Published online Apr 21, 2023. doi: 10.3748/wjg.v29.i15.2283
Peer-review started: December 29, 2022
First decision: February 1, 2023
Revised: February 13, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: April 21, 2023
Processing time: 105 Days and 23.2 Hours
Coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a major global pu
Core Tip: Coronavirus disease 2019 (COVID-19) has become a major global public health event, resulting in a significant social and economic burden. Although COVID-19 was initially characterized as an upper respiratory and pulmonary infection, recent evidence suggests that it is a complex disease including gastrointestinal symptoms. Moreover, it remains unclear whether the gastrointestinal symptoms are caused by direct infection of the gastrointestinal tract by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or are the result of systemic immune activation and subsequent dysregulation of homeostatic mechanisms. This review provides a brief overview of the mechanisms by which SARS-CoV-2 disrupts gastrointestinal barrier integrity.
- Citation: Xue W, Honda M, Hibi T. Mechanisms of gastrointestinal barrier dysfunction in COVID-19 patients. World J Gastroenterol 2023; 29(15): 2283-2293
- URL: https://www.wjgnet.com/1007-9327/full/v29/i15/2283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i15.2283
Since the advent of coronavirus disease 2019 (COVID-19), the disease has spread globally and had a profound impact on the lives and health of people around the world[1]. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), replicates and matures once it enters cells through the angiotensin-converting enzyme 2 (ACE2) receptor. SARS-CoV-2 can trigger an inflammatory response that involves the activation of immune cells by a variety of cytokines[2,3]. ACE2 receptors are present in multiple cell types throughout the body, including the oral and nasal mucosa, lung, and gastrointestinal tract, indicating that SARS-CoV-2 can cause multi-organ damage[4,5].
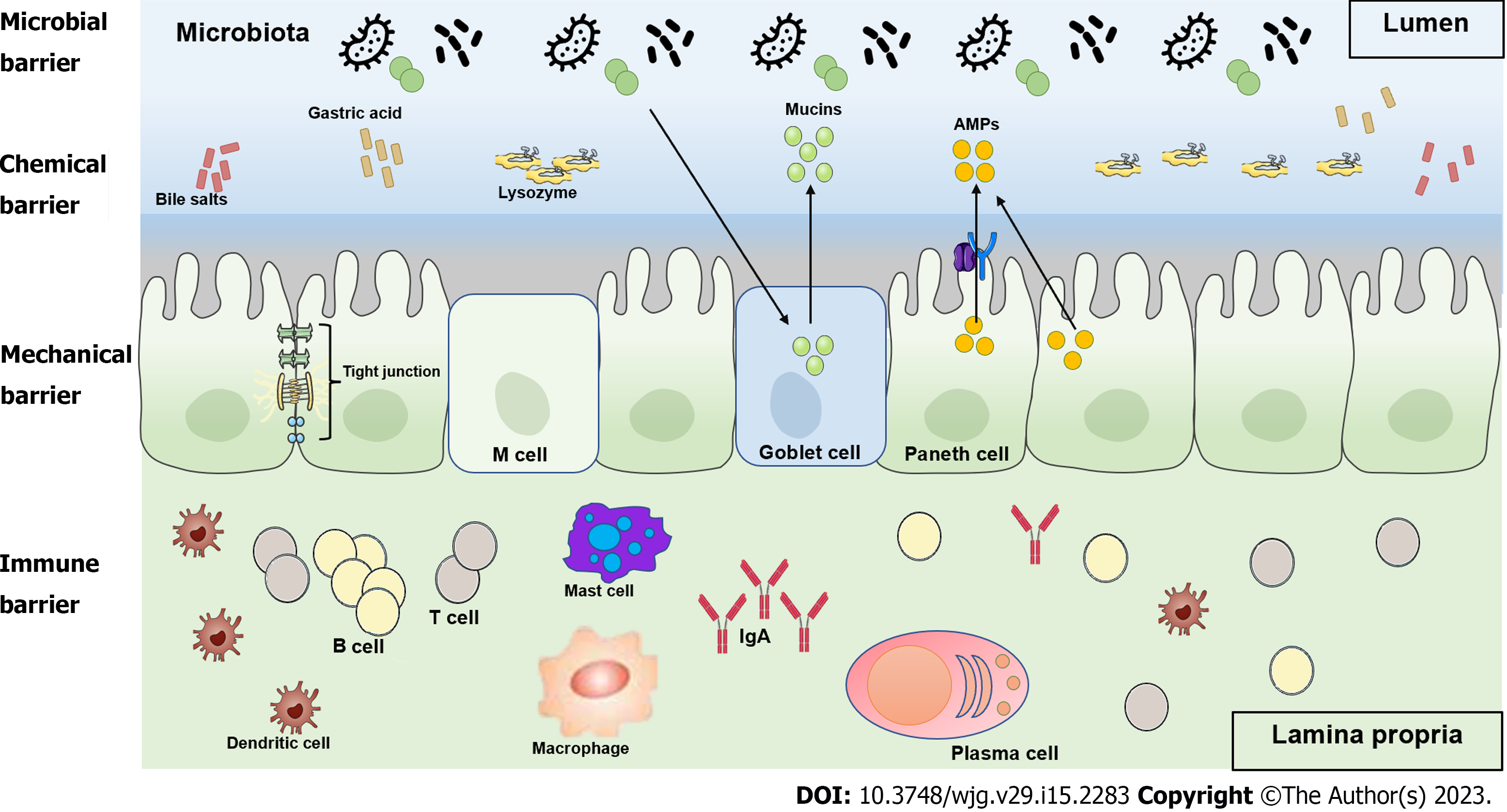
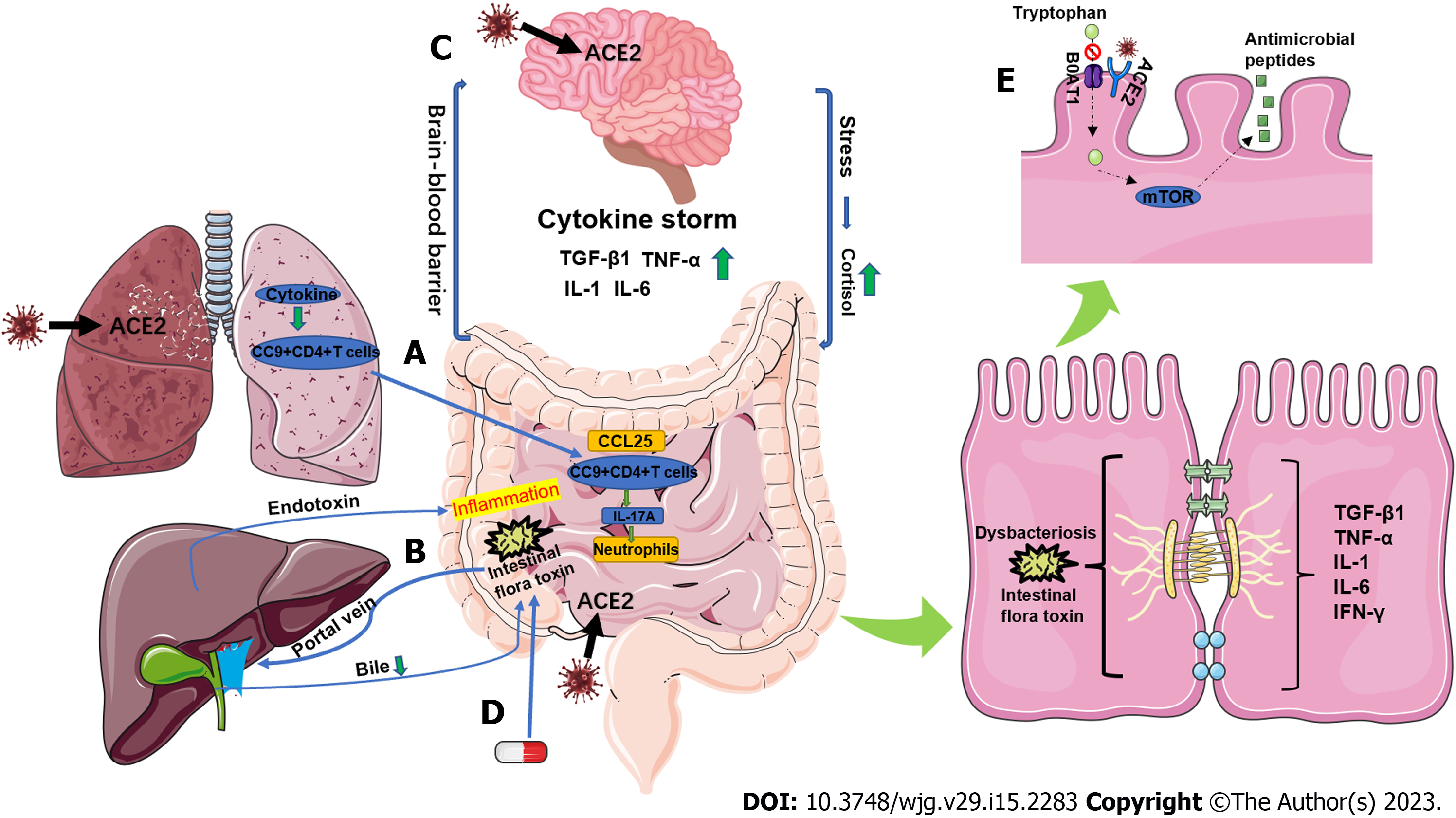
The intestinal tract is the digestive organ of the body, but also has endocrine and immune functions, and is the first line of defense against non-specific infections. However, the intestine is also the largest reservoir of bacteria and endotoxins in the body and is, therefore, a hidden source of infection. While digesting and absorbing nutrients, the intestine contains bacteria and their metabolites, and the gastrointestinal barrier plays a very important role in preventing systemic absorption of harmful microbes and substances[6,7]. The gastrointestinal barrier is composed of the intestinal epithelial cell layer, mucus layer, normal intestinal flora, intestinal immune system, and intestine-hepatic axis, which together perform the functions of mechanical barrier, chemical barrier, microbial barrier, and immune barrier (Figure 1). This barrier plays an important role in homeostasis by preventing harmful substances and pathogens in the gastrointestinal tract from entering the internal environment of the body[8]. In this review, we describe the pathophysiological mechanisms of gastrointestinal barrier dysfunction in COVID-19 patients (Figure 2).
The intestinal mechanical barrier consists of intestinal mucosal epithelial cells, tight junctions (TJs) between epithelial cells, and a layer of bacteria and mucous on the surface of epithelial cells. TJs are the most important component of the intestinal mechanical barrier[9] and are composed of four transmembrane proteins: Zonula occludens (ZO); occludin; claudins, and junctional adhesion molecules (JAMs). TJs form an important barrier against the translocation of bacteria and toxins in the intestine[10]. The TJs between cells close the gaps between adjacent intestinal epithelial cells, preventing bacteria and antigens in the intestinal lumen from entering the lamina propria and activating immune cells in the lamina propria. Therefore, TJs maintain the stability of the intestinal mucosal barrier and prevent abnormal immune reactions in the mucosa. Moreover, the TJs between intestinal epithelial cells play an important role in maintaining the morphological structure of epithelial cells, regulating the differentiation and repair of epithelial cells and intercellular material transport, and maintaining the barrier function of the intestinal mucosa and intestinal mucosal permeability[10-12].
The intestinal chemical barrier, also called the mucus layer, is a general term for the antimicrobial substances produced by the resident intestinal bacteria and host cells e.g., Paneth cells and the chemicals that inhibit bacterial adhesion and colonization, such as gastric acid, digestive enzymes, lysozyme, and mucin[13,14]. The mucus layer, which is mainly composed of mucins and secretory mucin MUC2, limits the contact of gastrointestinal tissues with the microbiota[15,16]. Therefore, the mucus layer separates bacteria from intestinal epithelial cells in the intestinal lumen while allowing nutrient absorption[17]. It also lubricates the luminal contents to prevent degradation of the gastrointestinal tissues[18,19]. Gastric acid acts mainly at the beginning of the small intestine to inactivate bacteria and other pathogenic microorganisms. Lysozyme exerts bactericidal and antibacterial effects on the intestinal epithelial surface and in the intestinal lumen[20,21]. Moreover, bile is an important chemical barrier to endotoxins, as bile salts bind to endotoxins in the intestine and prevent absorption from the intestine into the portal vein[22].
The normal intestine is inhabited by a large number of bacteria, with at least 500 different species, most of which are anaerobic[23]. Under normal conditions, the normal flora maintain a relatively stable proportional relationship with each other. They combine with the intestinal mucosa to form a micro-ecosystem that is both interdependent and interactive with the micro-spatial structure of the host and this micro-ecosystem forms the microbial barrier of the intestine[24]. Under normal microecological conditions, the dominant replication of non-pathogenic intestinal flora can hinder the survival of pathogenic bacteria. At the same time, non-pathogenic flora also secrete some antibacterial and antimicrobial substances, such as lactic acid and bacteriocins, which can interfere with and inhibit the vitality and function of pathogenic bacteria[25-27].
The intestine is both a digestive organ and the largest immune organ in the body and is, therefore, an important part of the systemic immune system. The intestine is constantly exposed to antigenic substances, such as microbial antigens and food antigens, but the gastrointestinal barrier, including the immune barrier, can effectively prevent the penetration of these antigens. The intestinal immune defense system is mainly composed of gut-associated lymphoid tissue (GALT) in the intestinal wall and secreted IgA, IgM, and IgE. GALT is the site of induction and activation of the immune response, mainly in the Peyer's patches that are distributed throughout the small intestine[28,29]. Most of the IgA in intestinal secretions is secretory IgA (sIgA), which is the most secreted immunoglobulin in the body and the main immunoglobulin on the intestinal mucosal surface. sIgA plays a leading role in humoral immunity and is the first line of defense against the adhesion and colonization of pathogens in the intestinal mucosa[30,31]. Furthermore, perivascular macrophages in the lamina propria constitute an anatomical barrier that functions to prevent bacterial translocation[32]. Also, neutrophils play an important role in capturing and killing pathogens in enterocolitis[33].
Damage to the gastrointestinal mechanical barrier can be caused by direct infection of intestinal cells with SARS-CoV-2. The spike protein of SARS-CoV-2 has a high affinity for ACE2, which is widely expressed in intestinal epithelial cells. SARS-CoV-2 enters target cells mainly through the ACE2 receptor thus causing primary damage to the intestine by altering the expression and function of TJ-related proteins, leading to disruption of the paracellular barrier function[34-37]. A study by Sun et al[38] that compared differentially enriched proteins in the stools of COVID-19 patients and normal participants found evidence of intestinal infection and intestinal damage caused by SARS-CoV-2. For example, certain protein components of human immunoglobulins and hemoglobin were upregulated in COVID-19 patients, suggesting an enhanced immune response and potential bleeding in the intestine.
Studies have also shown that ZO-1 interacts with: (1) Occludin, claudins, and JAM; (2) molecular components of intracellular TJ plaques, such as ZO-2, ZO-3, and cingulin; and (3) the actin cytoskeleton[39]. Thus, ZO-1 plays a key role in the structural and functional integrity of the paracellular barrier[39]. Furthermore, the cytoskeletal and barrier biomarkers KRT19 and ZO1 were respectively enriched in stool and blood samples from COVID-19 patients, suggesting increased intestinal paracellular permeability and injury[38,40]. Another group showed that the microbial metabolite d-lactate and the TJ regulator zonulin were increased in the serum of patients with severe COVID-19 and in COVID-19 patients with secondary infections[41]. These data suggest that biomarkers of intestinal permeability may also be early biomarkers for a fatal outcome. Indeed, treatment with larazotide, a zonulin inhibitor, significantly improved time-to-resolution of gastrointestinal symptoms and time-to-clearance of spike antigenemia in pediatric COVID-19 patients[42,43].
In addition, apoptosis of intestinal epithelial cells in COVID-19 patients is another important factor affecting the integrity of the gastrointestinal mechanical barrier. A study by Lehmann et al[44] demonstrated a significant increase in the number of apoptotic epithelial cells in COVID-19 patients by using immunohistochemistry to detect cleaved caspase-3. They also examined the histomorphological changes of epithelial cells and found apoptosis and subsequent regenerative proliferation of epithelial cells in the small intestine of COVID-19 patients[44].
In addition, infection of lung and small intestinal epithelial cells with SARS-CoV-2 stimulates secretion of large amounts of pro-inflammatory cytokines (chemokine-1, TGF-β1, TNF-α, IL-1, and IL-6) causing a cytokine storm and damage to the small intestine that disrupts the integrity of the gastr
Many COVID-19 patients have a significantly reduced appetite, and severely affected patients, such as those dependent on mechanical ventilation, may not be able to eat, resulting in reduced secretion of gastric acid and bile. Under normal conditions, the intestinal epithelial surface is less susceptible to damage by harmful substances due to the mucus cover, which plays an important role in the gastrointestinal barrier. Studies have shown that in rats receiving total parenteral nutrition, glands atrophy, the intestinal mucus layer is damaged and thinned, intestinal permeability increases, and the gastrointestinal barrier is disrupted[47,48]. In standard total parenteral nutrition, no food passes through the intestine in the loading state and certain essential intestinal nutrients such as glutamine are lacking. Numerous subsequent studies have confirmed that parenteral nutrition tends to damage the intestinal epithelial mucus layer, leading to disruption of the gastrointestinal barrier[49,50].
SARS-CoV-2 is known to be neuroinvasive, and when the body is infected with SARS-CoV-2, a cytokine storm is generated and the release of large amounts of pro-inflammatory cytokines leads to blood-brain barrier damage[51,52]. In addition, lipopolysaccharide (LPS) is recognized by Toll-like receptors, which transduce signals that stimulate release of inflammatory cytokines[53-55]. ACE2 is expressed in certain regions of the human brain as well as in neurons and SARS-CoV-2 can directly damage these target organs[56]. After SARS-CoV-2 infection, impairment of the gastrointestinal barrier causes leaky gut and increased translocation of bacterial metabolic components and toxins into the bloodstream, which activates the immune response and leads to systemic inflammation[57-59]. This can promote neuronal degeneration and the development of psychiatric and neurodegenerative disorders[60]. Furthermore, neurological damage can affect the gastrointestinal barrier via the neuroendocrine pathway; this occurs mainly through the hypothalamic–pituitary–adrenal (HPA) axis[61-63]. Stress activates the HPA axis, which ultimately leads to the release of glucocorticoids such as cortisol from the adrenal cortex. Cortisol can alter gastrointestinal dynamics, increase intestinal permeability, and affect the intestinal microbiota[61].
When the microbial balance in the intestine is altered, pathogenic bacteria adhere to the intestinal epithelium and grow dominantly to replace the normal flora[64]. Pathogenic bacteria can directly damage the microvillous membrane proteins of the intestinal epithelium by producing bacterial proteases. Furthermore, pathogenic bacteria can alter biochemical reactions in the intestinal epithelium, leading to damage, blunting, fusion, or obliteration of the villi[65]. In addition, pathogenic bacteria can produce various toxins or other metabolites that inhibit protein synthesis in the intestinal epithelium, thereby damaging the intestinal mucosal barrier. Some opportunistic pathogens can also produce proteases that degrade IgA, which can weaken or eliminate the immune function provided by sIgA and promote bacterial translocation, leading to bacteremia and endotoxemia, thereby further damaging gastrointestinal barrier integrity[66].
A study by Sun et al[38] using metagenomic and metaproteomic methods showed significant changes in the composition of the gut microbiome in COVID-19 patients, characterized by a decrease in commensal species and an increase in opportunistic pathogenic species. Moreover, other studies have shown that the microbiota of COVID-19 patients is enriched with opportunistic pathogens, compared with healthy individuals[64,67-69]. Furthermore, the plasma concentration of the gut permeability marker FABP2 and gut microbial antigens PGN and LPS were significantly elevated in COVID-19 patients in comparison to healthy controls[70].
Hashimoto et al[71] found that ACE2 acts as a binding partner of amino acid transporter B0AT1 (SLC6A19) in the intestine and plays an important role in amino acid transport. Tryptophan is mainly absorbed through the B0AT1/ACE2 transport pathway in enterocytes, which activates the mammalian target of the rapamycin (mTOR) pathway to regulate the expression of antimicrobial peptides. These antimicrobial peptides affect the composition of the intestinal microbiota[71]. Competitive binding of ACE2 receptors by the SARS-CoV-2 virus prevents the absorption of tryptophan through the B0AT1/ACE2 transport pathway in enterocytes, impairs the regulation of antimicrobial peptide expression, and causes an intestinal dysbiosis, leading to disruption of the gastrointestinal barrier.
In addition, when patients with COVID-19 are admitted to hospital for treatment, changes in gastrointestinal barrier dysfunction are often induced by bacterial translocation and gut microbiome dysbiosis as a result of early and heavy use of antimicrobial drugs, such as macrolides, fluor
After binding to ACE2 receptors expressed on intestinal epithelial cells, SARS-CoV-2 is transported to the nuclear endosome and releases its RNA[76,77]. Toll-like receptors recognize viral RNA and signal downstream mediators, which induce IFN-α and -β production and then activate the transcription factor NF-κB to produce pro-inflammatory cytokines[78]. During SARS-CoV-2 infection, small intestinal tissue and feces show increased pro-inflammatory markers, including neutrophil and monocyte accumulation, increased chemokine-1, TGF-β1, IL-1, IL-6, IL-8, and IFN-γ expression and decreased levels of the anti-inflammatory cytokine IL-10[45,79,80]. IFN-γ is produced by several types of immune cells, especially T helper type 1 (Th1) cells. IFN-γ acts as a major inducer of the cell-mediated response to infection by activating macrophages, enhancing antigen presentation and T cell differentiation[81], and interacting directly with epithelial cells, leading to chemokine expression and antimicrobial peptide secretion[82,83]. In addition, mucosal CD4+ and CD8+ T cells, Th17 cells, neutrophils, dendritic cells, and macrophages were activated and T regulatory cells were reduced after intestinal epithelial cells were infected by SARS-CoV-2. Therefore, SARS-CoV-2 infection promotes an over-activated immune response that further damages intestinal epithelium[84-86]. Interestingly, one study found that SARS-CoV-2 was not always measurable in the stools of COVID-19 patients with gastrointestinal symptoms. These data further suggest that the gastrointestinal barrier dysfunction may not be a direct result of viral infection of intestinal mucosal epithelial cells, but rather a result of the immune response[87].
CCL25 is expressed in enterocytes and the CCL25-CCR9 axis mediates recruitment of lung-derived effector CD4+ T cells to the small intestine[88]. CCR9+CD4+ T cells disrupt homeostasis of the intestinal flora, thereby promoting the in-situ polarization of small intestinal Th17 cells and the production of IL-17A, leading to neutrophil aggregation and ultimately mediating intestinal immune-mediated injury[88,89]. Moreover, damage to the intestinal mucosa and bacterial imbalance can lead to harmful metabolites in the intestine being transferred to the liver through the portal vein, affecting liver function[90]. With impaired liver function, endotoxin inactivation is reduced, resulting in endotoxin entering the systemic circulation[91-94]. This induces an inflammatory response, damages intestinal mucosa, and causes bacterial translocation and bacteremia, inducing a systemic inflammatory response and thus forming a vicious cycle[95].
Furthermore, antiviral drugs can also cause gastrointestinal barrier dysfunction. For example, severe and persistent diarrhea may be associated with the use of oseltamivir and arborol. Other drugs that can cause gastrointestinal barrier dysfunction include chloroquine phosphate, lopinavir, remdesivir, and some proprietary Chinese medicines, as well as immunosuppressors[96].
Gastrointestinal barrier dysfunction in COVID-19 patients is not an independent symptom, but rather a trigger of other diseases. In critically ill patients, gastrointestinal barrier dysfunction, if not treated early, may aggravate primary disease or even cause multi-organ dysfunction syndrome, thus endangering patients' lives. To assess gastrointestinal barrier dysfunction in COVID-19 patients, clinicians should not only consider the effect of the primary disease, but also the pathophysiological situation within the intestine. The pathological mechanisms that cause gastrointestinal barrier damage and dysfunction are complex and various mechanisms are often intertwined, interacting with each other. Therefore, the clinical diagnosis and treatment of gastrointestinal barrier dysfunction should be comprehensive and targeted.
| 1. | Bartoszko JJ, Siemieniuk RAC, Kum E, Qasim A, Zeraatkar D, Ge L, Han MA, Sadeghirad B, Agarwal A, Agoritsas T, Chu DK, Couban R, Darzi AJ, Devji T, Ghadimi M, Honarmand K, Izcovich A, Khamis A, Lamontagne F, Loeb M, Marcucci M, McLeod SL, Motaghi S, Murthy S, Mustafa RA, Neary JD, Pardo-Hernandez H, Rada G, Rochwerg B, Switzer C, Tendal B, Thabane L, Vandvik PO, Vernooij RWM, Viteri-García A, Wang Y, Yao L, Ye Z, Guyatt GH, Brignardello-Petersen R. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373:n949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14580] [Article Influence: 2430.0] [Reference Citation Analysis (3)] |
| 3. | Hussman JP. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front Pharmacol. 2020;11:1169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11613] [Article Influence: 1935.5] [Reference Citation Analysis (2)] |
| 5. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4194] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 6. | Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5700] [Cited by in RCA: 5684] [Article Influence: 270.7] [Reference Citation Analysis (3)] |
| 7. | Bouhnik Y, Alain S, Attar A, Flourié B, Raskine L, Sanson-Le Pors MJ, Rambaud JC. Bacterial populations contaminating the upper gut in patients with small intestinal bacterial overgrowth syndrome. Am J Gastroenterol. 1999;94:1327-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 670] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 9. | Raya-Sandino A, Luissint AC, Kusters DHM, Narayanan V, Flemming S, Garcia-Hernandez V, Godsel LM, Green KJ, Hagen SJ, Conway DE, Parkos CA, Nusrat A. Regulation of intestinal epithelial intercellular adhesion and barrier function by desmosomal cadherin desmocollin-2. Mol Biol Cell. 2021;32:753-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 453] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 11. | González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 858] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 12. | Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 679] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 13. | Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 283] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Nyström EEL, Martinez-Abad B, Arike L, Birchenough GMH, Nonnecke EB, Castillo PA, Svensson F, Bevins CL, Hansson GC, Johansson MEV. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science. 2021;372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 239] [Article Influence: 47.8] [Reference Citation Analysis (1)] |
| 15. | Schneider H, Pelaseyed T, Svensson F, Johansson MEV. Study of mucin turnover in the small intestine by in vivo labeling. Sci Rep. 2018;8:5760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 16. | Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4659-4665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 1058] [Article Influence: 66.1] [Reference Citation Analysis (1)] |
| 17. | Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CC, Graham M, Elinav E, Flavell RA. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163:1444-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 465] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 18. | van der Post S, Jabbar KS, Birchenough G, Arike L, Akhtar N, Sjovall H, Johansson MEV, Hansson GC. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68:2142-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 19. | Birchenough GM, Nyström EE, Johansson ME, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 463] [Article Influence: 46.3] [Reference Citation Analysis (2)] |
| 20. | Bel S, Hooper LV. Secretory autophagy of lysozyme in Paneth cells. Autophagy. 2018;14:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Nakimbugwe D, Masschalck B, Deckers D, Callewaert L, Aertsen A, Michiels CW. Cell wall substrate specificity of six different lysozymes and lysozyme inhibitory activity of bacterial extracts. FEMS Microbiol Lett. 2006;259:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 684] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 23. | Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat Immunol. 2004;5:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 24. | Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1209] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 25. | Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465-1483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 26. | Kayama H, Okumura R, Takeda K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu Rev Immunol. 2020;38:23-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 508] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 27. | Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3709] [Cited by in RCA: 3240] [Article Influence: 162.0] [Reference Citation Analysis (9)] |
| 28. | Ferguson A. Immunological functions of the gut in relation to nutritional state and mode of delivery of nutrients. Gut. 1994;35:S10-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Nagura H, Sumi Y. Immunological functions of the gut--role of the mucosal immune system. Toxicol Pathol. 1988;16:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Noval Rivas M, Wakita D, Franklin MK, Carvalho TT, Abolhesn A, Gomez AC, Fishbein MC, Chen S, Lehman TJ, Sato K, Shibuya A, Fasano A, Kiyono H, Abe M, Tatsumoto N, Yamashita M, Crother TR, Shimada K, Arditi M. Intestinal Permeability and IgA Provoke Immune Vasculitis Linked to Cardiovascular Inflammation. Immunity. 2019;51:508-521.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Nagalingam NA, Lynch SV. Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18:968-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 32. | Honda M, Surewaard BGJ, Watanabe M, Hedrick CC, Lee WY, Brown K, McCoy KD, Kubes P. Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat Commun. 2020;11:1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol. 2018;15:206-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 34. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14346] [Article Influence: 2391.0] [Reference Citation Analysis (10)] |
| 35. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2936] [Article Influence: 489.3] [Reference Citation Analysis (0)] |
| 36. | Chen L, Li L, Han Y, Lv B, Zou S, Yu Q. Tong-fu-li-fei decoction exerts a protective effect on intestinal barrier of sepsis in rats through upregulating ZO-1/occludin/claudin-1 expression. J Pharmacol Sci. 2020;143:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Fernández-Blanco JA, Estévez J, Shea-Donohue T, Martínez V, Vergara P. Changes in Epithelial Barrier Function in Response to Parasitic Infection: Implications for IBD Pathogenesis. J Crohns Colitis. 2015;9:463-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Sun Z, Song ZG, Liu C, Tan S, Lin S, Zhu J, Dai FH, Gao J, She JL, Mei Z, Lou T, Zheng JJ, Liu Y, He J, Zheng Y, Ding C, Qian F, Chen YM. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022;20:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 39. | Assimakopoulos SF, Papageorgiou I, Charonis A. Enterocytes' tight junctions: From molecules to diseases. World J Gastrointest Pathophysiol. 2011;2:123-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Assimakopoulos SF, Mastronikolis S, DE Lastic AL, Aretha D, Papageorgiou D, Chalkidi T, Oikonomou I, Triantos C, Mouzaki A, Marangos M. Intestinal Barrier Biomarker ZO1 and Endotoxin Are Increased in Blood of Patients With COVID-19-associated Pneumonia. In Vivo. 2021;35:2483-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Hernández-Solis A, Güemes-González AM, Ruiz-Gómez X, Álvarez-Maldonado P, Castañeda-Casimiro J, Flores-López A, Ramírez-Guerra MA, Muñoz-Miranda O, Madera-Sandoval RL, Arriaga-Pizano LA, Nieto-Patlán A, Estrada-Parra S, Pérez-Tapia SM, Serafín-López J, Chacón-Salinas R, Escobar-Gutiérrez A, Soria-Castro R, Ruiz-Sánchez BP, Wong-Baeza I. IL-6, IL-10, sFas, granulysin and indicators of intestinal permeability as early biomarkers for a fatal outcome in COVID-19. Immunobiology. 2022;227:152288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Yonker LM, Swank Z, Gilboa T, Senussi Y, Kenyon V, Papadakis L, Boribong BP, Carroll RW, Walt DR, Fasano A. Zonulin Antagonist, Larazotide (AT1001), As an Adjuvant Treatment for Multisystem Inflammatory Syndrome in Children: A Case Series. Crit Care Explor. 2022;10:e0641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Yonker LM, Gilboa T, Ogata AF, Senussi Y, Lazarovits R, Boribong BP, Bartsch YC, Loiselle M, Rivas MN, Porritt RA, Lima R, Davis JP, Farkas EJ, Burns MD, Young N, Mahajan VS, Hajizadeh S, Lopez XIH, Kreuzer J, Morris R, Martinez EE, Han I, Griswold K Jr, Barry NC, Thompson DB, Church G, Edlow AG, Haas W, Pillai S, Arditi M, Alter G, Walt DR, Fasano A. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 44. | Lehmann M, Allers K, Heldt C, Meinhardt J, Schmidt F, Rodriguez-Sillke Y, Kunkel D, Schumann M, Böttcher C, Stahl-Hennig C, Elezkurtaj S, Bojarski C, Radbruch H, Corman VM, Schneider T, Loddenkemper C, Moos V, Weidinger C, Kühl AA, Siegmund B. Human small intestinal infection by SARS-CoV-2 is characterized by a mucosal infiltration with activated CD8(+) T cells. Mucosal Immunol. 2021;14:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 45. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30479] [Article Influence: 5079.8] [Reference Citation Analysis (13)] |
| 46. | Yamada S, Noda T, Okabe K, Yanagida S, Nishida M, Kanda Y. SARS-CoV-2 induces barrier damage and inflammatory responses in the human iPSC-derived intestinal epithelium. J Pharmacol Sci. 2022;149:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Li J, Langkamp-Henken B, Suzuki K, Stahlgren LH. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability. JPEN J Parenter Enteral Nutr. 1994;18:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Iiboshi Y, Nezu R, Kennedy M, Fujii M, Wasa M, Fukuzawa M, Kamata S, Takagi Y, Okada A. Total parenteral nutrition decreases luminal mucous gel and increases permeability of small intestine. JPEN J Parenter Enteral Nutr. 1994;18:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. Am J Respir Crit Care Med. 1995;152:1545-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 197] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 278] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 258] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 52. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4761] [Cited by in RCA: 4766] [Article Influence: 794.3] [Reference Citation Analysis (1)] |
| 53. | Pourbadie HG, Sayyah M, Khoshkholgh-Sima B, Choopani S, Nategh M, Motamedi F, Shokrgozar MA. Early minor stimulation of microglial TLR2 and TLR4 receptors attenuates Alzheimer's disease-related cognitive deficit in rats: behavioral, molecular, and electrophysiological evidence. Neurobiol Aging. 2018;70:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Iwasa T, Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Kawami T, Yamasaki M, Murakami M, Kato T, Kuwahara A, Yasui T, Irahara M. Developmental changes in hypothalamic toll-like-receptor 4 mRNA expression and the effects of lipopolysaccharide on such changes in female rats. Int J Dev Neurosci. 2015;40:12-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Sabatino ME, Sosa Ldel V, Petiti JP, Mukdsi JH, Mascanfroni ID, Pellizas CG, Gutiérrez S, Torres AI, De Paul AL. Functional Toll-like receptor 4 expressed in lactotrophs mediates LPS-induced proliferation in experimental pituitary hyperplasia. Exp Cell Res. 2013;319:3020-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Lindskog C, Méar L, Virhammar J, Fällmar D, Kumlien E, Hesselager G, Casar-Borota O, Rostami E. Protein Expression Profile of ACE2 in the Normal and COVID-19-Affected Human Brain. J Proteome Res. 2022;21:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Saithong S, Worasilchai N, Saisorn W, Udompornpitak K, Bhunyakarnjanarat T, Chindamporn A, Tovichayathamrong P, Torvorapanit P, Chiewchengchol D, Chancharoenthana W, Leelahavanichkul A. Neutrophil Extracellular Traps in Severe SARS-CoV-2 Infection: A Possible Impact of LPS and (1→3)-β-D-glucan in Blood from Gut Translocation. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Smith N, Goncalves P, Charbit B, Grzelak L, Beretta M, Planchais C, Bruel T, Rouilly V, Bondet V, Hadjadj J, Yatim N, Pere H, Merkling SH, Ghozlane A, Kernéis S, Rieux-Laucat F, Terrier B, Schwartz O, Mouquet H, Duffy D, Di Santo JP. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat Immunol. 2021;22:1428-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 59. | Mizutani T, Ishizaka A, Koga M, Ikeuchi K, Saito M, Adachi E, Yamayoshi S, Iwatsuki-Horimoto K, Yasuhara A, Kiyono H, Matano T, Suzuki Y, Tsutsumi T, Kawaoka Y, Yotsuyanagi H. Correlation Analysis between Gut Microbiota Alterations and the Cytokine Response in Patients with Coronavirus Disease during Hospitalization. Microbiol Spectr. 2022;10:e0168921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 60. | Nelson DW, Granberg T, Andersen P, Jokhadar E, Kåhlin J, Granström A, Hallinder H, Schening A, Thunborg C, Walles H, Hagman G, Shams-Latifi R, Yu J, Petersson S, Tzortzakakis A, Levak N, Aspö M, Piehl F, Zetterberg H, Kivipelto M, Eriksson LI. The Karolinska NeuroCOVID study protocol: Neurocognitive impairment, biomarkers and advanced imaging in critical care survivors. Acta Anaesthesiol Scand. 2022;66:759-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Vodička M, Ergang P, Hrnčíř T, Mikulecká A, Kvapilová P, Vagnerová K, Šestáková B, Fajstová A, Hermanová P, Hudcovic T, Kozáková H, Pácha J. Microbiota affects the expression of genes involved in HPA axis regulation and local metabolism of glucocorticoids in chronic psychosocial stress. Brain Behav Immun. 2018;73:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 62. | Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591-599. [PubMed] |
| 63. | Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2097] [Cited by in RCA: 1930] [Article Influence: 87.7] [Reference Citation Analysis (1)] |
| 64. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-955.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1106] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 65. | Wang XD, Ar'Rajab A, Andersson R, Soltesz V, Wang W, Svensson M, Bengmark S. The influence of surgically induced acute liver failure on the intestine in the rat. Scand J Gastroenterol. 1993;28:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Diebel LN, Liberati DM, Diglio CA, Dulchavsky SA, Brown WJ. Synergistic effects of Candida and Escherichia coli on gut barrier function. J Trauma. 1999;47:1045-50; discussion 1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Gaibani P, D'Amico F, Bartoletti M, Lombardo D, Rampelli S, Fornaro G, Coladonato S, Siniscalchi A, Re MC, Viale P, Brigidi P, Turroni S, Giannella M. The Gut Microbiota of Critically Ill Patients With COVID-19. Front Cell Infect Microbiol. 2021;11:670424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 68. | Cao J, Wang C, Zhang Y, Lei G, Xu K, Zhao N, Lu J, Meng F, Yu L, Yan J, Bai C, Zhang S, Zhang N, Gong Y, Bi Y, Shi Y, Chen Z, Dai L, Wang J, Yang P. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut Microbes. 2021;13:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 69. | Yin YS, Minacapelli CD, Parmar V, Catalano CC, Bhurwal A, Gupta K, Rustgi VK, Blaser MJ. Alterations of the fecal microbiota in relation to acute COVID-19 infection and recovery. Mol Biomed. 2022;3:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 70. | Prasad R, Patton MJ, Floyd JL, Fortmann S, DuPont M, Harbour A, Wright J, Lamendella R, Stevens BR, Oudit GY, Grant MB. Plasma Microbiome in COVID-19 Subjects: An Indicator of Gut Barrier Defects and Dysbiosis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 71. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 1020] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 72. | Yacouba A, Olowo-Okere A, Yunusa I. Repurposing of antibiotics for clinical management of COVID-19: a narrative review. Ann Clin Microbiol Antimicrob. 2021;20:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021;19:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 74. | Bernard-Raichon L, Venzon M, Klein J, Axelrad JE, Zhang C, Sullivan AP, Hussey GA, Casanovas-Massana A, Noval MG, Valero-Jimenez AM, Gago J, Putzel G, Pironti A, Wilder E; Yale IMPACT Research Team, Thorpe LE, Littman DR, Dittmann M, Stapleford KA, Shopsin B, Torres VJ, Ko AI, Iwasaki A, Cadwell K, Schluter J. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat Commun. 2022;13:5926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 75. | Wu LH, Ye ZN, Peng P, Xie WR, Xu JT, Zhang XY, Xia HH, He XX. Efficacy and Safety of Washed Microbiota Transplantation to Treat Patients with Mild-to-Severe COVID-19 and Suspected of Having Gut Microbiota Dysbiosis: Study Protocol for a Randomized Controlled Trial. Curr Med Sci. 2021;41:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, Brewer JR, Han A, Steach HR, Israelow B, Blackburn HN, Velazquez SE, Chen YG, Halene S, Iwasaki A, Meffre E, Nussenzweig M, Lieberman J, Wilen CB, Kluger Y, Flavell RA. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606:585-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 367] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 77. | Liang W, Feng Z, Rao S, Xiao C, Xue X, Lin Z, Zhang Q, Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 269] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 78. | Yang JY, Kim MS, Kim E, Cheon JH, Lee YS, Kim Y, Lee SH, Seo SU, Shin SH, Choi SS, Kim B, Chang SY, Ko HJ, Bae JW, Kweon MN. Enteric Viruses Ameliorate Gut Inflammation via Toll-like Receptor 3 and Toll-like Receptor 7-Mediated Interferon-β Production. Immunity. 2016;44:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 79. | Jiao L, Li H, Xu J, Yang M, Ma C, Li J, Zhao S, Wang H, Yang Y, Yu W, Wang J, Yang J, Long H, Gao J, Ding K, Wu D, Kuang D, Zhao Y, Liu J, Lu S, Liu H, Peng X. The Gastrointestinal Tract Is an Alternative Route for SARS-CoV-2 Infection in a Nonhuman Primate Model. Gastroenterology. 2021;160:1647-1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 80. | Zhang F, Mears JR, Shakib L, Beynor JI, Shanaj S, Korsunsky I, Nathan A; Accelerating Medicines Partnership Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium, Donlin LT, Raychaudhuri S. IFN-γ and TNF-α drive a CXCL10+ CCL2+ macrophage phenotype expanded in severe COVID-19 lungs and inflammatory diseases with tissue inflammation. Genome Med. 2021;13:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 81. | Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166-6173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2443] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 82. | Walrath T, Malizia RA, Zhu X, Sharp SP, D'Souza SS, Lopez-Soler R, Parr B, Kartchner B, Lee EC, Stain SC, Iwakura Y, O'Connor W Jr. IFN-γ and IL-17A regulate intestinal crypt production of CXCL10 in the healthy and inflamed colon. Am J Physiol Gastrointest Liver Physiol. 2020;318:G479-G489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Farin HF, Karthaus WR, Kujala P, Rakhshandehroo M, Schwank G, Vries RG, Kalkhoven E, Nieuwenhuis EE, Clevers H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-γ. J Exp Med. 2014;211:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 84. | Grant RA, Morales-Nebreda L, Markov NS, Swaminathan S, Querrey M, Guzman ER, Abbott DA, Donnelly HK, Donayre A, Goldberg IA, Klug ZM, Borkowski N, Lu Z, Kihshen H, Politanska Y, Sichizya L, Kang M, Shilatifard A, Qi C, Lomasney JW, Argento AC, Kruser JM, Malsin ES, Pickens CO, Smith SB, Walter JM, Pawlowski AE, Schneider D, Nannapaneni P, Abdala-Valencia H, Bharat A, Gottardi CJ, Budinger GRS, Misharin AV, Singer BD, Wunderink RG; NU SCRIPT Study Investigators. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590:635-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 566] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 85. | Zhou R, To KK, Wong YC, Liu L, Zhou B, Li X, Huang H, Mo Y, Luk TY, Lau TT, Yeung P, Chan WM, Wu AK, Lung KC, Tsang OT, Leung WS, Hung IF, Yuen KY, Chen Z. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity. 2020;53:864-877.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 86. | Ouyang Y, Yin J, Wang W, Shi H, Shi Y, Xu B, Qiao L, Feng Y, Pang L, Wei F, Guo X, Jin R, Chen D. Downregulated Gene Expression Spectrum and Immune Responses Changed During the Disease Progression in Patients With COVID-19. Clin Infect Dis. 2020;71:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 87. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1144] [Article Influence: 190.7] [Reference Citation Analysis (2)] |
| 88. | Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397-2410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 89. | Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069-5076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 90. | Miyake Y, Yamamoto K. Role of gut microbiota in liver diseases. Hepatol Res. 2013;43:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 91. | Filliol A, Piquet-Pellorce C, Raguénès-Nicol C, Dion S, Farooq M, Lucas-Clerc C, Vandenabeele P, Bertrand MJM, Le Seyec J, Samson M. RIPK1 protects hepatocytes from Kupffer cells-mediated TNF-induced apoptosis in mouse models of PAMP-induced hepatitis. J Hepatol. 2017;66:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 92. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1327] [Article Influence: 102.1] [Reference Citation Analysis (2)] |
| 93. | Ni YH, Huo LJ, Li TT. [Effect of interleukin-22 on proliferation and activation of hepatic stellate cells induced by acetaldehyde and related mechanism]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 94. | Wu X, Wang Y, Wang S, Xu R, Lv X. Purinergic P2X7 receptor mediates acetaldehyde-induced hepatic stellate cells activation via PKC-dependent GSK3β pathway. Int Immunopharmacol. 2017;43:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1057] [Article Influence: 132.1] [Reference Citation Analysis (1)] |
| 96. | Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245-G252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liakina V, Lithuania; Xin YN, China S-Editor: Yan JP L-Editor: A P-Editor: Cai YX