Published online Apr 7, 2023. doi: 10.3748/wjg.v29.i13.2050
Peer-review started: December 8, 2022
First decision: February 15, 2023
Revised: February 21, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 7, 2023
Processing time: 119 Days and 23.7 Hours
Acute pancreatitis (AP) is a disease spectrum ranging from mild to severe disease. During the coronavirus disease 2019 (COVID-19) pandemic, numerous reports of AP have been published, with most authors concluding a causal relationship between COVID-19 and AP. Retrospective case reports or small case series are unable to accurately determine the cause-effect relationship between COVID-19 and AP.
To establish whether COVID-19 is a cause of AP using the modified Naranjo scoring system.
A systematic review was conducted on PubMed, World of Science and Embase for articles reporting COVID-19 and AP from inception to August 2021. Exclusion criteria were cases of AP which were not reported to be due to COVID-19 infection, age < 18 years old, review articles and retrospective cohort studies. The original 10-item Naranjo scoring system (total score 13) was devised to approximate the likelihood of a clinical presentation to be secondary to an adverse drug reaction. We modified the original scoring system into a 8-item modified Naranjo scoring system (total score 9) to determine the cause-effect relationship between COVID-19 and AP. A cumulative score was decided for each case presented in the included articles. Interpretation of the modified Naranjo scoring system is as follows: ≤ 3: Doubtful, 4-6: Possible, ≥ 7: Probable cause.
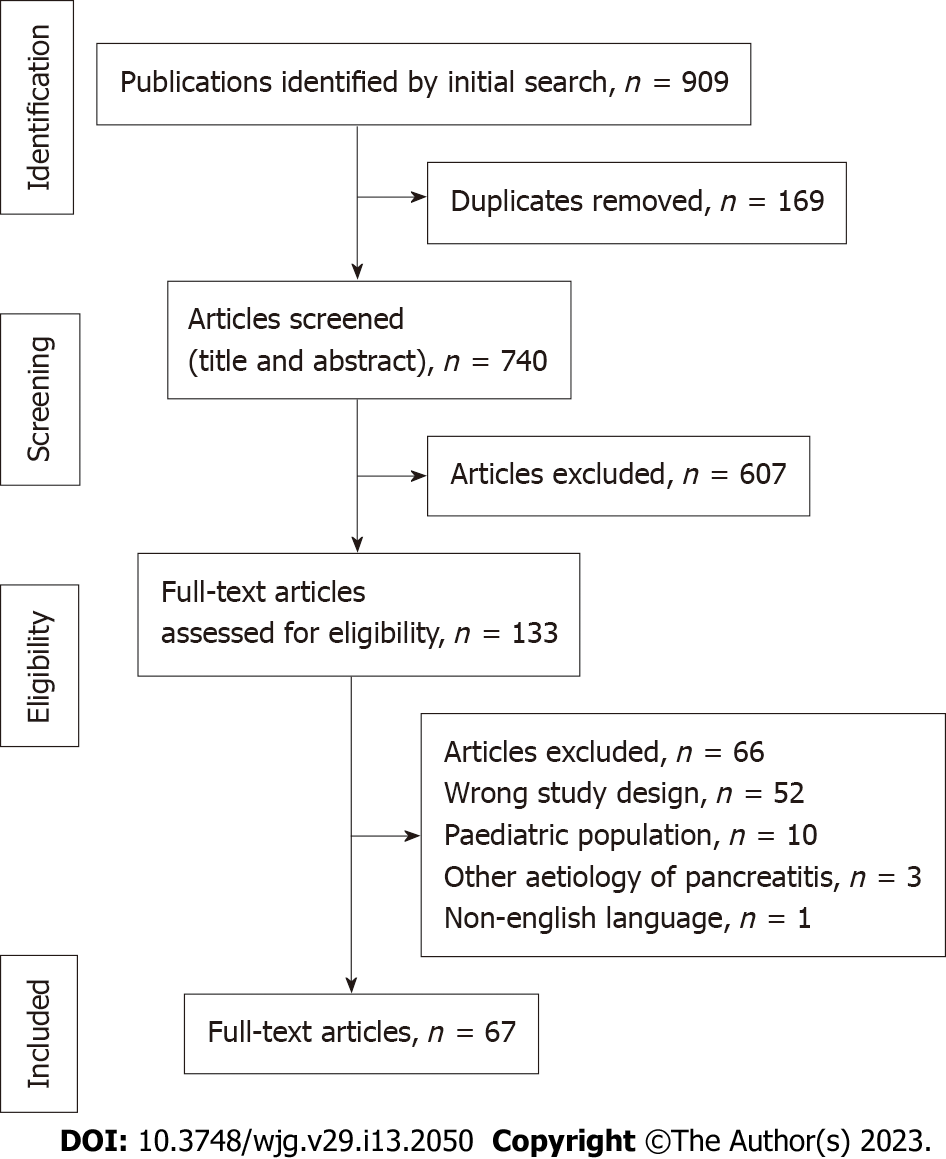
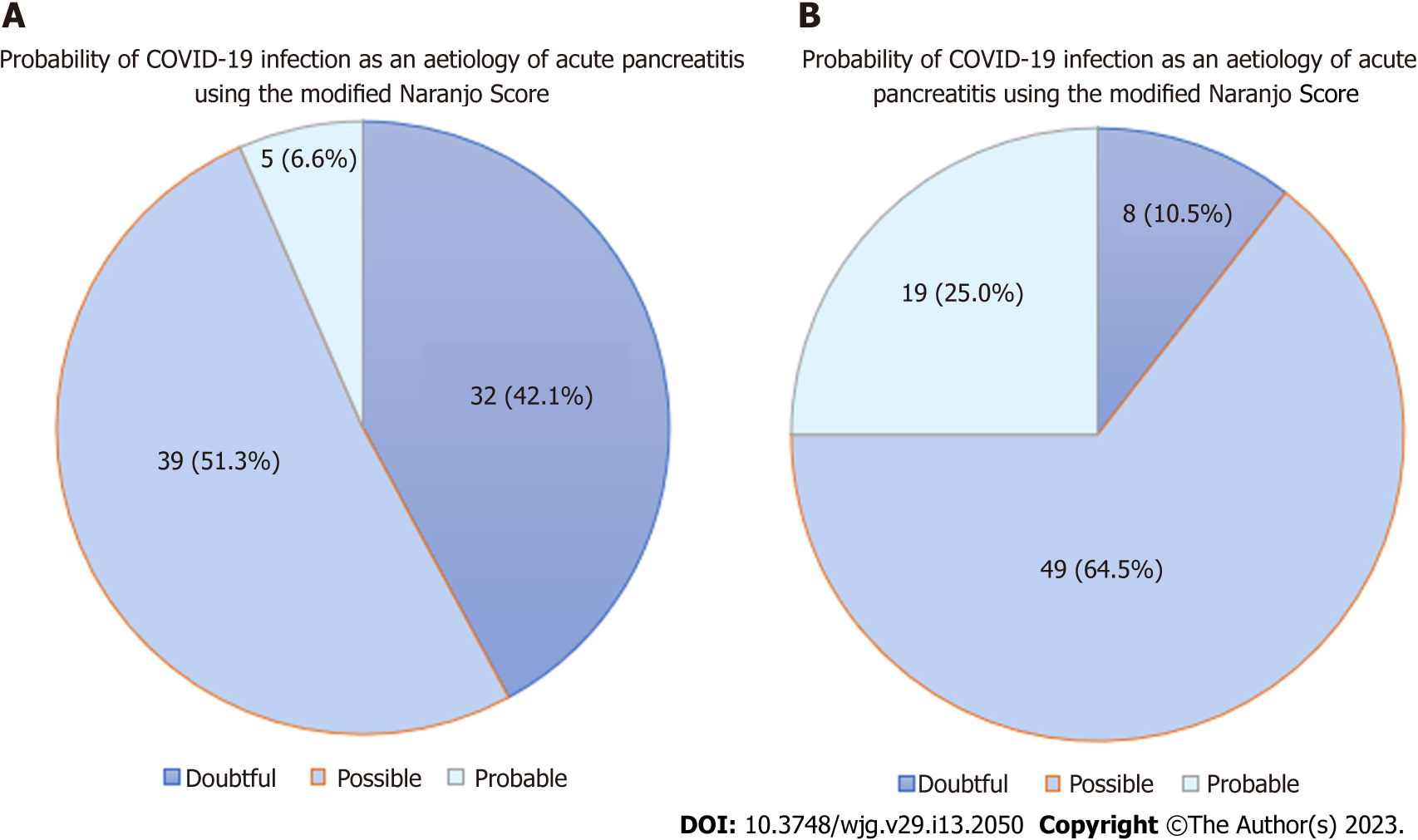
The initial search resulted in 909 articles, with 740 articles after removal of duplicates. A total of 67 articles were included in the final analysis, with 76 patients which had AP reported to be due to COVID-19. The mean age was 47.8 (range 18-94) years. Majority of patients (73.3%) had ≤ 7 d between onset of COVID-19 infection and diagnosis of AP. There were only 45 (59.2%) patients who had adequate investigations to rule out common aetiologies (gallstones, choledocholithiasis, alcohol, hypertriglyceridemia, hypercalcemia and trauma) of AP. Immunoglobulin G4 testing was conducted in 9 (13.5%) patients to rule out autoimmune AP. Only 5 (6.6%) patients underwent endoscopic ultrasound and/or magnetic resonance cholangiopancreatogram to rule out occult microlithiasis, pancreatic malignancy and pancreas divisum. None of the patients had other recently diagnosed viral infections apart from COVID-19 infection, or underwent genetic testing to rule out hereditary AP. There were 32 (42.1%), 39 (51.3%) and 5 (6.6%) patients with doubtful, possible, and probable cause-effect relationship respectively between COVID-19 and AP.
Current evidence is weak to establish a strong link between COVID-19 and AP. Investigations should be performed to rule out other causes of AP before establishing COVID-19 as an aetiology.
Core Tip: Numerous reports of acute pancreatitis (AP) have been published during the coronavirus disease 2019 (COVID-19) pandemic, citing COVID-19 as an aetiology of AP. However, COVID-19 has not been well-established to be a cause of AP. A total of 76 patients were included in our systematic review and were assessed using the modified Naranjo score; there were 32 (42.1%), 39 (51.3%) and 5 (6.6%) patients with doubtful, possible, and probable cause-effect relationship respectively between COVID-19 and AP. The link between COVID-19 and AP is weak based on current literature; COVID-19 should still remain a diagnosis of exclusion for AP until further evidence.
- Citation: Teng TZJ, Chua BQY, Lim PK, Chan KS, Shelat VG. Occam’s razor or Hickam’s dictum-COVID-19 is not a textbook aetiology of acute pancreatitis: A modified Naranjo Score appraisal. World J Gastroenterol 2023; 29(13): 2050-2063
- URL: https://www.wjgnet.com/1007-9327/full/v29/i13/2050.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i13.2050
Acute pancreatitis (AP) is a disease spectrum ranging from mild to severe, with an incidence of 50-80 per 100000 population[1]. Gallstone disorders and alcohol abuse remain the two commonest global causes of AP[2]. In rare circumstances, AP may be triggered by viral or parasitic infections[3,4]. Recently, there have been reports of AP caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of the time of this article, the coronavirus disease 2019 (COVID-19) pandemic is responsible for over 642 million infections and 6.6 million deaths worldwide[5]. While COVID-19 is primarily a respiratory disease, patients with COVID-19 infection may experience extra-pulmonary symptoms[6]. There has been an increase in reports of autoimmune and inflammatory conditions attributed to SARS-CoV-2[7], one example of which is AP. While there have been sporadic case reports and attempts at literature reviews on the potential cases of COVID-19-induced AP, the significant increase in cases reported raises concerns regarding COVID-19 as a definitive causal etiology for AP rather than an epiphenomenon[8].
The distinction between “association” vs “causation” can only be derived by prospective longitudinal studies involving a large population or by rigorous statistical analysis of large datasets and registries. It remains unproven if published reports of AP (effect) are beyond doubt due to COVID-19 infection (causal etiology). This is similar to determining whether symptoms experienced following ingestion of a new medication may be coined as an “adverse drug reaction” and attributed to that drug. Naranjo et al[9] designed a questionnaire to determine the likelihood of an adverse drug reaction (effect) due to a drug (causal etiology) rather than associated confounding factors[9]. Recently, we have reported the utility of modified Naranjo score to diagnose AP in patients prescribed with sulphasalazine[10]. This is a novel study which aims to systematically review case series and/or reports on COVID-19-induced AP and assess them using the modified Naranjo score to determine whether there is a cause-effect relationship.
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines[11]. A literature search was performed on PubMed, World of Science and Embase for articles on COVID-19 and AP from inception till August 8, 2021. The following search terms were used: ((“COVID-19” OR “Coronavirus 19” OR “SARS-CoV-2”) AND (“Pancreatitis” OR “Acute Pancreatitis” OR “Acute Edematous Pancreatitis” OR “Pancreatic Inflammation”)); MeSH terms were used where available. Inclusion criteria were case series and/or case reports on AP, where the aetiology was thought to be COVID-19 infection. Exclusion criteria were (1) reports on AP but aetiology was not attributed to COVID-19 infection; (2) cases with patients < 18 years old; and (3) based on article type (non-English language, review articles and retrospective cohort and/or case-control studies). After removing of duplicates, three authors (Tang ZJT, Chua BQY, and Lim PK) independently screened the articles for potential inclusion by title and abstract. Subsequently, the full texts of screened studies were obtained and reviewed for eligibility. As some case reports may not have abstracts published, full texts were reviewed if abstracts were not available. Conflicts were resolved by appeal to the senior author.
The original Naranjo score was described by Naranjo et al[9] in 1981 as a means of approximating the likelihood of attributing a patient’s presentation to a medication and labelling it as an adverse drug reaction[9]. It consists of ten closed-ended questions with the options for “Yes”, “No,” and “Do not know”, each with varying points allocated from “- 1” to “+ 2”. The total score is 13 and may be interpreted as such: ≤ 0: Doubtful, 1-4: Possible; 5-8: Probably and ≥ 9: Definite. Existing studies have modified the Naranjo score to determine the cause-effect relationship in various pathologies[10]. In this review, we propose a modification to the Naranjo score to determine the likelihood of COVID-19-induced AP (Table 1). This is a 8-item scoring system with a total of 9 points; a cumulative score was calculated for each case, and a probability classification was assigned based on their score: ≤ 3: Doubtful, 4-6: Possible, ≥ 7: Probable.
| Criteria | Yes | No | Unsure |
| Are there published reports of the COVID-19 causing acute pancreatitis? | + 1 | 0 | 0 |
| Was there short latency (≤ 7 d) between the onset of infection and the diagnosis of acute pancreatitis? | + 2 | - 1 | 0 |
| Was there a temporal relationship (≤ 1 mo) between onset of infection and onset of acute pancreatitis symptoms? | + 1 | - 1 | 0 |
| Did the acute pancreatitis resolve following resolution of the infection? | + 1 | - 1 | 0 |
| Were all commonly known causes of acute pancreatitis ruled out? (e.g., gallstones/choledocholithiasis, alcohol, hypertriglyceridaemia, hypercalcaemia, ERCP, trauma) | + 1 | - 1 | 0 |
| Was a serum IgG4 level checked? (To rule out autoimmune pancreatitis) | + 1 | 0 | 0 |
| Does the patient have or was the patient recently diagnosed with an infection (other than COVID-19) which could cause pancreatitis? | - 1 | + 1 | 0 |
| Was an EUS and/or MRCP performed? (e.g., to rule out occult microlithiasis, pancreatic malignancy and pancreas divisum) | + 1 | - 1 | 0 |
Our modified version of the Naranjo score retained some of the questions used in the original study: Whether reports suggest a causal relationship, if the resolution of COVID-19 led to the resolution of AP, and ruling out alternative causes of AP. Common causes of AP were defined as gallstones, choledocholithiasis, alcohol, hypertriglyceridaemia, hypercalcaemia or trauma. Investigations for these included history and physical examination (for alcohol and trauma), biochemical investigations (for hypertriglyceridaemia and hypercalcemia), imaging studies (ultrasonography and/or computed tomography of the abdomen and pelvis) (for gallstones or choledocholiathisis) and endoscopic retrograde cholangiopancreatogram for choledocholithiasis in the presence of abnormal liver function test and/or biliary dilation. The original Naranjo score included the need for drug challenge test or placebo, but this was not appropriate for this study, as a “drug challenge” would imply re-introduction of COVID-19 infection and identifying if the patient had recurrence of AP. Instead, whether the resolution of infection led to the resolution of acute pancreatitis was included as a criterion.
We additionally included questions on whether there is a cause-effect temporal relationship and a more in-depth approach in ruling out the other aetiologies of AP. This included ruling out autoimmune pancreatitis with testing for serum Immunoglobulin G4 (IgG4)[12], or whether an endoscopic ultrasound (EUS) or magnetic resonance cholangiopancreatography (MRCP) was performed to rule out occult microlithiasis, pancreatic malignancy or pancreatic divisum[13]. While genetic testing to rule out hereditary pancreatitis is a consideration[14], this was omitted in our modified Naranjo score as none of the included articles performed genetic tests for confirming/excluding hereditary pancreatitis. Additionally, there are no strict recommendations on the exact indications for genetic counselling and/or testing in AP, limiting its utility for inclusion in our proposed scoring system[15].
Three authors (Tang ZJT, Chua BQY, and Lim PK) performed all data extraction independently using the systematic review management tool Covidence (https://www.covidence.org). The following data were extracted: Year of study, age, and gender of the patient, and features relevant to the modified Naranjo score, as shown in Table 1.
The initial search resulted in 909 articles, with 740 articles after removal of duplicates. A total of 67 articles were included in the final analysis (Figure 1). There was a total of 76 patients which had AP reported to be due to COVID-19. Data extracted from all cases were reported in Table 2[16-82]. Seventy-six patients with a mean age of 47.8 years (range 18-94) were reported. There were 32 (42.1%), 39 (51.3%) and 5 (6.6%) patients with doubtful, possible, and probable cause-effect relationship respectively between COVID-19 and AP (Figure 2A). Majority of patients (73.7%, n = 56/76) had a short duration of latency (≤ 7 d) between the onset of infection and AP. Most reports did not explore the temporal relation between COVID-19 and AP; only 20 (26.3%) patients were described to have a temporal relationship between COVID-19 and AP. Common aetiologies of AP were ruled out in 45 (59.2%) patients. Serum IgG4 Levels were tested to rule out autoimmune pancreatitis in 9 (13.6%) patients. None of the patients were recently diagnosed with an infection known to cause AP, e.g., Coxsackie virus, cytomegalovirus, or herpes simplex virus. Only a minority (6.6%, n = 5/76) underwent either EUS or ERCP. None of the patients underwent genetic testing, e.g., SPINK-1, to rule out hereditary AP.
| No | First author | Year | Patient age/Sex | Scoring | Summed score | Result1 | |||||||
| 1 | Acherjya et al[16] | 2020 | 57/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 2 | Al-Douri et al[17] | 2020 | 45/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 3 | Al-Harmi et al[18] | 2021 | 52/F | 1 | 0 | 1 | 0 | 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 4 | Ali et al[19] | 2021 | 53/M | 1 | 0 | 1 | 0 | - 1 | 0 | 1 | - 1 | 1 | Doubtful |
| 5 | Aloysius et al[20] | 2020 | 36/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 6 | Alves et al[21] | 2020 | 56/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | Probable |
| 7 | Alwaeli et al[22] | 2020 | 30/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 8 | Amé and Balderramo[23] | 2022 | 42/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 9 | 65/F | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 6 | Possible | ||
| 10 | Anand et al[24] | 2020 | 59/F | 1 | 0 | 1 | 0 | 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 11 | Arjun et al[25] | 2020 | 34/M | 1 | 2 | 1 | 1 | 1 | 1 | 1 | - 1 | 7 | Probable |
| 12 | Bains et al[26] | 2020 | 68/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | 1 | 5 | Possible |
| 13 | Bokhari and Mahmood[27] | 2020 | 32/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 14 | Bouali et al[28] | 2021 | 60/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 15 | Brikman et al[29] | 2020 | 61/M | 1 | 0 | 1 | 0 | 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 16 | Canastar et al[30] | 2020 | 64/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 17 | Chandra et al[31] | 2021 | 53/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 18 | Cheung et al[32] | 2020 | 38/M | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | Probable |
| 19 | Chivato et al[33] | 2021 | 55/M | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 8 | Probable |
| 20 | Dufani et al[34] | 2020 | 27/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 21 | Elhence et al[35] | 2020 | 31/F | 1 | 0 | 1 | 0 | - 1 | 0 | 1 | - 1 | 1 | Doubtful |
| 22 | 40/M | 1 | 0 | 1 | 0 | - 1 | 0 | 1 | - 1 | 1 | Doubtful | ||
| 23 | 42/M | 1 | 0 | 1 | 0 | - 1 | 0 | 1 | - 1 | 1 | Doubtful | ||
| 24 | Fernandes et al[36] | 2020 | 36/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 25 | Fiore et al[37] | 2021 | 42/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 26 | 70/M | 1 | 0 | 1 | 0 | 1 | 0 | 1 | - 1 | 3 | Doubtful | ||
| 27 | Gadiparthi et al[38] | 2020 | 40/M | 1 | 2 | 1 | 1 | - 1 | 0 | 1 | - 1 | 4 | Possible |
| 28 | Gadiparthi et al[39] | 2021 | 74/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 29 | Gonzalo-Voltas et al[40] | 2020 | 76/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 30 | Gupta et al[41] | 2021 | 25/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | -1 | 6 | Possible |
| 31 | Hadi et al[42] | 2020 | 47/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 32 | 68/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful | ||
| 33 | Hanif et al[43] | 2021 | 30/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 34 | Hassani et al[44] | 2020 | 78/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 35 | Ibrahim et al[45] | 2020 | 33/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 36 | Jespersen Nizamic et al[46] | 2020 | 49/W | 1 | 0 | 1 | 0 | 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 37 | Kandasamy[47] | 2020 | 45/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 38 | Karimzadeh et al[48] | 2020 | 65/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 39 | Kataria and Sharif[49] | 2020 | 49/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 40 | Kolhe et al[50] | 2020 | 19/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 41 | Kumaran et al[51] | 2020 | 67/F | 1 | 2 | 1 | 1 | 1 | 1 | 1 | - 1 | 7 | Probable |
| 42 | Kurihara et al[52] | 2020 | 55/M | 1 | 0 | 1 | 0 | 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 43 | Lakshmanan and Malik[53] | 2020 | 68/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 44 | Maalouf et al[54] | 2021 | 62/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 45 | Mazrouei et al[55] | 2020 | 24/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 46 | Meireles et al[56] | 2020 | 36/F | 1 | 0 | 1 | 1 | 1 | 1 | 1 | - 1 | 5 | Possible |
| 47 | Merza et al[57] | 2020 | 57/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 48 | 70/M | 1 | 0 | 1 | 0 | - 1 | 0 | 1 | - 1 | 1 | Doubtful | ||
| 49 | Meyers et al[58] | 2020 | 67/M | 1 | 2 | 1 | 0 | - 1 | 1 | 1 | - 1 | 4 | Possible |
| 50 | Miao et al[59] | 2020 | 26/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 51 | Mobin et al[60] | 2020 | 18/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 52 | 66/M | 1 | 2 | 1 | 1 | - 1 | 0 | 1 | - 1 | 4 | Possible | ||
| 53 | Mohammadi Arbati and Molseghi[61] | 2021 | 28/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 54 | Muhammad Abrar Jeelani et al[62] | 2021 | 24/M | 1 | 0 | 1 | 1 | 1 | 0 | 1 | - 1 | 4 | Possible |
| 55 | Naqvi et al[63] | 2020 | 69/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 56 | Narang et al[64] | 2021 | 20/F | 1 | 2 | 1 | 0 | 1 | 1 | 1 | - 1 | 6 | Possible |
| 57 | Patnaik et al[65] | 2020 | 29/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 58 | Pinte and Baicus[66] | 2020 | 47/M | 1 | 0 | 1 | 0 | 1 | 0 | 1 | - 1 | 4 | Possible |
| 59 | Purayil et al[67] | 2020 | 58/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 60 | Rabice et al[68] | 2020 | 36/F | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible |
| 61 | Rotar et al[69] | 2020 | 39/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 62 | Sandhu et al[70] | 2021 | 25/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 63 | Shinohara et al[71] | 2020 | 58/M | 1 | 0 | 1 | 1 | - 1 | 0 | 1 | - 1 | 2 | Doubtful |
| 64 | Simou et al[72] | 2020 | 67/NM | 1 | 0 | 1 | 0 | - 1 | 0 | 1 | - 1 | 1 | Doubtful |
| 65 | Singh and Kharoud[73] | 2020 | 94/F | 1 | 2 | 1 | 0 | 1 | 1 | 1 | - 1 | 6 | Possible |
| 66 | 58/F | 1 | 0 | 1 | 0 | 1 | 1 | 1 | - 1 | 4 | Possible | ||
| 67 | Srinivasan et al[74] | 2021 | 52/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 68 | Tollard et al[75] | 2021 | 32/F | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 69 | Tomasi et al[76] | 2021 | 31/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 70 | Truscello et al[77] | 2020 | 71/M | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 71 | Wang et al[78] | 2020 | 42/M | 1 | 2 | 1 | 0 | 1 | 0 | 1 | - 1 | 5 | Possible |
| 72 | 35/M | 1 | 2 | 1 | 1 | 1 | 0 | 1 | - 1 | 6 | Possible | ||
| 73 | Wifi et al[79] | 2021 | 72/F | 1 | 2 | 1 | 0 | - 1 | 0 | 1 | - 1 | 3 | Doubtful |
| 74 | Yamamoto et al[80] | 2021 | 70/F | 1 | 0 | 1 | 0 | - 1 | 0 | 1 | - 1 | 1 | Doubtful |
| 75 | Zeng et al[81] | 2020 | 36/M | 1 | 2 | 1 | 0 | 0 | 0 | 1 | - 1 | 4 | Possible |
| 76 | Zielecki et al[82] | 2020 | 38/M | 1 | 0 | 1 | 1 | - 1 | 0 | 1 | 0 | 3 | Doubtful |
AP is a common cause of acute abdominal pain and hospital admissions and present as a disease spectrum[83]. In view of the COVID-19 pandemic, many retrospective case reports suggesting COVID-19 as a cause of AP have been published. It is an obligation to critically appraise these reports to define the strength of the association and evaluate if the co-occurrence is a mere association or actual causation. This novel study adopted the modified Naranjo scale and concluded the cause-effect relationship between COVID-19 and AP in most reports as doubtful (42.1%) or possible (51.3%).
While gallstones and alcohol consumption remain the most common aetiologies, in rare situations, viral infections have been reported to cause AP[3,4]. However, this should be a diagnosis of exclusion after ruling out of common aetiologies for AP. In our review, the reason why strong conclusions could not be drawn for most cases was the absence of serum IgG4 Levels (86.4%) to rule out autoimmune AP (score of + 1) and EUS or MRCP (93.4%) to rule out structural causes (e.g. occult microlithiasis or pancreatic divisum) (score of +1). Authors reporting further cases of AP where COVID-19 is suggested as an aetiology should perform these tests to rule out other aetiologies of AP prior to conclusion of COVID-19-induced AP. While the results do not confirm AP as a possible aetiology of COVID-19, it is still prudent to consider COVID-19 when patients present with AP, especially if there are concurrent clinical features of COVID-19 with temporal relation of symptoms and if other common causes are ruled out.
The current literature is torn on the idea that the SARS-CoV2 virus could have induced the recent cases of AP, where some propose a direct causal relationship and others claim the SARS-CoV2 virus as a bystander in the instances of idiopathic AP[84-86]. For the former, two possible mechanisms have been described: The direct effect of the SARS-CoV2 RNA on pancreatic tissue due to viral tropism and the indirect effect due to microthrombi formation. Regarding the direct mechanism, the expression of the angiotensin converting enzyme 2 (ACE2) protein on the SARS-CoV2 virus primes its entry into the pancreas. This expression of the ACE2 protein was similarly found in the islet and exocrine tissue microvasculature and in a subset of pancreatic ducts, as well as the TMPRSS2 proteins found in the ductal cells[84]; this suggests entry of SARS-CoV2 into pancreatic tissue resulting in AP. Regarding the indirect mechanism, SARS-CoV2 causes microthrombi formation due to its ability to invade endothelial cells via the ACE2 protein. This phenomenon is also observed in other virus infections, such as viral hepatitis[85]. Microthrombus in vasculature triggers hypoperfusion of the pancreas, resulting in ischemic pancreatitis. However, the above theories are merely conjectures made from parallel existing conditions superimposed on an under-researched virus. The possibility of COVID-19 being a pure bystander in the formation of AP also exists. This may be a situation of Occam’s razor– where we look to connect the dots between a relatively new disease and the patients’ manifestations– vs a pure case of Hickam’s dictum– where a patient happens to present with two mutually exclusive conditions. After all, it must be noted that idiopathic AP is a function of diagnostic workup efforts[86]. The lack of serological tests for IgG4, MRCP scan, and EUS imaging could inflate the association between COVID-19 and AP. As randomized studies cannot be conducted to establish COVID-19 as an aetiology for AP, critical appraisal of retrospective data is essential to discern association from causation.
The temporal relation of events is one of the important determinants of distinguishing causation from the association. Many authors did not explicitly mention a temporal relation of onset or resolution of COVID-19 and AP. While COVID-19 infections primarily involve the respiratory system, multi-systemic involvement have been reported. Since COVID-19 is a prerequisite for COVID-19 associated AP, we shall discuss the respiratory and imaging features first. COVID-19-induced AP may manifest in the absence of any respiratory symptoms or radiological evidence of lung involvement. Purayil et al[67] reported a 58-year-old male presenting with abdominal pain; a polymerase chain reaction (PCR) for COVID-19 was only done in response to his chest x-ray, which revealed bilateral infiltrates in the absence of respiratory symptoms[67]. A chest x-ray is routinely performed in patients with acute abdomen to rule out free air under the diaphragm secondary to hollow viscus perforation, which also can cause hyperamylasaemia and remain an important differential diagnosis of AP[87]. Additionally, chest x-ray findings form a part of scoring systems to predict severity and clinical outcomes in AP patients[88,89]. In the patient reported by Purayil et al[67], the chest x-ray was sensitive to detect COVID-19 changes and complemented the diagnostic work-up for epigastric pain. As most patients with AP will have a chest x-ray performed and due to the widespread prevalence of COVID-19 in the community, it is not possible to ascertain if COVID-19 resulted in AP or if the two diseases merely occurred simultaneously yet independently of each other.
The prevalence of gastrointestinal symptoms in COVID-19 infected patients ranges from 3.0%-79%, of which only 2.2%-6.0% of patients present with abdominal pain[90-92]. Abdominal pain is the most common symptom of AP and acute epigastric pain is one of the key diagnostic criteria of AP. Thus it remains unclear if gastrointestinal manifestations of COVID-19 are secondary to AP. Though resuscitation and early management of AP patients are not determined by aetiology, definite management for prevention of future occurences is determined by aetiology. Thus, knowledge of COVID-19 as an aetiology of AP is important as it stops the pursuit of aetiology identification, guides physician on the counselling of their patients, and impacts management decision for cholecystectomy. In patients with mild biliary pancreatitis, index admission laparoscopic cholecystectomy is considered good clinical practice while surgery should be delayed in patients with COVID-19 infection to minimize risk to healthcare workers and reduce patient morbidity[93,94]. Shao et al[95] analysed 589 patients with COVID-19 infection prior to surgery and concluded that postoperative mortality was nearly 6 times higher for patients infected with COVID-19 within 2 wk before surgery when adjusting for patient and procedure level factors[95]. Furthermore, delaying cholecystectomy in patients with biliary pancreatitis could increase the risk of future biliary events[88]. Thus, determining the aetiology of AP is essential as it impacts clinical decisions.
In addition to abdominal pain and imaging features, it is important to discuss the role of serum enzymes. While both amylase and lipase are usually measured in patients presenting with acute abdomen suggesting AP, the timing at which they rise can be cross-referenced to the time at which COVID-19 infection was diagnosed, which may shed some light on whether COVID-19 is a cause of AP, or merely coincidental. Amylase tends to rise within 3 to 6 h of AP and persists for up to 5 d. However, serum amylase has a relatively short half-life of 12 h and may return to normal limits within a day. Serum lipase rises similarly within 3 to 6 h, peaks at 24 h, and persists for 8 to 14 d. Hence, a patient with raised amylase or lipase alongside a concomitant COVID-19 infection before this time span of 12-24 h may still have AP caused by COVID-19. This clarity blurs if we consider the virus's incubation period and heterogeneity in the clinical presentation of both COVID-19 and AP. Stephens et al[96] reported 234 COVID-19 positive patients in the critical care unit, of which 52 (22.2%) patients had peak amylase three times the upper limit of normal, of which only 4 (1.7%) met the revised Atlanta criteria for diagnosis of AP[96]. Furthermore, some authors report COVID-19 associated hyperamylasemia secondary to pancreatic injury which does not amount to clinical AP[97,98]. This could be attributed to amylase release from other viscera like the gastrointestinal tract or elevated serum levels due to reduced renal excretion as a result of critical illness-related kidney injury[96]. A contrast-enhanced imaging remains an essential tool to diagnose AP, but it would not establish COVID-19 as a causative aetiology.
As the temporal occurrence of events (e.g. onset of abdominal pain, chest x-ray imaging, serum enzymes, and abdominal imaging) do not aid the distinction of causation or association, a different approach is essential. This situation is similar to the dilemma of attributing a clinical presentation towards an adverse drug reaction. The Naranjo score is a useful tool that aids clinical judgment in differentiating the cause-effect relationship between a drug and its possible adverse drug reaction[9]. While useful in determining the causality, it should be noted that the scale does not offer prognostic information. In addition, the Naranjo score includes isolating toxic concentrations of drugs in body fluids, clinical response to placebo administration, and a drug rechallenge to assess symptoms occurrence. While most authors report positive COVID-19 rapid antigen test and PCR, the response to placebo and re-infection are not reported. It is not possible to assess if the patients were re-infected with COVID-19 (after reporting of the case) with repeat AP and unethical to do this experimentally. Thus, no comment can be made on whether a ‘re-challenge’ would bring about similar symptoms. If these parameters are scored negative, many cases would be disadvantaged from the strength of causation, and thus, a modification of the Naranjo score that excludes placebo administration and re-infection was considered essential to determine COVID-19 as a cause of AP fairly. This approach has been reported in the past for sulphasalazine-induced AP and prednisolone induced pneumatosis coli[10,99]. Using this strategy, we determined the strength of association and possible causation as doubtful, possible or probable. Other established methods of determining a causal relationship between various aetiological agents and AP include the Badalov categorisation[100]. The Badalov categorisation was similarly designed to investigate association between drugs and adverse drug reactions; this involves assigning drugs into 5 categories (Class Ia, Ib, II, III and IV) based on the number of case reports published, drug rechallenge, latency and whether alternative causes were excluded. However, we have chosen to adopt the Naranjo score as it allows for a case-by-case evaluation of each report as opposed to a blanket categorization of COVID-19 as a possible aetiological agent of AP. Secondly, the Naranjo score also provides a better idea on the degree of association by generating data on a numerical scale which allows for in-depth analysis. Centers should continue reporting such occurrences of COVID-19-induced pancreatitis and consider incorporating our modified Naranjo score; artificial intelligence methods may subsequently be used to diagnose COVID-19-induced pancreatitis[101,102].
However, our study has its limitations. Firstly, given the nature of our study, prospective studies and systematic reviews were not analysed as they lack individual patient data. Additionally, interpretation of this study is limited by the small sample size of 76 patients. Secondly, a majority of included case reports date after the introduction of the COVID-19 vaccine. Many reports did not mention the vaccination status of the patient; COVID-19 vaccination has also been reported as a potential cause of AP which adds on to the dilemma[24,103]. Thirdly, with the advent of novel drugs used in COVID-19 treatment, the possibility of drug-induced AP needs to be considered as a differential diagnosis. Remdesivir is an antiviral drug widely used in the management of COVID-19 and it is increasingly reported to cause pancreatic injury with associated hyperamylasaemia as well as AP[104,105]. Fourthly, authors may not have reported cases where patients were reinfected by COVID-19. This is a potential limitation in the calculation of the modified Naranjo score. Fifthly, the determination of the criteria for “doubtful”, “possible” and “probable” is arbitrary. If we subtract one point from the minimum of each category (i.e. ≤ 2 for doubtful, 3-5 for possible and ≥ 6 for probable) to increase the causation strength, this would result in 8 (compared to 32) patients, 49 patients (compared to 39), and 19 patients (compared to 5) respectively (Figure 2A and B). As the median score was 4, if we use it as the cut-off, 32 patients (score < 4) will be categorized as “less likely” and 44 patients (score ≥ 4) as “more likely” to have COVID-19-induced AP. Thus, the assignment of scores, though partly arbitrary, the fact still prevails that existing reports have doubt about COVID-19 infection causing AP. Lastly, while some of the screened cases reported fatalities[13], our study did not assess the severity and mortality of COVID-19 induced AP.
The use of our proposed modified Naranjo score may help to determine whether COVID-19 is a likely aetiology of AP and may assist clinicians in making useful clinical decisions. The current evidence is weak to establish a strong causal link between COVID-19 and AP, and more evidence is necessary before COVID-19 should be incorporated as a “textbook aetiology” of AP.
Acute pancreatitis (AP) is a disease spectrum ranging from mild to severe disease. During the coronavirus disease 2019 (COVID-19) pandemic, numerous reports of AP have been published, with most authors concluding a causal relationship between COVID-19 and AP.
Published reports or case series of COVID-19-induced AP are retrospective in nature and are unable to accurately determine the cause-effect relationship between COVID-19 and AP.
This study aims to establish whether COVID-19 is a cause of AP by proposing a scoring system i.e. the modified Naranjo scoring system.
A systematic review was conducted on PubMed, World of Science and Embase for articles reporting COVID-19 and AP from inception to August 2021. Exclusion criteria were cases of AP which were not reported to be due to COVID-19 infection, age < 18 years old, review articles and retrospective cohort studies. The original 10-item Naranjo scoring system (total score 13) was devised to approximate the likelihood of a clinical presentation to be secondary to an adverse drug reaction. We modified the original scoring system into a 8-item modified Naranjo scoring system (total score 9) to determine the cause-effect relationship between COVID-19 and AP. A cumulative score was decided for each case presented in the included articles. Interpretation of the modified Naranjo scoring system is as follows: ≤ 3: Doubtful, 4-6: Possible, ≥ 7: Probable cause.
The initial search resulted in 909 articles, with 740 articles after removal of duplicates. A total of 67 articles were included in the final analysis, with 76 patients which had AP reported to be due to COVID-19. The mean age was 47.8 (range 18-94) years. Majority of patients (73.3%) had ≤ 7 d between onset of COVID-19 infection and diagnosis of AP. There were only 45 (59.2%) patients who had adequate investigations to rule out common aetiologies (gallstones, choledocholithiasis, alcohol, hypertriglyceridemia, hypercalcemia and trauma) of AP. Immunoglobulin G4 testing was conducted in 9 (13.5%) patients to rule out autoimmune AP. Only 5 (6.6%) patients underwent endoscopic ultrasound and/or magnetic resonance cholangiopancreatogram to rule out occult microlithiasis, pancreatic malignancy and pancreas divisum. None of the patients had other recently diagnosed viral infections apart from COVID-19 infection, or underwent genetic testing to rule out hereditary AP. There were 32 (42.1%), 39 (51.3%) and 5 (6.6%) patients with doubtful, possible, and probable cause-effect relationship respectively between COVID-19 and AP.
Current evidence is weak to establish a strong link between COVID-19 and AP. Investigations should be performed to rule out other causes of AP before establishing COVID-19 as an aetiology.
The use of our proposed modified Naranjo score may help to determine whether COVID-19 is a likely etiology of AP and may assist clinicians in making useful clinical decisions.
| 1. | Teng TZJ, Tan JKT, Baey S, Gunasekaran SK, Junnarkar SP, Low JK, Huey CWT, Shelat VG. Sequential organ failure assessment score is superior to other prognostic indices in acute pancreatitis. World J Crit Care Med. 2021;10:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Ong Y, Shelat VG. Ranson score to stratify severity in Acute Pancreatitis remains valid - Old is gold. Expert Rev Gastroenterol Hepatol. 2021;15:865-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Lee KH, Shelat VG, Low HC, Ho KY, Diddapur RK. Recurrent pancreatitis secondary to pancreatic ascariasis. Singapore Med J. 2009;50:e218-e219. [PubMed] |
| 4. | Teng TZJ, Shelat VG. Blastocystis hominis in Acute Pancreatitis: Innocent Bystander or Pathogen? Surg Infect (Larchmt). 2022;23:91-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2022. [cited 7 December 2022]. Available from: https://covid19.who.int/. |
| 6. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1144] [Article Influence: 190.7] [Reference Citation Analysis (2)] |
| 7. | Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 8. | Bonney GK, Gao Y, Chew CA, Windsor JA. SARS-COV-2 associated acute pancreatitis: Cause, consequence or epiphenomenon? Pancreatology. 2020;20:1017-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8464] [Article Influence: 188.1] [Reference Citation Analysis (5)] |
| 10. | Shelat VG. Sulfasalazine induced acute pancreatitis in a patient with prior cholecystectomy. Postgrad Med J. 2021;97:738-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51476] [Article Influence: 10295.2] [Reference Citation Analysis (2)] |
| 12. | Lee SC, Yang CH, Chang CT, Yu KH. Diagnostic Utility of Serum IgG4 in Autoimmune Pancreatitis: An Updated Comprehensive Systematic Review and Meta-analysis. J Clin Gastroenterol. 2022;56:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Li S, Tian B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine (Baltimore). 2017;96:e5908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Keiles S, Kammesheidt A. Identification of CFTR, PRSS1, and SPINK1 mutations in 381 patients with pancreatitis. Pancreas. 2006;33:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1095] [Article Influence: 84.2] [Reference Citation Analysis (10)] |
| 16. | Acherjya GK, Rahman MM, Islam MT, Alam AS, Tarafder K, Ali M, Deb SR. Acute pancreatitis in a COVID-19 patient: An unusual presentation. Clin Case Rep. 2020;8:3400-3407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Al-Douri A, John J, Agrawal D. S1442 Acute Pancreatitis as a Complication of COVID-19 Infection. AJG. 2020;115:S694. [DOI] [Full Text] |
| 18. | AlHarmi RAR, Fateel T, Sayed Adnan J, AlAwadhi K. Acute pancreatitis in a patient with COVID-19. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Ali E, Badawi M, Ahmed A, Abdelmahmuod E, Ibrahim W. Severe SARS-CoV-2 infection presenting with acute kidney injury and diabetic ketoacidosis complicated by pancreatitis in a 53-year man with hypertension. Clin Case Rep. 2021;9:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020;20:1026-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 21. | Alves AM, Yvamoto EY, Marzinotto MAN, Teixeira ACS, Carrilho FJ. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz J Infect Dis. 2020;24:561-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Alwaeli H, Shabbir M, Khamissi Sobi M, Alwaeli K. A Case of Severe Acute Pancreatitis Secondary to COVID-19 Infection in a 30-Year-Old Male Patient. Cureus. 2020;12:e11718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Amé RM, Balderramo D. Is necessary to rule out Severe Acute Respiratory Syndrome Coronavirus 2 infection in every patient admitted for acute pancreatitis? Gastroenterol Hepatol. 2022;45 Suppl 1:113-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Anand ER, Major C, Pickering O, Nelson M. Acute pancreatitis in a COVID-19 patient. Br J Surg. 2020;107:e182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Arjun S, Farraj K, Jacob B, Mustacchia P. S1425 An Atypical Presentation of COVID-19 as Acute Pancreatitis. AJG. 2020;115. [DOI] [Full Text] |
| 26. | Bains K, Seo DJ, Mahgoub A, Wang J. S1552 A Case of Roasted Pancreas From COVID-19 Infection. AJG. 2020;115:S787-S788. [DOI] [Full Text] |
| 27. | Bokhari SMMA, Mahmood F. Case Report: Novel Coronavirus-A Potential Cause of Acute Pancreatitis? Am J Trop Med Hyg. 2020;103:1154-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Bouali M, Ouchane M, Elbakouri A, Bensardi F, Elhattabi K, Fadil A. Total gastric necrosis following acute pancreatitis in a patient with COVID -19: Case report and literature review. Ann Med Surg (Lond). 2021;62:362-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Brikman S, Denysova V, Menzal H, Dori G. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ. 2020;192:E858-E859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Canastar M, Yick F, Pais S, Dhand A, Lebovics E. S1488 A Case of COVID-19-Associated Acute Pancreatitis. AJG. 2020;115:S750-S750. [DOI] [Full Text] |
| 31. | Chandra R, Lazar NJ, Goldman S, Imam Z, Mansour R. Novel Coronavirus (COVID-19) Infection-Attributed Acute Pancreatitis: A Case Report and Literature Review. Cureus. 2021;13:e15725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Cheung S, Delgado Fuentes A, Fetterman AD. Recurrent Acute Pancreatitis in a Patient with COVID-19 Infection. Am J Case Rep. 2020;21:e927076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Chivato Martín-Falquina I, García-Morán S, Jiménez Moreno MA. Acute pancreatitis in SARS-CoV-2 infection. Beyond respiratory distress. Rev Esp Enferm Dig. 2021;113:388-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Dufani J, Abuhamidah M, Buaisha HM, Mukherjee S. Acute Pancreatitis and Diabetic Ketoacidosis in a Patient With COVID-19: A Case Report. AJG. 2020;115:S750. [DOI] [Full Text] |
| 35. | Elhence A, Mahapatra SJ, Vajpai T, Garg PK. Acute pancreatitis and nosocomial COVID-19: Cause specific host responses may determine lung injury. Pancreatology. 2020;20:1258-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Fernandes DA, Yumioka AS, Filho HRM. SARS-CoV-2 and acute pancreatitis: a new etiological agent? Rev Esp Enferm Dig. 2020;112:890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Fiore V, Beretta R, De Vito A, Barac A, Maida I, Joeseph Kelvin DD, Piu C, Lai V, Madeddu G, Rubino S, Stevanovic G, Korica S, Babudieri S. Emerging Clinical Features of COVID-19 Related Pancreatitis: Case Reports and Review of the Literature. Front Med (Lausanne). 2021;8:779118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Gadiparthi C, Bassi M, Yegneswaran B, Ho S, Pitchumoni CS. Hyperglycemia, Hypertriglyceridemia, and Acute Pancreatitis in COVID-19 Infection: Clinical Implications. Pancreas. 2020;49:e62-e63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Gadiparthi C, Mohapatra S, Kanna S, Vykuntam V, Chen W. Acute pancreatitis in a patient with COVID-19: a case report. Transl Gastroenterol Hepatol. 2021;6:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Gonzalo-Voltas A, Uxia Fernández-Pérez-Torres C, Baena-Díez JM. Acute pancreatitis in a patient with COVID-19 infection. Med Clin (Engl Ed). 2020;155:183-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Gupta A, Bansal DP, Rijhwani P, Singh V. A Case Report on Acute Pancreatitis in a Patient With Coronavirus Disease 2019 (COVID-19) Pneumonia. Cureus. 2021;13:e14628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, Gluud LL. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology. 2020;20:665-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 43. | Hanif M, Khan AW, Ullah S, Sundas F, Khan SJ. Can COVID-19 Cause Pancreatitis? J Coll Physicians Surg Pak. 2021;31:S120-S122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Hassani AH, Beheshti A, Almasi F, Ketabi Moghaddam P, Azizi M, Shahrokh S. Unusual gastrointestinal manifestations of COVID-19: two case reports. Gastroenterol Hepatol Bed Bench. 2020;13:410-414. [PubMed] |
| 45. | Ibrahim YS, Karuppasamy G, Parambil JV, Alsoub H, Al-Shokri SD. Case Report: Paralytic Ileus: A Potential Extrapulmonary Manifestation of Severe COVID-19. Am J Trop Med Hyg. 2020;103:1600-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 46. | Jespersen Nizamic T, Huang Y, Alnimri M, Cheng M, Chen LX, Jen KY. COVID-19 Manifesting as Renal Allograft Dysfunction, Acute Pancreatitis, and Thrombotic Microangiopathy: A Case Report. Transplant Proc. 2021;53:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Kandasamy S. An unusual presentation of COVID-19: Acute pancreatitis. Ann Hepatobiliary Pancreat Surg. 2020;24:539-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Karimzadeh S, Manzuri A, Ebrahimi M, Huy NT. COVID-19 presenting as acute pancreatitis: Lessons from a patient in Iran. Pancreatology. 2020;20:1024-1025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Kataria S, Sharif A, Ur Rehman A, Ahmed Z, Hanan A. COVID-19 Induced Acute Pancreatitis: A Case Report and Literature Review. Cureus. 2020;12:e9169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Kolhe M, Sharma P, Anikhindi S, Bansal N, Saachdeva M, Arora A. Acute pancreatitis in a patient with COVID-19: A case report. IJHPD. 2020;10:1. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Kurihara Y, Maruhashi T, Wada T, Osada M, Oi M, Yamaoka K, Asari Y. Pancreatitis in a Patient with Severe Coronavirus Disease Pneumonia Treated with Veno-venous Extracorporeal Membrane Oxygenation. Intern Med. 2020;59:2903-2906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Lakshmanan S, Malik A. Acute Pancreatitis in Mild COVID-19 Infection. Cureus. 2020;12:e9886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Maalouf RG, Kozhaya K, El Zakhem A. SARS-CoV-2 induced necrotizing pancreatitis. Med Clin (Engl Ed). 2021;156:629-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Mazrouei SSA, Saeed GA, Al Helali AA. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep. 2020;15:1601-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 56. | Meireles PA, Bessa F, Gaspar P, Parreira I, Silva VD, Mota C, Alvoeiro L. Acalculous Acute Pancreatitis in a COVID-19 Patient. Eur J Case Rep Intern Med. 2020;7:001710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Merza N, Yousuf H, Mansi T, Naguib T. S1476 COVID-19-Associated Atypical Acute Pancreatitis! AJG. 2020;115:S750. [DOI] [Full Text] |
| 58. | Meyers MH, Main MJ, Orr JK, Obstein KL. A Case of COVID-19-Induced Acute Pancreatitis. Pancreas. 2020;49:e108-e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Miao Y, Lidove O, Mauhin W. First case of acute pancreatitis related to SARS-CoV-2 infection. Br J Surg. 2020;107:e270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Mobin N, Paracha N, Ilyas S, Khan A, Tokarski M, Schifter M, Mohanty SR. S1422 COVID-19-Associated Pancreatitis: It's Not All About the Lungs. AJG. 2020;115. [DOI] [Full Text] |
| 61. | Mohammadi Arbati M, Molseghi MH. COVID-19 Presenting as Acute Necrotizing Pancreatitis. J Investig Med High Impact Case Rep. 2021;9:23247096211009393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Muhammad Abrar Jeelani H, Sheikh MM, Samuel SS, Omotosho YB, Sharko A, Albetar R. Acute Pancreatitis in a Patient With COVID-19 After the Resolution of Respiratory Symptoms. J Investig Med High Impact Case Rep. 2021;9:23247096211024773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Naqvi SJ, Karamat S, Tzimas D, Dibble S, Clohosey S. S1532 Gastrointestinal Manifestation of COVID-19: A Case Report Describing Acute Pancreatitis in the Setting of SARS-CoV-2. AJG. 2020;115. [DOI] [Full Text] |
| 64. | Narang K, Szymanski LM, Kane SV, Rose CH. Acute Pancreatitis in a Pregnant Patient With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol. 2021;137:431-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Patnaik RNK, Gogia A, Kakar A. Acute pancreatic injury induced by COVID-19. IDCases. 2020;22:e00959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Pinte L, Baicus C. Pancreatic involvement in SARS-CoV-2: case report and living review. J Gastrointestin Liver Dis. 2020;29:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Purayil N, Sirajudeen J, Va N, Mathew J. COVID-19 Presenting as Acute Abdominal Pain: A Case Report. Cureus. 2020;12:e9659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 68. | Rabice SR, Altshuler PC, Bovet C, Sullivan C, Gagnon AJ. COVID-19 infection presenting as pancreatitis in a pregnant woman: A case report. Case Rep Womens Health. 2020;27:e00228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Rotar O, Khomiak I, Polanskyy O, Muskovsky Y, Railianu S, Fishbach M. Case Report of Fatal Acute Necrotizing Pancreatitis in Patient with COVID-19: We Should Be Aware Of Hemorrhagic Complications. JOP. 2020;167-171. [DOI] [Full Text] |
| 70. | Sandhu H, Mallik D, Lokavarapu MJ, Huda F, Basu S. Acute Recurrent Pancreatitis and COVID-19 Infection: A Case Report with Literature Review. Cureus. 2021;13:e13490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Shinohara T, Otani A, Yamashita M, Wakimoto Y, Jubishi D, Okamoto K, Kanno Y, Ikeda M, Ishigaki K, Nakai Y, Harada S, Okugawa S, Koike K, Moriya K. Acute Pancreatitis During COVID-19 Pneumonia. Pancreas. 2020;49:e106-e108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Simou EM, Louardi M, Khaoury I, Abidi MA, Mansour A, Louadghiri AE, Fahmaoui K, Ezzouine H, Charra B. Coronavirus disease-19 (COVID-19) associated with acute pancreatitis: case report. Pan Afr Med J. 2020;37:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Singh GD, Kharoud HK. S1573 Case Report and Literature Review of Acute Pancreatitis in COVID-19 Patient. AJG. 2020;115:S800. [DOI] [Full Text] |
| 74. | Srinivasan A, Wilson B, Pansuriya T, Qasmi ST, Kesavan RB. Diabetic Ketoacidosis and Acute Pancreatitis in Covid-19 Setting. JES. 2021;5 (Supplement_1):A375-A376. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 75. | Tollard C, Champenois V, Delemer B, Carsin-Vu A, Barraud S. An inaugural diabetic ketoacidosis with acute pancreatitis during COVID-19. Acta Diabetol. 2021;58:389-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Tomasi I, Scott L, Cullen J, di Maggio F, Ebied H, Wheatstone S. A rare case of heterotopic pancreatitis and intestinal malrotation in a COVID-19 positive patient. COVID-19, causative or coincidence? Int J Surg Case Rep. 2021;82:105917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 77. | Truscello DJ, Shah M, Murad FM. S1454 Severe Acute Pancreatitis Initial Presentation of COVID-19 Infection. AJG. 2020;115:S694. [DOI] [Full Text] |
| 78. | Wang K, Luo J, Tan F, Liu J, Ni Z, Liu D, Tian P, Li W. Acute Pancreatitis as the Initial Manifestation in 2 Cases of COVID-19 in Wuhan, China. Open Forum Infect Dis. 2020;7:ofaa324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Wifi MN, Nabil A, Awad A, Eltatawy R. COVID-induced pancreatitis: case report. Egypt J Intern Med. 2021;33:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Yamamoto K, Oka K, Sakae H, Otsuka F. Acute Pancreatitis Related to COVID-19 Infection. Intern Med. 2021;60:2159-2160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Zeng Y, Zhou X, Gang Y, Wang H, Song L, Luo S, Zhang X. A fatal outcome from SARS-CoV-2 infection: One case report of a young man with multiple organ damage. Radiol Infect Dis. 2020;7:208-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Zielecki P, Kaniewska M, Furmanek M, Bulski T, Rydzewska G. Effective treatment of severe acute pancreatitis and COVID-19 pneumonia with tocilizumab. Prz Gastroenterol. 2020;15:267-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Chan KS, Shelat VG. Diagnosis, severity stratification and management of adult acute pancreatitis-current evidence and controversies. World J Gastrointest Surg. 2022;14:1179-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (5)] |
| 84. | Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, Saunders DC, Bottino R, Aramandla R, Jenkins R, Stein R, Kaestner KH, Vahedi G; HPAP Consortium, Brissova M, Powers AC. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in β Cells. Cell Metab. 2020;32:1028-1040.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 85. | Panic N, Mihajlovic S, Vujasinovic M, Bulajic M, Löhr JM. Pancreatitis Associated with Viral Hepatitis: Systematic Review. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Lee JK, Enns R. Review of idiopathic pancreatitis. World J Gastroenterol. 2007;13:6296-6313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Anbalakan K, Chua D, Pandya GJ, Shelat VG. Five year experience in management of perforated peptic ulcer and validation of common mortality risk prediction models - are existing models sufficient? Int J Surg. 2015;14:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 88. | Kiat TTJ, Gunasekaran SK, Junnarkar SP, Low JK, Woon W, Shelat VG. Are traditional scoring systems for severity stratification of acute pancreatitis sufficient? Ann Hepatobiliary Pancreat Surg. 2018;22:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Chan EE, Shelat VG. Pancreaticopleural Fistula Causing Massive Right Hydrothorax and Respiratory Failure. Case Rep Surg. 2016;2016:8294056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1214] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 91. | Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 587] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 92. | Fang D, Ma JD, Guan JL, Wang MR, Song Y, Tian DA, Li PY. [Manifestations of digestive system of hospitalized patients with coronavirus disease 2019 in Wuhan, China: a single-center descriptive study]. Zhonghua Xiaohua Zazhi. 40:151-156. [DOI] [Full Text] |
| 93. | Mak MHW, Chew WL, Junnarkar SP, Woon WWL, Low JK, Huey TCW, Shelat VG. Patient reported outcomes in elective laparoscopic cholecystectomy. Ann Hepatobiliary Pancreat Surg. 2019;23:20-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Teo ZHT, Huey CWT, Low JK, Junnarkar SP, Shelat VG. The Impact of the COVID-19 Pandemic on Hepatobiliary and Pancreatic Surgical Services in Singapore: Retrospective Quantitative Study. JMIR Perioper Med. 2022;5:e29045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Shao CC, McLeod MC, Thogaripally S, Mugavero MJ, Gleason LT, Dos Santos Marques IC, Chu DI, Gunnells DJ. Increased Risk of Postoperative Mortality Associated With Prior COVID-19 Infection. Am J Prev Med. 2022;63:S75-S82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 96. | Stephens JR, Wong JLC, Broomhead R, Stümpfle R, Waheed U, Patel P, Brett SJ, Soni S. Raised serum amylase in patients with COVID-19 may not be associated with pancreatitis. Br J Surg. 2021;108:e152-e153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020;18:2128-2130.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 481] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 98. | Samanta J, Gupta R, Singh MP, Patnaik I, Kumar A, Kochhar R. Coronavirus disease 2019 and the pancreas. Pancreatology. 2020;20:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 99. | Goh SSN, Shelat V. Prednisolone induced pneumatosis coli and pneumoperitoneum. World J Gastroenterol. 2022;28:3739-3742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648-661; quiz 644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 101. | Jamshidi MB, Lalbakhsh A, Talla J, Peroutka Z, Hadjilooei F, Lalbakhsh P, Jamshidi M, Spada L, Mirmozafari M, Dehghani M, Sabet A, Roshani S, Bayat-Makou N, Mohamadzade B, Malek Z, Jamshidi A, Kiani S, Hashemi-Dezaki H, Mohyuddin W. Artificial Intelligence and COVID-19: Deep Learning Approaches for Diagnosis and Treatment. IEEE Access. 2020;8:109581-109595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 102. | Jamshidi MB, Talla J, Lalbakhsh A, Sharifi-Atashgah MS, Sabet A, Peroutka Z. A Conceptual Deep Learning Framework for COVID-19 Drug Discovery. In: Rajashree Paul, editors. Proceedings of the 2021 IEEE 12th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON). 2021 Dec 1-4; New York, United States. Piscataway, NJ: Institute of Electrical and Electronics Engineers (IEEE), 2021: 00030-00034. [DOI] [Full Text] |
| 103. | Ozaka S, Kodera T, Ariki S, Kobayashi T, Murakami K. Acute pancreatitis soon after COVID-19 vaccination: A case report. Medicine (Baltimore). 2022;101:e28471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Miyazaki K, Yoshimura Y, Miyata N, Sasaki H, Shiba A, Aga M, Hamakawa Y, Taniguchi Y, Misumi Y, Agemi Y, Shimokawa T, Okamoto H, Tachikawa N. Acute pancreatitis or severe increase in pancreatic enzyme levels following remdesivir administration in COVID-19 patients: an observational study. Sci Rep. 2022;12:5323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 105. | Khadka S, Williams K, Solanki S. Remdesivir-Associated Pancreatitis. Am J Ther. 2022;29:e444-e446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Jamshidi MB, Czech Republic S-Editor: Li L L-Editor: A P-Editor: Li L