Published online Sep 14, 2022. doi: 10.3748/wjg.v28.i34.4943
Peer-review started: April 11, 2022
First decision: May 12, 2022
Revised: June 29, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: September 14, 2022
Processing time: 148 Days and 18.2 Hours
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are rare tumors derived from the neuroendocrine cell system, which that have increased in incidence and prevalence in recent years. Despite improvements in radiological and metabolic imaging, endoscopy still plays a pivotal role in the number of GEP-NENs. Tumor detection, characterization, and staging are essential in management and treatment planning. Upper and lower gastrointestinal (GI) endoscopy is essential for correct localization of the primary tumor site of GI NENs. Endoscopic ultrasonography (EUS) has an important role in the imaging and tissue acquisition of pancreatic NENs and locoregional staging of GI neuroendocrine tumors. Correct staging and histological diagnosis have important prognostic implications. Endoscopic operating techniques allow the removal of small GI NENs in the early stage of mucosal or submucosal invasion of the intestinal wall. Preoperative EUS-guided techniques may help the surgeon locate small and deep tumors, thus avoiding formal pancreatic resections in favor of parenchymal-sparing surgery. Finally, locoregional ablative treatments have been proposed in recent studies with promising results in selected patients.
Core Tip: Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are complex neoplasms that present many clinical challenges. This review reports endoscopic management of patients with GEP-NENs. Endoscopic procedures allow diagnosis, local staging, and tissue acquisition. Early NENs of the stomach, duodenum, or rectum are generally removed by endoscopic operating techniques. New endoscopic ultrasonography-guided operative techniques may help the surgeon locate small and deep tumors or treat small pancreatic NENs.
- Citation: Iabichino G, Di Leo M, Arena M, Rubis Passoni GG, Morandi E, Turpini F, Viaggi P, Luigiano C, De Luca L. Diagnosis, treatment, and current concepts in the endoscopic management of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol 2022; 28(34): 4943-4958
- URL: https://www.wjgnet.com/1007-9327/full/v28/i34/4943.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i34.4943
Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are rare neoplasms arising from neuroendocrine cells distributed in the GEP tract. Most commonly, the primary lesion is located in the gastric mucosa, small and large intestine, rectum, and pancreas[1].
The incidence of GEP-NENs has substantially increased over the last decades. Data from the Surveillance, Epidemiology, and End Results registry show that the incidence of GEP-NENs has increased 6.4 fold since the program’s inception in 1973. This phenomenon may be related to the increasing number of radiological imaging and endoscopic examinations performed[1,2].
Approximately 15%-30% of GEP-NENs are functioning tumors with hormone-related symptoms; the remaining 70%-85% are nonfunctioning and detected incidentally or because the patient has symptoms of mass effects or distant metastases[3]. The management of GEP-NENs requires a multidisciplinary approach. Since GEP-NENs are less frequent than other malignancies, endoscopic management of these tumors may not be fully understood.
This review focuses on the endoscopic diagnosis and treatment of GEP-NENs.
The GEP tract represents the most common localization of NENs. Gastrointestinal NENs (GI-NENs) develop from the stomach (23%), appendix (21%), small bowel (15%), and rectum (14%). Esophageal and colonic NENs are rare tumors that account for a small percentage of GI-NENs. Pancreatic NENs (Pan-NENs) represent about 1% of all pancreatic neoplasms but their prevalence is around 10%[1].
The World Health Organization subclassifies GEP-NENs based on the mitotic count and Ki-67 index (Table 1). This classification defines both well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs). NETs are divided into grade 1 (Ki-67 index < 3, mitotic rate < 2), grade 2 (Ki-67 index 3-20, mitotic rate 2-20), and grade 3 (Ki-67 index > 20, mitotic rate > 20). NECs exhibit poorly differentiated morphology with significant atypia and frequently have geographic necrosis. Tumors involving both neuroendocrine and non-neuroendocrine cells are classified as mixed NENs and non-NENs[4].
| NENs | Differentiation | Grade | Mitotic rate | Ki-67 index % |
| NET, G1 | Well differentiated | Low | < 2 | < 3 |
| NET, G2 | Well differentiated | Intermediate | 2-20 | 3-20 |
| NET, G3 | Well differentiated | High | > 20 | > 20 |
| NEC, small cell type | Poorly differentiated | High | > 20 | > 20 |
| NEC, large cell type | Poorly differentiated | High | > 20 | > 20 |
| MiNEN | Well or poorly differentiated | Variable | Variable | Variable |
There are two major classifications in current clinical use: the European Neuroendocrine Tumor Society (ENETS) tumor, node and metastasis (TNM) system and the American Joint Committee on Cancer TNM system[5,6].
Clinically, GEP-NENs can be classified into nonfunctioning and functioning tumors. The functioning tumors secrete substances that cause appreciable clinical symptoms, whereas the nonfunctioning neoplasms do not secrete any substance or the substance produced is inactive. The hormones produced and clinical symptoms vary by site of the primary GEP-NENs. Clinical symptoms include hypoglycemic syndrome, carcinoid syndrome, Zollinger-Ellison syndrome, watery diarrhea-hypokalemia-achlorhydria syndrome, and glucagonoma (Table 2)[7].
| Tissue | Hormones | Symptoms/Syndrome |
| Gastric | Histamine, CGA | Atypical flush, wheeze, angioedema |
| Duodenal | CGA, somatostatin, gastrin | Cholelithiasis, steatorrhea, diabetes, ZE syndrome (gastrinoma) |
| Jejuno-ileal, appendiceal, cecal | Serotonin, CGA, pancreastatin | Carcinoid syndrome |
| Colorectal | Pancreatic polypeptide | No hormonal symptoms |
| Pancreatic | Insulin | Recurrent hypoglycemia |
| Glucagon | Diarrhea, glossitis, necrolytic migratory erythema, weight loss, hyperglycemia, blood clots | |
| VIP | Diarrhea, hypokalemia, achlorhydria | |
| ACTH | Cushingoid facies, weight gain, diabetes, hypertension | |
| GHRH | Acromegalic features, diabetes | |
| PTHRP | Hypercalcemia | |
| Gastrin | Pain, diarrhea (ZE syndrome) | |
| Somatostatin | Diabetes, cholelithiasis, steatorrhea, weight loss | |
| Serotonin | Flushing, diarrhea (carcinoid syndrome) |
Chromogranin A is currently the most commonly used biomarker for GEP-NENs. It has a 10%-35% specificity, and its sensitivity ranges from 32% to 92%. Serotonin and its metabolite 5-hydroxyindole acetic acid have been measured in blood and urine samples, respectively, as markers of carcinoid syndrome. However, the sensitivity of this biomarker is as low as 35% in the absence of carcinoid syndrome[8].
Several other potential biomarkers include neuron-specific enolase, human chorionic gonadotropin, alpha-fetoprotein, and pancreatic polypeptide. These circulating biomarkers are useful to aid diagnosis, but are of insufficient value to accurately identify the primary tumor site, correlate with tumor grade, and differentiate low-level malignancy from high-grade disease[9].
In recent years, new biomarkers have been evaluated that may correlate with clinical outcomes and be useful as better prognostic indicators. These include microRNAs, long noncoding RNAs, circulating tumor cells, and DNA methylation patterns[10].
Most GEP-NENs are sporadic, but they also can arise as part of inherited familial syndromes. About 5% of patients with GEP-NENs harbor genomic mutations with well-characterized familial syndromes such as multiple endocrine neoplasia type 1 (MEN1), von Hippel-Lindau (VHL) disease, tuberous sclerosis (TSC), and neurofibromatosis type 1 (NF1)[11].
MEN1 is an autosomal-dominant syndrome, characterized by NENs of the anterior pituitary, parathyroid glands, and pancreas. VHL syndrome is an autosomal-dominant syndrome characterized by a variety of benign and malignant neoplasms including clear renal cell carcinomas, pheochromocytomas, hemangioblastomas, retinal angiomas, paragangliomas, and pNENs. TSC is an autosomal-dominant syndrome characterized by widespread, low-grade tumors, and hamartomas in multiple organs including the brain, heart, skin, eyes, kidney, lung, and liver. pNENs are described in only 1% to 5% of cases. NF1 is an autosomal-dominant syndrome characterized by ubiquitous neurofibromas; multiple cafe-au-lait skin spots; and susceptibility to gliomas, myeloid leukemia, pheochromocytomas, and occasionally pNENs[12].
Gastric NENs (G-NENs) are neoplasms derived from the enterochromaffin-like cells of the gastric mucosa. They are classified into types I, II and III according to their clinical and pathophysiological characteristics (Table 3)[13].
| Characteristics | Type I | Type II | Type III |
| Prevalence | 70%-80% | 5%-10% | 10%-20% |
| Background | Autoimmune chronic atrophic gastritis | Gastrinomas (Zollinger-Ellison syndrome) | Normal mucosa |
| Number of lesions | Multiple | Multiple | Single |
| Size of tumors | 1-2 cm | 1 cm | > 2 cm |
| Site of tumor | Corpus and/or fundus | Corpus and/or fundus | Anywhere |
| Serum gastrin levels | Elevated | Elevated | Normal |
| Gastric pH | High | Low | Normal |
| Invasion | Rare | More common | Common |
| Prognosis (5-yr survival) | Excellent (90%-95%) | Good (70%-90%) | Worse (less than 35%) |
Type I G-NENs correspond to the majority of G-NENs found in the stomach (70%-80%) and are associated with autoimmune chronic atrophic gastritis and hypergastrinemia, type II G-NENs lesions are caused by gastrinomas and commonly found in patients with Zollinger-Ellison syndrome (ZES) and MEN1, and type III G-NENs lesions consist of a sporadic lesion and are unrelated to gastrin hypersecretion.
Patients with G-NENs typically present with nonspecific symptoms and the diagnosis is made by upper gastrointestinal (GI) endoscopy for symptoms such as abdominal pain, nausea, bleeding, and anemia. Despite the low incidence of G-NET, recent data have demonstrated an increase due to an expanding use of upper endoscopy, improvement of endoscopes, and more attention to identification and characterization of gastric lesion[14]. Type I G-NENs occur in the corpus and/or fundus and are multiple small reddish polyps, usually subcentimetrics. They are associated with a chronic atrophic gastritis with an excellent prognosis. Tumor extension is limited to the mucosa or submucosa.
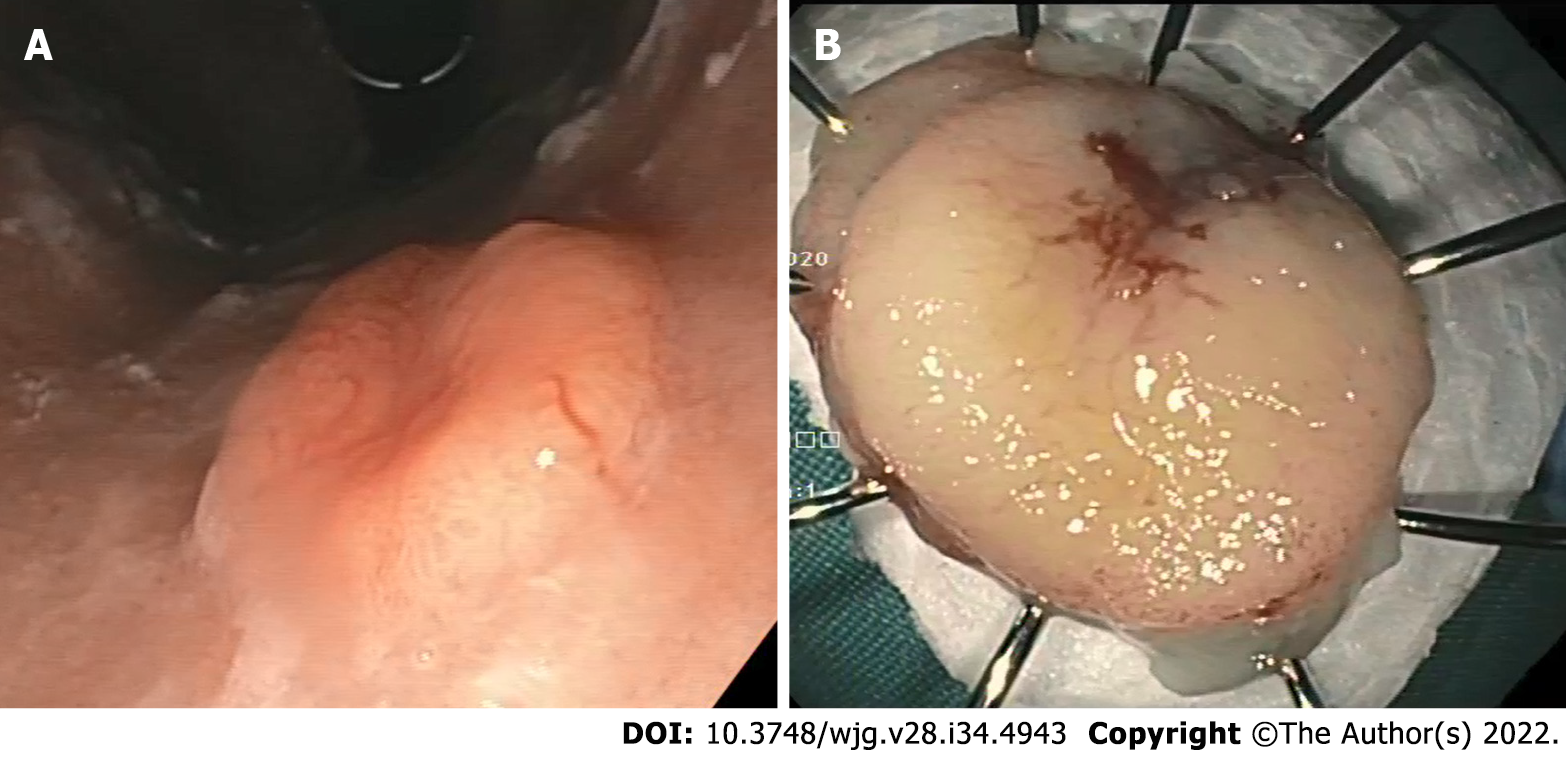
Type II G-NENs are similar to type I lesions but the adjacent gastric folds have a hypertrophic gastric mucosa and occasionally have multiple areas of ulceration of gastric and duodenal mucosa. Type II G-NENs are involved in MEN1 and ZES and have a good prognosis, similar to type I. Type III G-NENs are single, large (> 2 cm), sometimes ulcerated, more aggressive and associated with local and distant spread, and involve the muscular layer (Figure 1)[15]. The prognosis of this subgroup is poor.
At the time of diagnosis, biopsy samples should be taken from the lesions and multiple gastric biopsies (antrum, body, and fundus) should be performed for etiologic orientation.
Endoscopic ultrasonography (EUS) should be performed if the lesion is greater than 1 cm in order to assess the layer of origin, tumor size, echogenicity, margins, wall invasion, and regional lymph nodes. At EUS, G-NENs present as rounded isoechoic or hypoechoic tumors, localized in the second (deeper mucosal) or third (submucosal) echo layers. EUS diagnostic accuracy for evaluation of subepithelial lesion including G-NET is suboptimal (43%-67%)[16]. EUS is a very accurate technique for assessing tumor size and muscularis propria integrity, factors that seem to condition the potential for distant metastasis[17].
In type I and II G-NENs, staging EUS is frequently performed to evaluate indication to endoscopic treatment. For patients suspected of having type II G-NENs, EUS evaluation must be performed for the assessment of any duodenal or Pan-NENs. In Type III G-NENs, EUS is indicated to stage the disease by assessing the presence of regional lymph node involvement[18].
Duodenal NENs (D-NENs) are solitary, small lesions generally discovered incidentally on imaging studies or endoscopy. They are generally classified into five different tumor types: duodenal gastrinomas, somatostatinomas, nonfunctional D-NENs, duodenal gangliocytic paragangliomas, and high-grade poorly differentiated NEC[19].
Most D-NENs are localized predominantly in the first and second duodenal portion and are nonfunctioning with a low risk of local or distant metastases. Functioning D-NENs are typically associated with higher metastatic power. The presence of multiple D-NENs should raise suspicion of MEN1-ZES[19].
D-NENs located in the ampullary/periampullary region have more aggressive behavior and poorer overall survival than D-NENs located elsewhere in the duodenum[20,21].
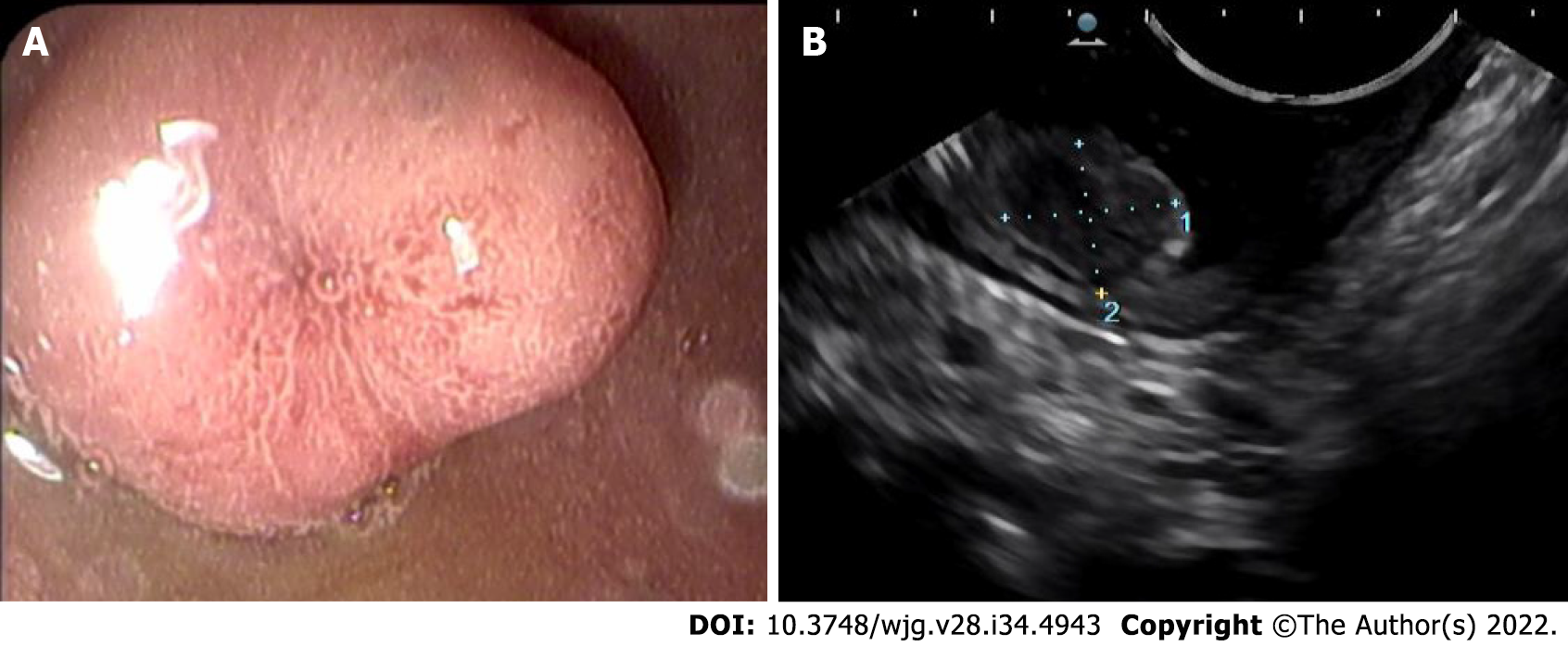
On upper GI endoscopy, D-NENs have the appearance of submucosal tumors with a surface color often identical to the surrounding mucosa (Figure 2). As the tumor enlarges, a depression may form in the center, which is eventually replaced by an ulcer crater[22].
EUS evaluation is useful for duodenal subepithelial lesions characterization; the accuracy of combined endoscopic/EUS imaging for all duodenal lesions is 84.9%[23].
EUS is also useful to exclude locoregional lymph node metastases and thus the indication for endoscopic mucosal resection in case of lesions > 1 cm. At EUS evaluation, D-NENs are usually located in the submucosal layer and are rounded, hypoechoic, well-demarcated small lesions. Fine needle aspiration (FNA) should also be performed in cases of nondiagnostic histopathology[18]. Duodenoscopy and EUS are indicated to identify the primary tumor in the case of ZES and in patients with MEN1[24].
Small bowel NENs (SB-NENs) are the most common NENs developing distant metastases; however, their prognosis remains favorable[25]. They tend to present in later stages because clinical symptoms are often insidious and the diagnosis often occurs in the course of an intervention for intestinal obstruction or bleeding[26].
Accurate localization and staging of SB-NENs often involve a combination of several imaging modalities. Endoscopic examinations such as capsule endoscopy and double-balloon enteroscopy are useful in identifying occult SB-NENs when no primary tumor was found on conventional imaging. Macroscopically, most SB-NENs are sessile nodules or ulcerated lesions usually measuring between 1 and 2 cm or as multiple tumors[27,28].
Appendiceal NENs (A-NENs) are usually discovered incidentally at final pathological examination after an appendectomy is performed for acute appendicitis. Colonoscopic examination of the entire large bowel is mandatory given the frequency of synchronous colorectal neoplasia[27]. A-NENs usually have a good prognosis. In most cases, appendicectomy alone is considered curative; however, in selected cases with high malignant potential, the right hemicolectomy could be considered[29].
Colonic NENs (C-NENs) and rectal NENs (R-NENs) are two different clinical entities. C-NENs have more aggressive features and much worse prognosis than R-NENs[30]. Nearly 70% of C-NENs are located in the ascending colon, particularly in the cecum with a mean size of 5 cm at presentation. The patients generally experience late symptoms such as abdominal pain, GI bleeding, and weight loss, and most of them have local or distant metastasis[15].
Most R-NENs are diagnosed through screening sigmoidoscopy and/or colonoscopy while only a small fraction have symptoms such as diarrhea, abdominal pain, or rectal bleeding.
The majority of R-NENs are small size lesions less than 1 cm and only 5% present are larger than 2 cm[31].
Endoscopically, R-NENs appear as sessile or semipedunculated polypoid lesions with normal or a yellow-discolored mucosa, frequently located in the midrectum. Larger lesions can have different endoscopic characteristics such as ulcerations, depressions or hyperemic color, which can be suggestive of aggressive disease[30].
At EUS, R-NENs have the aspect of nodular, hypoechoic, or isoechoic submucosal tumor clearly demarcated from the surrounding tissue[18].
EUS-FNA can be helpful to make a differential diagnosis with other subephitelial lesion[32] with a diagnostic accuracy of 85.1%[33].
According to the current ENETS consensus, an EUS should also be performed in order to assess the depth of rectal wall invasion and regional lymphadenopathy prior to endoscopic resection[34].
A complete colonoscopy is indicated after a diagnosis of a R-NENs to exclude concomitant colon cancer and other NENs[35].
Pan-NENs comprise 1% to 2% of pancreatic neoplasms[36]. Pan-NENs are classified as functioning or nonfunctioning depending on whether they cause hormonal overproduction syndrome. Functioning Pan-NENs include insulinoma, gastrinoma, VIPoma, and glucagonoma. Nonfunctioning Pan-NENs comprise the largest group of Pan-NENs and do not produce syndromes of hormonal excess. Nonfunctioning Pan-NENs often manifest later in the course of the disease or are discovered incidentally during abdominal imaging examinations performed for other diseases[37].
EUS is a very useful tool in the management of Pan-NENs and has been considered the imaging study of choice to be performed after other negative noninvasive imaging studies are negative[38].
Puli et al[39] assessed the diagnostic accuracy of EUS for detection of Pan-NENs. This meta-analysis demonstrated an excellent accuracy of EUS in this setting with a sensitivity of 87.2% and a specificity of 98.0%.
A meta-analysis of 2015 assessed incremental benefit of preoperative EUS for the detection of suspected Pan-NENs after other investigative modalities have been attempted. EUS increased the overall Pan-NENs detection by over 25% and was particularly useful in functioning Pan-NENs, typically smaller in size (i.e. insulinomas or gastrinoma)[40].
EUS is also very useful in assessing the presence of multiple lesions, size lesion and especially the distance between the lesion and the main pancreatic duct, a factor that can drive the decision on which surgical approach (i.e. enucleation vs resection)[18]. Pancreatic enucleation is commonly performed for Pan-NENs with a low risk of malignant progression but post-operative pancreatic fistula risk is higher for neoplasms located close to the duct[41].
EUS is of course useful for differential diagnosis of Pan-NENs with other solid pancreatic lesions. Pan-NENs are solid lesions and on EUS examination, appear as well-demarcated, hypoechoic lesions with a homogeneous pattern. However, because Pan-NENs grow expansively, they may also appear as cystic or indistinguishable from pancreatic adenocarcinomas.
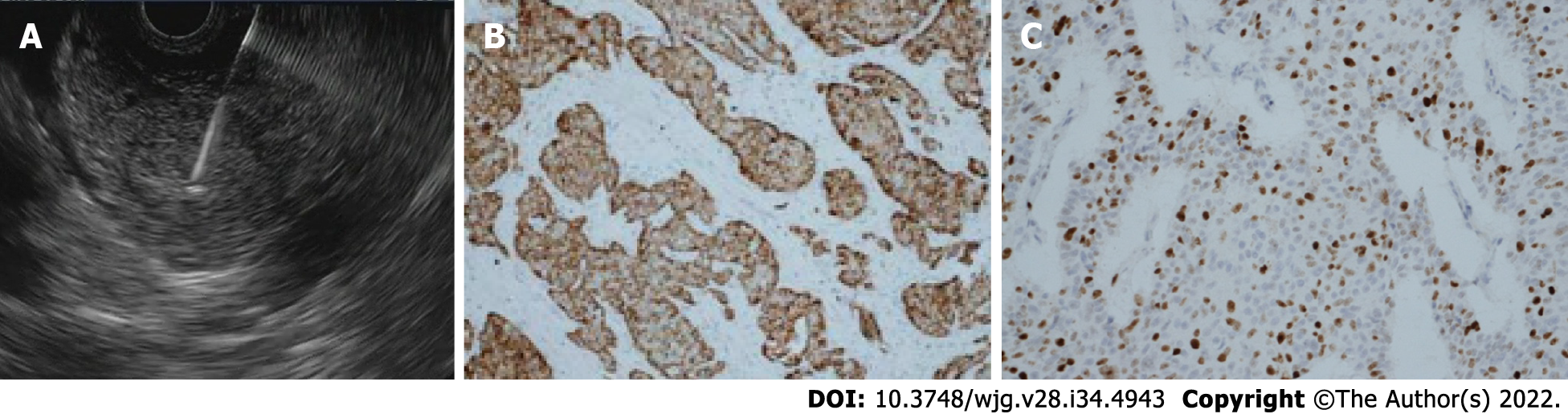
In Pan-NENs EUS guided tissue acquisition is considered the procedure of choice to reach cytologic diagnosis, but also ascertainment of tumor grade by determining the Ki-67 proliferation index and mitotic count (Figure 3).
EUS-FNA provides a cytological specimen with a sensitivity ranging between 80% and 90%, specificity at 96%[18]. The adequacy and concordance of Ki-67 evaluation of EUS-FNA compared with histology remains unclear especially for tumor > 20 mm[42,43]. To overcome these limitations of cytological Ki-67 determination, sampling with needles for EUS-guided fine-needle biopsy (EUS-FNB) have been introduced with good results[44,45]. In a recent retrospective study EUS-FNB outperformed EUS-FNA for Ki-67 proliferation index determination[46].
EUS elastography (EUS-E) is a newer tool of diagnostic EUS for differential diagnosis of solid pancreatic lesions. It is a technique that analyzes pancreatic stiffness being a useful tool for the differential diagnosis of pancreatic masses with a qualitative or quantitative elastographic assessment. A meta-analysis by Zhang et al[47] showed a sensitivity and specificity of quantitative EUS-E for the differentiation of benign and malignant pancreatic masses of 0.95 and 0.61, respectively. Iglesias-García et al[48] evaluated the accuracy of quantitative EUS-E for the differential diagnosis of solid pancreatic masses. EUS-E was helpful in differentiating pancreatic cancer from Pan-NENs with a sensitivity of 100% and a specificity of 88%. In another study, elastographic analysis was accurate in discriminating between benign and malignant pancreatic lesions, without difference between NENs and other nonmalignant lesions[49].
Recently, shear wave elastography (SWE) has been introduced as a quantitative absolute measurement of tissue hardness. Ohno et al[50] compared the diagnostic performances of EUS-SWE and conventional strain elastography for solid pancreatic lesions without significant differences.
To date EUS-E does not provide sufficient diagnostic accuracy to replace tissue diagnosis but it plays a significant role in those cases of suspected pancreatic cancer where biopsy sampling was inconclusive and can help to select the area of the pancreatic lesion to be sampled[51].
Contrast-enhanced EUS (CE-EUS) consists of an intravenous injection of contrast media during the EUS examination so parenchymal perfusion and the microvasculature of the pancreas can be visualized. Pancreatic cancer is observed as a hypoenhanced heterogeneous lesion whereas Pan-NENs are observed as well-demarcated lesions with hyperenhancement in the arterial phase.
CE-EUS increased the accuracy of EUS for both the detection and characterization of solid pancreatic lesions[52].
Kitano et al[53] prospectively evaluated how accurately CE-EUS characterizes pancreatic lesions and hyperenhanced lesions were diagnosed as Pan-NENs with a sensitivity of 79% and specificity of 99%. Several studies have examined CE-EUS in the differentiation between malignant and benign Pan-NENs[39,40]. Heterogeneous enhancement at an early arterial phase with fewer vessels and more fibrosis is associated with an aggressive tumor[54]. Palazzo et al[55] reported a sensitivity of 86% and a specificity of 96% in prediction tumor aggressiveness in Pan-NENs with the use of CE-EUS.
NET could be found also in the bile ducts[56]; however, the incidence is very low. In the literature, only 100 cases of biliary tree NETs were described and they have an excellent prognosis[57]. In these cases, complete surgical excision offers optimal treatment with no evidence of chemotherapy or radiotherapy’s role in the management.
In the last 10 years, the approach of GEP-NENs progressively included endoscopic resection technique. The choice of the treatment modality needs of a careful assessment and depends by multiple factors. The indication of endoscopic respective therapy does not include NET of appendix, colon and biliary tree while for pancreatic NET the suitable endoscopic approaches are ablative techniques.
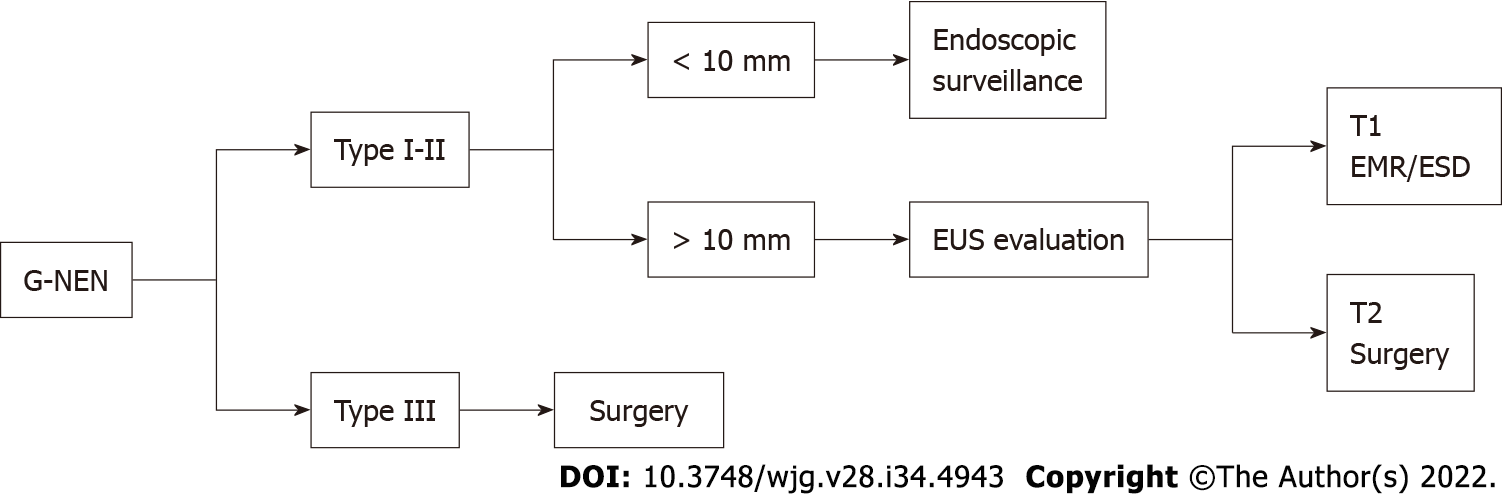
Most type I G-NENs are limited to the mucosa or submucosa and they rarely invade the muscularis propria or metastasize to local lymph nodes if < 10 mm. Current guidelines suggested removing all tumors ≥ 10 mm[16]. EUS is recommended to identify possible involvement of regional lymph nodes and invasion beyond the submucosa prior to resection. Endoscopic resection (ER) either by endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) is the treatment of choice when EUS demonstrates the lesion to be localized to the mucosa or submucosa[58].
Surgical treatment is the option for lesions which are predicted to be T2 (lesion larger than 20 mm or grading G3) or lesions with positive margins[58].
Sato et al[59] showed in a low number of cases that about 66% of type I G-NENs resected by EMR had a positive vertical margin, whereas no case with ESD had positive vertical or horizontal margins. Kim et al[60] evaluated the clinical usefulness of ESD with that of EMR for resection of type I G-NENs with an estimated size of ≤ 10 mm. ESD yielded a significantly higher histologic complete resection rate than EMR, particularly in the vertical resection margin.
Noh et al[61] evaluated outcomes of endoscopic treatment for type 1 G-NENs below 20 mm in diameter. The complete resection rate was significantly higher in the ESD group than in the EMR group with similar procedure-related adverse events. For type II G-NENs local or limited excision can be recommended and indication for treatment type is similar to type 1 G-NENs[24,62]. Type III G-NENs are more associated with deeper invasion of the gastric wall, higher risk of nodal metastasis than type I and II G-NENs and surgery is considered the initial therapeutic approach[58]. ER has been proposed for small lesions. Kwon et al[63] investigated the clinical outcomes of type 3 G-NENs (mean tumor size of 10.2 ± 6.3 mm) after endoscopic treatment with a median follow-up of 46 mo. Of the 45 included in the follow-up, no evidence of tumor recurrence was found. Authors concluded that endoscopic treatment could be applied for type 3 G-NENs smaller than 2 cm, confined in the submucosal layer and without lymphovascular invasion. In Figure 4, we summarize the current recommendations for G-NENs.
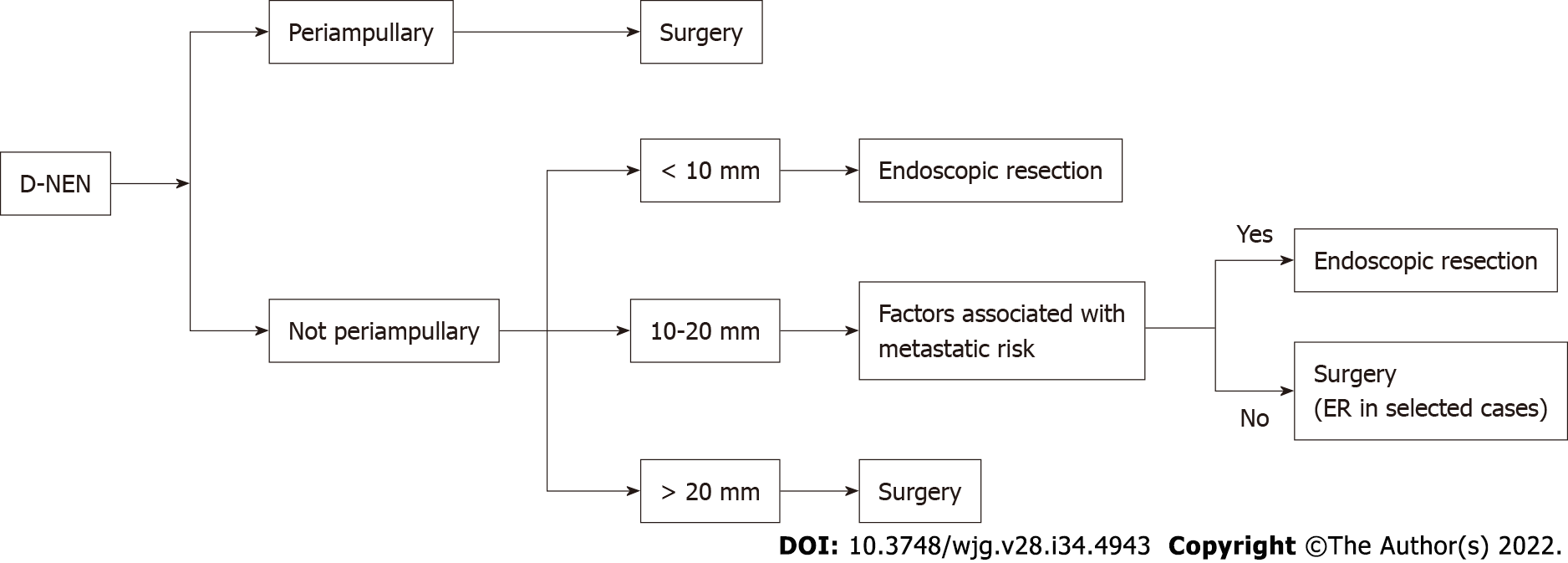
Most D-NENs are located in the first or second part of the duodenum, with 20% of them occurring in the periampullary region[21]. Current guidelines indicate ER for small (≤ 10 mm) nonperiampullary D-NENs confined to the submucosal layer, without lymph node or distant metastasis. Either endoscopic or surgical resection is allowed, for nonperiampullary D-NENs measuring 10-20 mm without metastatic risk (G1, no muscolaris invasion, no lymph-vascular invasion and no lymph-node metastasis)[24,58].
There are different ER techniques, such as cap technique, EMR, EMR with ligation device and ESD[58].
Gincul et al[64] evaluated the feasibility and outcome of endoscopic treatment of D-NENs (including 7 ampullary G1/G2 NETs with ≤ 20 mm) with EMR. The resection rate was R0 only in 51.6% (16/31) of patients. During a median follow-up period of 56 mo, 2 patients (8.3%) presented a tumor recurrence. Morbidity was 38 % (11/29) and mortality was 3% (one severe bleeding).
Nishio et al[65] assessed the efficacy, safety and the long-term outcomes of ESD for nonperiampullary D-NENs ≤ 10 mm in diameter. En bloc, R0 and curative resection were achieved in 100% (8/8), 88% (7/8), and 88% (7/8) of tumors, respectively. During a median follow-up of 34.0 mo none of the patients showed evidence of local recurrence or distant metastasis. Perforation occurred in 2 patients (16%) without need for surgery.
In a recent review, Brito et al[66] evaluated the effectiveness and complications of ER techniques in patients with D-NETs ≤ 20 mm. Polypectomy was associated with a high occurrence of incomplete resections. Among the mucosectomies, EMR with cap or EMR with injection was associated with lower frequencies of compromised margin and recurrent surgery. Endoscopic submucosal dissection was not associated with recurrence but it was associated with a higher occurrence of bleeding and perforation.
ER of D-NENs is complex and associated with significant morbidity. EMR is associated with a lower R0 resection rate than ESD. ESD is associated with better pathologically confirmed resection but with a higher morbidity rate and should be restricted to expert centers[67]. ESGE guidelines suggests choosing between EMR, ESD, and endoscopic full thickness resection (EFTR) to resect nonampullary, nonfunctional duodenal NENs of < 15 mm, depending on size, location, depth of invasion, and local expertise[16].
Ampullary D-NENs have a less favorable prognosis than nonampullary D-NENs and pancreaticoduodenectomy is recommended regardless of size[24].
Local resection might be sufficient for small highly differentiated ampullary D-NENs without node metastases especially in patients with comorbidities[24].
In one of the largest studies of EMR of D-NENs above reported, Gincul et al[64] included 7 ampullary G1/G2 D-NENs with ≤ 20 mm. In this subgroup of patients, R0 resection was achieved in 5 patients (71%) without recurrence with a median follow-up period of 56 mo.
Shimai et al[68] reported 3 patients with an ampullary D-NENS, who underwent endoscopic papillectomy without tumor recurrence or metastasis after a follow-up of 2 years. In Figure 5, we summarize the current recommendations for D-NENs.
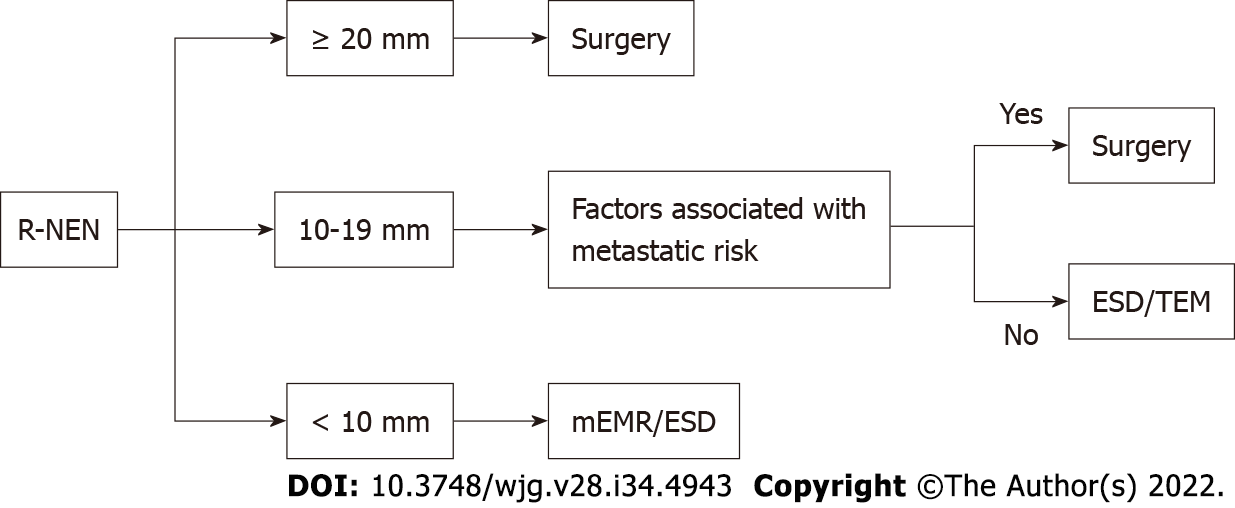
The current guidelines recommend ER for R-NENs < 10 mm in size with no risk factors for metastasis. R-NENs larger than 20 mm are candidates for surgical resection. There is controversy over R-NENs of intermediate size 10-19 mm (Figure 6). R-NENs with these sizes have a poorer prognosis compared with those < 10 mm[34]. The main factors associated with the risk of lymph-node metastases are atypical endoscopic aspect (presence of mucosal depression or ulceration), suspicious pararectal lymph node at EUS, invasion of the muscularis propria, G2, and lymphovascular invasion. Rectal NET with any risk factor for metastasis should be considered for surgical resection with lymphadenoctomy. For lesions measuring 10-19 mm without factors associated with metastatic risk the most appropriate resection techniques may be ESD or transanal endoscopic microsurgery (TEM)[24-34].
The appropriate endoscopic technique resection should allow for en bloc oncological excision. Optimal endoscopic treatment modality for R-NENs has not yet been achieved.
Conventional polypectomy is associated with a low rate of complete resection as most of the R-NENs are submucosal. Son et al[69] reported a complete resection rate by conventional polypectomy of 30.9%.
EMR is simple and has low complication rates but can sometimes cause incomplete resection and difficulty in pathologic evaluation because even small R-NENs can invade the submucosa. Several studies, assessing efficacy of EMR, have reported complete resection rates ranging from 30%-70%[70-74].
Therefore, several m-EMR methods have been reported and included cap-assisted EMR (EMR-C), EMR with a ligating device (EMR-L), and EMR after circumferential precutting (EMR-P).
EMR-L is performed suctioning the lesion into the ligating device and cutting by using a round snare after placing bands around the base of tissue suctioned. EMR-C is performed with a transparent cap fitted to the scope, followed by snare cautery resection. These techniques allow the cutting of the submucosal layer from the muscularis propria[75].
Lee et al[75] performed a retrospective study comparing EMR-L and EMR-C, concluding that EMR-L may achieve both a higher endoscopic rate and a histologic complete resection rate. An analysis of 17 studies reported that the complete resection rate of R-NENs using EMR-L was 94.8% compared with 72.4% for EMR-C[76].
EMR-P is performed by lifting the mucosa with a saline injection, making a circumferential incision using the tip of the snare or special endoknives and resecting the tumor with a snare. This technique has the advantages, unlike the other m-EMR procedures, of not being affected by lesion size. Several studies have investigated the usefulness of EMR-P for resection of R-NENs showing a complete resection rate from 81.2% to 96.7% with a short procedure time, and an acceptable safety profile[77-79].
Recently Park et al[80] evaluated the safety and efficacy of underwater endoscopic mucosal resection in the treatment of small R-NENs (< 10 mm) with high R0 resection rates similar to ESD.
ESD is an advanced endoscopic technique employing a submucosal injection to lift the lesion away from the muscularis propria layer. Dedicated devices are then used to dissect around the entire lesion in the submucosal plane. This technique results in a high en bloc resection rate although more complicated, time consuming and with higher risk of complications than EMR and m-EMR[81].
Zhou et al[82] performed a meta-analysis comparing ESD with EMR and m-EMR in the treatment of R-NENs smaller than 15 mm in diameter. Complete resection rate was significantly higher in the ESD group than in the EMR group and comparable between the ESD group and the m-EMR group. A recent meta-analysis compared efficacy of ESD and EMR in curing R-NENs. This study showed that ESD is more effective in curing R-NENs of 10-20 mm in size than EMR without significant differences for R-NENs smaller than 10 mm[83].
In patients with an incomplete resection from EMR techniques, ESD may be indicated as salvage therapy[24,84].
A recent new endoscopic technique is EFTR, which is performed using a full thickness resection device (FTRD). The FTRD uses a transparent cap with a modified over-the-scope clip (OTSC) mounted over a standard colonoscope. The lesion is pulled into the cap and the pseudopolyp created by the OTSC closure is then resected with the snare preloaded in the tip of the cap. Meier et al[85] conducted a study evaluating EFTR in 40 cases of R-NENs showing effectiveness of this method (R0 resection rate in 95%) in addition to feasibility (median time, 18.5 min) and safety.
Patients with small Pan-NENs are candidates for pancreatic-sparing procedures such as central pancreatectomy or enucleation. Small Pan-NENs can be difficult to detect intra-operatively by palpation only. EUS-guided techniques to facilitate small Pan-NENs localization especially during laparoscopy surgery are tattooing or fiducial markers implantation. These techniques allow a precise localization of lesions ensuring adequate margins of resection and preserving normal pancreatic parenchyma[86].
Endoscopic ultrasound-guided fine needle tattooing (EUS-FNT) is a safe, easy to perform and useful new method to mark preoperatively small Pan-NENs. Generally, a 22-gauge standard needle allows easy injection of the tattooing solution. The needle is inserted inside the target lesion or immediately near the lesion borders into the normal parenchyma. The most frequently solution utilized is a sterile carbon-based ink which is nondegradable and remains in the tissues indefinitely[87].
Recently Rosa et al[88] evaluated EUS-FNT in facilitating intra-operative detection of Pan-NENs (8 insulinoma and 8 nonfunctional Pan-NENs.) The tattoo mark was detected in all but one patient. Only a small hematoma secondary to the EUS-FNT was observed.
The placement of fiducial markers implantation under EUS guidance is another technique to facilitate Pan-NENs localization during surgery. Fiducials are implantable radiographic markers that have been used for many years to mark soft tissue in radiology. The fiducial is easily visible during an intraoperative ultrasound[86].
Law et al[89] reported on two consecutive patients with small Pan-NENs who underwent fiducial placement. In both of the reported cases, the fiducials were visualized by intraoperative ultrasound, and the surgical resection was successful without procedure-related complications.
In recent years endoscopic ultrasound-guided ablation therapy has emerged as a new therapeutic option for solid pancreatic tumors, especially for Pan-NENs in elderly patients and candidates unfit for surgery. There are two main techniques used, EUS-guided radiofrequency ablation (EUS-RFA) and EUS-guided ethanol ablation (EUS-EA)[90]. Functioning and multiple Pan-NENs seem to be the ideal target for endoscopic ultrasound -guided ablation therapy resolving symptomatic hormonal syndromes. In case of nonfunctioning Pan-NENs, these techniques could be a therapeutic option in the case of patients unfit to surgery[91].
EUS-EA can be safe and useful for the control of symptoms in patients with small insulinomas[91-93].
Jürgensen et al[92] reported the first case of EUS–EA of a 13 mm pancreatic insulinoma in a 78-year-old woman. The patient exhibited no further hypoglycemic episodes with no recurrence of the tumor on follow-up.
Levy et al[93] reported a small series of 5 patients for EUS-EA of insulinomas; they observed that symptomatic relief was relieved almost immediately after the procedure and maintained during the follow-up.
Qin et al[94] reported a series of 4 patients for EUS-EA of insulinomas with no recurrence of hypoglycemia and complications during follow-up.
Choi et al[95] reported the largest cohort of 32 patients with nonfunctioning Pan-NENs treated with EUS–EA. In 24 out of 40 tumors (60%) complete ablation was achieved.
The other less invasive locoregional therapy for Pan-NENs is EUS-RFA. Multiple case reports of EUS-RFA of insulinomas showed complete regression of the clinical syndrome[96-98].
Regarding nonfunctioning Pan-NENs, Barthet et al[99] conducted a prospective multicenter study including 12 patients with 14 Pan-NENs (mean size 13.1 mm) treated with EUS-RFA. Among the 14 Pan-NENs, at 1-year follow-up, 12 had completely disappeared (86% tumor resolution). Two adverse events occurred (one pancreatitis, one pancreatic ductal stenosis). Another case series included 11 patients with nonfunctioning Pan-NENs. A complete radiological response was achieved in 8 of 11 patients treated with EUS-RFA. Two cases of mild pancreatitis occurred[100].
GEP-NENs are on the rise. The reasons for this phenomenon are a better awareness of an increased and more widespread use of GI endoscopy and advanced radiological imaging[1]. The overall survival rate for patients with GEP-NENs has improved in the last years[2]. This achievement is due to both early detection and better therapeutic strategies of GEP-NENs.
Endoscopy is the only method of choice to detect asymptomatic GI-NENs at an early stage. Most patients with early, GI-NENs can be treated with ER or surveillance.
Pan-NENs are relatively rare tumors but their number is increasing, mainly because of the advances in various diagnostic imaging modalities as EUS. Newer advancement in the field of EUS such as the evolution of needles, EUS-E and CE-EUS can provide useful information with an improvement in the management of Pan-NENs. EUS-guided tumor ablation therapies can be a therapeutic option in selected patients unfit for surgery.
The literature evidence on this field is growing every day. For the nature of our study (mini-review) and the very wide issue, we collected and summarized the most important evidence regarding the endoscopic treatment underlying current guideline. We believe that a systematic review the literature with meta-analysis (when applicable) for every specific GI-NENs is needed to prove and confirm the role of endoscopy in diagnosis and therapy of many types of GI-NENs.
| 1. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 2. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2666] [Article Influence: 296.2] [Reference Citation Analysis (5)] |
| 3. | Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol. 2012;24:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2761] [Article Influence: 460.2] [Reference Citation Analysis (3)] |
| 5. | Luo G, Javed A, Strosberg JR, Jin K, Zhang Y, Liu C, Xu J, Soares K, Weiss MJ, Zheng L, Wolfgang CL, Cives M, Wong J, Wang W, Sun J, Shao C, Tan H, Li J, Ni Q, Shen L, Chen M, He J, Chen J, Yu X. Modified Staging Classification for Pancreatic Neuroendocrine Tumors on the Basis of the American Joint Committee on Cancer and European Neuroendocrine Tumor Society Systems. J Clin Oncol. 2017;35:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4704] [Article Influence: 522.7] [Reference Citation Analysis (4)] |
| 7. | Dillon JS. Workup of Gastroenteropancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am. 2020;29:165-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Fang JM, Li J, Shi J. An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol. 2022;28:1009-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (12)] |
| 9. | Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, Meyer T, Moss SF, Washington K, Wolin E, Liu E, Goldenring J. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435-e446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 10. | Sansone A, Lauretta R, Vottari S, Chiefari A, Barnabei A, Romanelli F, Appetecchia M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Fernandez CJ, Agarwal M, Pottakkat B, Haroon NN, George AS, Pappachan JM. Gastroenteropancreatic neuroendocrine neoplasms: A clinical snapshot. World J Gastrointest Surg. 2021;13:231-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 12. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 13. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 379] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39:1071-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (20)] |
| 16. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 17. | Varas MJ, Gornals JB, Pons C, Espinós JC, Abad R, Lorente FJ, Bargalló D. Usefulness of endoscopic ultrasonography (EUS) for selecting carcinoid tumors as candidates to endoscopic resection. Rev Esp Enferm Dig. 2010;102:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Zilli A, Arcidiacono PG, Conte D, Massironi S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: lights and shadows. Dig Liver Dis. 2018;50:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005;19:675-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 20. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3321] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 21. | Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg. 2014;18:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Sato Y, Hashimoto S, Mizuno K, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22:6817-6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (5)] |
| 23. | Pavlovic Markovic A, Rösch T, Alempijevic T, Krstic M, Tomic D, Dugalic P, Sokic Milutinovic A, Bulajic M. Endoscopic ultrasound for differential diagnosis of duodenal lesions. Ultraschall Med. 2012;33:E210-E217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | de Mestier L, Lepage C, Baudin E, Coriat R, Courbon F, Couvelard A, Do Cao C, Frampas E, Gaujoux S, Gincul R, Goudet P, Lombard-Bohas C, Poncet G, Smith D, Ruszniewski P, Lecomte T, Bouché O, Walter T, Cadiot G; Thésaurus National de Cancérologie Digestive (TNCD). Digestive Neuroendocrine Neoplasms (NEN): French Intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2020;52:473-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 25. | Scott AT, Howe JR. Management of Small Bowel Neuroendocrine Tumors. Surg Oncol Clin N Am. 2020;29:223-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Scott AT, Howe JR. Management of Small Bowel Neuroendocrine Tumors. J Oncol Pract. 2018;14:471-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Hirabayashi K, Zamboni G, Nishi T, Tanaka A, Kajiwara H, Nakamura N. Histopathology of gastrointestinal neuroendocrine neoplasms. Front Oncol. 2013;3:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Sivero L, Telesca DA, Ruggiero S, Russo T, Amato M, Bianco T, Amato B, Formisano C, Avellino M, Napolitano V. Endoscopic diagnosis and treatment of neuroendocrine tumors of the digestive system. Open Med (Wars). 2016;11:369-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Toumpanakis C, Fazio N, Tiensuu Janson E, Hörsch D, Pascher A, Reed N, O Apos Toole D, Nieveen van Dijkum E, Partelli S, Rinke A, Kos-Kudla B, Costa F, Pape UF, Grozinsky-Glasberg S, Scoazec JY; The ENETS 2016 Munich Advisory Board Participants; ENETS 2016 Munich Advisory Board Participants. Unmet Needs in Appendiceal Neuroendocrine Neoplasms. Neuroendocrinology. 2019;108:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Broecker JS, Ethun CG, Postlewait LM, Le N, McInnis M, Russell MC, Sullivan P, Kooby DA, Staley CA, Maithel SK, Cardona K. Colon and Rectal Neuroendocrine Tumors: Are They Really One Disease? Am Surg. 2018;84:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | McDermott FD, Heeney A, Courtney D, Mohan H, Winter D. Rectal carcinoids: a systematic review. Surg Endosc. 2014;28:2020-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Cazacu IM, Singh BS, Luzuriaga Chavez AA, Koduru P, Ejaz S, Weston BR, Ross WA, Lee JH, Roy-Chowdhuri S, Bhutani MS. EUS and EUS-guided FNA/core biopsies in the evaluation of subepithelial lesions of the lower gastrointestinal tract: 10-year experience. Endosc Ultrasound. 2020;9:329-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Chen HT, Xu GQ, Teng XD, Chen YP, Chen LH, Li YM. Diagnostic accuracy of endoscopic ultrasonography for rectal neuroendocrine neoplasms. World J Gastroenterol. 2014;20:10470-10477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, Plöckinger U, Papotti M, Salazar R, Pascher A; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Kamp K, Damhuis RA, Feelders RA, de Herder WW. Occurrence of second primary malignancies in patients with neuroendocrine tumors of the digestive tract and pancreas. Endocr Relat Cancer. 2012;19:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K, Doi R, Shimatsu A, Nishida T, Komoto I, Hirata Y, Nakamura K, Igarashi H, Jensen RT, Wiedenmann B, Imamura M. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 37. | Massironi S, Sciola V, Peracchi M, Ciafardini C, Spampatti MP, Conte D. Neuroendocrine tumors of the gastro-entero-pancreatic system. World J Gastroenterol. 2008;14:5377-5384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1022] [Article Influence: 102.2] [Reference Citation Analysis (1)] |
| 39. | Puli SR, Kalva N, Bechtold ML, Pamulaparthy SR, Cashman MD, Estes NC, Pearl RH, Volmar FH, Dillon S, Shekleton MF, Forcione D. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: a systematic review and meta analysis. World J Gastroenterol. 2013;19:3678-3684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | James PD, Tsolakis AV, Zhang M, Belletrutti PJ, Mohamed R, Roberts DJ, Heitman SJ. Incremental benefit of preoperative EUS for the detection of pancreatic neuroendocrine tumors: a meta-analysis. Gastrointest Endosc. 2015;81:848-56.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Giuliani T, Marchegiani G, Girgis MD, Crinò SF, Muthusamy VR, Bernardoni L, Pea A, Ramera M, Paiella S, Landoni L, Gabbrielli A, Salvia R, Donahue TR, Bassi C. Endoscopic placement of pancreatic stent for "Deep" pancreatic enucleations operative technique and preliminary experience at two high-volume centers. Surg Endosc. 2020;34:2796-2802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Paiella S, Landoni L, Rota R, Valenti M, Elio G, Crinò SF, Manfrin E, Parisi A, Cingarlini S, D'Onofrio M, Scarpa A, Lawlor RT, Bernardoni L, Capelli P, Nessi C, Miotto M, Gabbrielli A, Bassi C, Salvia R. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors: a retrospective analysis of 110 cases. Endoscopy. 2020;52:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Boutsen L, Jouret-Mourin A, Borbath I, van Maanen A, Weynand B. Accuracy of Pancreatic Neuroendocrine Tumour Grading by Endoscopic Ultrasound-Guided Fine Needle Aspiration: Analysis of a Large Cohort and Perspectives for Improvement. Neuroendocrinology. 2018;106:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | Leeds JS, Nayar MK, Bekkali NLH, Wilson CH, Johnson SJ, Haugk B, Darne A, Oppong KW. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc Int Open. 2019;7:E1281-E1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Kamata K, Ashida R, Yasukawa S, Chiba Y, Fukutake N, Nebiki H, Kurita A, Takaoka M, Ogura T, Shiomi H, Asada M, Yasuda H, Shigekawa M, Yanagisawa A, Kudo M, Kitano M. Histological diagnosis and grading of pancreatic neuroendocrine tumor by endoscopic ultrasound-guided fine needle biopsy using a 25-gauge needle with a core trap: A multicenter prospective trial. Pancreatology. 2020;20:1428-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Crinò SF, Ammendola S, Meneghetti A, Bernardoni L, Conti Bellocchi MC, Gabbrielli A, Landoni L, Paiella S, Pin F, Parisi A, Mastrosimini MG, Amodio A, Frulloni L, Facciorusso A, Larghi A, Manfrin E. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology. 2021;21:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 47. | Zhang B, Zhu F, Li P, Yu S, Zhao Y, Li M. Endoscopic ultrasound elastography in the diagnosis of pancreatic masses: A meta-analysis. Pancreatology. 2018;18:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Iglesias-Garcia J, Larino-Noia J, Abdulkader I, Forteza J, Dominguez-Munoz JE. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 49. | Carrara S, Di Leo M, Grizzi F, Correale L, Rahal D, Anderloni A, Auriemma F, Fugazza A, Preatoni P, Maselli R, Hassan C, Finati E, Mangiavillano B, Repici A. EUS elastography (strain ratio) and fractal-based quantitative analysis for the diagnosis of solid pancreatic lesions. Gastrointest Endosc. 2018;87:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Ohno E, Kawashima H, Ishikawa T, Iida T, Suzuki H, Uetsuki K, Yashika J, Yamada K, Yoshikawa M, Gibo N, Aoki T, Kataoka K, Mori H, Yamamura T, Furukawa K, Nakamura M, Hirooka Y, Fujishiro M. Diagnostic performance of endoscopic ultrasonography-guided elastography for solid pancreatic lesions: Shear-wave measurements versus strain elastography with histogram analysis. Dig Endosc. 2021;33:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Cosgrove D, Piscaglia F, Bamber J, Bojunga J, Correas JM, Gilja OH, Klauser AS, Sporea I, Calliada F, Cantisani V, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Fromageau J, Havre RF, Jenssen C, Ohlinger R, Săftoiu A, Schaefer F, Dietrich CF; EFSUMB. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 640] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 52. | He XK, Ding Y, Sun LM. Contrast-enhanced endoscopic ultrasound for differential diagnosis of pancreatic cancer: an updated meta-analysis. Oncotarget. 2017;8:66392-66401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, Murakami T, Takeyama Y. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 54. | Takada S, Kato H, Saragai Y, Muro S, Uchida D, Tomoda T, Matsumoto K, Horiguchi S, Tanaka N, Okada H. Contrast-enhanced harmonic endoscopic ultrasound using time-intensity curve analysis predicts pathological grade of pancreatic neuroendocrine neoplasm. J Med Ultrason (2001). 2019;46:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Palazzo M, Napoléon B, Gincul R, Pioche M, Pujol B, Lefort C, Fumex F, Hautefeuille V, Fabre M, Cros J, Felce M, Couvelard A, Sauvanet A, Lévy P, Ruszniewski P, Palazzo L. Contrast harmonic EUS for the prediction of pancreatic neuroendocrine tumor aggressiveness (with videos). Gastrointest Endosc. 2018;87:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | De Luca L, Tommasoni S, de Leone A, Bianchi ML, de Nictolis M, Baroncini D. Neuroendocrine tumor of the extrahepatic bile duct: a tumor in an unusual site visualized by cholangioscopy. Endoscopy. 2013;45 Suppl 2:E338-E339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Altiti M, Al-Sa'afin AJ, Al-Tawarah T, Suleihat A, Abulhaj S, Mahseeri M. Biliary tree neuroendocrine tumor, an incidental finding. Int J Surg Case Rep. 2021;82:105940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 366] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 59. | Sato Y, Takeuchi M, Hashimoto S, Mizuno K, Kobayashi M, Iwafuchi M, Narisawa R, Aoyagi Y. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology. 2013;60:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (1)] |
| 60. | Kim HH, Kim GH, Kim JH, Choi MG, Song GA, Kim SE. The efficacy of endoscopic submucosal dissection of type I gastric carcinoid tumors compared with conventional endoscopic mucosal resection. Gastroenterol Res Pract. 2014;2014:253860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 61. | Noh JH, Kim DH, Yoon H, Hsing LC, Na HK, Ahn JY, Lee JH, Jung KW, Choi KD, Song HJ, Lee GH, Jung HY. Clinical Outcomes of Endoscopic Treatment for Type 1 Gastric Neuroendocrine Tumor. J Gastrointest Surg. 2021;25:2495-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | De Luca L, Di Berardino M, Mangiavillano B, Repici A. Gastric endoscopic submucosal dissection in Western countries: Indications, applications, efficacy and training perspective. World J Gastrointest Surg. 2021;13:1180-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Kwon YH, Jeon SW, Kim GH, Kim JI, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD, Moon JS. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. 2013;19:8703-8708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 64. | Gincul R, Ponchon T, Napoleon B, Scoazec JY, Guillaud O, Saurin JC, Ciocirlan M, Lepilliez V, Pioche M, Lefort C, Adham M, Pialat J, Chayvialle JA, Walter T. Endoscopic treatment of sporadic small duodenal and ampullary neuroendocrine tumors. Endoscopy. 2016;48:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 65. | Nishio M, Hirasawa K, Ozeki Y, Sawada A, Ikeda R, Fukuchi T, Kobayashi R, Makazu M, Sato C, Maeda S. Short- and long-term outcomes of endoscopic submucosal dissection for non-ampullary duodenal neuroendocrine tumors. Ann Gastroenterol. 2020;33:265-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Brito HP, Torres IT, Turke KC, Parada AA, Waisberg J, Botelho RV. Comparison of endoscopic resection techniques for duodenal neuroendocrine tumors: systematic review. Endosc Int Open. 2021;9:E1214-E1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Amoyel M, Belle A, Dhooge M, Ali EA, Hallit R, Prat F, Dohan A, Terris B, Chaussade S, Coriat R, Barret M. Endoscopic management of non-ampullary duodenal adenomas. Endosc Int Open. 2022;10:E96-E108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Shimai S, Yamamoto K, Sofuni A, Tsuchiya T, Ishii K, Tanaka R, Tonozuka R, Honjo M, Mukai S, Fujita M, Nagai K, Asai Y, Matsunami Y, Kurosawa T, Kojima H, Honma H, Minami H, Yamaguchi H, Itoi T. Three Cases of Ampullary Neuroendocrine Tumor Treated by Endoscopic Papillectomy: A Case Report and Literature Review. Intern Med. 2020;59:2369-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Son HJ, Sohn DK, Hong CW, Han KS, Kim BC, Park JW, Choi HS, Chang HJ, Oh JH. Factors associated with complete local excision of small rectal carcinoid tumor. Int J Colorectal Dis. 2013;28:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Kim KM, Eo SJ, Shim SG, Choi JH, Min BH, Lee JH, Chang DK, Kim YH, Rhee PL, Kim JJ, Rhee JC, Kim JY. Treatment outcomes according to endoscopic treatment modalities for rectal carcinoid tumors. Clin Res Hepatol Gastroenterol. 2013;37:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Yang DH, Park Y, Park SH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2016;83:1015-22; quiz 1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Nakamura K, Osada M, Goto A, Iwasa T, Takahashi S, Takizawa N, Akahoshi K, Ochiai T, Nakamura N, Akiho H, Itaba S, Harada N, Iju M, Tanaka M, Kubo H, Somada S, Ihara E, Oda Y, Ito T, Takayanagi R. Short- and long-term outcomes of endoscopic resection of rectal neuroendocrine tumours: analyses according to the WHO 2010 classification. Scand J Gastroenterol. 2016;51:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Jeon JH, Cheung DY, Lee SJ, Kim HJ, Kim HK, Cho HJ, Lee IK, Kim JI, Park SH, Kim JK. Endoscopic resection yields reliable outcomes for small rectal neuroendocrine tumors. Dig Endosc. 2014;26:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Kim HH, Park SJ, Lee SH, Park HU, Song CS, Park MI, Moon W. Efficacy of endoscopic submucosal resection with a ligation device for removing small rectal carcinoid tumor compared with endoscopic mucosal resection: analysis of 100 cases. Dig Endosc. 2012;24:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Lee J, Park YE, Choi JH, Heo NY, Park J, Park SH, Moon YS, Nam KH, Kim TO. Comparison between cap-assisted and ligation-assisted endoscopic mucosal resection for rectal neuroendocrine tumors. Ann Gastroenterol. 2020;33:385-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | de Mestier L, Brixi H, Gincul R, Ponchon T, Cadiot G. Updating the management of patients with rectal neuroendocrine tumors. Endoscopy. 2013;45:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 77. | Cheung DY, Choi SK, Kim HK, Kim SS, Chae HS, Seo KJ, Cho YS. Circumferential submucosal incision prior to endoscopic mucosal resection provides comparable clinical outcomes to submucosal dissection for well-differentiated neuroendocrine tumors of the rectum. Surg Endosc. 2015;29:1500-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | So H, Yoo SH, Han S, Kim GU, Seo M, Hwang SW, Yang DH, Byeon JS. Efficacy of Precut Endoscopic Mucosal Resection for Treatment of Rectal Neuroendocrine Tumors. Clin Endosc. 2017;50:585-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Huang J, Lu ZS, Yang YS, Yuan J, Wang XD, Meng JY, Du H, Wang HB. Endoscopic mucosal resection with circumferential incision for treatment of rectal carcinoid tumours. World J Surg Oncol. 2014;12:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Park SS, Han KS, Kim B, Chang Kim B, Hong CW, Sohn DK, Chang HJ. Comparison of underwater endoscopic mucosal resection and endoscopic submucosal dissection of rectal neuroendocrine tumors (with videos). Gastrointest Endosc. 2020;91:1164-1171.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 81. | Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, Cassese G, Pagano G, Tropeano FP, Luglio G, De Palma GD. Diagnosis and Management of Rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 82. | Zhou X, Xie H, Xie L, Li J, Cao W, Fu W. Endoscopic resection therapies for rectal neuroendocrine tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 83. | Yong JN, Lim XC, Nistala KRY, Lim LKE, Lim GEH, Quek J, Tham HY, Wong NW, Tan KK, Chong CS. Endoscopic submucosal dissection versus endoscopic mucosal resection for rectal carcinoid tumor. A meta-analysis and meta-regression with single-arm analysis. J Dig Dis. 2021;22:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 84. | Ramage JK, De Herder WW, Delle Fave G, Ferolla P, Ferone D, Ito T, Ruszniewski P, Sundin A, Weber W, Zheng-Pei Z, Taal B, Pascher A; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 85. | Meier B, Albrecht H, Wiedbrauck T, Schmidt A, Caca K. Full-thickness resection of neuroendocrine tumors in the rectum. Endoscopy. 2020;52:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 86. | Melita G, Pallio S, Tortora A, Crinò SF, Macrì A, Dionigi G. Diagnostic and Interventional Role of Endoscopic Ultrasonography for the Management of Pancreatic Neuroendocrine Neoplasms. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 87. | Rimbas M, Larghi A, Fusaroli P, Dong Y, Hollerbach S, Jenssen C, Săftoiu A, Sahai AV, Napoleon B, Arcidiacono PG, Braden B, Burmeister S, Carrara S, Cui XW, Hocke M, Iglesias-Garcia J, Kitano M, Oppong KW, Sun S, Di Leo M, Petrone MC, B Teoh AY, Dietrich CF. How to perform EUS-guided tattooing? Endosc Ultrasound. 2020;9:291-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Rosa F, Rimbaș M, Rizzatti G, Quero G, Fiorillo C, Impagnatiello M, D'Aversa F, Costamagna G, Alfieri S, Larghi A. EUS-guided fine needle tattooing (EUS-FNT) for preoperative localization of small pancreatic neuroendocrine tumors (p-NETs): a single-center experience. Surg Endosc. 2021;35:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 89. | Law JK, Singh VK, Khashab MA, Hruban RH, Canto MI, Shin EJ, Saxena P, Weiss MJ, Pawlik TM, Wolfgang CL, Lennon AM. Endoscopic ultrasound (EUS)-guided fiducial placement allows localization of small neuroendocrine tumors during parenchymal-sparing pancreatic surgery. Surg Endosc. 2013;27:3921-3926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Zhang L, Tan S, Huang S, Zhong C, Lü M, Peng Y, Tang X. The safety and efficacy of endoscopic ultrasound-guided ablation therapy for solid pancreatic tumors: a systematic review. Scand J Gastroenterol. 2020;55:1121-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 91. | Testoni SGG, Healey AJ, Dietrich CF, Arcidiacono PG. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc Ultrasound. 2020;9:83-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 92. | Jürgensen C, Schuppan D, Neser F, Ernstberger J, Junghans U, Stölzel U. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc. 2006;63:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 93. | Levy MJ, Thompson GB, Topazian MD, Callstrom MR, Grant CS, Vella A. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointest Endosc. 2012;75:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Qin SY, Lu XP, Jiang HX. EUS-guided ethanol ablation of insulinomas: case series and literature review. Medicine (Baltimore). 2014;93:e85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Choi JH, Park DH, Kim MH, Hwang HS, Hong SM, Song TJ, Lee SS, Seo DW, Lee SK. Outcomes after endoscopic ultrasound-guided ethanol-lipiodol ablation of small pancreatic neuroendocrine tumors. Dig Endosc. 2018;30:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 97. | Limmer S, Huppert PE, Juette V, Lenhart A, Welte M, Wietholtz H. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol. 2009;21:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Rossi S, Viera FT, Ghittoni G, Cobianchi L, Rosa LL, Siciliani L, Bortolotto C, Veronese L, Vercelli A, Gallotti A, Ravetta V. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 99. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 100. | Oleinikov K, Dancour A, Epshtein J, Benson A, Mazeh H, Tal I, Matalon S, Benbassat CA, Livovsky DM, Goldin E, Gross DJ, Jacob H, Grozinsky-Glasberg S. Endoscopic Ultrasound-Guided Radiofrequency Ablation: A New Therapeutic Approach for Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab. 2019;104:2637-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alali AA, Kuwait; Hu HG, China; Shah OJ, India; Wang WQ, China S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H