Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6935
Peer-review started: May 18, 2022
First decision: August 1, 2022
Revised: October 2, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: December 28, 2022
Processing time: 210 Days and 5.4 Hours
Irritable bowel syndrome and bladder pain syndrome often overlap and are both characterized by visceral hypersensitivity. Since pelvic organs share common sensory pathways, it is likely that those syndromes involve a cross-sensitization of the bladder and the colon. The precise pathophysiology remains poorly understood.
To develop a model of chronic bladder-colon cross-sensitization and to investigate the mech-anisms involved.
Chronic cross-organ visceral sensitization was obtained in C57BL/6 mice using ultrasound-guided intravesical injections of acetic acid under brief isoflurane anesthesia. Colorectal sensitivity was assessed in conscious mice by measuring intracolonic pressure during isobaric colorectal distensions. Myeloperoxidase, used as a marker of colorectal inflammation, was measured in the colon, and colorectal permeability was measured using chambers. c-Fos protein expression, used as a marker of neuronal activation, was assessed in the spinal cord (L6-S1 level) using immunohistochemistry. Green fluorescent protein on the fractalkine receptor-positive mice were used to identify and count microglia cells in the L6-S1 dorsal horn of the spinal cord. The expression of NK1 receptors and MAPK-p38 were quantified in the spinal cord using western blot.
Visceral hypersensitivity to colorectal distension was observed after the intravesical injection of acetic acid vs saline (P < 0.0001). This effect started 1 h post-injection and lasted up to 7 d post-injection. No increased permeability or inflammation was shown in the bladder or colon 7 d post-injection. Visceral hypersensitivity was associated with the increased expression of c-Fos protein in the spinal cord (P < 0.0001). In green fluorescent protein on the fractalkine receptor-positive mice, intravesical acetic acid injection resulted in an increased number of microglia cells in the L6-S1 dorsal horn of the spinal cord (P < 0.0001). NK1 receptor and MAPK-p38 levels were increased in the spinal cord up to 7 d after injection (P = 0.007 and 0.023 respectively). Colorectal sensitization was prevented by intrathecal or intracerebroventricular injections of minocycline, a microglia inhibitor, by intracerebroventricular injection of CP-99994 dihydrochloride, a NK1 antagonist, and by intracerebroventricular injection of SB203580, a MAPK-p38 inhibitor.
We describe a new model of cross-organ visceral sensitization between the bladder and the colon in mice. Intravesical injections of acetic acid induced a long-lasting colorectal hypersensitivity to distension, mediated by neuroglial interactions, MAPK-p38 phosphorylation and the NK1 receptor.
Core Tip: A model of chronic cross-organ visceral sensitization in mice was developed using ultrasound-guided intravesical injections of acetic acid. Visceral hypersensitivity to colorectal distension was observed as early as 1 h post-injection and lasted up to 7 d. Visceral hypersensitivity was associated with an increased expression of c-Fos protein in the spinal cord. The NK1 receptor and MAPK-p38 levels were upregulated in the spinal cord 7 d post-injection. Colorectal sensitization was prevented by intrathecal or intracerebroventricular injections of minocycline, a microglia inhibitor, by intracerebroventricular injection of CP-99994, a NK1 antagonist, and by intracerebroventricular injection of SB203580, a MAPK-p38 inhibitor.
- Citation: Atmani K, Wuestenberghs F, Baron M, Bouleté I, Guérin C, Bahlouli W, Vaudry D, do Rego JC, Cornu JN, Leroi AM, Coëffier M, Meleine M, Gourcerol G. Bladder-colon chronic cross-sensitization involves neuro-glial pathways in male mice. World J Gastroenterol 2022; 28(48): 6935-6949
- URL: https://www.wjgnet.com/1007-9327/full/v28/i48/6935.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i48.6935
Irritable bowel syndrome (IBS) and bladder pain syndrome (BPS) are two functional disorders that affect the gastrointestinal tract and the urinary tract, respectively[1,2]. Their prevalence in the general population is 4.6%[3] and 4.2%[4], respectively, and recent studies have shown a strong overlap between both syndromes[1,2]. Indeed, BPS is found in 40%-60% of IBS patients[5], and IBS is observed in 25.4%-38.6% of BPS patients[1,2]. Both syndromes are characterized by visceral mechanical hypersensitivity at the urinary tract level for BPS and at the intestinal level for IBS[6,7]. The involvement of several mechanisms has been suggested in the onset and/or the maintenance of visceral hyperalgesia, including urothelial and/or intestinal epithelial permeability, mucosal immune activation and altered brain-gut interaction[8].
Despite increasing knowledge of the pathophysiology of IBS and BPS, limited mechanistic data is available in the context of BPS-IBS overlap. Based on the fact that pelvic organs share common sensory pathways, a few studies have offered evidence that cross-sensitization between the bladder and the colon may explain sensitization of both organs[9]. This may involve primary extrinsic afferents or central sensitization, both at the spinal and supraspinal levels[10]. All these studies involved acute sensitization models, often in anesthetized animals[11]; therefore, there is not yet any data regarding the chronicization of pelvic organ cross-sensitization.
The role of spinal glia has recently been highlighted in the sensitization of the bladder or colon[12] and even in cross-organ sensitization between both organs[13]. The role of spinal glia, however, has never been demonstrated in the maintenance of such sensitization using models of bladder-colon cross-sensitization induced chronic visceral hyperalgesia. The aims of our study were therefore to develop a model of chronic cross-organ sensitization between the bladder and the colon and to investigate the mechanisms involved in the development and persistence of this cross-sensitization.
The experiments were carried out in accordance with the ethical guidelines of the International Association for the Study of Pain[14] and in accordance with the guidelines of the French Ministry of Agriculture and Fisheries (Decree No. 874848). Our protocol was approved by the local Ethics Committee for Animal Experiments (CENOMEXA No: N/02-01-13/02/01-16).
Adult male wild type C57Bl/6 mice (Janvier Laboratories, Le Genest-Saint-Isle, France) and transgenic mice expressing the green fluorescent protein on the fractalkine receptor of microglial cells (Inserm Laboratory U1239, Dr. David Vaudry Team, Mont Saint Aignan, France[15]) were 8 wk old on the day of the experiment (weight range: 22-26 g). The animals were randomized by their weight in several cages, with five mice per cage, housed in a 12 h light/dark cycle in an animal housing facility free of specific pathogenic organisms and maintained at room temperature (22 ± 2 °C). The animals received a standard diet (RM1 diet; SDS, Witham, Essex, United Kingdom). Drinking water and food were available ad libitum. Each manipulation or experiment took place after at least 1 wk of acclimatization to the housing conditions. All animals were euthanized by cervical dislocation after anesthesia with ketamine (100 mg/kg, Imalgene 1000; Merial, Lyon, France) and xylazine (10 mg/kg, Rompun® 2%; Bayer, Berlin, Germany) administered intraperitoneally before tissue collection.
The animal protocol was designed to minimize pain or discomfort to the animals. Eight series of mice were used in this work. Each set was comprised of different groups of mice, and each group was formed of 5-8 mice. The first series was used to develop the cross-organ sensitization model. Once the model was validated, a second series was used to analyze inflammatory parameters in the colon and the bladder and to assess colonic and bladder permeabilities. We then focused on the central nervous system, especially at lamina I and II of the dorsal horn at the L6-S1 segment, where pelvic extrinsic primary afferent neurons form synapses with spinal neurons. Our third series assessed the neuronal activity in this model using c-Fos expression, and inflammatory parameters were measured in the spinal cord of a fourth series. The model was then transposed on transgenic mice to assess changes in microglia cells. Finally, three different inhibitors/antagonists were used in the sixth, seventh and eighth series to gain a better understanding of which pathway could be involved in the cross-sensitization process.
An injection of acetic acid (0.75%, 200 μL) or saline (NaCl 0.9%, 200 µL; Baxter, Deerfield, IL, United States) was made into the urinary bladder using ultrasound monitoring in mice under brief anesthesia (isoflurane: 3% in 1.5 L/min of air, Iso-Vet®; Piramal Critical Care, Voorschoten, The Netherlands).
We measured visceral pain to colorectal distension (CRD) using a non-invasive method, as previously reported in mice[16]. Changes in intracolonic pressure, reflecting viscero-motor responses induced by CRD (nociceptive stimulus), were used as a surrogate marker of colorectal sensitivity[16].
An infinitely compliant distension balloon (diameter 0.7 cm) was made using a polyethylene bag attached to a PE-50 catheter (Intramedics, France) drilled in its end and taped 2 cm below the pressure sensor of a miniaturized pressure transducer catheter (SPR-524 Mikro-Tip catheter; Millar Instruments, Houston, TX, United States). Polypropylene 4-0 Ligatures (Prolène®; Ethicon Inc., Somerville, NJ, United States) were covered with parafilm to prevent any air leak.
On the experimental day, mice were briefly anesthetized with isoflurane (3% in air), and the lubricated “balloon-pressure sensor” was introduced into the colorectum, so that the balloon was inserted 2 cm upstream of the anal margin into the colon. Each mouse was placed in an adjustable mouse restrainer (30 mm diameter × 90 mm length, LE5016; In Vivo Research Instruments, Perkin Elmer, Waltham, MA, United States) and left to rest for 30 min before the CRD procedure. The balloon was then secured to the tail with tape and connected to an electronic barostat (Distender Series II; G & J Electronics Inc., Toronto, ON, Canada) to perform isobaric CRD. Our distension protocol consisted of a set of graded phasic distensions of 15, 30, 45 and 60 mmHg (two times each, 20 s duration, 4 min inter-stimulus interval) (Protocol Plus Deluxe; G & J Electronics, Toronto, ON, Canada). Voltage output was converted digitally using CED digital-analogic converter (Micro 1401; Cambridge Electronic Design, Cambridge, United Kingdom) and Spike 2 software (CED, Ltd., Cambridge, United Kingdom). The pressure sensor allowed the assessment of visceral pain via a custom-made script that allowed signals to be specifically extracted from abdominal muscle contractions (excluding those from colonic contractions).
Colonic sensitivity was measured in awake mice at 60 min, 3 d and 7 d following acetic acid urinary bladder injections.
After euthanasia, bladder and distal colon samples were removed on day 7. Samples were cut along the mesenteric border. Bladder and colonic permeabilities were assessed by measuring fluorescein isothiocyanate (FITC)-dextran (4 kDa) fluxes in Ussing chambers with an exchange surface of 0.07 cm2 (Harvard Apparatus, Holliston, MA, United States) as previously described[17]. FITC-dextran (5 mg/mL) was loaded on the mucosal side. After 3 h at 37 °C, the medium from the contralateral side (serosa) was removed and stored at -80 °C. The fluorescence level of FITC-dextran (excitation at 485 nm, emission at 535 nm) was measured in a 96-well black plate using spectrometer Chameleon V (Hidex Co, Turku, Finland). The results were converted to concentrations of FITC-dextran (mg/mL) for analysis.
After euthanasia, bladder and distal colon samples were removed on day 7. Colonic and vesical tissues (around 50 mg) were washed in phosphate-buffered saline and then homogenized (50 mg/mL) in 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich, Steinheim, Germany) with 50 mmol/L of phosphate-buffered saline (pH 6.0). They were frozen at -80 °C and thawed at 37 °C three times, then sonicated (Vibra Cell ultrasonic processor 75115; Bioblock Scientific, Illkirch, France) and finally centrifuged (14000 rpm at 4 °C for 15 min). Myeloperoxidase (MPO) was assayed in the supernatant by adding 1 mg/mL of dianisidine dihydrochloride (Sigma-Aldrich) and 5 × 10-5% of hydrogen peroxide (Sigma-Aldrich). The change in optical density was measured at 450 nm. One unit of MPO activity was defined as the amount that degraded 1.0 µmol of hydrogen peroxide per minute at 25 °C, and human neutrophil MPO (Sigma-Aldrich) was used as standard, as previously described[18,19].
Seven days after the intrabladder injection of acetic acid 0.75%, c-Fos immunohistochemistry was performed after 120 min of CRD at 45 mmHg (20 s of distension every 4 min for 120 min). Under ketamine (100 mg/kg i.p.)/xylazine (10 mg/kg) anesthesia and upon thoracotomy, mice were perfused through a cardiac-aorta cannula with saline followed by 150 mL/mouse of ice-cold 4% paraformaldehyde and 14% saturated picric acid in a 0.1 M phosphate buffer solution (pH 7.2). After decapitation, the lumbo-sacral spinal cord (L6-S1) was post-fixed in the same fixative solution overnight at 4 °C, cryoprotected by immersion in 10% sucrose overnight and transferred to 30% sucrose overnight. The spinal cords were then embedded in Tissue-Tek® optimal cutting temperature compound (Sakura Finetek United States, Inc., Torrance, CA, United States), snap-frozen and cut with a cryostat. Fluorescent microscopy was used to identify activated neurons. The expression of c-Fos was assessed by immunofluorescence. We applied anti-c-Fos antibody (1:2000; Calbiochem, Darmstadt, Germany) overnight at 4 °C and then incubated Cy3-conjugated goat anti-rabbit IgG (1:400; Fisher, Invitrogen, Carlsbad, CA, United States) for 2 h at room temperature. Pictures were taken using a fluorescence microscope (DM5500 B; Leica Microsystem Ltd., Wetzlar, Germany) at magnification × 10, and the number of c-Fos immunoreactive cells in lamina I and II of the dorsal horn at the L6-S1 segment of the spinal cord was counted for each mouse. The average number of stained nuclei in three 20 μm thick slices for each mouse was used for analysis.
Total RNA from L6-S1 spinal cord segments was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). RNA was purified according to the manufacturer’s instructions. Total RNA was treated with DNase I (Invitrogen) to remove any contaminating DNA. DNase I was stopped with DNase inactivation reagent (Invitrogen) according to the manufacturer’s instructions. The quality and quantity of total RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, MA, United States). The ratio of absorbance at 260 nm and 280 nm was used to assess the purity of RNA. A ratio of ≥ 2.0 was accepted for analysis.
After reverse transcription of 1.5 μg total RNA into cDNA by using 200 units of SuperScript II Reverse Transcriptase (Invitrogen), quantitative PCR was performed using SYBR Green technology on a Bio-Rad CFX96 real time PCR system (Bio-Rad Laboratories, Marnes-la-Coquette, France). The GAPDH gene was chosen as the reference gene. All samples were performed in duplicate in a single 96-well reaction plate. Serially diluted cDNA samples were used as external standards. The absolute quantification of mRNA was performed by converting the sample Ct values to concentration (copies per µL) based on standard curves. The identity and purity of the amplified products were assessed using melting curve analysis at the end of amplification. The technique was used to assay TNF-α, IL-1β and IL-10 mRNA in the spinal cord. The primer sequences for the targeted mouse genes are presented in Supplementary Table 1.
Green fluorescent protein on the fractalkine receptor-positive transgenic mice were perfused as described for c-Fos immunofluorescence, and the dorsal horn of L6-S1 spinal cord was embedded in Tissue-Tek® optimal cutting temperature compound, snap-frozen and cut with a cryostat. Pictures were then taken with the Leica photonic microscope used for c-Fos experiments at magnification × 10, and the number of microglia cells expressing microglia green fluorescent protein in lamina I and II of L6-S1 dorsal spinal cord was counted using NIH ImageJ software (version 2.0.0-rc-43/1.51u)[20]. The average number of stained nuclei per field in three 20 μm thick slices for each mouse was used for statistics.
Minocycline (2.5 mg/mL; 1.25 mg/kg; Sigma-Aldrich), a microglia blocker, CP 99994 (15 mg/mL; 7.5 mg/kg; Sigma-Aldrich), a NK1R antagonist, SB203580 (5 mg/mL; 2.5 mg/kg; Calbiochem, Merck, EMD Millipore Corp., Billerica, MA, United States), a MAPK-p38 blocker, or saline were injected in the intracerebroventricular (ICV) region using a Hamilton syringe (NeurosTM, Gastight, 1705, 33 gauge; Dutscher, Bernolsheim, France) 1 h before injecting acetic acid (0.75%) into the bladder. Intrathecal injections of minocycline at the L6-S1 Level of the spinal cord were also performed to demonstrate that microglia activation occurred at that level.
After euthanasia, the L6-S1 segment of the mice spinal cord was removed on ice and quickly frozen in liquid nitrogen. After thawing on ice, the spinal cord samples were homogenized at 4 °C in a lysis buffer (100 μL of buffer A × 2, 2 μL of 100 mmol/L dithiothreitol, 50 μL of 1% NP40, 1 μL of 1 × P8340 protease inhibitors, 2 μL of 1 × P2850 phosphatase inhibitors and 200 mL of H2O). Samples were displayed on ice for 15 min, then centrifuged at 12000 × g for 15 min at 4 °C. The protein-containing supernatant was collected and stored at -80 °C until analysis. The proteins (25 μg) were loaded on a 4%-20% gradient polyacrylamide gel (Bio-Rad) and transferred onto a nitro-cellulose membrane (GE Healthcare, Orsay, France). Membranes were then blocked for 1 h at room temperature with 5% (w/v) nonfat dry milk in Tris buffered saline containing 0.05% Tween® 20 (Sigma-Aldrich). An overnight incubation at 4 °C was then performed with primary antibodies: anti-P-p38 (1:500; Cell Signaling Technology®, Leiden, The Netherlands; P/N 4511) or anti-NK1R (1:200; Atlas Antibodies, Bromma, Sweden; P/N HPA074573) from rabbit or anti-GAPDH (1:5000; Santa Cruz Biotechnology, Tebu-Bio, Le Perray en Yvelines, France) from goat. All antibodies were diluted in a blocking solution. After three washes, a 1-h incubation was performed with a peroxidase-conjugated IgG secondary antibody from goat anti-rabbit or from rabbit anti-goat (1:5000; Santa Cruz Biotechnology, Tebu-Bio, Le Perray en Yvelines, France). After three additional washes, immunocomplexes were revealed using the ECL detection system (GE Healthcare Life Sciences, Little Chalfont, United Kingdom). Proteins were quantified by densitometry using ImageScanner III and ImageQuant TL software (GE Healthcare Life Sciences), and standardized against the intensity of GAPDH.
The data were expressed as mean ± standard error of the mean. Quantitative data was compared between groups using an unpaired t-test, with Welch’s correction in case of unequal variances. Comparisons of multiple groups were performed using one-way analysis of variance (Kruskal-Wallis test) with post-hoc analysis (Dunn’s multiple comparison post-test to compare all pairs of columns to each other or Dunnett’s post-test to compare all pairs of columns to controls) to assess the difference among the groups, and two-way analysis of variance with post-hoc analysis (Bonferroni’s correction) was used to assess groups with repeated measures. Individual data for visceral sensitivity in mice, especially the kinetics of visceromotor responses to increasing CRD, was visually assessed for each experimental group separately; tracings of animals with aberrant or outlier responses to distensions were excluded from the analysis. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 9.3.1 for Windows (GraphPad Software Inc., San Diego, CA, United States, www.graphpad.com). The statistical methods used in this study were reviewed by Fabien Wuestenberghs from CHU UCL Namur.
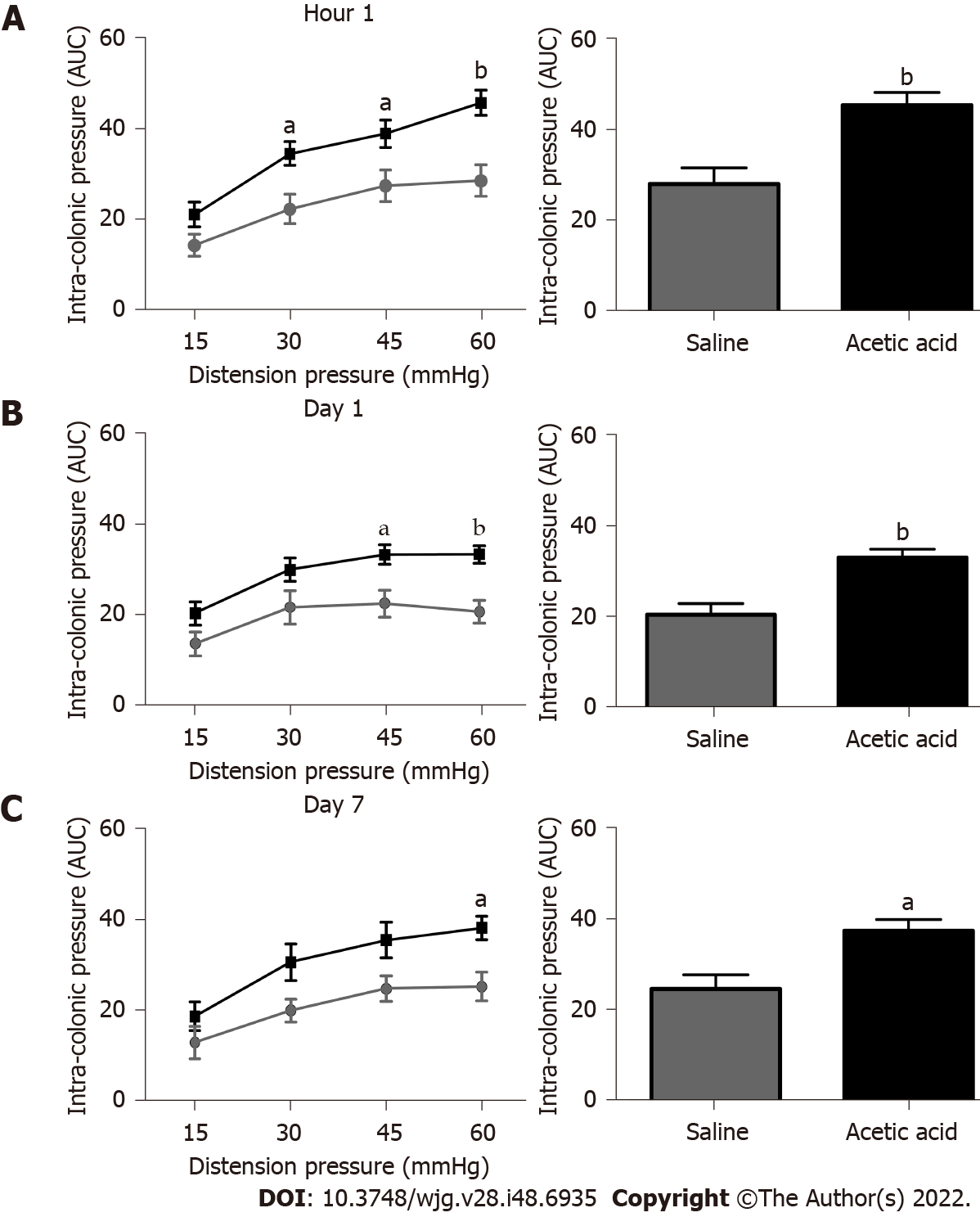
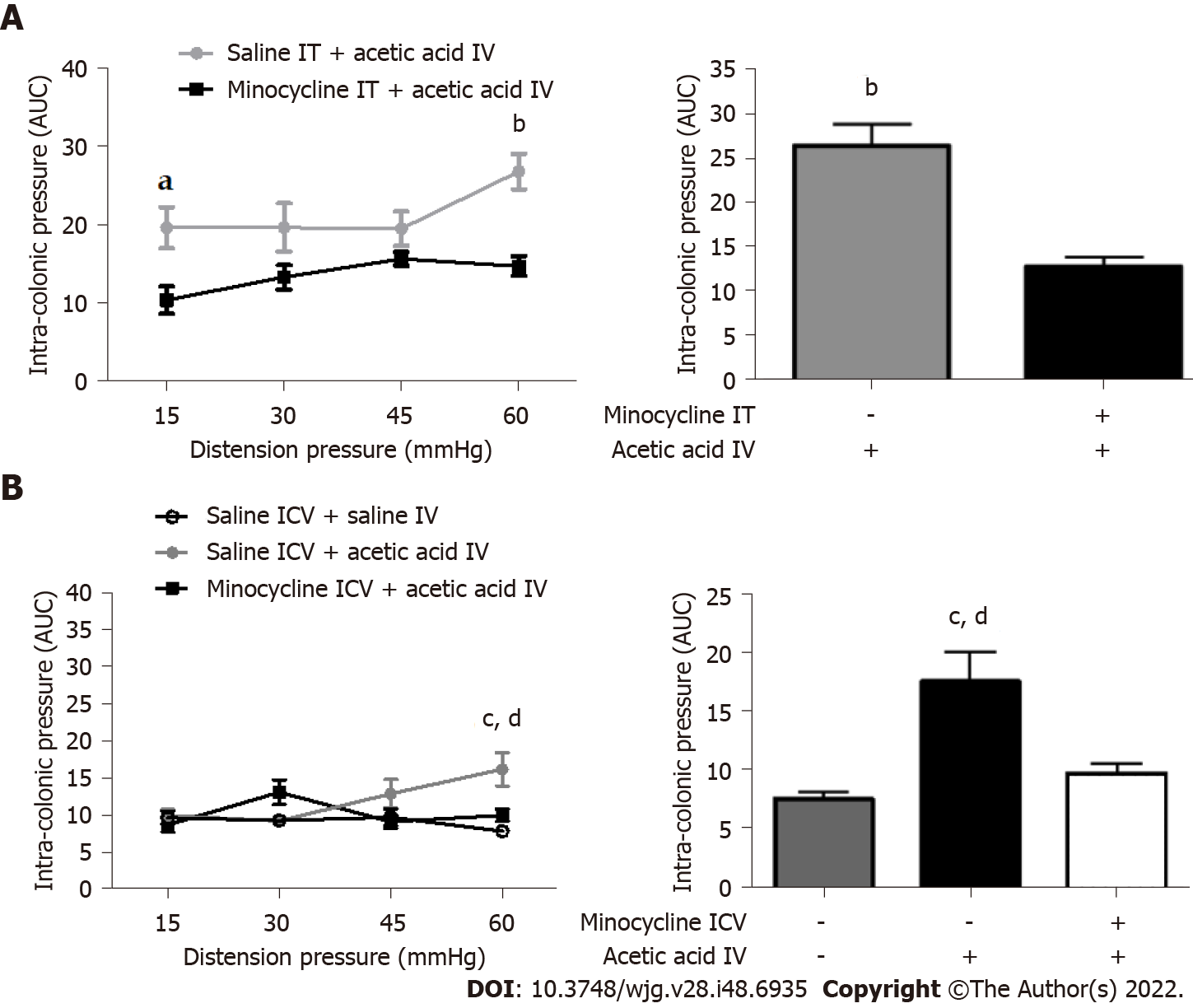
Based on our previous rat study[9], we tested the intravesical administration of a 0.75% acetic acid solution in mice. A single intravesical injection of 0.75% acetic acid under ultrasound monitoring induced an increase of the colonic nociceptive response during CRD at 30 mmHg (P < 0.05), 45 mmHg (P < 0.05) and 60 mmHg (P < 0.001) 1 h after injection (Figure 1). An increased colonic nociceptive response during CRD was still observed at 60 mmHg (P < 0.05) on 7th d in the acetic acid group compared to the control group (Figure 1), confirming that colonic hypersensitivity persists up to 7 d after the intravesical injection in our model. Further experiments were therefore designed to understand the mechanisms of the chronicization of cross-sensitization in this model. No serious adverse events occurred during the experiments.
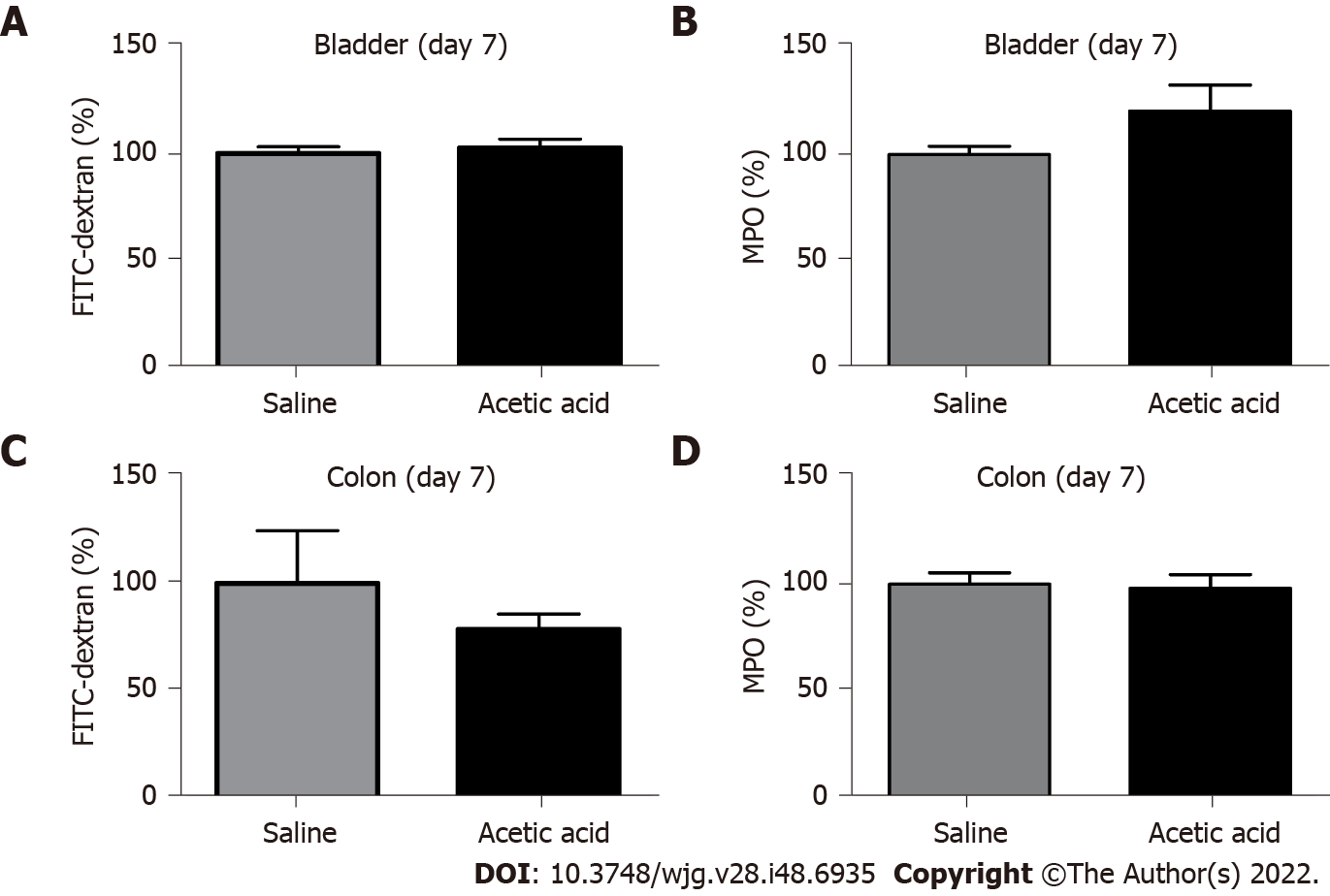
No differences were found at day 7 in either bladder or colon permeabilities or MPO activities between animals treated with acetic acid or saline intravesically (Figure 2).
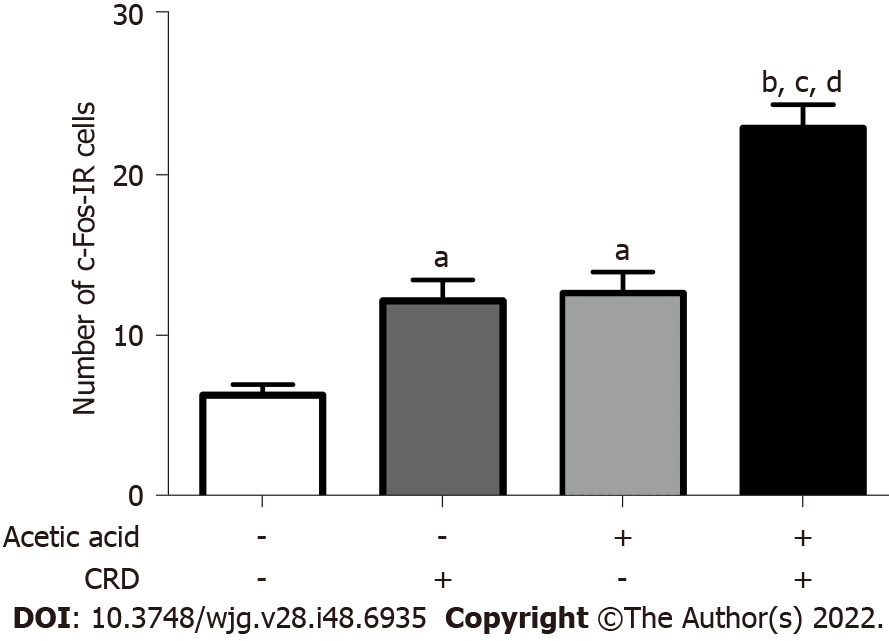
In the absence of CRD, the number of c-Fos immunoreactive cells in lamina I and II of the L6-S1 level of the dorsal horn was 6.5 ± 0.7 per slice in mice treated with saline. The CRD performed on the 7th d in these mice treated with saline increased the number of c-Fos immunoreactive cells to 12.4 ± 1.3 (P < 0.05). In mice treated with 0.75% acetic acid, the number of c-Fos immunoreactive cells was 12.9 ± 1.3 in the absence of CRD and increased to 23.2 ± 1.5 (P < 0.05 vs all other groups) after CRD (Figure 3).
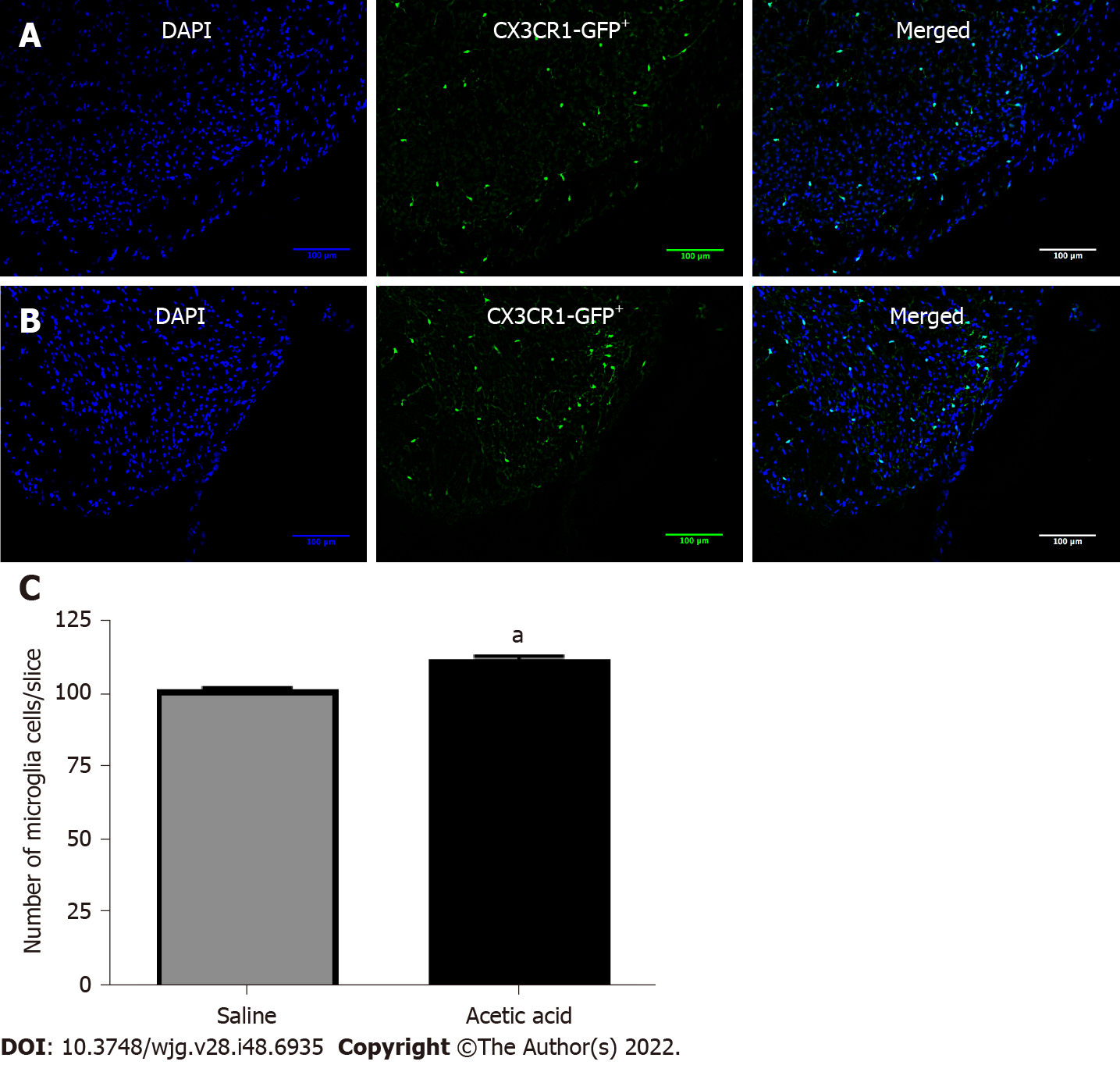
The number of microglial cells has been observed in a transgenic mouse model that specifically expresses green fluorescent protein associated with the fractalkine receptor (Figure 4A and B). Seven days after injection of a 0.75% acetic acid solution into the bladder, the number of microglial cells increased compared to mice injected with saline (113.4 ± 13.0 vs 102.9 ± 11.2 per field, respectively, P < 0.05) (Figure 4C).
We administered minocycline, a microglial inhibitor, at the central level 1 h before the intravesical injection of acetic acid or saline to demonstrate the involvement of microglia cells. Both intrathecal and ICV injections of minocycline prevented the development of cross-sensitization induced by intravesical administration of acetic acid (Figure 5A and B). ICV injections were favored for the following experiments because they are less traumatic and cause less stress to the animals.
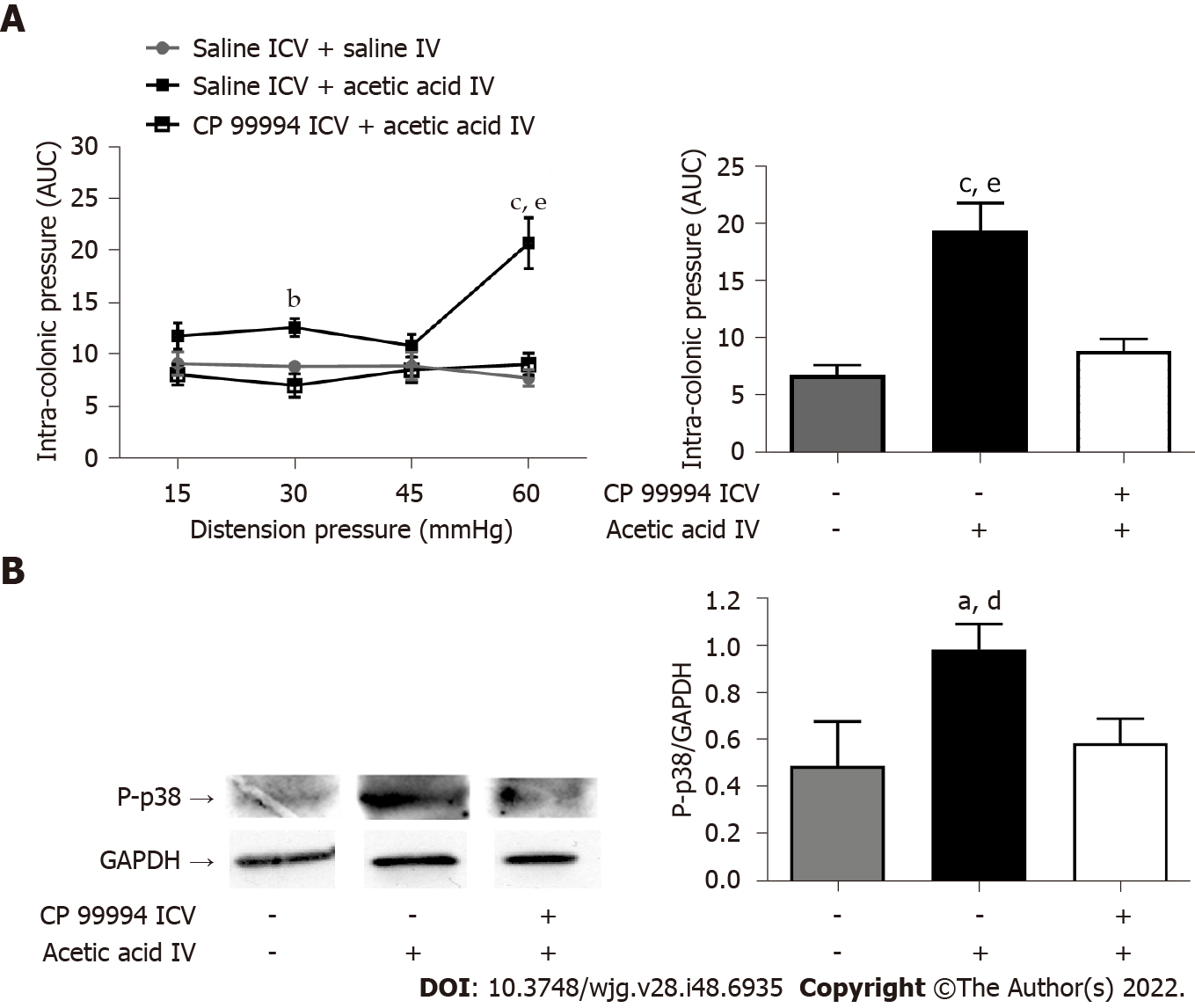
The ICV injection of CP 99994, a NK1R antagonist, 1 h before the intravesical injection of acetic acid, prevented the development of bladder-colon cross-sensitization compared to mice treated intracerebroventricularly by saline (Figure 6A). The intravesical administration of acetic acid increased the phosphorylation of MAPK-p38, a microglial protein involved in chronic pain generation[21], compared to mice injected intravesically with saline, but the ICV injection of CP 99994 prevented this phosphorylation, suggesting that microglial activation depends on the activation of NK1R (Figure 6B).
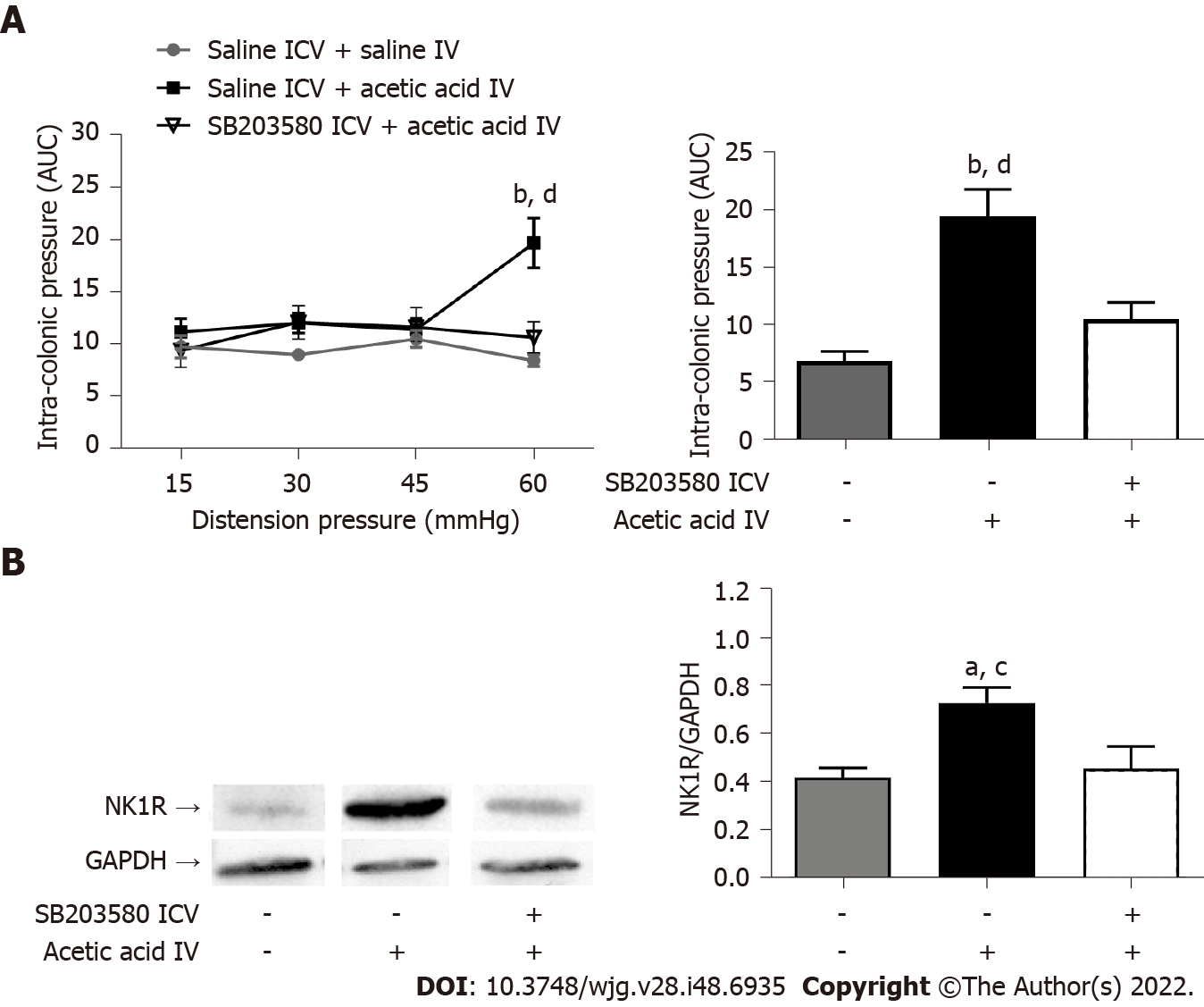
The ICV injection of SB203580, a MAPK-p38 inhibitor, 1 h before the intravesical injection of acetic acid prevented the occurrence of bladder-colon cross-sensitization (Figure 7A). The intravesical administration of acetic acid induced an increase in NK1R expression in the posterior horn of the L6-S1 level of the spinal cord compared to the administration of saline (Figure 7B). Acetic acid-induced spinal overexpression of NK1R was blocked by prior ICV administration of SB203580 (Figure 7B), suggesting that the MAPK-p38 pathway is involved in the development of bladder-colon cross-sensitization.
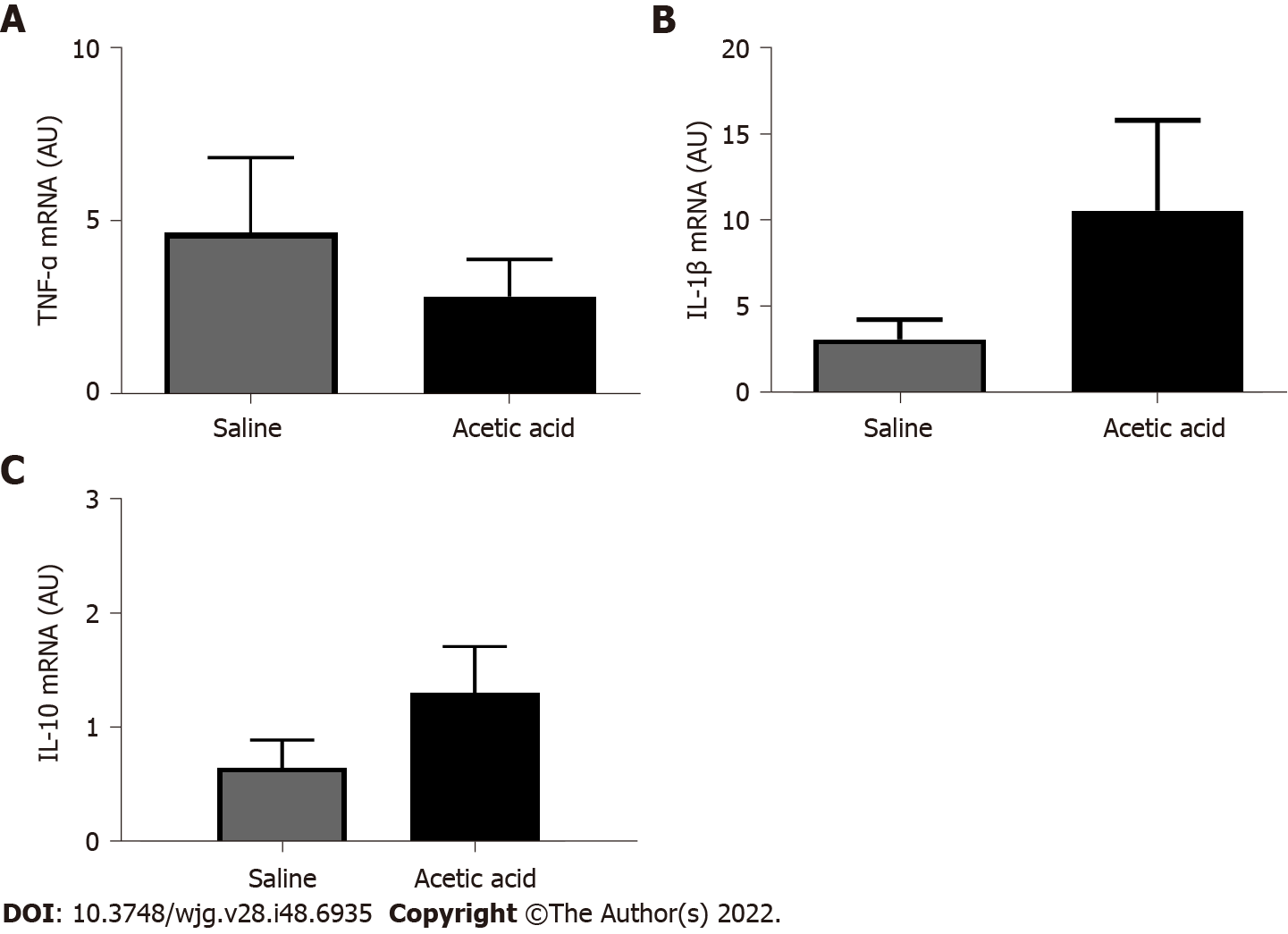
Expression levels of IL-1β, TNF-α and IL-10 mRNA did not differ between control and acetic acid groups (P = 0.22, 0.47 and 0.19, respectively) (Figure 8).
A mouse model of bladder to colon cross-sensitization with persistent visceral hypersensitivity in the colorectum has not been developed until now to our knowledge. Our study showed that pelvic visceral cross-sensitization involves central sensitization with microglia modulation following a peripheral inflammatory event and is mediated by the NK1 and MAPK-p38 pathways.
The pathophysiology of colon-bladder cross-sensitization is complex and probably involves both peripheral and central mechanisms including: the sensitization of sensory nerve terminals in both organs; cross-sensitization of adjacent sensory primary afferent neurons within dorsal root ganglia (involving satellite glia cells and macrophages); axon reflexes via primary sensory afferent neurons with a dichotomizing axon explaining neurogenic inflammation if there is a convergence of sensory information from distinct organs to a single neuron and antidromic release of inflammatory mediators in the unaffected organ (pre-spinal convergence); the sensitization of second order spinal neurons in which there is a convergence of inputs from both colon and bladder, involving spinal interneurons (convergence-projection theory or dorsal root reflex); and supraspinal mechanisms (modified central processing of visceral stimuli in the amygdala, etc.)[10]. Our work adds to the current understanding that central sensitization in the spinal cord involves microglia modulation and is mediated by the NK1 and MAPK-p38 pathways.
It is already known that an increase in proinflammatory factors within the bladder tissue can sensitize the colon[22-24]. Several mechanisms have been proposed to explain this inter-organ sensitization. These mechanisms include the increased mechanical sensitivity of visceral muscle afferents, a higher proportion of chemosensitive visceral afferents[23], brainstem neurons integrating somatovisceral messages after bladder irritation[25], and central sensitization resulting from stressful life events[26].
In our study, we used a mouse model of colonic visceral hypersensitivity induced by cross-organ sensitization to demonstrate that microglia play a central role in the development of this hypersensitivity. Indeed, we showed that the ICV injection of minocycline, which is known to be an inhibitor of microglial cells, blocks the cross-sensitization process. It has already been shown that minocycline also induces a decrease in neuronal excitability by preventing phosphorylation of the ERK protein and MAPK, which are expressed in the spinal cord[27]. The analgesic effect of minocycline is mediated by its action in both microglial activation and neuronal activation. Microglial cells have already been shown to be recruited during colonic sensitization induced by chronic stress in rats[12], but our study is the first to identify the crucial role of microglia in bladder to colon cross-sensitization and in a mouse model.
We showed that the role of microglia in the development of cross-sensitization from the bladder to the colon is mediated by the tachykininergic pathway, particularly involving NK1R. This receptor activates, directly or indirectly, the MAPK-p38 protein via its phosphorylation. Several studies have confirmed that MAPK-p38 is expressed only by microglial cells[21,28,29]. Its involvement in the course of chronic stress-induced colonic sensitization has already been demonstrated in rats[12]. Another study suggested that the activation of microglial MAPK-p38 protein at the ventromedial nucleus of the spinal cord (rostral ventromedial medulla) was responsible for uterocolonic cross-sensitization in an acute model[30]. In our work, we found that visceral hypersensitivity induced by chronic stress was associated with the phosphorylation of MAPK-p38 at the microglial cell level in mice and that this effect was inhibited by the ICV injection of a MAPK-p38 inhibitor (SB203580).
Bradesi et al[12] also showed that the fractalkine receptor potentiates the development of visceral hypersensitivity via a chemokine function on NK1R. The activation of MAPK-p38 is known to be associated with the increased synthesis and secretion of several neurotransmitters of inflammation, such as COX2, IL-1β, inducible nitric oxide synthase, PLA2 and PGE2[31]. The mediators of inflammation involved in our model are probably different from those involved in the trinitrobenzenesulfonic acid-induced colitis models, in which expression of IL-1β is upregulated in the spinal cord[32] since we did not demonstrate any changes at the TNF-α, IL-1β and IL-10 mRNA level.
We therefore propose a mechanistic view of the molecular and cellular mechanisms underlying the development of colonic hypersensitivity by bladder-to-colon cross-sensitization in which the inflammatory reaction following irritation of the urothelium induces the activation of extrinsic primary afferent neurons, some of them co-innervating the bladder and the colon and giving rise to axon reflexes, while others innervating the colon are activated by paracrine interactions. Convergent neurons in the dorsal root ganglia and the spinal cord, and those innervating the colon secondarily sensitize the colon. Some activated extrinsic primary afferent neurons relaying to other neurons in the dorsal horn of the spinal cord specifically secrete substance P, a member of the tachykinin family, which binds to NK1R activating the MAPK-p38 by phosphorylation. This in turn could either directly or indirectly induce the synthesis of mediators involved in neuroplasticity and neuroinflammation in the spinal cord.
A mini-invasive approach that specifically targets the bladder without inducing structural lesions in neighboring areas (especially the colon and peritoneum) is a prerequisite for cross-sensitization. Indeed, abdominal surgery is known to induce stress, involving a hormonal stress response at the peripheral and central levels, and implicating the corticotropin-releasing factor pathways[33]. It can induce visceral sensitization in the absence of signs of overt inflammation in mice[16]. Similarly, we decided to use measurements of the intracolonic pressure as a surrogate marker of visceromotor responses induced by CRD to assess visceral sensitivity in our study. This technique was an alternative to the measurement of the electromyographic activity of the abdominal muscles[34] and had the advantage of being minimally invasive.
When we planned our study, the available animal models for the study of bladder-bowel interactions included acute[11] and chronic[35,36] colon irritation with 2,4,6-trinitrobenzenesulfonic acid in mice[36] and in rats[11] and acute bladder irritation by cyclophosphamide, an antitumoral drug known to induce hemorrhagic cystitis in mice[35] and by infusing protamine sulphate and potassium chloride in rats[11]. In those studies, colonic sensitivity was assessed on day 5 after bladder irritation in mice and on the day of the irritation in rats.
The main limitation of our model is that the chronicity of the cross-organ sensitization was considered at day 7 following bladder irritation in mice. This period may seem short in view of clinical situations that evolve over several years. However, no other inflammatory model of cross-sensitization from the bladder to the colon published in the literature exceeds 48 h[35]. Furthermore, stress-induced chronic colonic sensitization models with 11 d of homotypic stress[12] were shown to be sufficient to induce changes in colonic tenderness and spinal microglia modulation. Our 7-d period in mice therefore seems to be long enough to attest to the chronicity of the process. Further studies with a longer longitudinal assessment of visceral sensitivity are necessary to confirm our results. Our results can only be extrapolated to males because sex-dependent differences in the responses to the sensitization process[37,38] and microglia[39] have been reported. Since pelvic neuroanatomy is similar in other rodents and in humans, we could expect similar results in those species, which could explain in part the common overlap between BPS and IBS in the clinic.
In conclusion, we have developed the first model of cross-organ chronic visceral sensitization between bladder and colon in mice. Pelvic cross-sensitization involves central sensitization with microglia modulation and is mediated by the NK1 receptor pathway and MAPK-p38 activation.
Limited mechanistic data is available in the context of overlap between bladder pain syndrome and irritable bowel syndrome. Based on the fact that pelvic organs share common sensory pathways, a few studies have offered evidence that cross-sensitization between the bladder and the colon may explain sensitization of both organs. This may involve primary extrinsic afferents or central sensitization, both at the spinal and supraspinal levels.
The precise pathophysiology involved in cross-sensitization of the bladder and the colon remains poorly understood.
The objectives of this study were to develop a model of chronic bladder-colon cross-sensitization and to investigate the mechanisms involved.
Chronic cross-organ visceral sensitization was obtained in C57BL/6 mice using ultrasound-guided intravesical injections of acetic acid under brief isoflurane anesthesia. Colorectal sensitivity was assessed in conscious mice by measuring intracolonic pressure during isobaric colorectal distension. Three different inhibitors/antagonists were assessed to gain a better understanding of which pathway could be involved in the cross-sensitization process.
Visceral hypersensitivity to colorectal distension was observed after the intravesical injection of acetic acid. This effect started 1 h post-injection and lasted up to 7 d post-injection. Colorectal sensitization was prevented by intrathecal or intracerebroventricular injections of minocycline, a microglia inhibitor, by intracerebroventricular injection of CP-99994 dihydrochloride, a NK1 antagonist, and by intracerebroventricular injection of SB203580, a MAPK-p38 inhibitor.
We described a new model of cross-organ visceral sensitization between the bladder and the colon in mice lasting up to 7 d. Intravesical injections of acetic acid induced colorectal hypersensitivity to distension, mediated by neuroglial interactions, MAPK-p38 phosphorylation and the NK1 receptor.
A bladder-colon chronic cross-sensitization mouse model using intravesical injections of acetic acid can be used as a preclinical model of overlap between bladder pain syndrome and irritable bowel syndrome.
The authors thank Professor Paul Mulder and Professor Vincent Richard (Inserm Unit 1096, Rouen, France) for sharing their ultrasound machine.
| 1. | Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 254] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, Doggweiler R, Yang CC, Mishra N, Nordling J. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol. 2010;184:1358-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158:1262-1273.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 4. | Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Mathieu N. [Somatic comorbidities in irritable bowel syndrome: fibromyalgia, chronic fatigue syndrome, and interstitial cystitis]. Gastroenterol Clin Biol. 2009;33 Suppl 1:S17-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 291] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Lai HH, Vetter J, Jain S, Gereau RW 4th, Andriole GL. The overlap and distinction of self-reported symptoms between interstitial cystitis/bladder pain syndrome and overactive bladder: a questionnaire based analysis. J Urol. 2014;192:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Barbara G, Cremon C, De Giorgio R, Dothel G, Zecchi L, Bellacosa L, Carini G, Stanghellini V, Corinaldesi R. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Langlois LD, Le Long E, Meleine M, Antor M, Atmani K, Dechelotte P, Leroi AM, Gourcerol G. Acute sacral nerve stimulation reduces visceral mechanosensitivity in a cross-organ sensitization model. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 10. | Brumovsky PR, Gebhart GF. Visceral organ cross-sensitization - an integrated perspective. Auton Neurosci. 2010;153:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Bradesi S, Svensson CI, Steinauer J, Pothoulakis C, Yaksh TL, Mayer EA. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology. 2009;136:1339-1348, e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Qiao LY, Tiwari N. Spinal neuron-glia-immune interaction in cross-organ sensitization. Am J Physiol Gastrointest Liver Physiol. 2020;319:G748-G760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5938] [Cited by in RCA: 6337] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 15. | Brifault C, Gras M, Liot D, May V, Vaudry D, Wurtz O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke. 2015;46:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Larauche M, Gourcerol G, Million M, Adelson DW, Taché Y. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress. 2010;13:343-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Beutheu S, Ouelaa W, Guérin C, Belmonte L, Aziz M, Tennoune N, Bôle-Feysot C, Galas L, Déchelotte P, Coëffier M. Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin Nutr. 2014;33:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Meleine M, Boudieu L, Gelot A, Muller E, Lashermes A, Matricon J, Silberberg C, Theodorou V, Eschalier A, Ardid D, Carvalho FA. Comparative effects of α2δ-1 Ligands in mouse models of colonic hypersensitivity. World J Gastroenterol. 2016;22:7111-7123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 19. | Maaser C, Kannengiesser K, Specht C, Lügering A, Brzoska T, Luger TA, Domschke W, Kucharzik T. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006;55:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Rasband WS. Image J. U.S. National Institutes of Health, Bethesda, Maryland, USA. Available from: https://imagejnihgov/ij/. |
| 21. | Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017-4022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 648] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 22. | Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 23. | Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1250-G1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Furuta A, Suzuki Y, Naruoka T, Asano K, Egawa S, Yoshimura N. Cross-sensitization mechanisms between colon and bladder via transient receptor potential A1 stimulation in rats. Int Urogynecol J. 2014;25:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Kaddumi EG, Hubscher CH. Changes in rat brainstem responsiveness to somatovisceral inputs following acute bladder irritation. Exp Neurol. 2007;203:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1116] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 27. | Cho IH, Lee MJ, Jang M, Gwak NG, Lee KY, Jung HS. Minocycline markedly reduces acute visceral nociception via inhibiting neuronal ERK phosphorylation. Mol Pain. 2012;8:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 471] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 29. | Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154 Suppl 1:S10-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 901] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 30. | Chen Z, Xie F, Bao M, Li X, Chao Y, Lin C, Guo R, Zhang C, Wu A, Yue Y, Guan Y, Wang Y. Activation of p38 MAPK in the rostral ventromedial medulla by visceral noxious inputs transmitted via the dorsal columns may contribute to pelvic organ cross-sensitization in rats with endometriosis. Neuroscience. 2015;291:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003;14:1153-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Majima T, Funahashi Y, Kawamorita N, Takai S, Matsukawa Y, Yamamoto T, Yoshimura N, Gotoh M. Role of microglia in the spinal cord in colon-to-bladder neural crosstalk in a rat model of colitis. Neurourol Urodyn. 2018;37:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (3)] |
| 34. | Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, Taché Y. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G215-G227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006;291:G658-G665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G451-G457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Ji Y, Tang B, Cao DY, Wang G, Traub RJ. Sex differences in spinal processing of transient and inflammatory colorectal stimuli in the rat. Pain. 2012;153:1965-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Yoshiyama M, Kobayashi H, Araki I, Du S, Zakoji H, Takeda M. Sex-related differences in activity of lower urinary tract in response to intravesical acid irritation in decerebrate unanesthetized mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R954-R960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, Maricos M, Jordan P, Buonfiglioli A, Gielniewski B, Ochocka N, Cömert C, Friedrich C, Artiles LS, Kaminska B, Mertins P, Beule D, Kettenmann H, Wolf SA. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018;24:2773-2783.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Société Nationale Française de Gastro-Entérologie.
Specialty type: Neurosciences
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Han J, China; Lu Q, China S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H