Published online Aug 28, 2022. doi: 10.3748/wjg.v28.i32.4726
Peer-review started: May 1, 2022
First decision: June 19, 2022
Revised: July 10, 2022
Accepted: July 31, 2022
Article in press: July 31, 2022
Published online: August 28, 2022
Processing time: 116 Days and 9.4 Hours
Timely differentiation of biliary atresia (BA) from other infantile cholestatic diseases can impact patient outcomes. Additionally, non-invasive staging of fibrosis after Kasai hepatoportoenterostomy has not been widely standardized. Shear wave elastography is an ultrasound modality that detects changes in tissue stiffness. The authors propose that the utility of elastography in BA can be elucidated through meta-analysis of existing studies.
To assess the utility of elastography in: (1) BA diagnosis, and (2) post-Kasai fibro
A literature search identified articles that evaluated elastography for BA diagnosis and for post-Kasai follow-up. Twenty studies met criteria for meta-analysis: Eleven for diagnosis and nine for follow-up post-Kasai. Estimated diagnostic odds ratio (DOR), sensitivity, and specificity of elastography were calculated through a random-effects model using Meta-DiSc software.
Mean liver stiffness in BA infants at diagnosis was significantly higher than in non-BA, with overall DOR 24.61, sensitivity 83%, and specificity 79%. Post-Kasai, mean liver stiffness was significantly higher in BA patients with varices than in patients without, with DOR 16.36, sensitivity 85%, and specificity 76%. Elastography differentiated stage F4 fibrosis from F0-F3 with DOR of 70.03, sensitivity 96%, and specificity 89%. Elastography also differentiated F3-F4 fibrosis from F0-F2 with DOR of 24.68, sensitivity 85%, and specificity 81%.
Elastography has potential as a non-invasive modality for BA diagnosis and surveillance post-Kasai. This paper’s limitations include inter-study method heterogeneity and small sample sizes. Future, standardized, multi-center studies are recommended.
Core Tip: Ultrasound elastography is an emerging, non-invasive imaging modality to detect organ stiffness. It may be a useful tool in biliary atresia (BA) diagnosis and post-Kasai fibrosis surveillance. In a meta-analysis of twenty existing studies (eleven for diagnosis and nine for follow-up), this paper shows that mean liver stiffness is significantly higher in BA patients compared to non-BA patients at time of diagnosis. Post-Kasai, elastography can differentiate between early and advanced fibrosis as well as help to discern the presence or absence of varices. While there are limitations to this analysis, elastography shows great promise for its utility as a non-invasive modality for BA patients.
- Citation: Wagner ES, Abdelgawad HAH, Landry M, Asfour B, Slidell MB, Azzam R. Use of shear wave elastography for the diagnosis and follow-up of biliary atresia: A meta-analysis. World J Gastroenterol 2022; 28(32): 4726-4740
- URL: https://www.wjgnet.com/1007-9327/full/v28/i32/4726.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i32.4726
Biliary atresia (BA) is a progressive, fibroinflammatory disease of the biliary system that exclusively affects infants. It accounts for about 30% of all cases of prolonged cholestasis in the first three months of life[1]. Its incidence is estimated to be approximately 1:10000-19000 live births, with increased prevalence in East Asian countries to up to 1:2700[2]. The initial clinical manifestations include progressive jaundice, deep-colored urine, light colored stools, and an increase in conjugated serum bilirubin levels. The similarity in clinical presentation and blood biochemistry results between patients with BA and those with other infantile cholestatic liver diseases represents a great challenge to clinicians in accurately establishing a final diagnosis in a timely fashion. Early identification of BA in an infant with cholestatic jaundice is crucial, as the Kasai portoenterostomy (KPE) procedure may help restore bile flow and prevent the otherwise rapid progression to biliary cirrhosis. A BA outcomes meta-analysis by Jimenez-Rivera et al[2] included fourteen studies that focused on native liver survival (NLS), all of which reported improved NLS when KPE was performed at an earlier age. Given its progressive nature, BA is the most common indication for liver transplantation in the pediatric population, with NLS ranging from 24.0% to 52.8% at ten years post-KPE[2]. Patients with BA who have undergone KPE need close clinical monitoring to assess for signs of chronic liver disease progression, particularly as they approach potential need for liver transplant. Currently, there are no widely accepted, validated non-invasive tests to pre-operatively diagnose BA nor to evaluate for progressive fibrosis after KPE.
While ultrasound findings, such as absence of a contractile gall bladder after feeding or the presence of a triangular cord sign, can be suggestive of BA, ultrasound alone is not diagnostic[3]. In order to diagnose BA, a liver biopsy is performed to assess for the histopathologic signs of biliary obstruction, portal edema, and fibrosis[4]. When performed at age 60 d or less, liver biopsy is 96.4% sensitive for the diagnosis of BA[3]. The progressive nature of the disease makes it imperative that a diagnosis is made as early as possible. The gold standard test to confirm BA is a formal cholangiogram. Assessment for progression of chronic liver disease post-KPE is usually achieved by the constellation of findings on laboratory and radiologic testing as well as consideration for repeat liver biopsy and/or upper gastrointestinal endoscopy, with no standardized guidelines for non-invasive staging of hepatic fibrosis nor timing for endoscopic evaluation for potential variceal evolution. Invasive procedures carry risks, both procedural as well as those related to sedation or anesthesia[4]. Delays at each juncture, both in diagnosis and in post-KPE surveillance, can have a potentially detrimental impact on patient outcome.
Ultrasound elastography (UE) is an emerging imaging modality that uses applied force via ultrasound probe to measure the elastic properties of tissue[5,6]. UE can be classified into strain imaging and shear wave imaging. Strain imaging relies on a normal external force (push) or intrinsic forces (i.e., respiration or cardiovascular movement) within the body. For this reason, strain imaging is difficult to quantify and reproduce, particularly in deeper organs, leading to limited applicability in the liver. As a result, strain imaging was not the focus of this analysis[7,8].
Shear wave imaging, also called shear wave elastography (SWE) utilizes a dynamic stress and can be further classified into transient elastography (TE), point shear wave elastography (pSWE; also called VTQ or Virtual Touch Quantification on some platforms), and two-dimensional shear wave elastography (2D-SWE; also called VTIQ or Virtual Touch IQ on some platforms)[8,9]. TE, which is performed using the FibroScan (Echosens, Paris, France) system, utilizes a mechanical punch to generate forces and measure unidirectional pressure waves[5]. pSWE and 2D-SWE differ from TE in that they utilize B mode ultrasound imaging guidance, which enables direct visualization of tissue, as well as an acoustic radiation force impulse (ARFI) in order to measure tissue stress. pSWE enables the measurement of average shear wave speed within a region of interest while 2D-SWE uses multiple foci of tissue displacement along an axis to generate a quantitative map of shear wave speed[5].
SWE has been studied extensively in adult liver literature with studies in pediatric liver disease emerging only recently[6]. And yet, while multiple authors have addressed the utility of SWE for diagnosis and management in BA, there have been no large-scale studies nor meta-analyses on this modality. The aim of this meta-analysis is to evaluate the utility of SWE in: (1) the differentiation of BA from other cholestatic liver diseases of infancy, and (2) the assessment of fibrosis progression in post-KPE follow-up.
This review was conducted in strict accordance with the Cochrane handbook of systematic reviews of interventions[10]. We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis of Diagnostic Test Accuracy studies (PRISMA-DTA statement)[11]. The review was publicly registered with the Open Science Framework (OSF). The statistical analysis was performed by co-authors H.A., who has over four years as a biostatistician in a clinical research organization, and M.L., who is a clinical research coordinator employed by the University of Chicago.
PubMed, Scopus, Web of Science, EBSCO, and Cochrane Central were searched for English language studies with information on BA and elastography from 2010 to 2021. Two of the authors performed the literature search using the keywords: “Elastography” AND “biliary atresia”.
Full texts were reviewed to select eligible studies for meta-analysis. Studies were included if the following criteria were met: (1) The study used SWE to differentiate BA from other cholestatic liver diseases of infancy; (2) the study used SWE in the follow-up of patients with BA for the development of fibrosis/cirrhosis post-KPE; and (3) the study assessed the accuracy of SWE for the diagnosis or follow-up of liver fibrosis based on the cut-off point for liver stiffness and/or spleen stiffness values.
Exclusion criteria were as follows: (1) Full text article was not available and/or abstract and article were not available in English; (2) the study was not specific to BA and/or did not utilize SWE (3) the study evaluated the accuracy of SWE combined with other diagnostic methods; (4) the study used SWE as a reference test to study novel biomarkers; and (5) the study was a review article, letter to the editor, case report, editorial, case series, or consensus statement. Only studies with reliable data for extraction and analysis were included in the meta-analysis.
Two authors independently reviewed and extracted the data from each included study. Data were extracted for study characteristics (e.g., first author and year of publication, study period, study setting, study design, and sample size), baseline characteristics of the included patients (e.g., age of patients, laboratory date, age at diagnosis), and diagnostic data (e.g., sensitivity, specificity, predictive values).
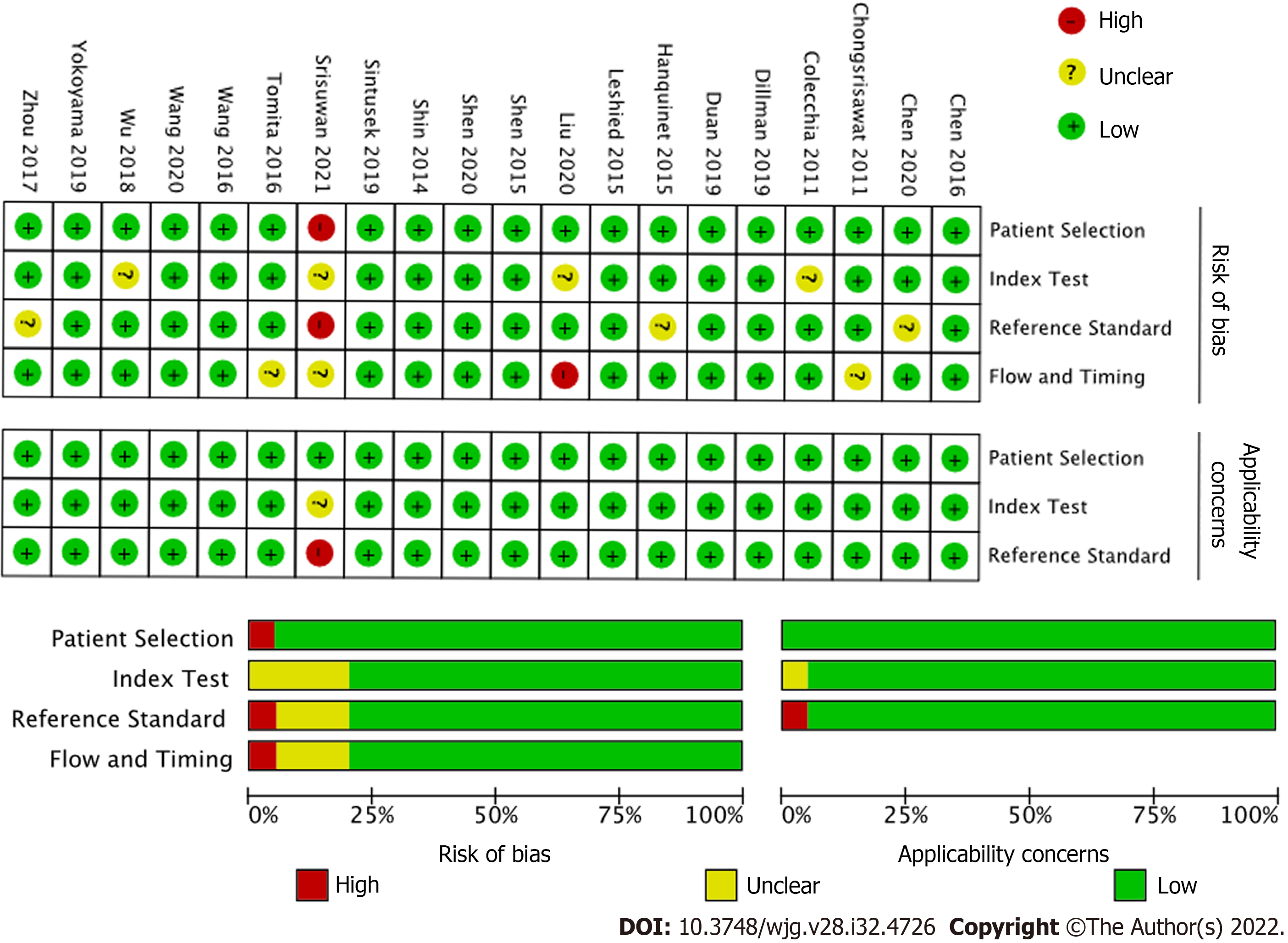
Two researchers assessed the risk of bias of studies included using version 2 of the Quality Assessment of Diagnostic Test Accuracy Studies (QUADAS-2) tool[12]. QUADAS-2 examines seven items on the representation of patient spectrum, selection criteria, reference standard, verification bias, timing, and study withdrawals. Both reviewers scored the tool independently; all disagreements were discussed and consensus was reached.
Heterogeneity was assessed by visual inspection of the forest plots and measured by I-square and Chi-Square tests. In case of significant heterogeneity (P < 0.1), a random effect model was used. Continuous data were pooled as standardized mean difference (SMD) in a meta-analysis model using inverse variance method with the respective 95% confidence intervals. We used Comprehensive Meta Analysis (CMA, United States) software version 3.3.070 for windows. Methods described in reference[13] were utilized to compute missing standard deviations. The diagnostic accuracy indicators of each test were pooled as sensitivity, specificity, and diagnostic odds ratio (DOR) with the corresponding 95% confidence intervals by the DerSimonian-Laird random effect model using MetaDiSc Beta-1.4 software. In addition, the Summary Receiver Operating Characteristic (SROC) curve was used for the evaluation of diagnostic tests and represents the relationship between true positive and negative rates considering the varying diagnostic thresholds among studies. The summary of the test performance was represented with an AUC of 1.0 (100%) indicating perfect discriminatory ability to distinguish BA from non-BA.
Seven studies used SWE machinery that reported results in units of m/s as opposed to kPa, with four being evaluated for diagnostic test accuracy[14-18] and two for utility of SWE for follow-up[19,20]. Values reported in m/s were converted to kPa as per reference[8] (Supplementary material).
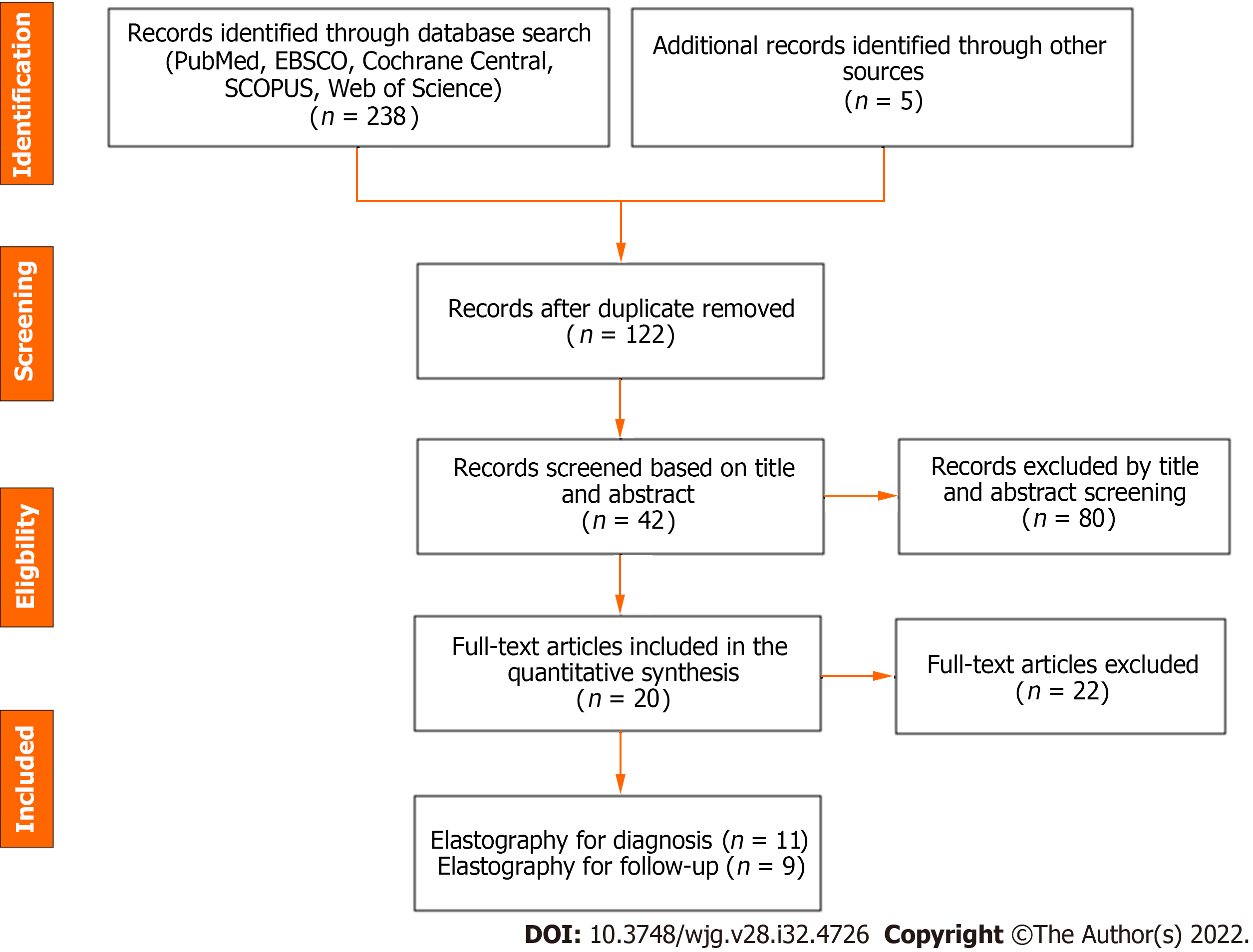
Electronic search yielded 243 studies with 42 identified as potentially meeting inclusion criteria (Figure 1). Full text articles were retrieved and assessed for final eligibility. After the review of all full text articles, 22 were excluded due to study design and unreliable data for extraction, the use of SWE as an adjunct to the primary study of a novel biomarker, and subject comparison to healthy controls (as opposed to other cholestatic infants). Eleven studies on the utility of SWE in accurately differentiating BA from other cholestatic liver diseases of infancy met criteria for inclusion, as well as nine studies that evaluated the utility of SWE in assessing and monitoring the development of hepatic fibrosis/cirrhosis post-KPE. The studies included were mostly single center, retrospective analyses conducted in infants and children (age 0-18 years) and published in peer-reviewed journals between 2010 and 2021. Detailed individual study characteristics are presented in Table 1.
| Ref. | Country | Study design | Elastography Method; machine; probe, if specified | Reference standard | Sample size | Type of patient | Main finding |
| Diagnosis | |||||||
| Hanquinet et al[14], Pediatric Radiology, 2015 | Switzerland | Single center retrospective analysis | pSWE (VTQ); Acuson S2000 or S3000 (Siemens Healthcare, Erlangen, Germany) | Liver biopsy; Cholangiogram | 20 | Cholestatic infants; mean age 52.1 d | Utilizing SWE in addition to standard abdominal ultrasound can provide useful information on liver fibrosis to aid in the diagnosis of BA |
| Leschied et al[15], Pediatric Radiology, 2015 | USA | Prospective cohort | pSWE (VTQ) and 2D-SWE (VTIQ), Acuson S3000 (Siemens Healthcare, Erlangen, Germany); 9L4 Transducer | Liver biopsy; Cholangiogram | 11 | Infants with suspected liver disease; mean age 3.8 mo | Shear wave speeds were significantly higher in children with BA than those without |
| Wang et al[31], Journal of Ultrasound Medicine, 2016 | China | Single center case control | pSWE; Aixplorer (SuperSonic Imagine SA, Aix-en-Provence, France); L15-4 linear probe | KPE | 38 | Cholestatic infants age 16 to 140 d | Mean shear wave speeds were higher for BA patients than non-BA cholestatic patients and control patients |
| Zhou et al [22], European Radiology, 2017 | China | Single center prospective analysis | pSWE; Aixplorer (SuperSonic Imagine SA, Aix-en-Provence, France); SL15-4 linear array transducer | Liver biopsy; Cholangiogram; surgical exploration | 172 | Cholestatic infants, age 2 to 140 d | SWE is useful to differentiate BA from non-BA; its performance does not outperform grey scale ultrasound |
| Wu et al[32], Hepatology, 2018 | Taiwan | Single center prospective analysis | TE; FibroScan 502 Touch (Echosens, Paris, France); S1 probe | Liver biopsy; cholangiogram | 48 | Cholestatic infants, age 35 to 61 d | Liver stiffness assessment during the work up of cholestatic infants may facilitate diagnosis of BA |
| Dillman et al[16], Journal of Pediatrics, 2019 | USA | Multiple center prospective analysis | 2D-SWE (VTIQ) and pSWE (VTQ); Acuson S2000 or S3000 (Siemens Healthcare, Erlangen, Germany); 9L4 linear transducer probe | Not specified | 41 | Cholestatic infants, age 24 to 52 d | SWE and GGT can help discriminate BA from other causes of cholestasis |
| Duan et al[33], BioMed Research International, 2019 | China | Single center case control | 2D-SWE; TUS-Aplio 500 (Canon Medical Systems, Tokyo, Japan); 14L5 linear array probe | Liver biopsy; KPE | 138 | Cholestatic infants, age 5-90 d | SWE can help distinguish BA from other cholestatic diseases; the diagnostic specificity increases when combined with grey-scale ultrasound |
| Chen et al[17], European Radiology, 2020 | China | Single center multiple method (prospective and retrospective) analysis | pSWE (VTQ); Acuson S2000 (Siemens Healthcare, Erlangen, Germany); 4-9MHz linear transducer | Liver biopsy; cholangiogram | 308 in subgroup 1; 187 in subgroup 2 | Cholestatic infants, age under 100 d | Shear wave speed, coupled with presence of triangular cord sign, provided moderate-to-high accuracy for BA diagnosis. This study also found high diagnostic performance in a risk stratification model built on five predictors (shear wave speed, triangular cord sign, GGT, abnormal gallbladder, clay-colored stool) |
| Liu et al[18], International Journal of Clinical Practice, 2020 | China | Single center retrospective analysis | 2D-SWE (VTIQ) and pSWE (VTQ); Acuson OXANA2 (Siemens Healthcare, Erlangen, Germany); 3-5.5 MHz-6C1 convex and 4-9MHz 9L4 linear array probe | Surgical exploration | 59 | Cholestatic infants, age 25 to 141 d | VTQ and VTIQ can help distinguish BA from non-BA in cholestatic infants; VTIQ has higher sensitivity and specificity than VTQ |
| Shen et al[34], BMC Pediatrics, 2020 | China | Single center retrospective analysis | pSWE; Aixplorer (SuperSonic Imagine SA, Aix-en-Provence, France); L15-4 linear probe | Not specified | 282 | Cholestatic infants, age under 120 d | Liver stiffness measurements and GGT values have the potential to decrease rates of BA misdiagnosis |
| Wang et al[35], Academic Radiology, 2020 | China | Single center prospective analysis | 2D-SWE; Aixplorer (SuperSonic Imagine SA, Aix-en-Provence, France); linear probe | Liver biopsy; Cholangiogram | 294 | Cholestatic infants, age under 70 d | Age, gallbladder morphology, and liver elasticity incorporated together into a nomogram shows an improved predictive value for BA diagnosis |
| Follow-up | |||||||
| Chongsrisawat et al[36], BMC Gastroenterology, 2011 | Thailand | Single center prospective analysis | TE; FibroScan 502 Touch (Echosens, Paris, France) | Endoscopy | 73 | BA patients after KPE, mean age 9.11 yr | TE is useful for predicting the presence of EV/GV in BA patients post-KPE |
| Colecchia et al[37], Digestive and Liver Disease, 2011 | Italy | Single center prospective analysis | TE; FibroScan (Echosens, Paris, France) | Endoscopy | 31 | BA patients after KPE, age 4 to 25 yr | Non-invasive studies, such as liver stiffness measurement, can predict the presence of EV in BA patients post-KPE |
| Shin et al[38], Journal of Ultrasound Medicine, 2014 | South Korea | Single center retrospective analysis | TE; FibroScan 502 Touch (Echosens, Paris, France); S or M probe | Liver biopsy | 47 | BA patients, mean age 60 d | TE may be a useful, non-invasive method for diagnosing severe fibrosis and cirrhosis; may predict outcomes before surgery or liver biopsy in infants with BA |
| Shen et al[39], World Journal of Gastroenterology, 2015 | China | Single center retrospective analysis | TE; FibroScan (Echosens, Paris, France); S probe | Liver biopsy | 31 | BA patients, age 34 to 121 d | TE can be a useful, non-invasive technique to assess liver fibrosis in children with BA. The cut-off value of 15.15 kPa can distinguish cirrhotic from non-cirrhotic patients |
| Chen et al[40], Nature Scientific Reports, 2016 | China | Single center retrospective analysis | 2D-SWE; Aixplorer (SuperSonic Imagine SA, Aix-en-Provence, France); SC-1 curvilinear probe | Liver biopsy | 24 | BA patients after KPE, mean age 6.6 yr | 2D-SWE has more promise as a means of assessing liver fibrosis in BA patients than APRI or FIB-4 scoring |
| Tomita et al[20], Pediatric Radiology, 2016 | Japan | Single center prospective analysis | pSWE (VTQ); Acuson S2000 (Siemens Healthcare, Erlangen, Germany); 4C1 probe | Liver biopsy; endoscopy | 28 | BA patients, age 0.1 to 33.6 yr | Liver and spleen stiffness measured via ARFI has potential as a non-invasive marker of liver fibrosis and esophageal varices in BA patients |
| Sintusek et al[41], Journal of Pediatric Gastroenterology and Nutrition, 2019 | Thailand | Single center prospective analysis | TE; FibroScan Compact 530 (Echosens, Paris, France); S or M probe | Endoscopy | 51 | BA patients after KPE, mean age 10.63 yr | Spleen stiffness can predict the presence of esophageal varices in children with BA; combination of spleen and liver stiffness measurements to diagnose varices increases diagnostic yield |
| Yokoyama et al[19], Hepatology Research, 2019 | Japan | Single center prospective study | 2D-SWE; Aplio i900 (Canon Medical Systems, Tokyo, Japan); i8CX1 transducer | Endoscopy | 34 | BA patients after KPE, age 1034 to 3940 d | Spleen stiffness (measured via 2D-SWE) is the most accurate predictor of high risk esophageal/gastric varices in BA patients |
| Srisuwan et al[21], Siriraj Medical Journal, 2021 | Thailand | Single center cross-sectional study | TE; FibroScan 502 Touch (Echosens, Paris, France); S or M probe | Endoscopy | 20 | BA patients after KPE, age 2.3 to 21.0 yr | There is correlation between liver stiffness measurement and clinical/radiological evidence of portal hypertension. TE can predict presence of esophageal varices with high sensitivity |
The overall quality of the included studies assessed by the QUADAS-2 (Figure 2), was moderate to high. All analyzed studies were found to have low risk of bias in five or more of the seven items, with the exception of[21], which showed unclear risk of bias in two of seven items and high risk of bias in patient selection. Unclear risk of bias was reported in patient selection due to lack of clear selection criteria in several studies. Potential risk of bias in the index test was recorded in several studies due to lack of reporting on blinding relative to reference standard results. Potential bias in reference standards emerged due to the use of multiple different reference standards (surgical exploration, intraoperative cholangiography, liver biopsy) with possibility for incorrect classification of BA. Finally, bias in the flow and timing was primarily due to a lack of reported details in some of the included studies.
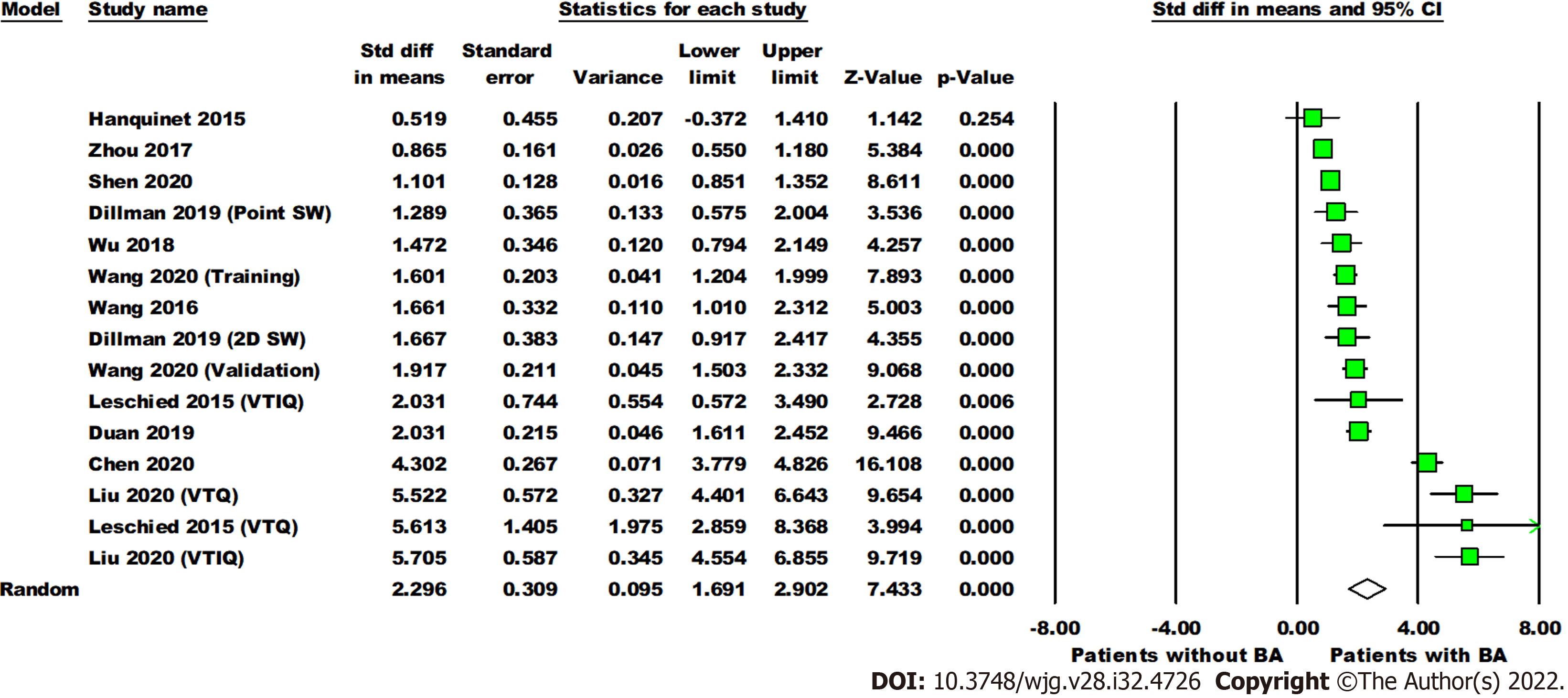
Data on SWE for distinguishing BA from other cholestatic neonatal liver diseases were collected from 11 studies with 1307 total patients (560 with BA and 747 without BA). The mean liver stiffness value was significantly higher in the BA group compared to the non-BA group (overall SMD = 2.30 kPa, 95%CI: 1.69, 2.90, P < 0.0001). Pooled studies were heterogeneous (P < 0.01,
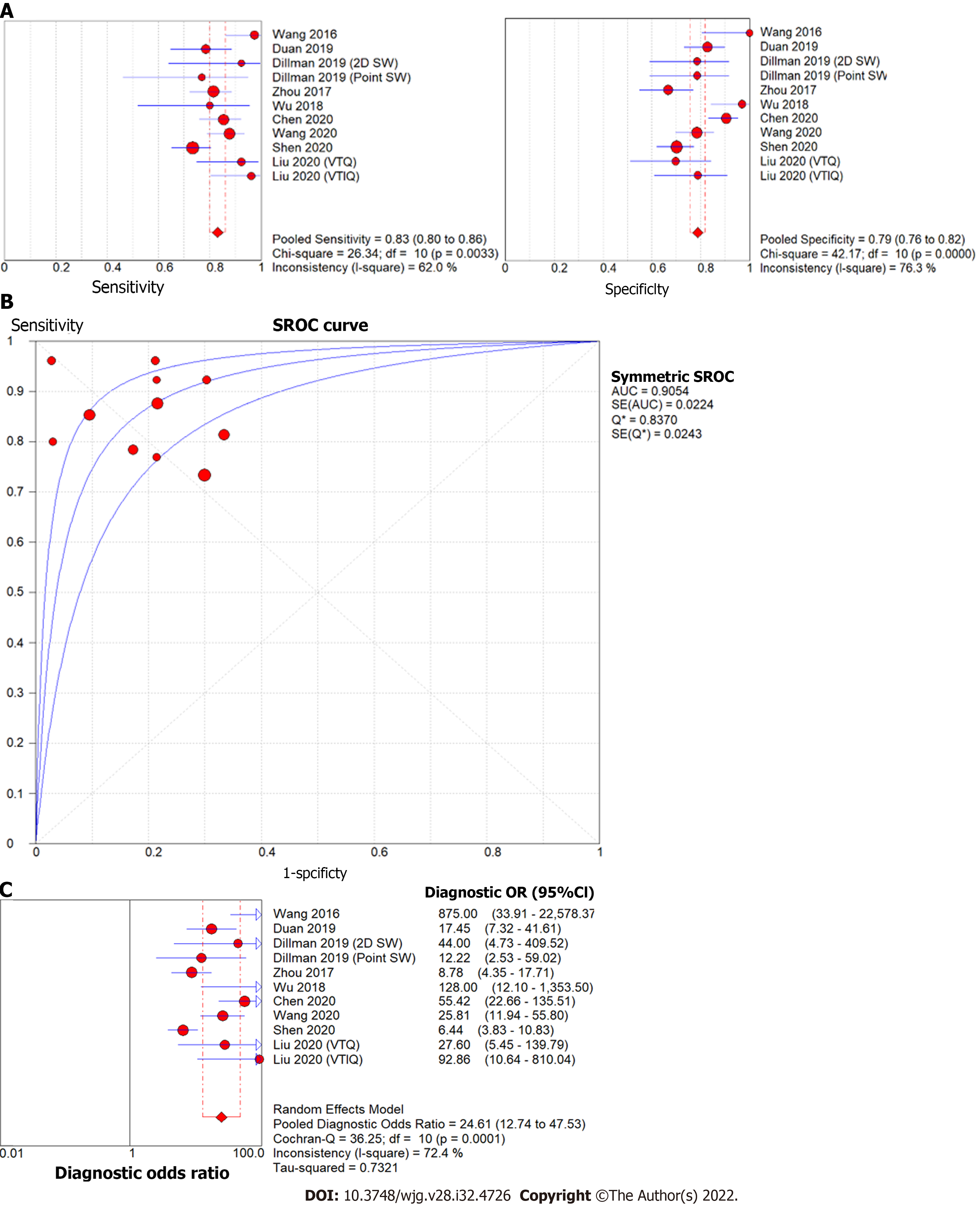
The forest plot of sensitivity and specificity for the diagnostic performance of the liver stiffness value measured by SWE for differentiating BA from other etiologies of neonatal cholestasis is shown in Figure 4A. The pooled sensitivity was 83% (95%CI: 80%, 86%) and specificity was 79%, (95%CI: 76%, 82%). The SROC curves of SWE for the diagnosis of BA are illustrated in Figure 4B. The SROC curve was symmetric, and the AUC was 0.91, Q was 0.84 (SE = 0.02). The DOR was 24.61, (95%CI: 12.74, 47.53,
Data on SWE to assess liver disease progression in post-KPE follow-up were collected from nine studies including 327 total patients [101 patients with varices, 124 patients without varices, 41 patients with fibrosis (F0-F2), and 61 patients with fibrosis (F3-F4)]. Fibrosis stage is reported via METAVIR standards, a scale from F0 to F4 where F0 shows no signs of histologic fibrosis, F2 represents moderate fibrosis with few bridges/septa, F3 is numerous septa without cirrhosis, and F4 signifies cirrhosis.
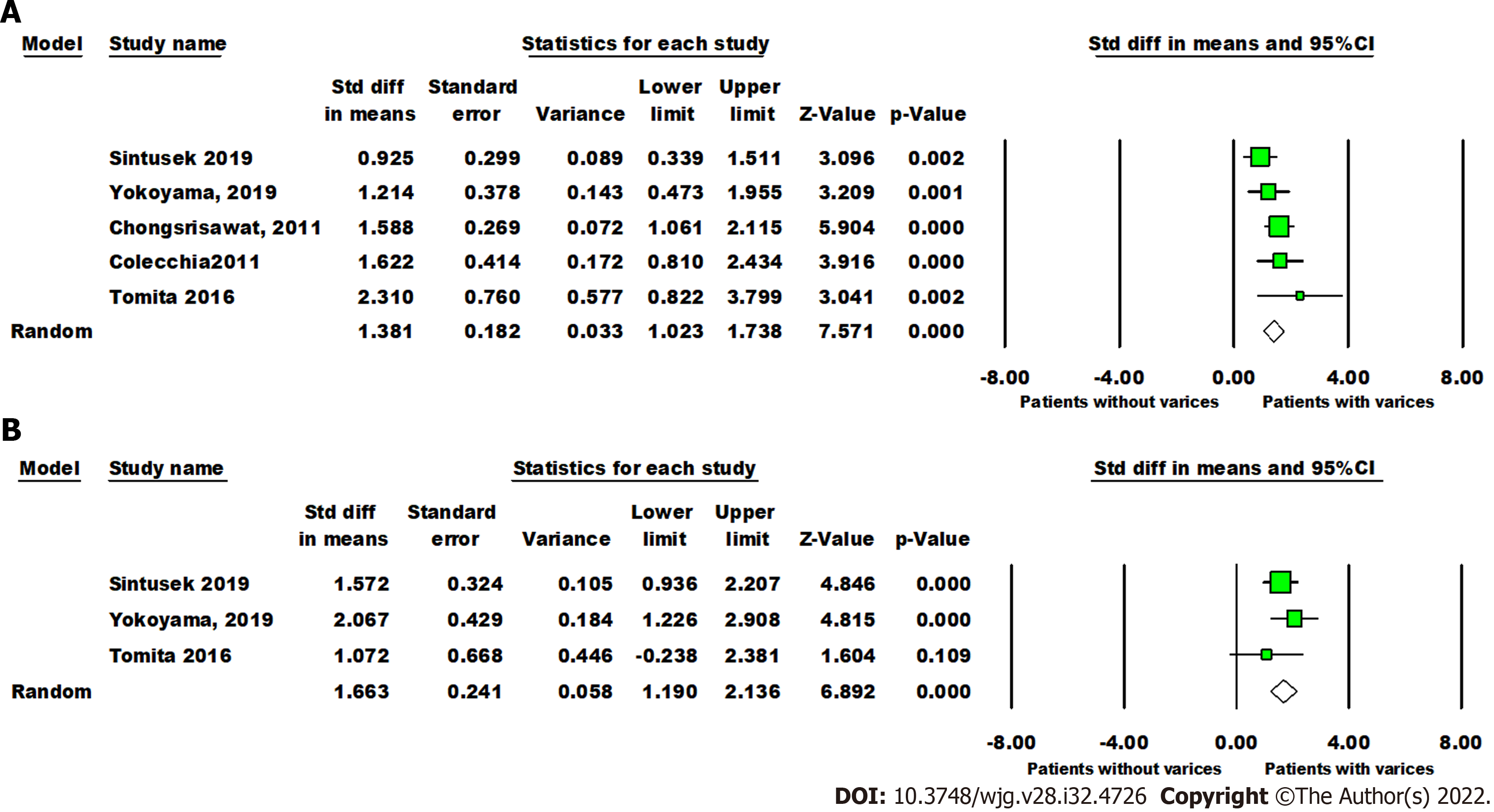
The mean liver stiffness value was significantly higher in patients with varices in comparison to patients without varices (overall SMD 1.38 kPa, 95%CI: 1.02, 1.74, P < 0.00001). Pooled studies were homogenous (P = 0.29,
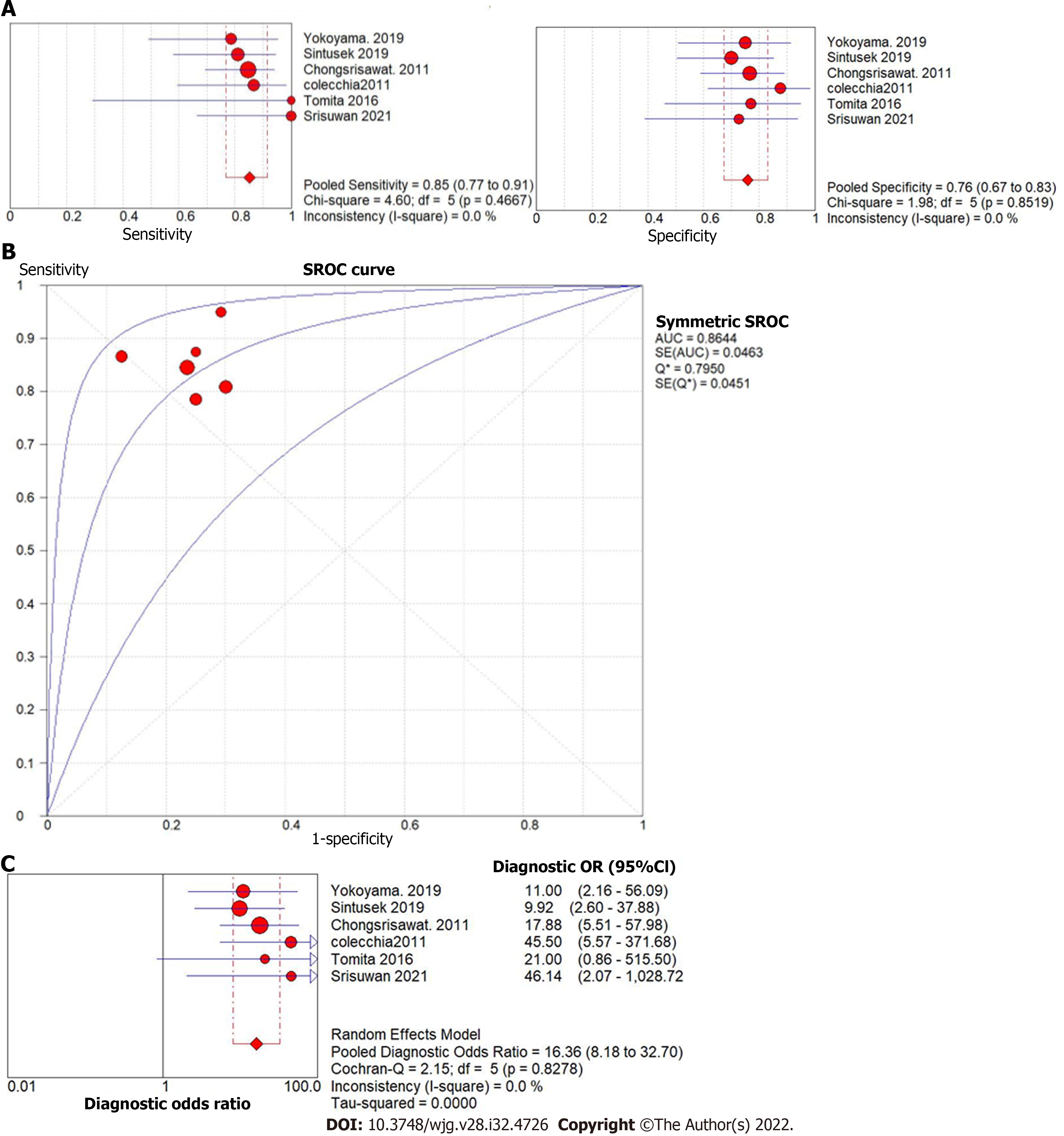
The forest plot of the sensitivity and specificity for the diagnostic performance of liver stiffness value measured by SWE to predict the presence of varices is shown in Figure 6A. The pooled sensitivity was 85% (95%CI: 77%, 91%), while the specificity was 76%, 95%CI (67%, 83%). Illustrated in Figure 6B, the SROC curve was symmetric and the AUC was 0.86 with Q value of 0.80 (SE = 0.05). The DOR was 16.36, 95%CI (8.18, 32.70),
Three studies utilized spleen stiffness measured by SWE to predict the presence of varices with endoscopy as a reference test. The pooled sensitivity was 84% (95%CI: 69%, 94%), while the specificity was 84%, 95%CI (73%, 92%). The AUC of the SROC curve was 0.91, with Q value of 0.85 (SE = 0.403). The DOR was 28.93, 95%CI (8.99, 93.14),
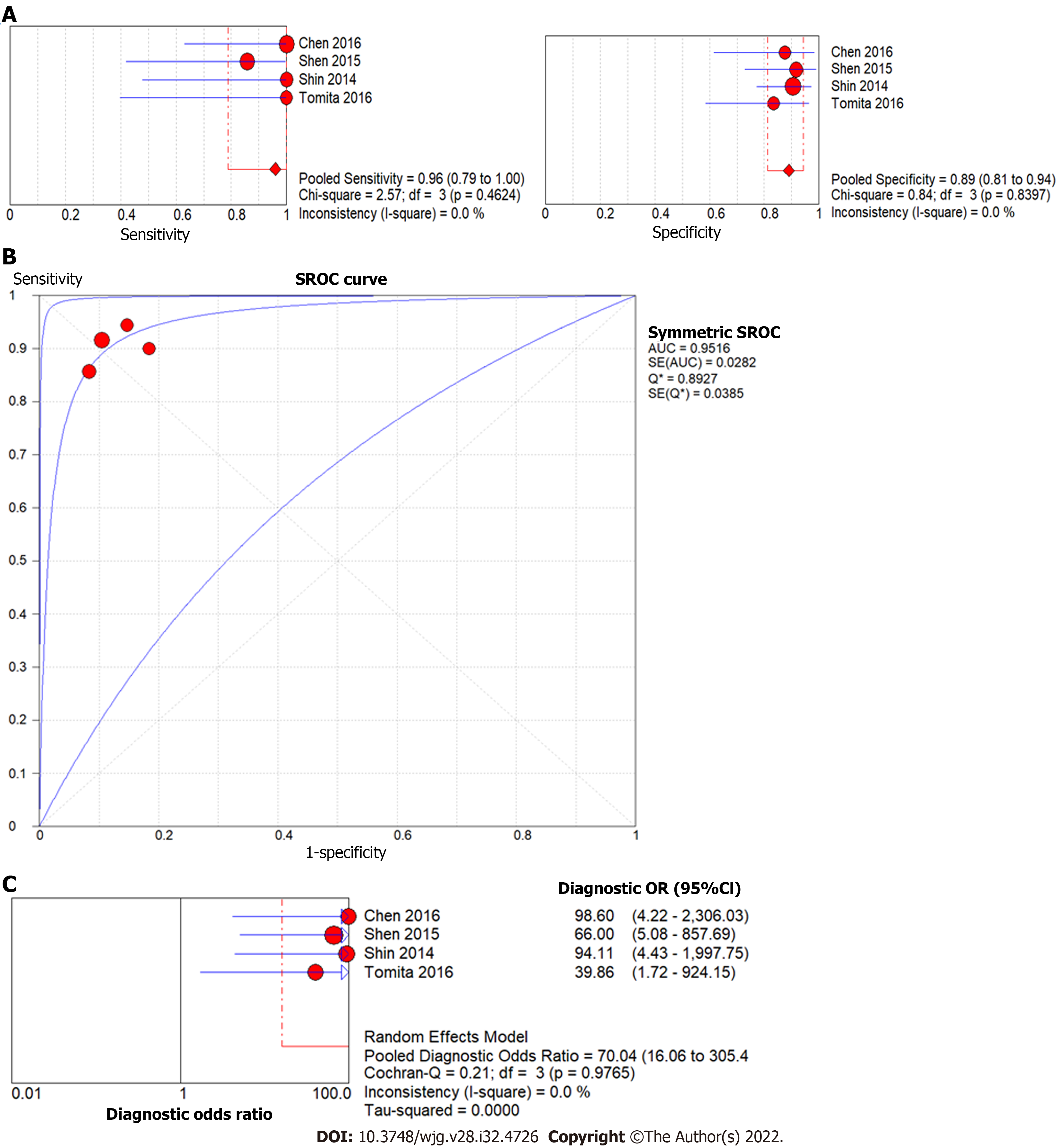
Four studies used liver stiffness value measured by SWE to predict the presence of liver fibrosis F4 (vs F0-3) when liver biopsy was the reference test. The forest plot for the sensitivity and specificity of the diagnostic performance of liver stiffness value measured by SWE to predict the presence of liver fibrosis F4 is shown in Figure 7A. The pooled sensitivity was 96% (95%CI: 79%, 100%), while the specificity was 89%, (95%CI: 81%, 94%). The SROC curves of liver stiffness value measured by SWE are illustrated in Figure 7B. The AUC of the SROC curve was 0.95, with Q value of 0.89 (SE = 0.04). The DOR was 70.04, (95%CI: 16.06, 305.40),
Three studies used the liver stiffness value measured by SWE to predict the presence of liver fibrosis F3-4 (vs F0-2) when liver biopsy was the reference test. The pooled sensitivity was 85% (95%CI: 71%, 94%), and specificity 81%, 95%CI (67%, 91%). The AUC of the SROC curve was 0.91, with Q value of 0.84 (SE = 0.04). The DOR was 24.68, 95%CI (7.43, 82.01),
Early diagnosis of BA is crucial to patient outcomes. At present, diagnosis depends on invasive liver biopsy and intraoperative cholangiogram. The present meta-analysis is the first to our knowledge to assess the potential utility of preoperative SWE in establishing the diagnosis of BA. In our meta-analysis, we observed that liver stiffness value measured by SWE had high diagnostic accuracy for differentiating BA from other neonatal cholestasis (AUC = 0.91) with high summary sensitivity of 83% as well as summary specificity of 79% and DOR of 24.61. Notably, several of the studies used different reference standards (five utilized liver biopsy, one utilized surgical exploration or cholangiography, and one did not specify its reference standard). When interpreted within the context of pathophysiology of BA, with fibrosis beginning at bile duct obliteration, in comparison to non-BA cholestasis which has variable degrees of fibrosis, these results reflect the potential for SWE to aid in the diagnostic process.
Post-KPE, a significant number of patients develop persistent cholestasis, portal hypertension, and progressive fibrosis. Risk stratification for these patients continues to be a clinical challenge for hepatologists. The second portion of this analysis explored the potential utility of SWE for accurately assessing the progression of hepatic fibrosis post-KPE. We observed significant differences in liver and spleen stiffness in patients with varices. The diagnostic accuracy of liver stiffness value measured by SWE was similar to spleen stiffness value to predict the presence of varices post-KPE (AUC = 0.86 and 0.92, respectively). The pooled sensitivity was comparable between liver stiffness and spleen stiffness (85% vs 84%), but the pooled specificity of spleen stiffness was higher than liver stiffness (84% vs 76%). Notably, only three studies investigated spleen stiffness and five investigated liver stiffness to predict the presence of post-KPE varices.
This analysis also demonstrated significant predictive efficacy for the presence and severity of biopsy-proven liver fibrosis in four studies. Liver stiffness value measured by SWE to determine advanced liver fibrosis (F4 vs F0-3) has high diagnostic accuracy (AUC = 0.95), sensitivity (96%) and specificity (89%). Liver stiffness value measured by SWE to differentiate between F3-4 and F0-2 fibrosis has a lower diagnostic accuracy (AUC = 0.91), with sensitivity (85%) and specificity (81%). This is in line with studies in the adult literature, which have found increased accuracy of SWE for high grade (stage F3-4) fibrosis in comparison to F0-F2[23].
Although the findings from this meta-analysis are compelling, there are notable limitations to the analysis. The studies included in this analysis were mostly small and single-center, which limits generalizability across large, non-homogenous populations. Furthermore, each study analyzed for BA diagnosis was performed on infants of different average age (with ranges from two to 140 d of life); given the rapid evolution of fibrosis in BA, the variety of ages analyzed may influence the results.
In addition to the use of different reference standards (liver biopsy vs surgical exploration or cholangiogram), there were notable differences in methodology from study to study, in particular regarding patients’ fasting status and sedation, sonographer characteristics (blinded vs non-blinded, level of expertise, etc.), number of measurements obtained, and measurement quality standards. Previous analyses of SWE technology have highlighted significant differences in results obtained depending on sedation and fasting status, operator dependence, and probe choice[5,24-27]. This demonstrates the importance of methodological unity in comparison between data sets. While our results are promising, this is an important implication in terms of broad applicability of our meta-analysis and the need for further, well-designed trials to address the potential utility of SWE in BA.
Patient factors aside, several study centers utilized different SWE platforms (TE vs pSWE vs 2D-SWE) and machines (detailed in Table 1), each of which reported results in either kPa or m/s. As such, analysis relied upon interconversion of results from Young’s modulus to shear modulus (kPa to m/s)[7]. While there is a well-documented mathematical proof enabling this interconversion, the proof relies upon assumptions about tissue characteristics, notably that the tissue has constant density and homogeneity, displays isotropic and elastic characteristics, and is linear under compressible stress (Supplementary material)[7,8]. The inherent characteristics of an inflamed, potentially fibrotic liver do not fit well into this description, which is important to mention given an analysis that relies heavily on interconversion between results[28,29]. In a similar vein, a recent study by Darweesh et al[30] found that cholestasis itself can increase liver stiffness measurements in adult patients, separate to histologic findings of fibrosis; future studies must take this potential confounder into account. This meta-analysis must be interpreted within the context of these limitations, with future studies taking important steps to address potential limitations and barriers to interpretation during the study design, data collection and analysis phases of work.
Despite non-standardized methodology across analyzed articles as well as reliance upon data interconversion, this meta-analysis highlights the potential of SWE as a non-invasive method to assist in both the diagnosis as well as post-KPE follow-up of BA. Further standardized, multi-center studies are needed in order to better elucidate this potential. By applying uniform methods, machinery, and data reporting (in either kPa or m/s), further studies may be able to identify SWE as a non-invasive tool with utility both in diagnosis of BA as well as post-KPE prediction of outcomes.
Biliary atresia (BA) is a progressive infantile cholestatic disease. Diagnosis is confirmed by intra-operative cholangiogram; any delays to diagnosis and palliating Kasai hepatoportoenterostomy can increase the odds of needing liver transplant. Following Kasai, patients will need surveillance for progression of liver disease, which often requires liver biopsy.
Because the diagnosis and surveillance of BA both involve invasive procedures, there are risks for delay and/or adverse outcomes at each stage. By using non-invasive shear wave elastography (SWE), which measures changes in tissue stiffness, to identify liver stiffness thresholds for BA diagnosis and evolution of fibrosis post-Kasai, clinicians may be able to accurately diagnose and surveil BA without invasive procedures.
The authors performed a meta-analysis on studies into the utility of SWE for BA diagnosis and post-Kasai surveillance in order to determine whether existing literature could help identify liver stiffness thresholds for BA diagnosis and development of fibrosis post-Kasai.
A literature search yielded twenty studies, eleven for diagnosis and nine for follow-up post-Kasai. Diagnostic odds ratio (DOR), sensitivity, and specificity of elastography were calculated through a random-effects model.
Mean liver stiffness in BA infants was higher than in cholestatic infants without BA with DOR 24.61, sensitivity 83%, specificity 79%. Mean liver stiffness post-Kasai was significantly higher in patients with varices than those without (DOR 16.36, sensitivity 85%, specificity 76%). SWE differentiated METAVIR F4 fibrosis from F0-F3 (DOR 70.03, sensitivity 96%, specificity 89%) as well as F3-F4 fibrosis from F0-F2 (DOR 24.68, sensitivity 85%, specificity 81%).
SWE may be a useful, non-invasive modality for the diagnosis and post-Kasai surveillance in BA. The analysis is limited by methodological heterogeneity between studies as well as small sample sizes.
In order for SWE to be useful for future BA cases, larger, standardized, multi-center studies are recommended to establish appropriate protocols.
| 1. | Fischler B, Lamireau T. Cholestasis in the newborn and infant. Clin Res Hepatol Gastroenterol. 2014;38:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr. 2013;56:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Lee JY, Sullivan K, El Demellawy D, Nasr A. The value of preoperative liver biopsy in the diagnosis of extrahepatic biliary atresia: A systematic review and meta-analysis. J Pediatr Surg. 2016;51:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Dezsőfi A, Baumann U, Dhawan A, Durmaz O, Fischler B, Hadzic N, Hierro L, Lacaille F, McLin VA, Nobili V, Socha P, Vajro P, Knisely AS; ESPGHAN Hepatology Committee. Liver biopsy in children: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2015;60:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 793] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 6. | Thumar V, Squires JH, Spicer PJ, Robinson AL, Chan SS. Ultrasound Elastography Applications in Pediatrics. Ultrasound Q. 2018;34:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 7. | Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 680] [Article Influence: 61.8] [Reference Citation Analysis (5)] |
| 8. | Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7:1303-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 1233] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 9. | Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol (NY). 2018;43:773-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 10. | Ferrari J, Higgins JP, Williams RL. Interventions for treating hallux valgus (abductovalgus) and bunions. Cochrane Database Syst Rev. 2000;CD000964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; and the PRISMA-DTA Group, Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 2332] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 12. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 10396] [Article Influence: 693.1] [Reference Citation Analysis (3)] |
| 13. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 8044] [Article Influence: 670.3] [Reference Citation Analysis (0)] |
| 14. | Hanquinet S, Courvoisier DS, Rougemont AL, Dhouib A, Rubbia-Brandt L, Wildhaber BE, Merlini L, McLin VA, Anooshiravani M. Contribution of acoustic radiation force impulse (ARFI) elastography to the ultrasound diagnosis of biliary atresia. Pediatr Radiol. 2015;45:1489-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Leschied JR, Dillman JR, Bilhartz J, Heider A, Smith EA, Lopez MJ. Shear wave elastography helps differentiate biliary atresia from other neonatal/infantile liver diseases. Pediatr Radiol. 2015;45:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Dillman JR, DiPaola FW, Smith SJ, Barth RA, Asai A, Lam S, Campbell KM, Bezerra JA, Tiao GM, Trout AT. Prospective Assessment of Ultrasound Shear Wave Elastography for Discriminating Biliary Atresia from other Causes of Neonatal Cholestasis. J Pediatr. 2019;212:60-65.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Chen Y, Zhao D, Gu S, Li Y, Pan W, Zhang Y. Three-color risk stratification for improving the diagnostic accuracy for biliary atresia. Eur Radiol. 2020;30:3852-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Liu YF, Ni XW, Pan Y, Luo HX. Comparison of the diagnostic value of virtual touch tissue quantification and virtual touch tissue imaging quantification in infants with biliary atresia. Int J Clin Pract. 2021;75:e13860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Yokoyama S, Ishigami M, Honda T, Kuzuya T, Ishizu Y, Ito T, Hirooka Y, Tanaka Y, Tainaka T, Shirota C, Chiba K, Uchida H, Fujishiro M. Spleen stiffness by 2-D shear wave elastography is the most accurate predictor of high-risk esophagogastric varices in children with biliary atresia. Hepatol Res. 2019;49:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Tomita H, Ohkuma K, Masugi Y, Hosoe N, Hoshino K, Fuchimoto Y, Fujino A, Shimizu T, Kato M, Fujimura T, Ishihama H, Takahashi N, Tanami Y, Ebinuma H, Saito H, Sakamoto M, Nakano M, Kuroda T. Diagnosing native liver fibrosis and esophageal varices using liver and spleen stiffness measurements in biliary atresia: a pilot study. Pediatr Radiol. 2016;46:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Srisuwan W, Laochareonsuk W, Wetwittayakhlang P, Kritsaneepaiboon S, Sangkhathat S. Correlation of Transient Elastography and Biliary Cirrhosis in Longterm Survivors of Biliary Atresia. Siriraj Med J. 2021;73:32-37. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Zhou LY, Jiang H, Shan QY, Chen D, Lin XN, Liu BX, Xie XY. Liver stiffness measurements with supersonic shear wave elastography in the diagnosis of biliary atresia: a comparative study with grey-scale US. Eur Radiol. 2017;27:3474-3484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Gharibvand MM, Asare M, Motamedfar A, Alavinejad P, Momeni M. Ultrasound shear wave elastography and liver biopsy to determine liver fibrosis in adult patients. J Family Med Prim Care. 2020;9:943-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Andersen ES, Christensen PB, Weis N. Transient elastography for liver fibrosis diagnosis. Eur J Intern Med. 2009;20:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Goldschmidt I, Streckenbach C, Dingemann C, Pfister ED, di Nanni A, Zapf A, Baumann U. Application and limitations of transient liver elastography in children. J Pediatr Gastroenterol Nutr. 2013;57:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, Castéra L, Sandrin L, Merrouche W, Lavrand F, Lamireau T. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Franchi-Abella S, Corno L, Gonzales E, Antoni G, Fabre M, Ducot B, Pariente D, Gennisson JL, Tanter M, Corréas JM. Feasibility and Diagnostic Accuracy of Supersonic Shear-Wave Elastography for the Assessment of Liver Stiffness and Liver Fibrosis in Children: A Pilot Study of 96 Patients. Radiology. 2016;278:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Barr RG. Shear wave liver elastography. Abdom Radiol (NY). 2018;43:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325-e331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Darweesh SK, Zayed N, Atef M, Ramzy E, Yousry A, Musa S. Increased liver stiffness by transient elastography and acoustic radiation force impulse imaging in patients with extrahepatic cholestasis. Eur J Gastroenterol Hepatol. 2021;33:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Wang X, Qian L, Jia L, Bellah R, Wang N, Xin Y, Liu Q. Utility of Shear Wave Elastography for Differentiating Biliary Atresia From Infantile Hepatitis Syndrome. J Ultrasound Med. 2016;35:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Wu JF, Lee CS, Lin WH, Jeng YM, Chen HL, Ni YH, Hsu HY, Chang MH. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology. 2018;68:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Duan X, Peng Y, Liu W, Yang L, Zhang J. Does Supersonic Shear Wave Elastography Help Differentiate Biliary Atresia from Other Causes of Cholestatic Hepatitis in Infants Less than 90 Days Old? Biomed Res Int. 2019;2019:9036362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Shen Q, Tan SS, Wang Z, Cai S, Pang W, Peng C, Chen Y. Combination of gamma-glutamyl transferase and liver stiffness measurement for biliary atresia screening at different ages: a retrospective analysis of 282 infants. BMC Pediatr. 2020;20:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Wang Y, Jia LQ, Hu YX, Xin Y, Yang X, Wang XM. Development and Validation of a Nomogram Incorporating Ultrasonic and Elastic Findings for the Preoperative Diagnosis of Biliary Atresia. Acad Radiol. 2021;28 Suppl 1:S55-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Chongsrisawat V, Vejapipat P, Siripon N, Poovorawan Y. Transient elastography for predicting esophageal/gastric varices in children with biliary atresia. BMC Gastroenterol. 2011;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Colecchia A, Di Biase AR, Scaioli E, Predieri B, Iughetti L, Reggiani ML, Montrone L, Ceccarelli PL, Vestito A, Viola L, Paolucci P, Festi D. Non-invasive methods can predict oesophageal varices in patients with biliary atresia after a Kasai procedure. Dig Liver Dis. 2011;43:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Shin NY, Kim MJ, Lee MJ, Han SJ, Koh H, Namgung R, Park YN. Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med. 2014;33:853-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Shen QL, Chen YJ, Wang ZM, Zhang TC, Pang WB, Shu J, Peng CH. Assessment of liver fibrosis by Fibroscan as compared to liver biopsy in biliary atresia. World J Gastroenterol. 2015;21:6931-6936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Chen S, Liao B, Zhong Z, Zheng Y, Liu B, Shan Q, Xie X, Zhou L. Supersonic shearwave elastography in the assessment of liver fibrosis for postoperative patients with biliary atresia. Sci Rep. 2016;6:31057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Sintusek P, Siriporn N, Punpanich D, Chongsrisawat V, Poovorawan Y. Spleen and Liver Stiffness to Detect Esophageal Varices in Children with Biliary Atresia. J Pediatr Gastroenterol Nutr. 2019;69:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Egypt; Pop TL, Romania; Redkar RG, India S-Editor: Chen YL L-Editor: A P-Editor: Cai YX