Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2747
Peer-review started: January 9, 2023
First decision: February 1, 2023
Revised: February 7, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: May 14, 2023
Processing time: 122 Days and 7.5 Hours
Acute pancreatitis (AP) is an inflammatory disease of the pancreas, which can progress to severe AP, with a high risk of death. It is one of the most complicated and clinically challenging of all disorders affecting the abdomen. The main causes of AP are gallstone migration and alcohol abuse. Other causes are uncommon, controversial and insufficiently explained. The disease is primarily characterized by inappropriate activation of trypsinogen, infiltration of inflammatory cells, and destruction of secretory cells. According to the revised Atlanta classification, severity of the disease is categorized into three levels: Mild, moderately severe and severe, depending upon organ failure and local as well as systemic complications. Various methods have been used for predicting the severity of AP and its outcome, such as clinical evaluation, imaging evaluation and testing of various biochemical markers. However, AP is a very complex disease and despite the fact that there are of several clinical, biochemical and imaging criteria for assessment of severity of AP, it is not an easy task to predict its subsequent course. Therefore, there are existing controversies regarding diagnostic and therapeutic modalities, their effectiveness and complications in the treatment of AP. The main reason being the fact, that the pathophysiologic mechanisms of AP have not been fully elucidated and need to be studied further. In this editorial article, we discuss the efficacy of the existing diagnostic and therapeutic modalities, complications and treatment failure in the management of AP.
Core Tip: Acute pancreatitis (AP) is an inflammatory disease of the pancreas, with abnormal trypsinogen activation as the primary pathogenesis and varies from clinically mild to fulminant form. Severe forms of AP are a relatively common cause of death. Progress in the establishment of biochemical, imaging and clinical criteria for the severity and prognosis of the disease has markedly influenced the therapeutic approach and the outcome of the disease. This article presents the diagnostic and therapeutic modalities with regard to their effectiveness, complications and treatment failure, as well as discussing some of the controversial issues in the treatment of AP.
- Citation: Zerem E, Kurtcehajic A, Kunosić S, Zerem Malkočević D, Zerem O. Current trends in acute pancreatitis: Diagnostic and therapeutic challenges. World J Gastroenterol 2023; 29(18): 2747-2763
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2747.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2747
Acute pancreatitis (AP) is an inflammatory pancreatic disease affecting all ages, with an annual incidence of 10-50 cases per 100000 persons[1]. The main causes of AP are gallstone migration and alcohol abuse. Among these, predominance of one cause over the other depends on socioeconomic, ethnic, and cultural differences[2-5].
Most patients with AP present with a mild and self-limited disease. Conversely, 15%-20% patients with AP develop local and/or systemic complications, frequently leading in multiple (respiratory, cardiovascular, renal, and hepatic) organ failure (MOF) and death. According to the revised Atlantic classification, the severity of AP is divided into three levels: Mild, moderately severe, and severe, based on organ failure as well as local and systemic complications[3]. However, the pathophysiologic mechanism of AP has not been fully elucidated and there are several controversies regarding the diagnostic and therapeutic modalities due to their effectiveness and complications in the treatment of the disease. These controversies primarily relate to the therapeutic treatment at the early stage of the disease, which includes fluid resuscitation, including the most appropriate type of fluid to use, as well as the time, volume, and rate of administration. Other controversies include the timing of restart and the importance of nutritional support, the role of prophylactic antibiotics, the timing of application of more aggressive methods including surgery, as well as the treatment of complications which can negatively impact the patient's prognosis and quality of life[4-6].
This editorial article presents the most important diagnostic and therapeutic modalities with respect to their efficacy, complications and treatment failure in the treatment of AP.
There are multiple causes and pathological conditions potentially associated with AP (Table 1). It is generally accepted that gallstones and alcohol abuse are responsible for about 90% of all cases of AP. The role of gallstones is very important in the etiopathogenesis of AP and any finding indicating the presence of gallstones in the gallbladder or biliary tract in patients with AP can be classified as the cause of the disease. Therefore, all patients with AP should be screened by ultrasound for the presence of cholecystolithiasis, common bile duct stones, or diagnose signs of biliary obstruction[2,7,8]. Alcohol-induced pancreatitis is more common in young and middle-aged people in whom an idiosyncratic sensitivity to alcohol may exist at levels of alcohol exceeding 80 g/dL. Other predisposing factors may be the level of alcohol dehydrogenase activity in the gastric mucosa and the liver[2,7,8].
| Causes of acute pancreatitis | |
| Toxic and metabolic | |
| Alcohol | |
| Hyperlipidemia (triglycerides > 600 mg) | |
| Hypercalcemia (hyperparathyroidism) | |
| Diabetes mellitus | |
| Hypothyroidism | |
| Uremia | |
| Drugs (medicaments) | |
| Scorpion venom | |
| Mechanical | |
| Gallstones, biliary sludge | |
| Ampullary obstruction (Crohn’s disease, villous tumors of the ampulla) | |
| Pancreatic obstruction (pancreatic tumor, chronic pancreatitis) | |
| Sphincter of Oddi dysfunction | |
| Pancreas divisium | |
| Post ERCP-pancreatitis | |
| Congenital malformation | |
| Trauma | |
| Others | |
| Ischemia | |
| Organ transplantation (bone marrow transplantation) | |
| Iatrogenic injury | |
| Infection | |
| Hereditary | |
| Autoimmune | |
| Cystic fibrosis | |
| Tropical (Ascaris lumbricoides) | |
| Idiopathic pancreatitis | |
Hypertriglyceridemia (triglycerides > 600 mg) is well-known cause of AP. One of the predominant causes of serum triglycerides elevation is alcohol intake. Therefore, it is sometimes challenging to assess whether the cause of AP is alcohol consumption or hypertriglyceridemia[9-11].
A large variety of drugs have been related to AP. Although, some drugs such as diuretics, azathioprine, sulfonamides, drugs used in the treatment of acquired immunodeficiency syndrome such as didanosine and zalcitabine, and steroids can cause AP through a direct toxic effect, most cases of drug-related pancreatitis are probably triggered by individual sensitivity. In large epidemiological studies, it has been proven that potentially pancreatotoxic drugs are not independent risk factors for the development of AP. The interval from the beginning of drug intake to the development of AP is highly variable and ranges from a few weeks, in a drug-induced immunologic reaction, to several months, when accumulation of toxic metabolites (e.g. valporic acid, pentamidine, didanosine) is required. The mechanism of AP for many of these medications is obscure[12-16].
AP is predominantly acute inflammatory process which involves the parenchyma of the pancreas, with involvement of other regional tissues or distant organ systems in severe forms of the disease. The pathogenic mechanism of AP is presented by inappropriate activation of trypsinogen and destruction of secretory cells followed by systemic release of cytokines and inflammatory mediators, causing the activation of inflammatory cells, fever and MOF. Calcium overload, mitochondrial dysfunction, impaired autophagy, endoplasmic reticulum stress, and exosomes are other factors in pathogenesis of the disease[16]. Edema of pancreatic and peripancreatic tissues and fat necrosis are common in all forms of AP (mild, moderately severe and severe) however, in the severe AP (SAP), there is a possibility of hemorrhage within the pancreas[4,7,17,18].
Cellular necrosis elements (acinar cells, duct cell, and islet cells) are considerable in SAP but necrosis is usually absent in mild and moderate forms of the disease. During SAP, pancreatic necrosis (PN) develops due to impairment in pancreatic microcirculation and its complete development takes usually several (approximately 4-7) day after the beginning of the disease but, the development of PN is not strictly fixed in time and may progress during the first 2 wk[4,16-18]. During that period, PN is usual sterile and its infection is extremely rare. After the first 1-2 wk, the development of secondary infection in PN, due to translocation of intestinal flora, is associated with increased morbidity and mortality[4].
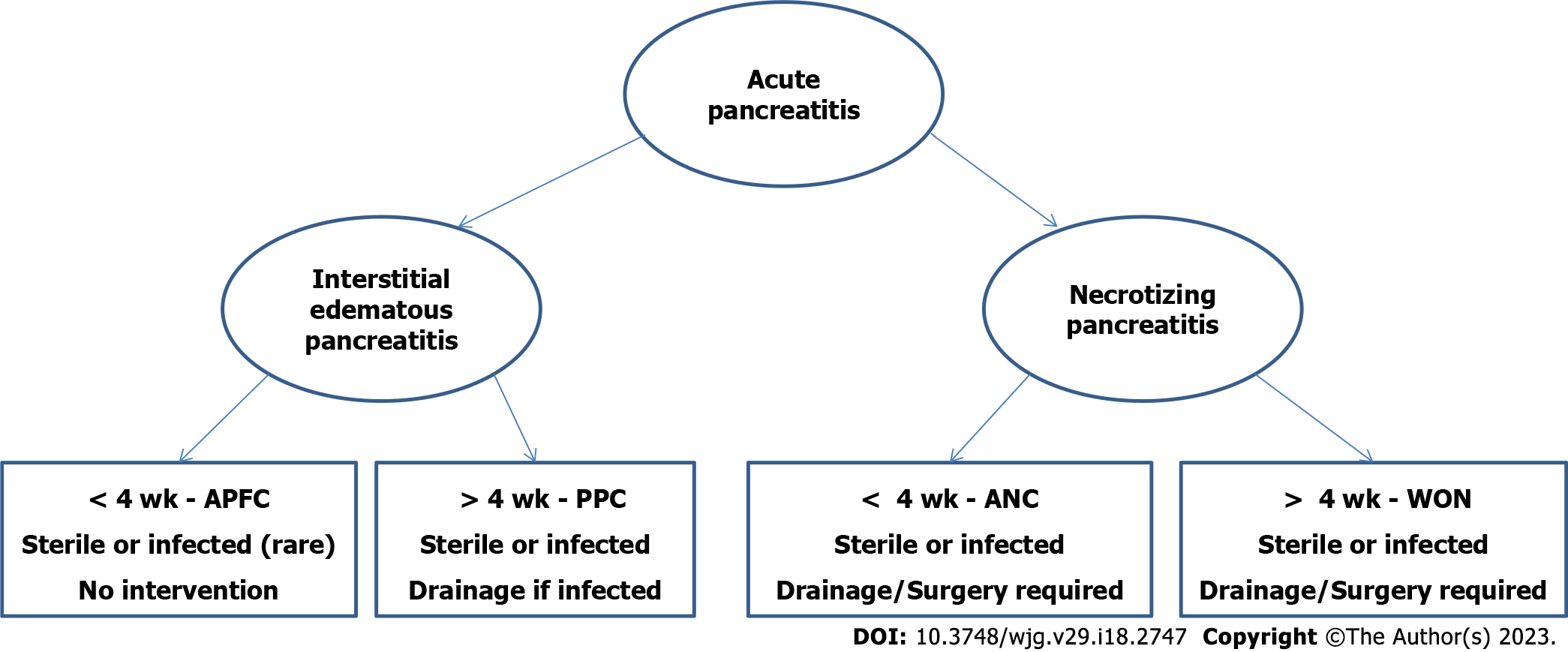
Early in the course of AP, acute pancreatic fluid collections (APFC) can occur as amylase-rich and protein-rich pancreatic juice collections and they usually resolve spontaneously. Pancreatic fluid collections, which present for more than 4 wk, are usually caused by disruption of pancreatic duct (PD) with extravasation of pancreatic juice and they are termed as pancreatic pseudocysts (PPC) or pancreatic walled-off necrosis (WON). Extra-pancreatic manifestations of SAP include systemic inflammatory response syndrome (SIRS) following by systemic MOF or exacerbation of serious pre-existing illness related to AP[4,16-18].
Diagnosis of AP is based on clinical presentation, laboratory tests, and imaging findings and requires two out of the following three criteria to be present: Clinical (acute pain attack in the upper abdomen spreading to the back), laboratory (serum lipase and/or amylase levels are three or more times higher than normal values) and typical imaging [computed tomography (CT), magnetic resonance imaging, ultrasonography] findings that are characteristic for AP[5].
The most common presenting symptoms of the disease are abdominal pain (80%–95%) followed by nausea and vomiting (40%–80%), rebound tenderness, breathlessness, impaired consciousness with pyrexia, distension and reduced bowel sounds[4,16].
The diagnosis of AP can be supported by serum and urinary laboratory tests to clarify its origin. They usually reflect organ dysfunction and metabolic disturbances. Amylase is traditionally the laboratory test of choice but, given its higher sensitivity and specificity, lipase (serum level greater than three times normal appears) is more valuable test for diagnosing of AP. The elevation of these enzymes in the serum occurs due to the leakage of pancreatic acinar cells into the interstitial space followed by their subsequent absorption into the circulation[19-21]. Some authors consider that the lipase/amylase ratio can be a crucial parameter in establishing alcohol as the etiology of AP. Transaminases are mostly used in differentiation biliary from other causes of AP[22]. The negative predictive value of urinary trypsinogen-2 level is 99%. Therefore, urinary trypsinogen-2 levels accurately diagnose AP and may be considered as useful markers to determine extra-pancreatic inflammation in AP[19,23,24]. Serum immunoreactive trypsin, chymotrypsin, elastase, phospholipase A2, alfa2-macroglobulin, methemalbumin, and carboxipeptides levels have been suggested in diagnosing AP. However, these tests are not in routine use and are not commercially available[25,26].
When the clinical presentation is typical but the laboratory parameters are ambiguous or inconclusive, imaging techniques are necessary. Contrast-enhanced CT (CECT) is the most performed imaging test and the modality of choice for the diagnosis of PN, the determination of its extent, and the diagnosis of local complications[4,27-29]. However, the complete development of PN takes usually several (approximately 4-7) days from the beginning of the disease and CECT cannot be applied to reliably assess the presence and extent of necrosis before that time[1,4].
Magnetic resonance imaging is a good alternative to CECT due to its superiority soft tissue contrast resolution and better evaluation of the biliary tree and PD. Also, this method can be used as a substitute for endoscopic retrograde cholangiopancreatography (ERCP) in the diagnostic evaluation of the PD[4,30-32].
Ultrasound, endoscopic ultrasound and ERCP are adjuncts to CECT and they are used for the diagnosis of cholelithiasis, disconnected PD, evaluation of the collection contents, and follow-up imaging. Imaging examinations are usually not necessary in an emergency when the clinical presentation and laboratory tests are consistent with features of AP[1,4,28,33,34].
AP varies from clinically mild to fulminating disease and has been recorded as a cause of sudden death. The outcome of acute attack depends in part on the etiology and in part on the severity of the attack. It is generally accepted that death from AP has a bimodal temporal distribution: Early mortality is the consequence of SIRS followed by MOF, while late mortality is caused by superinfection of PNs and peripancreatic fluid collections resulting in sepsis[4,7,34,35].
Advances made in establishing diagnostic criteria for the severity and prognosis of an attack have markedly influenced therapeutic approach. Several multifactorial scoring systems (Ranson, APACHE II, Glasgow-Imrie, SOFA, Balthazar, BISOP, etc.) have been extensively applied with the goal of predicting which patients might have a severe clinical course and which of them will recover without major complications[7,20,21,36-41].
A commonly accepted definition of SAP was not established until 1993, when the first version of the Atlanta classification for AP was published. The classification emphasizes the difference between milder interstitial form of AP from SAP related to 'local complications', but do not contain a clear definition of pancreatic and peripancreatic collections that was standardized worldwide[42]. The classification was revised in 2012[1,3], defining early and late AP which can be either edematous interstitial or necrotizing one. According to the severity of the disease, AP is divided into mild, moderately severe, and severe form, based on organ failure as well as local and systemic complications. AP collections are differentiated into APFC, PPC, acute necrotic collection, and pancreatic WON, according to the type of AP[1,3,7] (Figure 1).
However, the value of early prognostic evaluation of AP remains uncertain due to the fact that patients who have the same initial predicting scores often have very different subsequent clinical courses of the disease. Also, the relevance of the prognostic evaluation is markedly affected by the lack of widely accepted definition of severity and outcome of the disease[1,4,7].
AP is a dynamic disease process in which most attacks are mild (mild and moderately severe forms), AP is a dynamic disease process in which most attacks are mild (mild and moderately severe forms), with ongoing recovery after a few days of conservative supportive therapy. However, some patients with SAP can develop local and/or systemic complications and MOF. There are two treatment periods in AP: Early treatment of the acute attack should be applied in both mild and severe forms of AP, while late management includes treatment of SAP complications. The most important measures of intensive monitoring and supportive therapeutic procedures in AP are presented in Table 2.
| Cornerstone treatment measures | ||
| Intensive monitoring and support of cardiac, pulmonary, renal, and hepatobiliary functions | ||
| Fluid resuscitation with monitoring of vital constants and urine output | ||
| Electrolyte solutions and Plasma expanders | ||
| Humidified oxygen administration | ||
| Catecholamine (dopamine, dobutamine) to prevent renal failure | ||
| Appropriate nutritional support | ||
| Early treatment of systemic complications | ||
| Mechanical ventilation with positive end-expiratory pressure | ||
| Catecholamine (epinephrine) if shock develops | ||
| Hemofiltration, dialysis | ||
| Insulin and calcium substitution to treat metabolic complications | ||
| Prevention of infectious complications | ||
| Biliary tract management | ||
| Management of necrotizing pancreatitis | ||
| Conservative, Imaging, Endoscopic, Surgical management | ||
| Management of late complications | ||
| Pancreatic pseudocysts | ||
| Walled-off pancreatic necrosis | ||
| Disconnected pancreatic duct syndrome/pancreatic fistula | ||
| Acute non-infectious complications in acute pancreatitis | ||
| Intra-abdominal hypertension | ||
| Pseudoaneurysm | ||
| Venous thrombosis | ||
| Bowel fistula | ||
| Management of special types of acute pancreatitis | ||
| Pediatric | ||
| Hyperparathyroidism | ||
| Hypertriglyceridemia | ||
| Post-ERCP pancreatitis | ||
| Trauma | ||
| Pregnancy | ||
| Long term complications and long-term care | ||
In early stage of AP, all patients require appropriate conservative treatment and sufficient nutritional support. Most patients with mild or moderately SAP will recover with conservative treatment that includes correction of hypovolemia and hypoxemia, as well as pain relief. For a long time, correction of hypovolemia, even with mild AP, was carried out by early aggressive hydration with monitoring vital constants and urine output[3,5,6,42-44]. However, there is conflicting evidence regarding the fluid management strategy both in terms of fluid type, optimal volume and rate of administration, as well as severity of AP. Several recent randomized trials showed that early aggressive fluid resuscitation, in patients with AP, resulted in a higher incidence of fluid overload (with potentially increasing risk for acute kidney injury and pulmonary edema) without improvement in clinical outcomes[45-48]. Other, also randomized controlled trials, reported that early aggressive intravenous hydration hastens clinical improvement in patients with AP and that aggressive fluid strategy is beneficial especially for certain subsets of patients and some types of AP[48-51]. Most authors agree that these discrepancies are not fully elucidated and that future studies are needed to investigate which fluid management strategy is optimal for majority of the patients and which subsets of patients with AP may benefit from different management of fluid replacement[6,47].
Pain control is a very important therapeutic measure in the early stage of AP and can be provided by appropriate intravenous administration of a non-opiate analgesic. Opiates (e.g., meperidine) may also be given as required[52-54]. Some recent systematic reviews and meta-analysis suggest that epidural anesthesia is safe and effective in reducing pain severity, improving pancreatic perfusion, and decreasing mortality, within the first 24 h of AP onset. However, there is paucity of evidence to guide pain management in AP with small datasets per study[55,56] Hypoxemia is a rare event in mild and moderately SAP but, in severe forms of the disease, respiratory insufficiency with hypoxemia is often present as single organ failure. Hypoxemia could be avoided by ensuring airway patency and supplemental application of humidified oxygen, which would allow maintenance of arterial oxygen saturation above 95%. In case of development of respiratory insufficiency, mechanical ventilation with positive expiratory pressure is mandatory[4-6,43,44].
Conversely, patients with SAP are at high risk of developing PN, MOF, and septic complications. The most important therapeutic goals in the initial treatment of SAP are the provision of supportive therapy and the treatment of specific complications that may occur at the beginning of the disease[4].
The nutritional management strategy for patients with AP has generated intense debate over the past few decades. Oral nutrition should be restarted immediately in patients with preserved gastrointestinal peristalsis, without abdominal pain, nausea, vomiting, or evidence for intestinal obstruction or ileus[5,18]. Most patients with SAP have increased basal energy needs, pronounced protein catabolism and endogenous gluconeogenesis.
The purpose of nutritional support is the reduction of wasting, to support the structure and function of organs, and to have a positive influence on the clinical course of the disease. Also, if patients with SAP develop paralytic ileus as a complication of the disease, keeping the pancreas at rest is necessary. Since they require nutritional support to achieve a positive nitrogen balance, parenteral nutrition should be started as soon as possible with the aim to achieve a positive nitrogen balance within the first 72 h after the onset of the disease[4].
However, SAP represents a typical model of septic syndrome due to the gastrointestinal barrier failure, which reduces gastrointestinal motility and damages mucosal integrity with subsequent increases in its permeability. This leads to an increased risk of bacterial overgrowth and their translocation from the intestinal tract to peripancreatic necrosis. Therefore, one of the main therapeutic goals in SAP is to maintain intestinal integrity to prevent bacterial and endotoxin translocation and improve the immune system of the gastrointestinal tract. Administration of enteral feeding, with or without immunonutrition can maintain mucosal integrity and prevent or decrease bacterial translocation. Therefore, early nutritional support should be prioritized as soon as possible (optimally, within the first 24–72 h)[4,18,57-59].
The presence of infected PN is the most important negative indicator and is the main cause responsible for morbidity and mortality in SAP. The infection organisms that are responsible for PN infection are mostly Gram-negative bacteria of intestinal origin, and they can reach PN through a previously damaged intestinal mucosal barrier[4,18].
Broad-spectrum antibiotics with ability to pass into PN (e.g., carbapenems, quinolones, and metronidazole), should be prescribed only when infected necrosis is confirmed or strongly suspected[18]. However, the administration of antibiotic prophylaxis in order to prevent infection of sterile necrosis is controversial. Some authors advocate the use of prophylactic antibiotics in SAP considering that they can prevent the development of superinfection in necrotic tissues, which is the only measure of initial treatment of PNs, since their development are not preventable[4,18,60-65]. Some authors consider that there is a reduction in pancreatic infection in the subgroup of patients who received broad-spectrum antibiotics, concluding that more evidence is needed[60,61].
However, multiple prospective, randomized, placebo-controlled trials and most important guidelines have demonstrated that the routine use of prophylactic broad-spectrum antibiotics, in patients with SAP, has no influence on the development of infected necrosis, systemic complications, need for surgery, or mortality[18,62-65]. Besides, prolonged antibiotic therapy increases the prevalence of fungal infections[4,18,61]. Evidence supporting the prophylactic use of antifungal agents in patients with PN is lacking. Also, the use of probiotic prophylaxis is not suggested for the prevention of infectious complications in AP[18,62,63].
The development of extensive PN is a main cause of complications and mortality in patients with SAP. Therefore, it is very important to apply the best methods to identify patients with PN who require more aggressive interventions than those who could be treated with less aggressive measures. Different clinical entities, such as persistent pancreatic fluid collections, pancreatic fistula, persistent SIRS, obstructive jaundice, and ongoing symptoms can be detected during SAP and they can predict the severity of clinical course of the disease. Their management vary depending on the severity and the type of complication, since different complications require different treatment modalities. The revised Atlanta classification offers useful recommendations to determine the strategy for management of the SAP complications[1,3-5,18,66].
The step-up approach is used, in the treatment of infected PN, as a less invasive alternative approach compared to early surgical necrosectomy. This approach is based on the statement that surgical debridement should be delayed until demarcation of necrotic from normal tissue is established, which would reduce the risk of bleeding into the necrotic tissue during or after the surgical intervention. Several studies that have conducted long-term follow-up of clinical outcomes in patients with AP have shown that the step-up approach leads to a reduction in morbidity and mortality and should be preferred over the classic surgical approach if both methods are technically feasible[4,8,18,67-71].
However, there are still certain disagreements regarding the comparison of the endoscopic and surgical step-up approach. In a randomized trial, Bang et al[72], compared outcomes of the surgical vs endoscopic step-up approach and they reported that the endoscopic was superior to the surgical step-up approach in reducing major complications, lowered costs, and increased quality of life in patients with infected PN[72]. Dutch Pancreatitis Study Group, in the TENSION trial, reported that the endoscopic approach may be more suitable than the surgical step-up approach, in the treatment of infected PN, based on favorable short-term outcomes. However, they presented that the endoscopic was not superior to the surgical step-up approach in reducing death or major complications in patients with infected PN, while the rate of pancreatic fistulas and length of hospital stay were lower in the endoscopy group[73-75]. Most experts in the discussion of this issue point out that the endoscopic approach is the best management for patients with infected PN, but that it is necessary to develop common protocols for endoscopic approach, based on all the observations and suggestions[76-79]. Finally, it can be concluded that, some segments of the step-up approach can be changed and improved, but its basic concept (delay, drain, and debride) remains as the reference standard intervention for PN[71].
If necrotizing pancreatitis is associated with liquefied necrotic debris in the pancreatic and peripancreatic regions, with the presence of abdominal and/or pelvic fluid several authors[80-85] have advocated the concept of removing the peritoneal fluid. The rationale for removing the peritoneal fluid is to reduce inflammation and disease severity since the intra-abdominal fluid, accumulated during the disease, may contain factors that trigger and increase the severity of AP, including proinflammatory mediators and infection.
In general, abdominal paracentesis drainage (APD) has the role of a preparatory procedure before the application of PCD with the intent to achieve better result than through conventional step-up approach. Therefore, the integration of APD into a step-up approach is beneficial for patients, due to the fact that this procedure removes great number of inflammatory factors from the seroperitoneum[82].
Early in the course of AP, pancreatic inflammation, manifesting as partial or total PN, causes more liquefied areas and leads to extravasations of enzyme-rich pancreatic juice into the peripancreatic regions, with the consequent development of sterile necrosis and ANC. They usually resolve spontaneously and the vast majority of patients with sterile PN can be treated conservatively and without interventional procedures (i.e. catheter drainage or necrosectomy). However, if unresolved spontaneously, they can lead to a poor clinical course and allow active pancreatic enzymes to initiate physiologic pathways leading to MOF and sepsis[4,17].
Therefore, PCD or endoscopic drainage may be required in symptomatic patients with sterile PN and persistent malaise characterized by abdominal pain, nausea, vomiting, and nutritional failure or with associated complications, including gastrointestinal and/or biliary obstruction, persistent SIRS, or fistulas[5]. Continuous PCD may justify this approach based on the concept that elimination of cytokines and inflammatory mediators from initially sterile pancreatic juice collections may avoid or prevent systemic complications in severe forms of AP[4,17,86-90]. Besides, in cases whereby a high output of amylase-rich fluid continues to drain through the catheter, continued prolonged drainage is necessary despite the sterility of the collection[91].
The development of secondary infection in PN is associated with increased morbidity and mortality (15%–20%) and there is consensus that infected necrotic tissue should be removed in order to prevent the sepsis[4,8,33,92,93]. A small proportion of patients with documented infected PN who remain clinically stable can be managed with only conservative treatment and close monitoring, without intervention[5]. The traditional method of treating patients with infected PN by laparotomy retains its role in the treatment of the disease. However, surgical intervention is performed under general anesthesia and causes significantly greater trauma compared to minimally invasive methods, with possible consequent worsening of organ dysfunction, profuse bleeding and sepsis as well as post-operative mortality[8,94,95]. Therefore, laparotomy should be delayed for as long as possible or avoided in order to decrease mortality and morbidity rates[96]. Although there is no globally accepted treatment modality and it should be tailored to each individual patient, the step-up approach starting with monitoring and conservative measures, followed by PCD or endoscopic drainage and minimally invasive VARD has been shown to produce superior outcomes to the traditional open necrosectomy in the treatment of infected PN[4,8,18,66-72,97-99].
Disconnected PD syndrome (DPDS) is anatomic condition which is usually seen in PN. Early in the course of necrotizing pancreatitis, pancreatic inflammation causes extravasation of pancreatic secretion into the pancreatic and perpancreatic tissues with the consequent development of sterile PN which may lead to the PD disruption and interruption of continuity between the duct in the left sided pancreas and the luminal gastrointestinal tract. In such clinical setting, when the PD has no continuity with the viable tissue of the pancreas, this part no longer drains its contents into the duodenum, but produces a persistent pancreatic fistula, there is a high probability of consequent peripancreatic collection formation[1,18,100-102].
There are three types of DPDS: Concurrent (there is necrosis of the neck and body, but there is perfusion of the tail of the pancreas), delayed (PPC or WON occupy the middle part of the gland but with the perfused left-sided remnant of the pancreas) and DPDS associated with chronic pancreatitis (PD is blocked by a stricture or calculus in the proximal part, leading to atrophy of the distal segment of the duct and resulting in the formation of PPC)[1,102].
Standard treatment for DPDS is operative resection of the disconnected pancreas[103,104]. PN causing DPDS could be initially treated with percutaneous[105], endoscopic, or minimally invasive surgical techniques as temporary measures. However, elective distal pancreatectomy is mandatory in most patients as definitive treatment for DPDS[106-108].
Pancreatic fistulas are the consequence of pancreatic autodigestion or necrosis that results in a persistent PD disruption. Disruption of the PD secondary to PN causes the lack of continuity between PD and the viable pancreatic tissue, so that this segment of the pancreas no longer drains into the duodenum, but into the surrounding regions (usually in the pancreatic tail area) leading to its accumulation and PPC formation[1,4,18,100]. However, pancreatic secretions can also reach distant sites, causing pancreatic ascites, pleural effusion, distant PPC, or pancreatocutaneous fistula. According to that, pancreatic fistulas can be divided into two groups: (1) Internal, in which the PD communicates with the peritoneal or pleural cavity or some other hollow viscus; and (2) external, where the PD communicates with the skin[1,4,100,105,109].
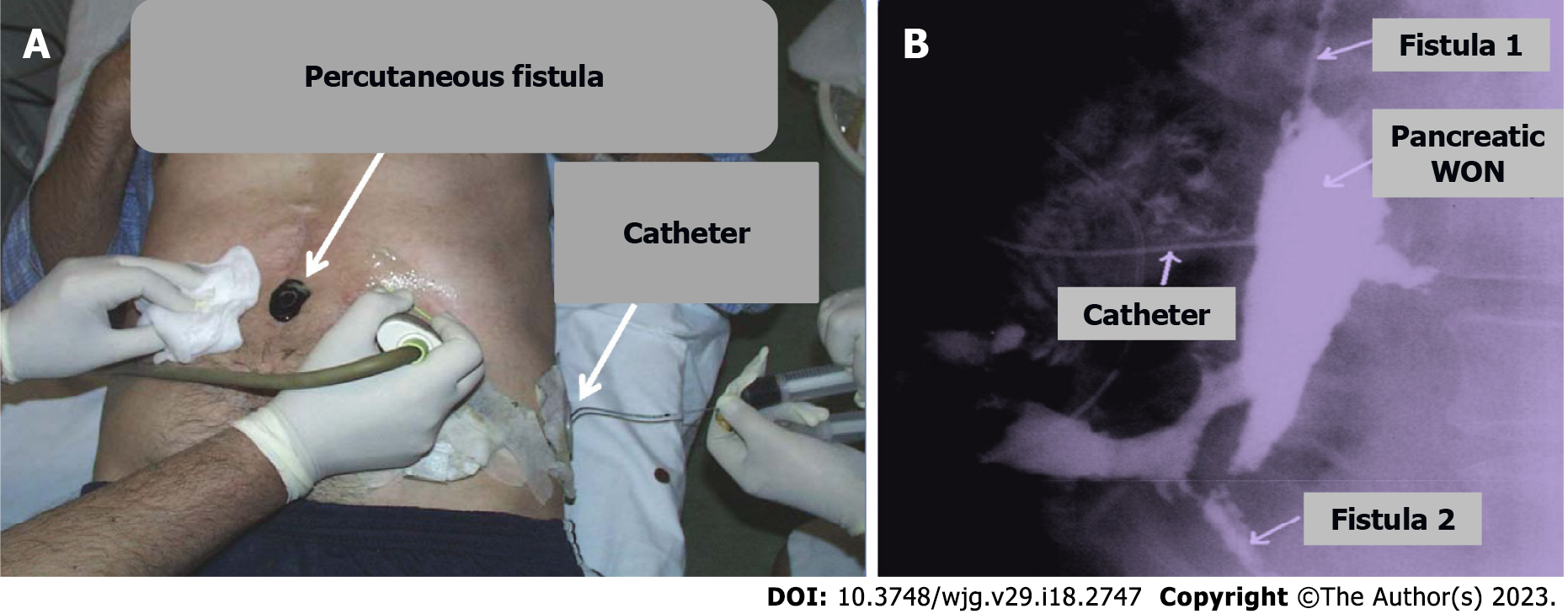
Treatment of pancreatic fistulas depends on both the site of duct disruption and presence or absence of downstream ductal obstruction or DPDS. In the beginning of the disease, the management is usually conservative including total parenteral nutrition and the administration of pancreatic secretory inhibitor octreotide. However, if this management fails interventional procedures (Figure 2) and surgery are the following options. Surgical intervention, for fistula treatment, is technically challenging and could be followed with major complications[100-102,106-112].
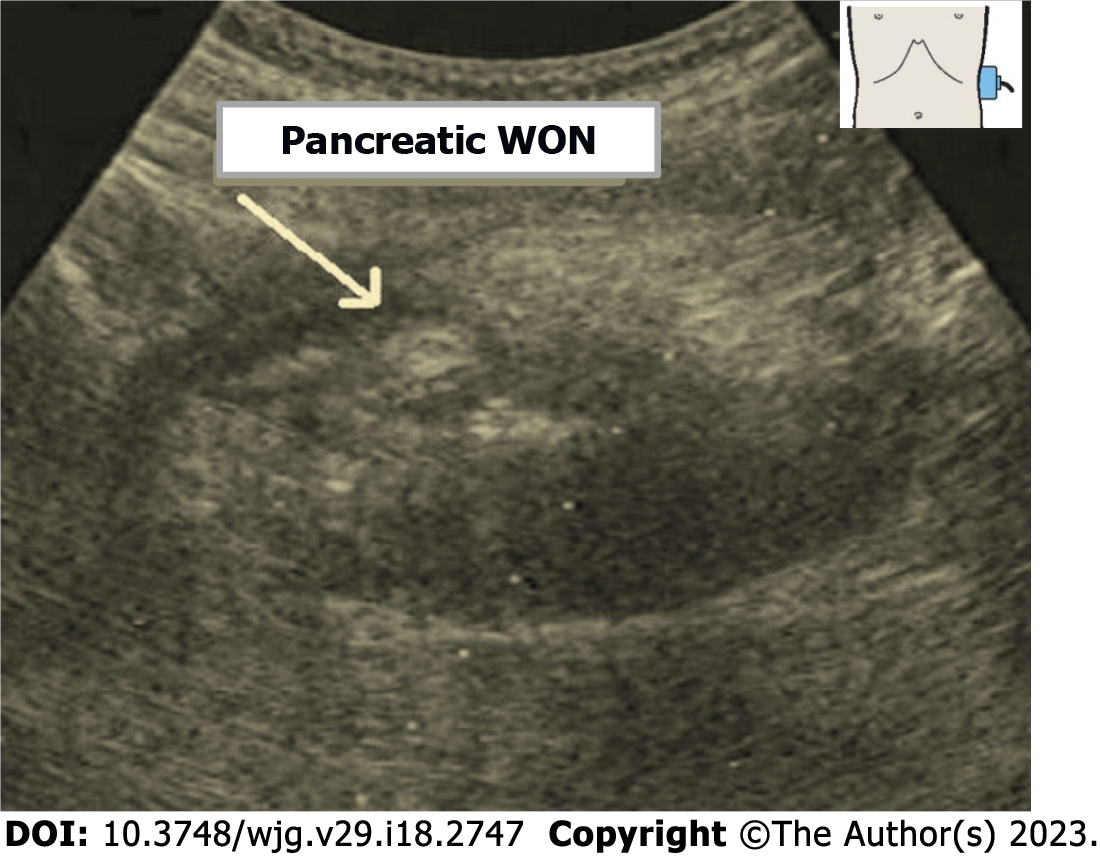
Pancreatic WON is a located WON of pus resulting from liquefaction of necrotic areas or secondary infection of acute PPC, with or without communication with main PD. WON occurs at a relatively late stage, most commonly three to five weeks after of onset of AP. APFCs tend to be poorly walled-off and can leak into retroperitoneum, the peritoneal cavity, the mediastinum, the pleura or the soft tissues. Pancreatic WON is a heterogeneous, low-density collection in a defined cavity containing gas bubbles (Figure 3). CECT scanning is the diagnostic test of choice. Diagnosis may be confirmed by percutaneous or endoscopic aspiration[113]. Asymptomatic WON does not mandate intervention and may resolve spontaneously over a period of time[29]. Symptomatic WON generally requires intervention. The most common treatment modality is PCD (Figure 2) or endoscopic drainage. Surgical drainage is done rarely, only when percutaneous drainage is not successful[114-116].
A PPC is a fluid collection usually found near the pancreas that is formed by the secretion of pancreatic juice from the inflamed parenchyma or from a disrupted duct. The PPC wall consists of fibrous non-epithelialized tissue[42]. PPC can sometimes appear at a great distance from the pancreas (e.g. thorax, groin) when the fluid dissects through tissue planes. At early stage of AP, APFC are common, but majority of them regress spontaneously and need no treatment. About 5% of patients with APFC develop PPCs, which are defined by their ellipsoidal shape and well-formed wall. Treatment for PPCs varies depending on their size and the presence of symptoms. Asymptomatic PPCs, less than 50 mm in diameter, should only be monitored by ultrasound. However, some of them may persist and progress to produce complications such as pain, infection, gastric outlet, intestinal or biliary obstruction. These PPCs are symptomatic and require treatment. The most common treatment modalities for symptomatic PPCs are minimally invasive approaches, such as PCD or endoscopic drainage. Surgical drainage is done rarely, only when percutaneous drainage is not successful[4,97,105,110,115-119].
Upper gastrointestinal bleeding is common in AP and usually results from stress ulcers, peptic ulcer disease, or hemorrhagic gastroduodenitis. Massive hemorrhage occurs rare in AP, most commonly into the gastrointestinal tract, the abdominal cavity or into the PD. Erosion of the great pancreatic or peripancreatic vessel leads to rupture or formation of a pseudoaneurysm. The splenic artery is most often affected vessel, followed by the pancreaticoduodenal and gastroduodenal arteries[42,63]. Pseudoaneurysm formation should be suspected if there are repeated episodes of gastrointestinal bleeding, an increasing pulsatile abdominal mass and in patients with bloating and increasing abdominal pain. Unfortunately, aneurysm rupture in these arteries mostly results in severe and life-threatening bleeding. The diagnosis can be made by angiography or angio-CT. Sometimes, arterio
The prevention of relapses of AP starts with the current episode treatment. Potential etiologic causes should be determined and adequately treated in order to prevent future relapses of the disease. This implies an adequate and prompt diagnostic and therapeutic approach to patients with biliary stones and sludge, as well as to patients with hyperlipidemia and hypercalcemia[1,5,7,16].
AP caused by hyperlipidemia tend to develop more severe forms of the disease and up to 50% of them develop SAP. Therefore, appropriate diet and drug management of the lipoprotein metabolic disorders as well as alcohol abstinence are crucial in preventing relapses of pancreatitis[9-11]. Morphologic abnormalities and tumors also have to be excluded.
After discharge from the hospital, patients with AP should follow a diet without alcohol and high fat food consumption, eating frequent small meals four to six times per day. A few months after the acute phase of AP, patients can try to introduce a diet with a slightly increased fat content and grilled meat. Patients should be warned that the next attack of AP could be more dangerous than the previous one, so lifestyle changes are necessary to reduce the risk of disease relapse[16,121,122].
AP is an inflammatory pancreatic disease that is characterized by inappropriate activation of trypsinogen and destruction of secretory cells which leads to activation of inflammatory cells, fever, and MOF. Diagnosis of AP is based on clinical, laboratory and imaging parameters, which are included into prognostic scoring system, developed with the aim of predicting the severity of the disease. Advances made in establishing diagnostic criteria for the severity and prognosis of AP have markedly influenced therapeutic approach and reduced the mortality rate of the disease.
There are two treatment periods in AP: Initial management remains supportive, consisting of conservative treatment which should be applied in both mild and severe forms of AP, while late management incorporates the treatment of the SAP complications. Currently, the treatment of SAP complications has shifted from early surgical approach to minimally invasive step-up strategy as the reference standard intervention. However, AP is a complex disease and despite the existence of numerous criteria, it is difficult to predict its clinical course. Additional researches, preferably randomized trials or prospective collaborative studies, are required to increase understanding of the pathophysiology of the disease and enable adequate responses to the diagnostic and therapeutic challenges, in order to improve the management of particularly severe forms of AP.
| 1. | Sureka B, Bansal K, Patidar Y, Arora A. Imaging lexicon for acute pancreatitis: 2012 Atlanta Classification revisited. Gastroenterol Rep (Oxf). 2016;4:16-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (3)] |
| 2. | Párniczky A, Kui B, Szentesi A, Balázs A, Szűcs Á, Mosztbacher D, Czimmer J, Sarlós P, Bajor J, Gódi S, Vincze Á, Illés A, Szabó I, Pár G, Takács T, Czakó L, Szepes Z, Rakonczay Z, Izbéki F, Gervain J, Halász A, Novák J, Crai S, Hritz I, Góg C, Sümegi J, Golovics P, Varga M, Bod B, Hamvas J, Varga-Müller M, Papp Z, Sahin-Tóth M, Hegyi P; Hungarian Pancreatic Study Group. Prospective, Multicentre, Nationwide Clinical Data from 600 Cases of Acute Pancreatitis. PLoS One. 2016;11:e0165309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4709] [Article Influence: 362.2] [Reference Citation Analysis (48)] |
| 4. | Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20:13879-13892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 238] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (5)] |
| 5. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1095] [Article Influence: 84.2] [Reference Citation Analysis (10)] |
| 6. | Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial Medical Treatment of Acute Pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology. 2018;154:1103-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 7. | Walkowska J, Zielinska N, Karauda P, Tubbs RS, Kurtys K, Olewnik Ł. The Pancreas and Known Factors of Acute Pancreatitis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (1)] |
| 8. | Zerem E, Imamović G, Sušić A, Haračić B. Step-up approach to infected necrotising pancreatitis: a 20-year experience of percutaneous drainage in a single centre. Dig Liver Dis. 2011;43:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Cruciat G, Nemeti G, Goidescu I, Anitan S, Florian A. Hypertriglyceridemia triggered acute pancreatitis in pregnancy - diagnostic approach, management and follow-up care. Lipids Health Dis. 2020;19:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | Wang L, Xu T, Wang R, Wang X, Wu D. Hypertriglyceridemia Acute Pancreatitis: Animal Experiment Research. Dig Dis Sci. 2022;67:761-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 11. | Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 12. | Chadalavada P, Simons-Linares CR, Chahal P. Drug-induced acute pancreatitis: Prevalence, Causative agents, and Outcomes. Pancreatology. 2020;20:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Abu-El-Haija M, Hornung L, Lin TK, Nathan JD, Thompson T, Vitale DS, Nasr A, Husain SZ, Denson L. Drug induced pancreatitis is the leading known cause of first attack acute pancreatitis in children. Pancreatology. 2020;20:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Gagnon AL, Lavoie A, Frigon MP, Michaud-Herbst A, Tremblay K. A Drug-Induced Acute Pancreatitis Retrospective Study. Can J Gastroenterol Hepatol. 2020;2020:1516493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chocair PR, Neves PDMM, Mohrbacher S, Neto MP, Sato VAH, Oliveira ÉS, Barbosa LV, Bales AM, da Silva FP, Cuvello-Neto AL, Duley JA. Case Report: Azathioprine: An Old and Wronged Immunosuppressant. Front Immunol. 2022;13:903012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Szatmary P, Grammatikopoulos T, Cai W, Huang W, Mukherjee R, Halloran C, Beyer G, Sutton R. Acute Pancreatitis: Diagnosis and Treatment. Drugs. 2022;82:1251-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 336] [Reference Citation Analysis (1)] |
| 17. | Zerem E, Imamovic G, Omerović S, Imširović B. Randomized controlled trial on sterile fluid collections management in acute pancreatitis: should they be removed? Surg Endosc. 2009;23:2770-2777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 466] [Article Influence: 77.7] [Reference Citation Analysis (3)] |
| 19. | Rompianesi G, Hann A, Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis. Cochrane Database Syst Rev. 2017;4:CD012010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 20. | Zerem D, Zerem O, Zerem E. Role of Clinical, Biochemical, and Imaging Parameters in predicting the Severity of Acute Pancreatitis. Euroasian J Hepatogastroenterol. 2017;7:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Zerem E, Imamović G, Latić F, Mavija Z. Prognostic value of acute fluid collections diagnosed by ultrasound in the early assessment of severity of acute pancreatitis. J Clin Ultrasound. 2013;41:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Bougard M, Barbier L, Godart B, Le Bayon-Bréard AG, Marques F, Salamé E. Management of biliary acute pancreatitis. J Visc Surg. 2019;156:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Yasuda H, Kataoka K, Takeyama Y, Takeda K, Ito T, Mayumi T, Isaji S, Mine T, Kitagawa M, Kiriyama S, Sakagami J, Masamune A, Inui K, Hirano K, Akashi R, Yokoe M, Sogame Y, Okazaki K, Morioka C, Kihara Y, Kawa S, Tanaka M, Andoh A, Kimura W, Nishimori I, Furuse J, Yokota I, Shimosegawa T. Usefulness of urinary trypsinogen-2 and trypsinogen activation peptide in acute pancreatitis: A multicenter study in Japan. World J Gastroenterol. 2019;25:107-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 24. | Mayumi T, Inui K, Maetani I, Yokoe M, Sakamoto T, Yoshida M, Ko S, Hirata K, Takada T; Urinary Trypsinogen-2 Dipstick for Acute Pancreatitis Study Group of Japanese Society of Abdominal Emergency Medicine (UtrAP Study Group). Validity of the urinary trypsinogen-2 test in the diagnosis of acute pancreatitis. Pancreas. 2012;41:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Staubli SM, Oertli D, Nebiker CA. Laboratory markers predicting severity of acute pancreatitis. Crit Rev Clin Lab Sci. 2015;52:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 26. | Staubli SM, Schäfer J, Rosenthal R, Zeindler J, Oertli D, Nebiker CA. The role of CRP and Pentraxin 3 in the prediction of systemic inflammatory response syndrome and death in acute pancreatitis. Sci Rep. 2019;9:18340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Bastati N, Kristic A, Poetter-Lang S, Messner A, Herold A, Hodge JC, Schindl M, Ba-Ssalamah A. Imaging of inflammatory disease of the pancreas. Br J Radiol. 2021;94:20201214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Brizi MG, Perillo F, Cannone F, Tuzza L, Manfredi R. The role of imaging in acute pancreatitis. Radiol Med. 2021;126:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Freeman ML, Werner J, van Santvoort HC, Baron TH, Besselink MG, Windsor JA, Horvath KD, vanSonnenberg E, Bollen TL, Vege SS; International Multidisciplinary Panel of Speakers and Moderators. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 30. | Ball CG, Correa-Gallego C, Howard TJ, Zyromski NJ, House MG, Pitt HA, Nakeeb A, Schmidt CM, Akisik F, Lillemoe KD. Radiation dose from computed tomography in patients with necrotizing pancreatitis: how much is too much? J Gastrointest Surg. 2010;14:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J, Matos C. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 198] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 32. | Pelaez-Luna M, Vege SS, Petersen BT, Chari ST, Clain JE, Levy MJ, Pearson RK, Topazian MD, Farnell MB, Kendrick ML, Baron TH. Disconnected pancreatic duct syndrome in severe acute pancreatitis: clinical and imaging characteristics and outcomes in a cohort of 31 cases. Gastrointest Endosc. 2008;68:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 33. | Maher MM, Lucey BC, Gervais DA, Mueller PR. Acute pancreatitis: the role of imaging and interventional radiology. Cardiovasc Intervent Radiol. 2004;27:208-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Vege SS, Fletcher JG, Talukdar R, Sarr MG. Peripancreatic collections in acute pancreatitis: correlation between computerized tomography and operative findings. World J Gastroenterol. 2010;16:4291-4296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Balthazar EJ. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am. 1989;27:19-37. [PubMed] |
| 36. | Ranson JH. The timing of biliary surgery in acute pancreatitis. Ann Surg. 1979;189:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 212] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | McKay CJ, Imrie CW. Staging of acute pancreatitis. Is it important? Surg Clin North Am. 1999;79:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Arif A, Jaleel F, Rashid K. Accuracy of BISAP score in prediction of severe acute pancreatitis. Pak J Med Sci. 2019;35:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Ma X, Li L, Jin T, Xia Q. [Harmless acute pancreatitis score on admission can accurately predict mild acute pancreatitis]. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Al-Qahtani HH, Alam MKh, Waheed M. Comparison of Harmless Acute Pancreatitis Score with Ranson's Score in Predicting the Severity of Acute Pancreatitis. J Coll Physicians Surg Pak. 2017;27:75-79. [PubMed] |
| 41. | Kui B, Pintér J, Molontay R, Nagy M, Farkas N, Gede N, Vincze Á, Bajor J, Gódi S, Czimmer J, Szabó I, Illés A, Sarlós P, Hágendorn R, Pár G, Papp M, Vitális Z, Kovács G, Fehér E, Földi I, Izbéki F, Gajdán L, Fejes R, Németh BC, Török I, Farkas H, Mickevicius A, Sallinen V, Galeev S, Ramírez-Maldonado E, Párniczky A, Erőss B, Hegyi PJ, Márta K, Váncsa S, Sutton R, Szatmary P, Latawiec D, Halloran C, de-Madaria E, Pando E, Alberti P, Gómez-Jurado MJ, Tantau A, Szentesi A, Hegyi P; Hungarian Pancreatic Study Group. EASY-APP: An artificial intelligence model and application for early and easy prediction of severity in acute pancreatitis. Clin Transl Med. 2022;12:e842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 42. | Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1929] [Cited by in RCA: 1746] [Article Influence: 52.9] [Reference Citation Analysis (1)] |
| 43. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 599] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 44. | Hirota M, Takada T, Kitamura N, Ito T, Hirata K, Yoshida M, Mayumi T, Kataoka K, Takeda K, Sekimoto M, Hirota M, Kimura Y, Wada K, Amano H, Gabata T, Arata S, Yokoe M, Kiriyama S. Fundamental and intensive care of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2010;17:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | de-Madaria E, Buxbaum JL, Maisonneuve P, García García de Paredes A, Zapater P, Guilabert L, Vaillo-Rocamora A, Rodríguez-Gandía MÁ, Donate-Ortega J, Lozada-Hernández EE, Collazo Moreno AJR, Lira-Aguilar A, Llovet LP, Mehta R, Tandel R, Navarro P, Sánchez-Pardo AM, Sánchez-Marin C, Cobreros M, Fernández-Cabrera I, Casals-Seoane F, Casas Deza D, Lauret-Braña E, Martí-Marqués E, Camacho-Montaño LM, Ubieto V, Ganuza M, Bolado F; ERICA Consortium. Aggressive or Moderate Fluid Resuscitation in Acute Pancreatitis. N Engl J Med. 2022;387:989-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (1)] |
| 46. | Cuéllar-Monterrubio JE, Monreal-Robles R, González-Moreno EI, Borjas-Almaguer OD, Herrera-Elizondo JL, García-Compean D, Maldonado-Garza HJ, González-González JA. Nonaggressive Versus Aggressive Intravenous Fluid Therapy in Acute Pancreatitis With More Than 24 Hours From Disease Onset: A Randomized Controlled Trial. Pancreas. 2020;49:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 47. | Gad MM, Simons-Linares CR. Is aggressive intravenous fluid resuscitation beneficial in acute pancreatitis? World J Gastroenterol. 2020;26:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (6)] |
| 48. | Angsubhakorn A, Tipchaichatta K, Chirapongsathorn S. Comparison of aggressive versus standard intravenous hydration for clinical improvement among patients with mild acute pancreatitis: A randomized controlled trial. Pancreatology. 2021;21:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 49. | Buxbaum JL, Quezada M, Da B, Jani N, Lane C, Mwengela D, Kelly T, Jhun P, Dhanireddy K, Laine L. Early Aggressive Hydration Hastens Clinical Improvement in Mild Acute Pancreatitis. Am J Gastroenterol. 2017;112:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 50. | Park CH, Paik WH, Park ET, Shim CS, Lee TY, Kang C, Noh MH, Yi SY, Lee JK, Hyun JJ. Aggressive intravenous hydration with lactated Ringer's solution for prevention of post-ERCP pancreatitis: a prospective randomized multicenter clinical trial. Endoscopy. 2018;50:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 51. | Wu M, Jiang S, Lu X, Zhong Y, Song Y, Fan Z, Kang X. Aggressive hydration with lactated ringer solution in prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e25598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 52. | Thompson DR. Narcotic analgesic effects on the sphincter of Oddi: a review of the data and therapeutic implications in treating pancreatitis. Am J Gastroenterol. 2001;96:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 119] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Peiró AM, Martínez J, Martínez E, de Madaria E, Llorens P, Horga JF, Pérez-Mateo M. Efficacy and tolerance of metamizole versus morphine for acute pancreatitis pain. Pancreatology. 2008;8:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Basurto Ona X, Rigau Comas D, Urrútia G. Opioids for acute pancreatitis pain. Cochrane Database Syst Rev. 2013;CD009179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Al-Leswas D, Baxter N, Lim WB, Robertson F, Ratnayake B, Samanta J, Capurso G, de-Madaria E, Drewes AM, Windsor J, Pandanaboyana S. The safety and efficacy of epidural anaesthesia in acute pancreatitis: a systematic review and meta-analysis. HPB (Oxford). 2023;25:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Thavanesan N, White S, Lee S, Ratnayake B, Oppong KW, Nayar MK, Sharp L, Drewes AM, Capurso G, De-Madaria E, Siriwardena AK, Windsor JA, Pandanaboyana S. Analgesia in the Initial Management of Acute Pancreatitis: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. World J Surg. 2022;46:878-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Oláh A, Romics L Jr. Enteral nutrition in acute pancreatitis: a review of the current evidence. World J Gastroenterol. 2014;20:16123-16131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 58. | Takeda K, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Sekimoto M, Hirota M, Kimura Y, Isaji S, Koizumi M, Otsuki M, Matsuno S; JPN. JPN Guidelines for the management of acute pancreatitis: medical management of acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Meier R, Ockenga J, Pertkiewicz M, Pap A, Milinic N, Macfie J; DGEM (German Society for Nutritional Medicine), Löser C, Keim V; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Pancreas. Clin Nutr. 2006;25:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (2)] |
| 60. | Sharma VK, Howden CW. Prophylactic antibiotic administration reduces sepsis and mortality in acute necrotizing pancreatitis: a meta-analysis. Pancreas. 2001;22:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Isenmann R, Rünzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, Malfertheiner P, Goebell H, Beger HG; German Antibiotics in Severe Acute Pancreatitis Study Group. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 62. | Kochhar R, Ahammed SK, Chakrabarti A, Ray P, Sinha SK, Dutta U, Wig JD, Singh K. Prevalence and outcome of fungal infection in patients with severe acute pancreatitis. J Gastroenterol Hepatol. 2009;24:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Cruz-Santamaría DM, Taxonera C, Giner M. Update on pathogenesis and clinical management of acute pancreatitis. World J Gastrointest Pathophysiol. 2012;3:60-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 64. | Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, Imrie CW, Johnson CD, Knaebel HP, Laterre PF, Maravi-Poma E, Kissler JJ, Sanchez-Garcia M, Utzolino S. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 65. | García-Barrasa A, Borobia FG, Pallares R, Jorba R, Poves I, Busquets J, Fabregat J. A double-blind, placebo-controlled trial of ciprofloxacin prophylaxis in patients with acute necrotizing pancreatitis. J Gastrointest Surg. 2009;13:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Isaji S, Takada T, Mayumi T, Yoshida M, Wada K, Yokoe M, Itoi T, Gabata T. Revised Japanese guidelines for the management of acute pancreatitis 2015: revised concepts and updated points. J Hepatobiliary Pancreat Sci. 2015;22:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL, van Ramshorst B, Nieuwenhuijs VB, Timmer R, Laméris JS, Kruyt PM, Manusama ER, van der Harst E, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, van Leeuwen MS, Buskens E, Gooszen HG; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1075] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 68. | da Costa DW, Boerma D, van Santvoort HC, Horvath KD, Werner J, Carter CR, Bollen TL, Gooszen HG, Besselink MG, Bakker OJ. Staged multidisciplinary step-up management for necrotizing pancreatitis. Br J Surg. 2014;101:e65-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 69. | Trikudanathan G, Tawfik P, Amateau SK, Munigala S, Arain M, Attam R, Beilman G, Flanagan S, Freeman ML, Mallery S. Early (<4 Weeks) Versus Standard (≥ 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am J Gastroenterol. 2018;113:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 70. | Aparna D, Kumar S, Kamalkumar S. Mortality and morbidity in necrotizing pancreatitis managed on principles of step-up approach: 7 years experience from a single surgical unit. World J Gastrointest Surg. 2017;9:200-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Besselink MG. The 'step-up approach' to infected necrotizing pancreatitis: delay, drain, debride. Dig Liver Dis. 2011;43:421-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Bang JY, Arnoletti JP, Holt BA, Sutton B, Hasan MK, Navaneethan U, Feranec N, Wilcox CM, Tharian B, Hawes RH, Varadarajulu S. An Endoscopic Transluminal Approach, Compared With Minimally Invasive Surgery, Reduces Complications and Costs for Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1027-1040.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 73. | van Brunschot S, van Grinsven J, Voermans RP, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, Dijkgraaf MG, van Eijck CH, Erkelens GW, van Goor H, Hadithi M, Haveman JW, Hofker SH, Jansen JJ, Laméris JS, van Lienden KP, Manusama ER, Meijssen MA, Mulder CJ, Nieuwenhuis VB, Poley JW, de Ridder RJ, Rosman C, Schaapherder AF, Scheepers JJ, Schoon EJ, Seerden T, Spanier BW, Straathof JW, Timmer R, Venneman NG, Vleggaar FP, Witteman BJ, Gooszen HG, van Santvoort HC, Fockens P; Dutch Pancreatitis Study Group. Transluminal endoscopic step-up approach versus minimally invasive surgical step-up approach in patients with infected necrotising pancreatitis (TENSION trial): design and rationale of a randomised controlled multicenter trial [ISRCTN09186711]. BMC Gastroenterol. 2013;13:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | van Brunschot S, van Grinsven J, van Santvoort HC, Bakker OJ, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SA, Bruno MJ, Cappendijk VC, Consten EC, Dejong CH, van Eijck CH, Erkelens WG, van Goor H, van Grevenstein WMU, Haveman JW, Hofker SH, Jansen JM, Laméris JS, van Lienden KP, Meijssen MA, Mulder CJ, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TE, Scheepers JJ, Schepers NJ, Schwartz MP, Seerden T, Spanier BWM, Straathof JWA, Strijker M, Timmer R, Venneman NG, Vleggaar FP, Voermans RP, Witteman BJ, Gooszen HG, Dijkgraaf MG, Fockens P; Dutch Pancreatitis Study Group. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. 2018;391:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 506] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 75. | Onnekink AM, Boxhoorn L, Timmerhuis HC, Bac ST, Besselink MG, Boermeester MA, Bollen TL, Bosscha K, Bouwense SAW, Bruno MJ, van Brunschot S, Cappendijk VC, Consten ECJ, Dejong CH, Dijkgraaf MGW, van Eijck CHJ, Erkelens WG, van Goor H, van Grinsven J, Haveman JW, van Hooft JE, Jansen JM, van Lienden KP, Meijssen MAC, Nieuwenhuijs VB, Poley JW, Quispel R, de Ridder RJ, Römkens TEH, van Santvoort HC, Scheepers JJ, Schwartz MP, Seerden T, Spanier MBW, Straathof JWA, Timmer R, Venneman NG, Verdonk RC, Vleggaar FP, van Wanrooij RL, Witteman BJM, Fockens P, Voermans RP; Dutch Pancreatitis Study Group. Endoscopic Versus Surgical Step-Up Approach for Infected Necrotizing Pancreatitis (ExTENSION): Long-term Follow-up of a Randomized Trial. Gastroenterology. 2022;163:712-722.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 76. | Rizzatti G, Rimbaş M, Larghi A. Endoscopic Ultrasound-Guided Drainage for Infected Necrotizing Pancreatitis: Better Than Surgery But Still Lacking Treatment Protocol Standardization. Gastroenterology. 2019;157:582-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Garg PK, Zyromski NJ, Freeman ML. Infected Necrotizing Pancreatitis: Evolving Interventional Strategies From Minimally Invasive Surgery to Endoscopic Therapy-Evidence Mounts, But One Size Does Not Fit All. Gastroenterology. 2019;156:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Boxhoorn L, Besselink MG, Voermans RP; Dutch Pancreatitis Study Group. Surgery Versus Endoscopy for Infected Necrotizing Pancreatitis: A Fair Comparison? Gastroenterology. 2019;157:583-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Bang JY, Varadarajulu S. Reply. Gastroenterology. 2019;157:584-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Liu RH, Wen Y, Sun HY, Liu CY, Zhang YF, Yang Y, Huang QL, Tang JJ, Huang CC, Tang LJ. Abdominal paracentesis drainage ameliorates severe acute pancreatitis in rats by regulating the polarization of peritoneal macrophages. World J Gastroenterol. 2018;24:5131-5143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 81. | Liu WH, Wang T, Yan HT, Chen T, Xu C, Ye P, Zhang N, Liu ZC, Tang LJ. Predictors of percutaneous catheter drainage (PCD) after abdominal paracentesis drainage (APD) in patients with moderately severe or severe acute pancreatitis along with fluid collections. PLoS One. 2015;10:e0115348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 82. | Zerem E, Kunosić S, Zerem D, Boloban A, Zerem O, Zlomužica E. Benefits of abdominal paracentesis drainage performed ahead of percutaneous catheter drainage as a modification of the step-up approach in acute pancreatitis with fluid collections. Acta Gastroenterol Belg. 2020;83:285-293. [PubMed] |
| 83. | Liu WH, Ren LN, Chen T, Liu LY, Jiang JH, Wang T, Xu C, Yan HT, Zheng XB, Song FQ, Tang LJ. Abdominal paracentesis drainage ahead of percutaneous catheter drainage benefits patients attacked by acute pancreatitis with fluid collections: a retrospective clinical cohort study. Crit Care Med. 2015;43:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Wang T, Liu LY, Luo H, Dai RW, Liang HY, Chen T, Yan HT, Cui JF, Li NL, Yang W, Liu WH, Tang LJ. Intra-Abdominal Pressure Reduction After Percutaneous Catheter Drainage Is a Protective Factor for Severe Pancreatitis Patients With Sterile Fluid Collections. Pancreas. 2016;45:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Liu L, Yan H, Liu W, Cui J, Wang T, Dai R, Liang H, Luo H, Tang L. Abdominal Paracentesis Drainage Does Not Increase Infection in Severe Acute Pancreatitis: A Prospective Study. J Clin Gastroenterol. 2015;49:757-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Zhang Y, Zhang SY, Gao SL, Liang ZY, Yu WQ, Liang TB. Successful Resolution of Gastric Outlet Obstruction Caused by Pancreatic Pseudocyst or Walled-Off Necrosis After Acute Pancreatitis: The Role of Percutaneous Catheter Drainage. Pancreas. 2015;44:1290-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Sugimoto M, Sonntag DP, Flint GS, Boyce CJ, Kirkham JC, Harris TJ, Carr SM, Nelson BD, Bell DA, Barton JG, Traverso LW. Biliary Stenosis and Gastric Outlet Obstruction: Late Complications After Acute Pancreatitis With Pancreatic Duct Disruption. Pancreas. 2018;47:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Bellam BL, Samanta J, Gupta P, Kumar M P, Sharma V, Dhaka N, Sarma P, Muktesh G, Gupta V, Sinha SK, Kochhar R. Predictors of outcome of percutaneous catheter drainage in patients with acute pancreatitis having acute fluid collection and development of a predictive model. Pancreatology. 2019;19:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Sugimoto M, Sonntag DP, Flint GS, Boyce CJ, Kirkham JC, Harris TJ, Carr SM, Nelson BD, Barton JG, Traverso LW. A percutaneous drainage protocol for severe and moderately severe acute pancreatitis. Surg Endosc. 2015;29:3282-3291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Sugimoto M, Sonntag DP, Flint GS, Boyce CJ, Kirkham JC, Harris TJ, Carr SM, Nelson BD, Bell DA, Barton JG, Traverso LW. Better Outcomes if Percutaneous Drainage Is Used Early and Proactively in the Course of Necrotizing Pancreatitis. J Vasc Interv Radiol. 2016;27:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 91. | Walser EM, Nealon WH, Marroquin S, Raza S, Hernandez JA, Vasek J. Sterile fluid collections in acute pancreatitis: catheter drainage versus simple aspiration. Cardiovasc Intervent Radiol. 2006;29:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | van Baal MC, Bollen TL, Bakker OJ, van Goor H, Boermeester MA, Dejong CH, Gooszen HG, van der Harst E, van Eijck CH, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. The role of routine fine-needle aspiration in the diagnosis of infected necrotizing pancreatitis. Surgery. 2014;155:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | van Grinsven J, van Dijk SM, Dijkgraaf MG, Boermeester MA, Bollen TL, Bruno MJ, van Brunschot S, Dejong CH, van Eijck CH, van Lienden KP, Boerma D, van Duijvendijk P, Hadithi M, Haveman JW, van der Hulst RW, Jansen JM, Lips DJ, Manusama ER, Molenaar IQ, van der Peet DL, Poen AC, Quispel R, Schaapherder AF, Schoon EJ, Schwartz MP, Seerden TC, Spanier BWM, Straathof JW, Venneman NG, van de Vrie W, Witteman BJ, van Goor H, Fockens P, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Postponed or immediate drainage of infected necrotizing pancreatitis (POINTER trial): study protocol for a randomized controlled trial. Trials. 2019;20:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG; Dutch Pancreatitis Study Group. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 95. | Horvath K, Freeny P, Escallon J, Heagerty P, Comstock B, Glickerman DJ, Bulger E, Sinanan M, Langdale L, Kolokythas O, Andrews RT. Safety and efficacy of video-assisted retroperitoneal debridement for infected pancreatic collections: a multicenter, prospective, single-arm phase 2 study. Arch Surg. 2010;145:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 96. | Bausch D, Wellner U, Kahl S, Kuesters S, Richter-Schrag HJ, Utzolino S, Hopt UT, Keck T, Fischer A. Minimally invasive operations for acute necrotizing pancreatitis: comparison of minimally invasive retroperitoneal necrosectomy with endoscopic transgastric necrosectomy. Surgery. 2012;152:S128-S134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Mallick B, Dhaka N, Gupta P, Gulati A, Malik S, Sinha SK, Yadav TD, Gupta V, Kochhar R. An audit of percutaneous drainage for acute necrotic collections and walled off necrosis in patients with acute pancreatitis. Pancreatology. 2018;18:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | van Grinsven J, van Brunschot S, Bakker OJ, Bollen TL, Boermeester MA, Bruno MJ, Dejong CH, Dijkgraaf MG, van Eijck CH, Fockens P, van Goor H, Gooszen HG, Horvath KD, van Lienden KP, van Santvoort HC, Besselink MG; Dutch Pancreatitis Study Group. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. HPB (Oxford). 2015;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 99. | Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, Buskens E, Dejong CH, van Duijvendijk P, van Eijck CH, Fockens P, van Goor H, van Grevenstein WM, van der Harst E, Heisterkamp J, Hesselink EJ, Hofker S, Houdijk AP, Karsten T, Kruyt PM, van Laarhoven CJ, Laméris JS, van Leeuwen MS, Manusama ER, Molenaar IQ, Nieuwenhuijs VB, van Ramshorst B, Roos D, Rosman C, Schaapherder AF, van der Schelling GP, Timmer R, Verdonk RC, de Wit RJ, Gooszen HG, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Superiority of Step-up Approach vs Open Necrosectomy in Long-term Follow-up of Patients With Necrotizing Pancreatitis. Gastroenterology. 2019;156:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 100. | van Dijk SM, Timmerhuis HC, Verdonk RC, Reijnders E, Bruno MJ, Fockens P, Voermans RP, Besselink MG, van Santvoort HC; Dutch Pancreatitis Study Group. Treatment of disrupted and disconnected pancreatic duct in necrotizing pancreatitis: A systematic review and meta-analysis. Pancreatology. 2019;19:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Sandrasegaran K, Tann M, Jennings SG, Maglinte DD, Peter SD, Sherman S, Howard TJ. Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics. 2007;27:1389-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 102. | Fischer TD, Gutman DS, Hughes SJ, Trevino JG, Behrns KE. Disconnected pancreatic duct syndrome: disease classification and management strategies. J Am Coll Surg. 2014;219:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 103. | Nealon WH, Bhutani M, Riall TS, Raju G, Ozkan O, Neilan R. A unifying concept: pancreatic ductal anatomy both predicts and determines the major complications resulting from pancreatitis. J Am Coll Surg. 2009;208:790-9; discussion 799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 104. | Howard TJ, Moore SA, Saxena R, Matthews DE, Schmidt CM, Wiebke EA. Pancreatic duct strictures are a common cause of recurrent pancreatitis after successful management of pancreatic necrosis. Surgery. 2004;136:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Zerem E, Imširović B, Loga-Zec S, Kunosić S, Hujdurović A, Zerem O. Treatment of Recurrent Pancreatic Pseudocysts with Proven Communication between Pseudocyst and Pancreatic Duct by Long-term Percutaneous Drainage. Ann Acad Med Singap. 2015;44:542-544. [PubMed] |
| 106. | Nadkarni NA, Kotwal V, Sarr MG, Swaroop Vege S. Disconnected Pancreatic Duct Syndrome: Endoscopic Stent or Surgeon's Knife? Pancreas. 2015;44:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 107. | Varadarajulu S, Wilcox CM. Endoscopic placement of permanent indwelling transmural stents in disconnected pancreatic duct syndrome: does benefit outweigh the risks? Gastrointest Endosc. 2011;74:1408-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Rana SS, Bhasin DK, Rao C, Sharma R, Gupta R. Consequences of long term indwelling transmural stents in patients with walled off pancreatic necrosis & disconnected pancreatic duct syndrome. Pancreatology. 2013;13:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 109. | Zerem E, Omerović S. Successful percutaneous drainage with iodine irrigation for pancreatic fistulas and abscesses after necrotizing pancreatitis. Med Princ Pract. 2012;21:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 110. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 338] [Article Influence: 26.0] [Reference Citation Analysis (2)] |
| 111. | Halttunen J, Kylänpää L. Treatment of Pancreatic Fistulas. Eur J Trauma Emerg Surg. 2007;33:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Seewald S, Brand B, Groth S, Omar S, Mendoza G, Seitz U, Yasuda I, Xikun H, Nam VC, Xu H, Thonke F, Soehendra N. Endoscopic sealing of pancreatic fistula by using N-butyl-2-cyanoacrylate. Gastrointest Endosc. 2004;59:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | van Grinsven J, van Brunschot S, van Baal MC, Besselink MG, Fockens P, van Goor H, van Santvoort HC, Bollen TL; Dutch Pancreatitis Study Group. Natural History of Gas Configurations and Encapsulation in Necrotic Collections During Necrotizing Pancreatitis. J Gastrointest Surg. 2018;22:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 114. | Zerem E, Pavlović-Čalić N, Sušić A, Haračić B. Percutaneous management of pancreatic abscesses: long term results in a single center. Eur J Intern Med. 2011;22:e50-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Nealon WH, Walser E. Surgical management of complications associated with percutaneous and/or endoscopic management of pseudocyst of the pancreas. Ann Surg. 2005;241:948-57; discussion 957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 116. | Zerem E, Hauser G, Loga-Zec S, Kunosić S, Jovanović P, Crnkić D. Minimally invasive treatment of pancreatic pseudocysts. World J Gastroenterol. 2015;21:6850-6860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 117. | Tyberg A, Karia K, Gabr M, Desai A, Doshi R, Gaidhane M, Sharaiha RZ, Kahaleh M. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol. 2016;22:2256-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 118. | Zerem E, Imamović G, Omerović S, Ljuca F, Haracić B. Percutaneous treatment for symptomatic pancreatic pseudocysts: Long-term results in a single center. Eur J Intern Med. 2010;21:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Will U, Wegener C, Graf KI, Wanzar I, Manger T, Meyer F. Differential treatment and early outcome in the interventional endoscopic management of pancreatic pseudocysts in 27 patients. World J Gastroenterol. 2006;12:4175-4178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Flati G, Andrén-Sandberg A, La Pinta M, Porowska B, Carboni M. Potentially fatal bleeding in acute pancreatitis: pathophysiology, prevention, and treatment. Pancreas. 2003;26:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 121. | Fonseca Sepúlveda EV, Guerrero-Lozano R. Acute pancreatitis and recurrent acute pancreatitis: an exploration of clinical and etiologic factors and outcomes. J Pediatr (Rio J). 2019;95:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 122. | Kumar S, Ooi CY, Werlin S, Abu-El-Haija M, Barth B, Bellin MD, Durie PR, Fishman DS, Freedman SD, Gariepy C, Giefer MJ, Gonska T, Heyman MB, Himes R, Husain SZ, Lin TK, Lowe ME, Morinville V, Palermo JJ, Pohl JF, Schwarzenberg SJ, Troendle D, Wilschanski M, Zimmerman MB, Uc A. Risk Factors Associated With Pediatric Acute Recurrent and Chronic Pancreatitis: Lessons From INSPPIRE. JAMA Pediatr. 2016;170:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bosnia and Herzegovina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Matsuo Y, Japan; Tellez-Avila F, United States S-Editor: Fan JR L-Editor: A P-Editor: Zhao S