Published online Jan 7, 2023. doi: 10.3748/wjg.v29.i1.61
Peer-review started: September 26, 2022
First decision: October 18, 2022
Revised: October 22, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 7, 2023
Processing time: 100 Days and 5.5 Hours
Cirrhosis is a leading cause of morbidity and mortality, impacting more than 120 million people worldwide. Although geographic differences exist, etiologic factors such as alcohol use disorder, chronic viral hepatitis infections, and non-alcoholic fatty liver disease are prevalent in nearly every region. Historically, significant effort has been devoted to modifying these risks to prevent disease progression. Nevertheless, more than 11% of patients with compensated cirrhosis experience hepatic decompensation each year. This transition signifies the most important prognostic factor in the natural history of the disease, corresponding to a decline in median survival to below 2 years. Over the past decade, the need for pharmacotherapies aimed at reducing the risk for hepatic decompensation has been emphasized, and non-selective beta-blockers have emerged as the most effective option to date. However, a critical therapeutic gap still exists, and additional therapies have been proposed, including statins, rifaximin, and sodium-glucose cotransporter-2 inhibitors. Based on the results of innovative retrospective analyses and small-scale prospective trials, these pharmacotherapies represent promising options, but further studies, including randomized controlled trials, are necessary before they can be incorporated into clinical use. This report highlights the potential impact of these agents and others in preventing hepatic decompensation and discusses how this paradigm shift may pave the way for guideline-directed medical therapy in cirrhosis.
Core Tip: Hepatic decompensation is the most important clinical predictor of morbidity and mortality among patients with cirrhosis. New pharmacotherapies aimed at preventing hepatic decompensation in high-risk patients are emerging, augmenting traditional management strategies. These treatments represent safe, accessible, and effective options that may improve quality of life and prolong transplant-free survival, regardless of the etiologic factors involved.
- Citation: Lee S, Saffo S. Evolution of care in cirrhosis: Preventing hepatic decompensation through pharmacotherapy. World J Gastroenterol 2023; 29(1): 61-74
- URL: https://www.wjgnet.com/1007-9327/full/v29/i1/61.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i1.61
Cirrhosis reflects the end stage of all chronic liver diseases. It impacts more than 120 million people globally, largely in the context of common risk factors such as alcohol use disorder, chronic viral hepatitis, and non-alcoholic fatty liver disease (NAFLD)[1]. It represents the 8th leading cause of mortality in the United States and 13th in the world, with the number of attributable deaths worldwide having increased by approximately 50% between 1990 and 2013[2]. Regardless of etiology, the most important prognostic factor for survival is the presence or absence of hepatic decompensation, which includes complications such as ascites, variceal hemorrhage, hepatic encephalopathy, and jaundice[3,4]. Although patients with cirrhosis may remain compensated for extended periods of time, especially if their underlying risk factors are mitigated, approximately 11% of those with compensated disease experience new decompensations each year[5], and the progression from compensated to decompensated cirrhosis is associated with a decline in median survival from 12 years to less than 2 years[6]. Among individuals with compensated disease, the 10-year risks of developing ascites, gastrointestinal hemorrhage, hepatic encephalopathy, and jaundice are 40%, 25%, 28%, and 33%, respectively[6].
Beyond its burden on patient health and quality of life, cirrhosis also represents a critical healthcare challenge. According to the Global Burden of Disease Study 2017[1], there are 112 million and 10.6 million cases of compensated and decompensated cirrhosis worldwide, respectively. In the United States, this is associated with significant healthcare costs, including approximately $2.5 billion annually for hospitalization and treatment and $10.6 billion annually for losses in work productivity and health-related quality of life[7]. These costs are disproportionately borne in the management of complications among those with decompensated disease. Despite the significant health and socioeconomic burdens, it is only recently that management strategies have pivoted from focusing primarily on risk factor modification and the treatment of complications towards the prevention of hepatic decompensation in high-risk patients. Unlike the management of other common chronic diseases such as congestive heart failure, in which the implementation of guideline-directed medical therapy has resulted in significant reductions in morbidity and mortality, the long-term management of cirrhosis has been historically limited by a lack of robust chemoprevention[8,9]. In this report, we discuss the management of cirrhosis, focusing on pharmacotherapies aimed at preventing hepatic decompensation.
Chronic hepatitis C virus (HCV) infection, NAFLD, and alcohol-related liver disease are the three leading causes of cirrhosis in the United States[10]. For each of these etiologies, a critical component of the long-term management involves risk factor modification. Among patients with chronic HCV infection and compensated disease, the advent of direct-acting antiviral agents represents a landmark achievement that has been shown to reduce the risk for liver-related complications[11-14]. Unfortunately, more than 40% of HCV infections are diagnosed after hepatic decompensation has already occurred[10], at which point antiviral treatment is less effective and associated with a higher risk for adverse events[15]. For those with NAFLD, a growing body of literature supports the efficacy of a multimodal approach which integrates dietary changes, weight reduction, and restoring insulin sensitivity[16]. Glucagon-like peptide 1 receptor agonists such as semaglutide have an evolving role in the treatment in of NAFLD, offering both weight reduction and possible attenuation of steatohepatitis, and a number of other drugs aimed at reducing fibrosis progression and the risk for complications are currently being evaluated in phase II and phase III clinical trials[17]. However, to date, disease-specific pharmacologic treatments have yet to be approved. The prevalence of NAFLD and its associated complications continue to increase[18] such that it is currently the second leading cause of cirrhosis among patients awaiting liver transplantation in the United States[14] and is expected to overtake HCV-related cirrhosis as the most frequent indication for liver transplantation[19]. Finally, alcohol-related liver disease remains highly prevalent worldwide[20] with increasing cirrhosis-related mortality in regions with high alcohol consumption[21]. Abstaining from alcohol significantly improves survival among patients with cirrhosis, but pharmacologic options for alcohol use disorder remain limited, partially due to concerns about hepatotoxicity[22]. For patients with less common causes of chronic liver disease, risk factor modification may include therapies such as immunosuppression (autoimmune hepatitis), ursodeoxycholic acid (primary biliary cholangitis), and phlebotomy or chelation (hemochromatosis). Unfortunately, the impact of some of these treatments is generally diminished in the context of cirrhosis.
Once hepatic decompensation has occurred, treatment strategies may be implemented to address specific complications. This includes pharmacotherapy to eliminate ascites (diuretics), reduce the risk of variceal rupture [non-selective beta-blockers (NSBBs)], and prevent recurrent encephalopathy (lactulose and rifaximin)[23-25]. Among patients with refractory ascites or variceal hemorrhage, transjugular intrahepatic portosystemic shunting (TIPS) reduces the risk of further decompensation and may improve survival in a subset of patients[26-28]. Finally, liver transplantation is the only durable curative option for patients with decompensated cirrhosis. However, resource limitations and host factors restrict the use of TIPS and liver transplantation to a relatively small number of patients in resource-rich settings. Furthermore, despite advances in the care of decompensated cirrhosis, the 30-d mortality following hospital discharges for hepatic decompensation remains largely unchanged[29]. Thus, significant interest exists in preventing the progression to decompensated cirrhosis.
NSBBs are currently the only agents recommended for the long-term management of portal hypertension in patients with compensated cirrhosis[30]. Traditional NSBBs such as propranolol and nadolol inhibit β1 and β2 receptors, mitigating the hyperdynamic response and splanchnic arteriolar vasodilation that occur in the context of cirrhosis. Carvedilol has additional α1 inhibition that reduces intrahepatic vascular resistance[31,32]. Together, these hemodynamic effects attenuate portal hypertension, reducing the risk for hepatic decompensation. In addition, NSBBs impact the risk for liver-related complications through other mechanisms. For example, NSBBs reduce abnormal gastrointestinal permeability and bacterial translocation[33] and upregulate the phagocytic activity of monocytes and granulocytes after exposure to bacterial DNA[34]. As such, a recent prospective study demonstrated that NSBBs reduce the risk for bacterial infections [odds ratio = 0.46, 95% confidence interval (CI): 0.3-0.7; P = 0.001][35].
The landmark trial demonstrating the utility of NSBBs in preventing decompensation was the study on Beta-Blockers to Prevent Decompensation of Cirrhosis with Portal Hypertension (PREDESCI)[36]. In this multicenter, double-blind, randomized controlled trial (RCT), 201 patients with compensated cirrhosis and clinically-significant portal hypertension (CSPH) without high-risk varices were randomized to receive NSBBs (propranolol up to 160 mg twice daily or carvedilol up to 25 mg daily) or placebo. Over a median follow-up of 37 mo, the risk of hepatic decompensation, including ascites, variceal hemorrhage, or hepatic encephalopathy, was significantly lower among patients receiving NSBBs relative to those receiving placebo [hazard ratio (HR) = 0.51, 95%CI: 0.26-0.97; P = 0.041]. This difference was driven primarily by a reduction in ascites (HR = 0.42, 95%CI: 0.19-0.92; P = 0.03), although non-significant trends towards decreased progression to high-risk varices and improved survival were also observed. The number needed to treat for the prevention of a decompensation event over the follow-up period was 9, and the incidence of adverse events was similar between the treatment and placebo groups.
Prior to PREDESCI, other RCTs consistently demonstrated that propranolol and nadolol were effective in preventing variceal hemorrhage among patients with cirrhosis and large esophageal varies but without prior bleeding episodes. Pascal and Cales[37] found that patients receiving propranolol up to 320 mg daily were less likely to develop bleeding episodes compared to those in the placebo group (74% vs 39%; P < 0.05); Idéo et al[38] also observed significantly lower rates among those receiving nadolol up to 120 mg daily (94.4%) relative to those receiving placebo (70.2%). Other studies have reported similar risk reductions in initial[39,40] and subsequent[41] variceal bleeds, although one reported a significant difference only among patients who lacked ascites[42]. Given these data, NSBBs are currently recommended for the primary and secondary prophylaxis of variceal hemorrhage[37,41]. Notably, however, the Prevention of Esophageal Varices by Beta-Adrenergic Blockers trial observed no differences in the use of timolol up to 80 mg daily vs placebo in the development of varices among patients with compensated cirrhosis[43].
The potential benefits of carvedilol beyond those of traditional NSBBs have also been of great interest. Four studies have evaluated the impact of carvedilol in preventing hepatic decompensation or disease progression among patients with compensated cirrhosis and CSPH. A subgroup analysis of the PREDESCI trial[36] found a non-significant reduction in the risk for hepatic decompensation or death (HR = 0.39, 95%CI: 0.10-1.49; P = 0.16) and Bhardwaj et al[44] observed a significantly higher likelihood of non-progression from small to large esophageal varices (79.4% vs 61.4%; P = 0.04). In comparing carvedilol to variceal band ligation, one study found carvedilol 12.5 mg daily to be associated with significantly lower rates of initial variceal hemorrhage (HR = 0.41, 95%CI: 0.19-0.96; P = 0.04) but similar rates of bleeding-related and overall mortality[45], while another reported comparable rates across all three outcomes with the same dosing[46]. Additionally, a recent meta-analysis of these four studies observed a significantly improved hazard ratio for decompensation among patients receiving carvedilol compared to control therapy (HR = 0.506, 95%CI: 0.289-0.887; P = 0.017)[47]. Finally, in comparison to propranolol, multiple studies, including PREDESCI, have suggested that carvedilol may be superior in reducing the hepatic venous pressure gradient[36,48]. However, it remains unclear whether this finding consistently translates into a difference in the risk for hepatic decompensation.
Although NSBBs have been extensively studied and represent the current treatment of choice for the prevention of hepatic decompensation, there is significant interest in identifying additional therapies for chemoprevention for a variety of reasons. First, most commonly, patients may have contraindications or intolerance to NSBBs. Common adverse effects include bradycardia, hypotension, and fatigue. Second, pharmacotherapy may be leveraged for pleiotropic benefits, and alternative therapies may provide an opportunity for enhanced personalized care. Finally, therapies may have additive or synergistic effects that may provide additional clinical benefit for high-risk patients. As such, a number of agents are currently under evaluation (Table 1).
| Agents | Mechanism of action | Primary benefits | Potential adverse effects | Other limitations | Supported by RCT |
| NSBBs | β1 and β2 blockade; α1 blockade (carvedilol) | Decreased portal pressure | Hypotension, bradycardia, fatigue | Dosing frequency (propranolol) | Yes |
| Statins | Inhibition of HMG-CoA reductase | Decreased inflammation and endothelial dysfunction | Myopathy, hepatitis, diabetes | Ongoing | |
| Rifaximin | Broad-spectrum, gut-specific antibiotic | Reduced dysbiosis and bacterial translocation | Gastrointestinal upset | Cost | Included patients with prior decompensation |
| Anticoagulants | Inactivation of clotting factors | Reduced endothelial dysfunction | Hemorrhage | SQ injection (enoxaparin), dosing (warfarin) | Included patients with prior decompensation |
| ACE inhibitors | Inhibition of angiotensin II production | Decreased portal pressure1 | Hypotension, AKI, electrolyte derangements, angioedema | No | |
| ARBs | Inhibition of angiotensin II type 1 receptor | Decreased portal pressure | Hypotension, AKI, electrolyte derangements | No | |
| MRAs | Inhibition of the aldosterone receptor in the distal nephron | Decreased portal pressure | Hypotension, AKI, electrolyte derangements | Gynecomastia (spironolactone) | Only in combination with NSBB |
| SGLT2 inhibitors | Inhibition of proximal tubule sodium-glucose cotransporter | Decreased portal pressure | Electrolyte derangements, mycotic genital infections | Cost, risk of ketoacidosis in AUD | No |
| Albumin | Anionic carrier protein with pleiotropic properties | Reduced inflammation; increased effective circulating volume | Volume overload | Cost, intravenous administration | Included patients with prior decompensation |
These cholesterol-lowering drugs are a mainstay for the treatment of dyslipidemia and atherosclerotic cardiovascular disease but have diverse pleiotropic effects that may impact a wide range of other disease processes. Concerns regarding hepatotoxicity have historically limited their use among patients with chronic liver disease[49], but emerging evidence indicates that their anti-fibrotic, immunomodulatory, and antioxidant effects may attenuate portal hypertension and limit disease progression[50-53] without posing an excess safety risk among patients with compensated disease[54-56].
Several retrospective studies have evaluated the role of statins in preventing hepatic decompensation. In one case-control study of patients with predominantly early-stage cirrhosis, statin use was associated with a decreased risk of hepatic decompensation over 36 mo (HR = 0.58; P = 0.04)[57]. Similar findings have been reported in patients with cirrhosis due to chronic viral hepatitis; among statin users, the HR for hepatic decompensation was 0.39 (95%CI: 0.25-0.62)[58] for hepatitis B virus-related cirrhosis and 0.51 (95%CI: 0.29-0.93)[58] to 0.55 (95%CI: 0.39-0.77)[59] for HCV-related cirrhosis. A trend towards decreased hepatic decompensation was observed with among patients with alcohol-related cirrhosis (HR = 0.69, 95%CI: 0.45-1.07)[58]. A recent meta-analysis of these three observational studies found the pooled HR for hepatic decompensation to be 0.54 (95%CI: 0.46-0.65) with minimal heterogeneity (I2 = 0%)[60]. A similar meta-analysis using these studies also demonstrated that the association between statin use and an improved decompensation was independent of cirrhosis etiology[61].
In light of the encouraging findings reported in observational studies, RCTs evaluating the impact of statins in preventing hepatic decompensation are currently in progress. In the United States, the Statins and Cirrhosis: Reducing Events of Decompensation trial is studying simvastatin at a dose of 40 mg daily (ClinicalTrials.gov, NCT03654053)[62]. In Denmark, the Statins for Prevention of Disease Progression and Hospitalization in Liver Cirrhosis trial is studying atorvastatin at doses of 10-20 mg daily (ClinicalTrials.gov, NCT04072601).
Patients with cirrhosis can experience increased bacterial translocation secondary to elevated portal pressures, increased gastrointestinal permeability, and altered gut microbiota, thereby contributing to the inflammatory milieu. Rifaximin is a safe poorly-absorbed oral antibiotic with broad gut-selective antimicrobial activity against gram-positive and gram-negative bacteria. Beyond its impacts on the intestinal microbiome, it may also attenuate inflammation, decrease bacterial interactions with enterocytes, and improve intestinal epithelial integrity[63,64]. Furthermore, the combination of propranolol and rifaximin compared to propranolol alone is associated with a more significant reduction in portal pressure[65]. Although rifaximin is currently approved for the prevention of recurrent hepatic encephalopathy[66], it may also have a role in preventing other hepatic decompensations.
A number of studies have evaluated the association between rifaximin use and liver-related complications, demonstrating that rifaximin may reduce the risk of further decompensation in patients with decompensated cirrhosis[63]. However, little is known about its role in those with high-risk compensated disease. Most notably, in a post-hoc analysis of a RCT comparing rifaximin 550 mg twice daily to placebo, Flamm et al[67] demonstrated that rifaximin reduces the risk for further hepatic decompensation (HR = 0.41, 95%CI: 0.25-0.67; P < 0.001). This finding was corroborated by Zeng et al[68] in a prospective randomized open-labelled study. Currently, there are no ongoing trials investigating the impact of rifaximin in patients with compensated disease, although the Simvastatin Plus Rifaximin in Decompensated Cirrhosis study is examining the role of statins and rifaximin among patients with pre-existing decompensated disease (ClinicalTrials.gov, NCT03780673). Historically, a critical barrier to the study and use of rifaximin has been cost.
In light of the impaired synthesis of clotting factors and the presence of thrombocytopenia associated with cirrhosis, the risk of bleeding has historically been prioritized over thrombosis[69]. However, more recent evidence suggests that a new state of rebalanced hemostasis is achieved among those with stable cirrhosis in which decompensation can lead to increased risks of both hemorrhage and thrombosis, which in turn, can increase the risk for further decompensation[69]. Thrombin has been proposed to activate hepatic stellate cells and lead to upregulation of hepatic fibrosis, suggesting a potential role for anticoagulation in slowing cirrhosis disease progression[70].
Several studies have evaluated the efficacy and safety of anticoagulation in preventing or managing venous thrombotic events in patients with cirrhosis, but only two have specifically addressed its association with preventing decompensation[71-75]. In a RCT of patients with advanced cirrhosis randomized to receive enoxaparin 4000 IU/d or no treatment, decompensation was significantly less common among those receiving enoxaparin (11.7% vs 59.4%; P < 0.0001)[75]. Enoxaparin was independently associated with a reduced risk for hepatic decompensation (HR = 0.331, 95%CI: 0.148-0.741; P = 0.007). Notably, following cessation of enoxaparin receipt, rates of hepatic decompensation were similar between those in the control and treatment groups. In contrast, in a retrospective study of patients with cirrhosis and portal vein thrombosis, the 1-year probability of hepatic decompensation was not significantly different between patients who did or did not receive warfarin (15.6% vs 17.9%; P = 0.847)[72]. The ongoing CIRROXABAN phase III RCT (ClinicalTrials.gov, NCT02643212) aims to evaluate the effect of rivaroxaban in the development of decompensation among patients with cirrhosis.
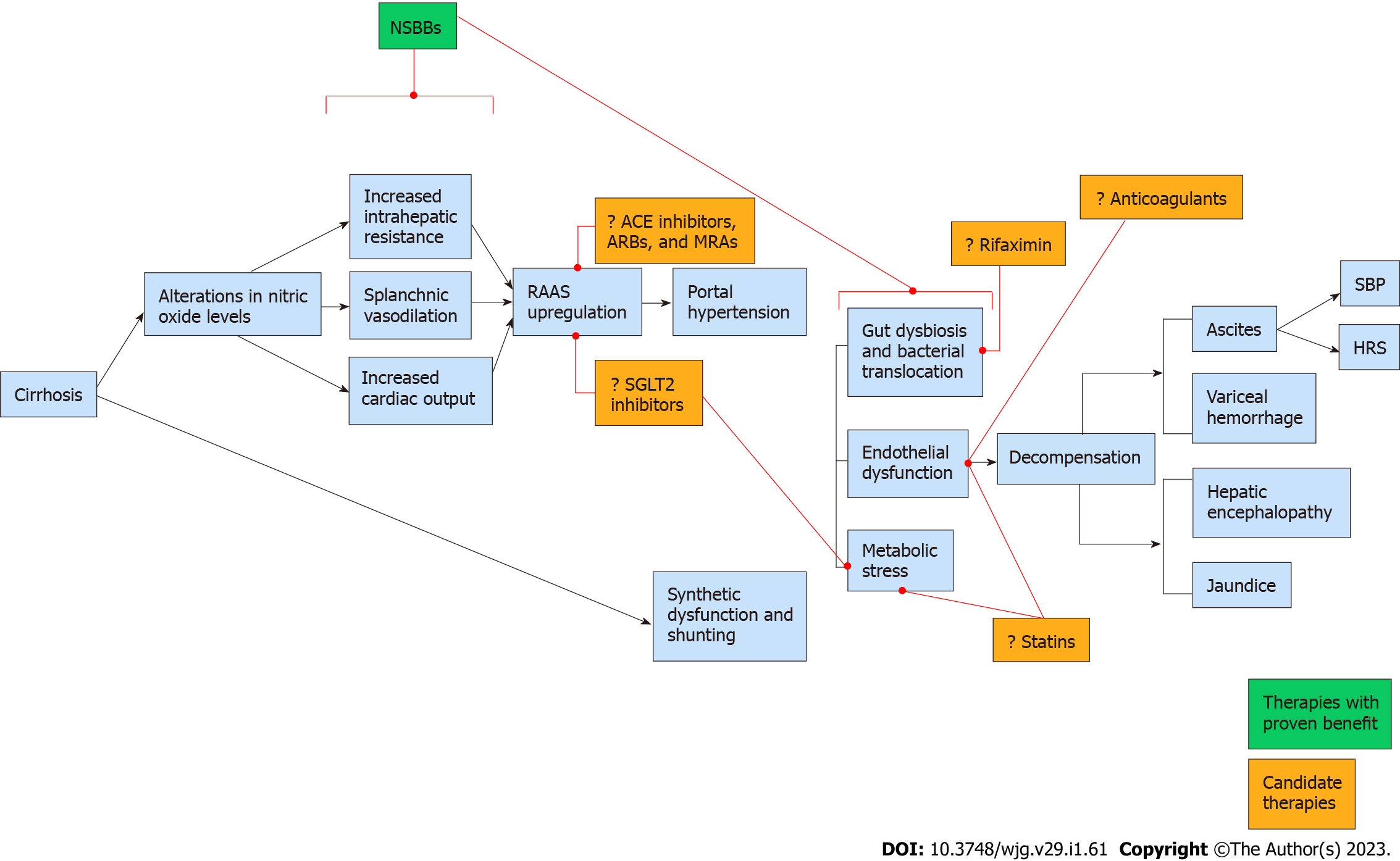
The renin-angiotensin-aldosterone system (RAAS) has a central role in the pathogenesis and progression of portal hypertension. Splanchnic and peripheral vasodilation in response to excess nitric oxide stimulate the renin-angiotensin-aldosterone and sympathetic nervous systems, triggering a number of mechanisms that further exacerbate portal hypertension. Mediators of these pathways have been directly linked to mortality in patients with cirrhosis[76,77]. Thus, a number of drugs which attenuate the RAAS have been evaluated with mixed results. These include angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists such as spironolactone, and sodium-glucose cotransporter-2 (SGLT2) inhibitors.
Both ACE inhibitors and ARBs have been studied in portal hypertension, but their impact in preventing hepatic decompensation remains unclear. Current evidence suggests that ARBs may reduce portal pressures[78-84], but they have yet to be evaluated in RCTs. In contrast, ACE inhibitors have not been shown to reduce portal pressures[85], but a recent large retrospective analysis suggest that they may reduce the risk for liver-related complications in patients with NAFLD[86]. Regardless, while both agents may be safe and potentially beneficial in patients with early-stage disease, their side-effect profile may be deleterious among patients with CSPH.
Spironolactone is critical in the management of cirrhotic ascites. However, the drug has been investigated as an adjunctive agent to NSBBs in the prevention of variceal hemorrhage. A prospective trial demonstrated that the addition of spironolactone 100 mg daily to nadolol reduced the risk of a combined endpoint of variceal hemorrhage and ascites (39% vs 20%; P < 0.04)[87]. Further trials exploring the utility of spironolactone in preventing hepatic decompensation among compensated patients have not been pursued, and thus the agent is currently not recommended for chemoprevention.
Initially introduced as antidiabetic drugs, SGLT2 inhibitors have become mainstays in the management of cardiovascular disease and chronic kidney disease among diabetic and nondiabetic patients due to their broad pleiotropic effects[88-93]. By inhibiting sodium and glucose reabsorption in the proximal convoluted tubule, SGLT2 inhibitors restore tubuloglomerular feedback, attenuate overactivation of the RAAS and the sympathetic nervous system, and promote natriuresis, thereby overcoming key mechanisms that are also implicated in the pathogenesis of portal hypertension in cirrhosis[9]. Unlike ACE inhibitors, ARBs, and spironolactone, SGLT2 inhibitors have limited effects on systemic blood pressure and may be better tolerated among patients with CSPH. Although RCTs in cirrhosis are currently lacking, retrospective studies suggest that these agents, namely empagliflozin, are likely safe and warrant further investigation[94-98].
Albumin has versatile anti-inflammatory and plasma expansion properties that may also mitigate mechanisms implicated in hepatic decompensation. To our knowledge, no study has investigated the role of albumin in preventing hepatic decompensation among patients with compensated disease. However, the Human Albumin for the Treatment of Ascites in Patients with Hepatic Cirrhosis study assessed the impact of human albumin in patients with uncomplicated ascites[99]. Although the primary outcome was mortality, secondary analyses demonstrated that weekly 40-gram albumin infusions reduced the risk for refractory ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, and hepatic encephalopathy in comparison to standard medical therapy alone. Notably, the study was limited by the fact that the treatment group received more frequent outpatient evaluations, allowing providers to address potential impending complications in a more proactive manner. Because of accessibility and cost limitations, in addition to other factors that restrict widespread use, it is unlikely that albumin will receive significant consideration as a potential therapeutic option in patients with compensated cirrhosis.
Numerous other agents hypothesized to decrease the risk of hepatic decompensation have been evaluated. Despite early studies suggesting indirect benefits, there is a paucity of consistent, rigorous clinical evidence demonstrating a direct role for nitrates[100-103], endothelin-A antagonists[104,105], farnesoid X receptor agonists[106], phosphodiesterease-5 inhibitors[107-111], serelaxin[112], sorafenib[113-115], taurine[116], and thalidomide[117] in the prevention of hepatic decompensation. Further
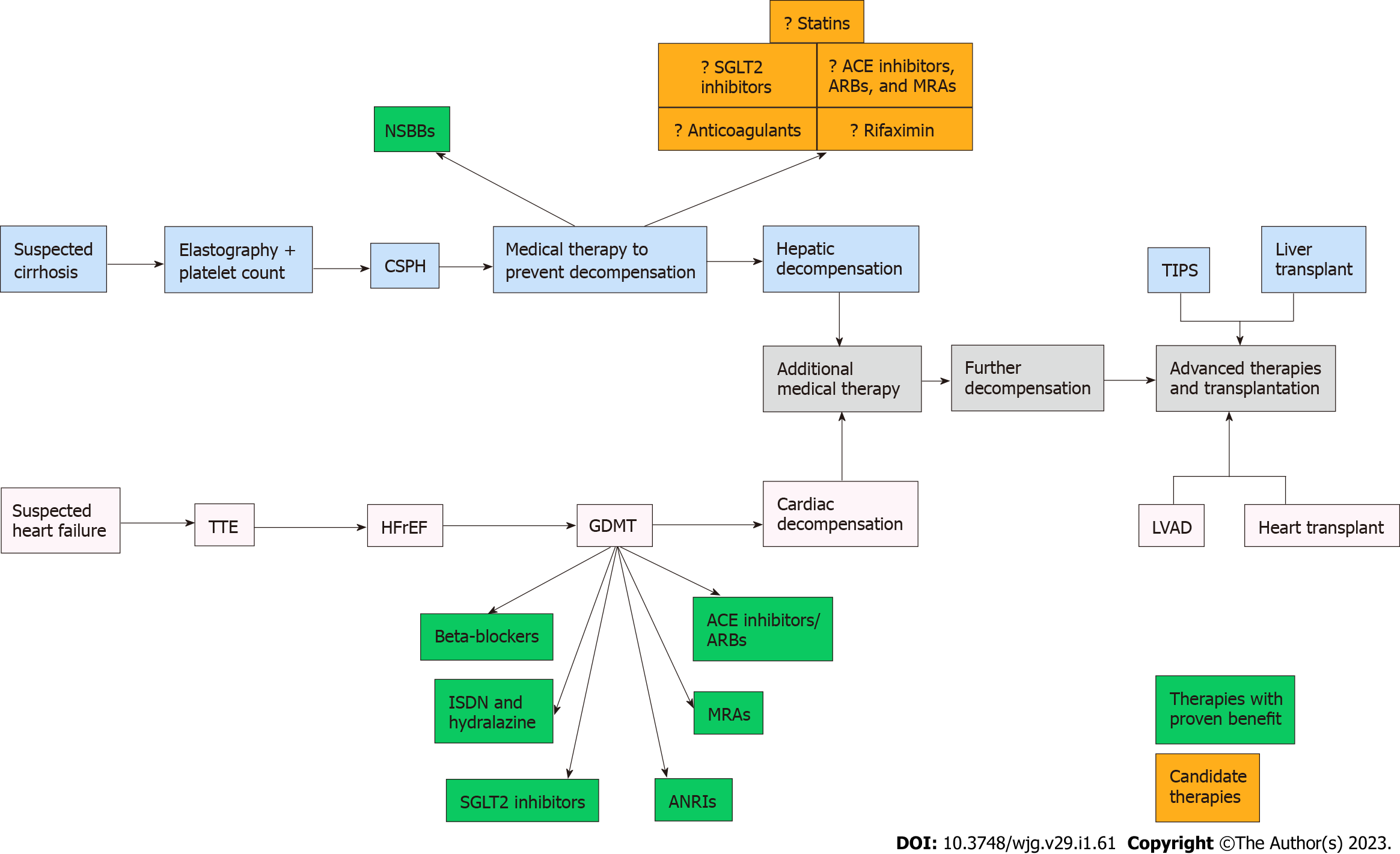
Until PREDESCI validated the use of NSBBs for chemoprevention, the pharmacologic management of cirrhosis had experienced little progress over the preceding half century. In contrast, over the same period of time, incremental advances in the medical management of systolic heart failure, a condition that mechanistically mimics portal hypertension, led to the widespread implementation of guideline-directed medical therapy, which significantly improved survival (Figure 1). In the past, the lack of non-invasive tools to identify high-risk patients hindered the development and application of chemoprevention in cirrhosis. However, with the advent of elastography and the validation of clinical prediction tools that incorporate laboratory markers such as platelet count, clinicians are now able to risk-stratify patients more efficiently, paving the way for more robust medical management. Based on the findings of the ANTICIPATE study and the guidelines proposed in the Baveno VII workshop[121,122], patients who have a high likelihood for CSPH based on a combination of liver stiffness measurements and platelet count should be initiated on appropriate chemoprevention with a NSBB in the absence of contraindications to therapy. As additional agents for chemoprevention are evaluated over the coming years, a diverse multi-targeted strategy (Figure 2) may become feasible, mimicking the approach currently utilized for congestive heart failure. Although there are a number of candidate drugs, statins, rifaximin, and SGLT2 inhibitors currently offer the most promise, combining potential efficacy with other important considerations such as safety, accessibility, and systemic benefits.
The development of hepatic decompensation represents the most important prognostic factor in the natural history of cirrhosis. Treatments that mitigate this risk have an important role in the management of patients with CSPH. In light of the development of robust non-invasive tools that allow for the timely and accurate risk stratification of patients with cirrhosis, the application of chemoprevention is becoming increasingly feasible. NSBBs are currently the mainstays of treatment in this regard, but emerging therapies such as statins, rifaximin, and SGLT2 inhibitors, may offer hope for personalized multimodal strategies in the future. This paradigm shift may ultimately reduce liver-related morbidity and mortality, improve quality of life, and limit the socioeconomic burden of cirrhosis regardless of the etiologic factors involved.
| 1. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1184] [Cited by in RCA: 1115] [Article Influence: 185.8] [Reference Citation Analysis (5)] |
| 2. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5495] [Cited by in RCA: 5353] [Article Influence: 486.6] [Reference Citation Analysis (0)] |
| 3. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G, Politi F, Luca A, Virdone R, Licata A, Pagliaro L. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 393] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 4. | Ripoll C, Bari K, Garcia-Tsao G. Serum Albumin Can Identify Patients With Compensated Cirrhosis With a Good Prognosis. J Clin Gastroenterol. 2015;49:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 719] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 7. | Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol (N Y). 2011;7:661-671. [PubMed] |
| 8. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1621] [Article Influence: 405.3] [Reference Citation Analysis (0)] |
| 9. | Saffo S, Taddei T. SGLT2 inhibitors and cirrhosis: A unique perspective on the comanagement of diabetes mellitus and ascites. Clin Liver Dis (Hoboken). 2018;11:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Berzigotti A. Advances and challenges in cirrhosis and portal hypertension. BMC Med. 2017;15:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, Marcellin P, Manns M, Nikitin I, Poordad F, Sherman M, Zeuzem S, Scott J, Gilles L, Lenz O, Peeters M, Sekar V, De Smedt G, Beumont-Mauviel M. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, Jacobson IM, Kowdley KV, Nyberg L, Subramanian GM, Hyland RH, Arterburn S, Jiang D, McNally J, Brainard D, Symonds WT, McHutchison JG, Sheikh AM, Younossi Z, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1330] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 13. | Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, Janczewska E, Villamil F, Scott J, Peeters M, Lenz O, Ouwerkerk-Mahadevan S, De La Rosa G, Kalmeijer R, Sinha R, Beumont-Mauviel M. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (1)] |
| 14. | Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090-1099.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 480] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 15. | Fernández Carrillo C, Lens S, Llop E, Pascasio JM, Crespo J, Arenas J, Fernández I, Baliellas C, Carrión JA, de la Mata M, Buti M, Castells L, Albillos A, Romero M, Turnes J, Pons C, Moreno-Planas JM, Moreno-Palomares JJ, Fernández-Rodriguez C, García-Samaniego J, Prieto M, Fernández Bermejo M, Salmerón J, Badia E, Salcedo M, Herrero JI, Granados R, Blé M, Mariño Z, Calleja JL. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: Analysis of data from the Hepa-C registry. Hepatology. 2017;65:1810-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and Its Management. Nutr Clin Pract. 2020;35:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 17. | Tapper EB, Ufere NN, Huang DQ, Loomba R. Review article: current and emerging therapies for the management of cirrhosis and its complications. Aliment Pharmacol Ther. 2022;55:1099-1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1832] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 19. | Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 20. | Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 550] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 21. | Leon DA, McCambridge J. Liver cirrhosis mortality rates in Britain, 1950 to 2002. Lancet. 2006;367:645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Veldt BJ, Lainé F, Guillygomarc'h A, Lauvin L, Boudjema K, Messner M, Brissot P, Deugnier Y, Moirand R. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 545] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 24. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1499] [Article Influence: 166.6] [Reference Citation Analysis (3)] |
| 25. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1490] [Article Influence: 124.2] [Reference Citation Analysis (1)] |
| 26. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 864] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 27. | Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, Bai W, Guo W, Yu T, Yuan X, Zhang H, Xie H, Yao L, Wang J, Li T, Wang Q, Chen H, Wang E, Xia D, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Cai H, Xia J, Yin Z, Wu K, Fan D, Han G; AVB-TIPS Study Group. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (1)] |
| 28. | Bureau C, Thabut D, Oberti F, Dharancy S, Carbonell N, Bouvier A, Mathurin P, Otal P, Cabarrou P, Péron JM, Vinel JP. Transjugular Intrahepatic Portosystemic Shunts With Covered Stents Increase Transplant-Free Survival of Patients With Cirrhosis and Recurrent Ascites. Gastroenterology. 2017;152:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 322] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 29. | Kanwal F, Tansel A, Kramer JR, Feng H, Asch SM, El-Serag HB. Trends in 30-Day and 1-Year Mortality Among Patients Hospitalized With Cirrhosis From 2004 to 2013. Am J Gastroenterol. 2017;112:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2355] [Article Influence: 214.1] [Reference Citation Analysis (4)] |
| 31. | Bañares R, Moitinho E, Piqueras B, Casado M, García-Pagán JC, de Diego A, Bosch J. Carvedilol, a new nonselective beta-blocker with intrinsic anti- Alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology. 1999;30:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Bañares R, Moitinho E, Matilla A, García-Pagán JC, Lampreave JL, Piera C, Abraldes JG, De Diego A, Albillos A, Bosch J. Randomized comparison of long-term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhosis. Hepatology. 2002;36:1367-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, Trauner M, Peck-Radosavljevic M, Vogelsang H; Vienna Hepatic Hemodynamic Lab. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 34. | Gimenez P, Garcia-Martinez I, Francés R, Gonzalez-Navajas JM, Mauri M, Alfayate R, Almenara S, Miralles C, Palazon JM, Carnicer F, Pascual S, Such J, Horga JF, Zapater P. Treatment with non-selective beta-blockers affects the systemic inflammatory response to bacterial DNA in patients with cirrhosis. Liver Int. 2018;38:2219-2227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Merli M, Lucidi C, Di Gregorio V, Giannelli V, Giusto M, Ceccarelli G, Riggio O, Venditti M. The chronic use of beta-blockers and proton pump inhibitors may affect the rate of bacterial infections in cirrhosis. Liver Int. 2015;35:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 495] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 37. | Pascal JP, Cales P. Propranolol in the prevention of first upper gastrointestinal tract hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1987;317:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 220] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Idéo G, Bellati G, Fesce E, Grimoldi D. Nadolol can prevent the first gastrointestinal bleeding in cirrhotics: a prospective, randomized study. Hepatology. 1988;8:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Poynard T, Calès P, Pasta L, Ideo G, Pascal JP, Pagliaro L, Lebrec D. Beta-adrenergic-antagonist drugs in the prevention of gastrointestinal bleeding in patients with cirrhosis and esophageal varices. An analysis of data and prognostic factors in 589 patients from four randomized clinical trials. Franco-Italian Multicenter Study Group. N Engl J Med. 1991;324:1532-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 313] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 40. | Groszmann RJ, Bosch J, Grace ND, Conn HO, Garcia-Tsao G, Navasa M, Alberts J, Rodes J, Fischer R, Bermann M. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 453] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 41. | Lebrec D, Poynard T, Hillon P, Benhamou JP. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis: a controlled study. N Engl J Med. 1981;305:1371-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 346] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Propranolol prevents first gastrointestinal bleeding in non-ascitic cirrhotic patients. Final report of a multicenter randomized trial. The Italian Multicenter Project for Propranolol in Prevention of Bleeding. J Hepatol. 1989;9:75-83. [PubMed] [DOI] [Full Text] |
| 43. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Gao H, Makuch R; Portal Hypertension Collaborative Group. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 662] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 44. | Bhardwaj A, Kedarisetty CK, Vashishtha C, Bhadoria AS, Jindal A, Kumar G, Choudhary A, Shasthry SM, Maiwall R, Kumar M, Bhatia V, Sarin SK. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut. 2017;66:1838-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Tripathi D, Ferguson JW, Kochar N, Leithead JA, Therapondos G, McAvoy NC, Stanley AJ, Forrest EH, Hislop WS, Mills PR, Hayes PC. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology. 2009;50:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 46. | Shah HA, Azam Z, Rauf J, Abid S, Hamid S, Jafri W, Khalid A, Ismail FW, Parkash O, Subhan A, Munir SM. Carvedilol vs. esophageal variceal band ligation in the primary prophylaxis of variceal hemorrhage: a multicentre randomized controlled trial. J Hepatol. 2014;60:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 47. | Villanueva C, Torres F, Sarin SK, Shah HA, Tripathi D, Brujats A, Rodrigues SG, Bhardwaj A, Azam Z, Hayes PC, Jindal A, Abid S, Alvarado E, Bosch J; Carvedilol-IPD-MA-group and the Baveno Cooperation: an EASL Consortium. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J Hepatol. 2022;77:1014-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 48. | Sinagra E, Perricone G, D'Amico M, Tinè F, D'Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Vargas JI, Arrese M, Shah VH, Arab JP. Use of Statins in Patients with Chronic Liver Disease and Cirrhosis: Current Views and Prospects. Curr Gastroenterol Rep. 2017;19:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 50. | Cabrera L, Abraldes JG. Statins: the Panacea of Cirrhosis? Curr Hepatol Rep. 2016;15:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 51. | Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garca-Pagán JC, Rodés J, Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 52. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 53. | Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, Marchiori RC, Rezende GF. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: A randomized controlled trial. Dig Liver Dis. 2015;47:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 54. | Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R; Pravastatin in Chronic Liver Disease Study Investigators. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 55. | Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 56. | Moctezuma-Velázquez C, Abraldes JG, Montano-Loza AJ. The Use of Statins in Patients With Chronic Liver Disease and Cirrhosis. Curr Treat Options Gastroenterol. 2018;16:226-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59:1958-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, Lu CL. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Mohanty A, Tate JP, Garcia-Tsao G. Statins Are Associated With a Decreased Risk of Decompensation and Death in Veterans With Hepatitis C-Related Compensated Cirrhosis. Gastroenterology. 2016;150:430-40.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 60. | Kamal S, Khan MA, Seth A, Cholankeril G, Gupta D, Singh U, Kamal F, Howden CW, Stave C, Nair S, Satapathy SK, Ahmed A. Beneficial Effects of Statins on the Rates of Hepatic Fibrosis, Hepatic Decompensation, and Mortality in Chronic Liver Disease: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:1495-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Kim RG, Loomba R, Prokop LJ, Singh S. Statin Use and Risk of Cirrhosis and Related Complications in Patients With Chronic Liver Diseases: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1521-1530.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 62. | Kaplan DE, Mehta R, Garcia-Tsao G, Albrecht J, Aytaman A, Baffy G, Bajaj J, Hernaez R, Hunt K, Ioannou G, Johnson K, Kanwal F, Lee TH, Monto A, Pandya P, Schaubel D, Taddei TH. SACRED: Effect of simvastatin on hepatic decompensation and death in subjects with high-risk compensated cirrhosis: Statins and Cirrhosis: Reducing Events of Decompensation. Contemp Clin Trials. 2021;104:106367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Caraceni P, Vargas V, Solà E, Alessandria C, de Wit K, Trebicka J, Angeli P, Mookerjee RP, Durand F, Pose E, Krag A, Bajaj JS, Beuers U, Ginès P; Liverhope Consortium. The Use of Rifaximin in Patients With Cirrhosis. Hepatology. 2021;74:1660-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 64. | Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther. 2016;43 Suppl 1:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 65. | Baik SK, Lim YL, Cho YZ, Kim MY, Jang YO, Suk KT, Cheon GJ, Kim YD, Choi DH. G03: Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy in the hepatic venous pressure gradient response and propranolol dose reduction – A pilot study. J Hepatol. 2015;62:S188. [DOI] [Full Text] |
| 66. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1982] [Article Influence: 247.8] [Reference Citation Analysis (2)] |
| 67. | Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Therap Adv Gastroenterol. 2018;11:1756284818800307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Zeng X, Sheng X, Wang PQ, Xin HG, Guo YB, Lin Y, Zhong JW, He CZ, Yin J, Liu TT, Ma WJ, Xiao X, Shi PM, Yuan ZL, Yang L, Ma X, Xu JM, Shen XZ, Yang CQ, Zhu X, Lv NH, Xie WF. Low-dose rifaximin prevents complications and improves survival in patients with decompensated liver cirrhosis. Hepatol Int. 2021;15:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | Leonardi F, Maria N, Villa E. Anticoagulation in cirrhosis: a new paradigm? Clin Mol Hepatol. 2017;23:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Bitto N, Liguori E, La Mura V. Coagulation, Microenvironment and Liver Fibrosis. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 71. | Senzolo M, M Sartori T, Rossetto V, Burra P, Cillo U, Boccagni P, Gasparini D, Miotto D, Simioni P, Tsochatzis E, A Burroughs K. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int. 2012;32:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 263] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 72. | Chen H, Liu L, Qi X, He C, Wu F, Fan D, Han G. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 73. | Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MV, García-Criado A, Abraldes JG, de la Peña J, Bañares R, Albillos A, Bosch J, García-Pagán JC. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 74. | Amitrano L, Guardascione MA, Menchise A, Martino R, Scaglione M, Giovine S, Romano L, Balzano A. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol. 2010;44:448-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 75. | Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S, Lei B, Bernabucci V, Vukotic R, De Maria N, Schepis F, Karampatou A, Caporali C, Simoni L, Del Buono M, Zambotto B, Turola E, Fornaciari G, Schianchi S, Ferrari A, Valla D. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143:1253-1260.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 552] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 76. | Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (12)] |
| 77. | Osawa L, Nakanishi H, Kurosaki M, Kirino S, Inada K, Yamashita K, Hayakawa Y, Sekiguchi S, Wang W, Okada M, Higuchi M, Komiyama Y, Takaura K, Takada H, Kaneko S, Maeyashiki C, Tamaki N, Yasui Y, Tsuchiya K, Itakura J, Takahashi Y, Enomoto N, Izumi N. Plasma Renin Activity Predicts Prognosis and Liver Disease-Related Events in Liver Cirrhosis Patients with Ascites Treated by Tolvaptan. Dig Dis. 2022;40:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | González-Abraldes J, Albillos A, Bañares R, Del Arbol LR, Moitinho E, Rodríguez C, González M, Escorsell A, García-Pagán JC, Bosch J. Randomized comparison of long-term losartan vs propranolol in lowering portal pressure in cirrhosis. Gastroenterology. 2001;121:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Schepke M, Werner E, Biecker E, Schiedermaier P, Heller J, Neef M, Stoffel-Wagner B, Hofer U, Caselmann WH, Sauerbruch T. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology. 2001;121:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Castaño G, Viudez P, Riccitelli M, Sookoian S. A randomized study of losartan vs propranolol: Effects on hepatic and systemic hemodynamics in cirrhotic patients. Ann Hepatol. 2003;2:36-40. [PubMed] [DOI] [Full Text] |
| 81. | De BK, Bandyopadhyay K, Das TK, Das D, Biswas PK, Majumdar D, Mandal SK, Ray S, Dasgupta S. Portal pressure response to losartan compared with propranolol in patients with cirrhosis. Am J Gastroenterol. 2003;98:1371-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Debernardi-Venon W, Martini S, Biasi F, Vizio B, Termine A, Poli G, Brunello F, Alessandria C, Bonardi R, Saracco G, Rizzetto M, Marzano A. AT1 receptor antagonist Candesartan in selected cirrhotic patients: effect on portal pressure and liver fibrosis markers. J Hepatol. 2007;46:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Hidaka H, Kokubu S, Nakazawa T, Okuwaki Y, Ono K, Watanabe M, Shibuya A, Saigenji K. New angiotensin II type 1 receptor blocker olmesartan improves portal hypertension in patients with cirrhosis. Hepatol Res. 2007;37:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Hidaka H, Nakazawa T, Shibuya A, Minamino T, Takada J, Tanaka Y, Okuwaki Y, Watanabe M, Koizumi W. Effects of 1-year administration of olmesartan on portal pressure and TGF-beta1 in selected patients with cirrhosis: a randomized controlled trial. J Gastroenterol. 2011;46:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Vlachogiannakos J, Tang AK, Patch D, Burroughs AK. Angiotensin converting enzyme inhibitors and angiotensin II antagonists as therapy in chronic liver disease. Gut. 2001;49:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Zhang X, Wong GL, Yip TC, Tse YK, Liang LY, Hui VW, Lin H, Li GL, Lai JC, Chan HL, Wong VW. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 87. | Abecasis R, Kravetz D, Fassio E, Ameigeiras B, Garcia D, Isla R, Landeira G, Dominguez N, Romero G, Argonz J, Terg R. Nadolol plus spironolactone in the prophylaxis of first variceal bleed in nonascitic cirrhotic patients: A preliminary study. Hepatology. 2003;37:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8720] [Article Influence: 792.7] [Reference Citation Analysis (2)] |
| 89. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2303] [Cited by in RCA: 2639] [Article Influence: 263.9] [Reference Citation Analysis (0)] |
| 90. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4860] [Article Influence: 694.3] [Reference Citation Analysis (0)] |
| 91. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3584] [Article Influence: 597.3] [Reference Citation Analysis (1)] |
| 92. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5652] [Article Influence: 628.0] [Reference Citation Analysis (0)] |
| 93. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 4302] [Article Influence: 614.6] [Reference Citation Analysis (0)] |
| 94. | Montalvo-Gordon I, Chi-Cervera LA, García-Tsao G. Sodium-Glucose Cotransporter 2 Inhibitors Ameliorate Ascites and Peripheral Edema in Patients With Cirrhosis and Diabetes. Hepatology. 2020;72:1880-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 95. | Kalambokis GN, Tsiakas I, Filippas-Ntekuan S, Christaki M, Despotis G, Milionis H. Empagliflozin Eliminates Refractory Ascites and Hepatic Hydrothorax in a Patient With Primary Biliary Cirrhosis. Am J Gastroenterol. 2021;116:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Miyamoto Y, Honda A, Yokose S, Nagata M, Miyamoto J. Weaning from concentrated ascites reinfusion therapy for refractory ascites by SGLT2 inhibitor. Clin Kidney J. 2022;15:831-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 97. | Saffo S, Garcia-Tsao G, Taddei T. SGLT2 inhibitors in patients with cirrhosis and diabetes mellitus: A tertiary center cohort study and insights about a potential therapeutic target for portal hypertension. J Diabetes. 2021;13:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Saffo S, Kaplan DE, Mahmud N, Serper M, John BV, Ross JS, Taddei T. Impact of SGLT2 inhibitors in comparison with DPP4 inhibitors on ascites and death in veterans with cirrhosis on metformin. Diabetes Obes Metab. 2021;23:2402-2408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 99. | Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, Levantesi F, Airoldi A, Boccia S, Svegliati-Baroni G, Fagiuoli S, Romanelli RG, Cozzolongo R, Di Marco V, Sangiovanni V, Morisco F, Toniutto P, Tortora A, De Marco R, Angelico M, Cacciola I, Elia G, Federico A, Massironi S, Guarisco R, Galioto A, Ballardini G, Rendina M, Nardelli S, Piano S, Elia C, Prestianni L, Cappa FM, Cesarini L, Simone L, Pasquale C, Cavallin M, Andrealli A, Fidone F, Ruggeri M, Roncadori A, Baldassarre M, Tufoni M, Zaccherini G, Bernardi M; ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 377] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 100. | García-Pagán JC, Feu F, Bosch J, Rodés J. Propranolol compared with propranolol plus isosorbide-5-mononitrate for portal hypertension in cirrhosis. A randomized controlled study. Ann Intern Med. 1991;114:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 172] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 101. | García-Pagán JC, Morillas R, Bañares R, Albillos A, Villanueva C, Vila C, Genescà J, Jimenez M, Rodriguez M, Calleja JL, Balanzó J, García-Durán F, Planas R, Bosch J; Spanish Variceal Bleeding Study Group. Propranolol plus placebo versus propranolol plus isosorbide-5-mononitrate in the prevention of a first variceal bleed: a double-blind RCT. Hepatology. 2003;37:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 102. | García-Pagán JC, Villanueva C, Vila MC, Albillos A, Genescà J, Ruiz-Del-Arbol L, Planas R, Rodriguez M, Calleja JL, González A, Solà R, Balanzó J, Bosch J; MOVE Group. Mononitrato Varices Esofágicas. Isosorbide mononitrate in the prevention of first variceal bleed in patients who cannot receive beta-blockers. Gastroenterology. 2001;121:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 103. | Borroni G, Salerno F, Cazzaniga M, Bissoli F, Lorenzano E, Maggi A, Visentin S, Panzeri A, de Franchis R. Nadolol is superior to isosorbide mononitrate for the prevention of the first variceal bleeding in cirrhotic patients with ascites. J Hepatol. 2002;37:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 104. | Tripathi D, Therapondos G, Ferguson JW, Newby DE, Webb DJ, Hayes PC. Endothelin-1 contributes to maintenance of systemic but not portal haemodynamics in patients with early cirrhosis: a randomised controlled trial. Gut. 2006;55:1290-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Zipprichi A, Schenkel E, Gittinger F, Winkler M, Michl P, Ripoll C. Selective endothelin-a blockade decreases portal pressure in patients with cirrhosis. A pilot study combining a local intraarterial and systemic administration. J Hepatol. 2016;64:S247. [DOI] [Full Text] |
| 106. | Mookerjee R, Rosselli M, Pieri G, Beecher-Jones T, Hooshmand-Rad R, Chouhan M, Mehta G, Jalan R, Shapiro D. Effects of the FXR agonist obeticholic acid on hepatic venous pressure gradient (HVPG) in alcoholic cirrhosis: a proof of concept phase 2a study. J Hepatol. 2014;60:S7-S8. [DOI] [Full Text] |
| 107. | Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rössle M, Kreisel W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver -- results of a pilot study. Aliment Pharmacol Ther. 2006;23:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | Clemmesen JO, Giraldi A, Ott P, Dalhoff K, Hansen BA, Larsen FS. Sildenafil does not influence hepatic venous pressure gradient in patients with cirrhosis. World J Gastroenterol. 2008;14:6208-6212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 109. | Lee KC, Yang YY, Wang YW, Hou MC, Lee FY, Lin HC, Lee SD. Acute administration of sildenafil enhances hepatic cyclic guanosine monophosphate production and reduces hepatic sinusoid resistance in cirrhotic patients. Hepatol Res. 2008;38:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Tandon P, Inayat I, Tal M, Spector M, Shea M, Groszmann RJ, Garcia-Tsao G. Sildenafil has no effect on portal pressure but lowers arterial pressure in patients with compensated cirrhosis. Clin Gastroenterol Hepatol. 2010;8:546-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 111. | Kreisel W, Deibert P, Kupcinskas L, Sumskiene J, Appenrodt B, Roth S, Neagu M, Rössle M, Zipprich A, Caca K, Ferlitsch A, Dilger K, Mohrbacher R, Greinwald R, Sauerbruch T. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Snowdon VK, Lachlan NJ, Hoy AM, Hadoke PW, Semple SI, Patel D, Mungall W, Kendall TJ, Thomson A, Lennen RJ, Jansen MA, Moran CM, Pellicoro A, Ramachandran P, Shaw I, Aucott RL, Severin T, Saini R, Pak J, Yates D, Dongre N, Duffield JS, Webb DJ, Iredale JP, Hayes PC, Fallowfield JA. Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial. PLoS Med. 2017;14:e1002248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 113. | Pinter M, Sieghart W, Reiberger T, Rohr-Udilova N, Ferlitsch A, Peck-Radosavljevic M. The effects of sorafenib on the portal hypertensive syndrome in patients with liver cirrhosis and hepatocellular carcinoma--a pilot study. Aliment Pharmacol Ther. 2012;35:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 114. | Coriat R, Gouya H, Mir O, Ropert S, Vignaux O, Chaussade S, Sogni P, Pol S, Blanchet B, Legmann P, Goldwasser F. Reversible decrease of portal venous flow in cirrhotic patients: a positive side effect of sorafenib. PLoS One. 2011;6:e16978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 115. | Garcia-Tsao G, Fallon MB, Reddy KR, Loo N, Bari K, Augustin S, Ciarleglio M, Deng Y, Taddei TH, Strazzabosco M. Placebo-controlled, randomized, pilot study of the effect of sorafenib on portal pressure in patients with cirrhosis, portal hypertension and ablated hepatocellular carcinoma (HCC). Hepatology. 2015;62:580A-581A. |
| 116. | Schwarzer R, Kivaranovic D, Mandorfer M, Paternostro R, Wolrab D, Heinisch B, Reiberger T, Ferlitsch M, Gerner C, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Randomised clinical study: the effects of oral taurine 6g/day vs placebo on portal hypertension. Aliment Pharmacol Ther. 2018;47:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Austin AS, Mahida YR, Clarke D, Ryder SD, Freeman JG. A pilot study to investigate the use of oxpentifylline (pentoxifylline) and thalidomide in portal hypertension secondary to alcoholic cirrhosis. Aliment Pharmacol Ther. 2004;19:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 118. | Zhou X, Zhang T, Sun Y, Li C, Ding X, Zhu Y, Li L, Fan Z. Systematic Review and Meta-analysis: Association of Aspirin With Incidence of Hepatocellular Carcinoma. Front Pharmacol. 2022;13:764854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 119. | Memel ZN, Arvind A, Moninuola O, Philpotts L, Chung RT, Corey KE, Simon TG. Aspirin Use Is Associated with a Reduced Incidence of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Hepatol Commun. 2021;5:133-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 120. | Kaplan DE, Serper M, John BV, Tessiatore KM, Lerer R, Mehta R, Fox R, Aytaman A, Baytarian M, Hunt K, Albrecht J, Taddei TH; Veterans Outcomes and Cost Associated with Liver disease Study Group. Effects of Metformin Exposure on Survival in a Large National Cohort of Patients With Diabetes and Cirrhosis. Clin Gastroenterol Hepatol. 2021;19:2148-2160.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 121. | Abraldes JG, Bureau C, Stefanescu H, Augustin S, Ney M, Blasco H, Procopet B, Bosch J, Genesca J, Berzigotti A; Anticipate Investigators. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The "Anticipate" study. Hepatology. 2016;64:2173-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 122. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1840] [Article Influence: 460.0] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Elshimi E, Egypt; Rabago LR, Spain; Zhuge YZ, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ