Published online Nov 28, 2022. doi: 10.3748/wjg.v28.i44.6271
Peer-review started: August 6, 2022
First decision: September 26, 2022
Revised: October 9, 2022
Accepted: November 9, 2022
Article in press: November 9, 2022
Published online: November 28, 2022
Processing time: 110 Days and 9 Hours
The selection criteria for Barcelona Clinic Liver Cancer (BCLC) intermediate-stage hepatocellular carcinoma (HCC) patients who would truly benefit from liver resection (LR) remain undefined.
To identify BCLC-B HCC patients more suitable for LR.
We included patients undergoing curative LR for BCLC stage A or B multi-nodular HCC (MNHCC) and stratified BCLC-B patients by the sum of tumor size and number (N + S). Overall survival (OS), recurrence-free survival (RFS), recur-rence-to-death survival (RTDS), recurrence patterns, and treatments after recurrence in BCLC-B patients in each subgroup were compared with those in BCLC-A patients.
In total, 143 patients who underwent curative LR for MNHCC with BCLC-A (n = 25) or BCLC-B (n = 118) were retrospectively analyzed. According to the N + S, patients with BCLC-B HCC were divided into two subgroups: BCLC-B1 (N + S ≤ 10, n = 83) and BCLC-B2 (N + S > 10, n = 35). Compared with BCLC-B2 patients, those with BCLC-B1 had a better OS (5-year OS rate: 67.4% vs 33.6%; P < 0.001), which was comparable to that in BCLC-A patients (5-year OS rate: 67.4% vs 74.1%; P = 0.250), and a better RFS (median RFS: 19 mo vs 7 mo; P < 0.001), which was worse than that in BCLC-A patients (median RFS: 19 mo vs 48 mo; P = 0.022). Further analysis of patients who developed recurrence showed that both BCLC-B1 and BCLC-A patients had better RTDS (median RTDS: Not reached vs 49 mo; P = 0.599), while the RTDS in BCLC-B2 patients was worse (median RTDS: 16 mo vs not reached, P < 0.001; 16 mo vs 49 mo, P = 0.042). The recurrence patterns were similar between BCLC-B1 and BCLC-A patients, but BCLC-B2 patients had a shorter recurrence time and a higher proportion of patients had recurrence with macrovascular invasion and/or extrahepatic metastasis, both of which were independent risk factors for RTDS.
BCLC-B HCC patients undergoing hepatectomy with N + S ≤ 10 had mild recurrence patterns and excellent OS similar to those in BCLC-A MNHCC patients, and LR should be considered in these patients.
Core Tip: Subgroups of Barcelona Clinic Liver Cancer (BCLC) intermediate-stage hepatocellular carcinoma (HCC) patients who would truly benefit from liver resection (LR) remain undefined. We demonstrated that the sum of tumor size and number (N + S) can predict not only prognosis in BCLC-B patients undergoing LR, but also the recurrence patterns and recurrence-to-death survival (RTDS) in these patients. In addition, we indicated that BCLC-B patients undergoing hepatectomy with N + S ≤ 10 had mild recurrence patterns, good RTDS and excellent overall survival similar to those in BCLC-A multinodular HCC patients. The results of this study are helpful in selecting BCLC-B patients more suitable for LR.
- Citation: Hu XS, Yang HY, Leng C, Zhang ZW. Postoperative outcomes and recurrence patterns of intermediate-stage hepatocellular carcinoma dictated by the sum of tumor size and number. World J Gastroenterol 2022; 28(44): 6271-6281
- URL: https://www.wjgnet.com/1007-9327/full/v28/i44/6271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i44.6271
As the sixth most common cancer globally, primary liver cancer accounted for 906,000 newly confirmed cancer cases and 830,000 cancer-related deaths worldwide in 2020, of which 75%-85% were hepatocellular carcinoma (HCC)[1].
Barcelona Clinic Liver Cancer (BCLC) staging system, which was proposed in 1999, has been widely used to guide treatment decisions in patients with HCC[2,3]. The 2022 version of the BCLC strategy recommends liver transplantation (LT), transarterial chemoembolization (TACE), and systemic therapy, respectively, for BCLC intermediate-stage HCC patients based on their expected survival time[4].
In addition, emerging studies have suggested that liver resection (LR) may also be a good treatment option for BCLC-B HCC patients[5,6]. Nevertheless, the subgroups of BCLC-B HCC patients who would truly benefit from LR have yet to be defined. Several previous studies found that some BCLC-B HCC patients undergoing LR had favorable long-term overall survival (OS) rates (5-year OS rates: 50%-75%); however, these selected patients still had high postoperative recurrence rates (2-year recurrence rate: ≥ 50%), which means that many of these patients had good recurrence-to-death survival (RTDS)[7,8]. Both recurrence patterns and treatments after recurrence can affect the RTDS of HCC patients who develop recurrence after LR[9-11]. However, previous studies did not analyze the main reasons why these selected patients had good RTDS, which may affect the judgment of the role of LR in these patients[7,8].
In this study, we retrospectively included patients undergoing curative LR for BCLC stage A or B multinodular HCC (MNHCC) and stratified the BCLC-B patients by the sum of tumor size and number (N + S), which combines the two main prognostic factors of BCLC-B patients into a continuous variable[7,8]. BCLC-B patients more suitable for LR were identified by comparing the outcomes, recurrence patterns, and treatments after recurrence in BCLC-B patients in each subgroup with those in BCLC-A patients.
We enrolled BCLC stage A or B MNHCC patients who underwent curative LR in Tongji Hospital from January 2010 to May 2018. The inclusion criteria were: (1) MNHCC pathologically diagnosed with two or more nodules, in which lesions less than 1 cm in diameter and less than 2 cm away from the main nodule were defined as satellite nodules[12]; (2) Curative resection, defined as complete macroscopic removal of all tumors with negative histologic resection margins for the tumors (R0 resection)[13,14]; and (3) No preoperative anticancer treatment other than TACE. The exclusion criteria were: (1) Re-current HCC or combined HCC and cholangiocarcinoma; and (2) Complicated with other malignancies.
Patient data at the time of initial hepatectomy including sex, age, body mass index, hepatitis B antigen status, liver function, tumor characteristics, surgical procedure, and preoperative treatment were recorded. Liver function in this study was classified by the albumin-bilirubin score[15]. Maximum tumor size was defined as the maximum diameter of the largest tumor. Microvascular invasion was defined as tumor within a vascular space lined by endothelium that was visible only on microscopy[16].
In addition, the recurrence patterns, which consisted of recurrence time and tumor characteristics at the time of recurrence, and treatments after recurrence in those patients who developed recurrence during follow-up were also recorded. Recurrence time was defined as the time between initial LR and the first recurrence.
In our center, we routinely estimated the residual liver volume in MNHCC patients before hepatectomy, and only patients with residual liver volume of more than 40% of the standard liver volume (for patients with liver cirrhosis) or more than 30% (for patients without liver cirrhosis) would receive LR[17,18]. The decision to perform anatomical or non-anatomical hepatectomy depended largely on the tumor distribution, and major resection was defined as the resection of three or more Couinaud liver segments[19]. Intraoperative ultrasound was routinely used to locate the tumor and screen the nodules. All nodules were completely resected intraoperatively and negative margin was determined according to postoperative pathology.
Patients were followed every month with measurement of serum alpha-fetoprotein (AFP), chest radiography and ultrasound or computed tomography (CT) or magnetic resonance imaging (MRI) in the first 6 mo after discharge from hospital, and every 3-6 mo thereafter. When HCC recurrence was confirmed by CT or MRI, patients were treated with repeated hepatectomy, ablation, TACE or systemic therapy. Follow-up was terminated on May 15, 2022.
Recurrence-free survival (RFS) was calculated from the date of hepatectomy until recurrence or last follow-up. OS was defined as the time from LR to death or last follow-up, and RTDS was defined as the time from recurrence to death or last follow-up.
Continuous variables were presented as mean ± SD or median (interquartile range; IQR). Categorical variables were described by frequency and percentage. In the comparison of different subgroups, continuous variables were compared using the Student’s t or Mann-Whitney U test, and categorical variables using the χ2 or Fisher’s exact test, as appropriate. Survival was analyzed by the Kaplan-Meier method, and survival curves were compared by the log-rank test. Univariate and multivariate analyses were based on the Cox proportional analysis. Variables with P values less than 0.1 identified by the univariate analysis were included in multivariate analysis. The cutoff value of N + S was determined by X-tile, a bioinformatics tool produced by Camp and colleagues[20]. The area under receiver operating characteristic (ROC) curve (AUC) was compared using DeLong test[21]. P < 0.05 was considered to indicate statistical significance. Both SPSS (version 23.0, SPSS, Inc., Chicago, IL, United States) and MedCalc software (version 20.115, MedCalc Software, Ostend, Belgium) were used for the analysis.
A total of 143 patients who underwent curative LR for BCLC stage A or B MNHCC were enrolled. Their mean age was 52.1 years and most patients were male (n = 134, 93.7%) and were hepatitis B surface antigen positive (n = 131, 91.6%). Median maximum tumor size in the entire cohort was 5.6 cm (IQR: 3.4–7.6) and tumor number in the vast majority of patients was ≤ 3 (n = 136, 95.1%). Overall, 17.5% (n = 25) of patients had BCLC-A MNHCC, and 82.5% (n = 118) had BCLC-B MNHCC (Table 1).
| Variables | Total (n = 143) | BCLC-A (n = 25) | BCLC-B (n = 118) | P value |
| Male gender | 134 (93.7) | 25 (100) | 109 (92.4) | 0.330 |
| Age (yr) | 52.1 ± 12.7 | 50.5 ± 14.5 | 52.4 ± 12.4 | 0.490 |
| BMI | 22.97 ± 3.15 | 23.05 ± 3.35 | 22.96 ± 3.12 | 0.895 |
| HBs-Ag positive | 131 (91.6) | 24 (96) | 107 (90.7) | 0.635 |
| Albumin (g/L) | 38.89 ± 4.51 | 39.95 ± 5.15 | 38.65 ± 4.34 | 0.194 |
| Bilirubin (μmol/L) | 13.8 (9.9, 18) | 12.9 (10.2, 20.9) | 13.9 (9.7, 17.8) | 0.568 |
| ALBI grade | 0.680 | |||
| 1 | 69 (48.3) | 13 (52) | 56 (47.5) | |
| 2/3 | 74 (51.7) | 12/0 (48/0) | 62/0 (52.5/0) | |
| AFP (μg/L) | 239 (13, 2338) | 74 (6, 390) | 483 (16, 2944) | 0.011 |
| Maximum tumor size (cm) | 5.6 (3.4, 7.6) | 2.5 (2.1, 2.9) | 6.2 (4.1, 8.4) | < 0.001 |
| Tumor number | 0.460 | |||
| ≤ 3 | 136 (95.1) | 25 (100) | 111 (94.1) | |
| > 3 | 7 (4.9) | 0 | 7 (5.9) | |
| Tumor distribution | 0.506 | |||
| Unilateral | 83 (58) | 16 (64) | 67 (56.8) | |
| Bilateral | 60 (42) | 9 (36) | 51 (43.2) | |
| Presence of microvascular invasion | 15 (10.5) | 1 (4) | 14 (11.9) | 0.420 |
| Edmondson-Steiner grade | 0.337 | |||
| I-II | 85 (59.4) | 17 (68) | 68 (57.6) | |
| III-IV | 58 (40.6) | 8 (32) | 50 (42.4) | |
| Major resection | 64 (44.8) | 4 (16) | 60 (50.8) | 0.001 |
| Anatomical hepatectomy | 22 (15.4) | 4 (16) | 18 (15.3) | 1.000 |
| Preoperative TACE | 0.690 | |||
| No | 121 (84.6) | 20 (80) | 101 (85.6) | |
| Yes | 22 (15.4) | 5 (20) | 17 (14.4) |
After a median follow-up of 54 mo (IQR 27–79), 5-year OS and RFS after R0 resection in all patients were 60.2% and 23.2%, respectively. Of note, BCLC-B patients had worse 5-year OS (57.2% vs 74.1%, P = 0.028, Supplementary Figure 1A) and RFS (19.4% vs 41.6%, P = 0.002, Supplementary Figure 1B).
Among patients undergoing LR for BCLC-B HCC, the median maximum tumor size was 6.2 cm (IQR: 4.1–8.4) and tumor number in 111 (94.1%) patients was ≤ 3. Of note, 43.2% (n = 51) of patients had bilateral disease and 14.4% (n = 17) of patients underwent TACE before initial LR (Table 1).
Using the X-tile program[20], patients with BCLC-B HCC were divided into two groups by N + S: BCLC-B1 (≤ 10, n = 83, 70.3%), BCLC-B2 (> 10, n = 35, 29.7%) (Supplementary Figure 2).
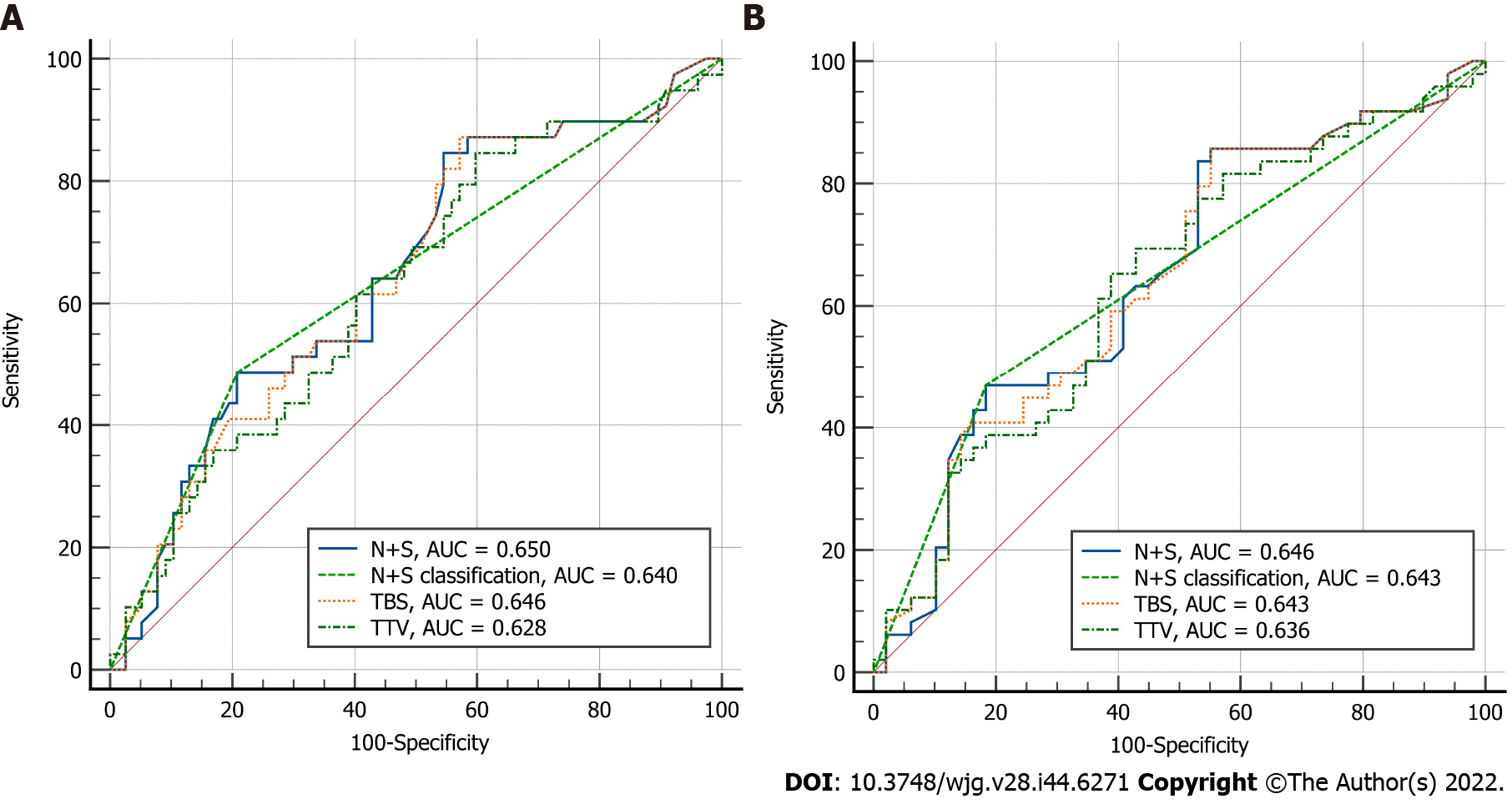
The prognostic ability of N + S and the rationality of the cut-off value of 10 were then verified by time-dependent ROC curves and Cox-regression analysis. The AUCs for 3-year and 5-year OS in BCLC-B patients were 0.650 and 0.646, respectively, for N + S, and 0.640 and 0.643, respectively, for stratification according to N + S (Figure 1). Multivariate analysis showed that N + S > 10 was an independent risk factor for OS [hazard ratio (HR) 2.996, 1.779 to 5.045; P < 0.001] (Table 2) and RFS (HR 1.657, 1.057 to 2.596; P = 0.028) (Table 3) in BCLC-B patients.
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender (male) | 1.128 (0.409-3.117) | 0.816 | ||
| Age (> 65 yr) | 0.668 (0.285-1.562) | 0.352 | ||
| BMI > 25 | 0.954 (0.513-1.772) | 0.880 | ||
| HBs-Ag positive | 1.084 (0.466-2.525) | 0.851 | ||
| ALBI grade | ||||
| 1 | 1.00 (Reference) | 1.00 (Reference) | ||
| 2 | 1.891 (1.112-3.217) | 0.019 | 2.279 (1.236-4.201) | 0.008 |
| AFP > 400 ng/mL | 1.969 (1.165-3.327) | 0.011 | ||
| Maximum tumor size > 5 cm | 2.510 (1.354-4.651) | 0.003 | ||
| Tumor number > 3 | 3.806 (1.716-8.444) | 0.001 | 5.519 (2.207-13.803) | < 0.001 |
| N + S > 10 | 3.403 (2.031-5.702) | < 0.001 | 2.996 (1.779-5.045) | < 0.001 |
| Bilateral tumor distribution | 2.201 (1.312-3.694) | 0.003 | ||
| Presence of MVI | 1.816 (0.855-3.858) | 0.120 | ||
| Edmondson-Steiner III-IV | 2.084 (1.248-3.480) | 0.005 | 2.051 (1.219-3.449) | 0.007 |
| Major resection | 1.886 (1.115-3.191) | 0.018 | ||
| NAH | 0.905 (0.458-1.788) | 0.775 | ||
| Preoperative TACE | ||||
| No | 1.00 (Reference) | |||
| Yes | 0.494 (0.198-1.238) | 0.132 | ||
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender (male) | 1.135 (0.526-2.450) | 0.747 | ||
| Age (> 65 yr) | 0.881 (0.514-1.511) | 0.646 | ||
| BMI > 25 | 1.157 (0.729-1.836) | 0.536 | ||
| HBs-Ag positive | 1.109 (0.558-2.202) | 0.769 | ||
| ALBI grade | ||||
| 1 | 1.00 (Reference) | |||
| 2 | 1.474 (0.988-2.198) | 0.057 | ||
| AFP > 400 ng/mL | 1.458 (0.984-2.162) | 0.060 | ||
| Maximum tumor size > 5 cm | 1.253 (0.830-1.891) | 0.283 | ||
| Tumor number > 3 | 2.449 (1.123-5.343) | 0.024 | ||
| N + S > 10 | 2.113 (1.385-3.224) | 0.001 | 1.657 (1.057-2.596) | 0.028 |
| Bilateral tumor distribution | 2.104 (1.409-3.140) | < 0.001 | 1.820 (1.187-2.791) | 0.006 |
| Presence of MVI | 1.757 (0.973-3.171) | 0.062 | ||
| Edmondson-Steiner III-IV | 1.709 (1.151-2.539) | 0.008 | 1.676 (1.127-2.493) | 0.011 |
| Major resection | 1.285 (0.867-1.904) | 0.211 | ||
| NAH | 1.126 (0.650-1.950) | 0.673 | ||
| Preoperative TACE | ||||
| No | 1.00 (Reference) | |||
| Yes | 0.784 (0.437-1.405) | 0.414 | ||
In addition, we compared the predictive accuracy of N + S with those of tumor burden score (TBS) and total tumor volume (TTV), both of which were previous prognostic models based on tumor size and number of HCC patients[22,23]. The results showed that the AUCs of N + S at 3 and 5 years were both similar to those of TBS (3-year AUC, 0.650 vs 0.646, P = 0.552; 5-year AUC, 0.646 vs 0.643, P = 0.762) and TTV (3-year AUC, 0.650 vs 0.628, P = 0.171; 5-year AUC, 0.646 vs 0.636, P = 0.535) (Figure 1).
Clinical characteristics, OS and RFS of patients with BCLC-B1, BCLC-B2, and BCLC-A were compared (Figure 2, Supplementary Table 1). The results showed that BCLC-B2 patients had a higher serum AFP level and a larger proportion of bilateral tumor distribution, compared to patients with BCLC-A and BCLC-B1. With an increase in N + S, the maximum tumor size gradually increased, and a larger proportion of patients underwent major resection (Supplementary Table 1).
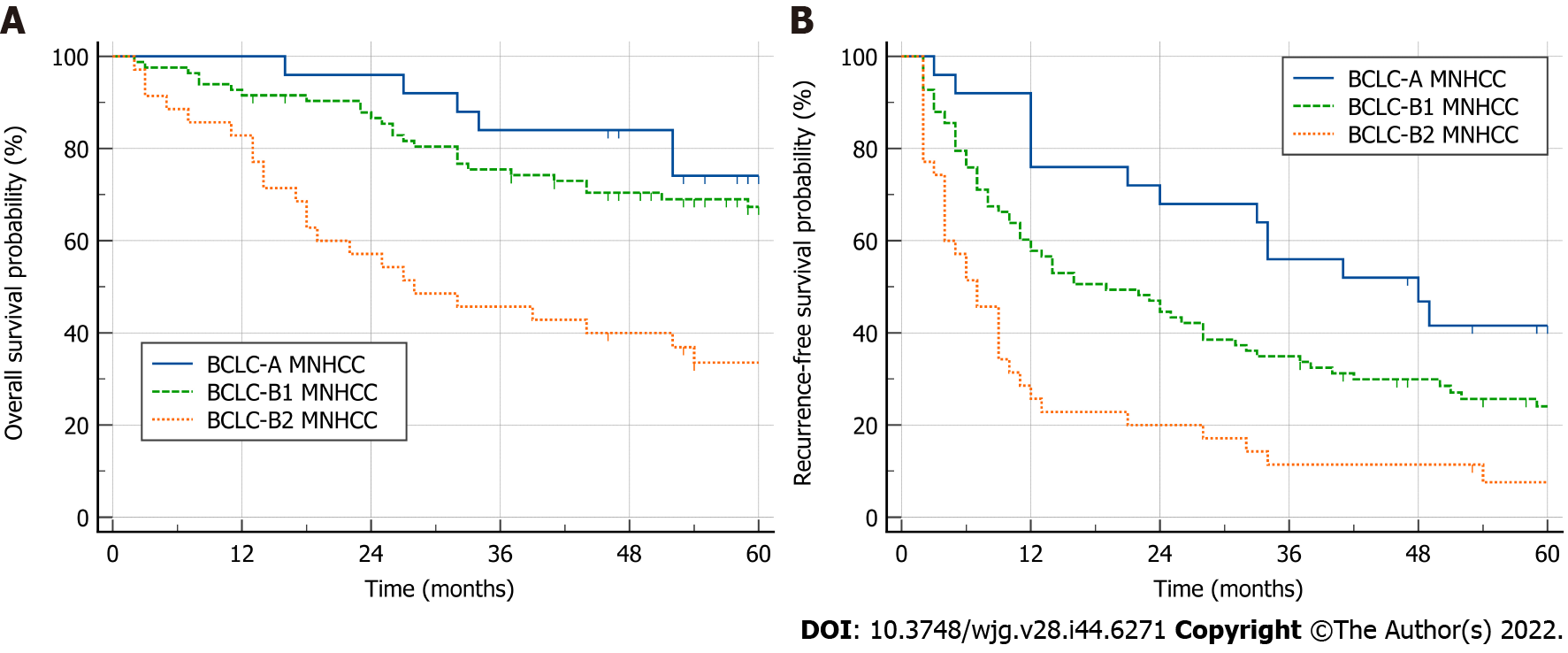
Both BCLC-A patients and BCLC-B1 patients had good 5-year OS (74.1% vs 67.4%, P = 0.250), which was better than that in BCLC-B2 patients (74.1% vs 33.6%, P < 0.001; 67.4% vs 33.6%, P < 0.001) (Figure 2A). Compared with BCLC-A patients, BCLC-B1 patients had a worse RFS (median RFS: 19 mo vs 48 mo; P = 0.022), which was better than that in BCLC-B2 patients (median RFS: 19 mo vs 7 mo; P < 0.001) (Figure 2B).
During follow-up, 14 (56%) BCLC-A, 66 (79.5%) BCLC-B1 and 34 (97.1%) BCLC-B2 patients developed recurrences (P < 0.001). Nine BCLC-B1 and 4 BCLC-B2 patients who lacked information on tumor characteristics at the time of recurrence and treatments after recurrence were excluded from the analysis. Ultimately, 14 BCLC-A, 57 BCLC-B1 and 30 BCLC-B2 patients with recurrence were included in the analysis. The recurrence patterns and treatments after recurrence in these patients are sum-marized in Supplementary Table 2.
Compared with BCLC-A and BCLC-B1 patients, BCLC-B2 patients had a shorter recurrence time and a higher proportion of recurrence with macrovascular invasion and/or extrahepatic metastasis. However, there were no significant statistical differences in recurrence patterns and treatment after recurrence between BCLC-B1 and BCLC-A patients. Fewer BCLC-B2 patients underwent curative treatments after recurrence than BCLC-A patients, but the treatment after recurrence was similar between BCLC-B2 patients and BCLC-B1 patients (Supplementary Table 2).
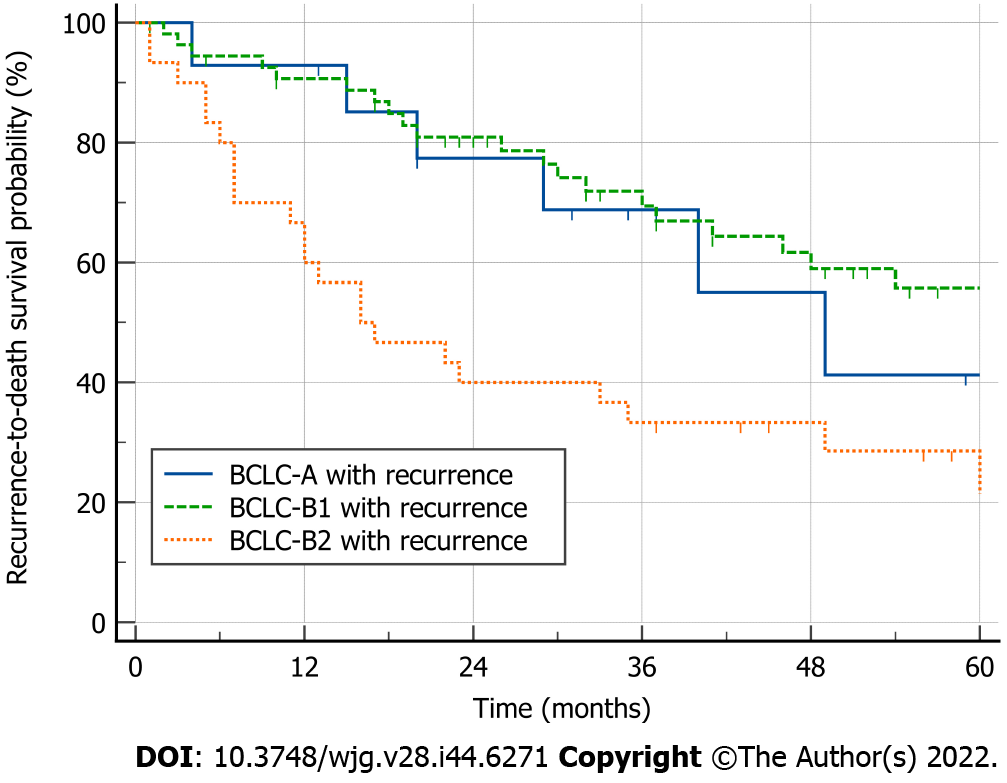
Both BCLC-B1 and BCLC-A patients had good RTDS (median RTDS: Not reached, vs 49 mo for BCLC-B1 and BCLC-A patients, respectively; P = 0.599), while BCLC-B2 patients had a worse RTDS (16 mo vs not reached, P < 0.001; 16 mo vs 49 mo, P = 0.042) (Figure 3).
We further conducted a multivariate analysis of the factors affecting RTDS of BCLC stage A or B MNHCC patients undergoing LR. Multivariate analysis showed that initial tumor with BCLC-B2 (N + S > 10) (HR 2.696, 1.468 to 4.953; P = 0.001), recurrence within 2-year (HR 4.353, 1.024 to 18.503; P = 0.046), recurrent tumor number > 3 (HR 3.247, 1.629 to 6.474; P = 0.001), recurrence with macrovascular invasion and/or extrahepatic spread (HR 2.894, 1.458 to 5.746; P = 0.002) and noncurative treatments after recurrence (HR 2.423, 1.209 to 4.854; P = 0.013) were independent risk factors for RTDS (Supplementary Table 3).
The role of LR in BCLC-B HCC patients is unclear. Although the latest BCLC staging system still does not recommend LR for BCLC-B patients, the results of emerging studies have indicated that LR resulted in a good 5-year OS for BCLC-B HCC patients[4-6]. In this study, patients who underwent LR for BCLC-B HCC had an overall 5-year OS rate of 57.2%, which demonstrated that LR was a good treatment option in these patients.
To select BCLC-B patients more suitable for LR, we stratified these patients according to N + S, which has been used to select HCC patients who are more suitable for LT and for TACE[24,25]. In fact, Matsukuma et al[26] suggested that N + S was a good prognostic factor for BCLC-B HCC patients undergoing hepatectomy. The present study increased the cutoff point of N + S from 8 to 10, which may be related to different study cohorts and different calculation methods used for the cutoff value[26]. Nevertheless, the results of this study and in the study by Matsukuma et al[26] demonstrated that N + S could predict the recurrence and OS of BCLC-B HCC patients undergoing hepatectomy. In addition, the present study showed that N + S had a predictive accuracy similar to TBS and TTV in predicting OS in BCLC-B patients. However, compared with the complicated calculation of TBS and TTV, the calculation of N + S is simpler and more suitable for clinical application.
Previous studies have focused on the OS when selecting BCLC-B patients for LR, and ignored that those selected patients still had a high postoperative recurrence rate[7,8]. In order to demonstrate that the selected BCLC-B HCC patients truly benefit from LR rather than remedial treatments after recurrence, and to better understand the tumor characteristics of the selected patients, we compared not only the OS and RFS, but also the RTDS, recurrence patterns, and treatments after recurrence.
In the present study, BCLC-B1 (BCLC-B with N + S ≤ 10) HCC patients were considered as BCLC-B HCC patients who likely benefitted most from LR. Although BCLC-B1 HCC patients were still more likely to develop recurrence after LR than BCLC-A MNHCC patients, these BCLC-B1 patients had mild recurrence pattern, good RTDS and excellent OS similar to BCLC-A MNHCC patients.
However, BCLC-B2 (BCLC-B with N + S > 10) HCC patients not only had a high postoperative recurrence rate, but also an aggressive recurrence pattern. Although the treatment after recurrence was similar between BCLC-B2 patients and BCLC-B1 patients, the BCLC-B2 patients still had a worse RTDS. The long-term OS of BCLC-B2 patients undergoing LR is not satisfactory.
To the best of our knowledge, this study is the first to demonstrate that N + S could predict not only prognosis in BCLC-B HCC patients, but also the recurrence patterns and RTDS in these patients.
In addition, it is interesting to note that patients with BCLC-B1 HCC had worse RFS but comparable OS than patients with BCLC-A MNHCC in this study. In fact, previous studies comparing LT vs LR in HCC patients found a similar phenomenon. These studies showed that although patients receiving LR had a higher rate of postoperative recurrence, the 5-year OS between LR and LT was comparable[27,28]. Previous studies have suggested that the reasons for this phenomenon may be related to noncancerous death in the LT group and treatment after recurrence in the LR group, and our results suggest that it may also be related to the recurrence patterns after LR.
As a single-center retrospective study, the present study has some limitations. Firstly, the sample size was small, which may have affected the accuracy of the results. Secondly, there was a lack of comparison of treatment options other than LR. Some patients with BCLC-B HCC and N + S ≤ 10 would meet the ‘Extended Liver Transplant criteria’, and the best treatment option for these patients remains to be explored[29]. Finally, the results of this study need to be verified by an external cohort.
N + S is a good measure that could predict the OS, RFS, RTDS and recurrence patterns in BCLC-B HCC patients undergoing LR. In particular, BCLC-B patients with N + S ≤ 10 had survivals similar to those of BCLC-A MNHCC patients. Given the computational simplicity of N + S, it is worth exploring the application of N + S to guide decision-making in the treatment of BCLC-B patients.
Emerging studies have shown that Barcelona Clinic Liver Cancer (BCLC) intermediate-stage hepatocellular carcinoma (HCC) patients had a good prognosis after liver resection (LR), but the subgroups of BCLC-B patients more suitable for LR have yet to be defined.
There is a lack of studies on whether the sum of tumor size and number (N + S) can be used to select BCLC-B patients who are more suitable for LR. The effect of recurrence patterns on long-term survival in BCLC-B patients undergoing LR is also poorly explored.
The present study aimed to identify BCLC-B patients more suitable for LR and to further analyze the reasons why these patients could benefit from LR.
BCLC stage A or B multinodular HCC (MNHCC) patients undergoing curative hepatectomy were enrolled. Overall survival (OS), recurrence-free survival (RFS), recurrence-to-death survival (RTDS), recurrence patterns, and treatments after recurrence in BCLC-B patients in each subgroup according to N + S were compared with those in BCLC-A patients.
N + S could predict not only the OS and RFS in BCLC-B HCC patients undergoing hepatectomy, but also the recurrence patterns and RTDS in these patients. BCLC-B patients with N + S ≤ 10 had mild recurrence patterns, good RTDS and excellent OS similar to those in BCLC-A MNHCC patients.
N + S can be used to select BCLC-B HCC patients who are more suitable for LR, and LR should be considered in BCLC-B patients with N + S ≤ 10.
As a measure that can be easily obtained and calculated in clinical practice, N + S can help with the clinical decision-making in the treatment of BCLC-B HCC patients.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68525] [Article Influence: 13705.0] [Reference Citation Analysis (201)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6413] [Article Influence: 801.6] [Reference Citation Analysis (9)] |
| 3. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3434] [Article Influence: 429.3] [Reference Citation Analysis (3)] |
| 4. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 3107] [Article Influence: 776.8] [Reference Citation Analysis (61)] |
| 5. | Kim H, Ahn SW, Hong SK, Yoon KC, Kim HS, Choi YR, Lee HW, Yi NJ, Lee KW, Suh KS; Korean Liver Cancer Association. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg. 2017;104:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Labgaa I, Taffé P, Martin D, Clerc D, Schwartz M, Kokudo N, Denys A, Halkic N, Demartines N, Melloul E. Comparison of Partial Hepatectomy and Transarterial Chemoembolization in Intermediate-Stage Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer. 2020;9:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Wada H, Eguchi H, Noda T, Ogawa H, Yamada D, Tomimaru Y, Tomokuni A, Asaoka T, Kawamoto K, Gotoh K, Marubashi S, Umeshita K, Nagano H, Doki Y, Mori M. Selection criteria for hepatic resection in intermediate-stage (BCLC stage B) multiple hepatocellular carcinoma. Surgery. 2016;160:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Zhang YF, Zhou J, Wei W, Zou RH, Chen MS, Lau WY, Shi M, Guo RP. Intermediate-stage hepatocellular carcinoma treated with hepatic resection: the NSP score as an aid to decision-making. Br J Cancer. 2016;115:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Zou Q, Li J, Wu D, Yan Z, Wan X, Wang K, Shi L, Lau WY, Wu M, Shen F. Nomograms for Pre-operative and Post-operative Prediction of Long-Term Survival of Patients Who Underwent Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg Oncol. 2016;23:2618-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Wang K, Liu G, Li J, Yan Z, Xia Y, Wan X, Ji Y, Lau WY, Wu M, Shen F. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Yao LQ, Chen ZL, Feng ZH, Diao YK, Li C, Sun HY, Zhong JH, Chen TH, Gu WM, Zhou YH, Zhang WG, Wang H, Zeng YY, Wu H, Wang MD, Xu XF, Pawlik TM, Lau WY, Shen F, Yang T. Clinical Features of Recurrence After Hepatic Resection for Early-Stage Hepatocellular Carcinoma and Long-Term Survival Outcomes of Patients with Recurrence: A Multi-institutional Analysis. Ann Surg Oncol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 13. | Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J, Cong WM, Wu MC, Lau WY, Cheng SQ. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol. 2016;23:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2167] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 16. | Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 562] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 17. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 199] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 617] [Article Influence: 102.8] [Reference Citation Analysis (3)] |
| 19. | Pol B, Campan P, Hardwigsen J, Botti G, Pons J, Le Treut YP. Morbidity of major hepatic resections: a 100-case prospective study. Eur J Surg. 1999;165:446-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 3081] [Article Influence: 146.7] [Reference Citation Analysis (8)] |
| 21. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 22. | Tsilimigras DI, Moris D, Hyer JM, Bagante F, Sahara K, Moro A, Paredes AZ, Mehta R, Ratti F, Marques HP, Silva S, Soubrane O, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Sasaki K, Rodarte AI, Aucejo FN, Pawlik TM. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br J Surg. 2020;107:854-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 23. | Zakaria HM, Macshut M, Gaballa NK, Sherif AE, Abdel-Samea ME, Abdel-Samiee M, Marwan I, Yassein T. Total tumor volume as a prognostic value for survival following liver resection in patients with hepatocellular carcinoma. Retrospective cohort study. Ann Med Surg (Lond). 2020;54:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 24. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1616] [Article Influence: 89.8] [Reference Citation Analysis (1)] |
| 25. | Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, Mu W, Yin G, Li H, Zhao H, Li J, Zhang C, Zhu X, Wu J, Gong W, Li Z, Lin Z, Pan X, Shi H, Shao G, Liu J, Yang S, Zheng Y, Xu J, Song J, Wang W, Wang Z, Zhang Y, Ding R, Zhang H, Yu H, Zheng L, Gu W, You N, Wang G, Zhang S, Feng L, Liu L, Zhang P, Li X, Chen J, Xu T, Zhou W, Zeng H, Huang W, Jiang W, Zhang W, Shao W, Li L, Niu J, Yuan J, Lv Y, Li K, Yin Z, Xia J, Fan D, Han G; China HCC-TACE Study Group. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol. 2019;70:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (6)] |
| 26. | Matsukuma S, Sakamoto K, Tokuhisa Y, Tokumitsu Y, Matsui H, Kanekiyo S, Tomochika S, Iida M, Suzuki N, Takeda S, Ueno T, Wada H, Kobayashi S, Saeki I, Eguchi H, Sakon M, Sakaida I, Nagano H. Outcomes following liver resection for multinodular Barcelona Clinic Liver Cancer-B hepatocellular carcinoma. Oncol Lett. 2018;16:6383-6392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Kaido T, Morita S, Tanaka S, Ogawa K, Mori A, Hatano E, Uemoto S. Long-term outcomes of hepatic resection versus living donor liver transplantation for hepatocellular carcinoma: a propensity score-matching study. Dis Markers. 2015;2015:425926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Michelakos T, Xourafas D, Qadan M, Pieretti-Vanmarcke R, Cai L, Patel MS, Adler JT, Fontan F, Basit U, Vagefi PA, Elias N, Tanabe KK, Berger D, Yeh H, Markmann JF, Chang DC, Ferrone CR. Hepatocellular Carcinoma in Transplantable Child-Pugh A Cirrhotics: Should Cost Affect Resection vs Transplantation? J Gastrointest Surg. 2019;23:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, Durand F, Ijzermans J, Polak W, Zheng S, Roberts JP, Sapisochin G, Hibi T, Kwan NM, Ghobrial M, Soin A. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang M, United States; Zimmitti G, Italy S-Editor: Liu GL L-Editor: A P-Editor: Liu GL