Published online Nov 28, 2022. doi: 10.3748/wjg.v28.i44.6258
Peer-review started: September 10, 2022
First decision: October 19, 2022
Revised: November 1, 2022
Accepted: November 17, 2022
Article in press: November 17, 2022
Published online: November 28, 2022
Processing time: 75 Days and 15.1 Hours
Short bowel syndrome (SBS) with intestinal failure (IF) is a rare but severe complication of Crohn’s disease (CD), which is the most frequent benign condition that leads to SBS after repeated surgical resections, even in the era of biologics and small molecules. Glucagon-like peptide-2 analogues have been deeply studied recently for the treatment of SBS-IF. These drugs have a significant intestinotrophic effect and the potential to reduce the chronic dependence of SBS-IF patients on parenteral support or nutrition. Teduglutide has been approved for the treatment of SBS-IF, and apraglutide is currently in clinical development. The use of these drugs was examined with a focus on their use in CD patients.
Core Tip: Short bowel syndrome with intestinal failure and chronic dependency on parenteral support are rare but severe complications of Crohn’s disease (CD) after repeated intestinal resections. New therapeutic options are available, including glucagon-like peptide-2 analogues. Their use in CD appears safe and efficacious, but more data from specifically designed studies are needed.
- Citation: Pizzoferrato M, Puca P, Ennas S, Cammarota G, Guidi L. Glucagon-like peptide-2 analogues for Crohn’s disease patients with short bowel syndrome and intestinal failure. World J Gastroenterol 2022; 28(44): 6258-6270
- URL: https://www.wjgnet.com/1007-9327/full/v28/i44/6258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i44.6258
Short bowel syndrome (SBS) in adults is a condition in which the normal length of the bowel (which ranges from 3 to 8 metres) is reduced to less than 2 metres[1]. It is classified anatomically into: (1) Type 1: End-jejunostomy; (2) Type 2: Jejuno-colonic anastomosis; and (3) Type 3: Jejuno-ileal anastomosis[2]. The onset of SBS may lead to intestinal failure (IF), which is defined according to the European Society for Clinical Nutrition and Metabolism as the reduction of gut function below the minimum necessary for the absorption of macronutrients and/or water and electrolytes, and intravenous supplementation is required to maintain health and/or growth. A reduction in gut absorptive function that does not require intravenous supplementation to maintain health and/or growth is considered intestinal deficiency. IF is categorised according to temporal and functional evolution into: (1) Type 1 - acute, short term and self-limiting; (2) Type 2 - a prolonged acute condition, often in metabolically unstable patients, that requires complex multidisciplinary care and intravenous supplementation over periods of weeks or months; and (3) Type 3 - a chronic condition in stable patients[3].
However, not all patients with SBS develop IF, and not all patients with IF have underlying SBS[1]. The anatomical classification of SBS is mirrored by different clinical presentations: (1) Type 1 (end-jejunostomy with no colon in continuity) shows a higher risk of dehydration and electrolyte imbalance, especially immediately after surgical resection; (2) Type 2 (jejuno-colonic anastomosis) patients may develop malnutrition in a long-term setting (months to years); and (3) Type 3 (jejuno-ileal anastomosis with ileo-caecal valve conservation) has a lower risk of malnutrition[4].
The causes of SBS-IF in 2919 patients with benign chronic IF were CD (22.4%), mesenteric ischaemia (17.7%), surgical complications (15.8%), primary chronic pseudo-obstruction (9.75%) and radiation enteritis (7.3%). The pathogenetic mechanisms of IF were heterogeneous in the same population, with most patients presenting (38.6%) with end jejunostomy, and approximately 20% of patients presenting with jejuno-colonic anastomosis[5]. The remainder of the cohort was divided into intestinal dysmotility (17.5%), fistulas (7%), mucosal disease (6.8%), jejuno-ileal anastomosis (5.9%), and mechanical obstruction (4.4%)[5]. However, other reports, such as the United States intestinal transplant (IT) registry, describe mesenteric ischaemia as the first cause of IT (24%) and CD as the second most frequent cause (11%)[6].
Following a large bowel resection, humans undergo a wide range of functional and anatomical modifications due to the reduction of the intestinal area dedicated to the absorption of nutrients[7]. Structural and anatomical changes include crypt hyperplasia, angiogenesis, bowel dilation and bowel elongation. Functional changes include accelerated crypt differentiation, a slower transit time, an increase in the number of transporters and a consequent increase in nutrient absorption[8]. After an early phase immediately following surgery, the adaptation phase starts 48 h after resection and lasts at least 1-2 years. Most of the intestinal adaptation described above occurs in this phase. Nutritional homeostasis may be achieved in the maintenance phase via oral autonomy or with parenteral support (PS)[3].
The clinical consequences of SBS are very heterogeneous and may considerably impact the patient’s quality of life. One frequent symptom is diarrhoea due to accelerated intestinal transit, intestinal and gastric increased secretion[9], intestinal bacterial overgrowth[10], and the malabsorption of fats and bile salts[11,12]. One common consequence of SBS is the formation of stones. Asymptomatic gallbladder stones were reported in a population of SBS patients[13]. Most of these stones consist of calcium bilirubinate. The formation of gallbladder stones is favored by altered enterohepatic circulation[14], gallbladder hypomobility[15], and the reduced secretion of cholecystokinin after meals[16]. The formation of calcium oxalate kidney stones is also common, especially in patients with SBS and colon in continuity[17]. Within the colon, unabsorbed long-chain fatty acids compete with oxalate for available luminal calcium, and a larger amount of free oxalic acid is absorbed via passive diffusion and ultimately excreted by the kidney[13]. Other mechanisms may be involved, such as regional differences in oxalate absorption[17].
A relatively rare but underestimated complication of patients with SBS, especially patients with colon preservation, is so-called D-lactic acidosis. When a carbohydrate meal is consumed, colonic bacteria metabolise non-absorbed carbohydrates to short-chain-fatty acid and lactate. Lactate lowers the intraluminal pH, which leads to the overgrowth of D-lactate-producing bacteria, Bifidobacterium and Lactobacillum. D-lactate is absorbed into the systemic circulation and is responsible for the onset of neurological symptoms[18]. From a clinical point of view, this condition is characterized by neurological symptoms, such as ataraxia, movement disorders and altered mental status[19].
The prevalence of IF due to benign disease ranges from 5 to 80 cases of patients on chronic home parenteral nutrition (HPN) per million population in Europe[3]. Benign chronic IF and secondary SBS are in the directory of rare diseases ORPHANET (ORPHA:294422 and ORPHA:95427).
A survey in 64 centres from 22 countries enrolling 2919 patients on HPN described CD as the most frequent benign cause of SBS-IF[5]. However, a long-term study from a single IF centre in the United Kingdom described a change in the causes of IF over time, and the prevalence of CD decreased from 44% in 1978-1988 to 22% in 2006-2012[20].
Surgery remains a common treatment for CD during the course of the disease[21]. The indications for small intestinal resection in CD range from complications, such as stenosis, fistulas and abscesses, to the treatment of medically refractory disease[22]. Surgery rates in CD declined in recent decades due to multifactorial reasons, including earlier diagnosis and treatment, the use of biological agents, a decline in smoking rates, and improved patient education[23,24].
Although a United States population study demonstrated that biological agents reduced the proportion of CD patients undergoing resection, this reduction was not observed in patients with SBS-IF, which likely represents a subgroup of CD patients characterized by a more severe disease course and resistance to treatments[25]. Predictors of SBS-IF in CD were reviewed and roughly correspond to predictors of severe disease course. Notably, the CD phenotype characterized by an ileocolonic location is associated with a greater risk, and the absence of the ileocecal valve increases the risk of HPN dependence. Patients with perianal disease at diagnosis have a higher risk of disabling disease course, including bowel resections, and patients with penetrating disease have longer bowel resections and more frequently depend on HPN. Patients younger than 40 years at the time of CD diagnosis were more likely to have a “very short bowel” (< 100 cm), and an older age at first surgery was associated with decreased odds of IF. Patients who had ever smoked were more likely to develop IF. A family history of inflammatory bowel disease (IBD), frequent corticosteroid use for flares, and the number of bowel resections and complications of surgery were also identified as risk factors for SBS-IF[26].
A recent case-control study of 410 CD patients (41 with SBS) at a single centre demonstrated that subjects with SBS underwent significantly more bowel resections than controls. Patients treated with IV steroids were at higher risk of SBS, and Montreal B1 (inflammatory) behavior and treatment with budesonide characterized patients at a lower risk of SBS[27].
Another study of 2456 IBD patients identified 25 patients who required long-term HPN (1%, 24 CD). They described that HPN use was significantly associated with smoking, narcotic use, IBD-related surgeries, and lower quality-of-life scores. They found that these refractory patients had a 15-fold increase in annual median health care charges compared to control IBD patients[28].
The management of SBS-IF is complex, expensive and requires a multidisciplinary approach. A multidisciplinary team consisting of at least gastroenterologists, nutritionists, surgeons, radiologists, stoma therapists, care managers, pharmacists, and home care nurses is useful to provide patients with the best management possible[29]. The process of intestinal rehabilitation may be improved with medical management and includes spontaneous adaptation (dietary intervention and oral rehydrating solutions) and pharmacological therapies to improve symptoms. Gastric hypersecretion may be reduced with Proton-pump inhibitors. The accelerated transit time may be slowed with loperamide. The reduced reabsorption of bile acids may be targeted with cholestyramine, and bacterial overgrowth may be modulated with antibiotics, particularly rifaximin. Other drugs commonly used in the symptomatic management of diarrhoea are diphenoxylate, atropine, codeine, and antihistamines (e.g., ranitidine and cimetidine).
Although symptom management provides an initial benefit in quality of life and the patient’s perception of disease, it does not provide any improvement in prognosis[30]. The cornerstone in the treatment of SBS-IF is HPN. HPN ensures the correct intake of micro- and macronutrients and provides a significant improvement in prognosis. HPN is a life-saving treatment for these patients, but it significantly lowers patients’ quality of life and is not free of complications[31].
HPN is generally administered in a single dose via a nutrition bag and infused over 10 to 12 h, typically overnight. Patients are encouraged to continue oral feeding with HPN[32]. Catheter infections [also called catheter-related blood stream infections (CRBSIs)] and central venous catheter (CVC) thrombosis are the most feared complications of parenteral nutrition. The rates of CRBSI range from 0 to 11.89 episodes per 1000 catheter days in systematic reviews and meta-analyses[33]. No significant difference was shown in catheter infection rates between peripherally inserted central catheters (PICCs) and tunneled catheters. However, PICCs showed lower CRBSI rates than ports[34]. The more frequently implicated pathogens are Gram-positive bacteria (Staphylococcus aureus, Enterococcus species, and Streptococcus species), Gram-negative bacteria (Klebsiella pneumoniae, Escherichia coli, and Enterobacter cloacae), and fungi (Candida parapsilosis, Candida albicans, and Candida glabrata)[35]. Antibiotic therapy is the mainstay of CRBSI management. An empiric regimen with broad-spectrum drugs is generally used until the responsible agents are identified[36]. Bacterial infections are generally treated with the catheter in place, but fungal infections are treated by removing the catheter[37]. Locking the catheter with ethanol[38,39], taurolidine[40] or antibiotics[41] has been proposed as a strategy for the prevention of CRBSI. However, the results from randomized controlled trials (RCTs) are nonunivocal, and no clear guidelines have been generated on this topic[42].
The incidence of CVC thrombosis is approximately 0.12 events/1000 catheter days[43]. The management of catheter-related thrombotic events is based on the administration of low-molecular-weight heparins (LMWH)[44] or thrombolytic agents, such as streptokinase[45]. Flushing the CVC with a saline solution or LMWH has been proposed as a measure to prevent the occurrence of thrombotic events[46,47].
One possible complication of HPN is progressive steatohepatitis and liver damage, also known as “IF-associated Liver Disease” (IFALD). Up to half of the patients receiving total parenteral nutrition develop severe liver disease after 5 years[48,49], and higher rates of incidence and prevalence are observed in children[50]. IFALD manifests as steatosis, steatohepatitis or a cholestatic pattern. It may lead to progressive liver damage with fibrosis and cirrhosis in some cases[51]. Therapeutic strategies include fish oil-based lipid emulsions[52], ursodeoxycholic acid[53], and lecithin administration[54].
Malabsorption-related anaemia may develop into microcytic anaemia due to iron deficiency or macrocytic anaemia due to malabsorption of vitamin B12 and folate, which lead to the need for iron and vitamin supplementation[3,55]. The occurrence of metabolic bone disease must be monitored[56]. Bone densitometry, the biochemical dosing of vitamin D and calcium profiles play a role in preventing the insurgency of metabolic bone disease[57]. Manganese toxicity must be noted. Manganese is a central element of HPN, but its requirement is low. This low requirement leads to an increase in blood concentration and accumulation in the central nervous system, which is detectable using magnetic resonance imaging. Manganese toxicity presents with neurological symptoms that are similar, but not identical, to Parkinson’s disease[58].
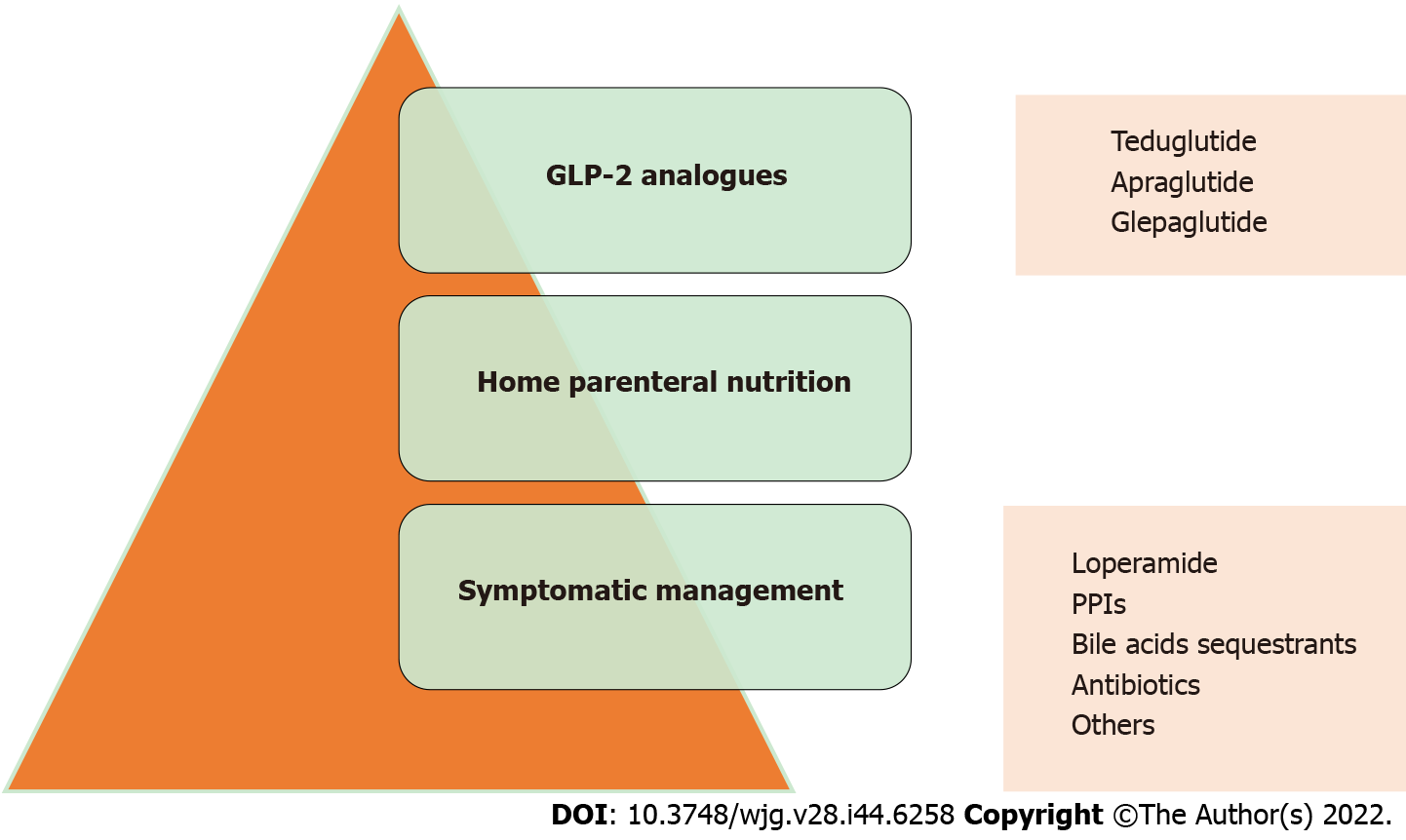
Hormonal manipulation may also increase intestinal absorption[59] (Figure 1). Only two molecules have been approved for SBS-IF therapy, growth hormone in the United States and the glucagon-like peptide-2 (GLP-2) analogue teduglutide (TED) in the United States and Europe[60,61]. TED is a recombinant analogue of GLP-2 that is resistant to degradation by DPD4. The elimination half-life of TED is 2 to 6 h, which allows administration as a daily subcutaneous injection. Notably, the elimination half-life of endogenous GLP-2 is approximately 7 min[62].
The mechanism of action of TED is complex and involves direct and indirect effects of interaction with a GLP-2 receptor and includes the following most relevant factors: Crypt cell proliferation, increase in bowel weight and villous growth, enhancement of intestinal barrier function, inhibition of motility of the gastrointestinal tract and gastric acid secretion, and increase of intestinal blood flow[60]. The effect of TED on intestinal epithelial stem cells is of particular relevance in the possible risk of the drug being tumorigenic[63].
The first open-label trial on TED was performed and published in 2005. Sixteen patients with end-jejunostomy or colon in continuity received s.c. TED for 21 d. Patients treated with TED showed an increased absorption of nutrients, urine output and sodium excretion. TED significantly increased villus height, crypt depth and mitotic index in the small intestines of patients with end-jejunostomy. Leg oedema and enlargement of the stoma nipple were the most common, but not severe, side effects[64].
The most significant RCT of TED effectiveness was performed from 2008 to 2011 and published in 2012 (STEPS study). Eighty-six patients receiving PS were enrolled: 43 patients underwent management with TED, and 43 were treated with placebo (PBO) for 24 wk. In the TED group, 63% of patients were responders who reduced their PS by at least 20% compared to 30% of patients in the PBO group (P = 0.02). The secondary outcomes of this study were the reduction of parenteral nutrition volume at week 24 (2.1 litres per week difference between the TED group and PBO, P < 0.05) and the number of patients achieving at least 1 d off parenteral nutrition per week (21/39 in the TED group vs 9/39 in the PBO group)[65].
The STEPS study was followed by STEPS-2, which was a 2-year, open-label extension of the prior study. The enrolled patients completed 24 wk of TED (TED/TED) or PBO (PBO/TED) in the initial PBO-controlled study or qualified for the original study but were not treated (NT/TED) because of full enrolment. Patients received 0.05 mg/kg/d subcutaneous TED for up to 24 mo (NT/TED and PBO/TED) or up to 30 mo (TED/TED). In patients who completed the study, clinical response (as defined in the STEPS study) was achieved in 28/30 (93%) TED/TED, 16/29 (55%) PBO/TED, and 4/6 (67%) NT/TED patients. The mean PS volume reductions from baseline were 7.6 (66%), 3.1 (28%), and 4.0 (39%) litres/week in the TED/TED, PBO/TED, and NT/TED groups, respectively. Thirteen patients achieved full enteral autonomy. This trial demonstrated the long-term effectiveness and safety of TED[66].
A further extension of STEPS and STEPS-2 studies is the STEPS-3 study. Long-term treatment with TED in this 1-year, open-label extension trial showed a safety profile consistent with previous studies, with prolonged effectiveness and a further reduction in HPN necessity[67]. A post hoc analysis determined factors associated with the response to TED. Notably, the remaining bowel anatomy, rather than the remaining bowel length, primarily affected the outcome of TED therapy. Patients with jejunostomy or ileostomy (SBS-IF type 1 according to the anatomical classification) experienced the most pronounced benefit from TED compared to patients with colon continuity. The same study outlined how patients with higher baseline PS volume had higher reductions in PS, which means that SBS–IF patients with more severe malabsorption benefit the most from therapy with TED[68].
Safety data from clinical trials of TED reported abdominal distension and gastrointestinal stoma complications as the most frequent adverse events (AEs), and the AE frequency was higher early in the course of treatment and declined thereafter[69]. The capacity of GLP-2 to accelerate the growth of experimental intestinal tumors was known before the introduction of TED[70,71]. A post hoc analysis of STEPS studies found that 50 of 65 patients with remnant colon (77%) underwent a protocol-mandated postexposure colonoscopy. Colon polyps were reported in 12% (9/73) of patients at baseline and 18% (9/50) of patients postexposure. Two patients had polyps at baseline and postexposure. Histology was available for 7 patients: 5 had adenomas (1 serrated, 4 tubular), and none had malignancies or high-grade dysplasia[72].
A large population analysis characterizing 170 patients treated with TED from the United States from 2015 to 2020 found that an overall 5.9% of patients developed posttreatment polyps of the large intestine. Twenty events (12%) of catheter infection were described in the same study within 1 year from the start of administration. Other documented adverse effects were abdominal pain (70 cases, 41.2%), nausea (40 cases, 23.5%), intestinal obstruction (30 cases, 17.6%) and stoma complications (20 cases, 11.8%). Ten patients (6%) developed colon polyps[73].
A French real-life study reported a population of 54 patients with SBS-IF treated with TED. The effectiveness and safety were fully confirmed. Twenty-four weeks after the start of treatment, 85% of patients experienced a reduction in PS of at least 20%, and 24% of patients were weaned off PS[74]. Another real-life cohort of 18 patients treated with TED for a median of 3.2 years was reported. Twelve of these patients achieved a clinical response at 12 mo, which was maintained in most of these patients at 36 mo. Five patients were able to wean off PS, but no predictor was identified due to the small sample[75].
New GLP-2 analogue molecules were studied recently. Apraglutide (TA799-007), which is characterized by high protein binding, resistance to DPP4 cleavage, low clearance and slow absorption after subcutaneous administration, has been highlighted[76]. These features make apraglutide clinically appealing, which suggests the possibility of more delayed dose administration[77].
Apraglutide showed efficacy in studies of animal models of SBS-IF in terms of increases in intestinal length and weight, crypt weight and villous height and lower faecal fat and energy losses[78,79]. The effects on intestinal length and mucosal hypertrophy after discontinuation of TED and apraglutide were also studied in piglets. These two molecules apparently showed similar results in this case: Intestinal growth appeared to be a lasting outcome of treatment with long-acting GLP-2 and persisted for at least 7 d after discontinuation. In contrast, mucosal hypertrophy appeared to regress 7 d after the end of treatment with both agents[80]. However, these results must be interpreted with caution and need further confirmation. The once weekly administration of apraglutide to neonatal piglets showed superior intestinotrophic effects than a single daily dose of TED and led to increased bowel length and villus height[81].
A recent PBO-controlled, randomized, phase II clinical trial treated eight adults with SBS-IF with once-weekly 5-mg apraglutide or PBO for 4 wk, followed by once-weekly 10-mg apraglutide doses for 4 wk, with a washout period of 6-10 wk between treatments. No severe AEs related to the drug were detected, and comparable safety profiles were observed between the low-dose and high-dose regimens. The main AE detected was an increase in urine output, and the following less frequent AEs were reported in decreasing order: Stoma complications, decreased thirst, oedema, increased weight, and injection site reactions[82]. A phase 3 study in SBS-IF patients treated with apraglutide vs PBO is currently ongoing[83].
Glepaglutide is another long-acting analogue of GLP-2 that chemically differs from native GLP-2 by 9 amino acid substitutions and a C-terminal tail consisting of six lysine residues. It has a half-life of approximately 50 h and has been evaluated in a phase II study, where it demonstrated good tolerance and the ability to improve intestinal absorption in SBS patients treated with 1 mg and 10 mg daily glepaglutide[84]. A phase 3 study comparing weekly or twice weekly glepaglutide and PBO administration to SBS patients is currently ongoing[85].
Other molecules are in the preclinical phase of their development. Elsiglutide is a GLP-2 analogue with a long half-life that reduced diarrhoea induced by lapatinib, a tyrosine kinase inhibitor, in a rat model[86]. Dapiglutide is a dual GLP-1 and GLP-2 agonist that showed beneficial effects in a rat model of SBS by improving body weight, promoting intestinal growth, increasing villous height and intestinal length, and reducing watery stool losses[87].
For the duration of therapy with GLP-2 analogues in patients with SBS-IF, the available data indicate that these drugs must be administered over a long life because reversal of the previous need for PS occurs if TED is discontinued[64]. A recent report described 13 patients (one with CD and one with ulcerative colitis) who discontinued TED after a successful clinical outcome. The volume of PS remained stable in the first 4 years but later increased in 12/13 patients up to 9 years after withdrawal[88]. These data support further studies exploring the possibility of the periodic use of GLP-2 analogues for selected SBS-IF patients.
Knowledge of GLP-2 analogue use in patients with CD is limited. No RCTs specifically address the efficacy and safety of GLP-2 in CD patients with SBS-IF. However, 18 patients with CD were included in the 86 subjects of the STEPS study, 10 in the TED arm and 8 in the PBO arm[65]. Sixteen CD patients were included in the STEPS-2 long-term study[66]. The CD patients in these studies were in the inactive phase of their inflammatory disease, and the concomitant use of immunosuppressants and biologics were exclusion criteria. The subsequent post hoc analysis of the STEPS studies analysing factors associated with the response to TED in patients with SBS-IF found that the effects of TED on PS volume reduction were more pronounced in patients with jejunostomy/ileostomy than in patients with partial colon continuity. Only 10.5% of CD patients had colon in continuity in this study. Therefore, CD patients were likely among the best responders according to this analysis[68].
Subsequent data from real-life studies reported the experiences of TED treatment for CD patients treated with immunosuppressants and biologics and patients with active inflammation. A retrospective cohort study published in 2017 analysed 13 patients with CD and SBS-IF treated with TED for an average of 1 year. Nine of these patients were under parenteral nutrition before beginning TED therapy, and only 1 of them needed parenteral nutrition after TED. All of these patients required intravenous fluids before TED, but only 7 of them required this supplementation after TED. Eight of these patients were treated with biological therapies and/or immunosuppressants. TED was well tolerated in most of these CD patients, with the exception of one patient who intermittently presented with obstructive symptoms that required the cessation of therapy[89].
Two other active CD cases treated with TED were described. The first case was a 38-year-old patient with relapsing perianastomotic disease treated with ustekinumab. This patient received PN daily for SBS and was weaned off after 7 mo of TED. The second patient was 39 years old and had received PN daily since 2003. This patient was treated with vedolizumab and 6-mercaptopurine. After multiple resections, he was left with a jejunocolic anastomosis and 60 cm of residual small bowel length. PN was reduced to 1 night per week after TED[90].
One case of long-term TED use (54 mo) was reported in a patient with multidrug-resistant CD, and beneficial effects on nutritional status, a significant anti-inflammatory effect and reduction in CD clinical activity were observed[91]. A recent real-life cohort of 52 patients successfully treated with TED found that 16 had CD[74]. Another real-life series of 18 patients on TED found that 10 patients had SBS-IF caused by CD, and the 5 who were able to withdraw PS were all CD patients[75]. The findings of these studies are presented in Table 1.
| Ref. | Molecule tested | Number of patients | Number of CD patients | Study type | Main results | Adverse events |
| Jeppesen et al[64], 2005 | Teduglutide | 16 | 12 (in clinical remission) | Pilot open label, phase II | Increased wet weight absorption; decreased urine weight and urine sodium excretion; decreased fecal wet weight and fecal energy content; increased villus height, crypt depth and mitotic index in end-jejunostomy patients | Enlargement of the stoma nipple; mild lower leg oedema; severe AE (dehydration, sepsis, CS) in 4/16, not judged to be related to the drug |
| Jeppesen et al[65], 2012 | Teduglutide | 43 (+ 43 PBO) | 10 (in clinical remission) | Multicenter, randomized, double blind, PBO-controlled phase III | 63% of TED patients had a ≥ 20% reduction of PS volume at week 24 (significant versus 30% of the PBO group); increased serum citrulline (index of intestinal mucosa mass) | Mostly mild GI symptoms (abdominal pain, nausea, stoma complication, or abdominal distension); 7/43 CS; not different from PBO |
| Schwartz et al[66], 2016 | Teduglutide | 88 | 16 | 2 yr open label extension study | Clinical response (≥ 20% reduction of PS volume) in 28/30 (93%) and 66% of PS volume reduction in TED/TED group; 13 reached enteral autonomy | 34%abdominal pain; 25% episodes of weight decrease; 39% infections (SAE); 2 CD exacerbations (12% of CD, SAE) |
| Kochar et al[89], 2017 | Teduglutide | 13 | 13 (8 on biologics and/or IS) | Retrospective cohort study (median duration 1 yr) | 9 patients on PN at the beginning of therapy; 1 patient still on PN at the end of therapy; PS reduced from median 9000 mL/wk to 3100 mL/wk, 6 patients no PS at the end | Among non-immunosuppressed (5) only 2 minor AE and 1 CS; among immunosuppressed (8) minor AE, 3 CS and 2 pancreatitis |
| Barberio et al[94], 2019 | Teduglutide | 1 | 1 (on EN and adalimumab) | Case report | EN reduction of 50% after 24 wk of TED; EN suspension after 72 wk of treatment | Transient nausea and mild abdominal pain and nausea |
| Al Draiweesh et al[90], 2019 | Teduglutide | 2 | 2 (on biologics) | Case report | Weaning off from PS after 7 mo of TED; improvement in oral intake, reduced stool output, and weight gain; reduction of PN to 1 night/wk | Any reported |
| Naimi et al[84], 2019 | Glepaglutide | 16 | 8 | Double-blind randomised phase II trial | 1 mg daily glepaglutide reduces the fecal output by 592 mg/d; 10 mg daily glepaglutide reduces the fecal output by 833 mg/d | Stoma complications (73%); injection site reactions (61%); peripheral edema (56%); nausea and abdominal pain (44%); SAEs (stoma obstruction, sepsis) in 4 patients |
| Joly et al[74], 2020 | Teduglutide | 54 | 16 | Retrospective multicenter real life cohort | 85% of patients were responders (PS reduction ≥ 20%) at week 24; 24% weaned off PS at week 24 | Not specifically collected |
| Borghini et al[91], 2020 | Teduglutide | 1 | 1 (clinically active) | Case report | At least 20% reduction in PS; daily Kcal intake reduction of 15%; reduction in CD activity and severity | Two mild CVC-related infections |
| Mouillot et al[93], 2020 | Teduglutide | 1 | 1 | Case report | Reduction of PS and increase of nutrients absorption; hypertrophy of villi at capsule endoscopy; increase in the size of intestinal villi and crypts assessed by biopsy | Not specifically assessed |
| Puello et al[75], 2021 | Teduglutide | 18 | 10 | Retrospective single center real life cohort | Response (reduction of > 20% PS) in: 16 (75%) patients at 12 mo; 10 of 13 (76.9%) patients at 24 mo; 7 of 10 (70%) patients at 36 mo; 3 of 3 (100%) patients at 60 mo; 5 patients (all CD) weaned off PS | Abdominal pain or cramping (3); volume overload (1); taste loss (1); legs edema (2); increase in ostomy size (2) |
| Eliasson et al[82], 2022 | Apraglutide | 8 | 3 (in clinical remission) | Open label phase I-II trial | Weekly 5 mg apraglutide increases urinary output by 714 mL/d; weekly 10 mg apraglutide increases urinary output by 795 mL/d | Polyuria (7/8); any stoma complication (6/8); thirst decrease (4/8) and appetite decrease (3/8); edema (4/8); no SAE related to the drug |
Other retrospective and prospective cohorts of SBS-IF patients treated with TED (including a variable proportion of CD patients) were described and recently summarized in a meta-analysis that confirmed the efficacy of TED in adult SBS-IF patients[92]. This analysis suggested a non-significant trend of CD aetiology of SBS-IF as a positive predictor of response and weaning from PS.
A case of intestinal adaptation induced by TED in CD was recently described. The case was a 40-year-old female patient suffering from SBS-IF due to CD since 2001. The patient underwent terminal jejunostomy, with 100 cm of jejunum remaining. SBS and IF resulted, and the woman needed continuous PS. She was recommended to start treatment with TED, and her intestinal status and disease activity were studied at baseline. No active CD was detected, and the intestinal villi appeared normal. After 1 year of continued TED treatment, a new biopsy of intestinal villi was performed and showed an increase of 33% in the length of villi[93].
The efficacy of off-label TED use in a 65-year-old CD patient treated with adalimumab with SBS and enteral nutrition was also described, and the patient was weaned off enteral nutrition after 72 wk[94]. Concerning TED discontinuation, a report described 2 CD patients on chronic TED treatment who were not able to tolerate even a few days withdrawal of the drug[95].
One line of research attempted to determine whether TED had anti-inflammatory activity in CD based on the results of the use of glucagon-like peptides in animal models of colitis[96]. An RCT was performed on 100 CD patients with moderately to severely active disease who were treated with TED (0.05, 0.10, or 0.20 mg/kg) daily for 8 wk or PBO. Seventy-one patients completed the study, and the results showed numerically higher response and remission rates in all treatment groups compared to PBO, but these differences were not statistically significant. The results were more substantial in the group treated with the higher dose (44% response and 32% remission vs 32% response and 20% remission in the PBO group). It is questionable whether the clinical disease activity indices modifications described in this study were mostly due to the effect of TED on diarrhoea because no significant modification of C-reactive protein was detected. AEs were not different between the PBO and treatment groups[97]. This last result is particularly relevant, because it came from a controlled study on a relevant number of patients with active CD, although not affected by SBS-IF. The good safety profile in this setting justifies the use of GLP-2 analogues in patients with SBS-IF and active CD, but more data on this specific population are needed. Data on the use of the new GLP-2 analogues (apraglutide and glepaglutide) in CD are lacking.
SBS-IF is a rare condition that affects patients who undergo several intestinal resections due to CD and other gastrointestinal conditions. Although surgery rates in CD have declined in recent decades due to improved diagnostic and pharmacological modalities, the rate of CD patients developing SBS-IF (albeit low) has not been reduced. Several mechanisms are adopted by organisms to adapt to intestinal functional reduction. However, these adaptations are often not sufficient to avoid the clinical impact and long-term complications of SBS-IF, and several patients require HPN. The main therapeutic approaches consist of PS and symptomatic therapy. Yet, PS is associated with several AEs that complicate patient management, such as catheter-related complications, liver and metabolic disease, iron deficiency anaemia, and manganese toxicity.
New molecules and therapeutic approaches have been studied in recent years. The GLP-2 analogue TED demonstrated a relevant clinical utility for SBS-IF patients, and it significantly reduced HPN volume and/or days of infusion, allowing enteral autonomy in some patients. However, because this therapy is likely life-long or of long duration, more data are needed on the long-term safety and cost-effectiveness. Recent further consideration has been given to new GLP-2 analogues that have undergone phase I and II studies, including apraglutide and glepaglutide. These agents showed low clearance and slow absorption after subcutaneous administration, which allowed for a single weekly administration. Relatively scant data are available on the use of GLP-2 analogues in CD patients with SBS-IF. However, signals from RCTs and real-life observations indicate that TED is efficacious and well tolerated by CD patients, even if they are being treated with immunosuppressants and/or biological agents. More data are needed on the use of GLP-2 analogues in patients with active CD to clarify their safety and efficacy in this setting. This new pharmacological approach may improve the quality of life of patients with CD and SBS-IF and reduce their dependence on artificial nutrition. Clinical studies specifically addressing this peculiar population are warranted.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Crenn PP, France; Reiner J, Germany; Sipos F, Hungary; Triantafillidis J, Greece S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Pironi L. Definitions of intestinal failure and the short bowel syndrome. Best Pract Res Clin Gastroenterol. 2016;30:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology. 2003;124:1111-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 318] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Pironi L, Arends J, Bozzetti F, Cuerda C, Gillanders L, Jeppesen PB, Joly F, Kelly D, Lal S, Staun M, Szczepanek K, Van Gossum A, Wanten G, Schneider SM; Home Artificial Nutrition & Chronic Intestinal Failure Special Interest Group of ESPEN. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35:247-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 510] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 4. | Tappenden KA. Pathophysiology of short bowel syndrome: considerations of resected and residual anatomy. JPEN J Parenter Enteral Nutr. 2014;38:14S-22S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Pironi L, Konrad D, Brandt C, Joly F, Wanten G, Agostini F, Chambrier C, Aimasso U, Zeraschi S, Kelly D, Szczepanek K, Jukes A, Di Caro S, Theilla M, Kunecki M, Daniels J, Serlie M, Poullenot F, Wu J, Cooper SC, Rasmussen HH, Compher C, Seguy D, Crivelli A, Pagano MC, Hughes SJ, Guglielmi FW, Kozjek NR, Schneider SM, Gillanders L, Ellegard L, Thibault R, Matras P, Zmarzly A, Matysiak K, Van Gossum A, Forbes A, Wyer N, Taus M, Virgili NM, O'Callaghan M, Chapman B, Osland E, Cuerda C, Sahin P, Jones L, Lee ADW, Bertasi V, Orlandoni P, Izbéki F, Spaggiari C, Díez MB, Doitchinova-Simeonova M, Garde C, Serralde-Zúñiga AE, Olveira G, Krznaric Z, Czako L, Kekstas G, Sanz-Paris A, Jáuregui EP, Murillo AZ, Schafer E, Arends J, Suárez-Llanos JP, Shaffer J, Lal S. Clinical classification of adult patients with chronic intestinal failure due to benign disease: An international multicenter cross-sectional survey. Clin Nutr. 2018;37:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, Farmer DG, Lacaille F, Iyer K, Fishbein T; Intestinal Transplant Association. Intestinal transplant registry report: global activity and trends. Am J Transplant. 2015;15:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 7. | Warner BW. The Pathogenesis of Resection-Associated Intestinal Adaptation. Cell Mol Gastroenterol Hepatol. 2016;2:429-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Seiler KM, Goo WH, Zhang Q, Courtney C, Bajinting A, Guo J, Warner BW. Adaptation of extracellular matrix to massive small bowel resection in mice. J Pediatr Surg. 2020;55:1107-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Kunkel D, Basseri B, Low K, Lezcano S, Soffer EE, Conklin JL, Mathur R, Pimentel M. Efficacy of the glucagon-like peptide-1 agonist exenatide in the treatment of short bowel syndrome. Neurogastroenterol Motil. 2011;23:739-e328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Dibaise JK, Young RJ, Vanderhoof JA. Enteric microbial flora, bacterial overgrowth, and short-bowel syndrome. Clin Gastroenterol Hepatol. 2006;4:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Camilleri M, Nurko S. Bile Acid Diarrhea in Adults and Adolescents. Neurogastroenterol Motil. 2022;34:e14287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Hvistendahl MK, Naimi RM, Hansen SH, Rehfeld JF, Kissow H, Pedersen J, Dragsted LO, Sonne DP, Knop FK, Jeppesen PB. Bile acid-farnesoid X receptor-fibroblast growth factor 19 axis in patients with short bowel syndrome: The randomized, glepaglutide phase 2 trial. JPEN J Parenter Enteral Nutr. 2022;46:923-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Nightingale JM, Lennard-Jones JE, Gertner DJ, Wood SR, Bartram CI. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut. 1992;33:1493-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 205] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | van de Peppel IP, Verkade HJ, Jonker JW. Metabolic consequences of ileal interruption of the enterohepatic circulation of bile acids. Am J Physiol Gastrointest Liver Physiol. 2020;319:G619-G625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Ledeboer M, Masclee AA, Biemond I, Lamers CB. Gallbladder motility and cholecystokinin secretion during continuous enteral nutrition. Am J Gastroenterol. 1997;92:2274-2279. [PubMed] |
| 16. | Ling PR, Sheikh M, Boyce P, Keane-Ellison M, Thibault A, Burke P, Freedman S, Bistrian BR. Cholecystokinin (CCK) secretion in patients with severe short bowel syndrome (SSBS). Dig Dis Sci. 2001;46:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Yang J, Sun H, Wan S, Mamtawla G, Gao X, Zhang L, Li Y, Wang X, Li J. Risk Factors for Nephrolithiasis in Adults with Short Bowel Syndrome. Ann Nutr Metab. 2019;75:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kowlgi NG, Chhabra L. D-lactic acidosis: an underrecognized complication of short bowel syndrome. Gastroenterol Res Pract. 2015;2015:476215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Petersen C. D-lactic acidosis. Nutr Clin Pract. 2005;20:634-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Dibb M, Soop M, Teubner A, Shaffer J, Abraham A, Carlson G, Lal S. Survival and nutritional dependence on home parenteral nutrition: Three decades of experience from a single referral centre. Clin Nutr. 2017;36:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, Ghosh S, Wiebe S, Kaplan GG. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 683] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 22. | Elriz K, Palascak-Juif V, Joly F, Seguy D, Beau P, Chambrier C, Boncompain M, Fontaine E, Laharie D, Savoye G, Lerebours E. Crohn's disease patients with chronic intestinal failure receiving long-term parenteral nutrition: a cross-national adult study. Aliment Pharmacol Ther. 2011;34:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Rungoe C, Langholz E, Andersson M, Basit S, Nielsen NM, Wohlfahrt J, Jess T. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2014;63:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Dittrich AE, Sutton RT, Haynes K, Wang H, Fedorak RN, Kroeker KI. Incidence Rates for Surgery in Crohn's Disease Have Decreased: A Population-based Time-trend Analysis. Inflamm Bowel Dis. 2020;26:1909-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Limketkai BN, Parian AM, Chen PH, Colombel JF. Treatment With Biologic Agents Has Not Reduced Surgeries Among Patients With Crohn's Disease With Short Bowel Syndrome. Clin Gastroenterol Hepatol. 2017;15:1908-1914.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Limketkai BN, Parian AM, Shah ND, Colombel JF. Short Bowel Syndrome and Intestinal Failure in Crohn's Disease. Inflamm Bowel Dis. 2016;22:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Vaillant S, Guillo L, Michot N, D'Amico F, Germain A, Danese S, Baumann C, Rousseau H, Quilliot D, Peyrin-Biroulet L. Predictors for short bowel syndrome in Crohn's disease. Dig Liver Dis. 2020;52:1455-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Kurin M, Anderson A, Ramos Rivers C, Koutroumpakis F, Centa P, Bender-Heine J, Kozak G, Kramer E, O'Keefe SJ, Whitcomb DC, Levinthal DJ, Koutroubakis IE, Dunn MA, Hashash JG, Binion DG. Clinical Characteristics of Inflammatory Bowel Disease Patients Requiring Long-Term Parenteral Support in the Present Era of Highly Effective Biologic Therapy. JPEN J Parenter Enteral Nutr. 2021;45:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Rhoda KM, Parekh NR, Lennon E, Shay-Downer C, Quintini C, Steiger E, Kirby DF. The multidisciplinary approach to the care of patients with intestinal failure at a tertiary care facility. Nutr Clin Pract. 2010;25:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Billiauws L, Maggiori L, Joly F, Panis Y. Medical and surgical management of short bowel syndrome. J Visc Surg. 2018;155:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Winkler MF, Smith CE. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38:32S-37S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Dibb M, Teubner A, Theis V, Shaffer J, Lal S. Review article: the management of long-term parenteral nutrition. Aliment Pharmacol Ther. 2013;37:587-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Reitzel RA, Rosenblatt J, Chaftari AM, Raad II. Epidemiology of Infectious and Noninfectious Catheter Complications in Patients Receiving Home Parenteral Nutrition: A Systematic Review and Meta-Analysis. JPEN J Parenter Enteral Nutr. 2019;43:832-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Mateo-Lobo R, Riveiro J, Vega-Piñero B, Botella-Carretero JI. Infectious Complications in Home Parenteral Nutrition: A Systematic Review and Meta-Analysis Comparing Peripherally-Inserted Central Catheters with Other Central Catheters. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Dreesen M, Foulon V, Spriet I, Goossens GA, Hiele M, De Pourcq L, Willems L. Epidemiology of catheter-related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr. 2013;32:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Santarpia L, Pasanisi F, Alfonsi L, Violante G, Tiseo D, De Simone G, Contaldo F. Prevention and treatment of implanted central venous catheter (CVC) - related sepsis: a report after six years of home parenteral nutrition (HPN). Clin Nutr. 2002;21:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Chaves F, Garnacho-Montero J, Del Pozo JL, Bouza E, Capdevila JA, de Cueto M, Domínguez MÁ, Esteban J, Fernández-Hidalgo N, Fernández Sampedro M, Fortún J, Guembe M, Lorente L, Paño JR, Ramírez P, Salavert M, Sánchez M, Vallés J. Diagnosis and treatment of catheter-related bloodstream infection: Clinical guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med Intensiva (Engl Ed). 2018;42:5-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Salonen BR, Bonnes SL, Vallumsetla N, Varayil JE, Mundi MS, Hurt RT. A prospective double blind randomized controlled study on the use of ethanol locks in HPN patients. Clin Nutr. 2018;37:1181-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | John BK, Khan MA, Speerhas R, Rhoda K, Hamilton C, Dechicco R, Lopez R, Steiger E, Kirby DF. Ethanol lock therapy in reducing catheter-related bloodstream infections in adult home parenteral nutrition patients: results of a retrospective study. JPEN J Parenter Enteral Nutr. 2012;36:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Wouters Y, Theilla M, Singer P, Tribler S, Jeppesen PB, Pironi L, Vinter-Jensen L, Rasmussen HH, Rahman F, Wanten GJA. Randomised clinical trial: 2% taurolidine versus 0.9% saline locking in patients on home parenteral nutrition. Aliment Pharmacol Ther. 2018;48:410-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Pittiruti M, Bertoglio S, Scoppettuolo G, Biffi R, Lamperti M, Dal Molin A, Panocchia N, Petrosillo N, Venditti M, Rigo C, DeLutio E. Evidence-based criteria for the choice and the clinical use of the most appropriate lock solutions for central venous catheters (excluding dialysis catheters): a GAVeCeLT consensus. J Vasc Access. 2016;17:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Labriola L. Antibiotic locks for the treatment of catheter-related blood stream infection: Still more hope than data. Semin Dial. 2019;32:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Puiggròs C, Cuerda C, Virgili N, Chicharro ML, Martínez C, Garde C, de Luis D; Grupo NADYA-SENPE. [Catheter occlusion and venous thrombosis prevention and incidence in adult home parenteral nutrition (HPN) programme patients]. Nutr Hosp. 2012;27:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Debourdeau P, Farge D, Beckers M, Baglin C, Bauersachs RM, Brenner B, Brilhante D, Falanga A, Gerotzafias GT, Haim N, Kakkar AK, Khorana AA, Lecumberri R, Mandala M, Marty M, Monreal M, Mousa SA, Noble S, Pabinger I, Prandoni P, Prins MH, Qari MH, Streiff MB, Syrigos K, Büller HR, Bounameaux H. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 45. | Steiger E. Dysfunction and thrombotic complications of vascular access devices. JPEN J Parenter Enteral Nutr. 2006;30:S70-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Zhong L, Wang HL, Xu B, Yuan Y, Wang X, Zhang YY, Ji L, Pan ZM, Hu ZS. Normal saline vs heparin for patency of central venous catheters in adult patients - a systematic review and meta-analysis. Crit Care. 2017;21:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Smith S, Dawson S, Hennessey R, Andrew M. Maintenance of the patency of indwelling central venous catheters: is heparin necessary? Am J Pediatr Hematol Oncol. 1991;13:141-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Pironi L, Sasdelli AS. Intestinal Failure-Associated Liver Disease. Clin Liver Dis. 2019;23:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 422] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 50. | Courtney CM, Warner BW. Pediatric intestinal failure-associated liver disease. Curr Opin Pediatr. 2017;29:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Buchman AL, Iyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/Liver transplantation. Hepatology. 2006;43:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Lee WS, Chew KS, Ng RT, Kasmi KE, Sokol RJ. Intestinal failure-associated liver disease (IFALD): insights into pathogenesis and advances in management. Hepatol Int. 2020;14:305-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 53. | De Marco G, Sordino D, Bruzzese E, Di Caro S, Mambretti D, Tramontano A, Colombo C, Simoni P, Guarino A. Early treatment with ursodeoxycholic acid for cholestasis in children on parenteral nutrition because of primary intestinal failure. Aliment Pharmacol Ther. 2006;24:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Buchman AL, Dubin M, Jenden D, Moukarzel A, Roch MH, Rice K, Gornbein J, Ament ME, Eckhert CD. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology. 1992;102:1363-1370. [PubMed] |
| 55. | Hwa YL, Rashtak S, Kelly DG, Murray JA. Iron Deficiency in Long-Term Parenteral Nutrition Therapy. JPEN J Parenter Enteral Nutr. 2016;40:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Buchman AL, Moukarzel A. Metabolic bone disease associated with total parenteral nutrition. Clin Nutr. 2000;19:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Massironi S, Cavalcoli F, Rausa E, Invernizzi P, Braga M, Vecchi M. Understanding short bowel syndrome: Current status and future perspectives. Dig Liver Dis. 2020;52:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 58. | Dastych M, Dastych M Jr, Senkyrík M. Manganese in Whole Blood and Hair in Patients with Long-Term Home Parenteral Nutrition. Clin Lab. 2016;62:173-177. [PubMed] |
| 59. | Jeppesen PB. Teduglutide, a novel glucagon-like peptide 2 analog, in the treatment of patients with short bowel syndrome. Therap Adv Gastroenterol. 2012;5:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Billiauws L, Joly F. Emerging treatments for short bowel syndrome in adult patients. Expert Rev Gastroenterol Hepatol. 2019;13:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 61. | Reiner J, Berlin P, Wobar J, Schäffler H, Bannert K, Bastian M, Vollmar B, Jaster R, Lamprecht G, Witte M. Teduglutide Promotes Epithelial Tight Junction Pore Function in Murine Short Bowel Syndrome to Alleviate Intestinal Insufficiency. Dig Dis Sci. 2020;65:3521-3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Billiauws L, Bataille J, Boehm V, Corcos O, Joly F. Teduglutide for treatment of adult patients with short bowel syndrome. Expert Opin Biol Ther. 2017;17:623-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Sipos F, Műzes G. Teduglutide-induced stem cell function in intestinal repair. J Invest Surg. 2018;31:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 64. | Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, Ziegler TR, Gregory J, Tappenden KA, Holst J, Mortensen PB. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 65. | Jeppesen PB, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O'keefe SJ, Forbes A, Heinze H, Joelsson B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473-1481.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 66. | Schwartz LK, O'Keefe SJ, Fujioka K, Gabe SM, Lamprecht G, Pape UF, Li B, Youssef NN, Jeppesen PB. Long-Term Teduglutide for the Treatment of Patients With Intestinal Failure Associated With Short Bowel Syndrome. Clin Transl Gastroenterol. 2016;7:e142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 67. | Seidner DL, Fujioka K, Boullata JI, Iyer K, Lee HM, Ziegler TR. Reduction of Parenteral Nutrition and Hydration Support and Safety With Long-Term Teduglutide Treatment in Patients With Short Bowel Syndrome-Associated Intestinal Failure: STEPS-3 Study. Nutr Clin Pract. 2018;33:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 68. | Jeppesen PB, Gabe SM, Seidner DL, Lee HM, Olivier C. Factors Associated With Response to Teduglutide in Patients With Short-Bowel Syndrome and Intestinal Failure. Gastroenterology. 2018;154:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 69. | Pape UF, Iyer KR, Jeppesen PB, Kunecki M, Pironi L, Schneider SM, Seidner DL, Lee HM, Caminis J. Teduglutide for the treatment of adults with intestinal failure associated with short bowel syndrome: pooled safety data from four clinical trials. Therap Adv Gastroenterol. 2020;13:1756284820905766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 70. | Thomas RP, Hellmich MR, Townsend CM Jr, Evers BM. Role of gastrointestinal hormones in the proliferation of normal and neoplastic tissues. Endocr Rev. 2003;24:571-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Thulesen J, Hartmann B, Hare KJ, Kissow H, Ørskov C, Holst JJ, Poulsen SS. Glucagon-like peptide 2 (GLP-2) accelerates the growth of colonic neoplasms in mice. Gut. 2004;53:1145-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Armstrong D, Forbes A, Jeppesen PB, Lee HM, Nagy P, Seidner DL. Colon polyps in patients with short bowel syndrome before and after teduglutide: Post hoc analysis of the STEPS study series. Clin Nutr. 2020;39:1774-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Loutfy A, Kurin M, Shah R, Davitkov P. Characterization of American teduglutide consumers from 2015 to 2020: A large database study. JPEN J Parenter Enteral Nutr. 2022;46:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 74. | Joly F, Seguy D, Nuzzo A, Chambrier C, Beau P, Poullenot F, Thibault R, Armengol Debeir L, Layec S, Boehm V, Lallemand J, Quilliot D, Schneider SM. Six-month outcomes of teduglutide treatment in adult patients with short bowel syndrome with chronic intestinal failure: A real-world French observational cohort study. Clin Nutr. 2020;39:2856-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | Puello F, Wall E, Herlitz J, Lozano ES, Semrad C, Micic D. Long-Term Outcomes With Teduglutide From a Single Center. JPEN J Parenter Enteral Nutr. 2021;45:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Hargrove DM, Alagarsamy S, Croston G, Laporte R, Qi S, Srinivasan K, Sueiras-Diaz J, Wiśniewski K, Hartwig J, Lu M, Posch AP, Wiśniewska H, Schteingart CD, Rivière PJ, Dimitriadou V. Pharmacological Characterization of Apraglutide, a Novel Long-Acting Peptidic Glucagon-Like Peptide-2 Agonist, for the Treatment of Short Bowel Syndrome. J Pharmacol Exp Ther. 2020;373:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 77. | Wiśniewski K, Sueiras-Diaz J, Jiang G, Galyean R, Lu M, Thompson D, Wang YC, Croston G, Posch A, Hargrove DM, Wiśniewska H, Laporte R, Dwyer JJ, Qi S, Srinivasan K, Hartwig J, Ferdyan N, Mares M, Kraus J, Alagarsamy S, Rivière PJ, Schteingart CD. Synthesis and Pharmacological Characterization of Novel Glucagon-like Peptide-2 (GLP-2) Analogues with Low Systemic Clearance. J Med Chem. 2016;59:3129-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Martchenko SE, Sweeney ME, Dimitriadou V, Murray JA, Brubaker PL. Site-Specific and Temporal Effects of Apraglutide, a Novel Long-Acting Glucagon-Like Peptide-2 Receptor Agonist, on Intestinal Growth in Mice. J Pharmacol Exp Ther. 2020;373:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Slim GM, Lansing M, Wizzard P, Nation PN, Wheeler SE, Brubaker PL, Jeppesen PB, Wales PW, Turner JM. Novel Long-Acting GLP-2 Analogue, FE 203799 (Apraglutide), Enhances Adaptation and Linear Intestinal Growth in a Neonatal Piglet Model of Short Bowel Syndrome with Total Resection of the Ileum. JPEN J Parenter Enteral Nutr. 2019;43:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 80. | Hinchliffe T, Pauline ML, Wizzard PR, Nation PN, Brubaker P, Campbell JR, Kim Y, Dimitriadou V, Wales PW, Turner JM. Durability of Linear Small-Intestinal Growth Following Treatment Discontinuation of Long-Acting Glucagon-Like Peptide 2 (GLP-2) Analogues. JPEN J Parenter Enteral Nutr. 2021;45:1466-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 81. | Pauline ML, Nation PN, Wizzard PR, Hinchliffe T, Wu T, Dimitriadou V, Turner JM, Wales PW. Comparing the Intestinotrophic Effects of 2 Glucagon-Like Peptide-2 Analogues in the Treatment of Short-Bowel Syndrome in Neonatal Piglets. JPEN J Parenter Enteral Nutr. 2021;45:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Eliasson J, Hvistendahl MK, Freund N, Bolognani F, Meyer C, Jeppesen PB. Apraglutide, a novel glucagon-like peptide-2 analog, improves fluid absorption in patients with short bowel syndrome intestinal failure: Findings from a placebo-controlled, randomized phase 2 trial. JPEN J Parenter Enteral Nutr. 2022;46:896-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 83. | VectivBio AG. Trial to Evaluate Efficacy and Safety of Apraglutide in SBS-IF (STARS). [accessed 2022 Jun 2]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04627025 ClinicalTrials.gov Identifier: NCT04627025. |
| 84. | Naimi RM, Hvistendahl M, Enevoldsen LH, Madsen JL, Fuglsang S, Poulsen SS, Kissow H, Pedersen J, Nerup N, Ambrus R, Achiam MP, Svendsen LB, Holst JJ, Hartmann B, Hansen SH, Dragsted LO, Steensberg A, Mouritzen U, Hansen MB, Jeppesen PB. Glepaglutide, a novel long-acting glucagon-like peptide-2 analogue, for patients with short bowel syndrome: a randomised phase 2 trial. Lancet Gastroenterol Hepatol. 2019;4:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 85. | Pharma Z. Efficacy And Safety Evaluation of Glepaglutide in Treatment of SBS (EASE SBS 1). [accessed 2018 Oct 1]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03690206 ClinicalTrials.gov Identifier: NCT03690206. |
| 86. | Mayo BJ, Secombe KR, Wignall AD, Bateman E, Thorpe D, Pietra C, Keefe DM, Bowen JM. The GLP-2 analogue elsiglutide reduces diarrhoea caused by the tyrosine kinase inhibitor lapatinib in rats. Cancer Chemother Pharmacol. 2020;85:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Reiner J, Berlin P, Held J, Thiery J, Skarbaliene J, Griffin J, Russell W, Eriksson PO, Berner-Hansen M, Ehlers L, Vollmar B, Jaster R, Witte M, Lamprecht G. Dapiglutide, a novel dual GLP-1 and GLP-2 receptor agonist, attenuates intestinal insufficiency in a murine model of short bowel. JPEN J Parenter Enteral Nutr. 2022;46:1107-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Zaczek Z, Jurczak-Kobus P, Panczyk M, Braszczyńska-Sochacka J, Majewska K, Kunecki M, Dąbrowska K, Sobocki J. Changes in Parenteral Nutrition Requirements and BMI in Patients with Parenteral Nutrition-Dependent Short Bowel Syndrome after Stopping Teduglutide-9 Years of Follow-Up. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Kochar B, Long MD, Shelton E, Young L, Farraye FA, Yajnik V, Herfarth H. Safety and Efficacy of Teduglutide (Gattex) in Patients With Crohn's Disease and Need for Parenteral Support Due to Short Bowel Syndrome-associated Intestinal Failure. J Clin Gastroenterol. 2017;51:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 90. | Al Draiweesh S, Ma C, Gregor JC, Rahman A, Jairath V. Teduglutide in Patients With Active Crohn's Disease and Short Bowel Syndrome. Inflamm Bowel Dis. 2019;25:e109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Borghini R, Caronna R, Donato G, Picarelli A. GLP-2 analog Teduglutide in active Crohn's disease and short bowel syndrome: Confirmation of anti-inflammatory role and future perspectives. Dig Liver Dis. 2020;52:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Bioletto F, D'Eusebio C, Merlo FD, Aimasso U, Ossola M, Pellegrini M, Ponzo V, Chiarotto A, De Francesco A, Ghigo E, Bo S. Efficacy of Teduglutide for Parenteral Support Reduction in Patients with Short Bowel Syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 93. | Mouillot T, Boehm V, Treton X, Ferrandi E, Kapel N, Cazals-Hatem D, Joly F. Small-Bowel Adaptation: A Case of Morphological Changes Induced by Teduglutide in Short-Bowel Syndrome With Intestinal Failure. JPEN J Parenter Enteral Nutr. 2020;44:940-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Barberio B, Sturniolo GC, D'Incà R, Farinati F, Bigotto MA, Ghisa M, Lorenzon G, Savarino E. Efficacy of teduglutide in a patient with Crohn's disease and short bowel syndrome on enteral nutrition: let's start to think out of the box. Gastroenterol Rep (Oxf). 2019;7:459-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 95. | Mazzuoli S, Regano N, Lamacchia S, Silvestri A, Guglielmi FW. Intestinal iatrogenic hyperadaptation in patients with short bowel syndrome and Crohn's disease: Is this an indication for mandatory lifelong injections of teduglutide? Nutrition. 2021;91-92:111396. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 96. | Zatorski H, Sałaga M, Fichna J. Role of glucagon-like peptides in inflammatory bowel diseases-current knowledge and future perspectives. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1321-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 97. | Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG; Teduglutide Study Group. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn's disease. Inflamm Bowel Dis. 2010;16:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |