Published online May 7, 2023. doi: 10.3748/wjg.v29.i17.2666
Peer-review started: December 27, 2022
First decision: January 22, 2023
Revised: February 6, 2023
Accepted: April 11, 2023
Article in press: April 11, 2023
Published online: May 7, 2023
Processing time: 130 Days and 11 Hours
Fecal microbial transplantation (FMT) is a promising new method for treating active ulcerative colitis (UC), but knowledge regarding FMT for quiescent UC is scarce.
To investigate FMT for the maintenance of remission in UC patients.
Forty-eight UC patients were randomized to receive a single-dose FMT or autologous transplant via colonoscopy. The primary endpoint was set to the maintenance of remission, a fecal calprotectin level below 200 μg/g, and a clinical Mayo score below three throughout the 12-mo follow-up. As secondary end
The main endpoint was achieved by 13 out of 24 (54%) patients in the FMT group and by 10 out of 24 (41%) patients in the placebo group (log-rank test, P = 0.660). Four months after FMT, the quality-of-life scores decreased in the FMT group compared to the placebo group (P = 0.017). In addition, the disease-specific quality of life measure was higher in the placebo group than in the FMT group at the same time point (P = 0.003). There were no differences in blood chemistry, fecal calprotectin, or endoscopic findings among the study groups at 12 mo. The adverse events were infrequent, mild, and distributed equally between the groups.
There were no differences in the number of relapses between the study groups at the 12-mo follow-up. Thus, our results do not support the use of a single-dose FMT for the maintenance of remission in UC.
Core Tip: This randomized controlled trial compared the efficacy of fecal microbial transplantation via colonoscopy and autologous placebo containing patients’ own feces for the maintenance of remission in 48 patients with ulcerative colitis. The colitis activity was measured with the clinical Mayo score and fecal calprotectin. There was no significant difference in relapses between the groups at the 12-mo follow-up. Remission remained in 54% of the patients in the fecal microbial transplantation group compared to 41% in the placebo group. There was no difference in the adverse events between the groups.
- Citation: Lahtinen P, Jalanka J, Mattila E, Tillonen J, Bergman P, Satokari R, Arkkila P. Fecal microbiota transplantation for the maintenance of remission in patients with ulcerative colitis: A randomized controlled trial. World J Gastroenterol 2023; 29(17): 2666-2678
- URL: https://www.wjgnet.com/1007-9327/full/v29/i17/2666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i17.2666
Ulcerative colitis (UC) is a chronic inflammatory disease with an uncertain etiology. The symptoms of UC include bloody diarrhea and abdominal cramps. The pathophysiology is thought to involve an altered and exaggerated inflammatory response to commensal bacteria in genetically predisposed individuals[1]. An increasing proportion of the population is affected by UC, and the prevalence is highest in North America and Northern Europe. For example, in Finland, the yearly incidence is over 25/100000 and is growing[2]. The lifelong risk of colectomy remains elevated despite new immune response-targeting treatment options[3]. Patients with UC show a reduced quality of life compared to the general population even if the disease is quiescent[4].
UC is associated with decreased gut microbial diversity and stability as well as altered microbiota composition and function[5]. In conditions such as Clostridioides difficile infection (CDI) and irritable bowel syndrome (IBS), fecal microbial transplantation (FMT) has been shown to alter the patients’ gut microbiota in the long term to resemble that of healthy donors[6-8]. During the last decade, FMT has become a recommended treatment option for recurrent Clostridioides difficile infection (rCDI)[9]. The efficacy of FMT for rCDI exceeds 90% using an optimal protocol[10,11]. On this basis, it is worthwhile to investigate FMT in UC patients.
FMT has shown promising efficacy for active UC in placebo-controlled trials, although the methodology has varied between studies[12-15]. Repetitious FMTs have been the most frequently applied approach among these studies, while the applied treatment protocols have been otherwise diverse. Some studies have applied a multidonor approach and prepared each transplant from the feces of multiple donors[14,15]. Anaerobic conditions for manufacturing the fecal transplant have been investigated and shown to yield good results[15], as has the administration of a transplant to each patient as many as 40 times[14]. One study showed a clear difference in efficacy between donors, as transplants from one donor were more effective than transplants from the other five donors[13].
A recent randomized placebo-controlled trial from India investigated the efficacy of FMT in the maintenance of UC remission[16]. In this study, FMT prevented relapses through the administration of transplants during bimonthly colonoscopies, making the implementation of the applied protocol very laborious in clinical practice. Additionally, the study population consisted of primary responders to FMT treatment; thus, the patients in the trial were a highly selective group.
Given that a single FMT alters the gut microbiota for the long term in rCDI[6] as well as in IBS[8] patients, we aimed to investigate the efficacy of a single FMT via colonoscopy to maintain remission in UC patients. Additionally, we aimed to investigate the potential differences in quality of life, fecal calprotectin, blood chemistry [blood count, liver enzymes, creatinine, and C-reactive protein (CRP)], and endoscopic findings during the 12-mo follow-up period.
We randomized patients with UC in remission into two groups in a 1:1 ratio to receive either FMT from a healthy donor (“FMT group”) or an autologous transplant made from the patient’s own feces (“placebo group”). To ensure blinding, all participants donated their stool for the preparation of the placebo transplant, and the FMT group samples were discarded. Bowel lavage was performed using a macrogol solution prior to colonoscopy. The transplant was administered into the cecum of the patient during colonoscopy at baseline.
After the baseline intervention, the patients were followed until a colonoscopy 12 mo later. During the follow-up period, the participants were contacted at 2 mo, 4 mo, and 8 mo after the intervention, at which times the clinical Mayo score[17] was recorded and blood samples were obtained. The quality of life was assessed at baseline as well as at 4 mo and 12 mo[4]. Fecal calprotectin samples were obtained at seven timepoints (baseline and at 2 mo, 4 mo, 6 mo, 8 mo, 10 mo, and 12 mo).
The primary endpoint was sustained remission through the 12-mo follow-up time. Remission was defined as a clinical Mayo score below three and a fecal calprotectin level below 200 μg/g. Additionally, an overt relapse between the measurement points leading to a course of steroids or escalation of maintenance therapy was considered a failure.
This randomized placebo-controlled study was conducted in Finland in the Gastroenterology Departments of Helsinki University Hospital, Helsinki and Päijät-Häme Central Hospital, Lahti. The ethical review board of Helsinki University Hospital approved the study (29/13/03/01/2014). The principles of the Declaration of Helsinki were followed. The trial was registered at ClinicalTrials.gov (NCT03561532).
Forty-eight patients (21-70-years-old) diagnosed with UC were recruited for the study. The inclusion criteria stated that the patients had to be in remission, and the eligibility criteria included fecal calprotectin levels below 100 μg/g and a clinical Mayo score < 3 at the time of screening. The exclusion criteria included the use of antibiotics within 3 mo prior to study entry, a history of tumor necrosis factor-α blockers or other biologics, the use of a high dose of corticosteroids (prednisolone ≥ 20 mg/d), and pregnancy. The patients were recruited from primary and secondary health care centers of the Helsinki and Lahti regions. At baseline, the majority of the patients were on mesalazine.
After the screening visit and before the start of the trial, some patients experienced minor activation of the disease; 8 patients, 4 in each group, had a clinical Mayo score ≥ 3, and 10 patients, 3 in the FMT group and 7 in the placebo group, had fecal calprotectin levels ≥ 200 μg/g. At baseline, none of these patients experienced significant symptoms, and they did not require escalation of medication. Participants with fecal calprotectin ≥ 200 μg/g or a clinical Mayo score ≥ 3 were analyzed separately as “subgroup B” (n = 15), and the participants without these signs of disease activity at baseline were included in “subgroup A” (n = 33). Among all the recruited patients, 16 patients had minor endoscopic colitis activity with an endoscopic Mayo score of 1 at baseline, while the rest of the patients had an endoscopic Mayo score of 0 at baseline.
Participant recruitment started in October 2014. At the beginning of the study, the inclusion criteria required a diagnosis of UC within 6 mo. However, due to very slowly proceeding recruitment, an amendment to the study protocol was made and approved by the ethical board in October 2016 (HUS/1652/2016). Thereafter, patients with any disease duration were eligible. Recruitment remained slow even after the amendment. The study proceeded using fewer than the desired 80 participants due to time constraints. The follow-up of the last included patient was completed in May 2020 (CONSORT flow diagram in Supplementary Figure 1).
Transplants from three healthy donors were used in this study. The donors had normal body weights and were healthy without any diagnosed long-term illnesses or medications. All donors had a healthy lifestyle and a diet that included animal products but was rich in vegetables. They were screened according to the best practice at the time[10]; however, the donor screening guidelines have evolved since the start of the trial[9]. The laboratory tests for donor screening are collectively presented in Supplementary Table 1. We applied transplants from a female in her forties (“Donor 1”) and a young adult male (“Donor 2”), both of whom had previously served as donors in our studies[6,8] and in routine clinical practice of FMT to treat rCDI. A male in his fifties (“Donor 3”) was a new donor recruited for this study.
Half of the participants, 24 out of 48, received FMT via colonoscopy into the cecum as described previously[10]. The fecal transplants were produced from 30 g of feces from a healthy donor. We used three universal donors, and the fecal suspensions were prepared as previously described and stored at -80 °C[10].
Briefly, feces were suspended in sterile saline and mixed with glycerol (final concentration of 10%) in a 250 mL screw cap container by using a spatula. The suspension was frozen at -80 °C immediately after preparation and within 2 h of defecation to minimize the time of exposure to oxygen. For FMT application, the suspension was thawed at 37 °C or room temperature, mixed briefly and loaded into syringes to avoid clogging by unsuspended particles. If necessary, the suspension was passed through a presterilized, stainless steel tea strainer to remove particles before loading the syringes. The remaining 24 participants in the placebo group were treated in an otherwise similar manner, but the fecal suspension was made using the participants’ own freshly donated stool. Autologous placebo was prepared from fresh feces for practical reasons to prevent an extra visit and minimize inconvenience to the patients.
The relapse rates during the 12-mo follow-up period were estimated to be 50% in the placebo group and 15% in the FMT group. Previous studies of FMT for maintenance of remission of UC were not published at the time of study design. Due to the lack of available studies, the estimation of outcomes was based on knowledge concerning the maintenance of remission using mesalazine[18] and extrapolating from FMT studies for rCDI[11], in which over 90% efficacy had been achieved.
The calculated sample size using the z-test (95% confidence interval, α = 0.05 and β = 0.1, 90% power) to find a significant treatment effect was 33 patients in each group, and to cover possible dropouts, we aimed for a sample size of 40 participants per group, 80 participants in total[19].
The participants were randomized 1:1 to receive FMT or placebo. The randomization was executed in blocks of 6 patients by a study nurse not involved in the treatment of the patients. The participants and the treating personnel were blinded to the type of feces transplanted. The randomization was decoded only after all the patients had completed the 12-mo follow-up.
The primary outcome was the maintenance of remission through the 12-mo follow-up period. A relapse of colitis was considered a failure to achieve the primary outcome. The patients were followed until the time of the recorded relapse, after which they were dropped from the follow-up. The patients who remained in remission were followed until the study endpoint of 12 mo after baseline.
The secondary endpoints were quality of life, endoscopic and histologic findings at 12 mo, fecal calprotectin, and blood chemistry. General quality of life was assessed with the 15 dimensions (15D) questionnaire, and disease-specific quality of life was assessed with the Inflammatory Bowel Disease Quality of Life Questionnaire (IBDQ) (McMaster University, Hamilton, Canada, license No. IBDQ22-081)[4]. The histologic activity was graded in four categories: histological remission; mild activity (lamina propria or intraepithelial neutrophils); moderate activity (presence of crypt abscess); and strong activity (presence of erosion or ulcer)[20].
The participants donated stool samples every 2nd mo (months 0, 2, 4, 6, 8, 10, and 12) for the detection of fecal calprotectin. Blood samples were obtained at baseline as well as at months 4, 8, and 12. The blood tests included complete blood counts, liver enzymes, creatinine, and CRP. Fecal calprotectin values below 50 μg/g and CRP values below ten were not reported by the laboratory and were coded as null accordingly.
Descriptive statistics are presented as the means with standard deviations (SDs) for continuous variables and as frequencies and percentages for qualitative variables. Differences between the study groups in the maintenance of remission during the follow-up were assessed using the Kaplan-Meier method. Associations of baseline characteristics with the maintenance of remission were analyzed with univariate Cox regression models. In addition, 15D scores were presented using profile plots, and differences between groups were assessed by t tests. Differences in endoscopic and histological colitis activity between the study groups were analyzed with the χ2 test. P values < 0.05 were considered statistically significant for all analyses. SPSS version 27 (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY, United States) was used for the statistical analysis.
Forty-nine patients were recruited for this study. After the screening visit and before randomization, 1 patient had overt activation of colitis and was excluded. This left 48 patients to be randomized, with 24 in each group. The patient flow of the study is presented in Supplementary Figure 1, and the baseline characteristics of the patients are presented in Table 1. The placebo group had a longer duration of disease than the FMT group (114 mo vs 39 mo, P = 0.006). At baseline, the mean fecal calprotectin level was 115.8 (SD: 184.7) in the placebo group and 66.4 (SD: 108.6) in the FMT group (P = 0.261). The majority of the patients were on mesalazine: 21 out of 24 patients in the FMT group and 22 out of 24 in the placebo group. Four patients in the placebo group were on thiopurine, but none were in the FMT group. At the study baseline, 2 patients in both groups were still on lower doses of tapering corticosteroid therapy. There were no statistically significant differences between the randomization groups within subgroups A and B, in which the patients had fecal calprotectin < 200 μg/g and a clinical Mayo score < 3 or fecal calprotectin ≥ 200 μg/g and a clinical Mayo score ≥ 3 at baseline, respectively (Table 1).
| Baseline variable | All patients (n = 48) | Pvalue | Subgroup A (n = 33) | Pvalue | Subgroup B (n = 15) | Pvalue | |||
| FMT | Placebo | FMT | Placebo | FMT | Placebo | ||||
| Sex as M/F | 14/10 | 12/12 | 0.562 | 8/9 | 8/8 | 0.866 | 4/4 | 5/2 | 0.608 |
| Age | 43.0 (12.9) | 43.1 (12.1) | 0.982 | 43.6 (13.0) | 44.8 (12.0) | 0.781 | 41.7 (13.6) | 39.8 (12.5) | 0.775 |
| Disease duration in mo | 39.2 (51.0) | 114.0 (117.6) | 0.006 | 41.0 (56.2) | 125.4 (121.7) | 0.015 | 34.9 (38.7) | 91.3 (113.2) | 0.233 |
| Fecal calprotectin | 66.0 (108.6) | 115.8 (184.7) | 0.261 | 34.7 (46.3) | 18.9 (44.9) | 0.330 | 142.3 (172.9) | 309.6 (208.3) | 0.117 |
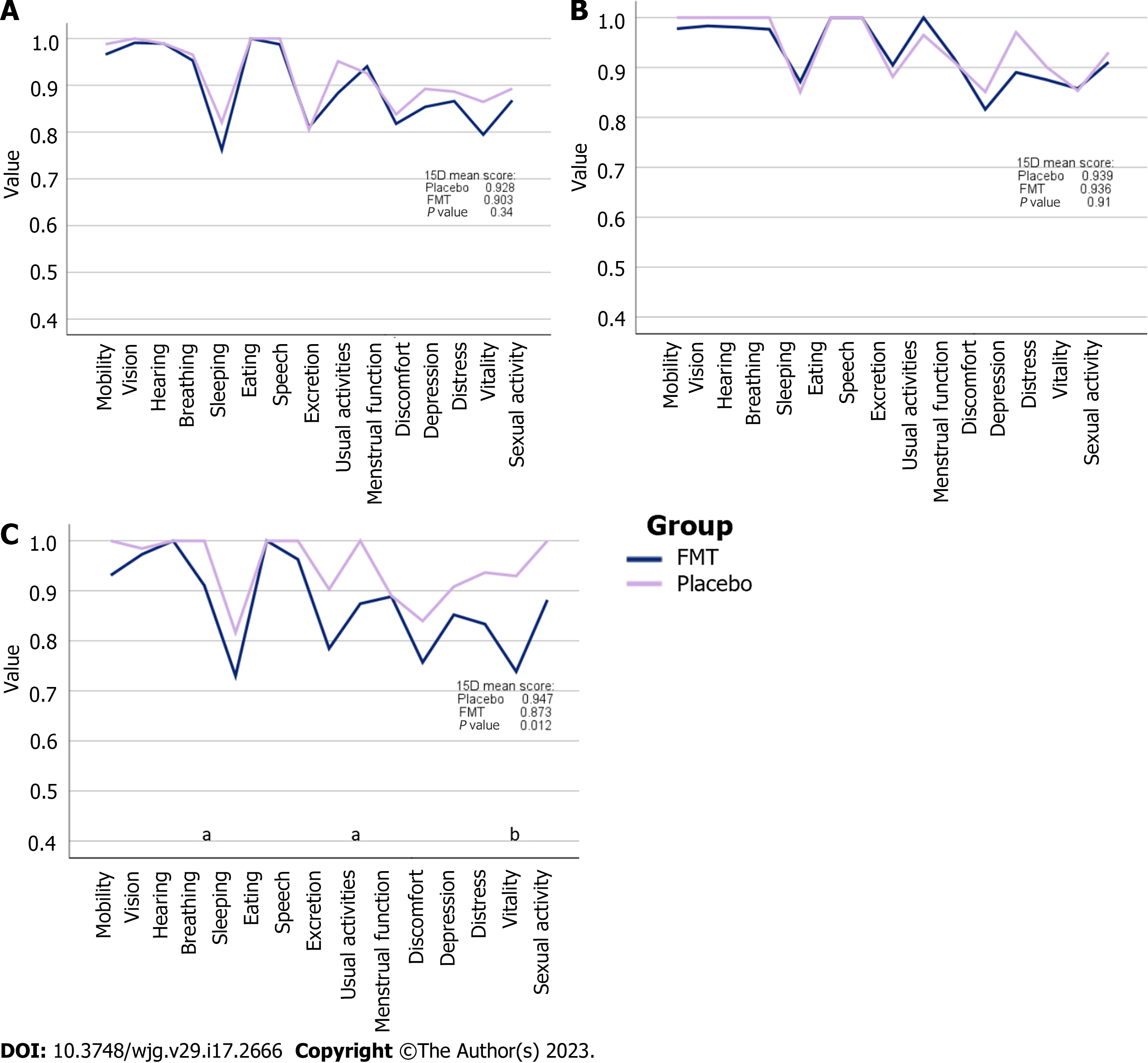
| 15D | 0.903 (0.095) | 0.928 (0.072) | 0.335 | 0.899 (0.106) | 0.939 (0.070) | 0.221 | 0.915 (0.070) | 0.907 (0.078) | 0.830 |
| IBDQ | 169.4 (28.8) | 162.7 (39.8) | 0.519 | 166.9 (28.6) | 171.4 (32.0) | 0.688 | 175.0 (30.5) | 147.4 (49.3) | 0.223 |
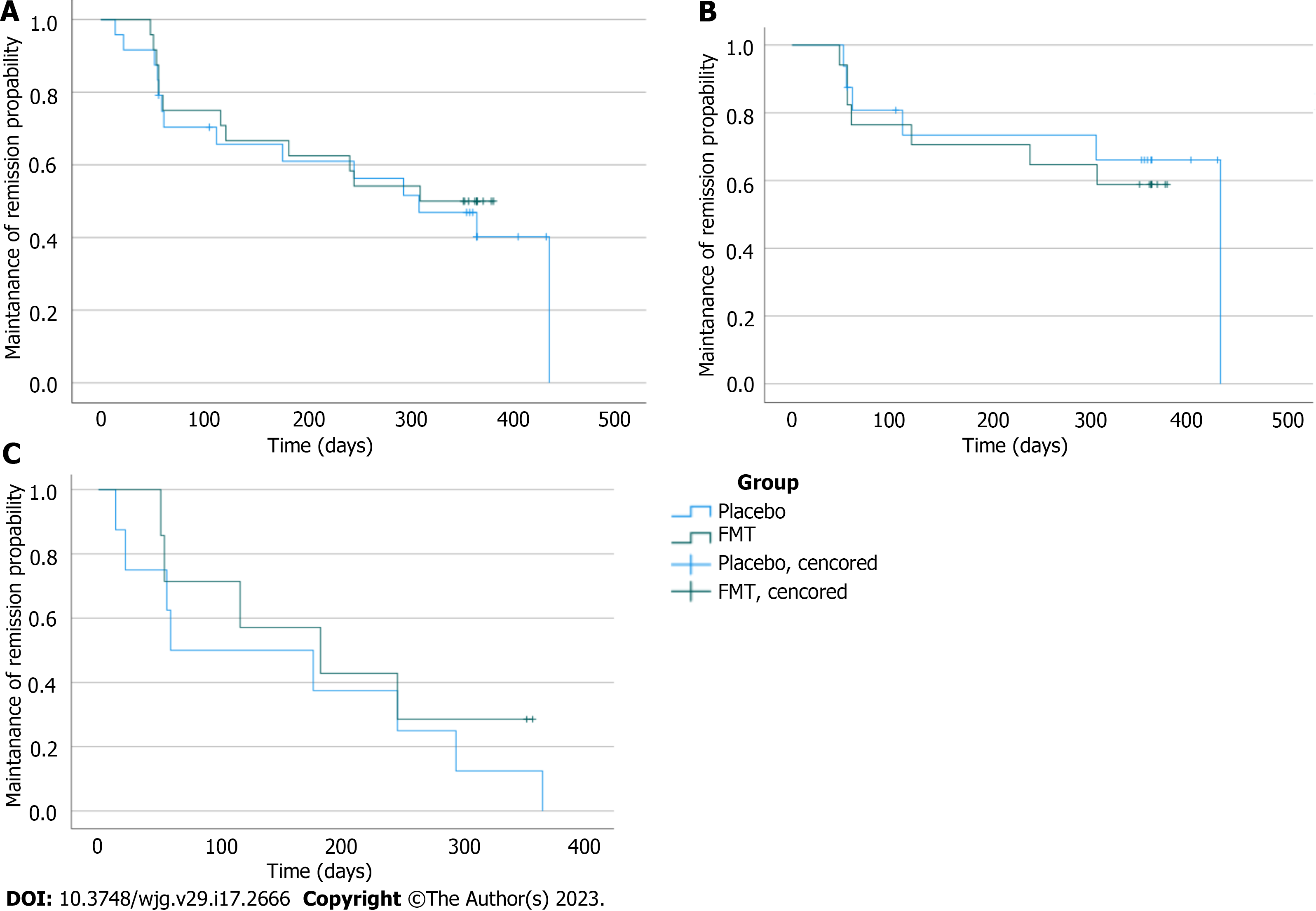
The primary endpoint of the study was the maintenance of remission through the 12-mo follow-up, which was achieved by 13 out of 24 (54%) patients in the FMT group and by 10 out of 24 (41%) patients in the placebo group. The difference between the groups was not statistically significant (log-rank test P = 0.660). A Kaplan-Meier survival curve of relapses in the FMT and placebo groups is presented in Figure 1A.
A similar result was obtained when the patients were divided into subgroups according to the clinical Mayo score and fecal calprotectin level at baseline. In subgroup A, 6 out of 16 patients relapsed in the placebo group, and 7 out of 17 patients relapsed in the FMT group (P = 0.703, Figure 1B). Similarly, subgroup B showed no difference between the placebo and FMT groups (P = 0.556) in the number of relapses; all 8 patients in the placebo group and 5 out of 7 patients in the FMT group relapsed (Figure 1C).
To study the possible effect of a specific donor on the patient’s outcome, we divided the patients into three groups according to the donor and compared these to the placebo. There were no statistically significant differences in the number of relapses between the different donors (log-rank, P = 0.517). At the 12-mo follow-up, 41.7% (10/24) of the patients in the placebo group remained in remission compared to 33.3% (2/6) from Donor 1, 50.0% (5/10) from Donor 2, and 62.5% (5/8) from Donor 3.
We also analyzed the effect of essential baseline characteristics on the maintenance of remission between these donor groups, which included the duration of disease status, fecal calprotectin, clinical Mayo score, total 15D score, and total IBDQ score (Supplementary Table 2). The mean duration of disease was 114 mo in the placebo group, 5 mo in the Donor 1 group, 52 mo in the Donor 2 group, and 49 mo in the Donor 3 group. The disease duration did not have a statistically significant effect on the maintenance of remission in any of the donor groups. There were some statistically significant relationships between baseline characteristics and maintenance of remission. In the placebo group, lower maintenance of remission time was associated with higher baseline fecal calprotectin [Cox regression, hazard ratio (HR): 1.003; 95% confidence interval (CI): 1.001-1.005; P = 0.010) and a higher baseline clinical Mayo score (Cox regression, HR: 1.498; 95%CI: 1.067-2.102; P = 0.020). In the Donor 2 group, a lower mean 15D total score at baseline was associated with lower maintenance of remission (Cox regression, HR: 0.000; CI: 0.000-0.374; P = 0.033). All other analyzed associations were statistically insignificant (Supplementary Table 2).
We investigated the impact of FMT on patient quality of life as measured with the 15D questionnaire and disease-specific quality of life as measured with the IBDQ questionnaire.
The 15D total score was similar between the placebo and FMT groups at baseline (t test, P = 0.335) (Figure 2A) and at the 12-mo follow-up after FMT treatment (P = 0.905) (Figure 2B). However, there was a significant difference in the 15D total score between the FMT and placebo groups (P = 0.017) 4 mo after treatment (Figure 2C). The mean change in the 15D total score from baseline to 4 mo was -0.032 (slightly worse) in the FMT group and -0.009 (no change) in the placebo group. The estimation of the importance of change was performed as presented previously[21]. The mean change in the 15D total score from baseline to 12 mo was -0.008 (no change) in the FMT group and -0.015 (slightly worse) in the placebo group. Additionally, of the 15D, there were statistically significant differences in breathing (P = 0.049), usual activities (P = 0.042), and vitality (P = 0.006), all favoring the placebo group.
The disease-specific quality of life as measured with the IBDQ[22] was also similar between the placebo and FMT groups at baseline (P = 0.519) and at 12 mo (P = 0.868), but at 4 mo, there was a difference in the IBDQ total score favoring the placebo group (P = 0.003). Of the four IBDQ subcategories, there were statistically significant differences in the emotions (P = 0.008) and systemic (P = 0.010) subcategories.
The blood chemistry, complete blood count, liver enzymes, and creatinine and CRP levels were analyzed at four different timepoints. Fecal calprotectin was measured every 2nd mo at six different timepoints. There were no clinically significant changes in any of the blood tests compared to the baseline. All laboratory tests at each timepoint showed no statistically significant differences between the FMT and placebo groups (P > 0.05). The blood chemistry and fecal calprotectin values are collectively presented in Supplementary Table 3.
A colonoscopy was performed at the end of the trial, and pinch biopsies were obtained from all 23 patients who reached the primary endpoint and remained in clinical remission throughout the follow-up period. Endoscopic colitis activity was detected in 2 out of 13 patients in the FMT group and in 2 out of 10 patients in the placebo group. Likewise, mild histological colitis activity was detected in the colon pinch biopsies in 2 out of 13 patients in the FMT group and 2 out of 10 patients in the placebo group, indicating chronic inflammation. Thus, the number of patients who were in endoscopic and histologic remission in the follow-up colonoscopy was 11 out of 13 in the FMT group and 8 out of 10 in the placebo group (χ2, P = 0.772).
A similar number of patients experienced UC activation in the FMT and placebo groups (Figure 1). In addition to colitis activation, other adverse events were recorded in 4 patients in the FMT group and 6 patients in the placebo group.
In the FMT group, the adverse events included fatigue through the follow-up period, gastroenteritis at 8 mo after FMT, constipation at 3 wk after FMT and a diagnosis of primary sclerosing cholangitis. In addition, 1 patient reported fatigue and episodes of atrial fibrillation at the 4-mo timepoint, for which he underwent ablation treatment. This patient subsequently developed pneumonia.
In the placebo group, 1 patient with fibromyalgia reported back pain and colitis symptoms simultaneously. Another patient visited the emergency room 6 mo after the procedure and was diagnosed with mitral valve insufficiency. One patient with spondylarthritis experienced arthralgia during the follow-up. One patient experienced an escalation of bloating after the procedure, and 2 patients experienced a prolonged mild respiratory infection. Possible hospitalizations were monitored in all the participants for 12 mo, but none were attributable to FMT.
In this placebo-controlled trial, we examined the effect of a single FMT via colonoscopy on the maintenance of remission in UC patients. The primary endpoint was sustained remission over a 12-mo follow-up period. A relapse of UC was regarded as a failure to achieve the primary endpoint. We set the cutoff values to differentiate between remission and relapse to a clinically significant level; thus, a clinical Mayo score above three and fecal calprotectin levels above 200 μg/g were considered to indicate colitis activation. There was no statistically significant difference in the number of patients with a relapse of UC during the follow-up period in the FMT and placebo groups. According to the results, a single FMT via colonoscopy was ineffective for maintaining UC in remission.
Previously, the impact of the donor on the outcome of FMT was demonstrated in patients with active UC[13]. Including more than one donor in FMT trials enables comparison between the donors. In this trial, we used three donors. Sustainable remission through the follow-up was achieved by 33.3% of the patients who received FMT from Donor 1, whereas the same was achieved by 62.5% of the patients treated with FMT from Donor 3. However, the number of patients in each group was small, and the differences did not reach statistical significance. Furthermore, the Donor 1 treatment was applied at the beginning of the trial when the inclusion criteria were different, requiring new-onset disease, and consequently the baseline activity markers, fecal calprotectin and clinical Mayo score were higher in the patients of Donor 1 than in the patients of Donor 3. For these reasons, the existence or magnitude of the donor effect could not be proven or disproven.
Studies evaluating FMT for active as well as quiescent UC have been encouraging[13,16], but the present data are not sufficient to justify treating UC patients with FMT in clinical practice. Our goal was to investigate whether manipulation of the gut microbiota with FMT early after UC diagnosis would help in the maintenance of remission and the effect on the course of the disease. When planning this study, we aimed to recruit patients whose UC was diagnosed within 6 mo prior to the study baseline. However, due to slow recruitment, we made a change in the study protocol and started including patients with any duration of the disease. Additionally, another center, Päijät-Häme Central Hospital, joined the study in addition to Helsinki University Hospital. Nevertheless, the recruitment remained slow, and we were only able to recruit 48 of the originally planned 80 patients within a reasonable time.
As a result of the change in the protocol, 31% of the patients fulfilled the initial inclusion criteria and had been diagnosed within the previous 6 mo, of whom 6 patients were in the FMT group and 9 patients were in the placebo group. Coincidentally, the patients with the longest duration of the disease were also randomized into the placebo group, resulting in a statistically significant difference in the duration of disease status between the randomization groups. The groups were similar to each other in all other parameters (Table 1). Patients with biologics were not included in the trial; thus, the participants did not have a history of severe and difficult-to-treat disease.
Previously published randomized, placebo-controlled FMT trials investigating patients with UC have included patients with active colitis or patients who have reached clinical remission after several FMT sessions[15,16,23]. The patients in our study had UC in clinical remission but had not previously received FMT therapy. Between the recruitment and the study baseline, a portion of the patients had elevated calprotectin and clinical Mayo score values without overt colitis symptoms and were included in a subgroup analysis (Table 1). Overall, the population of our study represented UC patients in real-world clinical practice.
As a secondary endpoint, we aimed to investigate the effect of FMT on patient quality of life. We evaluated this outcome with the disease-specific IBDQ questionnaire and with the 15D questionnaire, which measures general health-related quality of life. Both questionnaires measure the quality of life in IBD patients with equal reliability[4]. Interestingly, the placebo group presented higher quality-of-life scores 4 mo after the treatment. This may refer to the extraintestinal influence of the gut microbiota, although the difference between the groups may partly be explained by the longer duration of disease in the placebo group and consequently better adaptation to the fluctuating symptoms of the disease. Indeed, the statistically significant differences concerned vitality, usual activities, and breathing in the 15D questionnaire, while intestinal symptoms did not differ between the groups. Additionally, in the IBDQ questionnaire, the subcategories of emotions and systemic symptoms were statistically significantly better in the placebo group at the 4-mo timepoint. We found disease duration and adaptation to be the most plausible explanation for the observed differences since the subscores of the FMT group increased and the differences between treatment groups disappeared at 12 mo. However, changes in microbiota composition and activity extrapolating to extraintestinal effects should also be addressed in future investigations. Previously, we observed a possible link between microbiota, general mental health, and depression in our FMT studies on IBS and rCDI[8,24].
In line with our previous placebo-controlled FMT trial[8], the reported adverse events in this trial were evenly distributed between groups. There were no severe adverse events attributable to FMT, replicating previous reports stating that FMT was safe when performed with high standards[25]. Even as FMT appears safe in randomized controlled trials[8,13,15] and evidence of long-term safety appears encouraging[24], we find it highly important to continue gathering safety data on FMT from randomized trials as well as collecting registry data from clinical practice. The interindividual variability of donors is high concerning microbiota profiles as well as other characteristics, and therefore the scientific community and clinicians performing FMT for CDI need to stay alert for infectious complications and for possible rare short-term and long-term adverse effects of FMT[26].
Our study had some limitations. First, the number of studied patients remained rather low, with only 48 patients in total due to slow recruitment. Second, after patients experienced a relapse of UC, further data were not recorded. This decreased the amount of obtained data and complicated the comparison of secondary endpoints between the groups, as there were fewer cases left for the analysis with each successive time point. However, after a relapse, some of the patients were given corticosteroids or the medication was changed, which would have misrepresented the true effects of FMT or placebo. Another drawback was that the patients in the placebo group had UC for a longer duration than those in the FMT group and were likely in a more stable phase of the disease. This may have impacted the results of the primary endpoint as well as secondary endpoints; however, there were no statistically significant correlations between the duration of disease and the time to relapse or quality of life in either study group.
Our study also had clear advantages. Its blinded placebo-controlled design is a definite strength. We applied an autologous placebo, which assures very reliable blinding, and the same method has yielded valid results in FMT trials for rCDI[27] and in other conditions such as IBS[8] and Crohn’s disease[28]. However, it must be noted that the composition of the fecal microbiota may change when it is exposed to oxygen, and in the case of patient samples, the duration of oxygen exposure could not be carefully controlled, unlike for the donor samples, which were prepared and frozen within 2 h of defecation. The advantage of applying an autologous placebo is that it assures very reliable blinding. Other forms of placebo may be more easily detected by the patient or treating personnel. Another advantage of our study is the sufficiently long follow-up time, which enabled the treatment effect durability to be monitored.
Unlike in rCDI, clinical efficacy may not be achieved in UC by just a single FMT, possibly due to difficulties in modulating the microbiota in the longer term by only one FMT dose. Repeated FMT treatments could possibly enhance efficacy, as shown by Sood et al[16] where repeated FMT treatments were associated with maintenance of remission. In that trial, the study population was selected from responders to FMT given for induction of remission, and bimonthly colonoscopic FMTs for 1 year maintained UC in remission better than placebo. Thus, the positive effects of FMT may be maintained by repeated treatments. Repeated FMT treatments have also shown promising results in the induction of remission of active UC[13-15]. Engraftment of the transplanted microbes may be more difficult in an active colitis environment than in a state of remission. From this perspective, repeated FMT can be more justified in active disease.
Moreover, FMT may also exert its efficacy via host-derived biomolecules that exert immunoregulatory action or induce transcriptional changes in the affected intestinal epithelium. Action by nonpersisting biomolecules could also explain why multiple FMTs are needed for the induction of remission. On the other hand, if microbiota modulation is considered critical, a single FMT by colonoscopy with our protocol (applying 30 g of donor feces) can induce prolonged microbial engraftment in rCDI patients as well as in IBS patients[6,8].
To our knowledge, our trial is the first controlled trial to investigate a single FMT for the maintenance of remission in UC patients. In future FMT studies on the maintenance of UC remission, repeated treatments seem reasonable. However, other methods to enhance and prolong the effects should also be considered. For example, combining a dietary intervention may improve results and prolong remission[29]. In one recent trial, a dietary intervention resulted in a superior effect on the induction of remission in UC patients compared to FMT[30].
The optimization of donor selection could possibly improve outcomes even with a single FMT given in remission. Additionally, conditions for the engraftment and functioning of beneficial microbiota may be important, particularly when FMT is given to patients in remission. Diet has a great impact on gut microbiota and is likely an important factor affecting the survival and function of transplanted microbes[31]. We suggest prompt documenting of the diet in future FMT studies. Additionally, the combination of FMT with dietary modulation should be addressed in future studies[29,30].
There are many open questions to be answered before we can determine whether FMT may be applied for the maintenance of remission in UC. More research is needed to define the optimal donor characteristics, patient population, and timing for FMT. Additionally, the best route of FMT administration remains undefined. While the colonoscopic route has shown promise[16], FMT with capsules may be considered when high numbers of patients need to be treated[23]. Finally, we do not yet know which stool components are responsible for the positive effects of FMT, and there is much room for future innovative research.
In conclusion, there were no statistically significant differences in the number of UC relapses after a single FMT or placebo treatment; therefore, the main outcome of our study was negative. Our results do not support applying a single FMT for the maintenance of UC remission. However, these results must be interpreted with caution due to the small sample size, and larger studies are warranted.
Ulcerative colitis (UC) is associated with altered gut microbiota. The pathophysiology of UC is thought to involve an altered and exaggerated inflammatory response to commensal bacteria. Fecal microbiota transplantation has yielded good results in the induction of UC remission.
Despite the development in medications for UC, some patients do not respond sufficiently to current treatment options and new treatment modalities are needed. Modulation of gut microbiota via fecal microbial transplantation (FMT) is a potential new treatment option for UC patients.
The goal of this trial was to gather information of the role of gut microbiota in maintenance of remission in UC patients, and the aim was to investigate FMT for the maintenance of UC remission.
Forty-eight patients with quiescent UC were randomized 1:1 to receive a single FMT via colonoscopy or a placebo made from the patient’s own stool. The patients were followed for 12 mo, and colitis symptoms were measured as well as fecal calprotectin. As secondary endpoints, quality of life, blood chemistry, and endoscopic findings at 12 mo were measured.
UC remission was maintained by 13 out of 24 (54%) patients in the FMT group and by 10 out of 24 (41%) patients in the placebo group (log-rank test, P = 0.660). The quality of life was lower in the FMT group at 4 mo after FMT as compared to the placebo group (P = 0.017). There were no differences in blood chemistry, fecal calprotectin, or endoscopic findings at 12 mo between the groups.
There were no significant differences in the maintenance of remission between the groups during the 12-mo follow-up. Thus, our results do not support the use of a single-dose FMT for the maintenance of remission in UC patients.
Many open questions need to be answered before we can determine whether FMT may be applied for the maintenance of remission in UC. We do not yet know which stool components distribute the positive effects of FMT. More research is needed to define the optimal donor characteristics, patient population, and the optimal number and timing of FMT treatments.
Study nurses Pirkko Tuukkala (Helsinki University Hospital) and Virve Liukkonen (Päijät-Häme Central Hospital) are acknowledged for their contribution to the study with a high level of expertise and commitment.
| 1. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2703] [Article Influence: 300.3] [Reference Citation Analysis (2)] |
| 2. | Jussila A, Virta LJ, Kautiainen H, Rekiaro M, Nieminen U, Färkkilä MA. Increasing incidence of inflammatory bowel diseases between 2000 and 2007: a nationwide register study in Finland. Inflamm Bowel Dis. 2012;18:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1929] [Cited by in RCA: 1713] [Article Influence: 244.7] [Reference Citation Analysis (0)] |
| 4. | Haapamäki J, Roine RP, Sintonen H, Turunen U, Färkkilä MA, Arkkila PE. Health-related quality of life in inflammatory bowel disease measured with the generic 15D instrument. Qual Life Res. 2010;19:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 916] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 6. | Jalanka J, Mattila E, Jouhten H, Hartman J, de Vos WM, Arkkila P, Satokari R. Long-term effects on luminal and mucosal microbiota and commonly acquired taxa in faecal microbiota transplantation for recurrent Clostridium difficile infection. BMC Med. 2016;14:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 7. | Fuentes S, van Nood E, Tims S, Heikamp-de Jong I, ter Braak CJ, Keller JJ, Zoetendal EG, de Vos WM. Reset of a critically disturbed microbial ecosystem: faecal transplant in recurrent Clostridium difficile infection. ISME J. 2014;8:1621-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Lahtinen P, Jalanka J, Hartikainen A, Mattila E, Hillilä M, Punkkinen J, Koskenpato J, Anttila VJ, Tillonen J, Satokari R, Arkkila P. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther. 2020;51:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 9. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 842] [Article Influence: 93.6] [Reference Citation Analysis (1)] |
| 10. | Satokari R, Mattila E, Kainulainen V, Arkkila PE. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection--an observational cohort study. Aliment Pharmacol Ther. 2015;41:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 11. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2747] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 12. | Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, Löwenberg M, van den Brink GR, Mathus-Vliegen EM, de Vos WM, Zoetendal EG, D'Haens GR, Ponsioen CY. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015;149:110-118.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 702] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 13. | Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, Armstrong D, Marshall JK, Kassam Z, Reinisch W, Lee CH. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102-109.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 1122] [Article Influence: 102.0] [Reference Citation Analysis (1)] |
| 14. | Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 939] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 15. | Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, Rosewarne CP, Bickley C, Peters C, Schoeman MN, Conlon MA, Roberts-Thomson IC, Andrews JM. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019;321:156-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 664] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 16. | Sood A, Mahajan R, Singh A, Midha V, Mehta V, Narang V, Singh T, Singh Pannu A. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J Crohns Colitis. 2019;13:1311-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 765] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 18. | Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD000544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Chow SC. Sample Size Calculations in Clinical Research. 2nd Ed: Chapman & Hall/CRC Biostatistics Series, 2008: 52. |
| 20. | D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P, Schölmerich J, Sutherland LR. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 810] [Article Influence: 42.6] [Reference Citation Analysis (1)] |
| 21. | Alanne S, Roine RP, Räsänen P, Vainiola T, Sintonen H. Estimating the minimum important change in the 15D scores. Qual Life Res. 2015;24:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804-810. [PubMed] |
| 23. | Haifer C, Paramsothy S, Kaakoush NO, Saikal A, Ghaly S, Yang T, Luu LDW, Borody TJ, Leong RW. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2022;7:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 24. | Jalanka J, Hillamaa A, Satokari R, Mattila E, Anttila VJ, Arkkila P. The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2018;47:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Osman M, Budree S, Kelly CR, Panchal P, Allegretti JR, Kassam Z; OpenBiome Collaborators. Effectiveness and Safety of Fecal Microbiota Transplantation for Clostridioides Difficile Infection: Results From a 5344-Patient Cohort Study. Gastroenterology. 2022;163:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, Reinert SE, Machan JT, Brandt LJ. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann Intern Med. 2016;165:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 28. | Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, Bourrier A, Le Gall G, Lalande V, De Rougemont A, Kirchgesner J, Daguenel A, Cachanado M, Rousseau A, Drouet É, Rosenzwajg M, Hagege H, Dray X, Klatzman D, Marteau P; Saint-Antoine IBD Network, Beaugerie L, Simon T. Fecal microbiota transplantation to maintain remission in Crohn's disease: a pilot randomized controlled study. Microbiome. 2020;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (1)] |
| 29. | Kedia S, Virmani S, K Vuyyuru S, Kumar P, Kante B, Sahu P, Kaushal K, Farooqui M, Singh M, Verma M, Bajaj A, Markandey M, Sachdeva K, Das P, Makharia GK, Ahuja V. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 2022;71:2401-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 30. | Sarbagili Shabat C, Scaldaferri F, Zittan E, Hirsch A, Mentella MC, Musca T, Cohen NA, Ron Y, Fliss Isakov N, Pfeffer J, Yaakov M, Fanali C, Turchini L, Masucci L, Quaranta G, Kolonimos N, Godneva A, Weinberger A, Kopylov U, Levine A, Maharshak N. Use of Faecal Transplantation with a Novel Diet for Mild to Moderate Active Ulcerative Colitis: The CRAFT UC Randomised Controlled Trial. J Crohns Colitis. 2022;16:369-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 31. | Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 1206] [Article Influence: 172.3] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Finland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Triantafillidis J, Greece; Wang LH, China; Zhang Z, China; Zhang F, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Cai YX