Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6922
Peer-review started: September 25, 2022
First decision: October 30, 2022
Revised: November 1, 2022
Accepted: November 25, 2022
Article in press: December 18, 2022
Published online: December 28, 2022
Processing time: 92 Days and 22.2 Hours
Acute-on-chronic liver failure (ACLF) is a syndrome that occurs in patients with chronic liver disease and is characterized by acute decompensation, organ failure and high short-term mortality. Partially due to the lack of universal diagnostic criteria, the actual ACLF prevalence remains unclear; nevertheless, it is expected to be a highly prevalent condition worldwide. Earlier transplantation is an effective protective measure for selected ACLF patients. Besides liver trans-plantation, diagnosing and treating precipitant events and providing supportive treatment for organ failures are currently the cornerstone of ACLF therapy. Although new clinical specific therapies have been researched, more studies are necessary to assess safety and efficacy. Therefore, future ACLF management strategies must consider measures to improve access to liver transplantation because the time window for this life-saving therapy is frequently narrow. Thus, an urgent and global discussion about allocation and prioritization for transplantation in critically ill ACLF patients is needed because there is evidence suggesting that the current model may not portray their waitlist mortality. In addition, while donor organ quality is meant to be a prognostic factor in the ACLF setting, recent evidence suggests that machine perfusion of the liver may be a safe tool to improve the donor organ pool and expedite liver transplantation in this scenario.
Core Tip: Acute-on-chronic liver failure (ACLF) is characterized by high short-term mortality. Although new clinical specific therapies have been researched, more studies are necessary to assess safety and efficacy. Conversely, earlier transplantation is effective for selected patients. Therefore, future ACLF management strategies must consider measures to improve access to liver transplantation. Discussions about donor organ allocation and recipient prioritization are necessary because there is evidence suggesting the current model may not portray the waitlist mortality of these patients. In this scenario, machine perfusion of the liver may prove to be a safe tool to improve the donor organ pool.
- Citation: Della Guardia B, Boteon APCS, Matielo CEL, Felga G, Boteon YL. Current and future perspectives on acute-on-chronic liver failure: Challenges of transplantation, machine perfusion, and beyond. World J Gastroenterol 2022; 28(48): 6922-6934
- URL: https://www.wjgnet.com/1007-9327/full/v28/i48/6922.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i48.6922
Despite the heterogeneity in the diagnosis criteria, consensually, acute-on-chronic liver failure (ACLF) is a condition that occurs in patients with chronic liver disease developing multi-organ failure in the presence of one or more hepatic or extrahepatic precipitant events. In addition, it is associated with high 28-d mortality[1-4]. Several international consortiums proposed different definitions of ACLF, reflecting their own types of underlying liver disease and precipitant events. The Asian Pacific Association for the Study of the Liver (APASL), the European Association for the Study of the Liver (EASL), formed by the Chronic Liver Failure (CLIF) Consortium and the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) are the most widely accepted[1,5,6].
Because of the lack of universal diagnostic criteria and study design limitations, the actual ACLF prevalence remains unclear. However, it is expected to be a highly prevalent condition worldwide. For example, in the CANONIC study (EASL-CLIF Acute-on-Chronic Liver Failure in Cirrhosis study), a prospective evaluation of 1343 patients admitted for acutely decompensated cirrhosis, 22.6% met the EASL-ACLF criteria for ACLF, and 8.3% developed the condition during hospitalization. In another study using APASL criteria, 12% of complicated cirrhotic patients were diagnosed with ACLF[1,7]. Recently, a large meta-analysis of global epidemiological data found about 35% of ACLF in patients admitted due to acutely decompensated cirrhosis using EASL-ACLF criteria, with a higher prevalence in South Asia, reaching 65%[8]. Notably, the overall mortality in ACLF is about 32% and increases in parallel with the number of organ failure (OF). The 28-d mortality rate for ACLF - grades 1 to 3 - ranges from 20% to 80%, 49% to 77%, and 13% to 86% for EASL-CLIF Consortium, NACSELD, and APASL-ACLF, respectively[4,9].
In recent years, several studies have investigated the pathophysiology, prognosis, and treatment options for ACLF. Although the benefit of timing liver transplantation (LT) in ACLF is undeniable, issues still exist with assessing the mortality risk of patients on the waiting list to avoid futile LT. In addition, another urgent and global discussion is about allocation and prioritization for LT in critically ill ACLF patients, as the time window for transplantation is frequently narrow[2,10,11].
This review provides an overview of ACLF management, focusing on the current challenges of LT in this scenario and future perspectives, including machine perfusion of the liver.
The natural history of cirrhosis is characterized by a long asymptomatic phase called compensated cirrhosis. The increase of portal pressure above the hepatic venous pressure gradient of 10 mmHg plays a central role in the transition to decompensated cirrhosis. Acute decompensation (AD) is defined by the new development of ascites, hepatic encephalopathy, hepatorenal syndrome, variceal bleeding, or infection[1,9].
In the PREDICT study (PREDICTing ACLF), a prospective and observational analysis of 1071 patients hospitalized with AD, 218 (20%) developed ACLF in 90 d (3-mo mortality rate of 53.7%), called pre-ACLF. Two hundred and thirty-three patients (22%) required frequent hospitalization unrelated to ACLF, called unstable decompensated cirrhosis (3-mo mortality rate of 21.0%), and 620 (58%) reached a state called stable decompensated cirrhosis (1-year mortality rate of 9.5%). These three clinical courses revealed by the PREDICT Study group originated from data of the CANONIC study, which created the EASL-CLIF definition of ACLF - probably the most widely accepted definition used to date[1,12]. Therefore, cirrhotic patients with pre-ACLF should be promptly identified due to the high risk of death in the short term.
The EASL-CLIF definition considers ACLF in cirrhotic patients with or without prior episodes of decompensation and one or more OF. The CANONIC study enrolled 1343 patients from 29 centers in 12 European countries. Chronic hepatitis C virus and alcohol were the most frequent underlying causes of chronic liver diseases. The OF definitions were adapted from the Sequential Organ Failure Assessment (SOFA) score and called the CLIF-SOFA score. The CLIF-SOFA score includes subscores ranging from 0 to 4 for each of the six components (liver, kidney, brain, coagulation, circulation, and lungs). Aggregated scores range from 0 to 24 and provide information on overall severity[13].
OF was defined by liver failure (level of serum bilirubin) or extrahepatic failure. The latter includes kidney failure (defined by serum creatinine), cerebral (by grade of encephalopathy according to West-Haven classification), coagulation (by the international normalized ratio [INR]), circulatory (by blood pressure or need of vasopressors), and respiratory failure (by partial pressure of oxygen [PaO2]/fraction of inspired oxygen [FiO2] or oxygen saturation [SpO2]/FiO2) (Table 1). The most prevalent OF was kidney (43.6%), followed by coagulation (27.7%) and cerebral (24.1%). At presentation, the prevalence of ACLF grades 1, 2, and 3 were 49%, 35%, and 16%, respectively. The mortality in 28 d without LT was 32.8% in the seminal study.
| ACLF grades | Number of organ failures |
| 1 | Single organ kidney failure |
| Single liver, coagulation, circulatory or lung failure with creatine levels ranging from 1.5 mg/dL to 1.9 mg/dL or encephalopathy grade 1 or 2, or both | |
| Single brain failure with creatinine ranging from 1.5 mg/dL to 1.9 mg/dL | |
| 2 | 2 organ failures |
| 3 | 3 or more organ failures |
This definition was based on an expert opinion on ACLF from the APASL, published in 2009 with updates in 2014 and 2019, using data from 1402 and 3300 patients, respectively[5,14,15]. This definition considers patients with compensated cirrhosis (diagnosed or not) and non-cirrhotic liver disease patients with a first episode of acute liver deterioration due to an insult directed to the liver. This reflects most of the patient population seen in Asia. In Asia, there is mainly hepatitis B virus reactivation or superinfection with hepatitis viruses A, D, or E. Patients with extrahepatic precipitants and those with kidney, circulatory or respiratory failures are excluded, meaning the liver dysfunction is the basis of the APASL definition. Extrahepatic OF may subsequently develop but are not needed for diagnostic criteria. The acute hepatic insult can be manifested by jaundice (serum bilirubin ≥ 5 mg/dL), coagulopathy (INR ≥ 1.5) and complications within 4 wk such as ascites, encephalopathy, or both. With these criteria, there is an estimated 25% to 37% 30 d-mortality[16].
ACLF was defined as the development of two or more OF (maximum of four) in patients with AD cirrhosis with or without prior episodes of decompensation. They used a prospective multicenter Canadian and American cohort of 507 patients with non-elective hospitalization in 18 centers. The OF criteria were: Kidney if dialysis is required; brain if encephalopathy grades 3 or 4 as West Haven classification occurs; respiratory if mechanical ventilation is needed; circulatory if vasopressor support is required or a reduction in systolic blood pressure by 40 mmHg from baseline despite adequate fluid resuscitation. The most prevalent OF was cerebral (36%), followed by circulatory (16%), kidney (13%) and respiratory (9%)[6]. Lately, these criteria were validated in a cohort of 2675 patients with or without infections, and mortality was higher in patients with infections, whatever the number of OF[17].
This section will discuss the most frequent precipitants for ACLF, its pathophysiology, and prognosis; nevertheless, a more thorough review of these subjects is outside the scope of this manuscript. Regardless of the ACLF definition employed, the precipitant factors can be recognized in only about 50% of patients with ACLF[9]. In 2021, the EASL-CLIF Consortium published a second paper derived from the PREDICT study reporting the precipitant factors which could influence the clinical course and the prognosis of ACLF patients. The most prevalent, more than 96% of cases, were bacterial infections (documented) and severe alcoholic hepatitis, whereas gastrointestinal bleeding with shock and toxic encephalopathy were rare. Although not so common, other precipitant factors like drug-induced liver injury, surgery, viral hepatitis, and ischemia can also be considered[18]. Mezzano et al[8] in a recent review and meta-analysis using ACLF-EASL-CLIF criteria in patients from Europe, East/South Asia and North/South America, showed that bacterial infections (35%), followed by gastrointestinal bleeding (22%) and acute alcohol consumption (19%) were the most frequent triggers to ACLF, with kidney dysfunction being the most common organ failure (49%). Although it is crucial to identify precipitant factors for ACLF, a recent study reported that they could not be detected in up to one-third of patients[12,19].
Data from the CANONIC cohort showed that ACLF is a dynamic syndrome and may evolve to resolution, improvement or worsening in a short period[1]. ACLF grade 1 could be reversible in most patients (54.0%), while 21.0% remain stable in grade 1, and 24.5% progress to a higher grade. The clinical course after 3-7 d from diagnosis of ACLF may be a better predictor of outcome than its initial severity. Indeed, patients with grade 3 ACLF 3-7 d after diagnosis showed the worst prognosis[20,21]. This was called the opportunity window, wherein LT could reach the best results.
In severely ill patients, the prognosis differed according to the number of OF. For example, three OF had lower 28-d transplant-free mortality than those with four OF, 53.0% vs > 90.0%, respectively[20,21]. Indeed, the number of OF - according to the EASL-CLIF definition - along with age and white cell count compose the EASL-CLIF ACLF score. This predictive tool developed by the CANONIC study group proved superior to older models )Acute Physiology and Chronic Health Evaluation [APACHE] II, Child-Turcotte-Pugh, Model for End-stage Liver Disease [MELD]) to predict ACLF patients' mortality[22]. Accordingly, a recent study demonstrated that patients with an EASL-CLIF ACLF score of greater than 70 had 90% mortality at 90 d, regardless of care setting[23]. Another study reported a 28-d mortality rate of 100% for patients with a score greater than 70 at 48 h post-intensive care unit (ICU) admission[24].
Nevertheless, importantly, a 90-d and a 1-year post-LT survival rate of 90% and 81%, respectively, were reported for ACLF patients with 5 to 6 OF[25]. Conversely, without LT, survival rates dropped dramatically[25]. Therefore, although application and reassessment of the EASL-CLIF ACLF score may prevent prolongation of futile therapy, especially after a short trial of ICU stay, withdrawal of care must only be considered if the patient is not a LT candidate.
Recent evidence suggests systemic inflammation is the key to AD and ACLF disease progression[3,10,26]. Briefly, in patients with sepsis as the precipitant event, the inflammatory response is triggered by the recognition of pathogen-associated molecular patterns by pattern recognition receptors. The inflammatory response is then exacerbated, resulting in organ damage, cell death and release of damage-associated molecular patterns, which could aggravate and accelerate OF development in the ACLF setting. In addition, cirrhotic patients have portal hypertension, secondary intestinal congestion, and splanchnic endothelial dysfunction. These features can enhance gut permeability and facilitate bacterial translocation, driving local and systemic inflammation. Traditionally, inflammatory response causes organ dysfunction and stimulation of nitric oxide production, worsening pre-existing circulatory collapse and activation of immune cells. Another mechanism involved is mitochondrial metabolic impairment, resulting in metabolic disorder and cellular dysfunction with a preferential allocation of circulant nutrients to innate immune cells due to high metabolic demands. Secondarily, this process decreased mitochondrial energy production and enhanced organ dysfunction. So, inflammation and immunoparesis are thus key features of ACLF[3,26].
Besides LT, diagnosing and treating precipitant events and providing supportive treatment for OF are currently the cornerstone of ACLF therapy.
Infections: antimicrobial therapy should commence as quickly as possible based on the suspected site involved, existing culture results and local antimicrobial sensitivity patterns. Empirical broad-spectrum antimicrobials should be promptly initiated and deescalated once the results are available. Empirical antifungals should be considered in patients without clinical improvements within 48-72 h. In addition, antifungal therapy may also be used in ACLF patients with multiple risk factors, such as corticosteroid use, prolonged antimicrobial therapy, long-term central venous access devices, parenteral nutrition, renal replacement therapy, sarcopenia and malnutrition[4,17,21]. Recent studies also recommended avoiding proton pump inhibitors unless there is a clear indication (like stress-ulcer prophylaxis) because they increase the risk of infection, mainly due to Clostridioides. Nonselective beta-blockers, when tolerated, and rifaximin may be beneficial by reducing bacterial translocation and intestinal dysbiosis.
Alcoholic hepatitis: although corticosteroids are indicated in patients with alcoholic hepatitis, especially when Maddrey's discriminant function score is higher than 32, the response negatively correlates with the number of OF in ACLF. The risk of new infections is one of the most critical factors in the decision-making process for steroid therapy[4,21]. The response to steroids should be assessed with the Lille score on day 7.
Acute viral hepatitis or reactivation: potent nucleotide or nucleoside analogues should be started in the event of hepatitis B infection or reactivation.
Surgical procedures: surgery of any type in patients with cirrhosis is associated with a significant risk of OF and ACLF. Consequently, it must be carefully considered. For example, open abdominal non-liver surgery, high preoperative cardiovascular risk, or hepatic venous pressure gradient greater than 16 mmHg were frequently associated with ACLF. Recently, a new score, the VOCAL PENN score, demonstrates an excellent ability to predict 30-d mortality when surgery is needed in patients with ACLF[18,27].
ACLF patients frequently require admission to an ICU for advanced OF support and assistance from a multidisciplinary team.
Hemodynamic: Early goal-directed therapy using intravenous fluid resuscitation, preferably with crystalloids, must target mean arterial pressure > 65 mmHg. If vasopressors are required, nore-pinephrine is the first option, and a low dose of vasopressin can be necessary. Next, terlipressin or epinephrine can be added, though they are no longer the second option. Finally, intravenous hydrocortisone can be indicated in refractory septic shock, whereas no long-term survival benefit exists[28].
Acute kidney injury (AKI): It is essential to eliminate or avoid nephrotoxic drugs. The assessment of AKI severity using the modified Kidney Disease Improving Global Outcomes (KDIGO) criteria established the use of albumin in patients with stages 2-3 and albumin plus terlipressin or norepinephrine in type-1 hepatorenal syndrome. In non-responders, it should be necessary to start renal replacement therapy, mainly in patients with LT perspectives[29,30].
Lungs and respiratory failure: The airway should be protected in West-Haven grade 3 or 4 hepatic encephalopathy patients with elective intubation. Patients should be sedated with short-acting agents such as propofol. Benzodiazepines should be avoided. Hypoxemia (PaO2 < 80 mmHg) should be prevented, and paracentesis is clinically indicated in case of tense ascites[28].
Gastrointestinal: Consider the use of stress-ulcer prophylactic drugs. As soon as possible, initiate oral or enteral feedings[18].
Coagulation: Consider prophylaxis for deep-vein thrombosis in the absence of severe coagulopathy. However, avoid correction of INR alterations with fresh frozen plasma. Instead, assessing the risk of bleeding and thrombosis in ACLF patients should be done with viscoelastic testing (rotational thromboelastography or rotational thromboelastometry), which must also guide correction when needed or before invasive procedures[31,32].
The use of albumin has been well recommended to prevent AKI and renal failure in spontaneous bacterial peritonitis, besides preventing post-paracentesis circulatory dysfunction[18,26,33,34]. Recent data have highlighted the non-oncotic properties of albumin as homeostatic effects, antioxidants, immunomodulation, endothelial stabilization, and toxic binding metabolites, including bile acids. Three studies recently evaluated the routine outpatient administration of intravenous albumin. One of them, the ANSWER trial, which included outpatients in an early stage of liver disease, showed improvement in mortality and reduction in cirrhosis-related complications, mainly when the albumin level was maintained above 4 g/dL[35]. However, further studies are necessary to indicate albumin infusion use routinely[18,28].
Various artificial and bioartificial extracorporeal liver support systems have been attempted to treat ACLF. However, artificial liver support such as molecular adsorbent recirculating system and fractionated plasma separation and adsorption system (Prometheus) have failed to show any survival benefit in this setting. Some bioartificial liver supports with a source of cells, traditionally human or porcine hepatocytes, are under investigation, but the clinical benefit is still unclear[18,21].
While the use of plasma exchange has been shown to improve survival in acute liver failure, the actual effect in the ACLF scenario is unknown. Some studies in Asia using selected patients with ACLF showed improvement in 30 and 90-d survival in non-transplanted patients. Still, randomized trials are needed on the duration and amount of plasma exchange required[36].
One potential therapy for ACLF is the administration of granulocyte colony-stimulating factor (G-CSF). Several trials, mainly in patients with hepatitis B from Asia and India, have studied the efficacy of G-CSF and showed an increased leukocyte and neutrophil count, reduced severity of the disease and a protective effect on the development of sepsis, hepatorenal syndrome and hepatic encephalopathy[3]. However, trials in Western cohorts did not demonstrate survival benefits, CLIF OF scores modification or recurrence of infections. Therefore, to date, G-CSF cannot be recommended as part of routine treatment for ACLF[3,18,33,37].
Another promising therapy is mesenchymal stem cell transplantation, which can be a bridge to stabilization in patients with ACLF until LT. However, if the concept is interesting in theory, the studies were made with a few patients, and consequently, many questions remain open[38].
In addition to treatment for hypercholesterolemia, statins may have a role in ACLF therapy because of their potential hepatoprotective and anti-inflammatory properties. Currently, two clinical trials are underway to address the benefits and safety of statin (simvastatin and atorvastatin) in cirrhotic patients[26,39,40].
The gut microbiota plays an important role in complications associated with cirrhosis, with specific intestinal microbiomes being associated with adverse outcomes. The changes in the gut microbiome parallel the disease stages reaching their peak in ACLF. ACLF patients were shown to present an increase of Enterococcus and Peptostreptococcus sp and a reduction of some autochthonous bacteria[41]. Individual microbiome signatures could possibly identify ACLF patients and their prognosis, leading to more personalized treatment, a topic under investigation in the MICROB-PREDICT study (https://microb-predict.eu/). Manipulation of the gut microbiome using fecal microbiota tran-splantation may positively impact the course of cirrhosis, as shown in patients with severe alcoholic hepatitis. In addition, one novel-engineered carbon bead (Carbalive™) designed to absorb toxins from the gut and prevent translocation is under investigation[26]. Whereas promising, more studies are necessary to assess the clinical significance of gut microbiome manipulation in managing ACLF.
Although the MELD has enhanced equity in organ allocation in LT, there is evidence suggesting it may not portray the waitlist mortality of ACLF patients. This is in accordance with distinct pathological mechanisms in decompensated cirrhosis and ACLF[42].
Sundaram et al[43], in a large retrospective study, reported that irrespective of the MELD-Na score, ACLF-3 patients had a worse prognosis (ACLF grades refer to the EASL-CLIF classification hereafter unless contrarily stated). They found that 43.8% of patients with ACLF-3 and MELD-Na < 25 died or were removed from the waitlist at 28 d, having the worst prognosis among ACLF groups. This is probably associated with the more frequent occurrence of extrahepatic failures, which, although not fully captured by the MELD score, result in a high mortality rate. In addition, the authors report that LT within 30 d of listing was the only significant independent protective factor for 1-year patient survival after transplantation. More importantly, in this study, yet ACLF-3 patients had a greater mortality risk, they presented a similar probability of being transplanted than non-ACLF-3 patients with similar MELD scores[44].
In the CANONIC study, Jalan et al[22] developed an organ function scoring system (CLIF Consortium Organ Failure score, CLIF-C OFs) to diagnose ACLF and the prognostic EASL-CLIF ACLF score. The latter score discussed previously revealed significantly higher mortality 28-d predictive accuracy than the MELD and MELD-Na[22].
Allocation systems must privilege ACLF patients once so far earlier transplantation is the cornerstone of their successful management. The question remains whether ACLF-3 patients must be prioritized or whether a new scoring system that depicts better OF must be implemented for these cases[44-46].
Donor organ selection in the ACLF scenario can be a real conundrum for transplant teams to solve. This is because there is evidence that donor characteristics may be associated with a poor outcome after transplantation[25,47,48], which adds complexity to the decision of whether to accept or not a donor organ offer. This aspect is troubling, considering that strict donor organ selection may even postpone further transplantation to this threatened population, especially in regions with frequent high MELD recipients.
A recent retrospective analysis from the United Network for Organ Sharing (UNOS) involving 50552 transplanted ACLF patients reported the donor risk index (DRI) above 1.7 as an independent risk factor for mortality within 1 year after LT (hazard ratio [HR] 1.22; 95% confidence interval [CI] 1.09-1.35)[47]. On the other hand, independently of the MELD score, they described LT within 30 d of the ACLF diagnosis as a predictor of improved 1-year survival after transplantation[47]. Other authors also reported similar results[48,49]. These findings together pressurize even more transplant teams, which face the dilemma of whether to proceed with a high-risk donor or wait for an ideal donor which might not come on time.
It is ideally argued that the benefit of transplantation to this population surpasses the potential negative impact of a suboptimal graft when comparing this risk with a 1-year survival probability without transplantation. This is especially true for ACLF-3 patients, whose reported 1-year survival probability with and without transplantation is 83.9% and 7.9%, respectively[50,51]. Nevertheless, the scarce literature available also reports extra caution during donor organ selection for ACLF-3 patients in real life. For example, in the UNOS database retrospective study, ACLF-3 patients received organs from younger donors (mean age 38.7 years) with the cause of death predominantly related to head trauma (38.0%) and a small percentage of organs from high-risk donors with DRI ≥ 1.7 (22.9%)[47].
Kitajima et al[52] using the Organ Procurement and Transplantation Network and the UNOS registry, recently analyzed 17300 transplanted ACLF patients between 2002 and 2019. They grouped the patients by eras, Era 1 (2002-2007, n = 4,032), Era 2 (2008-2013, n = 6,130), and Era 3 (2014-2019, n = 7,138). Donor characteristics were classified according to the DRI (DRI < 1.2, 1.2-1.6, 1.6-2.0, and > 2.0). They have shown a significant improvement in overall patient survival and transplant outcomes throughout eras. However, although donors with DRI > 2.0 were associated with a lower risk of patient death in Eras 2 and 3 than Era 1 in ACLF-1 and 2, this was not confirmed for ACLF-3[52]. Therefore, the authors advise the need for particular caution for high-risk donors (DRI > 2.0) for ACLF-3 patients.
Comparatively, ACLF grades 1 and 2 are associated with lower mortality than ACLF grade 3. The reported 28-d and 90-d mortality rates for ACLF grades 1 and 2 are 25.8% and 28.6%, and 41.1% and 65.4%, respectively[1]. Therefore, more judicious donor organ selection could be applied, mainly when disease progression is evaluated concomitantly. Whereas data regarding the impact of donor organ selection on postoperative outcomes are scarcer for this population, the results of the study mentioned above support a wider acceptance of suboptimal grafts in ACLF-1 and 2 patients[52]. Nevertheless, the quality of evidence (retrospective study) must be considered before drawing definitive conclusions.
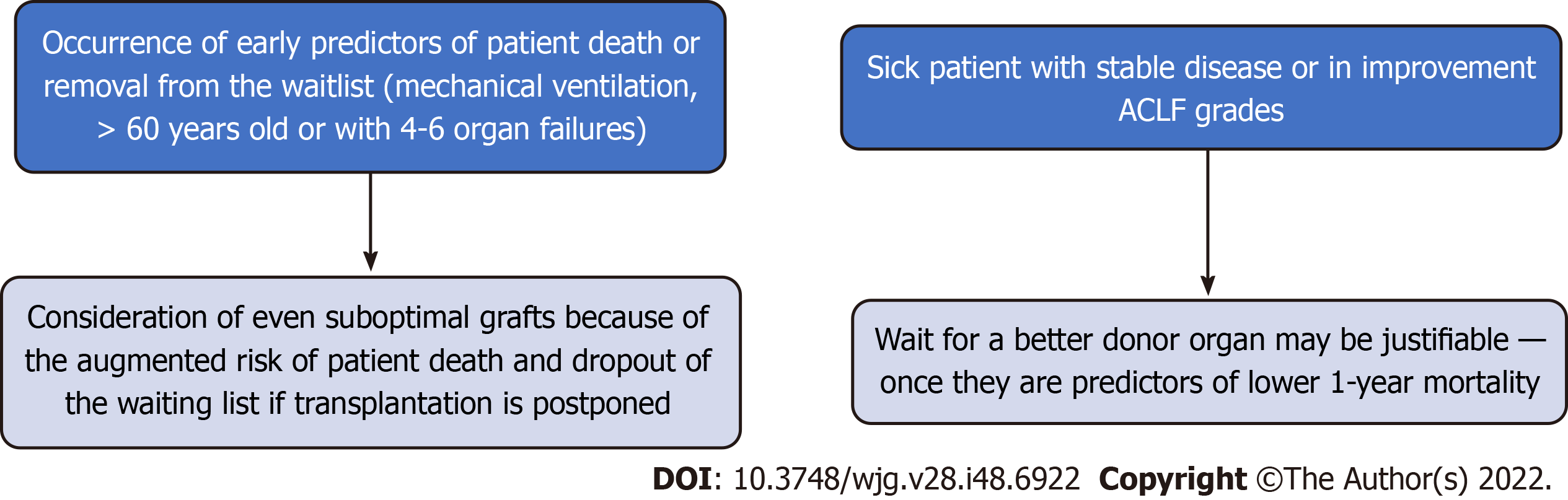
In the setting of donor organ shortage, another question to consider is the allocation of an ideal organ to a very sick recipient who may not survive the procedure. Again, the coexistence of additional risk factors and the real-time change in OF may guide the decision to accept a non-ideal donor organ offer rather than wait (Figure 1). Accordingly, there is evidence suggesting that in ACLF-3 patients, a better survival rate can be achieved if the transplant occurs after organ failure recovery[48].
Indeed, the timing for LT in ACLF patients is critical. In a recent study involving specifically ACLF-3 patients, authors investigated the optimal timing for transplantation and the impact of extended criteria donor (ECD) organs (defined exclusively by the DRI ≥ 1.7)[49]. They analyzed three variables to define the groups of patients (age ≤ 60 or > 60 years, 3 OF or more, and hepatic or extrahepatic ACLF-3). Through two-way sensitivity analyses, they found that overall survival is optimized by earlier transplantation, especially among candidates > 60-years-old or with 4-6 OF[49]. These findings are in accordance with the proposal mentioned above and reinforce the need to consider early transplantation, even with suitable suboptimal grafts, to this population of ACLF patients.
To date, just the DRI was evaluated as a donor parameter within ACLF studies. The DRI is a quantitative score developed to predict the risk of graft failure[53]. It identified seven donor characteristics associated with graft failure, donor age, donation after circulatory death (DCD), split/partial grafts, race, height, and cause of brain death[53]. Yet, the DRI has known limitations which must be considered in this analysis. First, the DRI does not account for steatosis, a known risk factor for postoperative graft dysfunction. Second, cold ischemia time cannot be anticipated in all cases, especially in challenging logistical scenarios, such as in countries with long territorial extensions and complex surgical cases. Consequently, a more thorough evaluation of the impact of donor features within ACLF studies is needed.
Living-donor liver transplantation (LDLT) could expand the donor organ pool and expedite transplantation in ACLF patients. Whilst studies in countries where deceased donors are scarce for cultural reasons demonstrated good LDLT postoperative outcomes[54,55], concerns regarding the prognosis of the sickest patients leading to stringent patient selection criteria hinder the applicability of this option thus far. In a retrospective analysis of 60 patients with EASL-CLIF grade 1 and 2 ACLF, LDLT transplanted patients exhibited a 1-year survival rate of 92% vs 11% in those who did not undergo transplantation[55]. In another retrospective study involving 218 ACLF patients, employing strict selection criteria -no high vasopressors or respiratory failure- for LDLT transplantation, the 1-year postoperative patient survival was 92.9% for EASL-CLIF grade 1, 85.4% for grade 2, and 75.6% for grade 3[54]. Despite suggesting the benefit of LDLD in this setting, the justifiable caution patient selection may have biased the conclusions. In addition, right lobe LDLT is most often required, leading to a right hepatectomy in the donor, which increases their morbidity and mortality.
While nowadays, consensually amongst experts, ECD organs must be considered in ACLF patients due to the transplant benefit, further studies detailing the real impact of donor characteristics on posttransplant patient survival are awaited. This is particularly valid for ACLF-3 patients, in which the limited literature available urges caution before proceeding with a high-risk donor. Thus far, this general concept is more theoretical than practical and originates from the need to provide timely transplantation to these patients. Therefore, this is a warranted subject of deeper investigation for future studies.
Machine perfusion of the liver (MPL) is currently a hot topic in LT. It has gained growing attention from the transplant community with the expansion of the ECD population and, therefore, the need to prevent the frequent ischemia-reperfusion injury (IRI)-ECD postoperative-related complications. IRI is an intrinsic consequence of solid organ transplantation and the basis of major postoperative complications. Although ECD organs are highly vulnerable to IRI, surpassing the protective capacity of traditional static cold storage (SCS) preservation solutions and, consequently, associated with higher morbidity and mortality rates after transplantation, their increased utilization is needed to attend to the rising number of patients on the waiting list. Thus far, the two most studied modalities of MPL in LT are ex situ hypothermic and normothermic machine perfusion.
The hypothermic oxygenated machine perfusion (HOPE) of the liver was shown to enhance mitochondrial respiratory function[56]. The optimized mitochondrial respiratory chain and oxidative phosphorylation system increase cellular energy production -replenishing the exhausted stores of adenosine triphosphate-, avoid the reverse flow of electrons with the production of reactive oxygen species, and prevent the activation of the inflammatory cascade with subsequent tissue damage[56].
The normothermic machine perfusion of the liver (NMP) allows the recovery of the full metabolism of the organ at 37 °C. Consequently, it requires an oxygen carrier to attend to the cellular metabolic demand. NMP permits the assessment of parameters that traditionally indicate appropriate liver function such as bile production, vascular flow, lactate metabolism, glucose metabolism, and hepatocellular injury such as transaminases released into the perfusate[57]. In addition, NMP enables prolonged organ preservation and, potentially, ex situ organ treatments[57,58].
So far, most clinical trials on MPL in LT were intended to demonstrate the safety and feasibility of the technique and were centered in European countries. Arguably, the sickest patients with very high morbidity and mortality risk were not included in these studies and were not even on the waiting list in many of these countries. Therefore, regional divergence and particularities amongst geographical areas, such as mean MELD on the waiting list and territorial extensions, must also be considered.
Hurdles to timely access of ACLF patients to LT and their disadvantage in receiving a donor organ offer in the MELD allocation system were presented herein. In addition, concerns about accepting ECD organs to these sick patients were discussed beforehand. Consequently, none of the MP clinical trials has encompassed ACLF patients thus far. Nevertheless, hypothetically, MPL can be even more advantageous for this population.
Studies suggest that MP may recondition ECD organs before transplantation, preventing further deterioration or even improving their quality. Although DCD LT has not been reported for ACLF patients, clinical trials described similar results for patients transplanted with DCD organs treated with HOPE and those transplanted with low-risk donors after brain death (DBD)[59]. Furthermore, in a randomized clinical trial, hypothermic machine perfusion reduced the occurrence of postreperfusion syndrome and early allograft dysfunction (EAD) after DCD LT. These factors contribute to the early recovery of the sickest patients after the procedure[60]. Accordingly, concerning the applicability of the technique in ECD DBD LT, a multicenter randomized clinical trial recently reported that, compared to SCS, HOPE led to a significant reduction in 90-d complications with a shorter hospital stay and a trend toward a reduced rate of EAD[61].
Prolonged organ preservation and assessment of organ viability are two critical features related to NMP which may benefit ACLF transplantation. Whereas it could not find a difference in graft survival or patient survival compared to SCS, the first randomized clinical trial on NMP demonstrated that it could safely extend the organ preservation time[62]. The median total preservation time was close to 12 h for NMP-preserved livers. Driven mainly by the difference in peak aspartate transaminase, NMP also reduced the occurrence of EAD[62]. The VITTAL clinical trial (NCT02740608), from Birmingham, United Kingdom, transplanted twenty-two donor livers discarded by all United Kingdom centers of 31 meeting specific high-risk criteria based on the lactate clearance to levels ≤ 2.5 mmol/L within 4 h on NMP with 100% 90-d patient and graft survival[63]. Nevertheless, applying the Birmingham criteria, NMP could not prevent non-anastomotic biliary strictures in DCD livers, and 4 (18%) patients needed re-transplantation[63].
Our group recently reported for the first time the successful transplantation of an ACLF patient using an ECD DBD liver graft treated with HOPE[64]. The autoimmune hepatitis-related cirrhosis ACLF-2 patient (liver and coagulation failure) with a MELD-Na score of 42 was offered an ECD DBD organ with a DRI of 2.79 after 8 d from hospital admission (well above the previously identified threshold of 1.7). HOPE started after 06 h and 19 min of cold ischemia time and lasted 5 h and 19 min. The flavine mononucleotide was measured in the perfusate to assess the viability of the organ and revealed a low value after 30 min of perfusion (3097 A.U.) - permissive for transplantation in any recipient. During transplantation, the reperfusion was uneventful. Postoperatively, the graft recovered well, without EAD, according to the Olthoff criteria, and the patient developed AKI KDIGO stage 3 with complete recovery after 1 wk[64].
While the case suggests the feasibility and safety of employing MP within this setting, more conclusive evidence to prove the benefit of the technique is still needed. So far, because of the scarce existing literature, the evidence of the impact of ECD transplantation in ACLF grades 1 and 2 is still anecdotal. In addition, there are no reports on the application of MPL in ACLF-3 patients, those with greater risk and the subject of more concerns in the literature. Earlier transplantation was shown to improve overall survival in ACLF-3 patients > 60-years-old or with 4-6 OF[49]. This population and those with the coexistence of additional risk factors and worsening organ failures may be the target population for future MPL studies.
Yet the evidence is very limited currently, MPL may play a game-changing role in ACLF tran-splantation. First, it can expedite LT because it allows more liberal acceptance of ECD organs based on the properties of the technique. The proven capacity of MPL to recondition ECD livers reassures surgeons in their decision to accept an ECD organ. This effect is amplified with the application of biomarkers for organ viability assessment during perfusion. Second, rescuing discarded high-risk organs or prolonging the preservation of organs compromised by logistics via MPL may increase the donor organ pool, which may also help tackle the shortage of donor organs for transplantation. This is especially important in countries with frequent high MELD score patients and long cold ischemia time, which may need to adapt their organ preservations systems.
Although new clinical specific therapies have been researched for ACLF management, earlier transplantation -within the frequently narrow opportunity window- is a proven effective therapy for selected ACLF patients. Thus far, other options encompass diagnosing and treating precipitant events and supportive treatment for organ failures. Therefore, current and future perspectives on ACLF management must envisage improved access to LT. Accordingly, discussions about allocation and prioritization for transplantation in critically ill ACLF patients are awaited because there is evidence suggesting the current model may not portray their waitlist mortality. Furthermore, whereas donor organ quality is meant to be a prognostic factor in the ACLF setting, recent evidence suggests that MPL may be a safe tool to improve the donor organ pool and expedite access to this life-saving procedure.
| 1. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2279] [Article Influence: 175.3] [Reference Citation Analysis (6)] |
| 2. | Li F, Thuluvath PJ. EASL-CLIF criteria outperform NACSELD criteria for diagnosis and prognostication in ACLF. J Hepatol. 2021;75:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Khanam A, Kottilil S. Acute-on-Chronic Liver Failure: Pathophysiological Mechanisms and Management. Front Med (Lausanne). 2021;8:752875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Zaccherini G, Weiss E, Moreau R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 2021;3:100176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P, Lau GK, Liu Q, Madan K, Mohamed R, Ning Q, Rahman S, Rastogi A, Riordan SM, Sakhuja P, Samuel D, Shah S, Sharma BC, Sharma P, Takikawa Y, Thapa BR, Wai CT, Yuen MF. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 651] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
| 6. | Bajaj JS, O'Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, Subramanian RM, Thacker LR, Kamath PS; North American Consortium For The Study Of End-Stage Liver Disease (NACSELD). Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 454] [Article Influence: 37.8] [Reference Citation Analysis (2)] |
| 7. | Zhang J, Gao S, Duan Z, Hu KQ. Overview on acute-on-chronic liver failure. Front Med. 2016;10:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Mezzano G, Juanola A, Cardenas A, Mezey E, Hamilton JP, Pose E, Graupera I, Ginès P, Solà E, Hernaez R. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut. 2022;71:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 9. | Durand F, Roux O, Weiss E, Francoz C. Acute-on-chronic liver failure: Where do we stand? Liver Int. 2021;41 Suppl 1:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Schulz MS, Gu W, Schnitzbauer AA, Trebicka J. Liver Transplantation as a Cornerstone Treatment for Acute-On-Chronic Liver Failure. Transpl Int. 2022;35:10108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 11. | Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, Sacleux SC, Pageaux GP, Radenne S, Trebicka J, Fernandez J, Perricone G, Piano S, Nadalin S, Morelli MC, Martini S, Polak WG, Zieniewicz K, Toso C, Berenguer M, Iegri C, Invernizzi F, Volpes R, Karam V, Adam R, Faitot F, Rabinovich L, Saliba F, Meunier L, Lesurtel M, Uschner FE, Fondevila C, Michard B, Coilly A, Meszaros M, Poinsot D, Schnitzbauer A, De Carlis LG, Fumagalli R, Angeli P, Arroyo V, Jalan R; ELITA/EF-CLIF working group. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol. 2021;75:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 12. | Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, Giovo I, Uschner FE, Jimenez C, Mookerjee R, Gustot T, Albillos A, Bañares R, Janicko M, Steib C, Reiberger T, Acevedo J, Gatti P, Bernal W, Zeuzem S, Zipprich A, Piano S, Berg T, Bruns T, Bendtsen F, Coenraad M, Merli M, Stauber R, Zoller H, Ramos JP, Solè C, Soriano G, de Gottardi A, Gronbaek H, Saliba F, Trautwein C, Özdogan OC, Francque S, Ryder S, Nahon P, Romero-Gomez M, Van Vlierberghe H, Francoz C, Manns M, Garcia E, Tufoni M, Amoros A, Pavesi M, Sanchez C, Curto A, Pitarch C, Putignano A, Moreno E, Shawcross D, Aguilar F, Clària J, Ponzo P, Jansen C, Vitalis Z, Zaccherini G, Balogh B, Vargas V, Montagnese S, Alessandria C, Bernardi M, Ginès P, Jalan R, Moreau R, Angeli P, Arroyo V; PREDICT STUDY group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 13. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 8128] [Article Influence: 270.9] [Reference Citation Analysis (11)] |
| 14. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2058] [Article Influence: 205.8] [Reference Citation Analysis (12)] |
| 15. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 697] [Cited by in RCA: 648] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 16. | Dhiman RK, Agrawal S, Gupta T, Duseja A, Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014;20:14934-14941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 17. | O'Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, Subramanian RM, Kamath PS, Thuluvath P, Vargas HE, Maliakkal B, Tandon P, Lai J, Thacker LR, Bajaj JS. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 2018;67:2367-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (1)] |
| 18. | Bajaj JS, O'Leary JG, Lai JC, Wong F, Long MD, Wong RJ, Kamath PS. Acute-on-Chronic Liver Failure Clinical Guidelines. Am J Gastroenterol. 2022;117:225-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 19. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 456] [Article Influence: 50.7] [Reference Citation Analysis (1)] |
| 20. | Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, Trebicka J, Elkrief L, Hopf C, Solís-Munoz P, Saliba F, Zeuzem S, Albillos A, Benten D, Montero-Alvarez JL, Chivas MT, Concepción M, Córdoba J, McCormick A, Stauber R, Vogel W, de Gottardi A, Welzel TM, Domenicali M, Risso A, Wendon J, Deulofeu C, Angeli P, Durand F, Pavesi M, Gerbes A, Jalan R, Moreau R, Ginés P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 494] [Article Influence: 44.9] [Reference Citation Analysis (2)] |
| 21. | Kumar R, Mehta G, Jalan R. Acute-on-chronic liver failure. Clin Med (Lond). 2020;20:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 780] [Article Influence: 65.0] [Reference Citation Analysis (2)] |
| 23. | Karvellas CJ, Garcia-Lopez E, Fernandez J, Saliba F, Sy E, Jalan R, Pavesi M, Gustot T, Ronco JJ, Arroyo V; Chronic Liver Failure Consortium and European Foundation for the Study of Chronic Liver Failure. Dynamic Prognostication in Critically Ill Cirrhotic Patients With Multiorgan Failure in ICUs in Europe and North America: A Multicenter Analysis. Crit Care Med. 2018;46:1783-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 24. | Engelmann C, Thomsen KL, Zakeri N, Sheikh M, Agarwal B, Jalan R, Mookerjee RP. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care. 2018;22:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 25. | Thuluvath PJ, Thuluvath AJ, Hanish S, Savva Y. Liver transplantation in patients with multiple organ failures: Feasibility and outcomes. J Hepatol. 2018;69:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Abbas N, Rajoriya N, Elsharkawy AM, Chauhan A. Acute-on-chronic liver failure (ACLF) in 2022: have novel treatment paradigms already arrived? Expert Rev Gastroenterol Hepatol. 2022;16:639-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 27. | Mahmud N, Fricker Z, Hubbard RA, Ioannou GN, Lewis JD, Taddei TH, Rothstein KD, Serper M, Goldberg DS, Kaplan DE. Risk Prediction Models for Post-Operative Mortality in Patients With Cirrhosis. Hepatology. 2021;73:204-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 28. | Nanchal R, Subramanian R, Karvellas CJ, Hollenberg SM, Peppard WJ, Singbartl K, Truwit J, Al-Khafaji AH, Killian AJ, Alquraini M, Alshammari K, Alshamsi F, Belley-Cote E, Cartin-Ceba R, Dionne JC, Galusca DM, Huang DT, Hyzy RC, Junek M, Kandiah P, Kumar G, Morgan RL, Morris PE, Olson JC, Sieracki R, Steadman R, Taylor B, Alhazzani W. Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU: Cardiovascular, Endocrine, Hematologic, Pulmonary and Renal Considerations: Executive Summary. Crit Care Med. 2020;48:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 29. | Sanyal AJ, Boyer TD, Frederick RT, Wong F, Rossaro L, Araya V, Vargas HE, Reddy KR, Pappas SC, Teuber P, Escalante S, Jamil K. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther. 2017;45:1390-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA, Sharma P, Sanyal AJ, Mayo MJ, Frederick RT, Escalante S, Jamil K; CONFIRM Study Investigators. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021;384:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 31. | Simonetto DA, Singal AK, Garcia-Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG Clinical Guideline: Disorders of the Hepatic and Mesenteric Circulation. Am J Gastroenterol. 2020;115:18-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 32. | Vuyyuru SK, Singh AD, Gamanagatti SR, Rout G, Gunjan D, Shalimar. A Randomized Control Trial of Thromboelastography-Guided Transfusion in Cirrhosis for High-Risk Invasive Liver-Related Procedures. Dig Dis Sci. 2020;65:2104-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (2)] |
| 33. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 752] [Article Influence: 107.4] [Reference Citation Analysis (2)] |
| 34. | Aday A, O'Leary JG. Acute on Chronic Liver Failure: Definition and Implications. Clin Liver Dis. 2020;24:521-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, Levantesi F, Airoldi A, Boccia S, Svegliati-Baroni G, Fagiuoli S, Romanelli RG, Cozzolongo R, Di Marco V, Sangiovanni V, Morisco F, Toniutto P, Tortora A, De Marco R, Angelico M, Cacciola I, Elia G, Federico A, Massironi S, Guarisco R, Galioto A, Ballardini G, Rendina M, Nardelli S, Piano S, Elia C, Prestianni L, Cappa FM, Cesarini L, Simone L, Pasquale C, Cavallin M, Andrealli A, Fidone F, Ruggeri M, Roncadori A, Baldassarre M, Tufoni M, Zaccherini G, Bernardi M; ANSWER Study Investigators. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391:2417-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 379] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 36. | Tan EX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: A systematic review. World J Gastroenterol. 2020;26:219-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (4)] |
| 37. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 38. | Xue R, Meng Q, Dong J, Li J, Yao Q, Zhu Y, Yu H. Clinical performance of stem cell therapy in patients with acute-on-chronic liver failure: a systematic review and meta-analysis. J Transl Med. 2018;16:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Kimer N, Grønbæk H, Fred RG, Hansen T, Deshmukh AS, Mann M, Bendtsen F. Atorvastatin for prevention of disease progression and hospitalisation in liver cirrhosis: protocol for a randomised, double-blind, placebo-controlled trial. BMJ Open. 2020;10:e035284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, Roux O, Uschner FE, de Wit K, Zaccherini G, Alessandria C, Angeli P, Bernardi M, Beuers U, Caraceni P, Durand F, Mookerjee RP, Trebicka J, Vargas V, Andrade RJ, Carol M, Pich J, Ferrero J, Domenech G, Llopis M, Torres F, Kamath PS, Abraldes JG, Solà E, Ginès P. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 41. | Solé C, Guilly S, Da Silva K, Llopis M, Le-Chatelier E, Huelin P, Carol M, Moreira R, Fabrellas N, De Prada G, Napoleone L, Graupera I, Pose E, Juanola A, Borruel N, Berland M, Toapanta D, Casellas F, Guarner F, Doré J, Solà E, Ehrlich SD, Ginès P. Alterations in Gut Microbiome in Cirrhosis as Assessed by Quantitative Metagenomics: Relationship With Acute-on-Chronic Liver Failure and Prognosis. Gastroenterology. 2021;160:206-218.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 42. | Clària J, Arroyo V, Moreau R. The Acute-on-Chronic Liver Failure Syndrome, or When the Innate Immune System Goes Astray. J Immunol. 2016;197:3755-3761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 43. | Sundaram V, Shah P, Mahmud N, Lindenmeyer CC, Klein AS, Wong RJ, Karvellas CJ, K Asrani S, Jalan R. Patients with severe acute-on-chronic liver failure are disadvantaged by model for end-stage liver disease-based organ allocation policy. Aliment Pharmacol Ther. 2020;52:1204-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Abdallah MA, Waleed M, Bell MG, Nelson M, Wong R, Sundaram V, Singal AK. Systematic review with meta-analysis: liver transplant provides survival benefit in patients with acute on chronic liver failure. Aliment Pharmacol Ther. 2020;52:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 659] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 46. | Karvellas CJ, Francoz C, Weiss E. Liver Transplantation in Acute-on-chronic Liver Failure. Transplantation. 2021;105:1471-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 47. | Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, Wong RJ. Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology. 2019;156:1381-1391.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (1)] |
| 48. | Sundaram V, Kogachi S, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Levitsky J, Rahimi RS, Jalan R. Effect of the clinical course of acute-on-chronic liver failure prior to liver transplantation on post-transplant survival. J Hepatol. 2020;72:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 49. | Zhang S, Suen SC, Gong CL, Pham J, Trebicka J, Duvoux C, Klein AS, Wu T, Jalan R, Sundaram V. Early transplantation maximizes survival in severe acute-on-chronic liver failure: Results of a Markov decision process model. JHEP Rep. 2021;3:100367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, Lassailly G, Dharancy S, Boleslawski E, Lebuffe G, Kipnis E, Ichai P, Coilly A, De Martin E, Antonini TM, Vibert E, Jaber S, Herrerro A, Samuel D, Duhamel A, Pageaux GP, Mathurin P, Saliba F. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 51. | Laique SN, Eghtesad B, Lindenmeyer CC. Surgical Considerations Regarding Transplantation for the Patient With Acute-on-Chronic Liver Failure. Clin Liver Dis (Hoboken). 2022;19:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Kitajima T, Kuno Y, Ivanics T, Lu M, Moonka D, Shimada S, Shamaa T, Abouljoud MS, Nagai S. Improved Survival With Higher-risk Donor Grafts in Liver Transplant With Acute-on-chronic Liver Failure. Transplant Direct. 2022;8:e1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 53. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1526] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 54. | Yadav SK, Saraf N, Choudhary NS, Sah JK, Sah SK, Rastogi A, Bhangui P, Saigal S, Soin AS. Living Donor Liver Transplantation for Acute-on-Chronic Liver Failure. Liver Transpl. 2019;25:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 55. | Bhatti ABH, Dar FS, Butt MO, Sahaab E, Salih M, Shah NH, Khan NY, Zia HH, Khan EU, Khan NA. Living Donor Liver Transplantation for Acute on Chronic Liver Failure Based on EASL-CLIF Diagnostic Criteria. J Clin Exp Hepatol. 2018;8:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Boteon YL, Laing RW, Schlegel A, Wallace L, Smith A, Attard J, Bhogal RH, Neil DAH, Hübscher S, Perera MTPR, Mirza DF, Afford SC, Mergental H. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018;24:1699-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 57. | Romero JM, Kalashnikov N. Normothermic Machine Perfusion Increases Donor Liver Use. JAMA Surg. 2022;157:742. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Boteon YL, Attard J, Boteon APCS, Wallace L, Reynolds G, Hubscher S, Mirza DF, Mergental H, Bhogal RH, Afford SC. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transpl. 2019;25:1007-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 59. | Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, DeOliveira ML, Kron P, Clavien PA. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann Surg. 2015;262:764-70; discussion 770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 60. | van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, Erdmann JI, Gilbo N, de Haas RJ, Heaton N, van Hoek B, Huurman VAL, Jochmans I, van Leeuwen OB, de Meijer VE, Monbaliu D, Polak WG, Slangen JJG, Troisi RI, Vanlander A, de Jonge J, Porte RJ; DHOPE-DCD Trial Investigators. Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med. 2021;384:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 448] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 61. | Czigany Z, Pratschke J, Froněk J, Guba M, Schöning W, Raptis DA, Andrassy J, Kramer M, Strnad P, Tolba RH, Liu W, Keller T, Miller H, Pavicevic S, Uluk D, Kocik M, Lurje I, Trautwein C, Mehrabi A, Popescu I, Vondran FWR, Ju C, Tacke F, Neumann UP, Lurje G. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann Surg. 2021;274:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 62. | Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, García-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malagò M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera MTPR, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ; Consortium for Organ Preservation in Europe. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 916] [Article Influence: 114.5] [Reference Citation Analysis (2)] |
| 63. | Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, Barton D, Curbishley S, Wilkhu M, Neil DAH, Hübscher SG, Muiesan P, Isaac JR, Roberts KJ, Abradelo M, Schlegel A, Ferguson J, Cilliers H, Bion J, Adams DH, Morris C, Friend PJ, Yap C, Afford SC, Mirza DF. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11:2939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 64. | Boteon APCS, Schlegel A, Carvalho MF, Boteon YL. Hypothermic oxygenated machine perfusion as a tool to facilitate liver transplantation in the acute-on-chronic liver failure scenario. Liver Transpl. 2022;28:1678-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Liver Transplantation Society; The Transplantation Society; Academia Nacional de Medicina; and Associação Brasileira de Transplante de Órgãos.
Specialty type: Transplantation
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Walabh P, South Africa; Wang Y, China S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM