Published online Sep 7, 2022. doi: 10.3748/wjg.v28.i33.4875
Peer-review started: May 4, 2022
First decision: June 2, 2022
Revised: July 7, 2022
Accepted: August 6, 2022
Article in press: August 6, 2022
Published online: September 7, 2022
Processing time: 118 Days and 22.9 Hours
Achalasia is a rare benign esophageal motor disorder characterized by incomplete relaxation of the lower esophageal sphincter (LES). The treatment of achalasia is not curative, but rather is aimed at reducing LES pressure. In patients who have failed noninvasive therapy, surgery should be considered. Myotomy with partial fundoplication has been considered the first-line treatment for non-advanced achalasia. Recently, peroral endoscopic myotomy (POEM), a technique that employs the principles of submucosal endoscopy to perform the equivalent of a surgical myotomy, has emerged as a promising minimally invasive technique for the management of this condition.
To compare POEM and laparoscopic myotomy and partial fundoplication (LM-PF) regarding their efficacy and outcomes for the treatment of achalasia.
Forty treatment-naive adult patients who had been diagnosed with achalasia based on clinical and manometric criteria (dysphagia score ≥ II and Eckardt score > 3) were randomized to undergo either LM-PF or POEM. The outcome measures were anesthesia time, procedure time, symptom improvement, reflux esophagitis (as determined with the Gastroesophageal Reflux Disease Questionnaire), barium column height at 1 and 5 min (on a barium esophagogram), pressure at the LES, the occurrence of adverse events (AEs), length of stay (LOS), and quality of life (QoL).
There were no statistically significant differences between the LM-PF and POEM groups regarding symptom improvement at 1, 6, and 12 mo of follow-up (P = 0.192, P = 0.242, and P = 0.242, res
POEM and LM-PF appear to be equally effective in controlling the symptoms of achalasia, shortening LOS, and minimizing AEs. Nevertheless, POEM has the advantage of improving all domains of QoL, and shortening anesthesia and procedure times but with a significantly higher rate of gastroesophageal reflux.
Core Tip: This randomized controlled trial compared the efficacy and outcomes of laparoscopic myotomy and partial fundoplication (LM-PF) with those of peroral endoscopic myotomy (POEM) for the treatment of patients with achalasia of any etiology. There were no statistically significant differences between the LM-PF and POEM groups regarding symptoms. However, the rates of reflux esophagitis were significantly higher in the POEM group. POEM and LM-PF appear to be equally effective in controlling the symptoms of achalasia, shortening length of hospital stay, and minimizing adverse events. However, POEM has the advantage of shortening anesthesia and procedure times.
- Citation: de Moura ETH, Jukemura J, Ribeiro IB, Farias GFA, de Almeida Delgado AA, Coutinho LMA, de Moura DTH, Aissar Sallum RA, Nasi A, Sánchez-Luna SA, Sakai P, de Moura EGH. Peroral endoscopic myotomy vs laparoscopic myotomy and partial fundoplication for esophageal achalasia: A single-center randomized controlled trial. World J Gastroenterol 2022; 28(33): 4875-4889
- URL: https://www.wjgnet.com/1007-9327/full/v28/i33/4875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i33.4875
Achalasia is a rare benign esophageal motor disorder characterized by incomplete relaxation of the lower esophageal sphincter (LES)[1-3]. For primary or idiopathic achalasia, the underlying etiology has yet to be clearly defined; secondary achalasia results from any one of several systemic diseases including infectious, autoimmune, and drug-induced disorders[4-6]. In both cases, the most common symptoms are progressive dysphagia, regurgitation, and weight loss. The symptom intensity and treatment response can be assessed with validated scales such as the Eckardt score[2,7,8]. The diagnosis requires the proper integration between reported symptoms and the interpretation of diagnostic tests such as a barium esophagogram, esophagogastroduodenoscopy (EGD), and manometry—either con
The treatment of achalasia is not curative but rather is aimed at reducing LES pressure[13-17]. In patients who have failed noninvasive therapy, surgery should be considered[18]. Myotomy with partial fundoplication has been considered the first-line treatment for non-advanced achalasia[19].
Recently, peroral endoscopic myotomy (POEM), a technique that employs the principles of submucosal endoscopy to perform the equivalent of a surgical myotomy, has emerged as a promising minimally invasive technique for the management of this condition[20]. This technique was first described in 1980 and subsequently modified to create what is now POEM[21,22].
This randomized controlled trial (RCT) compared the efficacy and outcomes of laparoscopic myotomy and partial fundoplication (LM-PF) with those of POEM for the treatment of patients with achalasia of any etiology. We also compared the two procedures in terms of the incidence of reflux esophagitis.
This was a single-center RCT in which we evaluated 40 treatment-naive patients with esophageal achalasia. We included patients ≥ 18 years of age who had been diagnosed with achalasia based on clinical and manometric criteria (dysphagia score ≥ II and Eckardt score > 3) and who provided informed consent. Patients who had previously undergone endoscopic or surgical procedures involving the esophagogastric junction (EGJ) were excluded, as were those with liver cirrhosis, esophageal varices, Barrett’s esophagus, esophageal strictures, premalignant or malignant EGJ lesions, coagulopathies, pseudoachalasia, esophageal diverticulum, severe cardiopulmonary diseases, or severe systemic diseases, as well as those who were at high surgical risk and those who were pregnant or lactating.
An investigator who was unaffiliated with the trial created the randomization list. Specific software (www.randomizer.org) was used, and participants were randomly allocated at a 1:1 ratio to the experimental (POEM) group or the comparison (LM-PF) group.
The sample size was calculated to identify statistical significance between LM-PF and POEM regarding reflux esophagitis rates, which were assumed to be 5% and 40% after LM-PF and POEM, respectively[23]. To achieve a power of 80% with an alpha of 0.05, we estimated the minimum sample size to be 38 (19 patients in each group). Taking potential losses into consideration, we chose to include a total of 40 patients.
POEM: All POEM procedures were performed by a single operator with extensive experience in the technique. Prophylactic intravenous antibiotics and a proton pump inhibitor (PPI) were administered 30 min before intubation and general anesthesia.
After the gastroscope was introduced, the esophageal lumen and mucosa were thoroughly cleaned. This was followed by submucosal injection of 10 mL of 0.5% indigo carmine. An incision was made into the mucosa of the posterior wall, between 5 and 6 o’clock, at 10 cm above the EGJ. The incision was made with a dual-function submucosal dissection knife (HybridKnife; Erbe, Tübingen, Germany) in Endocut Q mode (effect 2, width 3, and interval 5). Subsequently, spray coagulation (effect 2 at 40 W) was used to create a submucosal tunnel extending 3-4 cm beyond the EGJ into the proximal stomach. In all patients, full-thickness myotomy—including the circular and longitudinal muscle layers—was performed in Endocut Q mode. The myotomy was initiated 2 cm distal from the mucosal entry point and extended 3-4 cm into the proximal stomach. The mucosal incision was closed by using through-the-scope clips (Supplementary Figures 1 and 2).
A barium esophagogram was obtained on postoperative day 1. In the absence of complications, the patient was started on a clear liquid diet and subsequently advanced to a full liquid diet for 14 d.
LM-PF: All LM-PF procedures were performed by members of the foregut surgery group. After pneumoperitoneum had been established, five trocars were positioned, after which the left hepatic lobe was retracted to access the EGJ (Supplementary Figure 3). That was followed by division of the phrenoesophageal ligament, dissection, and isolation of the distal esophagus from adjacent structures; and anterolateral dislocation of the distal esophagus. The anterior gastric adipose tissue and the anterior vagus nerve were dissected and separated from the esophagus and stomach, after which myotomy of the circular and longitudinal muscle layers was performed, extending from 5-6 cm above the EGJ to 2-3 cm below the EGJ. Partial fundoplication between the esophagus and stomach was then performed by placing sutures in three planes: Posterior—two to three sutures; left lateral—four to five sutures (on the left side); and right anterior—a line of sutures covering the myotomy, thus interposed with the gastric fundus on the right. In the absence of complications, patients were started on a clear liquid diet on the morning following the procedure and maintained on a mechanical soft diet for 14 d after discharge.
Clinical assessments: Although achalasia subtyping is defined based on HRM, in this study, the achalasia subtype was evaluated according to the degree of esophageal dilation on the barium esophagogram and esophageal motor activity on EM or HRM. Given that Chagas disease, which often involves the esophagus, is common in Brazil, all patients were screened for anti-Trypanosoma cruzi antibodies by enzyme-linked immunosorbent assay and indirect immunofluorescence. Weight loss, dysphagia, and pain were assessed before the procedure, as well as at 6 and 12 mo after the procedure, by using the Eckardt score. Patients with an Eckardt score ≥ 3 were categorized as symptomatic. The clinical evaluation of gastroesophageal reflux (GER) and the diagnosis of GER disease (GERD) was based on the application of the GER Disease Questionnaire (GerdQ)[24] (Supplementary Figure 4).
EGD: We performed EGD before the procedure, as well as at 6 and 12 mo after. Esophagitis was graded according to the Los Angeles classification system[25]. We performed chromoendoscopy with narrow-band imaging and 2.5% Lugol’s solution to screen for esophageal cancer. Suspicious lesions were biopsied.
Barium esophagogram: To assess esophageal emptying before and 12 mo after the procedure, we used a timed barium esophagogram, as previously described[26]. The degree of esophageal emptying is qualitatively estimated by comparing 1- and 5-min images or by measuring the height and width (in centimeters) of the barium column at both times, calculating the approximate area, and determining the percentage change in the area.
EM: Conventional EM was performed before and 12 mo after the procedure. It should be noted that HRM technology was not available in Brazil when the trial began. To perform conventional EM, we used an eight-channel computerized polygraph under pneumohydraulic capillary infusion at a flow rate of 0.6 mL/min/channel. Preparation was required with a 12-h fast and suspension of medications that alter esophageal motility. The technique consists of passing a probe through the nostril and checking the position in the stomach through deep inspiration. With the patient in the supine position, the probe is pulled centimeter by centimeter to measure the mean respiratory pressure and pressure inversion point, and then one of the channels is positioned distal to 3 cm from the upper edge of the LES and the other channels are distant 5 cm apart. Finally, the catheter is pulled up to the upper esophageal sphincter. Through the average of the four distal radial channels of the conventional manometry catheter, the maximum expiratory pressure (MEP) was identified, which best represents the LES pressure itself.
Quality of life: To evaluate the quality of life (QoL), we used the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36)[27,28]. The SF-36 comprises 36 questions covering eight domains: Physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health.
Adverse events: Among the adverse events (AEs) recorded, pneumoperitoneum requiring drainage or puncture was categorized as a minor AE, as was minor mucosal damage requiring endoscopic closure. Major AEs were defined as pneumoperitoneum leading to hemodynamic instability and premature interruption of the procedure; bleeding requiring a blood transfusion and accompanied by hemo
For POEM and LM-PF, the following outcome measures were evaluated: Operative time; length of the myotomy in the esophagus and stomach; myotomy site; complications; and LOS. Patient data were collected on the Research Electronic Data Capture platform.
At 1, 6, and 12 mo after the interventions, the Eckardt score was determined, the SF-36 was applied, EGD was performed, and timed barium esophagograms were obtained. Conventional EM was performed at 6 and 12 mo. Patients received the maximum dose of PPI for the first 30 d postprocedure, and those who presented with erosive esophagitis at follow-up endoscopy were maintained on PPI treatment for 8 wk. Treatment success was defined as symptom improvement (≤ 3-point reduction in the Eckardt score), an LES pressure < 15 mmHg[30-32], and a > 50% reduction in the height of the barium column at 1 min. Treatment failure was defined as symptom persistence in patients with an Eckardt score ≥ 3.
We performed descriptive analyses of all study variables. Quantitative variables were expressed as means with standard deviations or as medians with interquartile ranges. Qualitative variables are expressed as absolute and relative frequencies. For the comparison of means between the two groups, the Student’s t-test was used. When the assumption of normality was rejected, the nonparametric Mann-Whitney test was used. To test the homogeneity between proportions, the chi-square test or Fisher’s exact test was used. Repeated-measures analysis of variance was used to compare the groups over the course of the study. When the assumption of normality was rejected, the nonparametric Mann-Whitney test and Friedman test were used. The data were processed with the SPSS Statistics software package, version 17.0 for Windows (SPSS Inc., Chicago, IL, United States). P < 0.05 was considered statistically significant.
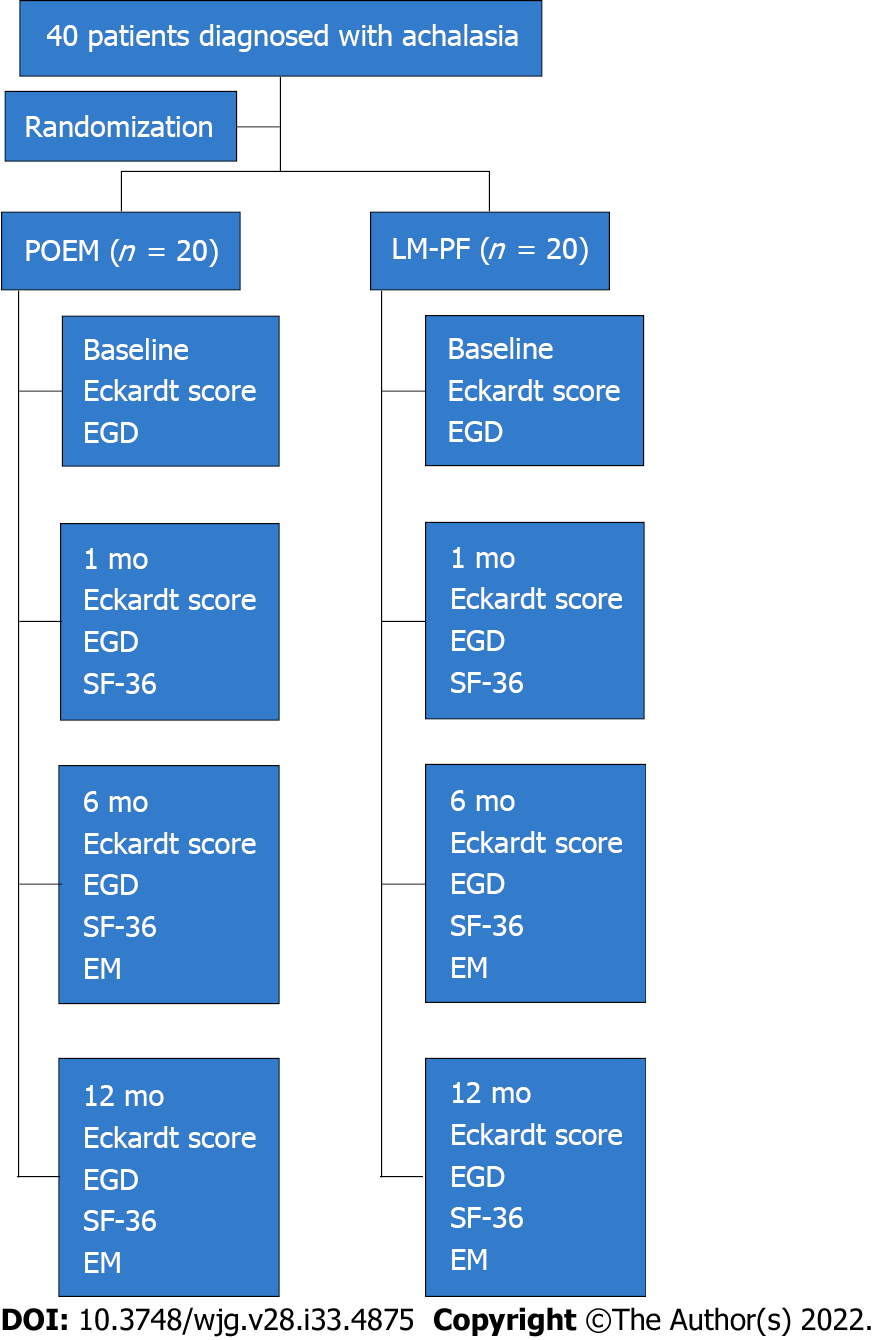
Between March 2017 and February 2018, 40 patients diagnosed with achalasia were enrolled and randomized to undergo LM-PF (n = 20) or POEM (n = 20), as detailed in Figure 1. Nine (22.5%) of the forty patients (five in the POEM group and four in the LM-PF group) tested positive for anti-T. cruzi antibodies, indicating exposure to Chagas disease. At baseline, there was no statistical difference between the two groups in terms of sex, age, etiology, grade, symptom duration, weight, body mass index, or Eckardt score (Table 1). The study was terminated after all patients had been followed for at least 12 mo.
| Variable | Total (n = 40) | Group | P value | |
| LM-PF (n = 20) | POEM (n = 20) | |||
| Male, n (%) | 26 (65) | 14 (70.0) | 12 (60.0) | 0.507 |
| Age in yr, mean ± SD | 44.55 (13.77) | 44.20 (13.21) | 44.90 (14.64) | 0.875 |
| Etiology of achalasia, n (%) | 1.000 | |||
| Chagas disease | 9 (22.5) | 4 (20.0) | 5 (25.0) | |
| Idiopathic | 31 (77.5) | 16 (80.0) | 15 (75.0) | |
| BMI in kg/m2, mean ± SD | 22.78 (4.49) | 22.79 (4.41) | 22.77 (4.70) | 0.988 |
| Eckardt score, median (IQR) | 8.00 (6.25-9.00) | 8.50 (7.25-9.75) | 8.00 (6.00-9.00) | 0.478 |
Eckardt scores at 1, 6, and 12 mo were lower than the baseline scores in both groups—1.0, 0.5, and 0.50, respectively, vs 8.0, in the POEM group; and 0.0, 0.0, and 0.0, respectively, vs 8.5, in the LM-PF group—and the differences were statistically significant (P < 0.001; Table 2). There were no statistical differences between the two groups for the Eckardt scores at 1, 6, and 12 mo of follow-up (P = 0.192, P = 0.242, and P = 0.242, respectively).
| Time point | Total (n = 40), median (IQR) | Group | P value1 | |
| LM-PF (n = 20), median (IQR) | POEM (n = 20), median (IQR) | |||
| Baseline | 8.00 (6.25-9.00) | 8.50 (7.25-9.75) | 8.00 (6.00-9.00) | 0.478 |
| 1 mo | 0.00 (0.00-1.00) | 0.00 (0.00-1.00) | 1.00 (0.00-1.00) | 0.192 |
| 6 mo | 0.00 (0.00-1.75) | 0.00 (0.00-1.00) | 0.50 (0.00-2.00) | 0.242 |
| 12 mo | 0.00 (0.00-1.75) | 0.00 (0.00-1.00) | 0.50 (0.00-2.00) | 0.242 |
In the POEM group, treatment success was confirmed at 1 mo in all 20 patients, at 6 mo in 18 of the patients (90%), and at 12 mo in 19 (95%). In the LM-PF group, treatment success was confirmed at 1 mo and was maintained at 6 and 12 mo in all 20 patients. As shown in Table 3, there was no statistical difference between the two groups regarding treatment success at 1, 6, or 12 mo (P = 0.487 and P = 1.000 for 6 and 12 mo, respectively).
| Time point | Total (n = 40), n (%) | Group | P value1 | |
| LM-PF (n = 20), n (%) | POEM (n = 20), n (%) | |||
| 1 mo | 40 (100.0) | 20 (100.0) | 20 (100.0) | - |
| 6 mo | 38 (95.0) | 20 (100.0) | 18 (90.0) | 0.487 |
| 12 mo | 39 (97.50) | 20 (100.0) | 19 (95.0) | 1.000 |
In both groups, there were significant postprocedural improvements in dysphagia, although the differences were not significant at 1, 6, or 12 mo (P = 0.602; P = 0.565, and P = 0.547, respectively). However, statistically significant improvements in weight loss, chest pain, and regurgitation were observed in both groups (Supplementary Tables 1 and 2). The postprocedure rate of GER, as assessed with the GerdQ, was higher in the POEM group than in the LM-PF group (64.6% vs 11.1%; P < 0.02).
At 1, 6, and 12 mo, only 20, 18, and 18 POEM group patients, respectively, underwent EGD, as did only 17, 16, and 17 LM-PF group patients, respectively. The remaining patients declined to undergo EGD because they were asymptomatic. The rates of esophagitis were significantly higher in the POEM group than in the LM-PF group at 1, 6, and 12 mo of follow-up (P = 0.014, P < 0.001, and P = 0.002, respectively). In the LM-PF group, 1 patient had esophagitis (classified as grade A) at 6 mo and 2 patients had esophagitis (classified as grades B and C, respectively) at 12 mo. In the POEM group, esophagitis was observed at 1 mo in 5 patients (being classified as grade A in one, grade B in three, and grade C in one), at 6 mo in 10 patients (being classified as grade A in three, grade B in two, and grade C in five), and at 12 mo in 11 patients (being classified as grade A in five, grade C in four, and grade D in two). At 1, 6, and 12 mo, the rates of esophagitis were 0.0%, 5.6%, and 11.1%, respectively, in the LM-PF group and 29.4%, 62.5%, and 64.6%, respectively, in the POEM group (Table 4).
| Time point | n (%) of Total | Group | P value1 | |
| LM-PF, n (%) of Total | POEM, n (%) of Total | |||
| Baseline | 1 (2.5) of 40 | 0 (0.0) of 20 | 1 (5.0) of 20 | 1.000 |
| 1 mo | 5 (13.5) of 37 | 0 (0.0) of 20 | 5 (29.4) of 17 | 0.014 |
| 6 mo | 11 (32.4) of 34 | 1 (5.6) of 18 | 10 (62.5) of 16 | < 0.001 |
| 12 mo | 13 (37.1) of 35 | 2 (11.1) of 18 | 11 (64.6) of 17 | 0.002 |
Table 5 shows the results of the barium esophagogram. In both groups, the heights of the barium column at 1 and 5 min were significantly lower at 1, 6, and 12 mo than at baseline (P < 0.001). There was no statistical difference between the two groups regarding the barium column height values at 1 and 5 min in the follow-up periods (intent-to-treat analysis: P = 0.429 and 0.773; per-protocol analysis: P = 0.505 and 0.922).
| Variable | Total (n = 40) | Group | |
| LM-PF (n = 20) | POEM (n = 20) | ||
| Height at 1 min | |||
| Baseline | 17.48 (8.11) | 16.97 (6.70) | 17.99 (9.46) |
| 1 mo | 9.90 (5.88) | 11.39 (4.18) | 8.42 (6.99) |
| 6 mo | 8.91 (4.49) | 9.61 (3.72) | 8.22 (5.15) |
| 12 mo | 10.35 (3.38) | 10.98 (2.73) | 9.73 (3.90) |
| Height at 5 min | |||
| Baseline | 15.31 (8.13) | 14.92 (7.06) | 15.69 (9.24) |
| 1 mo | 5.53 (5.41) | 6.30 (4.84) | 4.75 (5.95) |
| 6 mo | 5.69 (5.14) | 5.99 (4.56) | 5.39 (5.77) |
| 12 mo | 8.31 (4.96) | 8.66 (4.92) | 7.97 (5.10) |
In both groups, the MEP values were significantly lower at 6 and 12 mo than at baseline (Table 6). There was no statistical difference between the two groups at either of those time points (intention-to-treat analysis: P = 0.848).
| Variable | Time point | Total (n = 40) | Group | |
| LM-PF (n = 20) | POEM (n = 20) | |||
| Maximal expiratory pressure | Baseline | 25.98 (10.42) | 24.53 (9.90) | 27.43 (10.98) |
| 6 mo | 10.44 (5.86) | 9.93 (5.45) | 10.94 (6.34) | |
| 12 mo | 10.11 (5.09) | 9.34 (4.19) | 10.87 (5.85) | |
Table 7 describes the AEs, LOS, anesthesia time, and procedure time, in the sample as a whole and by groups. There was no statistical difference between the two groups regarding the rate of AEs (P = 0.605). The relevant complications observed in the immediate postprocedural period included empyema requiring thoracostomy in one (5%) of the LM-PF patients, and inadvertent intraoperative mucosal damage in three (15%) of the POEM patients (treated with endoscopic clipping). The clinical outcomes were favorable in all patients. The mean LOS was 3.95 ± 3.36 d in the LM-PF group, compared with 3.40 ± 0.75 d in the POEM group (P = 0.483). The mean anesthesia time and mean procedure time were both shorter in the POEM group than in the LM-PF group (185.00 ± 56.89 and 95.70 ± 30.47 min, respectively, vs 296.75 ± 56.13 and 218.75 ± 50.88 min, respectively; P < 0.001 for both).
| Variable | Total (n = 40) | Group | P value | |
| LM-PF (n = 20) | POEM (n = 20) | |||
| Adverse events, n (%) | 4 (10.0) | 1 (5.0) | 3 (15.0) | 0.605a |
| Length of hospital stay in d | 3.68 (2.42) | 3.95 (3.36) | 3.40 (0.75) | 0.483b |
| Anesthesia time in min | 240.88 (79.46) | 296.75 (56.13) | 185.00 (56.89) | < 0.001b |
| Procedure time in min | 157.23 (74.81) | 218.75 (50.88) | 95.70 (30.47) | < 0.001b |
Table 8 shows the results obtained with the SF-36. In the POEM group, there were postprocedural improvements in all SF-36 domains, whereas there were improvements in only three domains (physical functioning, energy/fatigue, and general health) in the LM-PF group.
| SF-36 domain | Baseline score | P value | Score at 12 mo | P value | ||
| LM-PF (n = 20), median (IQR) | POEM (n = 20), median (IQR) | LM-PF (n = 20), median (IQR) | POEM (n = 20), median (IQR) | |||
| Physical functioning | 95.00 (70.0-100.0) | 77.50 (62.25-95.0) | 0.165 | 100.0 (90.0-100.0) | 92.50 (66.25-100.0) | 0.183 |
| Role-physical | 100.0 (56.25-100.0) | 25.0 (0.00-68.75) | 0.012 | 100.0 (100.0-100.0) | 100.0 (56.25-100.0) | 0.445 |
| Role-emotional | 100.0 (33.30-100.0) | 33.30 (0.00-66.70) | 0.021 | 100.0 (66.70-100.0) | 100.0 (41.65-100.0) | 0.640 |
| Vitality | 67.5 (46.25-88.75) | 52.50 (36.25-65.00) | 0.052 | 85.0 (56.25-100.0) | 80.0 (56.25-90.0) | 0.341 |
| Mental health | 74.0 (54.0-95.0) | 58.0 (44.0-75.0) | 0.030 | 94.0 (69.0-100.0) | 80.0 (57.0-91.0) | 0.174 |
| Social functioning | 81.25 (62.50-100.0) | 56.25 (37.50-71.88) | 0.006 | 100.0 (78.13-100.0) | 93.75 (40.63-100.0) | 0.174 |
| Bodily pain | 73.75 (49.38-97.50) | 62.50 (45.0-79.38) | 0.201 | 90.0 (78.13-100.0) | 80.0 (55.0-97.50) | 0.142 |
| General health | 50.0 (31.25-83.75) | 55.0 (41.25-65.0) | 0.698 | 82.50 (61.25-100.0) | 67.50 (52.50-100.0) | 0.445 |
In this single-center RCT comparing POEM and LM-PF in treatment-naive patients with achalasia, a significant proportion of the patients evaluated had achalasia attributed to Chagas disease. In a study by Farias et al[33], no statistical difference was observed between idiopathic and Chagas disease-associated achalasia regarding treatment success and AEs with POEM.
For years, LM-PF has been considered the gold-standard treatment for achalasia[34], because it provides good clinical results, has a low reintervention rate, and has adequate reproducibility. In the first study involving the use of endoscopic myotomy[21], conducted in 1980, all 17 of the patients in the sample showed symptom improvement. Although, technical improvements proposed by Inoue et al[22] in 2010 and several cohort studies comparing POEM and LM-PF[35-45] over the last decade have proved its safety and efficacy in the management of achalasia, the POEM technique is still not fully standardized[22].
The first RCT comparing the two techniques in the treatment of idiopathic achalasia[46], including 221 patients, demonstrated clinical success rates at 1 year and 2 years of follow-up of 84.8% and 83.0%, respectively, in the POEM group, comparable to the 83.5% and 81.7%, respectively, observed for the LM-PF group. In our study, the clinical success rate at the end of the 1st year was 95% in the POEM group and 100% in the LM-PF group, with no statistical difference between the two techniques. This discrepancy between our results and those of the earlier trial may be related to the fact that approximately 35% of the patients evaluated in that trial had previously received some type of treatment, which could have increased the degree of technical difficulty in dissection secondary to submucosal fibrosis.
We observed no statistical differences between the two techniques concerning Eckardt scores for dysphagia, regurgitation, chest pain, and weight loss, at 1, 6, and 12 mo of follow-up, which demonstrates the noninferiority of POEM to the LM-PF.
Immediate postprocedural complications occurred in 10% of the 40 patients evaluated in the present study. There were no cases of death in our sample, and the rate of AEs did not differ significantly between the two techniques. In our study, all POEM procedures involved a full-thickness myotomy, which made pneumoperitoneum an expected event. Pneumoperitoneum is a common finding after POEM and is not indicative of an unfavorable outcome for the patient. We categorized pneumoperitoneum as an AE only if abdominal decompression was required.
Anesthesia and procedure times were shorter for POEM than for LM-PF. That can be explained by the fact that the POEM involved full-thickness myotomy and did not involve fundoplication. There was no difference between the two procedures in terms of LOS and QoL.
We found that POEM and LM-PF both resulted in significant decreases in the 1- and 5-min barium column heights at 1, 6, and 12 mo after the procedures, demonstrating a clear decrease in resistance to the passage of contrast at the level of the EGJ. Sanagapalli et al[47] showed an association of significant improvement in symptoms when there is a mean reduction in the residual barium column height by about 53%. The LES pressure (MEP) on conventional EM was significantly lower throughout the follow-up period than at baseline, and there was no significant difference between the two groups.
In this study, the rates of treatment success were comparable between surgical and endoscopic myotomy, both providing symptom improvement, as well as objective improvement in radiological and manometric parameters, at 1, 6, and 12 mo. A recent systematic review and meta-analysis demonstrated that the incidence of GER is higher after POEM than after laparoscopic Heller myotomy[48]. That is in agreement with our findings. The evaluation of GER in our study was based on the typical clinical manifestations of GERD or the identification of erosive esophagitis by EGD. All patients with symptoms and suggestive endoscopic findings of GER received PPI treatment with suspension or maintenance according to the clinical and endoscopic response. A significant limitation of our study was the absence of pHmetry evaluation, which is the main method for GERD evaluation. Prior to our study, we con
Erosive esophagitis, especially grade C or D, is considered indicative of GER after endoscopy in patients without a history of the condition[50]. We consider that patients undergoing POEM have a wider esophagogastric transition that favors a higher rate of GER compared to LM-PF, despite similar LES pressures between the groups. Werner et al[46] also showed more GER in patients undergoing POEM despite no differences in manometry compared to LM-PF.
The POEM technique has undergone numerous changes since its initial description by Inoue et al[22]. It has been shown that short- to medium-term efficacy is comparable between myotomy of the circular muscle layer only and full-thickness myotomy, as well as that the latter, despite significantly reducing the duration of POEM, may increase the risk of GERD[51,52]. Likewise, there is uncertainty about whether myotomy should be performed in the anterior or posterior wall, the latter technique being associated with a higher incidence of GER[53,54], although other studies have failed to demonstrate that[55,56]. In the present study, we chose a long posterior full-thickness myotomy, because of the greater technical ease[43,57,58].
The results obtained in our study corroborate those of a previous study demonstrating the noninferiority of POEM to LM-PF for symptom control in patients with achalasia, except for postprocedure GER[46]. That raises the question of which technical changes we should study. Therefore, it is valid to perform in-depth analyses of oblique fiber preservation techniques[59], as well as the use of POEM with fundoplication[60,61]. One study[58] demonstrated that preservation of the oblique muscle, using the two penetrating vessels as an anatomical landmark, can significantly reduce the frequency of post-POEM GER, although that should be interpreted with caution because it was a retrospective cohort study, without strict methodological criteria, and with limited reproducibility. In the present study, we employed the conventional POEM technique as previously described[62], and the preservation of the two penetrating vessels was not standardized. The postprocedural occurrence of GERD symptoms in our sample was > 50%, similar to what has been reported by other authors. Despite not including patients undergoing POEM, a recent study[63] showed that achalasia patients with post-treatment reflux symptoms demonstrate esophageal hypersensitivity to chemical and mechanical stimuli, which may determine symptom generation.
Another strategy proposed to minimize the occurrence of GER after POEM is performing transoral incisionless fundoplication. In one pilot study[60], that procedure was reported to have a 100% success rate in terms of symptom control, acid exposure time, and the need for antisecretory drugs. In another pilot study[61], standard POEM combined with endoscopic fundoplication (POEM-F) was employed, and no complications were observed. A recent retrospective study followed patients for 12 mo after POEM-F[64], and showed that the incidence of postprocedural GER was only 11.1%. Albeit attractive, POEM-F has several potential limitations[65]. First, it is necessary to perform POEM in the anterior wall, contrary to the current trend of using a posterior wall approach. Second, it may not be possible to perform POEM-F in patients who have previously undergone anterior myotomy and experience symptom recurrence due to submucosal fibrosis. Third, the long-term durability of this type of fundoplication is still unknown.
In our opinion, it will take some time for the literature to reveal whether endoscopic or surgical myotomy is the best long-term option for the treatment of achalasia. Two crucial points that weigh unfavorably on the POEM procedure, in terms of the possibility that it will come to be widely indicated for the treatment of achalasia[66,67]. The first is the paucity of high-quality (randomized) technical studies comparing POEM with the well-established techniques of pneumatic dilatation of the cardia and laparoscopic myotomy with fundoplication, which could show, at least, the noninferiority of POEM. The second is the lack of studies with long (> 5 years) follow-up periods, which could demonstrate the true reintervention rate, based on the identification of serious late complications, including GER requiring fundoplication and dysphagia resulting from an inadequate myotomy[68]. Currently, the results at 2-3 years are similar between the endoscopic and surgical myotomy techniques concerning the clinical parameters, except for the greater occurrence of GER after the endoscopic technique, which typically responds well to antisecretory drug treatment. However, those data are accompanied by uncertainties that will only be resolved over time.
Our results allow us to conclude that LM-PF and POEM are equally effective in controlling the clinical symptoms of achalasia at 1, 6, and 12 mo. Although the use of the POEM technique results in a significantly higher rate of postprocedure GER, it also shortens anesthesia and procedure times. We found no differences between the two methods regarding LOS, the occurrence of AEs, or QoL. In the POEM group, there was an improvement in all domains of QoL.
Achalasia is a rare benign esophageal motor disorder characterized by incomplete relaxation of the lower esophageal sphincter (LES). The treatment of achalasia is not curative but rather aimed at reducing the LES pressure. Surgical myotomy with partial fundoplication is traditional the gold standard method for the management of these patients. Peroral endoscopic myotomy (POEM) use its increasing due to it satisfactory results.
Since there is still no definition of the best treatment for achalasia, the objective of this study was to compare the techniques used.
This study aimed to compare POEM and laparoscopic myotomy and partial fundoplication (LM-PF) regarding their efficacy and outcomes for the treatment of achalasia.
This was a single-center randomized controlled clinical trial.
There were no significant differences between the LM-PF and POEM groups regarding symptom improvement at 1, 6, and 12 mo of follow-up. Rates of reflux esophagitis were significantly higher in the POEM group. There were also no statistical differences regarding manometry values or the occurrence of adverse events or length of stay. Anesthesia time and procedure time were significantly shorter in the POEM group than in the LM-PF group. In the POEM group, there was an improvement in all domains of quality of life.
POEM and LM-PF are equally effective in controlling symptoms of achalasia. POEM has the advantage of reducing anesthesia and procedure times, but with a significantly higher rate of gastroesophageal reflux.
Future research should focus on long-term follow-up and outcomes of different techniques. It is possible that the improvement in the POEM technique may contribute to new perspectives on reflux symptoms.
| 1. | Gregersen H, Lo KM. Pathophysiology and treatment of achalasia in a muscle mechanical perspective. Ann N Y Acad Sci. 2018;1434:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Mayberry JF. Epidemiology and demographics of achalasia. Gastrointest Endosc Clin N Am. 2001;11:235-248, v. [PubMed] |
| 3. | Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22:e256-e261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 4. | Reynolds JC, Parkman HP. Achalasia. Gastroenterol Clin North Am. 1989;18:223-255. [PubMed] |
| 5. | Yu L, Li J, Wang T, Zhang Y, Krasna MJ. Functional analysis of long-term outcome after Heller's myotomy for achalasia. Dis Esophagus. 2010;23:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 6. | Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238-49; quiz 1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 7. | Eckardt VF. Clinical presentations and complications of achalasia. Gastrointest Endosc Clin N Am. 2001;11:281-292, vi. [PubMed] |
| 8. | Winter H, Shukla R, Elshaer M, Riaz AA. Current management of achalasia – A review. Br J Med Pract. 2015;8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Ellis FH Jr, Olsen AM. Achalasia of the esophagus. Major Probl Clin Surg. 1969;9:1-221. [PubMed] |
| 10. | Gross R, Johnson LF, Kaminski RJ. Esophageal emptying in achalasia quantitated by a radioisotope technique. Dig Dis Sci. 1979;24:945-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Ehlers AP, Oelschlager BK, Pellegrini CA, Wright AS, Saunders MD, Flum DR, He H, Farjah F. Achalasia Treatment, Outcomes, Utilization, and Costs: A Population-Based Study from the United States. J Am Coll Surg. 2017;225:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Cappell MS, Stavropoulos SN, Friedel D. Updated Systematic Review of Achalasia, with a Focus on POEM Therapy. Dig Dis Sci. 2020;65:38-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Spiess AE, Kahrilas PJ. Treating achalasia: from whalebone to laparoscope. JAMA. 1998;280:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 445] [Article Influence: 37.1] [Reference Citation Analysis (1)] |
| 15. | Bonifácio P, de Moura DTH, Bernardo WM, de Moura ETH, Farias GFA, Neto ACM, Lordello M, Korkischko N, Sallum R, de Moura EGH. Pneumatic dilation versus laparoscopic Heller's myotomy in the treatment of achalasia: systematic review and meta-analysis based on randomized controlled trials. Dis Esophagus. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Vaezi MF, Felix VN, Penagini R, Mauro A, de Moura EG, Pu LZ, Martínek J, Rieder E. Achalasia: from diagnosis to management. Ann N Y Acad Sci. 2016;1381:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Herbella FA, Moura EG, Patti MG. Achalasia 2016: Treatment Alternatives. J Laparoendosc Adv Surg Tech A. 2017;27:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Martins RK, Ribeiro IB, DE Moura DTH, Hathorn KE, Bernardo WM, DE Moura EGH. PERORAL (POEM) OR SURGICAL MYOTOMY FOR THE TREATMENT OF ACHALASIA: A SYSTEMATIC REVIEW AND META-ANALYSIS. Arq Gastroenterol. 2020;57:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Jung HK, Hong SJ, Lee OY, Pandolfino J, Park H, Miwa H, Ghoshal UC, Mahadeva S, Oshima T, Chen M, Chua ASB, Cho YK, Lee TH, Min YW, Park CH, Kwon JG, Park MI, Jung K, Park JK, Jung KW, Lim HC, Jung DH, Kim DH, Lim CH, Moon HS, Park JH, Choi SC, Suzuki H, Patcharatrakul T, Wu JCY, Lee KJ, Tanaka S, Siah KTH, Park KS, Kim SE; Korean Society of Neurogastroenterology and Motility. 2019 Seoul Consensus on Esophageal Achalasia Guidelines. J Neurogastroenterol Motil. 2020;26:180-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Kahaleh M, Tyberg A, Suresh S, Lambroza A, Gaidhane M, Zamarripa F, Martínez GM, Carames JC, Moura ET, Farias GF, Porfilio MG, Nieto J, Rey M, Rodriguez Casas F, Mondragón Hernández OV, Vargas-Rubio R, Canadas R, Hani A, Munoz G, Castillo B, Lukashok HP, Robles-Medranda C, de Moura EG. How does per-oral endoscopic myotomy compare to Heller myotomy? Endosc Int Open. 2020;8:E1392-E1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Ortega JA, Madureri V, Perez L. Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc. 1980;26:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 102] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1287] [Article Influence: 80.4] [Reference Citation Analysis (2)] |
| 23. | de Pascale S, Repici A, Puccetti F, Carlani E, Rosati R, Fumagalli U. Peroral endoscopic myotomy versus surgical myotomy for primary achalasia: single-center, retrospective analysis of 74 patients. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Jones R, Junghard O, Dent J, Vakil N, Halling K, Wernersson B, Lind T. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 528] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 25. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1697] [Article Influence: 62.9] [Reference Citation Analysis (2)] |
| 26. | de Oliveira JM, Birgisson S, Doinoff C, Einstein D, Herts B, Davros W, Obuchowski N, Koehler RE, Richter J, Baker ME. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol. 1997;169:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [PubMed] |
| 28. | Ciconelli RM, Ferraz MB, Santos W, Meinão I. Tradução para a língua portuguesa e validação do questionário genérico de avaliação de qualidade de vida SF-36 (Brasil SF-36). Rev Bras Reumatol. 1999;39:143-50. s2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26179] [Article Influence: 1190.0] [Reference Citation Analysis (2)] |
| 30. | Müller M, Eckardt AJ, Wehrmann T. Endoscopic approach to achalasia. World J Gastrointest Endosc. 2013;5:379-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Müller M, Keck C, Eckardt AJ, Werling S, Wehrmann T, König J, Gockel I. Outcomes of pneumatic dilation in achalasia: Extended follow-up of more than 25 years with a focus on manometric subtypes. J Gastroenterol Hepatol. 2018;33:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Ghoshal UC, Rangan M. A review of factors predicting outcome of pneumatic dilation in patients with achalasia cardia. J Neurogastroenterol Motil. 2011;17:9-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Farias GFA, de Moura DTH, de Moura ETH, de Rezende DT, Hathorn KE, Nasi A, Queiroz NSF, de Moura EGH. Peroral endoscopic myotomy (POEM): a comparative study between Chagasic and idiopathic achalasia. Endosc Int Open. 2020;8:E506-E512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Zaninotto G, Bennett C, Boeckxstaens G, Costantini M, Ferguson MK, Pandolfino JE, Patti MG, Ribeiro U Jr, Richter J, Swanstrom L, Tack J, Triadafilopoulos G, Markar SR, Salvador R, Faccio L, Andreollo NA, Cecconello I, Costamagna G, da Rocha JRM, Hungness ES, Fisichella PM, Fuchs KH, Gockel I, Gurski R, Gyawali CP, Herbella FAM, Holloway RH, Hongo M, Jobe BA, Kahrilas PJ, Katzka DA, Dua KS, Liu D, Moonen A, Nasi A, Pasricha PJ, Penagini R, Perretta S, Sallum RAA, Sarnelli G, Savarino E, Schlottmann F, Sifrim D, Soper N, Tatum RP, Vaezi MF, van Herwaarden-Lindeboom M, Vanuytsel T, Vela MF, Watson DI, Zerbib F, Gittens S, Pontillo C, Vermigli S, Inama D, Low DE. The 2018 ISDE achalasia guidelines. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 35. | Hungness ES, Teitelbaum EN, Santos BF, Arafat FO, Pandolfino JE, Kahrilas PJ, Soper NJ. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg. 2013;17:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Ujiki MB, Yetasook AK, Zapf M, Linn JG, Carbray JM, Denham W. Peroral endoscopic myotomy: A short-term comparison with the standard laparoscopic approach. Surgery. 2013;154:893-7; discussion 897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Bhayani NH, Kurian AA, Dunst CM, Sharata AM, Rieder E, Swanstrom LL. A comparative study on comprehensive, objective outcomes of laparoscopic Heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia. Ann Surg. 2014;259:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 38. | Teitelbaum EN, Rajeswaran S, Zhang R, Sieberg RT, Miller FH, Soper NJ, Hungness ES. Peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy produce a similar short-term anatomic and functional effect. Surgery. 2013;154:885-91; discussion 891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Leeds SG, Burdick JS, Ogola GO, Ontiveros E. Comparison of outcomes of laparoscopic Heller myotomy versus per-oral endoscopic myotomy for management of achalasia. Proc (Bayl Univ Med Cent). 2017;30:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Hanna AN, Datta J, Ginzberg S, Dasher K, Ginsberg GG, Dempsey DT. Laparoscopic Heller Myotomy vs Per Oral Endoscopic Myotomy: Patient-Reported Outcomes at a Single Institution. J Am Coll Surg. 2018;226:465-472.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Chan SM, Wu JC, Teoh AY, Yip HC, Ng EK, Lau JY, Chiu PW. Comparison of early outcomes and quality of life after laparoscopic Heller's cardiomyotomy to peroral endoscopic myotomy for treatment of achalasia. Dig Endosc. 2016;28:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Kumagai K, Tsai JA, Thorell A, Lundell L, Håkanson B. Per-oral endoscopic myotomy for achalasia. Are results comparable to laparoscopic Heller myotomy? Scand J Gastroenterol. 2015;50:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Kumbhari V, Khashab MA. Peroral endoscopic myotomy. World J Gastrointest Endosc. 2015;7:496-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 44. | Ramirez M, Zubieta C, Ciotola F, Amenabar A, Badaloni A, Nachman F, Nieponice A. Per oral endoscopic myotomy vs. laparoscopic Heller myotomy, does gastric extension length matter? Surg Endosc. 2018;32:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Kahaleh M, Xu MM, Zamarripa F, Martínez G, Arantes VN, Rodriguez FC, Castillo B, Andalib I, Tyberg A, Lambroza A, Saumoy M, Carames JC, Baptista A, Robles-Medranda C, Lukashok H, Gaidhane M, Valencia JMB, Moura ETHD, Moura EGHD. POEM in Latin America: The Rise of a New Standard. J Clin Gastroenterol. 2019;53:e352-e355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Werner YB, Hakanson B, Martinek J, Repici A, von Rahden BHA, Bredenoord AJ, Bisschops R, Messmann H, Vollberg MC, Noder T, Kersten JF, Mann O, Izbicki J, Pazdro A, Fumagalli U, Rosati R, Germer CT, Schijven MP, Emmermann A, von Renteln D, Fockens P, Boeckxstaens G, Rösch T. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N Engl J Med. 2019;381:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 377] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 47. | Sanagapalli S, Plumb A, Maynard J, Leong RW, Sweis R. The timed barium swallow and its relationship to symptoms in achalasia: Analysis of surface area and emptying rate. Neurogastroenterol Motil. 2020;32:e13928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Schlottmann F, Luckett DJ, Fine J, Shaheen NJ, Patti MG. Laparoscopic Heller Myotomy Versus Peroral Endoscopic Myotomy (POEM) for Achalasia: A Systematic Review and Meta-analysis. Ann Surg. 2018;267:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (1)] |
| 49. | Smart HL, Foster PN, Evans DF, Slevin B, Atkinson M. Twenty four hour oesophageal acidity in achalasia before and after pneumatic dilatation. Gut. 1987;28:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Mota RCL, de Moura EGH, de Moura DTH, Bernardo WM, de Moura ETH, Brunaldi VO, Sakai P, Thompson CC. Risk factors for gastroesophageal reflux after POEM for achalasia: a systematic review and meta-analysis. Surg Endosc. 2021;35:383-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Gu L, Ouyang Z, Lv L, Liang C, Zhu H, Liu D. Safety and efficacy of peroral endoscopic myotomy with standard myotomy versus short myotomy for treatment-naïve patients with type II achalasia: a prospective randomized trial. Gastrointest Endosc. 2021;93:1304-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 52. | Duan T, Tan Y, Zhou J, Lv L, Liu D. A Retrospective Study of Peroral Endoscopic Full-Thickness Myotomy in Patients with Severe Achalasia. J Laparoendosc Adv Surg Tech A. 2017;27:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Tan Y, Lv L, Wang X, Zhu H, Chu Y, Luo M, Li C, Zhou H, Huo J, Liu D. Efficacy of anterior versus posterior per-oral endoscopic myotomy for treating achalasia: a randomized, prospective study. Gastrointest Endosc. 2018;88:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 54. | Ramchandani M, Nabi Z, Reddy DN, Talele R, Darisetty S, Kotla R, Chavan R, Tandan M. Outcomes of anterior myotomy versus posterior myotomy during POEM: a randomized pilot study. Endosc Int Open. 2018;6:E190-E198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 55. | Ichkhanian Y, Abimansour JP, Pioche M, Vosoughi K, Eleftheriadis N, Chiu PWY, Minami H, Ogihara K, Sanaei O, Jovani M, Khashab MA. Outcomes of anterior versus posterior peroral endoscopic myotomy 2 years post-procedure: prospective follow-up results from a randomized clinical trial. Endoscopy. 2021;53:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Rodríguez de Santiago E, Mohammed N, Manolakis A, Shimamura Y, Onimaru M, Inoue H. Anterior versus posterior myotomy during poem for the treatment of achalasia: systematic review and meta-analysis of randomized clinical trials. J Gastrointestin Liver Dis. 2019;28:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Swanström LL, Rieder E, Dunst CM. A stepwise approach and early clinical experience in peroral endoscopic myotomy for the treatment of achalasia and esophageal motility disorders. J Am Coll Surg. 2011;213:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 58. | Tanaka S, Toyonaga T, Kawara F, Watanabe D, Hoshi N, Abe H, Ariyoshi R, Ohara Y, Takao T, Morita Y, Umegaki E, Kodama Y. Novel per-oral endoscopic myotomy method preserving oblique muscle using two penetrating vessels as anatomic landmarks reduces postoperative gastroesophageal reflux. J Gastroenterol Hepatol. 2019;34:2158-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 59. | Tanaka S, Kawara F, Toyonaga T, Inoue H, Bechara R, Hoshi N, Abe H, Ohara Y, Ishida T, Morita Y, Umegaki E. Two penetrating vessels as a novel indicator of the appropriate distal end of peroral endoscopic myotomy. Dig Endosc. 2018;30:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 60. | Tyberg A, Choi A, Gaidhane M, Kahaleh M. Transoral incisional fundoplication for reflux after peroral endoscopic myotomy: a crucial addition to our arsenal. Endosc Int Open. 2018;6:E549-E552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Inoue H, Ueno A, Shimamura Y, Manolakis A, Sharma A, Kono S, Nishimoto M, Sumi K, Ikeda H, Goda K, Onimaru M, Yamaguchi N, Itoh H. Peroral endoscopic myotomy and fundoplication: a novel NOTES procedure. Endoscopy. 2019;51:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 62. | Zhou PH, Cai MY, Yao LQ, Zhong YS, Ren Z, Xu MD, Qin XY. Peroral Endoscopic Myotomy for Esophageal Achalasia by HybridKnife: A Case Report. Case Rep Gastrointest Med. 2012;2012:325479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Ponds FA, Oors JM, Smout AJPM, Bredenoord AJ. Reflux symptoms and oesophageal acidification in treated achalasia patients are often not reflux related. Gut. 2021;70:30-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 64. | Bapaye A, Dashatwar P, Dharamsi S, Pujari R, Gadhikar H. Single-session endoscopic fundoplication after peroral endoscopic myotomy (POEM+F) for prevention of post gastroesophageal reflux - 1-year follow-up study. Endoscopy. 2021;53:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | Reddy ND. Peroral endoscopic myotomy with fundoplication: are we there yet! Endoscopy. 2019;51:111-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Patti MG, Andolfi C, Bowers SP, Soper NJ. POEM vs Laparoscopic Heller Myotomy and Fundoplication: Which Is Now the Gold Standard for Treatment of Achalasia? J Gastrointest Surg. 2017;21:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Liu-Burdowski J, Duarte-Chavez R, Kahaleh M. Per-oral Endoscopic Myotomy: State of the Art. J Clin Gastroenterol. 2022;56:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | McKay SC, Dunst CM, Sharata AM, Fletcher R, Reavis KM, Bradley DD, DeMeester SR, Müller D, Parker B, Swanström LL. POEM: clinical outcomes beyond 5 years. Surg Endosc. 2021;35:5709-5716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bortolotti M, Italy; Sweis R, United Kingdom S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR