Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4186
Peer-review started: February 11, 2023
First decision: March 20, 2023
Revised: March 25, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: July 14, 2023
Processing time: 141 Days and 19.8 Hours
Radical resection remains an effective strategy for patients with hepatocellular carcinoma (HCC). Unfortunately, the postoperative early recurrence (recurrence within 2 years) rate is still high.
To develop a radiomics model based on preoperative contrast-enhanced computed tomography (CECT) to evaluate early recurrence in HCC patients with a single tumour.
We enrolled a total of 402 HCC patients from two centres who were diagnosed with a single tumour and underwent radical resection. First, the features from the portal venous and arterial phases of CECT were extracted based on the region of interest, and the early recurrence-related radiomics features were selected via the least absolute shrinkage and selection operator proportional hazards model (LASSO Cox) to determine radiomics scores for each patient. Then, the clinico
A total of 1915 radiomics features were extracted from CECT images, and 31 of them were used to determine the radiomics scores, which showed a significant difference between the early recurrence and nonearly recurrence groups. Univariate and multivariate Cox regression analyses showed that radiomics scores and serum alpha-fetoprotein were independent indicators, and they were used to develop a combined model to predict early recurrence. The area under the receiver operating characteristic curve values for the training and validation cohorts were 0.77 and 0.74, respectively, while the C-indices were 0.712 and 0.674, respectively. The calibration curves and decision curve analysis showed satisfactory accuracy and clinical utilities. Kaplan-Meier curves based on recurrence-free survival and overall survival showed significant differences.
The preoperative radiomics model was shown to be effective for predicting early recurrence among HCC patients with a single tumour.
Core Tip: Hepatocellular carcinoma (HCC) is a growing health issue worldwide, ranking sixth in incidence and third in mortality among all cancers. Moreover, due to the high malignancy and suppressive immune microenvironment of HCC, there remain high recurrence and metastasis rates. Therefore, we developed a radiomics model based on preoperative contrast-enhanced computed tomography to evaluate early recurrence in HCC patients with a single tumour.
- Citation: Li SQ, Su LL, Xu TF, Ren LY, Chen DB, Qin WY, Yan XZ, Fan JX, Chen HS, Liao WJ. Radiomics model based on contrast-enhanced computed tomography to predict early recurrence in patients with hepatocellular carcinoma after radical resection. World J Gastroenterol 2023; 29(26): 4186-4199
- URL: https://www.wjgnet.com/1007-9327/full/v29/i26/4186.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i26.4186
Hepatocellular carcinoma (HCC) is a growing health issue worldwide, ranking sixth in incidence and third in mortality among all cancers[1]. For early-stage HCC patients, liver resection and liver transplantation are considered the main strategies to prolong overall survival (OS)[2,3]. However, the majority of HCC patients are diagnosed at later disease stages, and thus, surgical care is no longer a treatment option for these individuals[4]. Moreover, due to the high malignancy and suppressive immune microenvironment of HCC[5], there remain high recurrence and metastasis rates even for patients who undergo resection. Therefore, it is of vital importance to conduct systematic surveillance of HCC recurrence, and there is an urgent need to precisely predict recurrence in patients with HCC.
Currently, the recurrence rate for postoperative HCC patients is over 50% within 5 years[6], and studies have shown that patients with early recurrence – i.e., recurrence occurring within 2 years – have worse outcomes and poorer prognosis than those with recurrence over 2 years[7]. Typically, the identification of primary and recurrent tumour depends on the detection of genotype, in other words, on the molecular scale[8,9]. However, its complexities have limited the widespread adoption of this method. To evaluate the prognosis of HCC patients, many staging systems have been introduced to clinical use, such as the Barcelona Clinic Liver Cancer (BCLC) staging system and albumin-bilirubin (ALBI) grading[4,10]. Given that the staging systems above are more suitable for use in evaluating liver function or guiding treatment, there is a lack of an efficient and accurate approach to evaluate recurrence.
Radiomics is an emerging discipline based on medical imaging. Medical imaging, which includes ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI), has played a crucial role in the screening and diagnosis of HCC[11]. However, the conventional imaging process relies on the experience and judgement of radiologists and might miss or be hard to quantify some crucial details. Many studies have shown that radiomics features may contain pathophysiological characteristics, which reflect tumour heterogeneity and are associated with patient prognosis[10]. Based on artificial intelligence technology, radiomics has shown certain potential in HCC related gene analysis, microvascular invasion, postoperative survival and recurrence prediction, etc[12-16]. However, there are few externally validated reports that studied on the use of radiomics to assess the risk of early recurrence in HCC patients after surgery.
In this study, we aimed to establish a radiomics model to predict early recurrence for patients who underwent radical resection based on contrast-enhanced CT (CECT) and clinical variables, evaluate this model’s performance, and verify its feasibility in clinical application.
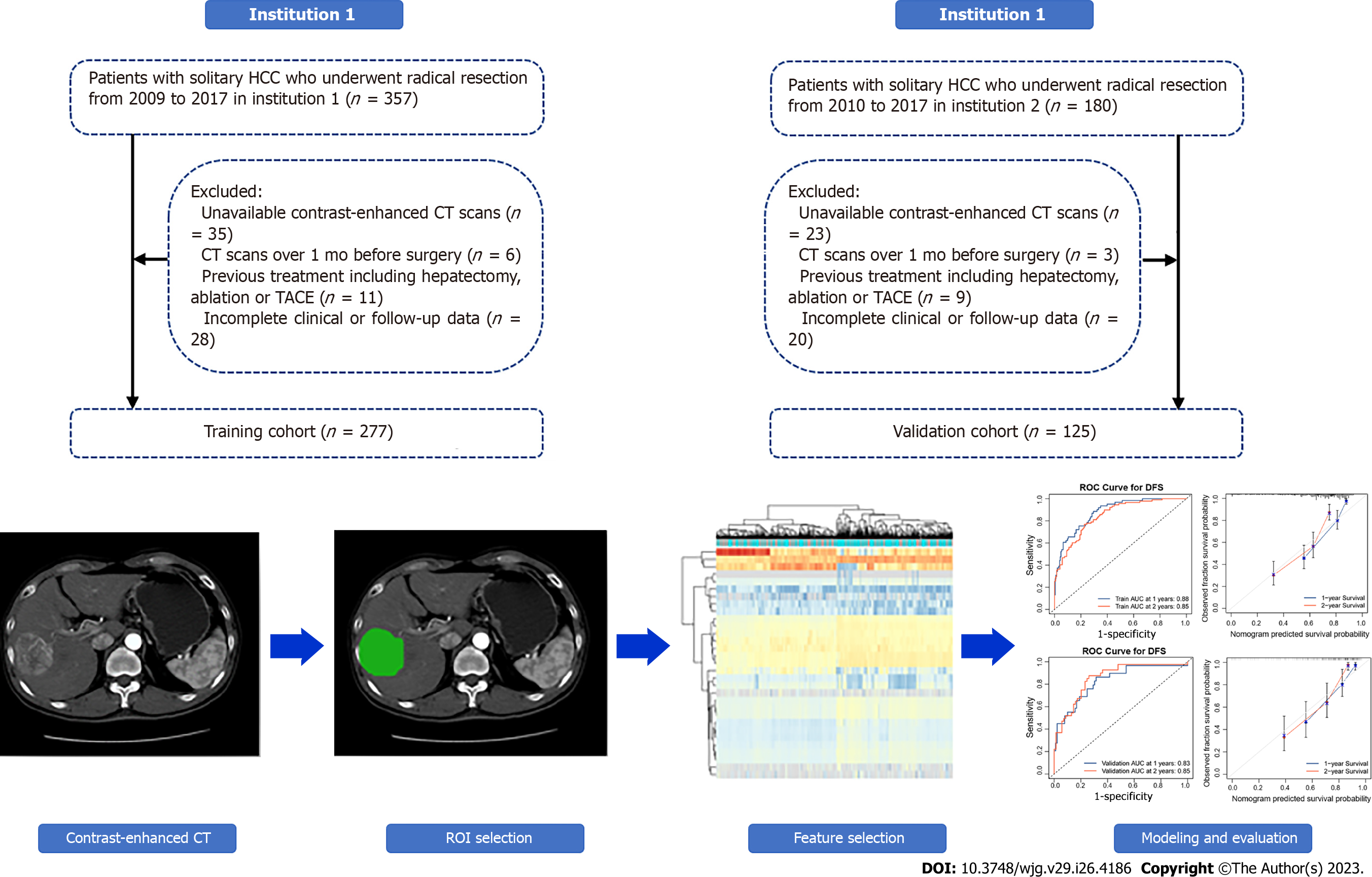
We retrospectively enrolled 537 HCC patients from two institutions (Affiliated Hospital of Guilin Medical University; Peking University People’s Hospital) who underwent radical resection. According to the exclusion criteria in Figure 1, 277 patients enrolled from October 2009 to May 2017 at the Affiliated Hospital of Guilin Medical University were set as the training cohort, while 125 patients enrolled from June 2010 to December 2017 at Peking University People's Hospital were set as the validation cohort. Along with the CECT images, information regarding demographic characteristics, clinicopathological data, and laboratory data were also retrospectively collected. Patient demographic characteristics included sex and age, while clinicopathological data were collected from electronic case records, containing tumour size and vascular invasion. Furthermore, laboratory data were collected before surgery, including routine blood test, liver function, and alpha-fetoprotein (AFP) level.
All enrolled patients were diagnosed with HCC by postoperative pathological examination; solitary HCC was confirmed by preoperative CECT and intraoperative palpation or US. In addition to CECT, at least US and MRI supported an HCC diagnosis. Radical resection was defined as complete removal of the tumour with no residual tumour or new lesion observed in two observations at an interval of no less than 4 wk. Two independent pathologists histopathologically examined resected tumour specimens. The main exclusion criteria were as follows: (1) CECT image unavailable; (2) CECT scan over 1 mo before operation; (3) previous treatment including hepatectomy, ablation, or transarterial chemoembolization; and (4) incomplete clinical or follow-up data.
Postoperative surveillance at each institution was conducted according to the protocol. Patients were followed every 2 mo within the first 2 years and every 3-6 mo after 2 years postoperatively (abdominal ultrasonography, serum AFP, and other serological tests were performed). CECT or MRI examination was performed if recurrence was suspected. OS was determined as the interval between the operation date and the date of death or last follow-up. Recurrence-free survival (RFS) was determined from the operation date of radical surgery to the date of the first recurrence at any site, death, or the last follow-up. Recurrence within 2 years after operation was considered as early recurrence.
The CECT images were obtained from each institution according to routine procedures, and the usage of contrast medium is described in the Supplementary material.
Regions of interest (ROIs) were defined as the tumour areas in both arterial and portal venous phases, which were delineated via 3D Slicer (v4.11, https://www.slicer.org) by two experienced radiologists (Reader 1: H.Y. with 10 years of working experience; reader 2: Z.Z. with 20 years of working experience). If they could not reach a consensus, the third radiologist intervened in the evaluation.
To minimize the variability and normalize the CT images, all the images were resampled to a voxel size of 1 mm × 1 mm × 1 mm. Then, the radiomics features were extracted from the ROI segments based on the Pyradiomics package (version 3.0.1). The categories of radiomics features were as follows: First-order statistics, shape-based features (2D and 3D), grey level cooccurrence matrix (GLCM), grey level run length matrix (GLRLM), grey level size zone matrix (GLZM), neighbouring grey tone difference matrix (NGTDM), and grey level dependence matrix (GLDM). LASSO Cox regression was performed to filter and obtain the early recurrence-related radiomics features according to the minimum criteria with 10-fold cross-validation. The radiomics score was determined according to the coefficients.
A radiomics and clinical combined model was developed via univariate and multivariate analyses, in which the optimal cut-off value of the radiomics score was determined via X-tile (Version 3.6.1). The candidate clinical indicators were hepatitis B surface antigen (HBsAg) (positive vs negative), cirrhosis (present vs absent), microvascular invasion (MVI) (present vs absent), serum AFP (> 200 vs ≤ 200 ng/mL), tumour size (cm), platelets (× 109/L), gamma-glutamyl transpeptidase (GGT) (U/L), and ALBI. The combined model was presented as a nomogram and validated in an independent cohort.
Continuous variables that follow a normal distribution are shown as the mean ± SD and were compared by using the Student’s t test. Otherwise, the Mann-Whitney U test was performed. Categorical variables were compared by chi-squared tests. RFS and OS analyses were conducted by using the Kaplan-Meier method and compared by log-rank test among different groups. Time-dependent receiver operating characteristic (ROC) curves were plotted based on the timeROC package. The concordance index (C-index) was determined to evaluate the predictive ability of the combined model. The calibration curves were plotted with the rms package, while decision curve analysis (DCA) was based on the rmda package. SPSS18.0 (SPSS Inc., Chicago, IL, United States) and R (version 4.0.3, https://www.rproject.org/) were used for statistical analyses. P < 0.05 was considered statistically significant.
A total of 402 patients who underwent preoperative CECT were enrolled according to the exclusion criteria from two institutions, and the baseline characteristics are illustrated in Table 1. There were no significant differences in the variables evaluated between the two cohorts except aspartate aminotransferase (AST); most patients were male (both 85.60%), with liver cirrhosis (92.06% and 93.60%) and HBV-related HCC (83.75% and 82.40%, respectively). In institution 1, the median follow-up time was 54.2 mo, the median OS time was 40.0 mo, and the early recurrence rate was 36.82% (102 recurred among 277). In institution 2, the median follow-up time was 50.7 mo, the OS time was 37.0 mo, and the early recurrence rate was 32.80% (41 recurred among 125).
| Parameter | Training cohort, n = 277 | Validation cohort, n = 125 | P value | |
| Gender | Female | 40 (14.44) | 18 (14.40) | 0.991 |
| Male | 237 (85.56) | 107 (85.60) | ||
| Age (yr) | 50.60 ± 11.27 | 51.92 ± 10.50 | 0.265 | |
| HBsAg | Negative | 45 (16.25) | 22 (17.60) | 0.736 |
| Positive | 232 (83.75) | 103 (82.40) | ||
| Child-Pugh stage | A | 252 (90.97) | 116 (92.80) | 0.543 |
| B | 25 (9.03) | 9 (7.20) | ||
| BCLC stage | 0 + A | 173 (62.45) | 83 (66.40) | 0.447 |
| B + C | 104 (37.55) | 42 (33.60) | ||
| Cirrhosis | Absent | 22 (7.94) | 8 (6.40) | 0.586 |
| Present | 255 (92.06) | 117 (93.60) | ||
| MVI | Absent | 162 (58.48) | 75 (60.00) | 0.775 |
| Present | 115 (41.52) | 50 (40.00) | ||
| AFP (ng/mL) | ≤ 200 | 130 (46.93) | 67 (53.60) | 0.204 |
| > 200 | 147 (53.07) | 58 (46.40) | ||
| Tumor size (cm) | 7.42 ± 4.51 | 7.07 ± 4.03 | 0.464 | |
| Platelets (× 109/L) | 198.26 ± 94.67 | 201.52 ± 86.77 | 0.743 | |
| GGT (U/L, median, IQR) | 73.90 (41.74, 130.23) | 80.35 (46.92, 151.00) | 0.198 | |
| ALT (U/L, median, IQR) | 32.80 (21.11, 51.70) | 29.51 (20.50, 48.82) | 0.176 | |
| AST (U/L, median, IQR) | 36.47 (26.75, 53.80) | 35.04 (25.90, 51.60) | 0.449 | |
| TB (mg/dL) | 17.41 ± 26.52 | 17.40 ± 23.84 | 0.995 | |
| DB (mg/dL) | 7.99 ± 17.54 | 8.92 ± 23.26 | 0.658 | |
| ALBI | -2.50 ± 0.47 | -2.53 ± 0.42 | 0.493 |
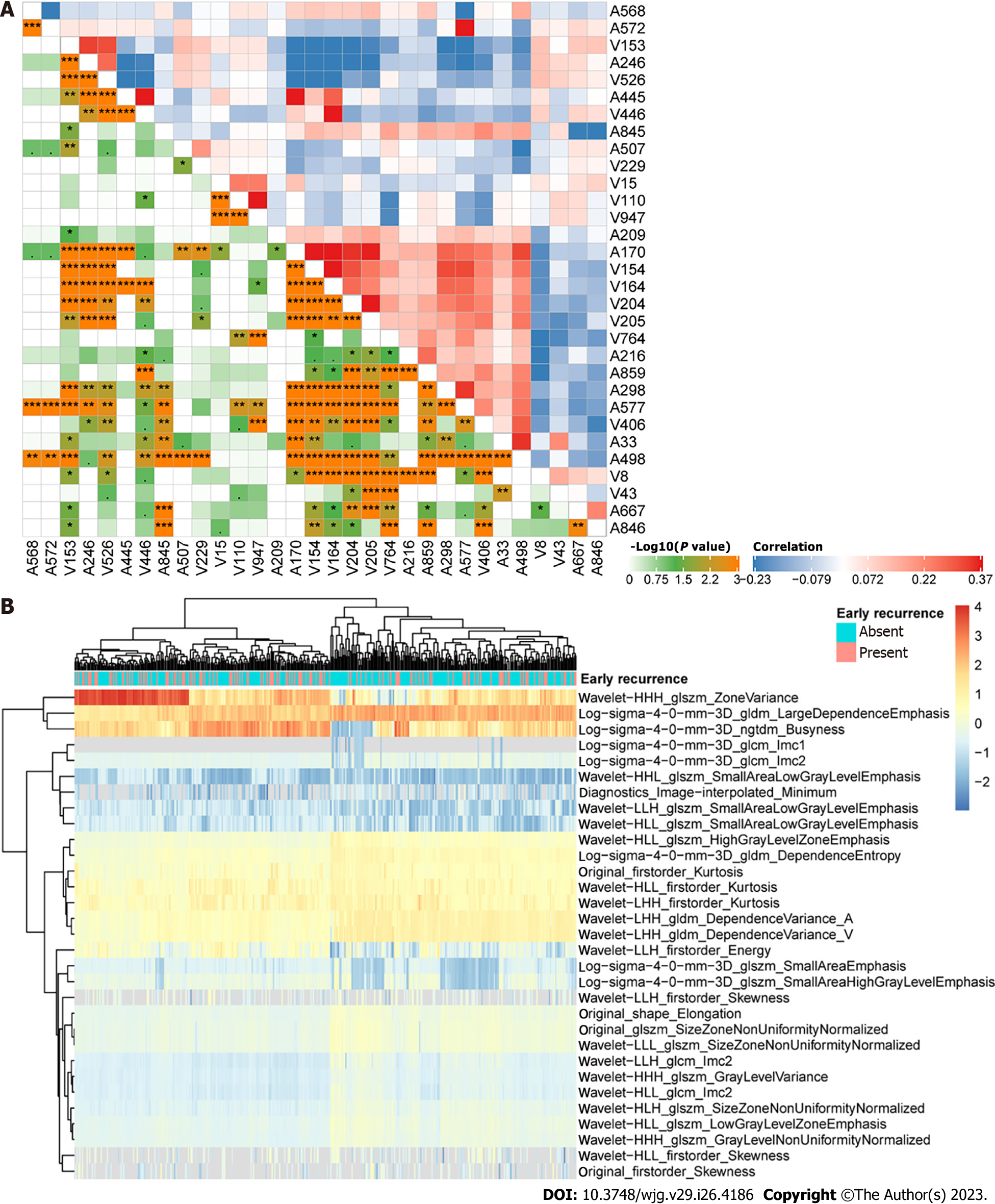
A total of 1915 CECT-based radiomics features were extracted from images acquired at both the arterial and portal venous phases among 277 patients in institution 1 as the training cohort. To filter early recurrence-related features, LASSO Cox regression was performed, and 31 features were obtained (Supplementary Figure 1). Figure 2 displays the distribution and correlations among these features. Then, a radiomics score was determined according to the coefficients in Supplementary Table 1 for each patient, and the optimal cut-off value was identified as 15.93, based on which the low and high radiomics risk groups were divided accordingly. As shown in Figure 3A, the radiomics scores were significantly different between the early and nonearly recurrence groups, while the area under curve (AUC) value was 0.73 for both institutions (Figure 3B), thus demonstrating the predictive value of the radiomics score for early recurrence HCC.
To further improve the predictive performance of the model, univariate and multivariate Cox regression analyses were conducted among the radiomics model and clinicopathologic indicators. The results from univariate analysis (Table 2) illustrated that the radiomics score, MVI, serum AFP, tumour size, and GGT were statistically significant and were included in the multivariate analysis. Multivariate analysis showed that radiomics score [hazard ratio (HR), 3.86; 95%CI: 2.41-6.16; P < 0.001] and serum AFP (HR, 1.52; 95%CI: 1.00-2.30; P = 0.048) were identified as independent predictive indicators for early HCC recurrence.
| Variable | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Rad-score (high vs low) (n) | 4.08 | 2.64-6.24 | < 0.001 | 3.86 | 2.41-6.16 | < 0.001 |
| HBsAg (present vs absent) (n) | 1.81 | 0.97-3.39 | 0.063 | |||
| Cirrhosis (present vs absent) (n) | 1.84 | 0.75-4.52 | 0.183 | |||
| MVI (present vs absent) (n) | 1.83 | 1.24-2.71 | 0.002 | 1.20 | 0.77-1.85 | 0.418 |
| AFP (> 200 vs ≤ 200) (ng/mL) | 1.73 | 1.16-2.58 | 0.007 | 1.52 | 1.00-2.30 | 0.048 |
| Tumor size (cm) | 1.05 | 1.01-1.09 | 0.005 | 1.00 | 0.94-1.04 | 0.621 |
| Platelets (× 109/L) | 1.00 | 1.00-1.00 | 0.160 | |||
| GGT (U/L) | 1.02 | 1.04-1.02 | 0.034 | 1.01 | 1.01-1.00 | 0.333 |
| DB (mg/dL) | 1.00 | 1.00-1.01 | 0.928 | |||
| ALBI | 0.96 | 0.64-1.44 | 0.838 | |||
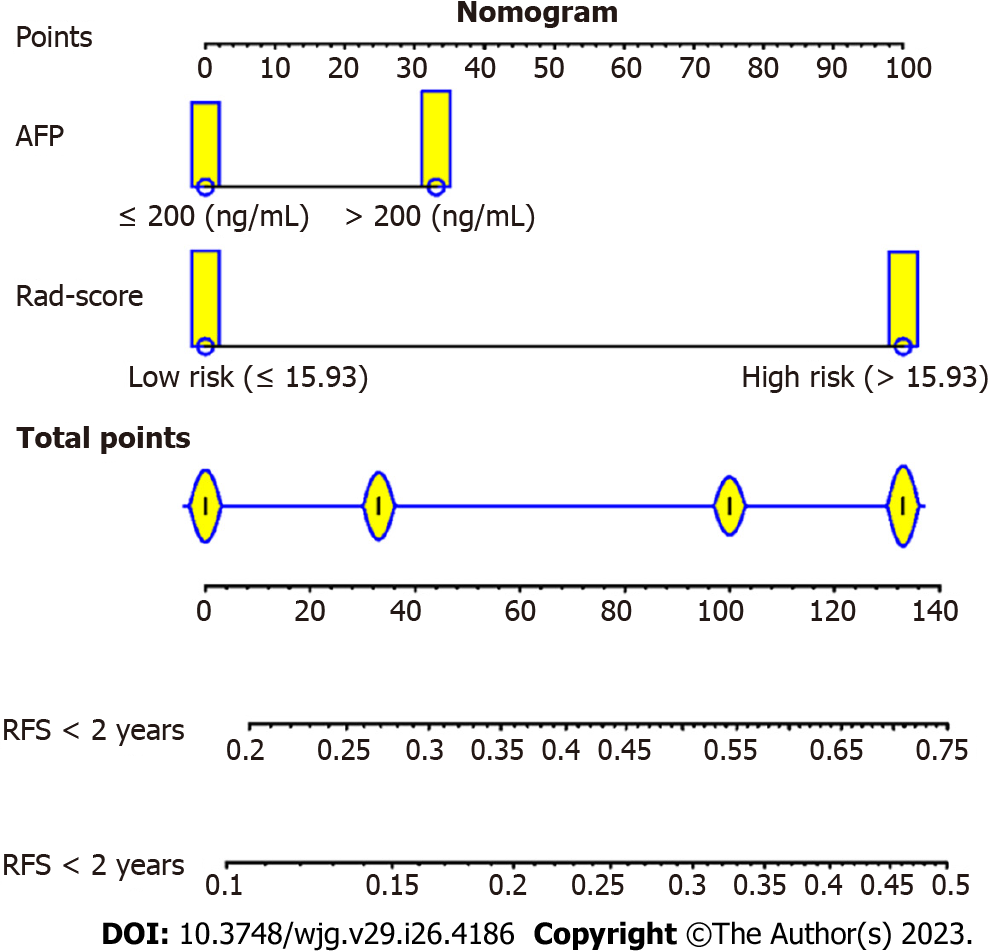
Next, a predictive and visible nomogram was developed based on the radiomics and clinical combined model (Figure 4), and the AUC values of the combined model were 0.79 (95%CI: 0.73-0.84) and 0.77 (95%CI: 0.70-0.83) for 1-year and 2-year recurrence prediction in the training cohort (Figure 5A), respectively. The AUC values of the validation cohort were 0.65 (95%CI: 0.58-0.73) and 0.74 (95%CI: 0.63-0.77) for 1-year and 2-year recurrence, respectively (Figure 5B). The C-index was 0.712 and 0.674 in the training and validation cohorts, respectively. Moreover, the calibration curves illustrated good agreement between predictive and observed outcomes (Figure 5C and D), while the DCA showed that the combined model had a relative high net benefit (Figure 5E and F).
To verify the discrimination ability of the combined model, survival curves for RFS and OS were plotted based on the median risk points in the training and validation cohorts. For early recurrence, the high-risk group was more likely to present recurrence than the low-risk group in both the training and validation cohorts (log-rank, P < 0.0001 for both) (Figure 6A and C). Furthermore, OS was significantly different (log-rank, P < 0.0001 for the training cohort and P = 0.0446 for the validation cohort) (Figure 6B and D).
Next, we performed subgroup analysis in AFP-negative (< 20 ng/mL) and AFP-positive (≥ 20 ng/mL) groups. Regardless of the AFP level (Figure 6E-H), the model calculated points were closely related to early recurrence and OS (log-rank, P < 0.0001 for all). The results above illustrated the discrimination ability of the combined model.
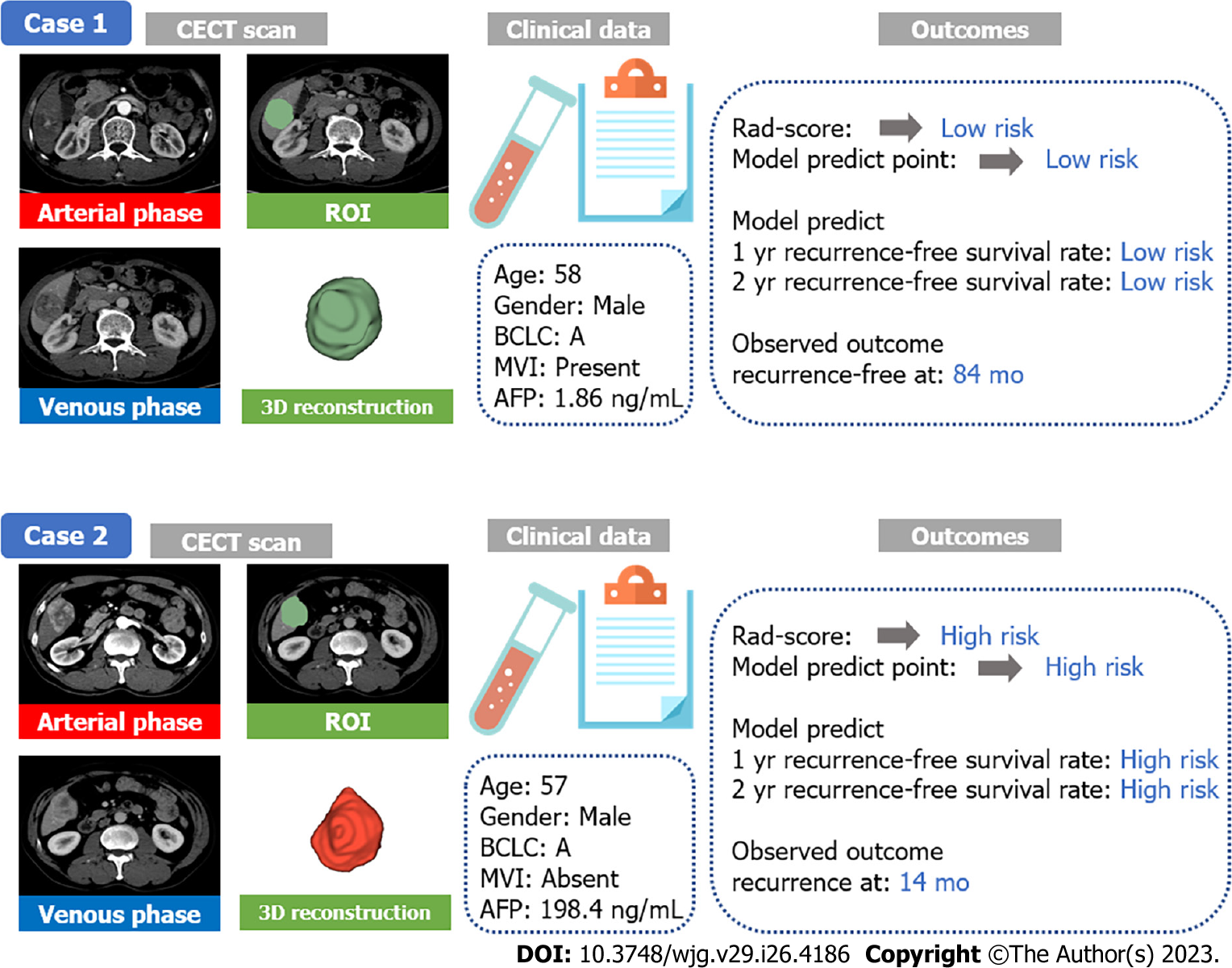
Two representative case reports were displayed to demonstrate the distinguishing ability for similar cases (Figure 7). Both of them were male with BCLC A stage HCC and similar ages, and the ROI, also known as the tumour area, was similar in tumour size and position. MVI was present in AFP-negative case 1, while MVI was absent in AFP-positive case 2. However, their outcomes were different. Case 1 was both low risk at the radiomics score and model prediction point and without recurrence until the last follow-up. Case 2 was high risk with worse outcomes, and recurrence was present at 14 mo after the operation. Thus, we concluded that the radiomics combined model could support to distinguish similar cases using CECT in clinical practice.
Due to the lack of indicators to predict early recurrence for patients who underwent radical resection, we determined a radiomics score based on preoperative CECT for patients from two independent centres, which was shown to be closely related to the occurrence of early recurrence. Next, serum AFP was included to establish a radiomics and clinical combined model, which elevated the accuracy of the model. The AUC values for the two cohorts were 0.77 and 0.74, respectively, and our findings supported that radiomics is able to be adopted to evaluate the RFS of HCC patients and might support preoperative risk stratification and postoperative surveillance management. Previous studies have illustrated the usage of radiomics in predicting recurrence of HCC[15,16], but few studies focused on early recurrence and conducted external validation, in which early recurrence was defined as recurrence occurring within 2 years. Furthermore, the model divided the HCC patients into two risk subgroups with a favourable distinction of recurrence and a significant difference in OS. AFP-negative subgroup analysis was also performed to evaluate the reliability of the model.
The heterogeneity in primary and recurrent HCC is quite different. Typically, the gene profile and microenvironments are supposed to be able to distinguish them[8,9]. Villanueva et al[17] developed a composite prognostic model for HCC recurrence based on gene expression in tumour tissues. However, the majority of recurrent HCC cases occur within 2 years after surgery, which is regarded as real recurrence. Many studies have indicated that patients with early recurrence have worse outcomes than those with late recurrence[18]. Hence, it is of vital significance to monitor early recurrence in clinical practice. Increasing evidence has illustrated that the immune microenvironment plays a crucial role in the early recurrence of HCC. Sun et al[19] revealed the unique characteristics of early relapse HCC at single-cell resolution. Additionally, metabolic microenvironments, such as lipogenesis and glycolysis, might promote oncogenic transformation[20-23]. The mechanism above reveals the complexity of recurrent HCC. Many staging systems have been applied in the diagnosis and treatment of HCC, including the AJCC TNM staging system and the BCLC and Child-Pugh staging systems. Nevertheless, none of them are capable of predicting recurrence. Serum AFP level has long been used as an indicator to predict recurrence in both hepatectomy and liver transplantation[24,25], which showed effect in our model, as well.
Preoperative imaging plays a very important role in the effective diagnosis and treatment of HCC, and CECT is part of this process. For BCLC stages A and B patients, liver resection is considered the main treatment strategy. However, the postoperative recurrence rate is extremely high. Hence, we employed radiomics to explore more details from CECT images. In this study, radiomics scores were determined based on the 31 features. There were 16 features extracted from images in the arterial phase and 15 features from images in the portal venous phase, which was consistent with the diagnosis of HCC depending on the dynamic changes in vascular findings from the arterial phase to portal venous phase[26-29]. Consistently, among these features, most of them were based on wavelet filtered features that accounted for the greatest weight, which coincided with the previous studies[15], and might represent tumour heterogeneity of HCC and peritumoral tissues that were hard to explain and easy to be ignored by the radiologist. The potential use of radiomics has been widely reported. Hence, we combined radiomics and serum indicators to evaluate the risk of early recurrence, which was validated in an independent cohort. Our findings showed favourable value in predicting early recurrence for patients with HCC via non-invasive indicators and might guide clinical decisions.
There were several limitations in our study. First, because this was a retrospective analysis, inherent biases were inevitable. Second, even though the radiomics features were extracted via a standard process, the features might differ and be critically dependent across CT machines and centres. Third, with the development of multimodality radiomics, Gd-EOB-DTPA-enhanced MR or 18F-FDG PET/CT has been widely used and have more favourable discrimination in adipose tissue and tumour capsules[30,31]. Therefore, we should conduct prospective trials with multimodality radiomics to confirm our results.
In summary, the radiomics scores calculated herein revealed significant differences between early and nonearly recurrence HCC patients. Then, we integrated the scores with serum AFP to develop a radiomics and clinical combined model that showed advantages and might serve as a powerful tool to predict early recurrence of HCC. We will perform further validation and translate the results into clinical application.
Hepatocellular carcinoma (HCC) seriously endangers human life and health, but there is still a lack of satisfactory treatment options. Even if it is diagnosed at early stage, the recurrence rate is still very high. The clinical monitoring strategy for HCC recurrence is limited, so there is a need to find a new and effective recurrence prediction model for HCC. And we developed a radiomics model based on preoperative contrast-enhanced computed tomography (CECT) to evaluate early recurrence in patients with a single tumour.
Due to the high malignancy and suppressive immune microenvironment of HCC, there are still high recurrence and metastasis rates even in HCC patients who have undergone radical resection. Therefore, it is of vital importance to conduct systematic surveillance of HCC recurrence, and there is an urgent need to precisely predict recurrence in patients with HCC. If tumour recurrence can be detected earlier, the survival and quality of life of HCC patients might be greatly improved.
Despite the rapid development in the treatment of HCC in recent decades, patients’ outcomes remain unsatisfactory. One of the reasons is that the early diagnosis system of HCC recurrence is not yet well developed, so our research team established a recurrence prediction model for HCC based on medical imaging such as computed tomography (CT) to predict HCC recurrence earlier, so that timely treatment measures can be taken.
We collected CT images from 537 clinical patients in two institutions and extracted valuable CT image features with 3D Slicer (v4.11, https://www.slicer.org). SPSS18.0 (SPSS Inc., Chicago, IL, United States) and R (version 4.0.3, https://www.rproject.org/) were used for statistical analyses and the prediction model of HCC recurrence was established jointly with AFP.
The radiomics scores calculated herein revealed significant differences between early and nonearly recurrence HCC patients. We combined radiomics and serum indicators to evaluate the risk of early recurrence, which was validated in an independent cohort. Our findings showed the value of predicting early recurrence in HCC patients by noninvasive indicators and might guide clinical decisions making. However, this is a retrospective analysis, and inherent biases are inevitable. We would conduct prospective trials with multimodality radiomics to confirm our results in future.
The preoperative radiomics model was shown to be effective for predicting early recurrence among patients with single HCC. Compared with pathological biopsy or other tests, this model is noninvasive and more convenient for HCC patients.
For HCC diagnosis, treatment, or prognosis assessment, more non-invasive methods are of great significance and needed.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68750] [Article Influence: 13750.0] [Reference Citation Analysis (201)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6431] [Article Influence: 803.9] [Reference Citation Analysis (10)] |
| 3. | Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 4. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3374] [Article Influence: 482.0] [Reference Citation Analysis (45)] |
| 5. | Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, Tang W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 321] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 6. | Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 725] [Article Influence: 65.9] [Reference Citation Analysis (1)] |
| 8. | Chen PJ, Chen DS, Lai MY, Chang MH, Huang GT, Yang PM, Sheu JC, Lee SC, Hsu HC, Sung JL. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology. 1989;96:527-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Ng IO, Guan XY, Poon RT, Fan ST, Lee JM. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol. 2003;199:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2183] [Article Influence: 198.5] [Reference Citation Analysis (0)] |
| 11. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 3956] [Article Influence: 439.6] [Reference Citation Analysis (0)] |
| 12. | Kitao A, Matsui O, Zhang Y, Ogi T, Nakada S, Sato Y, Harada K, Yoneda N, Kozaka K, Inoue D, Yoshida K, Koda W, Yamashita T, Kaneko S, Kobayashi S, Gabata T. Dynamic CT and Gadoxetic Acid-enhanced MRI Characteristics of P53-mutated Hepatocellular Carcinoma. Radiology. 2023;306:e220531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 13. | Xu X, Zhang HL, Liu QP, Sun SW, Zhang J, Zhu FP, Yang G, Yan X, Zhang YD, Liu XS. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 546] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 14. | Liu Q, Li J, Liu F, Yang W, Ding J, Chen W, Wei Y, Li B, Zheng L. A radiomics nomogram for the prediction of overall survival in patients with hepatocellular carcinoma after hepatectomy. Cancer Imaging. 2020;20:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Ji GW, Zhu FP, Xu Q, Wang K, Wu MY, Tang WW, Li XC, Wang XH. Radiomic Features at Contrast-enhanced CT Predict Recurrence in Early Stage Hepatocellular Carcinoma: A Multi-Institutional Study. Radiology. 2020;294:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Wang C, Zheng D, Liao Y, Wang X, Huang Z, Zhong Q. Radiomics nomogram based on multi-parametric magnetic resonance imaging for predicting early recurrence in small hepatocellular carcinoma after radiofrequency ablation. Front Oncol. 2022;12:1013770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M, Kumada H, Thung SN, Bruix J, Newell P, April C, Fan JB, Roayaie S, Mazzaferro V, Schwartz ME, Llovet JM. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140:1501-12.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 18. | Yamamoto Y, Ikoma H, Morimura R, Konishi H, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Kubota T, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Sakakura C, Ochiai T, Otsuji E. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol. 2015;21:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, Huang Y, Wei X, Shi Y, Zhao Z, Li Y, Guo Z, Yu Q, Xu L, Volpe G, Qiu S, Zhou J, Ward C, Sun H, Yin Y, Xu X, Wang X, Esteban MA, Yang H, Wang J, Dean M, Zhang Y, Liu S, Yang X, Fan J. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404-421.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 606] [Article Influence: 121.2] [Reference Citation Analysis (0)] |
| 20. | Zuo X, Chen Z, Gao W, Zhang Y, Wang J, Cao M, Cai J, Wu J, Wang X. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol. 2020;13:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 337] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 21. | Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, Brozzetti S, Staniscia T, Chen X, Dombrowski F, Evert M. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 492] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 22. | Gao Q, Zhu H, Dong L, Shi W, Chen R, Song Z, Huang C, Li J, Dong X, Zhou Y, Liu Q, Ma L, Wang X, Zhou J, Liu Y, Boja E, Robles AI, Ma W, Wang P, Li Y, Ding L, Wen B, Zhang B, Rodriguez H, Gao D, Zhou H, Fan J. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell. 2019;179:561-577.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 23. | Li Q, Pan X, Zhu D, Deng Z, Jiang R, Wang X. Circular RNA MAT2B Promotes Glycolysis and Malignancy of Hepatocellular Carcinoma Through the miR-338-3p/PKM2 Axis Under Hypoxic Stress. Hepatology. 2019;70:1298-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 24. | Ijichi M, Takayama T, Matsumura M, Shiratori Y, Omata M, Makuuchi M. alpha-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: a prospective study. Hepatology. 2002;35:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Notarpaolo A, Layese R, Magistri P, Gambato M, Colledan M, Magini G, Miglioresi L, Vitale A, Vennarecci G, Ambrosio CD, Burra P, Di Benedetto F, Fagiuoli S, Colasanti M, Maria Ettorre G, Andreoli A, Cillo U, Laurent A, Katsahian S, Audureau E, Roudot-Thoraval F, Duvoux C. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 26. | Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 27. | Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, Yi S, Shi W, Quan Q, Li K, Zheng L, Zhang H, Caughey BA, Zhao Q, Hou J, Zhang R, Xu Y, Cai H, Li G, Hou R, Zhong Z, Lin D, Fu X, Zhu J, Duan Y, Yu M, Ying B, Zhang W, Wang J, Zhang E, Zhang C, Li O, Guo R, Carter H, Zhu JK, Hao X, Zhang K. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 658] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 28. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4520] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 29. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 798] [Article Influence: 57.0] [Reference Citation Analysis (2)] |
| 30. | Vreugdenburg TD, Ma N, Duncan JK, Riitano D, Cameron AL, Maddern GJ. Comparative diagnostic accuracy of hepatocyte-specific gadoxetic acid (Gd-EOB-DTPA) enhanced MR imaging and contrast enhanced CT for the detection of liver metastases: a systematic review and meta-analysis. Int J Colorectal Dis. 2016;31:1739-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 31. | Tanimoto A, Lee JM, Murakami T, Huppertz A, Kudo M, Grazioli L. Consensus report of the 2nd International Forum for Liver MRI. Eur Radiol. 2009;19 Suppl 5:S975-S989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ampollini L, Italy; He D, China S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yu HG