Published online Apr 14, 2023. doi: 10.3748/wjg.v29.i14.2114
Peer-review started: December 23, 2022
First decision: January 3, 2023
Revised: January 9, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 14, 2023
Processing time: 111 Days and 2.1 Hours
The clinical scenario of pediatric liver disease is becoming more intricate due to changes in the disease spectrum, in which an increasing number of inherited/ metabolic liver diseases are reported, while infectious diseases show a decreasing trend. The similar clinical manifestations caused by inherited/metabolic diseases might be under-recognized or misdiagnosed due to nonspecific characteristics. A delayed visit to a doctor due to a lack of symptoms or mild symptoms at an early stage will result in late diagnosis and treatment. Moreover, limited diagnostic approaches, especially liver biopsy, are not easily accepted by pediatric patients, leading to challenges in etiological diagnosis. Liver dysfunction due to inherited/metabolic diseases is often caused by a variety of metabolites, so precision treatment is difficult; symptomatic treatment is a compelling option for inherited disorders.
Core Tip: The spectrum of diseases causing pediatric liver dysfunction has been changing, and an increasing number of inherited/metabolic disorders have been increasingly recognized as major contributors to liver disease in children. Etiological diagnosis remains challenging due to the frequent absence of symptoms or nonspecific signs and limited diagnostic approaches, especially liver biopsy, which is not easily accepted by pediatric patients. Consequently, the treatment of pediatric inherited/ metabolic liver disease is challenging. In this manuscript, we review here the challenges in pediatric inherited/metabolic liver disease, including epidemiological changes in the disease spectrum and challenges in etiological diagnosis and treatment.
- Citation: Zou YG, Wang H, Li WW, Dai DL. Challenges in pediatric inherited/metabolic liver disease: Focus on the disease spectrum, diagnosis and management of relatively common disorders. World J Gastroenterol 2023; 29(14): 2114-2126
- URL: https://www.wjgnet.com/1007-9327/full/v29/i14/2114.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i14.2114
Pediatric liver disease is a common entity in children resulting from different causes, including hepatocyte injury and cholestasis. Recently, the spectrum of diseases causing pediatric liver disease has been changing. Infectious diseases are decreasing due to improvement in hygienic conditions, while the proportions of metabolic disorders, inherited diseases and genetic defects are increasing. At an early stage, elevated liver transaminase may be a unique nonspecific manifestation[1]. Early diagnosis mainly depends on biochemistry, pathogen identification and imaging findings for patients without distinctive clinical features[2]. While new diagnostic methods have improved the diagnostic capability for liver disease in children, some novel challenges remain for pediatricians. Diet and/or lifestyle modifications are usually the primary treatment for some inherited/metabolic liver diseases. Where indicated, drug therapy, surgery and gene modification are necessary to provide lifelong functional correction of liver-based metabolic defects. We review here the changes in epidemiology and the challenges in etiological diagnosis and treatment of inherited/metabolic liver disease in children. Due to the broad spectrum of diseases, we focus on some of the most common conditions resulting in metabolic/inherited liver disease and easily misdiagnosed diseases without evident malformations.
The exact prevalence of pediatric liver disease is not clear. However, there have been some changes in the disease spectrum causing pediatric liver disease in the past decade. With the popularization of hepatitis B vaccines and the development of effective antiviral treatments, the global incidence of hepatitis B has declined. By 2019, the incidence of hepatitis B in children younger than 5 had fallen from 5% in the 1980s to less than 1% in the 2000s[3,4]. With the promotion of blood screening, the incidence of hepatitis C also declined slightly. By 2018, the global incidence of hepatitis C in children under 18 years old was 0.13%[5]. While the understanding of cholestatic liver diseases (CLD) has deepened in the field of inherited/metabolic liver diseases, an increasing number of noninfectious factors have been recognized as major contributors to liver dysfunction or cholestasis in children[6-10]. A single-center study in China showed that nonviral liver diseases accounted for 23.3% of pediatric liver diseases in 2011-2017, increasing from 17.53% in 2001-2010[11]. Nonalcoholic fatty liver disease (NAFLD) has rapidly become one of the most common liver diseases in the pediatric population. Approximately 2.6% to 9.6% of the population was affected by NAFLD in the Americas and Asia in 2006. The global prevalence of NAFLD in the pediatric population is approximately 10% currently[12]. Recent genetic diagnosis techniques and their easy accessibility raise the possibility of detecting some metabolic diseases involving Wilson’s disease (WD)[13], glycogen storage disease (GSD)[14] and α1-antitrypsin deficiency[15]. The incidence of WD is usually quoted as 1:30000, but recent genetic studies have shown a higher prevalence ranging from 1:2400 to 1:6500[16]. CLD affects approximately 1/2500 infants, and biliary atresia accounts for 1/3[17]; genetic or metabolic factors account for approximately one-third of cholestasis cases causing subsequent liver dysfunction, including citrin deficiency (CD)[18], progressive familial intrahepatic cholestasis (PFIC)[19] and Alagille syndrome (AGS)[20]. In the early 2000s, the incidence of CD was 1 in between 17000 and 34000 births[18], while its incidence increased to 1 in 7000 births in 2020[21]. The incidence of GSD is approximately 1:10,000 Live births[22]. AGS had an incidence of approximately 1 in 70000 Live births in 2020[23], an increase from approximately 1 in 100000 in the 1990s[24]. The estimated overall incidence of PFIC increased from 1/300000-1/500000 Live births in the 1990s[24] to 1/50000-1/100000 Live births in the 2010s[25].
It is difficult to determine an etiological diagnosis for liver diseases that are undetectable by newborn screening, especially at an early stage. A diversity of inherited/metabolic causes can result in pediatric liver disease[26]. Fatty liver disease (nonalcoholic and alcoholic), autoimmune hepatitis, WD, disorder of bile acid synthesis, AGS, hereditary hemochromatosis, and α-1 antitrypsin deficiencies are among the causes of pediatric liver disease[27]. Hepatocytes have similar responses to different impairments, while clinical and laboratory signs are frequently monomorphic. In addition, the absence of symptoms and nonspecific signs at an early stage contributes to the difficulty of diagnosis.
Liver disease is usually divided into two types - hepatocellular and cholestatic disease - which sometimes overlap. Alanine transferase (ALT), alkaline phosphatase (ALP), aspartate transferase (AST), γ-glutamyl transferase (GGT), prothrombin time, bilirubin and albumin are the most common biochemical markers for liver function, but they are susceptible to extrahepatic factors. A disproportionate increase in the level of ALT and AST compared with ALP suggests hepatocyte damage or muscle disorders; conversely, a disproportionate increase in ALP level compared to ALT and AST indicates cholestatic injury. Moreover, an elevated serum conjugated bilirubin level suggests hepatocellular or cholestatic disease. These markers can assist in roughly sorting the types of pediatric liver disease, but they are not helpful for accurate etiological diagnosis.
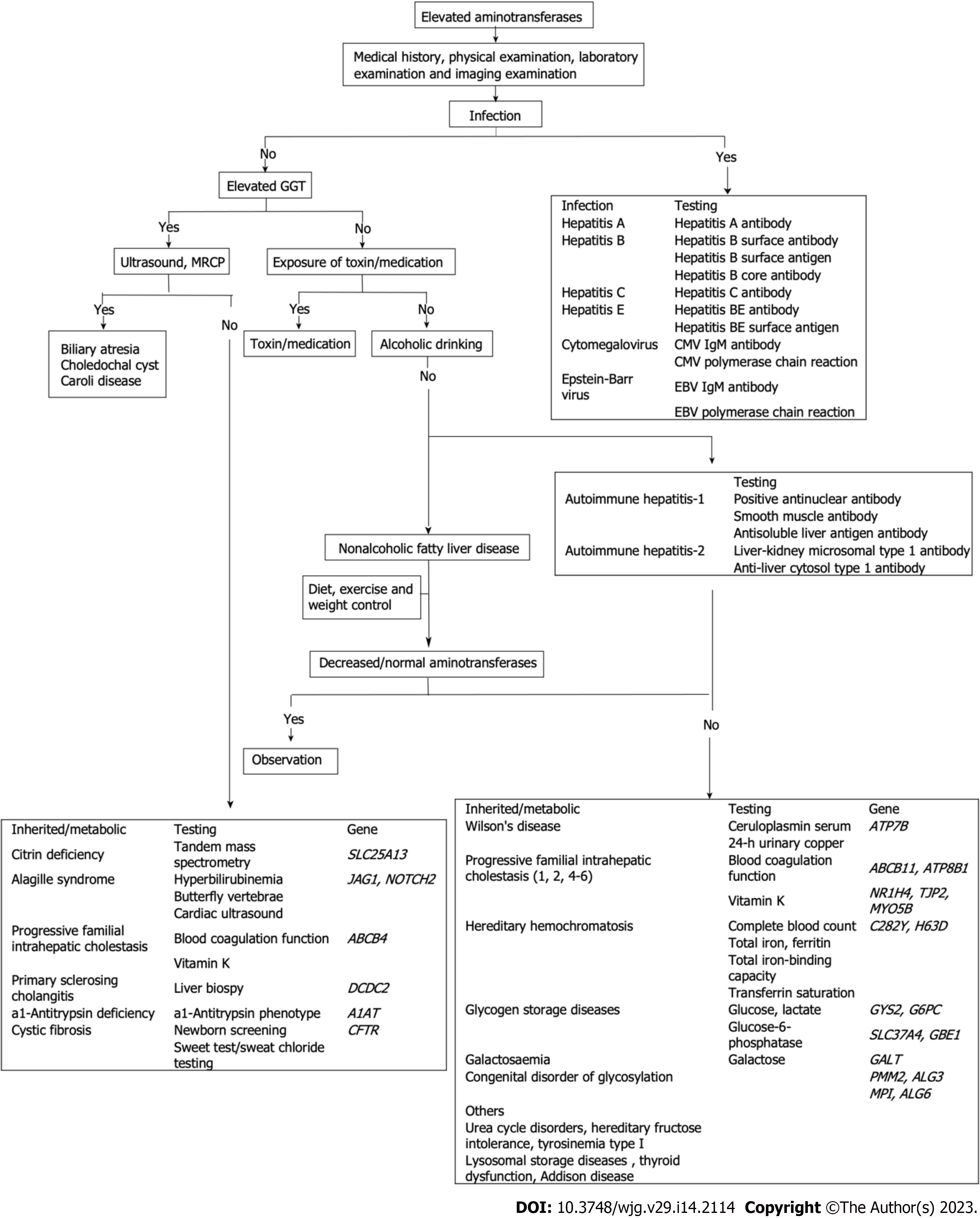
With advances in diagnostic tools for pediatric liver disease over the last few decades, some novel diagnostic modalities, such as ultrasound, magnetic resonance, and genetic testing, have been used to evaluate liver disease; however, percutaneous liver biopsy for histopathological evaluation remains crucial for assessment of the severity of hepatopathies[28]. We focus on the challenges in etiological diagnosis in the following section (Figure 1).
Pediatric NAFLD is frequently free of symptoms, and diagnosis is based on hepatic steatosis on ultrasound or evidence of abnormal liver enzymes and the exclusion of some known causes of hepatosteatosis other than NAFLD. As a standard diagnostic method in NAFLD, liver biopsy has some inherent limitations: the method is invasive; the accuracy depends on the experience of the pathologist; it is difficult to use for NAFLD staging due to the nonuniformity of the small liver specimen; and it may present special challenges in extremely obese children due to difficulty in evaluating the liver location and the depth of subcutaneous fat tissue. Finally, the optimal timing of liver biopsy for diagnosis and follow-up has not been determined[29].
Several noninvasive diagnostic tools have been proposed instead of hepatic histology, including the hepatic steatosis index, NAFLD fatty liver index, liver fat score, and pediatric prediction score, which are widely accepted clinically. However, surrogate markers and scores remain to be validated or are not sufficient to predict the presence of hepatic steatosis[30-32]. Ultrasound technology has been shown to be inaccurate for the diagnosis of hepatosteatosis due to its low specificity and sensitivity[33]. Due to radiation risk, computed tomography is not commonly recommended as a diagnostic tool despite being reasonably specific and sensitive for hepatic steatosis. Despite its accuracy for hepatic steatosis, magnetic resonance spectroscopy and imaging are not widely used due to cost, lack of availability and lack of validated cutoffs to determine NAFLD[34]. As a novel noninvasive tool, transient elastography (TE) has shown promise in the evaluation of steatosis in pediatric patients. TE was reported to perform better than ultrasound for the assessment of hepatic steatosis in children[35]. It is a good diagnostic tool for measuring liver stiffness and can differentiate advanced fibrosis. However, there are some limitations to this diagnostic modality, particularly abdominal adiposity, which increases the distance between the probe and liver; body mass index greater than 30 kg/m2; and operator experience[36]. Further validation of TE is necessary due to the limited data available for pediatric NAFLD patients. Controlled attenuation parameters are a promising imaging method to detect steatosis based on vibrationally controlled TE. The same probe is used to quantify steatosis by assessing the attenuation of ultrasound in liver fat. Some magnetic resonance-based methods, e.g., magnetic resonance spectroscopy/magnetic resonance proton density fat fraction, have been developed to quantify hepatic steatosis and fibrosis[37]. Its accuracy, cost effectiveness and ability to monitor changes in the level of steatosis in patients with NAFLD remain unknown[38]. Most challenging is that no reliable biomarkers have been identified to detect or predict inflammation in NAFLD. Recently, Chae et al[39] successfully developed machine learning-based NAFLD diagnostic models based on metabolome profiles and metabolic pathway changes. It can be utilized as a less-invasive approach for diagnosing the disease.
WD is an autosomal recessive copper metabolic disorder caused by mutations in the ATP7B gene[40]. The clinical manifestations regarding liver involvement range from incidental findings of liver function abnormalities to acute hepatic failure. Symptoms at any age are frequently nonspecific[41]. Early clinical symptoms in WD patients are diverse and atypical, and biochemical tests have false positives and false negatives, making early diagnosis more difficult. In most cases, its diagnosis is based on a combination of laboratory findings and clinical presentations[40]. The diagnosis might be difficult in children with WD, particularly at early stages of liver disease, given the absence of a single distinctive characteristic or available biochemical test to confirm or exclude the diagnosis. Indeed, many affected children do not have elevated liver copper levels, high urinary copper excretion or a Kayser-Fleischer ring[42].
Serum ceruloplasmin is typically decreased in patients with WD, although normal ceruloplasmin levels are also seen in some patients[40]. Notably, the levels can also be affected by other factors[43]. Therefore, WD cannot be diagnosed solely based on ceruloplasmin levels. Exchangeable copper is an experimental technique that might be able to determine bioavailable copper in plasma, but it does not reliably measure no ceruloplasmin-bound copper levels[44]. Twenty four-hour urinary copper excretion is a useful method to diagnose and monitor treatment in WD; however, it can be lower than 100 μg in some patients with WD, especially in children and their asymptomatic siblings[45]. Moreover, it might be difficult to interpret because of overlapping findings with other hepatopathies, especially acute hepatic damage or liver failure, and the reference limits vary among laboratories[45]. Although copper quantification in liver tissue has high specificity and diagnostic value, the test is invasive and involves risks; the concentration can be underestimated due to sampling errors or increased in cholestatic disorders[46]. These equivocal findings emphasize the importance of genetic testing for mutation identification. ATP7B gene mutational analysis is an important tool for diagnosing WD. However, it may take time to obtain the results of molecular genetic diagnosis; analysis is difficult due to more than 700 possible mutations, and many patients are compound heterozygotes[47]. Another difficulty of genetic testing research is that, except for a few hotspot mutations, most mutations are extremely low in frequency in the population, making it difficult to collect sufficient cases for statistical analysis, accurate analysis of ATP7B genotype-phenotype and clarification of the corresponding pathogenicity to aid diagnosis. Recently, a scoring system based on all available tests developed by the 8th International Meeting on WD and Menke Disease was found to have good diagnostic accuracy in pediatrics[48].
GSD refers to a group of inherited disorders of glycogen synthesis, degradation, or metabolism regulation. The hepatic forms usually have a wide spectrum (I, III, IV, VI, IX, and 0) but similar cardinal manifestations[49], although they may have variable expressivity. GSD type I is the most common; type Ia is present in 80% of cases, whereas type Ib accounts for the remaining 20%. The enzyme defect in GSD type Ia is primarily expressed in the liver, kidney, and intestine, while Ib is expressed ubiquitously. Consequently, both types Ia and Ib manifest as hepatomegaly, hypoglycemia, and lactic acidosis, but neutropenia and myeloid dysfunctions are unique to GSD type Ib[50]. GSD classification is sometimes difficult due to the common characteristics among different types. Newborn screening is currently not available for GSD. Encouragingly, some specific screening systems using dried blood spots were reported to be applicable for newborn screening for GSDIa in the real world[51]. A detailed clinical history and careful examination along with laboratory findings could suggest the diagnosis. Reduced enzymatic activity in peripheral blood can confirm the diagnosis of GSD types III, IV and IX; however, normal activity in leukocytes does not exclude the other forms[52]. According to related studies, urinary glucose tetrasaccharide (Glc4) excretion, a biomarker for GSD II, is also elevated in patients with hepatic GSD, so Glc4 might be a good biomarker for GSD, but more studies are still needed to confirm this hypothesis. In pediatric GSD III patients, decreasing Glc4 in urine reflects improved fasting tolerance[53]. Traditional invasive liver biopsy for the assessment of enzymatic activity in hepatic cells is no longer the gold standard and is neither cost effective nor convenient. As a robust alternative diagnostic tool that has emerged over the course of the past few years, gene examination has replaced liver biopsy. It is a noninvasive method for confirming the diagnosis and classification of GSDs, allowing for appropriate specific therapy for different types of GSDs[54]. For many years, the conventional Sanger sequencing method has been the gold standard for the detection and screening of mutations, but this laborious, costly and time-consuming method leads to delayed diagnosis which decelerates care and treatment[55]. Combined with clinical signs and biochemical indices, next-generation sequencing (NGS), as the gold standard, provides a high-throughput and accurate method for genetic diagnoses of GSDs. It bridges the difficulties of GSD diagnosis due to broad genetic heterogeneity and clinical manifestations. The much faster and more sensitive NGS makes clinical application more practicable. However, the identification of variants of uncertain significance poses a challenge in diagnosis owing to variable gene coverage, noncoding and structural variants that are unable to be captured and duplications/deletions that are missed[56]. Higher sequencing coverage increases the validation of findings as well as costs. Therefore, methods with the best accuracy, coverage, and cost are expected to be designed. Finally, traditional static biochemical markers might not adequately reflect the dynamic changes in response to metabolic stressors or treatment. Continuous glucose monitoring appears attractive as a highly informative noninvasive technique to monitor the dynamics of hypoglycemia in patients with GSDs, but it might be limited by regional availability[57].
CLD covers a spectrum of disorders caused by inborn errors of metabolism, primarily manifesting as a cholestatic syndrome. Intrahepatic cholestasis can be divided into hepatocellular and biliary forms[58]. Hepatic cholestatic disease can cause related clinical symptoms and secondary changes resulting from cholestasis. The diagnosis of CLD is challenging due to the nonspecific symptoms and the broad differential diagnosis. Biochemical indicators include increases in serum ALP and GGT in patients without symptoms at an early stage and an increase in conjugated bilirubin at advanced stages. Isolated elevation of serum GGT and/or ALP can result from certain rare disorders, rapid bone growth or bone disease, and drug intake; consequently, it is limited in the diagnosis of cholestasis due to low specificity. As a sensitive and specific, non-invasive, and relatively inexpensive tool, ultrasonography has some disadvantages in that abnormalities of bile ducts may be misdiagnosed. Moreover, the pancreas and the lower common bile duct are frequently not well depicted. Abdominal computed tomography holds the risk of radiation and might not be superior to ultrasound for biliary tree delineation. Routine new-born screening (NBS) using electrospray ionization tandem mass spectrometry technology has been widely used for inborn errors of metabolism[59]. Nevertheless, this strategy depends on complete clinical information and therefore is associated with false negatives. In addition, the selection of limited known metabolic pathways likely hampers the discovery of novel metabolic defects[60]. In contrast, NGS panels have recently emerged as an appealing tool to diagnose pediatric metabolic liver disease[60].
CD is the most common inherited autosomal recessive metabolic disorder and is caused by mutations in the SLC25 gene family encoding proteins[61]. There are mainly 3 age-dependent phenotypes[21,61], and neonatal intrahepatic cholestasis (NICCD) is the major pediatric CD phenotype. Neonates or infants with CD manifest with intrahepatic cholestasis, citrullinemia, dyslipidemia, hyperammonemia, galactosemia, and hypoglycemia[62]. In early infancy, these symptoms overlap with those of other CLD, e.g., biliary atresia and neonatal hepatitis, contributing to the difficulty in prompt and accurate diagnosis. Patients with adult-onset symptoms, hyperammonaemia and neuropsychosis can be misdiagnosed with other neurological entities. NBS for CD provides the opportunity to initiate early treatment in newborns with NICCD; however, NBS for CD is not performed in certain countries, and the sensitivity and specificity of NBS results are not satisfactory[63]. False negatives at the cutoff value in NBS have been reported in some studies; less than 30% of NICCD patients were detected by NBS using tandem mass spectrometry to measure amino acids[64]. Abnormal biochemical parameters are important clues for CD diagnosis. The increases in serum transaminase, total galactose, alkaline phosphatase, and direct hyperbilirubin levels, accompanied by the prolongation in coagulation parameters, lead to confusion of the disease with galactosemia and causes misdiagnosis. However, galactose-1-phosphate uridyl transferase activity is normal in these patients[65]. AFP elevation might be an important laboratory finding suggesting NICCD[66]. SLC25A13 genetic analysis is a reliable method for confirmation of NICCD. However, a percentage of SLC25A13 mutations are not detectable by conventional DNA analysis alone[67]. The verification of these mutations is challenging for the definite diagnosis of NICCD. Moreover, SLC25A13 mutations worldwide demonstrate remarkable heterogeneity. In such cases, some reliable evidence for molecular diagnostic strategies originated from sophisticated functional, molecular and in silico analysis of the SLC25A13 gene and its cDNA, although it is usually labor-intensive and expensive. Sanger sequencing, denaturing high-performance liquid chromatography and PCR restriction fragment length polymorphism are alternative tools for the analysis of gene mutations. However, these methods are too complex to be widely used in clinical practice.
A universal consensus on drug therapy for NAFLD has not been established. A balanced diet and lifestyle interventions based on exercise are the mainstay of NAFLD management[68]. The side effects of drugs, poor compliance of diet and exercise control, and the risks of surgical therapy remain challenges for pediatricians in the treatment of NAFLD. The provision of low-fat diets and hypocaloric, low-carbohydrate diets, e.g., a diet low in free sugar content[69] or a Mediterranean diet rich in fiber, polyunsaturated fats and antioxidants[70], provide reductions in liver fat content as long as weight loss is achieved. Although proven effective, this treatment modality has some inherent barriers. The patient's compliance, the lack of clinical nutritionists, and the therapeutic recipes juggling the nutrition requirements for development restrict the application. Moreover, no specific diet or program is recommended for the treatment of NAFLD in children from different regions and with different ethnicities. Increasing physical activity is important; however, the appropriate type of physical activity is controversial. There is no general consensus on the exercise category (aerobic, resistance exercise or combined) or the volume and intensity of physical activity. Some studies have demonstrated that combined exercise is more effective than aerobic exercise alone in improving NAFLD progression[71]. Additionally, affected children frequently fail to implement prescribed lifestyle changes due to a lack of motivation to alter contributory habits. The antioxidants metformin and ursodeoxycholic acid, initially used in the treatment of NAFLD in adults, have only limited effects in the treatment of pediatric NAFLD[72]. Similarly, omega-3 fatty acid supplementation was not effective in inducing a significant reduction in ALT or improving liver steatosis on ultrasonography[73]. Some randomized controlled trials indicated that probiotic supplementation might be beneficial in children with NAFLD, but the current evidence does not specify the exact beneficial strain of probiotics, requiring further studies[74]. Interestingly, the strategy of targeting the lysosome has seemed to encourage a new direction for future NAFLD treatment, e.g., inhibition of mTOR localized on the lysosome surface and restoration of normal lysosomal function and autophagy[75]. Nevertheless, all of the studies are limited to animal models.
While bariatric surgery has been indicated as a treatment option in severely obese children and those with comorbidities for the improvement of NAFLD-related liver damage[76-78], the unidentified evidence on nutritional deficiency and its impact on growth and development make it controversial in pediatric patients[76]. Intragastric balloons, a promising alternative temporary physical device, could improve metabolic parameters and liver alterations in pediatric patients with morbid obesity[79], but further clinical observational evidence is needed for validation in pediatric patients.
Nutritional and medicinal therapy regimens are important for CD patients, and the basic therapy is nutrition. The principle of therapy should be based on the specific food intake[61]. The common therapeutic diet for liver diseases is a high carbohydrate and low protein diet, although it is controversial[80]; other options include a low carbohydrate diet or additional high protein and a low carbohydrate-restricted formula with medium-chain triglyceride supplementation[81]. Dietetic treatment with medium-chain triglyceride (MCT) supplementation and lactose-free or low-carbohydrate formulas has been recommended. The clinical manifestations of NICCD often improve spontaneously within the first year, even without treatment for some patients. Most infants can be reintroduced to protein- and lipid-enriched food by 1 year of age. MCT supplements are suggested even after improvement of clinical manifestations based on the biochemical rationale[64]. In addition to dietary treatment, administration of sodium pyruvate might be effective in correcting growth restriction. However, some subjects develop severe hepatic dysfunction. Liver transplantation (LT) is the most effective option to correct metabolic disturbances in patients with an inefficient therapeutic diet[82]. However, LT also has some disadvantages, e.g., the shortage of liver donors, the cost and the possibility of failing to improve the lives of some CD patients due to immunological complications or other causes. It was reported that supplementation with nicotinamide might be worth trying as a supplemental therapy for CD. mRNA therapy was reported to improve metabolic and behavioral abnormalities in an animal model of CD. Further study is needed to develop safer and more effective treatments for CD patients[21].
The focus of drug therapy for children with WD is to remove excessive copper by promoting copper excretion using chelating agents, blocking intestinal copper absorption with zinc salts[13], or both. At the same time, WD patients should avoid copper-rich foods until they have normal liver biochemistry. D-penicillamine is utilized as a first-line therapeutic medication for acute and/or symptomatic patients with WD. Although it is highly effective, it is linked to serious adverse side effects and requires discontinuation of the drug in some patients[83]. As a second-line drug for patients intolerant to penicillamine, trientine dihydrochloride has equal effectiveness and less frequent adverse reactions, but its high costs limit its application. Trientine tetrahydrochloride is a hybrid with similar benefits and risks to trientine dihydrochloride. A good patient response with a lower dose makes it more cost effective. It has been used for the treatment of WD in adolescents and children older than 5 years old who are intolerant to penicillamine in the European Union, but it was reported that trientine was associated with a higher frequency of initial neurological worsening and increased transaminases[42]. Furthermore, chelation therapies that increase copper excretion can result in iatrogenic copper deficiency[84]. Zinc salts are used for asymptomatic children or in combination with penicillamine for the initial management of symptomatic patients, but some patients display progressive aggravation on zinc therapy[85]. The increased circulating Cu as a result of chelating therapy may lead to oxidative stress and disease deterioration. Melatonin, as an antioxidant, has been shown to strongly ameliorate liver and brain damage from oxidative stress[86]. In fact, treatment failure poses another challenge in the treatment of WD, which can occur with any WD medication early during treatment initiation or later while on maintenance therapy[41]. The main difficulty of WD treatment is to address Wilson's disease crises, including hemolytic crisis and acute liver failure. LT corrects the metabolic defect in the livers of patients with WD and restores normal copper metabolism. The indications for LT are chronic liver disease not responsive to medical treatment, advanced liver failure and fulminant liver failure[13,87]. The fact that acute liver failure is unpredictable, coupled with the shortage in liver donors, remains a challenge to liver transplantation for WD patients.
The focus of treatment for GSDs is to maintain blood glucose levels and control lipid and uric acid levels. Diet therapy has remained the primary treatment for GSDs. Cornstarch therapy can better control blood sugar, but infants younger than 6 mo who have not yet fully developed amylase are prone to indigestion and abdominal distention. Starch with a slower rate of absorption may extend sleep periods for children with GSDs. The new waxy maize starch (Glycosade) effectively increases safety through avoidance of overnight feeding and improved sleep and quality of life; however, there have been no dosing guidelines published. Tolerance to the volume required to maintain euglycemia through the night and its strong taste, granular texture, and cost are the main problems. Ketogenic diets were shown to have positive outcomes in the management of patients with some GSD types[88], but the optimal β-hydroxybutyrate concentration in ketogenic diets has not been defined. In addition, high ketones may contribute to osteoporosis, delayed growth, and elevated transaminase[89]. Impaired cortisol levels secondary to deregulation were observed in a cohort study, which could constitute a new potential therapeutic target in GSD I patients[90]. Dietary therapy not aimed at the cause of disease might not prevent long-term complications and might even trigger secondary metabolic manifestations. Hepatic GSDs are associated with microbial dysbiosis; therefore, supplementation with probiotics is recom
Therapy currently focuses on reducing damage to the liver and other organs caused by cholestasis and prevention of the progression of liver fibrosis by reducing toxic substances in hepatocytes. Although medications or dietary modifications are potentially effective for many of these conditions if recognized early, there are some challenges in therapy for CLD. Many metabolic and CLD are sufficiently rare such that pediatricians might have never before seen them.
In some instances, the etiology is unknown, and no specific therapy is available; however, in rare cholestatic disorders in which the cause is known, no specific treatments have been developed[93]. Ursodeoxycholic acid (UDCA) is presently the mainstay of therapy for most cholestatic hepatic diseases; however, all patients with primary sclerosing cholangitis and approximately one in three patients with primary biliary cholangitis do not respond to or intolerant to UDCA treatment[94,95]. As a strong farnesoid X nuclear receptor agonist that downregulates the intestinal bile acid transporter, obeticholic acid is currently the only approved second-line therapy for UDCA nonresponders or intolerance to UDCA; however, only half of these patients respond to obeticholic acid. Patients who do not respond to treatment with UDCA and/or obeticholic acid have an increased risk of progression to biliary cirrhosis, end-stage liver disease and death or liver transplantation[96,97]. Oral vancomycin has been reported to improve liver biochemical tests (ALT and γGTP) and symptoms in children with primary sclerosing cholangitis due to its minimal oral absorption, high concentration in the gut and inhibition of cytokine release from T cells[98]. Further clinical studies of vancomycin treatment for CLD are expected. Although liver transplantation (LT) remains the only curative therapy for patients who fail to respond to conservative management, disease recurrence and extrahepatic manifestations significantly affect quality of life, and long-term studies are needed to understand patient history after LT[99,100]. Hepatocyte transplantation, as an alternative to organ transplantation, is a promising treatment for patients with CLD arising from inherited metabolic disorders, e.g., PFIC[101,102]. The problem of low engraftment rates and long-term survival of transplanted hepatocytes is the most important obstacle regarding current techniques. Moreover, a major limitation is the lack of good-quality donor organs, and thus far, there have been no clinically applicable techniques to monitor the function and survival of engrafted cells[101].
Liver function injury in children is etiological heterogeneous. Early etiological diagnosis is critically important because it could help to determine the history of the disease and modify of the treatment regimen, but diagnosis is often delayed due to the presence of nonspecific presentations or the frequent absence of symptoms. It is frequently at the stage of progressive disease when typical symptoms appear. Therefore, recognition leading to diagnosis and treatment remains a great challenge for pediatricians. In this paper, we reviewed the changes in epidemiology and the challenges in the diagnosis and treatment of pediatric inherited/metabolic liver disease. We hope to assist pediatricians in understanding this condition better and identifying and treating this condition as early as possible.
| 1. | Tsunoda T, Inui A, Iwasawa K, Oikawa M, Sogo T, Komatsu H, Ito Y, Fujisawa T. Acute liver dysfunction not resulting from hepatitis virus in immunocompetent children. Pediatr Int. 2017;59:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Goldner D, Lavine JE. Nonalcoholic Fatty Liver Disease in Children: Unique Considerations and Challenges. Gastroenterology. 2020;158:1967-1983.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1261] [Article Influence: 157.6] [Reference Citation Analysis (6)] |
| 4. | Lanini S, Ustianowski A, Pisapia R, Zumla A, Ippolito G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect Dis Clin North Am. 2019;33:1045-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Indolfi G, Giometto S, Serranti D, Bettiol A, Bigagli E, De Masi S, Lucenteforte E. Systematic review with meta-analysis: the efficacy and safety of direct-acting antivirals in children and adolescents with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2020;52:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Zhang J, Yang Y, Gong JY, Li LT, Li JQ, Zhang MH, Lu Y, Xie XB, Hong YR, Yu Z, Knisely AS, Wang JS. Low-GGT intrahepatic cholestasis associated with biallelic USP53 variants: Clinical, histological and ultrastructural characterization. Liver Int. 2020;40:1142-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Cousin MA, Conboy E, Wang JS, Lenz D, Schwab TL, Williams M, Abraham RS, Barnett S, El-Youssef M, Graham RP, Gutierrez Sanchez LH, Hasadsri L, Hoffmann GF, Hull NC, Kopajtich R, Kovacs-Nagy R, Li JQ, Marx-Berger D, McLin V, McNiven MA, Mounajjed T, Prokisch H, Rymen D, Schulze RJ, Staufner C, Yang Y, Clark KJ, Lanpher BC, Klee EW. RINT1 Bi-allelic Variations Cause Infantile-Onset Recurrent Acute Liver Failure and Skeletal Abnormalities. Am J Hum Genet. 2019;105:108-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Overeem AW, Li Q, Qiu YL, Cartón-García F, Leng C, Klappe K, Dronkers J, Hsiao NH, Wang JS, Arango D, van Ijzendoorn SCD. A Molecular Mechanism Underlying Genotype-Specific Intrahepatic Cholestasis Resulting From MYO5B Mutations. Hepatology. 2020;72:213-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Qiu YL, Liu T, Abuduxikuer K, Hao CZ, Gong JY, Zhang MH, Li LT, Yan YY, Li JQ, Wang JS. Novel missense mutation in VPS33B is associated with isolated low gamma-glutamyltransferase cholestasis: Attenuated, incomplete phenotype of arthrogryposis, renal dysfunction, and cholestasis syndrome. Hum Mutat. 2019;40:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Kamath BM, Loomes KM, Piccoli DA. Medical management of Alagille syndrome. J Pediatr Gastroenterol Nutr. 2010;50:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Gan Y, Zhang M, Zhu S, Dong Y, Xu Z, Chen D, Wang L, Wang F, Yan J, Cao L, Wang P, Li A. Analysis of children non-viral liver disease spectrum in single center from 2011 to 2017. Chuanranbing Xinxi. 2019;32:109-112+135. [DOI] [Full Text] |
| 12. | Takahashi Y, Fukusato T. Pediatric nonalcoholic fatty liver disease: overview with emphasis on histology. World J Gastroenterol. 2010;16:5280-5285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Fernando M, van Mourik I, Wassmer E, Kelly D. Wilson disease in children and adolescents. Arch Dis Child. 2020;105:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Chou JY, Cho JH, Kim GY, Mansfield BC. Molecular biology and gene therapy for glycogen storage disease type Ib. J Inherit Metab Dis. 2018;41:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Lomas DA, Hurst JR, Gooptu B. Update on alpha-1 antitrypsin deficiency: New therapies. J Hepatol. 2016;65:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Avan A, Członkowska A, Gaskin S, Granzotto A, Sensi SL, Hoogenraad TU. The Role of Zinc in the Treatment of Wilson's Disease. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 17. | Bezerra JA, Balistreri WF. Cholestatic syndromes of infancy and childhood. Semin Gastrointest Dis. 2001;12:54-65. [PubMed] |
| 18. | Saheki T, Inoue K, Tushima A, Mutoh K, Kobayashi K. Citrin deficiency and current treatment concepts. Mol Genet Metab. 2010;100 Suppl 1:S59-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Baker A, Kerkar N, Todorova L, Kamath BM, Houwen RHJ. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2019;43:20-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 20. | Ma YL, Song YZ. [Advances in the diagnosis and treatment of Alagille syndrome]. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:1188-1192. [PubMed] |
| 21. | Hayasaka K. Metabolic basis and treatment of citrin deficiency. J Inherit Metab Dis. 2021;44:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Beyzaei Z, Geramizadeh B, Karimzadeh S. Diagnosis of hepatic glycogen storage disease patients with overlapping clinical symptoms by massively parallel sequencing: a systematic review of literature. Orphanet J Rare Dis. 2020;15:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Ayoub MD, Kamath BM. Alagille Syndrome: Current Understanding of Pathogenesis, and Challenges in Diagnosis and Management. Clin Liver Dis. 2022;26:355-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 24. | Emerick KM, Whitington PF. Clinical aspects of familial cholestasis (with molecular explanations). Curr Gastroenterol Rep. 1999;1:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Della Corte C, Mosca A, Vania A, Alterio A, Alisi A, Nobili V. Pediatric liver diseases: current challenges and future perspectives. Expert Rev Gastroenterol Hepatol. 2016;10:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Braun HA, Faasse SA, Vos MB. Advances in Pediatric Fatty Liver Disease: Pathogenesis, Diagnosis, and Treatment. Gastroenterol Clin North Am. 2018;47:949-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, Durmaz O, Lacaille F, McLin V, Nobili V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 29. | Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS, Xanthakos SA. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 784] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 30. | Koot BG, van der Baan-Slootweg OH, Bohte AE, Nederveen AJ, van Werven JR, Tamminga-Smeulders CL, Merkus MP, Schaap FG, Jansen PL, Stoker J, Benninga MA. Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity (Silver Spring). 2013;21:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Maffeis C, Banzato C, Rigotti F, Nobili V, Valandro S, Manfredi R, Morandi A. Biochemical parameters and anthropometry predict NAFLD in obese children. J Pediatr Gastroenterol Nutr. 2011;53:590-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Walker RW, Sinatra F, Hartiala J, Weigensberg M, Spruijt-Metz D, Alderete TL, Goran MI, Allayee H. Genetic and clinical markers of elevated liver fat content in overweight and obese Hispanic children. Obesity (Silver Spring). 2013;21:E790-E797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB. Evidence and recommendations for imaging liver fat in children, based on systematic review. Clin Gastroenterol Hepatol. 2014;12:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Tang A, Desai A, Hamilton G, Wolfson T, Gamst A, Lam J, Clark L, Hooker J, Chavez T, Ang BD, Middleton MS, Peterson M, Loomba R, Sirlin CB. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 35. | Ferraioli G, Calcaterra V, Lissandrin R, Guazzotti M, Maiocchi L, Tinelli C, De Silvestri A, Regalbuto C, Pelizzo G, Larizza D, Filice C. Noninvasive assessment of liver steatosis in children: the clinical value of controlled attenuation parameter. BMC Gastroenterol. 2017;17:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 37. | Schwimmer JB, Middleton MS, Behling C, Newton KP, Awai HI, Paiz MN, Lam J, Hooker JC, Hamilton G, Fontanesi J, Sirlin CB. Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology. 2015;61:1887-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Liu K, Wong VW, Lau K, Liu SD, Tse YK, Yip TC, Kwok R, Chan AY, Chan HL, Wong GL. Prognostic Value of Controlled Attenuation Parameter by Transient Elastography. Am J Gastroenterol. 2017;112:1812-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Chae W, Lee KJ, Huh KY, Moon JS, Ko JS, Cho JY. Association of Metabolic Signatures with Nonalcoholic Fatty Liver Disease in Pediatric Population. Metabolites. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Korman JD, Volenberg I, Balko J, Webster J, Schiodt FV, Squires RH Jr, Fontana RJ, Lee WM, Schilsky ML; Pediatric and Adult Acute Liver Failure Study Groups. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Schilsky ML, Roberts EA, Bronstein JM, Dhawan A, Hamilton JP, Rivard AM, Washington MK, Weiss KH, Zimbrean PC. A multidisciplinary approach to the diagnosis and management of Wilson disease: Executive summary of the 2022 Practice Guidance on Wilson disease from the American Association for the Study of Liver Diseases. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (1)] |
| 42. | Couchonnal E, Lion-François L, Guillaud O, Habes D, Debray D, Lamireau T, Broué P, Fabre A, Vanlemmens C, Sobesky R, Gottrand F, Bridoux-Henno L, Dumortier J, Belmalih A, Poujois A, Jacquemin E, Brunet AS, Bost M, Lachaux A. Pediatric Wilson's Disease: Phenotypic, Genetic Characterization and Outcome of 182 Children in France. J Pediatr Gastroenterol Nutr. 2021;73:e80-e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Suchy FJ, Sokol RJ, Balistreri WF. Liver disease in children. 3rd ed. Cambridge, New York: Cambridge University Press, 2007. |
| 44. | Poujois A, Trocello JM, Djebrani-Oussedik N, Poupon J, Collet C, Girardot-Tinant N, Sobesky R, Habès D, Debray D, Vanlemmens C, Fluchère F, Ory-Magne F, Labreuche J, Preda C, Woimant F. Exchangeable copper: a reflection of the neurological severity in Wilson's disease. Eur J Neurol. 2017;24:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Capone K, Azzam RK. Wilson's Disease: A Review for the General Pediatrician. Pediatr Ann. 2018;47:e440-e444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Ferenci P, Steindl-Munda P, Vogel W, Jessner W, Gschwantler M, Stauber R, Datz C, Hackl F, Wrba F, Bauer P, Lorenz O. Diagnostic value of quantitative hepatic copper determination in patients with Wilson's Disease. Clin Gastroenterol Hepatol. 2005;3:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Ferenci P. Wilson's Disease. Clin Gastroenterol Hepatol. 2005;3:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Koppikar S, Dhawan A. Evaluation of the scoring system for the diagnosis of Wilson's disease in children. Liver Int. 2005;25:680-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Beyzaei Z, Geramizadeh B. Molecular diagnosis of glycogen storage disease type I: a review. EXCLI J. 2019;18:30-46. [PubMed] |
| 50. | Wright TLF, Umaña LA, Ramirez CM. Update on glycogen storage disease: primary hepatic involvement. Curr Opin Pediatr. 2022;34:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Niba ETE, Wijaya YOS, Awano H, Taniguchi N, Takeshima Y, Nishio H, Shinohara M. DBS Screening for Glycogen Storage Disease Type 1a: Detection of c.648G>T Mutation in G6PC by Combination of Modified Competitive Oligonucleotide Priming-PCR and Melting Curve Analysis. Int J Neonatal Screen. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 52. | Massese M, Tagliaferri F, Dionisi-Vici C, Maiorana A. Glycogen storage diseases with liver involvement: a literature review of GSD type 0, IV, VI, IX and XI. Orphanet J Rare Dis. 2022;17:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 53. | Heiner-Fokkema MR, van der Krogt J, de Boer F, Fokkert-Wilts MJ, Maatman RGHJ, Hoogeveen IJ, Derks TGJ. The multiple faces of urinary glucose tetrasaccharide as biomarker for patients with hepatic glycogen storage diseases. Genet Med. 2020;22:1915-1916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Ahmed S, Afroze B. Glycogen storage diseases-time to flip the outdated diagnostic approach centered on liver biopsy with the molecular testing. Pak J Med Sci. 2020;36:290-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Vega AI, Medrano C, Navarrete R, Desviat LR, Merinero B, Rodríguez-Pombo P, Vitoria I, Ugarte M, Pérez-Cerdá C, Pérez B. Molecular diagnosis of glycogen storage disease and disorders with overlapping clinical symptoms by massive parallel sequencing. Genet Med. 2016;18:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Horton RH, Lucassen AM. Recent developments in genetic/genomic medicine. Clin Sci (Lond). 2019;133:697-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 57. | Derks TGJ, Rodriguez-Buritica DF, Ahmad A, de Boer F, Couce ML, Grünert SC, Labrune P, López Maldonado N, Fischinger Moura de Souza C, Riba-Wolman R, Rossi A, Saavedra H, Gupta RN, Valayannopoulos V, Mitchell J. Glycogen Storage Disease Type Ia: Current Management Options, Burden and Unmet Needs. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 58. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1228] [Article Influence: 72.2] [Reference Citation Analysis (1)] |
| 59. | Bodamer OA, Hoffmann GF, Lindner M. Expanded newborn screening in Europe 2007. J Inherit Metab Dis. 2007;30:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Coene KLM, Kluijtmans LAJ, van der Heeft E, Engelke UFH, de Boer S, Hoegen B, Kwast HJT, van de Vorst M, Huigen MCDG, Keularts IMLW, Schreuder MF, van Karnebeek CDM, Wortmann SB, de Vries MC, Janssen MCH, Gilissen C, Engel J, Wevers RA. Next-generation metabolic screening: targeted and untargeted metabolomics for the diagnosis of inborn errors of metabolism in individual patients. J Inherit Metab Dis. 2018;41:337-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 61. | Saheki T, Moriyama M, Funahashi A, Kuroda E. AGC2 (Citrin) Deficiency-From Recognition of the Disease till Construction of Therapeutic Procedures. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Pinto A, Ashmore C, Batzios S, Daly A, Dawson C, Dixon M, Evans S, Green D, Gribben J, Hunjan I, Jameson E, Newby C, Pierre G, Rajwal S, Robertson L, Santra S, Sharrard M, Vara R, White L, Wilcox G, Yilmaz O, MacDonald A. Dietary Management, Clinical Status and Outcome of Patients with Citrin Deficiency in the UK. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Shigetomi H, Tanaka T, Nagao M, Tsutsumi H. Early Detection and Diagnosis of Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency Missed by Newborn Screening Using Tandem Mass Spectrometry. Int J Neonatal Screen. 2018;4:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Kido J, Häberle J, Sugawara K, Tanaka T, Nagao M, Sawada T, Wada Y, Numakura C, Murayama K, Watanabe Y, Kojima-Ishii K, Sasai H, Kosugiyama K, Nakamura K. Clinical manifestation and long-term outcome of citrin deficiency: Report from a nationwide study in Japan. J Inherit Metab Dis. 2022;45:431-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 65. | Köse MD, Kagnici M, Özdemir TR, Erdur CB, Erdemir G, Karakoyun M, Guzin Y, Ceylaner S, Genel F. Clinical findings in five Turkish patients with citrin deficiency and identification of a novel mutation on SLC25A13. J Pediatr Endocrinol Metab. 2020;33:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Chong SC, Lo P, Chow CW, Yuen L, Chu WCW, Leung TY, Hui J, Scaglia F. Molecular and clinical characterization of citrin deficiency in a cohort of Chinese patients in Hong Kong. Mol Genet Metab Rep. 2018;17:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Tokuhara D, Iijima M, Tamamori A, Ohura T, Takaya J, Maisawa S, Kobayashi K, Saheki T, Yamano T, Okano Y. Novel diagnostic approach to citrin deficiency: analysis of citrin protein in lymphocytes. Mol Genet Metab. 2007;90:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3289] [Article Influence: 328.9] [Reference Citation Analysis (7)] |
| 69. | Schwimmer JB, Ugalde-Nicalo P, Welsh JA, Angeles JE, Cordero M, Harlow KE, Alazraki A, Durelle J, Knight-Scott J, Newton KP, Cleeton R, Knott C, Konomi J, Middleton MS, Travers C, Sirlin CB, Hernandez A, Sekkarie A, McCracken C, Vos MB. Effect of a Low Free Sugar Diet vs Usual Diet on Nonalcoholic Fatty Liver Disease in Adolescent Boys: A Randomized Clinical Trial. JAMA. 2019;321:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 70. | Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24:2083-2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 71. | de Piano A, de Mello MT, Sanches Pde L, da Silva PL, Campos RM, Carnier J, Corgosinho F, Foschini D, Masquio DL, Tock L, Oyama LM, do Nascimento CM, Tufik S, Dâmaso AR. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur J Gastroenterol Hepatol. 2012;24:1313-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Ünalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 872] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 73. | Janczyk W, Lebensztejn D, Wierzbicka-Rucińska A, Mazur A, Neuhoff-Murawska J, Matusik P, Socha P. Omega-3 Fatty acids therapy in children with nonalcoholic Fatty liver disease: a randomized controlled trial. J Pediatr. 2015;166:1358-63.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 74. | Gkiourtzis Ν, Kalopitas G, Vadarlis A, Bakaloudi DR, Dionysopoulos G, Karanika E, Tsekitsidi E, Chourdakis M. The Benefit of Probiotics in Pediatric Nonalcoholic Fatty Liver Disease: A Meta-analysis of Randomized Control Trials. J Pediatr Gastroenterol Nutr. 2022;75:e31-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Pu J. Targeting the lysosome: Mechanisms and treatments for nonalcoholic fatty liver disease. J Cell Biochem. 2022;123:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Nobili V, Vajro P, Dezsofi A, Fischler B, Hadzic N, Jahnel J, Lamireau T, McKiernan P, McLin V, Socha P, Tizzard S, Baumann U. Indications and limitations of bariatric intervention in severely obese children and adolescents with and without nonalcoholic steatohepatitis: ESPGHAN Hepatology Committee Position Statement. J Pediatr Gastroenterol Nutr. 2015;60:550-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Loy JJ, Youn HA, Schwack B, Kurian M, Ren Fielding C, Fielding GA. Improvement in nonalcoholic fatty liver disease and metabolic syndrome in adolescents undergoing bariatric surgery. Surg Obes Relat Dis. 2015;11:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Nobili V, Carpino G, De Peppo F, Caccamo R, Mosca A, Romito I, Overi D, Franchitto A, Onori P, Alisi A, Gaudio E. Laparoscopic Sleeve Gastrectomy Improves Nonalcoholic Fatty Liver Disease-Related Liver Damage in Adolescents by Reshaping Cellular Interactions and Hepatic Adipocytokine Production. J Pediatr. 2018;194:100-108.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Nobili V, Della Corte C, Liccardo D, Mosca A, Caccamo R, Morino GS, Alterio A, De Peppo F. Obalon intragastric balloon in the treatment of paediatric obesity: a pilot study. Pediatr Obes. 2015;10:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Fukushima K, Yazaki M, Nakamura M, Tanaka N, Kobayashi K, Saheki T, Takei H, Ikeda S. Conventional diet therapy for hyperammonemia is risky in the treatment of hepatic encephalopathy associated with citrin deficiency. Intern Med. 2010;49:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Hayasaka K, Numakura C, Yamakawa M, Mitsui T, Watanabe H, Haga H, Yazaki M, Ohira H, Ochiai Y, Tahara T, Nakahara T, Yamashiki N, Nakayama T, Kon T, Mitsubuchi H, Yoshida H. Medium-chain triglycerides supplement therapy with a low-carbohydrate formula can supply energy and enhance ammonia detoxification in the hepatocytes of patients with adult-onset type II citrullinemia. J Inherit Metab Dis. 2018;41:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Ikeda S, Yazaki M, Takei Y, Ikegami T, Hashikura Y, Kawasaki S, Iwai M, Kobayashi K, Saheki T. Type II (adult onset) citrullinaemia: clinical pictures and the therapeutic effect of liver transplantation. J Neurol Neurosurg Psychiatry. 2001;71:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Weiss KH, Thurik F, Gotthardt DN, Schäfer M, Teufel U, Wiegand F, Merle U, Ferenci-Foerster D, Maieron A, Stauber R, Zoller H, Schmidt HH, Reuner U, Hefter H, Trocello JM, Houwen RH, Ferenci P, Stremmel W; EUROWILSON Consortium. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11:1028-35.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 84. | Hedera P. Clinical management of Wilson disease. Ann Transl Med. 2019;7:S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 85. | Santiago R, Gottrand F, Debray D, Bridoux L, Lachaux A, Morali A, Lapeyre D, Lamireau T. Zinc Therapy for Wilson Disease in Children in French Pediatric Centers. J Pediatr Gastroenterol Nutr. 2015;61:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Sharma R, Reiter RJ, Ma Q. Melatonin: A hypothesis regarding its use to treat Wilson disease. Med Hypotheses. 2019;133:109408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Garoufalia Z, Prodromidou A, Machairas N, Kostakis ID, Stamopoulos P, Zavras N, Fouzas I, Sotiropoulos GC. Liver Transplantation for Wilson's Disease in Non-adult Patients: A Systematic Review. Transplant Proc. 2019;51:443-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Bhattacharya K. Investigation and management of the hepatic glycogen storage diseases. Transl Pediatr. 2015;4:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 89. | Ross KM, Ferrecchia IA, Dahlberg KR, Dambska M, Ryan PT, Weinstein DA. Dietary Management of the Glycogen Storage Diseases: Evolution of Treatment and Ongoing Controversies. Adv Nutr. 2020;11:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 90. | Rossi A, Simeoli C, Salerno M, Ferrigno R, Della Casa R, Colao A, Strisciuglio P, Parenti G, Pivonello R, Melis D. Imbalanced cortisol concentrations in glycogen storage disease type I: evidence for a possible link between endocrine regulation and metabolic derangement. Orphanet J Rare Dis. 2020;15:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Jauze L, Monteillet L, Mithieux G, Rajas F, Ronzitti G. Challenges of Gene Therapy for the Treatment of Glycogen Storage Diseases Type I and Type III. Hum Gene Ther. 2019;30:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Shimizu S, Sakamoto S, Yamada M, Fukuda A, Yanagi Y, Uchida H, Mimori K, Shoji K, Funaki T, Miyairi I, Nakano N, Haga C, Yoshioka T, Imadome KI, Horikawa R, Kasahara M. Immunological features and complications in patients with glycogen storage disease 1b after living donor liver transplantation. Pediatr Transplant. 2021;25:e14104. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 93. | Paumgartner G. Pharmacotherapy of cholestatic liver diseases. J Dig Dis. 2010;11:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Appanna G, Kallis Y. An update on the management of cholestatic liver diseases. Clin Med (Lond). 2020;20:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Wagner M, Fickert P. Drug Therapies for Chronic Cholestatic Liver Diseases. Annu Rev Pharmacol Toxicol. 2020;60:503-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 96. | Goldstein J, Levy C. Novel and emerging therapies for cholestatic liver diseases. Liver Int. 2018;38:1520-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 97. | Nevens F, Trauner M, Manns MP. Primary biliary cholangitis as a roadmap for the development of novel treatments for cholestatic liver diseases(†). J Hepatol. 2023;78:430-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 98. | Hasegawa S, Yoneda M, Kurita Y, Nogami A, Honda Y, Hosono K, Nakajima A. Cholestatic Liver Disease: Current Treatment Strategies and New Therapeutic Agents. Drugs. 2021;81:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 99. | Carrion AF, Bhamidimarri KR. Liver transplant for cholestatic liver diseases. Clin Liver Dis. 2013;17:345-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Oya Y, Sugawara Y, Honda M, Yoshii D, Isono K, Hayashida S, Yamamoto H, Inomata Y. Living Donor Liver Transplantation for Progressive Familial Intrahepatic Cholestasis Type 1: Two Reported Cases. Transplant Proc. 2017;49:1123-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272:201-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 102. | van der Woerd WL, Houwen RH, van de Graaf SF. Current and future therapies for inherited cholestatic liver diseases. World J Gastroenterol. 2017;23:763-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balaban YH, Turkey; Uebayashi EY, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP