Published online Oct 7, 2022. doi: 10.3748/wjg.v28.i37.5403
Peer-review started: July 21, 2022
First decision: August 19, 2022
Revised: August 27, 2022
Accepted: September 15, 2022
Article in press: September 15, 2022
Published online: October 7, 2022
Processing time: 69 Days and 23.8 Hours
Gastrointestinal cancer (GIC) is the most common cancer with a poor prognosis. Currently, surgery is the main treatment for GIC. However, the high rate of postoperative recurrence leads to a low five-year survival rate. In recent years, immunotherapy has received much attention. As the only immunotherapy drugs approved by the Food and Drug Administration (FDA), immune checkpoint blockade (ICB) drugs have great potential in cancer therapy. Nevertheless, the efficacy of ICB treatment is greatly limited by the low immunogenicity and immunosuppressive microenvironment of GIC. Therefore, the targets of immunotherapy have expanded from ICB to increasing tumor immunogenicity, increasing the recruitment and maturation of immune cells and reducing the proportion of inhibitory immune cells, such as M2-like macrophages, regulatory T cells and myeloid-derived suppressor cells. Moreover, with the development of nanotechnology, a variety of nanoparticles have been approved by the FDA for clinical therapy, so novel nanodrug delivery systems have become a research focus for anticancer therapy. In this review, we summarize recent advances in the appli
Core Tip: Recently, immunotherapy has received substantial attention. Although there are several Food and Drug Administration-approved immune checkpoint blockade (ICB) drugs, the efficacy remains limited, and the response rate is less than 20%. Because gastrointestinal cancer (GIC) is a group of immunosuppressive cancers, the efficacy of ICB treatment is also limited. Therefore, enhancing the immunogenicity of GIC or reversing the immunosuppressive microenvironment of GIC have become potential approaches for GIC immunotherapy. There are many studies on nanoparticle-based cancer therapy. However, there are only a few studies on immunotherapy-based nanoparticles in GIC. Here, we summarize recent advances in the application of immunotherapy-based nanoparticles in GIC and present our thoughts about this topic.
- Citation: Ding YN, Xue M, Tang QS, Wang LJ, Ding HY, Li H, Gao CC, Yu WP. Immunotherapy-based novel nanoparticles in the treatment of gastrointestinal cancer: Trends and challenges. World J Gastroenterol 2022; 28(37): 5403-5419
- URL: https://www.wjgnet.com/1007-9327/full/v28/i37/5403.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i37.5403
Gastrointestinal cancer (GIC) has been among the most commonly diagnosed cancers in recent decades[1-3]. In recent reports, the incidence and mortality rates have gradually decreased for gastric cancer (GC), hepatocellular carcinoma (HCC) and esophageal cancer in China; in contrast, the rates for colorectal cancer (CRC) have increased[4]. Regardless of the changes in the incidence and mortality rates of GIC, the disease has greatly affected the quality of life of many individuals.
Similar to other types of cancer, GIC has several therapies available. As the most conventional means of cancer treatment, surgery, chemotherapy and radiotherapy play important roles. Although traditional therapies effectively prolong survival for patients with GIC, there are still many drawbacks that cannot be ignored[5]. Surgery, especially minimally invasive surgery and radiotherapy, can effectively shrink the tumor and even make the local tumor disappear; chemotherapy can be administered systematically to kill cancer cells[6-8]. However, these treatments cannot prevent recurrence. Moreover, for GICs, the side effects of radiotherapy and chemotherapy on the digestive system seriously affect the quality of life of patients and cannot be ignored[9-11]. To improve the therapeutic effect and reduce the occurrence of adverse reactions, clinicians often try a variety of therapeutic combinations to achieve complementary advantages[12,13].
With progress in the concept of cancer treatment and the development of diagnosis and treatment technology, various precision treatment methods, such as targeted therapy, photodynamic therapy (PDT), photothermal therapy (PTT) and immunotherapy, have emerged as new sources of hope for patients[14-19]. Some scholars believe that the characteristics of the GIC immune microenvironment are related to the high mortality of patients with GIC; therefore, treatments that target the GIC immune microenvironment are gradually being recognized[20]. As one of the therapeutic methods that targets the cancer immune microenvironment, immune checkpoint blockade (ICB) treatment has achieved great success in clinical practice, laying a good foundation for the development of cancer immunotherapy[21].
Recently, a variety of nanobased drugs (such as Eligard[22], Marqibo[23], Onivyde[24], Doxil[25], Abraxane[26], Ontak[27] and Nanotherm[28]) have been widely used in clinical practice due to several characteristics, including their low toxicity, long circulation and passive targeting ability[29,30]. However, most of the nanobased drugs mentioned above are liposomes. In addition to liposomes, there are also other types of nanoparticles that possess the same potential for clinical translation. Similar to liposomes, small extracellular vesicles and cell membrane vesicles also have lipid bilayers, and they have better biocompatibility than liposomes due to their origin[31-34]. Furthermore, due to their simple production process and high drug loading efficiency, polymersomes are also considered candidate nanoparticles for clinical translation[35-37]. There are also many kinds of novel nanoparticles, such as gold nanoparticles, manganese dioxide nanoparticles, upconversion nanoparticles (UCNPs), metal organic framework nanoparticles and mesoporous silica nanoparticles (MSNPs), which can also play important roles in different diseases or cancers through their own characteristics[38-42]. Here, among the GICs, we focus on GC, HCC, CRC and pancreatic cancer and summarize the application trends of immunotherapy-based novel nanoparticles in these cancers as well as the challenges and opportunities in the future.
GC remains one of the most common causes of cancer-related death globally. Although a variety of treatments have been developed, the main treatment for GC is still surgery or endoscopic resection. The probability of patients experiencing recurrence after surgery is approximately 60%[43]. Currently, the median overall survival time with fluoropyrimidine-based combination chemotherapy is less than one year. In general, the overall clinical therapeutic effect of GC is not satisfactory[44,45]. In addition, immunotherapy for GC will become an important treatment option in the future, and nanoparticles, as highly efficient drug carriers, have played an important role in clinical practice[46-48]. Whether the combination of immunotherapy and nanoparticles can produce improved therapeutic effects is also worth examining.
Immune checkpoint inhibitors (ICIs), such as anti-programmed death receptor-1 (anti-PD-1) antibody and anti-programmed death receptor-ligand 1 (anti-PD-L1) antibody, can effectively block the PD-1/PD-L1 pathway and enhance the anticancer immune response[49]. Based on ICI treatment, Xu et al[50] prepared a novel nanoparticle named docetaxel (DOC)-PEG-PCL-monoclonal antibody (mAb) NP, which contained DOC as the chemotherapeutic drug and conjugated PD-L1 mAb on the surface of the nanoparticle. This nanodrug delivery system (NDDS) can effectively improve drug delivery efficiency and the solubility of hydrophobic drugs such as DOC. In addition, the system can target PD-L1-positive GC cells, exhibiting clinical translation potential. Recently, scientists found that a gradually acquired heritable de novo methylation program inhibited T-cell proliferation and clonal diversity during PD-1 blockade therapy[51]. Inspired by this study, Hu et al[52] designed copolymers loaded with the epigenetic agent 5-Aza-20-deoxycytidine (DAC), and an anti-PD-1 antibody was conjugated to the surface of the nanoparticles. The nanoparticles increased the stability of DAC and improved the therapeutic effect of ICI treatment in vivo.
Due to the characteristics of the cancer immune microenvironment, T-cell infiltration in GC patients is insufficient, which limits the effect of ICB treatment in GC[53]. Guo et al[54] constructed an NDDS named HMON@IR820/Pt-NPs, which coencapsulated platinum nanoparticles (chemo-prodrugs) and IR820 (photosensitizer) into hollow mesoporous organosilica nanoparticles. IR820-mediated PDT can lead to the release of oxidative mitochondrial DNA (mitoDNA). In addition, this oxidative process can oxidize Pt(0) to cytotoxic Pt(II), which can lead to the dysfunction of nuclear DNA (nDNA). The dual damage of mitoDNA and nDNA can activate the c-GAS/stimulator of interferon genes (STING) pathway, which can directly stimulate innate immunity and increase the infiltration of CD8+ T cells, thus improving the efficacy of immunotherapy for GC.
Multiple studies have confirmed that tumor-associated macrophages (TAMs) are also involved in the composition of the tumor immune microenvironment. Moreover, M2-like macrophages can inhibit tumor immunity and promote tumor immune escape[55,56]. Zhang et al[57]’s group designed a novel human serum albumin (HSA)-Au(III) thiosemicarbazone agent nanoparticle delivery system for chemotherapy and immunotherapy in GC. This NDDS can simultaneously directly kill GC cells and polarize TAMs into M1-like macrophages, providing a new immunotherapy strategy for clinical translation.
The majority of cancer patients are often unable to activate adequate levels of anticancer immunity, whereas therapeutic tumor vaccines can help patients proactively generate adequate anticancer immune responses against tumor-specific antigens (TSAs) and tumor-associated antigens[58]. Among the different types of tumor vaccines, dendritic cell (DC)-based tumor vaccines have been explored in clinical experiments[59,60]. Kohnepoushi et al[61] prepared poly(lactic-co-glycolic) acid nanoparticles to protect the human gastric tumor antigen against proteolytic enzymes. In addition, nanoparticles that contain human gastric tumor antigen can facilitate DC maturation and further enhance the efficacy of DC vaccines in clinical practice.
In addition to ICB treatment and other therapies that can improve the cancer immune microenvironment, immunoadjuvants can act as a potential adjunctive therapy to stimulate anticancer immunity[62,63]. Zhang et al[64] developed a gold nanoshell-based NDDS that can convert near-infrared (NIR) light into thermal energy, enabling PTT. Moreover, high temperature can also break thiol bonds to release gene therapy agents and oligooxynucleotides that contain cytosine-guanine (CpG) motifs (which are also known as immunoadjuvants). This study designed a novel NDDS combined with hyperthermia, gene therapy and immunotherapy, which exhibited encouraging anticancer efficacy against GC in vitro and in vivo (Table 1).
| Type of nanoparticle | Treatment strategy | Drugs or active substance involved | The main involvement of immune cells | Ref. |
| Copolymers | ICIs, chemotherapy | DOC, PD-L1 mAb | T cells | Xu et al[50] |
| Copolymers | ICIs, epigenetic treatment | DAC, nivolumab | PD1+CD8+ TILs | Hu et al[52] |
| Hollow mesoporous organosilica nanoparticles | Dual-damage to nDNA and mitoDNA activates the c-GAS/STING pathway to stimulate innate immunity | Platinum, IR820 | CD8+ T cells, DCs | Guo et al[54] |
| HSA nanoparticles | Targeted chemotherapy and immunotherapy | Au(III) thiosemicarbazone agent | TAMs | Zhang et al[57] |
| Polymers | DC vaccine | Human gastric tumor antigens | DCs | Kohnepoushi et al[61] |
| Gold nanoshell | Gene therapy, hyperthermia and immunoadjuvants therapy | HER-2 targeted siRNA, gold, CpG | DCs, T cells | Zhang et al[64] |
Primary liver cancer is among the most commonly diagnosed cancers, most of which are HCC[65,66]. Due to the high infection rate of hepatitis B virus, the incidence of HCC in China remains high[67]. Surgical resection of the liver is the main treatment for HCC. However, the prognosis after surgery is still poor. Recently, the development of molecular targeted therapy and immunotherapy for HCC has gained recognition in clinical studies[68]. Moreover, NDDSs can improve the efficiency of drug delivery into the tumor area and reduce side effects[69-71]. At present, a large number of studies using immunotherapy-based NDDSs have shown great potential for clinical translation.
ICB treatment has also emerged as a new option for advanced HCC[72]. However, ICB treatment alone has limited efficacy against HCC. Therefore, how to combine other kinds of therapies to improve the efficiency of ICB treatment has become a new academic topic. For example, Food and Drug Administration (FDA)-approved sorafenib-experienced patients used ipilimumab (anti-CTLA-4) combined with nivolumab (anti-PD-1) in March 2020[73]. In the last two decades, scientists have found that chemotherapeutic drugs, radiotherapy, PDT and some other treatments can induce immunogenic cell death (ICD), which can lead to the release of TSAs and increase tumor antigenicity[74]. Hence, ICD can improve the efficacy of ICB treatment by increasing tumor immunogenicity. According to the therapeutic strategies mentioned above, Xu et al[75] designed a cyclic arginine-glycine-aspartic acid peptide-modified self-assembling polymer-based NDDS. Cancer cells were damaged by PDT and chemotherapy, while induced ICD and enhanced tumor immunogenicity provided a suitable immune microenvironment for ICB treatment. Previous studies found that a lack of the p53 tumor suppressor gene leads to tumorigenesis and drug resistance[76-78]. With the development of research on the p53 tumor suppressor gene, an increasing body of evidence indicates that the p53 protein plays an important role in anticancer immunity by regulating the cancer immune microenvironment[79-81]. Furthermore, a recent study suggested that ICD induced by cytotoxic agents, such as chemotherapy drugs, may be involved in the activation of the p53 pathway[82]. Xiao et al[83] developed a novel lipid-polymer hybrid nanoplatform for mRNA delivery that can induce the expression of p53, effectively reprogramming the immune microenvironment of HCC. Moreover, combination with anti-PD-1 therapy can reverse the inhibitory immune microenvironment of HCC. To solve the problem of HCC recurrence after surgery, Li et al[84] designed a bionic NDDS consisting of MSNPs loaded with anti-PD-L1 and sorafenib and coated with platelet membranes at the surface of the MSNPs. This NDDS can target wounds and generate potent anti-HCC immunity, providing a new therapeutic idea for preventing recurrence in postsurgery HCC patients.
As we mentioned before, chemotherapy-based ICD can cause cancer cells to be more easily recognized by the immune system. However, the effect of single-drug-mediated ICD is very limited. Some studies have attempted to enhance the effect of ICD by combining two different ICD inducers to solve this problem. Yu et al[85] evaluated the potential of icaritin as an ICD inducer and utilized NDDS to deliver low doses of icaritin and doxorubicin simultaneously to the tumor area. This NDDS can reprogram the immune microenvironment and induce satisfactory anti-HCC effects. Furthermore, NDDS can lower the dose of chemotherapy to reduce the side effects.
TAMs play a major role in the immunosuppressive microenvironment of HCC[86]. Wang et al[87] screened chemokine C-C motif ligand (CCL)2 and CCL5 as two major chemokines responsible for the polarization of M2-like macrophages and designed a CCL2 and CCL5 dual-target lipid nanoparticle system. The combination of TAMs targeting lipid nanoparticles with ICB treatment achieved long-term survival in HCC mice. Similarly, as a common feature of the tumor microenvironment, hypoxia is also common in HCC. Hypoxia can lead to radioresistance and the formation of an immunosuppressive microenvironment, including the accumulation of TAMs and depletion of effector T cells, which are closely related to the occurrence and development of cancer[88-90]. Dai et al[91] synthesized polydopamine-nanoparticle-stabilized oxygen microcapsules that can deliver oxygen to the tumor region and rapidly increase the concentration of oxygen. In this study, oxygen microcapsules increased HCC sensitivity to radiotherapy and polarized M2-like macrophages into M1-like macrophages, consequently activating anti-HCC immunity. In addition to conventional immune cells, liver sinusoidal endothelial cells (LSECs) can also play a significant role in immunosuppressive regulation[92]. Yu et al[93] designed a simvastatin-loaded NDDS to target LSECs in HCC patients. This NDDS can reduce the capillarization of LSECs to improve the stromal microenvironment and recruit natural killer T cells to inhibit tumor progression.
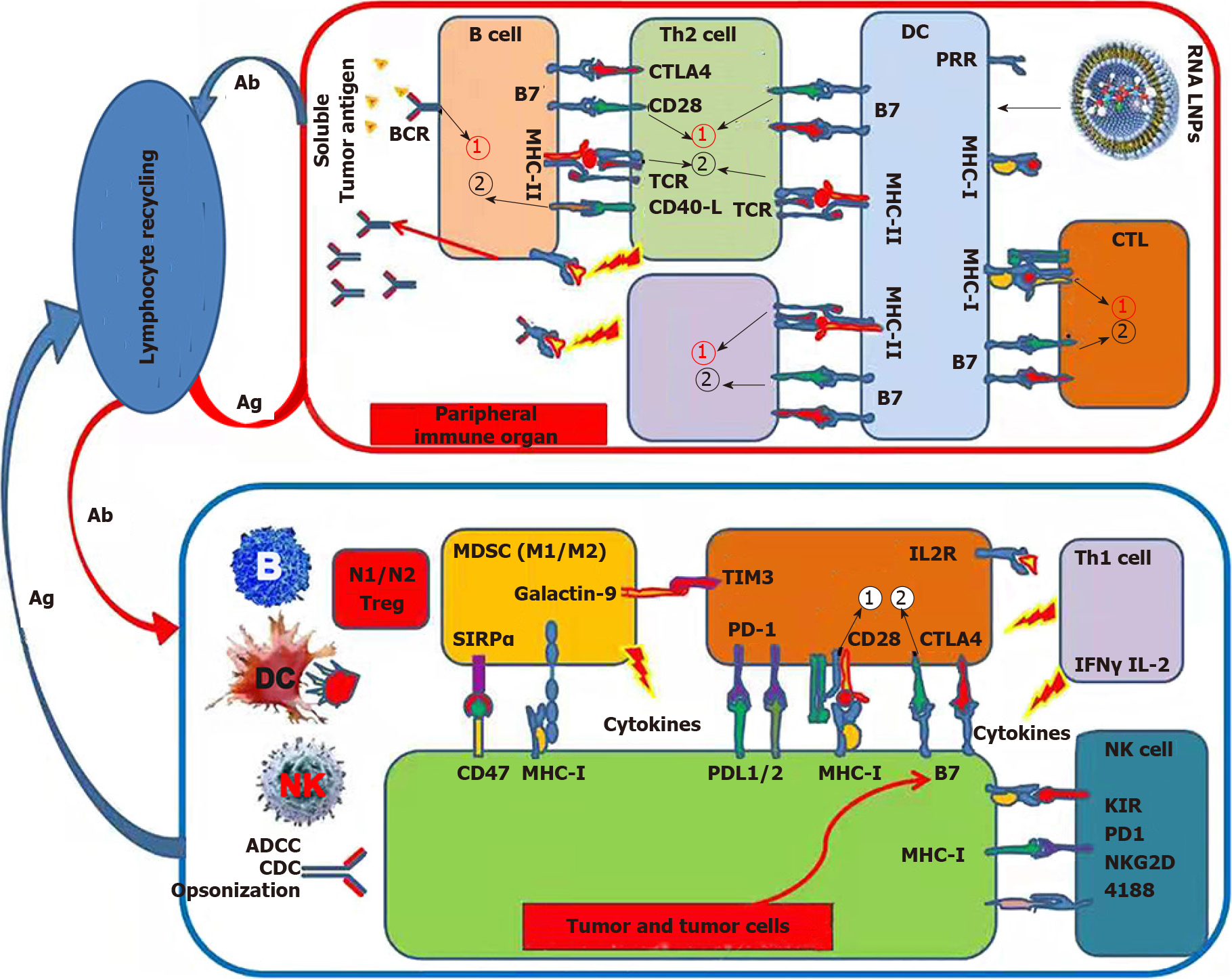
Cationic lipid nanoparticles have been suggested to be suitable delivery vectors for RNA, and several messenger RNA vaccines are based on lipid nanotechnology that was approved by the FDA during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic[94-97]. Zhang et al[98] developed a total HCC-derived RNA-loaded lipid nanoparticle vaccine to target DCs and activate anticancer immunity (Figure 1 and Table 2).
| Type of nanoparticle | Treatment strategy | Drugs or active substance involved | The main involvement of immune cells | Ref. |
| Nano-micelles | ICD, chemotherapy, PDT | PTX, TPABDTO | CTLs, MDSCs, Tregs, DCs | Xu et al[75] |
| Polymers | p53 gene reprograms the immune microenvironment | p53 mRNA | T cells, NK cells | Xiao et al[83] |
| MSNPs | Anti-angiogenic drugs, ICIs | Sorafenib, PD-L1 antibody | T cells | Li et al[84] |
| Copolymers | ICD, chemotherapy | Icaritin, DOX | T cells, DCs | Yu et al[85] |
| Lipid nanoparticle | CCL2 and CCL5 dual-target | BisCCL2/5i mRNA | TAMs | Wang et al[87] |
| Microcapsules | Improving hypoxia | Oxygen | TAMs | Dai et al[91] |
| Copolymers | Mitigates LSEC capillarization | Simvastatin | NKT cells | Yu et al[93] |
| LNPs | Antigen specific vaccine | Tumor-derived RNA | T cells, DCs | Zhang et al[98] |
CRC is the third leading cause of cancer-related deaths globally[99]. CRC is the only cancer that can be reduced by screening. Most CRC patients can be screened by flexible sigmoidoscopy or guaiac-based fecal occult blood tests[100]. However, approximately 25% of CRC patients are at stage 4, and the 5-year survival rate is only 11%[101,102]. To improve the survival rate of advanced CRC patients, immunotherapy and nanoparticle-based drug delivery systems have become the focus of basic and clinical research for the past few years[103-105].
Similar to GC and HCC, ICB treatment is more widely used in CRC patients, but its curative effect is extremely limited, especially for mismatch repair-proficient/microsatellite stability/microsatellite instability-low CRC patients[106]. As we reported earlier, ICB treatment combined with ICD can achieve a “1 + 1 > 2” effect. A similar treatment strategy has also been applied in CRC research. For example, Yuan et al[107] utilized the ability of PDT to induce ICD and developed a photosensitive NDDS combined with ICB treatment that can enhance the response rate of anti-PD-L1 therapy in CRC. Zhu et al[108] also designed an oxaliplatin prodrug-conjugated photosensitive NDDS that can be stimulated by the NIR-II window (1000-1700 nm) for PTT, which is a proven to induce ICD. Moreover, oxaliplatin, a chemotherapy drug, is also known as an ICD inducer. This novel NDDS can induce ICD through both PTT and chemotherapy, which may provide a promising immunotherapy strategy for advanced CRC treatment. Shikonin (SK), a major active ingredient isolated from traditional Chinese medicine, has also been proven to induce ICD. Li et al[109] designed a versatile nanoparticle that can deliver knockdown siRNA for both the ICD inducer SK and PD-L1, which presents potential for CRC immunotherapy. Recently, ferroptosis was discovered as a nonapoptotic form of regulated cell death[110]. In addition, Duan et al[111]’s group proved that dihydroartemisinin (DHA), as a reactive oxygen species (ROS)-producing drug and ferroptosis inducer, can also induce ICD to potentiate anticancer immunity. Therefore, the same research group developed a Zn-pyrophosphate core-shell NDDS codeliver DHA and pyropheophorbide-iron (pyro-Fe). Glutathione and other thiol-based reductants in cancer cells can reduce Pyro-FeIII to Pyro-FeII, which can catalyze the decomposition of DHA to induce ICD and ferroptosis. This novel NDDS overcame the deficiency of iron in solid tumors, enhanced the ability of DHA to induce ferroptosis and ICD, and increased the infiltration of CD8+ T cells in CRC.
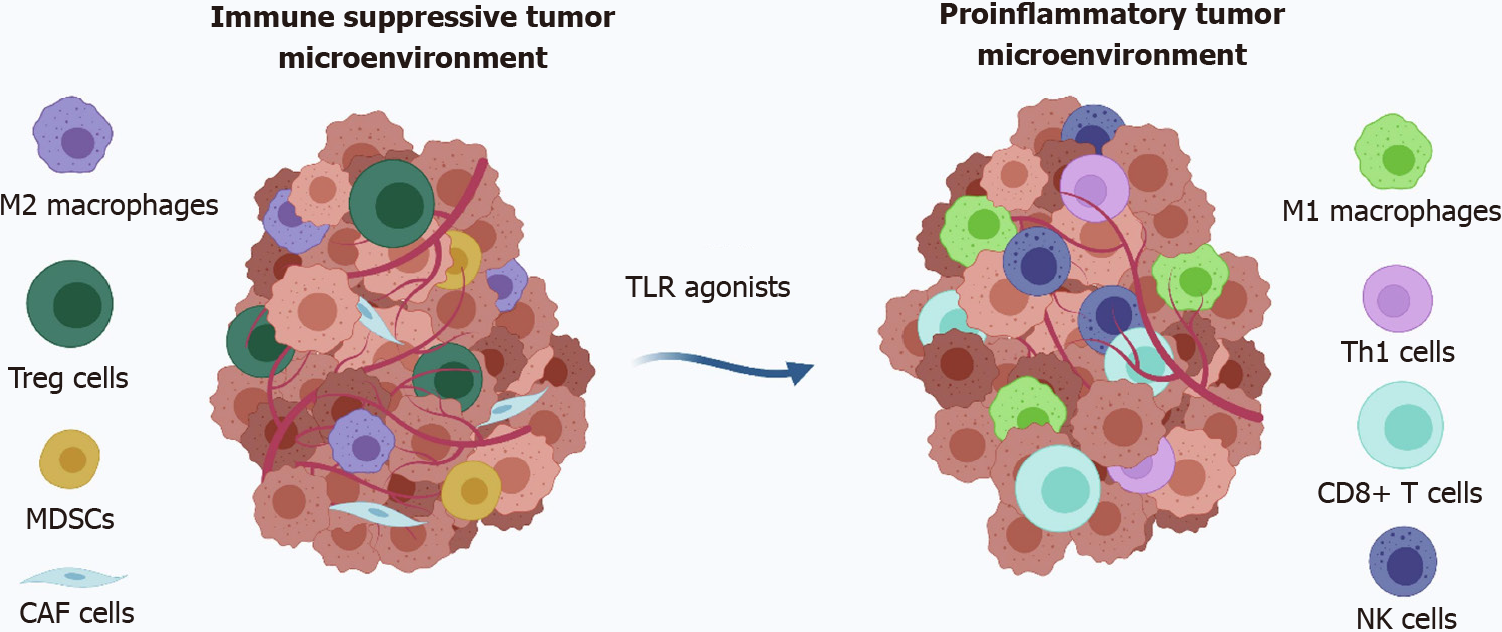
In addition to actively increasing the immunogenicity of CRC, stimulating immune cells can also activate anti-CRC immunity. Immune cells can be activated by stimulating toll-like receptors (TLRs), such as DCs and macrophages. Several TLR agonists have been approved by the FDA. However, none are currently approved for CRC treatment. The major problem for TLR agonists is the small size of the drugs, which allows the drugs to spread rapidly from the administration site and cause severe systemic side effects (Figure 2)[112]. Fortunately, nanoparticle-based delivery systems can solve this problem. Bahmani et al[113] prepared a platelet membrane-coated nanoparticle loaded with the TLR7 agonist R848. This biomimetic NDDS enhanced the retention of the drug in the tumor and effectively stimulated the maturation of DCs, resulting in complete tumor eradication in a murine model of CRC.
Notably, long noncoding RNAs (lncRNAs) have recently been reported to be involved in the formation of the immunosuppressive cancer microenvironment and have become a potential immunotherapy target[114-116]. Liu et al[117] designed a bioscaffold loaded with a lncRNA-targeting biomimetic NDDS that modulated the cancer immune microenvironment against CRC recurrence after surgery. The biomimetic NDDS coated with a CRC membrane, which provides NDDS with a tumor-homing capacity and carries TSAs into the tumor area, promotes the maturation of DCs. Moreover, a plasmid-encoding short hairpin RNA against Pvt1 was encapsulated inside the NDDS to enhance ICD and ameliorate granulocytic-myeloid-derived suppressor cell (G-MDSC)-mediated immunosuppression. This work provides a new perspective for NDDS-based lncRNA-targeted immunotherapy.
In recent years, as an important component of the immunosuppressive cancer microenvironment, MDSCs have also been identified as potential targets for cancer immunotherapy. Additionally, recent studies reported that MDSCs could be selectively enlarged because of the enrichment of Fusobacterium nucleatum (Fn) in CRC tissue, resulting in a cancer immunosuppressive microenvironment[118-121]. Dong et al[122] proposed a phage-based antibacterial system that used the broad-spectrum antibacterial effect of silver nanoparticles (AgNPs) for antibacterial activity and then transported phage M13 into the tumor and utilized the recognition mechanism of phages to selectively kill Fn, thus preventing the recruitment of MDSCs. In addition, phages are highly immunogenic and can directly stimulate the maturation of DCs and promote the activation of M1-like macrophages, significantly enhancing the anti-CRC immune response.
Over the years, CRC vaccines have been a focus of scientific research. Zhang et al[123] designed an in situ cancer vaccine. They reported a supramolecular assembled programmable immune activation nanomedicine (PIAN) that can produce strong and durable anticancer immunity in situ. PIAN entered the tumor area through enhanced permeability and retention (EPR) after tail vein injection and was then disassembled by the high ROS within the tumor tissue. The release of poly-[(N-2-hydroxyethyl)-aspartamide]-Pt(IV)/beta-cyclodextrin simultaneously mediated tumor cell death and antigen release. In addition, CpG/polyamidoamine (CpG/PAMAM) captured the released antigen and entered the tumor draining lymph node to stimulate DC maturation, thus activating anti-CRC-specific immunity. This excellent work provides a new idea for designing nanomedicine-based programmable in situ cancer vaccines for cancer immunotherapy (Table 3).
| Type of nanoparticle | Treatment strategy | Drugs or active substance involved | The main involvement of immune cells | Ref. |
| Copolymers | PDT induces HIF-1α expression, leading to the upregulation of PD-L1 expression, ICIs | Photosensitizer, PD-L1 antibody | DCs, CD8+T cells, memory T cells | Yuan et al[107] |
| Polymeric nanoparticle | PTT, chemotherapy, ICD | PBOXA, donor–spacer–acceptor–spacer–donor type fluorophore | DCs, T cells, CTLs | Zhu et al[108] |
| Copolymers | ICD, ICIs | SK, PD-L1 knockdown siRNA | DCs, TAMs, Tregs, T cells | Li et al[109] |
| Polymers | ICD, ferroptosis | DHA | DCs, T cells | Duan et al[111] |
| Platelet membrane-coated nanoparticle | TLR7 treatment | R848 | DCs | Bahmani et al[113] |
| Liposomes with cell membrane | ICD, chemotherapy, lncRNA-targeting therapy | Oxaliplatin, shPvt1 | DCs, MDSCs, CD8+T cells | Liu et al[117] |
| Silver nanoparticles | Anti-Fn | Phage M13 | MDSCs, DCs, TAMs | Dong et al[122] |
| Supramolecular assembled programmable immune activation nanomedicine | In-situ cancer vaccine, ICD | PPCD, CpG/PAMAM | DCs, CD8+T cells | Zhang et al[123] |
As one of the most aggressive and fatal cancers, pancreatic cancer has been the leading cause of cancer-related deaths worldwide in the last few decades[124,125]. Most patients experience no obvious symptoms during the development of the disease. Therefore, it is difficult to diagnose the disease in the early stage, and patients often miss the optimal treatment time after they have been diagnosed with pancreatic cancer. Moreover, the majority of patients eventually relapse, even if they receive potentially radical treatment[126]. In contrast to other malignant tumors, stromal hyperplasia is the main feature of the pancreatic cancer microenvironment[127]. As a result, pancreatic cancer does not have a sufficient blood supply, so antiangiogenic drugs are not suitable for pancreatic cancer[128]. In addition, the tumor stroma of pancreatic cancer acts as a natural physical barrier between the tumor tissue and the body’s immune system, which also limits the application of immunotherapy[129,130]. Until now, most phase I and II clinical trials of immunotherapy in pancreatic cancer have failed. Interestingly, ICB treatment combined with chemotherapy and/or radiotherapy has shown encouraging clinical efficacy[131]. In recent years, with the continuous development of nanotechnology, scientists have proposed a variety of nanodelivery systems aimed at the unique pathological characteristics of pancreatic cancer. They attempted to utilize NDDSs to achieve synergistic therapy and improve the tumor microenvironment to reverse the current situation of pancreatic cancer[132].
As we mentioned above, the tumor stroma of pancreatic cancer limits the efficacy of immunotherapy. Wang et al[133] reported a pH-responsive clustered nanoparticle (iCluster) loaded with both siPD-L1 and transforming growth factor-β (TGF-β) receptor inhibitors (LY2157299). iCluster can deliver siPD-L1 and LY2157299 to tumor blood vessels and then release small PAMAM at acidic tumor extracellular pH (pHe). Therefore, siPD-L1 can penetrate into tumor tissue as deeply as possible to activate anticancer immunity, and a TGF-β receptor inhibitor can reduce the barrier function of the tumor stroma to help more drugs penetrate into the tumor tissue, further promoting the activation of anticancer immunity. Similarly, Yu et al[134] designed a size-adjustable nanoparticle consisting of IR780 containing the thermosensitive ICB drug (BMS-202) conjugated to HSA-BMS. Under mild hyperthermia therapy, this novel nanoparticle releases the small HSA-BMS into the tumor site and relieves the immunosuppressive environment to normalize immunity. In recent years, some studies have reported that RNA interference (RNAi) has emerged as a better agent for inducing anticancer immunity than antibodies or small molecules in vivo[135]. PLGA polymers have been proven to be a potentially excellent siRNA delivery vector exhibiting low toxicity, sustained release and the EPR effect[136,137]. Jung et al[138] developed a poly(lactic-co-glycolic) acid (PLGA)-based siRNA nanoparticle named siPD-L1@PLGA. siPD-L1@PLGA increased the infiltration of CD8+ T cells and significantly inhibited tumor growth.
The poor immunogenicity and excessive immunosuppressive cancer microenvironment of pancreatic cancer result in a lack of adequate antigen-presenting cells in the tumor microenvironment. Lorkowski et al[139] reported a dual-immunostimulatory nanoparticle that was simultaneously loaded with a STING agonist and TLR4 agonist. These dual-immunostimulatory nanoparticles can be taken up by DCs in the tumor site to significantly increase the number of mature DCs and activate anticancer immunity in pancreatic cancer. Theoretically, cancer immunosuppressive cells mainly include TAMs, MDSCs and regulatory T cells (Tregs). Recent studies have shown that MDSCs are the major inhibitory immune cells in the immunosuppressive microenvironment of pancreatic cancer[140]. A previous study found that low-molecular-weight heparin-D-α-tocopheryl (LMWH) could significantly inhibit G-MDSC recruit
Pyroptosis is a mode of programmed cell death[143]. Recent studies have shown that pyrophosis can induce powerful anticancer immunity[144-146]. However, pyrophosis is usually induced by chemo
Gemcitabine is among the most effective FDA-approved chemotherapy drugs to prolong survival in patients with pancreatic cancer. However, the immunosuppressive cancer microenvironment, especially the presence of TAMs, significantly weakens the efficacy of gemcitabine. It has even been reported that gemcitabine can induce an increase in TAMs and promote the establishment of a tumor-suppressive immune microenvironment, which further increases gemcitabine drug resistance[149]. Furthermore, gemcitabine can even induce an increase in TAMs and promote the establishment of an immunosuppressive tumor microenvironment, which further leads to gemcitabine drug resistance[150]. Thus, Wang et al[151] developed a biomimetic nanoparticle named PG@KMCM consisting of gemcitabine-loaded PLGA nanoparticles coated with stable M2-like macrophage targeting peptides (M2pep). Pancreatic cancer cell membranes can deliver PG@KMCM to pancreatic cancer and target M2-like macrophages by M2pep to reprogram TAMs and reverse gemcitabine drug resistance. Cao et al[152] also considered TAMs to be a therapeutic target and reported a reduction-responsive RNAi NDDS to regulate the function of TAMs and reprogram tumor lipid metabolism. On the one hand, this novel NDDS can block the activity of monoacylglycerol lipase (MGLL) by MGLL siRNA to reduce the production of free fatty acids and thus cut off the tumor’s nutrition supply. On the other hand, MGLL blockade may lead to the accumulation of 2-arachidonoylglycerol, which can be secreted into the cancer microenvironment and activate the endocannabinoid receptor-2 (CB-2), which can transform TAMs into M2-like macrophages. Therefore, they prepared CB-2 siRNA to block CB-2 expression, preventing the transition of M2-like macrophages. The dual-RNAi NDDS developed in this research shows significant enhancement of the immunological environment in pancreatic cancer.
PTT has achieved satisfactory results in animal experiments, but it is difficult to apply widely in the clinic. The main reason is poor light penetration. It is harder to achieve the desired therapeutic effect for pancreatic cancer due to the depth of pancreatic cancer and the presence of the tumor stroma. To solve this conundrum, Wang et al[153] proposed magnetic resonance imaging (MRI)-guided interventional PTT (IPTT). They designed an iron oxide-based nanoparticle loaded with indocyanine green for PTT and imiquimod (IMQ) as an immunostimulant. IPTT can induce in situ cancer vaccination, which can be amplified by IMQ. In addition, iron oxide is a widely used MRI contrast agent. A recent study reported that iron oxide can modulate the cancer microenvironment by transforming M2-like macrophages into M1-like macrophages[154]. Overall, these novel iron oxide-based nanoparticles can improve therapeutic effects by directly killing cancer cells and activating the long-lasting immune effect by in situ vaccination and regulation of the immune microenvironment (Table 4).
| Type of nanoparticle | Treatment strategy | Drugs or active substance involved | The main involvement of immune cells | Ref. |
| Clustered nanoparticle | ICIs, TGF-β receptor inhibitors | LY2157299, siPD-L1 | T cells | Wang et al[133] |
| HAS-Liposomes | ICD, ICIs, PTT | BMS-202, IR780 | DCs, CTLs, T cells | Yu et al[134] |
| Copolymers | ICIs | siPD-L1 | CD8+T cells, NK cells | Jung et al[138] |
| LNPs | STING and TLR4 therapy | STING agonist, R4 agonist | DCs, Tregs, TAMs | Lorkowski et al[139] |
| Micellar nanoparticle | Inhibit G-MDSCs recruitment, chemotherapy | LMWH, PTX | G-MDSC, CD8+T cells, CD4+T cells | Lu et al[142] |
| UCNPs | Pyroptosis | K3ZrF7:Yb/Er UCNPs | DCs, memory T cells | Ding et al[148] |
| Cancer cell membrane with copolymers | ICIs, M2-macrophages targeting | M2pep, TAAs, PD-L1 antibody | TAMs, CD8+T cells | Wang et al[151] |
| PDSA-based nanoplatform | Suppression of FFAs, repolarization of TAMs | siMGLL, siCB-2 | TAMs | Cao et al[152] |
| Copolymers | PTT, immunotherapy | ICG, IMQ, IONs | TAMs, CD8+T cells, CD4+T cells, CD4+T cells | Wang et al[153] |
| IONs | Repolarization of TAMs | Ferumoxytol | TAMs | Zanganeh et al[154] |
In recent years, the increased development of immunotherapy has provided hope to patients with advanced cancer. Several ICB drugs have been approved by the FDA for clinical application in cancer treatment. However, due to the immunosuppressive microenvironment, only approximately 20% of patients can benefit from ICB treatment. In addition to ICB treatment, some conventional therapies, such as chemotherapy and radiotherapy, are also closely related to the immunosuppressive tumor microenvironment. The facts we mentioned above also exist in GIC. Therefore, we believe that in addition to ICB treatment, we should focus on reversing the immunosuppressive microenvironment in the future. Moreover, advances in nanotechnology have made drug delivery more efficient, allowing us to deliver drugs at specific times and locations based on the characteristics of the cancer and the drugs. We wondered whether the combination of nanotechnology and immunotherapy could achieve satisfactory therapeutic efficacy in GIC. Here, we summarize recent advances in immunotherapy-based novel nanoparticles in the treatment of GIC.
Since GC, HCC and CRC share similar tumor immune microenvironments, we will discuss the application of immunotherapy-based nanoparticles in these three kinds of GICs in the following paragraphs. Due to the limited monotherapy effect of ICB treatment, nanoparticles, as drug delivery vehicles, cannot significantly improve the therapeutic effect of ICB treatment. Thus, basically all ICB-based nanoparticles are combined with other therapeutic strategies. ICB treatment can reverse tumor immune escape from T cells. However, the low immunogenicity of the tumor results in insufficient T-cell infiltration in the tumor tissue. Hence, most studies have attempted to promote the therapeutic effect of ICB-based nanoparticles by inducing ICD.
ICD can increase tumor immunogenicity, but similar to ICB treatment, the immune-stimulating effect of ICD is limited. To amplify the ICD effect, some studies utilized the drug-loading capacity of nanoparticles and adopted a combination of multiple ICD inducers to enhance the immune response. Even so, we still do not recommend the combination of multiple ICD inducers to promote anticancer immunity. On the one hand, this strategy does not solve the problem of insufficient T-cell infiltration; on the other hand, ICD inducers themselves can directly kill tumor cells. It is difficult to determine whether tumor inhibition is due to cytotoxicity or ICD-induced anticancer immunity.
Compared with ICD and ICB treatment, we believe that reprogramming the immunosuppressive tumor microenvironment by targeting inhibitory immune cells (e.g., TAMs, Tregs and MDSCs) will be a revolutionary breakthrough in cancer immunotherapy in the future. Recently, many studies have attempted to successfully reprogram the tumor immune microenvironment by polarizing M2-like macrophages into M1-like macrophages. However, few reports have designed NDDSs to target Tregs and MDSCs in the tumor microenvironment. Therefore, it is of great significance to develop NDDSs for Tregs and MDSCs. In addition, the relationship between the intestinal flora and the immunosuppressive microenvironment of CRC also deserves future attention.
TLR agonists have also emerged as a promising treatment for cancer immunotherapy. However, due to the lack of targeting of TLR agonists, free TLR agonists often lead to serious systemic side effects. Therefore, it is necessary to deliver TLR agonists by nanoparticles. Numerous TLR agonists have been proven to be effective in stimulating anticancer immunity. In addition, combination therapies of TLR agonists with other immunotherapies are also anticipated. However, how to deliver TLR agonists to the tumor site stably and accurately and reduce serious systemic side effects are still problems that need prompt solutions.
With the successful large-scale clinical application of SARS-CoV-2 mRNA vaccines, research on cancer vaccines is also imminent. Due to the high heterogeneity of cancer, RNA vaccines are the best option. However, RNA is highly unstable. Liposomes, as mature NDDSs, can prepare cancer vaccines by encapsulating RNA. In addition, to improve the vaccine effect, NDDSs can be encapsulated with immune adjuvants to promote immune activation. RNA-based cancer vaccines, as a personalized cancer treatment strategy, can effectively improve anticancer immunity.
Next, we will discuss the application of immunotherapy-based nanoparticles in pancreatic cancer. Pancreatic cancer has several characteristics that are not found in other kinds of GICs, including the following: (1) Pancreatic cancer is surrounded by a tumor stroma, resulting in a physical barrier that isolates pancreatic cancer from the surrounding immune microenvironment; (2) Due to the anatomic position of the pancreas, pancreatic cancer is located deep in the abdominal cavity and therefore is not sensitive to PDT and PTT; and (3) Unlike other GICs, pancreatic cancer lacks blood supply and can adapt to nutrient deficiency and in a long-term hypoxic state.
To pass through the physical barrier of pancreatic cancer, size-adjusted NDDSs are the best option. Due to the deep location of pancreatic cancer, PTT has limited efficacy. Inspired by a previous study, we believe that IPTT and interventional PDT can be widely applied in the treatment of pancreatic cancer. Additionally, interventional light-mediated therapy can be extended to GC, esophageal cancer and CRC, as well as HCC.
Compared with GC, HCC and CRC, pancreatic cancer has a similar immunosuppressive microenvironment, and the immunosuppressive situation is even worse. Most of the treatment strategies mentioned in GC, HCC and CRC can also be applied in pancreatic cancer. In previous reports, immunotherapy-based nanoparticles mainly used liposomes and copolymer nanoparticles, which are chemical synthesis products. Therefore, the nanoparticles can be designed according to demand. To increase biocompatibility and deliver tumor antigens, some literature has used tumor cell membranes to prepare biomimetic NDDSs, which have also achieved good results. In addition to the abovementioned nanoparticles, we particularly recommend small extracellular vesicles (also known as exosomes) as immunotherapy-based nanoparticles. First, exosomes are naturally nanosized. Second, similar to cell membrane vesicles, exosomes derived from tumor cells can carry tumor antigens. Third, exosomes can use surface modification to achieve biological functions, such as targeting. Last, exosomes have a certain drug delivery capacity. Thus, exosomes are potential immunotherapy-based nanoparticles for GIC that have not been reported in previous studies.
GIC is a common tumor worldwide. The immune microenvironments of GC, HCC, CRC and pancreatic cancer have similarities and differences. There are still many mechanisms of immune escape in GIC that are not well understood. Therefore, we need an in-depth understanding of the characteristics of each kind of GIC to take advantage of its characteristics and design immunotherapy-based nanoparticles.
| 1. | Huang F, Wang BR, Wu YQ, Wang FC, Zhang J, Wang YG. Oncolytic viruses against cancer stem cells: A promising approach for gastrointestinal cancer. World J Gastroenterol. 2016;22:7999-8009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11975] [Article Influence: 2993.8] [Reference Citation Analysis (9)] |
| 3. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 3313] [Article Influence: 662.6] [Reference Citation Analysis (9)] |
| 4. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2503] [Article Influence: 625.8] [Reference Citation Analysis (2)] |
| 5. | Neumann PA, Berlet MW, Friess H. Surgical oncology in the age of multimodality therapy for cancer of the upper and lower gastrointestinal tract. Expert Rev Anticancer Ther. 2021;21:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Hamed OH, Gusani NJ, Kimchi ET, Kavic SM. Minimally invasive surgery in gastrointestinal cancer: benefits, challenges, and solutions for underutilization. JSLS. 2014;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Pofahl WE, Pories WJ. Current status and future directions of geriatric general surgery. J Am Geriatr Soc. 2003;51:S351-S354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ciabatti S, Cammelli S, Frakulli R, Arcelli A, Macchia G, Deodato F, Cilla S, Giaccherini L, Buwenge M, Morganti AG. Radiotherapy of pancreatic cancer in older patients: A systematic review. J Geriatr Oncol. 2019;10:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Grabenbauer GG, Holger G. Management of radiation and chemotherapy related acute toxicity in gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2016;30:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Sipaviciute A, Sileika E, Burneckis A, Dulskas A. Late gastrointestinal toxicity after radiotherapy for rectal cancer: a systematic review. Int J Colorectal Dis. 2020;35:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Marin JJ, Romero MR, Blazquez AG, Herraez E, Keck E, Briz O. Importance and limitations of chemotherapy among the available treatments for gastrointestinal tumours. Anticancer Agents Med Chem. 2009;9:162-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Kelly RJ. Emerging Multimodality Approaches to Treat Localized Esophageal Cancer. J Natl Compr Canc Netw. 2019;17:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Metzger R, Bollschweiler E, Hölscher AH, Warnecke-Eberz U. ERCC1: impact in multimodality treatment of upper gastrointestinal cancer. Future Oncol. 2010;6:1735-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Tazawa H, Kagawa S, Fujiwara T. MicroRNAs as potential target gene in cancer gene therapy of gastrointestinal tumors. Expert Opin Biol Ther. 2011;11:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Rosenbaum MW, Gonzalez RS. Targeted therapy for upper gastrointestinal tract cancer: current and future prospects. Histopathology. 2021;78:148-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Yano T, Wang KK. Photodynamic Therapy for Gastrointestinal Cancer. Photochem Photobiol. 2020;96:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Hao M, Kong C, Jiang C, Hou R, Zhao X, Li J, Wang Y, Gao Y, Zhang H, Yang B, Jiang J. Polydopamine-coated Au-Ag nanoparticle-guided photothermal colorectal cancer therapy through multiple cell death pathways. Acta Biomater. 2019;83:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Lee SY, Shieh MJ. Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl Mater Interfaces. 2020;12:4254-4264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Ding Y, Yang R, Yu W, Hu C, Zhang Z, Liu D, An Y, Wang X, He C, Liu P, Tang Q, Chen D. Chitosan oligosaccharide decorated liposomes combined with TH302 for photodynamic therapy in triple negative breast cancer. J Nanobiotechnology. 2021;19:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Zhang Y, Xu J, Zhang N, Chen M, Wang H, Zhu D. Targeting the tumour immune microenvironment for cancer therapy in human gastrointestinal malignancies. Cancer Lett. 2019;458:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | He M, Yang T, Wang Y, Wang M, Chen X, Ding D, Zheng Y, Chen H. Immune Checkpoint Inhibitor-Based Strategies for Synergistic Cancer Therapy. Adv Healthc Mater. 2021;10:e2002104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | FDA approves liposomal vincristine (Marqibo) for rare leukemia. Oncology (Williston Park). 2012;26:841. [PubMed] |
| 24. | Frampton JE. Liposomal Irinotecan: A Review in Metastatic Pancreatic Adenocarcinoma. Drugs. 2020;80:1007-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 463] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 26. | Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release. 2013;170:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 27. | Duvic M, Talpur R. Optimizing denileukin diftitox (Ontak) therapy. Future Oncol. 2008;4:457-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Rivera Gil P, Hühn D, del Mercato LL, Sasse D, Parak WJ. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharmacol Res. 2010;62:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Ding Y, Wang L, Li H, Miao F, Zhang Z, Hu C, Yu W, Tang Q, Shao G. Application of lipid nanovesicle drug delivery system in cancer immunotherapy. J Nanobiotechnology. 2022;20:214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | DaunoXome approved. AIDS Patient Care STDS. 1996;10:263. [PubMed] |
| 31. | Bost JP, Barriga H, Holme MN, Gallud A, Maugeri M, Gupta D, Lehto T, Valadi H, Esbjörner EK, Stevens MM, El-Andaloussi S. Delivery of Oligonucleotide Therapeutics: Chemical Modifications, Lipid Nanoparticles, and Extracellular Vesicles. ACS Nano. 2021;15:13993-14021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 32. | Zou S, Wang B, Wang C, Wang Q, Zhang L. Cell membrane-coated nanoparticles: research advances. Nanomedicine (Lond). 2020;15:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 33. | Huang R, Cai GQ, Li J, Li XS, Liu HT, Shang XL, Zhou JD, Nie XM, Gui R. Platelet membrane-camouflaged silver metal-organic framework drug system against infections caused by methicillin-resistant Staphylococcus aureus. J Nanobiotechnology. 2021;19:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Jing B, Qian R, Jiang D, Gai Y, Liu Z, Guo F, Ren S, Gao Y, Lan X, An R. Extracellular vesicles-based pre-targeting strategy enables multi-modal imaging of orthotopic colon cancer and image-guided surgery. J Nanobiotechnology. 2021;19:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Moulahoum H, Ghorbanizamani F, Zihnioglu F, Timur S. Surface Biomodification of Liposomes and Polymersomes for Efficient Targeted Drug Delivery. Bioconjug Chem. 2021;32:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 36. | He C, Ding H, Chen J, Ding Y, Yang R, Hu C, An Y, Liu D, Liu P, Tang Q, Zhang Z. Immunogenic Cell Death Induced by Chemoradiotherapy of Novel pH-Sensitive Cargo-Loaded Polymersomes in Glioblastoma. Int J Nanomedicine. 2021;16:7123-7135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | He C, Zhang Z, Ding Y, Xue K, Wang X, Yang R, An Y, Liu D, Hu C, Tang Q. LRP1-mediated pH-sensitive polymersomes facilitate combination therapy of glioblastoma in vitro and in vivo. J Nanobiotechnology. 2021;19:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Han Y, An Y, Jia G, Wang X, He C, Ding Y, Tang Q. Facile assembly of upconversion nanoparticle-based micelles for active targeted dual-mode imaging in pancreatic cancer. J Nanobiotechnology. 2018;16:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Xu W, Qing X, Liu S, Chen Z, Zhang Y. Manganese oxide nanomaterials for bacterial infection detection and therapy. J Mater Chem B. 2022;10:1343-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Connor DM, Broome AM. Gold Nanoparticles for the Delivery of Cancer Therapeutics. Adv Cancer Res. 2018;139:163-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Zhang X, Lu Y, Jia D, Qiu W, Ma X, Zhang X, Xu Z, Wen F. Acidic microenvironment responsive polymeric MOF-based nanoparticles induce immunogenic cell death for combined cancer therapy. J Nanobiotechnology. 2021;19:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Iranpour S, Bahrami AR, Nekooei S, Sh Saljooghi A, Matin MM. Improving anti-cancer drug delivery performance of magnetic mesoporous silica nanocarriers for more efficient colorectal cancer therapy. J Nanobiotechnology. 2021;19:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3308] [Article Influence: 551.3] [Reference Citation Analysis (6)] |
| 44. | Menges M, Hoehler T. Current strategies in systemic treatment of gastric cancer and cancer of the gastroesophageal junction. J Cancer Res Clin Oncol. 2009;135:29-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Sasaki Y, Nishina T, Yasui H, Goto M, Muro K, Tsuji A, Koizumi W, Toh Y, Hara T, Miyata Y. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105:812-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (4)] |
| 46. | Avgustinovich AV, Bakina OV, Afanas'ev SG, Cheremisina OV, Spirina LV, Dobrodeev AY, Buldakov M, Choynzonov EL. Nanoparticles in Gastric Cancer Management. Curr Pharm Des. 2021;27:2436-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Zhao Q, Cao L, Guan L, Bie L, Wang S, Xie B, Chen X, Shen X, Cao F. Immunotherapy for gastric cancer: dilemmas and prospect. Brief Funct Genomics. 2019;18:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 48. | Nakamura M, Ojima T, Katsuda M, Hayata K, Kitadani J, Nakamori M, Yamaue H. Phase 1 Study of Combined Chemotherapy of Nab-Paclitaxel, S-1, and Oxaliplatin for Gastric Cancer with Peritoneal Metastasis (NSOX Study). Oncology. 2021;99:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727-742. [PubMed] |
| 50. | Xu S, Cui F, Huang D, Zhang D, Zhu A, Sun X, Cao Y, Ding S, Wang Y, Gao E, Zhang F. PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. Int J Nanomedicine. 2019;14:17-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 51. | Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1871] [Article Influence: 170.1] [Reference Citation Analysis (0)] |
| 52. | Hu N, Li W, Hong Y, Zeng Z, Zhang J, Wu X, Zhou K, Wu F. A PD1 targeted nano-delivery system based on epigenetic alterations of T cell responses in the treatment of gastric cancer. Mol Ther Oncolytics. 2022;24:148-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, Sato E, Kuwata T, Kinoshita T, Yamamoto M, Nomura S, Tsukamoto T, Mano H, Shitara K, Nishikawa H. An Oncogenic Alteration Creates a Microenvironment that Promotes Tumor Progression by Conferring a Metabolic Advantage to Regulatory T Cells. Immunity. 2020;53:187-203.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 54. | Guo W, Chen Z, Li Z, Huang H, Ren Y, Zhao B, Li G, Hu Y. Improved immunotherapy for gastric cancer by nanocomposites with capability of triggering Dual-Damage of Nuclear/Mitochondrial DNA and cGAS/STING-Mediated innate immunity. Chem Eng J. 2022;443:136428. [DOI] [Full Text] |
| 55. | Pan Y, Yu Y, Wang X, Zhang T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol. 2020;11:583084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 1400] [Article Influence: 233.3] [Reference Citation Analysis (0)] |
| 56. | Cheng N, Bai X, Shu Y, Ahmad O, Shen P. Targeting tumor-associated macrophages as an antitumor strategy. Biochem Pharmacol. 2021;183:114354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 57. | Zhang J, Jiang M, Li S, Zhang Z, Sun H, Yang F, Liang H. Developing a Novel Anticancer Gold(III) Agent to Integrate Chemotherapy and Immunotherapy. J Med Chem. 2021;64:6777-6791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | Morse MA, Gwin WR 3rd, Mitchell DA. Vaccine Therapies for Cancer: Then and Now. Target Oncol. 2021;16:121-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 59. | Liu BY, Chen XH, Gu QL, Li JF, Yin HR, Zhu ZG, Lin YZ. Antitumor effects of vaccine consisting of dendritic cells pulsed with tumor RNA from gastric cancer. World J Gastroenterol. 2004;10:630-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Wu Y, Wang L, Zhang Y. Dendritic cells as vectors for immunotherapy of tumor and its application for gastric cancer therapy. Cell Mol Immunol. 2004;1:351-356. [PubMed] |
| 61. | Kohnepoushi C, Nejati V, Delirezh N, Biparva P. Poly Lactic-co-Glycolic Acid Nanoparticles Containing Human Gastric Tumor Lysates as Antigen Delivery Vehicles for Dendritic Cell-Based Antitumor Immunotherapy. Immunol Invest. 2019;48:794-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Banstola A, Jeong JH, Yook S. Immunoadjuvants for cancer immunotherapy: A review of recent developments. Acta Biomater. 2020;114:16-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 63. | Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 674] [Cited by in RCA: 651] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 64. | Zhang J, Zhao T, Han F, Hu Y, Li Y. Photothermal and gene therapy combined with immunotherapy to gastric cancer by the gold nanoshell-based system. J Nanobiotechnology. 2019;17:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 65. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 438] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 66. | Wang DX, Yang X, Lin JZ, Bai Y, Long JY, Yang XB, Seery S, Zhao HT. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: A retrospective, real-world study conducted in China. World J Gastroenterol. 2020;26:4465-4478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | Lou T, Li B, Xiong P, Jin C, Chen Y. External validation of hepatocellular carcinoma risk scores in patients with chronic hepatitis B virus infection in China. J Viral Hepat. 2021;28:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Qing X, Xu W, Zong J, Du X, Peng H, Zhang Y. Emerging treatment modalities for systemic therapy in hepatocellular carcinoma. Biomark Res. 2021;9:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2016;24:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 379] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 70. | Jia G, Han Y, An Y, Ding Y, He C, Wang X, Tang Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 553] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 71. | An Y, Yang R, Wang X, Han Y, Jia G, Hu C, Zhang Z, Liu D, Tang Q. Facile Assembly of Thermosensitive Liposomes for Active Targeting Imaging and Synergetic Chemo-/Magnetic Hyperthermia Therapy. Front Bioeng Biotechnol. 2021;9:691091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front Immunol. 2020;11:568759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 73. | Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou M-M, Matilla A, Tovoli F, Knox JJ, He AR, El-Rayes BF, Acosta-Rivera M, Neely J, Shen Y, Baccan C, Cruz CMD, Hsu C. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J Clin Oncol. 2019;37:4012-4012. [RCA] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 74. | Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, Draganov D, Edelson RL, Formenti SC, Fucikova J, Gabriele L, Gaipl US, Gameiro SR, Garg AD, Golden E, Han J, Harrington KJ, Hemminki A, Hodge JW, Hossain DMS, Illidge T, Karin M, Kaufman HL, Kepp O, Kroemer G, Lasarte JJ, Loi S, Lotze MT, Manic G, Merghoub T, Melcher AA, Mossman KL, Prosper F, Rekdal Ø, Rescigno M, Riganti C, Sistigu A, Smyth MJ, Spisek R, Stagg J, Strauss BE, Tang D, Tatsuno K, van Gool SW, Vandenabeele P, Yamazaki T, Zamarin D, Zitvogel L, Cesano A, Marincola FM. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 756] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 75. | Xu J, Zheng Q, Cheng X, Hu S, Zhang C, Zhou X, Sun P, Wang W, Su Z, Zou T, Song Z, Xia Y, Yi X, Gao Y. Chemo-photodynamic therapy with light-triggered disassembly of theranostic nanoplatform in combination with checkpoint blockade for immunotherapy of hepatocellular carcinoma. J Nanobiotechnology. 2021;19:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 76. | Chang F, Syrjänen S, Syrjänen K. Implications of the p53 tumor-suppressor gene in clinical oncology. J Clin Oncol. 1995;13:1009-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Harris CC. Structure and function of the p53 tumor suppressor gene: clues for rational cancer therapeutic strategies. J Natl Cancer Inst. 1996;88:1442-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 416] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 78. | Xu L, Pirollo KF, Chang EH. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J Control Release. 2001;74:115-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Cui Y, Guo G. Immunomodulatory Function of the Tumor Suppressor p53 in Host Immune Response and the Tumor Microenvironment. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 80. | Blagih J, Buck MD, Vousden KH. p53, cancer and the immune response. J Cell Sci. 2020;133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 232] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 81. | Bezzi M, Seitzer N, Ishikawa T, Reschke M, Chen M, Wang G, Mitchell C, Ng C, Katon J, Lunardi A, Signoretti S, Clohessy JG, Zhang J, Pandolfi PP. Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat Med. 2018;24:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 82. | Guo G, Yu M, Xiao W, Celis E, Cui Y. Local Activation of p53 in the Tumor Microenvironment Overcomes Immune Suppression and Enhances Antitumor Immunity. Cancer Res. 2017;77:2292-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 83. | Xiao Y, Chen J, Zhou H, Zeng X, Ruan Z, Pu Z, Jiang X, Matsui A, Zhu L, Amoozgar Z, Chen DS, Han X, Duda DG, Shi J. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat Commun. 2022;13:758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 84. | Li B, Zhang X, Wu Z, Chu T, Yang Z, Xu S, Wu S, Qie Y, Lu Z, Qi F, Hu M, Zhao G, Wei J, Zhao Y, Nie G, Meng H, Liu R, Li S. Reducing Postoperative Recurrence of Early-Stage Hepatocellular Carcinoma by a Wound-Targeted Nanodrug. Adv Sci (Weinh). 2022;9:e2200477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 85. | Yu Z, Guo J, Hu M, Gao Y, Huang L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano. 2020;14:4816-4828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 86. | Li Z, Wu T, Zheng B, Chen L. Individualized precision treatment: Targeting TAM in HCC. Cancer Lett. 2019;458:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 87. | Wang Y, Tiruthani K, Li S, Hu M, Zhong G, Tang Y, Roy S, Zhang L, Tan J, Liao C, Liu R. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv Mater. 2021;33:e2007603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 88. | Kabakov AE, Yakimova AO. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 89. | Manoochehri Khoshinani H, Afshar S, Najafi R. Hypoxia: A Double-Edged Sword in Cancer Therapy. Cancer Invest. 2016;34:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 90. | Boutilier AJ, Elsawa SF. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 1186] [Article Influence: 237.2] [Reference Citation Analysis (0)] |
| 91. | Dai X, Ruan J, Guo Y, Sun Z, Liu J, Bao X, Zhang H, Li Q, Ye C, Wang X, Zhao CX, Zhou F, Sheng J, Chen D, Zhao P. Enhanced radiotherapy efficacy and induced anti-tumor immunity in HCC by improving hypoxia microenvironment using oxygen microcapsules. Chem Eng J. 2021;422:130109. [DOI] [Full Text] |
| 92. | Yang M, Zhang C. The role of liver sinusoidal endothelial cells in cancer liver metastasis. Am J Cancer Res. 2021;11:1845-1860. [PubMed] |
| 93. | Yu Z, Guo J, Liu Y, Wang M, Liu Z, Gao Y, Huang L. Nano delivery of simvastatin targets liver sinusoidal endothelial cells to remodel tumor microenvironment for hepatocellular carcinoma. J Nanobiotechnology. 2022;20:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 94. | Kon E, Elia U, Peer D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr Opin Biotechnol. 2022;73:329-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 95. | Gebre MS, Brito LA, Tostanoski LH, Edwards DK, Carfi A, Barouch DH. Novel approaches for vaccine development. Cell. 2021;184:1589-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 96. | Huang H, Zhang C, Yang S, Xiao W, Zheng Q, Song X. The investigation of mRNA vaccines formulated in liposomes administrated in multiple routes against SARS-CoV-2. J Control Release. 2021;335:449-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 97. | Szebeni J, Storm G, Ljubimova JY, Castells M, Phillips EJ, Turjeman K, Barenholz Y, Crommelin DJA, Dobrovolskaia MA. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol. 2022;17:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 98. | Zhang Y, Xie F, Yin Y, Zhang Q, Jin H, Wu Y, Pang L, Li J, Gao J. Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles Against Hepatocellular Carcinoma. Int J Nanomedicine. 2021;16:1553-1564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 99. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15528] [Article Influence: 2588.0] [Reference Citation Analysis (6)] |
| 100. | Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for Colorectal Cancer Screening. Gastroenterology. 2020;158:418-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 472] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 101. | Sagaert X, Vanstapel A, Verbeek S. Tumor Heterogeneity in Colorectal Cancer: What Do We Know So Far? Pathobiology. 2018;85:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 102. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2937] [Article Influence: 326.3] [Reference Citation Analysis (7)] |
| 103. | Bai J, Chen H, Bai X. Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J Clin Lab Anal. 2021;35:e23810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 104. | Krasteva N, Georgieva M. Promising Therapeutic Strategies for Colorectal Cancer Treatment Based on Nanomaterials. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 105. | Liu H, Xu C, Meng M, Li S, Sheng S, Zhang S, Ni W, Tian H, Wang Q. Metal-organic framework-mediated multifunctional nanoparticles for combined chemo-photothermal therapy and enhanced immunotherapy against colorectal cancer. Acta Biomater. 2022;144:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 106. | Wangmo D, Premsrirut PK, Yuan C, Morris WS, Zhao X, Subramanian S. ACKR4 in Tumor Cells Regulates Dendritic Cell Migration to Tumor-Draining Lymph Nodes and T-Cell Priming. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Yuan Z, Fan G, Wu H, Liu C, Zhan Y, Qiu Y, Shou C, Gao F, Zhang J, Yin P, Xu K. Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles. Mol Ther. 2021;29:2931-2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 108. | Zhu Q, Sun F, Li T, Zhou M, Ye J, Ji A, Wang H, Ding C, Chen H, Xu Z, Yu H. Engineering Oxaliplatin Prodrug Nanoparticles for Second Near-Infrared Fluorescence Imaging-Guided Immunotherapy of Colorectal Cancer. Small. 2021;17:e2007882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Li J, Zhao M, Sun M, Wu S, Zhang H, Dai Y, Wang D. Multifunctional Nanoparticles Boost Cancer Immunotherapy Based on Modulating the Immunosuppressive Tumor Microenvironment. ACS Appl Mater Interfaces. 2020;12:50734-50747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 110. | Liang C, Zhang X, Yang M, Dong X. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv Mater. 2019;31:e1904197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 1128] [Article Influence: 161.1] [Reference Citation Analysis (0)] |
| 111. | Duan X, Chan C, Han W, Guo N, Weichselbaum RR, Lin W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat Commun. 2019;10:1899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 112. | Haegebaert RMS, Kempers M, Ceelen W, Lentacker I, Remaut K. Nanoparticle mediated targeting of toll-like receptors to treat colorectal cancer. Eur J Pharm Biopharm. 2022;172:16-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 113. | Bahmani B, Gong H, Luk BT, Haushalter KJ, DeTeresa E, Previti M, Zhou J, Gao W, Bui JD, Zhang L, Fang RH, Zhang J. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12:1999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 114. | Luo Y, Yang J, Yu J, Liu X, Yu C, Hu J, Shi H, Ma X. Long Non-coding RNAs: Emerging Roles in the Immunosuppressive Tumor Microenvironment. Front Oncol. 2020;10:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 115. | Huang D, Chen J, Yang L, Ouyang Q, Li J, Lao L, Zhao J, Liu J, Lu Y, Xing Y, Chen F, Su F, Yao H, Liu Q, Su S, Song E. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat Immunol. 2018;19:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 352] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 116. | Zheng Y, Tian X, Wang T, Xia X, Cao F, Tian J, Xu P, Ma J, Xu H, Wang S. Long noncoding RNA Pvt1 regulates the immunosuppression activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Mol Cancer. 2019;18:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 117. | Liu F, Dai Z, Cheng Q, Xu L, Huang L, Liu Z, Li X, Wang N, Wang G, Wang L, Wang Z. LncRNA-targeting bio-scaffold mediates triple immune effects for postoperative colorectal cancer immunotherapy. Biomaterials. 2022;284:121485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 118. | Tilg H, Adolph TE, Gerner RR, Moschen AR. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell. 2018;33:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 582] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 119. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 2059] [Article Influence: 171.6] [Reference Citation Analysis (1)] |
| 120. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 2053] [Article Influence: 157.9] [Reference Citation Analysis (1)] |
| 121. | Chen T, Li Q, Wu J, Wu Y, Peng W, Li H, Wang J, Tang X, Peng Y, Fu X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol Immunother. 2018;67:1635-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 122. | Dong X, Pan P, Zheng DW, Bao P, Zeng X, Zhang XZ. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020;6:eaba1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 123. | Zhang Y, Ma S, Liu X, Xu Y, Zhao J, Si X, Li H, Huang Z, Wang Z, Tang Z, Song W, Chen X. Supramolecular Assembled Programmable Nanomedicine As In Situ Cancer Vaccine for Cancer Immunotherapy. Adv Mater. 2021;33:e2007293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 124. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 476] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 125. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 862] [Article Influence: 172.4] [Reference Citation Analysis (0)] |
| 126. | Zhao Z, Liu W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol Cancer Res Treat. 2020;19:1533033820962117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 127. | Nishida T, Yoshitomi H, Takano S, Kagawa S, Shimizu H, Ohtsuka M, Kato A, Furukawa K, Miyazaki M. Low Stromal Area and High Stromal Microvessel Density Predict Poor Prognosis in Pancreatic Cancer. Pancreas. 2016;45:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao HL, Li H, Zhang SR, Xu JZ, Qi ZH, Ni QX, Yu XJ, Liu L. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019;22:15-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 129. | Selvanesan BC, Meena K, Beck A, Meheus L, Lara O, Rooman I, Gravekamp C. Nicotinamide combined with gemcitabine is an immunomodulatory therapy that restrains pancreatic cancer in mice. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Rocha FG. Landmark Series: Immunotherapy and Targeted Therapy for Pancreatic Cancer. Ann Surg Oncol. 2021;28:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E, Ziogas D, Psyrri A, Karamouzis MV. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat Rev. 2020;86:102016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (1)] |
| 132. | Jia M, Zhang D, Zhang C, Li C. Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy. J Nanobiotechnology. 2021;19:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 133. | Wang Y, Gao Z, Du X, Chen S, Zhang W, Wang J, Li H, He X, Cao J. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8:5121-5132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 134. | Yu Q, Tang X, Zhao W, Qiu Y, He J, Wan D, Li J, Wang X, He X, Liu Y, Li M, Zhang Z, He Q. Mild hyperthermia promotes immune checkpoint blockade-based immunotherapy against metastatic pancreatic cancer using size-adjustable nanoparticles. Acta Biomater. 2021;133:244-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 135. | Wang C, Shi X, Song H, Zhang C, Wang X, Huang P, Dong A, Zhang Y, Kong D, Wang W. Polymer-lipid hybrid nanovesicle-enabled combination of immunogenic chemotherapy and RNAi-mediated PD-L1 knockdown elicits antitumor immunity against melanoma. Biomaterials. 2021;268:120579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 136. | Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2117] [Cited by in RCA: 2531] [Article Influence: 180.8] [Reference Citation Analysis (0)] |
| 137. | Allavena P, Palmioli A, Avigni R, Sironi M, La Ferla B, Maeda A. PLGA Based Nanoparticles for the Monocyte-Mediated Anti-Tumor Drug Delivery System. J Biomed Nanotechnol. 2020;16:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 138. | Jung JY, Ryu HJ, Lee SH, Kim DY, Kim MJ, Lee EJ, Ryu YM, Kim SY, Kim KP, Choi EY, Ahn HJ, Chang S. siRNA Nanoparticle Targeting PD-L1 Activates Tumor Immunity and Abrogates Pancreatic Cancer Growth in Humanized Preclinical Model. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 139. | Lorkowski ME, Atukorale PU, Bielecki PA, Tong KH, Covarrubias G, Zhang Y, Loutrianakis G, Moon TJ, Santulli AR, Becicka WM, Karathanasis E. Immunostimulatory nanoparticle incorporating two immune agonists for the treatment of pancreatic tumors. J Control Release. 2021;330:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 140. | Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 227] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 141. | Long Y, Lu Z, Xu S, Li M, Wang X, Zhang Z, He Q. Self-Delivery Micellar Nanoparticles Prevent Premetastatic Niche Formation by Interfering with the Early Recruitment and Vascular Destruction of Granulocytic Myeloid-Derived Suppressor Cells. Nano Lett. 2020;20:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 142. | Lu Z, Long Y, Wang Y, Wang X, Xia C, Li M, Zhang Z, He Q. Phenylboronic acid modified nanoparticles simultaneously target pancreatic cancer and its metastasis and alleviate immunosuppression. Eur J Pharm Biopharm. 2021;165:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 143. | Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 996] [Cited by in RCA: 1646] [Article Influence: 329.2] [Reference Citation Analysis (0)] |
| 144. | Li J, Anraku Y, Kataoka K. Self-Boosting Catalytic Nanoreactors Integrated with Triggerable Crosslinking Membrane Networks for Initiation of Immunogenic Cell Death by Pyroptosis. Angew Chem Int Ed Engl. 2020;59:13526-13530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 145. | Liu Y, Zhen W, Wang Y, Song S, Zhang H. Na2S2O8 Nanoparticles Trigger Antitumor Immunotherapy through Reactive Oxygen Species Storm and Surge of Tumor Osmolarity. J Am Chem Soc. 2020;142:21751-21757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 146. | Zhao P, Wang M, Chen M, Chen Z, Peng X, Zhou F, Song J, Qu J. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials. 2020;254:120142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 147. | Fan JX, Deng RH, Wang H, Liu XH, Wang XN, Qin R, Jin X, Lei TR, Zheng D, Zhou PH, Sun Y, Zhang XZ. Epigenetics-Based Tumor Cells Pyroptosis for Enhancing the Immunological Effect of Chemotherapeutic Nanocarriers. Nano Lett. 2019;19:8049-8058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 148. | Ding B, Sheng J, Zheng P, Li C, Li D, Cheng Z, Ma P, Lin J. Biodegradable Upconversion Nanoparticles Induce Pyroptosis for Cancer Immunotherapy. Nano Lett. 2021;21:8281-8289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 149. | D'Errico G, Alonso-Nocelo M, Vallespinos M, Hermann PC, Alcalá S, García CP, Martin-Hijano L, Valle S, Earl J, Cassiano C, Lombardia L, Feliu J, Monti MC, Seufferlein T, García-Bermejo L, Martinelli P, Carrato A, Sainz B Jr. Tumor-associated macrophage-secreted 14-3-3ζ signals via AXL to promote pancreatic cancer chemoresistance. Oncogene. 2019;38:5469-5485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 150. | Bulle A, Dekervel J, Deschuttere L, Nittner D, Libbrecht L, Janky R, Plaisance S, Topal B, Coosemans A, Lambrechts D, Van Cutsem E, Verslype C, van Pelt J. Gemcitabine Recruits M2-Type Tumor-Associated Macrophages into the Stroma of Pancreatic Cancer. Transl Oncol. 2020;13:100743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 151. | Wang M, Hu Q, Huang J, Zhao X, Shao S, Zhang F, Yao Z, Ping Y, Liang T. Engineered a dual-targeting biomimetic nanomedicine for pancreatic cancer chemoimmunotherapy. J Nanobiotechnology. 2022;20:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 152. | Cao S, Saw PE, Shen Q, Li R, Liu Y, Xu X. Reduction-responsive RNAi nanoplatform to reprogram tumor lipid metabolism and repolarize macrophage for combination pancreatic cancer therapy. Biomaterials. 2022;280:121264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 153. | Wang M, Li Y, Wang M, Liu K, Hoover AR, Li M, Towner RA, Mukherjee P, Zhou F, Qu J, Chen WR. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2022;138:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 154. | Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1233] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manojlovic N, Serbia; Serban ED, Romania S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ