Published online Jun 14, 2023. doi: 10.3748/wjg.v29.i22.3497
Peer-review started: May 8, 2023
First decision: May 11, 2023
Revised: May 12, 2023
Accepted: May 19, 2023
Article in press: May 19, 2023
Published online: June 14, 2023
Processing time: 30 Days and 4.9 Hours
Per-oral endoscopic myotomy (POEM) is emerging as a prefer treatment option for pediatric achalasia. However, data are limited on the long-term efficacy of POEM in children and adolescents with achalasia.
To evaluate the safety and long-term efficacy of POEM for pediatric patients with achalasia and compare those outcomes with adult patients.
This retrospective cohort study was conducted in patients with achalasia who underwent POEM. Patients aged under 18 years were included in the pediatric group; patients aged between 18 to 65 years who underwent POEM in the same period were assigned to the control group. For investigation of long-term follow-up, the pediatric group were matched with patients from the control group in a 1:1 ratio. The procedure-related parameters, adverse events, clinical success, gas
From January 2012 to March 2020, POEM was performed in 1025 patients aged under 65 years old (48 in the pediatric group, 1025 in the control group). No significant differences were observed in the occurrence of POEM complications between the two groups (14.6% vs 14.6%; P = 0.99). Among the 34 pediatric patients (70.8%) who underwent follow-up for 5.7 years (range 2.6-10.6 years), clinical success was achieved in 35 patients (35/36; 97.2%). No differences were observed in post-POEM GERD occurrence (17.6% vs 35.3%; P = 0.10). QoL was significantly improved in both groups after POEM.
POEM is safe and effective for pediatric patients with achalasia. It can achieve significant symptoms relief and improve QoL.
Core Tip: Per-oral endoscopic myotomy (POEM) is widely accepted in adult patients with achalasia. However, there is limited data on the application of POEM in pediatric patients, particularly regarding the long-term outcomes. In this study, we evaluated the safety and long-term efficacy of POEM in pediatric patients and compared those results with adult patients. The results show that POEM is safe and effective for pediatric patients with achalasia.
- Citation: Bi YW, Lei X, Ru N, Li LS, Wang NJ, Zhang B, Yao Y, Linghu EQ, Chai NL. Per-oral endoscopic myotomy is safe and effective for pediatric patients with achalasia: A long-term follow-up study. World J Gastroenterol 2023; 29(22): 3497-3507
- URL: https://www.wjgnet.com/1007-9327/full/v29/i22/3497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i22.3497
Achalasia is a rare disorder of the esophageal smooth muscle characterized by impaired relaxation of the lower esophageal sphincter (LES) and absent or spastic contractions in the esophageal body[1]. Recent studies have estimated that the annul incidence of achalasia in pediatric population is approximately 0.02-0.31 per 100000 individuals[2-4]. Despite this modest incidence rate, the disease burden of achalasia in pediatric patients is substantial owing to the extreme disruption caused to the patients’ childhood and education. Children with achalasia suffer from dysphagia, regurgitation, vomiting, chest pain, weight loss, and respiratory symptoms (nocturnal cough, aspiration), with frequent emergency department visits, hospitalizations, and malnutrition caused by decreased dietary intake of these children[5].
The traditional management of achalasia includes medication, endoscopy, and laparoscopic Heller myotomy (LHM)[6]. Per-oral endoscopic myotomy (POEM) is a minimally invasive method of myotomy first applied in clinical since 2010[7]. Since then, numerous large scales, retrospective and prospective with long-term follow-up studies have shown that POEM is safe and effective in the treatment of achalasia in adult patients[8,9]. However, studies on the safety and long-term effectiveness of POEM in the treatment of pediatric population are limited[10-13]. Owning to the low incidence of achalasia in pediatric population, the sample sizes that can be included in studies are usually small which may affect the credibility of the results. POEM has been proved to be safe and effective in adult patients, comparing the results in pediatric patients with adult patients may further validated the effectiveness of POEM in pediatric patients. Furthermore, the long-term outcome of POEM is needed to be studied as children have a long-life expectancy. Therefore, the aim of this study was to comprehensively evaluate and analyze the safety and long-term outcomes of POEM in pediatric patients and compare with those in adults.
We performed a retrospective cohort study to evaluate the outcome of pediatric patients with achalasia who underwent POEM in comparison to the outcome of all adult patients who underwent POEM within the same period. Written informed consent was obtained from each patient, and the study was approved by the Medical Ethics Committee of The First Medical Center of Chinese PLA General Hospital. Informed consent for patients aged less than 18 years was provided by their guardian.
The inclusion criteria in this study included: (1) Achalasia diagnosed by Eckardt score ≥ 4 and further confirmed by esophagogastroduodenoscopy (EGD), barium esophagram, and/or esophageal manometry; and excluding others secondary to tumor, autoimmune diseases, etc.; and (2) Patients with age ≤ 65 when performing POEM. Those patients with severe cardiopulmonary disease, blood coagulation disorders, or other underlying diseases were excluded from this study.
Patients aged less than 18 years who underwent POEM in The First Medical Center of Chinese PLA General Hospital, Beijing, China from January 2012 to March 2020 were included in the pediatric group, whereas patients between the ages of 18-65 who underwent POEM in the same period were assigned to the control group. Demographic data and the disease course of the achalasia, including the manifestations, diagnosis, and prior treatments, were recorded in detail upon admission to the hospital. In addition, the Urbach scale questionaries were completed to assess the quality of life (QoL) of achalasia patients[14].
To evaluate the long-term outcomes of POEM in pediatric patients, we matched the pediatric group with patients from the control group in a 1:1 ratio. The matching principles were based on identical gender, the same type of Chicago classification and Ling classification, a surgery date that did not differ by more than three months, and the involvement of the same operating physician.
Patients were scheduled for follow-up visits at 3 mo, 6 mo, and 1 year after POEM, then yearly. EGD was performed to assess wound healing and to determine whether or not post-POEM reflux esophagitis existed. Moreover, esophageal high-resolution manometry, an X-ray barium meal, and 24-h esophageal pH monitoring were performed if possible. Patients were also contacted via telephone and the post-POEM complications, Eckardt score and the Urbach questionaries were recorded.
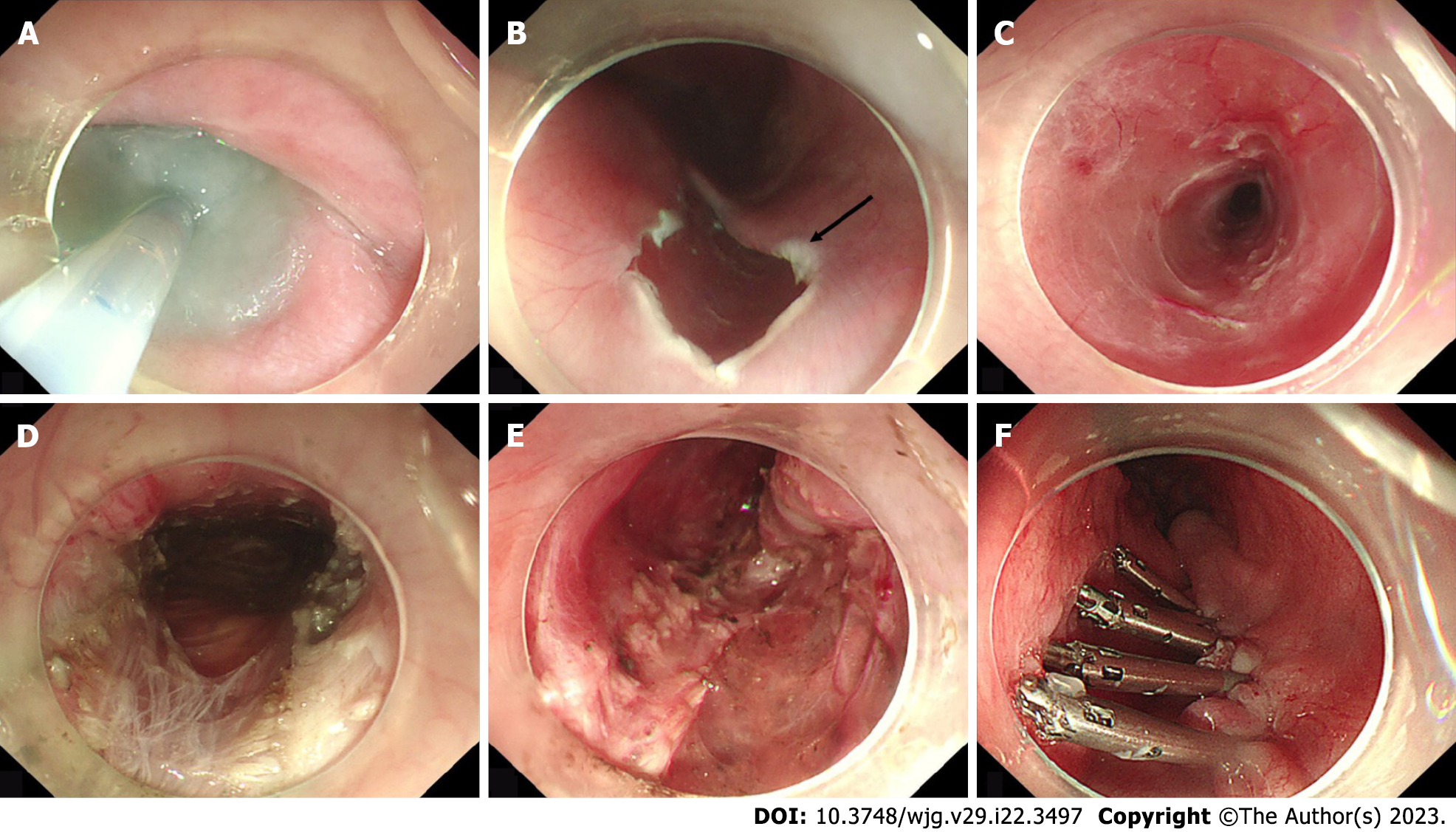
POEM was performed after the patients fasted for 48 h and underwent EGD to ensure that no food residue remained in the esophageal lumen. The standard POEM procedure was performed as previously described and generally consisted of the following major steps: First, a submucosal injection of methylene blue saline solution (1:10000) was administered, then a mucosal incision was made approximately 6–10 cm above the gastroesophageal junction (GEJ); second, a submucosal tunnel was established, passing over the GEJ and 2–3 cm into the proximal stomach; third, a myotomy started 2 cm distal to the incision and extended 2–3 cm into the stomach; and finally, after assuring hemostasis and verifying that the endoscope could easily pass the cardia, the mucosal incision was closed with clips (Figure 1). Adequate extension of the submucosal tunnel across the GEJ was confirmed by visualizing blanched gastric mucosa and a vascular pattern. Carbon dioxide gas was used for insufflation with a CO2 insufflator (UCR; Olympus) during procedures.
All patients were kept fasting for 3 d after the procedure. A liquid diet was started for an additional day. Antibiotics and proton pump inhibitor (PPI) were administered intravenously during the fasting period. An oral PPI was required for at least 4 wk subsequently. X-ray or chest and abdominal computed tomography was performed to evaluate gas-related adverse events.
The Ling classification was based on the endoscopic morphology of the esophageal lumen[15]. According to multiring structures, crescent-like structures, and diverticula, the Ling classification was recorded as Ling I, Ling IIa, Ling Ib, Ling IIc, and Ling III.
Perioperative outcomes and clinical follow-up were evaluated. Perioperative outcomes included technical success, and perioperative adverse events. Technical success was defined as the successful completion of the entire POEM procedure; if the procedure was not completed, it was defined as technical failure. Perioperative adverse events include mucosa injury, gas-related adverse events (pneumothorax, pneumoperitoneum and subcutaneous emphysema), pleural effusion, and bleeding. Procedure time was defined as the time from submucosal injection to the end of incision closure with clips. Clinical success was defined as a post-POEM Eckardt score ≤ 3. QoL was accessed by Urbach questionaries.
Depending on the distributional properties, outcome measures were expressed as the mean ± SD or as median values with ranges. For normally distributed continuous data, statistical significance was assessed by the Student’s t-test; for categorical data, significance was assessed by the χ2 test with Yates’ correction when appropriate or by the Fisher’s exact test; and for non-normally distributed continuous data, statistical significance was assessed by the Wilcoxon test. A P value of < 0.05 was established as the significance level.
From January 2012 to March 2020, POEM was performed in 1073 patients aged under 65 years old (48 in the pediatric group and 1025 in the control group).
The demographic and clinical characteristics of the patients in the two groups are shown in Table 1. There was no significant difference in gender distribution between the two groups. The median age of the pediatric patients who underwent POEM in our hospital was 16 years (range 7-18 years). Compared with control group, body weight index of pediatric group was significantly lower (17.7 vs 20.9, P < 0.001). Although the median disease course was significantly shorter in the pediatric group (median 17.5 mo vs 48 mo, P < 0.001), no significant differences were observed between the two groups regarding the type of Chicago classification, type of Ling classification and residual LES pressure. Twelve pediatric patients and 183 adult patients had undergone prior treatments, including botulinum toxin injection, pneumatic balloon dilation, endoscopic stent placement, POEM and LHM. In terms of prior treatment history, there were 4 (8.3%) patients received botulinum toxin injection, 5 (10.4%) patients received pneumatic balloon dilation, 1 (2.1%) patient had POEM and 2 (4.2%) patients had more than one treatment method in pediatric group. While 46 (4.5%) patients had botulinum toxin injection, 92 (9.0%) patients had pneumatic balloon dilation, 9 (0.9%) patients had POEM, 10 (1.0%) had LHM, 14 (1.4%) patients had endoscopic stent placement, and 12 (1.2%) had more than one treatment method in control group.
| Pediatric group (n = 48) | Control group (n = 1025) | P value | ||
| Sex, n (%) | ||||
| Male | 26 (54.2) | 469 (45.8) | 0.253 | |
| Female | 22 (45.8) | 556 (54.2) | ||
| Age, median (range), years | 16 (7-18) | 43 (19-65) | < 0.001 | |
| BMI, median (range), kg/m2 | 17.7 (11.2-26.8) | 20.9 (12.9-40.4) | < 0.001 | |
| Chicago classification, n (%) | n = 42, 87.5 | n = 1003, 97.8 | 0.928 | |
| Type I | 7 (16.7) | 156 (15.5) | ||
| Type II | 32 (76.2) | 787 (78.5) | ||
| Type III | 3 (7.1) | 60 (6.0) | ||
| Ling classification, n (%) | 0.076 | |||
| Ling I | 9 (18.8) | 189 (17.0) | ||
| Ling IIa | 17 (35.4) | 340 (30.6) | ||
| Ling IIb | 18 (37.5) | 249 (22.4) | ||
| Ling IIc | 4 (8.3) | 206 (18.5) | ||
| Ling III | 0 (0.0) | 41 (1.35) | ||
| Disease course, median (range), month | 17.5 (2-120) | 48 (1-540) | < 0.001 | |
| LES pressure, median (range), mmHg | n = 42, 87.5 | n = 1003, 97.8 | 0.214 | |
| 25.8 (8.4-65.9) | 24.5 (1-83.2) | |||
| Prior treatment | 0.210 | |||
| Patients without prior treatment, n (%) | 36 (75.0) | 842 (82.1) | ||
| Patients with prior treatment, n (%) | 12 (25.0) | 183 (17.9) | ||
| BTI | 4 (8.3) | 46 (4.5) | ||
| PBD | 5 (10.4) | 92 (9.0) | ||
| POEM | 1 (2.1) | 9 (0.9) | ||
| LHM | 0 (0.0) | 10 (1.0) | ||
| ESP | 0 (0.0) | 14 (1.4) | ||
| PBD + BTI | 1 (2.1) | 3 (0.3) | ||
| ESP + BTI | 1 (2.1) | 1 (0.1) | ||
| POEM + PBD | 0 (0.0) | 2 (0.2) | ||
| PBD + ESP | 0 (0.0) | 3 (0.3) | ||
| PBD + LHM | 0 (0.0) | 1 (0.1) | ||
| BTI + LHM | 0 (0.0) | 1 (0.1) | ||
| ESP + LHM | 0 (0.0) | 1 (0.1) | ||
The procedure results in the two groups are shown in Table 2. All pediatric patients successfully underwent POEM. Of the 1025 patients in control group, 1016 (99.1%) had technique success; nine patients could not complete the POEM procedure because of serious submucosal adhesions. No significant differences were observed between the pediatric and control groups regarding the length of tunnel, length of esophageal myotomy, length of gastric myotomy, types of myotomy and procedure time. The mean lengths of the tunnel were 10.8 cm (range, 7–14 cm) and 11.2 cm (range, 5-20 cm), respectively, whereas the length of the esophageal and gastric tunnels were 5.3 cm (range, 1-9 cm) and 1.9 cm (range, 0-4 cm) in pediatric group and were 5.2 cm (range, 3-15 cm) and 1.9 cm (range, 0-4 cm) in control group. A progressive full-thickness myotomy was the most common type of myotomy in both group (87.5% in pediatric group and 78.0% in control group). The types of myotomy in the remaining pediatric patients involved full thickness muscle myotomy (6.3%), circular muscle myotomy (2.1%), and other types (4.2%). In the control group, full-thickness myotomy accounted for 9.6%, circular myotomy accounted for 5.5%, and other incision types accounted for 6.9%. The mean procedure time was 43.9 min (range, 24-116 min) and 47.1 min (14-160 min) respectively.
| Pediatric group | Control group | P value | |||
| Technique success | 48 (100.0) | 1016 (99.1) | > 0.999 | ||
| Length of tunnel, mean (range), cm | 10.8 (7-14) | 11.2 (5-20) | 0.206 | ||
| Length of esophageal myotomy, mean (range), cm | 5.3 (1-9) | 5.2 (3-15) | 0.529 | ||
| Length of gastric myotomy, mean (range), cm | 1.9 (0-4) | 1.9 (0-4) | 0.648 | ||
| Types of myotomy | 0.458 | ||||
| Progressive full-thickness myotomy | 42 (87.5) | 792 (78.0) | |||
| Full-thickness muscle myotomy | 3 (6.3) | 98 (9.6) | |||
| Circular muscle myotomy | 1 (2.1) | 56 (5.5) | |||
| Others1 | 2 (4.2) | 70 (6.9) | |||
| Procedure time, mean (range), min | 43.9 (24-116) | 47.1 (14-160) | 0.394 | ||
| Complications | 7 (14.6) | 148 (14.6) | 0.997 | ||
| Mucosa injury | 3 (6.2) | 84 (8.3) | |||
| Gas-related adverse event | |||||
| Pneumothorax | 0 (0.0) | 3 (0.3) | |||
| Pneumoperitoneum | 2 (4.2) | 27 (2.7) | |||
| Subcutaneous emphysema | 2 (4.2) | 32 (3.2) | |||
| Pleural effusion | 0 (0.0) | 1 (0.1) | |||
| Bleeding | 0 (0.0) | 1 (0.1) | |||
No significant differences were observed between the pediatric and control groups regarding the overall complications of POEM (14.6 % vs 14.6 %; P = 0.997). All of the mucosa injuries were closed with titanium clips and porcine fibrin glue. Patients with pneumoperitoneum treated by puncture with a 10-mL syringe intra-operatively and patients with subcutaneous emphysema gradually absorbed the gas without special intervention. In the pediatric group, three complications of POEM occurred in seven patients (7/48; 14.6%), with three of these patients presenting with mucosa injury, two presenting with pneumoperitoneum, and two presenting with subcutaneous emphysema. In control group, in addition to mucosa injury and gas-related complications, a 27-year-old male patient had delayed bleeding with hematemesis 11 d after the POEM and he underwent gastroscopic hemostasis. The patient with pleural effusion was treated with closed thoracic drainage. None of the patients with complications required surgical intervention or intensive care unit (ICU) transfer.
The median follow-up period was 5.7 years (range 2.6-10.6 years) for the pediatric group and 14 patients were lost for long-term follow-up. Significant differences were shown in the Eckardt score, QoL, height, weight, residual LES pressure and numbers of months absent from school of the pediatric patients before and after POEM (Table 3). The Eckardt score after median 5.7 years of follow-up was significantly declined compared with the score before POEM (8.0 vs 1.1, P < 0.001) and all symptom component of the Eckardt score decreased significantly. QoL was assessed by Urbach scale questionaries, which is a 10-item measure of disease-specific health-related QoL that sampled the concepts of food tolerance, dysphagiarelated behavior modifications, pain, heartburn, distress, lifestyle limitation, and satisfaction. Scores on the Urbach scale questionaries range from 10-33, and lower scores indicate better QoL. Urbach scores were significantly lower in pediatric patients at long-term follow-up after POEM treatment compared with pre-treatment scores (24.7 vs 12.8, P < 0.001). The height and weight of pediatric patients also improved significantly at follow-up (163.7 cm vs 170.5 cm and 49.9 kg vs 64.3 kg, P < 0.001). Moreover, the absences from school decreased significantly (median 3.3 mo vs 0.1 mo, P < 0.001).
| Pre-POEM | Post-POEM | P value | |
| Eckardt score, mean (range) | 8.0 (4-11) | 1.1 (0-4) | < 0.001 |
| Dysphagia, mean ± SD | 2.8 ± 0.7 | 0.6 ± 0.7 | < 0.001 |
| Regurgitation, mean ± SD | 1.7 ± 0.8 | 0.1 ± 0.2 | < 0.001 |
| Chest pain, mean ± SD | 1.0 ± 0.9 | 0.4 ± 0.6 | < 0.001 |
| Weight loss, mean ± SD | 1.5 ± 1.2 | 0.0 ± 0.0 | < 0.001 |
| Urbach score, mean (range) | 12.8 (10-20) | 24.7 (18-30) | < 0.001 |
| Height, mean (range), cm | 163.7 (120-184) | 170.5 (159-185) | < 0.001 |
| Weight, mean (range), kg | 49.9 (22.2-82) | 64.3 (47-100) | < 0.001 |
| Number of months absent from school, mean (range), months | 3.3 (0.5-24) | 0.1 (0-1) | < 0.001 |
To compare the long-term outcomes of POEM in pediatric patients and adult patients, we matched the pediatric group with patients from the control group in a 1:1 ratio. The matching principles were based on identical gender, the same type of Chicago classification and Ling classification, a surgery date that did not differ by more than three months, and the involvement of the same operating physician. The long-term outcomes of POEM in pediatric patients and matched adult patients were shown in Table 4. The median follow-up time in pediatric group and matched adult group was 5.7years (range 2.6-10.6 years) and 6.0 years (range 2.7-10.7 years) respectively. Patients in both groups had clinical symptoms relief and QoL improvement after POEM. No significant differences were observed between the two groups regarding the Eckardt scores, Urbach scores, clinical failure and clinical reflux evaluation. Before POEM treatment and at post-operative follow-up, the body mass index (BMI) of matched control group was significantly greater than that of pediatric group (17.7 vs 20.0; 20.8 vs 24.0). However, the difference in BMI before and after POEM was similar in both groups (3.7 vs 3.5, P = 0.502).
| Pediatric group (n = 34) | Matched control group (n = 34) | P value | ||
| Follow-up time, median (range), years | 5.7 (2.6-10.6) | 6.0 (2.7-10.7) | 0.954 | |
| Pre-POEM, Eckardt score, mean (range) | 8.0 (4-11) | 7.9 (4-12) | 0.975 | |
| Post-POEM (3 mo), Eckardt score, mean (range) | 0.3 (0-3) | 0.3 (0-2) | 0.978 | |
| Post-POEM, Eckardt score, mean (range) | 1.1 (0-4) | 1.4 (0-3) | 0.088 | |
| Eckardt score1 (pre-post), mean (range) | 6.9 (2-10) | 6.4 (0-12) | 0.578 | |
| Pre-POEM BMI, median (range), kg/m2 | 17.7 (11.2-26.8) | 20.0 (16.4-28.7) | < 0.001 | |
| Post-POEM BMI, median (range), kg/m2 | 20.8 (15.8-29.5) | 24.0 (19.5-29.4) | < 0.001 | |
| BMI (Post-pre)2, median (range), kg/m2 | 3.7 (-1.7-13) | 3.5 (0.7-12.9) | 0.502 | |
| Pre-POEM, Urbach score, mean (range) | 12.8 (10-20) | 12.7 (10-20) | 0.842 | |
| Post-POEM, Urbach score, mean (range) | 24.7 (18-30) | 25.7 (17-33) | 0.221 | |
| Urbach score3 (pre-post), mean (range) | 11.9 (1-18) | 13.0 (4-23) | 0.254 | |
| Clinical success, n (%) | 33 (97.1) | 34 (100.0) | > 0.999 | |
| Clinical reflux evaluation | 6 (17.6) | 12 (35.3) | 0.099 | |
| Reflux esophagitis by gastroscopy | 2 (5.9) | 2 (5.9) | ||
| Symptomatic reflux | 4 (11.8) | 10 (29.4) | ||
All adult patients in matched group received clinical success. One pediatric patient was considered clinical failure because his Eckardt score was 4. The patient mainly has substernal pain and need to drink water to when eating dry food without weight loss and chest pain.
Gastroesophageal reflux disease (GERD) is the most common adverse event after POEM treatment[16]. Reflux esophagitis identified by EGD and assessed by the Los Angeles classification. A GerdQ score of GERD with a score of > 7 was defined as symptomatic reflux[11,13]. At median 5.7 years follow-up, 6 patients (6/34, 17.6%) were suffered from symptomatic reflux, with 2 patients (5.9%) showed reflux esophagitis (1 Los Angeles type A, 1 Los Angeles type B) on endoscopy and 4 patients (11.8%) were suffered from clinical reflux. In matched control group, 2 patients (5.9%) showed reflux esophagitis (1 Los Angeles type A, 1 Los Angeles type C) on endoscopy, 10 patients (29.4%) were suffered from clinical reflux. All patients were well controlled with medical therapy.
In this study, we included 48 pediatric patients and 1025 adult patients with achalasia and patients had a median 5.7 years followed-up. The risk of complications relating to POEM was not increased in pediatric patients, and the long-term efficacy of POEM in pediatric patients were similar to the results of adult group. Our results indicated that POEM is a safe and effective procedure for the management of pediatric achalasia.
Achalasia is a primary esophageal motility disorder with unknown etiology. While adults and children both present with progressive dysphagia initially to solids and in some cases to liquids, the manifestations can be more protean and challenging to diagnose in children. A majority of pediatric patients, especially those under 6-7 years of age, are often misdiagnosed as GERD and given acid-suppressive therapy[17]. Patients have dysphagia due to esophageal motility disorder, and some children may show reluctance to eat and be misdiagnosed as anorexia[18]. Up to 50% of children are treated with antacids or prokinetics before the diagnosis of achalasia is identified[19]. Dysphagia and regurgitation are the main symptoms of achalasia, and patients often suffer from malnutrition due to insufficient intake[20]. Upon majority of patients present normal BMI or even obese, there are almost 50% of patients to be at moderate or high risk for malnutrition at presentation[21]. Compared with adult patients, malnutrition in a pediatric patient may negatively affect long-term growth and development. Furthermore, psychology of children is immature, and the decline in QoL caused by achalasia can lead to a series of mental illnesses in pediatric patients such as low self-esteem, depression, anxiety, school stress or decreased performance[22]. Therefore, it is urgent to find an effective treatment method to relieve the symptoms of achalasia in pediatric patients. POEM is a is a less invasive therapy with promising treatment effect. Since the clinical application of POEM in 2010, a large number of studies have been conducted and shown that POEM is a safe and effective method for the treatment of achalasia in adult patients[7,23,24]. However, data of POEM for pediatric patients are limited. Due to the low incidence of achalasia in children and adolescents, the sample size is small in most studies which is difficult to systematically evaluate and avoid statistical bias. Therefore, comparing its outcomes with those in adults seems particularly necessary. In addition, pediatric patients have a long-life expectancy and the long-term outcomes of POEM should be investigated. To our best knowledge, this is the first study evaluated the safety and long-term efficacy of POEM in children and compared those with adults with the median follow-up time over 5 years.
In our study, we compare 48 pediatric patients with 1025 adults with a median follow-up over 5 years. The technique success achieved 100% in pediatric group and 99.1% in control group. Standard tunnel and the length of myotomy were applied during POEM procedure in both groups. According to the previous studies, pediatric patients were all received standard tunnel and myotomy length[11,13,25]. POEM-related parameters such as length of tunnel, length of myotomy, types of myotomy and operation time were similar to those of adult patients.
Our results showed that there are no significant differences between the pediatric and control groups regarding the overall complications of POEM. All pediatric patients who developed complications recovered with conservative management. While one adult patient had delayed bleeding with hematemesis need another endoscopic invention and one pleural effusion which treated with closed thoracic drainage. Liu et al[13] showed only five children (3.8%) experienced major adverse events, which were resolved by conservative treatment[13]. Peng et al[11] found two children (8.3%) developed perioperative subcutaneous emphysema during the procedure and were spontaneously absorbed without any intervention[11]. Wood et al[25] showed that among 21 pediatric patients who received POEM, one had mucosa injury and one had pneumoperitoneum. None of them need for reintervention[25]. Choné et al[26] indicated that 1 case (1%) of significant per-procedure bleeding, 7 cases (6%) had postoperative adverse events. None of them need surgery or transfer to ICU[26]. All these results indicate that POEM is a safe treatment method for pediatric patients.
In our study, all the pediatric patients with preoperative dysphagia and regurgitation reported improved or resolved symptoms at the median 5.7 years follow-up. All of them were free from the further intervention for achalasia after POEM. Compared with adult patients, POEM also achieved satisfactory long-term outcomes in pediatric patients. One pediatric patient was considered clinical failure at last time follow-up as his Eckardt score was 4. The patient began to experience intermittent dysphagia symptoms again in the second year after POEM and at last time follow-up the patient mainly has substernal pain and need to drink water to when eating dry food every day. In this patient, a submucosal tunnel of standard length was established, but the end of the tunnel was located at the EGJ, and only esophagomyotomy was performed. Studies had showed that in order to ensure the curative effect of POEM, the length of myotomy is routinely 8-10 cm, at least 2 cm below the EGJ[27]. Therefore, symptom recurrence of the patient may be related to insufficient myotomy and we suggested him could receive a re-POEM.
Several factors could limit the extent to which the results can be generalized in our study. First, we did not analysis the risk factors for complications of POEM in pediatric patients due to the small sample size. Second, the mean age was slightly older in pediatric group (median age was 16). This shortage is due to younger children tend to receive treatment in children’s hospitals in China. Third, the study was retrospective and a single-center analysis.
In summary, POEM is safe treatment method and could achieve satisfactory long-term outcomes in pediatric patients. Furthermore, prospective, multicenter, and large sample studies are warranted to validate our conclusions.
Achalasia is a rare disorder of the esophageal smooth muscle which cause dysphagia, regurgitation, chest pain and weight loss. In recent years, per-oral endoscopic myotomy (POEM) is emerging as a prefer treatment option for patients achalasia.
Although POEM was proved to be safe and efficacy for adult patients with achalasia, data of POEM in pediatric patients are limited.
To evaluate the safety and long-term efficacy of POEM for pediatric patients with achalasia.
We performed a retrospective cohort study to evaluate the outcome of pediatric patients with achalasia who underwent POEM in comparison to the outcome of all adult patients who underwent POEM within the same period. To evaluate the safety of POEM, we compare the operation-related parameters and complications in the two groups. To evaluate the long-term efficacy of POEM, we compare the body mass index, clinical symptoms, quality of life in pediatric group before and after POEM; and we also compare those outcomes in pediatric group and matched adult group.
The risk of complications relating to POEM was not increased in pediatric patients, and the long-term efficacy of POEM in pediatric patients were similar to the results of adult group.
POEM is safe treatment method and could achieve satisfactory long-term outcomes in pediatric patients.
POEM can be an an effective treatment option for achalasia in pediatric patients.
| 1. | Savarino E, Bhatia S, Roman S, Sifrim D, Tack J, Thompson SK, Gyawali CP. Achalasia. Nat Rev Dis Primers. 2022;8:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 2. | Sato H, Yokomichi H, Takahashi K, Tominaga K, Mizusawa T, Kimura N, Kawata Y, Terai S. Epidemiological analysis of achalasia in Japan using a large-scale claims database. J Gastroenterol. 2019;54:621-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Miller J, Khlevner J, Rodriguez L. Upper Gastrointestinal Functional and Motility Disorders in Children. Pediatr Clin North Am. 2021;68:1237-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Smits M, van Lennep M, Vrijlandt R, Benninga M, Oors J, Houwen R, Kokke F, van der Zee D, Escher J, van den Neucker A, de Meij T, Bodewes F, Schweizer J, Damen G, Busch O, van Wijk M. Pediatric Achalasia in the Netherlands: Incidence, Clinical Course, and Quality of Life. J Pediatr. 2016;169:110-5.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Meyer A, Catto-Smith A, Crameri J, Simpson D, Alex G, Hardikar W, Cameron D, Oliver M. Achalasia: Outcome in children. J Gastroenterol Hepatol. 2017;32:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am J Gastroenterol. 2020;115:1393-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (1)] |
| 7. | Werner YB, Hakanson B, Martinek J, Repici A, von Rahden BHA, Bredenoord AJ, Bisschops R, Messmann H, Vollberg MC, Noder T, Kersten JF, Mann O, Izbicki J, Pazdro A, Fumagalli U, Rosati R, Germer CT, Schijven MP, Emmermann A, von Renteln D, Fockens P, Boeckxstaens G, Rösch T. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N Engl J Med. 2019;381:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 376] [Article Influence: 53.7] [Reference Citation Analysis (2)] |
| 8. | de Moura ETH, Jukemura J, Ribeiro IB, Farias GFA, de Almeida Delgado AA, Coutinho LMA, de Moura DTH, Aissar Sallum RA, Nasi A, Sánchez-Luna SA, Sakai P, de Moura EGH. Peroral endoscopic myotomy vs laparoscopic myotomy and partial fundoplication for esophageal achalasia: A single-center randomized controlled trial. World J Gastroenterol. 2022;28:4875-4889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (3)] |
| 9. | Shiwaku H, Inoue H, Sato H, Onimaru M, Minami H, Tanaka S, Sato C, Ogawa R, Okushima N, Yokomichi H. Peroral endoscopic myotomy for achalasia: a prospective multicenter study in Japan. Gastrointest Endosc. 2020;91:1037-1044.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Nabi Z, Ramchandani M, Basha J, Goud R, Darisetty S, Reddy DN. POEM Is a Durable Treatment in Children and Adolescents With Achalasia Cardia. Front Pediatr. 2022;10:812201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Peng D, Tan Y, Li C, Lv L, Zhu H, Liang C, Li R, Liu D. Peroral Endoscopic Myotomy for Pediatric Achalasia: A Retrospective Analysis of 21 Cases With a Minimum Follow-Up of 5 Years. Front Pediatr. 2022;10:845103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Miao S, Wu J, Lu J, Wang Y, Tang Z, Zhou Y, Huang Z, Ying H, Zhou P. Peroral Endoscopic Myotomy in Children With Achalasia: A Relatively Long-term Single-center Study. J Pediatr Gastroenterol Nutr. 2018;66:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Wang Y, Fang Y, Huang Y, Yang H, Ren X, Xu M, Chen S, Chen W, Zhong Y, Zhang Y, Qin W, Hu J, Cai M, Yao L, Li Q, Zhou P. Short-term safety and efficacy of peroral endoscopic myotomy for the treatment of achalasia in children. J Gastroenterol. 2020;55:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Urbach DR, Tomlinson GA, Harnish JL, Martino R, Diamant NE. A measure of disease-specific health-related quality of life for achalasia. Am J Gastroenterol. 2005;100:1668-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Li HK, Linghu EQ. New endoscopic classification of achalasia for selection of candidates for peroral endoscopic myotomy. World J Gastroenterol. 2013;19:556-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Familiari P, Greco S, Gigante G, Calì A, Boškoski I, Onder G, Perri V, Costamagna G. Gastroesophageal reflux disease after peroral endoscopic myotomy: Analysis of clinical, procedural and functional factors, associated with gastroesophageal reflux disease and esophagitis. Dig Endosc. 2016;28:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Hallal C, Kieling CO, Nunes DL, Ferreira CT, Peterson G, Barros SG, Arruda CA, Fraga JC, Goldani HA. Diagnosis, misdiagnosis, and associated diseases of achalasia in children and adolescents: a twelve-year single center experience. Pediatr Surg Int. 2012;28:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Franklin AL, Petrosyan M, Kane TD. Childhood achalasia: A comprehensive review of disease, diagnosis and therapeutic management. World J Gastrointest Endosc. 2014;6:105-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (4)] |
| 19. | Lee CW, Kays DW, Chen MK, Islam S. Outcomes of treatment of childhood achalasia. J Pediatr Surg. 2010;45:1173-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Mari A, Sweis R. Assessment and management of dysphagia and achalasia. Clin Med (Lond). 2021;21:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Newberry C, Vajravelu RK, Pickett-Blakely O, Falk G, Yang YX, Lynch KL. Achalasia Patients Are at Nutritional Risk Regardless of Presenting Weight Category. Dig Dis Sci. 2018;63:1243-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Frankhuisen R, van Herwaarden MA, Heijkoop R, Smout AJ, Baron A, Vermeijden JR, Gooszen HG, Samsom M. Persisting symptoms and decreased health-related quality-of-life in a cross-sectional study of treated achalasia patients. Aliment Pharmacol Ther. 2007;26:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Bechara R, Ikeda H, Inoue H. Peroral endoscopic myotomy: an evolving treatment for achalasia. Nat Rev Gastroenterol Hepatol. 2015;12:410-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 24. | Xu S, Chai N, Tang X, Linghu E, Li L, Wang S, Zhang X. Outcomes of peroral endoscopic myotomy in challenging achalasia patients: a long-term follow-up study. Surg Endosc. 2021;35:3732-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Wood LS, Chandler JM, Portelli KE, Taylor JS, Kethman WC, Wall JK. Treating children with achalasia using per-oral endoscopic myotomy (POEM): Twenty-one cases in review. J Pediatr Surg. 2020;55:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Choné A, Familiari P, von Rahden B, Desai P, Inoue H, Shimamura Y, Eleftheriadis N, Yamashita K, Khashab MA, Shiwaku H, Seewald S, Draganov PV, Alvarez LBM, Chaussade S, Tantau M, Abraham M, Marks J, Arevalo G, Albéniz E, Mion F, Roman S, Rivory J, Dubois R, Lachaux A, Benech N, Subtil F, Ponchon T, Barret M, Pioche M. Multicenter Evaluation of Clinical Efficacy and Safety of Per-oral Endoscopic Myotomy in Children. J Pediatr Gastroenterol Nutr. 2019;69:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Li L, Chai N, Linghu E, Li Z, Du C, Zhang W, Zou J, Xiong Y, Zhang X, Tang P. Safety and efficacy of using a short tunnel versus a standard tunnel for peroral endoscopic myotomy for Ling type IIc and III achalasia: a retrospective study. Surg Endosc. 2019;33:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee HS, South Korea; Topi S, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR