Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3971
Peer-review started: February 21, 2022
First decision: April 16, 2022
Revised: April 30, 2022
Accepted: July 5, 2022
Article in press: July 5, 2022
Published online: August 7, 2022
Processing time: 163 Days and 4.8 Hours
Microwave ablation (MWA) is an effective treatment option for patients with primary liver cancer. However, it has been reported that the MWA procedure induces a hepatic inflammatory response and injury, which may negatively affect the efficacy of MWA. As such, the discovery of reliable markers to monitor the patient’s response to MWA is needed. Golgi protein 73 (GP73) has been shown to be associated with chronic liver disease. To date, the potential value of serum GP73 in the dynamic monitoring during MWA of liver cancer remains unclear.
To examine the effects of MWA on the serum levels of GP73 in patients with primary liver cancer.
A total of 150 primary liver cancer patients with a single small lesion (≤ 3 cm in diameter) were retrospectively enrolled spanning the period between January 2016 and October 2018. All of the patients received MWA for the treatment of primary liver cancer. Serum GP73, alpha-fetoprotein (AFP), and widely used liver biochemical indicators [serum albumin, total bilirubin (TBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST)] were compared before MWA and at different time points, including 1, 2, and 4 wk following the ablation procedure.
Complete tumor ablation was achieved in 95.33% of the patients at 1 mo after MWA. The 1-, 2-, and 3-year disease-free survival rates were 74.67%, 59.33%, and 54.00%, respectively. The serum AFP levels were significantly decreased at 1, 2, and 4 wk after MWA; they returned to the normal range at 12 wk after MWA; and they remained stable thereafter during follow-up in those cases without recurrence. In contrast, the serum GP73 levels were significantly increased at 1 and 2 wk after MWA. The serum GP73 levels reached the peak at 2 wk after MWA, started to decline after hepatoprotective treatment with glycyrrhizin and reduced glutathione, and returned to the pretreatment levels at 12 and 24 wk after MWA. Notably, the changes of serum GP73 in response to MWA were similar to those of TBIL, ALT, and AST.
Serum GP73 is markedly increased in response to MWA of liver cancer. Thus, serum GP73 holds potential as a marker to monitor MWA-induced inflammatory liver injury in need of amelioration.
Core Tip: Microwave ablation (MWA) has become an effective modality of cancer treatment, including primary liver cancer. However, the MWA procedure induces a hepatic inflammatory response and injury, which may diminish the efficacy of MWA. Therefore, the discovery of reliable markers to monitor the response to MWA is still needed. In this study, we examined the effects of MWA on the serum levels of Golgi protein 73 (GP73). The resulting data suggest that serum GP73 is markedly elevated in response to the MWA procedure. Importantly, our novel findings may have the clinical implication that serum GP73 could be a potential marker to monitor MWA-induced inflammatory liver injury.
- Citation: Xu ZJ, Wei MJ, Zhang XM, Liu HG, Wu JP, Huang JF, Li YF, Huang ZJ, Yan YY. Effects of microwave ablation on serum Golgi protein 73 in patients with primary liver cancer. World J Gastroenterol 2022; 28(29): 3971-3980
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3971.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3971
Liver cancer is one of the most common malignant tumors throughout the world, and its incidence is relatively high in Asian countries and Pacific islands[1,2]. In China, nearly 500000 cases of primary liver cancer are newly diagnosed annually, accounting for approximately 50% of all new primary liver cancer cases worldwide, mainly due to a particularly high prevalence of hepatitis B virus (HBV) infection[1-3]. Primary liver cancer mainly includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined hepatocellular-cholangiocarcinoma, of which HCC makes up 75%-85% and ICC accounts for 10%-15% of all primary liver cancer cases[4]. For patients with primary liver cancer at an early stage, ablation therapy has been shown to be an effective treatment option. Microwave ablation (MWA) is an ablation modality that destroys cancer cells by using heat from microwave energy. Over the last decade, extensive studies have shown that MWA is effective and safe for treating small primary liver cancer, which is usually less than 3 cm in diameter[5-9]. However, it has been reported that the MWA procedure induces a hepatic inflammatory response and injury, which may negatively affect its efficacy[10]. Therefore, the discovery of reliable markers to monitor the patient’s response to MWA is still needed.
Golgi protein 73 (GP73) is a transmembrane glycoprotein with a molecular weight of 73 kDa. Under normal conditions in the liver, GP73 is mainly expressed in the epithelial cells of the bile duct, while its expression in hepatocytes is considerably lower[11]. Previous studies have shown that hepatic GP73 expression is upregulated in a variety of acute and chronic liver diseases[12]. Our previous study also has demonstrated that GP73 is expressed in the cytoplasm of hepatocytes, but not in the infiltrating inflammatory cells in patients with chronic HBV infection, and that changes in the hepatic and serum levels of GP73 are positively correlated with hepatic necroinflammatory activity in CHB patients[13]. Few hepatocytes expressed GP73, and the serum GP73 levels were low in patients with chronic HBV infection but without indications of liver injury. However, once hepatic necrosis was triggered, the affected hepatocytes started to release more GP73 into the blood, resulting in elevated hepatic and serum levels of GP73. Our previous study also found that elevated serum GP73 levels were positively associated with a higher hepatic necroinflammatory activity grade[14]. To date, the potential value of serum GP73 in the dynamic monitoring and assessment of patient response to MWA during the treatment of liver cancer remains to be further investigated.
Intrigued by our previous findings, we aimed to examine the effects of MWA of liver cancer on the serum GP73 levels before and at different time points after ablation therapy in patients with primary liver cancer. The findings may help to identify potential markers to be used in the dynamic monitoring of the response to MWA for the treatment of primary liver cancer.
A total of 150 patients with primary liver cancer were retrospectively enrolled from the Liver Disease Center at the 910th Hospital of the PLA Logistics Support Force between January 2016 and October 2018. All of the study subjects were diagnosed as having primary liver cancer, in accordance with the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer in China (2019 edition) issued by the National Health and Health Commission of the People’s Republic of China[15], and fulfilled the following inclusion criteria: (1) Age > 18 years old; (2) A single lesion ≤ 3 cm in diameter; and (3) No invasion into the portal vein, hepatic vein, or extrahepatic distant metastasis on imaging examinations [e.g., color Doppler ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI)]. All of the 150 patients with primary liver cancer had a history of HBV infection. Chronic hepatitis B (CHB) and CHB-associated liver cirrhosis were diagnosed in accordance with the diagnostic criteria as reported in the Guidelines of Prevention and Treatment for Chronic Hepatitis B (2019 version)[16]. The exclusion criteria were as follows: (1) Age ≤ 18 years old; (2) The number of lesions ≥ 2 or the size > 3 cm in diameter; (3) The tumor invaded adjacent organs or extrahepatic metastases occurred; (4) Child–Pugh score of grade C; (5) Uncorrectable coagulation dysfunction; (6) Complicated with active infection; (7) Massive ascites and cachexia; (8) Major organ failure (heart, brain, lung, kidney, or other important organs); (9) Eastern Cooperative Oncology Group (ECOG) score > 2; and (10) Disorders of consciousness or inability to cooperate with medical treatments. Among the 150 patients with primary liver cancer, 143 patients with space-occupying lesions with typical imaging features of liver cancer did not receive a liver biopsy, and the remaining 7 patients underwent a liver biopsy and had a pathological diagnosis of primary HCC. In terms of the status of the background liver, 115 patients had background liver cirrhosis, and 35 patients showed no clinical signs of liver cirrhosis.
This study was approved by the Ethics Committee of the 910th Hospital of the PLA Joint Logistics Support Force (Quanzhou, Fujian Province, China). All of the patients provided a signed informed consent.
All of the patients refused liver resection surgery and were willing to undergo MWA of liver cancer on a microwave therapeutic apparatus (Nanjing, Jiangsu Province, China). The procedure was performed under the guidance of CT or color Doppler ultrasound. The power and ablation time were designed for each patient based on the size of the liver cancer and the surrounding tissues. The ablation zone size was 0.5-1 cm to the margin of the tumor.
Enhanced CT or MRI examinations were performed on the patients at 1 mo after MWA to determine the complete or partial tumor ablation rate. The patients were followed up for more than 3 years, during which the patients were scheduled for enhanced CT or MRI to determine the cumulative recurrence rate, survival time, local tumor progression, presence of new tumors, disease-free survival (DFS), and overall survival (OS). Complete tumor ablation was defined as no residual tumor on enhanced CT or MRI within the ablation zone and adequate ablation margins of 0.5-1.0 cm. Partial tumor ablation was defined as the presence of a residual tumor on enhanced CT or MRI in the zone of ablation.
Enzyme-linked immunosorbent assay (ELISA) was performed to determine the serum GP73 levels at the Laboratory of Liver Diseases, the 910th Hospital of the PLA Joint Logistics Support Force. The GP73 ELISA detection kit was purchased from Hotgen Biotech Co. Ltd. (Beijing, China) and used for the determination of serum GP73 levels on a Model 680 Microplate Reader (BIO-RAD, United States), according to the manufacturer’s protocol. A serum GP73 concentration of 45 mg/L was used as a cut-off value for the general healthy population.
Serum alpha-fetoprotein (AFP) was examined using an AFP detection kit (Roche Diagnostics, Basel, Switzerland), and a value of < 7 mg/L was considered to be normal. Liver biochemical tests, including serum albumin (ALB), total bilirubin (TBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), were determined on a TBA120FR automatic biochemistry analyzer (Toshiba Medical System Co., Ltd., Japan) in the Clinical Laboratory of the 910th Hospital of the PLA Joint Logistics Support Force (Quanzhou, Fujian Province, China).
Statistical analysis was performed using GraphPad Prism 5.0 software (Graphpad Software Inc., La Jolla, CA, United States). Count data were expressed as percentages (%). Continuous variables were presented as the median (25%, 75%). For paired data following a normal distribution, a paired t-test was used to evaluate differences between the pairs. For non-normally distributed data, a paired Wilcoxon signed-rank test was applied to determine the difference between matched samples. The Kaplan–Meier method was used for survival analysis. P < 0.05 indicated that the difference was statistically significant. The statistical methods of this study were reviewed by Zhu YL from the Department of Medical Statistics, Huaqiao University.
The study cohort was comprised of 150 primary liver cancer patients with one small lesion (average size, 2.03 cm in diameter; range, 0.7-3 cm), including 130 men (86.67%) and 20 women (13.33%), and the median age was 56.00 years old. All of the enrolled patients had a medical history of chronic HBV infection, of which 97 patients (97/150, 64.7%) were positive for the HBV e antigen. Of note, 91 patients (91/150, 60.7%) received antiviral treatment with entecavir or tenofovir disoproxil fumarate for more than 1 year before undergoing MWA and had an HBV DNA concentration of < 500 IU/L, while the remaining 59 patients (59/150, 39.33%) who did not receive the antiviral treatment had an average HBV DNA level of 6.32 (4.52, 7.15) log10 IU/L. Of the enrolled primary liver cancer patients, 115 patients had compensated liver cirrhosis, accounting for 76.7% of the study subjects. Prior to MWA, the 150 patients with primary liver cancer had an ECOG Performance Status score of 0 and a Child–Pugh classification of Class A. The majority of the patients with primary liver cancer had abnormally high levels of AFP, accounting for 68.00% (102/150) of the study subjects. The liver biochemical characteristics, including serum ALB, TBIL, ALT, and AST, as well as the AFP and serum GP73 Levels of the study subjects before MWA are summarized in Table 1.
| ALB (g/L) | TBIL (μmol/L) | ALT (U/L) | AST (U/L) | GP73 (mg/L) | AFP (mg/L) | |
| Cases | 150 | 150 | 150 | 150 | 150 | 1021 |
| Before MWA | 42.10 (38.83, 45.20) | 16.20 (12.70, 23.35) | 30.10 (20.55, 41.55) | 28.30 (22.05, 35.40) | 90.83 (54.49, 110.60) | 110.40 (32.71, 267.30) |
| 1 wk after MWA | 38.40 (36.65, 41.15)a | 24.80(16.25, 30.60)a | 102.20(88.65, 166.30)a | 81.70(68.25, 91.30)a | 127.10(84.66, 175.50)a | 37.61(23.30, 95.48)a |
| 2 wk after MWA | 40.30 (38.36, 42.00) | 15.00 (12.90, 18.05) | 29.40 (25.65, 36.85) | 29.00 (24.10, 36.05) | 130.70 (88.39, 163.60) | 27.34 (6.32, 81.59)b |
| 4 wk after MWA | 43.75 (37.12, 47.31) | 17.35 (13.62, 25.76) | 33.21 (21.57, 43.82) | 26.29 (19.70, 40.61) | 102.20 (59.15, 121.90) | 7.32 (3.87, 16.25)c |
| at/W | 7.037 (t) | 3.991 (t) | 6.703 (t) | 5.768 (t) | 5.150 (t) | -157 (W) |
| aP | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
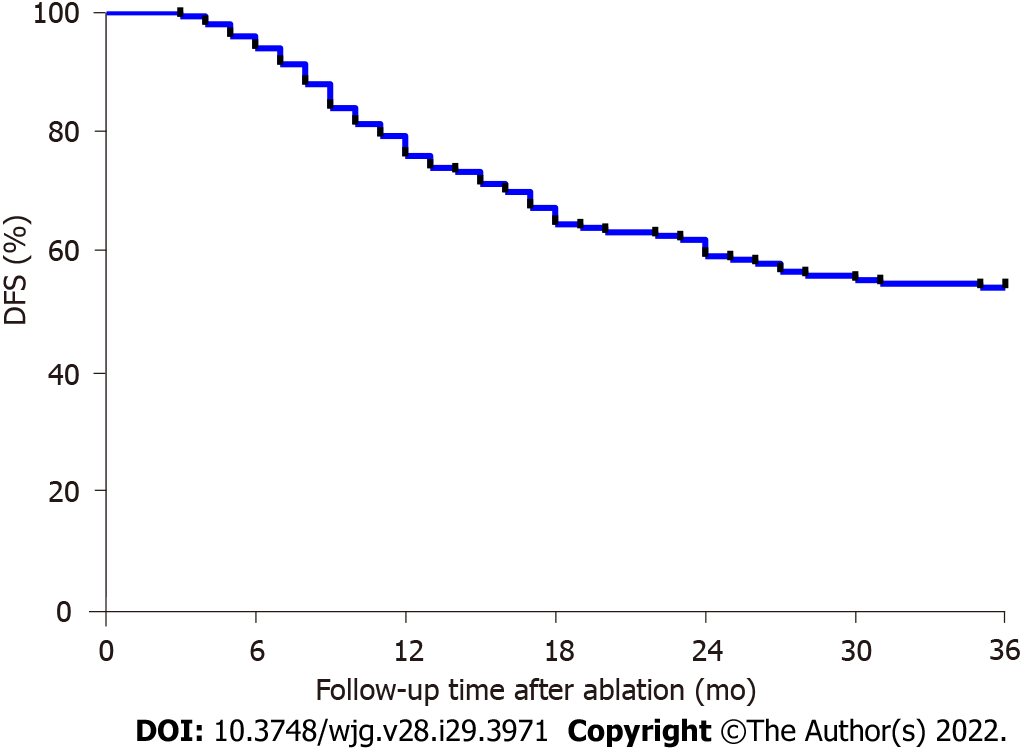
All of the patients received MWA for primary liver cancer, and the effectiveness was evaluated, including the complete tumor ablation rate at 1 mo after MWA, recurrence rate, and survival time. At 1 month after MWA, enhanced CT/MRI examinations showed that 143 patients (143/150, 95.33%) achieved complete tumor ablation. A small proportion of the patients (7/150, 4.67%) who had partial tumor ablation underwent reablation treatment with MWA and achieved complete tumor ablation. All of the 150 patients were followed up for more than 3 years, and the results were as follows: 134 patients (134/150, 89.33%) survived and 16 patients (16/150, 10.67%) died due to gastrointestinal bleeding in 5 patients (5/150, 3.33%) and liver failure in 11 patients (11/150, 7.33%). The OS rates at 1, 2, and 3 years after MWA were 100.00%, 96.00%, and 89.33%, respectively. In terms of tumor recurrence, cumulative recurrence occurred in 69 patients (69/150, 46.00%) at 3 years after MWA, including 13 patients (13/150, 8.67%) with local tumor progression and 57 patients (57/150, 37.33%) with a new tumor. Among the 69 patients with tumor recurrence, the overall 1-, 2-, and 3-year recurrence rates after MWA were 55.07% (38/150), 33.33% (23/150), and 11.59% (8/150), respectively. As presented in Figure 1, the 1-, 2-, and 3-year DFS rates after MWA were 74.67%, 59.33%, and 54.00%, respectively, in the patients with MWA treatment of primary liver cancer.
MWA for primary liver cancer may cause some adverse effects, including inflammatory injury in the liver. We analyzed the effects of MWA treatment on the widely used liver biochemical indicators ALB, TBIL, ALT, and AST by comparing their values of the enrolled patients before MWA and at different time points (1, 2, and 4 wk) after the treatment procedure. As shown in Table 1, the serum levels of ALB were significantly decreased, while the levels of serum TBIL, ALT, and AST were significantly increased at 1 wk after MWA treatment compared with those levels prior to MWA as a control (all P < 0.001). We found that increases in the biochemical indicators (TBIL, ALT, and AST) were related to the hepatocyte injury in response to MWA treatment. There were no significant differences in ALB, TBIL, ALT, or AST between before and at 2 or 4 wk after the MWA treatment (Table 1), which was mainly attributed to the implementation of hepatoprotective therapy using the active compounds glycyrrhizin and reduced glutathione for nearly 2 wk.
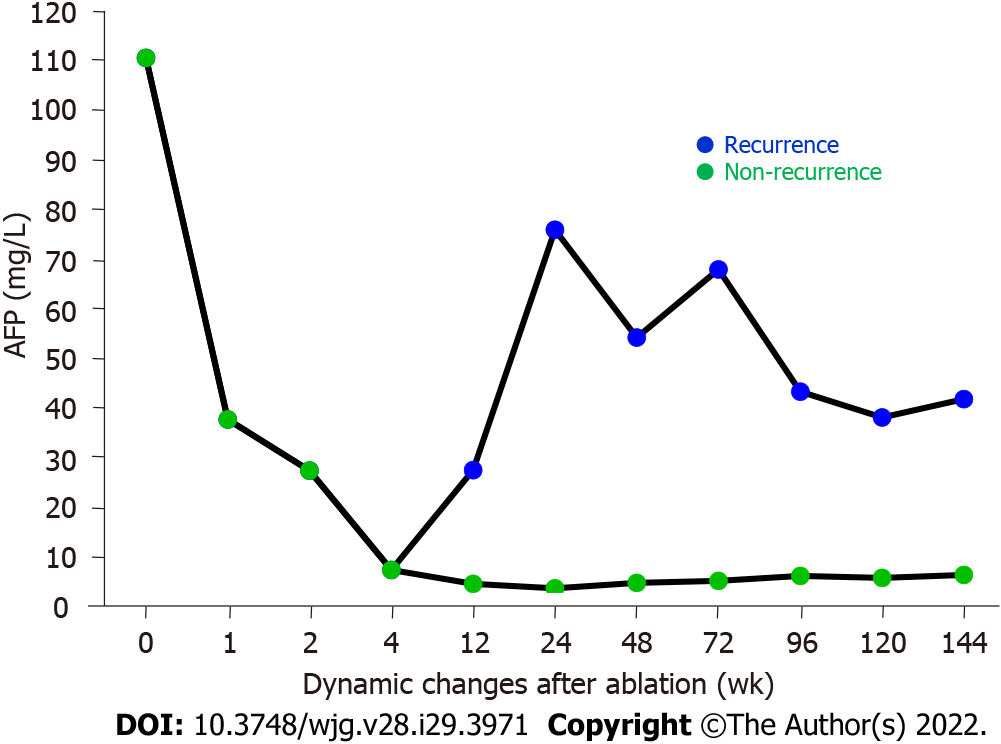
We examined the effects of MWA on the serum AFP levels before and after MWA treatment in the enrolled patients with primary liver cancer. As illustrated in Figure 2 and Table 1, the patients had an abnormally high AFP level of 110.40 (32.71, 267.30) mg/L before MWA treatment (pre-MWA), which was decreased sharply to 37.61 (23.30, 95.48) mg/L at 1 wk after MWA treatment. Notably, the AFP level continued to decrease at 2 and 4 wk, returned to the normal range at 12 wk after MWA, and remained stable thereafter during follow-up in those cases without recurrence (Figure 2, Table 1). Statistical analysis revealed that the serum AFP levels at all time points after the MWA treatment were significantly lower compared with the value prior to MWA treatment as a control (all P < 0.001). For the recurrent cases with primary liver cancer, however, the serum AFP levels started to increase at 12 wk post MWA; the values were 75.85 mg/L (range, 38.32-86.70 mg/L), 54.17 mg/L (range, 37.83-82.60 mg/L), 67.80 mg/L (range, 37.67-97.32 mg/L), 43.20 mg/L (range, 29.80-58.96 mg/L), 38.05 mg/L (range, 30.85-93.93 mg/L), and 66.73 mg/L (range, 51.90-81.56 mg/L) at 24, 48, 72, 96, 120, and 144 wk following the MWA treatment, respectively (Figure 2).
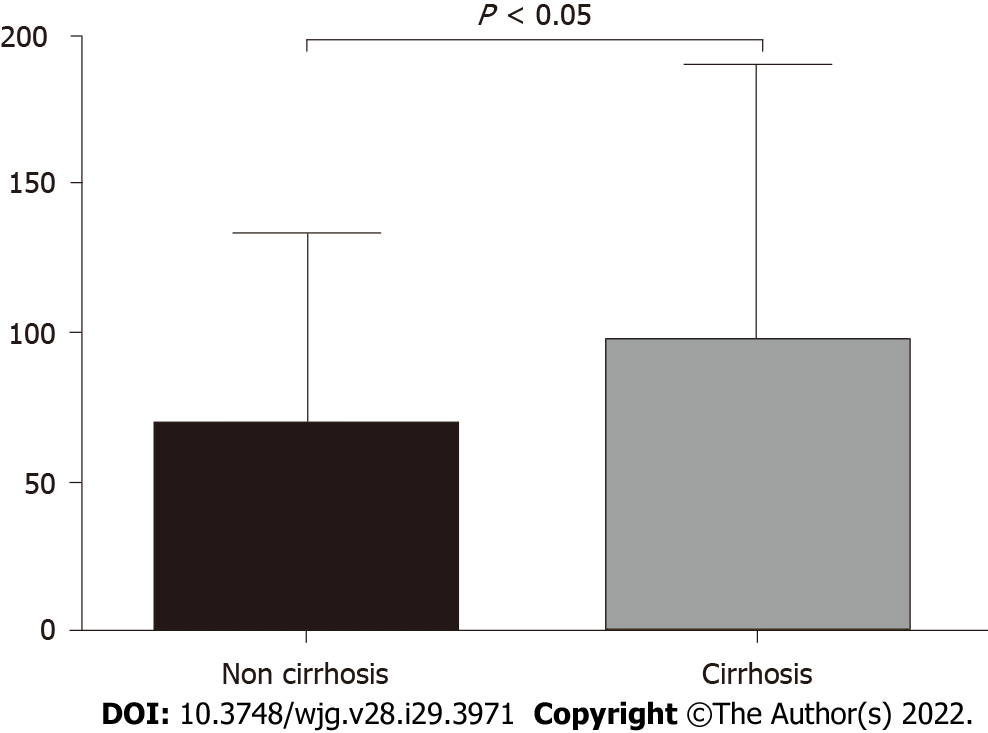
We compared the serum GP73 levels in the enrolled liver cancer patients with vs without background liver cirrhosis. As shown in Figure 3, the average level of serum GP73 in the 115 patients with liver cirrhosis was 97.76 mg/L (range, 70.65-133.10 mg/L), which was significantly greater than 69.02 mg/L (range, 45.48-101.40 mg/L) in the 35 patients without liver cirrhosis (t = 2.477, P < 0.05) (Figure 3).
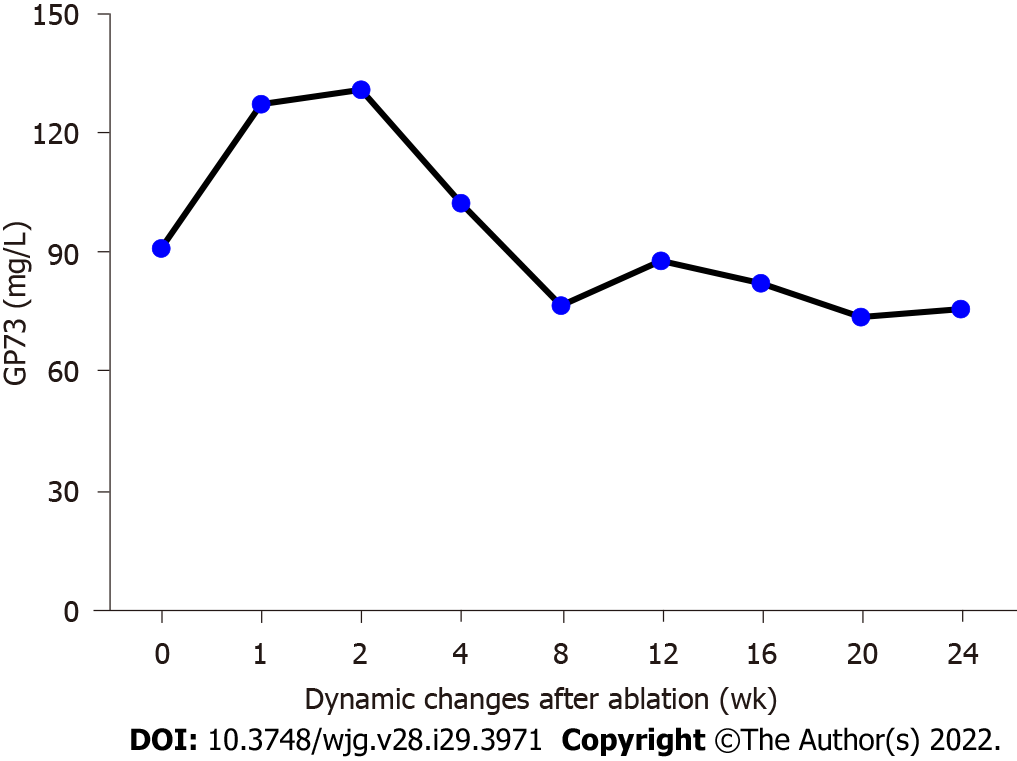
To evaluate the potential value of serum GP73 measurement for inflammatory injury associated with MWA treatment, we analyzed the serum GP73 levels in the primary liver cancer patients before and at different times after the MWA treatment. As shown in Figure 4 and Table 1, the average serum GP73 level was 90.83 mg/L (range, 54.49-110.6 mg/L) before MWA, which was increased to 127.1 mg/L (range, 84.66-175.50 mg/L) and 130.70 mg/L (range, 88.39-163.60 mg/L) at 1 and 2 wk following the MWA treatment, respectively. Notably, the serum GP73 level started to decline after hepatoprotective treatment with glycyrrhizin and reduced glutathione at 4 wk post MWA; the values were 102.20 mg/L (range, 59.15-121.90 mg/L), 85.73 mg/L (range, 61.42-105.70 mg/L), and 76.09 mg/L (range, 59.26-102.66 mg/L) at 8, 12, and 24 wk following the MWA treatment, respectively (Figure 4). Statistical analysis revealed that the serum GP73 levels were significantly greater at 1 and 2 wk after the MWA procedure vs before MWA (1 wk after MWA vs before MWA: t = 5.150, P < 0.001; 2 wk after MWA vs before MWA: t = 6.182, P < 0.001) (Figure 4). It is worth noting that the serum GP73 level reached the peak at 2 wk after MWA and then started to decline at 4 wk after MWA (Figure 4). During the follow-up of the patients at 12 and 24 wk following the MWA treatment, the serum GP73 levels were similar to the pretreatment level (Figure 4).
This retrospective study has the following major findings: (1) MWA was highly efficacious in treating patients with small primary liver cancer, with as high as 95.33% of the patients achieving complete tumor ablation at 1 mo after MWA; (2) The serum AFP level was significantly decreased following the MWA treatment and returned to the normal range at 3 mo after MWA, which was in agreement with the observation for the complete tumor ablation rate; (3) The serum GP73 levels were significantly elevated at 1 and 2 wk following MWA, with the peak reached at 2 wk after completion of the treatment; (4) The serum GP73 level declined starting at 4 wk after MWA and continued to decrease to the pretreatment level at 12 and 24 wk after MWA; and (5) The effect of MWA on the serum GP73 level was similar to those of TBIL, ALT, and AST. These findings suggest that measurement of the serum GP73 level has the potential to monitor MWA-mediated inflammatory injury in patients with primary liver cancer.
In the last decade, percutaneous ablation therapy has been used as a radical treatment method for treating patients with liver cancer[6,17]. In contrast to other types of ablation therapy, MWA has a number of advantages (e.g., a faster ablation time, the capability of simultaneous ablation of multiple lesions, and larger tumor ablation volumes)[18]. Compared with traditional radiofrequency ablation for the treatment of small primary liver cancer nodules, MWA is less affected by the heat sink effect[19-21]. Therefore, MWA is widely accepted as an effective nonsurgical treatment option for liver cancer of a small size and at an early stage[19-23]. Of note, MWA has been demonstrated to completely destroy tumor cells and has been proposed as a radical treatment for small primary liver cancer (≤ 3 cm in diameter) with a single lesion[6,22,24]. In addition, Zhang et al[25] performed a meta-analysis of 1480 patients and showed that the therapeutic effectiveness of MWA was superior to that of surgical resection for the treatment of small liver cancer (< 3 cm in diameter). There were no significant differences in the OS, DFS rate, and recurrence rate between MWA and surgical resection for small liver cancer. Our results revealed a high therapeutic effectiveness of MWA for primary liver cancer as supported by several lines of evidence, including 95.33% of patients reaching complete tumor ablation at 1 mo after MWA, high OS rates (1- and 3-year OS rates of 100.00% and 89.33%, respectively), and high DFS rates (1-, 2-, and 3-year DFS rates of 74.67%, 59.33%, and 54.00%, respectively), which were similar to those values previously reported for surgical resection[26]. Despite the high therapeutic effectiveness of MWA for small primary liver cancer, the procedure may trigger an inflammatory response and cause liver injury, which will need to be monitored and alleviated properly.
It merits attention that the serum GP73 level in the present study was significantly elevated at 1 wk after MWA, reached the peak at 2 wk after completion of the procedure, and then declined at 4 wk following MWA. During follow-up of the patients with primary liver cancer at 12 and 24 wk after MWA, the serum GP73 levels nearly returned to the pretreatment level. Consistent with the increase of the serum GP73 levels at 2 and 4 wk after MWA, the serum TBIL, ALT, and AST levels were also significantly increased. Moreover, previous studies have reported that MWA is associated with liver injury due to the high temperature generated in the procedure, induction of hepatocyte apoptosis, and stimulation of the intrahepatic macrophage-related inflammatory response[10,21]. After the MWA-mediated liver injury was alleviated with the implementation of hepatoprotective therapy using the active compounds glycyrrhizin and reduced glutathione for nearly 2 wk, the serum GP73 level was significantly decreased and returned to a level similar to that before MWA. The change of the serum GP73 level in the early response to MWA as well as anti-inflammatory and hepatoprotective therapy using glycyrrhizin and reduced glutathione suggests that serum GP73 is related to the aseptic inflammatory injury of hepatocytes after MWA. The results of this study are also in agreement with those of our previous study demonstrating that the serum GP73 level is increased in liver inflammatory injury[13,14]. Therefore, these findings suggest that the serum GP73 level could be a useful diagnostic approach to monitor the liver inflammatory injury caused by MWA during the treatment of primary liver cancer.
This study does have some limitations that must be addressed. For instance, this was a retrospective study that selected liver cancer patients with one small lesion (≤ 3 cm); therefore, bias in patient selection may have occurred. In addition, due to the retrospective nature of this study, we were unable to examine the correlation between the initial level of GP73 after MWA and the AFP level, recurrence rate, or survival rate. Further prospective studies are needed in the future to validate the interesting findings and to assess the diagnostic accuracy of serum GP73 for monitoring liver injury following MWA treatment for primary liver cancer.
Taken together, this study demonstrated that serum GP73 is markedly elevated in response to MWA treatment for primary liver cancer. Therefore, the findings have a clinical implication that measurement of serum GP73 holds promise for monitoring MWA-induced inflammatory liver injury that requires alleviation.
Microwave ablation (MWA) has been proven to be highly effective in treatment of small primary liver cancer. However, the procedure may trigger an inflammatory response and cause liver injury in primary liver cancer patients undergoing MWA. As such, it is needed to find reliable markers to monitor and evaluate patient response to MWA. Previous studies have shown that Golgi protein 73 (GP73) are associated with liver inflammatory injury.
This study was designed to test our hypothesis that serum GP73 levels altered in response to MWA in patients with primary liver cancer, and thereby could be used as a potential marker for MWA-induced liver inflammation and injury.
The main objective of this study was to examine effects of MWA on the serum levels of GP73 before and at different time points after the ablation procedure in patients with primary liver cancer.
Patients with primary liver cancer (≤ 3 cm in diameter) receiving MWA were retrospectively enrolled in this study. Serum GP73 levels were compared before and 1, 2, and 4 wk after the ablation procedure.
The serum GP73 levels were significantly elevated at 1 and 2 wk after MWA with the peak at 2 wk after completion of the treatment. The serum GP73 levels decreased starting at 4 wk after MWA and continued to decline to the pretreatment level at 12 and 24 wk after MWA. It was worthy to note that the alterations of serum GP73 levels in response to MWA were similar to those of liver biochemical indicators.
The findings of this study have demonstrated that serum GP73 levels altered in response to MWA in patients with primary liver cancer, and thereby measurement of serum GP73 level holds potential as a biomarker for monitoring and assessment of MWA-mediated inflammatory injury in patients with primary liver cancer.
Future prospective studies are needed to validate the findings and to assess the diagnostic accuracy of serum GP73 for monitoring liver injury following MWA treatment for primary liver cancer.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68439] [Article Influence: 13687.8] [Reference Citation Analysis (201)] |
| 2. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1799] [Cited by in RCA: 1826] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 3. | Fung J, Lai CL, Yuen MF. Hepatitis B and C virus-related carcinogenesis. Clin Microbiol Infect. 2009;15:964-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56638] [Article Influence: 7079.8] [Reference Citation Analysis (134)] |
| 5. | Hasegawa K, Aoki T, Ishizawa T, Kaneko J, Sakamoto Y, Sugawara Y, Kokudo N. Comparison of the therapeutic outcomes between surgical resection and percutaneous ablation for small hepatocellular carcinoma. Ann Surg Oncol. 2014;21 Suppl 3:S348-S355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Liang P, Yu J, Lu MD, Dong BW, Yu XL, Zhou XD, Hu B, Xie MX, Cheng W, He W, Jia JW, Lu GR. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19:5430-5438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 8. | Vogl TJ, Nour-Eldin NA, Hammerstingl RM, Panahi B, Naguib NNN. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms - Review Article. Rofo. 2017;189:1055-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Shiina S, Sato K, Tateishi R, Shimizu M, Ohama H, Hatanaka T, Takawa M, Nagamatsu H, Imai Y. Percutaneous Ablation for Hepatocellular Carcinoma: Comparison of Various Ablation Techniques and Surgery. Can J Gastroenterol Hepatol. 2018;2018:4756147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Zhou H, Sun Y, Wang Q, Li Z, Zhong W, Wang X, Dai X, Kong L. N-acetylcysteine alleviates liver injury by suppressing macrophage-mediated inflammatory response post microwave ablation. Int Immunopharmacol. 2020;85:106580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene. 2000;249:53-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Liu X, Wan X, Li Z, Lin C, Zhan Y, Lu X. Golgi protein 73(GP73), a useful serum marker in liver diseases. Clin Chem Lab Med. 2011;49:1311-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Xu Z, Liu L, Pan X, Wei K, Wei M, Yang H, Liu Q. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver disease. Medicine (Baltimore). 2015;94:e659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (3)] |
| 14. | Xu Z, Shen J, Pan X, Wei M, Liu L, Wei K, Yang H, Huang J. Predictive value of serum Golgi protein 73 for prominent hepatic necroinflammation in chronic HBV infection. J Med Virol. 2018;90:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (3)] |
| 15. | Department of Medical Administration, National Health and Health Commission of the People's Republic of China. . [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 16. | Chinese Society of Infectious Diseases; Chinese Medical Association. ; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 123] [Reference Citation Analysis (1)] |
| 17. | Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 18. | Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (3)] |
| 19. | Ierardi AM, Giorlando F, Piacentino F, Fontana F, Novario R, Angileri SA, Duka E, Carrafiello G. Factors predicting outcomes of microwave ablation of small hepatocellular carcinoma. Radiol Med. 2017;122:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7:2578-2589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (2)] |
| 21. | Bhardwaj N, Dormer J, Ahmad F, Strickland AD, Gravante G, West K, Dennison AR, Lloyd DM. Microwave ablation of the liver: a description of lesion evolution over time and an investigation of the heat sink effect. Pathology. 2011;43:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Luo W, Zhang Y, He G, Yu M, Zheng M, Liu L, Zhou X. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Wang ZL, Liang P, Dong BW, Yu XL, Yu DJ. Prognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective study. J Gastrointest Surg. 2008;12:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Rao Z, Ling W, Dai X, Zhang H, Pu L, Wu J, Zhu D, Yang X, Li Z, Lu L, Wang X, Zhou H, Kong L. Precoagulation with microwave ablation for hepatic parenchymal transection during liver partial resection. Int J Hyperthermia. 2019;36:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Zhang M, Ma H, Zhang J, He L, Ye X, Li X. Comparison of microwave ablation and hepatic resection for hepatocellular carcinoma: a meta-analysis. Onco Targets Ther. 2017;10:4829-4839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Zhang EL, Yang F, Wu ZB, Yue CS, He TY, Li KY, Xiao ZY, Xiong M, Chen XP, Huang ZY. Therapeutic efficacy of percutaneous microwave coagulation vs liver resection for single hepatocellular carcinoma ≤3 cm with Child-Pugh A cirrhosis. Eur J Surg Oncol. 2016;42:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koganti S, United States; Sultana N, Bangladesh; Zhang X, United States S-Editor: Yan JP L-Editor: A P-Editor: Yan JP