Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6522
Peer-review started: July 20, 2022
First decision: October 22, 2022
Revised: November 4, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 14, 2022
Processing time: 140 Days and 18.8 Hours
3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2) is a benzothiophene carboxylate derivative that can suppress the catabolism of branched-chain amino acid (BCAA)-associated mammalian target of rapamycin complex 1 (mTORC1) activation. Previous studies have demonstrated the therapeutic effects of BT2 on arthritis, liver cancer, and kidney injury. However, the effects of BT2 on ulcerative colitis (UC) are unknown.
To investigate the anti-UC effects of BT2 and the underlying mechanism.
Mouse UC models were created through the administration of 3.5% dextran sodium sulfate (DSS) for 7 d. The mice in the treated groups were administered salazosulfapyridine (300 mg/kg) or BT2 (20 mg/kg) orally from day 1 to day 7. At the end of the study, all of the mice were sacrificed, and colon tissues were removed for hematoxylin and eosin staining, immunoblot analyses, and immunohistochemical assays. Cytokine levels were measured by flow cytometry. The contents of BCAAs including valine, leucine, and isoleucine, in mouse serum were detected by liquid chromatography-tandem mass spectrometry, and the abundance of intestinal flora was analyzed by 16S ribosomal DNA sequencing.
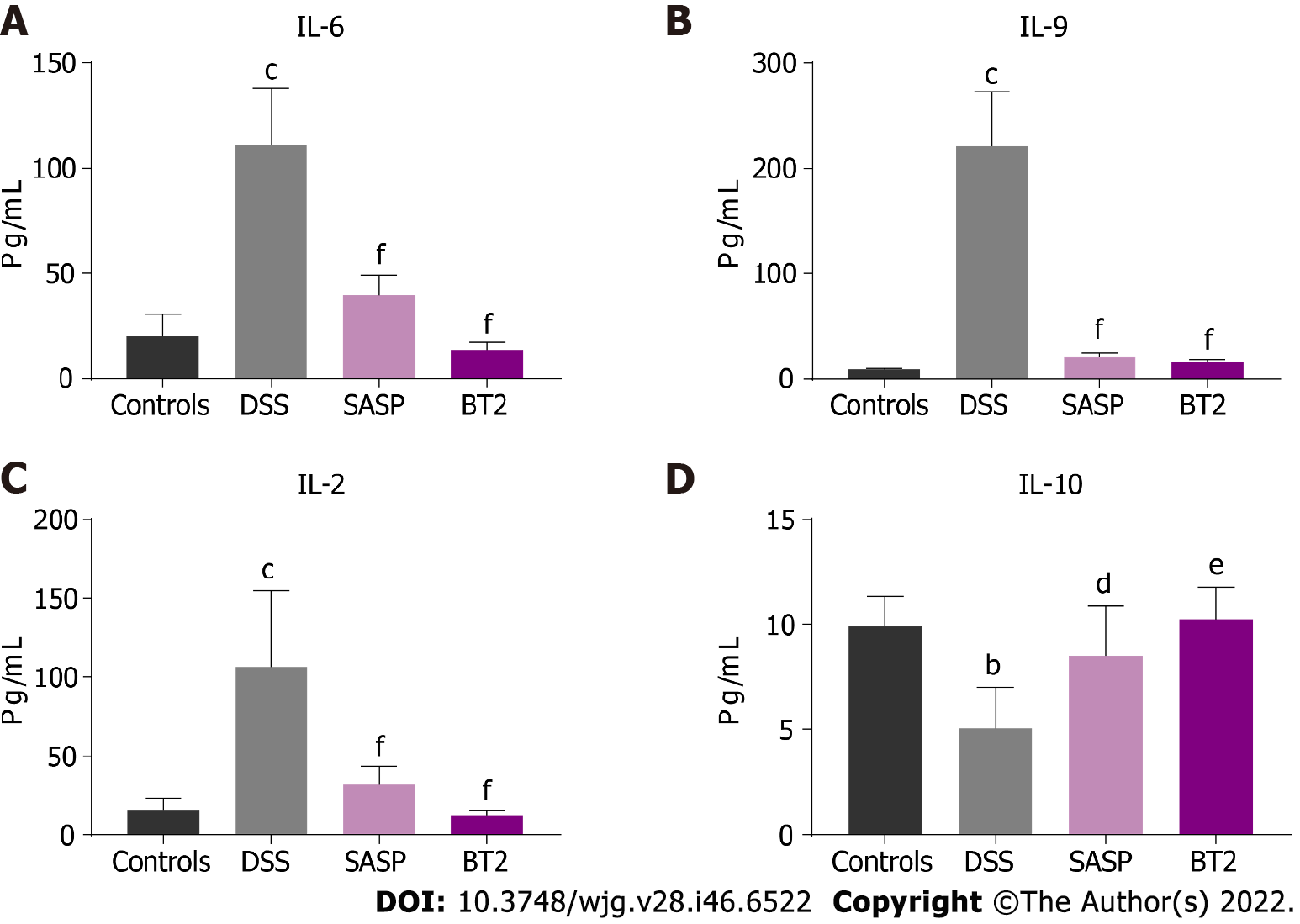
Our results revealed that BT2 significantly ameliorated the inflammatory symptoms and pathological damage induced by DSS in mice. BT2 also reduced the production of the proinflammatory cytokines interleukin 6 (IL-6), IL-9, and IL-2 and increased the anti-inflammatory cytokine IL-10 level. In addition, BT2 notably improved BCAA catabolism and suppressed mTORC1 activation and cyclooxygenase-2 expression in the colon tissues of UC mice. Furthermore, high-throughput sequencing revealed that BT2 restored the gut microbial abundance and diversity in mice with colitis. Compared with the DSS group, BT2 treatment increased the ratio of Firmicutes to Bacteroidetes and decreased the abundance of Enterobacteriaceae and Escherichia-Shigella.
Our results indicated that BT2 significantly ameliorated DSS-induced UC and that the latent mechanism involved the suppression of BCAA-associated mTORC1 activation and modulation of the intestinal flora.
Core Tip: Gut microbiota dysbiosis and hyperactivated mammalian target of rapamycin complex 1 (mTORC1) make great contributions to the pathogenesis of ulcerative colitis (UC). In our study, 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2) exerted significant therapeutic effects on the amelioration of dextran sodium sulfate-induced acute colitis. Our work reported that BT2 played an anti-UC role through suppression of branched amino acid-associated mTORC1 activation and modulation of the intestinal flora. Overall, BT2 could represent a potential candidate drug in the design of treatment strategies for UC.
- Citation: He QZ, Wei P, Zhang JZ, Liu TT, Shi KQ, Liu HH, Zhang JW, Liu SJ. 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid alleviates ulcerative colitis by suppressing mammalian target of rapamycin complex 1 activation and regulating intestinal microbiota. World J Gastroenterol 2022; 28(46): 6522-6536
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6522.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6522
Ulcerative colitis (UC) is a chronic idiopathic intestinal disease characterized by persistent mucosal inflammation extending from the proximal rectum[1]. Due to colonic mucosal damage and intestinal inflammation, UC patients generally experience abdominal pain, diarrhea, and bloody stools and are at a high risk for colorectal cancer[2,3]. UC is traditionally regarded as a disease of Western nations, but recent data have shown that the incidence of UC is increasing worldwide, including in Asia and the Middle East, which indicates its emergence as a global public health challenge[4,5]. Although the explicit etiology remains unclear, UC is primarily considered the result of gut microbiota dysbiosis, dysregulated immune responses, and disrupted intestinal epithelial function. The intestinal flora is involved in intestinal maturation, mucosal physiological functions, nutrient production, nutrient metabolism, and other processes. Patients with UC exhibit clearly disordered intestinal flora compared with healthy people[6-8], which reveals that gut flora imbalance plays an essential role in the pathogenesis of UC.
Mammalian target of rapamycin (mTOR), a crucial regulatory center of cell proliferation and growth, functions via two complexes called mTOR complex 1 (mTORC1) and mTOR complex 2. Previous studies have shown that mTORC1 makes a great contribution to the pathogenesis of UC and promotes the cyclooxygenase-2 (COX-2)-mediated inflammatory infiltration of T helper 17 cells to aggravate colitis[9-12]. A wide range of intracellular signals such as amino acids, growth factors, energy, and hypoxia activate the mTORC1 pathway. Notably, high levels of branched-chain amino acids (BCAAs) including valine, leucine and isoleucine can maintain the persistent activation of mTORC1, leading to a series of diseases such as liver cancer[13-15]. In general, the first step in BCAA catabolism is the branched-chain aminotransferase (BCAT)-mediated transamination reaction, which generates branched-chain α-keto acids (BCKAs). The BCKAs are then oxidatively decarboxylated by the branched-chain α-keto acid dehydrogenase complex (BCKDC) to produce substrates for the tricarboxylic acid cycle[16]. 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2) is a potent inhibitor of branched-chain α-keto acid dehydrogenase kinase (BCKDK), which can specifically phosphorylate and inactivate BCKDC. BCKDC is highly dephosphorylated and activated in the heart, muscle, kidney, and liver of mice treated with BT2, which results in reductions in plasma BCAA levels[17]. Additionally, a previous study revealed that BT2 suppressed mTORC1 activation both in vitro and in vivo and caused no toxicity damage to mice following gavage administration[18,19]. However, the effects of BT2 on UC and its potential mechanism remain unknown.
In our study, we not only evaluated the anti-UC effect of BT2 but also emphasized its impact on the gut microbiota and the inhibition of BCAA-related mTORC1 activation in UC mice.
Dextran sodium sulfate (DSS) was purchased from MP Biomedicals (Irvine, CA, United States), and salazosulfapyridine (SASP) was purchased from Xinyi Pharmaceutical Group (Shanghai, China). BT2 was obtained from Yuanye Biotechnology Co., Ltd. (Shanghai, China). The LEGENDplex™ Multi-Analyte Flow Assay Kit was supplied by BioLegend (San Diego, CA, United States). The BCA Protein Quantification Kit and Supper ECL Detection Reagent were purchased from Yeasen Biotechnology Co., Ltd. (Shanghai, China). The antibodies used for these experiments were as follows: Ribosomal protein S6 (S6) [1:1000, #2317; Cell Signaling Technology (CST), Danvers, MA, United States], phosphorylated S6Ser235/236 (p-S6, 1:1000, 4858; CST), eukaryotic translation initiation factor 4E binding protein 1 (4EBP1) (9644, 1:1000; CST), p-4EBP1Thr37/46 (1:1000, 2855; CST), COX-2 (1:1000, 12282; CST), mTOR (1:1000, 66878-1-Ig; Proteintech, Rosemont, IL, United States), p-mTORSer2448 (1:1000, 67778-1-Ig; Proteintech), branched-chain amino transferase 2 (BCAT2) (1:1000, 16417-1-AP; Proteintech), rabbit antibody (1:10000, SA00001-2; Proteintech), mouse antibody (1:10000, SA00001-1; Proteintech), BCKDK (1:500, 374424; Santa Cruz, Dallas, TX, United States), and branched-chain α–keto acid dehydrogenase (BCKDHA) (1:500, 271538; Santa Cruz).
Forty female C57BL/6 J mice (aged 7-8 wk; 18-20 g) were purchased from the Model Animal Research Center of Nanjing University, Nanjing, China (License No: 202124233). All experiments were performed in accordance with the regulations of the pharmacology laboratory and Animal Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine (2022DW-16-02).
The animals were maintained five per cage and housed in a specific sterile environment with a temperature of 23 °C ± 2 °C, relative humidity of 50%, and 12-h light/dark cycle. After 2 wk of acclimatization, the mice were randomly divided into four groups: Control group, model group, SASP group, and BT2 group. A total of 10 mice were included in each group. The mice in the control group were given normal drinking water, and the mice in the other groups were given 3.5% DSS in drinking water to induce colitis. The mice in the BT2 group and SASP group were treated with 20 mg/kg BT2 and 300 mg/kg SASP, respectively, by oral gavage in a volume of 0.2 mL. The mice in the control group and DSS group were given 0.5% carboxymethylcellulose sodium. The daily body weight loss and stool characteristics were recorded, and the mice were sacrificed 7 d later. On the 8th day, fecal samples and orbital blood samples were collected. The blood samples were clotted for at least 30 min and centrifuged for 20 min at 4 °C and 3000 r/min. Colon segments were extracted, and the lengths from the middle cecum to the end of the colon were measured. All abovementioned samples were stored at -80 °C until required for further investigation.
The assay preparations were carried out according to the LEGENDplex™ Multi-Analyte Flow Assay Kit, and a flow cytometer (Beckman Coulter, Brea, CA, United States) was used for the detection of cytokine levels.
The disease activity index (DAI) score comprehensively evaluates the severity of colonic inflammation from three aspects[20]: (1) Body weight loss, 1 point for every 5% decrease in the mouse body weight up to 4 points; (2) Stool consistency, the stool form was scored from 0 (normal) to 4 points (watery diarrhea); and (3) Hematochezia, scores of 0-4 points were given for negative hemoccult to gross bleeding.
Colon tissue samples were lysed with RIPA buffer (Solarbio, Beijing, China), protease inhibitor (Biosharp, Beijing, China), and phosphatase inhibitors (Biosharp). The protein concentration was detected using the BCA protein assay reagent, and 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed using 30 µg total protein samples at a constant voltage of 100 V. Separated proteins were blotted onto nitrocellulose membranes and then blocked in 5% skim milk for 1 h. The membranes were incubated overnight at 4 °C with primary antibodies diluted to appropriate concentrations and then incubated with secondary antibodies for 1 h at room temperature. The protein bands were visualized with enhanced chemiluminescence detection reagent and quantified using ImageJ software.
Total RNA was extracted from colon tissues using the Total RNA Extraction Kit (Yeasen, Shanghai, China). The concentration and purity of the RNA samples were detected with an ultra-micro fluorescence UV spectrophotometer (DeNovix Inc., Wilmington, DE, United States). A kit (Yeasen) was used for the reverse transcription of total RNA into cDNA, and polymerase chain reaction (qPCR) was performed with a real-time fluorescence quantitative PCR detection system (Thermo Fisher Scientific, Waltham, MA, United States). The primers were as follows: β-actin forward, CTGTGCCCATCTACGAGGGCTAT and reverse, TTTGATGTCACGCACGATTTCC; COX-2 forward, GCGACATACTCAAGCAGGAGCA and reverse, AGTGGTAACCGCTCAGGTGTTG. All primers were provided by Tsingke Biotechnology Co., Ltd. (Beijing, China).
Colon tissue sections were dewaxed and dehydrated with different grades of alcohol and xylene. The tissue sections were placed in a repair box filled with citric acid (pH 6.0) antigen retrieval buffer for antigen retrieval in a microwave oven and then washed three times with phosphate-buffered saline (PBS). The sections were placed in 3% hydrogen peroxide to block endogenous peroxidase activity and then sealed with 3% bovine serum albumin for 30 min at room temperature. The sections were incubated with primary antibody overnight at 4 °C. The slices were washed three times with PBS, incubated with secondary antibody for 50 min, washed three times with PBS, and subjected to a diaminobenzidine color developing solution. The nuclei were counterstained with hematoxylin for 3-4 min. Images were collected by light microscopy and analyzed by expert pathologists. The immunohistochemistry score was calculated based on the proportion of cells with an unequivocal positive reaction (0: < 5%; 1: 5%-20%; 2: 21%-40%; 3: 41%-60%; 4: 61%-80%; and 5: > 80%).
Fresh colon tissues were fixed in fixing solution for more than 24 h and then embedded in paraffin blocks. Then the wax blocks were trimmed, cut into 4-μm-thick sections, and subjected to hematoxylin and eosin (H&E) staining. Histopathological changes were observed by a light microscope (Nikon, Tokyo, Japan). The histopathological score was divided into epithelial injury and inflammatory cell infiltration according to specific evaluation criteria described elsewhere[21].
Total genomic DNA from fecal samples was extracted using the PowerSoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, United States) following the manufacturer’s protocol. The DNA quality and concentration were detected using a Nanodrop spectrophotometer (Thermo Fisher Scientific). The V3-V4 regions of bacterial 16S ribosomal DNA (rDNA) were amplified by PCR using the primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACNNGGGTATCTAAT-3’). The target bands were detected by 1% agarose gel electrophoresis, and the PCR products were purified by the magnetic bead method. Then microbial diversity sequencing library was established, and deep sequencing was performed with the Illumina MiSeq PE300 high-throughput sequencing platform at the Allwegene Company (Beijing, China). Pear (v0.9.6) software was used to filter and splice the data for the optimized sequence. Qualified reads were clustered into operational taxonomic units (OTUs) at a similarity level of 97% using the Uparse algorithm of Vsearch (v2.7.1) software. Taxonomic analysis at different levels (kingdom, phylum, class, order, family, genus, and species) was obtained using the Ribosomal Database Project classifier algorithm. QIIME1 (v1.8.0) software was used for α diversity index analysis (including the Shannon and Chao1 indexes), and nonmetric multidimensional scaling analysis (NMDS) was performed using the R package (v3.6.0).
Serum amino acid concentrations were determined by liquid chromatography-tandem mass spectrometry with the Q-life Lab 9000 mass spectrometer at Shenzhen Institute of Life and Health Medicine Co., Ltd. (Jiangsu, China). Serum samples were loaded and separated online using the InfinityLab Poroshell 120 EC-C18 column (2.1 mm × 100 mm, 2.7 μm; Agilent, Santa Clara, CA, United States). The column temperature was set to 45 °C. The mobile phase consisted of 0.01% formic acid in water (solvent A) and acetonitrile (solvent B). The flow rate was 0.3 mL/min, and the injection volume was 2 μL. Gradient elution was used as follows: 0-1.5 min, 8% B; 3.5 min, 16% B; 6.5 min, 20% B; 8.0 min, 95% B; and 8.01-11.0 min, 8% B. The electrospray ionization settings were as follows: Capillary voltage, 3.5 kV; spray voltage, 300 V; drying gas temperature, 250 °C; drying gas flow rate, 7 L/min; sheath gas flow rate, 11 L/min; and sheath gas temperature, 250 °C.
The experimental data are expressed as the mean ± SD, and one-way analysis of variance was used to evaluate the significance among multiple groups. P < 0.05 was considered statistically significant. Data analysis and picture drawing were performed using GraphPad Prism 7.0 software (GraphPad, San Diego, CA, United States).
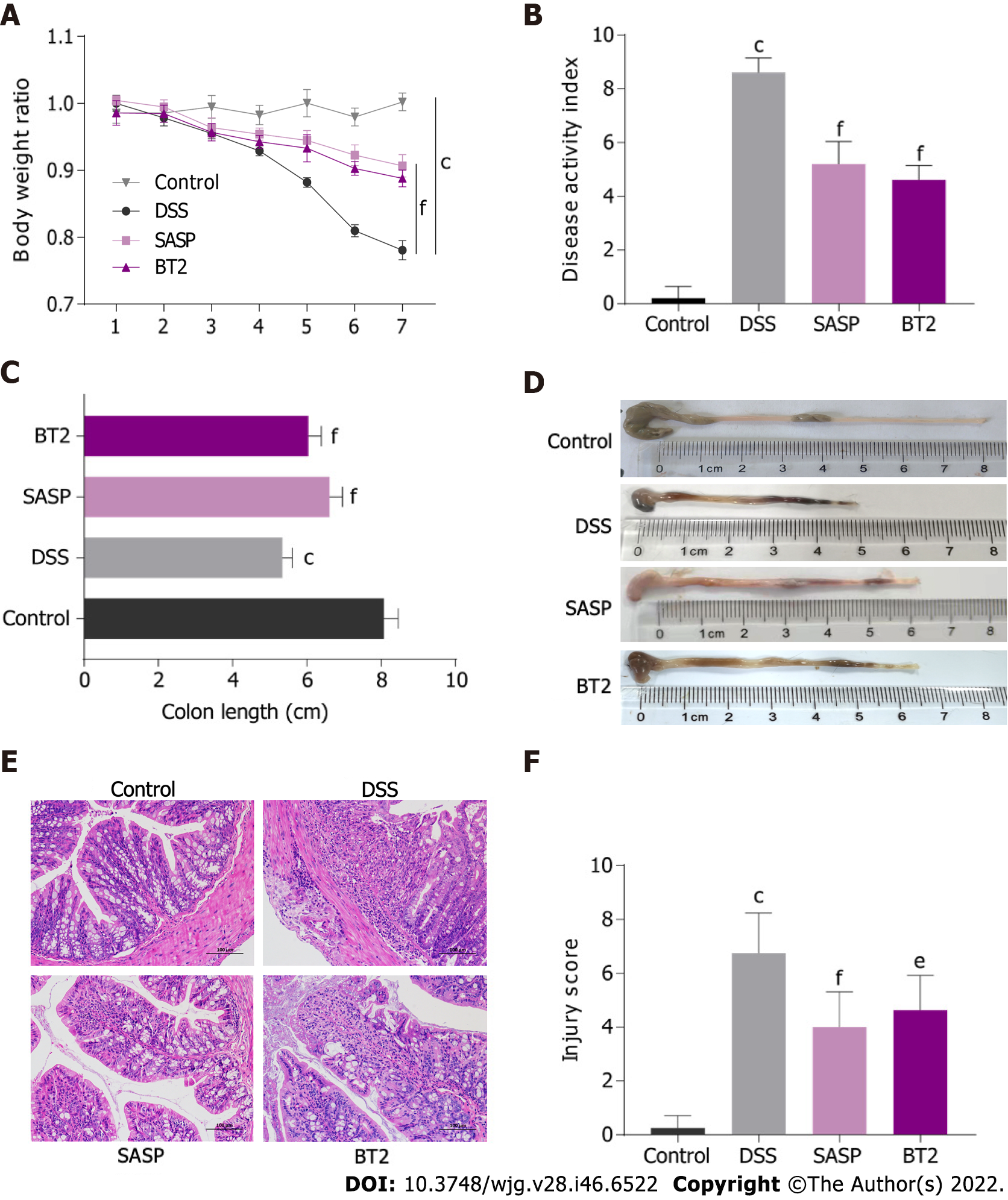
The effects of BT2 on DSS-induced UC mice were assessed based on the DAI score, body weight loss, and colon length. Oral 3.5% DSS treatment led to a significant reduction in body weight (Figure 1A), but the weight loss was reversed by BT2 and SASP treatment. The DAI score including weight loss rate, stool consistency and hematochezia was markedly increased in colitis mice, whereas the BT2- and SASP-treated groups showed improvements in this metric (Figure 1B). Then we examined the changes in the colon length. DSS treatment evidently shortened the colon length compared with that of the control group, whereas 7 d of BT2 and SASP administration extended the length (Figure 1C and D). These data showed that BT2 could improve the clinical symptoms of UC mice.
Representative H&E staining images and the pathological scores of colon tissues from each group are shown in Figure 1E and F. The mucosal epithelium of the mice in the control group was intact, and the morphology of the cells was normal and showed no inflammatory infiltration. The tissues of the mice in the DSS-treated group displayed evident epithelial damage and inflammatory infiltration. However, the histological injury score revealed that BT2 and SASP treatment reduced the severity of UC. Furth
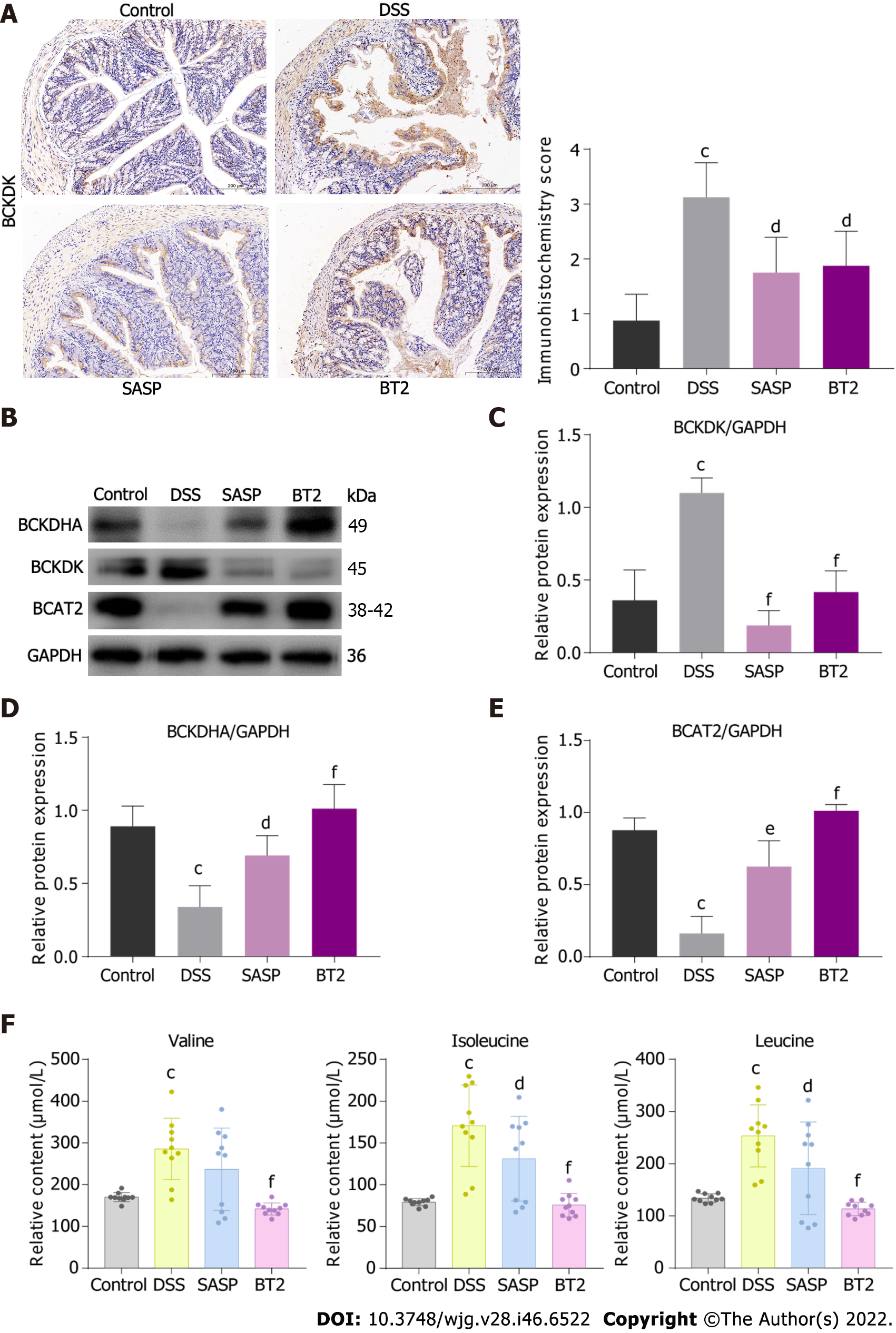
To verify the effects of BT2 on BCAA catabolism in DSS-induced UC mice, the expression levels of the catabolic enzymes BCKDHA, BCAT2, and BCKDK in colon tissues were detected by immunoblotting (Figure 3). The DSS group showed increased BCKDK expression and decreased expression of BCKDHA and BCAT2, but these changes were notably reversed by BT2 administration (Figure 3B-E). Consistently, immunohistochemistry analysis showed that BCKDK expression was increased in colon tissues with severe inflammation but reduced in BT2-treated mice (Figure 3A). Moreover, higher concentrations of leucine, isoleucine, and valine were observed in the serum of DSS-treated mice, whereas BT2 treatment resulted in downregulation of these amino acids (Figure 3F). In summary, BCAA catabolism was impaired in UC mice but rescued by BT2 treatment.
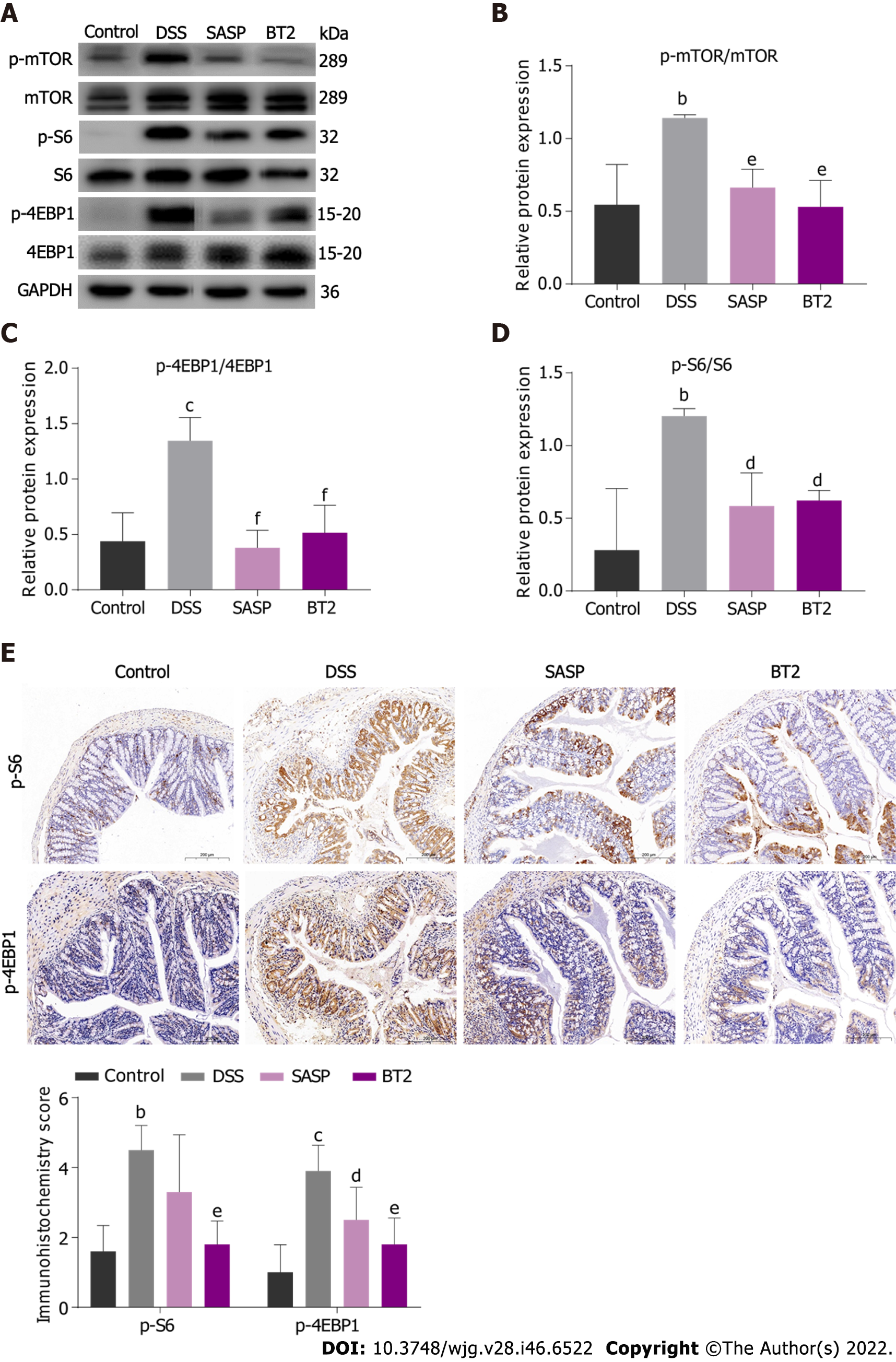
Based on the beneficial effect of BT2 on BCAA metabolism, we hypothesized that BT2 could suppress BCAA-related mTORC1 activation in UC mice. In accordance with this assumption, the phosphorylation of mTOR and its characterized downstream substrates 4-EBP1 and S6 were explored by Western blot analysis. As shown in Figure 4A-D, the expression levels of these phosphorylated proteins were markedly elevated in the colon tissues from the DSS group but decreased in the colon tissues from the BT2 group. Additionally, immunohistochemical staining showed that BT2 treatment significantly reversed the upregulation of phosphorylated 4-EBP1 and p-S6 expression in colon tissues induced by DSS (Figure 4E). Collectively, these results suggested that BT2 could inhibit BCAA-related mTORC1 activation in the colon tissues of UC mice.
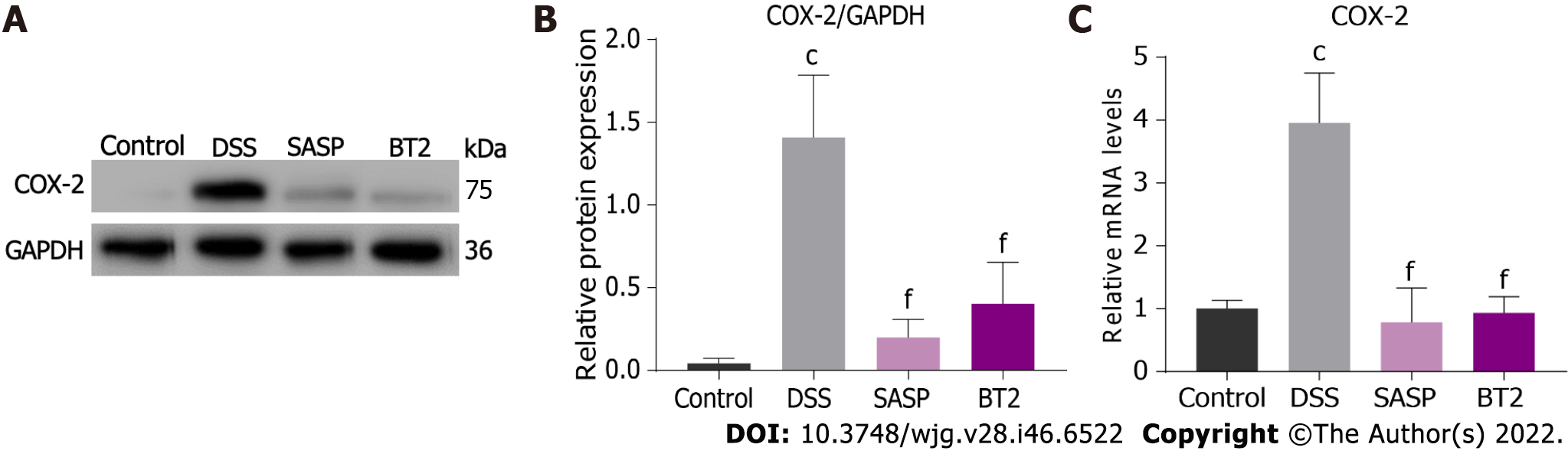
The overexpression of COX-2 in experimental colitis has been widely reported; therefore, we investigated the effect of BT2 on COX-2 expression in colon tissues of UC mice. As shown in Figure 5A and B, COX-2 expression was markedly induced by DSS but reduced in the BT2 group. As depicted in Figure 5C, mice in the DSS group showed higher mRNA levels of COX-2 but BT2 treatment reversed this effect.
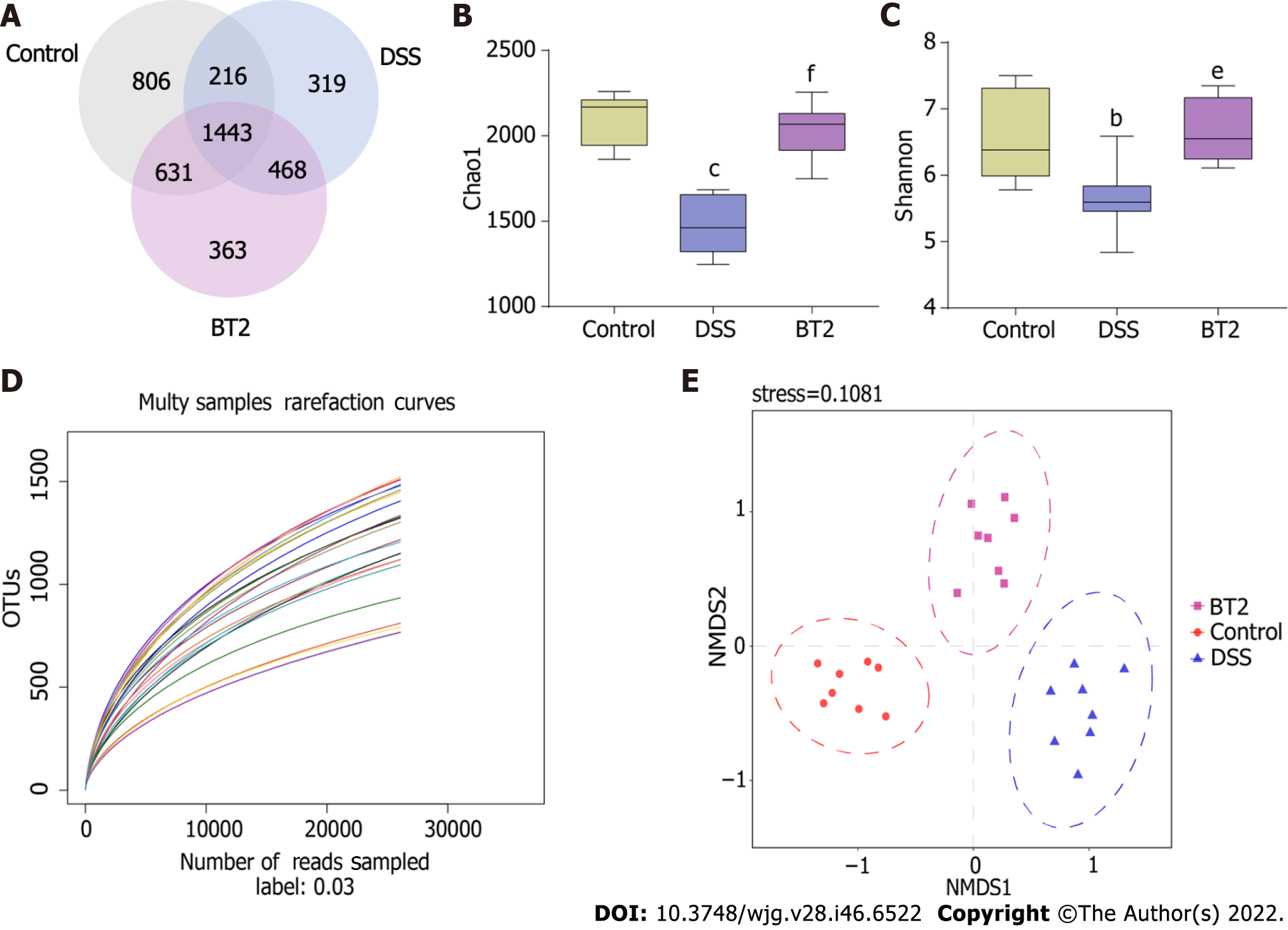
The gut microbiota is involved in various physiological functions of the intestinal mucosa and is a new target for the prevention, diagnosis, and treatment of colonic inflammation. Therefore, 16S rDNA sequencing was performed to investigate whether BT2 regulates the composition of intestinal microflora in colitis mice (Figure 6). The rarefaction curve, which was used to estimate the sample size and the species richness, showed that the sequencing amount was reasonable (Figure 6D). NMDS analysis based on β diversity revealed that the flora structure was evidently different between the DSS group and the control group but that BT2 treatment improved this dissimilarity (Figure 6E). The Venn diagram was created to illustrate the number of common and unique OTUs among multiple samples. The overlapping part indicates the common OTUs, and the nonoverlapping areas represent the unique OTUs in each group. As shown in Figure 6A, 1443 OTUs were present in all groups, whereas 806, 319, and 363 OTUs were uniquely present in the control group, DSS group, and BT2 group, respectively. The α diversity index, including the Chao1 and Shannon indexes, showed that the gut microbiota diversity and richness were lower in colitis mice than in the control group. However, these alterations were normalized by BT2 treatment (Figure 6B and C).
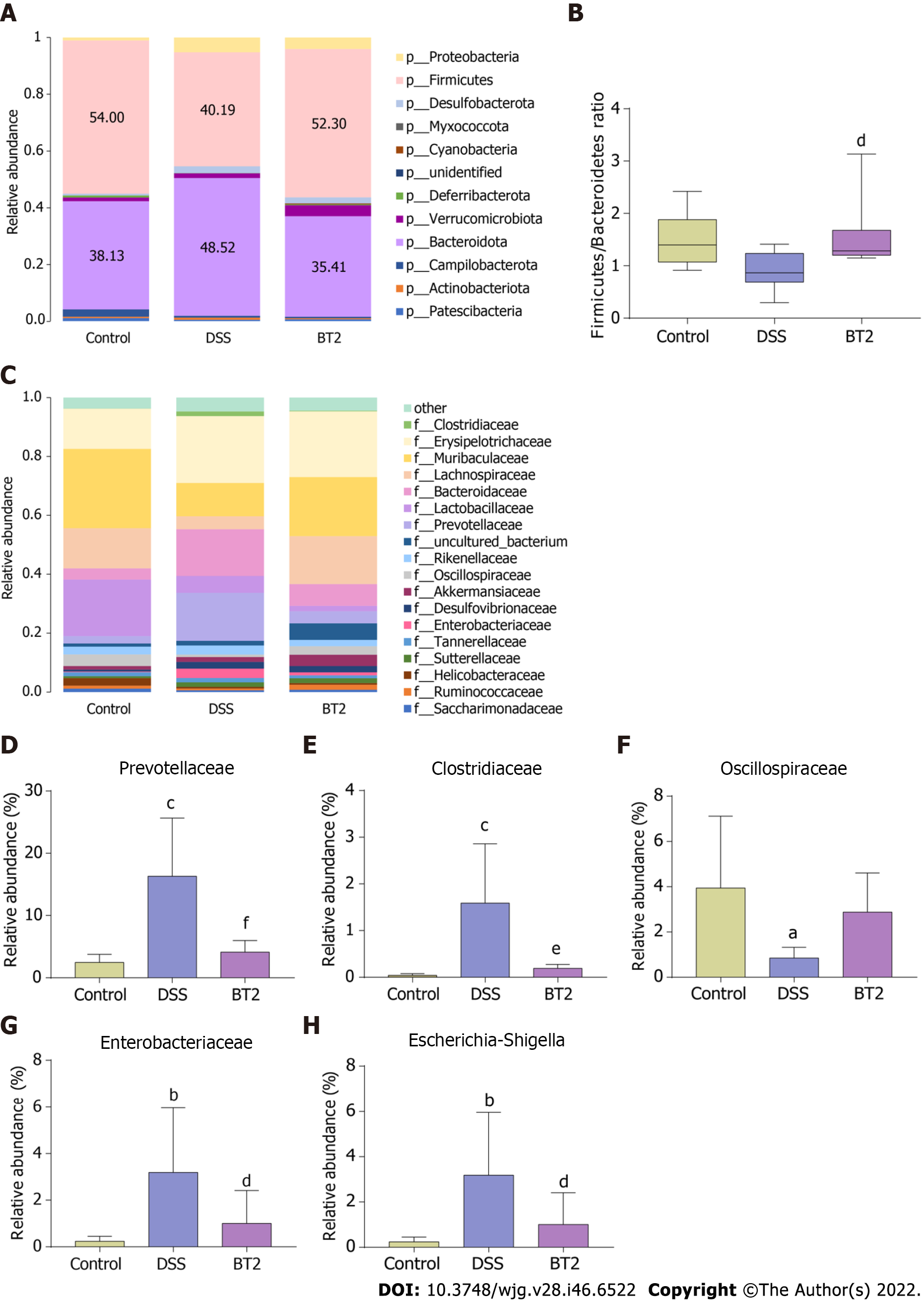
As shown in Figure 7A, the intestinal flora mainly consisted of Firmicutes, Bacteroidetes, and Proteobacteria at the phylum level. The relative abundance levels of Bacteroidetes and Proteobacteria were increased in the DSS group, whereas the abundance of Firmicutes was reduced. However, BT2 intervention significantly restored the abundance of the three phyla. Moreover, the ratio of Firmicutes to Bacteroidetes (F/B) was decreased in the fecal samples from the DSS group relative to the control group, but this decrease was markedly reversed by BT2 (Figure 7B). At the family level, UC mice displayed evidently higher populations of Clostridiaceae and Prevotellaceae, which are related to the synthesis of BCAAs compared with normal mice. As expected, BT2 treatment notably increased the abundance of Clostridiaceae and Prevotellaceae (Figure 7C-E). Furthermore, the administration of BT2 prevented the increases in Enterobacteriaceae and Escherichia-Shigella caused by DSS and slightly restored the downregulation of Oscillospiraceae (Figure 7F-H).
UC has emerged as one of the most common chronic recurrent intestinal diseases in recent years and is treated with 5-aminosalicylic acid preparations, glucocorticoids, anti-tumor necrosis factor agents and thiopurines. These drugs can relieve colonic inflammation to a certain extent but are usually accompanied by adverse reactions, intolerance, and treatment limitations[22,23]. Therefore, research on new drug targets and therapeutics for UC has received increasing attention. A large number of studies have shown that disorder of the intestinal microbiota is crucial to the initiation and progression of UC. The metabolites produced by intestinal flora, such as BCAAs and short-chain fatty acids, can be absorbed by the host intestinal tract to affect its physiological and pathological functions, whereas an imbalance in the intestinal flora affects these functional metabolites[15]. Bosch et al[24] reported that the levels of leucine, isoleucine, and valine were significantly elevated in the feces of inflammatory bowel disease (IBD) patients. BT2 is a specific inhibitor of BCKDK that can improve BCAA catabolism and limit the tumor burden by inhibiting mTORC1 in mouse hepatocellular carcinoma models[18], but the impact of BT2 on UC has not been explored to date.
This study showed that BT2 exerted obvious therapeutic effects on DSS-induced UC. BT2 intervention led to a reduction in body weight loss; improved the pathological injury of colon tissues; decreased the content of proinflammatory cytokines including IL-6, IL-9 and IL-2; and decreased the level of IL-10 compared with the results obtained with DSS treatment. In particular, we found that BT2 could ameliorate the gut microbiota disruption caused by DSS. The diversity and richness of the fecal microbiota in the DSS group were decreased, but this downregulation was reversed in the BT2 group. At the phylum level, the fecal flora of mice was mainly composed of Firmicutes, Bacteroidetes, and Proteobacteria. In addition, upregulation of the phylum Proteobacteria and downregulation of the F/B value in the DSS group were normalized by BT2 treatment. In accordance with previous reports, Proteobacteria represent the largest phylum in the bacterial domain and play a key role in driving proinflammatory changes and inducing IBD development due to their adhesiveness and invasiveness[25]. The F/B value has been used as an index for evaluating the disordered intestinal microbiome in UC and was significantly reduced in the fecal microbiota of IBD patients compared with controls[26,27]. At the family level, it is worth noting that two BCAA-producing bacteria, Clostridiaceae and Prevotellaceae, were increased in the DSS group but were downregulated in the BT2 group. Notably, Prevotella copri is a representative species that drives the synthesis of BCAAs, which are capable of inducing insulin resistance, aggravating glucose intolerance, and increasing the circulating level of BCAAs[28]. Intestinal microorganisms have great BCAA biosynthetic potential, and the increase in BCAA levels is related to the change in bacteria involved in BCAA biosynthesis[28]. A previous study showed that the relative abundance of microbes associated with BCAA synthesis was increased in mice fed a high-fat diet including the order Clostridiales, family Clostridiaceae, and Prevotellaceae[29]. The abundance of Clostridiaceae is positively correlated with the concentration of serum BCAAs in mice[30]. We also found that the level of Oscillospiraceae was slightly increased in the BT2 group. Oscillospiraceae is widely present in the intestinal microorganisms of humans and animals and is associated with inflammatory diseases. A previous meta-analysis showed that Oscillospiraceae was significantly reduced in the intestines of patients with Crohn’s disease but increased in those with gallstones[31]. Moreover, downregulation of Enterobacteriaceae and Escherichia-Shigella was also observed in the BT2 group. A recent report showed that host-induced inflammation disrupts gut microbes, leading to overgrowth of some aerobic bacteria, particularly Enterobacteriaceae, which is elevated in the intestinal tract of IBD patients and acts as an initiation inducer for inflammation[32]. Shigella is a gram-negative short bacillus that is highly contagious and regarded as a pathogen of IBD[33].
mTORC1 is a complex of mTOR that jointly promotes protein synthesis, cell growth, and proliferation by phosphorylating its direct downstream substrates S6 kinase 1 and 4E-BP1[34,35]. A growing number of findings have revealed the involvement of the mTORC1 pathway in the pathogenesis of UC. mTORC1 is hyperactivated in the colon tissues of UC patients and mice, and the overexpression of mTORC1 in colonic epithelial cells aggravates the inflammatory symptoms of UC mice[12]. Supplementation with mTORC1 inhibitors such as rapamycin, AZD8055, and everolimus markedly improves experimental UC by reducing leukocyte extravasation in the mucosa, suppressing T-cell proliferation and blocking the release of interferon from lymphocytes[36-38]. In our study, the activity of mTORC1 was enhanced in DSS-stimulated colitis mice, and BT2 significantly decreased mTORC1 activity. COX-2 is one of the isoforms of COX and is strongly associated with the development of IBD. COX-2 is highly expressed in the colon tissues of UC patients and animals and induces UC by producing prostaglandins (PGs), which promote inflammation and pain. The COX-2 inhibitor rofecoxib is potentially effective in improving experimental colitis by reducing PGE2 production, proinflammatory cytokines, and neutrophil infiltration[39,40]. In clinical UC samples, a positive correlation has been detected between COX-2 expression and mTORC1 activity[12]. Consistently, our study demonstrated that COX-2 expression was enhanced in UC mice but inhibited by BT2 intervention.
Amino acids, particularly BCAAs, serve as essential signaling factors for mTORC1 activation. The reduction of BCAA production by blocking BCAT1, a cytosolic aminotransferase for BCAAs, can significantly inhibit mTORC1 activation in chronic myeloid leukemia cells[41]. Rag guanine triphosphate (Rag GTPase) has been identified as a critical activator of mTORC1 in response to amino acid signals[42]. GATOR1, GATOR2, and Sestrin2 are three proteins involved in the regulation of recombination-activating genes. Sestrin2 is reportedly a leucine receptor for the mTORC1 signaling pathway[43]. Previous studies have shown that BCAA-induced mTORC1 activation is associated with the development of hepatocellular carcinoma, liver cancer, and prostate cancer[18,44,45]. Recently, Huangqin decoction relieves experimental colitis by regulating the metabolism of amino acids such as leucine and the mTOR pathway[46]. These results revealed that BCAA-mediated mTORC1 activation is a key research topic in the field of UC. Our data established an unreported link between BCAA-related mTORC1 activation and UC and thus elucidate a novel mechanism of BT2 against UC. Further investigations need to combine gene knockout mouse models and fecal microbiota transplantation to validate the specific mechanism of BT2-regulated BCAA metabolism in the anti-UC effect.
In summary, our data showed that the intestinal microbiota was disordered and BCAA catabolism was impaired in DSS-induced colitis mice, resulting in activation of the mTORC1 signaling pathway and overexpression of COX-2. Importantly, BT2 substantially reversed these phenomena and thus demonstrates potential value in the treatment of UC.
Ulcerative colitis (UC) is a chronic inflammatory disorder caused by multiple factors. As a novel inhibitor, 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2) was recently shown to play an important role in improving amino acid metabolism and anti-inflammation. However, no relevant studies have reported the protective effect of BT2 on UC.
To investigate the therapeutic effect of BT2 on UC and the potential pharmacological mechanism.
To clarify the role of BT2 in UC treatment and its underlying mechanism.
The acute colitis model was induced by 3.5% dextran sodium sulfate (DSS). The protective effect of BT2 was evaluated via clinical manifestations and colonic pathological changes. Flow cytometry was used to detect the contents of cytokines. Western blot analysis, immunohistochemical assays and liquid chromatography-tandem mass spectrometry were employed to estimate the mechanistic target of rapamycin complex 1 (mTORC1) activity and branched-chain amino acid (BCAA) catabolism. Moreover, the abundance of intestinal microbiota in feces was measured by 16S ribosomal DNA sequencing.
BT2 markedly alleviated colonic damage and reduced the release of proinflammatory cytokines in the DSS colitis model. BT2 also obviously improved BCAA catabolism in UC mice, as evidenced by the detection of related kinases in colon tissues and the targeted quantification of amino acids in serum. mTORC1 activity was enhanced in UC mice but inhibited by BT2 treatment. Moreover, 16S rRNA sequencing revealed that BT2 could prevent the decrease in the Firmicutes/Bacteroidetes ratio and the increase in harmful bacteria induced by DSS.
BT2 relieved colitis by inhibiting BCAA-related mTORC1 activation and restoring gut microbiota metabolism homeostasis.
BT2 showed significant efficacy on colitis and could serve as a promising therapeutic agent for UC treatment.
| 1. | Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (4)] |
| 2. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 877] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 3. | Han YD, Al Bandar MH, Dulskas A, Cho MS, Hur H, Min BS, Lee KY, Kim NK. Prognosis of ulcerative colitis colorectal cancer vs. sporadic colorectal cancer: propensity score matching analysis. BMC Surg. 2017;17:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3604] [Article Influence: 257.4] [Reference Citation Analysis (6)] |
| 5. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4493] [Article Influence: 499.2] [Reference Citation Analysis (111)] |
| 6. | Macfarlane S, Furrie E, Cummings JH, Macfarlane GT. Chemotaxonomic analysis of bacterial populations colonizing the rectal mucosa in patients with ulcerative colitis. Clin Infect Dis. 2004;38:1690-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3511] [Article Influence: 184.8] [Reference Citation Analysis (1)] |
| 8. | Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 956] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 9. | Gao S, Liu W, Zhuo X, Wang L, Wang G, Sun T, Zhao Z, Liu J, Tian Y, Zhou J, Yuan Z, Wu Y. The activation of mTOR is required for monocyte pro-inflammatory response in patients with coronary artery disease. Clin Sci (Lond). 2015;128:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Lyons J, Ghazi PC, Starchenko A, Tovaglieri A, Baldwin KR, Poulin EJ, Gierut JJ, Genetti C, Yajnik V, Breault DT, Lauffenburger DA, Haigis KM. The colonic epithelium plays an active role in promoting colitis by shaping the tissue cytokine profile. PLoS Biol. 2018;16:e2002417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Cheon SY, Cho K. Lipid metabolism, inflammation, and foam cell formation in health and metabolic disorders: targeting mTORC1. J Mol Med (Berl). 2021;99:1497-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Lin X, Sun Q, Zhou L, He M, Dong X, Lai M, Liu M, Su Y, Jia C, Han Z, Liu S, Zheng H, Jiang Y, Ling H, Li M, Chen J, Zou Z, Bai X. Colonic epithelial mTORC1 promotes ulcerative colitis through COX-2-mediated Th17 responses. Mucosal Immunol. 2018;11:1663-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Yoon MS. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 14. | Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al Batran R, Pherwani S, Ho KL, Boisvenue J, Karwi QG, Altamimi T, Wishart DS, Dyck JRB, Ussher JR, Oudit GY, Lopaschuk GD. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. 2019;18:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 15. | Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2638] [Cited by in RCA: 2550] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 16. | Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids. 2012;43:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Tso SC, Gui WJ, Wu CY, Chuang JL, Qi X, Skvora KJ, Dork K, Wallace AL, Morlock LK, Lee BH, Hutson SM, Strom SC, Williams NS, Tambar UK, Wynn RM, Chuang DT. Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain α-ketoacid dehydrogenase kinase. J Biol Chem. 2014;289:20583-20593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Ericksen RE, Lim SL, McDonnell E, Shuen WH, Vadiveloo M, White PJ, Ding Z, Kwok R, Lee P, Radda GK, Toh HC, Hirschey MD, Han W. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab. 2019;29:1151-1165.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 19. | Yeh MC, Wu BJ, Li Y, Elahy M, Prado-Lourenco L, Sockler J, Lau H, Day RO, Khachigian LM. BT2 Suppresses Human Monocytic-Endothelial Cell Adhesion, Bone Erosion and Inflammation. J Inflamm Res. 2021;14:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1293] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 21. | Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. 2012;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 351] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 22. | Alexakis C, Saxena S, Chhaya V, Cecil E, Curcin V, Pollok R. Do Thiopurines Reduce the Risk of Surgery in Elderly Onset Inflammatory Bowel Disease? Inflamm Bowel Dis. 2017;23:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94:1357-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 301] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 24. | Bosch S, Struys EA, van Gaal N, Bakkali A, Jansen EW, Diederen K, Benninga MA, Mulder CJ, de Boer NKH, de Meij TGJ. Fecal Amino Acid Analysis Can Discriminate De Novo Treatment-Naïve Pediatric Inflammatory Bowel Disease From Controls. J Pediatr Gastroenterol Nutr. 2018;66:773-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 613] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 26. | Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Doré J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 931] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 27. | Kabeerdoss J, Jayakanthan P, Pugazhendhi S, Ramakrishna BS. Alterations of mucosal microbiota in the colon of patients with inflammatory bowel disease revealed by real time polymerase chain reaction amplification of 16S ribosomal ribonucleic acid. Indian J Med Res. 2015;142:23-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K; MetaHIT Consortium, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1542] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 29. | Yue SJ, Liu J, Wang AT, Meng XT, Yang ZR, Peng C, Guan HS, Wang CY, Yan D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am J Physiol Endocrinol Metab. 2019;316:E73-E85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 30. | Yang Y, Zhao M, He X, Wu Q, Li DL, Zang WJ. Pyridostigmine Protects Against Diabetic Cardiomyopathy by Regulating Vagal Activity, Gut Microbiota, and Branched-Chain Amino Acid Catabolism in Diabetic Mice. Front Pharmacol. 2021;12:647481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223-4233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 650] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 32. | Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 33. | Kang E, Crouse A, Chevallier L, Pontier SM, Alzahrani A, Silué N, Campbell-Valois FX, Montagutelli X, Gruenheid S, Malo D. Enterobacteria and host resistance to infection. Mamm Genome. 2018;29:558-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 34. | Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014;36:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 35. | Wang X, Proud CG. mTORC1 signaling: what we still don't know. J Mol Cell Biol. 2011;3:206-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Hu S, Chen M, Wang Y, Wang Z, Pei Y, Fan R, Liu X, Wang L, Zhou J, Zheng S, Zhang T, Lin Y, Zhang M, Tao R, Zhong J. mTOR Inhibition Attenuates Dextran Sulfate Sodium-Induced Colitis by Suppressing T Cell Proliferation and Balancing TH1/TH17/Treg Profile. PLoS One. 2016;11:e0154564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Farkas S, Hornung M, Sattler C, Guba M, Steinbauer M, Anthuber M, Herfarth H, Schlitt HJ, Geissler EK. Rapamycin decreases leukocyte migration in vivo and effectively reduces experimentally induced chronic colitis. Int J Colorectal Dis. 2006;21:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Matsuda C, Ito T, Song J, Mizushima T, Tamagawa H, Kai Y, Hamanaka Y, Inoue M, Nishida T, Matsuda H, Sawa Y. Therapeutic effect of a new immunosuppressive agent, everolimus, on interleukin-10 gene-deficient mice with colitis. Clin Exp Immunol. 2007;148:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 39. | Martín AR, Villegas I, Alarcón de la Lastra C. The COX-2 inhibitor, rofecoxib, ameliorates dextran sulphate sodium induced colitis in mice. Inflamm Res. 2005;54:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Martín AR, Villegas I, La Casa C, Alarcón de la Lastra C. The cyclo-oxygenase-2 inhibitor, rofecoxib, attenuates mucosal damage due to colitis induced by trinitrobenzene sulphonic acid in rats. Eur J Pharmacol. 2003;481:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Hattori A, Tsunoda M, Konuma T, Kobayashi M, Nagy T, Glushka J, Tayyari F, McSkimming D, Kannan N, Tojo A, Edison AS, Ito T. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature. 2017;545:500-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 42. | Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1115] [Cited by in RCA: 1106] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 43. | Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 1009] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 44. | Melnik BC. Dairy consumption and hepatocellular carcinoma risk. Ann Transl Med. 2021;9:736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Zhang BK, Moran AM, Bailey CG, Rasko JEJ, Holst J, Wang Q. EGF-activated PI3K/Akt signalling coordinates leucine uptake by regulating LAT3 expression in prostate cancer. Cell Commun Signal. 2019;17:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Li MX, Li MY, Lei JX, Wu YZ, Li ZH, Chen LM, Zhou CL, Su JY, Huang GX, Huang XQ, Zheng XB. Huangqin decoction ameliorates DSS-induced ulcerative colitis: Role of gut microbiota and amino acid metabolism, mTOR pathway and intestinal epithelial barrier. Phytomedicine. 2022;100:154052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Gu L, China S-Editor: Fan JR L-Editor: Filipodia CL P-Editor: Zhang XD