Published online Sep 28, 2022. doi: 10.3748/wjg.v28.i36.5313
Peer-review started: July 14, 2022
First decision: July 31, 2022
Revised: August 11, 2022
Accepted: September 9, 2022
Article in press: September 9, 2022
Published online: September 28, 2022
Processing time: 70 Days and 21.4 Hours
Magnetic compression anastomosis (MCA) is a novel suture-free reconstruction of the digestive tract. It has been used in gastrointestinal anastomosis, jejunal anastomosis, cholangioenteric anastomosis and so on. The traditional operative outcomes of congenital esophageal atresia and benign esophageal stricture are poor, and there are too many complications postoperatively.
To test MCA technology to reconstruct the esophagus in dogs, prior to studying the feasibility and safety of MCA in humans.
Thirty-six dogs were randomized into either the study or control group (n = 18 per group). The dogs in the study group were subjected to end-to-end esophageal anastomosis with the magnetic compression device, while those in the control group underwent hand-sewn anastomosis with 4-0 absorbable multifilament Vicryl. We used interrupted single-layer inverting sutures. The anastomosis time, gross appearance, weight and pathology of the anastomosis were evaluated at one month, three months and six months postoperatively.
The anastomosis time of the MCA group was shorter than that of the hand-sewn group (7.5 ± 1.0 min vs 12.5 ± 1.8 min, P < 0.01). In the MCA group, X-ray examination was performed every day to locate the magnetic device in the esophagus before the magnetic device fell off from the esophagus. In the hand-sewn group, dogs did not undergo X-ray examination. One month after the surgeries, the mean weight of the dogs in the hand-sewn group had decreased more than that of the dogs in the MCA group (11.63 ± 0.71 kg vs 12.73 ± 0.80 kg, P < 0.05). At 3 mo and 6 mo after the operation, the dogs’ weights were similar between the two groups (13.75 ± 0.84 kg vs 14.03 ± 0.82 kg, 14.93 ± 0.80 kg vs 15.44 ± 0.47 kg). The number of inflammatory cells in MCA group was lower than that in hand-sewn group on 1 mo after operation.
MCA is an effective and safe method for esophageal reconstruction. The anastomosis time of the MCA group was less than that of the hand-sewn group. This study shows that MCA technology may be applied to human esophageal reconstruction, provided these favorable results are confirmed by more publications.
Core Tip: We used magnetic compression anastomosis (MCA) technology to reconstruct the esophagus. The anastomosis time of the MCA group was shorter than that of the hand-sewn group (7.5 ± 1.0 min vs 12.5 ± 1.8 min, P < 0.01). One month after the surgeries, the mean weight of the dogs in the hand-sewn group had decreased more than that of the dogs in the MCA group (11.63 ± 0.71 kg vs 12.73 ± 0.80 kg, P < 0.05). At 3 mo and 6 mo after the operation, the dogs’ weights were similar between the two groups. MCA is an effective and safe method for esophageal reconstruction. This study shows that MCA technology may be applied to human esophageal reconstruction, provided these favorable results are confirmed by more publications.
- Citation: Xu XH, Lv Y, Liu SQ, Cui XH, Suo RY. Esophageal magnetic compression anastomosis in dogs. World J Gastroenterol 2022; 28(36): 5313-5323
- URL: https://www.wjgnet.com/1007-9327/full/v28/i36/5313.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i36.5313
Esophageal atresia (EA) is a serious and fatal gastrointestinal developmental malformation in the neonatal period. It has a worldwide prevalence of 2.4 to 3.8 per 10000 newborns[1-4]. Because of the development of surgical and good neonatal intensive care, the survival is about 90% in those born with EA with severe associated anomalies and is even higher in those born with EA alone[5]. However, the postoperative complications of traditional hand-sewn anastomosis are numerous, and the therapeutic effect is not good. The primary complications during the postoperative period are leakage (incidence 15%-20%), stenosis of the anastomosis (30%-40%), gastroesophageal reflux (40%-65%), esophageal dysmotility, fistula recurrence, scoliosis, deformities of the thoracic wall and respiratory disorders[6-8]. After surgery, children suffer from various complications, which seriously affect their development and quality of life.
Magnetic compression anastomosis (MCA) is a novel suture-free reconstruction of the digestive tract. Since Kanshin et al[9] reported using a magnetic device to anastomose gastroduodenal and cecojejunal in dogs in 1978, there have been many reports of this technique using animal experiments and regarding its clinical applications. At present, clinically, it has been used in gastrointestinal anastomosis[10,11], jejunal anastomosis[12,13], cholangioenteric anastomosis[14-16] and so on. Fourteen patients underwent MCA for gastrointestinal anastomosis, and the technical success of MCA was achieved in 100% of the cases. Two patients underwent anastomotic restenosis, and 1 patient had an anastomotic perforation due to balloon dilatation to prevent restenosis. Fifteen patients underwent MCA for jejunal anastomosis, of which five patients had severe systemic disease and underwent complex open urinary reconstruction procedures. The device was successfully placed and effectively formed a side-to-side, functional end-to-end jejunal anastomosis. Forty-seven patients underwent MCA for cholangioenteric anastomosis. Thirty-eight patients had a malignant primary disease, while nine had benign disease. With a median follow-up of 547.5 d (range 223-1042 d), no patients had biliary fistula, while two developed anastomotic stricture at 4 mo and 14 mo after surgery. Magnetic materials have noncontact suction characteristics that will greatly simplify the process of gastrointestinal anastomosis, especially in the case of gastrointestinal stenosis or atresia. Recently, Kamada et al[10] reported that MCA without general anesthesia is a valuable alternative to surgery for gastrointestinal obstruction. Some patients with biliary obstruction are not eligible for conventional endoscopic procedures or are unsuitable for surgery. The MCA technique is an available method for performing choledochocholedochostomy and choledochoenterostomy interventionally[16]. The mean age of the patients in these two reports was above 60 years. Therefore, MCA technology can be designed as a minimally invasive technique and is tolerated by elderly patients.
Because of the many complications of traditional operations and the ability to obtain minimally invasive surgery by MCA, MCA could be a superior method to treat congenital EA. There have been several clinical reports on esophageal reconstruction using MCA[17-19]. Muensterer et al[17] reported that they used esophageal MCA for staged EA repair in 3 patients (Gross type A, B, and C) at high risk for conventional surgical repair. Surgeries were all successful, and there were no perioperative complications. Liu et al[18] reported that two patients who had severe stricture after simultaneous EA and duodenal obstruction repair underwent magnetic compression stricturoplasty. Magnetic compression stricturoplasty successfully established the patency of the esophagus in these two patients with refractory EA stricture. These two cases required multiple additional procedures, but durable esophageal patency with absence of dysphagia was achieved at 15 or 10 mo after magnetic compression stricturoplasty. Dorman et al[19] reported a case in which EA was repaired with a proximal fistula using endoscopic MCA after staged lengthening. Magnetic coupling occurred at 4 d, and after magnet removal at 13 d, an esophagram demonstrated a 10 French channel without leakage. A series of studies on MCA technology have been carried out in our laboratory. The technology has been used in choledochojejunostomy, rectovaginal fistula repair, laparoscopic pancreatoduodenectomy (LPD) and liver transplantation. Twenty-six mongrel dogs underwent choledochojejunostomy magnamosis with different magnetic pressure magnets. The surgical procedures were all successful[20]. There was a comparative study with 12 pigs for rectovaginal fistula repair. Eight animals were in the MCA group, and four animals were in the hand-sewn group. The rectovaginal fistula site was smooth, and healing was complete. This technology was used in one case clinically and achieved success[21]. Seven patients received MCA technology in LPD. LPD was successfully completed in all seven patients, of which seven underwent laparoscopic magnetic compression choledochojejunostomy and two received laparoscopic magnetic compression pancreatojejunostomy. No leakages were observed after the operation. After a median follow-up period of 11 mo (range 4-18 mo), there was no incidence of anastomotic stricture[22]. We also used MCA to reconstruct vessels in liver transplantation. In pig liver transplantation, we used MCA to reconstruct the suprahepatic vena cava, infrahepatic vena cava and portal vein. They were all successful, and the pig lived for over one month[23]. Currently, we want to use MCA technology to treat EA, but there is a paucity of published research data and animal studies. Therefore, the purpose of this study was to study the difference between MCA and hand-sewn anastomosis of the esophagus in dogs.
The protocol of the animal study was reviewed and approved by the committee for Ethics of Animal Experiments of Xi’an Jiaotong University (No. XJTULAC2020-1441). Thirty-six mongrel dogs (male = 18, female = 18) with body weights of 10-15 kg were provided by the Laboratory Animal Center of Xi’an Jiaotong University. Dogs were acclimatized to laboratory conditions (23 °C, ad libitum access to food and water) for 2 wk prior to experimentation. In this study, we selected mongrel dogs because the esophagus in the neck is long enough for reconstruction. This purpose is to avoid performing surgery in the thoracic cavity and to improve the survival rate.
Thirty-six dogs were randomized into either the MCA group or the hand-sewn group, n = 18 per group, with the same male to female ratio. The dogs in the MCA group were subjected to end-to-end esophageal anastomosis with the magnetic device, while those in the hand-sewn group underwent hand-sewn anastomosis with 4-0 absorbable multifilament Vicryl. We used interrupted single-layer sutures. In both groups, there was no surgical left in place. Postoperative complications and weight were observed. The anastomosis time, gross appearance and pathology of the anastomoses were evaluated at one month, three months and six months after the operation.
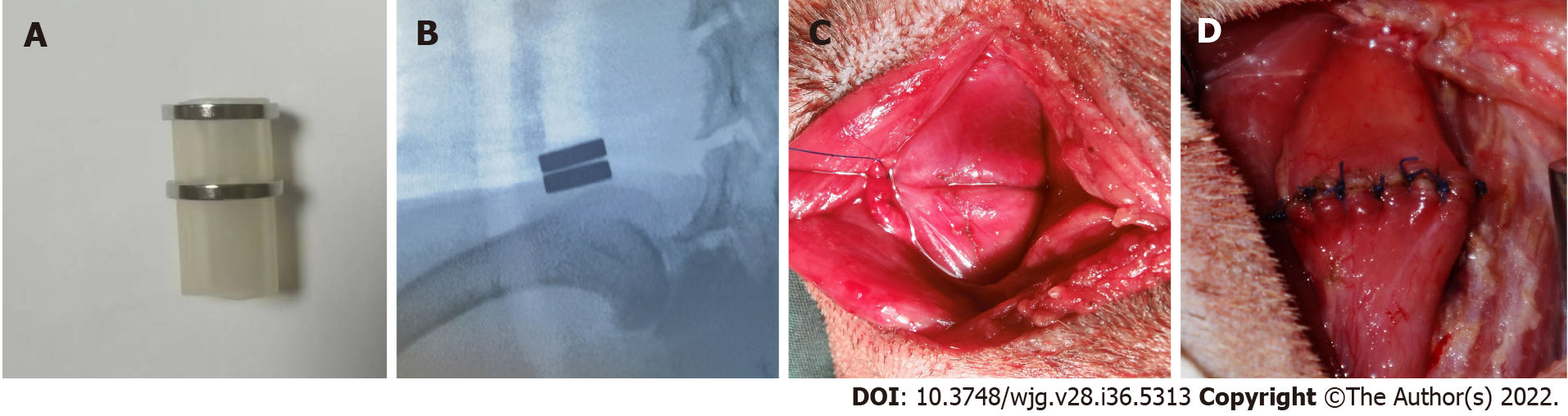
The magnetic anastomosis device used in the study possessed a parent magnetic ring (PMR) and daughter magnetic ring (DMR), which were made of rare earth neodymium iron boron (NdFeB, N40) and plated with nickel from Northwest Institute for Nonferrous Metal Research, Xi’an, China. A special drainage tube was placed between the PMR and DMR, and both the PMR and DMR had outer diameters of 20 mm, inner diameters of 14 mm, and thicknesses of 2 mm. The magnetic force between the PMR and DMR is 30 Newton at zero distance with a magnetic density of 8000 GS.
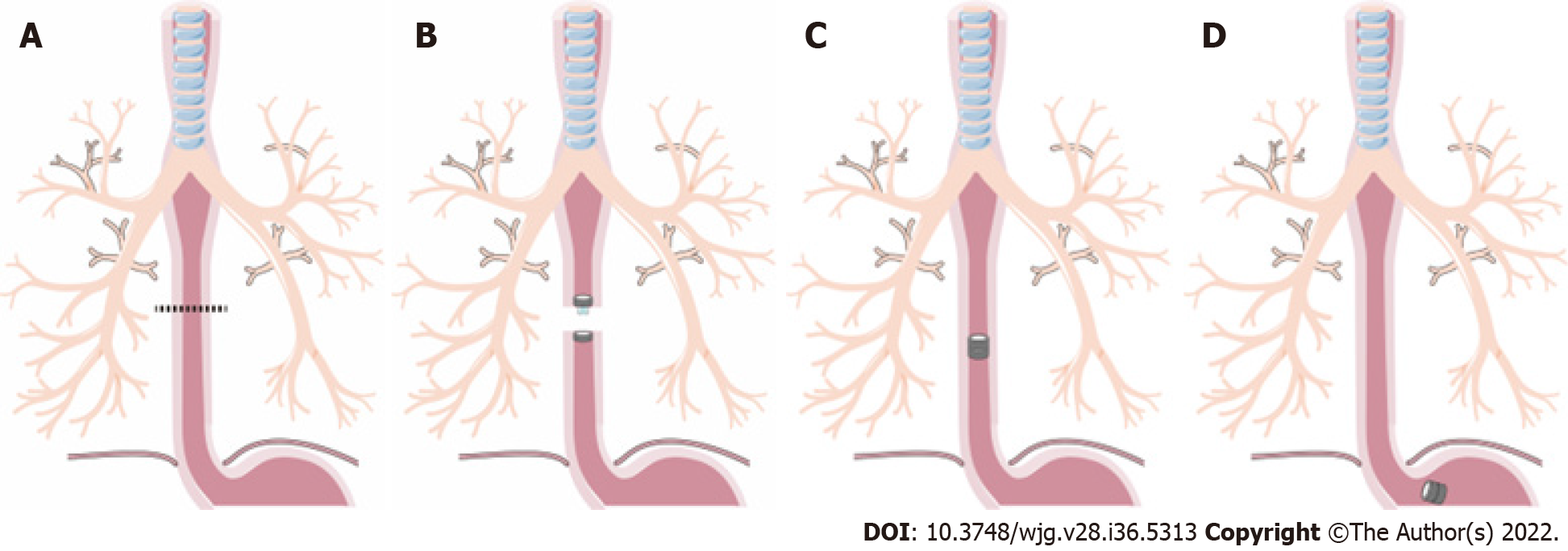
The dogs were first anesthetized with an intraperitoneal injection of 30 mg/kg pentobarbital sodium solution. The dogs were anesthetized and then placed in the right lateral position on a temperature-controlled operating table with their cervical region shaved and sterilized. The experimental process is shown in Figure 1. A 5 cm incision was made along the anterior edge of the sternocleidomastoid muscle in the neck. Then, the muscle was separated layer by layer, and a 3 cm long portion of the esophagus was found. Next, the esophagus was transected.
In the MCA group, the PMR and DMR were placed in the upper and lower esophagus, respectively, after transecting the esophagus (Figure 1A). The magnetic anastomosis device was shown in Figure 2A. The upper and lower ends of the esophageal anastomosis were sutured with 4-0 silk thread and tightened to the position of the special drainage tube. Under the action of attraction, the magnetic rings at both ends are attracted to each other automatically and press the anastomotic tissue together, and the esophageal anastomosis is then completed (Figure 2C). When the anastomotic tissue is necrotic and falls off, the magnetic device is discharged into the stomach with the digesta and is discharged out of the body. In the control group, the operation was the same as that in the study group, except that end-to-end anastomosis was performed with 4-0 absorbable sutures (Figure 2D).
An X-ray (Perlove Medical Equipment incorporated company, Nanjing, China) was performed on the dogs in the study group to confirm the target location and precise mating of the magnetic compression rings (Figure 2B). X-ray was performed every day before the magnets fell off the esophagus. Postoperatively, every dog was kept in a cage. After the surgery, the dogs in the two groups were fasted for two days and then started on enteral nutrition. However, in the hand-sewn group, dogs generally showed poor appetite and even refused to eat. They started enteral nutrition at 4 d, 5 d, or even longer. During this period, we can only keep them alive with parenteral nutrition. The dogs in the MCA group started enteral nutrition earlier than those in the hand-sewn group. All dogs in both groups were given cefazolin sodium intramuscularly for 3 d postoperatively to prevent infection. Intramuscular injection (0.5 g) was administered twice a day. After a week, dogs were given enteral nutrition, a liquid diet, a semiliquid diet, and a normal diet according to their recovery. Six dogs were randomly selected in each group at 1 mo, 3 mo and 6 mo after the operation. They were weighed and intravenously injected with an overdose of anesthetic.
All anastomosis segments with a sufficient length were harvested. The anastomotic stoma was found and severed 2 cm above and below the anastomosis. The gross information was observed, and all samples were immersed in 10% buffered formalin overnight. The samples were embedded in paraffin after fixation. Four-micrometer-thick sections were cut at the anastomosis site. Sections were stained with hematoxylin and eosin and Masson’s trichrome dye. Then, we observed the changes in the tissue and cells under a light microscope.
Data were analyzed using GraphPad Prism v8 software (GraphPad, La Jolla, CA, United States). Quantitative data are expressed as the mean ± SD, and differences between the two groups were analyzed with the independent samples t test. The anastomosis time and weight data conformed to a normal distribution and homogeneity of variance. Statistical significance was set at P < 0.05.
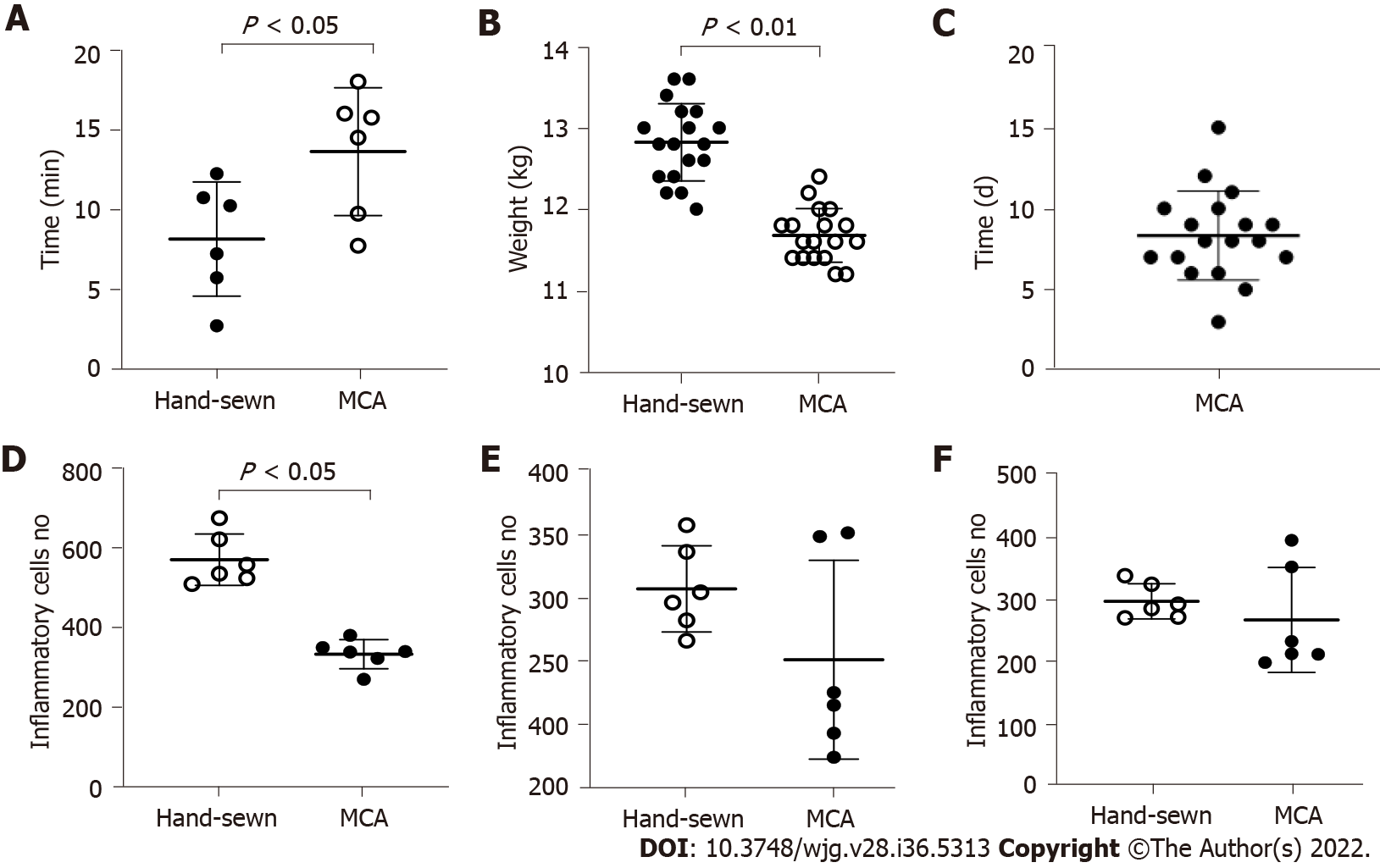
Anastomosis was successfully created in all dogs. The anastomosis time of the MCA group was shorter than that of the hand-sewn group (7.5 ± 1.0 vs 12.5 ± 1.8, P < 0.01) (Figure 3A). X-ray examinations showed that the esophagi were all unobstructed and that the contrast agent could pass through the anastomosis in the MCA group. All dogs survived. There was one dog in the hand-sewn group and one dog in the MCA group with stenosis after the operation. These were found when we obtained anastomotic stoma tissue. We observed one dog in the hand-sewn group who suddenly refused to eat, and the incision skin was red, swollen and purulent 8 d after the operation. We performed surgical exploration and performed a second esophageal reconstruction.
We observed how long the magnetic rings took to fall off into the stomach. The mean time was 8.3 ± 2.7 d (range 3-15 d) (Figure 3C). The weights of dogs in the MCA group (13.87 ± 0.63 kg) and the hand-sewn group (13.98 ± 0.80 kg) were similar at the beginning of the experiment. The difference in weight between the two groups was not statistically significant. However, the weights decreased more in the hand-sewn group than in the MCA group 1 mo after the operation. The mean weight of the MCA group was 12.73 ± 0.80 kg, while the mean weight of the hand-sewn group was 11.63 ± 0.71 kg, P < 0.05. At 3 mo and 6 mo after the operation, the dog weights were similar between the two groups (13.75 ± 0.84 kg vs 14.03 ± 0.82 kg, 14.93 ± 0.80 kg vs 15.44 ± 0.47 kg).
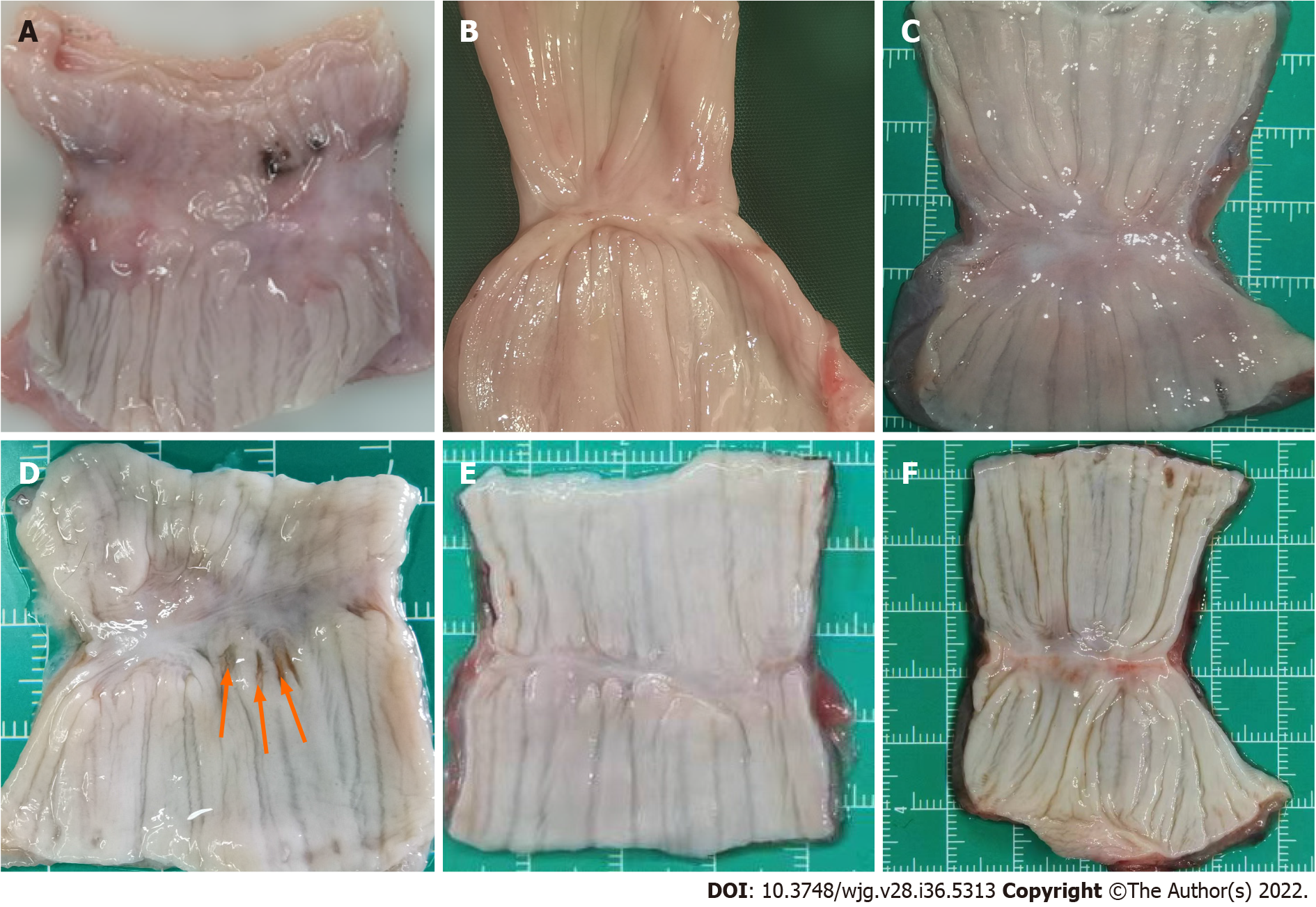
The esophagi grew well, and there were no ulcerations or fistulas. The mucosa layers were intact. However, the anastomotic stomas were slightly thinner than the peripheral tissue at 1 mo. We could see that the muscle layer tissues were not completely covered. In the hand-sewn anastomosis group, the suture plots were still visible 1 mo after the operation (Figure 4D). With the passage of time, the submucosa and muscle layers were gradually covered, and the anastomoses became increasingly smooth and flat. However, at 1 mo, 3 mo and 6 mo, the anastomoses of the MCA group were smoother than those of the hand-sewn group.
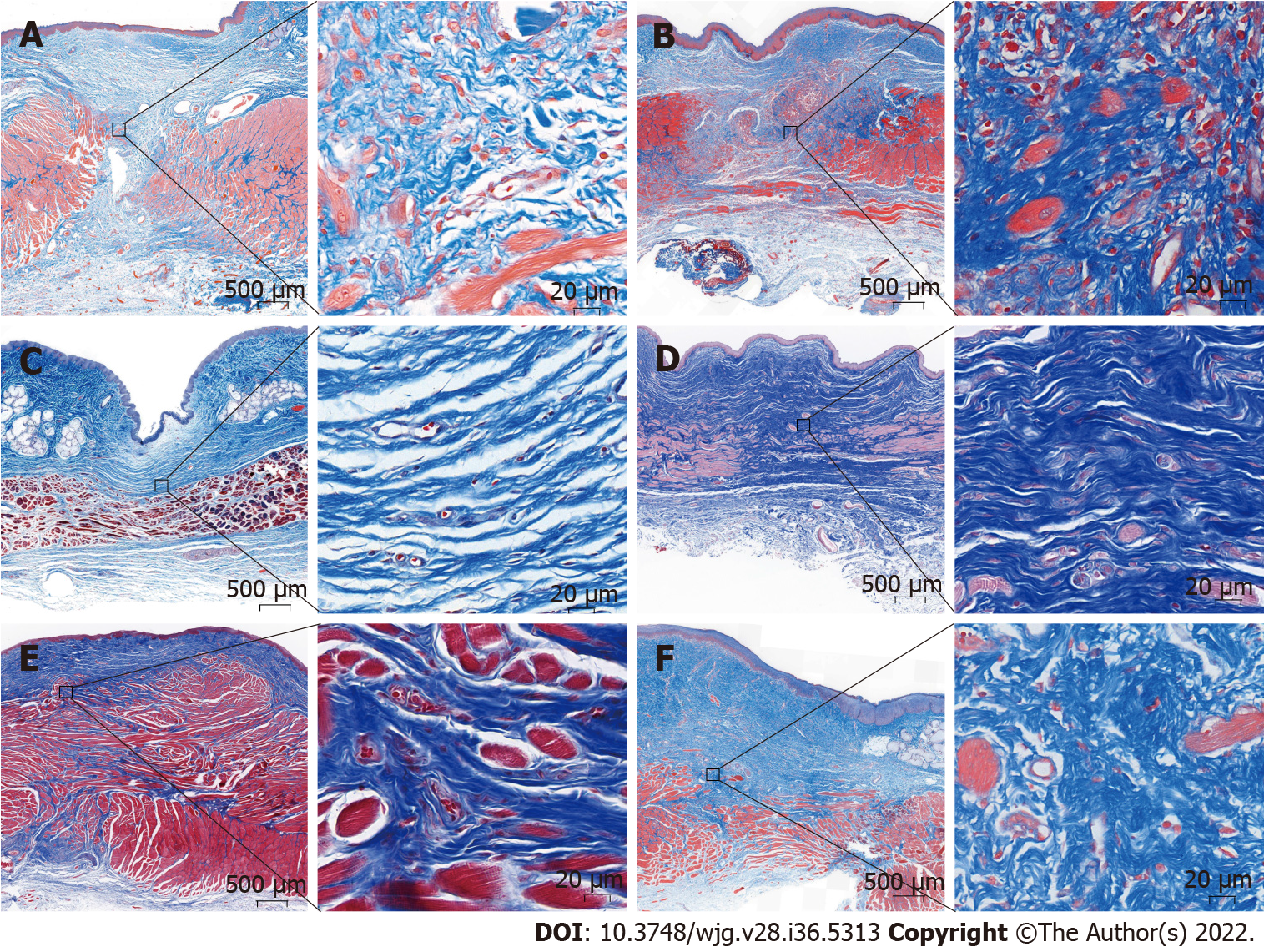
Light microscopy showed that the anastomotic mucosa of the MCA group had grown well during the first month after surgery, while the submucosal and muscular layers were still fractured (Figure 5A). Three months after the operation, the submucosal and muscular layers had completely covered the anastomoses, and the anastomotic tissue was similar to the normal esophageal tissue under light microscopy (Figure 5C). In the hand-sewn group, the mucosa was continuous one month after surgery, but the submucosa and muscle layers were still broken, similar to the findings in the MCA group at one month (Figure 5B). At 3 mo postoperatively, the anastomotic site was covered by the submucosa and muscularis (Figure 5D). The number of inflammatory cells in the hand-sewn group was greater than that in the MCA group at 1 mo (Figures 5A and B). We counted the number of inflammatory cells in one high-power field (40 ×) in both groups (334 ± 37 vs 572 ± 65, P < 0.01). At 3 mo and 6 mo, the number of inflammatory cells was less than that at 1 mo. Between the two groups, the number of inflammatory cells was equal at 3 mo and 6 mo (Figures 5C-F). Masson staining showed that the number of collagen fibers in the hand-sewn group was greater than that in the MCA group. The blue fibers are collagen fibers (Figures 6A-F).
We studied the tissue status of the anastomotic esophagus after esophageal reconstruction by magnetic anastomosis technology and clarified the safety of MCA surgery. A comparative study was conducted between magnetic anastomosis and hand-sewn anastomosis for esophageal reconstruction to determine whether the effects of MCA were superior to those of the hand-sewn method.
By observing gross specimens and tissue sections at different time periods in the MCA and hand-sewn groups, we found that in both the MCA group and the hand-sewn anastomosis group, the anastomotic tissues were not completely healed at 1 mo after surgery. The mucosal surface of the two groups was smooth without ischemia or necrosis, while some submucosa and muscularis were still missing. After 3 mo, the anastomotic sites of the two groups had healed well, and the mucosal layer, submucosal layer and muscular layer were continuous. Liquid food is still the main diet in the early stage to reduce the stimulation of esophageal mucosa and prevent the occurrence of ulcers. Based on the results of the experiment, we suggest that MCA patients should consume a liquid diet or a semiliquid diet for at least one month.
The tissue healing of the MCA group was faster and better than that of the hand-sewn group. Six months after the operation, the specimens of the MCA group were smoother than those in the hand-sewn group, and a slight amount of scar tissue was observed in the hand-sewn group. There are several reasons for this. Magnetic anastomosis technology applies the mutual attraction between magnetic rings to maintain a constant and balanced pressure on the tissues at the anastomosis, resulting in slow ischemic necrosis of the tissues between the magnetic rings and the growth and healing of the surrounding tissues. This is a slow process that allows time for esophageal compensation to grow. Hand-sewn sutures use Vicryl to quickly tighten the tissues at both ends of the esophagus. There is a certain tension at both ends of the esophagus, which may lead to shearing forces placed on the esophageal tissue from Vicryl. Second, Vicryl, as an absorbable thread, will exist in the tissue for a long time as a foreign body, which may cause infection and scar hyperplasia. The suture material could play an important role in the healing of esophageal anastomosis. Vicryl is an absorbable material, but the time of absorption is more than 1 mo. During this period, Vicryl may act as a foreign body, leading to foreign body granuloma or anastomosis edema. Braided sutures are more prone to infection than monofilament sutures. Vicryl is the braided suture and perhaps in this study Vicryl is not the best selection.
At six months postoperatively, the magnetic anastomosis group grew slightly better than the hand-sewn group. Before surgery, the weights of the dogs in both groups were the same. One month after surgery, the body weight of the hand-sewn group was significantly lower than that of the magnetic anastomosis group. Three months after surgery, the weights of the dogs were similar in both groups. This suggests that MCA may contribute to enhanced recovery after esophageal reconstruction. There are two reasons that may contribute to the enhanced recovery with MCA technology: (1) The magnetic device has a gap in the middle, through which the liquid can enter the stomach, while it will not contact the anastomotic tissue. It may relieve pain and reduce the incidence of infection; and (2) The shedding time of the magnetic device is shorter than the time of the suture being discharged or absorbed by the tissues. The absorbable time of Vicryl RapideR is approximately 40 d[24], while the magnetic device discharge time is approximately 8 d. We observed that the dogs in the MCA group could feed earlier than those in the hand-sewn group. The MCA group of dogs recovered faster and better than the dogs in the hand-sewn group.
During 6 mo of observation, there was 1 stenosis in the MCA group and 1 stenosis and 1 Leakage in the hand-sewn group. Because the sample size was small, there was no statistically significant difference between the two groups. The rate of stenosis and leakage of anastomosis using the traditional hand-sewn operation is high. This has seriously affected the postoperative growth of children. We hope that this novel MCA technology can improve EA. The complication rate needs to be verified by more animal experiments and clinical case reports.
One clinical report showed that anastomosis was achieved in an average of 6 d (range 3 to 7 d) in 5 patients (age 6 mo to 5.9 years) with severe recurrent postsurgical esophageal stenosis refractory to dilatation[25]. Another MCA was achieved on day 36[26]. Clinical reports have shown that the anastomosis time ranges from 3 d to 36 d[26-28]. It involves many factors, such as the age of the child, type of EA, size of the magnetic ring and field strength of the magnetic device. This will be our next experimental purpose. Based on a large amount of experimental and clinical data, a model was established to estimate various parameters of magnetic devices preoperatively.
There are also some limitations of the study. First, our surgery location was on the dog’s neck, not in the thoracic cavity. This is not consistent with the clinicopathology. The position of EA is generally flush with the bifurcation of the main bronchus and may have a tracheoesophageal fistula. Second, in this study, we did not consider the effect of different structural sutures on the anastomosis. Does monofilament have less effect on anastomotic healing than multifilament? Third, this is a model of esophageal anastomosis, and not of anastomosis in the setting of an atresia, there are several differences.
MCA is an effective and safe method for esophageal reconstruction in dogs. The anastomosis with MCA is faster than the hand-sewn anastomosis. Postoperatively, some aspects of the recovery of the MCA group were faster and better than those of the hand-sewn group. We provide some information useful for the future clinical application of the device in selected cases.
Magnetic compression anastomosis (MCA) is a novel suture-free reconstruction of the digestive tract. It has been used in gastrointestinal anastomosis, jejunal anastomosis, cholangioenteric anastomosis and so on. The traditional operative outcomes of congenital esophageal atresia and benign esophageal stricture are poor, and there are too many complications postoperatively.
There are several case reports of using MCA to treat esophageal stenosis. However, systematic animal experimental studies are scarce. This has restricted further clinical application of MCA.
This study was conducted to demonstrate the feasibility and safety of MCA for esophageal reconstruction and studied the difference between MCA and hand-sewn esophageal reconstruction.
Thirty-six dogs were randomized into either the study or control group (n = 18 per group). The dogs in the study group were subjected to end-to-end esophageal anastomosis with the magnetic compression device, while those in the control group underwent hand-sewn anastomosis with 4-0 absorbable multifilament Vicryl. We used interrupted single-layer sutures. The anastomosis time, gross appearance, weight and pathology of the anastomosis were evaluated at one month, three months and six months postoperatively.
The anastomosis time of the MCA group was shorter than that of the hand-sewn group (7.5 ± 1.0 min vs 12.5 ± 1.8 min, P < 0.01). One month after the surgeries, the mean weight of the dogs in the hand-sewn group had decreased more than that of the dogs in the MCA group (11.63 ± 0.71 kg vs 12.73 ± 0.80 kg, P < 0.05). At 3 mo and 6 mo after the operation, the dogs’ weights were similar between the two groups (13.75 ± 0.84 kg vs 14.03 ± 0.82 kg, 14.93 ± 0.80 kg vs 15.44 ± 0.47 kg). Under an optical microscope, the number of inflammatory cells in MCA group was lower than that in hand-sewn group on 1 mo after operation.
MCA is an effective and safe method for esophageal reconstruction. The anastomosis time of the MCA was less than that of the hand-sewn group. This study shows that MCA technology may be applied to human esophageal reconstruction, provided these favorable results are confirmed by more publications.
MCA for esophageal reconstruction in the thoracic cavity needs to be tested, and further clinical trials are needed to test its safety and guide its clinical application.
The authors would like to acknowledge the Laboratory Animal Center of Xi’an Jiaotong University for skillful technical assistance.
| 1. | Comella A, Tan Tanny SP, Hutson JM, Omari TI, Teague WJ, Nataraja RM, King SK. Esophageal morbidity in patients following repair of esophageal atresia: A systematic review. J Pediatr Surg. 2021;56:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Nassar N, Leoncini E, Amar E, Arteaga-Vázquez J, Bakker MK, Bower C, Canfield MA, Castilla EE, Cocchi G, Correa A, Csáky-Szunyogh M, Feldkamp ML, Khoshnood B, Landau D, Lelong N, López-Camelo JS, Lowry RB, McDonnell R, Merlob P, Métneki J, Morgan M, Mutchinick OM, Palmer MN, Rissmann A, Siffel C, Sìpek A, Szabova E, Tucker D, Mastroiacovo P. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2012;94:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Oddsberg J, Lu Y, Lagergren J. Aspects of esophageal atresia in a population-based setting: incidence, mortality, and cancer risk. Pediatr Surg Int. 2012;28:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Pedersen RN, Calzolari E, Husby S, Garne E; EUROCAT Working group. Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch Dis Child. 2012;97:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Smith N. Oesophageal atresia and tracheo-oesophageal fistula. Early Hum Dev. 2014;90:947-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Pinheiro PF, Simões e Silva AC, Pereira RM. Current knowledge on esophageal atresia. World J Gastroenterol. 2012;18:3662-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 187] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (3)] |
| 7. | van Lennep M, Singendonk MMJ, Dall'Oglio L, Gottrand F, Krishnan U, Terheggen-Lagro SWJ, Omari TI, Benninga MA, van Wijk MP. Oesophageal atresia. Nat Rev Dis Primers. 2019;5:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Schmedding A, Wittekindt B, Schloesser R, Hutter M, Rolle U. Outcome of esophageal atresia in Germany. Dis Esophagus. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kanshin NN, Permiakov NK, Dzhalagoniia RA, Nikulin BI, Kuznetsov AA. [Sutureless anastomoses in gastrointestinal surgery with and without steady magnetic field (experimental study)]. Arkh Patol. 1978;40:56-61. [PubMed] |
| 10. | Kamada T, Ohdaira H, Takeuchi H, Takahashi J, Ito E, Suzuki N, Narihiro S, Yoshida M, Yamanouchi E, Suzuki Y. New Technique for Magnetic Compression Anastomosis Without Incision for Gastrointestinal Obstruction. J Am Coll Surg. 2021;232:170-177.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Marrache MK, Itani MI, Farha J, Fayad L, Sharara SL, Kalloo AN, Khashab MA, Kumbhari V. Endoscopic gastrointestinal anastomosis: a review of established techniques. Gastrointest Endosc. 2021;93:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Graves CE, Co C, Hsi RS, Kwiat D, Imamura-Ching J, Harrison MR, Stoller ML. Magnetic Compression Anastomosis (Magnamosis): First-In-Human Trial. J Am Coll Surg. 2017;225:676-681.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Machytka E, Bužga M, Zonca P, Lautz DB, Ryou M, Simonson DC, Thompson CC. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc. 2017;86:904-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Liu XM, Li Y, Xiang JX, Ma F, Lu Q, Guo YG, Yan XP, Wang B, Zhang XF, Lv Y. Magnetic compression anastomosis for biliojejunostomy and pancreaticojejunostomy in Whipple's procedure: An initial clinical study. J Gastroenterol Hepatol. 2019;34:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Liu XM, Yan XP, Zhang HK, Ma F, Guo YG, Fan C, Wang SP, Shi AH, Wang B, Wang HH, Li JH, Zhang XG, Wu R, Zhang XF, Lv Y. Magnetic Anastomosis for Biliojejunostomy: First Prospective Clinical Trial. World J Surg. 2018;42:4039-4045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Itoi T, Kasuya K, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ikeuchi N, Takeuchi M, Nagano T, Iwamoto H, Yamanouchi E, Shimazu M, Tsuchida A. Magnetic compression anastomosis for biliary obstruction: review and experience at Tokyo Medical University Hospital. J Hepatobiliary Pancreat Sci. 2011;18:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Muensterer OJ, Evans LL, Sterlin A, Sahlabadi M, Aribindi V, Lindner A, König T, Harrison MR. Novel Device for Endoluminal Esophageal Atresia Repair: First-in-Human Experience. Pediatrics. 2021;148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Liu S, Fang Y, Lv Y, Zhao J, Luo R, Cheng J, Yang H, Zhang A, Shen Y, Jiang N. Magnetic compression stricturoplasty in patients with severe stricture after simultaneous esophageal atresia and duodenal obstruction repair: A case report. Exp Ther Med. 2022;23:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Dorman RM, Vali K, Harmon CM, Zaritzky M, Bass KD. Repair of esophageal atresia with proximal fistula using endoscopic magnetic compression anastomosis (magnamosis) after staged lengthening. Pediatr Surg Int. 2016;32:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Xue F, Guo HC, Li JP, Lu JW, Wang HH, Ma F, Liu YX, Lv Y. Choledochojejunostomy with an innovative magnetic compressive anastomosis: How to determine optimal pressure? World J Gastroenterol. 2016;22:2326-2335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | She ZF, Yan XP, Ma F, Wang HH, Yang H, Shi AH, Wang L, Qi X, Xiao B, Zou YL, Lv Y. Treatment of rectovaginal fistula by magnetic compression. Int Urogynecol J. 2017;28:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Li Y, Liu XM, Zhang HK, Zhang XF, Tang B, Ma F, Lv Y. Magnetic Compression Anastomosis in Laparoscopic Pancreatoduodenectomy: A Preliminary Study. J Surg Res. 2021;258:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Liu K, Yang H, Huang G, Shi A, Lu Q, Wang S, Qiao W, Wang H, Ke M, Ding H, Li T, Zhang Y, Yu J, Ren B, Wang R, Wang K, Feng H, Suo Z, Tang J, Lv Y. Adhesive anastomosis for organ transplantation. Bioact Mater. 2022;13:260-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Al-Qattan MM. Vicryl Rapide versus Vicryl suture in skin closure of the hand in children: a randomized prospective study. J Hand Surg Br. 2005;30:90-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Zaritzky M, Ben R, Johnston K. Magnetic gastrointestinal anastomosis in pediatric patients. J Pediatr Surg. 2014;49:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Liu SQ, Lv Y, Fang Y, Luo RX, Zhao JR, Luo RG, Li YM, Zhang J, Zhang PF, Guo JZ, Li QH, Han MX. Magnetic compression for anastomosis in treating an infant born with long-gap oesophageal atresia: A case report. Medicine (Baltimore). 2020;99:e22472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Slater BJ, Borobia P, Lovvorn HN, Raees MA, Bass KD, Almond S, Hoover JD, Kumar T, Zaritzky M. Use of Magnets as a Minimally Invasive Approach for Anastomosis in Esophageal Atresia: Long-Term Outcomes. J Laparoendosc Adv Surg Tech A. 2019;29:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Woo R, Wong CM, Trimble Z, Puapong D, Koehler S, Miller S, Johnson S. Magnetic Compression Stricturoplasty For Treatment of Refractory Esophageal Strictures in Children: Technique and Lessons Learned. Surg Innov. 2017;24:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abass M, Egypt; Trébol J, Spain S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ