cox regression model.
AZA discontinuation was due to primary failure or disease relapse in 30% of patients and due to disease remission in 25.2% of patients. We found that patients who discontinued AZA after a sustained remission of an average time of 5 years and 4 mo had a low risk of relapse (10%) in 1 year.
This study addresses an unanswered question: “When is it possible to discontinue AZA? How long should we wait before AZA cessation?”. Our study proves that AZA could be safely discontinued after 5 years of sustained remission because we have observed a lower relapse rate at 1-year follow-up. The main limitation of the study was the small size of patients.
For advanced evidence, future prospective research should be conducted to evaluate the long-term natural history of IBD after withdrawal of AZA.
| 1. | Mantzaris GJ. Thiopurines and Methotrexate Use in IBD Patients in a Biologic Era. Curr Treat Options Gastroenterol. 2017;15:84-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 2. | Sandborn W, Sutherland L, Pearson D, May G, Modigliani R, Prantera C. Azathioprine or 6-mercaptopurine for inducing remission of Crohn's disease. Cochrane Database Syst Rev. 2000;CD000545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 67] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Pearson DC, May GR, Fick G, Sutherland LR. Azathioprine for maintaining remission of Crohn's disease. Cochrane Database Syst Rev. 2000;CD000067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;2016:CD000478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2020;14:4-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 977] [Article Influence: 162.8] [Reference Citation Analysis (2)] |
| 6. | Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Spinelli A, Panis Y, Doherty G. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohns Colitis. 2022;16:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 663] [Article Influence: 165.8] [Reference Citation Analysis (1)] |
| 7. | Iborra M, Herreras J, Boscá-Watts MM, Cortés X, Trejo G, Cerrillo E, Hervás D, Mínguez M, Beltrán B, Nos P. Withdrawal of Azathioprine in Inflammatory Bowel Disease Patients Who Sustain Remission: New Risk Factors for Relapse. Dig Dis Sci. 2019;64:1612-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Kennedy NA, Kalla R, Warner B, Gambles CJ, Musy R, Reynolds S, Dattani R, Nayee H, Felwick R, Harris R, Marriott S, Senanayake SM, Lamb CA, Al-Hilou H, Gaya DR, Irving PM, Mansfield J, Parkes M, Ahmad T, Cummings JR, Arnott ID, Satsangi J, Lobo AJ, Smith M, Lindsay JO, Lees CW. Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther. 2014;40:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | O'Donoghue DP, Dawson AM, Powell-Tuck J, Bown RL, Lennard-Jones JE. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn's disease. Lancet. 1978;2:955-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 203] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Vilien M, Dahlerup JF, Munck LK, Nørregaard P, Grønbaek K, Fallingborg J. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn's disease: increased relapse rate the following year. Aliment Pharmacol Ther. 2004;19:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Hawthorne AB, Logan RF, Hawkey CJ, Foster PN, Axon AT, Swarbrick ET, Scott BB, Lennard-Jones JE. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 319] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 12. | Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 210] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Avallone EV, Pica R, Cassieri C, Zippi M, Paoluzi P, Vernia P. Azathioprine treatment in inflammatory bowel disease patients: type and time of onset of side effects. Eur Rev Med Pharmacol Sci. 2014;18:165-170. [PubMed] |
| 14. | Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Teml A, Schaeffeler E, Herrlinger KR, Klotz U, Schwab M. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet. 2007;46:187-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis. 2010;28:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Long MD, Herfarth HH, Pipkin CA, Porter CQ, Sandler RS, Kappelman MD. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 18. | Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (1)] |
| 19. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 767] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 20. | Narula N, Pray C, Wong ECL, Colombel JF, Marshall JK, Daperno M, Reinisch W, Dulai PS. Categorising Endoscopic Severity of Crohn's Disease Using the Modified Multiplier SES-CD [MM-SES-CD]. J Crohns Colitis. 2022;16:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Sharara AI, Malaeb M, Lenfant M, Ferrante M. Assessment of Endoscopic Disease Activity in Ulcerative Colitis: Is Simplicity the Ultimate Sophistication? Inflamm Intest Dis. 2022;7:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Rivière P, Vermeire S, Irles-Depe M, Van Assche G, Rutgeerts P, Denost Q, Wolthuis A, D'Hoore A, Laharie D, Ferrante M. Rates of Postoperative Recurrence of Crohn's Disease and Effects of Immunosuppressive and Biologic Therapies. Clin Gastroenterol Hepatol. 2021;19:713-720.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Dart RJ, Irving PM. Optimising use of thiopurines in inflammatory bowel disease. Expert Rev Clin Immunol. 2017;13:877-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1577] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 25. | Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2172] [Article Influence: 98.7] [Reference Citation Analysis (2)] |
| 26. | Yu YR, Rodriguez JR. Clinical presentation of Crohn's, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 27. | Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J, Buderus S, Martín-de-Carpi J, De Ridder L, Fagerberg UL, Hugot JP, Kierkus J, Kolacek S, Koletzko S, Lionetti P, Miele E, Navas López VM, Paerregaard A, Russell RK, Serban DE, Shaoul R, Van Rheenen P, Veereman G, Weiss B, Wilson D, Dignass A, Eliakim A, Winter H, Turner D; European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 869] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 28. | Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, Veres G, Aloi M, Strisciuglio C, Braegger CP, Assa A, Romano C, Hussey S, Stanton M, Pakarinen M, de Ridder L, Katsanos K, Croft N, Navas-López V, Wilson DC, Lawrence S, Russell RK. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67:257-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 360] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 29. | Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Holtmann MH, Krummenauer F, Claas C, Kremeyer K, Lorenz D, Rainer O, Vogel I, Böcker U, Böhm S, Büning C, Duchmann R, Gerken G, Herfarth H, Lügering N, Kruis W, Reinshagen M, Schmidt J, Stallmach A, Stein J, Sturm A, Galle PR, Hommes DW, D'Haens G, Rutgeerts P, Neurath MF. Long-term effectiveness of azathioprine in IBD beyond 4 years: a European multicenter study in 1176 patients. Dig Dis Sci. 2006;51:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Gordon M, Grafton-Clarke C, Akobeng A, Macdonald J, Chande N, Hanauer S, Arnott I. Pancreatitis associated with azathioprine and 6-mercaptopurine use in Crohn's disease: a systematic review. Frontline Gastroenterol. 2021;12:423-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2445] [Article Influence: 152.8] [Reference Citation Analysis (1)] |
| 33. | Connell WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343:1249-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 317] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 34. | Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: a UK population-based case-control study. Am J Gastroenterol. 2010;105:1604-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 36. | Fortes FML, Rocha R, Santana GO. Thiopurines are an independent risk factor for active tuberculosis in inflammatory bowel disease patients. World J Gastroenterol. 2023;29:1536-1538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Cassinotti A, Actis GC, Duca P, Massari A, Colombo E, Gai E, Annese V, D'Albasio G, Manes G, Travis S, Porro GB, Ardizzone S. Maintenance treatment with azathioprine in ulcerative colitis: outcome and predictive factors after drug withdrawal. Am J Gastroenterol. 2009;104:2760-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 38. | Boyapati RK, Torres J, Palmela C, Parker CE, Silverberg OM, Upadhyaya SD, Nguyen TM, Colombel JF. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease. Cochrane Database Syst Rev. 2018;5:CD012540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Lémann M, Mary JY, Colombel JF, Duclos B, Soule JC, Lerebours E, Modigliani R, Bouhnik Y; Groupe D'Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. A randomized, double-blind, controlled withdrawal trial in Crohn's disease patients in long-term remission on azathioprine. Gastroenterology. 2005;128:1812-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Wenzl HH, Primas C, Novacek G, Teml A, Öfferlbauer-Ernst A, Högenauer C, Vogelsang H, Petritsch W, Reinisch W. Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn's disease. Dig Dis Sci. 2015;60:1414-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alex G, Australia; Moon W, South Korea; Yu CH, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yu HG