Published online May 7, 2023. doi: 10.3748/wjg.v29.i17.2534
Peer-review started: December 23, 2022
First decision: February 16, 2023
Revised: February 26, 2023
Accepted: April 11, 2023
Article in press: April 11, 2023
Published online: May 7, 2023
Processing time: 121 Days and 14.7 Hours
In the world, nonalcoholic fatty liver disease (NAFLD) accounts for majority of diffuse hepatic diseases. Notably, substantial liver fat accumulation can trigger and accelerate hepatic fibrosis, thus contributing to disease progression. Moreover, the presence of NAFLD not only puts adverse influences for liver but is also associated with an increased risk of type 2 diabetes and cardiovascular diseases. Therefore, early detection and quantified measurement of hepatic fat content are of great importance. Liver biopsy is currently the most accurate method for the evaluation of hepatic steatosis. However, liver biopsy has several limitations, namely, its invasiveness, sampling error, high cost and moderate intraobserver and interobserver reproducibility. Recently, various quantitative imaging techniques have been developed for the diagnosis and quantified measurement of hepatic fat content, including ultrasound- or magnetic resonance-based methods. These quantitative imaging techniques can provide objective continuous metrics associated with liver fat content and be recorded for compa
Core Tip: Accurate evaluation of the hepatic steatosis is important. The conventional gray scale ultrasound has the limitation of low diagnostic accuracy for mild hepatic steatosis and inability to make quantification evaluations. Quantification imaging techniques including ultrasound-based techniques and magnetic resonance imaging-based techniques can provide objective continuous numbers associated with liver fat content and past records can be found when patients receiving check-ups to evaluate change of liver fat content, which is useful for the longitudinal follow-up to monitor the impact of clinical interventions.
- Citation: Zeng KY, Bao WYG, Wang YH, Liao M, Yang J, Huang JY, Lu Q. Non-invasive evaluation of liver steatosis with imaging modalities: New techniques and applications. World J Gastroenterol 2023; 29(17): 2534-2550
- URL: https://www.wjgnet.com/1007-9327/full/v29/i17/2534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i17.2534
There are several types of chronic liver diseases, but nonalcoholic fatty liver disease (NAFLD) is the broadest state[1,2]. NAFLD represents a wide range of liver abnormalities[3]. Over 25% of general population is influenced by NAFLD, while affected proportion of type 2 diabetes population is 55%-80%[1]. Simple fatty liver may progress to non-alcoholic steatohepatitis (NASH), which is a severe form of fatty liver characterized by inflammation of hepatocyte. This form may result in cirrhosis with portal hypertension or liver dysfunction or even to hepatocellular carcinoma (HCC)[4]. Notably, there is an annual incidence of 0.4 cases of HCC per 1000 population-years among patients with NAFLD, making it the third most common cause of HCC in the United States[5]. A diagnosis of NAFLD is associated with not only adverse effects on the liver, but also an increased risk of type 2 diabetes and cardiovascular disease[6,7]. A study suggested that there was a significant increase in mortality associated with liver-specific diseases or cardiovascular diseases in patients with NAFLD compared to controls[8]. For patients who received hepatectomy, hepatic steatosis can increase incidence of postoperative complications and death[9]. The risk of graft failure for patients undergoing liver transplantation increases when hepatic steatosis exceeds 30%[10].
As NAFLD poses substantial risks of HCC, liver-associated complications and other adverse events to patients, it is of great importance to diagnose and quantify hepatic fat content early[4]. NAFLD is likely to be reversible in its early stage even with simple treatments, for example, lifestyle changes[11]. In addition, the main factor contributing to disease progression in patients with NAFLD is liver fibrosis[1,3]. Abundant liver fat accumulation can trigger and accelerate hepatic fibrosis, thus contributing to disease progression[12,13]. Therefore, in patients with NAFLD, quantitative measurements of liver steatosis could be useful for prognostic assessment and treatment[4]. Although high level of liver fat can lead to fibrosis progression, it is of note that the level of liver fat is not always parallel to the grade of fibrosis. It has been suggested that patients without fibrosis or in the early stages of fibrosis may demonstrate obvious disease progression with high level of liver fat content; however, the fat content decreases when disease progresses to advanced fibrosis or cirrhosis[14-16]. Therefore, when evaluating the value of measuring hepatic steatosis for assessing disease progression and prognosis, the fibrosis status should first be taken into account[17].
When it comes to diagnosing diffuse hepatic disease, liver histopathologic examination is the most precise method. With liver biopsy, quantification of the liver fat level is classified into four grades (grade 0, < 5%; grade 1, 5%-33%; grade 2, 33%-66%; grade 3, > 66%)[18]. Although the use of liver biopsy correctly evaluates liver steatosis, its limitations include its invasiveness, sampling error, which make biopsy impractical for patients who have only simple steatosis[19,20]. Therefore, noninvasive methods to diagnose the presence of steatosis and to monitor changes in hepatic steatosis are needed (Table 1). Conventional gray-scale ultrasound can be applied to diagnose liver steatosis. However, its inability to provide accurate quantification of liver fat has limited its use in the diagnostic pathway of liver steatosis[21]. At present, a number of imaging techniques for the evaluation of hepatic fat content, including MR- and ultrasound-based methods, have been developed. In this review, we summarize the available imaging methods for the quantified measurement of hepatic fat content. In addition, we briefly discuss the clinical performance of these methods.
| Techniques | Clinical characteristics | Limitations |
| CAP | Low cost; High availability; Time-saving | High measurement failure rate |
| Allows simultaneous evaluation of steatosis and fibrosis | Measurement without B-mode ultrasound image | |
| Moderate to high diagnostic accuracy for detecting and grading steatosis | The cutoff value for diagnosing steatosis is poorly standardized | |
| Moderate to high repeatability and reproducibility | ||
| Well validated | ||
| ATI, ATT and UGAP | Outperform or have comparable diagnostic accuracy compared with CAP | The measurement may be influenced by liver fibrosis |
| High repeatability and reproducibility | Fairly small number of studies | |
| Strong correlation with liver histology or MRI-PDFF | ||
| Low measurement failure rate | ||
| Measured on B-mode ultrasound images | ||
| Att. PLUS | Measurement is obtained at the same time as the sound speed measurement | Fairly small number of studies |
| Comparable diagnostic accuracy with CAP | No study comparing this technique with liver histology or MRI-PDFF | |
| TAI and TSI | High diagnostic accuracy for detecting and grading steatosis | Fairly small number of studies |
| Strong correlation with MRI-PDFF | ||
| High repeatability and reproducibility | ||
| BSC | Uses a reference phantom to reduce sources of variability due to ultrasound systems or operators | Fairly small number of studies |
| High diagnostic accuracy for detecting and grading steatosis | ||
| Strong correlation with liver histology or MRI-PDFF | ||
| High repeatability and reproducibility | ||
| UDFF | Is a combination of both attenuation coefficient and backscatter coefficient | Fairly small number of studies |
| UDFF approximates MRI-PDFF | ||
| ASQ and NLV | Moderate to high diagnostic accuracy for detecting and grading steatosis | Weak correlation with liver histology |
| Strong correlation with CAP | The correlation with MR-based techniques is controversial | |
| The influence of fibrosis on measurement is controversial | ||
| Fairly small number of studies | ||
| SS | Moderate to high diagnostic accuracy for detecting and grading steatosis | Fairly small number of studies |
| Strong correlation with CAP | ||
| MRS and MRI-PDFF | High diagnostic accuracy for detecting and grading steatosis | High cost; low availability |
| Considered as the reference standard | Time-consuming |
Table 2 summarizes published diagnostic utility metrics and optimal cutoff values of quantitative ultrasound methods for quantified measurement of hepatic fat content.
| Ref. | No. | Method | Reference standard | Grade of steatosis | Optimal cutoff value | AUROC |
| Bae et al[59], 2019 | 108 | ATI | LB | ≥ S1 | 0.64 | 0.84 |
| ≥ S2 | 0.70 | 0.89 | ||||
| ≥ S3 | 0.75 | 0.93 | ||||
| Bae et al[60], 2022 | 120 | ATI | LB | ≥ S1 | 0.66 | 0.91 |
| ≥ S2 | 0.66 | 0.91 | ||||
| Tada et al[62], 2019 | 148 | ATI | LB | ≥ S1 | 0.66 | 0.85 |
| ≥ S2 | 0.67 | 0.91 | ||||
| ≥ S3 | 0.68 | 0.91 | ||||
| Tada et al[63], 2020 | 119 | ATI | MRI-PDFF | ≥ S1 | 0.63 | 0.81 |
| ≥ S2 | 0.73 | 0.87 | ||||
| ≥ S3 | 0.75 | 0.94 | ||||
| Jeon et al[61], 2019 | 87 | ATI | MRI-PDFF | ≥ S1 | 0.59 | 0.76 |
| Ferraioli et al[65], 2019 | 129 | ATI | MRI-PDFF | ≥ S1 | 0.63 | 0.91 |
| ≥ S2 | 0.72 | 0.95 | ||||
| Ferraioli et al[66], 2021 | 72 | ATI-GEN | MRI-PDFF | ≥ S1 | 0.62 | 0.92 |
| ATI-PEN | MRI-PDFF | ≥ S1 | 0.69 | 0.90 | ||
| Sugimoto et al[67], 2021 | 111 | ATI | LB | ≥ S1 | 0.67 | 0.88 |
| ≥ S2 | 0.72 | 0.86 | ||||
| ≥ S3 | 0.86 | 0.79 | ||||
| Hsu et al[70], 2021 | 28 | ATI | LB | ≥ S1 | 0.69 | 0.97 |
| ≥ S2 | 0.78 | 0.99 | ||||
| ≥ S3 | 0.82 | 0.97 | ||||
| Kwon et al[57], 2021 | 100 | ATI | MRI-PDFF | ≥ S1 | 0.62 | 0.91 |
| ≥ S2 | 0.72 | 0.94 | ||||
| Jang et al[58], 2022 | 57 | ATI | LB | ≥ S1 | 0.62 | 0.81 |
| Koizumi et al[73], 2019 | 89 | ATT | LB | ≥ S1 | 0.68 | 0.74 |
| ≥ S2 | 0.72 | 0.80 | ||||
| ≥ S3 | 0.78 | 0.96 | ||||
| Tamaki et al[54], 2018 | 351 | ATT | LB | ≥ S1 | 0.63 | 0.79 |
| ≥ S2 | 0.69 | 0.87 | ||||
| ≥ S3 | 0.85 | 0.96 | ||||
| Fujiwara et al[75], 2018 | 163 | UGAP | LB | ≥ S1 | 0.53 | 0.90 |
| ≥ S2 | 0.60 | 0.95 | ||||
| ≥ S3 | 0.65 | 0.96 | ||||
| Imajo et al[76], 2022 | 1010 | UGAP | MRI-PDFF | ≥ S1 | 0.65 | 0.91 |
| ≥ S2 | 0.71 | 0.91 | ||||
| ≥ S3 | 0.77 | 0.89 | ||||
| Kuroda et al[79], 2021 | 202 | UGAP | LB | ≥ S1 | 0.49 | 0.89 |
| ≥ S2 | 0.65 | 0.91 | ||||
| ≥ S3 | 0.69 | 0.92 | ||||
| Tada et al[80], 2019 | 126 | UGAP | MRI-PDFF | ≥ S1 | 0.60 | 0.92 |
| ≥ S2 | 0.69 | 0.87 | ||||
| ≥ S3 | 0.69 | 0,89 | ||||
| Jeon et al[83], 2021 | 120 | TAI | MRI-PDFF | ≥ S1 | 0.88 | 0.86 |
| TSI | MRI-PDFF | ≥ S1 | 91.2 | 0.96 | ||
| Rónaszéki et al[84], 2022 | 110 | TAI | MRI-PDFF | ≥ S1 | 0.59 | 0.92 |
| TSI | MRI-PDFF | ≥ S1 | 99.7 | 0.91 | ||
| Şendur et al[85], 2023 | 80 | TAI | MRI-PDFF | ≥ S1 | 0.75 | 0.95 |
| ≥ S2 | 0.86 | 0.97 | ||||
| ≥ S3 | 0.96 | 0.97 | ||||
| TSI | MRI-PDFF | ≥ S1 | 92.44 | 0.96 | ||
| ≥ S2 | 96.64 | 0.91 | ||||
| ≥ S3 | 99.45 | 0.94 | ||||
| Lin et al[91], 2015 | 204 | BSC | MRI-PDFF | ≥ S1 | 0.0038 | 0.98 |
| Dillman et al[94], 2022 | 56 | UDFF | MRI-PDFF | ≥ S1 | 5% | 0.90 |
| Labyed et al[37], 2020 | 101 | UDFF | LB | ≥ S1 | 8.1% | 0.94 |
| ≥ S2 | 15.9% | 0.88 | ||||
| ≥ S3 | 16.1% | 0.83 |
Due to its low price and availability, gray scale ultrasound is a traditional diagnostic method for diagnosing and monitoring liver steatosis[22]. When using this method, fatty infiltration is indicated by the following signs: Hyperechogenicity of the liver parenchyma, liver-to-kidney comparison, ultrasound beam attenuation, and impaired visualization of the intrahepatic structures[23]. However, it is difficult for operators to grade liver steatosis solely based on the gray scale ultrasound[24]. Degree of liver fat content can be classified into 4 grades (normal, mild, moderate, and severe)[22]. For moderate to severe hepatic steatosis, gray scale ultrasound has a high diagnostic accuracy. A meta-analysis enrolling a total of 2815 patients and using hepatic histopathologic results as the golden standard demonstrated that the overall sensitivity and specificity of gray scale ultrasound to distinguish normal liver and moderate steatosis were 85% and 93%[25]. However, gray scale ultrasound has restricted diagnostic performance for mild steatosis[24]. Another limitation is that gray scale ultrasound is based on qualitative visual features, and the intraobserver and interobserver reproducibility vary with different operators[26,27].
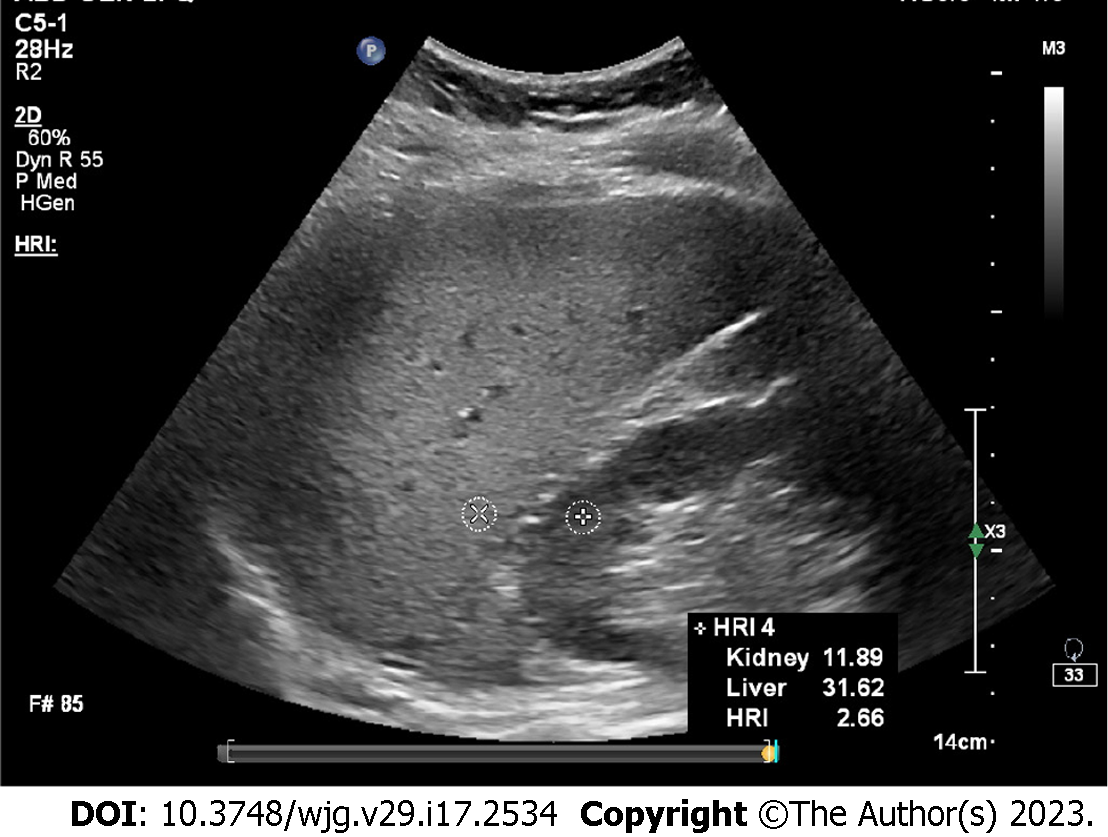
To improve the diagnostic performance of using gray scale ultrasound for the measurement of liver content, hepatorenal index (HRI) was developed[28] (Figure 1). This metric calculates the rate of parenchymal echo of the liver and the renal cortex[28]. Previous studies found that HRI had a significant correlation with histologic steatosis[29-31]. Marshall et al[32] reported a sensitivity, confirmed by liver biopsy, of 100% with an HRI cutoff of 1.27 for detecting more than 5% steatosis. Borges et al[33] reported for diagnosing fatty liver, the cutoff value of 1.24 revealed 93% sensitivity and specificity, but this study only used healthy volunteers as the control group. Stahlschmidt et al[34] suggested in livers with advanced fibrosis, HRI should not be used to measure steatosis because fibrosis replaces fat as NAFLD progresses. Similarly, patients suffering from chronic kidney disease may present increased echo of the renal cortex, which makes the HRI unreliable for grading steatosis[35]. Furthermore, Kjaergaard et al[36] found that HRI presented a higher incidence of failure (12%) compared to controlled attenuation parameter (CAP, 2%). In addition, it can be challenging to diagnose mild steatosis by HRI[33].
Mechanism of quantitative ultrasound techniques: Conventional gray scale ultrasound and HRI cannot provide quantitative information about liver fat content. Essentially, quantitative methods are used to model the relationship between physical properties of hepatic tissue and the echo signals that are scattered by it. The impedance difference of fat vesicles in hepatocytes causes increased scattering magnitudes and signal attenuation. A frequency-dependent analysis of signal attenuation and backscatter is performed on signals returned by tissue[37].
The quantitative ultrasound techniques used for the measurement of hepatic fat quantification included the spectral-based techniques and the techniques based on envelope statistics. Estimation of either attenuation coefficient (AC) or the backscatter coefficient (BSC) is used for spectral based techniques. The AC measures energy loss as ultrasound wave passes through tissue and the BSC measures the returned ultrasound energy when ultrasound wave strikes the microstructure of tissue. Techniques based on the envelope statistics of the backscattered ultrasound include the acoustic structure quantification (ASQ), normalized local variance (NLV), and estimation of sound speed[38]. Techniques according to envelope statistics are relatively novel. Microstructural characteristics of tissues can be determined by the shape and attributes of backscattered ultrasound[38].
Current commercial techniques and their mechanism of hepatic steatosis quantification are presented in Table 3.
| Technique | Mechanism for liver fat quantification | Principle of the techniques |
| CAP | Spectral based technique (AC) | CAP measures the attenuation of or reduction in the amplitude of the ultrasound waves on their way through the liver |
| ATI | Spectral based technique (AC) | ATI quantifies the degree of the ultrasound beam attenuation. The attenuation of the ultrasound beam is calculated by analyzing echo signals received by the transducer |
| ATT | Spectral based technique (AC) | Two ultrasonic waves of different frequencies (F0, F1; F0 < F1) are transmitted to the same beamline and the received signal is obtained. ATT estimates the attenuation coefficient it by calculating the slope of the received signal ratio (F0/F1) |
| UGAP | Spectral based technique (AC) | UGAP compares the measured liver signal and the referential signal (measured on the reference phantom with known attenuation and backscatter coefficients) |
| Att. PLUS | Spectral based technique (AC) | Att. PLUS measures the decrease in amplitude of ultrasound waves as they propagate throughout the tissue |
| TAI | Spectral based technique (AC) | TAI is determined based on the attenuation properties of different frequency components in the tissue, and the spectrum of radiofrequency signals provides a downshift of the center frequency according to depth. The TAI parameter indicates the slope of the ultrasound center frequency downshift |
| BSC | Spectral based technique (BSC) | BSC measures the ultrasound energy returned from the tissue |
| UDFF | Spectral based technique (BSC) | UDFF is obtained by combining both AC and BSC and the result is presented as the percentage of hepatic steatosis. Reference phantom data is integrated into the ultrasound system and fixed-acquisition region of interest is applied |
| TSI | Envelope Statistic based technique | The TSI is based on the shape parameter of the Nakagami distribution which reflects the local concentration and arrangement of ultrasound scatterers |
| ASQ | Envelope Statistic based technique | ASQ measures the FD ratio, which is based on the difference between theoretical and real echo amplitude distributions |
| NLV | Envelope Statistic based technique | NLV parameter was derived from ASQ, which analyzed ultrasound amplitudes sampled from gray-scale ultrasound images |
| SS | Envelope Statistic based technique | SS calculates the speed of sound through the liver |
| SSp.PLUS | Envelope Statistic based technique | SSp.PLUS is a novel technique that allows quantification of the intrahepatic speed of sound which is correlated with the liver fat content |
CAP: CAP was the initial available technique for quantified measurement of hepatic fat content. Attenuation of the ultrasound beam is applied to generate the CAP amount[39,40]. Typically, two types of probes, the medium probe and the extra-large probe, can be utilized. The choice of optimal probe is automatically controlled according to skin-to-liver capsule distance (SCD). When the SCD exceeds 2.5 cm, the extra-large probe is more effective than the M probe. The CAP is presented in units of decibels per meter (dB/m)[41].
More than 160 studies have discussed the efficacy of CAP as a metric for quantified measurement of liver fat content, and acceptable accuracy was found. The general diagnostic accuracy evaluated by the area under the receiver operating curve (AUROC) of CAP for detecting presence of steatosis has been displayed to range from 0.64 to 0.97[42-44]. A meta-analysis including 19 studies found that CAP had good diagnostic performance with AUROCs of 0.823 for distinguishing steatosis grade > S0, 0.865 for distinguishing steatosis grade > S1, 0.882 for distinguishing steatosis grade > S2. The corresponding optimal cutoff values for > S0, > S1, > S2 were 248, 268 and 280 dB/m. Moreover, they found that there was a potential link between NAFLD, diabetes mellitus, and body mass index with the CAP value[45]. Although the diagnostic utility of CAP for differentiating patients with and without hepatic steatosis has been fully validated, the optimal cutoff value to determine the presence of steatosis varies significantly between studies[17]. A meta-analysis of 2346 participants with different diffused hepatic diseases demonstrated that CAP cutoffs varied according to the etiology of the hepatic diseases, including NAFLD, chronic viral hepatitis, alcoholic liver disease[46].
The CAP value demonstrated a moderate to strong correlation with magnetic resonance (MR)-based techniques for liver steatosis quantification[47,48]. However, compared with MR-based methods, the CAP has inferior diagnostic ability in grading liver steatosis. Diagnostic effectiveness of MR spectroscopy (MRS) over CAP for diagnosing S1 was significantly higher (AUROC, 0.77 vs 0.99)[49]. Imajo et al[50] demonstrated suboptimal diagnostic performance of CAP compared to MRI-proton density fat fraction (MRI-PDFF) in grading liver steatosis.
However, CAP has the limitation of failure rate up to 7.7%. According to previous reports, an association was found between measurement failure and sex, body mass index, and metabolic syndrome[51]. Use of the extra-large probe can reduce the failure rate because it is designed for patients with obesity[4].
Quantification of attenuation using ultrasound imaging: Several techniques aiming to evaluate the attenuation coefficient applying ultrasound guidance have been exploited, including attenuation imaging (ATI), attenuation measurement function (ATT), and ultrasound guided attenuation parameter (UGAP). CAP has a disadvantage that it lacks the guidance of gray scale ultrasound images in choosing the area for measurement. In contrast, the ATI, ATT, and UGAP techniques are characterized by evaluating liver steatosis on gray scale ultrasonography images with accurate placement of region of interest[17]. When using these techniques, conventional gray scale ultrasound images can be evaluated simultaneously, and the exact region of interest can be placed to avoid the vessels, bile duct, masses or cysts. Therefore, the technical success rate using these methods is high[52-55]. Another advantage of ATI, ATT, and UGAP is that these techniques have high intraobserver and interobserver agreement. A range of 0.81 to 0.98 is found for the intraobserver agreement of ATI, and a range of 0.79 to 0.92 is found for the interobserver agreement. Although there are few studies investigating the topic, the intraobserver and interobserver agreement of UGAP is reported to be 0.86 and 0.84, respectively. In addition, ATI measurements among different operators demonstrated high agreement (intraclass correlation coefficients: 0.91)[17,44,56].
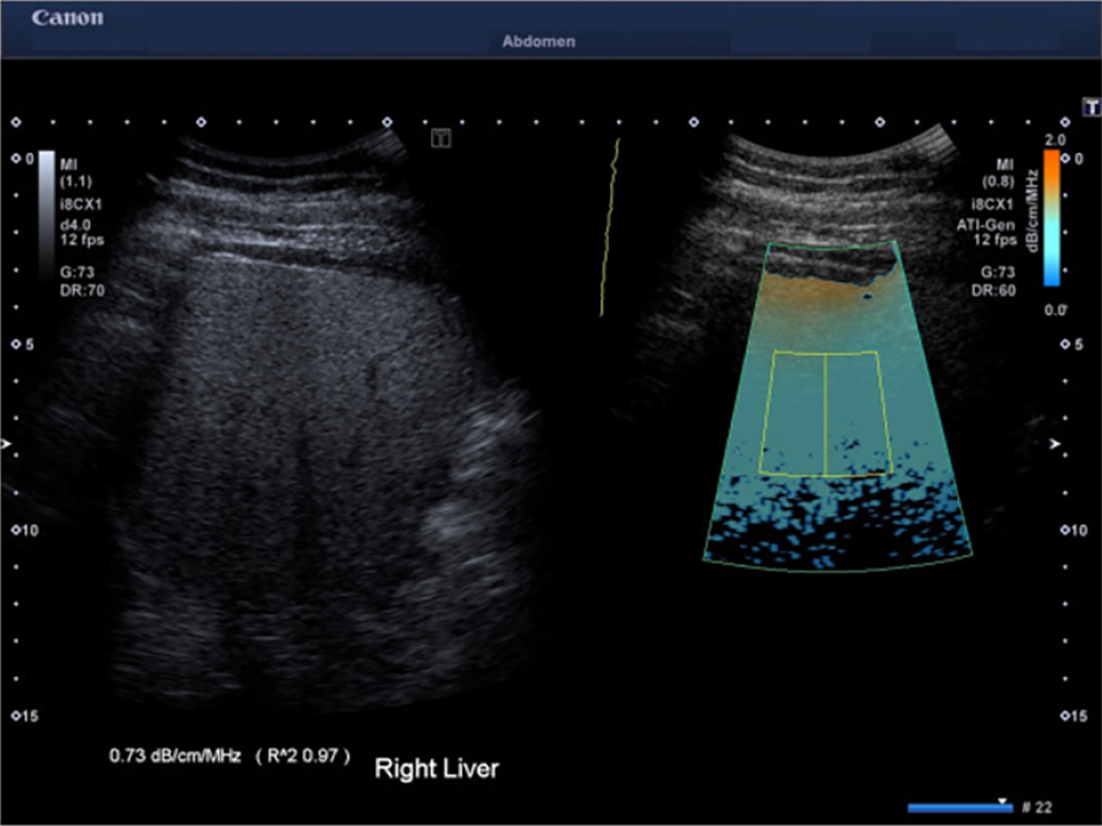
ATI is a kind of two-dimensional attenuation imaging technique (Figure 2)[57,58]. ATI assesses the attenuation of ultrasound beams in a region of interest using color-coded maps in real time. dB/cm/MHz is the unit of measurement for the attenuation coefficient[35]. In addition, to ensure a high technique success rate, the ATI is equipped with a reliability index (R2), and an R2 value ≥ 0.80 is considered a reliable measurement[59-61]. In the reported measurements, the cutoff values ranged from 0.63 to 0.69 dB/cm/MHz for detecting ≥ S1, 0.66-0.72 dB/cm/MHz for detecting ≥ S2, and 0.68-0.86 dB/cm/MHz for detecting = S3. The reported AUROCs were 0.80-0.97 for detecting ≥ S1, 0.86-0.99 for detecting ≥ S2, and 0.79-0.99 for detecting = S3[59-70]. It has been found that ATI measurements have a significant correlation with histological steatosis grade determined by liver biopsy[59,60,67]. Addi
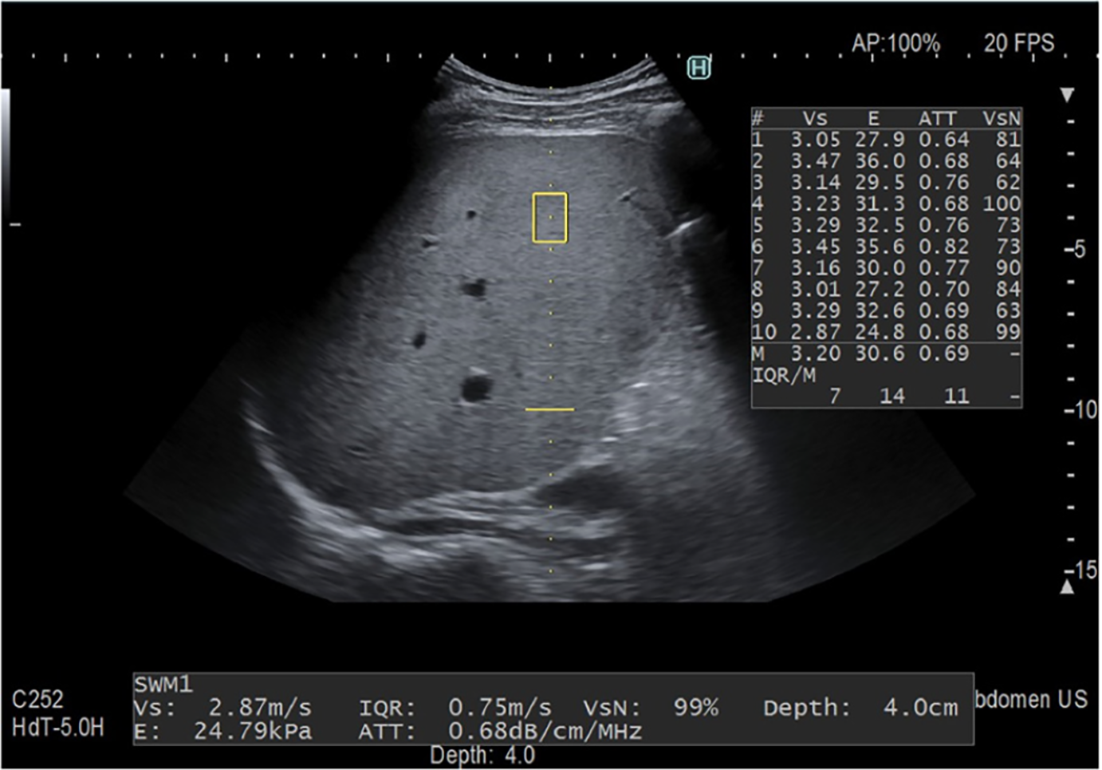
ATT is a technique developed by Fujifilm Health Care company (previously Hitachi Medical Systems, Japan) (Figure 3). In ATT, a beamline is connected to an ultrasonic transmitter with two ultrasonic waves of different frequencies (F0, F1) at once. The received signal is obtained, and attenuation coefficients are determined by the slope of the received signal ratio (F0/F1). The results are presented in units of dB/cm/MHz[54,55,71-73]. A study enrolled 351 patients and biopsy specimens were examined quantitatively for fat content. In terms of fat area, ATT had a significant correlation (r = 0.50, P < 0.001). The cutoff values were 0.62 dB/cm/MHz for S ≥ 1, 0.67 dB/cm/MHz for S ≥ 2 and 0.73 dB/cm/MHz for S ≥ 3 and corresponding AUROCs were 0.79, 0.87 and 0.96[54]. An analysis of 94 patients who received both ATT and CAP examinations when undergoing liver histopathologic examination revealed that ATT exhibited diagnostic accuracy equivalent to that of CAP for grading histological steatosis[73].
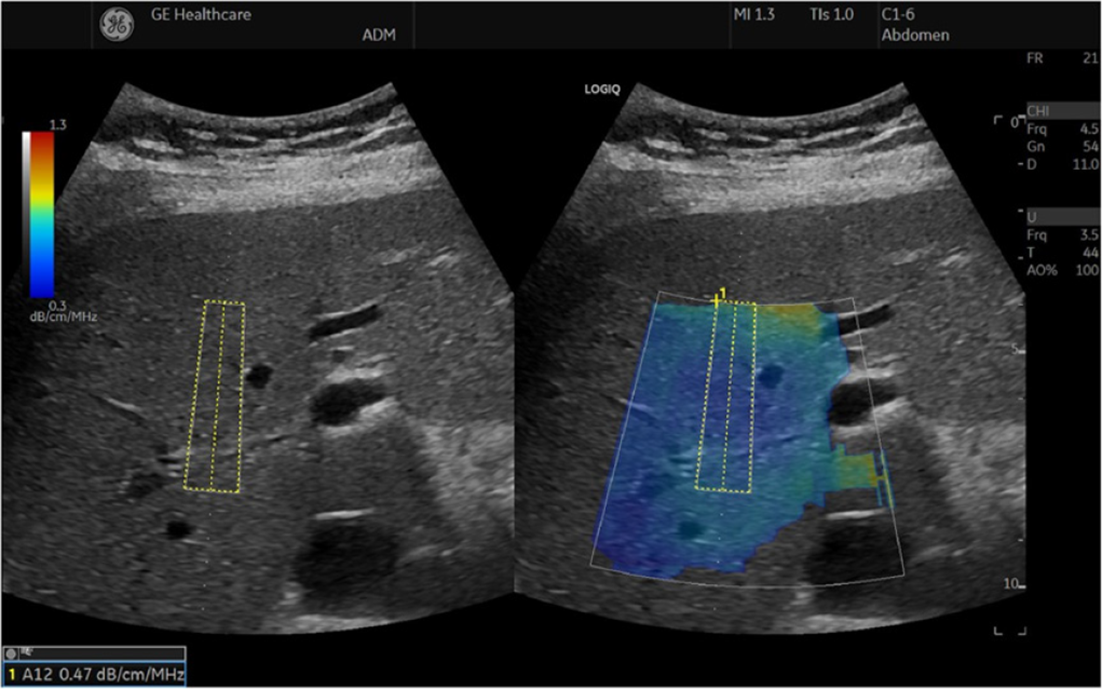
With known attenuation and BSC, an ultrasound system uses a phantom method to calculate attenuation coefficients measurement implemented in UGAP (Figure 4). Using this method, the US system's transmitting and receiving beamforming characteristics can be compensated. The result is presented in units of dB/cm/MHz[4,74-76]. Several studies reported good diagnostic efficacy of UGAP for liver fat content quantification applying hepatic histological results as the gold standard, and a positive association was found between UGAP and steatosis percentage (correlation coefficient: 0.78-0.81). The reported AUROCs were 0.89-0.92 for detecting steatosis grade ≥ S1, 0.90–0.95 for detecting steatosis grade ≥ S2, and 0.88-0.96 for detecting steatosis grade = S3[75,77-79]. Several other studies compared UGAP with MR-based methods, and a significant correlation between MR-based methods and attenuation coefficient values by UGAP was found (correlation coefficient: 0.72-0.77)[76,80]. Imajo et al[76] conducted a multicentric study with 1010 patients and reported that UGAP had good diagnostic efficacy for making quantified measurement of liver fat content. In their study, the AUROCs were 0.910 for detecting MRI-PDFF ≥ 5.2%, 0.912 for MRI-PDFF ≥ 11.3%, and 0.894 for MRI-PDFF ≥17.1%[76]. Fujiwara et al[75] reported that as compared to CAP, UGAP achieved significantly higher AUROCs for identifying ≥ S2 (0.950 vs 0.841) and ≥ S3 (0.959 vs 0.817). In addition, they also reported 5.2% of CAP patients had measurement failures, while no UGAP patients did. Tada et al[81] reported that there was no effect of liver stiffness on UGAP attenuation coefficient values.
Attenuation plane-wave ultrasound: Attenuation Plane-Wave Ultrasound (Att. PLUS) presents information on ultraphonic beam attenuation through a region of interest. The ultrasound beam attenuation is calculated in a region of interest at a constant depth. The attenuation coefficient results are displayed in units of dB/cm/MHz[56]. The Att. PLUS measurement is combined with the sound speed measurement for each acquisition. It is the median of five measurements taken consecutively that determines the final result[35]. Only one published study regarding this method was found. Popa et al[82] carried out a study aiming to assess the clinical value of Att. PLUS of noninvasive measurement of fatty liver with the CAP value considered as control. They reported that the cutoff value to detect S2-S3 was 0.5 dB/cm/MHz (sensitivity 53.1%, specificity 82.0%), and the AUROC was 0.72.
Tissue attenuation imaging and tissue scatter distribution imaging: Tissue attenuation imaging (TAI) parameter indicates slope of the ultrasound central frequency downshift along depth, which is able to be utilized to calculate acoustic attenuation. The tissue scatter distribution imaging (TSI) parameter is a measurement of the Nakagami parameters in the region of interest, which reflects the concentration of ultrasound scatterers and their arrangement locally[35,83].
We found three studies comparing TAI and TSI with MRI-PDFF, and these studies revealed that both TAI and TSI revealed correlation with MRI-PDFF[84-86]. Jeon et al[86] enrolled 120 patients to assess feasibility of TAI and TSI for hepatic steatosis quantification utilizing MRI-PDFF as the reference. According to MRI-PDFF, the participants were classified into three groups (≤ 5%, 5%-10%, and ≥ 10%). They found that both methods had excellent utility for diagnosing and evaluating the degree of hepatic steatosis. For diagnosing fatty quantification of ≥ 5% and ≥ 10%, the AUROCs of TAI were 0.861 and 0.835, and those of TSI were 0.964 and 0.935, respectively[86]. Rónaszéki et al[84] compared TAI with TSI utilizing MRI-PDFF as gold standard enrolling 101 participants and found that TAI provided better diagnostic performance than TSI for diagnosing ≥ 5% MRI-PDFF (AUROC: 0.89 vs 0.87) and ≥ 10% (AUROC: 0.93 vs 0.86). TAI and TSI revealed good intra- and interobserver agreement. In TAI, the intra- and interobserver ICCs were reported at 0.994 and 0.975, respectively, while in TSI, they were reported at 0.991 and 0.947[87].
Techniques based on ultrasound BSC: Using the BSC, we can determine amount of ultrasound energy reflected by the tissue. Applying computer algorithm and a reference phantom, the BSC can be estimated with less changeability resulted from ultrasound systems and operators. The right liver lobe was used to obtain gray scale images, and in the same liver region, a continuous series of 10 frames of transducer signals was captured. Then, in the tissue-imitating reference phantom, which mimics the acoustic properties of human hepatic tissue, consecutive frames were noted without changing scanner settings[35,88-90].
The diagnostic accuracy of the BSC has been evaluated by Lin et al[91] by analyzing 204 participants. They found that BSC was positively correlated with MRI-PDFF (Spearman’s ρ = 0.80; P < 0.0001). BSC had an AUROC of 0.98 with a cutoff value of 0.00381/cm-steradian for detecting patients with hepatic steatosis. In addition, when using the optimal BSC cutoff value, in the training group, hepatic steatosis was detected with 93% sensitivity and 97% specificity, while in the validation group, it was detected with 87% sensitivity and 91% specificity[91].
Han et al[89,90,92,93] published several studies focusing on the use of the BSC. In a study including 102 participants, they revealed moderate correlation of the BSC with MRI-PDFF (Pearson’s r = 0.58, P < 0.001)[93]. In addition, they enrolled 41 participants to study the repeatability and reproducibility of BSC and found that ICC were 0.87-0.95 for BSC acquired without participant repositioning and 0.69-0.82 with participant repositioning, suggesting that BSC measurement is repeatable and reproducible in patients with NAFLD[89].
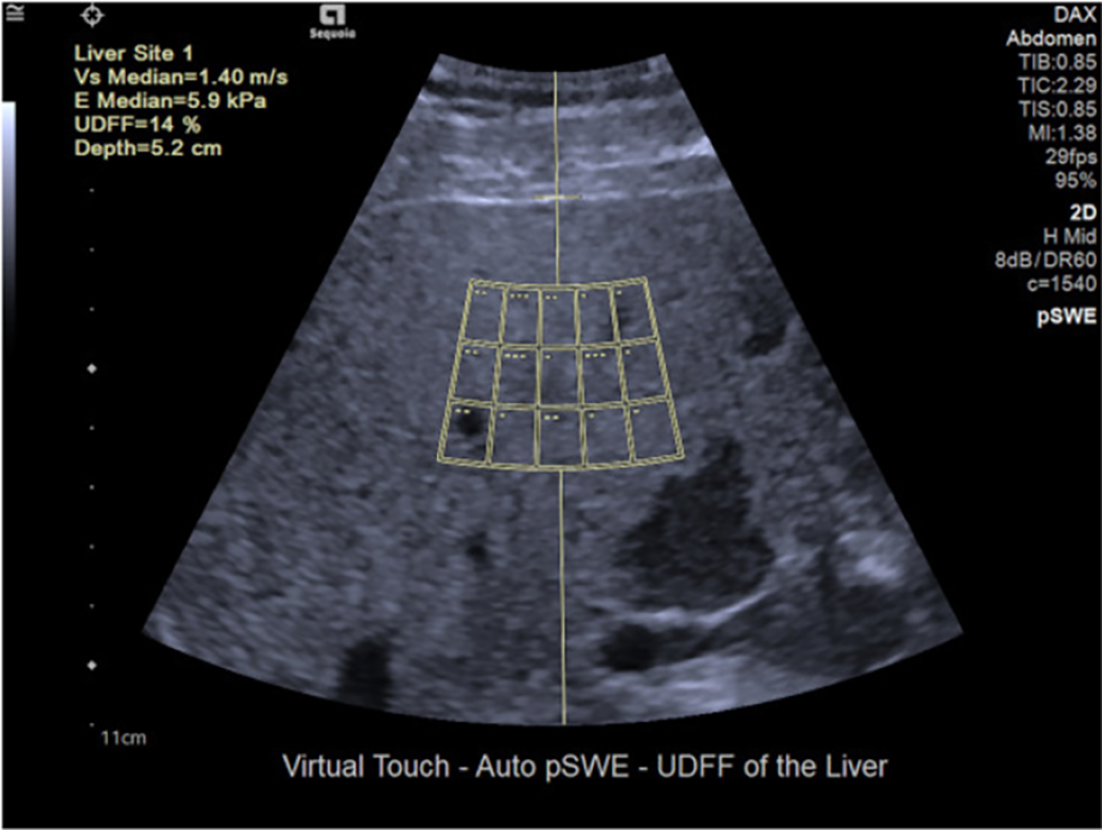
The ultrasound-derived fat fraction (UDFF) technique is a coalition of attenuation coefficient and BSC, and a percentage of liver fat content is reported as the result. Data from reference phantoms is integrated into the ultrasound system, and a fixed-acquisition region of interest is utilized[4] (Figure 5). Labyed and Milkowski[37] designed the UDFF method and conducted a study including 101 participants. They found that the UDFF was positively correlated with the MRI-PDFF (Pearson’s r = 0.87). Using the histology results as the gold standard, the AUROCs of UDFF were 0.94 for detecting S ≥ 1, 0.88 for S ≥ 2 and 0.83 for S = 3. When using MRI-PDFF to be the gold standard, AUROCs of UDFF were 0.97 for diagnosing MRI-PDFF higher than 5%, 0.95 for diagnosing MRI-PDFF higher than 10%[37]. Similarly, Dillman et al[94] reported that liver fat content quantification applying UDFF showed a significant correlation with MRI-PDFF (Spearman’s ρ = 0.82; P < 0.001).
ASQ: Quantifying the acoustic structure of an environment by comparing theoretical and real echo amplitude distributions is referred to as ASQ. In order to compute the theoretical echo amplitude distribution of the hepatic section imaged, the Rayleigh distribution function, assuming that solely ultrasound beam interference from small scattering objects generates the speckle pattern, is applied. However, actual echo amplitude distribution of the liver parenchyma does not follow the Rayleigh distribution. Because ultrasound beams are scattered by small structures, for example the walls of hepatic vessels, resulting in heterogeneity in echo amplitudes[95,96]. However, when diffuse liver diseases cause changes in parenchymal echotexture, ASQ can provide quantitative information by comparing theoretical echo amplitude distribution to a real distribution[97]. Kuroda et al[98] tested the ASQ-derived focal disturbance ratio (FD ratio) with 9 Leptin-deficient mice in comparison with histopathological results and found that the FD ratio had significant negative correlations with the fat droplet area (Spearman r = -0.72, P = 0.0017) and fat droplet size (Spearman r = -0.98, P = 0.0052), suggesting that the FD ratio can be used to quantify steatosis grade in an animal model and may be a quantitative metric of hepatic steatosis[98]. Karlas et al[95] conducted a cohort study to compare ASQ with MRS, and negative correlation was found between FD ratio and MRS (Spearman r = -0.43, P = 0.004). Similarly, in a prospective study including 36 patients with suspected fatty liver disease, the FD ratio showed a strong, negative correlation with the MRS in 36 patients[99]. Son et al[100] also reported FD ratio is comparable to hepatic fat fraction by MRS to make quantified measurement of liver fat content and diagnose liver fat content more than 10% in donor liver patients. Keller et al[96] found significant negative correlation between ASQ and steatosis level obtained by histological examination (r = -0.55, P < 0.0001). Nevertheless, they found no correlation between histologically determined fibrosis stage and any measurements of ASQ.
NLV: The NLV is derived from ASQ and analyzes ultrasound amplitudes sampled from grayscale ultrasound images[4,101]. Bae et al[102] assessed the clinical value of the NLV in the measurement of liver fat content in comparison with MRS in 40 male mice using histopathology as the golden standard and found that the AUROCs for diagnosing mild, moderate, and severe hepatic steatosis were 0.953, 0.896, and 0.735, and the NLV value performed similarly to MRS in detecting mild or moderate hepatic steatosis. The same authors also conducted a study with 194 patients to assess the diagnostic efficacy of the NLV for diagnosing and grading liver fat content using liver histopathology as the reference standard. They demonstrated the NLV had excellent diagnostic efficacy in detecting and grading fatty liver with AUROCs of 0.911 for ≥ steatosis grade 1, 0.974 for ≥ steatosis grade 2, and 0.954 ≥ steatosis grade 3[103].
Speed of sound: A speed of sound (SS) estimation is based on the fact that sound speed varies with fat content in soft tissues, and that the relationship between sound speed and liver fat percentage can be identified[104]. Dioguardi Burgio et al[104] carried out a study aiming to explore the value of SS for detecting and quantifying liver steatosis and included 100 patients who underwent both SS and abdominal MR. They found that, in the training cohort, a cut-off value of less than 1.537mm/s led to 87% sensitivity and 95.7% specificity for diagnosing any steatosis with an AUROC of 0.882%. Based on an SS cut-off value of 1.511mm/s, the sensitivity was 100% and specificity was 95.6% for detecting moderate to severe steatosis and the AUROC was 0.989[104].
Sound speed plane-wave ultrasound (SSp.PLUS) is a novel technique for measuring intrahepatic sound speed which is correlated with the liver fat content. The measurement of SSp.PLUS is expressed in m/s[82]. Popa et al[82] performed a study with 215 patients to test the value of SSp.PLUS in detecting and grading hepatic fat level applying the CAP value as the gold standard. As a first finding, SSp.PLUS is more closely correlated with CAP values than Att.PLUS: (r = -0.74) vs (r = 0.45). Furthermore, the SSp.PLUS cut-off of less than 1516 m/s indicated 98.36% specificity and 58.74% sensitivity for predicting the presence of significant steatosis (S2-S3)[82].
Quantification ultrasound techniques can provide objective continuous number associated with liver fat content and past records can be found when patients receiving check-ups to evaluate change of degree of fatty liver, which is useful for follow-up to monitor the impact of any clinical interventions. Besides, as hepatic steatosis may pose adverse effects to prognosis of patients, quantification of liver fat holds clinical significance. For example, substantial hepatic fat accumulation may contribute to rapid disease progression toward NASH or liver fibrosis[105]. Patients with liver resections are more likely to suffer postoperative complications and die due to liver fat accumulation. Compared with patients without steatosis, those with ≤ 30% steatosis have a significantly increased risk of postoperative complications and patients with > 30% steatosis have an increased risk of postoperative death[9,106,107]. It is worth to be mentioned that simple steatosis may lead to poor prognosis. A study carried out in a nationwide Swedish cohort from 1966 to 2017 including 10568 patients found that simple steatosis, non-fibrotic NASH, non-cirrhotic fibrosis, and cirrhosis were associated with significant higher hazard ratio for mortality risk compared with controls. The all-cause mortalities of cohorts with simple steatosis, non-fibrotic NASH, non-cirrhotic fibrosis, and cirrhosis were 2.52% person-years, 3.03% person-years, 3.53% person-years, and 7.05% person-years respectively whereas the mortality of population comparators was 1.69% person-years[108]. Association between imaging quantification method and clinical prognosis is another issue. In patients with chronic hepatitis C, CAP value ≤ 221 dB/m is associated with higher risk of HCC and in patients with NAFLD, CAP value ≤ 265 dB/m is associated with higher risk of HCC[109]. Similarly, in another cross-sectional study including 130 patients (HCC) and 54 patients (chronic hepatitis C), the authors reported that CAP value of chronic hepatitis C group was significantly higher than that of HCC group (259.96 dB/m vs 209.57 dB/m, P < 0.001)[110].
While serving as the conventional reference standard, liver histopathologic test has the limitations of invasiveness, sampling error, and high cost. Issues including availability, cost, accuracy and reliability should be taken into consideration when choosing the optimal noninvasive methods. The further application of noninvasive methods is desirable for detecting and grading hepatic steatosis at the initial diagnosis and monitoring changes in liver fat content during follow-up after receiving clinical therapies.
MRS and MRI-PDFF are reported to be the most accurate imaging modalities for quantified measurement of liver fat content. However, their low accessibility and high cost make it impossible to use MR-based techniques as repeatable methods to monitor the process of liver steatosis. Therefore, ultrasound-based techniques are more desirable with the advantages of portability and cost-effectiveness. CAP is the first method based on attenuation of the ultrasound beam, and its performance has been validated in several studies. However, the limitations of CAP are nonnegligible in that due to its blindness, it has a high rate of measurement failures because it cannot determine the exact location of the region of interest. UGAP, ATT and ATI have been developed to improve this situation, and these metrics can be used to evaluate degree of fatty liver on gray scale ultrasonography in real-time with a correct region of interest. The CAP measurement also showed suboptimal performance in quantifying liver fat content especially in mild steatosis, which limited its use as a golden standard to evaluate the efficacy of novel imaging methods for liver fat content quantification. In addition, techniques derived from other principles, such as ASQ, TSI and UDFF, have been developed. These techniques are reported to have nice clinical efficacy for liver fat quantification. Nevertheless, studies exploring value of such techniques enrolled a small number of participants. Therefore, future studies enrolling more participants are needed to test the utility of such techniques. Besides, imaging-based techniques may have some limitations. For example, CAP, ATI and MRI-PDFF may be unable to differentiate grade 2 with grade 3 liver steatosis.
Several hepatic steatosis quantification tools are launched by commercial platforms. Larger clinical studies are needed to compare the efficacy among different products. For patients with NAFLD, except for steatosis, inflammation and fibrosis are also significant features which are associated with prognosis. The steatosis measurement is able to be obtained together with the stiffness value by some tools. In this way, comprehensive evaluation of patients with NAFLD can be made. Except for elastography tools, ASQ has also been studied to evaluate liver stiffness. Hepatic steatosis measurement and stiffness measurement, in conjunction with other ultrasound methods, are promising tools for patients with diffuse liver disease to supervise curative effect and disease progression. Developing such a multi-parametric ultrasound modality will require future studies.
We thank all medical staff and technicians who agreed to participate in this review.
| 1. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 4028] [Article Influence: 503.5] [Reference Citation Analysis (2)] |
| 2. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1949] [Article Influence: 389.8] [Reference Citation Analysis (33)] |
| 3. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5235] [Article Influence: 654.4] [Reference Citation Analysis (9)] |
| 4. | Ferraioli G, Berzigotti A, Barr RG, Choi BI, Cui XW, Dong Y, Gilja OH, Lee JY, Lee DH, Moriyasu F, Piscaglia F, Sugimoto K, Wong GL, Wong VW, Dietrich CF. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med Biol. 2021;47:2803-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7961] [Article Influence: 796.1] [Reference Citation Analysis (8)] |
| 6. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 900] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 7. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2092] [Cited by in RCA: 2150] [Article Influence: 102.4] [Reference Citation Analysis (1)] |
| 8. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 934] [Article Influence: 62.3] [Reference Citation Analysis (1)] |
| 9. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A, Terrault N, Pazienza V, Giordani MT, Giostra E, Sonzogni A, Ruggiero G, Marcellin P, Powell EE, George J, Negro F; HCV Meta-Analysis (on) Individual Patients' Data Study Group. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 409] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Linares I, Hamar M, Selzner N, Selzner M. Steatosis in Liver Transplantation: Current Limitations and Future Strategies. Transplantation. 2019;103:78-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Hossain N, Kanwar P, Mohanty SR. A Comprehensive Updated Review of Pharmaceutical and Nonpharmaceutical Treatment for NAFLD. Gastroenterol Res Pract. 2016;2016:7109270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Ajmera V, Park CC, Caussy C, Singh S, Hernandez C, Bettencourt R, Hooker J, Sy E, Behling C, Xu R, Middleton MS, Valasek MA, Faulkner C, Rizo E, Richards L, Sirlin CB, Loomba R. Magnetic Resonance Imaging Proton Density Fat Fraction Associates With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018;155:307-310.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Stine JG, Munaganuru N, Barnard A, Wang JL, Kaulback K, Argo CK, Singh S, Fowler KJ, Sirlin CB, Loomba R. Change in MRI-PDFF and Histologic Response in Patients With Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2021;19:2274-2283.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 14. | Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quiñones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443-457.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 642] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 15. | Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Wildman-Tobriner B, Middleton MM, Moylan CA, Rossi S, Flores O, Chang ZA, Abdelmalek MF, Sirlin CB, Bashir MR. Association Between Magnetic Resonance Imaging-Proton Density Fat Fraction and Liver Histology Features in Patients With Nonalcoholic Fatty Liver Disease or Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1428-1435.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol. 2022;18:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 18. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8585] [Article Influence: 408.8] [Reference Citation Analysis (9)] |
| 19. | Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, Colca JR, Iwashita J, Koch GG, Dittrich HC. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 20. | Wong VW, Adams LA, de Lédinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. 2018;15:461-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 462] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 21. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1123] [Article Influence: 160.4] [Reference Citation Analysis (1)] |
| 22. | Barr RG. Ultrasound of Diffuse Liver Disease Including Elastography. Radiol Clin North Am. 2019;57:549-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1468] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 24. | Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 499] [Article Influence: 29.4] [Reference Citation Analysis (3)] |
| 25. | Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, Clark JM. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 1175] [Article Influence: 78.3] [Reference Citation Analysis (2)] |
| 26. | Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189:W320-W323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 329] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 27. | Cengiz M, Sentürk S, Cetin B, Bayrak AH, Bilek SU. Sonographic assessment of fatty liver: intraobserver and interobserver variability. Int J Clin Exp Med. 2014;7:5453-5460. [PubMed] |
| 28. | Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996;24:25-29. [PubMed] [DOI] [Full Text] |
| 29. | Mancini M, Prinster A, Annuzzi G, Liuzzi R, Giacco R, Medagli C, Cremone M, Clemente G, Maurea S, Riccardi G, Rivellese AA, Salvatore M. Sonographic hepatic-renal ratio as indicator of hepatic steatosis: comparison with (1)H magnetic resonance spectroscopy. Metabolism. 2009;58:1724-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Webb M, Yeshua H, Zelber-Sagi S, Santo E, Brazowski E, Halpern Z, Oren R. Diagnostic value of a computerized hepatorenal index for sonographic quantification of liver steatosis. AJR Am J Roentgenol. 2009;192:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Shiralkar K, Johnson S, Bluth EI, Marshall RH, Dornelles A, Gulotta PM. Improved method for calculating hepatic steatosis using the hepatorenal index. J Ultrasound Med. 2015;34:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Marshall RH, Eissa M, Bluth EI, Gulotta PM, Davis NK. Hepatorenal index as an accurate, simple, and effective tool in screening for steatosis. AJR Am J Roentgenol. 2012;199:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Borges VF, Diniz AL, Cotrim HP, Rocha HL, Andrade NB. Sonographic hepatorenal ratio: a noninvasive method to diagnose nonalcoholic steatosis. J Clin Ultrasound. 2013;41:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Stahlschmidt FL, Tafarel JR, Menini-Stahlschmidt CM, Baena CP. Hepatorenal index for grading liver steatosis with concomitant fibrosis. PLoS One. 2021;16:e0246837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Bozic D, Podrug K, Mikolasevic I, Grgurevic I. Ultrasound Methods for the Assessment of Liver Steatosis: A Critical Appraisal. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Kjaergaard M, Lindvig KP, Hansen CD, Detlefsen S, Krag A, Thiele M. Hepatorenal Index by B-Mode Ratio Versus Imaging and Fatty Liver Index to Diagnose Steatosis in Alcohol-Related and Nonalcoholic Fatty Liver Disease. J Ultrasound Med. 2023;42:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Labyed Y, Milkowski A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J Ultrasound Med. 2020;39:2427-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 38. | Oelze ML, Mamou J. Review of Quantitative Ultrasound: Envelope Statistics and Backscatter Coefficient Imaging and Contributions to Diagnostic Ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2016;63:336-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 39. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 656] [Article Influence: 41.0] [Reference Citation Analysis (3)] |
| 40. | Seneviratne N, Fang C, Sidhu PS. Ultrasound-based hepatic fat quantification: current status and future directions. Clin Radiol. 2023;78:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25:6053-6062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 324] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (8)] |
| 42. | de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 43. | Caussy C, Brissot J, Singh S, Bassirian S, Hernandez C, Bettencourt R, Rizo E, Richards L, Sirlin CB, Loomba R. Prospective, Same-Day, Direct Comparison of Controlled Attenuation Parameter With the M vs the XL Probe in Patients With Nonalcoholic Fatty Liver Disease, Using Magnetic Resonance Imaging-Proton Density Fat Fraction as the Standard. Clin Gastroenterol Hepatol. 2020;18:1842-1850.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Park J, Lee JM, Lee G, Jeon SK, Joo I. Quantitative Evaluation of Hepatic Steatosis Using Advanced Imaging Techniques: Focusing on New Quantitative Ultrasound Techniques. Korean J Radiol. 2022;23:13-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC, Ferraioli G, Chan WK, Wong VW, Myers RP, Chayama K, Friedrich-Rust M, Beaugrand M, Shen F, Hiriart JB, Sarin SK, Badea R, Jung KS, Marcellin P, Filice C, Mahadeva S, Wong GL, Crotty P, Masaki K, Bojunga J, Bedossa P, Keim V, Wiegand J. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 933] [Article Influence: 103.7] [Reference Citation Analysis (2)] |
| 46. | Petroff D, Blank V, Newsome PN, Shalimar, Voican CS, Thiele M, de Lédinghen V, Baumeler S, Chan WK, Perlemuter G, Cardoso AC, Aggarwal S, Sasso M, Eddowes PJ, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Cobbold JF, Naveau S, Lupsor-Platon M, Mueller S, Krag A, Irles-Depe M, Semela D, Wong GL, Wong VW, Villela-Nogueira CA, Garg H, Chazouillères O, Wiegand J, Karlas T. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 47. | Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, Ajmera V, Bettencourt R, Collier S, Hooker J, Sy E, Rizo E, Richards L, Sirlin CB, Loomba R. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67:1348-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 48. | Shao CX, Ye J, Dong Z, Li F, Lin Y, Liao B, Feng S, Zhong B. Steatosis grading consistency between controlled attenuation parameter and MRI-PDFF in monitoring metabolic associated fatty liver disease. Ther Adv Chronic Dis. 2021;12:20406223211033119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Runge JH, Smits LP, Verheij J, Depla A, Kuiken SD, Baak BC, Nederveen AJ, Beuers U, Stoker J. MR Spectroscopy-derived Proton Density Fat Fraction Is Superior to Controlled Attenuation Parameter for Detecting and Grading Hepatic Steatosis. Radiology. 2018;286:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 50. | Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H, Sumida Y, Ono M, Eguchi Y, Inoue T, Yamanaka T, Wada K, Saito S, Nakajima A. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626-637.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 628] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 51. | de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 52. | Yoo J, Lee JM, Joo I, Lee DH, Yoon JH, Kang HJ, Ahn SJ. Reproducibility of ultrasound attenuation imaging for the noninvasive evaluation of hepatic steatosis. Ultrasonography. 2020;39:121-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 53. | Yuri M, Nishimura T, Tada T, Yoshida M, Fujiwara A, Kawata S, Yoshihara K, Yoshioka R, Ota S, Nakano R, Yuri Y, Takashima T, Aizawa N, Ikeda N, Shiomi H, Ide YH, Enomoto H, Yasuhiro F, Yano H, Iijima H. Diagnosis of hepatic steatosis based on ultrasound attenuation imaging is not influenced by liver fibrosis. Hepatol Res. 2022;52:1009-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Tamaki N, Koizumi Y, Hirooka M, Yada N, Takada H, Nakashima O, Kudo M, Hiasa Y, Izumi N. Novel quantitative assessment system of liver steatosis using a newly developed attenuation measurement method. Hepatol Res. 2018;48:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 55. | Tamaki N, Kurosaki M, Yasui Y, Tsuchiya K, Izumi N. Attenuation coefficient (ATT) measurement for liver fat quantification in chronic liver disease. J Med Ultrason (2001). 2021;48:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Ferraioli G, Kumar V, Ozturk A, Nam K, de Korte CL, Barr RG. US Attenuation for Liver Fat Quantification: An AIUM-RSNA QIBA Pulse-Echo Quantitative Ultrasound Initiative. Radiology. 2022;302:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 57. | Kwon EY, Kim YR, Kang DM, Yoon KH, Lee YH. Usefulness of US attenuation imaging for the detection and severity grading of hepatic steatosis in routine abdominal ultrasonography. Clin Imaging. 2021;76:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Jang JK, Kim SY, Yoo IW, Cho YB, Kang HJ, Lee DH. Diagnostic performance of ultrasound attenuation imaging for assessing low-grade hepatic steatosis. Eur Radiol. 2022;32:2070-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 59. | Bae JS, Lee DH, Lee JY, Kim H, Yu SJ, Lee JH, Cho EJ, Lee YB, Han JK, Choi BI. Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol. 2019;29:6499-6507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (2)] |
| 60. | Bae JS, Lee DH, Suh KS, Kim H, Lee KB, Lee JY, Han JK. Noninvasive assessment of hepatic steatosis using a pathologic reference standard: comparison of CT, MRI, and US-based techniques. Ultrasonography. 2022;41:344-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Jeon SK, Lee JM, Joo I, Yoon JH, Lee DH, Lee JY, Han JK. Prospective Evaluation of Hepatic Steatosis Using Ultrasound Attenuation Imaging in Patients with Chronic Liver Disease with Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Ultrasound Med Biol. 2019;45:1407-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 62. | Tada T, Iijima H, Kobayashi N, Yoshida M, Nishimura T, Kumada T, Kondo R, Yano H, Kage M, Nakano C, Aoki T, Aizawa N, Ikeda N, Takashima T, Yuri Y, Ishii N, Hasegawa K, Takata R, Yoh K, Sakai Y, Nishikawa H, Iwata Y, Enomoto H, Hirota S, Fujimoto J, Nishiguchi S. Usefulness of Attenuation Imaging with an Ultrasound Scanner for the Evaluation of Hepatic Steatosis. Ultrasound Med Biol. 2019;45:2679-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 63. | Tada T, Kumada T, Toyoda H, Nakamura S, Shibata Y, Yasuda S, Watanuki Y, Tsujii K, Fukuda N, Fujioka M, Takeshima K, Niwa F, Ogawa S, Hashinokuchi S, Kataoka S, Ichikawa H, Iijima H. Attenuation imaging based on ultrasound technology for assessment of hepatic steatosis: A comparison with magnetic resonance imaging-determined proton density fat fraction. Hepatol Res. 2020;50:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 64. | Jeon SK, Lee JM, Joo I, Yoon JH. Assessment of the inter-platform reproducibility of ultrasound attenuation examination in nonalcoholic fatty liver disease. Ultrasonography. 2022;41:355-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Ferraioli G, Maiocchi L, Raciti MV, Tinelli C, De Silvestri A, Nichetti M, De Cata P, Rondanelli M, Chiovato L, Calliada F, Filice C. Detection of Liver Steatosis With a Novel Ultrasound-Based Technique: A Pilot Study Using MRI-Derived Proton Density Fat Fraction as the Gold Standard. Clin Transl Gastroenterol. 2019;10:e00081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 66. | Ferraioli G, Maiocchi L, Savietto G, Tinelli C, Nichetti M, Rondanelli M, Calliada F, Preda L, Filice C. Performance of the Attenuation Imaging Technology in the Detection of Liver Steatosis. J Ultrasound Med. 2021;40:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 67. | Sugimoto K, Abe M, Oshiro H, Takahashi H, Kakegawa T, Tomita Y, Yoshimasu Y, Takeuchi H, Itoi T. The most appropriate region-of-interest position for attenuation coefficient measurement in the evaluation of liver steatosis. J Med Ultrason (2001). 2021;48:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Lee DH. Quantitative assessment of fatty liver using ultrasound attenuation imaging. J Med Ultrason (2001). 2021;48:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Hsu PK, Wu LS, Su WW, Su PY, Chen YY, Hsu YC, Yen HH, Wu CL. Comparing the controlled attenuation parameter using FibroScan and attenuation imaging with ultrasound as a novel measurement for liver steatosis. PLoS One. 2021;16:e0254892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Hsu PK, Wu LS, Yen HH, Huang HP, Chen YY, Su PY, Su WW. Attenuation Imaging with Ultrasound as a Novel Evaluation Method for Liver Steatosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 71. | Cerit M, Şendur HN, Cindil E, Erbaş G, Yalçın MM, Cerit ET, Allahverdiyeva S, Oktar SÖ, Yücel C. Quantification of liver fat content with ultrasonographic attenuation measurement function: Correlation with unenhanced multidimensional computerized tomography. Clin Imaging. 2020;65:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Ferraioli G, Raimondi A, Maiocchi L, De Silvestri A, Filice C. Quantification of Liver Fat Content with the iATT Algorithm: Correlation with Controlled Attenuation Parameter. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Koizumi Y, Hirooka M, Tamaki N, Yada N, Nakashima O, Izumi N, Kudo M, Hiasa Y. New diagnostic technique to evaluate hepatic steatosis using the attenuation coefficient on ultrasound B mode. PLoS One. 2019;14:e0221548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Bende F, Sporea I, Șirli R, Bâldea V, Lazăr A, Lupușoru R, Fofiu R, Popescu A. Ultrasound-Guided Attenuation Parameter (UGAP) for the quantification of liver steatosis using the Controlled Attenuation Parameter (CAP) as the reference method. Med Ultrason. 2021;23:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Fujiwara Y, Kuroda H, Abe T, Ishida K, Oguri T, Noguchi S, Sugai T, Kamiyama N, Takikawa Y. The B-Mode Image-Guided Ultrasound Attenuation Parameter Accurately Detects Hepatic Steatosis in Chronic Liver Disease. Ultrasound Med Biol. 2018;44:2223-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 76. | Imajo K, Toyoda H, Yasuda S, Suzuki Y, Sugimoto K, Kuroda H, Akita T, Tanaka J, Yasui Y, Tamaki N, Kurosaki M, Izumi N, Nakajima A, Kumada T. Utility of Ultrasound-Guided Attenuation Parameter for Grading Steatosis With Reference to MRI-PDFF in a Large Cohort. Clin Gastroenterol Hepatol. 2022;20:2533-2541.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 77. | Ogino Y, Wakui N, Nagai H, Igarashi Y. The ultrasound-guided attenuation parameter is useful in quantification of hepatic steatosis in non-alcoholic fatty liver disease. JGH Open. 2021;5:947-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Kuroda H, Abe T, Fujiwara Y, Nagasawa T, Takikawa Y. Diagnostic accuracy of ultrasound-guided attenuation parameter as a noninvasive test for steatosis in non-alcoholic fatty liver disease. J Med Ultrason (2001). 2021;48:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 79. | Kuroda H, Fujiwara Y, Abe T, Nagasawa T, Oguri T, Noguchi S, Kamiyama N, Takikawa Y. Two-dimensional shear wave elastography and ultrasound-guided attenuation parameter for progressive non-alcoholic steatohepatitis. PLoS One. 2021;16:e0249493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 80. | Tada T, Kumada T, Toyoda H, Kobayashi N, Sone Y, Oguri T, Kamiyama N. Utility of Attenuation Coefficient Measurement Using an Ultrasound-Guided Attenuation Parameter for Evaluation of Hepatic Steatosis: Comparison With MRI-Determined Proton Density Fat Fraction. AJR Am J Roentgenol. 2019;212:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 81. | Tada T, Kumada T, Toyoda H, Yasuda S, Sone Y, Hashinokuchi S, Ogawa S, Oguri T, Kamiyama N, Chuma M, Akita T, Tanaka J. Liver stiffness does not affect ultrasound-guided attenuation coefficient measurement in the evaluation of hepatic steatosis. Hepatol Res. 2020;50:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Popa A, Bende F, Șirli R, Popescu A, Bâldea V, Lupușoru R, Cotrău R, Fofiu R, Foncea C, Sporea I. Quantification of Liver Fibrosis, Steatosis, and Viscosity Using Multiparametric Ultrasound in Patients with Non-Alcoholic Liver Disease: A "Real-Life" Cohort Study. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 83. | Jeon SK, Lee JM, Joo I. Clinical Feasibility of Quantitative Ultrasound Imaging for Suspected Hepatic Steatosis: Intra- and Inter-examiner Reliability and Correlation with Controlled Attenuation Parameter. Ultrasound Med Biol. 2021;47:438-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 84. | Rónaszéki AD, Budai BK, Csongrády B, Stollmayer R, Hagymási K, Werling K, Fodor T, Folhoffer A, Kalina I, Győri G, Maurovich-Horvat P, Kaposi PN. Tissue attenuation imaging and tissue scatter imaging for quantitative ultrasound evaluation of hepatic steatosis. Medicine (Baltimore). 2022;101:e29708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 85. | Şendur HN, Özdemir Kalkan D, Cerit MN, Kalkan G, Şendur AB, Özhan Oktar S. Hepatic Fat Quantification With Novel Ultrasound Based Techniques: A Diagnostic Performance Study Using Magnetic Resonance Imaging Proton Density Fat Fraction as Reference Standard. Can Assoc Radiol J. 2023;74:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 86. | Jeon SK, Lee JM, Joo I, Park SJ. Quantitative Ultrasound Radiofrequency Data Analysis for the Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Using Magnetic Resonance Imaging Proton Density Fat Fraction as the Reference Standard. Korean J Radiol. 2021;22:1077-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 87. | Şendur HN, Cerit MN, Ibrahimkhanli N, Şendur AB, Özhan Oktar S. Interobserver Variability in Ultrasound-Based Liver Fat Quantification. J Ultrasound Med. 2023;42:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Coila A, Oelze ML. Effects of acoustic nonlinearities on the ultrasonic backscatter coefficient estimation. J Acoust Soc Am. 2019;146:85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Han A, Andre MP, Deiranieh L, Housman E, Erdman JW Jr, Loomba R, Sirlin CB, O'Brien WD Jr. Repeatability and Reproducibility of the Ultrasonic Attenuation Coefficient and Backscatter Coefficient Measured in the Right Lobe of the Liver in Adults With Known or Suspected Nonalcoholic Fatty Liver Disease. J Ultrasound Med. 2018;37:1913-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 90. | Han A, Zhang YN, Boehringer AS, Andre MP, Erdman JW Jr, Loomba R, Sirlin CB, O'Brien WD Jr. Inter-platform reproducibility of ultrasonic attenuation and backscatter coefficients in assessing NAFLD. Eur Radiol. 2019;29:4699-4708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 91. | Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, Erdman JW Jr, O'Brien WD Jr, Andre MP, Sirlin CB, Loomba R. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease and Quantification of Liver Fat Using a New Quantitative Ultrasound Technique. Clin Gastroenterol Hepatol. 2015;13:1337-1345.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 92. | Han A, Labyed Y, Sy EZ, Boehringer AS, Andre MP, Erdman JW Jr, Loomba R, Sirlin CB, O'Brien WD Jr. Inter-sonographer reproducibility of quantitative ultrasound outcomes and shear wave speed measured in the right lobe of the liver in adults with known or suspected non-alcoholic fatty liver disease. Eur Radiol. 2018;28:4992-5000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 93. | Han A, Zhang YN, Boehringer AS, Montes V, Andre MP, Erdman JW Jr, Loomba R, Valasek MA, Sirlin CB, O'Brien WD Jr. Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease by Using Quantitative US. Radiology. 2020;295:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 94. | Dillman JR, Thapaliya S, Tkach JA, Trout AT. Quantification of Hepatic Steatosis by Ultrasound: Prospective Comparison With MRI Proton Density Fat Fraction as Reference Standard. AJR Am J Roentgenol. 2022;219:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 95. | Karlas T, Berger J, Garnov N, Lindner F, Busse H, Linder N, Schaudinn A, Relke B, Chakaroun R, Tröltzsch M, Wiegand J, Keim V. Estimating steatosis and fibrosis: Comparison of acoustic structure quantification with established techniques. World J Gastroenterol. 2015;21:4894-4902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 96. | Keller J, Kaltenbach TE, Haenle MM, Oeztuerk S, Graeter T, Mason RA, Seufferlein T, Kratzer W. Comparison of Acoustic Structure Quantification (ASQ), shearwave elastography and histology in patients with diffuse hepatopathies. BMC Med Imaging. 2015;15:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Lin YH, Wan YL, Tai DI, Tseng JH, Wang CY, Tsai YW, Lin YR, Chang TY, Tsui PH. Considerations of Ultrasound Scanning Approaches in Non-alcoholic Fatty Liver Disease Assessment through Acoustic Structure Quantification. Ultrasound Med Biol. 2019;45:1955-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 98. | Kuroda H, Kakisaka K, Kamiyama N, Oikawa T, Onodera M, Sawara K, Oikawa K, Endo R, Takikawa Y, Suzuki K. Non-invasive determination of hepatic steatosis by acoustic structure quantification from ultrasound echo amplitude. World J Gastroenterol. 2012;18:3889-3895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Lee DH, Lee JY, Park MS, Han JK. Non-invasive monitoring of hepatic steatosis via acoustic structure quantification of ultrasonography with MR spectroscopy as the reference standard. Ultrasonography. 2020;39:70-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Son JY, Lee JY, Yi NJ, Lee KW, Suh KS, Kim KG, Lee JM, Han JK, Choi BI. Hepatic Steatosis: Assessment with Acoustic Structure Quantification of US Imaging. Radiology. 2016;278:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | Zhao Y, Zhang C, Xu S, Zhang H, Wei S, Huang P, Zhang L, Wong YN, Xu W. Quantitative evaluation of hepatic steatosis using novel ultrasound technology normalized local variance (NLV) and its standard deviation with different ROIs in patients with metabolic-associated fatty liver disease: a pilot study. Abdom Radiol (NY). 2022;47:693-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Bae JS, Lee JY, Lee DH, Kim H, Lee Y, Han JK. Quantitative Evaluation of Hepatic Steatosis Using Normalized Local Variance in a Rat Model: Comparison with Histopathology as the Reference Standard. Korean J Radiol. 2019;20:1399-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Bae JS, Lee DH, Lee JY, Kim H, Yu SJ, Lee JH, Cho EJ, Lee YB, Han JK, Choi BI. Quantitative Assessment of Fatty Liver using Ultrasound with Normalized Local Variance Technique. Ultraschall Med. 2021;42:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Dioguardi Burgio M, Imbault M, Ronot M, Faccinetto A, Van Beers BE, Rautou PE, Castera L, Gennisson JL, Tanter M, Vilgrain V. Ultrasonic Adaptive Sound Speed Estimation for the Diagnosis and Quantification of Hepatic Steatosis: A Pilot Study. Ultraschall Med. 2019;40:722-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 105. | Suliman I, Abdelgelil N, Kassamali F, Hassanein TI. The Effects of Hepatic Steatosis on the Natural History of HBV Infection. Clin Liver Dis. 2019;23:433-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Abdalla EK, Vauthey JN. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;246:340-1; author reply 341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 107. | Spitzer AL, Lao OB, Dick AA, Bakthavatsalam R, Halldorson JB, Yeh MM, Upton MP, Reyes JD, Perkins JD. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16:874-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 108. | Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 498] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 109. | Izumi T, Sho T, Morikawa K, Shigesawa T, Suzuki K, Nakamura A, Ohara M, Kawagishi N, Umemura M, Shimazaki T, Kimura M, Nakai M, Suda G, Natsuizaka M, Ogawa K, Kudo Y, Nishida M, Ono K, Baba M, Furuya K, Sakamoto N. Assessing the risk of hepatocellular carcinoma by combining liver stiffness and the controlled attenuation parameter. Hepatol Res. 2019;49:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 110. | Abdelaziz AO, Shousha HI, Said EM, Soliman ZA, Shehata AA, Nabil MM, Abdelmaksoud AH, Elbaz TM, Abdelsalam FM. Evaluation of liver steatosis, measured by controlled attenuation parameter, in patients with hepatitis C-induced advanced liver fibrosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2018;30:1384-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Losurdo G, Italy; Tai DI, Taiwan; Uhlmann D, Germany S-Editor: Yan JP L-Editor: A P-Editor: Zhao S