Published online Jun 7, 2023. doi: 10.3748/wjg.v29.i21.3292
Peer-review started: March 5, 2023
First decision: April 3, 2023
Revised: April 14, 2023
Accepted: April 28, 2023
Article in press: April 28, 2023
Published online: June 7, 2023
Processing time: 88 Days and 8.2 Hours
Gastroesophageal reflux is associated with poorer outcomes after lung transplant, likely through recurrent aspiration and allograft injury. Although prior studies have demonstrated a relationship between impedance-pH results and transplant outcomes, the role of esophageal manometry in the assessment of lung transplant patients remains debated, and the impact of esophageal dysmotility on transplant outcomes is unclear. Of particular interest is ineffective esophageal motility (IEM) and its associated impact on esophageal clearance.
To assess the relationship between pre-transplant IEM diagnosis and acute rejection after lung transplantation.
This was a retrospective cohort study of lung transplant recipients at a tertiary care center between 2007 and 2018. Patients with pre-transplant anti-reflux surgery were excluded. Manometric and reflux diagnoses were recorded from pre-transplant esophageal function testing. Time-to-event analysis using Cox proportional hazards model was applied to evaluate outcome of first episode of acute cellular rejection, defined histologically per International Society of Heart and Lung Transplantation guidelines. Subjects not meeting this endpoint were censored at time of post-transplant anti-reflux surgery, last clinic visit, or death. Fisher’s exact test for binary variables and student’s t-test for continuous variables were performed to assess for differences between groups.
Of 184 subjects (54% men, mean age: 58, follow-up: 443 person-years) met criteria for inclusion. Interstitial pulmonary fibrosis represented the predominant pulmonary diagnosis (41%). During the follow-up period, 60 subjects (33.5%) developed acute rejection. The all-cause mortality was 16.3%. Time-to-event univariate analyses demonstrated significant association between IEM and acute rejection [hazard ratio (HR): 1.984, 95%CI: 1.03-3.30, P = 0.04], confirmed on Kaplan-Meier curve. On multivariable analysis, IEM remained independently associated with acute rejection, even after controlling for potential confounders such as the presence of acid and nonacid reflux (HR: 2.20, 95%CI: 1.18-4.11, P = 0.01). Nonacid reflux was also independently associated with acute rejection on both univariate (HR: 2.16, 95%CI: 1.26-3.72, P = 0.005) and multivariable analyses (HR: 2.10, 95%CI: 1.21-3.64, P = 0.009), adjusting for the presence of IEM.
Pre-transplant IEM was associated with acute rejection after transplantation, even after controlling for acid and nonacid reflux. Esophageal motility testing may be considered in lung transplant to predict outcomes.
Core Tip: While gastroesophageal reflux (GER) has been associated with poorer outcomes after lung transplant, the impact of esophageal dysmotility remains unclear. Our study found that ineffective eso
- Citation: Lo WK, Hiramoto B, Goldberg HJ, Sharma N, Chan WW. Ineffective esophageal motility is associated with acute rejection after lung transplantation independent of gastroesophageal reflux. World J Gastroenterol 2023; 29(21): 3292-3301
- URL: https://www.wjgnet.com/1007-9327/full/v29/i21/3292.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i21.3292
Lung transplantation survival remains the lowest among solid organ transplants despite small gains over the past decade. Current 5-year survival rates are estimated at 59.2%[1]. Gastroesophageal reflux disease (GERD) and esophageal dysmotility are commonly found in patients undergoing lung transplant evaluation and have been associated with worsened transplant outcomes. For example, GERD has been implicated in the development of acute rejection and chronic lung allograft dysfunction (CLAD). Acute rejection is an early risk factor for CLAD, an important mediator of mortality after the first year post-transplant[2,3]. While esophageal dysmotility may be associated with worsened severity of GERD due to aberrant peristaltic clearance of refluxed gastroduodenal contents, less is known regarding its independent effects on lung transplant outcomes[4-6].
Acute lung rejection is common within the first year post-transplant with rates as high as > 50%[7]. Prior work from our group demonstrated an association between pre-transplant impedance measures of reflux and early allograft injury post-transplant as well as early hospital readmission[8,9]. These measures included prolonged bolus clearance, and increased total proximal and distal reflux episodes[10]. Studies in humans and mouse models have demonstrated that markers of refluxate such as pepsin and bile acids are also associated with allograft dysfunction, and result in impaired innate immune responses[11-14].
Despite the established connection between esophageal motility and reflux clearance, few studies have analyzed transplant outcomes in patients with esophageal dysmotility. Notably, the International Society of Heart and Lung Transplantation (ISHLT) guidelines consider severe esophageal dysmotility to be a risk factor associated with a substantially increased risk of a poor outcome[15]. A few single center studies to date have demonstrated disorders of esophageal motility impacting lung transplant outcomes like CLAD in esophagogastric junction outflow obstruction (EGJOO), as well as 1-, 3-, and 5-year survival in the more severe phenotype of esophageal aperistalsis[16,17]. Thus far, limited data has impeded our understanding of less severe phenotypes of impaired peristalsis on lung transplant outcomes. The goal of our study is to determine the impact of pre-transplant esophageal dysmotility on lung transplant outcomes of acute rejection, specifically subjects with weak or impaired, but not fully absent, contractility, characterized as ineffective esophageal motility (IEM). We hypothesized that measures of esophageal dysmotility such as IEM are associated with increased rates of acute rejection in lung transplant patients.
This was a retrospective cohort study of adult patients age > 18 who underwent lung transplantation between 2007 and 2018 at a tertiary care referral center. Patients with pre-transplant high-resolution manometry (HRM) and multichannel intraluminal impedance and pH (MII-pH) testing were included, and patients with a history of pre-transplant antireflux surgery were excluded. Study subjects meeting inclusion and exclusion criteria were reviewed for collection of baseline characteristics and outcomes data.
Baseline demographics included age at time of transplantation, sex, body mass index, primary pulmonary diagnosis, and pre-transplant cardiac ejection fraction. Covariates of interest included IEM on HRM, and presence of acid reflux and non-acid reflux on MII-pH study. The primary outcome of interest was development of first episode of acute cellular rejection. This was defined by clinical and histologic criteria per ISHLT guidelines[18]. Other measured outcomes included all-cause mortality during the study period, use of proton pump inhibitor (PPI) medication post-transplant, and development of pulmonary infection.
All patients included in the study underwent HRM (Diversatek Healthcare, Milwaukee, WI, United States) prior to transplant. This system utilized a solid-state catheter with 32 circumferential pressure sensors spaced 1 cm apart. Transnasal catheter placement was performed with distal sensor placement directed into the proximal stomach, ensuring that the catheter is properly positioned across the lower esophageal sphincter (LES). After a brief accommodation period, patients were asked to perform ten 5-mL liquid swallows in the supine position. Results were analyzed utilizing a dedicated software package (BioView 5.6.3.0; Diversatek Healthcare, Milwaukee, WI, United States). IEM was defined by ≥ 50% weak or failed swallows using Chicago Classification v3.0 criteria[19]. Presence of IEM was classified as a dichotomous variable for data analysis.
All patients included in the study also MII-pH monitoring (Diversatek Healthcare, Milwaukee, WI, United States) off PPI prior to transplant. This system includes a portable data collection device, as well as the MII-pH catheter with two pH sensors (0, 15 cm) and eight impedance electrodes (-3, -1, 1, 3, 5, 9, 11, 13 cm). Transnasal catheter placement was performed and positioned with the distal pH sensor localized to 5 cm above the LES. Patients were asked to continue their normal daily activities during the 24-h study and to record meal periods, which were excluded from the analysis. MII-pH tracings were manually reviewed and analyzed utilizing a dedicated software package (BioView 5.6.3.0; Diversatek Healthcare, Milwaukee, WI, United States). Increased acid reflux was defined by acid exposure time (AET) > 4%, while increased non-acid reflux was defined as > 27 weakly acidic or alkaline (pH > 4) episodes per prior publications[20].
Patients were placed on a standard immunosuppressive regimen consisting of azathioprine or mycophenolate, tacrolimus, and methylprednisolone. Surveillance bronchoscopy and biopsies were obtained according to standardized post-transplant protocol at 1, 3, 6, and 12 mo. Additional diagnostic bronchoscopies were triggered by development of clinical symptoms concerning for infection or rejection. Acute rejection was categorized according to ISHLT criteria. Minimal rejection grades of A1B0 were counted as acute rejection if the patient presented with suggestive clinical symptoms and received treatment with pulsed steroids, or had persistent grade A rejection on repeat bronchoscopy.
All statistical analyses were performed utilizing SAS 9.3 statistical package (SAS Institute Inc., Cary, NC, United States). Baseline characteristics were compared using student’s t-test for continuous variables and Fisher’s exact test for dichotomous variables. Time-to-event analysis using Cox proportional hazards model was utilized to analyze the primary outcome of first episode of acute rejection. Cox proportional hazards regression was used to adjust for baseline covariates in the final analysis.
Of the 181 patients met inclusion criteria for the study with a total of 439 person-years of follow-up. The mean age of the cohort was 58 with a slight male predominance (54%), and the most common pulmonary diagnosis was idiopathic pulmonary fibrosis which accounted for 41% of patients. Acute rejection was demonstrated in 59 patients (33.5% of those receiving at least one bronchoscopy with biopsy) during the follow-up period for this study. There were 30 deaths during the study period reflecting an all-cause mortality rate of 16.6%.
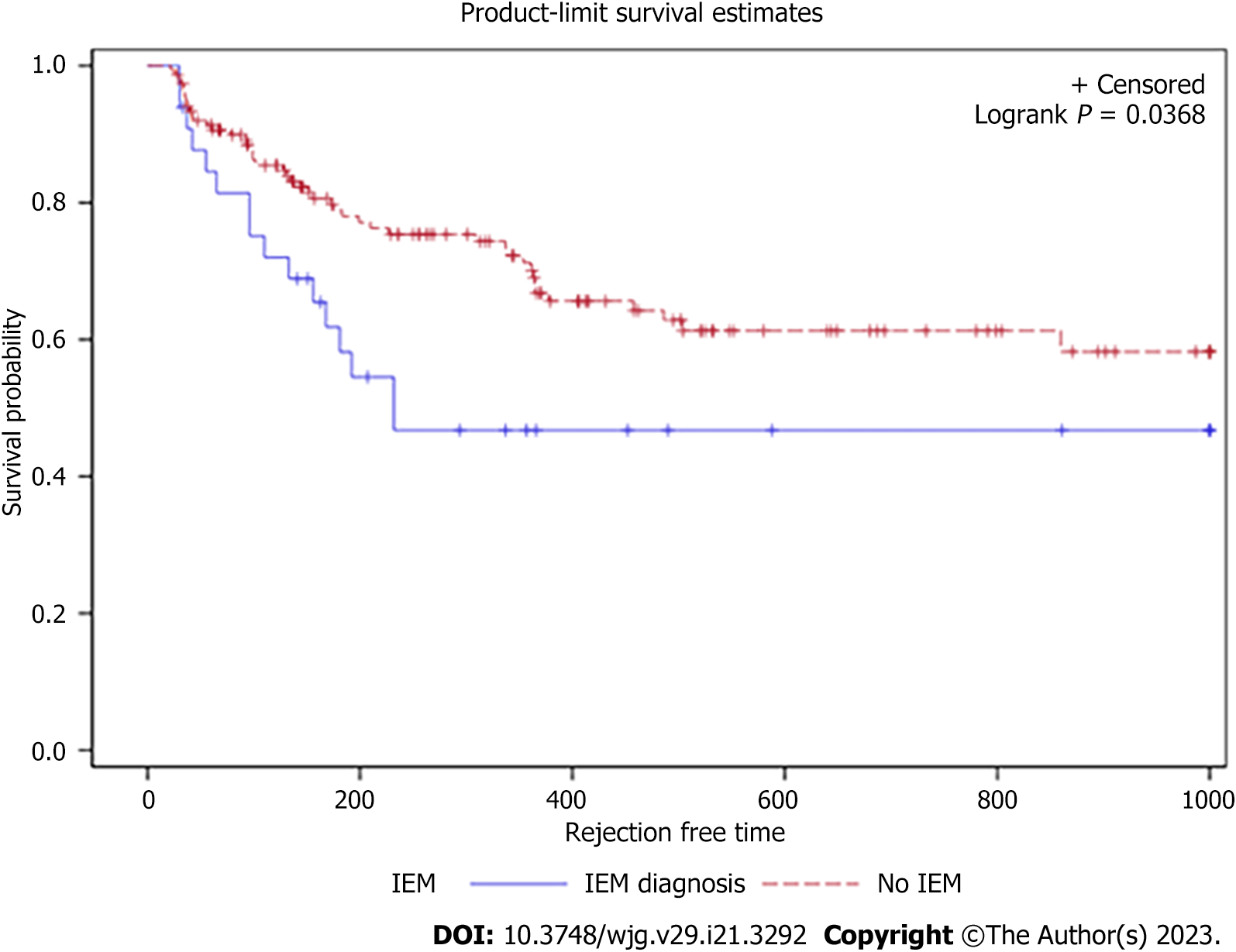
Pre-transplant HRM revealed normal esophageal motility in 130 patients (71.8%) and IEM in 31 patients (17.1%). The remaining 20 patients had abnormal manometry of other causes (7 distal esophageal spasm, 7 Jackhammer, 6 EGJOO). No patients had achalasia or absent contractility. The IEM group had slightly fewer Caucasian patients, but the remaining demographics were statistically similar compared to the normal group (Table 1). For the primary outcome on univariate analysis, IEM was associated with a decreased time to acute rejection [hazard ratio (HR): 1.984, 95%CI: 1.03-3.30, P = 0.04). The Kaplan-Meier survival curve trended toward significance with 40% of the IEM cohort developing acute rejection within approximately 250 d, compared to 500 d for the normal esophageal motility group (Figure 1). On multivariable analysis after adjusting for potential confounders including the presence of acid and nonacid reflux, IEM remained independently associated with acute rejection (HR: 2.20, 95%CI: 1.18-4.11, P = 0.01) (Table 2).
| Total (n = 181) | IEM (n = 31) | Normal motility (n = 130) | Other motility disorder (n = 20) | |
| Follow-up (years) | 2.43 ± 2.45 | 2.85 ± 2.77 | 2.43 ± 2.41 | 1.76 ± 2.11 |
| Male sex | 97 (53.6%) | 20 (64.5%) | 70 (53.8%) | 7 (35.0%) |
| BMI | 26.6 ± 4.37 | 25.8 ± 3.88 | 26.6 ± 4.55 | 28.0 ± 3.66 |
| Age at transplant (years) | 58.4 ± 10.2 | 57.9 ± 12.1 | 58.1 ± 10.2 | 61.6 ± 7.17 |
| White race1 | 166 (92.2%) | 24 (77.4%) | 123 (94.6%) | 20 (100%) |
| Pulmonary diagnosis | ||||
| ILD | 99 (54.7%) | 19 (61.3%) | 69 (53.1%) | 11 (55.0%) |
| IPF | 74 (40.9%) | 14 (45.2%) | 51 (39.2%) | 9 (45.0%) |
| COPD | 57 (31.5%) | 7 (22.6%) | 43 (33.1%) | 7 (35.0%) |
| CF | 17 (9.39%) | 4 (12.9%) | 13 (10.0%) | 0 (0%) |
| Cardiac function2 | ||||
| LVEF (%) | 61.7 ± 5.73 | 62.2 ± 5.79 | 61.4 ± 5.42 | 62.6 ± 7.55 |
| PaP (mmHg) | 27.7 ± 10.3 | 28.6 ± 11.0 | 27.3 ± 10.3 | 29.2 ± 8.91 |
| PCWP (mmHg) | 10.8 ± 4.96 | 9.06 ± 4.01 | 11.2 ± 5.15 | 11.5 ± 4.65 |
| PVR (dynes/sec/cm-5) | 252 ± 194 | 300 ± 238 | 233 ± 175 | 300 ± 217 |
| Pulmonary function, baseline2 | ||||
| FVC | 1.93 ± 0.82 | 1.97 ± 0.92 | 1.94 ± 0.81 | 1.77 ± 0.78 |
| FVC, %-pred | 50.1 ± 47.0 | 49.2 ± 18.4 | 50.1 ± 19.0 | 51.3 ± 24.4 |
| FEV1 | 1.24 ± 0.68 | 1.39 ± 0.74 | 1.22 ± 0.68 | 1.15 ± 0.57 |
| FEV1, %-pred | 40.5 ± 20.4 | 44.1 ± 21.1 | 39.4 ± 20.1 | 42.6 ± 21.6 |
| FEV1/FVC | 0.66 ± 0.25 | 0.72 ± 0.22 | 0.64 ± 0.25 | 0.67 ± 0.26 |
| Manometry results | ||||
| Normal | 130 | 0 | 130 | 0 |
| IEM | 31 | 31 | 0 | 0 |
| DES | 7 | 0 | 0 | 7 |
| Jackhammer | 7 | 0 | 0 | 7 |
| EGJOO | 6 | 0 | 0 | 6 |
| Reflux monitoring | ||||
| Acid reflux | 69 (38.1%) | 13 (41.9%) | 50 (38.5%) | 6 (30.0%) |
| Nonacid reflux2 | 42 (26.6%) | 11 (39.3%) | 30 (26.8%) | 1 (5.56%) |
| Lungs transplanted | ||||
| Unilateral | 43 (23.8%) | 6 (19.3%) | 34 (26.1%) | 3 (15.0%) |
| Bilateral | 138 (76.2%) | 25 (80.6%) | 96 (73.8%) | 17 (85.0%) |
| CMV mismatch | 53 (29.3%) | 7 (22.6%) | 42 (32.3%) | 4 (20.0%) |
| High-risk donor | 69 (38.1%) | 7 (22.6%) | 52 (40.0%) | 10 (50.0%) |
| Post-transplant PPI | 128 (70.7%) | 24 (77.4%) | 92 (70.8%) | 12 (60.0%) |
| Covariate | Cox univariate analysis | P value | Cox multivariable analysis | P value |
| Ineffective esophageal motility | 1.84 (1.03-3.30) | 0.04 | 2.20 (1.18-4.11) | 0.01 |
| Nonacid reflux | 2.16 (1.26-3.72) | 0.005 | 2.10 (1.21-3.64) | 0.009 |
| Acid reflux | 1.06 (0.63-1.76) | 0.83 | 0.92 (0.53-1.61) | 0.77 |
| Body-mass Index | 1.01 (0.95-1.06) | 0.85 | 1.02 (0.96-1.09) | 0.43 |
| Age at transplant | 1.00 (0.98-1.02) | 0.94 | 1.00 (0.97-1.02) | 0.93 |
| Male gender | 0.85 (0.51-1.41) | 0.53 | 0.75 (0.44-1.29) | 0.30 |
The presence of pathologic acid or nonacid reflux per MII-pH testing was also analyzed. Notably, pathologic acid reflux defined by AET > 4.2% was not associated with acute rejection on univariate (HR: 1.06, 95%CI: 0.63-1.76, P = 0.83) or multivariable analyses (HR: 0.92, 95%CI: 0.53-1.61, P = 0.77). On the other hand, increased non-acid reflux was associated with decreased time to acute rejection in the IEM group on univariate analysis (HR: 2.16, 95%CI: 1.26-3.72, P = 0.005) and multivariable analysis (HR: 2.10, 95%CI: 1.21-3.64, P = 0.009) (Table 2). This relationship occurred independent of the presence of IEM.
GERD and esophageal dysmotility are frequent comorbid conditions in patients with end-stage lung disease. There is increasing recognition of the role these esophageal dysfunctions play in the pathogenesis and clinical progression of specific etiologies of end-stage lung disease, as well as their role in the clinical outcomes of lung transplantation. Our study sought to determine the impact of esophageal hypomotility in the development of acute rejection after lung transplantation. We found that IEM demonstrated on pre-transplant testing was associated with increased risk of acute rejection after lung transplantation. This relationship remained after controlling for covariates including pre-transplant measures of acid and non-acid reflux. The magnitude of association was increased after controlling for these baseline factors on multivariate analysis. This suggests that esophageal motility may play a role in lung transplant outcomes that is independent of reflux-related allograft injury and rejection.
Acute rejection was demonstrated in 33.5% of the cohort in the study follow up. This is consistent with prior estimates which have ranged from 28% in the ISHLT registry to 53.3%[7,21]. Baseline demographics did not differ significantly between the IEM and control groups. Amongst the other covariates of interest, non-acid reflux was independently associated with decreased time to the development of acute rejection, and did not substantially alter the association between IEM and acute rejection.
Abnormal esophageal motility was found in 29.3% of our cohort. Of those with abnormal esophageal motility, 59% of these patients were classified as having IEM (or 17% of the total cohort). Prior studies have demonstrated esophageal dysmotility in as high as 78% of patients undergoing lung transplant evaluation, though it is important to note significant heterogeneity in how esophageal dysmotility has been categorized[22-25]. The prevalence in our cohort was consistent with another study that also categorized HRM diagnoses based on Chicago Classification v3.0, which found IEM in 32.7% of patients undergoing lung transplant evaluation[25], supporting the generalizability of our findings.
Recent studies have begun to characterize the impact of esophageal dysmotility on lung transplant outcomes. In a single center study of 31 patients with pre-transplant esophageal aperistalsis, defined as ≥ 90% failed swallows without any effective peristalsis on HRM, the 1-, 3-, and 5-year post-lung transplant survivals were lower than that of the control group with normal esophageal motility[17]. This study also demonstrated that recovery of peristaltic function post-transplant was associated with improved transplant survival outcomes matching that of the control group. Another study from the same group noted HRM diagnoses of esophageal dysmotility frequently changed post-lung transplant (51.4%) and that peristaltic vigor tends to increase, implicating a dynamic relationship between esophageal motility and pulmonary function[25]. These studies suggest that chronic lung diseases and the resultant altered respiratory mechanics may impact esophageal motility, most commonly associated with hypomotility that may improve with recovery of pulmonary function after transplantation. Two other single center studies utilizing post-lung transplant HRM also demonstrated associations between esophageal dysmotility and outcomes of acute and chronic rejection[16,26].
The mechanism through which esophageal dysmotility may impact lung transplantation outcomes is not completely clear, although it is speculated to largely be related to increased risk of microaspiration due to reduced esophageal clearance. Esophageal hypomotility may result in decreased clearance and increased proximal migration of gastric refluxate, thereby leading to higher risk for exposure to the airway. Reduced esophageal bolus transit and clearance may also be associated with elevated risk of esophago-pharyngeal reflux, with potential resultant injury to the lung allograft. On the other hand, abnormal reflux, which has already been previously linked with worse lung transplant outcomes, may also lead to esophageal hypomotility. However, our results suggested that esophageal hypomotility may be associated with higher risk of allograft rejection independent of reflux burden.
There remains significant heterogeneity in reflux and esophageal motility testing in the pre- and post-lung transplant settings. HRM is standardized within the pre-transplant evaluation at our institution. The results of this study indicate that results of pre-transplant HRM are informative for risk stratification and prognostication for lung transplant outcomes. This information, in turn, may also guide post-transplant care and monitoring for acute rejection.
There are several notable strengths to our study. Pre-transplant evaluation of esophageal motility on HRM and reflux measurements on MII-pH were standardized across all lung transplant candidates. Ascertainment bias for determination of acute rejection was minimized by surveillance bronchoscopy per standard protocol with biopsy at 1, 3, 6, and 12 months, though clinical symptoms in between these intervals could trigger additional diagnostic bronchoscopies. Baseline characteristics of the study cohort were consistent with previously published data for rates of acute rejection, prevalence of esophageal dysmotility during pre-transplant evaluation, and indication for lung transplantation. Lastly, distinct HRM diagnoses were categorized according to established classification criteria for analysis, and we were able to control for potential confounding of specific measures of reflux based on MII-pH monitoring results that were collected on all patients.
There are also several limitations to our study. This is a retrospective cohort study with results that are limited to a single academic institution with high volume of lung transplantation. The sample size is relatively limited within the IEM group, though consistent with prior studies published on the association between esophageal motility and lung transplant outcomes. While a small number of recent studies have suggested dynamic changes in esophageal motility post-transplant, post-transplant motility measurements were not obtained routinely as part of our study. Finally, due to the retrospective nature and inclusion period of our study cohort, Chicago classification v3.0 was used to define IEM. However, the most current Chicago classification v4.0 mainly further restricted the diagnosis of IEM with more stringent criteria than v3.0. Therefore, the use of Chicago classification v3.0 to define IEM would likely have biased our results towards the null, as some patients in our IEM group would have been classified as normal under v4.0. The fact that our results remained significant despite this potential bias would strengthen the observed relationship between IEM and acute allograft rejection.
In summary, our study demonstrated that IEM on pre-transplant esophageal motility testing was associated with decreased time to development of acute rejection after lung transplantation. Our study provides additional evidence for the association between esophageal dysmotility and poor lung transplant outcomes. It builds upon prior studies on esophageal aperistalsis and survival outcomes in lung transplantation by providing additional evidence for acute rejection in the less severe phenotype of IEM. It also suggests esophageal dysmotility may mediate long-term lung transplant outcomes through a pathway starting with acute rejection. Further studies are needed in delineating transplant outcomes by underlying pulmonary diagnosis, analyzing longer term outcomes such as chronic rejection and 3- and 5-year survival outcomes in the context of esophageal dysmotility, and comparing pre- and post-transplant esophageal function testing results on lung transplant outcomes.
Gastroesophageal reflux is associated with poor outcomes after lung transplantation. However, the impact of esophageal dysmotility and role of esophageal manometry remains unclear. Ineffective esophageal motility (IEM) is a disorder of esophageal motility associated with decreased esophageal clearance that may worsen transplant outcomes.
Esophageal evaluation remains poorly standardized in lung transplantation, and this work suggests that routine esophageal motility testing to identify IEM may help identify patients at risk for acute rejection.
To evaluate the relationship between IEM and acute rejection after lung transplantation, controlling for confounders including coexisting pathologic acid and nonacid reflux.
This was a retrospective cohort study of lung transplant recipients that underwent pre-transplant esophageal testing including manometry and pH at a tertiary referral center.
IEM on pre-transplant esophageal manometry was associated with higher risk of acute rejection on time-to-event analysis. On multivariable Cox regression analysis, IEM remains independently associated with increased acute rejection, even after controlling for pathologic reflux. In addition, increased non-acid reflux was also an independent risk factor for acute rejection in the multivariable model.
Lung transplant candidates with IEM had a greater risk of developing acute rejection, independent of pathologic acid and nonacid reflux. Additionally, nonacid reflux was independently associated with acute rejection. These findings suggest that IEM and other disorders affecting esophageal clearance may contribute to the pathophysiology of allograft injury, independent of a reflux-associated pathway.
Future research should focus on the implementation of standardized esophageal motility testing in lung transplantation, investigation of the impact of IEM and other disorders of esophageal motility on longer term transplant outcomes including chronic rejection and survival, and assessment of changes in esophageal motility after transplant and its effect on transplant outcomes.
| 1. | Valapour M, Lehr CJ, Skeans MA, Smith JM, Miller E, Goff R, Foutz J, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2019 Annual Data Report: Lung. Am J Transplant. 2021;21 Suppl 2:441-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 2. | Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Khalifah AP, Hachem RR, Chakinala MM, Yusen RD, Aloush A, Patterson GA, Mohanakumar T, Trulock EP, Walter MJ. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant. 2005;5:2022-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Savarino E, Gemignani L, Pohl D, Zentilin P, Dulbecco P, Assandri L, Marabotto E, Bonfanti D, Inferrera S, Fazio V, Malesci A, Tutuian R, Savarino V. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:476-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Mittal R, Vaezi MF. Esophageal Motility Disorders and Gastroesophageal Reflux Disease. N Engl J Med. 2020;383:1961-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Ribolsi M, Balestrieri P, Emerenziani S, Guarino MP, Cicala M. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol. 2014;109:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Todd JL, Neely ML, Kopetskie H, Sever ML, Kirchner J, Frankel CW, Snyder LD, Pavlisko EN, Martinu T, Tsuang W, Shino MY, Williams N, Robien MA, Singer LG, Budev M, Shah PD, Reynolds JM, Palmer SM, Belperio JA, Weigt SS. Risk Factors for Acute Rejection in the First Year after Lung Transplant. A Multicenter Study. Am J Respir Crit Care Med. 2020;202:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Lo WK, Burakoff R, Goldberg HJ, Feldman N, Chan WW. Pre-lung transplant measures of reflux on impedance are superior to pH testing alone in predicting early allograft injury. World J Gastroenterol. 2015;21:9111-9117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Lo WK, Goldberg HJ, Burakoff R, Feldman N, Chan WW. Increased proximal acid reflux is associated with early readmission following lung transplantation. Neurogastroenterol Motil. 2016;28:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Lo WK, Burakoff R, Goldberg HJ, Feldman N, Chan WW. Pre-transplant impedance measures of reflux are associated with early allograft injury after lung transplantation. J Heart Lung Transplant. 2015;34:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hartwig MG, Appel JZ, Li B, Hsieh CC, Yoon YH, Lin SS, Irish W, Parker W, Davis RD. Chronic aspiration of gastric fluid accelerates pulmonary allograft dysfunction in a rat model of lung transplantation. J Thorac Cardiovasc Surg. 2006;131:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | D'Ovidio F, Mura M, Ridsdale R, Takahashi H, Waddell TK, Hutcheon M, Hadjiliadis D, Singer LG, Pierre A, Chaparro C, Gutierrez C, Miller L, Darling G, Liu M, Post M, Keshavjee S. The effect of reflux and bile acid aspiration on the lung allograft and its surfactant and innate immunity molecules SP-A and SP-D. Am J Transplant. 2006;6:1930-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Olson MT, Liu W, Mohanakumar T, Bremner RM. A potential mechanism by which aspiration of duodenogastric fluid augments the risk for bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 2023;165:e23-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Zhang CYK, Ahmed M, Huszti E, Levy L, Hunter SE, Boonstra KM, Moshkelgosha S, Sage AT, Azad S, Zamel R, Ghany R, Yeung JC, Crespin OM, Frankel C, Budev M, Shah P, Reynolds JM, Snyder LD, Belperio JA, Singer LG, Weigt SS, Todd JL, Palmer SM, Keshavjee S, Martinu T; CTOT-20 investigators. Bronchoalveolar bile acid and inflammatory markers to identify high-risk lung transplant recipients with reflux and microaspiration. J Heart Lung Transplant. 2020;39:934-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M, Campos SV, Christon LM, Cypel M, Dellgren G, Hartwig MG, Kapnadak SG, Kolaitis NA, Kotloff RM, Patterson CM, Shlobin OA, Smith PJ, Solé A, Solomon M, Weill D, Wijsenbeek MS, Willemse BWM, Arcasoy SM, Ramos KJ. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021;40:1349-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 558] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 16. | Tangaroonsanti A, Lee AS, Crowell MD, Vela MF, Jones DR, Erasmus D, Keller C, Mallea J, Alvarez F, Almansa C, DeVault KR, Houghton LA. Impaired Esophageal Motility and Clearance Post-Lung Transplant: Risk For Chronic Allograft Failure. Clin Transl Gastroenterol. 2017;8:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Masuda T, Mittal SK, Csucska M, Kovacs B, Walia R, Huang JL, Smith MA, Bremner RM. Esophageal aperistalsis and lung transplant: Recovery of peristalsis after transplant is associated with improved long-term outcomes. J Thorac Cardiovasc Surg. 2020;160:1613-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, McNeil KD, Reed EF, Reinsmoen NL, Scott JP, Studer SM, Tazelaar HD, Wallwork JL, Westall G, Zamora MR, Zeevi A, Yousem SA. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 917] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 19. | Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1487] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 20. | Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, Zhang X, Adhami T, Murray J, Peters J, Castell D. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 386] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Chambers DC, Yusen RD, Cherikh WS, Goldfarb SB, Kucheryavaya AY, Khusch K, Levvey BJ, Lund LH, Meiser B, Rossano JW, Stehlik J; International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant. 2017;36:1047-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 473] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 22. | Sweet MP, Herbella FA, Leard L, Hoopes C, Golden J, Hays S, Patti MG. The prevalence of distal and proximal gastroesophageal reflux in patients awaiting lung transplantation. Ann Surg. 2006;244:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Gasper WJ, Sweet MP, Golden JA, Hoopes C, Leard LE, Kleinhenz ME, Hays SR, Patti MG. Lung transplantation in patients with connective tissue disorders and esophageal dysmotility. Dis Esophagus. 2008;21:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Seccombe J, Mirza F, Hachem R, Gyawali CP. Esophageal motor disease and reflux patterns in patients with advanced pulmonary disease undergoing lung transplant evaluation. Neurogastroenterol Motil. 2013;25:657-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Masuda T, Mittal SK, Kovács B, Smith MA, Walia R, Huang JL, Bremner RM. Foregut function before and after lung transplant. J Thorac Cardiovasc Surg. 2019;158:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Burlen J, Chennubhotla S, Ahmed S, Landes S, Ramirez A, Stocker AM, Abell TL. Investigating Defects of Esophageal Motility in Lung Transplant Recipients. Gastroenterology Res. 2022;15:120-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ladic A, Croatia; Morozov S, Russia S-Editor: Fan JR L-Editor: A P-Editor: Chen YX