Published online Mar 28, 2023. doi: 10.3748/wjg.v29.i12.1765
Peer-review started: June 20, 2022
First decision: August 1, 2022
Revised: August 4, 2022
Accepted: March 9, 2023
Article in press: March 9, 2023
Published online: March 28, 2023
Processing time: 281 Days and 7.3 Hours
Nonalcoholic fatty liver disease (NAFLD) or metabolic-associated fatty liver disease has been characterized by the lipid accumulation with injury of hepatocytes and has become one of the most common chronic liver diseases in the world. The complex mechanisms of NAFLD formation are still under identification. Carnitine palmitoyltransferase-II (CPT-II) on inner mitochondrial membrane (IMM) regulates long chain fatty acid β-oxidation, and its abnormality has had more and more attention paid to it by basic and clinical research in NAFLD. The sequences of its peptide chain and DNA nucleotides have been identified, and the catalytic activity of CPT-II is affected on its gene mutations, deficiency, enzymatic thermal instability, circulating carnitine level and so on. Recently, the CPT-II dysfunction has been discovered in models of liver lipid accumulation. Meanwhile, the malignant transformation of hepatocyte-related CD44+ stem T cell activation, high levels of tumor-related biomarkers (AFP, GPC3) and abnormal activation of Wnt3a expression as a key signal molecule of the Wnt/β-catenin pathway run parallel to the alterations of hepatocyte pathology. This review focuses on some of the progress of CPT-II inactivity on IMM with liver fatty accumulation as a possible novel pathogenesis for NAFLD in hepatocarcinogenesis.
Core Tip: The complex mechanisms of nonalcoholic fatty liver disease formation are still under identification. Hepatic carnitine palmitoyl transferase-II (CPT-II) on inner mitochondrial membrane regulates long chain fatty acid β-oxidation and this abnormality has had more attention paid to it by basic and clinical research. The sequences of its peptide chain and DNA nucleotides have been identified and the catalytic activity of CPT-II is affected on its gene mutations, deficiency, enzymatic thermal instability, circulating carnitine level and so on. CPT-II dysfunction has been discovered in models of lipid accumulation. Meanwhile, the malignant transformation of hepatocyte-related CD44+ stem T cell activation, high levels of tumor-related biomarkers and abnormal Wnt3a expression as a key signal molecule of the Wnt/β-catenin pathway run parallel to the alterations of hepatocyte pathology.
- Citation: Yao M, Zhou P, Qin YY, Wang L, Yao DF. Mitochondrial carnitine palmitoyltransferase-II dysfunction: A possible novel mechanism for nonalcoholic fatty liver disease in hepatocarcinogenesis. World J Gastroenterol 2023; 29(12): 1765-1778
- URL: https://www.wjgnet.com/1007-9327/full/v29/i12/1765.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i12.1765
Non-alcoholic fatty liver disease (NAFLD) or metabolic-associated fatty liver disease (MAFLD) is a general term of liver diseases characterized by inflammation, fatty accumulation and hepatocyte dysfunction, except of alcohol or other clear liver injury factors[1-3]. Up until now, NAFLD has become a potentially serious liver disease that affects approximately 25% of the adult population in the world[4], and is divided into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH) with or without liver fibrosis[4,5]. It has been shown that balloon like hepatocyte injury is based on NAFL. Most patients have no obvious symptoms and may not be diagnosed until they develop into liver cirrhosis or progress to hepatocellular carcinoma (HCC), and the effect of early clinical screening is poor[6]. Once NAFLD progresses to liver cirrhosis, it is difficult to reverse and there is a risk that HCC can’t be ignored. Not only that, it also involves the occurrence of multiple systemic diseases in the body which are closely related to cardiovascular disease, chronic kidney disease and colorectal tumor which all threaten human health[7,8]. Therefore, finding the monitoring target in the malignant transformation of NAFLD has practical clinical significance for the prevention of NAFLD-related liver malignant diseases[9].
Lipid metabolism rearrangements in NAFLD contribute to disease progress that has emerged as one of the most risks for HCC, where metabolic reprogramming is a hallmark[10]. Hepatic carnitine palmitoyl transferases (CPTs) are critical for long-chain fatty acids (LCFAs) -oxidation, as they are capable of transport through the mitochondrial membrane[11]. CPT is made up of two separate proteins (CPT-I and CPT-II). CPT-I is located in the outer mitochondrial membrane (OMM) with three isoforms (liver CPT1a, muscle CPT1b and brain CPT1c) and CPT-II is in the inner mitochondrial membrane (IMM)[12,13]. The amino acid and cDNA nucleotide sequences of the ubiquitous CPT-II have been elucidated. The mutations or dysregulation of the CPTs, which are associated with many serious and even fatal diseases, are promising targets for developing drugs to treat type 2 diabetes (T2D) and obesity[14,15]. Dysregulated lipid metabolism is involved in human diseases, including chronic inflammatory diseases and inflammatory-related tumors[16]. CPTs play an important role in lipid metabolism and fatty acid oxidation (FAO) in mitochondria. CPT-II has been confirmed as a rate-limiting enzyme and in regulation of host immune responses[17,18]. However, the pathological role of CPT-II alteration with NAFLD remains to be identified[19]. This review summarizes the latest research findings of CPT-II, which are important for accurate or early monitoring of NAFLD malignant transformation.
The most important function of the CPT family is to ensure that fatty acids enter the mitochondria for -oxidation. Transmembrane protein CPT-I is located in OMM and CPT-II is in IMM. Human CPT-II gene (CPT2) as an autosomal recessive trait encoded gene localizes on chromosomes 1 (1p32) and the gene full length contains 3090 nucleotides and 5 exons, which can encode the enzyme protein peptide chain composed of 658 amino acids[20]. Summaries of CPT2, CPT-II and total numbers of its reported mutated sites are shown in Table 1. Human CPT-II (NM_000098) is a mitochondrial protein in IMM. CPT-II together with CPT-I oxidize LCFA in the mitochondria and play pivotal roles in the LCFA transport across the mitochondrial membrane for β-oxidation[21]. In molecular genetic aspects, CPT2 is identified in about 70% of mutant alleles. There are variations in the CPT2 genome, most of which are single-base substitutions, small insertions or discrete deletions[22,23]. Among the enzymatic system, CPT-II plays a rate-limiting role in the entry of fatty acids into mitochondrial FAO and is considered to be a key component of cellular metabolic homeostasis[24]. Anti-cancer drug oxaliplatin can activate CPT-II in gastrointestinal cancer cells and promote the catabolism of fatty acids[18]. Knocking down of CPT2 by patient-derived xenograft models confirmed the regulating role of mitochondrial FAO in Src activation and metastasis of breast cancer[25]. However, a subset of substitutions, insertion or deletion, tend to cluster in all exons, especially in exon 4 and exon 5, suggesting that CPT2 clustering is due to a combination of factors such as the rate of heterogeneous mutations in the genome, biophysical characteristics of exogenous carcinogens, endogenous dysregulation and large mutation events related to genome instability[26].
| Exon | Size in bp | Nucleotides | Amino acids | Mut. no. | Mutation sites | |
| Amino acids | Others | |||||
| 1 | 668 | 1-668 | 1-51 | 5 | 2 | 3 |
| 2 | 81 | 669-749 | 52-78 | 4 | 2 | 2 |
| 3 | 107 | 750-856 | 79-113 | 5 | 3 | 2 |
| 4 | 1305 | 857-2161 | 114-548 | 59 | 49 | 10 |
| 5 | 929 | 2162-3090 | 549-658 | 16 | 12 | 4 |
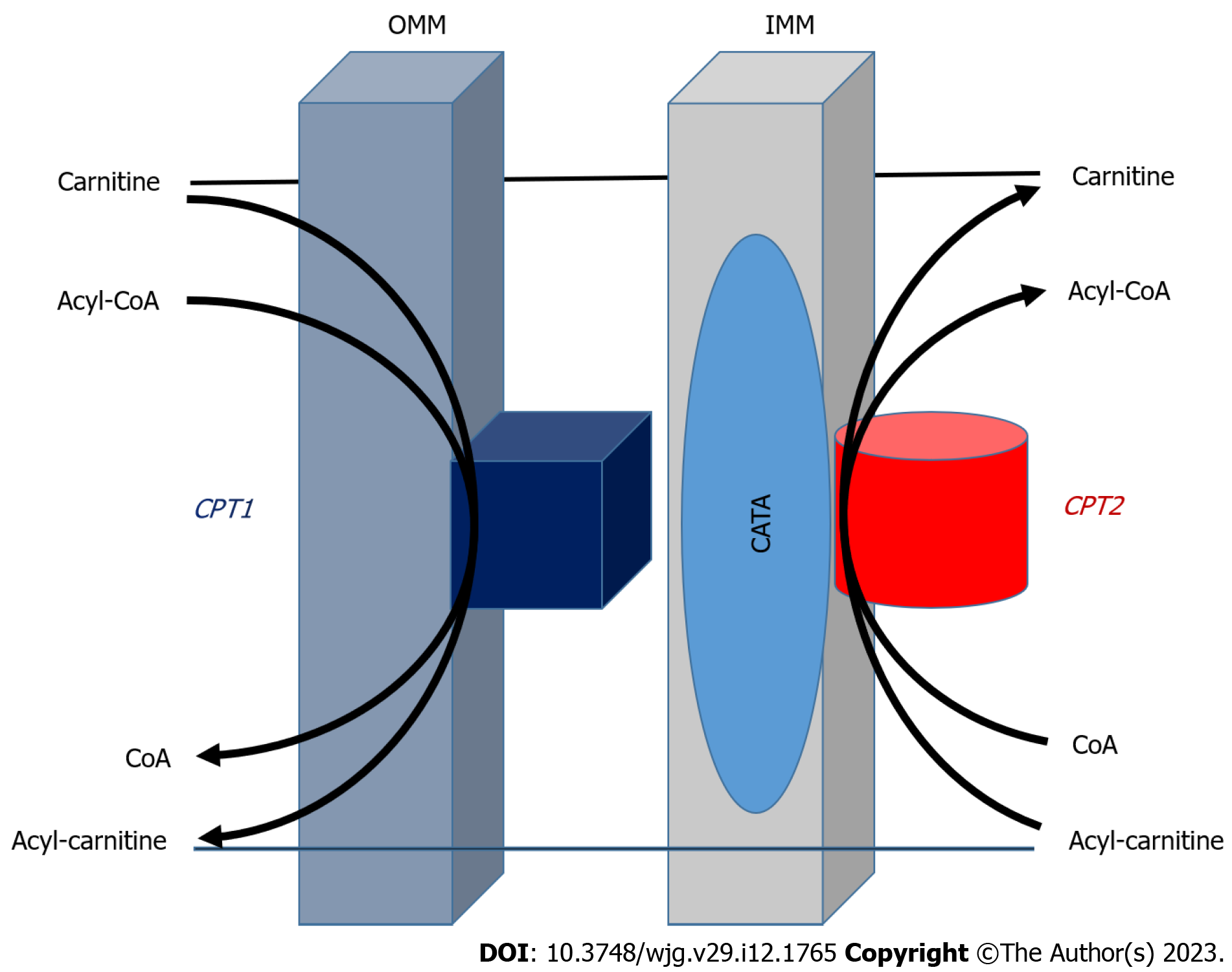
The enzymatic system that facilitates the transfer is known as CPT mainly in OMM or IMM and plays an important role in maintaining its structural and functional integrity. Liver cells must keep related metabolic homeostasis in a wide range of conditions and meet their ATP needs depending on FAO[27,28]. CPT-II catalyzes transesterified acylcarnitine’s transferred from cytosol into the intermembrane space (IMS) and the remaining acyl of acylcarnitine is changed back to CoA on IMM, which is next available for FAO. Meanwhile, the released carnitine is returned to the IMS of the mitochondrion via CACT and is available for fatty acid re-transport[29]. However, the deficiency or gene mutation of CPT-II can significantly affect mitochondrial FAO. Bezafibrate, as a well-known hypolipidemic drug, was tested to stimulate CPT2 mutation, but it should be a challenge to restore normal LCFA oxidation from a series of other fatty acid mitochondrial diseases[30,31], indicated that CPT-II not only provides ATP for liver cells via FAO, but also its down-regulated expression affects the growth and malignant transformation of hepatocytes via cell damage, related signal molecules, stem cells, immunology and so on[32]. Therefore, the study of CPT-II will help to understand the pathogenesis and to develop a promising treatment of NAFLD.
Previous studies of CPT2 mutations have identified the presence of single-base substitutions, and many other events such as double-base and multiple-base substitutions, insertions or deletions. The reported mutations among all five exons of CPT2 and 89 mutated sites are shown in Table 2[33,34]. Most of CPT2 or CPT-II mutations are located in exon 4 or exon 5. Biochemical consequences of these mutations are still controversial. The c.338 C>T (P.S113L) variant can be detected in most cases of Caucasians; in Japanese, c.1148 T>A (P.F383Y) is the most frequent variant allele and can obviously cause severe infant forms of symptoms. Among them, it may include deficiency of enzyme protein, enzyme inactivity or abnormality of enzymatic regulation. The protein encoded by this gene is a nuclear protein which is transported to the IMM. Due to the low activity, thermal instability, and short half-life of CPT-II, the CPT II variant exerts a dominant negative effect on homologous tetrameric proteins associated with mitochondrial LCFA oxidation impairment[35]. Recently, based on animal models or clinical studies, the crystal structures of CPT-II were determined in uninhibited forms and in complexes with inhibiting substrate analogs with anti-diabetic features. The crystal structures have a deep understanding of the enzymatic structure-function relationship which is conducive to the discovery of new inhibitors through structure-based drug design[36].
| Exon | Amino acid substitution | Others |
| 1 | Pro41Leu; Pro50His | 36-38 insGC; 6_43dupGGGCCC; 113_114dupGC |
| 2 | Pro55Arg; Ala67Gly | 182_203del 22; 153-1G>A (Intron 2)1 |
| 3 | Cys84Arg; Ala101Val; Ser113Leu | 256_257delAG; 232+1G>A (Intron 3)1 |
| 4 | Tyr120Cys; Leu121Gln; Arg124Gln; Arg124Ter; Asn146Thr; Arg151Gln; Arg151Trp; Arg161Trp; Lys164Ter; Arg167Gln; Pro173Ser; Glu174Lys; Tyr210Asp; Asp213Gly; Met214Thr; Gln216Arg; Pro227Leu; Arg231Trp; Arg247Trp; Lys274Met; Arg296Gln; Arg296Leu; Arg296Ter; Gly310Gly; Cys326Tyr; Asp328Gly; Met342Thr; Phe352Cys; Val368Ile; His369Gln; Arg382Lys; Phe383Tyr; Gln413Gln; Phe448Leu; Arg450Ter; Gly451Glu; Glu454Ter; Lys457Ter; Tyr479Phe; Tyr479Cys; Gly480Arg; Glu487Lys; Gly497Ser; Ile502Thr; Arg503Cys; Pro504Leu; Phe516Ser; Glu545Ala | 1569_1570delCA; 1444_1447delACAG; 1634_1636delAAG; 1646_49del; 1273_1274delAC; 1238_1239delAG; 1543_1546delGCCT; 907_918ins11; 533insT; 534-558del25; 1645+5G>A (Intron 5)1 |
| 5 | Arg560Gln; Leu575Pro; Asp576Gly; Ser588Cys; Ser590Asn; Gly600Arg; Pro604Ser; Val605Leu; Asp608His; Tyr628Ser; Arg631Cys; Leu644Ser | 1816_1817delGT; 1923_1935del; 340+1G>A (Intron 4)1; 340+5G>A (Intron 4)1 |
Using fatty acids as ATP requires more than 20 enzymes and transporters, which are involved in the activation and transport of fatty acids into the mitochondria. The membrane transport system of fatty acid β-oxidation in mitochondria is shown in Figure 1. Mitochondrial FAO is one of the major pathways for fatty acid degradation and is critical for maintaining ATP balance in the human body[37,38]. When the glucose supply is limited, fatty acids are an important source of energy after absorption and during fasting. But even when glucose is sufficient, FAO still is the main source of energy for human tissues. A series of enzymes, transporters and other facilitating proteins with biochemistry and physiological functions are involved in FAO (Figure 1). The role of CPT in the LCFA oxidation, and this system includes CPT-I, carnitine-acylcarnitine translocase (CACT) and CPT-II. The acyl-CoA synthetase located in OMM catalyzes fatty acids to form acyl-CoA with ATP and CoA participation, and then, transports long-chain acyl-CoA by the delivery system into mitochondria, that is a carnitine shuttle system to enter the process for β-oxidation. Most genes encoding CPT-II are known to be recessive genetic defects and the clinical manifestations of the related diseases may include hypoglycemia, cardiomyopathy, arrhythmias and rhabdomyolysis; It also illustrates the importance of FAO during fasting and in liver and (heart) muscle function[39,40].
The carnitine shuttle system controls fatty acid translocation across the mitochondrial membrane. Key enzymes determine the competition of glycolysis vs mitochondrial FAO defined by the Randle cycle. This transport system with CACT is an important part of fatty acid esterification through OMM and IMM of mitochondria (Figure 1). First, CPT-I at OMM catalyzes long-chain acyl-CoA along with carnitine conversion to long-chain acylcarnitine and CoA is transported to the mitochondrial interior with help of translocase on the mitochondrial intima[41,42]. After that, the transesterified acylcarnitine’s are transported from the cytosol into the IMM space as CACT acylcarnitine releases carnitine by CPT-II catalysis, and converts it to an acyl group from carnitine to acyl-CoA, available for β-oxidation. Released carnitine returns to the IMM space of the mitochondria for fatty acid re-transport[43]. CPT-II plays an important regulatory role in FAO and its function affects fatty acid metabolism. More importantly, CPTs in the carnitine shuttle system can be used as a drug target to reduce gluconeogenesis or restore liposome balance. Therefore, it has the potential value of gene therapy or immunotherapy, and the further study of the mechanism of CPTs could provide useful ideas for clinical treatment of related diseases[44,45].
Lipid metabolism involves a variety of biological processes, including the most important lipid metabolic pathway (FAO with carnitine shuttle system). The mutations or dysregulation of hepatic CPT-II have been linked to many serious or even fatal human diseases, and it should be a promising target for developing drugs to treat T2D or obesity[46]. However, the deficiency, over-expression or inactivation of liver CPT-II might ultimately lead to disruption of immune homeostasis, thereby increasing the risk of various inflammatory diseases and even tumors. There is some evidence that CPT-II or the associated mitochondrial LCFA are involved in the development and progression of these related diseases. Thus, the agonists or inhibitors targeting the CPTs or carnitine shuttle system have emerged as novel therapies for these diseases[47]. Normal function of FAO in IMM is closely dependent on the catalytic activity of CPT-II that could be affected by CPT2 variation, the amino acid substitution of enzyme, inhibition of enzyme activity, circulating carnitine level and so on.
Hepatic CPT-II deficiency is one of the most common forms of mitochondrial FAO disorders (FAODs) and have several clinical presentations that have been known for a long time. However, its phenotypic variability remains fascinating[48]. The clinical phenotypes of CPT-II deficiency are classified into muscular, severe infantile and fatal neonatal types. In addition, neonatal-onset CPT-II deficiency is often accompanied by brain and kidney organ dysfunction features, such as in the 1st mo of life, and is almost always fatal. Three different phenotypes (neonatal, infant and adult onset) have been identified, all with autosomal recessive inheritance patterns[49]. The clinical phenotype of adult CPT-II deficiency is mostly benign, and only with additional external stimuli, such as high-intensity exercise, can lead to major myopathy symptoms. However, the perinatal and infantile CPT-II deficiency usually involves multiple organ systems, especially when occurring in the perinatal period as it is the most serious form and is often fatal[50]. The application of mass spectrometry technology to analyze acylcarnitine profiles in blood has revolutionized the FAOD diagnosis, including CPT-II deficiency. In most cases, the number of CPT2 mutations is increasing and there is a clear genotype-phenotype correlation. However, the clinical variants in some patients might contain other genetic or environmental factors[51].
In the clinical setting, the manifestations of patients with CPT-II deficiency include severe infant liver disorders, myocardial infarction, fatality in neonates and myopathy (usually mild, from infancy to adulthood). Some patients have serious multi-system disease that includes liver function failure, cardiomyopathy, epilepsy, hypoglycemia and premature death, while others are characterized by muscle pain and weakness which is sometimes accompanied by myoglobinuria[52]. The proband was diagnosed for CPT-II deficiency by finding a decrease in muscle CPT activity or by identifying a biallelic variant of CPT2 in a molecular genetics test. A total of six mutations have been identified, including four new ones. Among those mutations, the S113 L mutation is common in about 50% of the mutant alleles. Three of the six mutations (3/6) have been found in a few unrelated patients, while others have been found in only one family with genetic heterogeneity. To date, about 100 CPT2 mutations have been discovered. Prenatal diagnosis is provided when the risk of infant/severe CPT-II deficiency is 1/4. Infantile CPT-II presents as a severe hypoglycemic episode of ketoacidosis, occasionally associated with heart damage and usually resulting in sudden death before age 1[50]. Treatment for CPT2 deficiency includes a low-fat diet in rich triglyceride or carnitine and avoiding fasting or hyperkinesis[53].
CPT-II activation is associated with disorders of mitochondrial β-oxidation of LCFA in IMM. Based on the crystal structure of mouse CAT, the active site of CPT-II is located at the interface between two domains, extending in tunnels through the enzyme protein centers, alone or in complex with its substrate carnitine or CoA[54]. In this tunnel, carnitine combines with CoA and its opposite is catalytic His343 residue. The information of CPT-II structure provides a molecular basis to understand the catalytic activity of CAT or to design their inhibitors. In addition, the carnitine might contribute to the catalytic stabilization of oxygen ions in the reaction intermediates. Hepatic CPT-II is sensitive to inhibition by metabolites of fatty acids, Triton X-100, or malonyl-CoA[55].
Artificially recombinant His6-N-hCPT2 and His6-N-hCPT2/S113L showed the same enzymatic activity for wild-type or S113L variants of CPT-II[56]. However, the mutant CPT-II exhibited abnormal destabilization at 40 °C or 45 °C and was more sensitive to be inhibited by malonyl-CoA. The thermal solubility of mutant CPT-II, which may explain the symptoms of CPT-II deficiency may mainly occur during prolonged exercise, infection, and exposure to cold. In addition, CPT II abnormalities are likely to be largely suppressed when fatty acid metabolism is stressed[54]. The unstable CPT II variants with enzymatic inactivity might lower mitochondrial fuel utilization under the phenotypic threshold during patients with hyperthermia, thus suggesting that hepatic CPT-II should play a pathological role in NAFLD progression.
High-risk patients have thermolabile genetic backgrounds of CPT-II in LCFA metabolism. However, until now, no related mutation of CPT-II was reported in NAFLD patients[57,58]. Almost fatal or handicapped virus-associated encephalopathy cases exhibited transiently higher serum LCAC levels during fever more than 40 °C. The specific activity of patients’ CPT-II (0.4 ± 0.06 nMol/min/mg) was 36% of normal control (1.1 ± 0.3 nMol/min/mg protein) at 37 °C. The CPT-II specific activity in the patient group was down to 50% for 2 h at 41 °C, and CPT-II in the normal control group still was 91.4%, and the sequencing analysis of patients’ CPT2 gene revealed compound (1055T>G/F352C) + (1102G>A/V368I) heterozygous variations[46,59]. F352C substitution was only reported in the Japanese study, and V368I polymorphic variation has relatively mild effects related to CPT-II deficiency[47,60]. The CPT-II mutation or dysregulation has been linked to more serious, even fatal diseases, and these data should be promising molecule targets to develop therapeutic agents for NAFLD in future.
Carnitine as a substance has a wide range of biological functions, including transport of LCAD from the cytoplasm to the mitochondrial matrix, regulation of acetyl-CoA/CoA, control of acyl transport between organelles and prevention of oxidative stress[58]. Maintaining normal fat metabolism depends on carnitine concentration that is synthesized in most eucaryotic organisms[61]. The methylation of lysine initiates the biosynthesis of carnitine. The formed trimethyllysine is then converted to butylbetaine in all tissues and finally hydroxylated to carnitine in the liver and released from the tissues, which are then actively absorbed by all other tissues[62,63]. This transfer requires the enzyme and transporter that accumulates carnitine within the cell (OCTN2 carnitine transporter), which is conjugated to LCFA (CPT-I), to transfer acylcarnitine’s through IMM (CACT), and to transfer carnitines through the IMM (CACT), fatty acids were conjugated back to CoA for subsequent -oxidation (CPT-II). The regulation of carnitine synthesis is still incompletely understood because the turnover of carnitine in the human body is slow[64].
Carnitine is essential for proper fat metabolism, producing ATP, and the transport of LFAC or medium fatty acid chains (MFAC). It attracts LFAC and MFAC, after it breaks them down, and then takes them to the cell's mitochondria for FAO. And as it turns out, the body burns more fat, providing the body with more natural energy in the process[65,66]. According to the previous study, using a carnitine antagonist 3-(2,2,2-trimethylpropionate hydrazine dihydrate, THP) resulted in lipid accumulation with increased liver weight in wild-type mice. The competition between THP and carnitine inhibited CPT-II activity, resulting in carnitine deficiency, acyl CoA and fat accumulation[67]. Clinical data showed that the blood carnitine concentration in NAFLD patients was lower than those in healthy people, and the level in NAFLD cases with liver cirrhosis accounted for only 22% of normal people[68,69]. The concentration of carnitine in patients with liver disease is low, the fat accumulation in rat liver tissue, the content of total fatty acids, free fatty acids, short chain fatty acids (SCFC) and LCFA in liver, and the content of chain, long chain, short chain and total fatty acids in circulating blood also change. During patients with hepatitis B or hepatitis C virus infection, or with mitochondrial FAODs present with NAFLD or severe liver diseases, enough carnitine should play an important role in the mitochondrial carnitine shuttle system, suggesting that circulating carnitine level affects FAO, ameliorates mitochondrial dysfunction, reduces insulin resistance and improves NAFLD progression[70,71].
NAFLD pathogenesis is much more complicated with multi-factorial events. Recently, the low activity of CPT-II on IMM during NAFLD progression has attracted much attention both in basic and clinical aspects[72,73]. Although many theories of NAFLD with abnormal lipid metabolism[8,74,75] such as insulin resistance (IR), lipid peroxidation, cytokine expression, iron overload, genetics, environment, immunity, drugs, living habits and so on. However, there are still many problems in the study of NAFLD pathogenesis. According to these theories, the IR stimulates liver fat accumulation and triglycerides, resulting in the first strike to NAFLD formation; then oxidative stress and lipid peroxidation aggravate hepatocyte injury to develop into the second strike that starts with asymptomatic steatosis, and continues to cell inflammation, steatohepatitis, fibrosis or hepatocyte malignant transformation[76,77]; hence a "multiple hit" hypothesis seems a more accurate proposal[78,79]. Up to now, the new discovery of loss of CPT-II activity has been confirmed in lipid accumulating models that should be one of the NAFLD mechanisms.
Ideal NAFLD models should correctly reflect both histopathology and pathophysiology, and imitate certain aspects of NAFLD which are divided into genetic, dietary and combination models referring to advantages and disadvantages[80,81]. Also, the models based on biological knowledge are reliable and reproducible, having low mortality, and being compatible with simple and feasible methods, not only in elucidating pathogenesis for understanding NAFLD but also in examining therapeutic effects of various agents to develop tools and giving crucial information. Inhibiting CPT-II activity is related to a disorder of lipid metabolism, which may be related to NAFLD pathogenesis and down-regulating CPT-II in liver tissues. Gene defects are associated with mitochondrial LCFA oxidation disorders[82,83].
In order to determine the independent and interdependent roles of triglyceride (TG) hydrolysis and FAO, liver-specific defects in mice were generated in TG hydrolysis (AtglL-/-) , FAO (CPT2L-/-) , or both (double knockout)[73,84]. Loss of a single component of FAO [CPT2, adipose TG lipase (Atgl), and peroxisome proliferators-activated receptor-α (PPAR-α)] resulting in a major independent effect on the morphology of liver cells, gene expression, and intermediate metabolism in response to fasting[84]. However, the mice in the high-fat diet (HFD) model revealed an interdependent role for Atgl and CPT2, as deletion of only one gene lead to NAFLD; But loss of both components leads to significant hepatocyte inflammation and liver fibrosis[85].
During NAFLD progression, the transcription factors E2f1 and E2f2 contributed to NAFLD-associated mice HCC and their involvement in metabolic recombination[72,85]. The expressions of E2f1 and E2f2 were significantly increased in the NAFLD-associated HCC in mice induced with HFD plus diethylnitrosamine (Den). However, the E2f1-/-and E2f2-/-mice were resistant to DEN-HFD-induced hepatocarcinogenesis and were associated with lipid accumulation. The administration of DEN-HFD in the E2f1-/-and E2f2-/- mice enhanced FAO and increased expression of CPT2 because of CPT2 as an essential enzyme for FAO, whose down-regulation was linked to the NAFLD-related hepatocarcinogenesis[86]. The mouse models of obesity-driven and NASH-driven HCC typically exhibit robust steatosis in HCC cells, as seem to be seen in the human NASH-HCC. The livers and HCC tissues from diethylnitrosamine-injected mice fed either control or HFD were subjected to comprehensive metabolome analysis[80,87]. Extensive acylcarnitine’s accumulation was seen in liver cancer tissue and in the sera of HFD-fed mice. A similar increasing level was seen in sera from patients with NASH-associated liver cancer. The increase of acylcarnitine might be related to the CPT-II down-regulation, suggesting that acylcarnitine is a surrogate marker of the down-regulation of CPT-II and directly participates in the development of hepatocarcinogenesis.
The down-regulation of CPT-II caused FAO inhibition which might be the cause of steatosis in HCC. The knockdown of the CPT2 gene in HCC cells could inhibit the Src-mediated activation of JNK and produce anti-lipotoxicity. Furthermore, oleylcarnitine promotes spheroid formation in HCC cells through STAT3 activation[88,89]. HFD feeding and carnitine supplementation synergistically enhance hepatocarcinogenesis with acylcarnitine accumulation in vivo. The CPT-II level in HCC mice was significantly lower than those in control or NAFLD mice, and was negatively correlated with the degree of hepatocyte malignant transformation[90,91]. A series of experiments confirmed that CPT-II was inactivated, suggesting that low CPT-II expression in IMM might lead to liver lipid accumulation and participate in promoting NAFLD malignant transformation[92].
Based on clinical and basic evidences, abnormal lipid accumulation was associated with NAFLD malignant transformation. However, only a few studies have been reported on the relationship between CPT-II activation and HCC progression. The alteration of CPT-II expression might be an important link in the obstruction of FAO and abnormal lipid accumulation[93]. Based on above findings, some scholars sequenced the whole gene of the mitochondrial CPT-II in NAFLD patients, and found that CPT2 variation was significantly associated with CPT-II activity that might be the key factor of NAFLD or related cirrhosis/HCC because its inactivation is closely related to an energy production disorder in models of NAFLD[94,95]. The dynamic alterations of CPT-II expression located on IMM during malignant transformation of hepatocytes in SD rats induced by chemical carcinogens (2-flu-orenylacetamide, 2-FAA) were investigated under lipid accumulation[19]. For the first time, the progressively decreasing expression of CPT-II at mRNA or protein level were reported, and the significantly increasing HCC related to molecular markers were confirmed during the rat hepatocarcinogenesis.
There has been a rise in the prevalence of NAFLD, paralleling a worldwide increase in MAFLD and HCC[96]. According to the dynamic pathological alterations of the model, a continuum of morphological abnormalities on liver sections has a variable course, from normal hepatocytes, lipid accumulation, cell denaturation, precancerous lesion and HCC formation. Compared with the control liver sections, there was a large amount of fat in the hepatocytes of the model rats by the oil red O staining; In the meantime, transmembrane glycoprotein CD44 activation promotes inflammatory cell recruitment and plays a key role of being linked to NAFLD progression to HCC[97-99]. CPT-II has been demonstrated to interact in forming supramolecular complexes that facilitate the passage of acylcarnitine and its expression was gradually decreased in malignant trans-formation of hepatocytes in NAFLD. However, the reported HCC biomarkers such as AFP, GPC3[100] and Wnt3a[101] were significantly increasing expressions in hepatocarcinogenesis except for CD44 as one of the most frequently reported cancer stem-like cell markers[102]. These data suggested that the alteration of CPT-II expression should be associated with the malignant progression of NAFLD.
Metabolically related NAFLD is emerging as a major cause of HCC in Western countries[103-105]. This presents an additional challenge, as NAFLD-related HCC tend to be advanced in elderly patients with comorbidities and their prognosis is very poor[106]. The pathogenesis of NAFLD-associated HCC is multifactorial and remains to be identified, although the risk of hepatocarcinogenesis is undoubtedly increased as NAFLD progresses to NASH and cirrhosis[107]. The new findings of CPT-II are useful for understanding NAFLD and HCC and should hopefully lead to the development of clinically relevant biomarkers and strategies to help identify high-risk patients, early use of preventive measures or better treatment[108]. Energy metabolism is a prerequisite for maintaining normal life activities. NAFLD is caused by excessive lipid accumulation in hepatocytes and is regarded as one of the most common liver diseases[109,110]. The down-regulation of CPT2 in IMM was one of the main causes of acyl carnitine accumulation, which was also seen in malignant transformation of hepatocytes, suggesting that CPT-II inactivity or dysfunction might become a new mechanism of blocked lipid oxidation for HCC.
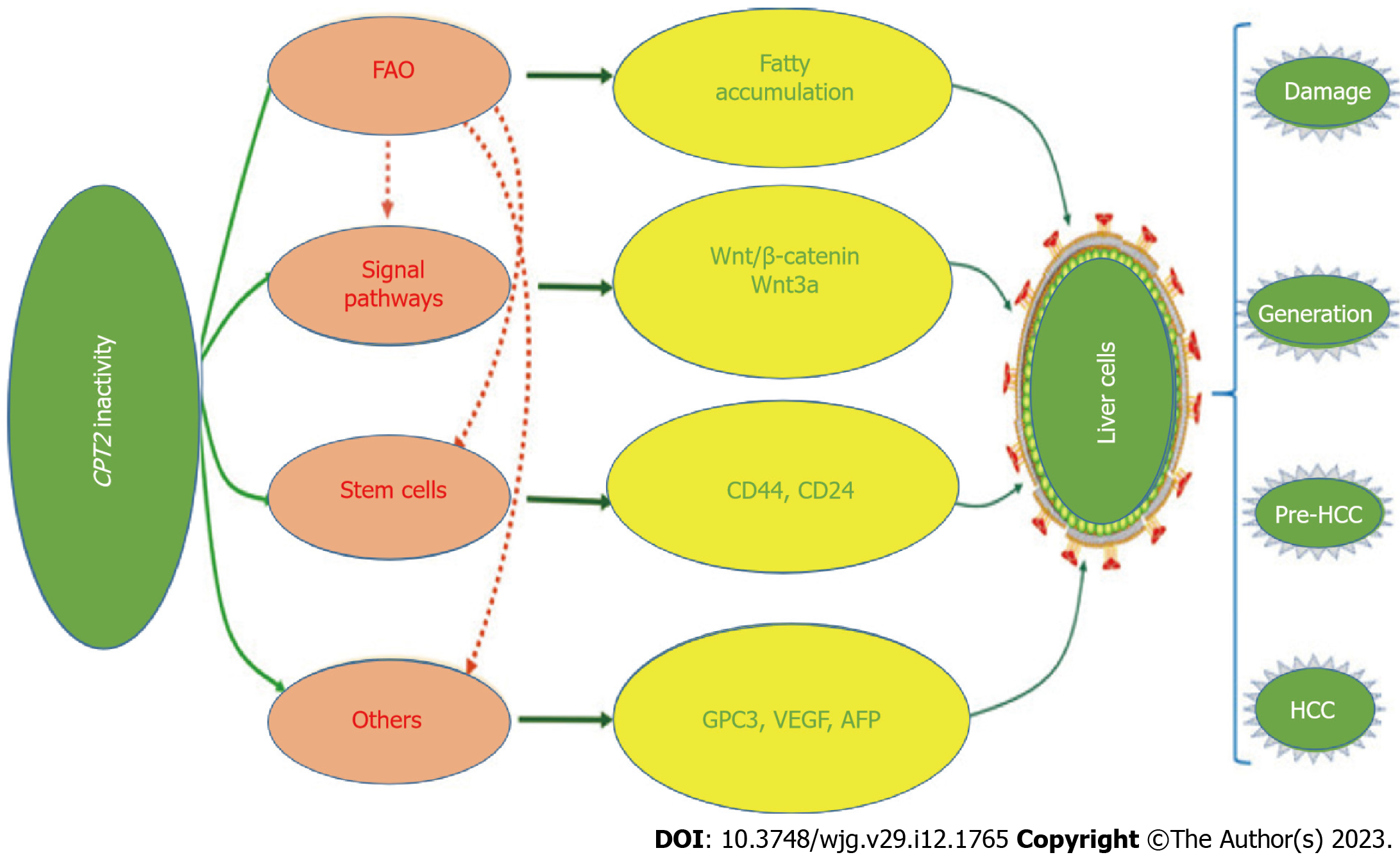
Owing to its high prevalence and potential risk, NAFLD has become a major health concern worldwide. Hepatocyte CPT-II variation or activity alteration undoubtedly has a significant impact on aggravating liver fatty accumulation, inducing activation of cancer-related stem cells, and malignant transformation of hepatocytes (Figure 2), especially in patients with HBV chronic infection. The phenomenon of CPT-II inactivation as warning signs of NAFLD malignant transformation needs attention. However, its specific regulatory mechanism is unknown, so this is a good research prospect. At present, the exact relationship between CPT-II and NAFLD remains to be explored. It is believed that with the vigorous development of molecular biological theory and technology, understanding the function of CPT-II physiology will continue to deepen, which has guided significant knowledge for CPT-II alteration during NAFLD progression. At the same time, it also brings hope and provides a theoretical basis for the assumption of early intervention of NAFLD or human related diseases and CPT-II can be used as a molecular target for monitoring or therapy.
Hepatocyte CPT-II variation or activity alteration undoubtedly has a significant impact on aggravating liver fatty accumulation, inducing activation of cancer-related stem cells, and malignant transformation of hepatocytes.
| 1. | Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 394] [Article Influence: 98.5] [Reference Citation Analysis (1)] |
| 2. | Segura-Azuara NLÁ, Varela-Chinchilla CD, Trinidad-Calderón PA. MAFLD/NAFLD Biopsy-Free Scoring Systems for Hepatic Steatosis, NASH, and Fibrosis Diagnosis. Front Med (Lausanne). 2021;8:774079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, Cortez-Pinto H, Crespo J, Cusi K, Dirac MA, Francque S, George J, Hagström H, Huang TT, Ismail MH, Kautz A, Sarin SK, Loomba R, Miller V, Newsome PN, Ninburg M, Ocama P, Ratziu V, Rinella M, Romero D, Romero-Gómez M, Schattenberg JM, Tsochatzis EA, Valenti L, Wong VW, Yilmaz Y, Younossi ZM, Zelber-Sagi S; NAFLD Consensus Consortium. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol. 2022;19:60-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 510] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 5. | Tacke F, Weiskirchen R. Non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH)-related liver fibrosis: mechanisms, treatment and prevention. Ann Transl Med. 2021;9:729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol. 2022;18:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 7. | Scorletti E, Carr RM. A new perspective on NAFLD: Focusing on lipid droplets. J Hepatol. 2022;76:934-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 8. | Foerster F, Gairing SJ, Müller L, Galle PR. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. J Hepatol. 2022;76:446-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 9. | Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, Hao H, Xiong J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B. 2022;12:558-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 10. | Metwally M, Berg T, Tsochatzis EA, Eslam M. Translation Reprogramming as a Novel Therapeutic Target in MAFLD. Adv Biol (Weinh). 2022;6:e2101298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 473] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 12. | Weber M, Mera P, Casas J, Salvador J, Rodríguez A, Alonso S, Sebastián D, Soler-Vázquez MC, Montironi C, Recalde S, Fucho R, Calderón-Domínguez M, Mir JF, Bartrons R, Escola-Gil JC, Sánchez-Infantes D, Zorzano A, Llorente-Cortes V, Casals N, Valentí V, Frühbeck G, Herrero L, Serra D. Liver CPT1A gene therapy reduces diet-induced hepatic steatosis in mice and highlights potential lipid biomarkers for human NAFLD. FASEB J. 2020;34:11816-11837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Sangineto M, Bukke VN, Bellanti F, Tamborra R, Moola A, Duda L, Villani R, Romano AD, Serviddio G. A Novel Nutraceuticals Mixture Improves Liver Steatosis by Preventing Oxidative Stress and Mitochondrial Dysfunction in a NAFLD Model. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Li L, Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid Med Cell Longev. 2018;2018:7580707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 371] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 15. | Zhou R, Lin C, Cheng Y, Zhuo X, Li Q, Xu W, Zhao L, Yang L. Liraglutide Alleviates Hepatic Steatosis and Liver Injury in T2MD Rats via a GLP-1R Dependent AMPK Pathway. Front Pharmacol. 2020;11:600175. [PubMed] [DOI] [Full Text] |
| 16. | Dall M, Hassing AS, Treebak JT. NAD+ and NAFLD - caution, causality and careful optimism. J Physiol. 2022;600:1135-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Petrick HL, Holloway GP. Cytosolic reverse CrAT activity in cardiac tissue: potential importance for fuel selection. Biochem J. 2018;475:1267-1269. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Lu JH, Wang F, Wang YN, He MM, Wu QN, Lu YX, Yu HE, Chen ZH, Zhao Q, Liu J, Chen YX, Wang DS, Sheng H, Liu ZX, Zeng ZL, Xu RH, Ju HQ. Inhibition of fatty acid catabolism augments the efficacy of oxaliplatin-based chemotherapy in gastrointestinal cancers. Cancer Lett. 2020;473:74-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Gu JJ, Yao M, Yang J, Cai Y, Zheng WJ, Wang L, Yao DB, Yao DF. Mitochondrial carnitine palmitoyl transferase-II inactivity aggravates lipid accumulation in rat hepatocarcinogenesis. World J Gastroenterol. 2017;23:256-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Rufer AC, Thoma R, Hennig M. Structural insight into function and regulation of carnitine palmitoyltransferase. Cell Mol Life Sci. 2009;66:2489-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Rufer AC, Thoma R, Benz J, Stihle M, Gsell B, De Roo E, Banner DW, Mueller F, Chomienne O, Hennig M. The crystal structure of carnitine palmitoyltransferase 2 and implications for diabetes treatment. Structure. 2006;14:713-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Trauner M, Fuchs CD. Novel therapeutic targets for cholestatic and fatty liver disease. Gut. 2022;71:194-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 23. | Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, Proikas-Cezanne T, Reggiori F. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy. 2022;18:50-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 24. | Han S, Wei R, Zhang X, Jiang N, Fan M, Huang JH, Xie B, Zhang L, Miao W, Butler AC, Coleman MA, Vaughan AT, Wang Y, Chen HW, Liu J, Li JJ. CPT1A/2-Mediated FAO Enhancement-A Metabolic Target in Radioresistant Breast Cancer. Front Oncol. 2019;9:1201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 25. | Park JH, Vithayathil S, Kumar S, Sung PL, Dobrolecki LE, Putluri V, Bhat VB, Bhowmik SK, Gupta V, Arora K, Wu D, Tsouko E, Zhang Y, Maity S, Donti TR, Graham BH, Frigo DE, Coarfa C, Yotnda P, Putluri N, Sreekumar A, Lewis MT, Creighton CJ, Wong LC, Kaipparettu BA. Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer. Cell Rep. 2016;14:2154-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 26. | de Carvalho Ribeiro M, Szabo G. Role of the Inflammasome in Liver Disease. Annu Rev Pathol. 2022;17:345-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 205] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 27. | Rufer AC, Lomize A, Benz J, Chomienne O, Thoma R, Hennig M. Carnitine palmitoyltransferase 2: analysis of membrane association and complex structure with a substrate analog. FEBS Lett. 2007;581:3247-3252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Song A, Park Y, Kim B, Lee SG. Modulation of Lipid Metabolism by Trans-Anethole in Hepatocytes. Molecules. 2020;25:4946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Wang J, Xiang H, Lu Y, Wu T, Ji G. The role and therapeutic implication of CPTs in fatty acid oxidation and cancers progression. Am J Cancer Res. 2021;11:2477-2494. [PubMed] |
| 30. | Yao M, Cai M, Yao D, Xu X, Yang R, Li Y, Zhang Y, Kido H. Abbreviated half-lives and impaired fuel utilization in carnitine palmitoyltransferase II variant fibroblasts. PLoS One. 2015;10:e0119936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Yao M, Yao D, Yamaguchi M, Chida J, Kido H. Bezafibrate upregulates carnitine palmitoyltransferase II expression and promotes mitochondrial energy crisis dissipation in fibroblasts of patients with influenza-associated encephalopathy. Mol Genet Metab. 2011;104:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Gu JJ, Yao M, Cai Y, Fang M, Wang L, Zheng WJ, Yao DB, Dong ZZ, Yao DF. [Dynamic expression of carnitine palmitoyltransferase II in the mitochondrial inner membrane during hepatocyte malignant transformation induced by lipid accumulation]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:279-284. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Stenson PD, Ball EV, Mort M, Phillips AD, Shaw K, Cooper DN. The Human Gene Mutation Database (HGMD) and its exploitation in the fields of personalized genomics and molecular evolution. Curr Protoc Bioinformatics. 2012;Chapter 1:Unit1.13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 34. | Xue Y, Chen Y, Ayub Q, Huang N, Ball EV, Mort M, Phillips AD, Shaw K, Stenson PD, Cooper DN, Tyler-Smith C; 1000 Genomes Project Consortium. Deleterious- and disease-allele prevalence in healthy individuals: insights from current predictions, mutation databases, and population-scale resequencing. Am J Hum Genet. 2012;91:1022-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 35. | Lehmann D, Zierz S. Normal protein content but abnormally inhibited enzyme activity in muscle carnitine palmitoyltransferase II deficiency. J Neurol Sci. 2014;339:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Yao D, Mizuguchi H, Yamaguchi M, Yamada H, Chida J, Shikata K, Kido H. Thermal instability of compound variants of carnitine palmitoyltransferase II and impaired mitochondrial fuel utilization in influenza-associated encephalopathy. Hum Mutat. 2008;29:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Houten SM, Violante S, Ventura FV, Wanders RJ. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu Rev Physiol. 2016;78:23-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 680] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 38. | Fucho R, Casals N, Serra D, Herrero L. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J. 2017;31:1263-1272. [PubMed] [DOI] [Full Text] |
| 39. | Angajala A, Lim S, Phillips JB, Kim JH, Yates C, You Z, Tan M. Diverse Roles of Mitochondria in Immune Responses: Novel Insights Into Immuno-Metabolism. Front Immunol. 2018;9:1605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 40. | Serra D, Mera P, Malandrino MI, Mir JF, Herrero L. Mitochondrial fatty acid oxidation in obesity. Antioxid Redox Signal. 2013;19:269-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 41. | Schlaepfer IR, Joshi M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology. 2020;161:bqz046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 508] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 42. | Casals N, Zammit V, Herrero L, Fadó R, Rodríguez-Rodríguez R, Serra D. Carnitine palmitoyltransferase 1C: From cognition to cancer. Prog Lipid Res. 2016;61:134-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | Isackson PJ, Bennett MJ, Vladutiu GD. Identification of 16 new disease-causing mutations in the CPT2 gene resulting in carnitine palmitoyltransferase II deficiency. Mol Genet Metab. 2006;89:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 405] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 45. | Eng JM, Estall JL. Diet-Induced Models of Non-Alcoholic Fatty Liver Disease: Food for Thought on Sugar, Fat, and Cholesterol. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 46. | Cacciola NA, Sgadari M, Sepe F, Petillo O, Margarucci S, Martano M, Maiolino P, Restucci B. Metabolic Flexibility in Canine Mammary Tumors: Implications of the Carnitine System. Animals (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Wang M, Wang K, Liao X, Hu H, Chen L, Meng L, Gao W, Li Q. Carnitine Palmitoyltransferase System: A New Target for Anti-Inflammatory and Anticancer Therapy? Front Pharmacol. 2021;12:760581. [PubMed] [DOI] [Full Text] |
| 48. | Mccormick BJ, Chirila RM. Carnitine palmitoyltransferase-II deficiency: case presentation and review of the literature. Rom J Intern Med. 2021;59:420-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Sigauke E, Rakheja D, Kitson K, Bennett MJ. Carnitine palmitoyltransferase II deficiency: a clinical, biochemical, and molecular review. Lab Invest. 2003;83:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Tajima G, Hara K, Yuasa M. Carnitine palmitoyltransferase II deficiency with a focus on newborn screening. J Hum Genet. 2019;64:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Lehmann D, Motlagh L, Robaa D, Zierz S. Muscle Carnitine Palmitoyltransferase II Deficiency: A Review of Enzymatic Controversy and Clinical Features. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Kilfoyle D, Hutchinson D, Potter H, George P. Recurrent myoglobinuria due to carnitine palmitoyltransferase II deficiency: clinical, biochemical, and genetic features of adult-onset cases. N Z Med J. 2005;118:U1320. [PubMed] |
| 53. | Taggart RT, Smail D, Apolito C, Vladutiu GD. Novel mutations associated with carnitine palmitoyltransferase II deficiency. Hum Mutat. 1999;13:210-220. [PubMed] [DOI] [Full Text] |
| 54. | Joshi PR, Deschauer M, Zierz S. Carnitine palmitoyltransferase II (CPT II) deficiency: genotype-phenotype analysis of 50 patients. J Neurol Sci. 2014;338:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Jogl G, Hsiao YS, Tong L. Structure and function of carnitine acyltransferases. Ann N Y Acad Sci. 2004;1033:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Motlagh L, Golbik R, Sippl W, Zierz S. Stabilization of the thermolabile variant S113L of carnitine palmitoyltransferase II. Neurol Genet. 2016;2:e53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Dang Y, Xu J, Zhu M, Zhou W, Zhang L, Ji G. Gan-Jiang-Ling-Zhu decoction alleviates hepatic steatosis in rats by the miR-138-5p/CPT1B axis. Biomed Pharmacother. 2020;127:110127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Xu A, Wang B, Fu J, Qin W, Yu T, Yang Z, Lu Q, Chen J, Chen Y, Wang H. Diet-induced hepatic steatosis activates Ras to promote hepatocarcinogenesis via CPT1α. Cancer Lett. 2019;442:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Yao D, Yao M, Yamaguchi M, Chida J, Kido H. Characterization of compound missense mutation and deletion of carnitine palmitoyltransferase II in a patient with adenovirus-associated encephalopathy. J Med Invest. 2011;58:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003;112:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 61. | Abolfathi M, Mohd-Yusof BN, Hanipah ZN, Mohd Redzwan S, Yusof LM, Khosroshahi MZ. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2020;48:102273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Li N, Zhao H. Role of Carnitine in Non-alcoholic Fatty Liver Disease and Other Related Diseases: An Update. Front Med (Lausanne). 2021;8:689042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Savic D, Hodson L, Neubauer S, Pavlides M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | Baker PR 2nd, Friedman JE. Mitochondrial role in the neonatal predisposition to developing nonalcoholic fatty liver disease. J Clin Invest. 2018;128:3692-3703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 65. | Poulos JE, Kalogerinis PT, Milanov V, Kalogerinis CT, Poulos EJ. The Effects of Vitamin E, Silymarin and Carnitine on the Metabolic Abnormalities Associated with Nonalcoholic Liver Disease. J Diet Suppl. 2022;19:287-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 66. | Sogabe M, Okahisa T, Kurihara T, Takehara M, Kagemoto K, Okazaki J, Kida Y, Hirao A, Tanaka H, Tomonari T, Taniguchi T, Okamoto K, Nakasono M, Takayama T. Differences among patients with and without nonalcoholic fatty liver disease having elevated alanine aminotransferase levels at various stages of metabolic syndrome. PLoS One. 2020;15:e0238388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Gu J, Yao M, Yao D, Wang L, Yang X. Nonalcoholic Lipid Accumulation and Hepatocyte Malignant Transformation. J Clin Transl Hepatol. 2016;4:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Enooku K, Nakagawa H, Fujiwara N, Kondo M, Minami T, Hoshida Y, Shibahara J, Tateishi R, Koike K. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci Rep. 2019;9:10663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 69. | da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. Novel insights on interactions between folate and lipid metabolism. Biofactors. 2014;40:277-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Giannakoulis VG, Dubovan P, Papoutsi E, Kataki A, Koskinas J. Senescence in HBV-, HCV- and NAFLD- Mediated Hepatocellular Carcinoma and Senotherapeutics: Current Evidence and Future Perspective. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Das UN. Beneficial role of bioactive lipids in the pathobiology, prevention, and management of HBV, HCV and alcoholic hepatitis, NAFLD, and liver cirrhosis: A review. J Adv Res. 2019;17:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | González-Romero F, Mestre D, Aurrekoetxea I, O'Rourke CJ, Andersen JB, Woodhoo A, Tamayo-Caro M, Varela-Rey M, Palomo-Irigoyen M, Gómez-Santos B, de Urturi DS, Núñez-García M, García-Rodríguez JL, Fernández-Ares L, Buqué X, Iglesias-Ara A, Bernales I, De Juan VG, Delgado TC, Goikoetxea-Usandizaga N, Lee R, Bhanot S, Delgado I, Perugorria MJ, Errazti G, Mosteiro L, Gaztambide S, Martinez de la Piscina I, Iruzubieta P, Crespo J, Banales JM, Martínez-Chantar ML, Castaño L, Zubiaga AM, Aspichueta P. E2F1 and E2F2-Mediated Repression of CPT2 Establishes a Lipid-Rich Tumor-Promoting Environment. Cancer Res. 2021;81:2874-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, Tanaka Y, Tateishi R, Hikiba Y, Misumi K, Tanaka M, Hayashi A, Shibahara J, Fukayama M, Arita J, Hasegawa K, Hirschfield H, Hoshida Y, Hirata Y, Otsuka M, Tateishi K, Koike K. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67:1493-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 74. | Cheung KP, Taylor KR, Jameson JM. Immunomodulation at epithelial sites by obesity and metabolic disease. Immunol Res. 2012;52:182-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Dhamija E, Paul SB, Kedia S. Non-alcoholic fatty liver disease associated with hepatocellular carcinoma: An increasing concern. Indian J Med Res. 2019;149:9-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 76. | Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017;37 Suppl 1:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 77. | Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 612] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 78. | Pinweha P, Rattanapornsompong K, Charoensawan V, Jitrapakdee S. MicroRNAs and oncogenic transcriptional regulatory networks controlling metabolic reprogramming in cancers. Comput Struct Biotechnol J. 2016;14:223-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 79. | Yi YS. Regulatory Roles of Caspase-11 Non-Canonical Inflammasome in Inflammatory Liver Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Lee J, Choi J, Selen Alpergin ES, Zhao L, Hartung T, Scafidi S, Riddle RC, Wolfgang MJ. Loss of Hepatic Mitochondrial Long-Chain Fatty Acid Oxidation Confers Resistance to Diet-Induced Obesity and Glucose Intolerance. Cell Rep. 2017;20:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 81. | Selen ES, Choi J, Wolfgang MJ. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 82. | Liu X, Zhang J, Ming Y, Chen X, Zeng M, Mao Y. The aggravation of mitochondrial dysfunction in nonalcoholic fatty liver disease accompanied with type 2 diabetes mellitus. Scand J Gastroenterol. 2015;50:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Jun DW, Cho WK, Jun JH, Kwon HJ, Jang KS, Kim HJ, Jeon HJ, Lee KN, Lee HL, Lee OY, Yoon BC, Choi HS, Hahm JS, Lee MH. Prevention of free fatty acid-induced hepatic lipotoxicity by carnitine via reversal of mitochondrial dysfunction. Liver Int. 2011;31:1315-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Amato A, Caldara GF, Nuzzo D, Baldassano S, Picone P, Rizzo M, Mulè F, Di Carlo M. NAFLD and Atherosclerosis Are Prevented by a Natural Dietary Supplement Containing Curcumin, Silymarin, Guggul, Chlorogenic Acid and Inulin in Mice Fed a High-Fat Diet. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Lee Y, Kim BR, Kang GH, Lee GJ, Park YJ, Kim H, Jang HC, Choi SH. The Effects of PPAR Agonists on Atherosclerosis and Nonalcoholic Fatty Liver Disease in ApoE-/-FXR-/- Mice. Endocrinol Metab (Seoul). 2021;36:1243-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Kim JH, Lee BR, Choi ES, Lee KM, Choi SK, Cho JH, Jeon WB, Kim E. Reverse Expression of Aging-Associated Molecules through Transfection of miRNAs to Aged Mice. Mol Ther Nucleic Acids. 2017;6:106-115. [PubMed] [DOI] [Full Text] |
| 87. | Schulien I, Hasselblatt P. Diethylnitrosamine-induced liver tumorigenesis in mice. Methods Cell Biol. 2021;163:137-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 88. | Attia YM, Tawfiq RA, Gibriel AA, Ali AA, Kassem DH, Hammam OA, Elmazar MM. Activation of FXR modulates SOCS3/Jak2/STAT3 signaling axis in a NASH-dependent hepatocellular carcinoma animal model. Biochem Pharmacol. 2021;186:114497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 89. | Zhou M, Mok MT, Sun H, Chan AW, Huang Y, Cheng AS, Xu G. The anti-diabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcino- genesis through cAMP-PKA-EGFR-STAT3 axis. Oncogene. 2017;36:4135-4149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Khan H, Ullah H, Nabavi SM. Mechanistic insights of hepatoprotective effects of curcumin: Therapeutic updates and future prospects. Food Chem Toxicol. 2019;124:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 91. | Holczbauer Á, Wangensteen KJ, Shin S. Cellular origins of regenerating liver and hepatocellular carcinoma. JHEP Rep. 2022;4:100416. [PubMed] [DOI] [Full Text] |
| 92. | Brown ZJ, Fu Q, Ma C, Kruhlak M, Zhang H, Luo J, Heinrich B, Yu SJ, Zhang Q, Wilson A, Shi ZD, Swenson R, Greten TF. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4+ T cell apoptosis promoting HCC development. Cell Death Dis. 2018;9:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 93. | Paul B, Lewinska M, Andersen JB. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022;4:100479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 94. | Ham JR, Lee HI, Choi RY, Sim MO, Seo KI, Lee MK. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat diet-induced obese mice. Food Funct. 2016;7:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 95. | Liu Q, Pan R, Ding L, Zhang F, Hu L, Ding B, Zhu L, Xia Y, Dou X. Rutin exhibits hepatoprotective effects in a mouse model of non-alcoholic fatty liver disease by reducing hepatic lipid levels and mitigating lipid-induced oxidative injuries. Int Immunopharmacol. 2017;49:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 96. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 3195] [Article Influence: 399.4] [Reference Citation Analysis (2)] |
| 97. | Patouraux S, Rousseau D, Bonnafous S, Lebeaupin C, Luci C, Canivet CM, Schneck AS, Bertola A, Saint-Paul MC, Iannelli A, Gugenheim J, Anty R, Tran A, Bailly-Maitre B, Gual P. CD44 is a key player in non-alcoholic steatohepatitis. J Hepatol. 2017;67:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 98. | Egan CE, Daugherity EK, Rogers AB, Abi Abdallah DS, Denkers EY, Maurer KJ. CCR2 and CD44 promote inflammatory cell recruitment during fatty liver formation in a lithogenic diet fed mouse model. PLoS One. 2013;8:e65247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Fang M, Yao M, Yang J, Zheng WJ, Wang L, Yao DF. Abnormal CD44 activation of hepatocytes with nonalcoholic fatty accumulation in rat hepatocarcinogenesis. World J Gastrointest Oncol. 2020;12:66-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Yao M, Yao DF, Bian YZ, Zhang CG, Qiu LW, Wu W, Sai WL, Yang JL, Zhang HJ. Oncofetal antigen glypican-3 as a promising early diagnostic marker for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Yao M, Yang JL, Wang DF, Wang L, Chen Y, Yao DF. Encouraging specific biomarkers-based therapeutic strategies for hepatocellular carcinoma. World J Clin Cases. 2022;10:3321-3333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 102. | Wu XF, Sha CX, Yang JL, Liu Y, Zhou P, Yao DF, Yao M. [Abnormal expression of CD44 aggravates liver disease progression in patients with non-alcoholic fatty liver disease accompanied with hepatitis B virus replication]. Zhonghua Gan Zang Bing Za Zhi. 2021;29:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 103. | Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 1050] [Article Influence: 150.0] [Reference Citation Analysis (1)] |
| 104. | Meroni M, Longo M, Rustichelli A, Dongiovanni P. Nutrition and Genetics in NAFLD: The Perfect Binomium. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 105. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 851] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 106. | Geh D, Anstee QM, Reeves HL. NAFLD-Associated HCC: Progress and Opportunities. J Hepatocell Carcinoma. 2021;8:223-239. [PubMed] [DOI] [Full Text] |
| 107. | Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: An evidence-based approach. World J Gastroenterol. 2019;25:1550-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (5)] |
| 108. | Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 109. | Márquez-Quiroga LV, Arellanes-Robledo J, Vásquez-Garzón VR, Villa-Treviño S, Muriel P. Models of nonalcoholic steatohepatitis potentiated by chemical inducers leading to hepatocellular carcinoma. Biochem Pharmacol. 2022;195:114845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 110. | Clutton-Brock TH, Iason GR. Sex ratio variation in mammals. Q Rev Biol. 1986;61:339-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ulasoglu C, Turkey; Liu QJ, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM