BIOGRAPHY
Fabrício Freire de Melo (Figure 1), PhD, is a professor at the Multidisciplinary Institute of Health of the Universidade Federal da Bahia, Brazil. He is currently a Research and Extension Coordinator at the aforementioned institute. He holds a bachelor's degree in Biological Sciences from the Pontifícia Universidade Católica de Minas Gerais (2004), in Brazil, and a master's degree (2007), a PhD (2011), and a postdoctoral fellowship (2013) in Biological Sciences (Microbiology) from the Universidade Federal de Minas Gerais (UFMG), Brazil. Moreover, he was a visiting researcher at the Medical Entomology Laboratory at the René Rachou Institute, Fiocruz, Brazil.
Figure 1 Fabrício Freire de Melo, PhD, Professor at the Universidade Federal da Bahia - Campus Anísio Teixeira, Brazil.
He divides his professional activity between research work and academic teaching. His main research areas include Helicobacter pylori infection, arboviruses, and, currently, SARS-CoV-2. He has developed extensive work on Helicobacter pylori, which includes investigations on the differences between the immune responses observed in children and adults infected with the bacterium. His work in several areas has already been recognized and awarded worldwide, being the cover of the World Journal of Clinical Oncology (Volume 11, Issue 11).
He is a member of the editorial board of the World Journal of Clinical Oncology, an academic editor of the World Journal of Gastroenterology, and a reviewer for journals including the World Journal of Gastroenterology, World Journal of Clinical Cases, and World Journal of Gastrointestinal Oncology.
INTRODUCTION
Helicobacter pylori (H. pylori) is a microaerophilic, Gram-negative, rod-shaped, mobile bacterium of great clinical importance that is able to colonize the extremely hostile stomach environment[1].
Studies analyzing populations suggest that approximately 50% of the world population are infected with H. pylori. In addition, most H. pylori infections appear to be acquired during childhood, and estimates suggest that a third of the child population are or will be infected with the bacterium[2,3].
H. pylori infection is associated with the development of peptic ulcer and gastric cancer (GC), and the interactions between the virulence factors of the pathogen and the host immune response seem to be crucial in the development of those diseases[1,4]. Reviews show that 10% of those infected with H. pylori develop a peptic ulcer and 1%-3% develop GC[3]. H. pylori is a Group I carcinogen according to the International Agency for Research on Cancer (IARC), with 89% of all gastric cancers being attributable to this infection[2].
The host immune response to H. pylori is complex and changeable. It is possible to notice during childhood a predominantly regulatory inflammatory pattern (Treg), with higher concentrations of TGF-β1 and IL-10 than colonized adults, in addition to the greater number of FOXP3+ Treg cells observed in the gastric mucosa of children. This predominantly regulatory pattern makes the gastric mucosa of children more vulnerable to H. pylori colonization, but with milder inflammation when compared to what occurs in the mucosa of infected adults. As a result, the immune system of pediatric patients is not able to eliminate the H. pylori infection, and the bacterium persists in the gastric environment if left untreated. Moreover, damage to the gastric mucosa is less frequent during childhood, despite persistent mucosal colonization[5-8].
In adults, there is a predominant Th1 response, with higher levels of IFN-γ and IL-12p70 in the gastric mucosa, in contrast to the predominance of the regulatory response found during childhood. Besides, adults have a more intense Th17 response when compared to children. This can be verified by the higher mucosal concentrations of cytokines such as IL-17A and IL-23 and lower concentrations of TGF-β1, which, despite participating in the Treg response, when expressed at lower levels, seems to synergize with IL-6, promoting the expression of IL-23 receptors (IL-23r) and favoring an intense Th17 response. This cytokine profile is closely associated with the occurrence of damage to the gastric epithelium. Therefore, adults have a higher susceptibility to developing peptic ulcers, gastric atrophy, and intestinal metaplasia, a well-known precancerous lesion[5,6,9].
Of note, an increase in pro-inflammatory cytokines such as TNFα, IL-1α, IL-1β, IL-6, IL-2, and IL-17A is observed in H. pylori-positive children compared to H. pylori-negative infants. However, the Treg profile seems to overcome the inflammatory responses promoted by Th1 and Th17 cytokines in those individuals. This predominance of a regulatory immune response observed in infants might favor the colonization and persistence of the infection in the gastric mucosa, whereas the Th1 and Th17 responses induce a higher inflammatory activity in adults, leading to a higher risk of H. pylori-related gastric damage.
PREVALENCE
H. pylori infect about 4.4 billion people worldwide[2]. The prevalence of the infection is variable around the world and has changed over the last few years, with a notable reduction of the H. pylori-infected population, especially in developed countries[2,10-12]. Hooi et al[2] showed, through a meta-analysis, that the seroprevalence is higher in underdeveloped regions, and the highest prevalences were found in Africa (79.1%), Latin America and the Caribbean (63.4%), and Asia (54.7%). Otherwise, developed regions such as North America (37.1%) and Oceania (24.4%) have lower prevalence rates[3].
The infection is mainly acquired during childhood, and this phenomenon is predominantly observed in countries with a higher prevalence of H. pylori-positive individuals[13-15]. Moreover, higher prevalences of H. pylori infection are associated with lower socioeconomic status, household overcrowding, and lower educational levels[11]. Sex may also influence the risk of acquiring the infection. A higher prevalence of the disease is usually observed among male subjects than in females. This may be related to hormonal factors, especially estrogen, which stimulates the immune response, and to a lower exposure to environmental factors such as smoking among women[16,17].
Furthermore, the prevalence may vary based on ethnic groups: Indigenous people in most countries are more susceptible to being infected[2]; a study in the United Arab Emirates showed a higher H. pylori prevalence among Africans than in Asian and Arabic populations, and, despite living in similar conditions to other ethnic groups, Malays were notably less affected by H. pylori infection than other people in that country[18-20]. In another study, Jonaityte et al[21] found a decline in the seroprevalence of H. pylori among medical students from Lithuania, with seroprevalences of 51.7, 30.4, 26.3, and 14.2% in 1995, 2012, 2016, and 2020, respectively. Besides, Africa, Western Asia, and South America are the regions with the highest incidence of H. pylori, while Oceania, North America, and Western Europe have lower prevalences of the bacterium[2].
BACTERIAL DENSITY AND GASTRIC INFLAMMATION
Despite being able to colonize all regions of the stomach, H. pylori proliferates better in certain anatomical areas, and higher bacterial densities are found in the antrum and cardia. Many factors can be responsible for this difference, such as the different levels of acid production in each portion of the stomach. In this sense, the regions with slightly lower acidity (antrum and cardia) are the regions with the highest H. pylori density[22,23].
Margarida et al[24], when studying 21 children infected with H. pylori, found infiltration of mononuclear (MN) cells in 50% of the cases. Furthermore, they did not find any neutrophil infiltrate in 40% of the participants, and, in 60% of the individuals, there was a slight eosinophilic infiltrate. Moreover, they have also found a relationship between bacterial density and MN and neutrophil cell counts in the stomach. Besides, they concluded that the infiltration of MN cells and neutrophils is lower in children infected with H. pylori than in H. pylori-negative adults. These findings were probably due to the differences between the immune response profiles predominating in each age group[2,9]. Thus, it is evident that the host immune response directed to the H. pylori can be influenced by several factors such as age and bacterial density, being complex and changeable.
CYTOKINE CONCENTRATIONS IN THE GASTRIC MUCOSA OF CHILDREN AND ADULTS
Given that H. pylori colonization is established mainly during childhood, that severer clinical outcomes related to the infection tend to occur as age advances, and that the immune system plays pivotal roles in H. pylori-related diseases, the following question is raised: Is the cytokine pattern observed in the immune response against the bacterium influenced by the age of colonized patients?
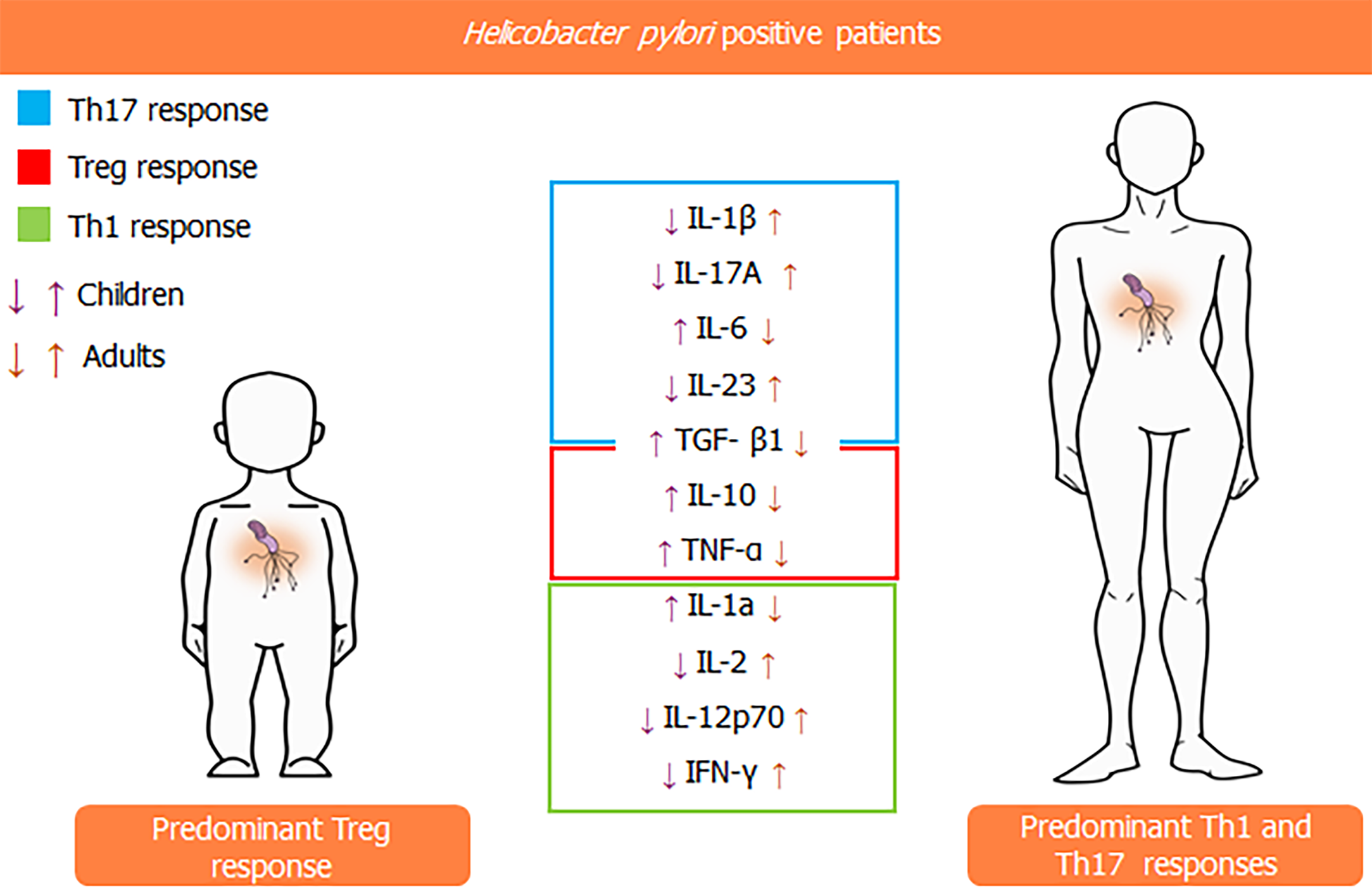
In an investigation enrolling Brazilian children and adults, our group has demonstrated that, among H. pylori-infected persons, infants tend to present a gastric Treg-polarized cytokine profile instead of the significant expression of Th17-related cytokines observed in older individuals. The analysis of the expression of cytokines in the gastric environment evidenced that IL-10 and TGF-β1 are expressed at higher levels in the former group, whereas the contrary was observed regarding the expression of IL-1β, IL-17A, and IL-23[5]. Those findings corroborate a precedent study by Harris et al, which showed more intense expression of the Treg-related cytokines TGF-β1 and IL-10 in children than in adults in a Chilean population[8]. Another study carried out by our group evaluated cytokines associated with innate and Th1 immune response in H. pylori-positive patients from various age groups[9]. We found that the gastric levels of IL-1α and TNF-α were significantly higher in children than in adults, whereas IL-2, IL-12p70, and IFN-γ were less expressed in infants than in older individuals (Figure 2). Interestingly, a drop in the gastric concentrations of IFN-γ and IL-12p70 in adults and an increase in the gastric mucosa levels of IL-1, IL-2, IL-12p70, and IFN-γ in children were observed with aging.
Figure 2 Comparison between gastric cytokines levels in children and adults.
Taken together, the aforementioned results show that age, indeed, influences the immune response against the bacterium and strongly suggest the occurrence of significant anti-inflammatory patterns among H. pylori-infected children, which might affect not only the development of gastric diseases but also other health-related aspects during the initial years of life. This hypothesis becomes even more relevant when considering that environmental stimuli are crucial for the development of the immune system in that life period, a position supported by the so-called hygiene hypothesis, which claims that the contact with microorganisms in early life is determinant for the maturation of the immune system[25]. Interestingly, a recent study by León et al[26] suggests that H. pylori may induce atopy modulation in children since they found that H. pylori-infected infants had higher expression of high-affinity IgE receptor (FcεRI) by peripheral dendritic cells and enhanced levels of FOXP3 and Latency Associated Peptide by T reg cells. The FcεRI is related to a regulatory dendritic cell profile since the interaction of IgE with that molecule fails to induce the maturation of these cells[27]. The possibility of systemic effects by H. pylori infection through the induction of immune system regulatory mechanisms makes us question the possible impacts of H. pylori eradication among children over the development of future immune system-related disorders. Although we understand and support the need for eliminating the bacterium, it has to be emphasized that this infection has been negatively correlated to the development of relevant immune system-linked diseases that are relevant among young people, including asthma[28]. In addition, the current scenario of widespread use of antibiotics and growing antimicrobial resistance among H. pylori strains should not be ignored[29]. Therefore, we hope that, along with the advances in the clinical analysis of genetic and epigenetic backgrounds, the future approaches to H. pylori infections and the decision on the necessity of bacterial eradication should be carried out in a more individualized manner, instead of the generalized, but necessary, treatments preconized by current guidelines.
Some studies have emphasized that immunizing agents against H. pylori should be able to induce a Th17 response to achieve satisfactory effectiveness. In that context, Velin et al induced mouse immunization using mucosally administered cholera toxin followed by H. felis challenge and observed that it induced a remarkable peak of CD4+IL-17+ T cells in the gastric mucosa[30]. Recently, a study by Chen et al[31] tried to immunize mice using a cyclic guanosine monophosphate-adenosine monophosphate as an adjuvant for the anti-H. pylori vaccine and observed that its effectiveness depended on high levels of antigen-specific Th1 and, mainly, Th17 responses. These findings draw attention to the aforementioned results showing low levels of Th17-related cytokines among H. pylori-infected children, which could represent an obstacle in the development of effective immunizing agents for that population. This is an important issue to be considered since the H. pylori infection is mainly acquired during childhood[31].
GASTRIC HISTOLOGY AND CYTOKINE CONCENTRATIONS
In our aforementioned study evaluating the variations of the Th1 immune response to the infection by H. pylori according to age, we observed that the increased levels of IFN-γ and IL-12p70 in the gastric environment were associated with an increase in MN cells in the gastric corpus and antrum. Moreover, when considering the group of young adults, IL-12p70 was linked to an increase in the count of both MN and PMN cells in the gastric antrum[9]. Interestingly, another study observed that the levels of IFN-γ and IL-12 were higher in infected children than in uninfected children (P < 0.001). In addition, these cytokines were positively correlated with the inflammation score (P < 0.01) and PMN infiltration, corroborating our findings[32]. In an analysis of polyclonal responses in CD4+ T cells in H. pylori-positive children, a potent production of IFN-γ was also observed. However, the responses were stronger in adults, due to their higher frequency of memory T cells[33]. Curiously, some authors have observed that the levels of IFN-γ mRNA in infected children were lower when compared to infected adults[8,34]. These data suggest an increased regulatory response conducted by Treg cells in children, thus reducing the inflammatory Th1 response in the gastric mucosa[5,8]. In a recent prospective Brazilian study, it was observed that IL-27 is increased in individuals with H. pylori-related duodenal ulcer and absent in patients with GC. Moreover, higher gastric concentrations of IL-12p70 (P < 0.001) and IFN-γ (P = 0.004) were observed in patients with duodenal ulcers than in those with GC. In addition, IL-27 is positively correlated to the expression of IL-12p70, an important cytokine in Th1 responses that directly influences the pattern of inflammation in the antral mucosa of patients with duodenal ulcer[35]. The relationship between IL-12p70 and IFN-γ is well elucidated in the context of H. pylori infection. In a study that added neutralizing antibodies to IL-12 in gastric biopsy cultures, authors observed a negative regulation of signal transducer and activator of transcription 4 (STAT4), an important factor for the production of IFN-γ, leading to a significant decrease in the concentrations of this cytokine (P < 0.001)[36]. Therefore, considerable progress has been achieved in the understanding of these important interplays between cytokine variations between different age groups and among regions of the gastric mucosa. Although the presence of MN and PMN cell infiltration associated with Th1 responses has been described, further studies are needed to aid in the understanding of the dynamics and frequency of these cells in the context of the H. pylori-induced gastric diseases.
As aforementioned, the H. pylori gastric environment colonization leads to a polarization toward Th1/Th17 responses, whereas Treg cells are responsible for the induction of anti-inflammatory responses. Of note, the Treg cells can be divided into IL-10-secreting Tr1 cells, TGF-β1-producing Tr3 cells, and FOXP3-expressing CD4+CD25high Treg cells[37]. The latter cells seem to be crucial in the setting of H. pylori infection. As long as they suppress the immune response against the bacterium, the pathogen persistence in the gastric mucosa might be favored. In that context, when evaluating the host immune response against H. pylori in adults and children, our group found that the expression of FOXP3+ Treg cells was significantly higher in the antrum of H. pylori-positive patients than in H. pylori-negative individuals[38]. This finding corroborates a previous study by Kandulski et al[39], which reported that H. pylori infection leads to a remarkable proportional enhancement of FOXP3+ Treg cells in the gastric cardia and antrum. In addition, the study by Silva et al[40], in its turn, reported that the levels of FOXP3-positive cells depend on the presence of gastritis. They observed that individuals with active chronic gastritis have lower expression of this molecule than persons without gastritis. Against this background, it is possible to infer that those cells are crucial for the occurrence of H. pylori-related diseases since they are directly associated with the levels of gastric mucosa inflammation.
Another finding in our study was the significantly higher levels of Treg FOXP3+ cells in children than in adults in the setting of H. pylori infection. Along with the cytokine pattern in pediatric patients previously discussed in this paper, this data indicates a milder infection with the bacterium in infants than in older individuals. Furthermore, a recent investigation using animals observed that mice infected during the neonatal period are more intensely colonized with the bacterium than those infected during adulthood. The neonatally infected mice had an immune response characterized by an intense infiltration of FOXP3+ Treg cells, and this result was VacA-dependent. Moreover, the study identified that the presence of VacA led to enhanced expression of IL-10 and TGF-β in macrophages whereas it suppressed the production of IL-23 in dendritic cells[41]. Another subsequent study by Altobelli et al[41] corroborates our hypothesis that the younger the host, the milder the inflammatory response against the bacterium with increased levels of FOXP3+ Treg cells. They used mice to evaluate the role of the induction of the co-inhibitory receptor B7-H1 in the chronic H. pylori infection and demonstrated that the induction of the Treg profile as well as the inhibition of T cell proliferation and IL-2 production are mediated by the B7-H1 expression, which results from the H. pylori type 4 secretion system (T4SS) action through the activation of the p38 MAPK pathway[42,43]. Interestingly, a recent study reported that animals infected with the H. pylori PMSS1 strain had higher levels of Treg cells and lower levels of Th17 cells than animals infected with the SS1 H. pylori strain[44]. Taken together, these studies show the plurality of factors influencing the induction of Treg cells in the gastric environment of H. pylori-positive individuals.
Notably, Wei et al[45] suggested that the immune response against H. pylori characterized by the expression of Treg FOXP3+ cells and IL-10 is not only observed in the gastric mucosa, but it is also enhanced in both superior and inferior gastrointestinal tracts after 10 wk of infection, suggesting a systemic character of this regulatory immune response. Data from another study identified significant enhancement of FOXP3 expression in patients with MALT lymphoma compared to individuals with active chronic gastritis. Interestingly, H. pylori-positive MALT lymphoma patients with increased expression of Treg FOXP3+ cells were significantly more responsive to the H. pylori eradication therapy than those with lower expression of Treg FOXP3+ cells[46]. In addition, Sen et al[47] reported significant enhancement in the levels of FOXP3 expressed by T CD25+ CD127 low/- cells in the peripheral blood of patients with GC compared to the control group, and the T CD25+ CD127 low/- cells were also present in the tumor microenvironment and contributed to the suppression of T effector cells against the tumor. These results suggest a relevant role of the H. pylori-induced immune system regulation by FOXP3-expressing cells in the scenario of the development and progression of malignancies associated with H. pylori gastric infection.
Finally, we demonstrated that children infected with H. pylori had Treg FOXP3 cell levels positively correlated with IL-10 expression in the gastric antrum and negatively correlated with the count of mononuclear and polymorphonuclear cells. Moreover, the levels of FOXP3+ Treg cells were also negatively correlated with mononuclear cells in adults. In that context, Gil et al[4] evaluated the expression of FOXP3, IL-10, TGF, and IL-17A as well as the dynamics of Th17/Treg FOXP3+ cells in the gastric mucosa of H. pylori-positive children. Their data showed that FOXP3, TGF-β1, and IL-10 were remarkably expressed in the infection and the number of FOXP3+ Treg cells was significantly enhanced among H. pylori-positive individuals compared to H. pylori-negatives. Moreover, FOXP3 was positively related to the bacterial density as well as with the number of polymorphonuclear and mononuclear cells among H. pylori-positive persons with gastritis. Therefore, the data provided by Gil et al[4] reinforce the influence of FOXP3 expression in the control of H. pylori-induced gastric inflammation and in the recruitment of mononuclear and polymorphonuclear cells, important components of the immune response against the pathogen and in the pathogenesis of diseases associated with this infection.
CONCLUSION
H. pylori infection remains an important determinant for gastric illness. Several factors can alter the host inflammation pattern directed to the bacterium, and it is evident that age is one of the most important variables in that setting. A better understanding of the immune system behavior at different ages, favoring, during childhood, the persistence of the infection and then, in adulthood, the gastric damage, can aid in the development of strategies aiming at the reduction of H. pylori prevalence, such as vaccines, and at the prevention of unfavorable infection-related clinical outcomes.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Higashida-Konishi M, Jonaitis LV, Krzyżek P S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ