Published online Jun 7, 2023. doi: 10.3748/wjg.v29.i21.3280
Peer-review started: March 6, 2023
First decision: March 18, 2023
Revised: March 31, 2023
Accepted: May 8, 2023
Article in press: May 8, 2023
Published online: June 7, 2023
Processing time: 87 Days and 5 Hours
Fibroblast growth factor (FGF) 15/19, which is expressed in and secreted from the distal ileum, can regulate hepatic glucose metabolism in an endocrine manner. The levels of both bile acids (BAs) and FGF15/19 are elevated after bariatric surgery. However, it is unclear whether the increase in FGF15/19 is induced by BAs. Moreover, it remains to be understood whether FGF15/19 elevations contribute to improvements in hepatic glucose metabolism after bariatric surgery.
To investigate the mechanism of improvement of hepatic glucose metabolism by elevated BAs after sleeve gastrectomy (SG).
By calculating and comparing the changes of body weight after SG with SHAM group, we examined the weight-loss effect of SG. The oral glucose tolerance test (OGTT) test and area under the curve of OGTT curves were used to assess the anti-diabetic effects of SG. By detecting the glycogen content, expression and activity of glycogen synthase as well as the glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (Pepck), we evaluated the hepatic glycogen content and gluconeogenesis activity. We examined the levels of total BA (TBA) together with the farnesoid X receptor (FXR)-agonistic BA subspecies in systemic serum and portal vein at week 12 post-surgery. Then the histological expression of ileal FXR and FGF15 and hepatic FGF receptor 4 (FGFR4) with its corresponding signal pathways involved in glucose metabolism were detected.
After surgery, food intake and body weight gain of SG group was decreased compare with the SHAM group. The hepatic glycogen content and glycogen synthase activity was significantly stimulated after SG, while the expression of the key enzyme for hepatic gluconeogenesis: G6Pase and Pepck, were depressed. TBA levels in serum and portal vein were both elevated after SG, the FXR-agonistic BA subspecies: Chenodeoxycholic acid (CDCA), lithocholic acid (LCA) in serum and CDCA, DCA, LCA in portal vein were all higher in SG group than that in SHAM group. Consequently, the ileal expression of FXR and FGF15 were also advanced in SG group. Moreover, the hepatic expression of FGFR4 was stimulated in SG-operated rats. As a result, the activity of its corresponding pathway for glycogen synthesis: FGFR4-Ras-extracellular signal regulated kinase pathway was stimulated, while the corresponding pathway for hepatic gluconeogenesis: FGFR4- cAMP regulatory element-binding protein- peroxisome proliferator-activated receptor γ coactivator-1α pathway was suppressed.
Elevated BAs after SG induced FGF15 expression in distal ileum by activating their receptor FXR. Furthermore, the promoted FGF15 partly mediated the improving effects on hepatic glucose metabolism of SG.
Core Tip: Sleeve gastrectomy (SG) improves hepatic glucose metabolism and alleviates type 2 diabetes mellitus through the intestine-liver crosstalk mediated by fibroblast growth factor 15 (FGF15). Following SG, bile acids are elevated, inducing the expression and secretion of FGF15 via the activation of farnesoid X receptor in the ileum. FGF15 then acts as an endocrine factor to promote glycogen synthesis and inhibit gluconeogenesis in the liver by specifically stimulating hepatic FGF receptor 4 and its corresponding signaling pathways.
- Citation: Wei M, Cao WB, Zhao RD, Sun DP, Liang YZ, Huang YD, Cheng ZW, Ouyang J, Yang WS, Yu WB. Fibroblast growth factor 15, induced by elevated bile acids, mediates the improvement of hepatic glucose metabolism after sleeve gastrectomy. World J Gastroenterol 2023; 29(21): 3280-3291
- URL: https://www.wjgnet.com/1007-9327/full/v29/i21/3280.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i21.3280
The incidence of obesity along with the relative metabolic abnormalities[1], especially type 2 diabetes mellitus (T2DM)[2], is on the rise globally. Bariatric surgery becomes the most efficient treatment option for obese patients with diabetes[3] due to its prominent weight reduction and durable metabolic improvement effect[4,5]. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are the most commonly performed surgical procedures currently[3]. Moreover, SG has now exceeded RYGB as the preferred approach for most surgeons[6,7]. Still, the mechanisms underlying effects of bariatric surgery remain to be fully elucidated.
Bile acid (BA) has been considered as prospective factor for the metabolic actions of bariatric surgery[8] because of the considerable significance of its receptors, Takeda G protein-coupled receptor 5[9] and farnesoid X receptor (FXR)[10]. Through intestinal FXR, BAs could induce the expression and secretion of fibroblast growth factor (FGF) 15/19 in the distal ileum[11,12]. Recent studies illustrate that both BAs and FGF19 -the human ortholog of rodent FGF15[13]- are elevated after bariatric surgery[14-17]. Hence, the elevation of FGF19 after bariatric surgery probably results from the induction of FXR by elevated BAs, which needs further investigation. Moreover, FGF15/19 also plays a role in regulating the metabolic homeostasis, especially the hepatic glucose metabolism[18]. Similar to insulin’s actions, FGF15/19 could act to lower the blood glucose level by repressing gluconeogenesis[19] and promoting glycogen synthesis[20] in the liver. Taken together, the ileal FXR-FGF15/19 pathway triggered by BAs might be another potential mechanism underlying the metabolic effects of bariatric surgery. However, to prove this, we need to confirm that the elevation of FGF15/19 results from the induction of FXR by elevated BAs and FGF15/19 participates in the improvement of hepatic glucose metabolism after bariatric surgery.
As insulin and FGF15/19 can exert comparable metabolic actions on liver, only to detect the activities of glycogen synthesis and gluconeogenesis of liver could not distinguish the action of FGF15/19 from insulin. Previous studies demonstrated that FGF15/19 functions in an insulin-independent manner through activating FGF receptor 4 (FGFR4)[21] and its corresponding signal pathways. FGF15/19 could promote glycogen synthesis by activating the Ras- extracellular signal regulated kinase (ERK) pathway[20] and inhibit hepatic gluconeogenesis by suppressing the cAMP regulatory element-binding protein (CREB)- peroxisome proliferator-activated receptor 𝛾 coactivator-1α (PGC-1α) pathway[19]. However, insulin induces these effects through phosphoinositide 3-kinase (PI3K)-Akt pathway. The signaling pathways engaged in regulation of hepatic glucose metabolism by FGF15/19 and insulin permit overlapping, but can distinct the biological effects of these two hormones. Hence, to detect the expression of FGFR4 and the activities of its corresponding signaling pathways after bariatric surgery would help to confirm the action of FGF15/19 on hepatic glucose metabolism.
We conducted SHAM and SG operations on an obese diabetic rat model induced by high-fat diet (HFD) and streptozotocin (STZ) in this study. BAs levels in serum and portal vein, and the ileal expression of FXR and FGF15 were detected to confirm the elevation of the FGF15 as well as the underlying mechanism after surgery. Moreover, we detected the hepatic expression of FGFR4 and the activity of its corresponding signaling pathways involved in glucose metabolism to investigate whether FGF15 mediates the improvement of hepatic glucose metabolic after SG.
We used Wistar rats in this study, and the rats (8-wk-old, 200 g on average) were purchased and housed in Laboratory Animal Center of Shandong University (Jinan, Shandong Province, China). The obese diabetic rat model was induced as previously reported[22,23]. The rats received a HFD (40% fat, 42% carbohydrate, 18% protein, Huafukang Biotech, China) for 1 mo, followed by low dose intraperitoneal STZ injection (35 mg/kg, Sigma, United States). The glucose level of peripheral blood obtained from tail vein was tested using a glucometer (Roche Diagnostics, Germany). Diabetes induction was confirmed based on the Oral Glucose Tolerance Test (OGTT) results and blood glucose levels examined at random time-points (≥ 16.7 mmol/L). The diabetic rats then randomly divided into matched SHAM (n = 10) and SG (n = 10) group. All actions and interventions applied on rats were approved and guided by Laboratory Animal Ethical and Welfare Committee of Shandong University Cheeloo College of Medicine.
Before operation, rats were fasted for about 12 h after two-days feeding of 10% Ensure (Abbott, United States). Moreover, anesthesia for rats was induced by 10% chloral hydrate (3 mL/kg).
SG: First, the relevant vessels and gastro-epiploic structures were ligated and transected to externalize the greater curvature of the stomach. Then, we conducted gastrectomy of 80% of the whole stomach from greater curvature, including the whole gastric fundus. The residual stomach was closed and continuously sutured with a 5-0 silk suture (Ningbo medical, China). The cardia, pylorus of stomach and continuity of stomach with esophagus and duodenum were all remained unaffected.
SHAM: In order to minimize the concomitant influence and bias triggered by surgical operation and anesthesia, we set SHAM group as previously reported. First, we isolated the greater curvature of stomach similarly as what we did in SG group. Then we clamped the stomach along the greater curvature with a pair of blunt forceps between cardia and pylorus to exert a similar pressure on the stomach. The exposed area and time of surgical field were extended as that of SG group to eliminate the undeserving influence of operation and anesthesia.
Postsurgical care: After surgery, the rats were only supplied with some water during the first 24 h. Then following 3-d feeding of 10% Ensure, the rats resumed the HDF diet till the end of the study. We recorded the body weight and calorie intake of every rat in both groups every day. Antibiotics were unnecessary and all rats in both groups survived and were sacrificed until the end of the study (week 12 post-surgery).
We conducted OGTTs preoperatively to confirm diabetes induction and to evaluate the anti-diabetic effects of SG at week 12 post-surgery. After 12-h fasting, the rats received 20% glucose (1 g/kg) chow via oral gavage. Blood glucose levels were measured at time point of 0 min, 10 min, 30 min, 60 min, and 120 min after administration.
At 12 wk after surgery, total BAs (TBA) levels were examined in both the systemic serum and portal vein. In addition, the specific levels of the following four FXR-agonist BA species were also examined: Chenodeoxycholic acid (CDCA), DCA, lithocholic acid (LCA), and cholic acid (CA). One hour before sacrificed, all rats were gavaged with 10% Ensure after 12-h fasting. When sacrificing the rats, we collected blood samples from the retrobulbar venous plexus and portal vein. Portal and systemic serum levels of TBA were detected on the Roche Cobas 8000 system using the enzyme cycling method. As previously described[22,23], the levels of BA species (CDCA, DCA, LCA, and CA) in these samples were evaluated using high-pressure liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). Standard substrates for these four BA species were purchased from Sigma Aldrich (United States).
After sacrificing, ileum and liver tissues in both groups were divided into three parts. Some were fixed in 4% paraformaldehyde and then embedded in paraffin for Periodic Acid-Schiff (PAS) staining and immunohistochemistry staining; some were stored at -80 °C for western blotting and hepatic glycogen content detection; and the others were stored in RNAlater™ Stabilization Solution (Thermo Fisher Scientific™) for real-time PCR.
The levels of FGF15, FXR in ileum and FGFR4, glucose-6-phosphatase (G6Pase), phosphoenolpyruvate carboxykinase (Pepck) in liver were measured using RT-PCR. Total RNA was extracted from the tissue using TRIzol reagent (Invitrogen, United States) and then reversely transcribed to cDNA by the High Capacity cDNA Reverse Transcription Kit (TOYOBO, Japan). Subsequently, the relative level of mRNA amplification was determined through the SYBR Green Real-time PCR Master Mix Kit (TOYOBO, Japan) according to manufacturer’s instructions. The following primers were used for analysis: FGF15: 5’-GCCATCAAGGACGTCAGCA-3’(F), 5’-CTTCCTCCGAGTAGCGAATCAG-3’ (R); FXR: 5’-TCCGGACATTCAACCATCAC-3’ (F), 5’-TCACTGCACATCCCAGATCTC-3’ (R); FGFR4: 5’-GGCCAGGTATACGGACATCA-3’ (F), 5’-GAGTCAGGCTGTCACATGTG-3’ (R); G6Pase: 5’-GTGGCAGTGGTCGGAGACT-3’ (F), 5’-ACGGGCGTTGTCCAAAC-3’ (R); Pepck: 5’-CACCATCACCTCCTGGAAGA-3’ (F), 5’-GGGTGCAGAATCTCGAGTTG-3’ (R).
We detected the hepatic glycogen content using the Glycogen Assay kit (ab65620, Abcam, United States) according to manufacturer’s protocol. Sections of the paraffin-embedded tissues were prepared for PAS and immunohistochemistry staining. We stained the glycogen in hepatocytes with the PAS Stain Kit (Mucin Stain) (ab150680, Abcam, United States) according to the manufacturer’s protocol. And we also examined the ileal expression of FGF15 and hepatic expression of FGFR4 by immunohistochemistry staining. The antibody for FGF15 (sc-16816) and FGFR4 (sc-136988) were both purchased from Santa Cruz Biotechnology (United States). Percentage of PAS- positive cells was calculated and quantification of immunoreactive signal was performed using Image J software.
We detected the hepatic expression of ERK1/2, p-ERK1/2, glycogen synthase (GS), p-GS, CREB, p-CREB and PGC-1α by western blotting. Anti-ERK1/2, anti-p-ERK1/2, anti-GS, anti-p-GS, anti-CREB, and anti-p-CREB for western blotting were purchased from Cell Signaling Technology (CST, United States), and anti-PGC-1α, anti-Actin were purchased from Abcam (United States).
All quantitative data are presented in the pattern of mean ± SEM. The area under the curve (AUC) was evaluated using trapezoidal integration. We used unpaired Student’s t test to analyzed statistical significance, and P < 0.05 indicated statistical significance. All statistical analyses were performed by SPSS version 20.0.
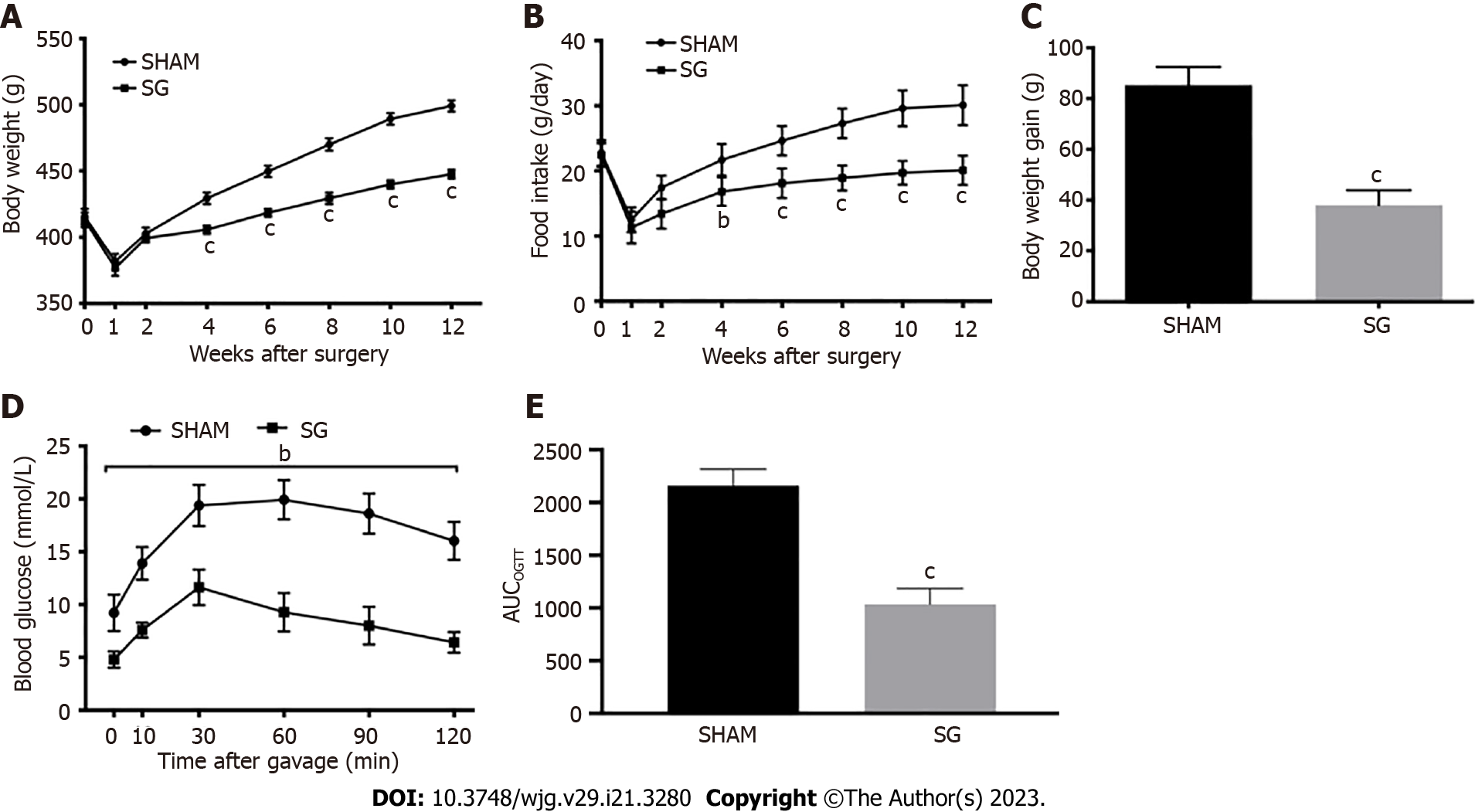
According to our previous studies, we evaluated the weight-loss and anti-diabetic effects of SG postoperatively. First, we assessed the weight-loss action by tracking the body weight and daily food intake of the rats in SHAM and SG group. After a sharp decrease during the first week after surgery, the body weight and food intake gradually increased in both groups. Since week 4 post-surgery, the increase of body weight and food intake in SG group became significantly slower than that in SHAM group (Figure 1A and B). And the body weight gain of SG group was correspondingly lower than that in SHAM group till week 12 post-surgery (Figure 1C). Then, we conducted the OGTT in both groups to evaluate the anti-diabetic effects of SG. The OGTT curves and result of AUCOGTT showed much better glucose tolerance in SG group than SHAM group (Figure 1D and E), which indicated a considerable anti-diabetic effect of SG.
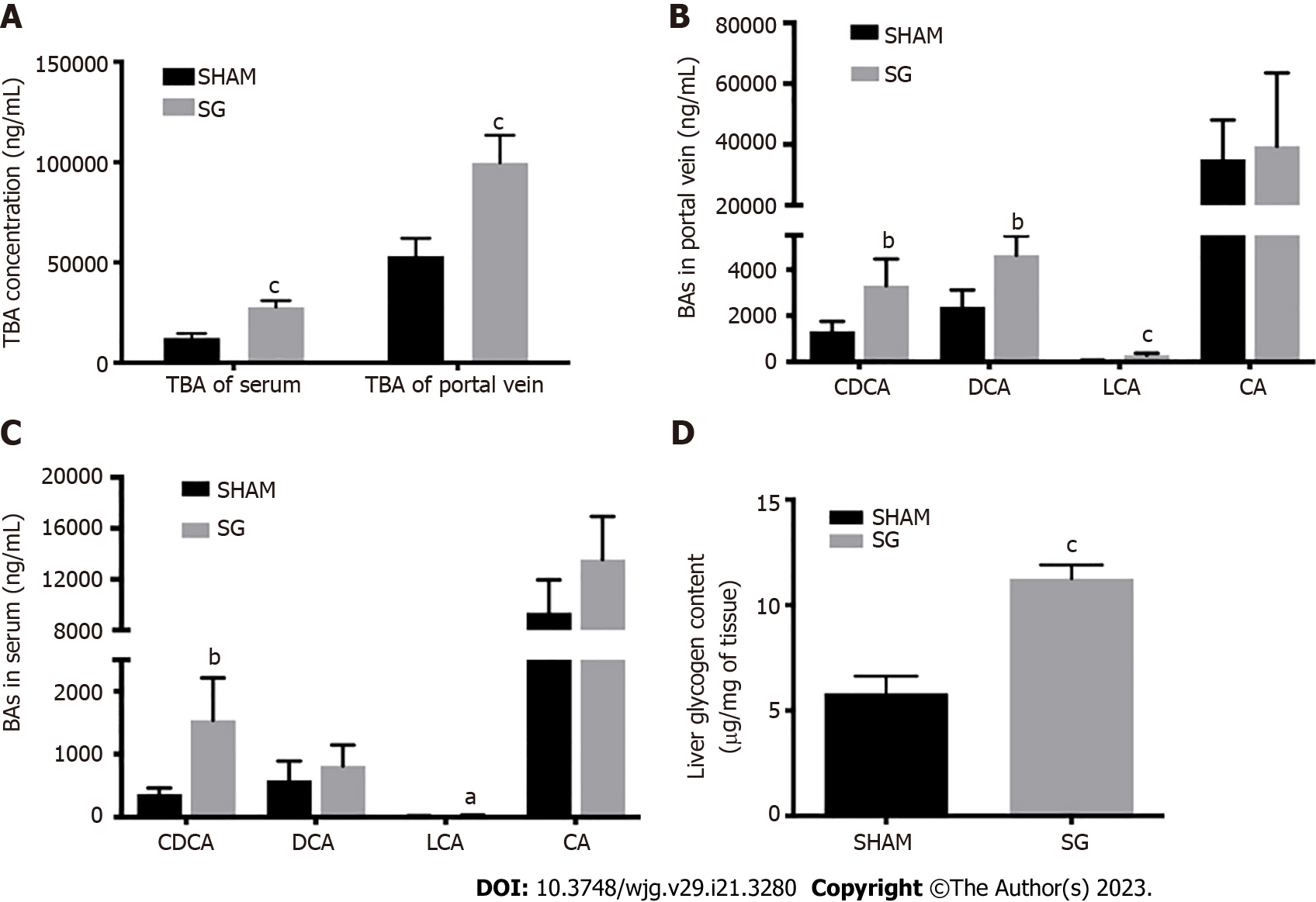
We detected the TBA levels both in systemic serum and portal vein at week 12 post-surgery, together with four BA species: CDCA, DCA, LCA, and CA, which are all FXR agonists. TBA levels of SG group in serum and portal vein were significantly higher than that in SHAM group (Figure 2A and B). Both CDCA and LCA levels in these two kinds samples were elevated and the portal vein DCA level was also higher in SG group (Figure 2C and D).
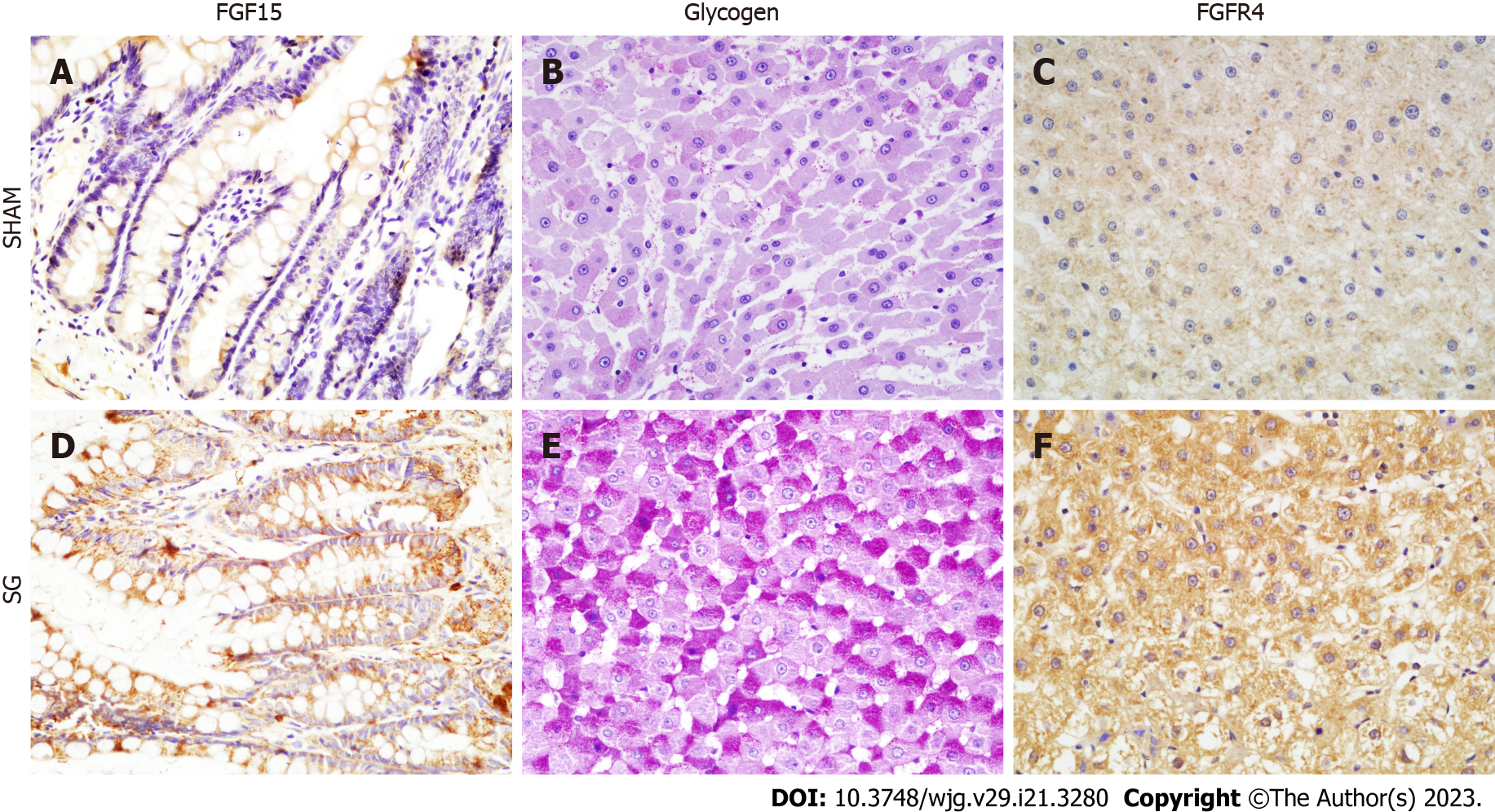
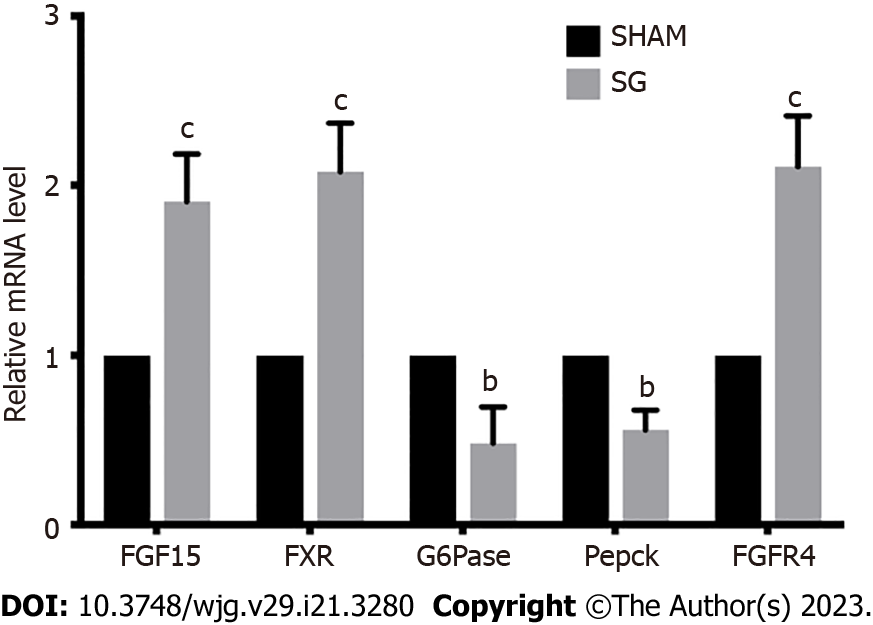
Then, we detected the influence of SG on FGF15 and FXR expressions in distal ileum. Both mRNA and protein expression levels of FGF15 in SG group were significantly higher than that in SHAM group (Figure 3A and D; Figure 4). Also, FXR mRNA level was also considerably elevated after SG (Figure 4).
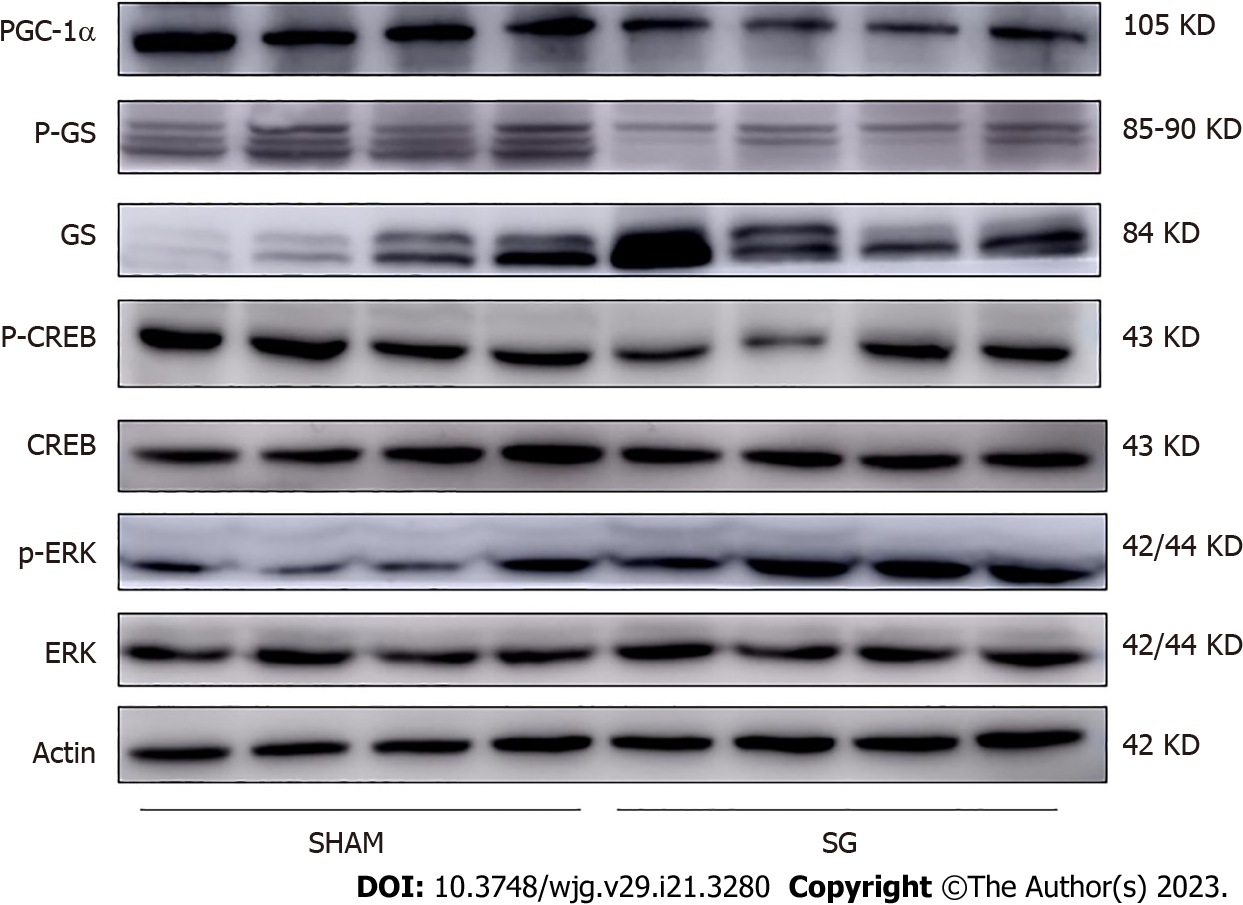
To evaluate the hepatic glucose metabolism, we detected the activities of hepatic glycogen synthesis and gluconeogenesis, together with the glycogen content in both groups after SG. The expression of glycogen synthase (GS) was significantly higher in the SG-operated rats, while the phosphorylation degree of GS (p-GS) was declined (Figure 5). In other words, the hepatic glycogen synthetic activity was significantly promoted after SG. Correspondingly, the glycogen content and the percentage of PAS-positive cells in liver were significantly higher in SG-operated rats (Figures 2A, 3B and E).
We then measured two key enzymes within hepatic gluconeogenesis, G6Pase and Pepck mRNA levels of both enzymes were significantly declined in SG group compared with SHAM group, which illustrated an inhibited gluconeogenesis process in SG-operated rats (Figure 4).
FGF15/19 can activate its receptor, FGFR4 in the liver with unique specificity[25]. The expression of FGFR4 was significantly promoted at both mRNA and protein levels in SG group (Figure 4). FGF15-FGFR4 regulates hepatic glycogen synthesis and gluconeogenesis through Ras-ERK pathway and CREB-PGC-1α pathway, respectively. ERK1/2 regulates the phosphorylation degree of GS. We detected no difference in the expression of total ERK1/2 protein level. Whereas, the phosphorylation of ERK1/2 was increased in SG-operated rats, which indicates the activation of ERK1/2 pathway (Figure 5). On the contrary, the declined phosphorylation degree of CREB and decreased expression of PGC-1α (Figure 5) together with the inhibition of G6Pase and Pepck (G6Pase and Pepck are two target genes of PGC-1α) illustrated the inhibition of CREB-PGC-1α pathway in SG group.
In this study, SG induced considerable weight loss and significantly improved the glucose homeostasis, especially the hepatic glucose metabolism in an obese diabetic rat model. Levels of TBA and FXR-agonistic BA subspecies were all increased in the serum of both peripheral circulation and portal vein. Besides, the expression of FGF15 which was induced by FXR activation in the ileum was increased after SG. And the FGF15 receptor FGFR4 in the liver together with its corresponding signal pathways involved in hepatic glycogen synthesis and gluconeogenesis were also activated.
Liver plays a key role in the metabolic regulation of the whole body, especially in maintaining homeostasis of blood glucose level through synthesis or breaking down of glycogen and the action of gluconeogenesis. One of the insulin’s actions is to promote glycogen synthesis and inhibit hepatic gluconeogenesis which is impaired in diabetic patients. While, we found that SG improved the glucose tolerance of diabetic rats in this study, the hepatic glycogen content was elevated and the expression of two key enzymes[19] (G6pase and Pepck) responsible for hepatic gluconeogenesis were decreased in the SG-operated rats. In other words, SG increased the insulin sensitivity by promoting the synthesis of glycogen and inhibiting the hepatic gluconeogenesis. Moreover, recent studies found that FGF15/19 could also exert the similar effects as insulin on hepatic metabolism in an insulin-independent pattern[19,20]. Furthermore, the serum FGF19 Level of diabetic patients was elevated after bariatric surgery[14-17]. Therefore, except the improvement of insulin sensitivity, the improvement of glucose tolerance post-surgery may also be partly mediated by the elevated FGF15/19.
Consistent with previous findings of FGF 19 in human, the ileal expression of FGF15 was increased in SG-operated rats in our study and the TBA levels in systemic serum and portal vein were also increased after SG. This may inconsistent with previous reports that FGF15 acts as an endocrinal factor to repress BA synthesis in the liver[11]. However, our previous studies have already proved that the elevation of serum BA level was mainly a result of the promoted ileal reabsorption rather than hepatic synthesis[22,23]. According to the process of the enterohepatic circulation, systemic serum BAs is a combinational result of hepatic uptake and ileal reabsorption of BAs and portal vein BAs levels can reflect the ileal reabsorption and the BA concentration of distal ileum[26,27]. Hence, the elevation of TBAs in systemic serum and portal vein in this study must be a result of the promoted ileal reabsorption. Then, we also explored the cause for the elevated expression of FGF15 in our study. FGF15/19 is mainly expressed in distal ileum and can be induced by FXR[28,29]. The increased ileal mRNA level of FXR in our study should be responsible for the elevation of FGF15 after SG. Moreover, FXR can be activated by different BA subspecies, among which CDCA is the most efficacious[30]. Furthermore, oral or intraduodenal infusion of CDCA triggers increase of serum FGF19 Level in humans[31,32]. Consistent with our previous study[22,23], CDCA levels as well as other FXR agonists were elevated after SG both in systemic serum and portal vein. Hence, activation of ileal FXR in this study would result from the elevation of these agonists among the SG-operated rats. Taken together, enhanced reabsorption of BAs after SG activated FXR in the ileum, which then promoted the expression and secretion of FGF15.
Stimulated by ileal FXR, FGF15/19 is promoted and secreted into the enterohepatic circulation. In addition of the regulation of BA homeostasis, FGF15/19 also plays a role in the hepatic glucose metabolism. Evidence have shown that FGF 15 knockout mice showed impaired glucose tolerance with decreased hepatic glycogen storage and advanced hepatic gluconeogenesis[20]. Diet-induced obesity and insulin resistance was failed in FGF19 transgenic mice[33,34]. These effects of FGF15/19 are all mediated through FGFR[35-37], the receptor of FGF15/19 with an unique specificity[21,25]. Moreover, expression of FGFR4 in liver was decreased among STZ-induced diabetes[36]. FGFR4-deficient mice displayed glucose intolerance and insulin resistance as well as increased weight gain[35]. As described above, FGF15/19-FGFR4 plays an important role in hepatic glucose metabolism. Considering the elevation of FGF15, we then examined whether FGF15-FGFR4 functions in the improvement of hepatic glucose metabolism after SG. The hepatic expression of FGFR4 among the HFD and STZ-induced diabetic rats was elevated after SG in this study, which indicates that FGF15-FGFR4 was activated in SG-operated rats. However, we still can’t conclude that FGF15-FGFR4 acts in improving the hepatic glucose metabolism because the regulation of BAs synthesis by FGF15 also needs activation of FGFR4.
To further confirm whether FGF15-FGFR4 contributes to the improvement of glucose metabolism in SG-operated rats, we detected the corresponding pathways of FGF15-FGFR4 involved in the hepatic glucose metabolism. Comparable to insulin, FGF15/19 could regulate hepatic glucose metabolism by promoting glycogen synthesis and inhibiting hepatic gluconeogenesis. But unlike the insulin-inducing PI3K-Akt patway, FGF15/19 acts through two different pathways. Moreover, FGF15/19 the inhibiting effect on BA synthesis of FGF15/19 was through a c-Jun N-terminal kinase-dependent pathway[12]. FGF15/19 promotes the hepatic glycogen synthesis by activating Ras-ERK pathway[20]. FGF15/19-FGFR4 triggers the phosphorylation of ERK1/2, which then increase the activity of GS by inhibiting the phosphorylation of this enzyme. Therefore, the increased phosphorylation of ERK1/2 and the declined phosphorylation of GS observed in this study indicated that FGF15-FGFR4-Ras-ERK pathway was activated in SG-operated rats. Together with the elevated glycogen content, we can conclude that the action of FGF15 (and its corresponding pathways) contributed to the glycogen synthesis promoting effect of SG. Moreover, FGF15/19 inhibits hepatic gluconeogenesis by supressing the CREB-PGC-1α pathway[19]. FGF15/19 could induce dephosphorylate and suppress the transcription factor CREB and correspondingly inhibited the expression of PGC-1α. Besides, G6Pase, and Pepck, the two key enzymes for hepatic gluconeogenesis, are two target genes of PGC-1α. So, integrating together the depressed phosphorylation of CREB, reduced expression of PGC-1α and the inhibited expression of G6Pase and Pepck, we could predicate that FGF15 inhibited the CREB-PGC-1α pathway and participated in suppressing the hepatic gluconeogenesis in SG-operated rats. Hence, the activated Ras-ERK1/2 pathway and inhibited CREB-PGC-1α pathway indicated that elevated FGF15 after SG played a role in regulating the hepatic glucose metabolism in parallel to insulin.
In this study, we confirmed the effect of elevated BAs and FGF15 on the hepatic glucose metabolism. FGF15, induced by elevated BAs in ileum, acts as an endocrine factor on the liver through portal vein. By specifically binding and activating the FGFR4 and its corresponding signal pathways, FGF15 improves the hepatic glucose metabolism through the intestine-liver crosstalk. Similarly, human FGF19, which is an ortholog of rodent FGF15, was also elevated after SG together with BAs. Besides, FGF19 can also bind and activate the FGFR 4 specifically. Thus, the results of this study presented basics for the research of human FGF19 after SG, and the FGF19 would be developed to be a target for clinical treatment of T2DM in the future.
Certainly, this study has several limitations. First, we did not directly detect the FGF15 Levels in serum and portal vein because no ELISA kits for FGF15 was available at that time. However, we detected the expression of FGF15 in the ileum at both mRNA and protein levels. As FGF15 is mainly expressed and secreted from the ileum into enterohepatic circulation, the ileal expression could reflect the FGF15 Levels in serum and portal vein. Second, the postprandial FGF15 Level varies with time, it is better to trace the FGF15 Levels at different time points after gavage. However, we can only sacrifice the rat and detect the expression of FGF15 in the ileum once. And, as previously reported, the expression level of FGF15 mRNA in ileum and the phosphorylation degree of downstream hepatic ERK1/2 reach the peak 1h after feeding in rodent[19]. So, we sacrificed the rats 1h after gavage and the following results then may be more meaningful and advisable. Third, the improvement of hepatic glucose metabolism after SG should surely be mainly mediated by insulin. Though the action of FGF15 underlying the effects of SG was proved in our study, the proportion of these effects accounted by FGF15 still needs further evaluation. Finally, the data presented in this study only show associations, but no causal relationships between the alteration of FGF15 and improvement of hepatic glucose metabolism after SG, which would have to be further investigated by using KO models.
BAs levels were elevated in the SG-operated rats and induced FGF15 expression by activating their receptor, FXR in distal ileum. Moreover, the action of FGF15 on hepatic glycogen synthesis and gluconeogenesis further contributes to the anti-diabetic effects of SG.
Bariatric surgery can significantly ameliorate type 2 diabetes mellitus (T2DM) through its rapid and durable weight-loss and hypoglycemic action. Both biles acids (BAs) and fibroblast growth factor (FGF) 15/19 are increased after surgery. Whether BAs and FGF15/19 participates in amelioration of T2DM after bariatric surgery and the underlying mechanism remain incompletely illuminated.
FGF15 which is induced by farnesoid X receptor (FXR) in ileum can improve the hepatic glucose metabolism through entero-hepatic circulation. Our previous study confirms that BA profiles within peripheral circulation and portal vein have changed after SG, with significant increase of FXR- activated BAs levels.
This study aimed to evaluate the effect of FGF15 on improvement of T2DM triggered by elevated BAs after sleeve gastrectomy (SG) and investigate the underlying mechanism.
The weight-loss and hypoglycemic action of SG were detected in a diabetic rat model induced by High-fat diet and streptozotocin (STZ), as well as the hepatic glycogen content and gluconeogenesis activity. Total BA (TBA) together with the FXR-agonistic BA subspecies levels in systemic serum and portal vein were examined at week 12 post-surgery. Then the expression and activity of ileal FXR and FGF15 and hepatic FGFR4 with its corresponding signal pathways involved in glucose metabolism were detected.
Compared with SHAM group, SG induced sustained weight loss and improved the hepatic glucose metabolism by promoting hepatic glycogen synthesis and inhibiting the gluconeogenesis. TBA levels and the FXR-agonistic subspecies in serum and portal vein were elevated after SG. Consequently, the ileal expression of FXR and FGF15 were also advanced. Moreover, the hepatic expression of FGFR4 and the activities of its corresponding pathways were stimulated in SG-operated rats.
FGF15, triggered by elevated BAs after SG, acts as an endocrine factor to induce the improvement of hepatic glucose metabolism through the intestine-liver crosstalk.
FXR-agonistic BA subspecies and FGF15 participate in improvement of hepatic glucose metabolism, and may be developed as new targets for the treatment of T2DM.
| 1. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 5843] [Article Influence: 1460.8] [Reference Citation Analysis (37)] |
| 2. | Magliano DJ, Chen L, Islam RM, Carstensen B, Gregg EW, Pavkov ME, Andes LJ, Balicer R, Baviera M, Boersma-van Dam E, Booth GL, Chan JCN, Chua YX, Fosse-Edorh S, Fuentes S, Gulseth HL, Gurevicius R, Ha KH, Hird TR, Jermendy G, Khalangot MD, Kim DJ, Kiss Z, Kravchenko VI, Leventer-Roberts M, Lin CY, Luk AOY, Mata-Cases M, Mauricio D, Nichols GA, Nielen MM, Pang D, Paul SK, Pelletier C, Pildava S, Porath A, Read SH, Roncaglioni MC, Lopez-Doriga Ruiz P, Shestakova M, Vikulova O, Wang KL, Wild SH, Yekutiel N, Shaw JE. Trends in the incidence of diagnosed diabetes: a multicountry analysis of aggregate data from 22 million diagnoses in high-income and middle-income settings. Lancet Diabetes Endocrinol. 2021;9:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 3. | Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 2016] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 5. | Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 580] [Cited by in RCA: 565] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 6. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, Chamseddine G, Bornstein SR, Rubino F. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 7. | Horwitz D, Padron C, Kelly T, Saunders JK, Ude-Welcome A, Schmidt AM, Parikh M. Long-term outcomes comparing metabolic surgery to no surgery in patients with type 2 diabetes and body mass index 30-35. Surg Obes Relat Dis. 2020;16:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Cai J, Rimal B, Jiang C, Chiang JYL, Patterson AD. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol Ther. 2022;237:108238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 299] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 9. | Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1447] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 10. | Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 765] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 11. | Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1297] [Cited by in RCA: 1473] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 12. | Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 554] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 13. | Wright TJ, Ladher R, McWhirter J, Murre C, Schoenwolf GC, Mansour SL. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol. 2004;269:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Docherty NG, Le Roux CW. Physiological adaptations following Roux-en-Y gastric bypass and the identification of targets for bariatric mimetic pharmacotherapy. Curr Opin Pharmacol. 2015;25:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, le Roux CW. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 293] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 16. | Sachdev S, Wang Q, Billington C, Connett J, Ahmed L, Inabnet W, Chua S, Ikramuddin S, Korner J. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes Surg. 2016;26:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Thöni V, Pfister A, Melmer A, Enrich B, Salzmann K, Kaser S, Lamina C, Ebenbichler CF, Hackl H, Tilg H, Moschen AR. Dynamics of Bile Acid Profiles, GLP-1, and FGF19 After Laparoscopic Gastric Banding. J Clin Endocrinol Metab. 2017;102:2974-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Cicione C, Degirolamo C, Moschetta A. Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology. 2012;56:2404-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S, Suino-Powell K, Xu HE, Gerard RD, Finck BN, Burgess SC, Mangelsdorf DJ, Kliewer SA. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 20. | Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 503] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 21. | Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-O M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687-26695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 642] [Cited by in RCA: 629] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Wang Y, Zhong M, Liu T, Han H, Zhang G, Liu S, Wei M, Wu Q, Hu S. Duodenal-Jejunal Bypass Preferentially Elevates Serum Taurine-Conjugated Bile Acids and Alters Gut Microbiota in a Diabetic Rat Model. Obes Surg. 2016;26:1890-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Wu Q, Zhang X, Zhong M, Han H, Liu S, Liu T, Wei M, Guo W, Xie H, Hu S, Zhang G. Effects of Bariatric Surgery on Serum Bile Acid Composition and Conjugation in a Diabetic Rat Model. Obes Surg. 2016;26:2384-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Ding L, Sousa KM, Jin L, Dong B, Kim BW, Ramirez R, Xiao Z, Gu Y, Yang Q, Wang J, Yu D, Pigazzi A, Schones D, Yang L, Moore D, Wang Z, Huang W. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. 2016;64:760-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 25. | Xie MH, Holcomb I, Deuel B, Dowd P, Huang A, Vagts A, Foster J, Liang J, Brush J, Gu Q, Hillan K, Goddard A, Gurney AL. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1783] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 27. | Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 733] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 28. | Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 611] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 29. | Stroeve JH, Brufau G, Stellaard F, Gonzalez FJ, Staels B, Kuipers F. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest. 2010;90:1457-1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1717] [Cited by in RCA: 1792] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 31. | Borup C, Wildt S, Rumessen JJ, Bouchelouche PN, Graff J, Damgaard M, McQuitty C, Rainteau D, Munck LK. Chenodeoxycholic acid stimulated fibroblast growth factor 19 response - a potential biochemical test for bile acid diarrhoea. Aliment Pharmacol Ther. 2017;45:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Meyer-Gerspach AC, Steinert RE, Keller S, Malarski A, Schulte FH, Beglinger C. Effects of chenodeoxycholic acid on the secretion of gut peptides and fibroblast growth factors in healthy humans. J Clin Endocrinol Metab. 2013;98:3351-3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 465] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 34. | Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 436] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 35. | Huang X, Yang C, Luo Y, Jin C, Wang F, McKeehan WL. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet induced fatty liver. Diabetes. 2007;56:2501-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 36. | Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem. 2009;284:11110-11120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Wu X, Li Y. Role of FGF19 induced FGFR4 activation in the regulation of glucose homeostasis. Aging (Albany NY). 2009;1:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Higashi Y, Japan; Pergamo M, United States S-Editor: Chen YL L-Editor: A P-Editor: Ma YJ