Published online Oct 28, 2022. doi: 10.3748/wjg.v28.i40.5881
Peer-review started: April 25, 2022
First decision: May 30, 2022
Revised: June 21, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: October 28, 2022
Processing time: 185 Days and 18.2 Hours
Liver transplantation for the most critically ill remains controversial; however, it is currently the only curative treatment option.
To assess immediate posttransplant outcomes and compare the short (1 year) and long-term (6 years) posttransplant survival among cirrhotic patients stratified by disease severity.
We included cirrhotic patients undergoing liver transplantation between 2015 and 2019 and categorized them into compensated cirrhosis (CC), decompensated cirrhosis (DC), and acute-on-chronic liver failure (ACLF). ACLF was further divided into severity grades. Our primary outcomes of interest were total days of intensive care unit (ICU) and hospital stay, development of complications and posttransplant survival at 1 and 6 years.
235 patients underwent liver transplantation (CC = 11, DC = 129 and ACLF = 95). Patients with ACLF had a significantly longer hospital stay [8.0 (6.0-13.0) vs CC, 6.0 (3.0-7.0), and DC 7.0 (4.5-10.0); P = 0.01] and developed more infection-related complications [47 (49.5%), vs CC, 1 (9.1%) and DC, 38 (29.5%); P < 0.01]. Posttransplant survival at 1- and 6-years was similar among groups (P = 0.60 and P = 0.90, respectively). ACLF patients stratified according to ACLF grade [ACLF-1 n = 40 (42.1%), ACLF-2 n = 33 (34.7%) and ACLF-3 n = 22 (23.2%)], had similar ICU and hospital stay length (P = 0.68, P = 0.54), as well as comparable frequencies of overall and infectious post-transplant complications (P = 0.58, P = 0.80). There was no survival difference between ACLF grades at 1 year and 6 years (P = 0.40 and P = 0.15).
Patients may benefit from liver transplantation regardless of the cirrhosis stage. ACLF patients have a longer hospital stay and frequency of infectious complications; however, excellent, and comparable 1 and 6-year survival rates support their enlisting and transplantation including those with ACLF-3.
Core Tip: Cirrhotic patients classified into compensated or decompensated cirrhosis and acute-on-chronic liver failure (ACLF) underwent liver transplantation. Patients with ACLF have a longer hospital stay and a higher frequency of infectious complications, but despite that, have similar posttransplant survival at one year and up to 6 years of follow-up.
- Citation: Cervantes-Alvarez E, Vilatoba M, Limon-de la Rosa N, Mendez-Guerrero O, Kershenobich D, Torre A, Navarro-Alvarez N. Liver transplantation is beneficial regardless of cirrhosis stage or acute-on-chronic liver failure grade: A single-center experience. World J Gastroenterol 2022; 28(40): 5881-5892
- URL: https://www.wjgnet.com/1007-9327/full/v28/i40/5881.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i40.5881
Cirrhosis is the consequence of chronic liver disease originated by a variety of etiological factors, characterized by the disruption of the normal hepatic architecture due to fibrosis with consequent hemodynamic repercussions. Unless the hepatic insult is removed, patients with this condition will suffer progression and transition from a stage of compensated cirrhosis (CC) to a stage of decompensated cirrhosis (DC) with the occurrence of portal hypertension-related symptoms[1]. An entity of recent definition known as acute-on-chronic liver failure (ACLF) is now recognized[2,3], which imposes the highest mortality risk given by a state of profound cirrhosis-associated immune dysfunction and the development of organ failures additional to that of the liver[4,5].
Currently, liver transplantation (LT) is the only definitive therapeutic measure for any of these patients, albeit with the implied risks including posttransplant complications and the long-term use of immunosuppressive drugs. However, patients benefit in general from excellent posttransplant survival.
There is controversial literature, and uncertainty prevails concerning the possible futility of assigning a liver to a patient with advanced cirrhosis and systemic alterations such as in those with ACLF. Some studies have demonstrated the presence of ACLF at the time of LT as a risk factor for mortality and graft loss, and that these patients may have lower short and long-term survival after transplant[6-8]. However, others have shown non-significant survival differences between ACLF and non-ACLF patients including a marked improvement in the prognosis of those with the highest severity (ACLF-3)[9-12]. The development of early allograft dysfunction and renal dysfunction is also comparable between these groups, as well as long-term liver and kidney function[12,13]. However, unfavorable outcomes can be expected and a higher frequency of perioperative and postoperative complications has been reported[8,9]. Differences may be found when ACLF patients are subdivided by ACLF grade, however, survival disparities are still controversial[7,9].
We here report our transplant center’s experience in an effort to further contribute to the evidence on the benefit of LT in ACLF. The aim was to assess immediate posttransplant outcomes and to compare the short (1 year) and long-term (6 years) posttransplant survival among cirrhotic patients stratified by disease severity. Unlike other studies so far, this study specifically compares survival and outcomes between compensated, decompensated cirrhosis and ACLF, thus distinctly contrasting the extremes of disease severity. Additional analyses were performed to determine possible differences between ACLF grades. These results should encourage further transplantation in those with this severe form of cirrhosis and even in patients with ACLF grade 3.
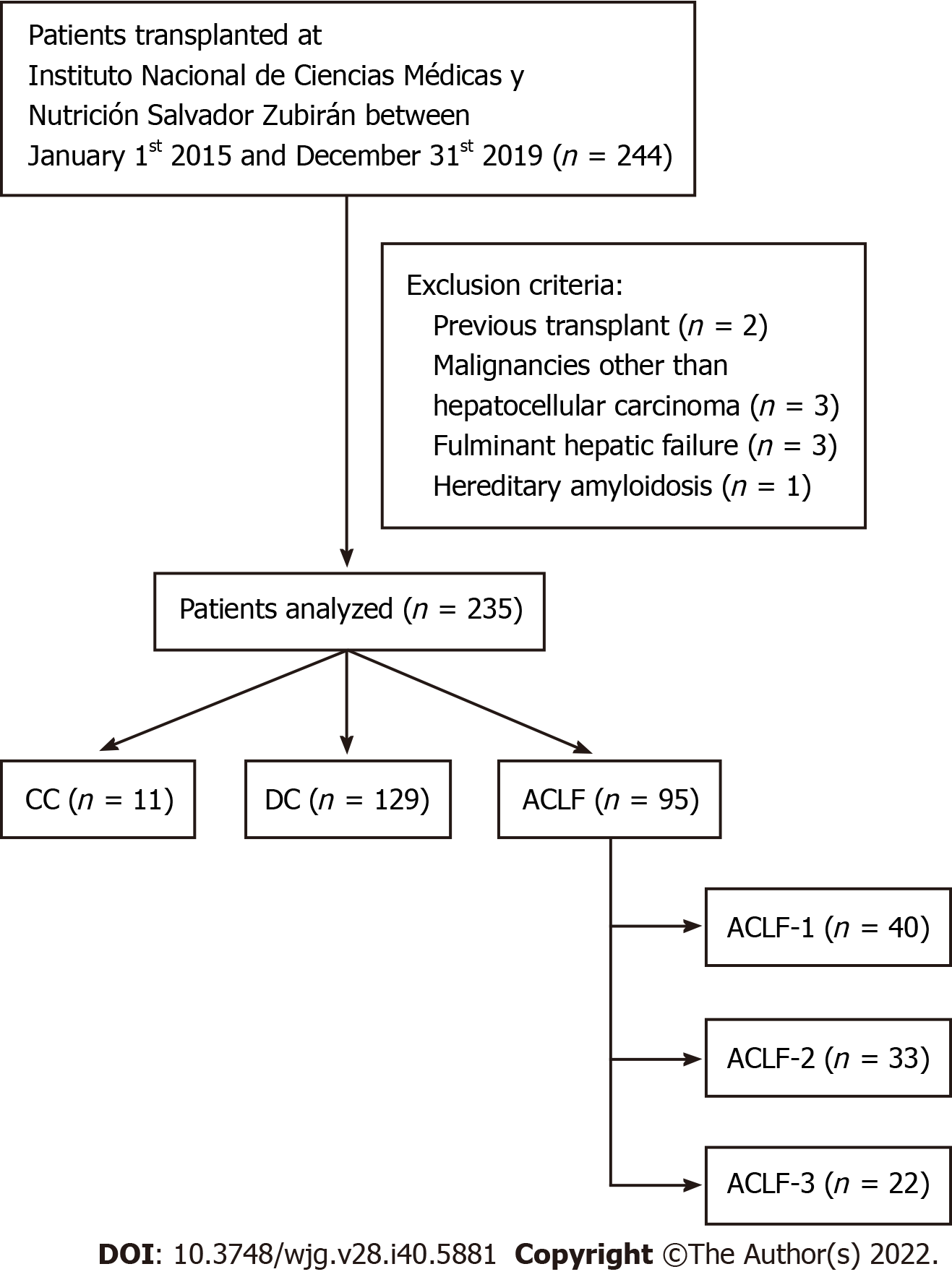
This study included all patients undergoing LT between January 1st 2015 and December 31st 2019. Patients with a previous transplant, malignancies other than hepatocellular carcinoma, fulminant hepatic failure, and amyloidosis were excluded. Patients were classified into compensated cirrhosis (CC), decompensated cirrhosis (DC), and ACLF, and the latter were further subdivided into ACLF grades 1, 2 and 3 (Figure 1). Diagnoses of CC and DC were based on the absence or presence of symptoms related to portal hypertension, including ascites, encephalopathy, or variceal bleeding, respectively, as previously described[14]. All CC patients received liver transplant because of hepatocarcinoma mainly due to hepatitis C virus (HCV) infection.
ACLF was diagnosed in a patient that fulfilled ACLF criteria any time during their clinical course while waiting to receive a LT according to the EASL-CLIF consortium criteria[2] which state the following organ failure (OF) definitions: liver (total bilirubin ≥ 12 mg/dL); kidney (creatinine ≥ 2 mg/dL); brain (encephalopathy grade 3 or 4 according to West-Haven criteria); coagulation (INR ≥ 2.5); circulation (vasopressor use due to circulatory failure); and lung (PaO2/FiO2 ≤ 200 or SpO2/FiO2 ≤ 214 or mechanical ventilation due to lung failure). ACLF grading was performed as follows: ACLF-1, patients with single kidney OF or non-renal OF plus kidney dysfunction (creatinine between 1.5-1.9 mg/dL) and/or brain dysfunction (encephalopathy grade 1 or 2 according to West-Haven criteria); ACLF-2, patients with two OFs; and ACLF-3, patients with three or more OFs.
Patients at our center are considered for LT based on cirrhosis disease severity according to an unrestricted evaluation of the Model for End-Stage Liver Disease (MELD), MELD-Na and CLIF-C scores and are ultimately listed according to an interdisciplinary consensus reached by the gastroenterology, cardiology, pneumology, infectology, otorhinolaryngology, psychiatry, surgery, anesthesiology, and stomatology specialties. Liver transplants were carried out in their majority with classic technique. Briefly, recipient hepatectomy involved a bilateral subcostal incision with or without midline extension. Then dissection and clamping of the portal vein, hepatic artery, bile duct, and superior and inferior vena cava were done. Implantation of the donor's liver was attained by anastomosing first the superior vena cava from the donor with that of the recipient, followed by the inferior vena cava, and portal vein, after which reperfusion of the donor liver was begun. Total reperfusion was then obtained by anastomosing the hepatic artery of the graft with the junction of the gastroduodenal artery and the common hepatic artery of the recipient. The procedure was completed after performing cholecystectomy and duct to-duct anastomosis.
The immunosuppressive regimen in all patients following the procedure consisted of all or a combination of the following drugs: a calcineurin inhibitor (tacrolimus or cyclosporine), corticosteroids, mycophenolate mofetil and the interleukin-2 (IL-2) receptor antagonist basiliximab. In the event of renal disease in the post-orthotopic liver transplant period, modifications to the immunosuppression regimen including CNI dose reduction with the addition of MMF, were performed.
No donor organs were obtained from executed prisoners or other institutionalized people.
This study was approved by the ethics committee of our institution (GAS-2368-17-20) and conforms to the provisions of the Declaration of Helsinki. Requirement of informed consent was waived due to its observational nature.
Medical records of all patients were examined to extract the following demographic and clinical variables [gender, age, cirrhosis etiology, presence of ascites and encephalopathy, vasopressor use, PaO2/FiO2 or SpO2/FiO2 relation, requirement of mechanical ventilation and precipitant event (bacterial infection, gastrointestinal hemorrhage, active alcoholism, other or unknown)]. Laboratory data measured at the time of LT necessary to determine disease severity and for the computation of the MELD-Na score was further registered: total bilirrubin (mg/dL), creatinine (mg/dL), INR, and leukocyte count (× 109/L). Our primary outcomes of interest were: the development of immediate posttransplant infectious complications, defined as any type of nosocomial-acquired, donor-derived or surgery-related infection presented during the immediate hospital stay following LT until the patients’ discharge; the development of any type of immediate postoperative complication according to Clavien-Dindo classification[15]; and post-LT survival at 1 year and 6 years. Similarities in donor liver graft quality were assessed by evaluation of the donor risk index (DRI[16]) which considers the donor’s age, height, race and cause of death, donation after cardiac death, split/partial graft, organ allocation and cold ischemia time.
Results of categorical variables are presented as frequencies and percentages, and as means and standard deviations or medians with interquartile range (IQR) for normally or not normally distributed continuous variables, respectively. Univariate statistical comparisons between categorical variables were performed with Pearson’s Chi-squared test or Fisher's exact test and between continuous variables with the analysis of variance test or the Kruskal-Wallis test according to the normal distribution. Paired t-tests were carried out between disease severity scores among ACLF groups. Posttransplant survival was analyzed with the Kaplan-Meier method and survival curves were compared with the log-rank test. Statistical analyses were done with SPSS version 28.0 for Windows, IBM Corp., Armonk, NY, United States and survival curves were plotted using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org) with the survminer package. Statistical significance was considered at a P value less than 0.05.
A total of 235 patients that underwent LT from 2015 to 2019 were included in this study, of which 95 (38.9%) fulfilled ACLF criteria, 129 (52.9%) were classified as DC, and 11 (4.5%) as CC (Table 1). When compared to the CANONIC study, we identified an overall younger population with ACLF patients being even younger than those with DC and CC [50.0 years (IQR 37.0-59.0) vs 52.0 years (IQR 43.0-61.0) and 57.0 years (IQR 53.0-59.0), respectively; P = 0.02]. Autoimmune etiologies (autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis and overlapping syndromes) were the most frequent in ACLF patients (44.2% vs 27.9% DC, and 9.1% CC; P < 0.01), whereas the leading cause in DC and CC patients was HCV infection (72.7% CC, 32.6% DC, and 13.7% ACLF; P < 0.0001). With regard to comorbidities, no statistical differences were observed between cirrhosis groups for frequencies of either type 2 diabetes mellitus or primary hypertension (P = 0.44 and P = 0.06, respectively). ACLF patients had the highest MELD-Na score (25 ± 6 vs 19 ± 4 and 11 ± 3, DC and CC respectively), and accordingly the highest bilirubin and creatinine values. The presence of clinical ascites and encephalopathy (including West-Haven grade 3-4 encephalopathy) was also significantly higher in ACLF patients (Table 1).
| CC, n = 11 (4.5%) | DC, n= 129 (52.9%) | ACLF, n = 95 (38.9%) | P value | |
| Male gender, n (%) | 6 (54.5) | 64 (49.6) | 47 (49.5) | 0.95 |
| Age, yr | 57.0 (53.0-59.0)a | 52.0 (43.0-61.0)c | 50.0 (37.0-59.0)a,c | 0.02 |
| Liver cirrhosis etiology, n (%) | ||||
| Autoimmune | 1 (9.1)a | 36 (27.9)c | 42 (44.2)a,c | < 0.01 |
| HCV | 8 (72.7)a,b | 42 (32.6)b,c | 13 (13.7)a,c | < 0.0001 |
| Alcoholic liver disease | 1 (9.1) | 11 (8.5) | 9 (9.5) | 0.93 |
| NASH | 0 (0.0) | 7 (5.4) | 6 (6.3) | 0.88 |
| Cryptogenic | 1 (9.1) | 22 (17.1) | 18 (18.9) | 0.76 |
| Other1 | 0 (0.0) | 11 (8.5) | 7 (7.4) | 0.84 |
| Comorbidities, n (%) | ||||
| Type 2 diabetes mellitus | 3 (27.3) | 22 (17.1) | 13 (13.7) | 0.44 |
| Primary hypertension | 2 (18.2) | 20 (15.5) | 6 (6.3) | 0.06 |
| Pre-transplant clinical data | ||||
| MELD-Na | 11 ± 3a,b | 19 ± 4b,c | 25 ± 6a,c | < 0.0001 |
| Total bilirubin (mg/dL) | 1.18 (1.03-1.45)a,b | 3.39 (2.3-5.46)b,c | 7.70 (4.14-16.63)a,c | < 0.0001 |
| INR | 1.1 (1.1-1.2)a,b | 1.5 (1.3-1.7)b | 1.5 (1.3-2.0)a | < 0.0001 |
| Serum creatinine (mg/dL) | 0.67 (0.57-0.71)a | 0.73 (0.61-0.88)c | 0.97 (0.75-1.35)a,c | < 0.0001 |
| Leukocyte count (× 109/L) | 2.9 (2.4-3)a | 4 (3.1-5)c | 5.4 (3.8-6.8)a,c | < 0.0001 |
| Disease manifestations2, n (%) | ||||
| Clinical ascites | 0 (0.0)a,b | 88 (68.2)b,c | 88 (92.6)a,c | < 0.0001 |
| Encephalopathy | 0 (0.0)a,b | 69 (53.5)b,c | 80 (84.2)a,c | < 0.0001 |
| Grade 3-4 encephalopathy (West- Haven) | 0 (0.0)a,b | 7 (5.4)b,c | 37 (38.9)a,c | < 0.0001 |
When assessing the last ACLF event of these patients before undergoing LT, the majority were classified as ACLF grade 1 [n = 40 (42.1%)] followed by ACLF-2 [n = 33 (34.7%)] and ACLF-3 [n = 22 (23.2%)] (Table 2). Overall median time to LT since their ACLF event was 31 d (IQR 11.0-88.0). However, those with ACLF-1 had a significantly longer time to LT compared to ACLF-2 and ACLF-3 [54.0 (IQR 21.3-122.8) vs 31.0 (IQR 7.0-59.5) and 22.0 (IQR 9.5-46.8), respectively; P = 0.03]. Demographic data and etiologies were similar within these three groups, with autoimmune etiologies being the most frequent among all ACLF grades (P = 0.30). The most common ACLF precipitant overall were bacterial infections and the absence of an identifiable factor (unknown). Other precipitants including pharmacological and procedure-related complications, were more frequent in ACLF-1 patients (P = 0.01). Kidney OF was the only one that did not differ significantly between ACLF groups [18 (45.0%) ACLF-1, 11 (33.3%) ACLF-2, and 14 (63.6%) ACLF-3; P = 0.09], whereas liver, brain, coagulation, circulation and lung failure were significantly higher in patients with ACLF-3 (Table 2).
| ACLF-1,n = 40 (42.1%) | ACLF-2, n = 33 (34.7%) | ACLF-3, n = 22 (23.2%) | P value | |
| Male gender, n (%) | 21 (52.5) | 17 (51.5) | 11 (50.0) | 0.98 |
| Age, yr | 55.0 (39.8-60.0) | 44.0 (36.5-53.0) | 49.0 (37.5-59.3) | 0.14 |
| Time to LT since ACLF event | 54.0 (21.3-122.8)a,b | 31.0 (7.0-59.5)b | 22.0 (9.5-46.8)a | 0.03 |
| Liver cirrhosis etiology, n (%) | ||||
| Autoimmune | 14 (35.0) | 17 (51.5) | 11 (50.0) | 0.30 |
| HCV | 9 (22.5) | 2 (6.1) | 2 (9.1) | 0.14 |
| Alcoholic liver disease | 2 (5.0) | 4 (12.1) | 3 (13.6) | 0.44 |
| NASH | 3 (7.5) | 2 (6.1) | 1 (4.5) | 0.99 |
| Cryptogenic | 10 (25.0) | 5 (15.2) | 3 (13.6) | 0.50 |
| Other1 | 2 (5.0) | 3 (9.1) | 2 (9.1) | 0.69 |
| ACLF precipitant, n (%) | ||||
| Bacterial infection | 14 (35.0) | 16 (48.5) | 11 (50.0) | 0.39 |
| Gastrointestinal hemorrhage | 1 (2.5) | 3 (9.1) | 1 (4.5) | 0.44 |
| Active alcoholism | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.99 |
| Other | 10 (25.0)a | 2 (6.1) | 0 (0.0)a | 0.01 |
| Unknown | 15 (37.5) | 12 (36.4) | 10 (45.5) | 0.77 |
| Organ failures, n (%) | ||||
| Liver | 14 (35.0)a,b | 22 (66.7)b | 16 (72.7)a | < 0.01 |
| Kidney | 18 (45.0) | 11 (33.3) | 14 (63.6) | 0.09 |
| Brain | 5 (12.5)a | 10 (30.3) | 11 (50.0)a | < 0.01 |
| Coagulation | 3 (7.5)a,b | 12 (36.4)b | 11 (50.0)a | < 0.001 |
| Circulation | 0 (0.0)a,b | 8 (24.2)b,c | 14 (63.6)a,c | < 0.0001 |
| Lung | 1 (2.5)a | 3 (9.1) | 7 (31.8)a | < 0.01 |
| ACLF event clinical data | ||||
| MELD-Na | 27 ± 4a,b | 29 ± 5b,c | 35 ± 4a,c | < 0.0001 |
| CLIF-C OF | 9 ± 1a,b | 10 ± 1b,c | 12 ± 2a,c | < 0.0001 |
| CLIF-C ACLF | 39 ± 8a | 43 ± 6c | 52 ± 6a,c | < 0.0001 |
| Total bilirubin (mg/dL) | 6.31 (2.99-12.91)a,b | 13.07 (6.39-22.31)b | 23.08 (10.56-27.76)a | < 0.001 |
| INR | 1.5 (1.2-1.9)a,b | 1.9 (1.4-2.5)b | 2.2 (1.7-2.8)a | < 0.001 |
| Serum creatinine (mg/dL) | 1.86 (1.18-2.27)b | 0.93 (0.71-1.99)b,c | 2.25 (1.42-2.87)c | < 0.01 |
| Leukocyte count (× 109/L) | 6.55 (4.73-9.43)a | 6.60 (4.55-8.25)c | 8.75 (6.88-13.05)a,c | < 0.01 |
| Pre-transplant clinical data | ||||
| MELD-Na | 23 ± 4a | 25 ± 5c | 29 ± 8a,c | < 0.0001 |
| CLIF-C OF | 8 ± 2a | 9 ± 2 | 10 ± 2a | 0.01 |
| CLIF-C ACLF | 37 ± 9 | 37 ± 8 | 41 ± 12 | 0.18 |
| Total bilirubin (mg/dL) | 4.58 (2.94-8.60)a | 9.75 (5.40-16.62) | 19.59 (5.48-34.11)a | < 0.01 |
| INR | 1.4 (1.3-1.7) | 1.6 (1.3-2.2) | 1.9 (1.4-2.6) | 0.05 |
| Serum creatinine (mg/dL) | 0.96 (0.75-1.29)a | 0.88 (0.72-1.17)c | 1.23 (0.94-1.95)a,c | < 0.01 |
| Leukocyte count (× 109/L) | 4.40 (3.23-6.70) | 4.80 (2.90-6.30) | 4.75 (2.48-10.45) | 0.92 |
Parameters reflecting disease severity including MELD-Na, CLIF-C OF and CLIF-C ACLF scores, total bilirubin, INR and leukocyte count were higher in ACLF-3 and lower in ACLF-1. We observed a generalized improvement of clinical parameters in ACLF patients at the time of LT, with a concomitant reduction of the disease severity scores evaluated. For instance, MELD-Na decreased significantly among all three ACLF grades (P < 0.01), and an improvement in the CLIF-C OF score was also observed. Interestingly, the CLIF-C ACLF score became similar within ACLF-1, 2 and 3 patients with no significant difference among them (P = 0.18), as well as INR and leukocyte count (P = 0.05 and P = 0.92, respectively) (Table 2).
Although severity of cirrhosis clearly differed between CC, DC and ACLF patients, posttransplant outcomes were mostly similar. While total days at the intensive care unit (ICU) were comparable and non-significant among these groups, patients with ACLF had a significantly longer hospital stay [8.0 d (IQR 6.0-13.0) vs 6.0 d (IQR 3.0-7.0) and 7.0 d (IQR 4.5-10.0), CC and DC, respectively; P = 0.01]. The frequency of patients who developed any type of complication (Clavien-Dindo I-V complications[15]) during their immediate hospital stay following LT was also similar, however those with ACLF more commonly presented an infectious complication (P < 0.01) (Table 3). When comparing days of hospital stay and posttransplant outcomes between ACLF-grades no significant differences were observed, thus the clinical course after LT of ACLF-3 patients was similar to that of those with ACLF-1 and 2 (Table 4).
| CC, n = 11 (4.5%) | DC, n = 129 (52.9%) | ACLF, n = 95 (38.9%) | P value | |
| ICU stay (d) | 2.0 (1.0-4.0) | 2.0 (1.5-4.0) | 3.0 (2.0-5.0) | 0.05 |
| Hospital stay (d) | 6.0 (3.0-7.0)a | 7.0 (4.5-10.0) | 8.0 (6.0-13.0)a | 0.01 |
| Any type of complication, n (%) | 7 (63.6) | 105 (81.4) | 85 (89.5) | 0.05 |
| Infectious complications, n (%) | 1 (9.1)a | 38 (29.5)b | 47 (49.5)a,b | < 0.01 |
| Complications (Clavien-Dindo), n (%) | ||||
| I | 4 (36.4) | 17 (13.2) | 19 (20.0) | 0.08 |
| II | 2 (18.2) | 51 (39.5) | 32 (33.7) | 0.31 |
| III | 1 (9.1) | 14 (10.9) | 17 (17.9) | 0.27 |
| IV | 0 (0.0) | 9 (7.0) | 12 (12.6) | 0.23 |
| V | 0 (0.0) | 14 (10.9) | 5 (5.3) | 0.24 |
| ACLF-1, n = 40 (42.1%) | ACLF-2, n = 33 (34.7%) | ACLF-3, n = 22 (23.2%) | P value | |
| ICU stay (d) | 3.0 (2.0-4.0) | 3.0 (2.0-6.0) | 3.0 (2.0-6.0) | 0.68 |
| Hospital stay (d) | 8.0 (5.0-11.8) | 8.0 (6.0-15.5) | 6.0 (5.8-14.3) | 0.54 |
| Any type of complication, n (%) | 35 (87.5) | 31 (93.9) | 19 (86.4) | 0.58 |
| Infectious complications, n (%) | 20 (50.0) | 15 (45.5) | 12 (54.5) | 0.80 |
| Complications (Clavien-Dindo), n (%) | ||||
| I | 7 (17.5) | 6 (18.2) | 6 (27.3) | 0.62 |
| II | 17 (42.5) | 11 (33.3) | 4 (18.2) | 0.15 |
| III | 5 (12.5) | 7 (21.2) | 5 (22.7) | 0.50 |
| IV | 4 (10.0) | 6 (18.2) | 2 (9.1) | 0.49 |
| V | 2 (5.0) | 1 (3.0) | 2 (9.1) | 0.61 |
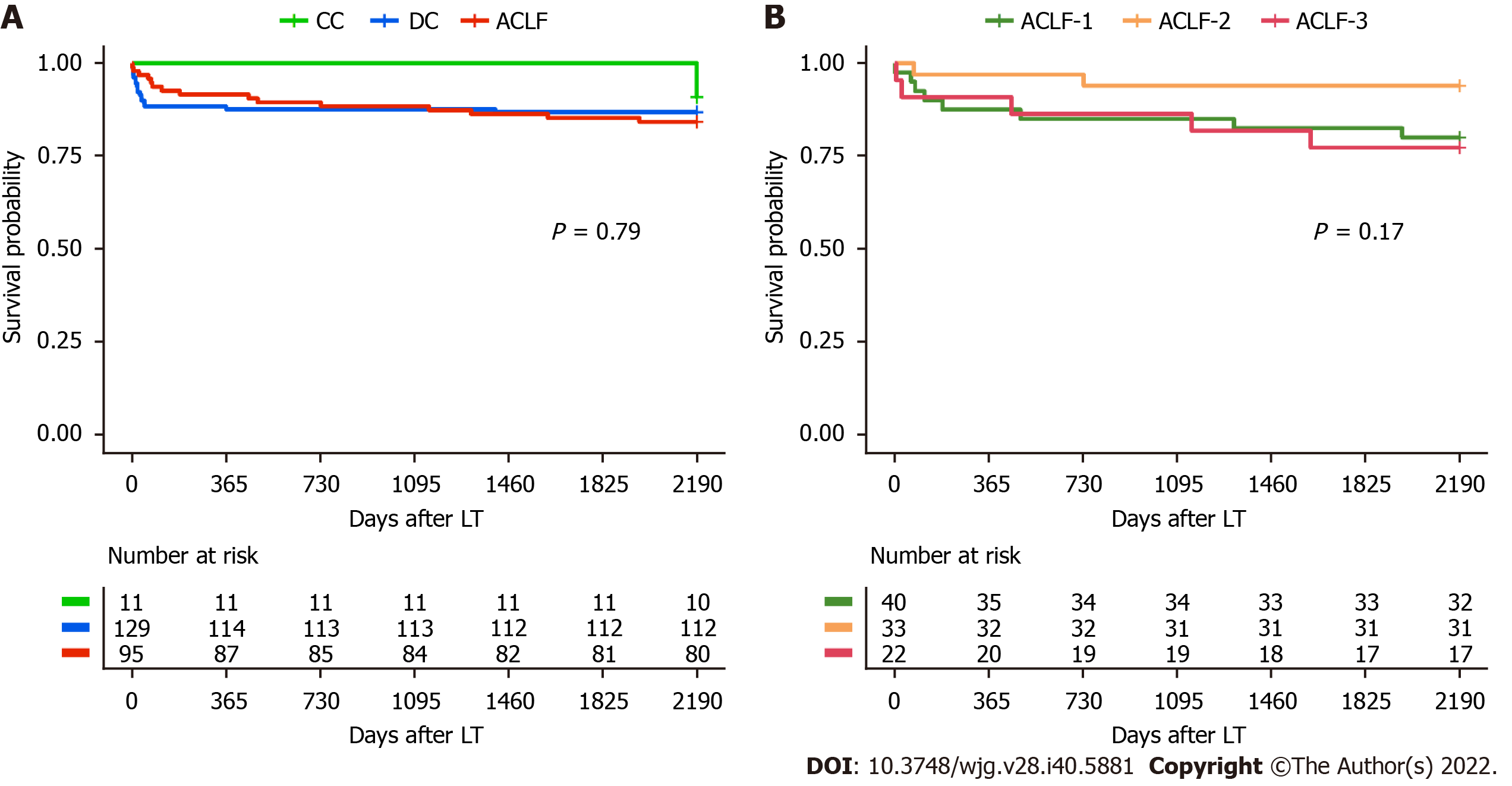
Assessment of posttransplant mortality revealed that ACLF, DC and CC patients have a comparable survival at 1 and 6 years after LT [87 (91.6%), 114 (88.4%), 11 (100%) at 1 year, respectively; P = 0.60. 80 (84.2%), 112 (86.8%), and 10 (90.9%) at 6 years, respectively; P = 0.90]. Early transplant mortality at the critical periods of 30 d and 3 mo was also non-significant (P = 0.38 and P = 0.30, respectively).
All groups received the same quality grafts as there were no significant differences between groups in the DRI (P = 0.13) (Table 5). Survival as assessed by Kaplan-Meier analysis showed no significant differences among groups (P = 0.79; Figure 2A). These analyses were additionally performed in the ACLF population by subdividing them into their severity grades and no significant differences were observed at 30-d and 3-mo mortality (P = 0.17 and P = 0.65, respectively), 1-year and overall survival (P = 0.40 and P = 0.15, respectively). Likewise, no differences were observed in the DRI index (P = 0.08) (Table 5). This was reflected in a non-significant Kaplan-Meier analysis (P = 0.17; Figure 2B), which confirms similar posttransplant outcomes even among ACLF-3 patients.
| CC, n = 11 (4.5%) | DC, n = 129 (52.9%) | ACLF, n = 95 (38.9%) | P value | ACLF-1, n = 40 (42.1%) | ACLF-2, n = 33 (34.7%) | ACLF-3, n = 22 (23.2%) | P value | |
| DRI | 1.41 (1.36-2.26) | 1.38 (1.21-1.53) | 1.32 (1.19-1.54) | 0.13 | 1.43 (1.24-1.62) | 1.27 (1.20-1.43) | 1.38 (1.20-1.69) | 0.08 |
| 30-d mortality | 0 (0.0) | 10 (7.8) | 3 (3.2) | 0.38 | 1 (2.5) | 0 (0.0) | 2 (9.1) | 0.17 |
| 3-mo mortality | 0 (0.0) | 15 (11.6) | 6 (6.3) | 0.30 | 3 (7.5) | 1 (3.0) | 2 (9.1) | 0.65 |
| 1-yr survival | 11 (100) | 114 (88.4) | 87 (91.6) | 0.60 | 35 (87.5) | 32 (97.0) | 20 (90.9) | 0.40 |
| Overall survival1 | 10 (90.9) | 112 (86.8) | 80 (84.2) | 0.90 | 32 (80.0) | 31 (93.9) | 17 (77.3) | 0.15 |
Despite controversies, LT has been increasingly encouraged in patients with ACLF, including those with the highest severity grade. Hemodynamic derangements and systemic inflammation may restrain clinicians from considering an ACLF patient as a candidate for this procedure; however, the decision is so urgent that mortality on the waiting list may be even higher than that of status-1a patients[17]. In support of LT benefit for critically ill patients, this study demonstrates that according to our single-center experience, posttransplant outcomes in ACLF are favorable and in fact comparable with those of CC and DC patients. Moreover, even when comparing between ACLF grades a worse prognosis was not observed in those with ACLF-3.
In contrast to the CANONIC study[2], our patient population was in general younger, and inte-restingly ACLF patients were also the youngest even though no differences were found by ACLF grade. However, the main etiology in this group was of autoimmune nature. Although autoimmune diseases in cirrhosis follow a progressive and complicated clinical course, autoimmune ACLF patients in our center showed non-significant posttransplant survival differences in comparison with non-ACLF patients regardless of ACLF grade, which goes accordingly to the reported excellent survival observed in ACLF patients with autoimmune etiology[18]. A clear clinical difference between ACLF, CC and DC patients was evident by a significantly higher MELD-Na score and leukocyte count at the time of the ACLF event. These two parameters along with the CLIF-C and CLIF C-ACLF decrease at the time of LT, indicating improvement of the ACLF syndrome and hence a more favorable profile that allowed eventual transplantation. Indeed, Kim et al[19] has previously reported that both lower MELD scores and no ACLF progression are considered independent factors associated with a high survival rate after LT. Moreover, we also observed that the CLIF-C ACLF score at the time of LT was now similar between ACLF grades, which may further explain improvement and thus equally excellent posttransplant outcomes within these subgroups.
Compared to other studies[9,20-22], ACLF-3 patients in our center benefited from an even greater 1-year survival rate (90.9%) which remained higher even after our 6 year follow-up (77.3%). There are several risk factors associated with worse 1-year posttransplant mortality in ACLF-3 patients, such as older age (≥ 53 years), high pretransplant arterial lactate levels, mechanical ventilation and high leukocyte count (≤ 10 g/L)[23]. Contributing to the favorable outcome observed in our ACLF population, including those with ACLF-3, several of the above mentioned reported risk factors for worse posttransplant mortality were not present in our patients. First, a younger age characterized our ACLF population and clinical parameters were mostly stable across all severity grades at the time of LT. Leukocyte counts were higher than in DC and CC patients, but generally always lower than 10 × 109/L either during the ACLF event or at LT. While bacterial infections were the main ACLF precipitant followed by unknown factors, important differences regarding other cohorts can be found with the frequency of certain OFs. Respiratory failure which is a risk factor for lower posttransplant survival[11,20,23] was uncommon as lung OF seldom occurred. Instead, liver OF prevailed in those with severe ACLF although closely followed by extrahepatic OFs including kidney OF, which was the most frequent in those with ACLF-1.
Inevitably, ACLF patients will have a longer and more complicated hospital stay after LT as has been reported thus far[9,22]. This was true in our center, where the latter required more days of ICU and hospital stay. Posttransplant complications by the Clavien-Dindo classification[15], were not different between ACLF and non-ACLF patients (CC and DC) in accordance with a systematic review[22]. Despite this encouraging finding, infectious complications were specifically more common in the former, occurring in over half of them, which is also in agreement with the study of Artru et al[9]. This may warrant a more directed antibiotic regimen in ACLF patients and physicians should be aware of this frequent outcome to promote a longer posttransplant survival. Interestingly, infections were equally prevalent in ACLF-3 patients according to our experience, which may be due to the similar pretransplant profile identified among severity grades including non-significant CLIF-C ACLF score differences. A good donor liver graft quality which was comparable between CC, DC and ACLF patients is another factor that may have contributed to an overall excellent outcome; however, optimal graft quality must not impede the decision for LT given its lesser impact compared to early transplantation, as has been recently reported[24]. Overall, our results encourage further transplantation in those with ACLF, considering that this procedure is the only effective treatment option and that survival was not significantly different compared to patients with less advanced cirrhosis, despite a more complicated posttransplant clinical course.
This study is limited by its retrospective nature and its single-center design; hence, findings must be compared to those of other authors. We report here the experience of one of the largest transplant centers in Mexico; however, demographics in this center will certainly vary with those seen in the rest of the country. This may explain the high proportion of autoimmune patients compared to HCV or alcoholic hepatitis. Regardless, during the five-year study period we have found a comparable proportion of ACLF patients who undergo LT, whose disease severity is markedly different from CC and DC patients. In spite of these differences, we observed a clear LT benefit as has been supported by previous studies.
In conclusion, out of 235 liver transplantation procedures that were carried out between 2015 and 2019 in our center, 38.9% corresponded to ACLF patients. Although important clinical differences were found with non-ACLF patients (CC and DC) and among each other when divided by severity grade, posttransplant survival was uniformly excellent. A longer hospital stay and frequency of infectious complications is to be expected, however, this should not restrain the decision to transplant those with ACLF. Furthermore, our observations support benefit even in the most critically ill patients (ACLF-3), given comparable 1-year and 6-year survival rates.
Currently, liver transplantation (LT) is the only definitive therapeutic measure for patients with cirrhosis, albeit with the implied risks including posttransplant complications and the long-term use of immunosuppressive drugs. However, these patients benefit in general from excellent posttransplant survival. The benefit and survival of this procedure for patients with more advanced cirrhosis such as those with acute-on-chronic liver failure (ACLF), still remains controversial, with some reports showing a clear benefit, while others reporting lower short and long-term survival after transplant.
In order to contribute to the current literature regarding the benefit of LT even in those with more severe diseases, we evaluate the immediate posttransplant outcomes and compared the posttransplant survival in patients stratified by disease severity.
To assess immediate posttransplant outcomes and compare the short (1 year) and long-term (6 years) posttransplant survival among cirrhotic patients stratified by disease severity.
We included cirrhotic patients undergoing liver transplantation between 2015 and 2019 and categorized them into compensated cirrhosis (CC), decompensated cirrhosis (DC), and ACLF. ACLF was further divided into severity grades. Medical records of all patients were examined to extract demographic and clinical variables as well as laboratory data measured at the time of LT and in the posttransplant period. Our primary outcomes of interest were: the development of immediate posttransplant infectious complications, defined as any type of nosocomial-acquired, donor-derived or surgery-related infection presented during the immediate hospital stay following LT until the patients’ discharge; the development of any type of immediate postoperative complication according to Clavien-Dindo classification; and post-LT survival at 1 year and 6 years. Posttransplant survival was analyzed with the Kaplan-Meier method and survival curves were compared with the log-rank test.
A total of 235 patients underwent liver transplantation (CC = 11, DC = 129 and ACLF = 95). Patients with ACLF had a significantly longer hospital stay and developed more infection-related complications. Posttransplant survival at 1- and 6-years was similar among groups. When ACLF patients were stratified according to ACLF grade, similar intensive care unit and hospital stay lengths were found, as well as comparable frequencies of overall and infectious posttransplant complications. Despite that, there was no survival difference between ACLF grades at 1 year and 6 years.
Patients may benefit from liver transplantation regardless of the cirrhosis stage. Despite having a longer hospital stay and a higher frequency of infectious complications, ACLF patients have excellent and comparable 1 and 6-year survival rates.
A multicenter study would be required to determine the value of LT in advanced disease patients such as those with ACLF according to disease etiology.
We would like to thank Elizabeth Costello for her important contribution in editing the English language text of this manuscript.
| 1. | Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 401] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2279] [Article Influence: 175.3] [Reference Citation Analysis (6)] |
| 3. | Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, Gines P, Kim WR, Kamath PS; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 4. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 897] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 5. | Laleman W, Claria J, Van der Merwe S, Moreau R, Trebicka J. Systemic Inflammation and Acute-on-Chronic Liver Failure: Too Much, Not Enough. Can J Gastroenterol Hepatol. 2018;2018:1027152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Levesque E, Winter A, Noorah Z, Daurès JP, Landais P, Feray C, Azoulay D. Impact of acute-on-chronic liver failure on 90-day mortality following a first liver transplantation. Liver Int. 2017;37:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Agbim U, Sharma A, Maliakkal B, Karri S, Yazawa M, Goldkamp W, Podila PSB, Vanatta JM, Gonzalez H, Molnar MZ, Nair SP, Eason JD, Satapathy SK. Outcomes of Liver Transplant Recipients With Acute-on-Chronic Liver Failure Based on EASL-CLIF Consortium Definition: A Single-center Study. Transplant Direct. 2020;6:e544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Huebener P, Sterneck MR, Bangert K, Drolz A, Lohse AW, Kluge S, Fischer L, Fuhrmann V. Stabilisation of acute-on-chronic liver failure patients before liver transplantation predicts post-transplant survival. Aliment Pharmacol Ther. 2018;47:1502-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, Lassailly G, Dharancy S, Boleslawski E, Lebuffe G, Kipnis E, Ichai P, Coilly A, De Martin E, Antonini TM, Vibert E, Jaber S, Herrerro A, Samuel D, Duhamel A, Pageaux GP, Mathurin P, Saliba F. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 10. | Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, Sacleux SC, Pageaux GP, Radenne S, Trebicka J, Fernandez J, Perricone G, Piano S, Nadalin S, Morelli MC, Martini S, Polak WG, Zieniewicz K, Toso C, Berenguer M, Iegri C, Invernizzi F, Volpes R, Karam V, Adam R, Faitot F, Rabinovich L, Saliba F, Meunier L, Lesurtel M, Uschner FE, Fondevila C, Michard B, Coilly A, Meszaros M, Poinsot D, Schnitzbauer A, De Carlis LG, Fumagalli R, Angeli P, Arroyo V, Jalan R; ELITA/EF-CLIF working group. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol. 2021;75:610-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 11. | Finkenstedt A, Nachbaur K, Zoller H, Joannidis M, Pratschke J, Graziadei IW, Vogel W. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl. 2013;19:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | O'Leary JG, Bajaj JS, Tandon P, Biggins SW, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, Lai J, Fallon M, Vargas HE, Thuluvath P, Subramanian R, Thacker LR, Reddy KR. Outcomes After Listing for Liver Transplant in Patients With Acute-on-Chronic Liver Failure: The Multicenter North American Consortium for the Study of End-Stage Liver Disease Experience. Liver Transpl. 2019;25:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Marciano S, Mauro E, Giunta D, Torres MC, Diaz JM, Bermudez C, Gutierrez-Acevedo MN, Narvaez A, Ortíz J, Dirchwolf M, Pollarsky F, Rojas-Saunero LP, Gadano A. Impact of acute-on-chronic liver failure on post-transplant survival and on kidney outcomes. Eur J Gastroenterol Hepatol. 2019;31:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Cervantes-Alvarez E, Limon-de la Rosa N, Vilatoba M, Pérez-Monter C, Hurtado-Gomez S, Martinez-Cabrera C, Argemi J, Alatorre-Arenas E, Yarza-Regalado S, Tejeda-Dominguez F, Lizardo-Thiebaud MJ, Mendez-Guerrero O, Gamboa-Dominguez A, Aguilar-Salinas CA, Huang CA, Kershenobich D, Bataller R, Torre A, Navarro-Alvarez N. Galectin-3 is overexpressed in advanced cirrhosis and predicts post-liver transplant infectious complications. Liver Int. 2022;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26173] [Article Influence: 1189.7] [Reference Citation Analysis (2)] |
| 16. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1526] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 17. | Sundaram V, Shah P, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Kuo A, Jalan R. Patients With Acute on Chronic Liver Failure Grade 3 Have Greater 14-Day Waitlist Mortality Than Status-1a Patients. Hepatology. 2019;70:334-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Singal AK, Wong RJ, Jalan R, Asrani S, Kuo YF. Primary biliary cholangitis has the highest waitlist mortality in patients with cirrhosis and acute on chronic liver failure awaiting liver transplant. Clin Transplant. 2021;35:e14479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Kim JE, Sinn DH, Choi GS, Kim JM, Joh JW, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Predictors and outcome of emergent Liver transplantation for patients with acute-on-chronic liver failure. Dig Liver Dis. 2021;53:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Sundaram V, Patel S, Shetty K, Lindenmeyer CC, Rahimi RS, Flocco G, Al-Attar A, Karvellas CJ, Challa S, Maddur H, Jou JH, Kriss M, Stein LL, Xiao AH, Vyhmeister RH, Green EW, Campbell B, Cranford W, Mahmud N, Fortune BE; Multi-Organ Dysfunction and Evaluation for Liver Transplantation (MODEL) Consortium. Risk Factors for Posttransplantation Mortality in Recipients With Grade 3 Acute-on-Chronic Liver Failure: Analysis of a North American Consortium. Liver Transpl. 2022;28:1078-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Sundaram V, Mahmud N, Perricone G, Katarey D, Wong RJ, Karvellas CJ, Fortune BE, Rahimi RS, Maddur H, Jou JH, Kriss M, Stein LL, Lee M, Jalan R; Multi-Organ Dysfunction, Evaluation for Liver Transplantation (MODEL) Consortium. Longterm Outcomes of Patients Undergoing Liver Transplantation for Acute-on-Chronic Liver Failure. Liver Transpl. 2020;26:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Abdallah MA, Waleed M, Bell MG, Nelson M, Wong R, Sundaram V, Singal AK. Systematic review with meta-analysis: liver transplant provides survival benefit in patients with acute on chronic liver failure. Aliment Pharmacol Ther. 2020;52:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Artzner T, Michard B, Weiss E, Barbier L, Noorah Z, Merle JC, Paugam-Burtz C, Francoz C, Durand F, Soubrane O, Pirani T, Theocharidou E, O'Grady J, Bernal W, Heaton N, Salamé E, Bucur P, Barraud H, Lefebvre F, Serfaty L, Besch C, Bachellier P, Schneider F, Levesque E, Faitot F. Liver transplantation for critically ill cirrhotic patients: Stratifying utility based on pretransplant factors. Am J Transplant. 2020;20:2437-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 24. | Zhang S, Suen SC, Gong CL, Pham J, Trebicka J, Duvoux C, Klein AS, Wu T, Jalan R, Sundaram V. Early transplantation maximizes survival in severe acute-on-chronic liver failure: Results of a Markov decision process model. JHEP Rep. 2021;3:100367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases, No. 135418.
Specialty type: Transplantation
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cassese G, Italy; Mogahed EA, Egypt; Sahin TT, Turkey S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM