Published online Jan 7, 2023. doi: 10.3748/wjg.v29.i1.126
Peer-review started: September 20, 2022
First decision: November 26, 2022
Revised: November 26, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 7, 2023
Processing time: 105 Days and 10.1 Hours
The metabolic syndrome as a consequence of the obesity pandemic resulted in a substantial increase in the prevalence of metabolic-associated fatty live disease (MAFLD) and type 2 diabetes mellitus (T2DM). Because of the similarity in pathobiology shared between T2DM and MAFLD, both disorders coexist in many patients and may potentiate the disease-related outcomes with rapid progression and increased complications of the individual diseases. In fact, awareness about this coexistence and the risk of complications are often overlooked by both hepatologists and diabetologists. Management of these individual disorders in a patient should be addressed wholistically using an appropriate multidisciplinary team approach involving both the specialists and, when necessary, liaising with dieticians and surgeons. This comprehensive review is to compile the current evidence from a diabetologist's perspective on MAFLD and T2DM and to suggest optimal management strategies.
Core Tip: The prevalence of metabolic-associated fatty live disease (MAFLD) and type 2 diabetes mellitus (T2DM) has increased exponentially as a consequence of the obesity pandemic across the globe. The pathobiology of T2DM and MAFLD are similar because both these disorders occur as a consequence of metabolic syndrome, and often coexist in many patients potentiating adverse outcomes and progression of individual diseases. However, the awareness about this coexistence is still inadequate even among hepatologists and diabetologists. A multidisciplinary team approach involving both the specialists is crucial in the optimal and wholistic management of both the disorders which is the theme of this comprehensive review.
- Citation: Jeeyavudeen MS, Khan SKA, Fouda S, Pappachan JM. Management of metabolic-associated fatty liver disease: The diabetology perspective. World J Gastroenterol 2023; 29(1): 126-143
- URL: https://www.wjgnet.com/1007-9327/full/v29/i1/126.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i1.126
The global prevalence of obesity is increasing daily because of adverse lifestyles such as overconsumption of a high-energy diet and lack of adequate physical activity. This is associated with various metabolic disorders, particularly metabolic-associated fatty liver disease (MAFLD) and type 2 diabetes mellitus (T2DM). MAFLD, previously termed as nonalcoholic fatty liver disease (NAFLD), is now identified as the most common cause of chronic liver disease in the world, affecting more than 30% of the global population[1].
Due to the similarity in pathogenesis, MAFLD is often associated with other lifestyle-related disorders such as hypertension, dyslipidemia, and most importantly T2DM, which significantly increases cardiovascular disease (CVD). MAFLD has also been found to be associated with excess risk of extrahepatic cancers, gallstones, gastro-oesophageal reflux disease, hypothyroidism, urolithiasis, chronic kidney disease (CKD), depression and worse maternal and foetal outcomes during pregnancy[2]. MAFLD is also associated with a substantial increase in cardiovascular morbidity and mortality, with a 3.5 times higher risk of heart failure and 1.93 times excess risk of all-cause CVD mortality as per recent study data in patients with T2DM[3].
In fact, the new definition for disease (MAFLD) was proposed in 2020 by replacing the old terminology NAFLD to reflect its highly remarkable link to metabolic syndrome (MetS) and the consequences such as T2DM[4]. While NAFLD was originally defined based on the imaging and/or histological evidence of steatosis in the absence of significant alcohol consumption and the exclusion of hepatitis from other etiologies, MAFLD diagnosis requires only the presence of metabolic dysfunction in persons with steatosis, and does not need excluding other etiologies of hepatitis. When MAFLD/ NAFLD only involves liver steatosis without significant damage to the hepatocytes, nonalcoholic steatohepatitis (NASH) involves inflammation of liver cells with varying degrees of hepatocyte destruction, which may lead on the cirrhosis and end stage liver failure[5].
The prevalence of MAFLD in patients with T2DM varies widely in the published literature, with a recent study reporting a rate of 68% among those with obesity and T2DM[4]. The global epidemiologic data suggests that the pooled prevalence of T2DM in those with MAFLD is 22.5%, with 43.6% of those with advanced disease (NASH)[5]. Therefore, management strategies for each disease entity should definitely address the coexistence of MAFLD and T2DM, for optimal disease outcomes. In this evidence-based review, we update the management of MAFLD with due consideration of the interlink between MAFLD and diabetes to enable physicians to approach patients having better diagnostic and therapeutic perspectives.
To compile the best and most up-to-date evidence, we performed a PubMed search using the MeSH terms/key words: “metabolic-associated fatty liver disease/MAFLD”, “nonalcoholic fatty liver disease/NAFLD”, “type 2 diabetes mellitus/T2DM/T2D”, “diabetes/diabetology/ diabetologist”, “obesity” “nonalcoholic steatohepatitis/NASH”, “metabolic syndrome/ MetS”, “fatty liver”, “steatosis”, “pathophysiology”, “lifestyle intervention”, “exercise”, “diet”, “pharmacotherapy”, “bariatric/metabolic surgery”, “pregnancy”, “children”, and “elderly/ old age”. The last date of search was 25th November 2022 and we used a Boolean search strategy using terms ‘AND’ or ‘OR’ where necessary to limit the search output. We limited the use of published literature in English language preferably from the most recent clinical guidelines, systematic reviews, randomised controlled trials, and high-quality review articles to procure the best available evidence on the topic of discussion while writing this narrative review paper.
MetS, which encompasses central adiposity, insulin resistance, hypertension and atherogenic dyslipidemia, is the common consequence of obesity. MAFLD is considered as the hepatic manifestation of MetS, and has a bidirectional and solid relationship with other comorbidities[6]. T2DM, the predominant sequel of MetS, is therefore commonly associated with MAFLD and vice versa because of the similarity in pathogenesis. Various genetic predisposing factors, insulin resistance (IR), proatherogenic dyslipidemia [low levels of high-density lipoprotein cholesterol (HDL-C) as well as elevated concentrations of very low-density lipoproteins, triglycerides, and apolipoprotein B100], and alterations in gut microbiota resulting in intestinal dysbiosis are implicated in the development MAFLD[7].
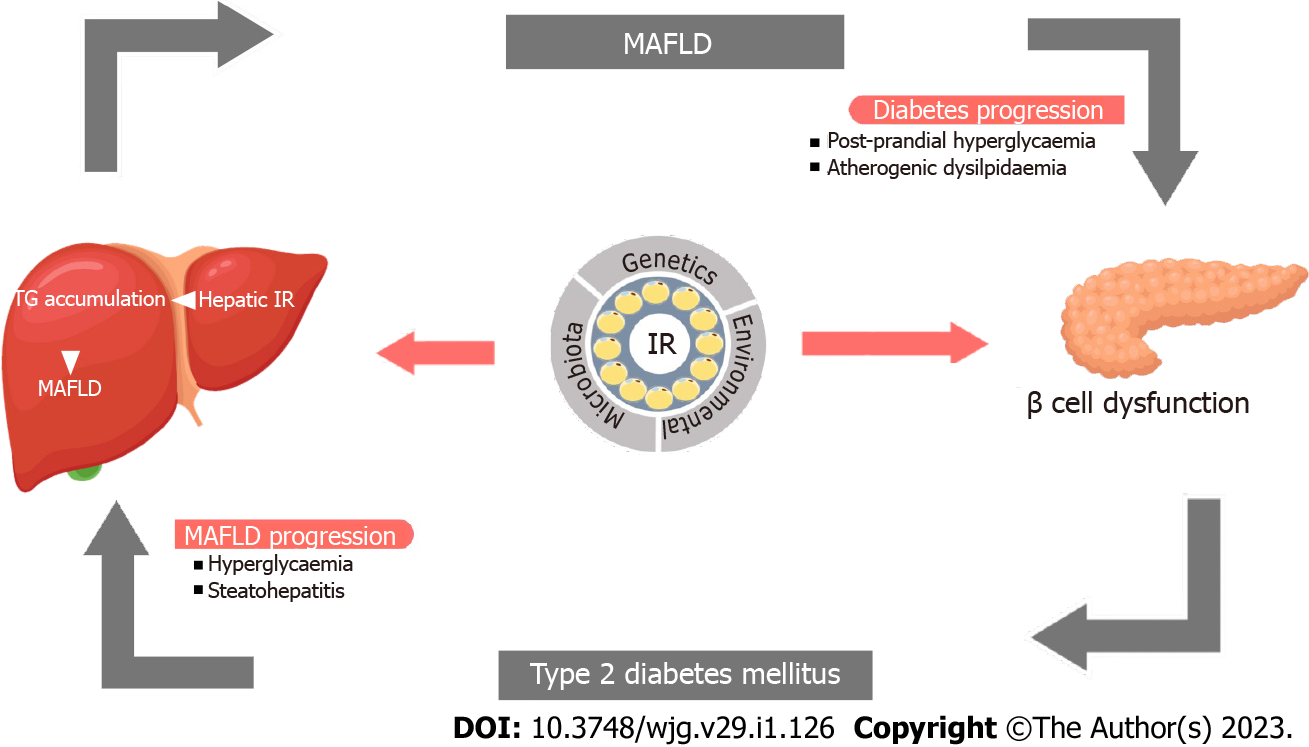
IR, the key factor in the development of both disorders, grossly alters the hepatic glucose and lipid metabolism[7-9]. Fasting hyperglycemia as a consequence of IR and postprandial hyperglycemia from the inability of the liver to store glucose as glycogen are both implicated in the development of hepatic steatosis. IR also increases the de novo lipogenesis and triglyceride production in the liver as a result of carbohydrate-rich diet in experimental models[10]. Increased liver fat, in turn, reduces hepatic insulin clearance, insulin sensitivity and augments total body insulin resistance[11]. These factors aggravate the risk of T2DM, and in those with pre-existing T2DM, a worsening of the disease. Advanced stages of MAFLD such as NASH and cirrhosis can increase the risk of development and worsening of T2DM[5,12-14]. Therefore, without addressing both the disease states, we cannot manage patients with MAFLD and T2DM. Figure 1 demonstrates the pathobiological interlink between T2DM and MAFLD.
By definition, MAFLD is associated with the accumulation of fat in > 5% of hepatocytes on histological evaluation or using imaging evidence of > 5.6% of liver fat on magnetic resonance spectroscopy[12-14]. Various professional bodies such as the American Association for the Study of Liver Diseases (AASLD)[13], European Association for the Study of the Liver (EASL)[12], the American Association of Clinical Endocrinology (AACE)[14], and the American Diabetes Association (ADA)[15], recommend screening for MAFLD in patients with diabetes. While the EASL guideline recommends screening patients using liver function tests (LFT) and/or ultrasound scan in all cases with obesity/MetS/T2DM, the AASLD suggests screening only those with a high index of suspicion for MAFLD or NASH because of the uncertainties about the diagnosis, treatment, and the natural history of the disease. The AASLD also recommends non-invasive screening strategies like NAFLD fibrosis score, fibrosis-4 (FIB-4) score, or transient elastography (TE) for the evaluation of risk of liver fibrosis.
Based on grade B, intermediate/high strength of evidence, the latest guideline by AACE in 2022 recommends that more rigorous screening strategy should be considered for patients with T2DM using FIB-4 score, even if they have normal LFTs[14]. This guideline also advises that clinicians should consider screening for MAFLD and advanced fibrosis in those with obesity and/or features of MetS, prediabetes, and those with liver steatosis on imaging study and/or high liver transaminase levels > 6 mo as they have "high risk" for advanced fibrosis. Those with intermediate to high FIB-4 scores are considered "high risk" and should be recommended a liver stiffness measurement by TE or enhanced lifer fibrosis (ELF) test[14].
The most validated and cost-effective screening test to assess the risk of fibrosis, to identify advanced disease and liver-related outcomes in MAFLD is the vibration controlled transient elastography[14]. The most accurate imaging technique, magnetic resonance elastography, should be reserved for selected cases because of the cost implications. Alternative tests such as shear wave elastography and steatosis, activity and fibrosis (SAF) score and NAFLD activity scoring (NAS) system are also useful for screening and risk stratification. The AACE guidelines (2022) also suggests considering screening for patients with type 1 diabetes mellitus (T1DM), using FIB-4 to exclude MAFLD with clinically significant fibrosis (stages F2-F4) in presence of obesity, features of MetS, deranged LFTs with transaminase levels (> 30 U/L), or liver steatosis on imaging studies.
Because of the close association of MAFLD with diabetes and the risk of worsening of either condition among the sufferers, all patients with one of these disease entities should be evaluated for the other disease and regularly monitored using clinical and biochemical markers for each condition. Patients with either T2DM or T1DM and cardiometabolic risk factors and/or elevated transaminases (> 30 U/L) should be further risk stratified using the FIB-4 scoring, TE, and/or ELF test to exclude advanced liver disease related to MAFLD[14]. Those with persistently elevated liver transaminases and/or the presence of steatosis upon imaging evaluation and categorized as intermediate or high risk based on biochemistry and/or imaging should be referred to a hepatologist for further assessment. Liver biopsy is now considered only rarely in the diagnostic evaluation of MAFLD because of the invasiveness of the test, the high risk of complications, and the availability of high quality non-invasive diagnostic strategies in recent years for all categories of patients[14,16-18]. Sequential testing for MAFLD using blood test and non-invasive testing does reduce the number of liver biopsies required for the confirmation. In the data from the STELLAR studies, sequential testing alone had a reasonable specificity and sensitivity with area under the curve between 0.75 to 0.80 to discriminate advanced fibrosis in MAFLD patients[19]. Our recommendations are in line with AACE/AASLD 2022 guidelines where liver biopsy is required only for intermediate to high-risk fibrosis patients or NASH diagnosis or exclude other coexisting diseases[14]. Bariatric surgery provides an opportunity to perform liver biopsy and can be potentially utilised, if the diagnosis of MAFLD is uncertain (with the other non-invasive tests) prior to the procedure, while being performed for the management of obesity.
Being a metabolic disorder having significant pathogenic association with adverse lifestyles, including dietary energy imbalance and sedentary behaviors, main strategies in the management of MAFLD should be tailored around lifestyle interventions and behavioral adaptations. However, the likelihood of success with these measures is often modest because of lack of adherence and therefore, physicians are often forced to opt for pharmacotherapeutic interventions. Considering the lack of availability of many approved therapeutic agents for the treatment of MAFLD, and the well-proven additional benefits such as improvement of prediabetes/diabetes, cardiometabolic risks, and the improvement physical and emotional quality of life, the importance of lifestyle interventions cannot be underscored.
The most recent AACE guidelines (2022) reinforces the importance of lifestyle interventions as part of the standard care for patients with MAFLD and obesity, MetS, diabetes/prediabetes, hypertension, dyslipidemia, and CVD[14]. Lifestyle intervention-related weight loss of 3%-5% has shown to improve steatosis[13], with a reversal of hepatic IR[20]. The resolution of NASH in a proportion of cases with improved liver inflammation has been observed with 7%-10% weight reduction[21]. Further, fibrosis regression has been observed in those losing > 10% body weight by lifestyle changes[21,22]. However, approximately 70% of these clinical trials only achieved the target weight loss of 5% from lack of compliance and poor adherence to these weight management strategies.
Various nutritional interventions targeting a weight loss ≥ 5% has shown to be effective in the management of MAFLD[14,23,24]. Aim of all forms of nutritional interventions for MAFLD is to reduce the proportion of macronutrient content of the diet to achieve total energy deficit by restricting intake of simple carbohydrates, saturated fat, and added sugars, along with adoption of healthier eating options like a Mediterranean diet. Recent research data also suggests that intermittent fasting and time-restricted eating behavior are associated with improvement of MAFLD[25,26].
Nutritional intervention with energy restricted anti-inflammatory diets are also associated with reduction of inflammation along with better MAFLD outcomes in a recent randomized controlled trial[27]. The trial also showed significant improvements in body weight (-7.1%), visceral adiposity (-22.3%), metabolic parameters [homeostatic model assessment of IR (HOMA-IR): 15.5%; cholesterol: -5.3%; low-density lipoprotein cholesterol (LDL-C): -4.6%; and triglycerides: -12.2%], and various biomarkers of inflammation. Strict nutritional interventions for MAFLD have also been found to be associated with improvements of CVD risk (blood pressure and QRISK2), metabolic health [fasting glucose, glycated hemoglobin (HbA1c), and insulin levels], body composition and quality of life along with improved liver-related outcomes[18,28]. However, the long-term effects and the sustainability of these nutritional interventions are still not clear in the absence of sufficiently long-term follow-up scientific research data. Although various dietary regimens are available starting from low carbohydrate/very low carbohydrate diet, ketogenic diet and Mediterranean diet, the ultimate goal for all these dietary regimens is to aim for calorie deficiency due to restriction of macronutrient (sugar and saturated fat). Of all the dietary regimens, the evidence base is strongest for the low glycaemic index Mediterranean diet which has shown to reduce the NAFLD score (median score: -4.14, 95%CI; -6.78, -1.49) when compared to the regular diet within six months duration[29]. Mediterranean diet also has cardiovascular protection property. Due to multiple health benefits with the Mediterranean diet, several professional societies recommend it as the preferred first line approach for MAFLD patients[12,14].
Physical activity is associated with improved muscle metabolism, resulting in energy dissipation and negative energy balance, especially with a calorie-restricted dietary intake. Exercise interventions have been demonstrated to significantly improve the liver transaminases regardless of the age, although more profound benefits have been observed in younger adults[30]. Along with improvements in MAFLD, various exercise programs have also been associated with improvements in metabolic parameters such as plasma levels of glucose, insulin, HbA1c, LDL-C, triglycerides, and HOMA-IR[31,32].
Apart from improvements in metabolic parameters, exercise interventions are also expected to improve all-cause morbidity and mortality associated with MAFLD. Both aerobic exercise and resistance training exercise result in reduction in the intrahepatic triglycerides (IHTG) content. In a randomised control trial from Thailand, a 12-wk regimen of moderate intensity aerobic exercise resulted in similar reduction of intrahepatic fat and improvement of insulin resistance in MAFLD patients when compared with resistance exercise and dietary modification[33]. A metanalysis involving six studies with 94 participants suggest that having a structured regimen results in greater reduction of IHTG and body weight[34]. Hence regular aerobic physical activity for 150-300 min/week of moderate-intensity or 75-150 min/week of vigorous-intensity coupled with some resistance training, aiming for a weight loss of > 5% in patients with MAFLD, can improve other comorbidities such as hypertension, diabetes, obstructive sleep apnea, dyslipidemia, CVD and possibly mortality[14,18,35]. Exercise interventions should ideally be coupled with nutritional changes to achieve hypocaloric energy balance to improve all these benefits. However, a recent Cochrane review concluded that there is considerable uncertainty in the evidence for benefits achieved by such interventions as the trials were of short follow-up duration of 2-24 mo[36]. Patients should be followed up for at least eight years to arrive at meaningful conclusions from these trials. Finally maintaining compliance in any chronic disorder is always challenging, different models like health behavioural model and protectional motivation theory have been proposed to improve the compliance, but all the models highlight the importance of explaining the effectiveness of the lifestyle changes to the patient and emphasising the importance of maintaining the target achieved for prevention of progression of MAFLD[37].
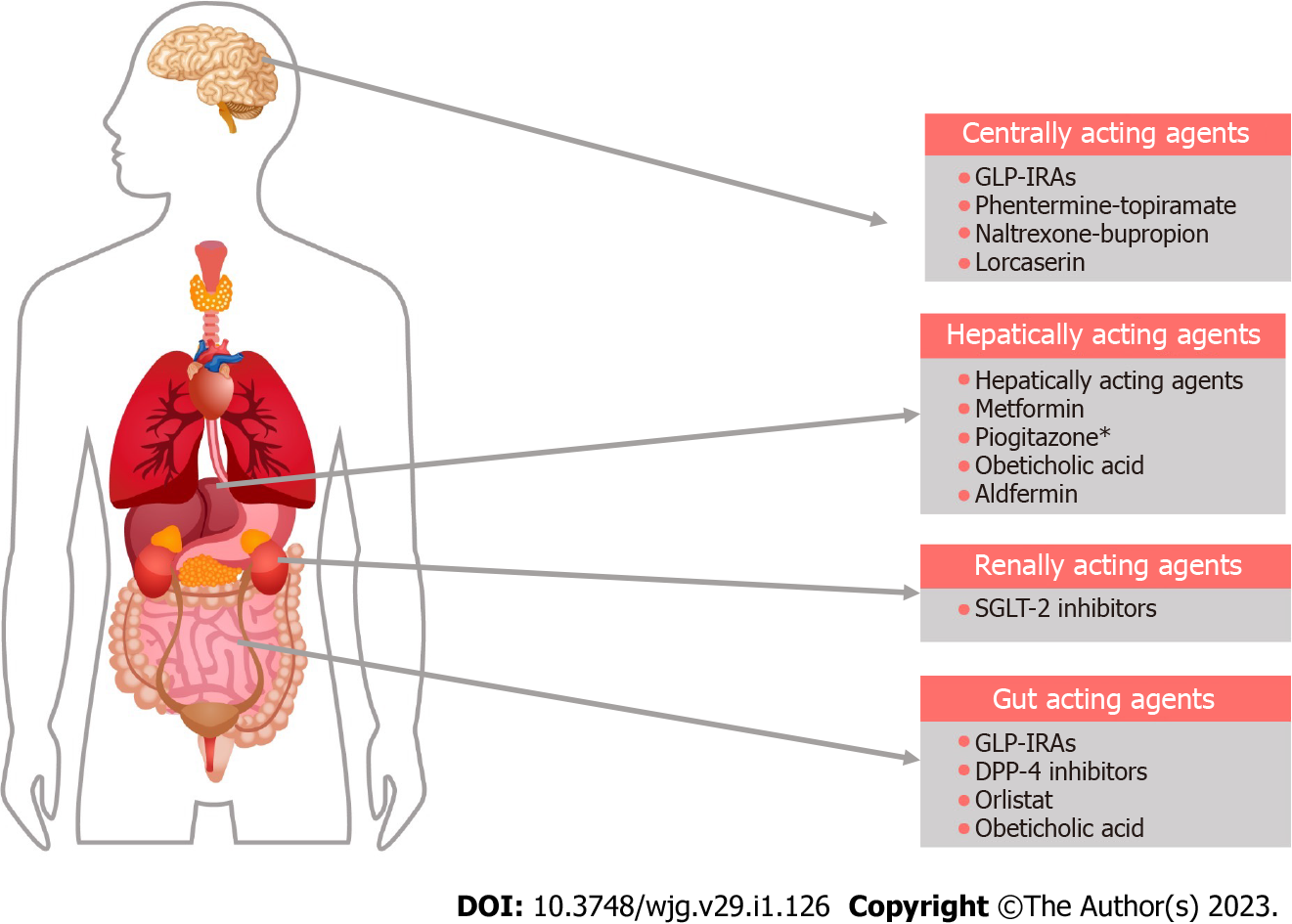
Although multiple clinical trials on the pharmacotherapy for MAFLD have been completed to date, there are no drugs approved by the United States Food and Drug Administration (FDA) for the management of the disease. Most of the therapeutic agents used currently in clinical practice are based on low-moderate certainty of evidence or expert recommendations though we can expect some very promising molecules soon. Figure 2 shows the major sites of action of the drugs currently used in the management of MAFLD.
Pioglitazone: Pioglitazone belongs to the thiazolidinedione group of antidiabetic drugs, and acts as a peroxisome proliferator-activated receptor gamma agonist which modulates the insulin sensitivity in liver, muscles, and adipose tissues, having a marked improvement of IR[38]. Based on grade A and high strength evidence, pioglitazone is currently recommended for treatment of MAFLD when there is evidence of NASH (raised transaminases and/or suggestive non-invasive tests) in presence of T2DM[14,18,39]. Significant improvement in NASH without worsening of fibrosis was seen with the use of pioglitazone dose of 45 mg daily (relative risk: 2.64, 95%CI: 1.36; 5.12). An HbA1c reduction ranging from 1%-1.6% has been observed with neutral cardiovascular (CV) safety profile in patients with T2DM treated with pioglitazone though the use of the molecule is now less common owing to the availability of newer antidiabetic medications with better CV benefits[40]. Weight gain potential, probable risks of worsening heart failure and diabetic maculopathy from fluid retention and elevated fracture risk are the hindrances for widespread use of pioglitazone in clinical practice. The association between pioglitazone and bladder cancer is controversial. FDA has issued safety warning and advise against the use of pioglitazone in patients with active bladder cancer and to exercise caution when used in patient with previous history of bladder cancer[41]. In a meta-analysis of 26 studies, the hazard ratio for developing bladder cancer in patient with type 2 diabetes with pioglitazone exposure was 1.07 (95%CI: 0.96-1.18) and was not statistically significant with the number needed to treat for one patient to develop bladder cancer was 899 to 6380 individuals[42]. Hence detailed history about the bladder symptoms if present, warrants further investigation before the start of the medication.
Metformin: Historically, metformin has been the drug of first choice in managing patients with T2DM over the past few decades and continues to be the first-line agent even today. By reducing hepatic gluconeogenesis and increasing skeletal muscle uptake of glucose, metformin improves insulin sensitivity and ameliorates IR. Metformin therapy in patients with MAFLD has been associated with therapeutic benefits such as alanine transaminase (ALT) and aspartate transaminase (AST) reductions of -2.84 (95%CI: -11.09 to 5.28) and -2.39 (95%CI: -7.55, 2.49), respectively, along with improvements in other biological indicators like lipid abnormalities and body mass index (BMI)[43]. In patients with T2DM and MAFLD, metformin therapy and lifestyle intervention were associated with a mean weight loss of 4.3%-7.9%, leading to improvements in hepatic IR and glycemic control[44], though the drug had no efficacy in improving NASH histology[7,45]. Due to the lack of evidence for benefits, the AACE guidelines (2022) do not recommend metformin therapy for patients with NASH though the treatment for diabetes with the drug may be continued in patients with MAFLD and NASH. In the absence of sufficient clinical data on the use of the drug in T1DM and MAFLD, metformin therapy may not be recommended in such patients though the drug may be continued in those already using it.
Incretins are gastrointestinal (GI) mucosal hormones secreted in response to the presence of nutrient stimuli in the GI lumen. Glucagon-like insulinotropic peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are the two gut hormones in this class, and both these molecules possess profound multisystemic effects on human energy balance, insulin pharmacokinetic and dynamic equilibrium, and metabolic homeostasis. Pharmacological manipulation of incretin physiology has been an area of immense global scientific research input over the past three decades that revolutionized not only diabetes therapeutics but also the knowledge regarding mechanisms of origin and evolution of a variety of metabolic and multiorgan disorders affecting human health[46,47].
The endogenously secreted GLP-1 is rapidly degraded by the gastrointestinal luminal protease enzyme dipeptidyl peptidase-4 (DPP-4) with the termination of its biological effects soon after secretion[48], with a very short elimination half-life of ≈2 min[49]. To augment the biological effects (incretin effects), and thereby improve the actions of incretin hormones, several drug molecules (incretin mimetics) belonging to GLP-1 receptor agonist (GLP-1RA) and DPP-4 inhibitor classes were subsequently developed in the early part of twenty-first century making a major paradigm shift in the management of T2DM. By augmenting the endogenous insulin production and modulating satiety, these molecules alter the pathobiology of T2DM with weight loss potential (GLP-1RAs) or weight neutrality (DPP-4 inhibitors) making them favorite drug choices to diabetologists. Cardiovascular morbidity and mortality benefits of some of the GLP-1RAs make them more attractive choices than other antidiabetic molecules, especially because of the CVD risks associated with diabetes.
GLP-1RAs: There are several molecules of this class currently available in the market, and newer drug molecules are under development. Exenatide, Liraglutide, Lixisenatide, Dulaglutide, Semaglutide, and Albiglutide are the widely available molecules in the GLP-1RA class. The new recent agent added to this group is Tirzepatide which also possesses GIP agonist property in addition to much higher weight loss potential and improvement of HbA1c in individuals with T2DM[50]. The mean HbA1c reduction achieved with different GLP-1RAs ranges from 0.66%-2.3%[50,51], while the mean weight loss attained varies from 2.0-11.4 kg (with Tirzepatide having maximum effect)[51,52].
Along with significant improvements in body weight and glycemic control, treatment with GLP-1RAs is also associated with improvement of cardiometabolic parameters like reduction of blood pressure, positive changes in adverse lipid profile (reduction of LDL-C and triglyceride and increase in HDL-C) and reduction of proteinuria, making this antidiabetic medication class a very desirable option for physicians[53]. Many of these benefits are also highly useful in managing patients with MAFLD, especially with diabetes.
Weight loss and the improvements in diabetes control and lipid abnormalities associated with GLP-1RA therapy may be the reasons for improvement of MAFLD. Based on grade A, high strength of evidence, the 2022 AACE guidelines recommends GLP-1RA therapy for patients with T2DM and biopsy-proven NASH, encouraging clinicians to consider the drug when there is high suspicion of NASH (high transaminases and positive non-invasive tests) and to offer these molecules for cardiometabolic benefits in presence of T2DM and MAFLD[14]. Judicious use of GLP-1RAs, in particular the long acting, once weekly molecules such as Dulaglutide and Semaglutide, are expected to improve the composite outcomes such as the activity scores, transaminases and progression of disease. A recent meta-analysis showed improvements in AST [weight mean difference (WMD) = -3.29 IU/L, 95%CI: -5.98, -0.61 IU/L, P = 0.02) , ALT (WMD = -9.92 IU/L, 95%CI: -19.89, 0.05 IU/L, P = 0.05), and GGT (WMD = -12.38 IU/L, 95%CI: -15.69, -9.07 IU/L, P < 0.00001) and reduction in FIB-4 score (WMD = -0.15, 95%CI: -0.29, 0.00, P = 0.05) when compared to placebo favoring use of GLP-1RAs for treatment of MAFLD[54]. Even though there is not enough data on the benefit of Tirzepatide in managing MAFLD, the drug must be associated with more profound effects considering remarkable improvements in cardiometabolic parameters, BMI, and diabetes control with this agent. Although GLP-1RA therapy is associated with improvements in body weight, total insulin requirements and cardiometabolic parameters in patients with T1DM[55], which might potentially benefit coexisting MAFLD, recommendations cannot be made in the absence of adequate scientific proof. GI intolerance in the form of nausea, vomiting and constipation (occasionally diarrhea and abdominal pain) are common side effects of this class of medications, with a discontinuation rate of 5%-10% within a few weeks of therapy (though clinical trials report only < 5%) observed in clinical practice[56]. Higher discontinuation rates of up to 47% being reported after 12 mo and 70% by 24 mo of treatment in some reports, mostly from lack of adequate benefit and cost implications[57].
DPP-4 inhibitors: DPP-4 inhibitors are useful for the management of T2DM with the added advantage of weight neutrality when compared to many other oral hypoglycemic agents. A recent meta-analysis showed an overall HbA1c reduction of -0.53% compared to placebo with slightly better benefit in Asians compared to Whites (-0.62% vs -0.49%) when this drug class was used as monotherapy in T2DM[58]. However, this class of medications is not recommended by the recent AACE guidelines for MAFLD because of lack of evidence on benefits except in managing patients with coexistent T2DM. Reinforcing these recommendations, a recent meta-analysis also did not show beneficial effects on the biochemical and imaging parameters of MAFLD in patients treated with DPP4 inhibitors[54].
Sodium glucose cotransporter-2 inhibitors (SGLT-2i) are available for the management of T2DM for nearly a decade. Noninsulin dependent glucose lowering effect with the added advantage of energy deficit induced by glycosuria make this class of drugs unique with positive cardiometabolic outcomes and modest weight loss potential. Empagliflozin, Canagliflozin, Dapagliflozin and Ertugliflozin are the common agents in this class available widely in the global market, with few other molecules available in Asia and several ones under development now. Although the AACE guidelines (2022) suggested a lack of evidence of benefit in treating steatohepatitis[14], a more recent systematic review showed remarkable benefits with the use of SGLT-2i[54]. They observed improvements in AST (WMD = -2.31 IU/L, 95%CI: -3.16, -1.47 IU/L, P < 0.00001), ALT (WMD = -5.93 IU/L, 95%CI: -7.70, -4.16 IU/L, P < 0.00001), and GGT (WMD = -6.49 IU/L, 95%CI: -11.09, -1.89 IU/L, P = 0.006) and reduction in FIB-4 score (WMD = -0.21, 95%CI: -0.40, -0.03, P = 0.02) when compared to placebo favoring the use of SGLT-2i in the management of MAFLD especially in the presence of T2DM and cardiometabolic disease.
The AACE guidelines (2022) do not recommend treatment of NASH patients with antidiabetic agents such as metformin, DPP-4 inhibitors, acarbose, and insulins because of the lack of evidence for their use on improving hepatocyte necrosis and inflammation[14]. However, these agents may be continued to manage T2DM optimally in these patients. Sulphonylureas are to be used with caution as some of these medications are metabolized by the liver.
Dyslipidemia, whether it is primary or secondary, is associated with a significantly higher risk of worsening of MAFLD because of metabolic dysregulation and abnormal lipid handling by the liver. Hence, the treatment of dyslipidemia is part of the routine management of MAFLD. Statins are useful in patients for the improvement of cardiometabolic outcomes, and treatment may also slow down the disease process[59,60]. Most recent data suggests that statin treatment is associated with a reduction in the steatosis grade and NAS score[61]. Therefore, statin therapy should be considered in all patients, including those with compensated cirrhosis, except when the transaminase levels are > 3 times the upper limit of normal[60]. Although Omega-3 fatty acids and fenofibrate were found to offer potential benefits in patients with hypertriglyceridemia and MAFLD in a recent small clinical trial[62], firm conclusions cannot be reached without large-scale studies. Pro-protein convertase subtilisin/kexin type 9 inhibitors are used to treat hypercholesterolemia in patients with statin intolerance or in those unable to achieve target reduction of LDL-C with tolerated doses of statins. Although these drugs are very useful for CVD protection in patients with uncontrolled dyslipidemia on conventional therapy without affecting diabetes management[63], there is not enough data on their use in patients with MAFLD to suggest recommendations for use.
α-tocopherol has been found to be useful in reducing liver transaminases with improvements in histological parameters of NASH, including ballooning, inflammation, and steatohepatitis in adults[64]. PIVEN study utilised a two by two factorial study design to evaluate the efficacy of α-tocopherol, at a dosage of 800 international units daily and pioglitazone in patients with biopsy proven NASH without diabetes[64]. At the end of 96 wk, patients on vitamin E therapy showed improvement in NASH as assessed by liver biopsy when compared to placebo (43% vs 19%, P = 0.001). The long-term efficacy of vitamin E to delay the progression of NASH is yet to be determined and there are some reports of increased all-cause mortality with high dose vitamin E but this is not proven and a meta-analysis have failed to confirm this association[65]. Also, we have to bear in mind that high dose vitamin E is associated with worsening insulin resistance; therefore, the drug is not recommended in patients with T2DM and those with advanced fibrosis[14].
Orlistat: Orlistat is a pancreatic and gastric lipase inhibitor that reduces dietary fat absorption and consequently causes energy malabsorption. The efficacy and side effects depend on the dietary fat content, and the discontinuation rate is high because of steatorrhea in those consuming high-fat diets. Conflicting results were observed in the review of clinical trials examining the efficacy of orlistat in a recent meta-analysis which showed improvements in transaminases and steatosis (on ultrasonographic imaging) in a few trials where drug use resulted in weight loss, between 5%-10%, with improvements in insulin resistance also[65]. The common drug dose is 120 mg three times daily, and steatorrhea may result in discontinuation in up to 25% of patients.
A meta-analysis of 330 patients with MAFLD treatment with orlistat showed marked improvements in the BMI (mean difference = -1.97; P = 0.02), reduction of ALT, and improvement of insulin resistance but with no significant changes to the liver fibrosis score[66]. A randomized, placebo-controlled clinical trial from Israel noted a higher reversal rate of fatty liver (24% vs 17%) in orlistat-treated group for 24 wk compared to the control group[67]. Similarly, orlistat treatment for 24 wk significantly reduced liver fat content as compared to the routine treatment. In an open label parallel group study of 170 patient who were either enrolled into orlistat or conventional care in 1:1.5 proportion, showed that the level of fat content decreased to a greater extent in the orlistat treated patients compared to the conventional care, which was [-5.45% vs -1.96%, where < 0.001 [intention to treat (ITT) analysis] and -6.66% vs -2.68%, P < 0.001 [per-protocol (PP) analysis][68]. Higher rates of improvement in steatosis grades were seen in the orlistat treatment group [45.6% vs 22.5% (ITT analysis), 57.4% vs 30.3% (PP analysis), both P < 0.001][68]. In the same study, multivariate logistic regression analysis showed that the benefit of orlistat treatment was an independent source of improvement of steatosis. In experimental animal models, orlistat has shown protective and therapeutic effects against high-fat diet-induced MAFLD via modulation of signaling pathways involved in improved metabolism, especially the Nrf2 signaling pathway[69]. In experimental studies, orlistat treatment in high fat diet-fed rats up-regulated several antioxidant enzymes, down-regulated cell death and inflammation and apoptosis in the testis and thus, ameliorated testicular dysfunction[70]. Overall, these data suggest that orlistat could influence the metabolism of patients, and this may offer therapeutic benefits against MAFLD.
Lesser studied anti-obesity medications: The less studied anti-obesity medications in MAFLD are phentermine-topiramate, naltrexone-bupropion and lorcaserin. These agents have predominant central action on pathways to control appetite and craving for food. Although weight loss benefit with these agents is similar or, in some cases, more than orlistat but these agents are fraught with cardiovascular complication. Hence, these agents are less preferred compared to the recent GLP-1RAs which have a better cardiovascular safety profile. In a post-hoc analysis, extended-release naltrexone-bupropion combination promoted improvement in FIB-4 score with no statistical change in the ALT when treated for six months[71]. In another study, lorcaserin treatment for six months improved fatty liver index and reduced energy intake without affecting lean mass[72]. More studies are needed specifically in MAFLD patients to clarify these agents' efficacy across various stages of MAFLD.
Obeticholic acid: Obeticholic acid (OCA), or 6α-ethyl-chenodeoxycholic acid, is a semisynthetic chemical compound that mimics bile acid (BA), chenodeoxycholic acid (CDCA) and is the first farnesoid X receptor (FXR) agonist to be approved by the FDA and European medicine agency (EMA) for primary biliary cholangitis also known as "Ocaliva"[73]. BA binds through G-protein-coupled BA receptor (TGR5/Gpbar-1) and activates the nuclear receptor (FXR), which regulates lipid and glucose metabolism. Hence, FXRs showcase an ideal target for managing MAFLD by modulating the homeostasis of cholesterol, triglyceride, glucose, energy, and BA synthesis[74]. OCA binds FXR with 100-fold affinity compared to its natural ligand CDCA and proves to be a practical analog of BA[75]. A phase 2 study on 64 humans showed that OCA administration (dose of 25 or 50 mg) for 6 wk increased insulin sensitivity, and reduced liver inflammation and fibrosis biomarkers in patients with MAFLD and T2DM[76]. In this double-blind, placebo-controlled, proof-of-concept study, patients were treated with either placebo (23 people), 25 mg OCA (20 people), or 50 mg OCA (21 people) once daily for 6 wk. Insulin sensitivity was measured by a 2-stage hyperinsulinemic-euglycemic insulin clamp before and after the 6-wk treatment period along with liver enzymes, fibroblast growth factor 19, lipid analytes, 7α-hydroxy-4-cholesten-3-one (a BA precursor), endogenous BAs, and markers of liver fibrosis. The insulin sensitivity increased by 28.0% and 20.1% from baseline in the group (20 people) treated with 25 mg OCA (P = 0.019) and in the group (21 people) treated with 50 mg OCA (P = 0.060) respectively. In the combined OCA groups, insulin sensitivity increased by 24.5% (P = 0.011) with a decrease of 5.5% in the placebo group[76]. In animal models, OCA treatment resulted in increased weight loss and improved insulin sensitivity in rabbits[77]. Similarly, OCA treatment improved the metabolic profile in MAFLD rats [Zucker (fa/fa) rats], while reducing visceral adiposity and hepatic steatosis[78]. Along similar lines, OCA treatment markedly affected the gene expression profile involved in hepatic lipotoxicity, including fatty acid synthesis, lipogenesis and gluconeogenesis genes[78]. In the FLINT trial, which involved 283 patients randomly assigned to receive OCA 25 mg daily or placebo for 72 wk showed 35% of patients treated with OCA showed marked reduction in fibrosis compared to 19% of patients treated with placebo[79]. These findings indicate that OCA would be a potential treatment option for patients with MAFLD, considering that OCA treatment improves the metabolic profile and reduces fatty liver and fibrosis. It should be noted that OCA is effective in obesity and type 2 diabetes patients, but the long-term safety data and also head-to-head comparison with other newer antidiabetic agents are still awaited.
Aldafermin: Aldafermin, also known as M70 or NGM282, is an analog of fibroblast growth factor 19 (FGF19), a human gut hormone secreted from the ileum in response to activation of FXR. This hormone is essential for bile acids, carbohydrates, and energy metabolism[80,81]. Elevations of primary and secondary bile acid in circulation are linked with primary liver disease[82]. It has been reported that FGF19, upon FXR activation, acts on the FGFR1c-KLB and FGFR4-KLB receptor complex on hepatocytes to block bile acids synthesis. Aldafermin, the FGF19 analog, acts similar way on this receptor complex. Upon activation, FGFR1c-KLB receptor suppresses the CYP7A1 expression. This gene encodes an enzyme, cholesterol 7a-hydroxylase, which is the first and a rate-limiting enzyme for the de novo synthesis of bile acids. In the phase 2 study of 176 patients with NASH and fibrosis (biopsy-confirmed) and increased fat in the liver (> 8% by magnetic resonance imaging-proton density fat fraction), it has been shown to have a significant reduction in the serum bile acid level[83]. These patients were administered with aldafermin 0.3 mg (23 patients), 1 mg (49 patients), 3 mg (49 patients), 6 mg (28 patients) or placebo (27 patients) for 12 wk. Similarly, 62 patients with PSC and > 1.5× upper limit of normally increased alkaline phosphatase were administered with 1 mg (21 patients), 3 mg (21 patients) aldafermin, or placebo (20 patients) for 12 wk. Serum bile acid profile and neoepitope-specific N-terminal pro-peptide of type III collagen or Pro-C3 (a direct measure of fibrogenesis) were measured for metabolic and cholestatic liver diseases. The results suggested that treatment with aldafermin caused dose-dependent reductions in serum bile acids, including deoxycholic acid, lithocholic acid, glycodeoxycholic acid, glycochenodeoxycholic acid, and glycocholic acid in patients with metabolic and cholestatic liver diseases[84]. In addition, the treatment of aldafermin resulted in the reduction of glycine-conjugated bile acids but not the taurine-conjugated bile acids. Overall, these findings suggest the possibility of using aldafermin for 24 wk as a treatment option for fatty liver disease, fibrosis regression, and resolution of NASH and stage 2 or stage 3 fibrosis.
Silymarin: Silymarin, extracted from the medicinal plant Silybum marianum, is a complex mixture of different flavonolignan isomers. Silymarin possesses an antioxidative effect and is mainly due to an isomer silybin diastereomers that is 50% of the mixture. Silybin undergoes biotransformation, forming glucuronide derivatives[85]. The mechanism of action of this drug on MAFLD involves antioxidative, choleretic, antifibrotic, regenerative, anti-inflammatory, and immunomodulatory effects[86]. Silymarin is now known as an antioxidant for reducing lipogenesis by downregulating the fatty acid synthase, peroxisome proliferator-activated receptor γ, and acetyl-CoA carboxylase[87,88]. MAFLD-induced insulin resistance and steatosis can be reduced by Silymarin which has shown the potential to restore the insulin action pathway through insulin receptor substrate-1/PI3K/Akt[89]. In a metanalysis involving 587 patients mostly from randomized controlled trials, results showed that Silymarin significantly decreased the AST and ALT levels more than the control group[90]. In a randomized, double-blind, placebo-controlled trial in NASH patients who had NAFLD activity scores of 4 or more, an intake of 700 mg per day of Silymarin for 48 wk caused a significant reduction in liver fibrosis[91]. Collectively, these studies have demonstrated that Silymarin is a safe drug, even at high doses, well tolerated, and it improves the functions of liver enzymes, activates liver metabolism, reduces oxidative stress, total cholesterol synthesis, and endothelial dysfunction in patients with MAFLD, but long-term data on different MAFLD stages are yet to come[92].
One of the effective means of obtaining durable weight loss is through surgical intervention in obese people. It aims not only for weight loss but includes improvements in incretin profiles, insulin secretion, and insulin sensitivity; hence it is a promising tool for MAFLD management. Two commonly used bariatric or metabolic surgeries are Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG). The fundal portion of the stomach is surgically removed during SG, whereas RYGB entails making a small pouch; this new pouch is then connected to the small intestine. While both surgical procedures result in decreased stomach volume, decreased production of acid, and changed gut hormones, RYGB involves architectural reorganization of the gastrointestinal tract, potentially impacting bile acid reabsorption[93]. As the primary bile acids (PBA) are made and conjugated in the liver, excreted in the intestine, deconjugated and/or transformed to secondary bile acids (SBA) by the gut microbiota, and recycled through reabsorption in the terminal ileum, changes in stomach due to the RYGB leads reorganization of the microbiota leading to change in PBA and SBA composition[94]. It was shown that MAFLD patients' serum had more PBA/SBA ratio compared to healthy people[94]. According to the AACE recent guidelines, metabolic surgeries are reserved for patients with MAFLD and BMI 35 kg/m2 especially if they have type 2 diabetes mellitus (level of recommendation grade B)[14]. It can be used as an opportunity for liver biopsy for histologic staging of liver fibrosis for further prognosis.
Many bariatric surgeries come with certain complications and limitations, including anastomotic disruption leading to leaks and fistulae, stomal stenosis, dumping syndrome, etc. The endoscopically implanted and removably attached duodenal-jejunal sleeve bypass (DJSB) or EndoBarrier® (GI Dynamics Inc., Lexington MA) can also be used for the same purpose. It is anchored in the initial section of the duodenum, where it is connected to a 60-cm long polymer sleeve by a nitinol stent anchor. The sleeve shields the proximal upper small intestine's mucosa from food that has been swallowed. DJSB offers functional similarities with RYGB as it mimics some of the physiological effects. These include food excluded from the proximal small intestine, and pancreatic and biliary secretions mixed together after food has passed through the sleeve. Endo-barrier treatment has been shown to promote significant weight loss in a patient with obesity and type 2 diabetes who are otherwise eligible for bariatric surgery but declined due to personal choice. Over the 12-mo study period, the weight loss was 15 kg (95%CI: 0.62-29.38; P < 0.05) and BMI 4.9 kg/m2 (95%CI: 1.1-8.7; P < 0.005), the confidence intervals are wide as expected for a small sample size of forty five patients[95]. The major limitation of the endo-barrier therapy is the attended complication like gastrointestinal intolerance, bleeding, and liver abscess. Although this can be reduced by improving the dietary compliance following the procedure and there are ongoing trials on how these complications can be effectively reduced so that endo-barrier can be a safe option for MAFLD management who fail medical therapy and decline surgery.
Obese patient who undergoes bariatric surgery may need to demonstrate initial weight loss for pre-operative surgery optimization of metabolic profile for safe surgical outcome. Most of these patients can get benefit from endoscopic therapy. In a study of individuals with obesity and MAFLD, bariatric endoscopy improved analytical and ultrasound parameters of insulin resistance, hepatic fat, and hypertriglyceridemia[96]. It can be suggested during short-term follow-up as an efficient and secure option, but the long-term benefits are yet to come as these are relatively newer modalities.
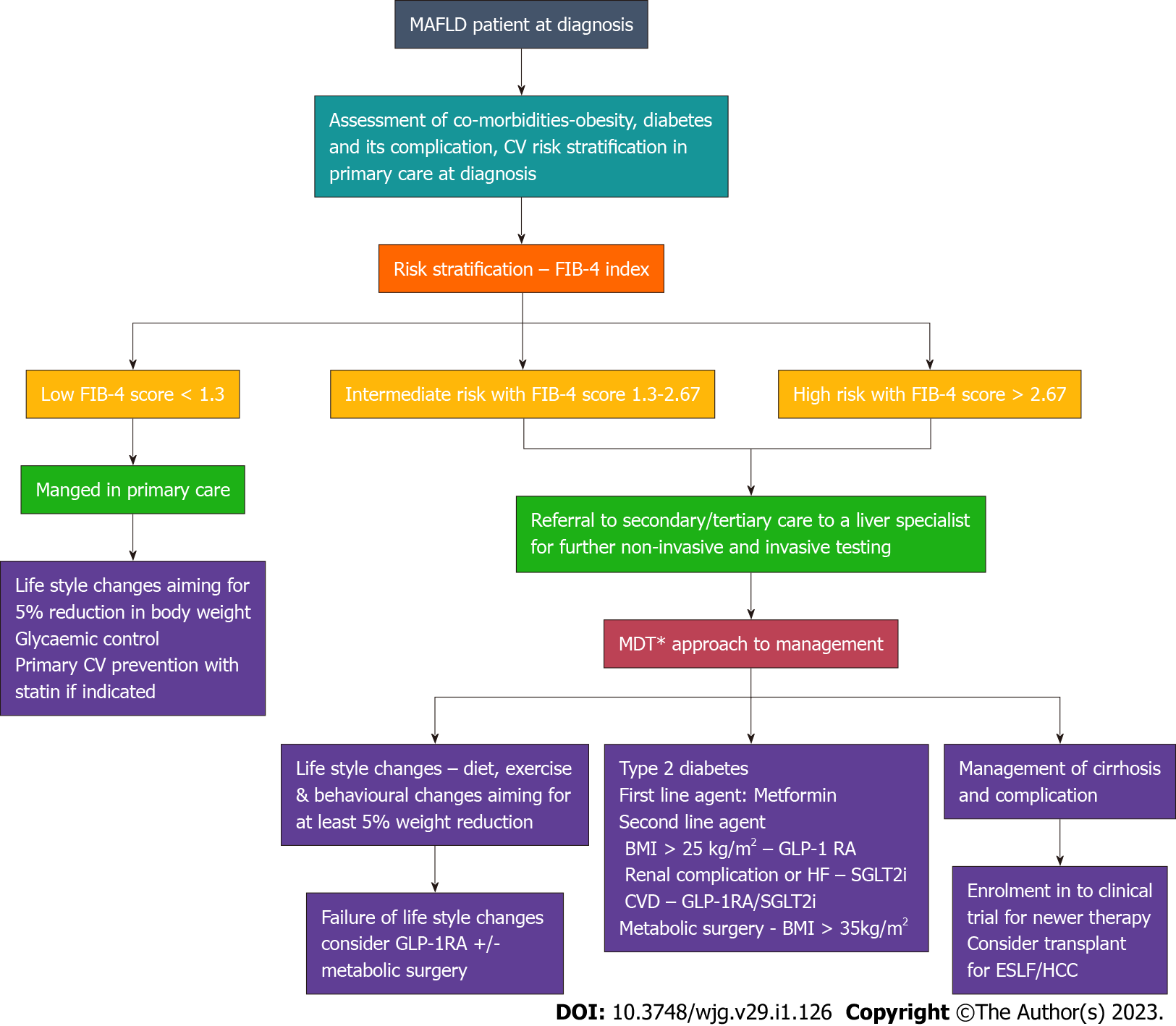
A broad approach for the management and referral system pathway for patients with MAFLD is shown in the Figure 3.
Pregnancy-related liver diseases occur in a trimester-specific fashion, as there is biotransformation of almost the whole body in pregnant women throughout the pregnancy. Developments of primary liver diseases are common during pregnancy. MAFLD in pregnancy can result in the development of various health issues in the mother, such as complications of hypertension, postpartum hemorrhage, and premature birth of the baby[97]. In a multicentric prospective study from Korea, Lee et al[98] showed that 18.4% (112/608) women had MAFLD during their early pregnancy, and MALFD is an independent risk factor in the development of gestational diabetes mellitus (GDM; 36/608). The participants with GDM development showed higher prevalence of radiological steatosis (55.6% vs 16.1%; P < 0.001) and higher fatty liver index (40.0 vs 10.7; P < 0.001) and hepatic steatosis index (35.5 vs 29.0; P < 0.001)[98]. In utero, MAFLD exposed children have a greater risk of obesity at an early age and pediatric MAFLD development[99].
Management of MAFLD during pregnancy is a multi-model approach to control complications, which should recognize the population at high risk and control hyperglycemia, prevent excessive weight gain, and avoid the development of GDM. To our best knowledge, there is no single specific medication for the treatment of MAFLD during pregnancy. Managing weight and lifestyle during the postpartum period is mandatory for reversing the MAFLD effects and avoiding complications during the next pregnancy. Study conducted in 316 individuals (not only pregnant women), exercise without loss of weight significantly lowered intrahepatic lipid content (SMD: -0.76, 95%CI: -1.04, -0.48) and ALT concentration (SMD: -0.52, 95%CI: -0.90, -0.14), AST content (SMD: -0.68, 95%CI: -1.21, -0.15), LDL-C concentration (SMD: -0.34, 95%CI: -0.66, -0.02), and triglycerides content (SMD: -0.59, 95%CI: -1.16, -0.02)[100]. Hence, pregnant women with MAFLD should be encouraged to exercise regularly within the safe limit to improve the metabolic profile with proper guidance from the expert obstetric team.
Due to a lack of recognition, screening, and appreciation of complications associated with MAFLD, the disease often remains undiagnosed among children. In a study of 2256 children with 715 children overweight, MAFLD was diagnosed in 23% among 715 with overweight (P < 0.01)[101]. As pediatric treatment options are limited due to inadequate number of clinical trials and insufficient knowledge, the overall goal is to improve the life quality of children and reduce liver morbidity and mortality. Lifestyle management is one of the best ways to manage MAFLD in children. In a study of 84 children of the age group between 3 and 18.8 years, over two years, with proper diet and physical exercise, participants who lost 5% or more of body weight had more significant improvements in the level of ALT when compared with the participants with < 5% weight loss (-35 ± 33 vs -20 ± 20 IU/L, respectively; P < 0.05)[102].
Between 40-50 years of age in males and 60-69 years in females, the prevalence of fatty liver is high and seems slightly low in older (> 70 years) cohorts. Hypertension, diabetes, hyperlipidemia, and obesity risk factors for MAFLD development are higher in elderly individuals. The diagnosis and management strategies are challenging in the elderly with other age-related comorbidities[103]. For the management of MAFLD, the underlying etiology must be treated parallel to other diseases including obesity, hyperlipidemia, and IR. Lifestyle modifications and pharmacological treatment are the two methods considered safe for managing MAFLD in elderly individuals. In a study with 261 elderly individuals for 52 wk, 72 (25%) achieved steatohepatitis resolution, 138 (47%) reduced NAS score, and 56 (19%) achieved fibrosis regression. During last week, 88 subjects (30%) reduced ≥ 5% of their weight. Degree of weight loss and improvements in all NASH-related histologic parameters are independently associated (odds ratios = 1.1-2.0; P < 0.01). A major proportion of participants with ≥ 5% loss in weight had [51 of 88 (58%)] NASH resolution and a 2-point reduction in NAS [72 of 88 (82%)] than participants with < 5% of loss in their weight (P < 0.001)[104]. In some elderly patients, these modifications might be incompatible. So, it is necessary to ensure that the introduced dietary instructions are not excessively aggressive and provide adequate nutrition to the elderly. Currently, no particular drug is approved for the treatment of MAFLD in the elderly. Therefore, there are no specific recommendations for drug usage in elderly individuals with MAFLD alone, but if they have concomitant T2DM, metformin, GLP-1RAs and SGLT-2i are the best available options.
MAFLD is a significant cause of chronic liver disease with reported increase in hepatocellular carcinoma (HCC), and a leading contributor to various systemic complications such as T2DM, CVD, and CKD. A certain degree of uncertainty is present regarding the natural history and prognosis of MAFLD. In some patients, the long evolution time for MAFLD progression from steatohepatitis to fibrosis is noted, but some progress faster from cirrhosis to hepatocellular carcinoma[105]. The exact reason for the development of HCC among MAFLD patients remains uncertain to date. When compared with chronic hepatitis C infection, the cumulative incidence of MAFLD patient developing HCC is lower (4% vs 2.5%) at 1 year and (30% vs 11%) at 5 years[106]. Moreover, patients with MAFLD has a tendency to develop HCC even without cirrhosis and the risk is five times when compared those with chronic hepatitis C infection[107]. As these patients without cirrhosis are not in the surveillance program, they tend to present with bigger tumours with reduced median survival[108]. Recent data suggest that patients who develop HCC due to MAFLD cirrhosis live longer than hepatitis C related HCC after curative treatment[109]. Liver transplantation is used either for end stage liver disease or HCC due to MAFLD. The transplant related mortality and morbidity is high compared to the HCC due to other aetiologies such as very high BMI and MAFLD related cardiovascular complications[110]. But it is interesting to note that post-transplant 5-year survival rate is not different between MAFLD and non-MALFD aetiology because the lower risk of graft failure balances the higher risk of sepsis and cardiovascular disease in MAFLD patients when compared to other aetiology[111].
More clinical trials involving different ethnicity having divergent gene pools are required to get insights into the contribution of the genetic basis of MAFLD and the genetic basis of successful treatment outcomes and failure in treatment. The effect of environmental factors should also be a primary focus of work in the next decade. The growing human population and the environmental impact have dramatically changed human life. Especially lifestyle diseases are emerging in this decade due to the impact of the environment on human life. Future studies also need to be focused on understanding this phenomenon.
Different pharmacological approaches are tried to reduce the risk of MAFLD. However, current strategies remain ineffective. The asymptomatic time intervals in MAFLD and NASH progression to cirrhosis and ultimately a liver failure and gaps in knowledge regarding modifiers of the disease contribute to significant challenges in the drug design for the clinical trials. The application of nanoparticles for drug delivery demonstrated potentially promising for enhancing drug bioavailability for MAFLD treatment. Various types of liver-targeting nanoparticles are exploited for MAFLD management[112].
Table 1 shows a summary of therapeutic strategy for MAFLD targeting risk factors.
| No | Disease/condition | Directed therapy | Supportive therapies |
| 1 | Overweight and obesity | Anti-obesity drugs, Bariatric surgery, lifestyle intervention (Calorie-restriction, dietary pattern, etc.) | MAFLD: Newer agents targeting on cellular inflammation and oxidative stress. |
| 2 | T2DM | Hypoglycemic agents like Metformin, GLP-1RAs, SGLT-2i, Thiazolidinediones and DPP-4 inhibitors | Fibrotic MAFLD: Potential future anti-fibrotic agents. |
| 3 | > 2 metabolic risks | Modulators of metabolism (Farnesoid X receptor agonsit, Peroxisome proliferator-activated receptor, fibroblast growth receptors, statins, aspirin) | Cirrhosis Complications: Control portal hypertension and bacterial peritonitis prophylaxis. End-stage liver diseases: Liver transplantation. |
MAFLD is a growing global health burden among the population with high susceptibility to obesity and insulin resistance. Notably, MAFLD is the most common cause of chronic liver disease in children and adolescents, exhibited by fatty liver disease and severe fibrosis. The most effective prevention strategy for MAFLD is lifestyle modification. Considering the prevalence and its impact, several novel therapies, including several novel therapeutic and surgical approaches, are currently under investigation. Pharmacological interventions, including treatment with antidiabetic medications, and anti-obesity drugs, can be considered for MAFLD patients but should be chosen wisely on a case-to-case basis. In addition, bariatric surgeries aimed to obtain durable weight loss are options for MAFLD patients who either fail medical management or have associated comorbidities. Novel approaches in drug delivery would be ideal for managing MAFLD in the future. We need to improve the knowledge of the personalized treatment options with targeted drugs, which would be effective in that subset of patients who fail or have difficulty adhering to the routine management pathways.
| 1. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 458] [Article Influence: 114.5] [Reference Citation Analysis (2)] |
| 2. | Yi M, Peng W, Feng X, Teng F, Tang Y, Kong Q, Chen Z. Extrahepatic morbidities and mortality of NAFLD: an umbrella review of meta-analyses. Aliment Pharmacol Ther. 2022;56:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Minhas AMK, Jain V, Maqsood MH, Pandey A, Khan SS, Fudim M, Fonarow GC, Butler J, Khan MS. Non-Alcoholic Fatty Liver Disease, Heart Failure, and Long-Term Mortality: Insights From the National Health and Nutrition Examination Survey. Curr Probl Cardiol. 2022;47:101333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Rojas YAO, Cuellar CLV, Barrón KMA, Arab JP, Miranda AL. Non-alcoholic fatty liver disease prevalence in Latin America: A systematic review and meta-analysis. Ann Hepatol. 2022;27:100706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7943] [Article Influence: 794.3] [Reference Citation Analysis (8)] |
| 6. | Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 1943] [Article Influence: 388.6] [Reference Citation Analysis (33)] |
| 7. | Diaconu CT, Guja C. Nonalcoholic Fatty Liver Disease and Its Complex Relation with Type 2 Diabetes Mellitus-From Prevalence to Diagnostic Approach and Treatment Strategies. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 8. | Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 9. | Basu R, Noureddin M, Clark JM. Nonalcoholic Fatty Liver Disease: Review of Management for Primary Care Providers. Mayo Clin Proc. 2022;97:1700-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Rabøl R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci U S A. 2011;108:13705-13709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Utzschneider KM, Kahn SE, Polidori DC. Hepatic Insulin Extraction in NAFLD Is Related to Insulin Resistance Rather Than Liver Fat Content. J Clin Endocrinol Metab. 2019;104:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3286] [Article Influence: 328.6] [Reference Citation Analysis (7)] |
| 13. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5228] [Article Influence: 653.5] [Reference Citation Analysis (9)] |
| 14. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 681] [Article Influence: 170.3] [Reference Citation Analysis (1)] |
| 15. | Vieira Barbosa J, Lai M. Nonalcoholic Fatty Liver Disease Screening in Type 2 Diabetes Mellitus Patients in the Primary Care Setting. Hepatol Commun. 2021;5:158-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 16. | Popa SL, Ismaiel A, Cristina P, Cristina M, Chiarioni G, David L, Dumitrascu DL. Non-Alcoholic Fatty Liver Disease: Implementing Complete Automated Diagnosis and Staging. A Systematic Review. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Yu Q, Liu Y, Hu P, Gao F, Huang G. Performance of Imaging Techniques in Non-invasive Diagnosis of Non-alcoholic Fatty Liver Disease in Children: A Systematic Review and Meta-Analysis. Front Pediatr. 2022;10:837116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Long MT, Noureddin M, Lim JK. AGA Clinical Practice Update: Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Lean Individuals: Expert Review. Gastroenterology. 2022;163:764-774.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 218] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 19. | Anstee QM, Lawitz EJ, Alkhouri N, Wong VW, Romero-Gomez M, Okanoue T, Trauner M, Kersey K, Li G, Han L, Jia C, Wang L, Chen G, Subramanian GM, Myers RP, Djedjos CS, Kohli A, Bzowej N, Younes Z, Sarin S, Shiffman ML, Harrison SA, Afdhal NH, Goodman Z, Younossi ZM. Noninvasive Tests Accurately Identify Advanced Fibrosis due to NASH: Baseline Data From the STELLAR Trials. Hepatology. 2019;70:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 20. | Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 665] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 21. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1786] [Article Influence: 162.4] [Reference Citation Analysis (3)] |
| 22. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 435] [Article Influence: 72.5] [Reference Citation Analysis (1)] |
| 23. | Varkaneh HK, Poursoleiman F, Al Masri MK, Alras KA, Shayah Y, Masmoum MD, Alangari FA, Alras AA, Rinaldi G, Day AS, Hekmatdoost A, Abu-Zaid A, Kutbi E. Low fat diet vs low carbohydrate diet for management of non-alcohol fatty liver disease: A systematic review. Front Nutr. 2022;9:987921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Haigh L, Kirk C, El Gendy K, Gallacher J, Errington L, Mathers JC, Anstee QM. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Clin Nutr. 2022;41:1913-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 25. | Różański G, Pheby D, Newton JL, Murovska M, Zalewski P, Słomko J. Effect of Different Types of Intermittent Fasting on Biochemical and Anthropometric Parameters among Patients with Metabolic-Associated Fatty Liver Disease (MAFLD)-A Systematic Review. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Yin C, Li Z, Xiang Y, Peng H, Yang P, Yuan S, Zhang X, Wu Y, Huang M, Li J. Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis. Front Nutr. 2021;8:709683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Kenđel Jovanović G, Mrakovcic-Sutic I, Pavičić Žeželj S, Benjak Horvat I, Šuša L, Rahelić D, Klobučar Majanović S. Metabolic and Hepatic Effects of Energy-Reduced Anti-Inflammatory Diet in Younger Adults with Obesity. Can J Gastroenterol Hepatol. 2021;2021:6649142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Scragg J, Avery L, Cassidy S, Taylor G, Haigh L, Boyle M, Trenell MI, Anstee QM, McPherson S, Hallsworth K. Feasibility of a Very Low Calorie Diet to Achieve a Sustainable 10% Weight Loss in Patients With Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2020;11:e00231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Misciagna G, Del Pilar Díaz M, Caramia DV, Bonfiglio C, Franco I, Noviello MR, Chiloiro M, Abbrescia DI, Mirizzi A, Tanzi M, Caruso MG, Correale M, Reddavide R, Inguaggiato R, Cisternino AM, Osella AR. Effect of a Low Glycemic Index Mediterranean Diet on Non-Alcoholic Fatty Liver Disease. A Randomized Controlled Clinici Trial. J Nutr Health Aging. 2017;21:404-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 30. | Hong F, Liu Y, Lebaka VR, Mohammed A, Ye W, Chen B, Korivi M. Effect of Exercise Training on Serum Transaminases in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Physiol. 2022;13:894044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Ghaffari M, Sadeghiyan S, Faramarzi M, Moghaddam M, Baghurst T. The effect of aerobic exercise on metabolic parameters of patients with non-alcoholic fatty liver disease: systematic review and meta-analysis. J Sports Med Phys Fitness. 2022;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Zhou BJ, Huang G, Wang W, Zhu LH, Deng YX, He YY, Ma FH. Intervention effects of four exercise modalities on nonalcoholic fatty liver disease: a systematic review and Bayesian network meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:7687-7697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Charatcharoenwitthaya P, Kuljiratitikal K, Aksornchanya O, Chaiyasoot K, Bandidniyamanon W, Charatcharoenwitthaya N. Moderate-Intensity Aerobic vs Resistance Exercise and Dietary Modification in Patients With Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. Clin Transl Gastroenterol. 2021;12:e00316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Sargeant JA, Gray LJ, Bodicoat DH, Willis SA, Stensel DJ, Nimmo MA, Aithal GP, King JA. The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: a systematic review and meta-analysis. Obes Rev. 2018;19:1446-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 369] [Article Influence: 73.8] [Reference Citation Analysis (2)] |
| 36. | Buzzetti E, Linden A, Best LM, Madden AM, Roberts D, Chase TJG, Freeman SC, Cooper NJ, Sutton AJ, Fritche D, Milne EJ, Wright K, Pavlov CS, Davidson BR, Tsochatzis E, Gurusamy KS. Lifestyle modifications for nonalcohol-related fatty liver disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;6:CD013156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Zelber-Sagi S, Bord S, Dror-Lavi G, Smith ML, Towne SD Jr, Buch A, Webb M, Yeshua H, Nimer A, Shibolet O. Role of illness perception and self-efficacy in lifestyle modification among non-alcoholic fatty liver disease patients. World J Gastroenterol. 2017;23:1881-1890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (2)] |
| 38. | Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 443] [Article Influence: 18.5] [Reference Citation Analysis (12)] |
| 39. | Kovalic AJ. Pharmacotherapeutic Impact on Nonalcoholic Steatohepatitis Histology: A Systematic Review and Network Meta-analysis. J Clin Exp Hepatol. 2022;12:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Raveendran AV, Fernandez CJ, Jacob K. Efficacy and Cardiovascular Safety of Thiazolidinediones. Curr Drug Saf. 2021;16:233-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Tang H, Shi W, Fu S, Wang T, Zhai S, Song Y, Han J. Pioglitazone and bladder cancer risk: a systematic review and meta-analysis. Cancer Med. 2018;7:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 42. | Filipova E, Uzunova K, Kalinov K, Vekov T. Pioglitazone and the Risk of Bladder Cancer: A Meta-Analysis. Diabetes Ther. 2017;8:705-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 43. | Lian J, Fu J. Efficacy of Various Hypoglycemic Agents in the Treatment of Patients With Nonalcoholic Liver Disease With or Without Diabetes: A Network Meta-Analysis. Front Endocrinol (Lausanne). 2021;12:649018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 45. | Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 46. | Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1396] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 47. | Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A; SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022;387:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 2053] [Article Influence: 513.3] [Reference Citation Analysis (0)] |
| 48. | Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 532] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 49. | Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol. 1996;271:E458-E464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 125] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide vs Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021;385:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 1266] [Article Influence: 253.2] [Reference Citation Analysis (0)] |
| 51. | Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE; EFC6018 GetGoal-Mono Study Investigators. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care. 2012;35:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 52. | Nauck MA, D'Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol. 2022;21:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (1)] |
| 53. | Yoshiji S, Minamino H, Tanaka D, Yamane S, Harada N, Inagaki N. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular and renal outcomes: A meta-analysis and meta-regression analysis. Diabetes Obes Metab. 2022;24:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Zafar Y, Rashid AM, Siddiqi AK, Ellahi A, Ahmed A, Hussain HU, Ahmed F, Menezes RG, Siddiqi TJ, Maniya MT. Effect of novel glucose lowering agents on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2022;46:101970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 55. | Avgerinos I, Manolopoulos A, Michailidis T, Kitsios K, Liakos A, Karagiannis T, Dimitrakopoulos K, Matthews DR, Tsapas A, Bekiari E. Comparative efficacy and safety of glucose-lowering drugs as adjunctive therapy for adults with type 1 diabetes: A systematic review and network meta-analysis. Diabetes Obes Metab. 2021;23:822-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Sikirica MV, Martin AA, Wood R, Leith A, Piercy J, Higgins V. Reasons for discontinuation of GLP1 receptor agonists: data from a real-world cross-sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2017;10:403-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 57. | Weiss T, Carr RD, Pal S, Yang L, Sawhney B, Boggs R, Rajpathak S, Iglay K. Real-World Adherence and Discontinuation of Glucagon-Like Peptide-1 Receptor Agonists Therapy in Type 2 Diabetes Mellitus Patients in the United States. Patient Prefer Adherence. 2020;14:2337-2345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 58. | Gan S, Dawed AY, Donnelly LA, Nair ATN, Palmer CNA, Mohan V, Pearson ER. Efficacy of Modern Diabetes Treatments DPP-4i, SGLT-2i, and GLP-1RA in White and Asian Patients With Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care. 2020;43:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Tzanaki I, Agouridis AP, Kostapanos MS. Is there a role of lipid-lowering therapies in the management of fatty liver disease? World J Hepatol. 2022;14:119-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J Gastroenterol. 2018;24:3361-3373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 412] [Cited by in RCA: 409] [Article Influence: 51.1] [Reference Citation Analysis (8)] |
| 61. | Boutari C, Pappas PD, Anastasilakis D, Mantzoros CS. Statins' efficacy in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin Nutr. 2022;41:2195-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 62. | Camacho-Muñoz D, Kiezel-Tsugunova M, Kiss O, Uddin M, Sundén M, Ryaboshapkina M, Lind L, Oscarsson J, Nicolaou A. Omega-3 carboxylic acids and fenofibrate differentially alter plasma lipid mediators in patients with non-alcoholic fatty liver disease. FASEB J. 2021;35:e21976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Monami M, Sesti G, Mannucci E. PCSK9 inhibitor therapy: A systematic review and meta-analysis of metabolic and cardiovascular outcomes in patients with diabetes. Diabetes Obes Metab. 2019;21:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2550] [Article Influence: 159.4] [Reference Citation Analysis (18)] |
| 65. | Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4:158-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 66. | Pan CS, Stanley TL. Effect of Weight Loss Medications on Hepatic Steatosis and Steatohepatitis: A Systematic Review. Front Endocrinol (Lausanne). 2020;11:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Wang H, Wang L, Cheng Y, Xia Z, Liao Y, Cao J. Efficacy of orlistat in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2018;9:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Zelber-Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, Leshno M, Blendis L, Halpern Z, Oren R. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 215] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 69. | Ye J, Wu Y, Li F, Wu T, Shao C, Lin Y, Wang W, Feng S, Zhong B. Effect of orlistat on liver fat content in patients with nonalcoholic fatty liver disease with obesity: assessment using magnetic resonance imaging-derived proton density fat fraction. Therap Adv Gastroenterol. 2019;12:1756284819879047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Zakaria Z, Othman ZA, Bagi Suleiman J, Jalil NAC, Ghazali WSW, Mohamed M. Protective and Therapeutic Effects of Orlistat on Metabolic Syndrome and Oxidative Stress in High-Fat Diet-Induced Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) in Rats: Role on Nrf2 Activation. Vet Sci. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Suleiman JB, Nna VU, Zakaria Z, Othman ZA, Bakar ABA, Mohamed M. Obesity-induced testicular oxidative stress, inflammation and apoptosis: Protective and therapeutic effects of orlistat. Reprod Toxicol. 2020;95:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 72. | Bajaj HS, Burrows M, Blavignac J, Paron E, Camacho F, Gould E, Barakat M. Extended-release naltrexone/bupropion and liver health: Pooled, post hoc analysis from four randomized controlled trials. Diabetes Obes Metab. 2021;23:861-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Tuccinardi D, Farr OM, Upadhyay J, Oussaada SM, Mathew H, Paschou SA, Perakakis N, Koniaris A, Kelesidis T, Mantzoros CS. Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: A six-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes Metab. 2019;21:1487-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 74. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D; POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 854] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 75. | Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1083] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 76. | Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 77. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574-82.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 750] [Article Influence: 57.7] [Reference Citation Analysis (4)] |
| 78. | Vignozzi L, Morelli A, Filippi S, Comeglio P, Chavalmane AK, Marchetta M, Toce M, Yehiely-Cohen R, Vannelli GB, Adorini L, Maggi M. Farnesoid X receptor activation improves erectile function in animal models of metabolic syndrome and diabetes. J Sex Med. 2011;8:57-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 355] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 80. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1841] [Article Influence: 167.4] [Reference Citation Analysis (3)] |
| 81. | DePaoli AM, Zhou M, Kaplan DD, Hunt SC, Adams TD, Learned RM, Tian H, Ling L. FGF19 Analog as a Surgical Factor Mimetic That Contributes to Metabolic Effects Beyond Glucose Homeostasis. Diabetes. 2019;68:1315-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Kliewer SA, Mangelsdorf DJ. Bile Acids as Hormones: The FXR-FGF15/19 Pathway. Dig Dis. 2015;33:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 83. | Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Barritt AS 4th. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:3318-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 84. | Sanyal AJ, Ling L, Beuers U, DePaoli AM, Lieu HD, Harrison SA, Hirschfield GM. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep. 2021;3:100255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 85. | Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern Med Rev. 2011;16:239-249. [PubMed] |
| 86. | Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 365] [Article Influence: 22.8] [Reference Citation Analysis (2)] |
| 87. | Akhtar DH, Iqbal U, Vazquez-Montesino LM, Dennis BB, Ahmed A. Pathogenesis of Insulin Resistance and Atherogenic Dyslipidemia in Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2019;7:362-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 88. | Cui CX, Deng JN, Yan L, Liu YY, Fan JY, Mu HN, Sun HY, Wang YH, Han JY. Silibinin Capsules improves high fat diet-induced nonalcoholic fatty liver disease in hamsters through modifying hepatic de novo lipogenesis and fatty acid oxidation. J Ethnopharmacol. 2017;208:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Zhang Y, Hai J, Cao M, Zhang Y, Pei S, Wang J, Zhang Q. Silibinin ameliorates steatosis and insulin resistance during non-alcoholic fatty liver disease development partly through targeting IRS-1/PI3K/Akt pathway. Int Immunopharmacol. 2013;17:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Zhong S, Fan Y, Yan Q, Fan X, Wu B, Han Y, Zhang Y, Chen Y, Zhang H, Niu J. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine (Baltimore). 2017;96:e9061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 91. | Wah Kheong C, Nik Mustapha NR, Mahadeva S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:1940-1949.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 92. | Aghemo A, Alekseeva OP, Angelico F, Bakulin IG, Bakulina NV, Bordin D, Bueverov AO, Drapkina OM, Gillessen A, Kagarmanova EM, Korochanskaya NV, Kucheryavii UA, Lazebnik LB, Livzan MA, Maev IV, Martynov AI, Osipenko MF, Sas EI, Starodubova A, Uspensky YP, Vinnitskaya EV, Yakovenko EP, Yakovlev AA. Role of silymarin as antioxidant in clinical management of chronic liver diseases: a narrative review. Ann Med. 2022;54:1548-1560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 93. | Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 94. | Talavera-Urquijo E, Beisani M, Balibrea JM, Alverdy JC. Is bariatric surgery resolving NAFLD via microbiota-mediated bile acid ratio reversal? Surg Obes Relat Dis. 2020;16:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Patel N, Mohanaruban A, Ashrafian H, Le Roux C, Byrne J, Mason J, Hopkins J, Kelly J, Teare J. EndoBarrier®: a Safe and Effective Novel Treatment for Obesity and Type 2 Diabetes? Obes Surg. 2018;28:1980-1989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Espinet Coll E, Vila Lolo C, Díaz Galán P, Gómez Valero JA, Bacchiddu S, Quintana Tomás C, Irigoyen D, Gunnard K, Juan-Creix Comamala A. Bariatric and metabolic endoscopy in the handling of fatty liver disease. A new emerging approach? Rev Esp Enferm Dig. 2019;111:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Sarkar M, Grab J, Dodge JL, Gunderson EP, Rubin J, Irani RA, Cedars M, Terrault N. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol. 2020;73:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 98. | Lee SM, Kwak SH, Koo JN, Oh IH, Kwon JE, Kim BJ, Kim SM, Kim SY, Kim GM, Joo SK, Koo BK, Shin S, Vixay C, Norwitz ER, Park CW, Jun JK, Kim W, Park JS. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62:238-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 99. | Mosca A, De Cosmi V, Parazzini F, Raponi M, Alisi A, Agostoni C, Nobili V. The Role of Genetic Predisposition, Programing During Fetal Life, Family Conditions, and Post-natal Diet in the Development of Pediatric Fatty Liver Disease. J Pediatr. 2019;211:72-77.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Babu AF, Csader S, Lok J, Gómez-Gallego C, Hanhineva K, El-Nezami H, Schwab U. Positive Effects of Exercise Intervention without Weight Loss and Dietary Changes in NAFLD-Related Clinical Parameters: A Systematic Review and Meta-Analysis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 101. | Riley MR, Bass NM, Rosenthal P, Merriman RB. Underdiagnosis of pediatric obesity and underscreening for fatty liver disease and metabolic syndrome by pediatricians and pediatric subspecialists. J Pediatr. 2005;147:839-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 102. | Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, Sartorelli MR, Angulo P. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 103. | Alqahtani SA, Schattenberg JM. NAFLD in the Elderly. Clin Interv Aging. 2021;16:1633-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 104. | Mobasheri N, Ghahremani L, Fallahzadeh Abarghooee E, Hassanzadeh J. Lifestyle Intervention for Patients with Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial Based on the Theory of Planned Behavior. Biomed Res Int. 2022;2022:3465980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 105. | Nath P, P Singh S. Nonalcoholic Fatty Liver Disease: Time to Take the Bull by the Horns. Euroasian J Hepatogastroenterol. 2018;8:47-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Oda K, Uto H, Mawatari S, Ido A. Clinical features of hepatocellular carcinoma associated with nonalcoholic fatty liver disease: a review of human studies. Clin J Gastroenterol. 2015;8:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 107. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-31.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 513] [Article Influence: 51.3] [Reference Citation Analysis (1)] |
| 108. | Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, Sumpter K, Stewart S, Rose J, Hudson M, Manas D, Reeves HL. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 458] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 109. | Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, Quek J, Xiao J, Dan YY, Siddiqui MS, Sanyal AJ, Muthiah MD, Loomba R, Huang DQ. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 110. | Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 381] [Article Influence: 63.5] [Reference Citation Analysis (1)] |
| 111. | Cholankeril G, Wong RJ, Hu M, Perumpail RB, Yoo ER, Puri P, Younossi ZM, Harrison SA, Ahmed A. Liver Transplantation for Nonalcoholic Steatohepatitis in the US: Temporal Trends and Outcomes. Dig Dis Sci. 2017;62:2915-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 112. | Moosavian SA, Sathyapalan T, Jamialahmadi T, Sahebkar A. The Emerging Role of Nanomedicine in the Management of Nonalcoholic Fatty Liver Disease: A State-of-the-Art Review. Bioinorg Chem Appl. 2021;2021:4041415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Italy; Kaewdech A, Thailand; Meng QH, China; Moriyama K, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H