Published online Jun 21, 2023. doi: 10.3748/wjg.v29.i23.3703
Peer-review started: February 8, 2023
First decision: March 7, 2023
Revised: March 14, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 21, 2023
Processing time: 128 Days and 3.4 Hours
Shear wave speed (SWS), shear wave dispersion (SWD), and attenuation imaging (ATI) are new diagnostic parameters for non-alcoholic fatty liver disease. To differentiate between non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver (NAFL), we developed a clinical index we refer to as the “NASH pen
To investigate whether the area of the NASH pentagon we propose is useful in discriminating between NASH and NAFL.
This non-invasive, prospective, observational study included patients diagnosed with fatty liver by abdominal ultrasound between September 2021 and August 2022 in whom shear wave elastography, SWD, and ATI were measured. Histolo
One hundred-seven patients (61 men, 46 women; mean age 55.1 years; mean BMI 26.8 kg/m2) were assessed. The LP group was significantly older (mean age: 60.8 ± 15.2 years vs 46.4 ± 13.2 years; P < 0.0001). Twenty-five patients who underwent liver biopsies were diagnosed with NASH, and 6 were diagnosed with NAFL. On ROC curve analyses, the areas under the ROC curves for SWS, dispersion slope, ATI value, BMI, Fib-4 index, and the area of the NASH pentagon were 0.88000, 0.82000, 0.58730, 0.63000, 0.59333, and 0.93651, respectively; the largest was that for the area of the NASH pentagon.
The NASH pentagon area appears useful for discriminating between patients with NASH and those with NAFL.
Core Tip: The non-alcoholic steatohepatitis (NASH) pentagon is a novel clinical index consisting of the five parameters of shear wave speed, dispersion slope, attenuation imaging value, Fib-4 index, and body mass index. It is simple and the calculation of its area is easy. The area of the NASH pentagon is useful for discriminating between patients with NASH and those with non-alcoholic fatty liver.
- Citation: Funada K, Kusano Y, Gyotoku Y, Shirahashi R, Suda T, Tamano M. Novel multi-parametric diagnosis of non-alcoholic fatty liver disease using ultrasonography, body mass index, and Fib-4 index. World J Gastroenterol 2023; 29(23): 3703-3714
- URL: https://www.wjgnet.com/1007-9327/full/v29/i23/3703.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i23.3703
Non-alcoholic fatty liver disease (NAFLD) is a global public health problem[1,2]. It is a grouping of diseases consisting of either non-alcoholic fatty liver (NAFL) or non-alcoholic steatohepatitis (NASH)[3]. If NASH progresses, it can lead to liver cirrhosis and hepatocellular carcinoma; thus, a clinical index needs to be developed that can efficiently discriminate NASH patients from NAFL patients[4,5]. Assessments based on multiple parameters using ultrasound, including shear wave elastography (SWE), are reported to be useful in stratifying the risk of advanced NASH[6].
SWE measures the speed of shear waves (shear wave speed; SWS) generated by push pulses. SWS is slow in soft objects and fast in hard objects. Measurements of SWS by SWE are considered useful in assessing liver fibrosis in viral hepatitis and NAFLD[7-11].
The shear wave dispersion (SWD) developed in recent years obtains the “frequency dependence” (dispersion slope; DS), which is the extent of the changes in the speed of shear waves, from changes in their frequency. Some preliminary evidence has been reported that DS measurements with SWD are an index of necroinflammation of the liver[12,13].
Attenuation imaging (ATI) was developed as a new testing method for evaluation of hepatic steatosis with ultrasound technology. ATI is a technique that uses the principle of attenuation due to phenomena such as absorption and diffusion of ultrasonic pulses emitted within the body when they pass through body tissue, and it produces a value referred to as the attenuation coefficient. Magnetic resonance imaging (MRI)-determined proton density fat fraction (PDFF) values have been reportedly used for assessing histological hepatic steatosis grades[14], and Tada et al[15] reported a good correlation between ATI values and MRI-determined PDFF values.
On the other hand, it is important to have parameters that do not require special equipment to measure. The prevalence of NAFLD in Japanese non-obese patients is 15%[16]. In other words, 85% of NAFLD patients are obese. Therefore, the body mass index (BMI), which can be easily used to determine obesity, is one such parameter of importance.
Similarly, the scoring system for liver fibrosis by the biochemical examination of blood is also relevant. The Fib-4 index, calculated by aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelets (Plts), and age, is simple and easy to use[17].
In this study, a pentagon consisting of five parameters was prepared with the addition of the BMI and the Fib-4 index to the three parameters of SWS, SWD, and ATI. Whether the area of this pentagon is useful in discriminating NASH from NAFL was then investigated.
This was a non-invasive, prospective, observational study that conformed with the ethical guidelines of the Declaration of Helsinki in its 2008 revision and was approved by the ethics review board of the hospital with which the authors are affiliated (No. 21057). The study was conducted after complete information disclosure on the website of the authors’ hospital.
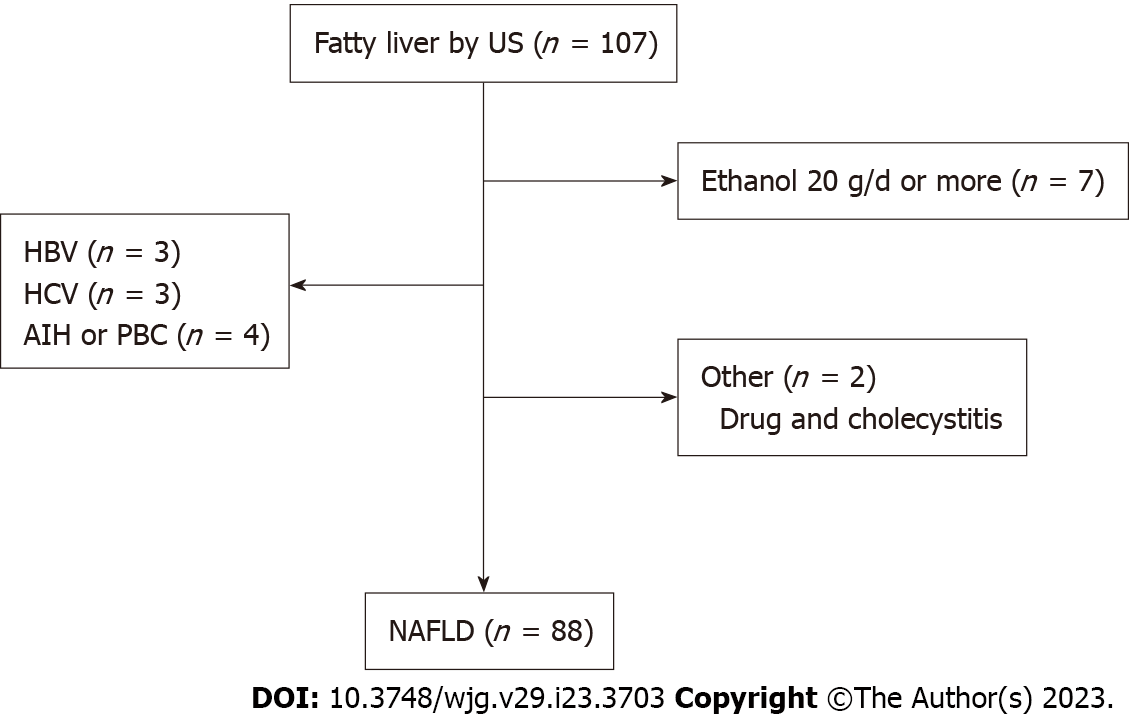
Of the patients diagnosed with fatty liver by abdominal ultrasound between September 2021 and August 2022, the subjects were patients in whom SWE, SWD, and ATI were measured. Patients with a history of alcohol intake of ethanol ≥ 20 g/d, who had hepatitis B, hepatitis C, or autoimmune liver disease, and patients with concurrent drug-induced liver injury or cholangitis were excluded.
The ultrasound scanner used was an Aplio i800 from Canon Medical Systems Corp. (Otawara, Tochigi, Japan). Fatty liver was diagnosed if the following were seen on B-mode ultrasound examination: (1) Bright liver[18]; (2) Positive hepatorenal echo contrast[19]; (3) Deep ultrasound attenuation in the liver[20]; and (4) Vascular blurring in the liver[21]. Specialists with ten or more years of ultrasonographic experience (Suda T and Tamano M) performed the B-mode ultrasound examination and measured SWE, SWD, and ATI.
All SWE measurements were done following B-mode scans, using the same diagnostic equipment and transducers. The measurement results are shown as SWS (m/s). A 1-cm-diameter, circular region of interest was placed on the sample box.
SWD was measured at the same time as SWE. Simultaneous measurements could be taken by switching to quad view mode including a SWS map and a SWD slope map. This test was also perform
After SWE and SWD were measured, the same physician performed the ATI test. The measurement result is shown as the ATI value [(dB/cm)/MHz]. A sample box with the default settings was used to acquire the data, set at least 1.5 cm below the liver capsule to avoid reverberation artifacts. The reliabi
AST, ALT, and Plts were measured on the same day as the abdominal ultrasound. The Fib-4 index was calculated from these values and age. The BMI was calculated from height and weight on the day on which abdominal ultrasound was performed.
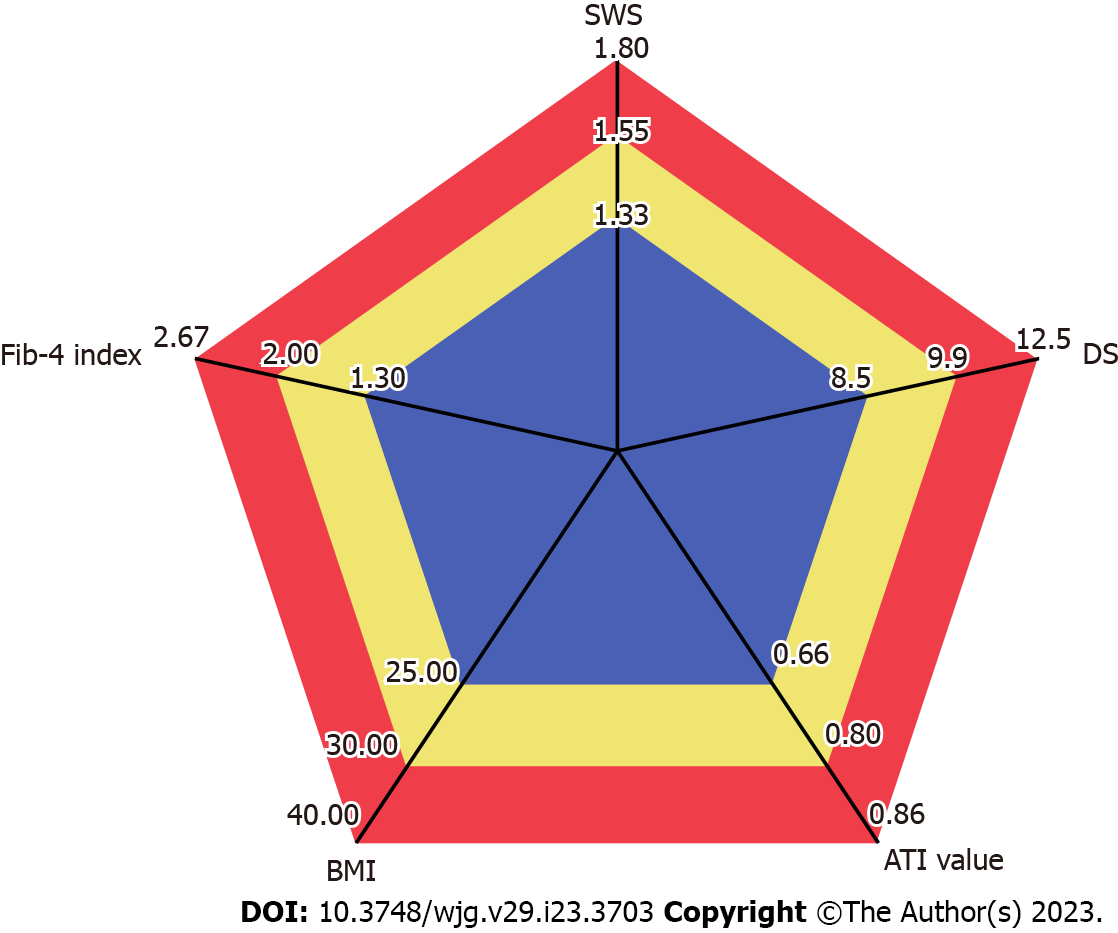
A pentagon consisting of these five parameters (SWS, DS, ATI value, BMI, and Fib-4 index) was created. This was defined as the NASH pentagon. The area of the NASH pentagon was calculated automatically by inputting a calculation formula into an Aplio i800 work station.
The standard value for each item was set according to previous studies as follows. The standard value of SWS was set as 1.33 m/s (cutoff value of fibrosis stage 1), that of DS was set to 8.5 (m/s)/KHz (cutoff value of lobular inflammation grade 1)[22]. Similarly, the standard value of ATI was set to 0.66 (dB/cm)/MHz, defining steatosis grade 1[16,22]. The standard value for BMI was set at 25.0 kg/m2 based on the index of the Japanese Society for the Study of Obesity[1]. The standard value for the Fib-4 index was set to 1.30, which is the low cutoff value for advanced fibrosis (stage 3-4), based on a report by Shah et al[24] The investigation was done taking the area of this pentagon as 100 (Figure 1).
Histological diagnosis based on liver biopsy was performed within three months from SWE measure
Patient characteristics and individual parameters were compared between a large pentagon group (LP group), in which the area of the NASH pentagon was 100 or greater, and a small pentagon group (SP group), in which the area was less than 100. In addition, the NASH diagnosis rate was investigated when the NASH pentagon area of 100 was taken as the reference.
In patients for whom liver biopsies were performed during the study period and a histologically confirm
Continuous data for SWS, DS, ATI value, and other clinical parameters are expressed as mean ± SD. A Spearman rank-order correlation coefficient test was used to test the independency of these clinical parameters. A non-paired Wilcoxon test was used in comparisons of each parameter between the two groups divided by NASH pentagon area, with P < 0.05 taken to indicate a significant difference.
The NASH diagnostic performance of SWS, DS, ATI value, BMI, Fib-4 index, and the area of the pentagon was investigated using the ROC curve. The area under the ROC curve (AUROC) was used to evaluate NASH diagnostic performance.
The number of patients who were diagnosed with fatty liver based on abdominal ultrasound and in whom SWE, SWD, and ATI were measured was 126. After excluding 7 patients with an alcohol intake history of ≥ 20 g ethanol/d, 10 patients with concurrent hepatitis B, hepatitis C, or autoimmune liver injury, and 2 patients for other reasons, the investigation was conducted with the remaining 107 patients (Figure 2).
The characteristics for these 107 patients are shown in Table 1. There were 61 men and 46 women, with a mean age of 55.1 years. The mean BMI was 26.8 kg/m2. Of the 107 patients, diabetes mellitus was seen in 25, dyslipidemia in 35, and hypertension in 37. Mean AST, ALT, and γ-glutamyltransferase concentrations were all mildly elevated at 44.9 U/L, 70.6 U/L, and 99.6 U/L, respectively. The mean Fib-4 index was 1.53. Twenty-five of the 107 patients were diagnosed with NASH, and the remaining 82 were diagnosed with NAFL.
| Patients’ characteristics | n = 107 |
| M/F | 61/46 |
| Age (yr) | 55.1 ± 15.9 |
| BMI (kg/m2) | 26.8 ± 4.1 |
| Metabolic diseases | |
| Diabetes mellitus (yes/no) | 25/82 |
| Dyslipidemia (yes/no) | 35/72 |
| Hypertension (yes/no) | 37/70 |
| Concomitant drugs | |
| SGLT2 inhibitor | 12 |
| DPP-4 inhibitor | 13 |
| Thiazolidinedione | 2 |
| GLP-1 agonist | 2 |
| Statin | 19 |
| Bezafibrate | 0 |
| Pemafibrate | 8 |
| EPA and DHA preparation | 1 |
| AST (U/L) | 44.9 ± 38.0 |
| ALT (U/L) | 70.6 ± 57.7 |
| GGT (U/L) | 99.6 ± 139.7 |
| T-B (mg/dL) | 0.9 ± 0.5 |
| Alb (mg/dL) | 4.5 ± 0.4 |
| eGFR (mL/min) | 73.0 ± 15.0 |
| HbA1c (%) | 6.5 ± 1.2 |
| T-chol (mg/dL) | 202.1 ± 52.6 |
| TG (mg/dL) | 180.0 ± 119.0 |
| WBC (103/μL) | 6731.8 ± 1882.5 |
| Hb (g/dL) | 14.7 ± 1.3 |
| Plts (104/μL) | 23.9 ± 7.6 |
| Fib-4 index | 1.53 ± 1.90 |
The Spearman rank correlation coefficient between SWS and the Fib-4 index was 0.3486; no strong correlation was found. Similarly, the rank correlation coefficient between ATI and BMI was 0.1955, showing no correlation.
The LP group with a NASH pentagon area ≥ 100 had 64 patients, and the SP group with an area < 100 had 43 patients. A comparison of the two groups is shown in Table 2.
| Large pentagon group (n = 64) | Small pentagon group (n = 43) | P value | |
| M/F | 33/31 | 28/15 | 0.165 |
| Age (yr) | 60.8 ± 15.2 | 46.4 ± 13.2 | < 0.0001 |
| Metabolic diseases | |||
| Diabetes mellitus (yes/no) | 18/46 | 6/37 | 0.0849 |
| Dyslipidemia (yes/no) | 27/37 | 8/35 | 0.0108 |
| Hypertension (yes/no) | 29/35 | 7/36 | 0.0183 |
| AST (U/L) | 52.6 ± 44.2 | 40.3 ± 24.4 | 0.0521 |
| ALT (U/L) | 61.1 ± 45.2 | 83.0 ± 71.6 | 0.1699 |
| GGT (U/L) | 97.3 ± 123.2 | 102.9 ± 162.0 | 0.7238 |
| T-B (mg/dL) | 1.01 ± 0.6 | 0.77 ± 0.3 | 0.0044 |
| Alb (mg/dL) | 4.5 ± 0.4 | 4.6 ± 0.4 | 0.2843 |
| eGFR (mL/min) | 71.8 ± 16.4 | 74.7 ± 12.1 | 0.1639 |
| HbA1c (%) | 6.5 ± 1.1 | 6.3 ± 1.2 | 0.2847 |
| T-chol (mg/dL) | 188.5 ± 59.4 | 221.3 ± 33.8 | 0.008 |
| TG (mg/dL) | 172.9 ± 126.5 | 189.9 ± 108.8 | 0.1278 |
| WBC (103/μL) | 6225.0 ± 1586.9 | 7463.9 ± 2050.9 | 0.008 |
| Hb (g/dL) | 14.7 ± 1.3 | 14.7 ± 1.5 | 0.6836 |
| Plts (104/μL) | 21.0 ± 6.5 | 28.8 ± 6.6 | < 0.0001 |
| SWS (m/s) | 1.78 ± 0.36 | 1.39 ± 0.14 | < 0.0001 |
| DS [(m/s)/kHz] | 13.3 ± 3.7 | 10.8 ± 2.0 | < 0.0001 |
| ATI value [(dB/cm)/MHz] | 0.78 ± 0.13 | 0.74 ± 0.13 | 0.1047 |
| Fib-4 index | 2.2 ± 2.3 | 0.7 ± 0.4 | < 0.0001 |
| BMI (kg/m2) | 27.8 ± 4.4 | 25.6 ± 3.2 | 0.0249 |
| NASH/NAFL | 22/42 | 3/40 | 0.0042 |
There was no difference in sex between the two groups, but mean age was 60.8 ± 15.2 years in the LP group and 46.4 ± 13.2 years in the SP group, with the LP group significantly older (P < 0.0001).
Concurrent diabetes mellitus was seen in 18/64 patients (28.1%) in the LP group and 6/43 patients (13.9%) in the SP group. While this represents a higher tendency in the LP group, no significant difference was seen (P = 0.0849). Concurrent dyslipidemia was seen in 27/64 patients (42.2%) in the LP group and 8/43 patients (18.6%) in the SP group, and concurrent hypertension was seen in 29/64 patients (45.3%) and 7/43 patients (16.3%), respectively; both were significantly higher in the LP group (P = 0.0108, P = 0.0183).
On blood biochemistry tests, total bilirubin was significantly higher in the LP group (P = 0.0044), whereas total cholesterol, white blood cells, and Plts were significantly lower in the LP group (P = 0.0080, P = 0.0080, P < 0.0001). There were no significant differences between the two groups in any of the other test results.
Comparisons of the five parameters that make up the NASH pentagon showed no significant difference between the groups in the ATI value (P = 0.1407), and significantly higher values in the LP group for SWS, DS, Fib-4 index, and BMI (P < 0.0001, P = 0.0004, P < 0.0001, P = 0.0249).
The number of patients diagnosed with NASH was 22 (34.3%) of 64 in the LP group and 3 (7.0%) of 43 in the SP group. The LP group had a significantly higher percentage of NASH patients (P = 0.0042).
Liver biopsy was performed during the period of this study in 31 patients. Twenty-five of these 31 patients were diagnosed with NASH, and six were diagnosed with NAFL. The ROC curves indicating the NASH diagnostic performance in these 31 patients are shown in Figure 3. The AUROCs for SWS, DS, ATI value, BMI, Fib-4 index, and the area of the NASH pentagon were 0.88000, 0.82000, 0.58730, 0.63000, 0.59333, and 0.93651, respectively. Of these six items, the largest AUROC was that for the area of the NASH pentagon.
Ultrasound elastography has been used to evaluate fibrosis noninvasively. SWE, one type of ultrasound elastography, includes transient elastography, point SWE, and other techniques[28-30]. The Aplio i800 used in the present study can measure SWE, SWD, and ATI with the same transducer. This machine is also equipped with a function that automatically creates radar charts from these measured values. The radar charts are created using five parameters. The three parameters of SWS, DS, and ATI value are set in the initial settings, and the remaining two can be freely set by the user. In the present study, these two were set as BMI and Fib-4 index, and it was thought that the area of this pentagon (NASH pentagon) may be useful for the diagnosis of NASH.
There are various reports about the cutoff values of SWE and DS[31]. In the present study, the cutoff values of SWE and DS were set from the report of Sugimoto et al[22] based on 120 liver biopsies. The cutoff values for steatosis grade 1 of ATI are reported to be 0.62-0.79[32-34]. In the present study, it was set to 0.66 from the report of Tada et al[35] based on 148 liver biopsies.
Obesity is the most important factor in the development of NAFLD. In NASH patients, there is a positive correlation between visceral fat and the amount of fat in hepatocytes[36]. Thus, obesity can lead to a diagnosis of NASH. In the present study, the reference value for BMI was set at 25.0 kg/m2 based on the index of the Japanese Society for the Study of Obesity and a WHO recommendation[23,37].
The Fib-4 index calculated from age, AST, ALT, and platelet count is regularly used as a noninvasive parameter of hepatic fibrosis[38]. In this study, a value of 1.30, which is the low cutoff value for advan
SWE and the Fib-4 index are indices of liver fibrosis. However, SWE makes use of ultrasound tech
In a comparison of the LP group with a NASH pentagon area of 100 or more and the SP group with an area of less than 100, the LP group was older and had a lower platelet count. This is thought to be a result of the strong involvement of the Fib-4 index among the constituent elements of the pentagon. In fact, a very large difference was seen in the Fib-4 index between the groups, which was 2.2 in the LP group and 0.7 in the SP group (P < 0.0001).
The mean BMI, on the other hand, was 27.8 kg/m2 in the LP group and 25.6 kg/m2 in the SP group, tending to be higher in the SP group. Obesity is an important element of NAFLD, but as NAFL progresses to NASH, there is a tendency for weight loss. Moreover, even in the SP group, the mean BMI of 25.6 kg/m2 was only slightly above the reference of 25.0 kg/m2. This result is also consistent with reports that nonobese NAFLD is more common in Asia than in western countries[39,40].
The onset and progression of NAFLD/NASH are related to metabolic syndrome, and they have an especially strong relationship with diabetes mellitus[41]. In the present study, no difference was seen in concurrent diabetes mellitus by differences in the NASH pentagon area, but concurrent dyslipidemia and hypertension were significantly higher in the LP group.
The NASH pentagon consists of five parameters: Fibrosis based on SWS, inflammation as assessed by DS, steatosis as assessed by ATI value, obesity assessed by BMI, and the level of serological and clinical progression based on the Fib-4 index. Once this pentagon is programmed in the ultrasound machine, it can be automatically created and its area subsequently calculated, making it very simple to use. We thus consider it to be a useful method for identifying NASH among NAFLD patients.
In fact, when ROC curves were made for the six parameters of SWS, DS, ATI value, BMI, Fib-4 index, and the area of the NASH pentagon in 31 patients who underwent liver biopsy during the study period, the largest AUROC was the one for the area of the NASH pentagon.
In the early stage of NASH, liver fibrosis is mild, and in these patients, SWS is expected to be either normal or only mildly elevated. In young patients, the Fib-4 index also tends to be low. Even in these patients, however, DS, ATI value, and BMI are expected to be high, so it may be possible to identify them using the NASH pentagon.
Conversely, BMI tends to decrease when NASH progresses to cirrhosis, and even histologically, a state is present in which reduced fat deposition is seen (so-called “burned-out NASH”)[42,43]. Such patients are expected to have low BMI and ATI values in conjunction with high SWS and Fib-4 index values. DS may have different values depending on the individual case, but the NASH pentagon is thought to be useful even in these patients.
This was a prospective study, but only 107 patients were included, and only 31 underwent liver biopsy during the study period. These numbers are too small to obtain sufficient results. Additional studies with a larger number of patients will be needed to confirm our results. It may also be necessary to conduct a more detailed pathological investigation and a review of the reference values for the five parameters.
The preparation of a NASH pentagon consisting of five parameters, SWS, DS, ATI value, Fib-4 index, and BMI, is simple and the calculation of its area is easy. The authors believe that this multi-parametric index will be useful for identifying NASH patients among NAFLD patients.
Non-alcoholic fatty liver disease (NAFLD) is a major problem throughout the world. If non-alcoholic steatohepatitis (NASH) progresses, it can lead to hepatocellular carcinoma. Novel clinical index needs to be developed that can efficiently discriminate NASH patients from non-alcoholic fatty liver (NAFL) patients.
Shear wave speed (SWS), shear wave dispersion (SWD), and attenuation imaging (ATI) are new ultrasound diagnostic parameters for NAFLD. We developed a novel clinical index as the “NASH pentagon” consisting of the 3 abovementioned parameters, body mass index (BMI), and Fib-4 index.
Objective of this study is to prove the utility of NASH pentagon area in the differentiation of NASH and NAFL.
Patients diagnosed with fatty liver by abdominal ultrasound between September 2021 and August 2022 were enrolled. Histological diagnosis based on liver biopsy was performed in 31 patients. The large pentagon group (LP group) and the small pentagon group (SP group), using an area of 100 as the cutoff, were compared; the NASH diagnosis rate was also investigated. In patients with a histologically confirmed diagnosis, receiver-operating characteristic curve analyses were performed.
The preparation of a NASH pentagon consisting of five parameters, SWS, DS, ATI value, Fib-4 index, and BMI, is simple and the calculation of its area is easy. The LP group with a NASH pentagon area ≥ 100 had 64 patients, and the SP group with an area < 100 had 43 patients. The number of patients diagnosed with NASH was 22 (34.3%) of 64 in the LP group and 3 (7.0%) of 43 in the SP group. The LP group had a significantly higher percentage of NASH patients (P = 0.0042).
The NASH pentagon is a novel multi-parametric index, and will be useful for identifying NASH patients among NAFLD patients.
NASH pentagon should be tried in more patients. Besides, the area of NASH pentagon and the details of the histologic diagnosis should be considered.
The authors would like to thank the institutions that participated in the working group and everyone who helped collect clinical data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: The Japanese Society of Gastroenterology, No. 8501.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pécsi D, Hungary; Sempokuya T, United States S-Editor: Gao CC L-Editor: A P-Editor: Wang JJ
| 1. | Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin Liver Dis. 2016;20:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 406] [Article Influence: 40.6] [Reference Citation Analysis (3)] |
| 2. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2452] [Article Influence: 350.3] [Reference Citation Analysis (1)] |
| 3. | Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 4. | Tsochatzis EA, Newsome PN. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. 2018;3:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1555] [Article Influence: 222.1] [Reference Citation Analysis (2)] |
| 6. | Kuroda H, Fujiwara Y, Abe T, Nagasawa T, Oguri T, Noguchi S, Kamiyama N, Takikawa Y. Two-dimensional shear wave elastography and ultrasound-guided attenuation parameter for progressive non-alcoholic steatohepatitis. PLoS One. 2021;16:e0249493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Suda T, Okawa O, Masaoka R, Gyotoku Y, Tokutomi N, Katayama Y, Tamano M. Shear wave elastography in hepatitis C patients before and after antiviral therapy. World J Hepatol. 2017;9:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Okuda S, Tsuji N, Imayoshi Y, Yasuda E. Utility of real-time shear wave elastography for assessing liver fibrosis in patients with chronic hepatitis C infection without cirrhosis: Comparison of liver fibrosis indices. Hepatol Res. 2015;45:E122-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Meyer G, Dauth N, Grimm M, Herrmann E, Bojunga J, Friedrich-Rust M. Shear Wave Elastography Reveals a High Prevalence of NAFLD-related Fibrosis even in Type 1 Diabetes. Exp Clin Endocrinol Diabetes. 2022;130:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Bâldea V, Bende F, Popescu A, Șirli R, Sporea I. Comparative study between two 2D-Shear Waves Elastography techniques for the non-invasive assessment of liver fibrosis in patients with chronic hepatitis C virus (HCV) infection. Med Ultrason. 2021;23:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 11. | Podrug K, Sporea I, Lupusoru R, Pastrovic F, Mustapic S, Bâldea V, Bozin T, Bokun T, Salkic N, Șirli R, Popescu A, Puljiz Z, Grgurevic I. Diagnostic Performance of 2-D Shear-Wave Elastography with Propagation Maps and Attenuation Imaging in Patients with Non-Alcoholic Fatty Liver Disease. Ultrasound Med Biol. 2021;47:2128-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Yoshimasu Y, Kasai Y, Furuichi Y, Itoi T. Viscoelasticity Measurement in Rat Livers Using Shear-Wave US Elastography. Ultrasound Med Biol. 2018;44:2018-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Lee DH, Lee JY, Bae JS, Yi NJ, Lee KW, Suh KS, Kim H, Lee KB, Han JK. Shear-Wave Dispersion Slope from US Shear-Wave Elastography: Detection of Allograft Damage after Liver Transplantation. Radiology. 2019;293:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Jeon SK, Lee JM, Joo I, Yoon JH, Lee DH, Lee JY, Han JK. Prospective Evaluation of Hepatic Steatosis Using Ultrasound Attenuation Imaging in Patients with Chronic Liver Disease with Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Ultrasound Med Biol. 2019;45:1407-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Tada T, Kumada T, Toyoda H, Nakamura S, Shibata Y, Yasuda S, Watanuki Y, Tsujii K, Fukuda N, Fujioka M, Takeshima K, Niwa F, Ogawa S, Hashinokuchi S, Kataoka S, Ichikawa H, Iijima H. Attenuation imaging based on ultrasound technology for assessment of hepatic steatosis: A comparison with magnetic resonance imaging-determined proton density fat fraction. Hepatol Res. 2020;50:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Nishioji K, Sumida Y, Kamaguchi M, Mochizuki N, Kobayashi M, Nishimura T, Yamaguchi K, Itoh Y. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011-2012. J Gastroenterol. 2015;50:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 464] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 18. | Joseph AE, Dewbury KC, McGuire PG. Ultrasound in the detection of chronic liver disease (the "bright liver"). Br J Radiol. 1979;52:184-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 96] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J Exp Med. 1983;139:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K, Kawahito Y, Yoshikawa T, Okanoue T. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 696] [Article Influence: 36.6] [Reference Citation Analysis (3)] |
| 21. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1466] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 22. | Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Abe M, Yoshimasu Y, Kasai Y, Sakamaki K, Hara T, Itoi T. The Role of Multiparametric US of the Liver for the Evaluation of Nonalcoholic Steatohepatitis. Radiology. 2020;296:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (1)] |
| 23. | Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002;66:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 1450] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 24. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1228] [Article Influence: 72.2] [Reference Citation Analysis (1)] |
| 25. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3739] [Article Influence: 155.8] [Reference Citation Analysis (4)] |
| 26. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2359] [Article Influence: 87.4] [Reference Citation Analysis (1)] |
| 27. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8538] [Article Influence: 406.6] [Reference Citation Analysis (7)] |
| 28. | Tamano M, Kojima K, Akima T, Murohisa T, Hashimoto T, Uetake C, Sugaya T, Nakano M, Hiraishi H, Yoneda M. The usefulness of measuring liver stiffness by transient elastography for assessing hepatic fibrosis in patients with various chronic liver diseases. Hepatogastroenterology. 2012;59:826-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56:1330-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Fang C, Konstantatou E, Romanos O, Yusuf GT, Quinlan DJ, Sidhu PS. Reproducibility of 2-Dimensional Shear Wave Elastography Assessment of the Liver: A Direct Comparison With Point Shear Wave Elastography in Healthy Volunteers. J Ultrasound Med. 2017;36:1563-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Suda T, Kanefuji T, Abe A, Nagayama I, Hoshi T, Morita S, Yagi K, Hatakeyama S, Hayatsu M, Hasegawa N, Terai S. A cut-off value of shear wave speed to distinguish nonalcoholic steatohepatitis candidates. Medicine (Baltimore). 2019;98:e13958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Sporea I, Bâldea V, Lupușoru R, Bende F, Mare R, Lazăr A, Popescu A, Șirli R. Quantification of Steatosis and Fibrosis using a new system implemented in an ultrasound machine. Med Ultrason. 2020;22:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 33. | Hsu PK, Wu LS, Yen HH, Huang HP, Chen YY, Su PY, Su WW. Attenuation Imaging with Ultrasound as a Novel Evaluation Method for Liver Steatosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Jang JK, Lee ES, Seo JW, Kim YR, Kim SY, Cho YY, Lee DH. Two-dimensional Shear-Wave Elastography and US Attenuation Imaging for Nonalcoholic Steatohepatitis Diagnosis: A Cross-sectional, Multicenter Study. Radiology. 2022;305:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 35. | Tada T, Iijima H, Kobayashi N, Yoshida M, Nishimura T, Kumada T, Kondo R, Yano H, Kage M, Nakano C, Aoki T, Aizawa N, Ikeda N, Takashima T, Yuri Y, Ishii N, Hasegawa K, Takata R, Yoh K, Sakai Y, Nishikawa H, Iwata Y, Enomoto H, Hirota S, Fujimoto J, Nishiguchi S. Usefulness of Attenuation Imaging with an Ultrasound Scanner for the Evaluation of Hepatic Steatosis. Ultrasound Med Biol. 2019;45:2679-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Koda M, Kawakami M, Murawaki Y, Senda M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J Gastroenterol. 2007;42:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7065] [Cited by in RCA: 8584] [Article Influence: 390.2] [Reference Citation Analysis (0)] |
| 38. | Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Fujita K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 39. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 843] [Article Influence: 93.7] [Reference Citation Analysis (2)] |
| 40. | Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, Chan HL, Chim AM, Woo J, Chu WC, Wong VW. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306-14; quiz 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 41. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 899] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 42. | Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, Akuta N, Yoneda M, Iwasa M, Otsuka M, Tamaki N, Kogiso T, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol. 2021;56:951-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 43. | Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y, Akuta N, Yoneda M, Iwasa M, Otsuka M, Tamaki N, Kogiso T, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol Res. 2021;51:1013-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |