Published online Mar 14, 2023. doi: 10.3748/wjg.v29.i10.1614
Peer-review started: November 30, 2022
First decision: December 20, 2022
Revised: December 24, 2022
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 14, 2023
Processing time: 99 Days and 21.1 Hours
Programmed death receptor-1 (PD-1) inhibitors have been approved as second-line treatment regimen in hepatocellular carcinoma (HCC), but it is still worth studying whether patients can benefit from PD-1 inhibitors as first-line drugs combined with targeted drugs and locoregional therapy.
To estimate the clinical outcome of transarterial chemoembolization (TACE) and lenvatinib plus PD-1 inhibitors for patients with unresectable HCC (uHCC).
We carried out retrospective research of 65 patients with uHCC who were treated at Peking Union Medical College Hospital from September 2017 to February 2022. 45 patients received the PD-1 inhibitors, lenvatinib, TACE (PD-1-Lenv-T) therapy, and 20 received the lenvatinib, TACE (Lenv-T) therapy. In terms of the dose of lenvatinib, 8 mg was given orally for patients weighing less than 60 kg and 12 mg for those weighing more than 60 kg. Of the patients in the PD-1 inhibitor combi
Patients with uHCC who received PD-1-Lenv-T therapy (n = 45) had a clearly longer overall survival than those who underwent Lenv-T therapy (n = 20, 26.8 vs 14.0 mo; P = 0.027). The median progression-free survival time between the two treatment regimens was also measured {11.7 mo [95% confidence interval (CI): 7.7-15.7] in the PD-1-Lenv-T group vs 8.5 mo (95%CI: 3.0-13.9) in the Lenv-T group (P = 0.028)}. The objective response rates of the PD-1-Lenv-T group and Lenv-T group were 44.4% and 20% (P = 0.059) according to the mRECIST criteria, meanwhile the disease control rates were 93.3% and 64.0% (P = 0.003), respectively. The type and frequency of AEs showed little distinction between patients received the two treatment regimens.
Our results suggest that the early combination of PD-1 inhibitors has manageable toxicity and hopeful efficacy in patients with uHCC.
Core Tip: This retrospective research was designed to evaluate the treatment outcome and safety of transarterial chemoembolization (TACE) and lenvatinib plus programmed death receptor-1 (PD-1) inhibitors in the treatment of patients with unresectable hepatocellular carcinoma (uHCC). Patients with uHCC who underwent PD-1 inhibitors, lenvatinib, TACE therapy (n = 45) had a evidently longer overall survival than those who underrwent lenvatinib, TACE therapy (n = 20, 26.8 vs 14.0 mo; P = 0.027). Early combination of PD-1 inhibitors has manageable toxicity and hopeful efficacy in patients with uHCC.
- Citation: Wang YY, Yang X, Wang YC, Long JY, Sun HS, Li YR, Xun ZY, Zhang N, Xue JN, Ning C, Zhang JW, Zhu CP, Zhang LH, Yang XB, Zhao HT. Clinical outcomes of lenvatinib plus transarterial chemoembolization with or without programmed death receptor-1 inhibitors in unresectable hepatocellular carcinoma. World J Gastroenterol 2023; 29(10): 1614-1626
- URL: https://www.wjgnet.com/1007-9327/full/v29/i10/1614.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i10.1614
Primary liver carcinoma is the sixth most common cancer type worldwide and leads to the third most cancer-related deaths[1]. The proportion of hepatocellular carcinoma (HCC) is approximately 80% in primary liver cancers[2]. Due to the powerful compensatory power of the liver, most patients with liver carcinoma are already in the advanced stage when they develop symptoms. Therefore, the systemic therapies of advanced HCC have attracted much attention.
Lenvatinib is an oral small molecule inhibitor of receptor tyrosine kinases [platelet-derived growth factor receptor (PDGFR), KIT, rearranged in transfection, fibroblast growth factor receptor (FGFR) 1-4 and vascular endothelial growth factor receptor (VEGFR) 1-3] that was applied for the first-line therapy of patients with unresectable HCC on the basis of the clinical outcomes from a randomized, multinational, open-label, noninferiority phase III trial[3]. In this clinical trial, lenvatinib demonstrated a better treatment effect than sorafenib. The overall survival (OS) with lenvatinib was 5 mo longer than that with sorafenib and the objective response rate (ORR) was approximately 2 times higher than that of sorafenib in hepatitis B virus (HBV) background subgroup analysis[3].
The main nutrients for liver cancer growth are supplied by the hepatic artery, which can lead to tumor ischemic necrosis by embolization[4]. Therefore, transarterial chemoembolization (TACE) is proposed as the standard therapeutic regimen for stage B HCC as classified by the Barcelona Clinic Liver Cancer (BCLC) staging system[5,6]. However, TACE also aggravates tumor hypoxia, resulting in the accumulation of hypoxia-inducible factor 1α, upregulating the expression of PDGF and VEGF, which facilitates angiogenesis-induced collateral vessel formation, and contributes to tumor revascularization and locoregional recurrence[7-9]. Therefore, several clinical trials have made an attempt to associate TACE with systemic antiangiogenic treatment[10-12]. A randomized, multicenter prospective trial comparing TACE plus sorafenib and TACE alone in patients with liver cancer confirmed that in patients with unresectable HCC (uHCC), TACE plus sorafenib clearly improved progression-free survival (PFS) compared with TACE alone[12]. As another antiangiogenic drug, lenvatinib has significantly improved clinical efficacy in the remedy of uHCC with TACE plus lenvatinib compared with TACE monotherapy in previous studies[13,14].
Immunotherapy, as a systemic therapy that has attracted much attention in recent years, has also made significant progress in liver cancer[15]. Programmed death receptor-1 (PD-1) expresses on the surface of T cells, and is a key immunosuppressive transmembrane protein[16]. In the tumor microenvironment, cancer cells can express PD ligand 1 (PD-L1), which can bond to PD-1 to inhibit the function of T cells and reduce their killing effect on tumor cells[17]. Studies have shown that lenvatinib has a synergistic effect with immune checkpoint inhibitors (ICIs)[18]. VEGF is a highly expressed angiogenic factor in the tumor microenvironment of HCC that supports tumor growth and promotes immune rejection and is a key mediator in the immunosuppressive microenvironment[19]. Previous researches have suggested that lenvatinib can alleviate immunosuppression in the tumor microenvironment by inhibiting VEGF and can also increase T lymphocyte infiltration in the immunosuppressive microenvironment, providing an effective immunotherapeutic microenvironment for ICIs to function[20].
In conclusion, the combination of systemic and local treatment for liver cancer can synergistically kill tumors and prolong the survival of patients in various ways. Currently, PD-1 inhibitors have been applied as second-line remedy in HCC[21], and it is still worth studying whether patients can obtain clinical benefit from PD-1 inhibitors as first-line drugs combined with targeted drugs and local therapy. This retrospective research was designed to evaluate the clinical outcome and safety of TACE and lenvatinib plus PD-1 inhibitors in the treatment of uHCC and to determine whether the early combination of PD-1 inhibitors could benefit uHCC patients.
In this retrospective research, the clinical information and image data of HCC patients were collected from Peking Union Medical College Hospital from September 2017 to February 2022. uHCC was confirmed by more than two clinical experts according to the National Comprehensive Cancer Network guidelines. The main inclusion criteria were as the following: (1) Histologically or clinically confirmed HCC; (2) 1 or more measurable lesion by the modified Response Evaluation Criteria in Solid Tumors (mRECIST criteria); (3) BCLC stage A, B or C HCC; (4) Child-Pugh class scored as A (score 5-6) or B (score 7); (5) Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0-1; and (6) Prior resection or ablation was allowed. The exclusion criteria were as the following: (1) Secondary malignant tumor of liver; (2) Child-Pugh class C; and (3) Any contraindication to TACE, lenvatinib or PD-1 inhibitors.
In this study, patients weighing less than 60 kg received a dose of 8 mg of lenvatinib, and those weighing more than 60 kg received a dose of 12 mg of lenvatinib orally once a day. Of the patients in the PD-1 inhibitor combination group, 15 received Toripalimab, 14 received Toripalimab, 14 received Camrelizumab, 4 received Pembrolizumab, 9 received Sintilimab, and 2 received Nivolumab, 1 with Tislelizumab. Camrelizumab, Sintilimab, Pembrolizumab and Tislelizumab were all given 200 mg every 3 wk. The dose of Toripalimab was 240 mg once every 3 wk. The dose of Nivolumab was 240 mg once every 2 wk.
TACE was performed under local anesthesia. After successful femoral artery puncture, 5-fluorouracil perfusion was performed through the celiac trunk. The tumor-supplying artery was superselected by a microcatheter, and chemoembolization was performed with a mixture of lipiodol and pirarubicin (25-40 mg/m2). According to the investigators’ assessment, TACE was performed every 4-6 wk when the patient had good hepatic function (Child-Pugh class A or B) until disease progression occurred.
According to Common Terminology Criteria for Adverse Events (CTCAE), v5.0, adverse events of patients during treatment were evaluated and graded. For grade 1 to grade 2 adverse events, there was no need to stop medication and symptomatic treatment was performed. If grade 3 or higher adverse reactions occur, medication should be suspended until the adverse reactions improve to grade 0-1 or baseline. For grade 4 or more adverse reactions, medication should be stopped and symptomatic treatment should be carried out actively.
The primary outcomes were OS and PFS. The definition of OS was the time from the first TACE therapy to death or the last follow-up.Tumor response was evaluated according to contrast-enhanced computed tomography or magnetic resonance imaging. The definition of PFS was the time between the first TACE therapy and disease recurrence or the last follow-up. The secondary outcome was the frequency of main adverse events (AEs), which were evaluated by the CTCAE, v 5.0. The key AEs after the initiation of combination therapy were observed. We conducted follow-up every 3 wk to assess outcome variables until tumor progression, intolerable AEs, or death.
We used T tests to compare continuous data conforming to the normal distribution. χ2 tests were performed to compare categorical variables. We used Mann-Whitney U tests to compare the continuous variables that did not conform to the normal distribution. Fisher’s exact test was used when the sample size is less than 40 or the theoretical frequency was less than 1. The survival rates were evaluated by the Kaplan-Meier curve. We identified independent prognostic factors related to OS by univariate and multivariate analyses based on the Cox regression model. All statistical analyses were performed by SPSS, v 25.0.
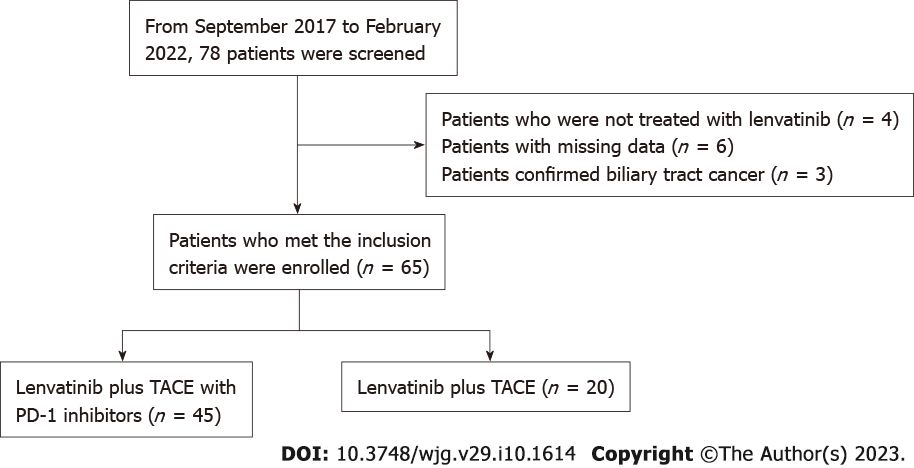
We finally enrolled 65 patients with uHCC according to the criteria; 45 received the PD-1 inhibitors, lenvatinib, TACE therapy (PD-1-Lenv-T), and 20 received the lenvatinib, TACE (Lenv-T therapy) (Figure 1). The clinical information and data at baseline of all patients are shown in Table 1. The median age in the PD-1-Lenv-T group was 54 years old and in the Lenv-T group was 62 years old. At baseline, tumors were considered BCLC stage A in 6.67%, stage B in 17.78% and stage C in 75.56% of patients receiving PD-1-Lenv-T and stage A in 10%, stage B in 15% and stage C in 75% of patients receiving Lenv-T (P = 0.88). The ECOG-PSs were 0 in 57.78% and 1 in 42.22% of patients treated with PD-1-Lenv-T and 0 in 35.0% and 1 in 65.0% of patients treated with Lenv-T (P = 0.154). The numbers of TACE treatments were < 3 in 62.22% and ≥ 3 in 37.78% of patients received PD-1-Lenv-T and < 3 in 80.0% and ≥ 3 in 20.0% of patients received Lenv-T therapy (P = 0.26). The tumor numbers were 1 in 37.78% and more than 1 in 62.22% of patients who were treated with PD-1-Lenv-T and 1 in 20.00% and more than 1 in 80.00% of patients who were treated with Lenv-T (P = 0.26). Baseline characteristics of tumor burden score (TBS) group, extrahepatic metastasis rate and portal vein tumor thrombus rate were similar between the two groups. Median administration time of lenvatinib was 5.9 mo (range: 1.0-15.9 mo) and 7.8 mo (range: 1.1-28.2 mo) in the Lenv-T group and the PD-1-Lenv-T group (P = 0.07), respectively. The median injection timesof PD-1 inhibitors in the group that received PD-1 inhibitor therapy was 6 (range: 1-29).
| Overall | Lenvatinib + TACE + PD-1 | Lenvatinib + TACE | P value | |
| Patient characteristics | ||||
| Number (n) | 65 | 45 | 20 | |
| Age, median (range), yr | 57 (18-79) | 54 (18-79) | 62 (26-75) | 0.0661 |
| < 65 | 50 (76.92) | 38 (84.44) | 12 (60.00) | |
| ≥ 65 | 15 (23.08) | 7 (15.56) | 8 (40.00) | |
| Gender | 0.0951 | |||
| Female | 8 (12.31) | 3 (6.67) | 5 (25.00) | |
| Male | 57 (87.69) | 42 (93.33) | 15 (75.00) | |
| ECOG-PS | 0.1541 | |||
| 0 | 33 (50.77) | 26 (57.78) | 7 (35.00) | |
| 1 | 32 (49.23) | 19 (42.22) | 13 (65.00) | |
| Etiology | 0.0021 | |||
| HBV | 54 (83.08) | 42 (93.33) | 12 (60.00) | |
| HCV | 4 (6.15) | 2 (4.44) | 2 (10.00) | |
| Non-HBV, non-HCV | 7 (10.77) | 1 (2.22) | 6 (30.00) | |
| Hepatic cirrhosis | 0.8791 | |||
| Yes | 43 (66.15) | 29 (64.44) | 14 (70.00) | |
| No | 22 (33.85) | 16 (35.56) | 6 (30.00) | |
| AFP | 0.5511 | |||
| < 400 | 21 (32.31) | 13 (28.89) | 8 (40.00) | |
| ≥ 400 | 44 (67.69) | 32 (71.11) | 12 (60.00) | |
| Child-Pugh score | 0.0951 | |||
| A5 | 48 (73.85) | 30 (66.67) | 18 (90.00) | |
| A6 or B7 | 17 (26.15) | 15 (33.33) | 2 (10.00) | |
| BCLC stage | 1.0001 | |||
| A or B | 16 (24.62) | 11 (24.44) | 5 (25.00) | |
| C | 49 (75.38) | 34 (75.56) | 15 (75.00) | |
| Tumor characteristics | ||||
| Tumor size | 0.0351 | |||
| < 7 cm | 25 (38.46) | 13 (28.89) | 12 (60.00) | |
| ≥ 7 cm | 40 (61.54) | 32 (71.11) | 8 (40.00) | |
| Tumor number | 0.2601 | |||
| Single | 21 (32.31) | 17 (37.78) | 4 (20.00) | |
| Multiple | 44 (67.69) | 28 (62.22) | 16 (80.00) | |
| TBS group | 0.1731 | |||
| L | 23 (35.38) | 13 (28.89) | 10 (50.00) | |
| H | 42 (64.62) | 32 (71.11) | 10 (50.00) | |
| Portal vein tumor thrombus | 0.1081 | |||
| Presence | 24 (36.92) | 20 (44.44) | 4 (20.00) | |
| Absence | 41 (63.08) | 25 (55.56) | 16 (80.00) | |
| Extrahepatic metastasis | 0.3761 | |||
| Yes | 23 (35.38) | 18 (40.00) | 5 (25.00) | |
| No | 42 (64.62) | 27 (60.00) | 15 (75.00) | |
| TACE times | 0.2601 | |||
| < 3 | 44 (67.69) | 28 (62.22) | 16 (80.00) | |
| ≥ 3 | 21 (32.31) | 17 (37.78) | 4 (20.00) |
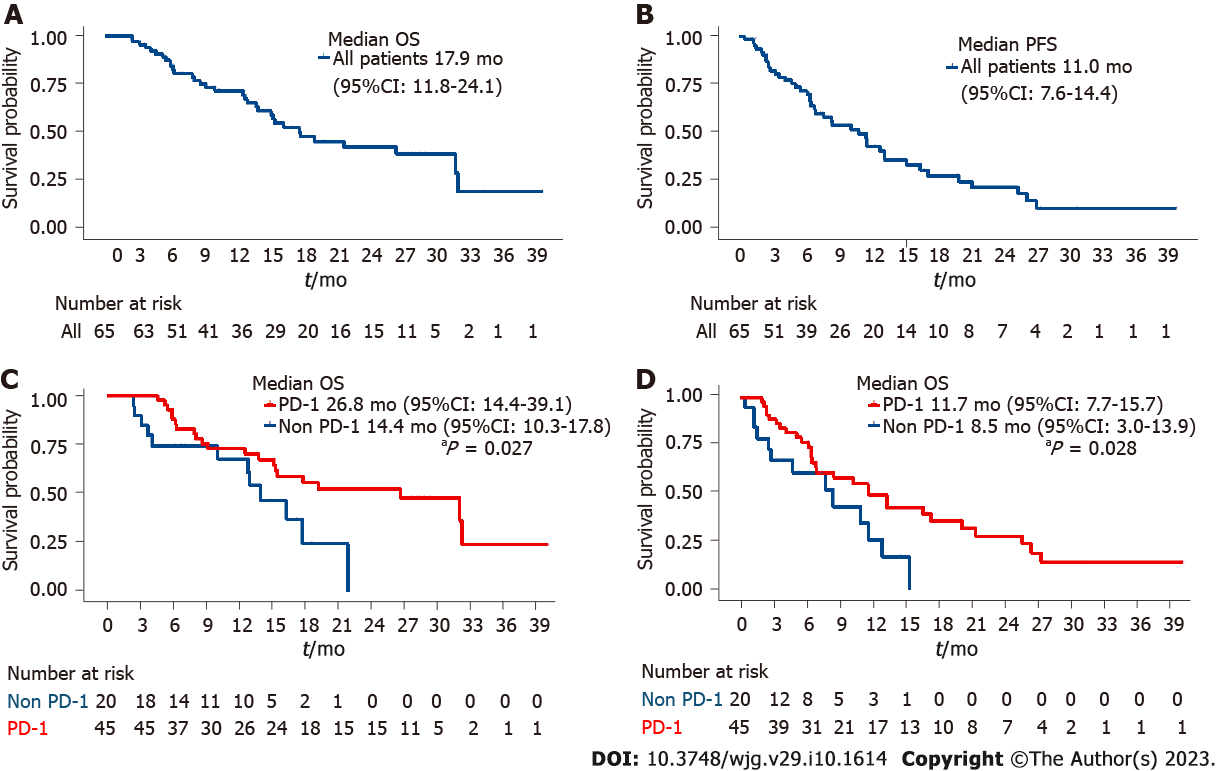
The primary clinical outcomes of this research were OS and PFS. The median follow-up time for all enrolled patients was 25.2 mo [95% confidence interval (CI): 18.8-31.7]. The duration of OS and PFS for all included patients were 17.9 mo (95%CI: 11.8-24.1) and 11.0 mo (95%CI: 7.6-14.4), respectively (Figure 2). The median OS time in the PD-1-Lenv-T group was 26.8 mo (95%CI: 14.4-39.1), while that in the Lenv-T group was 14.0 mo [(95%CI: 10.3-17.8), P = 0.027] (Figures 2A and C). A remarkable median OS improvement of 12.8 mo was observed, suggesting that the PD-1-Lenv-T regimen may have advantage over the Lenv-T regimen. In addition, the median PFS time was different between the two treatment groups [11.7 mo (95%CI: 7.7-15.7) in the PD-1-Lenv-T group vs 8.5 mo (95%CI: 3.0-13.9) in the Lenv-T group (P = 0.028)] (Figures 2B and D).
The best tumor responses of all patients with uHCC are shown in Table 2. The ORR in the PD-1-Lenv-T group was 44.4%, which was obviously higher than the ORR of 20% in the Lenv-T group (P = 0.059) according to the mRECIST criteria (Table 2). The disease control rates (DCRs) were 93.3% in the PD-1-Lenv-T group and 64.0% in the Lenv-T group (P = 0.003). When stratified by BCLC stage, the DCR differed between the two groups (patients with BCLC stage A or B vs patients with BCLC stage C) (Table 3). A total of nine patients changed medications after disease progression, with two patients switching from lenvatinib to apatinib, two receiving donafenib, one receiving regofenib, and three receiving bevacizumab. Another patient stopped TACE and switched to HAIC (hepatic arterial infusion chemotherapy).
| BCLC A or B | BCLC C | |||||
| Lenvatinib + TACE + PD-1 (n = 11) | Lenvatinib + TACE (n = 5) | P value | Lenvatinib + TACE + PD-1 (n = 34) | Lenvatinib + TACE (n = 15) | P value | |
| CR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| PR | 8 (72.7) | 1 (20) | 12 (35.3) | 3 (20.0) | ||
| SD | 3 (27.3) | 2 (40) | 19 (55.9) | 7 (46.7) | ||
| PD | 0 (0) | 2 (40) | 3 (8.8) | 5 (33.3) | ||
| ORR | 8 (72.7) | 1 (20) | 0.0772 | 12 (35.3) | 3 (20) | 0.2352 |
| DCR | 11(100) | 3 (60) | 0.0832 | 31 (91.2) | 10 (66.7) | 0.0472 |
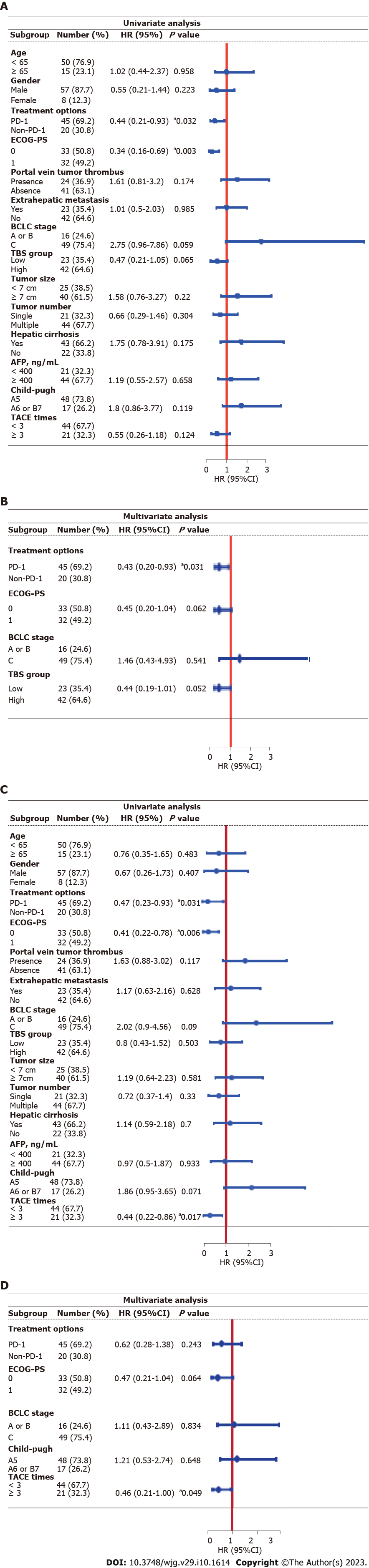
We identified independent prognostic factors related to OS by univariate and multivariate analyses based on the Cox regression model (Figure 3). Basic clinical characteristics (gender, age, etc.), tumor characteristics (tumor size, tumor number, etc.) and treatment status (number of TACE treatments, etc.) were included in the analyses. Univariate analysis suggested that OS was related to the treatment option [P = 0.032, hazard ratio (HR) = 0.44, 95%CI: 0.21-0.93), ECOG-PS score (P = 0.003, HR = 0.34, 95%CI: 0.16-0.69), BCLC stage (P = 0.059, HR = 2.75, 95%CI: 0.96-7.86) and TBS group (P = 0.065, HR = 0.47, 95%CI: 0.21-1.05). We subsequently included factors with a P value < 0.1 in the multivariate analysis and found that only the treatment option was an independent prognostic factor for OS (P = 0.031, HR = 0.43, 95%CI: 0.2-0.93). Similarly, univariate analysis revealed that PFS was related to the treatment option (P = 0.031, HR = 0.47, 95%CI: 0.23-0.93), ECOG-PS score (P = 0.006, HR = 2.42, 95%CI: 1.29-4.55), BCLC stage (P = 0.09, HR = 2.02, 95%CI: 0.9-4.56), Child-Pugh score (P = 0.071, HR = 1.86, 95%CI: 0.95-3.65) and number of TACE treatments (P = 0.017, HR = 0.44, 95%CI: 0.22-0.86). We subsequently included factors with a P value < 0.1 in the multivariate analysis and found that only the number of TACE treatments was an independent prognostic factor for PFS (P = 0.049, HR = 0.46, 95%CI: 0.21-1).
In total, 61 patients (93.8%) experienced AEs of any grade (Table 4). The top five most frequent treatment-related AEs in the PD-1-Lenv-T group were decreased appetite (42.2%), elevated aspartate aminotransferase (AST) (40.0%), decreased albumin (40.0%), hypertension (28.9%) and diarrhea (28.9%). In the Lenv-T group, fatigue (40.0%), decreased appetite (35.0%), and decreased albumin (30.0%) were the most frequent treatment-related AEs. Diarrhea (11.1%), decreased appetite (6.7%), elevated AST (6.7%), fatigue (6.7%), and hypertension (6.7%) were the most frequent grade 3/4 AEs in the PD-1-Lenv-T group. Decreased appetite (10.0%), decreased albumin (5.0%), diarrhea (5.0%), fatigue (5.0%), decreased platelet count (5.0%) and abdominal pain (5.0%) were the most frequent grade 3/4 AEs in the Lenv-T group. A total of three patients, two in the PD-1-Lenv-T group and one in the Lenv-T group, stopped treatment or changed treatment regimens because of intolerable AEs. Overall, the type and frequency of AEs was relatively similar between the two groups.
| AEs | Lenvatinib + TACE + PD-1 | Lenvatinib + TACE | ||
| All grades | Grade 3/4 | All grades | Grade 3/4 | |
| Decreased appetite | 19 (42.2) | 3 (6.7) | 7 (35.0) | 2 (10.0) |
| Elevated AST | 18 (40) | 3 (6.7) | 5 (25.0) | 0 (0) |
| Decreased albumin | 18 (40) | 2 (4.4) | 6 (30.0) | 1 (5.0) |
| Fatigue | 17 (37.8) | 3 (6.7) | 8 (40.0) | 1 (5.0) |
| Diarrhoea | 13 (28.9) | 5 (11.1) | 4 (20.0) | 1 (5.0) |
| Hypertension | 13 (28.9) | 3 (6.7) | 3 (15.0) | 0 (0) |
| Elevated blood bilirubin | 13 (28.9) | 2 (4.4) | 5 (25.0) | 0 (0) |
| Decreased platelet count | 13 (28.9) | 2 (4.4) | 5 (25.0) | 1 (5.0) |
| Hypothyroidism | 12 (26.7) | 2 (4.4) | 3 (15.0) | 0 (0) |
| Abdominal pain | 11 (24.4) | 2 (4.4) | 4 (20.0) | 1 (5.0) |
| Elevated ALT | 11 (24.4) | 2 (4.4) | 4 (20.0) | 0 (0) |
| Rash | 8 (17.8) | 2 (4.4) | 2 (10.0) | 0 (0) |
| Decreased WBC | 7 (15.6) | 0 (0) | 4 (20.0) | 0 (0) |
| Vomiting | 7 (15.6) | 0 (0) | 3 (15.0) | 0 (0) |
| Hypocalcemia | 6 (13.3) | 0 (0) | 5 (25.0) | 0 (0) |
| Palmar-plantar erythrodysaesthesia | 5 (11.1) | 0 (0) | 1 (5.0) | 0 (0) |
| Nausea | 4 (8.9) | 0 (0) | 4 (20.0) | 0 (0) |
| Elevated WBC | 4 (8.9) | 0 (0) | 3 (15.0) | 0 (0) |
| Gingival bleeding | 4 (8.9) | 0 (0) | 1 (5.0) | 0 (0) |
| Gastrointestinal hemorrhage | 4 (8.9) | 1 (2.2) | 0 (0) | 0 (0) |
| Decreased weight | 3 (6.7) | 0 (0) | 2 (10.0) | 0 (0) |
| Dysphonia | 3 (6.7) | 0 (0) | 2 (10.0) | 0 (0) |
| Proteinuria | 1 (2.2) | 1 (2.2) | 1 (5.0) | 0 (0) |
With the progress of new tyrosine kinase inhibitors and immunotherapy, individualized strategies for uHCC have improved. A recent randomized phase III trial, LAUNCH, comparing local therapy plus lenvatinib with lenvatinib monotherapy, demonstrated that TACE plus lenvatinib showed better overall survival in patients with uHCC (median OS: 17.8 vs 11.5 mo; HR = 0.45, P < 0.001)[22]. In our real-world study, patients in the TACE plus lenvatinib group had shorter OS and PFS than those in the LAUNCH trial (median OS: 17.8 vs 14.0 mo; median PFS: 10.6 vs 8.5 mo). A preclinical study showed that lenvatinib can blocke FGFR4 to reduce tumor PD-L1 expression and Treg differentiation, thus improving anti-PD-1 efficacy[18]. In addition to local therapies such as TACE, systemic therapy with PD-1 inhibitors is also being explored as a first-line therapy for uHCC. At the ESMO congress 2022, LEAP-002, a randomized phase III trial, enrolled 794 patients with advanced HCC who were not systematically treated and received lenvatinib plus pembrolizumab or lenvatinib alone in a 1:1 ratio[23]. Although the survival curve was initially higher in the Len + pembro group than in the monotherapy group approximately 15 mo after treatment, the prespecified statistical end point was not reached (median OS: 21.2 mo vs 19.0 mo, HR = 0.840, 95%CI: 0.708-0.997, P = 0.0227). However, in the subgroup analysis, HCC patients with HBV background benefited more in the Len + pembro group (HR = 0.75, 95%CI: 0.58-0.97). In our study, HBV-related HCC patients accounted for 93.33% in the PD-1-lenvatinib-TACE group, indicating that in the real world, Chinese HBV-related HCC patients are more likely to benefit from PD-1 inhibitors. This retrospective study estimated the clinical outcomes and safety of TACE in combination with lenvatinib and PD-1 inhibitors vs lenvatinib plus TACE in the remedy of uHCC. The results suggested that the PD-1-Lenv-T regimen significantly prolonged survival time in patients with uHCC, without unexpected safety-related complications.
After stratification according to BCLC stage, the ORR and DCR were 72.7% and 100%, respectively, in the PD-1-Lenv-T group of uHCC patients with BCLC stage A or B, indicating that combination early treatment with PD-1 inhibitors has a good control effect on lesions. In contrast to proportions in previous studies, 75.4% of patients in this study had BCLC stage C tumors, and 67.7% of patients had multiple tumors, which indicates greater clinical significance for the treatment of patients with advanced HCC. When identifying independent factors associated with OS, similar to previous studies, univariate log-rank analysis indicated that the treatment option (P = 0.032, HR = 0.44, 95%CI: 0.21-0.93) and ECOG-PS score (P = 0.003, HR = 0.34, 95%CI: 0.16-0.69) were associated with OS. This might be accounted for the fact that patients with a good ECOG-PS score have enough physical strength to tolerate treatment. The subsequent multivariate analysis showed that the treatment option was an independent prognostic risk factor for OS. When determining independent prognostic factors associated with PFS, the multivariate analysis suggested that only the number of TACE treatments was an independent prognostic factor for PFS (P = 0.049, 95%CI: 0.21-1); specifically, undergoing TACE ≥ 3 times prolonged the PFS of patients with uHCC. It may be that patients who respond to TACE treatment are more likely to undergo repeated TACE treatments.
According to our data, the PD-1-Lenv-T regimen performed well in terms of safety, and grade 3 or 4 AEs were rare. AEs of any grade occurred more frequently in the PD-1-Lenv-T group than in the pembrolizumab monotherapy group in the KEYNOTE-240 clinical trial, but there was no obvious difference in grade 3/4 AEs. The timely monitoring of and intervention for AEs by the treatment team also played a key role in the remedy of all patients. From the information collected, more symptomatic AEs, such as decreased appetite and fatigue, occurred with this treatment regimen. This study also exists several limitations. For example, this research was a retrospective study based on a single medical center with a limited number of patients.
In conclusion, the study results suggest that early combination treatment with PD-1 inhibitors has manageable toxicity and promising efficacy in patients with uHCC.
Programmed death receptor-1 (PD-1) inhibitors have been approved as second-line treatment regimen in hepatocellular carcinoma (HCC), and it is still worth studying whether patients can benefit from PD-1 inhibitors as first-line drugs combined with targeted drugs and local therapy.
To provide more options and references for the therapy of patients with unresectable HCC (uHCC).
Aim to evaluate the clinical outcomes and safety of transarterial chemoembolization (TACE) and lenvatinib plus PD-1 inhibitors for patients with uHCC.
We carried out a retrospective investigation of 65 patients with uHCC who were treated at Peking Union Medical College Hospital from September 2017 to February 2022.
Patients with uHCC who received PD-1 inhibitors, lenvatinib, TACE therapy (n = 45) had a clearly longer overall survival than those who underwent lenvatinib, TACE therapy (n = 20, 26.8 vs 14.0 mo; P = 0.027). The type and frequency of adverse events showed little difference between the two treatment groups.
Our results suggest that the early combination of PD-1 inhibitors has manageable toxicity and promising efficacy in patients with uHCC.
Patients with uHCC may obtain benefit a lot from the early combination of PD-1 inhibitors with TACE and lenvatinib.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68647] [Article Influence: 13729.4] [Reference Citation Analysis (201)] |
| 2. | Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007. Int J Cancer. 2016;139:1534-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 3. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4112] [Article Influence: 514.0] [Reference Citation Analysis (5)] |
| 4. | Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969-977. [PubMed] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6421] [Article Influence: 802.6] [Reference Citation Analysis (9)] |
| 6. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4366] [Article Influence: 545.8] [Reference Citation Analysis (6)] |
| 7. | Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 8. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 413] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 9. | Shim JH, Park JW, Kim JH, An M, Kong SY, Nam BH, Choi JI, Kim HB, Lee WJ, Kim CM. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 555] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 11. | Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A, Hacking N, Evans TRJ, Collins P, Hubner RA, Cunningham D, Primrose JN, Johnson PJ, Palmer DH. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 387] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 12. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 543] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 13. | Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, Liu L, Zhang X, Zhai J, Qu Z. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 14. | Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, Liu X, Zheng L, Chen J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer. 2021;127:3782-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 15. | Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 952] [Article Influence: 190.4] [Reference Citation Analysis (0)] |
| 16. | Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 17. | Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019;76:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 786] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 18. | Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, Lin J, Zhang J, Zhu W, Jia H, Qin L, Lu L, Chen J. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology. 2021;74:2544-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 19. | Deng H, Kan A, Lyu N, Mu L, Han Y, Liu L, Zhang Y, Duan Y, Liao S, Li S, Xie Q, Gao T, Li Y, Zhang Z, Zhao M. Dual Vascular Endothelial Growth Factor Receptor and Fibroblast Growth Factor Receptor Inhibition Elicits Antitumor Immunity and Enhances Programmed Cell Death-1 Checkpoint Blockade in Hepatocellular Carcinoma. Liver Cancer. 2020;9:338-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 20. | Lu M, Zhang X, Gao X, Sun S, Wei X, Hu X, Huang C, Xu H, Wang B, Zhang W, Li Z, Feng X, Zheng J, Zhang Q. Lenvatinib enhances T cell immunity and the efficacy of adoptive chimeric antigen receptor-modified T cells by decreasing myeloid-derived suppressor cells in cancer. Pharmacol Res. 2021;174:105829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1398] [Article Influence: 233.0] [Reference Citation Analysis (1)] |
| 22. | Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 308] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 23. | Finn R, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo B, Ren Z, Cheng A, Galle P, Kaneko S, Kumada H, Wang A, Mody K, Dubrovsky L, Siegel A, Liovet J. LEAP-002 - Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S808-S869. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Albillos A, Spain; McDowell HR, United Kingdom; Noverati N, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ