Published online May 21, 2023. doi: 10.3748/wjg.v29.i19.3013
Peer-review started: January 12, 2023
First decision: February 4, 2023
Revised: February 13, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: May 21, 2023
Processing time: 123 Days and 13.3 Hours
Prolonged symptoms after corona virus disease 2019 (Long-COVID) in dialysis-dependent patients and kidney transplant (KT) recipients are important as a possible risk factor for organ dysfunctions, especially gastrointestinal (GI) problems, during immunosuppressive therapy.
To identify the characteristics of GI manifestations of Long-COVID in patients with dialysis-dependent or KT status.
This observational, prospective study included patients with COVID-19 infection, confirmed by reverse transcription polymerase chain reaction, with the onset of symptoms between 1 January 2022 and 31 July 2022 which was explored at 3 mo after the onset, either through the out-patient follow-up or by telephone interviews.
The 645 eligible participants consisted of 588 cases with hemodialysis (HD), 38 patients with peritoneal dialysis (PD), and 19 KT recipients who were hospitalized with COVID-19 infection during the observation. Of these, 577 (89.5%) cases agreed to the interviews, while 64 (10.9%) patients with HD and 4 (10.5%) cases of PD were excluded. The mean age was 52 ± 11 years with 52% women. The median dialysis duration was 7 ± 3 and 5 ± 1 years for HD and PD groups, respectively, and the median time post-transplantation was 6 ± 2 years. Long-COVID was identified in 293/524 (56%) and 21/34 (62%) in HD and PD, respectively, and 7/19 (37%) KT recipients. Fatigue was the most prevalent (96%) of the non-GI tract symptoms, whereas anorexia (90.9%), loss of taste (64.4%), and abdominal pain (62.5%) were the first three common GI manifestations of Long-COVID. Notably, there were 6 cases of mesenteric panniculitis from 19 patients with GI symptoms in the KT group.
Different from patients with non-chronic kidney disease, there was a high prevalence of GI manifestations of Long-COVID in dialysis-dependent patients and KT recipients. An appropriate long-term follow-up in these vulnerable populations after COVID-19 infection is possibly necessary.
Core Tip: Prolonged symptoms after coronavirus disease 2019 (COVID-19) or prolonged symptoms after COVID-19 (Long-COVID) in dialysis-dependent patients and kidney transplant (KT) recipients are important as a possible risk factor for organ dysfunctions, especially gastrointestinal (GI) problems. In this study, we observed that a GI manifestation of Long-COVID is a frequent condition in patients with dialysis-dependence and kidney-transplant recipients. Long-COVID was significantly more prevalent in peritoneal dialysis patients than in hemodialysis patient or KT cases. We also found that patients who experienced either abdominal pain or diarrhea had a longer duration of other GI manifestations of Long-COVID, suggesting a need for closer observation of these patients during COVID-19 infection.
- Citation: Chancharoenthana W, Kamolratanakul S, Leelahavanichkul A, Ariyanon W, Chinpraditsuk S, Saelim R, Vadcharavivad S, Phumratanaprapin W, Wilairatana P. Gastrointestinal manifestations of long-term effects after COVID-19 infection in patients with dialysis or kidney transplantation: An observational cohort study. World J Gastroenterol 2023; 29(19): 3013-3026
- URL: https://www.wjgnet.com/1007-9327/full/v29/i19/3013.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i19.3013
The coronavirus disease (COVID-19) pandemic has a significant impact on the management of dialysis-dependent patients and kidney transplant (KT) recipients, while the chronic kidney disease (CKD) condition in these patients is also affecting the clinical manifestation of COVID-19 infection. The persistence of post-COVID-19 syndrome for weeks to months after the infection is a growing public health concern worldwide[1]. Currently, the definition of post-acute COVID-19 syndrome (PACS), also known as the post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (PASC) or prolonged symptoms after COVID-19 (Long-COVID) syndrome, depends on the population being studied, the post-infection timing, and the assessment tools[2,3]. Moreover, the overlap in its pathophysiology between overwhelming pro-inflammatory immune responses and direct viral cytopathic effects remains inconclusive[4]. In general, PACS mainly includes fatigue, pain, headache, neurological and cognitive impairments, cardio-pulmonary symptoms, and anosmia-dysgeusia[5]. The British National Institute for Health and Care Excellence (NICE) defines Long-COVID as any signs and symptoms that develop during or after an infection consistent with COVID-19, continue for over 12 wk, and cannot be explained by an alternative diagnosis[6]. A clinical case definition of Long-COVID by a Delphi consensus has crystallized the case definition of Long-COVID as clinical symptoms that occur in individuals with a history of probable or confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, usually 3 mo from the onset of COVID-19 with symptoms, that last for at least 2 mo without an alternative explainable condition[7].
Both dialysis-dependent patients and KT recipients are classified as vulnerable populations due to their immunosuppressive status derived from their CKD condition and the high number of comorbidities[8]. Thus, a more frequent prevalence of long-term after-effects of COVID-19 infection than in the general population is possible. Accordingly, aggressive approaches, along with prompt management of acute illness, should be used in these populations, making the subject of Long-COVID even more challenging. Recent reports have revealed an incidence of post-COVID-19 syndrome in dialysis patients and KT recipients of approximately 40%-70% of those who experience a COVID-19 infection[9-13]. The Long-COVID symptoms include respiratory-related symptoms, fatigue, peripheral neuropathy, venous thromboembolism, memory impairment, and de novo diabetes mellitus[9-11]. Notably, 60% of dialysis patients vs 10% of KT recipients had residual symptoms at 6 mo post-COVID-19 infection[11,14].
Even without the gastrointestinal (GI) symptoms, the severity of COVID-19 is associated with the GI tract as the translocation of pathogen molecules from the gut into the blood circulation (leaky gut) is reported[15], possibly from a quiescent the SARS-CoV-2 infection in the intestine[16]. Indeed, the cell entry of SARS-CoV-2 virus through angiotensin-converting enzyme 2 (ACE2) receptors on the squamous and columnar epithelial cells, including enterocytes, is well-known[17]. One large study of hospitalized COVID-19 revealed that 30% of the patients reported GI symptoms, such as abdominal pain, nausea and vomiting, and diarrhea, in addition to their respiratory tract symptoms[18]. Nevertheless, the impacts of COVID-19 infection, and particularly Long-COVID-19, in the GI spectrum is not fully understood in either dialysis patients or KT patients, and data on this topic remains scarce. Of note, data from the most recent report on post-acute SARS-CoV-2 infection sub-phenotype by Zhang et al[19] found that GI tract-related symptoms are one of the four most common characteristics in post-acute viral symptoms.
Hence, the aim of the present study was to determine the prevalence and characteristics of Long-COVID in a cohort of these patients. We hypothesize that Long-COVID, especially in the GI symptoms, may be underestimated in these populations and may need more clarification, particularly in the post-pandemic period.
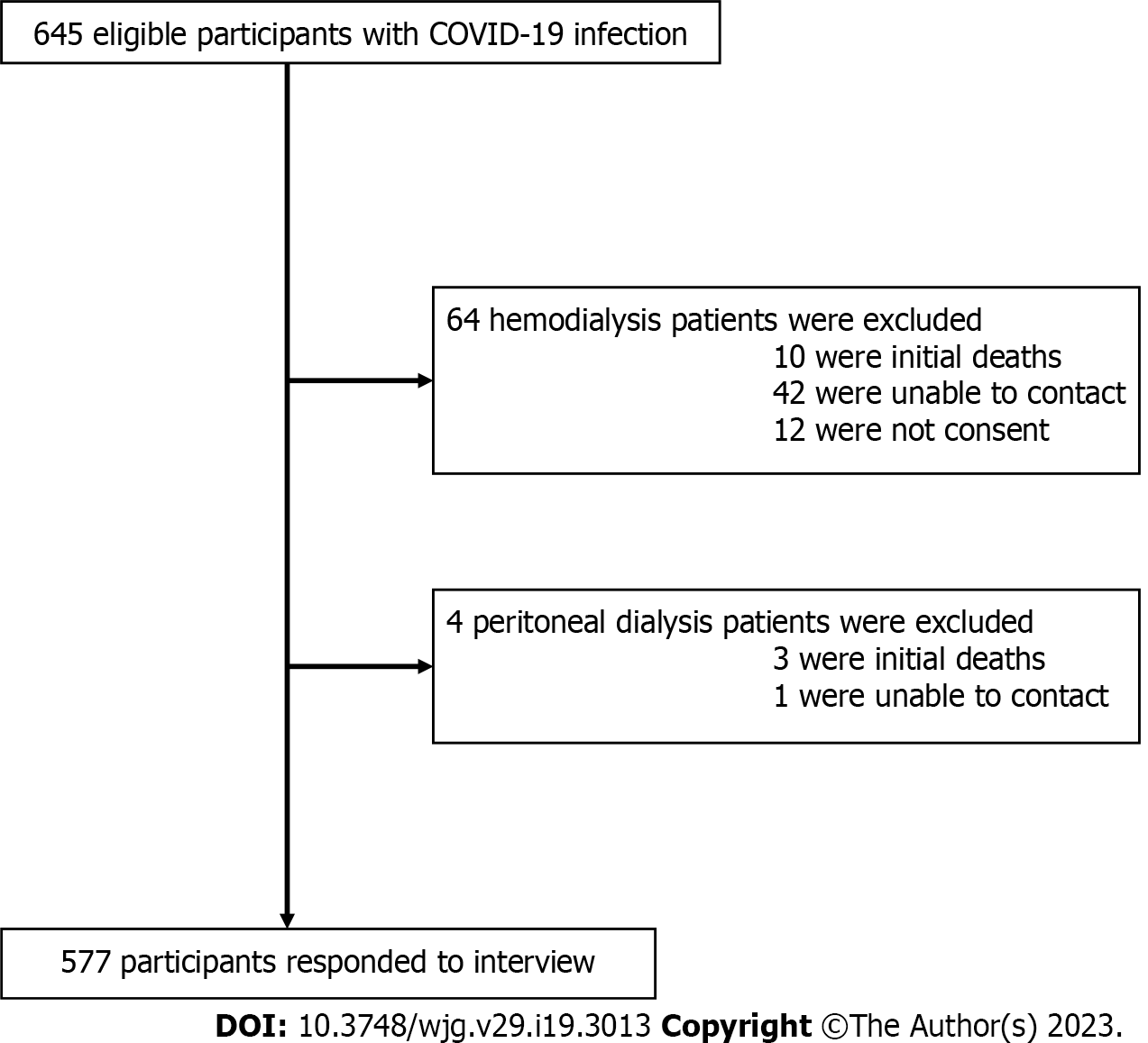
The study is a cohort longitudinal study performed in dialysis-dependent patients and KT recipients with COVID-19 infection under the care of three renal referral tertiary care centers. Eligible participants were those with a diagnosis of COVID-19 confirmed by an RT-PCR test from oro-nasopharyngeal swabs from January 2022 to 31 July 2022. The KT recipients with the following conditions were excluded: (1) Those who died before the follow-up interview, (2) those we were unable to contact, and (3) those without or unable to provide informed consent. The remaining dialysis-dependent patients and KT recipients who had experienced a post-COVID-19 infection for at least 3 mo were included in the study. Purposive sampling was used in order to ensure the representation of a range of characteristics and experiences of analytic relevance. Informed consent for participation in interviews was obtained either written or verbally over the phone from all participants in the study and the study was approved by the Research Ethics Commission of the Faculty of Tropical Medicine, Mahidol University, Thailand (MUTM 2022-081-01) along with adhered to STROBE guideline.
An interview consisting of a set of open-ended questions regarding symptoms during COVID-19 and post-COVID-19 infection periods. Then, interviews were designed to explore the specific persistent or emerging symptoms potentially due to GI tract-associated Long COVID-19 syndrome, as previously described[20]. Participants were interviewed either in person or by telephone by trained research nurses. Participants were considered to have GI tract-associated symptoms of Long COVID-19 if they showed one of the following signs: loss of appetite, nausea, weight loss, abdominal pain, heartburn, dysphagia, diarrhea, constipation, altered bowel motility, or irritable bowel syndrome[20]. In addition, the participant’s electronic medical records were used to obtain clinical data, including baseline demographics and transplant-related immunological risk, comorbidity, and data about COVID-19 admission. Although abnormal laboratory tests, such as elevated alanine aminotransferase, can present as GI tract-associated Long COVID-19 syndrome[21], only clinical signs and symptoms were explored in the present study.
Descriptive characteristics were presented as means and standard deviation (means ± SD) unless otherwise noted. The Kolmogorov-Smirnov and Levene’s tests were performed to establish data distribution and homogeneity, respectively. Chi-square tests were performed to compare categorical variables, whereas Tukey-Kramer multiple comparisons were used for continuous variables. Independent risk factors were assessed by applying a backward elimination stepwise binary regression and removing the least significant variables at each step. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. P < 0.05 was considered statistically significant. Data analysis was performed using the PASW 18.0.0 statistical software package (SPSS Inc., Chicago, IL, United States) and GraphPad Prism 9.3.1 software (GraphPad Software, Inc., La Jolla, CA, United States).
This study enrolled 645 eligible participants with COVID-19 infection, including 588 cases with hemodialysis (HD), 38 patients with peritoneal dialysis (PD), and 19 KT recipients. Of these, 577 (89.5%) participants agreed to interviews (Figure 1). All eligible KT recipients were enrolled in the study, and none of the transplant recipients in the KT cohort died or returned to dialysis.
The mean patient age was 52 ± 11 years, 300 (52%) were women, and the median dialysis duration was 7 ± 3 and 5 ± 1 years in the HD and PD groups, respectively. Hypertension (92%) and type 2 diabetes mellitus (77%) were the two most common comorbidities among the three groups of participants. The mean post-transplantation time was 6 ± 2 years (Table 1). Most of the HD patients had three dialysis sessions per week while continuous ambulatory PD (CAPD) was the most treatment modality used in PD patients. The three most common initial symptoms detected in both the dialysis and KT cohorts were fever (98%), coryza (96%), and cough (94%). Of note, the PD patients had a significant predominance of all symptoms compared to the HD and KT groups (P <0.0001); this could be because the highest comorbidities and uttermost severity of COVID-19 were observed in the PD group. For this reason, the combination therapy of Remdesivir (88.2%) and tocilizumab (63.1%) was prescribed significantly more frequently in this group with also correspondent the highest mean levels of both high-sensitivity C-reactive protein and D-dimer compared with HD and KT groups (P < 0.0001) (Table 1).
| Variables | Hemodialysis (n = 524) | Peritoneal dialysis (n = 34) | Kidney Transplantation (n = 19) | P value |
| Age, yr, mean ± SD | 48 ± 9 | 71 ± 12 | 44 ± 12 | < 0.001a,b |
| Female sex, n (%) | 278 (53.1) | 14 (41.2) | 8 (42.1) | NS |
| Body mass index, kg/m2, mean ± SD | 26 ± 4 | 23 ± 2 | 24 ± 4 | < 0.001a |
| Comorbidities, n (%) | ||||
| Hypertension | 487 (92.9) | 34 (100) | 12 (63.2) | < 0.0001b, 0.0002c |
| Diabetes | 408 (77.9) | 30 (88.2) | 6 (31.6) | < 0.0001b,c |
| Cardiovascular disease | 450 (85.9) | 28 (82.4) | 9 (47.4) | < 0.0001b, 0.008c |
| Pulmonary disease | 52 (9.9) | 5 (14.7) | 1 (5.3) | NS |
| Hepatic disease | 14 (2.7) | 4 (11.8) | 0 (0) | 0.004a |
| Renal replacement therapy | ||||
| Dialysis vintage, years | 7 ± 3 | 5 ± 1 | 6 ± 2 | < 0.001a |
| Frequency, 2× per week, n (%) | 84 (16.0) | N/A | N/A | |
| Frequency, 3× per week, n (%) | 440 (84.0) | N/A | N/A | |
| CAPD, n (%) | N/A | 32 (94.1) | N/A | |
| APD, n (%) | N/A | 2 (5.9) | N/A | |
| Deceased donor transplant, n (%) | N/A | N/A | 11 (57.9) | |
| Time from transplant, yr, mean ± SD | N/A | N/A | 6 ± 2 | < 0.001b,c |
| Maintenance immunosuppressive regimen by drug, n (%) | ||||
| Calcineurin inhibitors | ||||
| TAC | N/A | N/A | 11 (57.9) | |
| CsA | N/A | N/A | 8 (42.1) | |
| Prednisolone | N/A | N/A | 19 (100) | |
| Antimetabolites | ||||
| MPA | N/A | N/A | 16 (84.2) | |
| Azathioprine | N/A | N/A | 0 (0) | |
| mTOR inhibitors | N/A | N/A | 3 (15.8) | |
| Baseline creatinine, mean ± SD | 8 ± 2 | 11 ± 2 | 2.5 ± 0.8 | < 0.001a,b,c |
| Baseline creatinine > 1.5 mg/dL, n (%)1 | N/A | N/A | 7 (36.8) | |
| Day(s) of illness, mean ± SD | 4 ± 2 | 3 ± 1 | 3 ± 1 | < 0.001a |
| Initial symptoms, n (%) | ||||
| Fever or chills | 511 (97.5) | 34 (100) | 19 (100) | NS |
| Cough | 488 (93.1) | 34 (100) | 18 (94.7) | NS |
| Dyspnea | 321 (61.3) | 30 (88.2) | 16 (84.2) | 0.002a, 0.043b |
| Chest pain | 152 (29.0) | 11 (32.4) | 8 (42.1) | NS |
| Coryza | 501 (95.6) | 34 (100) | 19 (100) | NS |
| Headache | 161 (30.7) | 5 (14.7) | 6 (31.6) | 0.048a |
| Nasal congestion | 359 (68.5) | 9 (26.5) | 11 (57.9) | < 0.0001a, 0.025c |
| Fatigue | 209 (40.0) | 34 (100) | 9 (47.4) | < 0.0001a, < 0.0001c |
| Myalgia | 386 (73.7) | 31 (91.2) | 12 (63.2) | 0.023a, 0.013c |
| Nausea or vomiting | 137 (26.1) | 30 (88.2) | 4 (21.1) | < 0.0001a,c |
| Diarrhea | 83 (15.8) | 25 (73.5) | 3 (15.8) | < 0.0001a,c |
| Anosmia | 66 (12.6) | 7 (20.6) | 1 (5.3) | NS |
| Ageusia | 25 (4.8) | 6 (17.6) | 2 (10.5) | 0.002a |
| Number of symptoms, mean ± SD | 7 ± 2 | 9 ± 1 | 4 ± 2 | < 0.0001a,b,c |
| COVID-19 severity, n (%) | ||||
| Mild | 252 (48.1) | 2 (5.9) | 0 (0) | < 0.0001a,b |
| Moderate | 137 (26.1) | 7 (20.6) | 4 (21.1) | NS |
| Severe | 135 (25.8) | 25 (73.5) | 0 (0) | < 0.0001a,c, 0.011b |
| High-sensitivity C-reactive protein (mg/L) | 32 ± 14 | 59 ± 11 | 17 ± 9 | < 0.0001a,b,c |
| D-dimer (ng/mL) | 2749 ± 578 | 5339 ± 786 | 1699 ± 175 | < 0.0001a,b,c |
| Treatments, n (%) | ||||
| Remdesivir | 352 (67.2) | 30 (88.2) | 8 (42.1) | 0.011a, 0.023b, 0.0004c |
| Favipiravir | 172 (32.8) | 4 (11.8) | 11 (57.9) | 0.011a, 0.023b, < 0.001c |
| Tocilizumab | 39 (7.4) | 12 (63.1) | 0 (0) | < 0.0001a,c |
| Corticosteroids | 429 (81.9) | 34 (100) | 19 (100) | 0.007a, 0.041b |
| Low-molecular weight heparin | 482 (92.0) | 32 (94.1) | 7 (36.8) | < 0.0001b,c |
| Outcomes during the acute phase, n (%) | ||||
| Hospitalization | 524 (100) | 34 (100) | 19 (100) | - |
| Intensive care unit | 204 (38.9) | 27 (79.4) | 4 (21.1) | < 0.0001a,c |
| Oxygen therapy | 272 (51.9) | 32 (94.1) | 4 (21.1) | < 0.0001a,c, 0.008b |
| Invasive mechanical ventilation | 104 (19.8) | 23 (67.6) | 0 (0) | < 0.0001a,c, 0.031b |
| Increased dialysis frequency | 178 (34.0) | 0 (0) | N/A | < 0.0001a |
| Immunosuppression suspended except for steroids1 | N/A | N/A | 2 (10.5) | |
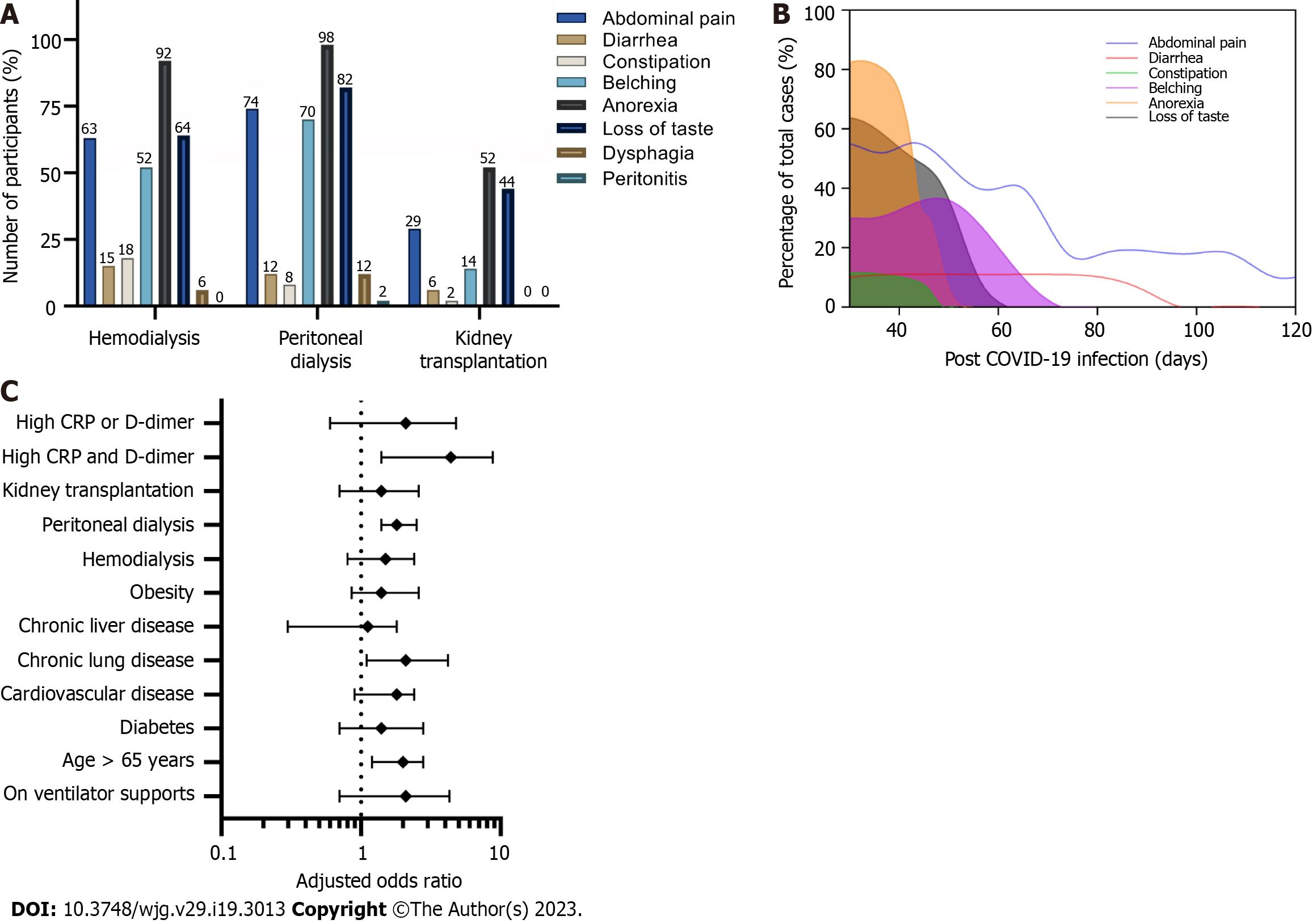
The Thai national guidelines for COVID-19 management in high-risk patients (during this study period) stipulate that all patients with CKD or CKD-equivalent disorders must be hospitalized for intensive care and monitoring during acute COVID-19 infection. As such, all participants in the study were hospitalized. During the early post-COVID-19 infection period, Long-COVID was identified in 293/524 (56%) of the HD, 21/34 (62%) of the PD, and 7/19 (37%) of the KT groups. Fatigue was the most prevalent symptom (96%) of the non-GI tract symptoms and was accompanied by loss of appetite or anorexia (81%), loss of taste (63%), hoarse voice (28%), unusual muscle pains (23%), hair loss (22%), a persistent cough (22%), headache (11%), and impaired cognitive and memory function (9%). Among the GI manifestations of Long-COVID, anorexia was the most prevalent symptom (525 cases, 90.9% from all groups), followed by loss of taste (372 cases, 64% from all groups), and abdominal pain (367 cases, 62.5% from all groups) (Figure 2A). Although anorexia and loss of taste were common in all three groups, they were more predominant in dialysis patients, but most cases showed much improvement by two months after the onset of COVID-19 infection. Abdominal pain and diarrhea were the symptoms that persisted for over 3 mo (Figure 2B).
Notably, our investigation of the causes of abdominal pain, which was reported by 87% of the patients, revealed that non-specific abdominal pain or probably acute gastritis was the main etiology in dialysis-dependent patients, whereas mesenteric panniculitis was the main etiology of abdominal pain (6 from 19 cases) in the KT group with a good response to oral corticosteroids (20 mg prednisolone), which were slowly tapered off in 8 wk. All six cases of mesenteric panniculitis had complete resolution, as indicated by the follow-up abdominal computer tomography.
Figure 2C shows the factors associated with the GI manifestation of Long-COVID. We found that COVID-19 patients who were older than 65 years (ORs 2.00, 95%CIs 1.2-2.8), who had chronic lung disease (ORs 2.10, 95%CIs 1.1-4.2), who were on PD (ORs 1.80, 95%CIs 1.4-2.5), or who had high levels of both C-reactive protein (CRP) and D-dimer at the onset (ORs 4.40, 95%CIs 1.4-8.8) were significantly likely to have GI manifestations of Long-COVID.
In this study, we observed that a GI manifestation of Long-COVID is a frequent condition in patients with dialysis dependence and KT recipients. Long-COVID was significantly more prevalent in PD patients than in HD or KT cases. Notably, patients who experienced either abdominal pain or diarrhea had a longer duration of other GI manifestations of Long-COVID, suggesting a need for closer observation of these patients during COVID-19 infection.
COVID-19 has brought forth a multitude of challenges to healthcare systems across the globe. Apart from the significant morbidity and mortality associated with COVID-19 during its initial phase, recognition and concern are growing regarding the long-term consequences of COVID-19[2,3,22]. Dialysis-dependent patients and KT recipients are clearly high-risk groups associated with higher numbers of comorbidities and immunosuppressive issues and require more aggressive courses of COVID-19 treatment in terms of acute and chronic complications[8,23,24]. Although the most visible manifestation of Long-COVID in the general population is asthenia, or brain fog[22,25], we found that anorexia was the most common GI manifestation of Long-COVID in both dialysis-dependent patients and KT recipients, followed by abdominal pain and loss of taste (Figure 2A).
The prevalence and characteristics of Long-COVID in the present cohort seem to differ from its manifestations in other populations, in which diarrhea was the most persistently encountered GI symptom[26-31]. This difference could be explained by the combination of pre-existing uremic toxins in dialysis-dependent patients, as well as a delayed clearance of inflammatory cytokines[32] and enhanced oxidative stress associated with end-stage renal disease (ESRD)[33]. As such, restoration of renal function in KT recipients resulted in a decrease in the incidence of GI manifestations of Long-COVID compared with ESRD patients (Figure 2A). However, kidney transplantation does not entirely reverse T cell functions[34], and the underlying mechanisms of epigenetic changes induced by any combination of inflammation and oxidative stress associated with uremia are not easily reversible[35]. For these reasons, KT recipients with COVID-19 infection still have a persistently increased risk for Long-COVID.
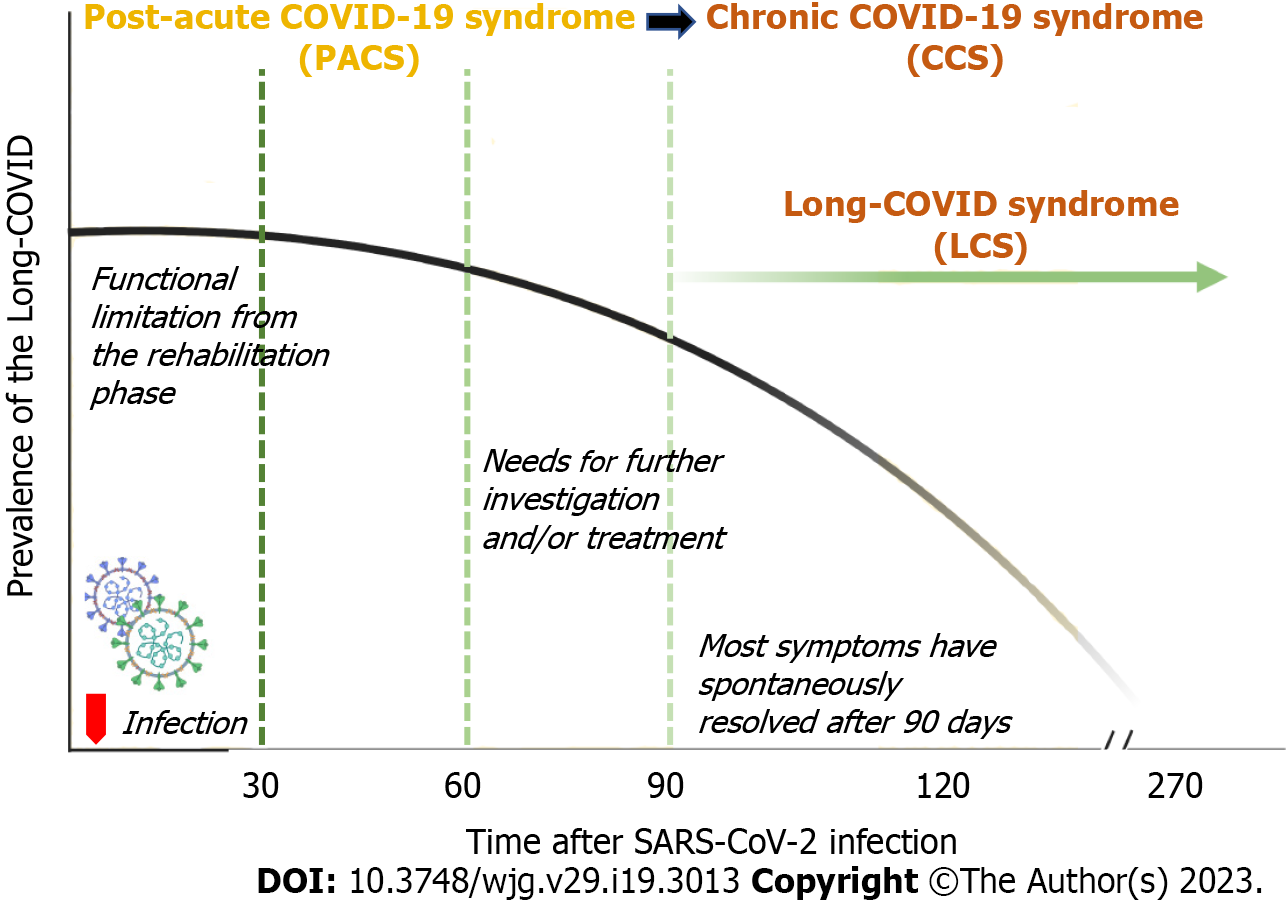
As shown in Figure 2B, most of the participants experienced much improvement in the manifestations of Long-COVID after 4 wk and nearly complete resolution by three months. However, the differential diagnosis between the functional limitation during the COVID rehabilitation phase vs the Long-COVID syndrome may be difficult. Accordingly, the need for a robust clarification of the sequelae after COVID-19 infection is another concern in the post-pandemic era. The British NICE suggests that the term PASC must refer to any clinical signs and symptoms that develop during or after an infection consistent with COVID-19, that continue for more than 12 wk, and that cannot be explained by any other conditions[6]. Similarly, the World Health Organization (WHO) defines the PASC syndrome as any symptoms without an alternative diagnosis from three months onwards and that last for at least 2 mo[7]. Based on our findings, we found a significant difference in the clinical spectrum between patients with a symptom duration longer than 3 mo vs less than 3 mo post-COVID-19 infection (Figure 2B), in agreement with the COVID Symptom Study[36]. One explanation for the persistence of clinical signs and symptoms following COVID-19 infection may involve the underlying biological factors, such as an aberrant immune response[37], diverse functional autoantibodies[38], or gut dysbiosis[39], that drive other virus-initiated chronic syndromes.
We support using the 12-week cut-off duration as recommended by NICE and WHO for the diagnosis of PASC, and we propose an additional revision of the specific nomenclature for early and late Long-COVID syndrome. We propose using the term “post-acute COVID-19 syndrome (PACS)” for the clinical syndrome that develops three months from post-COVID-19 infection and using “chronic COVID-19 syndrome” thereafter (Figure 3). We further recommend reserving the term “Long-COVID syndrome” for the clinical syndrome that develops beyond three months post-COVID-19 infection and lasts for at least six months, because Long-COVID syndrome may be another post-viral illness spectrum, like myalgic encephalomyelitis/chronic fatigue syndrome[40].
The mechanisms underlying the GI manifestations of Long-COVID are not completely understood. One plausible explanation might be that an impairment of gut homeostasis is explained by disruption of gut-lung communication[41]. The manifestations during acute COVID-19 are believed to be related to an increased expression of ACE2 on the small bowel mucosa[17], endotoxemia[16,42], leaky gut[16], and alterations in hepatic blood flow due to sinusoidal thrombi[43], all triggered by an increased proinflammatory state and intestinal dysbiosis. Undoubtedly, the greater severity of COVID-19 infection in the elderly (high C-reactive protein > 5 mg/L, high D-dimer > 500 ng/mL with > 65 years old), as shown in Figure 2C, also leads to a greater risk of GI manifestations of Long-COVID[44]. Although COVID-19 outcomes are comparable between PD and HD patients[45], the findings of the present study demonstrated that PD patients have a greater risk of developing GI manifestations of Long-COVID. Being elderly and having more symptoms (Table 1) may constitute key risk factors for developing Long-COVID in PD patients[46].
Irritable bowel syndrome, a condition with diverse symptoms, including diarrhea, constipation, and mixed bowel habits according to the Rome criteria[47], has been recently proposed as a possible consequence of COVID-19 infection due to disruption of the diversity and stability of the gut microbiota[48]. For this reason, evaluation of whether diarrhea and indigestion manifestations of Long-COVID alter the gut microbiome would be worth investigating[49]. This possibility also suggests that the use of specific probiotics and prebiotics in COVID-19 clinical treatment may help KT recipients with COVID-19 infections to rebalance their gut and lung microbial ecology, thereby boosting their immune responses against the virus in response to a new metabolic milieu[50].
Moreover, little is known about the pathophysiology of the abdominal pain manifestation of Long-COVID-19. Prolonged shedding of SARS-CoV-2 from the GI tract has been observed and could be responsible for some of the GI manifestations of Long-COVID[51]. Interestingly, we found that over one-third of our KT recipients had been diagnosed with mesenteric panniculitis-related Long-COVID. Although this is a rare condition, concern is growing regarding the conditional pain associated with COVID-19[52,53]. Notably, all of our recipients with mesenteric panniculitis fully recovered after corticosteroid administration, suggesting that systemic inflammation is the process involved here[54]. Although renal allograft dysfunction and graft loss following COVID-19 infection are possibly resulted from direct toxicity of SARS-CoV-2, cytokine storm-induced tubular injury, reduced immunosuppressive drugs during infection and decreased renal allograft blood flow from multiple organ failure[55-57], there was no reported case of acute kidney injury in the cohort.
The strengths of the present study are that we compiled the data available on the prevalence, symptomatology, and specific treatment of the particular GI manifestations of Long-COVID symptoms; this will help to guide clinicians in dealing with the pandemic. The identification of GI manifestations of Long-COVID in KT recipients could also help to define the contours of this new SARS-CoV-2 virus. Some limitations of the present study should also be acknowledged. This was a renal referral center study with a limited diversity of patient characteristics. No non-COVID-19 patients were included in the study, and the small number of participants made the study underpowered for investigating the risk factors associated with GI symptoms. Thus, the results need confirmation with larger cohorts that can apply the structural equation modeling for analysis, which has greater statistical power in terms of the probability of rejecting of a false null hypothesis than multiple regression analysis does[58]. In addition, the influence of different SARS-CoV-2 variants on GI manifestations of Long-COVID in dialysis-dependent and KT patients was not clarified, nor was the vaccination status against different variants addressed in our cohorts. However, based on the timing of the pandemic, the main strain circulating at the time of our study was the Delta (B.1.617) strain, accompanied by an early wave of the Omicron (B.1.1.529) variants[59,60]. Accordingly, an in-depth analysis of confounders should also be performed in larger, multinational cohorts. It is also challenging to find unrecovered pathophysiology of the long-term GI effects of COVID-19. Future research should not overlook other organ interactions to GI manifestations of Long-COVID, for instance, mental health symptoms (e.g., depression and anxiety symptoms) nor additional post-infectious symptoms that were not assessed in the present study (e.g., cardiovascular disease), as depression[61-63], gut-brain axis[64] or cardiovascular diseases[65] are the main etiology of long-term comorbidity of COVID-19[5], especially in HD patients[66]. In addition to lack of appetite, ESRD is recognized as a high risk of pre-existing undernutrition (malnutrition), including micronutrient deficiencies from malnutrition-inflammation-cachexia complex[67], which has been linked to increased mortality in patients with COVID-19[68]. Thus, nutrition support could be another critical intervention during COVID-19 infection in ESRD that robust research is needed for clarification as, vice versa, reduced long-term GI sequelae is probably part of the overall benefit from nutritional support.
In conclusion, at 3 mo after infection with SARS-CoV-2, renal replacement therapy patients and KT recipients with COVID-19 show high rates of GI manifestations of Long-COVID after discharge following their initial episode. These data point to optimized management as a potential line of research for decreasing Long-COVID syndrome in these populations.
The characteristics of persistent coronavirus disease 2019 (COVID-19) symptoms or Long-COVID in dialysis-dependent patients and kidney transplant (KT) is remain underestimate and urgent needs for investigation to prevent long-term complication in these vulnerable population.
End stage renal disease is a well-known condition for high mortality risk following COVID-19 infection. Thus, it is essential to explore the Long-COVID in these population as an early preventive strategy for preventing further morbidity and mortality.
To identify the characteristics of gastrointestinal (GI) manifestations of Long-COVID in patients with dialysis-dependent or KT status.
A prospective, observational study was conducted during January 2022 to July 2022 in patients with COVID-19 infection to explore the Long-COVID symptoms in 3-months after the onset by interviewing.
As of 577 cases agreed to the interviews, the mean age was 52±11 years with 52% women. Long-COVID was identified in 56%, 62% and 37% in hemodialysis, peritoneal dialysis, and KT respectively. While fatigue was the most prevalent (96%) of the non-GI tract symptoms, anorexia (90.9%), loss of taste (64.4%), and abdominal pain (62.5%) were the first three common GI manifestations of Long-COVID. Of note, there were 6 cases of mesenteric panniculitis from 19 patients with GI symptoms in the KT group.
Renal replacement therapy patients and KT recipients with COVID-19 show high rates of GI manifestations of Long-COVID after discharge following their initial episode.
Further study should aim to explore the pathophysiology of the long-term GI effects of COVID-19 in renal replacement therapy and KT patients, which may have different immune response to Long-COVID symptoms compared to those with immunocompetent.
| 1. | Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 2. | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 3154] [Article Influence: 630.8] [Reference Citation Analysis (0)] |
| 3. | Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: An overview. Diabetes Metab Syndr. 2021;15:869-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 427] [Cited by in RCA: 582] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 4. | Maltezou HC, Pavli A, Tsakris A. Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Visco V, Vitale C, Rispoli A, Izzo C, Virtuoso N, Ferruzzi GJ, Santopietro M, Melfi A, Rusciano MR, Maglio A, Di Pietro P, Carrizzo A, Galasso G, Vatrella A, Vecchione C, Ciccarelli M. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 6. | National Institute for Health and Care Excellence: Clinical Guidelines. COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence (NICE), 2020. |
| 7. | Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102-e107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 1669] [Article Influence: 417.3] [Reference Citation Analysis (0)] |
| 8. | Modelli de Andrade LG, de Sandes-Freitas TV, Requião-Moura LR, Viana LA, Cristelli MP, Garcia VD, Alcântara ALC, Esmeraldo RM, Abbud Filho M, Pacheco-Silva A, de Lima Carneiro ECR, Manfro RC, Costa KMAH, Simão DR, de Sousa MV, Santana VBBM, Noronha IL, Romão EA, Zanocco JA, Arimatea GGQ, De Boni Monteiro de Carvalho D, Tedesco-Silva H, Medina-Pestana J; COVID-19-KT Brazil. Development and validation of a simple web-based tool for early prediction of COVID-19-associated death in kidney transplant recipients. Am J Transplant. 2022;22:610-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Basic-Jukic N, Juric I, Furic-Cunko V, Katalinic L, Radic J, Bosnjak Z, Jelakovic B, Kastelan Z. Follow-up of renal transplant recipients after acute COVID-19-A prospective cohort single-center study. Immun Inflamm Dis. 2021;9:1563-1572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Malinowska A, Muchlado M, Ślizień Z, Biedunkiewicz B, Heleniak Z, Dębska-Ślizień A, Tylicki L. Post-COVID-19 Sydrome and Decrease in Health-Related Quality of Life in Kidney Transplant Recipients after SARS-COV-2 Infection-A Cohort Longitudinal Study from the North of Poland. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Chauhan S, Meshram HS, Kute V, Patel H, Desai S, Dave R. Long-term follow-up of SARS-CoV-2 recovered renal transplant recipients: A single-center experience from India. Transpl Infect Dis. 2021;23:e13735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Amorim CEN, Gomes VLT, Cristelli MP, Viana LA, de Luca Correa H, Lima GBB, de Sousa Silva FS, de Castro Lima GS, Rosa TDS, Nakamura MR, Quintino PM, Tedesco-Silva H, Medina-Pestana J. High Prevalence of Long-COVID Among Kidney Transplant Recipients: A Longitudinal Cohort Study. Transplantation. 2022;106:2408-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 13. | Och A, Tylicki P, Polewska K, Puchalska-Reglińska E, Parczewska A, Szabat K, Biedunkiewicz B, Dębska-Ślizień A, Tylicki L. Persistent Post-COVID-19 Syndrome in Hemodialyzed Patients-A Longitudinal Cohort Study from the North of Poland. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Demiray A, Kanbay A, Kanbay M. Long-term effect of COVID-19 infection on hemodialysis patients: Should we follow hemodialysis patients more closely? Clin Kidney J. 2022;15:369-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Zuo T, Liu Q, Zhang F, Lui GC, Tso EY, Yeoh YK, Chen Z, Boon SS, Chan FK, Chan PK, Ng SC. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 16. | Saithong S, Worasilchai N, Saisorn W, Udompornpitak K, Bhunyakarnjanarat T, Chindamporn A, Tovichayathamrong P, Torvorapanit P, Chiewchengchol D, Chancharoenthana W, Leelahavanichkul A. Neutrophil Extracellular Traps in Severe SARS-CoV-2 Infection: A Possible Impact of LPS and (1→3)-β-D-glucan in Blood from Gut Translocation. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14579] [Article Influence: 2429.8] [Reference Citation Analysis (3)] |
| 18. | Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2061] [Cited by in RCA: 2091] [Article Influence: 348.5] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Zang C, Xu Z, Zhang Y, Xu J, Bian J, Morozyuk D, Khullar D, Nordvig AS, Schenck EJ, Shenkman EA, Rothman RL, Block JP, Lyman K, Weiner MG, Carton TW, Wang F, Kaushal R. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat Med. 2023;29:226-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 124] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 20. | Meringer H, Mehandru S. Gastrointestinal post-acute COVID-19 syndrome. Nat Rev Gastroenterol Hepatol. 2022;19:345-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 1007] [Article Influence: 201.4] [Reference Citation Analysis (0)] |
| 22. | Burke MJ, Del Rio C. Long COVID has exposed medicine's blind-spot. Lancet Infect Dis. 2021;21:1062-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Requião-Moura LR, Sandes-Freitas TV, Viana LA, Cristelli MP, Andrade LGM, Garcia VD, Oliveira CMC, Esmeraldo RM, Abbud Filho M, Pacheco-Silva A, Sousa KC, Vicari AR, Costa KMAH, Simão DR, Sousa MV, Campos JB, Almeida RAMB, Deboni LM, Neto MM, Zanocco JA, Tedesco-Silva H, Medina-Pestana J; COVID-19-KT Brazil. High mortality among kidney transplant recipients diagnosed with coronavirus disease 2019: Results from the Brazilian multicenter cohort study. PLoS One. 2021;16:e0254822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | Medina-Pestana J, Cristelli MP, Foresto RD, Tedesco-Silva H, Requião-Moura LR. The Higher COVID-19 Fatality Rate Among Kidney Transplant Recipients Calls for Further Action. Transplantation. 2022;106:908-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Amin-Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med. 2021;27:1129-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 26. | Romero-Duarte Á, Rivera-Izquierdo M, Guerrero-Fernández de Alba I, Pérez-Contreras M, Fernández-Martínez NF, Ruiz-Montero R, Serrano-Ortiz Á, González-Serna RO, Salcedo-Leal I, Jiménez-Mejías E, Cárdenas-Cruz A. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: the ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021;19:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 27. | Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, Crooks M, Gabbay M, Brady M, Hishmeh L, Attree E, Heightman M, Banerjee R, Banerjee A; COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 28. | Blackett JW, Wainberg M, Elkind MSV, Freedberg DE. Potential Long Coronavirus Disease 2019 Gastrointestinal Symptoms 6 Months After Coronavirus Infection Are Associated With Mental Health Symptoms. Gastroenterology. 2022;162:648-650.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA. 2020;324:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2398] [Cited by in RCA: 2892] [Article Influence: 482.0] [Reference Citation Analysis (1)] |
| 30. | Moreno-Pérez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jiménez J, Asensio S, Sanchez R, Ruiz-Torregrosa P, Galan I, Scholz A, Amo A, González-delaAleja P, Boix V, Gil J; COVID19-ALC research group. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infect. 2021;82:378-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 402] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 31. | Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, Flament T, Ferreira-Maldent N, Bruyère F, Stefic K, Gaudy-Graffin C, Grammatico-Guillon L, Bernard L. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 485] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 32. | Caso JR, MacDowell KS, Soto M, Ruiz-Guerrero F, Carrasco-Díaz Á, Leza JC, Carrasco JL, Díaz-Marsá M. Dysfunction of Inflammatory Pathways and Their Relationship With Psychological Factors in Adult Female Patients With Eating Disorders. Front Pharmacol. 2022;13:846172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Chancharoenthana W, Udompronpitak K, Manochantr Y, Kantagowit P, Kaewkanha P, Issara-Amphorn J, Leelahavanichkul A. Repurposing of High-Dose Erythropoietin as a Potential Drug Attenuates Sepsis in Preconditioning Renal Injury. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Meijers RW, Litjens NH, de Wit EA, Langerak AW, Baan CC, Betjes MG. Uremia-associated immunological aging is stably imprinted in the T-cell system and not reversed by kidney transplantation. Transpl Int. 2014;27:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 36. | Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, Molteni E, Modat M, Jorge Cardoso M, May A, Ganesh S, Davies R, Nguyen LH, Drew DA, Astley CM, Joshi AD, Merino J, Tsereteli N, Fall T, Gomez MF, Duncan EL, Menni C, Williams FMK, Franks PW, Chan AT, Wolf J, Ourselin S, Spector T, Steves CJ. Author Correction: Attributes and predictors of long COVID. Nat Med. 2021;27:1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Vibholm LK, Nielsen SSF, Pahus MH, Frattari GS, Olesen R, Andersen R, Monrad I, Andersen AHF, Thomsen MM, Konrad CV, Andersen SD, Højen JF, Gunst JD, Østergaard L, Søgaard OS, Schleimann MH, Tolstrup M. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine. 2021;64:103230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 38. | Wang EY, Mao T, Klein J, Dai Y, Huck JD, Liu F, Zheng NS, Zhou T, Israelow B, Wong P, Lucas C, Silva J, Oh JE, Song E, Perotti ES, Fischer S, Campbell M, Fournier JB, Wyllie AL, Vogels CBF, Ott IM, Kalinich CC, Petrone ME, Watkins AE; Yale IMPACT Team, Cruz CD, Farhadian SF, Schulz WL, Grubaugh ND, Ko AI, Iwasaki A, Ring AM. Diverse Functional Autoantibodies in Patients with COVID-19. medRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 39. | Thomas RM, Jobin C. Microbiota in pancreatic health and disease: the next frontier in microbiome research. Nat Rev Gastroenterol Hepatol. 2020;17:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 235] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 40. | Astin R, Banerjee A, Baker MR, Dani M, Ford E, Hull JH, Lim PB, McNarry M, Morten K, O'Sullivan O, Pretorius E, Raman B, Soteropoulos DS, Taquet M, Hall CN. Long COVID: mechanisms, risk factors and recovery. Exp Physiol. 2023;108:12-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 41. | Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, Delhaes L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front Cell Infect Microbiol. 2020;10:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 529] [Article Influence: 88.2] [Reference Citation Analysis (1)] |
| 42. | Sirivongrangson P, Kulvichit W, Payungporn S, Pisitkun T, Chindamporn A, Peerapornratana S, Pisitkun P, Chitcharoen S, Sawaswong V, Worasilchai N, Kampunya S, Putcharoen O, Thawitsri T, Leelayuwatanakul N, Kongpolprom N, Phoophiboon V, Sriprasart T, Samransamruajkit R, Tungsanga S, Tiankanon K, Lumlertgul N, Leelahavanichkul A, Sriphojanart T, Tantawichien T, Thisyakorn U, Chirathaworn C, Praditpornsilpa K, Tungsanga K, Eiam-Ong S, Sitprija V, Kellum JA, Srisawat N. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med Exp. 2020;8:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 43. | Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27:377-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (2)] |
| 44. | Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, Heightman M, Hillman TE, Jacob J, Jarvis HC, Lipman MCI, Naidu SB, Nair A, Porter JC, Tomlinson GS, Hurst JR; ARC Study Group. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 569] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 45. | Ghonimi TAL, Alkad MM, Abuhelaiqa EA, Othman MM, Elgaali MA, Ibrahim RAM, Joseph SM, Al-Malki HA, Hamad AI. Mortality and associated risk factors of COVID-19 infection in dialysis patients in Qatar: A nationwide cohort study. PLoS One. 2021;16:e0254246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Robineau O, Zins M, Touvier M, Wiernik E, Lemogne C, de Lamballerie X, Blanché H, Deleuze JF, Saba Villarroel PM, Dorival C, Nicol J, Gomes-Rima R, Correia E, Coeuret-Pellicer M, Druesne-Pecollo N, Esseddik Y, Ribet C, Goldberg M, Severi G, Carrat F; Santé, Pratiques, Relations et Inégalités Sociales en Population Générale Pendant la Crise COVID-19–Sérologie (SAPRIS-SERO) Study Group. Long-lasting Symptoms After an Acute COVID-19 Infection and Factors Associated With Their Resolution. JAMA Netw Open. 2022;5:e2240985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1147] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 48. | Settanni CR, Ianiro G, Ponziani FR, Bibbò S, Segal JP, Cammarota G, Gasbarrini A. COVID-19 as a trigger of irritable bowel syndrome: A review of potential mechanisms. World J Gastroenterol. 2021;27:7433-7445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (2)] |
| 49. | Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC, Ng SSS, Zhang F, Li AYL, Lu W, Hui DS, Chan PK, Chan FKL, Ng SC. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71:544-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (1)] |
| 50. | Tungsanga S, Panpetch W, Bhunyakarnjanarat T, Udompornpitak K, Katavetin P, Chancharoenthana W, Chatthanathon P, Somboonna N, Tungsanga K, Tumwasorn S, Leelahavanichkul A. Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (1→3)-β-D-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1050] [Cited by in RCA: 1157] [Article Influence: 192.8] [Reference Citation Analysis (1)] |
| 52. | Mandala S, Kodati R, Tadepalli A, Reddy C, Kalyan S. An Unusual Cause of Acute Abdominal Pain in Coronavirus Disease (COVID-19): Report of Two Cases. Indian J Crit Care Med. 2022;26:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 53. | Alyousef IA, Alsaileek ZA, Alabdulsalam MA, Almohanna MA, Alshaqhaa NA, Alqahtani MM, Al Alyany AA, Alzahrani MA, Fallatah HA, Alqadhib JI, Alhawsawi AM, Alsuhaymi AD, Alasmari AM, Alshareef AJ, Al-Hawaj F. Mesenteric Panniculitis and COVID-19: A Rare Association. Cureus. 2022;14:e21314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Jiang QW, Wang FD, Wang WZ, Wu X, Shu HJ, Li JN, Yang AM, Qian JM, Wu D. [An analysis of clinical characteristics of twelve cases of mesenteric panniculitis]. Zhonghua Nei Ke Za Zhi. 2017;56:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, Redondo-Pachón MD, Murphy B, Florman S, Cyrino LG, Grafals M, Venkataraman S, Cheng XS, Wang AX, Zaza G, Ranghino A, Furian L, Manrique J, Maggiore U, Gandolfini I, Agrawal N, Patel H, Akalin E, Riella LV. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140-3148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 56. | Mohamed IH, Chowdary PB, Shetty S, Sammartino C, Sivaprakasam R, Lindsey B, Thuraisingham R, Yaqoob MM, Khurram MA. Outcomes of Renal Transplant Recipients With SARS-CoV-2 Infection in the Eye of the Storm: A Comparative Study With Waitlisted Patients. Transplantation. 2021;105:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Hadi YB, Naqvi SFZ, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in Solid Organ Transplant Recipients: A Propensity-matched Analysis of a Large Research Network. Transplantation. 2021;105:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 58. | Beran TN, Violato C. Structural equation modeling in medical research: a primer. BMC Res Notes. 2010;3:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 59. | Chantasrisawad N, Puthanakit T, Kornsitthikul K, Jaru-Ampornpan P, Tawan M, Matapituk P, Sophonphan J, Anugulruengkitt S, Tangsathapornpong A, Katanyutanon A; KIDSBOOST study team. Immunogenicity to SARS-CoV-2 Omicron variant among school-aged children with 2-dose of inactivated SARS-CoV-2 vaccines followed by BNT162b2 booster. Vaccine X. 2022;12:100221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 60. | Chancharoenthana W, Leelahavanichkul A, Chinpraditsuk S, Pongpirul K, Kamolratanakul S, Phumratanaprapin W, Wilairatana P, Pitisuttithum P. Social restriction versus herd immunity policies in the early phase of the SARS-CoV-2 pandemic: A mathematical modelling study. Asian Pac J Allergy Immunol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Nooripour R, Ghanbari N, Radwin LE, Hosseinian S, Hassani-Abharian P. Development and validation of COVID-19 Stress Scale (CSS) in an Iranian non-clinical population. Zahedan J Res Med Sci. 2022;24:e118719. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Nooripour R, Hosseinian S, Farmani F, Abtahi Foroshani N, Ghanbari N, Farkhojasteh VS. Relationship Between Hardiness and Stress of COVID-19 Through the Mediating Role of Mindfulness in Iranian Students. PCP. 2022;10:193-202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Nooripour R, Hosseinian S, Hussain AJ, Annabestani M, Maadal A, Radwin LE, Hassani-Abharian P, Pirkashani NG, Khoshkonesh A. How Resiliency and Hope Can Predict Stress of Covid-19 by Mediating Role of Spiritual Well-being Based on Machine Learning. J Relig Health. 2021;60:2306-2321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Chen J, Vitetta L. Gut-brain axis in the neurological comorbidity of COVID-19. Brain Commun. 2021;3:fcab118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Elseidy SA, Awad AK, Vorla M, Fatima A, Elbadawy MA, Mandal D, Mohamad T. Cardiovascular complications in the Post-Acute COVID-19 syndrome (PACS). Int J Cardiol Heart Vasc. 2022;40:101012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 66. | Carriazo S, Mas-Fontao S, Seghers C, Cano J, Goma E, Avello A, Ortiz A, Gonzalez-Parra E. Increased 1-year mortality in haemodialysis patients with COVID-19: a prospective, observational study. Clin Kidney J. 2022;15:432-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 67. | Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Barazzoni R, Breda J, Cuerda C, Schneider S, Deutz NE, Wickramasinghe K; COVID-19 Call Editorial Board. COVID-19: Lessons on malnutrition, nutritional care and public health from the ESPEN-WHO Europe call for papers. Clin Nutr. 2022;41:2858-2868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Awad AK, Egypt; Nooripour R, Iran S-Editor: Chang KL L-Editor: A P-Editor: Zhao S