Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3066
Peer-review started: January 26, 2023
First decision: February 16, 2023
Revised: March 1, 2023
Accepted: April 28, 2023
Article in press: April 28, 2023
Published online: May 28, 2023
Processing time: 119 Days and 13.2 Hours
The widespread uptake of different machine perfusion (MP) strategies for liver transplant has been driven by an effort to minimize graft injury. Damage to the cholangiocytes during the liver donation, preservation, or early posttransplant period may result in stricturing of the biliary tree and inadequate biliary drainage. This problem continues to trouble clinicians, and may have catastrophic conse
Core Tip: In recent years, the development of different machine perfusion (MP) strategies has generated interest in their use for both the assessment of grafts and optimization during the preservation period. The different mechanisms behind the diverse array of MP strategies may reduce the extent of cholangiocyte and may have the subsequent clinical effect of preventing the development of ischemic type biliary lesions (ITBL). This review summarizes the strength and limitations of clinical studies that have been undertaken, their results, and provides a summary of the available literature on MP and the prevention of ITBL.
- Citation: Durán M, Calleja R, Hann A, Clarke G, Ciria R, Nutu A, Sanabria-Mateos R, Ayllón MD, López-Cillero P, Mergental H, Briceño J, Perera MTPR. Machine perfusion and the prevention of ischemic type biliary lesions following liver transplant: What is the evidence? World J Gastroenterol 2023; 29(20): 3066-3083
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3066.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3066
In recent decades, liver transplantation has made several forward strides. These have been in the area of surgical technique, immunosuppressive drug strategies, treatment and prevention of recurrent viral infections, and the increasing use of alternative preservation techniques. Consequently, recipient and graft survival are greater than 90% at 1 year and long-term survival is considered the norm[1]. A longstanding problem that liver transplantation has faced is the mismatch between the number of donors and the higher number of patients listed for transplant. This has led to long waiting lists for a graft, and up to 20% of patients do not survive until transplantation[2,3]. Furthermore, the recent expansion of transplant indications to include certain oncological scenarios may further aggravate this issue[4]. As a response to this shortage in supply, living donor liver transplantation and using more marginal organs, including those donated after circulatory death (DCD) are strategies that have been used to increase the donor pool[5]. Improving long-term graft survival is vital, as the need for retransplantation creates additional demand on a scarce resource.
Adequate biliary drainage is paramount for the success of a liver transplant, and was previously labeled the ‘Achilles heel’ of this procedure[6]. Although vascular complications have the largest impact on short-term graft outcomes, biliary complications are the main source of long-term morbidity. These conditions often require costly interventions, cause suffering, and adversely affect patients’ quality of life[7]. The incidence of these complications is increasing as a result of the growing utilization of extended criteria donor organs, mainly from DCD donors[8].
In recent years, the development of numerous machine perfusion (MP) strategies has generated interest in their use for both the assessment of grafts and optimization during the preservation period[9]. Different techniques of MP have been described and vary in their application, with in situ MP occurring during organ procurement while the graft is within the donor and ex situ MP which occurs after the donor hepatectomy is completed. Both hepatocytes and cholangiocytes are vulnerable to ischemia-reperfusion injury (IRI), and injury to the latter during organ procurement and preservation lies behind the pathogenesis for biliary dysfunction[10]. Consequently, a current trend of research in the MP field is focused on how these different MP perfusion regimens influence post-transplant biliary complications and more specifically, ischemic type biliary lesions (ITBL).
This narrative literature review describes the strength and limitations of clinical studies that have been undertaken, their results, and provides a summary of the available literature on MP and the prevention of ITBL.
Postliver transplantation biliary complications can comprise one (or both) of the following entities; strictures (anastomotic and non-anastomotic), and biliary leaks[11]. Anastomotic biliary strictures occur at the site of biliary reconstruction and the surgical technique, and/or local tissue ischemia likely play a role. These strictures are usually managed with endoscopic or radiological procedures, however surgical revision may be required depending on the timing and type of anastomosis[12]. Nonanastomotic strictures (NAS), as the name implies, occur at a site away from the anastomosis and are most common within the initial 12 mo posttransplant. NAS are one of most feared late complications due to it being associated with high rates of graft loss and mortality, and minimal treatment options except re-transplantation[8,13].
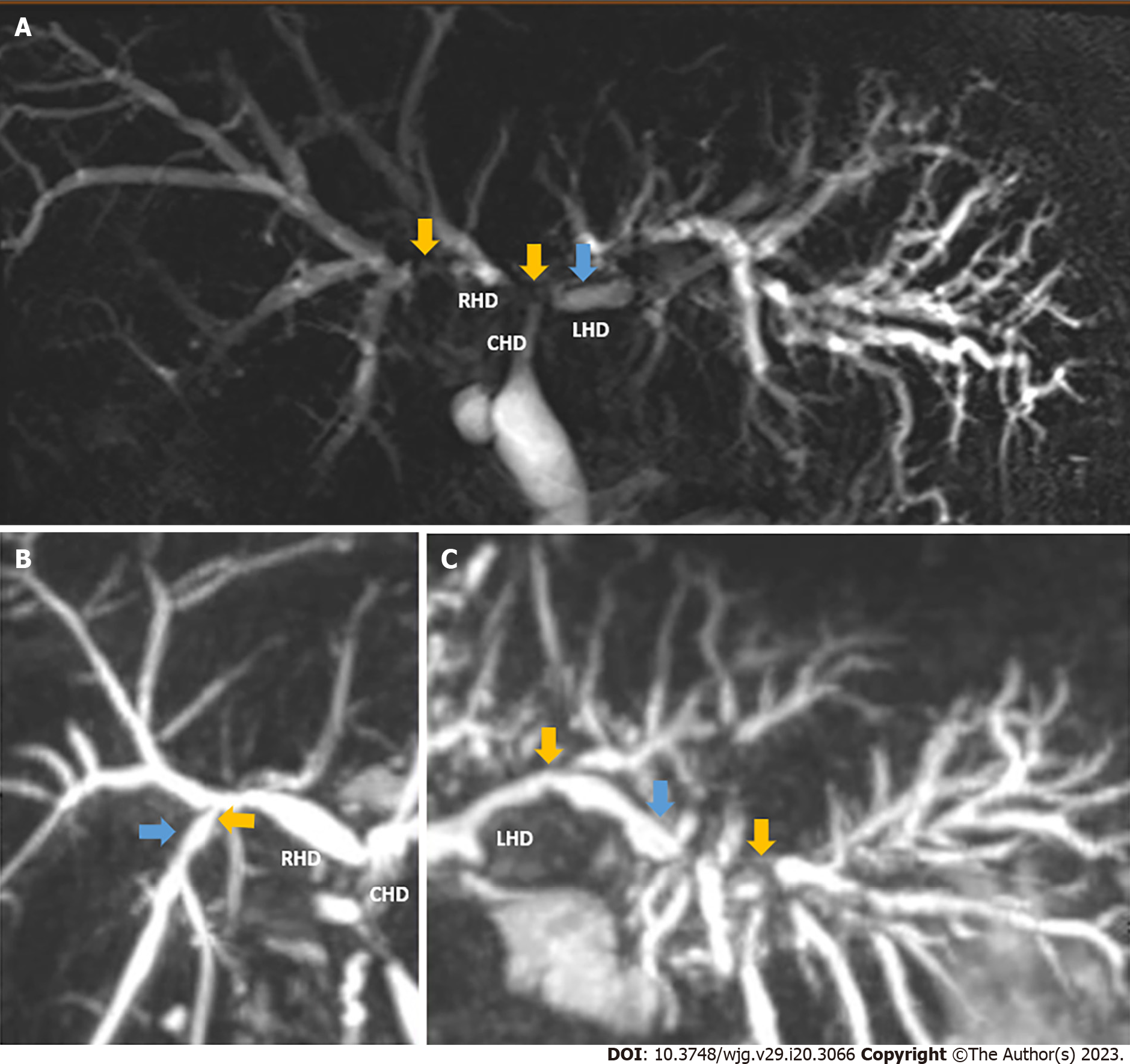
NAS is characterized by diffuse fibrotic strictures and dilatation of the biliary tree at any location from the liver periphery to the main extrahepatic ducts (Figure 1). In addition, with the disease evolution, the formation of biliary casts and intrahepatic bilomas may occur in its severest form[14]. The radiological manifestations of NAS are highly variable and range from a peripheral abscess, individual or multiple strictures around the hilum and first order biliary branches, to vanishing ducts seen along the entire biliary tree[15]. NAS can occur as a direct result of ischemia from an identifiable hepatic artery stenosis or thrombosis (HAT). However, very similar lesions can develop in the setting of an entirely patent hepatic artery and this specific situation is termed ITBL, given its similarity to an actual ischemic cholangiopathy (Figure 1).
In a seminal paper by authors from Groningen which excluded patients with HAT, ITBL was classified according to the affected area of the biliary tree[16]. In this study, they demonstrated that the anatomical location of ITBL varied between those that presented early (< 1 year) as opposed to late (> 1 year). In those presenting late, the peripheral liver (zone D) was involved more frequently and there was an association with immunological risk factors. By contrast, patients with an early presentation had lesions around the bifurcation and the common bile duct (zone A). This early group had a longer period of both cold and warm ischemia; therefore, hypoxia is thought to be one of the underlying mechanisms. More recently, a United States group from the Mayo Clinic proposed a radiologic classification of ITBL into four distinct patterns that correlate with a distinct natural evolution and prognosis[17]. In this case, those patients with diffuse necrosis and multifocal progressive patterns experienced more episodes of cholangitis and almost all required stents and eventual retransplant.
Multiple factors have been associated with ITBL development and are generally divided into three categories: ischemia-related injury; bile salt mediated injury; and immune-mediated injury[18,19]. The cold ischemia time (CIT) and donor warm ischemia time (dWIT), and inadequate microvasculature preservation predispose cholangiocytes to a subsequent IRI[10,18]. The increased incidence of ITBL in DCD grafts, which are characterized by a period of dWIT is well established, with ITBL rates of up to 39% following controlled DCD liver transplantation[13,20,21]. The toxic effect of bile salts on cholangiocytes at low temperatures during preservation is well known. Certain bile acids have been shown to promote the secretion of inflammatory mediators, and others are directly cytotoxic to cholangiocytes[22]. In addition, new bile after implantation and its altered bile salt/phospholipid ratio have detrimental effects on biliary epithelium and induce NAS[23-25]. An immune-mediated component to the cholangiocyte injury has also been proposed due to several clinical associations, but the mechanism remains under investigation. ITBL has been demonstrated in association with ABO incompatibility, recurrence of immune-mediated liver diseases, cytomegalovirus infection, presence of C-C chemokine receptor type 5, polymorphism, and acute or chronic rejection[18].
The pathogenesis of ITBL is multifactorial. Both the cold and warm ischemic periods induce the formation of reactive oxygen species (ROS). These ROS are generated as a result of Kupffer and polymorphonuclear cells activation, mitochondrial permeability transition production, oxidative changes in the structure of the biliary canaliculus and adenosine triphosphate adenosine triphosphate (ATP) depletion. These all lead to the apoptosis and/or necrosis of the cholangiocytes. This mechanism is interrelated with the cytotoxic effect of bile salts, the cholestasis status induced by ischemia-reperfusion and different immune-mediated mechanisms, which all further propagate the biliary injury. Appropriate donor selection, the use of preservation fluids and MP are just some of the strategies which are thought to prevent this condition[26].
The different mechanisms behind the diverse array of MP strategies may reduce the extent of cholangiocyte injury during the transplantation process, in comparison with static cold storage (SCS). This may have the subsequent clinical effect of preventing the development of ITBL. The number of clinical studies on MP strategies continues to increase. With only a few exceptions, the primary outcomes focus mainly on graft and patient survival. In this review, we focus on the available clinical evidence for abdominal normothermic regional perfusion (A-NRP), and ex situ hypothermic oxygenated perfusion (HOPE), and normothermic MP (NMP) in relation to the outcome of ITBL.
The incidence of ITBL posttransplant varies considerably between institution and nations. These differences likely relate to the type of organ donation permitted, ranging from donor after brain death (DBD) donors only in some parts of the world, to uncontrolled DCD donors in others. Different national laws regarding the donor age and the ‘no-touch’ period following asystole in DCD donors likely influences outcome, and these matters also complicate the results and interpretation of clinical trials on MP. In addition, it should be noted that the lack of clear and consistent definitions of ITBL in the currently available literature makes the assessment of the true impact challenging. Many studies either do not differentiate between anastomotic, ischemic NAS and ITBL; furthermore they may include both symptomatic and nonsymptomatic cases[27]. With these limitations in mind, a literature search was performed in October 2022 using PUBMED®, Embase®, and Medline®. Publications were restricted to those in English, however no further search filters were applied. The following search terms were used (in different combinations with Boolean operators): Liver, transplant, biliary stricture, ITBL, MP, machine preservation, normothermic, hypothermic, and NRP. Both prospective and retrospective studies were included if they included outcome data pertaining to biliary strictures. Studies without a control (or comparator) group were excluded. A pooled analysis was not performed due to a significant variation in study methodology, MP application technique, and outcome definitions. Institutional ethical approval was not required.
A-NRP is an in situ preservation technique using an extracorporeal oxygenated membrane, restoring the perfusion of abdominal organs after the donor is declared deceased. This technique may lead to a reduction in the dWIT depending on the timing of cannulation; however, it provides a period of resuscitation immediately after the cellular injury incurred from the dWIT. The provision of near physiological conditions during A-NRP provides a period in which cells can recover their energy stores, therefore they tolerate the CIT with minimal additional injury. It can also provide information about graft viability and reduce the effects of the IRI process[28].
In recent years, countries such as Spain, France, United Kingdom, and Italy have developed different A-NRP protocols as a strategy to improve the outcomes of DCD grafts[29-33]. These protocols differ in the liver viability criteria utilized “no-touch” periods required, pre-mortem substance administration, and vessel cannulation. All countries aforementioned have a mandatory no-touch period of 5 min except Italy, which requires 20 min. This inevitably lengthens the dWIT. Sedative analgesia administration is not allowed in the United Kingdom, in contrast to other countries. In Spain, premortem cannulation is allowed while in Italy and France only the identification of femoral vessels to ease cannulation is permitted. The obvious benefit of premortem cannulation is that it minimizes the dWIT and could achieve better outcomes as a result[34]. During A-NRP, certain viability parameters are measured, the most common among the different protocols are transaminase levels. Despite transaminase levels being widely accepted, it only reflects hepatocyte injury and not cholangiocytes. Other viability criteria employed include A-NRP duration[31], lactate clearance[30], and/or the presence of macrosteatosis[30,31]. However, some of these parameters are considered controversial[28].
To date, there has been no randomized controlled trial (RCT) comparing A-NRP to the standard retrieval method, known as the super rapid retrieval (SRR) technique. The clinical studies investigating A-NRP that include a comparator group, and provide data on biliary strictures are summarized in Table 1. The definition of ITBL used in these studies is relatively homogeneous as most authors have considered ITBL to have occurred when NAS developed in the context of a patent hepatic artery. However, two studies did not include an ITBL definition[35,36] and two authors[31,37] considered NAS regardless of the presence of concomitant HAT. Other limitations of these studies include variation in the A-NRP protocols, small sample size, and variability in follow-up periods. These trials can be put into three categories, according to the comparator groups: DCD-A-NRP vs DBD-SCS; DCD-A-NRP vs DCD-SRR; and DCD-A-NRP vs DCD with other MP.
| Ref. | Study design | Groups (n) | Control group | NRP protocol and viability criteria | Definition of ITBL | Follow up | ITBL in intervention (DCD NRP) | ITBL in control (DCD) | ITBL in control (DBD) |
| Schurink et al[42], 2022 | Cohort | NRP1 (20) vs DCD (49) vs DBD (81) | DCD/DBD | Dutch protocol2 | Symptomatic radiologically NAS without the presence of a HAT | Median-NRP 23 mo, DCD25 mo and DBD 26 mo | 1/15 (7%); 1/5 (20%)3 | 8/30 (26%) | 6/78 (7%) |
| Mohkam et al[45], 2022 | Cohort | NRP (157) vs NMP (34) | DCD | France protocol4 | NAS that were unrelated to any hepatic artery complications | Median-NRP 22 mo; NMP 24 mo | 2/68 (2.9%)5 | 3/34 (8.8%)5 | NA |
| Gaurav et al[44], 2022 | Cohort | NRP (69) vs NMP (67) vs SCS (97) | DCD | United Kingdom protocol6 | Presence of any biliary stricture, dilatation, or irregularity of the intra- or extrahepatic bile ducts and/or cast on MRCP away from the biliary anastomosis in the presence of patent arterial vasculature | Median-54 mo (SCS), 28 mo (NRP) and 24 mo (NMP) | 0/69 (0%)7 | 7/67 (11%)7 NMP and 12/97 (14%)7 SCS | NA |
| Hessheimer et al[34], 2022 | Cohort | NRP (545) vs SRR (258) | DCD | Spain protocol8 | Patient with patent hepatic artery, signs or symptoms of cholestasis, and direct or indirect cholangiographic imaging reflecting strictures of the intra- and/or extrahepatic biliary tree proximal to the transplant anastomosis | Median–31 mo | 6/545 (1%) | 24/258 (9%) | NA |
| Ruiz et al[40], 2021 | Cohort | NRP (100) vs DBD (200) | DBD | Spain protocol8 | Non-anastomotic biliary stricture in the presence of a patent hepatic artery and confirmed based on cholangiographic evidence (T-tube cholangiogram or magnetic resonance) | Mean-36 mo | 0/100 (0%) | NA | 0/200 (0%) |
| Muñoz et al[36], 2020 | Cohort | NRP (23) vs SRR (22) | DCD | Spain protocol8 | NR | Mean-33.9 mo (SRR) and 14.2 mo (NRP) | 0/23 (0%) | 3/22 (13.6%) | NA |
| Savier et al[31], 2020 | Cohort | NRP (50) vs DBD (100) | DBD | France protocol4 | Presence of any disseminated biliary stricture on magnetic resonance and endoscopic retrograde cholangiopancreatography, regardless of the presence or absence of arterial thrombosis or stenosis | Mean-34.8 mo (cDCD NRP) and 51.7 mo (DBD) | 1/50 (2%) | NA | 1/100 (1%) |
| Miñambres et al[35], 2020 | Cohort | NRP (16) vs DBD (29) | DBD | Spain protocol8 | NR | Median-6 mo (cDCD) and 16 mo (DBD) | 0/16 (0%) | NA | 0/29 (0%) |
| De carlis et al[43], 2021 | Cohort | DCD NRP + D-HOPE (37) vs DCD SRR SCS (37) | DCD | Italy protocol9 | Cholangiographic evidence of diffuse intrahepatic, hilar, or extrahepatic biliary strictures in the presence of a patent hepatic artery. Isolated anastomotic strictures were excluded from IC | Median-17 mo (NRP + D-HOPE) and all transplants were followed at least 1 yr | 1/37 (3%) | 3/37 (8%) | NA |
| Muller et al[37], 2020 | Cohort | NRP (132) vs HOPE (93) | DCD | France protocol4 | NAS was defined as either multifocal, unifocal intrahepatic, or hilar strictures with or without the presence of concomitant HAT or arterial complications. NAS was detected clinically and confirmed by magnetic resonance cholangiography | Median-20 mo (NRP) and 28 mo (HOPE) | 2/32 (6.3%)5 | 4/32 (12.5%)5 | NA |
| Hessheimer et al[41], 2019 | Cohort | NRP (95) vs SRR (117) | DCD | Spain protocol8 | Cholestasis and confirmed based on cholangiographic evidence (typically coming from magnetic resonance cholangiopancreatography) of diffuse non-anastomotic biliary strictures, with or without prestenotic dilatations, in the presence of a patent hepatic artery | Median-20 mo | 2/95 (2%) | 15/117 (13%) | NA |
| Rodríguez-Sanjuán et al[39], 2019 | Cohort | NRP (11) vs DBD (51) | DBD | Spain protocol8 | Diffuse stenosis of the intrahepatic biliary tree–suspected by jaundice, cholangitis, abnormal biochemical liver test, or abnormal findings on ultrasound or T-tube cholangiography- provided there is no hepatic artery thrombosis | Ranges between 7-27 mo. Minimum follow-up of 3 mo | 2/11 (13.3%) | NA | 13/51 (27.7%) |
| Watson et al[33], 2019 | Cohort | NRP (43) vs SRR (187) | DCD | United Kingdom protocol6 | Presence of any non-anastomotic biliary stricture on ERCP or MRCP in the absence of arterial thrombosis or stenosis | Up to 5 yr of follow-up | 0/42 (0%) | 47/171 (27%) | NA |
| De Carlis et al[38], 2018 | Cohort | NRP (20) vs DBD ECMO SCS (17) vs DBD non-ECMO SCS (52) | DBD-ECMO DBD-non-ECMO | Italy protocol9 | Strictures, irregularities, or dilatations of the intrahepatic bile duct. Isolated anastomotic biliary strictures were not included in the definition of IC. The diagnosis of IC was confirmed with at least 1 adequate imaging study of the biliary tree, and concomitant hepatic artery thrombosis was excluded by Doppler ultrasound or computed tomography | Median-14 mo (cDCD), 20 mo (DBD-ECMO) and 17 mo (DBD-non-ECMO) | 2/20 (10%) | NA | DBD-ECMO 0/17 0%; DBD-non-ECMO 2/52 (4%) |
In the first group of studies[31,35,38-40] that compared DCD-NRP-A grafts vs DBD-SCS grafts, these authors aimed to demonstrate that DCD grafts after NRP could have similar results of DBD grafts and therefore should not be considered marginal. These studies showed a similar incidence of ITBL between both groups, and the results in regard to early allograft dysfunction were promising. However, they had small sample sizes[35,38,39], differences in follow-up periods[31,35,38,39], and a high proportion of uncontrolled DCD donors[38]. Other authors have assessed the outcomes of DCD NRP grafts vs DCDs grafts recovered by via the SRR technique and subsequent SCS[33,34,36,41]. Watson et al[33] compared two groups, DCD-A-NRP (n = 43) vs DCD-SRR (n = 187), and reported both early allograft dysfunction and biliary complication rates. A significantly lower rate of early allograft dysfunction (12% vs 32%) and ischemic cholangiopathy (IC) (0% vs 27%) occurred in the DCD-A-NRP group. Similar comparisons have been performed by Spanish transplant groups. Muñoz et al[36] did not demonstrate significant differences in their cohort, probably due to the short follow-up period in the A-NRP group and the low number of patients. However, Hessheimer et al[41] subsequently found an ITBL incidence of 2% in the NRP group (n = 95) vs 13% in the SRR group (n = 117). These findings were repeated in a second study[34] with a larger sample size (545 NRP vs 258 SRR). In this second study the ITBL incidence was 1% vs 9% in favor of A-NRP, and this is at present the largest cohort of patients with A-NRP with premortem cannulation in the literature. Recently, Schurink et al[42] have reported the safety of NRP to rescue DCD grafts that were declined by the Eurotransplant region for transplantation, with no differences in primary nonfunction or IC compared to DBD and standard, non-NRP DCD grafts[42].
Finally, other authors[37,43-45] have compared A-NRP with ex situ preservation techniques such as NMP or HOPE. However, in many of these trials, accurate data on ITBL are lacking. The only study comparing HOPE vs NRP was conducted by Muller et al[37]. These authors reported the incidence of NAS regardless of hepatic artery status, rather than ITBL specifically. The rate of NAS was reported to be 6.3% in the NRP group and 12.5% in the HOPE group, but was not significantly different. DCD grafts that underwent A-NRP have been compared to DCD grafts undergoing ex situ NMP in a study by Gaurav et al[44] This study showed significantly lower rates of ITBL in the NRP group (6.3% vs 12.5%) after propensity score matching (PSM)[44]. However, in another study that used PSM analysis[45] (34 NMP vs 68 NRP), there were no significant differences in the ITBL rate of both groups (2.9% NRP vs 8.8% NMP). In this latter study, NMP was applied at source as opposed to the study by Gaurav et al[44], which applied it at the recipient hospital in the majority of cases[44].
A-NRP is a widely used in situ perfusion technique that has improved outcomes and graft utilization in DCD grafts. Protocol aspects such as the “no-touch” period or premortem cannulation may impact in these results, due to its association with WIT. Despite the differences between the protocols adopted in different countries, NRP has still achieved superior outcomes in comparison to SRR in regards to ITBL.
Hypothermic MP (HMP) was first introduced in clinical practice in 2010 by Guarrera et al[46], who demonstrate in their pilot case-controlled series that it was a feasible and safe preservation method[46]. Subsequently, in preclinical studies that investigated the active oxygenation of the hypothermic perfusate over a short period, it demonstrated restored mitochondrial integrity. This indicated a reduction of oxygen free radicals and damage-associated molecular patterns after transplantation[47-49]. Clinical studies have also reported promising results in preventing biliary complication and graft function compared to standard SCS[50,51].
HOPE is employed as an end-ischemic treatment after SRR or standard procurement in the case of DCD and DBD livers respectively, followed by a variable period of SCS. The devices available are not portable and HOPE needs to be applied at the recipient hospital. Its use limits the CIT and extends graft preservation time, avoiding the damage associated with extended periods of preservation. Two main perfusion strategies are currently employed which combine hypothermia with a highly oxygenated perfusate: (1) Single HOPE or ‘HOPE’, which consists of single perfusion through the portal vein; and (2) dual or ‘D-HOPE’ consisting of dual perfusion through the portal vein and the hepatic artery[15,52]. Currently, there are no clinical studies comparing the two strategies, although preclinical studies performed on pigs did not find differences regarding the preservation of hepatobiliary or endothelial function when comparing both strategies[53]. Advocates of D-HOPE emphasize that dominant vasculature of the bile ducts comes from arterial supply and single portal perfusion may not provide optimal preservation of the biliary tree. On the other hand, many argue the potential risk of mechanical damage to the hepatic artery intima that may occur during cannulation could cause a higher incidence of acute HAT following liver transplant[51]. Researchers from Zurich have proposed methods for liver graft assessment during HOPE using real-time quantification of flavin mononucleotide (FMN) in the perfusate[54]. FMN is a molecule part of complex I of the mitochondrial respiratory chain[37]. Its concentration is determined by fluorescence spectroscopy and levels in perfusate correlate with graft function, complications, and graft survival in DCD livers[54].
The available studies on the use of HOPE as a preservation method, with a control group and reporting data on biliary strictures are summarized in Table 2. At present, these include four RCTs and five retrospective cohort studies with an appropriate control group of SCS grafts, and a single study comparing HOPE against NRP. The influence of HOPE on ITBL prevention has been studied only to a limited extent because in the majority, the primary endpoint was not related to biliary complications.
| Ref. | Study design | Group (n) | DBD/DCD | HOPE duration (median) | Definition of ITBL | Follow up | ITBL-intervention | ITBL-control | ||
| DCD | DBD | DCD | DBD | |||||||
| Schlegel et al[61], 2023 | RCT | HOPE (85) vs SCS (85) | DBD | 95.5 min | NR | 12 mo | NA | 1/85 (1.2%) | NA | 3/85 (3.5%) |
| Ravaioli et al[56], 2022 | RCT | HOPE (66) vs SCS (69) | DBD | 145 min | Nonspecifically provided: Biliary strictures; Biliary others | 12 mo | NA | 5/55 (9%) | NA | 6/55 (11%) |
| van Rijn et al[52], 2021 | RCT | D-HOPE (78) vs SCS (78) | DCD | 132 min | Symptomatic NAS diagnosed with the use of 6-mo cholangiography in the presence of a patent HA | 6 mo | 5/78 (6%) | NA | 14/78 (18%) | NA |
| Czigany et al[57], 2021 | RCT | HOPE (23) vs SCS (23) | DBD | 145 min | Biliary complications (clinical; radiological) | 12 mo | NA | 4/23 (17%) | NA | 6/23 (26%) |
| Patrono et al[60], 2022 | Cohort | D-HOPE (121) vs SCS (723) | DBD | 138 min | Biliary complications 3-mo cholangiography if clinically indicated | Median 21.6 (D-HOPE) and 51.1 (SCS) mo | NA | 5/121 (4%) | NA | 35/723 (5%) |
| Rayar et al[58], 2021 | Cohort | HOPE (25) vs SCS (69) | DBD | 117 min | NR | 12 mo | NA | 0/25 (0%) | NA | 1/69 (1.5%)1 |
| Muller et al[37], 2020 | Cohort | NRP (132) vs HOPE (93) | DCD | 132 min | NAS was defined as strictures with or without HA thrombosis or arterial complications. | Median 20 (NRP) 28 mo (HOPE) mo | 2/32 (6.3%) | NA | 4/32 (12.5%) | NA |
| Ravaioli et al[59], 2020 | Cohort | HOPE (10) vs SCS (30) | DBD | 132 min | NR | 12 mo | NA | NP | NA | NP |
| Schlegel et al[55], 2019 | Cohort | HOPE (50) vs SCS DBD (50) vs SCS DCD (50) | Both | 120 min | Ischemic cholangiopathy defined radiologically, as intrahepatic or hilar BS and dilatations with patent HA | 5 yr | 4/50 (8%) | NA | 11/50 (22%) | 1/50 (2%) |
| van Rijn et al[51], 2017 | Cohort | D-HOPE (10) vs SCS (20) | DCD | 126 min | NAS was defined as bile duct stenosis in the biliary tree as detected by ERCP or MRCP with clinical signs of cholestasis and/or cholangitis in the presence of a patent HA | 12 mo | 1/10 (10%) | NA | 9/20 (45%)2 | NA |
The relationship between ITBL and DCD livers is well established[20]. However, only 4/10 published cohort studies and 1 RCT have studied the influence of HOPE on DCD graft outcomes[51,52,55]. Thus, most published studies included only DBD livers so there is little or no ITBL incidence, and are not powered for this endpoint[56-60]. The most relevant study on ITBL prevention by ex situ MP was the multicenter clinical trial led by the Groningen group[52]. This European trial had symptomatic NAS within 6 mo after transplantation as the primary outcome. The study included a clear definition of NAS, which included the presence of a patent hepatic artery and therefore the NAS in this study were all ITBL. The authors demonstrated that two hours of end-ischemic D-HOPE led to a lower risk of symptomatic NAS after liver transplantation of grafts from DCD donors. NAS occurred in 5/78 (6%) of the patients in the HOPE group compared to 14/78 (18%) of SCS grafts with 2 patients from the SCS group requiring a retransplant because of severe NAS. Furthermore, the D-HOPE group showed a reduction by a factor of almost four in treatment interventions required for NAS.
Trials published by Italian and German groups have reported the efficacy of HOPE for the prevention of complications and a shorter intensive care unit stay[56,57]. There was no mention of ITBL in both trials, and grafts studied were from DBD donors so one could assume that most of the biliary complications were from an anastomotic origin. While the primary endpoint in the German[57] study was the peak alanine transaminase within 1 wk after transplantation and demonstrated a 47% reduction in the serum peak of this enzyme in the HOPE group, the authors did not find any difference in biliary complication. rate (4/23, 17% vs 26% 6/23). A trial[56] demonstrated benefits of HOPE, with a reduction in EAD and better graft survival rates. This study did provide a clear definition of biliary complications, although reported a rate of biliary complication different from bile leak of 5/55 (9%) in the HOPE group compared to 6/55 (11%). Two patients in each group (4%) had biliary strictures, but it was unclear if these were ITBL. A recent multicenter trial on DBD livers from the Zurich group[61] reported the effects of HOPE on preventing postoperative complications. Although the primary endpoint was not reached, and the proportion of patients with at least one Clavien³ III complication did not differ, findings suggested that HOPE may decrease the risk of severe liver graft related events. The study did not include a clear definition of NAS and state if hepatic artery patency was required. In this study the NAS incidence was 1/85 (1.2%) in the HOPE group compared to 3/85 (3.5%) in the SCS group.
The only study comparing A-NRP and HOPE for liver grafts from DCD donors included an A-NRP cohort from six high-volume French centers and the HOPE cohort from the Zurich group[37]. In the A-NRP cohort, femoral artery cannulas were introduced post-mortem after a ‘‘no-touch’’ period of 5 min. NAS was defined as strictures with or without the presence of concomitant HAT or arterial complications and both groups showed similar rates of HAT, PNF and NAS (6/132 in the A-NRP group vs 8/93 in the HOPE group). D-HOPE has an encouraging role in preventing symptomatic ITBL in liver transplants from DCD grafts, due to mitochondrial injury prevention and ATP recovery under the hypothermic aerobic conditions.
NMP provides oxygenated blood at a physiological temperature via both the hepatic artery and portal vein, whilst the liver graft is ex situ[62]. Several different NMP devices are commercially available, and the majority are transportable even with graft connected. The only major differences between the devices are the nature of the arterial flow (pulsatile or non-pulsatile) and the caval outflow (open or closed). Since the phase 1 first-in-man study that demonstrated safety and feasibility of NMP, there has been a significant uptake in this preservation modality around the world and two large RCTs[63-65]. In contrast to ex situ HMP, the more physiological nature of NMP allows cellular function to continue and this can be assessed via surrogate parameters[66,67]. Numerous biochemical parameters within the NMP perfusate or bile have been used to predict hepatocellular and cholangiocyte function respectively, in an effort to identify the grafts destined to demonstrate severe EAD or biliary complications[68]. Therefore, an accurate discussion of the impact NMP on ITBL posttransplant needs to consist of two components: (1) The incidence of ITBL in NMP preserved grafts in comparison to other modalities; and (2) the accuracy of NMP parameters to identify livers destined to develop clinically significant ITBL.
Published outcomes for NMP are confounded by the fact that this preservation modality is more frequently applied to organs of marginal quality to facilitate transplantation[69], and it may be applied for the entirety of the preservation period from the donor hospital (“at source”) or following a period of cold storage during transport to the recipient center (“back to base”). The early clinical trials of NMP included both DBD and DCD grafts, with a focus on its feasibility, safety, and impact on the IRI as indicated by markers of early graft function[63,64]. These multicenter trials included standard risk donors that were accepted for transplant regardless of the preservation modality (NMP or SCS). Subsequently, clinical practice and research shifted to investigate the ability of NMP to resuscitate poor quality grafts that were otherwise deemed untransplantable with SCS[70-72]. Complicating matters further, supposedly untransplantable livers are a heterogenous group with a varying overall risk of ITBL[71,73]. As an example, a DBD graft with severe macrosteatosis has a different risk of ITBL than a DCD graft with 60 min of dWIT, but both will fall into the declined group due to differing clinical concerns. The increased utilization of NMP for livers otherwise deemed to high risk for transplant following SCS has pragmatic consequences for trial design. This loss in equipoise over the safety of randomizing high-risk livers to SCS results in a greater reliance on cohort studies.
The trials that have investigated NMP in the clinical setting, via either an RCT or cohort study with a representative control group, are listed in Table 3. Many of these trials unfortunately lacked clear definitions of what was considered ITBL, and whether complete artery patency was required for the diagnosis. To date, three RCTs of NMP have been completed[64,65,74] (Table 3). The first multicenter RCT published by Nasralla et al[64] in 2018 included standard risk grafts from both DBD and DCD donors and applied NMP at source. This study included a protocol magnetic resonance cholangiopancreatography at 6 mo to assess for biliary complications of which only a small proportion of subjects completed. The difference in stricture incidence was greatest in the DCD subgroup, with a rate of 26.3% in the DCD-SCS group as opposed to 11.1% in the DCD-NMP group. Whether these ischemic strictures were ITBL or the result of a vascular lesion is unclear, as HA patency was not possible to determine from the data provided[64]. The smaller RCT conducted by Ghinolfi et al[74] comprised a sample of elderly DBD donors (≥ 70 years) and applied NMP in a back-to-base approach[74]. However, the small sample size and lower overall risk of ITBL in DBD grafts makes the findings of this study less informative. In this study, only one patient developed a biliary stricture, and they were in the NMP group. A recent large RCT from the United States randomized nearly 300 livers (both DBD and DCD) to either at-source NMP or SCS[65]. These authors did provide a definition of what they considered an ischemic biliary complication (Table 3) and this occurred in 4 and 14 of the NMP and SCS livers respectively. Once again, the granularity of the data presented does not allow determination of the proportion of patients with ischemic biliary complications that were truly ITBL. It should be noted that none of these RCTs performed any formal viability testing of the grafts prior to transplant and were not powered appropriately to assess for biliary complications.
| Ref. | Study design | Intervention group (n) | Control group (n) | DBD, DCD intervention | DBD, DCD control | NMP duration1 | Viability testing | Definition of ITBL | Follow u | ITBL-intervention | ITBL-control | ||
| DCD | DBD | DCD | DBD | ||||||||||
| Markmann et al[65], 2022 | RCT | NMP at source (153) | SCS (146) | 125, 28 | 133, 13 | 4.5 h | NR | IBC defined as NAS or bile leaks, confirmed with ERCP or MRCP | 12 mo | 4/153 (2.6%) (DBD and DCD) | 14/146 (9.5%) (DBD and DCD) | ||
| Nasralla et al[64], 2018 | RCT | NMP at source (121) | SCS (101) | 87, 34 | 80, 21 | 9.1 h | No viability testing | Protocol MRCP at 6 mo. No distinction between IC and ITBL | 6 mo | 3/27 (11.1%)2 | 4/54 (7.4%)2 | 5/19 (26.3%)2 | 3/55 (5.5%)2 |
| Ghinolfi et al[74], 2019 | RCT | NMP back-to-base (10) | SCS (10) | All DBD | All DBD | 4.2 h | NR | NR | 6 mo | NA | 1/10 (10%) | NA | 0/10 |
| Gaurav et al[44], 2022 | Cohort | NMP back to base OR at-source (67) | SCS (97); NRP (69) | All DCD | All DCD | 7.6 h | Cambridge criteria | NAS defined as any BS, dilatation, or irregularity of the bile ducts and/or cast on MRCP away from the anastomosis with patent HA | 6 mo minimum | 12/67 (17.9%) [7/67, 10.4%3] | NA | NRP-4/69 (5.7%) [03] SCS-22/97 (22.6%) [12/97, 12.3%3] | NA |
| Hann et al[82], 2022 | Cohort | NMP back to base (26) | SCS (56) | All DBD | All DBD | 12 h | Birmingham criteria | Not reported | 6 mo minimum | NA | 1/26 (3.8%) | NA | 6/56 (10.7%) |
| Fodor et al[75], 2021 | Cohort | NMP back to base (59) | SCS (59) | 49, 9 | 55, 4 | 15 h | Certain parameters signs of "good organ function", others considered "warning" signs | ITBL was defined as BS, dilatation or irregularity of the intra- or extrahepatic bile ducts with or without biliary cast formation in the absence of HAS or HAT | 3 mo minimum | 0/9 | 2/49 (4%) | 1/4 (25%) | 7/55 (12.7%) |
| Mohkam et al[45], 2022 | Cohort | NMP at source (34) | NRP (68) | All DCDs | All DCD | 8.8 h | Not applied | Refers to BS requiring a specific treatment or resulting to graft loss and/or death | 23 mo | 1/34 (2.9%) | NA | 1/68 (1.5%) | NA |
| Mergental et al[71], 2020 | Cohort | NMP back-to-base (22) | SCS (44) | 12, 10 | 24, 204 | 9.8 h | Birmingham criteria | NR | 6 mo | 7/10 (70%) | 0/12 | NR | NR |
| Bral et al[83], 2019 | Cohort | NMP back-to-base (26) | NMP at source (17) | 20, 6 | 13, 4 | 7.8 h (back-to-base) 10.3 h (at-source) | Parameters included opening lactate level, lactate clearance, necessity of bicarbonate supplementation, and bile production | IC defined as diffuse BS in the absence of significant arterial stenosis | 6 mo | 0/6 | 0/20 | 0/4 | 0/13 |
| Ceresa et al[62], 2019 | Cohort | NMP back-to-base (31) | NMP at-source (104) | 23, 8 | 73, 31 | 8.4 h (mean) | No viability criteria | NR | 12 mo | 0/8 | 0/23 | NR | NR |
| Liu et al[84], 2019 | Cohort | NMP back to base OR at-source (21) | SCS (84) | 13, 8 | 52, 32 | 4 h 52 | No viability testing | NR | 12 mo minimum | 0/8 | 0/13 | NR | NR |
| Bral et al[85], 2017 | Cohort | NMP at-source (9) | SCS (30) | 6, 3 | 22, 8 | 11.5 h | No viability testing | NR | 6 mo | 0/3 | 0/6 | NR | NR |
| Ravikumar et al[63], 2016 | Cohort | NMP at-source (20) | SCS (40) | 16, 4 | 32, 8 | 9.3 h | No viability testing | NR | 30 d | 0/4 | 0/16 | NR | NR |
Both prospective and retrospective cohort studies have investigated biliary complications following NMP (Table 3). In the VITTAL trial, Mergental et al[71] reported the outcomes of a prospective cohort of 22 livers (12 DBD, 10 DCD) destined for discard that were transplanted following back-to-base NMP. After 6 mo follow-up, 3/10 of the DCD livers had developed symptomatic nonanastomotic biliary strictures. Requiring retransplantation[71]. Fodor et al[75] subsequently reported a retrospective cohort study of predominantly DBD grafts (49 DBD and 9 DCD) preserved via back-to-base NMP and reported both a lower incidence and severity of ITBL in the NMP group in comparison to SCS[75]. This study had a clear definition of ITBL and applied viability testing, which included bile output and pH, however specific parameters for these were not reported. Recently, Gaurav et al[44] published a single center retrospective cohort study of DCD grafts preserved by NMP (back-to-base and at-source), NRP and CS with the primary outcome being NAS[44]. These authors also provided a clear definition of NAS and this required hepatic artery patency. In the NMP group, 12/69 developed NAS and in 7/12 it was clinically significant. This was comparable to the SCS group (22/97, 12/22 clinically significant) but higher than the NRP group (4/69, 0/4 clinically significant). It must be noted that this study applied biliary viability testing to NMP preserved livers and 77% of these NMP preserved DCD livers proceeded to transplant.
Utilizing NMP perfusate and bile parameters to predict (and avoid) certain outcomes remains controversial. The risks associated with liberal use of NMP preserved livers is associated morbidity and mortality. The risks associated with being too stringent on the NMP viability criteria, when predictive accuracy is less than perfect, is the discard of a liver that would have resulted in acceptable outcomes. NMP indicators of biliary injury and/or function have been studied by several groups in human livers[76,77]. The early experience of associating bile pH, bicarbonate and glucose with cholangiopathies was reported by Watson et al[77] in 2018, from 16 transplanted livers. In this group of livers, a biliary pH < 7.4 occurred in 3/16, and all three of these developed a cholangiopathy. A lower biliary bicarbonate and higher biliary glucose concentration was also associated with subsequent cholangiopathy. Recently, the same group have reported outcomes in a much larger cohort of 144 transplanted livers[78]. Interestingly, 15 of these transplanted livers did not meet their previously reported cholangiocyte viability criteria and in a further three livers no bile was produced which precluded this assessment. Clinically significant NAS developed in 9/144 recipients and all of these had a bile pH > 7.5. Matton et al[76] investigated biliary NMP parameters in both the laboratory and a small clinical trial (n = 6)[76]. These authors did not have any cases of ITBL in the recipients within the trial, but demonstrated an inverse correlation between bile pH and bicarbonate concentration with histological evidence of biliary injury in a group of non-transplanted livers. A pH and bicarbonate concentration of < 7.48 and < 18 mmol/L had a positive predictive value of 75% and 91% for significant biliary injury. In summary, the prediction (and avoidance) of ITBL using NMP requires further research. This will undoubtedly be of assistance to the transplant community, however at present the accuracy remains sub-optimal and it must be improved to avoid the unnecessary discard of grafts.
In theory, the different rationale behind the various MP strategies could work synergistically to prevent ITBL. A-NRP abbreviates the dWIT induced damage and provides in-situ resuscitation prior to the cold ischemic period, whereas the ex situ techniques may dampen IRI. Conversely, the application of sequential MP strategies may follow the rule of diminishing returns and the resources may not be justified. Despite the report that individual grafts had the combination of A-NRP and NMP, clarity in the ITBL rate with this combination is lacking as they were excluded from the analysis of this study[44,79]. The Groningen group have also reported their experience with sequential ex-situ end-ischemic D-HOPE, controlled oxygenated rewarming and NMP for high-risk livers (mostly DCD grafts)[80]. The application of this protocol is proposed to protects these livers against IRI (D-HOPE phase) and enables viability assessment (NMP phase) prior to transplantation, resulting in promising outcomes.
In Italy, as aforementioned circulatory death during DCD procurement is declared after a stand-off period of 20 min. This prolonged dWIT time has been a general reluctance to use such DCD grafts for transplantation due to the probable high risk of graft failure. The combination of A-NRP and D-HOPE has contributed to increase the donor pool in this high-risk donor context. In a retrospective cohort study, this combination has reported satisfactory outcomes in terms of ITBL when compared with relatively low-risk donor control group (A-NRP + D-HOPE 3% vs SCS 8%)[43]. Recently, a new procedure called ischemia-free liver transplantation has been proposed, during which liver grafts are procured, preserved and implanted under continuous NMP. Its applicability and clinical impact are yet to be determined[81]. Further studies are required to determine if additional benefits are achieved by combining different techniques.
The volume of data on MP and its impact on the development of ITBL following liver transplant is growing. However, with only a few exceptions, ITBL was not the primary outcome under investigation in these studies and therefore were not designed and reported with this entity in mind. It is difficult in many published studies to tease out what biliary complications represent the development of ITBL, as opposed to strictures of another cause. With this considered, the evidence for prevention of ITBL with MP is distinctly different for DCD as opposed to DBD grafts.
The highest quality evidence for ITBL prevention with MP in DCD grafts is D-HOPE, in comparison to SRR and SCS. This is based on finding from a RCT[52]. However, a larger quantity of lower quality evidence supports A-NRP in comparison to both SRR with SCS, and NMP via a ‘back to base’ approach. At present, there is no evidence that D-HOPE is superior to A-NRP or that the combination of these two MP techniques results in a further reduction of ITBL than each one in isolation. Based on the DCD subgroup from one randomized trial[64], there is a suggestion that NMP applied ‘at source’ reduces ITBL in comparison to SRR and SCS[64]. There is no good quality evidence that NMP applied in a ‘back to base’ approach for DCD grafts prevents ITBL. In DBD donors, the incidence of ITBL is lower and therefore studies with a large sample size will be required to demonstrate a noticeable effect. The available evidence to suggest that either NMP ‘at source’ or ‘back to base’ for DBD grafts is weak, and consists of only one cohort study[75]. There is no trial evidence to support a reduction in ITBL for DBD grafts with HMP strategies.
ITBL remains an ongoing issue and the notion that biliary complications are the ‘achilles heel’ of liver transplantation remains true. However, with the introduction of MP technology, gains are being made in the prevention of this highly morbid condition. The greatest area of improvement is for DCD grafts with RCT evidence for D-HOPE, and large cohort studies supporting A-NRP. Given the demonstrated benefits these modalities have over SCS for DCD grafts, a loss of equipoise within the transplant community is diminishing the opportunity for further RCTs that include a SCS group. As an organ donor often generously gives numerous organs, the impact A-NRP has on other abdominal viscera may influence the decision regarding the most appropriate MP strategies. Multiple factors likely interact to cause ITBL, and a greater understanding of these will undoubtedly help refine both preventative and treatment interventions.
Angus Hann would like to acknowledge the funding received in the form of the Catherine Marie Enright research scholarship from the Royal Australasian College of Surgeons to support his program of research. Figure created with biorender.com, accessed on January 2023.
| 1. | Muller X, Marcon F, Sapisochin G, Marquez M, Dondero F, Rayar M, Doyle MMB, Callans L, Li J, Nowak G, Allard MA, Jochmans I, Jacskon K, Beltrame MC, van Reeven M, Iesari S, Cucchetti A, Sharma H, Staiger RD, Raptis DA, Petrowsky H, de Oliveira M, Hernandez-Alejandro R, Pinna AD, Lerut J, Polak WG, de Santibañes E, de Santibañes M, Cameron AM, Pirenne J, Cherqui D, Adam RA, Ericzon BG, Nashan B, Olthoff K, Shaked A, Chapman WC, Boudjema K, Soubrane O, Paugam-Burtz C, Greig PD, Grant DR, Carvalheiro A, Muiesan P, Dutkowski P, Puhan M, Clavien PA. Defining Benchmarks in Liver Transplantation: A Multicenter Outcome Analysis Determining Best Achievable Results. Ann Surg. 2018;267:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | MacConmara M, Hanish SI, Hwang CS, De Gregorio L, Desai DM, Feizpour CA, Tanriover B, Markmann JF, Zeh H 3rd, Vagefi PA. Making Every Liver Count: Increased Transplant Yield of Donor Livers Through Normothermic Machine Perfusion. Ann Surg. 2020;272:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Briceño J, Ciria R, de la Mata M. Donor-recipient matching: myths and realities. J Hepatol. 2013;58:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Panayotova G, Lunsford KE, Latt NL, Paterno F, Guarrera JV, Pyrsopoulos N. Expanding indications for liver transplantation in the era of liver transplant oncology. World J Gastrointest Surg. 2021;13:392-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Kollmann D, Sapisochin G, Goldaracena N, Hansen BE, Rajakumar R, Selzner N, Bhat M, McCluskey S, Cattral MS, Greig PD, Lilly L, McGilvray ID, Ghanekar A, Grant DR, Selzner M. Expanding the donor pool: Donation after circulatory death and living liver donation do not compromise the results of liver transplantation. Liver Transpl. 2018;24:779-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Calne RY. A new technique for biliary drainage in orthotopic liver transplantation utilizing the gall bladder as a pedicle graft conduit between the donor and recipient common bile ducts. Ann Surg. 1976;184:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Boteon YL, Boteon AP, Attard J, Wallace L, Bhogal RH, Afford SC. Impact of machine perfusion of the liver on post-transplant biliary complications: A systematic review. World J Transplant. 2018;8:220-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (2)] |
| 8. | Hessheimer AJ, Cárdenas A, García-Valdecasas JC, Fondevila C. Can we prevent ischemic-type biliary lesions in donation after circulatory determination of death liver transplantation? Liver Transpl. 2016;22:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Durán M, Hann A, Lembach H, Nutu A, Clarke G, Patel I, Sneiders D, Hartog H, Mirza DF, Perera MTPR. Normothermic Machine Perfusion as a Tool for Safe Transplantation of High-Risk Recipients. Transplantology. 2022;3:169-183. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Kochhar G, Parungao JM, Hanouneh IA, Parsi MA. Biliary complications following liver transplantation. World J Gastroenterol. 2013;19:2841-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 186] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 12. | Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH, Haagsma EB. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 14. | Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D'Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 321] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 15. | Schlegel A, Porte R, Dutkowski P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J Hepatol. 2022;76:1330-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Croome KP, Mathur AK, Aqel B, Yang L, Taner T, Heimbach JK, Rosen CB, Paz-Fumagalli R, Taner CB. Classification of Distinct Patterns of Ischemic Cholangiopathy Following DCD Liver Transplantation: Distinct Clinical Courses and Long-term Outcomes From a Multicenter Cohort. Transplantation. 2022;106:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | Op den Dries S, Sutton ME, Lisman T, Porte RJ. Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation. 2011;92:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | de Vries Y, von Meijenfeldt FA, Porte RJ. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1507-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | O'Neill S, Roebuck A, Khoo E, Wigmore SJ, Harrison EM. A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation. Transpl Int. 2014;27:1159-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Laing RW, Scalera I, Isaac J, Mergental H, Mirza DF, Hodson J, Wilkin RJ, Perera MT, Muiesan P. Liver Transplantation Using Grafts From Donors After Circulatory Death: A Propensity Score-Matched Study From a Single Center. Am J Transplant. 2016;16:1795-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Lamireau T, Zoltowska M, Levy E, Yousef I, Rosenbaum J, Tuchweber B, Desmoulière A. Effects of bile acids on biliary epithelial cells: proliferation, cytotoxicity, and cytokine secretion. Life Sci. 2003;72:1401-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Knoop M, Schnoy N, Keck H, Neuhaus P. Morphological changes of human common bile ducts after extended cold preservation. Transplantation. 1993;56:1572-1573. [PubMed] |
| 24. | Buis CI, Geuken E, Visser DS, Kuipers F, Haagsma EB, Verkade HJ, Porte RJ. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol. 2009;50:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Geuken E, Visser D, Kuipers F, Blokzijl H, Leuvenink HG, de Jong KP, Peeters PM, Jansen PL, Slooff MJ, Gouw AS, Porte RJ. Rapid increase of bile salt secretion is associated with bile duct injury after human liver transplantation. J Hepatol. 2004;41:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 26. | Cursio R, Gugenheim J. Ischemia-Reperfusion Injury and Ischemic-Type Biliary Lesions following Liver Transplantation. J Transplant. 2012;2012:164329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Ramírez-Del Val A, Guarrera J, Porte RJ, Selzner M, Spiro M, Raptis DA, Friend PJ, Nasralla D; ERAS4OLT. org Working Group. Does machine perfusion improve immediate and short-term outcomes by enhancing graft function and recipient recovery after liver transplantation? A systematic review of the literature, meta-analysis and expert panel recommendations. Clin Transplant. 2022;36:e14638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Melandro F, Basta G, Torri F, Biancofiore G, Del Turco S, Orlando F, Guarracino F, Maremmani P, Lazzeri C, Peris A, De Simone P, Ghinolfi D. Normothermic regional perfusion in liver transplantation from donation after cardiocirculatory death: Technical, biochemical, and regulatory aspects and review of literature. Artif Organs. 2022;46:1727-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 29. | Abdel-Misih SR, Wei L, Benson AB 3rd, Cohen S, Lai L, Skibber J, Wilkinson N, Weiser M, Schrag D, Bekaii-Saab T. Neoadjuvant Therapy for Rectal Cancer Affects Lymph Node Yield and Status Without Clear Implications on Outcome: The Case for Eliminating a Metric and Using Preoperative Staging to Guide Therapy. J Natl Compr Canc Netw. 2016;14:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | De Carlis L, De Carlis R, Lauterio A, Di Sandro S, Ferla F, Zanierato M. Sequential Use of Normothermic Regional Perfusion and Hypothermic Machine Perfusion in Donation After Cardiac Death Liver Transplantation With Extended Warm Ischemia Time. Transplantation. 2016;100:e101-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 31. | Savier E, Lim C, Rayar M, Orlando F, Boudjema K, Mohkam K, Lesurtel M, Mabrut JY, Pittau G, Begdadi N, Cherqui D, Adam R, Dondero F, Sepulveda A, Soubrane O, Bucur P, Barbier L, Salame E, Jasseron C, Antoine C, Riou B, Scatton O. Favorable Outcomes of Liver Transplantation from Controlled Circulatory Death Donors Using Normothermic Regional Perfusion Compared to Brain Death Donors. Transplantation. 2020;104:1943-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 32. | Trasplantes ONT. Protocolo nacional de donación y trasplante hepático en donación en asistolia controlada. 2015. Available from: https://www.enfermeria21.com/revistas/metas/articulo/81185/. |
| 33. | Watson CJE, Hunt F, Messer S, Currie I, Large S, Sutherland A, Crick K, Wigmore SJ, Fear C, Cornateanu S, Randle LV, Terrace JD, Upponi S, Taylor R, Allen E, Butler AJ, Oniscu GC. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant. 2019;19:1745-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 34. | Hessheimer AJ, de la Rosa G, Gastaca M, Ruíz P, Otero A, Gómez M, Alconchel F, Ramírez P, Bosca A, López-Andújar R, Atutxa L, Royo-Villanova M, Sánchez B, Santoyo J, Marín LM, Gómez-Bravo MÁ, Mosteiro F, Villegas Herrera MT, Villar Del Moral J, González-Abos C, Vidal B, López-Domínguez J, Lladó L, Roldán J, Justo I, Jiménez C, López-Monclús J, Sánchez-Turrión V, Rodríguez-Laíz G, Velasco Sánchez E, López-Baena JÁ, Caralt M, Charco R, Tomé S, Varo E, Martí-Cruchaga P, Rotellar F, Varona MA, Barrera M, Rodríguez-Sanjuan JC, Briceño J, López D, Blanco G, Nuño J, Pacheco D, Coll E, Domínguez-Gil B, Fondevila C. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: Outcomes and risk factors for graft loss. Am J Transplant. 2022;22:1169-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 35. | Miñambres E, Ruiz P, Ballesteros MA, Álvarez C, Cifrián JM, Atutxa L, Ventoso A, Castillo F, Gastaca M. Combined lung and liver procurement in controlled donation after circulatory death using normothermic abdominal perfusion. Initial experience in two Spanish centers. Am J Transplant. 2020;20:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Muñoz DC, Pérez BS, Martínez MP, León Díaz FJ, Fernández Aguilar JL, Pérez Daga JA, Santoyo J. Does Normothermic Regional Perfusion Improve the Results of Donation After Circulatory Death Liver Transplantation? Transplant Proc. 2020;52:1477-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Muller X, Mohkam K, Mueller M, Schlegel A, Dondero F, Sepulveda A, Savier E, Scatton O, Bucur P, Salame E, Jeddou H, Sulpice L, Pittau G, Allard MA, Mabrut JY, Dutkowski P, Clavien PA, Lesurtel M. Hypothermic Oxygenated Perfusion Versus Normothermic Regional Perfusion in Liver Transplantation From Controlled Donation After Circulatory Death: First International Comparative Study. Ann Surg. 2020;272:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 38. | De Carlis R, Di Sandro S, Lauterio A, Botta F, Ferla F, Andorno E, Bagnardi V, De Carlis L. Liver Grafts From Donors After Circulatory Death on Regional Perfusion With Extended Warm Ischemia Compared With Donors After Brain Death. Liver Transpl. 2018;24:1523-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Rodríguez-Sanjuán JC, Ruiz N, Miñambres E, Toledo E, González-Noriega M, Fernández-Santiago R, Castillo F. Liver Transplant From Controlled Cardiac Death Donors Using Normothermic Regional Perfusion: Comparison With Liver Transplants From Brain Dead Donors. Transplant Proc. 2019;51:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Ruiz P, Valdivieso A, Palomares I, Prieto M, Ventoso A, Salvador P, Senosiain M, Fernandez JR, Testillano M, Bustamante FJ, Gastaca M. Similar Results in Liver Transplantation From Controlled Donation After Circulatory Death Donors With Normothermic Regional Perfusion and Donation After Brain Death Donors: A Case-Matched Single-Center Study. Liver Transpl. 2021;27:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Hessheimer AJ, Coll E, Torres F, Ruíz P, Gastaca M, Rivas JI, Gómez M, Sánchez B, Santoyo J, Ramírez P, Parrilla P, Marín LM, Gómez-Bravo MÁ, García-Valdecasas JC, López-Monclús J, Boscá A, López-Andújar R, Fundora-Suárez J, Villar J, García-Sesma Á, Jiménez C, Rodríguez-Laíz G, Lladó L, Rodríguez JC, Barrera M, Charco R, López-Baena JÁ, Briceño J, Pardo F, Blanco G, Pacheco D, Domínguez-Gil B, Sánchez Turrión V, Fondevila C. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol. 2019;70:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 42. | Schurink IJ, de Goeij FHC, Habets LJM, van de Leemkolk FEM, van Dun CAA, Oniscu GC, Alwayn IPJ, Polak WG, Huurman VAL, de Jonge J. Salvage of Declined Extended-criteria DCD Livers Using In Situ Normothermic Regional Perfusion. Ann Surg. 2022;276:e223-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 43. | De Carlis R, Schlegel A, Frassoni S, Olivieri T, Ravaioli M, Camagni S, Patrono D, Bassi D, Pagano D, Di Sandro S, Lauterio A, Bagnardi V, Gruttadauria S, Cillo U, Romagnoli R, Colledan M, Cescon M, Di Benedetto F, Muiesan P, De Carlis L. How to Preserve Liver Grafts From Circulatory Death With Long Warm Ischemia? A Retrospective Italian Cohort Study With Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation. 2021;105:2385-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 44. | Gaurav R, Butler AJ, Kosmoliaptsis V, Mumford L, Fear C, Swift L, Fedotovs A, Upponi S, Khwaja S, Richards J, Allison M, Watson CJE. Liver Transplantation Outcomes From Controlled Circulatory Death Donors: SCS vs in situ NRP vs ex situ NMP. Ann Surg. 2022;275:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 45. | Mohkam K, Nasralla D, Mergental H, Muller X, Butler A, Jassem W, Imber C, Monbaliu D, Perera MTPR, Laing RW, García-Valdecasas JC, Paul A, Dondero F, Cauchy F, Savier E, Scatton O, Robin F, Sulpice L, Bucur P, Salamé E, Pittau G, Allard MA, Pradat P, Rossignol G, Mabrut JY, Ploeg RJ, Friend PJ, Mirza DF, Lesurtel M; Consortium for Organ Preservation in Europe (COPE). In situ normothermic regional perfusion versus ex situ normothermic machine perfusion in liver transplantation from donation after circulatory death. Liver Transpl. 2022;28:1716-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Guarrera JV, Henry SD, Samstein B, Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF, Lee HT, Brown RS Jr, Emond JC. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 426] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 47. | Schlegel A, Kron P, Graf R, Clavien PA, Dutkowski P. Hypothermic Oxygenated Perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg. 2014;260:931-7; discussion 937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Dutkowski P, Furrer K, Tian Y, Graf R, Clavien PA. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Ann Surg. 2006;244:968-76; discussion 976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | de Rougemont O, Breitenstein S, Leskosek B, Weber A, Graf R, Clavien PA, Dutkowski P. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009;250:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, DeOliveira ML, Kron P, Clavien PA. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann Surg. 2015;262:764-70; discussion 770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 51. | van Rijn R, Karimian N, Matton APM, Burlage LC, Westerkamp AC, van den Berg AP, de Kleine RHJ, de Boer MT, Lisman T, Porte RJ. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104:907-917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 52. | van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, Erdmann JI, Gilbo N, de Haas RJ, Heaton N, van Hoek B, Huurman VAL, Jochmans I, van Leeuwen OB, de Meijer VE, Monbaliu D, Polak WG, Slangen JJG, Troisi RI, Vanlander A, de Jonge J, Porte RJ; DHOPE-DCD Trial Investigators. Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med. 2021;384:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 53. | de Vries Y, Brüggenwirth IMA, Karangwa SA, von Meijenfeldt FA, van Leeuwen OB, Burlage LC, de Jong IEM, Gouw ASH, de Meijer VE, Lisman T, Porte RJ. Dual Versus Single Oxygenated Hypothermic Machine Perfusion of Porcine Livers: Impact on Hepatobiliary and Endothelial Cell Injury. Transplant Direct. 2021;7:e741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Muller X, Schlegel A, Kron P, Eshmuminov D, Würdinger M, Meierhofer D, Clavien PA, Dutkowski P. Novel Real-time Prediction of Liver Graft Function During Hypothermic Oxygenated Machine Perfusion Before Liver Transplantation. Ann Surg. 2019;270:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 55. | Schlegel A, Muller X, Kalisvaart M, Muellhaupt B, Perera MTPR, Isaac JR, Clavien PA, Muiesan P, Dutkowski P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. 2019;70:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 248] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 56. | Ravaioli M, Germinario G, Dajti G, Sessa M, Vasuri F, Siniscalchi A, Morelli MC, Serenari M, Del Gaudio M, Zanfi C, Odaldi F, Bertuzzo VR, Maroni L, Laurenzi A, Cescon M. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am J Transplant. 2022;22:2401-2408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 57. | Czigany Z, Pratschke J, Froněk J, Guba M, Schöning W, Raptis DA, Andrassy J, Kramer M, Strnad P, Tolba RH, Liu W, Keller T, Miller H, Pavicevic S, Uluk D, Kocik M, Lurje I, Trautwein C, Mehrabi A, Popescu I, Vondran FWR, Ju C, Tacke F, Neumann UP, Lurje G. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann Surg. 2021;274:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 58. | Rayar M, Beaurepaire JM, Bajeux E, Hamonic S, Renard T, Locher C, Desfourneaux V, Merdrignac A, Bergeat D, Lakehal M, Sulpice L, Houssel-Debry P, Jezequel C, Camus C, Bardou-Jacquet E, Meunier B. Hypothermic Oxygenated Perfusion Improves Extended Criteria Donor Liver Graft Function and Reduces Duration of Hospitalization Without Extra Cost: The PERPHO Study. Liver Transpl. 2021;27:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Ravaioli M, De Pace V, Angeletti A, Comai G, Vasuri F, Baldassarre M, Maroni L, Odaldi F, Fallani G, Caraceni P, Germinario G, Donadei C, Malvi D, Del Gaudio M, Bertuzzo VR, Siniscalchi A, Ranieri VM, D'Errico A, Pasquinelli G, Morelli MC, Pinna AD, Cescon M, La Manna G. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci Rep. 2020;10:6063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 60. | Patrono D, Cussa D, Sciannameo V, Montanari E, Panconesi R, Berchialla P, Lepore M, Gambella A, Rizza G, Catalano G, Mirabella S, Tandoi F, Lupo F, Balagna R, Salizzoni M, Romagnoli R. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am J Transplant. 2022;22:1382-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 61. | Schlegel A, Mueller M, Muller X, Eden J, Panconesi R, von Felten S, Steigmiller K, Sousa Da Silva RX, de Rougemont O, Mabrut JY, Lesurtel M, Cerisuelo MC, Heaton ND, Allard MA, Adam R, Monbaliu D, Jochmans I, Haring MPD, Porte RJ, Parente A, Muiesan P, Kron P, Attia M, Kollmann D, Berlakovich G, Rogiers X, Petterson K, Kranich AL, Amberg S, Müllhaupt B, Clavien PA, Dutkowski P. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J Hepatol. 2023;78:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 62. | Ceresa CDL, Nasralla D, Coussios CC, Friend PJ. The case for normothermic machine perfusion in liver transplantation. Liver Transpl. 2018;24:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, Quaglia A, Holroyd D, Vogel T, Coussios CC, Friend PJ. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant. 2016;16:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (1)] |
| 64. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 908] [Article Influence: 113.5] [Reference Citation Analysis (2)] |
| 65. | Markmann JF, Abouljoud MS, Ghobrial RM, Bhati CS, Pelletier SJ, Lu AD, Ottmann S, Klair T, Eymard C, Roll GR, Magliocca J, Pruett TL, Reyes J, Black SM, Marsh CL, Schnickel G, Kinkhabwala M, Florman SS, Merani S, Demetris AJ, Kimura S, Rizzari M, Saharia A, Levy M, Agarwal A, Cigarroa FG, Eason JD, Syed S, Washburn WK, Parekh J, Moon J, Maskin A, Yeh H, Vagefi PA, MacConmara MP. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 66. | Hann A, Lembach H, Nutu A, Mergental H, Isaac JL, Isaac JR, Oo YH, Armstrong MJ, Rajoriya N, Afford S, Bartlett D, Mirza DF, Hartog H, Perera MTPR. Assessment of Deceased Brain Dead Donor Liver Grafts via Normothermic Machine Perfusion: Lactate Clearance Time Threshold Can Be Safely Extended to 6 Hours. Liver Transpl. 2022;28:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Mergental H, Stephenson BTF, Laing RW, Kirkham AJ, Neil DAH, Wallace LL, Boteon YL, Widmer J, Bhogal RH, Perera MTPR, Smith A, Reynolds GM, Yap C, Hübscher SG, Mirza DF, Afford SC. Development of Clinical Criteria for Functional Assessment to Predict Primary Nonfunction of High-Risk Livers Using Normothermic Machine Perfusion. Liver Transpl. 2018;24:1453-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 68. | Hann A, Nutu A, Clarke G, Patel I, Sneiders D, Oo YH, Hartog H, Perera MTPR. Normothermic Machine Perfusion-Improving the Supply of Transplantable Livers for High-Risk Recipients. Transpl Int. 2022;35:10460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 69. | Hann A, Raza SS, Sneiders D, Nutu A, Mergental H, Mirza DF, Hartog H, Perera MTP. Comment on: Static cold storage compared with normothermic machine perfusion of the liver and effect on ischaemic-type biliary lesions after transplantation: a propensity-score matched study. Br J Surg. 2021;109:e12-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Mergental H, Perera MT, Laing RW, Muiesan P, Isaac JR, Smith A, Stephenson BT, Cilliers H, Neil DA, Hübscher SG, Afford SC, Mirza DF. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am J Transplant. 2016;16:3235-3245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 71. | Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, Barton D, Curbishley S, Wilkhu M, Neil DAH, Hübscher SG, Muiesan P, Isaac JR, Roberts KJ, Abradelo M, Schlegel A, Ferguson J, Cilliers H, Bion J, Adams DH, Morris C, Friend PJ, Yap C, Afford SC, Mirza DF. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 366] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 72. | Reiling J, Butler N, Simpson A, Hodgkinson P, Campbell C, Lockwood D, Bridle K, Santrampurwala N, Britton L, Crawford D, Dejong CHC, Fawcett J. Assessment and Transplantation of Orphan Donor Livers: A Back-to-Base Approach to Normothermic Machine Perfusion. Liver Transpl. 2020;26:1618-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Marcon F, Schlegel A, Bartlett DC, Kalisvaart M, Bishop D, Mergental H, Roberts KJ, Mirza DF, Isaac J, Muiesan P, Perera MT. Utilization of Declined Liver Grafts Yields Comparable Transplant Outcomes and Previous Decline Should Not Be a Deterrent to Graft Use. Transplantation. 2018;102:e211-e218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Ghinolfi D, Rreka E, De Tata V, Franzini M, Pezzati D, Fierabracci V, Masini M, Cacciatoinsilla A, Bindi ML, Marselli L, Mazzotti V, Morganti R, Marchetti P, Biancofiore G, Campani D, Paolicchi A, De Simone P. Pilot, Open, Randomized, Prospective Trial for Normothermic Machine Perfusion Evaluation in Liver Transplantation From Older Donors. Liver Transpl. 2019;25:436-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 75. | Fodor M, Cardini B, Peter W, Weissenbacher A, Oberhuber R, Hautz T, Otarashvili G, Margreiter C, Maglione M, Resch T, Krendl F, Meszaros AT, Bogensperger C, Gasteiger S, Messner F, Henninger B, Zoller H, Tilg H, Öfner D, Schneeberger S. Static cold storage compared with normothermic machine perfusion of the liver and effect on ischaemic-type biliary lesions after transplantation: a propensity score-matched study. Br J Surg. 2021;108:1082-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 76. | Matton APM, de Vries Y, Burlage LC, van Rijn R, Fujiyoshi M, de Meijer VE, de Boer MT, de Kleine RHJ, Verkade HJ, Gouw ASH, Lisman T, Porte RJ. Biliary Bicarbonate, pH, and Glucose Are Suitable Biomarkers of Biliary Viability During Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation. 2019;103:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 77. | Watson CJE, Kosmoliaptsis V, Pley C, Randle L, Fear C, Crick K, Gimson AE, Allison M, Upponi S, Brais R, Jochmans I, Butler AJ. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18:2005-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 78. | Watson CJE, Gaurav R, Fear C, Swift L, Selves L, Ceresa CDL, Upponi SS, Brais R, Allison M, Macdonald-Wallis C, Taylor R, Butler AJ. Predicting Early Allograft Function After Normothermic Machine Perfusion. Transplantation. 2022;106:2391-2398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 79. | Ghinolfi D, Dondossola D, Rreka E, Lonati C, Pezzati D, Cacciatoinsilla A, Kersik A, Lazzeri C, Zanella A, Peris A, Maggioni M, Biancofiore G, Reggiani P, Morganti R, De Simone P, Rossi G. Sequential Use of Normothermic Regional and Ex Situ Machine Perfusion in Donation After Circulatory Death Liver Transplant. Liver Transpl. 2021;27:385-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 80. | van Leeuwen OB, Bodewes SB, Lantinga VA, Haring MPD, Thorne AM, Brüggenwirth IMA, van den Berg AP, de Boer MT, de Jong IEM, de Kleine RHJ, Lascaris B, Nijsten MWN, Reyntjens KMEM, de Meijer VE, Porte RJ. Sequential hypothermic and normothermic machine perfusion enables safe transplantation of high-risk donor livers. Am J Transplant. 2022;22:1658-1670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 81. | Guo Z, Zhao Q, Huang S, Huang C, Wang D, Yang L, Zhang J, Chen M, Wu L, Zhang Z, Zhu Z, Wang L, Zhu C, Zhang Y, Tang Y, Sun C, Xiong W, Shen Y, Chen X, Xu J, Wang T, Ma Y, Hu A, Chen Y, Zhu X, Rong J, Cai C, Gong F, Guan X, Huang W, Ko DS, Li X, Tullius SG, Huang J, Ju W, He X. Ischaemia-free liver transplantation in humans: a first-in-human trial. Lancet Reg Health West Pac. 2021;16:100260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 82. | Hann A, Lembach H, Nutu A, Dassanayake B, Tillakaratne S, McKay SC, Boteon APCS, Boteon YL, Mergental H, Murphy N, Bangash MN, Neil DAH, Issac JL, Javed N, Faulkner T, Bennet D, Moore R, Vasanth S, Subash G, Cuell J, Rao R, Cilliers H, Russel S, Haydon G, Mutimer D, Roberts KJ, Mirza DF, Ferguson J, Bartlett D, Isaac JR, Rajoriya N, Armstrong MJ, Hartog H, Perera MTPR. Outcomes of normothermic machine perfusion of liver grafts in repeat liver transplantation (NAPLES initiative). Br J Surg. 2022;109:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Bral M, Dajani K, Leon Izquierdo D, Bigam D, Kneteman N, Ceresa CDL, Friend PJ, Shapiro AMJ. A Back-to-Base Experience of Human Normothermic Ex Situ Liver Perfusion: Does the Chill Kill? Liver Transpl. 2019;25:848-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 84. | Liu Q, Hassan A, Pezzati D, Soliman B, Lomaglio L, Grady P, Del Angel Diaz L, Simioni A, Maikhor S, Etterling J, D'Amico G, Iuppa G, Diago Uso T, Hashimoto K, Aucejo F, Fujiki M, Eghtesad B, Sasaki K, Kwon CHD, Cywinski J, Irefin S, Bennett A, Baldwin W, Miller C, Quintini C. Ex Situ Liver Machine Perfusion: The Impact of Fresh Frozen Plasma. Liver Transpl. 2020;26:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 85. | Bral M, Gala-Lopez B, Bigam D, Kneteman N, Malcolm A, Livingstone S, Andres A, Emamaullee J, Russell L, Coussios C, West LJ, Friend PJ, Shapiro AM. Preliminary Single-Center Canadian Experience of Human Normothermic Ex Vivo Liver Perfusion: Results of a Clinical Trial. Am J Transplant. 2017;17:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabbous H, Egypt; Xu X, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Yu HG