Published online Aug 7, 2022. doi: 10.3748/wjg.v28.i29.3838
Peer-review started: December 4, 2021
First decision: April 16, 2022
Revised: April 28, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: August 7, 2022
Processing time: 242 Days and 1.6 Hours
Obesity is associated with an increased risk of developing Crohn’s disease (CD), higher disease activity, and comparatively worse clinical outcomes.
To investigate the role of mesenteric adipose tissue-derived exosomes in the pathogenesis of CD aggravation in obese individuals.
First, we induced colitis in mice initiated on high-fat and normal diets and compared the severity of colitis. We then extracted and identified exosomes from mesenteric adipose tissue and determined the levels of metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) in mesenteric adipose tissue-derived exosomes and the colon. Next, we demonstrated an interaction between MALAT1 and the miR-15a-5p/activating transcription factor 6 (ATF6) axis. Finally, we explored the effects of mesenteric adipose tissue-derived exosomes extracted from mice fed a high-fat or normal diet on the severity of 2,4,6-trinitrobe-nzenesulfonic acid (TNBS)-induced colitis and ATF6-related endoplasmic reticulum stress pathways.
High-fat diet was found to aggravate TNBS-induced colitis in mice. The expression of MALAT1 in mesenteric adipose tissue-derived exosomes of high-fat diet-fed mice increased. The increased expression of MALAT1 in colon tissue exacerbated TNBS-induced colitis and activated the ATF6 endoplasmic reticulum stress pathway. This effect was partially reversed by the reduced expression of MALAT1 and overexpression of miR-15a-5p.
Mesenteric adipose tissue-derived exosome-encapsulated long noncoding RNAs MALAT1 targets the colon and aggravates TNBS-induced colitis in obese mice, which may potentially act on the miR-15a-5p/ATF6 axis and activate endoplasmic reticulum stress.
Core Tip: A higher visceral adipose tissue ratio has been associated with increased disease activity in patients with Crohn’s disease. However, the mechanisms underlying this effect remain unclear. Our study indicates that a high-fat diet increases the mesenteric adipose tissue content and aggravates colitis in mice. Mesenteric adipose tissue-derived exosome long noncoding RNAs metastasis-associated lung adenocarcinoma transcript 1 can be absorbed by the colon, leading to the activation of the endoplasmic reticulum stress pathway by targeting the miR-15a-5p/activating transcription factor 6 axis to aggravate colitis.
- Citation: Chen D, Lu MM, Wang JH, Ren Y, Xu LL, Cheng WX, Wang SS, Li XL, Cheng XF, Gao JG, Kalyani FS, Jin X. High-fat diet aggravates colitis via mesenteric adipose tissue derived exosome metastasis-associated lung adenocarcinoma transcript 1. World J Gastroenterol 2022; 28(29): 3838-3853
- URL: https://www.wjgnet.com/1007-9327/full/v28/i29/3838.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i29.3838
Crohn’s disease (CD) is a chronic relapsing inflammatory disease of the gastrointestinal tract that results from the interplay between genetic susceptibility and various environmental exposures[1]. The incidence of CD is higher in developed countries and urban areas than in developing and rural areas[2]. Similarly, the prevalence of CD is increasing in areas with rapid urbanization[3], paralleling the obesity epidemic, possibly in conjunction with western-style diets characterized by high fat content.
The conventional understanding is that CD is a chronic disease characterized by wasting, low weight, and malnutrition. Nevertheless, based on real world data, 15%-40% of patients with inflammatory bowel disease (IBD), including CD and ulcerative colitis (UC), are obese and 20%-40% are overweight[4]. Several studies have shown that obesity is associated with an increased risk of developing CD[5,6], a higher disease activity, and comparatively worse clinical outcomes[7]. For instance, Jain et al[8] observed that obesity was not only associated with an increased risk of persistent disease activity and relapse in patients with IBD but also with higher rates of anxiety, depression, fatigue, pain, and inferior social function scores. Although these studies on the association between obesity and the course of CD have some limitations, and the above-mentioned outcomes have not been observed consistently, accumulating evidence indicates that visceral adiposity better captures the association between obesity and CD than body mass index[4]. A higher baseline visceral adipose tissue/total fat mass ratio was found to be associated with increased disease activity[9]. Although the influence of obesity on the course of CD has been confirmed, the underlying mechanism remains elusive.
Significant expansion of adipose tissue is a common phenomenon in obese individuals. In addition to storing energy, adipose tissue is an endocrine organ that secretes various adipokines, lipokines, and exosomes, contributing to the state of chronic inflammation in obese individuals[10]. Localized mesenteric fat accumulation in CD patients, known as creeping fat, is not only related to overall obesity but also has a systemic pro-inflammatory effect[11]. Adipose tissue and visceral adipose tissue-derived endocrine hormones are involved in multiple diseases, including cancer[12], cardiovascular disease[13], and nonalcoholic steatohepatitis (NASH)[14]. Exosomes are extracellular vesicles secreted by various cells, including adipocytes[15]. Visceral adipose tissue-derived exosomes display tissue affinity, preferentially targeting the colonic lamina propria[16]. Mesenteric adipose tissue can directly invade the lamina propria. Therefore, we hypothesized that obesity exacerbates IBD, partially due to mesenteric adipose tissue-derived exosomes.
Previous studies have indicated that exosomes contain multiple non-coding RNAs such as circular RNAs, long noncoding RNAs (lncRNAs), and microRNAs, which serve as messengers in cell-to-cell communication. Among these, lncRNAs are non-coding RNAs containing more than 200 nucleotides[17]. LncRNAs can interact with transcription factors to regulate transcription, bind to proteins to promote the formation of ribonucleoproteins, guide chromatin-modifying protein complexes targeting genes, and act as sponges for downstream microRNAs, participating in a variety of pathophysiological processes[18]. To our knowledge, few studies have explored the potential functions and mechanisms of adipocyte-derived exosomal lncRNAs in the exacerbation of CD.
The present study aimed to investigate the role of lncRNAs transferred by mesenteric adipose tissue-derived exosomes in obesity aggravated CD, and thereby assess the potential of these IncRNAs as therapeutic targets for the treatment of CD in this subpopulation.
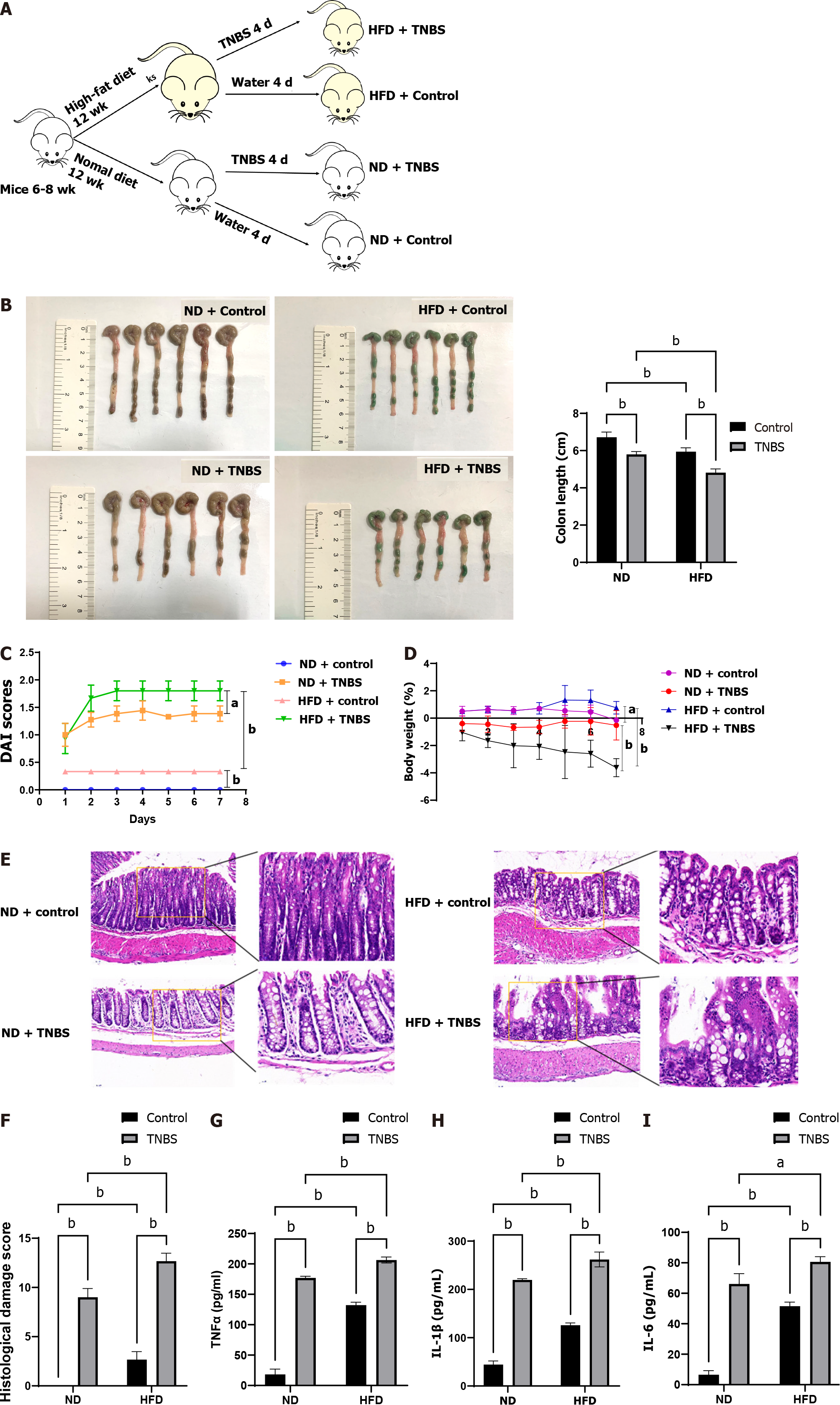
C57BL/6 mice (6-8 wk old) were purchased from Chengdu Dossy Experimental Animals Co.,Ltd. and fed either a normal diet (ND) (#D12451, Research Diets, United States) or a high-fat diet (HFD) (#D12492, Research Diets, United States) for 12 wk. To induce acute experimental colitis, 2,4,6-trinitrobe-nzenesulfonic acid (TNBS) (Beyotime, China; 100 μg/g, 175 μL) was injected into the colon for 4 d. The daily disease activity index (DAI) score was calculated in mice in accordance with a well-established method[19]. The body weight of the mice in each group was also recorded. On day 8, the mice were sacrificed for colon and mesenteric adipose tissue collection. Rectum tissue, 3 cm away from the anal margin, was collected for hematoxylin and eosin (HE) staining and inflammatory pathological score determination using the following steps. Briefly, mouse colon tissue was fixed in 10% formaldehyde, embedded in paraffin, sectioned, and stained with HE. Details of histological scoring have been described previously[20]. The remaining colon tissue was placed in liquid nitrogen for subsequent analyses. Mice were randomly divided into the following four groups (n = 6 each): ND + control, HFD + control, normal diet + TNBS, and HFD + TNBS.
Total RNA was isolated using Trizol (Invitrogen, United States) and reverse-transcribed into cDNA using the First Strand cDNA Synthesis Kit (TransGen, China), in accordance with the manufacturer’s instructions. Real-time reverse transcription-PCR (qRT-PCR) was performed using the SYBR Premix Ex Taq qPCR kit (TaKaRa, Japan). Primer sequences used are listed in Supplementary Table 1.
The concentrations of mouse interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) were detected using enzyme linked immunosorbent assay (ELISA) kits (Elabscience, China) according to the manufacturer’s instructions. Additionally, total protein was isolated using radio
Mesenteric adipose tissue (25 g) was isolated and placed into a sterile 50 mL polypropylene test tube containing 15 mL of collagenase [1 mg collagenase/1 mL phosphate belanced solution (PBS); 3 mL solution/1 g tissue]. After grinding, the solution was swirled for 20 s and the test tube was incubated at 37 °C in a rocking water bath at 100 rpm for 40 min. The solution was vortexed and filtered through a funnel containing a double gauze. Thereafter, the homogenate was centrifuged at 1000 rpm for 10 min, followed by collection of the top fat layer, which was washed three times with PBS. The precipitate was then resuspended in 10 mL of RBC lysis buffer (154 mmol/L NH4Cl, 10 mmol/L KHCO3, 1 mmol/L EDTA) in a water bath at 37 °C and 100 rpm for 5 min, followed by centrifugation at 1000 rpm for 10 min. The precipitates were resuspended in 10 mL of plate culture medium (DMEM, 0.1 mmol/L penicillin, 0.06 mmol/L streptomycin, 10% HI-FBS, pH 7.4), swirled, spread on 100 mm culture dishes, and incubated at 37 °C. After 20 h, the cells were washed with 10 mL of PBS three times, and supplemented with 1 mL of trypsin. Finally, the cells were resuspended and subcultured for further analysis.
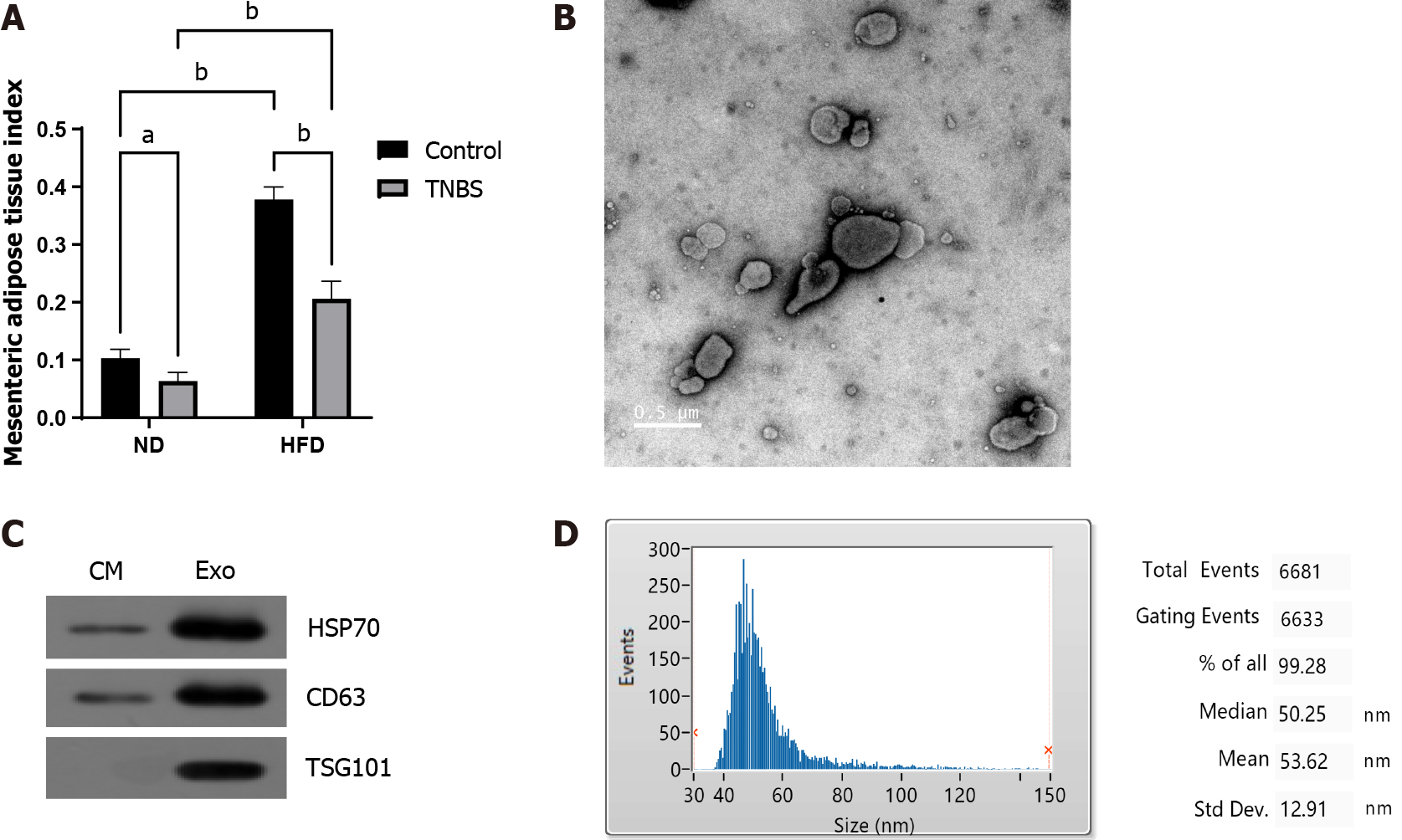
The supernatant of adipocytes was collected and centrifuged at 2000 × g for 30 min, followed by filtration through a 0.22 μm membrane to remove apoptotic cells, debris, and large particles. Exosomes were extracted from the filtrate according to the manufacturer’s instructions as described previously[21]. For further confirmation, the diluted exosomes were subjected to NanoFCM (China) for transmission electron microscopy and size distribution analysis.
The luciferase reporter assay was performed using the Dual-Luciferase Reporter Assay System (Promega, United States), as described previously[22]. The RNA pulldown assay was performed using the Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo, United States). Briefly, miR-15a-5p was biotinylated and transfected into the Caco-2 cells. Cultured cells were collected for lysis 48 h later. Thereafter, the RNA bound to miR-15a-5p was captured using Pierce nucleic acid-compatible strep
Adipocytes were transfected with shMALAT1 and miR-15a-5p using Plko.1-EGFP-puro. ShMALAT1 was designed based on information from Thermo Fisher (http://rnaidesigner.thermofisher.com/rnaiexpress/). The sequence of shMALAT1 was 5’-TGTACTATCCCATCACTGAAG-3’.
All data are presented as mean ± SD. Differences between two groups were analyzed using the Student’s t-test. Two-way analysis of variance was used to compare the differences between multiple groups with two factors. All statistical analyses were performed using GraphPad 9.0.2 software, and P < 0.05 was considered to indicate a significant difference between groups.
Accumulating evidence has shown that CD is more severe in obese individuals. We established a model of obesity in mice fed a HFD for 12 wk, while mice on a ND were used as controls. Thereafter, TNBS was used to induce colitis in the mice (Figure 1A). Compared with the control group, colon length was significantly shortened in the TNBS treatment group and the effect was further exacerbated by HFD (Figure 1B). To determine the degree of colitis activity index in different groups of mice, the DAI score was calculated daily for 8 d after starting TNBS treatment. HFD mice had a higher DAI score than the ND mice (Figure 1C). In addition, HFD significantly aggravated the TNBS-induced weight loss in mice (Figure 1D). To observe the degree of tissue damage in the mouse colon more intuitively, we used the colon tissue for HE staining and determined the histological injury scores (Figure 1E and F), which showed that HFD aggravated TNBS-induced colitis and tissue damage.
In addition, we measured the expression levels of pro-inflammatory cytokines in the colon tissues of mice in each group. The expression of TNF-α, IL-1β, and IL-6 in the colon of HFD mice treated with TNBS was significantly higher than that in ND mice treated with TNBS (Figure 1G-I). Notably, HFD itself increased the levels of pro-inflammatory cytokines in the colon of mice, which may be related to chronic inflammatory states in the body caused by obesity. These results suggest that HFD aggravates the severity of TNBS-induced colitis.
The mesenteric adipose tissue index was significantly increased in HFD mice (Figure 2A). MAT-Exos were isolated from mesenteric adipose tissue cultured in vitro, and the typical mitochondrial morphology was observed using transmission electron microscopy (Figure 2B). Using Western blotting, we demonstrated that the isolated exosomes expressed exosomal marker proteins CD63 and TSG101 and the adipocyte exosomal membrane protein HSP70 (Figure 2C). The properties of exosomes were further demonstrated by particle size analysis (Figure 2D). In summary, we successfully harvested and identified exosomes from mesenteric adipose tissue.
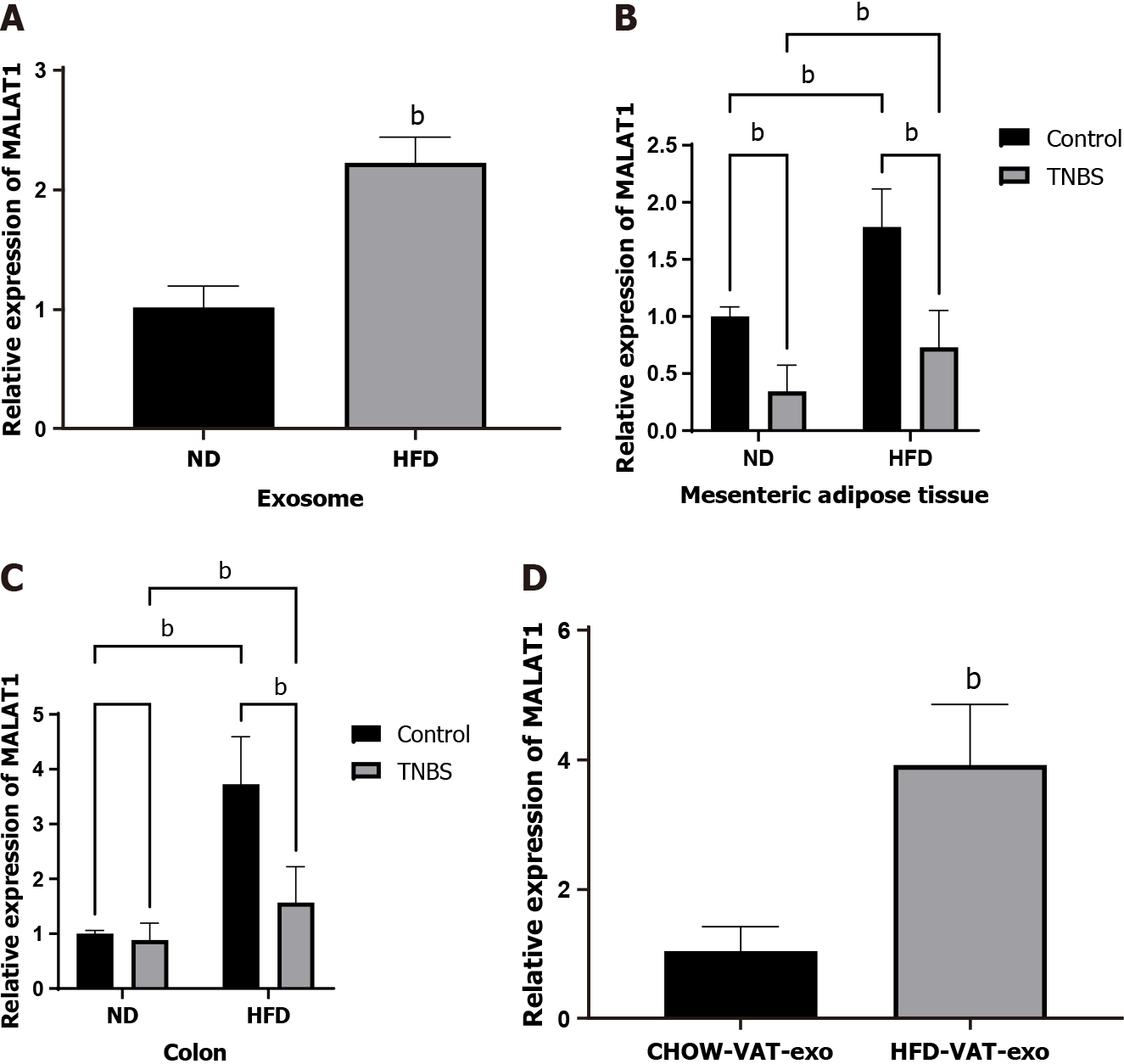
Existing literature indicates increased levels of lncRNA MALAT1 in HFD-fed mice[23]. In this study, the expression of lncRNA MALAT1 in MAT-Exos was found to be significantly increased in HFD-fed mice using qRT-PCR (Figure 3A). The relative expression of lncRNA MALAT1 in mesenteric adipose and colon tissue in HFD mice treated with TNBS was significantly increased compared to that in ND mice treated with TNBS (Figure 3B and C). Therefore, we focused our attention on lncRNA MALAT1 and speculated that the increase in the relative expression of MALAT1 in the colon tissue of HFD mice may be partially attributed to exosomes from the mesenteric adipose tissue.
For further verification, MAT-Exos labeled with a PKH26 marker were incubated with Caco2 cells in vitro. PKH26 fluorescence in Caco2 cells was detected using a laser scanning confocal microscope, indicating that Caco2 cells can absorb MAT-Exos (Supplementary Figure 1). In addition, exosomes isolated from the mesenteric adipose tissue of HFD-fed and ND mice were injected into the mice via the tail vein, followed by lncRNA MALAT1 detection in the mouse colon tissue. MAT-Exos of HFD-fed mice increased the relative expression of lncRNA MALAT1 in the colon tissues of recipient mice (Figure 3D). We speculate that in HFD mice, the MAT-Exos-encapsulated lncRNA MALAT1 might target the colon to increase its local expression.
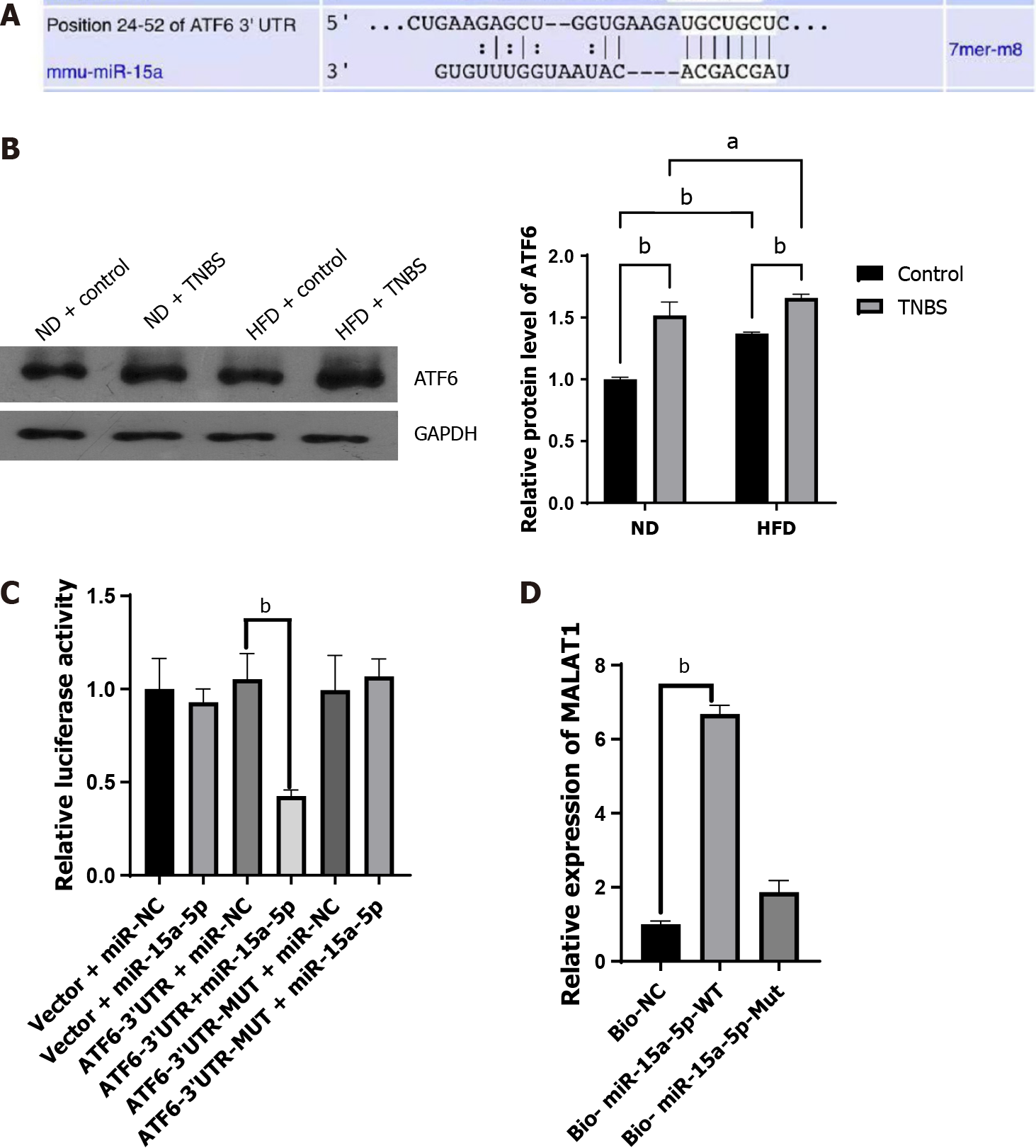
Numerous miRNAs with the ability to interact with lncRNA MALAT1 have been reported in literature[24], among which miR-15a-5p has attracted our attention. After searching miRDB (http://mirdb.org)[25] and TargetScan, we identified many potential mRNAs downstream of miR-15a-5p. Combining the literature review and bioinformatic prediction (Figure 4A), we chose ATF6 as the target mRNA of miR-15a-5p. ATF6 is an important molecule associated with endoplasmic reticulum (ER) stress[26] involved in gut homeostasis and IBD processes[27-29]. Western blotting was used to detect ATF6 expression in the colon tissues of mice in each group. The ATF6 Levels in the colon tissues of ND mice treated with TNBS were higher than those in ND mice without TNBS treatment. In addition, HFD was found to upregulate the expression of ATF6 in the colon tissues (Figure 4B). Subsequently, we used a dual-luciferase assay to confirm the interaction between ATF6 and miR-15a-5p. Compared with the control group, miR-15a-5p reduced the relative expression of ATF6 when placed downstream of the wild-type 3’ untranslated regions (3’ UTRs), but not when it was downstream of the mutant 3’ UTR, indicating a regulatory relationship between miR-15a-5p and ATF6 (Figure 4C). We then verified the interaction between miR-15a-5p and MALAT1 using RNA pull-down assays. We found that miR-15a-5p could bind to MALAT1, whereas the mutant miR-15a-5p could not pull down MALAT1 (Figure 4D). Based on these direct and indirect evidences, we can conclude that lncRNA MALAT1 can act on the miR-15a-5p/ATF6 axis.
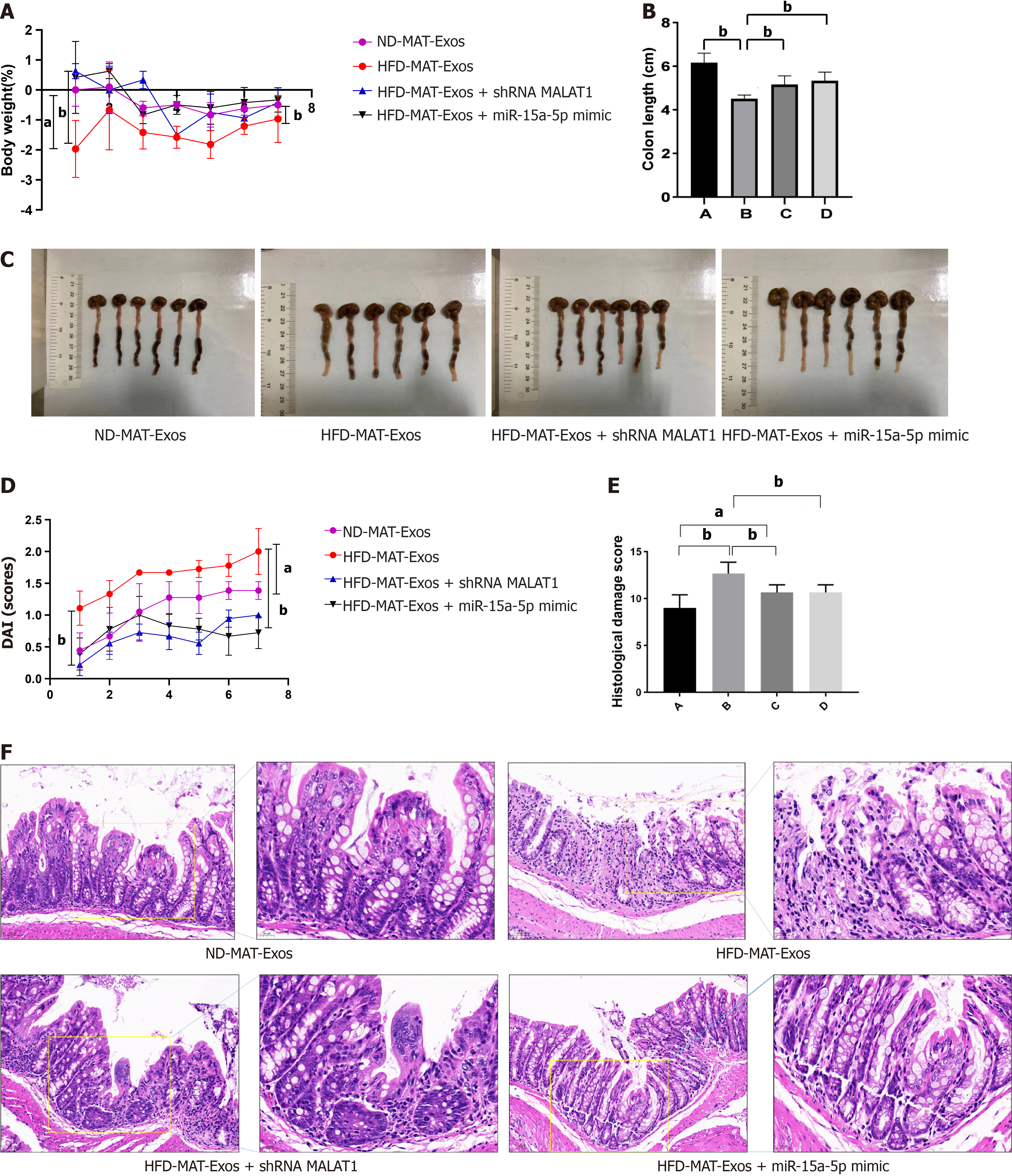
We designed animal experiments to further demonstrate that mesenteric adipose tissue derived exosomes and their encapsulated lncRNA-MALAT1 can aggravate TNBS-induced colitis in HFD mice. First, MAT-Exos from HFD mice were isolated, and treated with either a short hairpin RNA targeting MALAT1 (shMALAT) or a miR-15a-5p mimic before injection. MAT-Exos from ND mice were used as a control. Thereafter, the mice were randomly divided into four groups and treated with ND-MAT-Exos, HFD-MAT-Exos, HFD-MAT-Exos + shMALAT1, or HFD-MAT-Exos + miR-15a-5p mimic, i.e., A, B, C, and D groups, respectively. Mice were injected with MAT-Exos (100 μg/mouse) via the tail vein on days 1, 4, and 8. On day 3, TNBS was administered to induce colitis. On day 9, the mice were sacrificed and the colon tissue was collected. Compared to MAT-Exos from ND mice, MAT-EXOSs from HFD mice significantly increased the body weight loss (Figure 5A), DAI (Figure 5D), and histological damage score (Figure 5E and F), and decreased colon length (Figure 5B and C). Transfection with shMALAT1 to knockdown the expression of MALAT1 or treatment with the miR-15a-5p mimic to enhance the function of miR-15a-5p partially reversed the effects of MAT-Exos on HFD mice. In conclusion, lncRNA MALAT1 may sponge miR-15a-5p and aggravate TNBS-induced colitis in HFD mice.
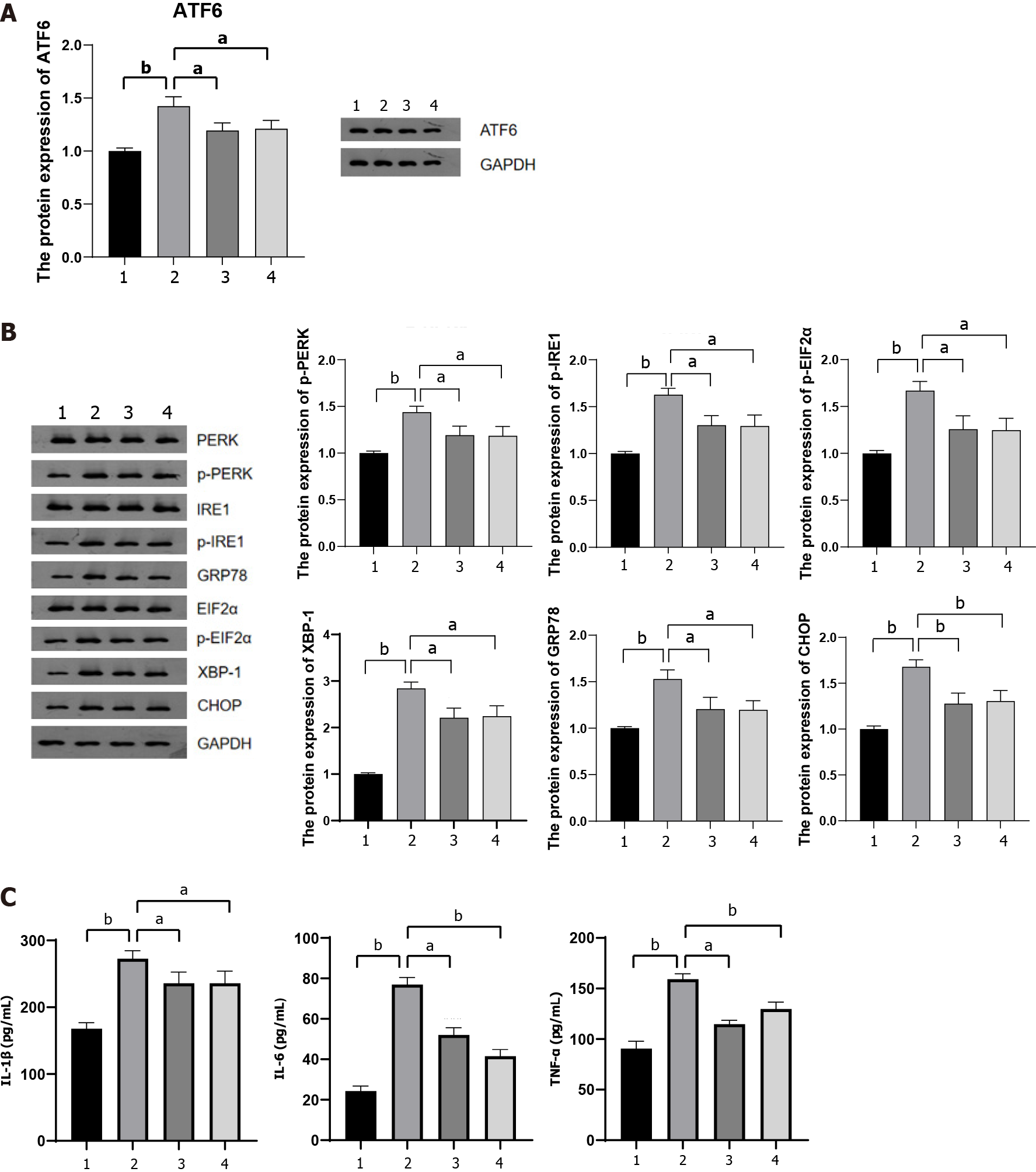
To further investigate whether lncRNA MALAT1 encapsulated within MAT-Exos of HFD mice aggravated TNBS-induced colitis via the miR-15a-5p/ATF6 axis, we detected the protein content of ATF6 in the colon tissue of mice in each group. MAT-Exos of HFD mice increased ATF6 expression in the colon tissue, whereas transfection with shMALAT1 or (A6) treatment with the miR-15a-5p mimic resulted in reduced expression of ATF6 in the colon tissue (Figure 6A). ATF6 is involved in IBD that develops in response to ER stress[30]. We further examined the expression of proteins associated with the ATF6-related ER stress pathway in the colon tissue. The expression of GRP78 and XBP-1 was increased in the colon of mice injected with MAT-Exos from HFD mice, a phenomenon that was antagonized by shMALAT1 and the miR-15a-5p mimic (Figure 6B; Supplementary Figure 2). In addi
Currently, almost one-third of the patients with IBD are obese and have a poor quality of life[8], great difficulty in inducing remission[33-35], increased risk of disease recurrence[8], and a higher burden of hospitalization and disease expenditure[36]. Therefore, it is necessary to explore the mechanism by which obesity is induced in IBD to improve its treatment. Previous studies have shown that the increased severity of IBD in obese patients is related to increased intestinal permeability, intestinal microflora translocation, and changes in the intestinal microflora spectrum[37,38]. In addition, the increase in plasma leptin levels and decrease in adiponectin levels were also found to be involved in obesity-mediated IBD aggravation, but the mechanisms underlying these processes need further exploration[39]. In this study, we propose a novel mechanism through which obesity aggravates IBD via exosome-secreted lncRNAs.
First, we demonstrated that a high-fat diet could aggravate TNBS-induced colitis in mice and explored the underlying mechanisms. One of the main characteristics of obesity is the accumulation of adipose tissue. As adipose tissue is an endocrine organ, it plays an important role in a variety of metabolism-related diseases, especially visceral adipose tissue[40]. We focused our attention on the mesenteric adipose tissue, a type of visceral adipose tissue, as it is closest to the colon tissue and can communicate with the colon in various ways. In patients with CD, the lesion is often surrounded by a special type of mesenteric adipose tissue called creeping fat, which is associated with the translocation of gut microbiota[41]. These findings suggest that the mesenteric adipose tissue may play an important role in IBD progression. We further found that TNBS treatment reduced the content of the mesenteric adipose tissue. However, the content of the mesenteric adipose tissue in HFD mice treated with TNBS was still significantly higher than that in ND mice treated with TNBS.
Exosomes serve as important means of intercellular communication. Previous reports have shown that exosomes secreted by visceral fat could target the colon tissue[16]. Therefore, we hypothesized that mesenteric-derived exosomes in obese mice may aggravate TNBS-induced colitis. Exosomes from the mesenteric adipose tissue were isolated and identified. Subsequently, exosomes extracted from the mesenteric adipose tissue of HFD and ND mice were separately injected into TNBS-treated ND mice via the tail vein. The results showed that exosomes from the mesenteric adipose tissue of HFD mice aggravated TNBS-induced colitis.
We then investigated the molecular mechanism by which exosomes derived from the mesenteric adipose tissue of HFD mice aggravated TNBS-induced colitis. A recent study indicated that visceral adipose tissue-derived exosomes can exacerbate colitis severity through proinflammatory noncoding RNAs such as miR-155[16]. In this study, we focused on the lncRNAs in exosomes. Using qRT-PCR, we identified that lncRNA expression is strongly associated with HFD in mesenteric adipose exosomes, as well as mesenteric adipose and colon tissues. In the same structures or tissues, the expression of MALAT1 was higher in TNBS-treated HFD mice than in TNBS-treated ND mice. An additional injection of MAT-Exos from HFD mice via the tail vein increased the relative expression of MALAT1 in the colon tissue, indicating that the increased expression of lncRNA MALAT1 in the colon tissue can be partially attributed to MAT-Exos arising from increased creeping fat. In fact, shMALAT1 partially reversed the effect of exosomes derived from the mesenteric adipose tissue in HFD mice, suggesting that exosomes derived from the mesenteric adipose tissue in HFD mice aggravated TNBS-induced enteritis in part due to the high expression of lncRNA MALAT1 in exosomes.
The mechanism by which lncRNA aggravates colitis was further investigated. We revealed that lncRNA MALAT1 could bind miR-15a-5p in vivo, and that the miR-15a-5p mimic could reverse the aggravation of colitis. Furthermore, the miR-15a-5p mimic and sh-MALAT1 reversed the HFD mice MAT-Exos-induced increase in the expression of ER stress-related proteins, including ATF6. We further verified the interaction between miR-15-15p and ATF6 using a dual-luciferase reporter assay. These results indicate that lncRNA MALAT1 may activate the ATF6-related ER stress signaling pathway by binding miR-15a-5p.
MALAT1 is abundantly expressed and highly conserved in 33 species of mammals, with the earliest study linking it to metastasis in non-small cell lung cancer[42]. However, recent studies have shown that MALAT1 plays an important role in a variety of tumors[18] as well as in a variety of autoimmune[43], infectious[44,45], and metabolism-related diseases[46]. Previous studies have shown that MALAT1 Levels are reduced in the plasma of patients with CD[47], suggesting that MALAT1 may be a protective factor with respect to the occurrence and development of CD. The results of our study indicated that TNBS treatment decreased the expression of MALAT1, in accordance with finding of previous reports, but the differences were not significant. Moreover, Li et al[48] indicated that MALAT1 targets intestinal epithelial cells and enhances the intestinal epithelial barrier, which plays a protective role by sequestering miR-146b-5p and maintaining the expression of AJC proteins, NUMB, and CLDN11 in IBD. However, they did not demonstrate an effect of MALAT1 overexpression on colitis in vivo.
Intriguingly, our study showed that obesity-induced increases in MALAT1 expression in colon tissue can aggravate colitis, suggesting that MALAT1 may be a double-edged sword with respect to IBD. Mechanistically, the contrary effect of MALAT1 in colitis was mainly due to the fact that exosomes derived from the mesenteric adipose tissue targeted the lamina propria of the colon rather than intestinal epithelial cells[16]. Many immune cells are distributed within the lamina propria of the colon, including dendritic cells, macrophages, and lymphoid cells, which supplement the barrier function of intestinal epithelial cells[49]. In addition, many adaptive immune system-related lymphocytes are recruited from peripheral circulation to the lamina propria and are involved in the onset and progression of IBD. LncRNA MALAT1 encapsulated in exosomes from the mesenteric adipose tissue may act on the abovementioned immune cells in the lamina propria and activate the ATF6-related ER stress pathway to promote the release of pro-inflammatory cytokines[50].
ER stress plays a role in the occurrence and development of metabolic syndrome-related diseases in the obese population[51]. Our study demonstrates that ER stress is one of the potential mechanisms by which obesity aggravates IBD, thereby highlighting the importance of weight loss in obese patients with IBD and the treatment of metabolic syndrome-related diseases. In addition, we preliminarily identified a new signaling pathway, namely, the MALAT1-miR-15a-5p/ATF6 axis, revealing a new regulatory mechanism, i.e., the ATF6-related ER stress signaling pathway. Whether this signaling pathway plays a role in other tissues or diseases requires further investigation.
This study has several limitations that should be acknowledged. First, the effect of exosomes derived from the mesenteric adipose tissue was only studied in the colon as a whole, and the specific target cell types remain unclear and require further elucidation. Second, it would be more beneficial if the regulation of the lncRNAMALAT1-miR-15a-5p-ATF6 pathway could be confirmed in a more in-depth investigation. Finally, we speculated that the divergent effect of lncRNA MALAT1 on IBD severity may be due to its various targeting areas, where endogenous MALAT1 may exert its effect on intestinal epithelial cells, while extrinsic mesenteric adipose tissue-derived EXO-MALAT1 may act on the intestinal lamina propria through its gut-homing property. However, this theory requires further exploration in the future.
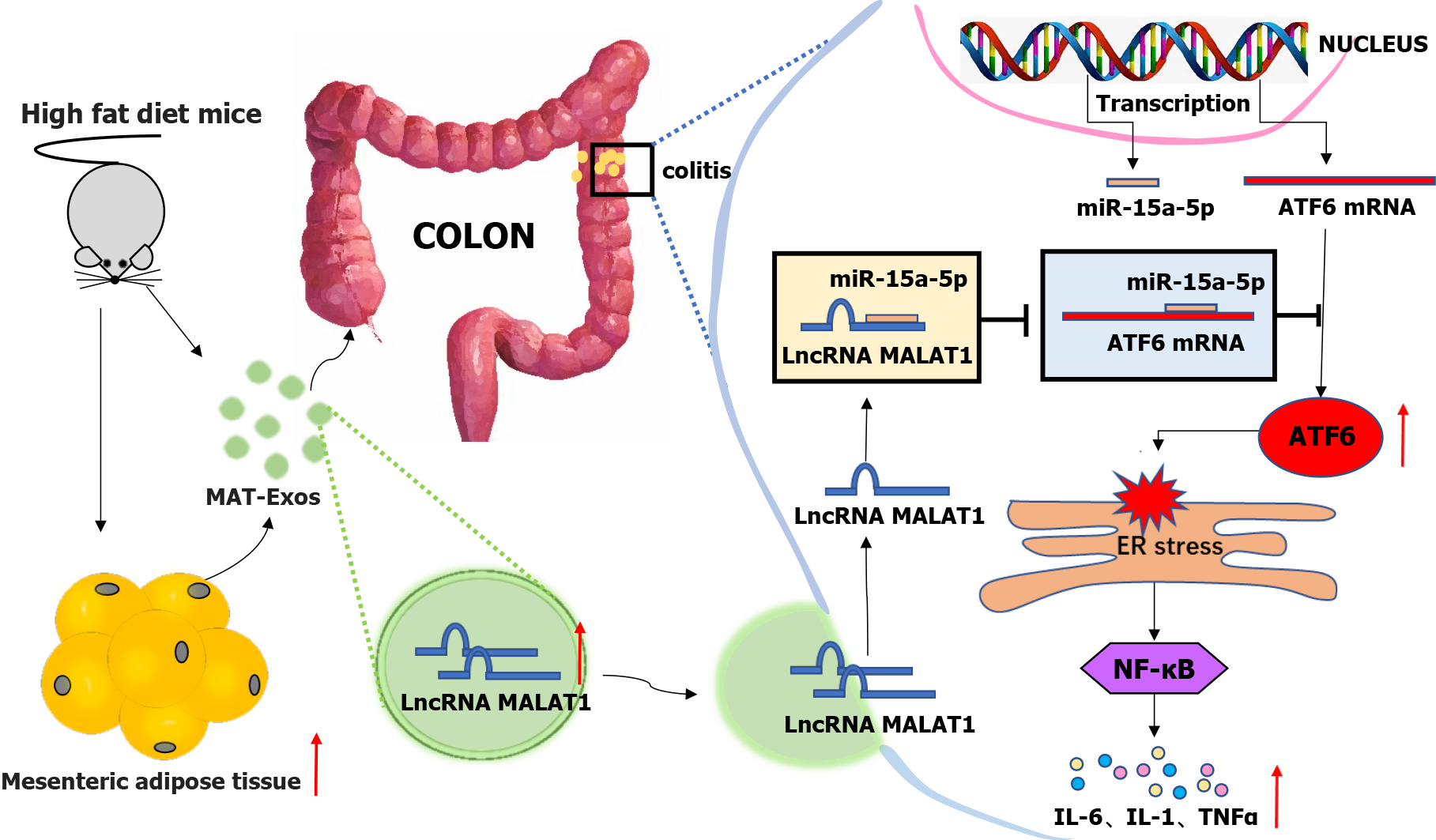
Overall, HFD increased the content of the mesenteric adipose tissue and associated EXO lncRNA MALAT1, which was then absorbed by the colon where it targeted the miR-15a-5p/ATF6 axis, thereby leading to the activation of the ER stress pathway. Further, downstream activation of the inflammatory factor signaling pathway may partially contribute to the aggravation of colitis in obese IBD patients.
A higher visceral adipose-tissue ratio was associated with an increased disease activity in CD patients. But the underlining mechanism still remains elusive. Our study indicated that high-fat diet increased the content of mesenteric adipose tissue and aggravated colitis in mice. Mesenteric adipose tissue derived exosome lncRNA metastasis-associated lung adenocarcinoma transcript 1 can be absorbed by the colon leading to activation of the endoplasmic reticulum stress pathway by way of targeting the miR-15a-5p/ATF6 axis to aggravate colitis (Figure 7).
Obesity is associated with an increased risk of developing Crohn’s disease (CD), higher disease activity, and comparatively worse clinical outcomes, especially in CD patients with a high visceral adipose tissue ratio. However, the underlying mechanisms remain unclear.
Exosomes contain multiple non-coding RNAs such as long non-coding RNAs (lncRNAs), which serve as messengers in cell-cell communication. Visceral adipose tissue derived exosomes display tissue affinity and preferentially target the colonic lamina propria. We hypothesized that obesity exacerbates inflammatory bowel disease, partially through mesenteric adipose tissue-derived exosomes.
To investigate the role of mesenteric adipose tissue-derived exosomes in CD aggravation through obesity, thereby providing a potential therapeutic target for CD in this subpopulation.
A 2,4,6-trinitrobe-nzenesulfonic acid (TNBS) was used to induce colitis in mice fed a high-fat diet (HFD) and normal diet (ND). Exosomes from the mesenteric adipose tissue were extracted and identified, followed by the investigation of lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) expression. Luciferase reporter and RNA pull-down assays were performed to verify the interaction between MALAT1 and miR-15a-5p/activating transcription factor 6 (ATF6) axis. Finally, mesenteric adipose tissue-derived exosomes extracted from HFD-fed mice were isolated and treated with either a short hairpin RNA targeting MALAT1 (shMALAT) or an miR-15a-5p mimic before being injected into mice to explore their influence on TNBS-induced colitis.
HFD can aggravate TNBS-induced colitis in mice, and increase the expression of MALAT1 in mesenteric adipose tissue-derived exosomes. Increased expression of MALAT1 in the colon tissue exacerbated TNBS-induced colitis and activated the ATF6-related endoplasmic reticulum stress pathway. Moreover, this effect was partially reversed by the reduced expression of MALAT1 and overexpression of miR-15a-5p.
Mesenteric adipose tissue-derived exosome-encapsulated lncRNA MALAT1 targets the colon and aggravates TNBS-induced colitis in obese mice, which may potentially act on the miR-15a-5p/ATF6 axis and indice endoplasmic reticulum stress.
Obesity-mediated aggravation of colitis might involve the mesenteric adipose tissue-derived exosome lncRNA MALAT1, but the specific cells of the intestine targeted by MALAT1 deserve further explo
| 1. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1968] [Article Influence: 218.7] [Reference Citation Analysis (113)] |
| 2. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3593] [Article Influence: 256.6] [Reference Citation Analysis (6)] |
| 3. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MNF, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJY, Chan FKL; Asia–Pacific Crohn's and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 615] [Article Influence: 47.3] [Reference Citation Analysis (2)] |
| 4. | Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 5. | Khalili H, Ananthakrishnan AN, Konijeti GG, Higuchi LM, Fuchs CS, Richter JM, Chan AT. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Harpsøe MC, Basit S, Andersson M, Nielsen NM, Frisch M, Wohlfahrt J, Nohr EA, Linneberg A, Jess T. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43:843-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Pringle PL, Stewart KO, Peloquin JM, Sturgeon HC, Nguyen D, Sauk J, Garber JJ, Yajnik V, Ananthakrishnan AN, Chan AT, Xavier RJ, Khalili H. Body Mass Index, Genetic Susceptibility, and Risk of Complications Among Individuals with Crohn's Disease. Inflamm Bowel Dis. 2015;21:2304-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Jain A, Nguyen NH, Proudfoot JA, Martin CF, Sandborn WJ, Kappelman MD, Long MD, Singh S. Impact of Obesity on Disease Activity and Patient-Reported Outcomes Measurement Information System (PROMIS) in Inflammatory Bowel Diseases. Am J Gastroenterol. 2019;114:630-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Büning C, von Kraft C, Hermsdorf M, Gentz E, Wirth EK, Valentini L, Haas V. Visceral Adipose Tissue in Patients with Crohn's Disease Correlates with Disease Activity, Inflammatory Markers, and Outcome. Inflamm Bowel Dis. 2015;21:2590-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Scheja L, Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat Rev Endocrinol. 2019;15:507-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 11. | Siegmund B. Mesenteric fat in Crohn's disease: the hot spot of inflammation? Gut. 2012;61:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 613] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 13. | Packer M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J Am Coll Cardiol. 2018;71:2360-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 463] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 14. | Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 723] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 15. | Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Xu J, Xia K, Chang Y, Liu J, Yuan W. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 16. | Wei M, Gao X, Liu L, Li Z, Wan Z, Dong Y, Chen X, Niu Y, Zhang J, Yang G. Visceral Adipose Tissue Derived Exosomes Exacerbate Colitis Severity via Pro-inflammatory MiRNAs in High Fat Diet Fed Mice. ACS Nano. 2020;14:5099-5110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Röder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigó R, Gingeras TR. Landscape of transcription in human cells. Nature. 2012;489:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3924] [Cited by in RCA: 4062] [Article Influence: 290.1] [Reference Citation Analysis (0)] |
| 18. | Amodio N, Raimondi L, Juli G, Stamato MA, Caracciolo D, Tagliaferri P, Tassone P. MALAT1: a druggable long non-coding RNA for targeted anti-cancer approaches. J Hematol Oncol. 2018;11:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 19. | Camuesco D, Comalada M, Rodríguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Gálvez J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol. 2004;143:908-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 1143] [Article Influence: 127.0] [Reference Citation Analysis (0)] |
| 21. | Gao J, Li X, Wang Y, Cao Y, Yao D, Sun L, Qin L, Qiu H, Zhan X. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol (Oxf). 2020;228:e13339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 22. | Xie L, Chen Z, Liu M, Huang W, Zou F, Ma X, Tao J, Guo J, Xia X, Lyu F, Wang H, Zheng C, Jiang J. MSC-Derived Exosomes Protect Vertebral Endplate Chondrocytes against Apoptosis and Calcification via the miR-31-5p/ATF6 Axis. Mol Ther Nucleic Acids. 2020;22:601-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | Liu SX, Zheng F, Xie KL, Xie MR, Jiang LJ, Cai Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol Ther Nucleic Acids. 2019;18:34-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Ji Q, Cai G, Liu X, Zhang Y, Wang Y, Zhou L, Sui H, Li Q. MALAT1 regulates the transcriptional and translational levels of proto-oncogene RUNX2 in colorectal cancer metastasis. Cell Death Dis. 2019;10:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127-D131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 855] [Cited by in RCA: 2196] [Article Influence: 366.0] [Reference Citation Analysis (0)] |
| 26. | Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 3181] [Article Influence: 227.2] [Reference Citation Analysis (0)] |
| 27. | Powell N, Pantazi E, Pavlidis P, Tsakmaki A, Li K, Yang F, Parker A, Pin C, Cozzetto D, Minns D, Stolarczyk E, Saveljeva S, Mohamed R, Lavender P, Afzali B, Digby-Bell J, Tjir-Li T, Kaser A, Friedman J, MacDonald TT, Bewick GA, Lord GM. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut. 2020;69:578-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 28. | Ma X, Dai Z, Sun K, Zhang Y, Chen J, Yang Y, Tso P, Wu G, Wu Z. Intestinal Epithelial Cell Endoplasmic Reticulum Stress and Inflammatory Bowel Disease Pathogenesis: An Update Review. Front Immunol. 2017;8:1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 29. | Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 Links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1177] [Cited by in RCA: 1168] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 30. | Stengel ST, Fazio A, Lipinski S, Jahn MT, Aden K, Ito G, Wottawa F, Kuiper JWP, Coleman OI, Tran F, Bordoni D, Bernardes JP, Jentzsch M, Luzius A, Bierwirth S, Messner B, Henning A, Welz L, Kakavand N, Falk-Paulsen M, Imm S, Hinrichsen F, Zilbauer M, Schreiber S, Kaser A, Blumberg R, Haller D, Rosenstiel P. Activating Transcription Factor 6 Mediates Inflammatory Signals in Intestinal Epithelial Cells Upon Endoplasmic Reticulum Stress. Gastroenterology. 2020;159:1357-1374.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 31. | Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161-10168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 532] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 32. | Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 615] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 33. | Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Bhalme M, Sharma A, Keld R, Willert R, Campbell S. Does weight-adjusted anti-tumour necrosis factor treatment favour obese patients with Crohn's disease? Eur J Gastroenterol Hepatol. 2013;25:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2118-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60:2436-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (4)] |
| 37. | Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, Darfeuille-Michaud A, Barnich N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 412] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 38. | Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 483] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 39. | Bilski J, Mazur-Bialy AI, Brzozowski B, Magierowski M, Jasnos K, Krzysiek-Maczka G, Urbanczyk K, Ptak-Belowska A, Zwolinska-Wcislo M, Mach T, Brzozowski T. Moderate exercise training attenuates the severity of experimental rodent colitis: the importance of crosstalk between adipose tissue and skeletal muscles. Mediators Inflamm. 2015;2015:605071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Kahn CR, Wang G, Lee KY. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129:3990-4000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 41. | Spencer SP, Sonnenburg JL. When Gut Microbiota Creep into Fat, the Fat Creeps Back. Cell. 2020;183:589-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Müller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031-8041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1584] [Cited by in RCA: 1822] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 43. | Li GQ, Fang YX, Liu Y, Meng FR, Wu X, Zhang CW, Zhang Y, Liu D, Gao B. MALAT1-Driven Inhibition of Wnt Signal Impedes Proliferation and Inflammation in Fibroblast-Like Synoviocytes Through CTNNB1 Promoter Methylation in Rheumatoid Arthritis. Hum Gene Ther. 2019;30:1008-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Gu H, Zhu Y, Zhou Y, Huang T, Zhang S, Zhao D, Liu F. LncRNA MALAT1 Affects Mycoplasma pneumoniae Pneumonia via NF-κB Regulation. Front Cell Dev Biol. 2020;8:563693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Yong H, Wu G, Chen J, Liu X, Bai Y, Tang N, Liu L, Wei J. lncRNA MALAT1 Accelerates Skeletal Muscle Cell Apoptosis and Inflammatory Response in Sepsis by Decreasing BRCA1 Expression by Recruiting EZH2. Mol Ther Nucleic Acids. 2020;19:97-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Cremer S, Michalik KM, Fischer A, Pfisterer L, Jaé N, Winter C, Boon RA, Muhly-Reinholz M, John D, Uchida S, Weber C, Poller W, Günther S, Braun T, Li DY, Maegdefessel L, Perisic Matic L, Hedin U, Soehnlein O, Zeiher A, Dimmeler S. Hematopoietic Deficiency of the Long Noncoding RNA MALAT1 Promotes Atherosclerosis and Plaque Inflammation. Circulation. 2019;139:1320-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 47. | Yarani R, Mirza AH, Kaur S, Pociot F. The emerging role of lncRNAs in inflammatory bowel disease. Exp Mol Med. 2018;50:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 48. | Li Y, Zhu L, Chen P, Wang Y, Yang G, Zhou G, Li L, Feng R, Qiu Y, Han J, Chen B, He Y, Zeng Z, Chen M, Zhang S. MALAT1 Maintains the Intestinal Mucosal Homeostasis in Crohn's Disease via the miR-146b-5p-CLDN11/NUMB Pathway. J Crohns Colitis. 2021;15:1542-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019;94:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 702] [Article Influence: 100.3] [Reference Citation Analysis (2)] |
| 50. | So JS. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol Cells. 2018;41:705-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 108] [Reference Citation Analysis (0)] |
| 51. | Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, Shao M, Zhao F, He S, Yang L, Zhang M, Nan F, Li J, Liu J, Jia W, Qiu Y, Song B, Han JJ, Rui L, Duan SZ, Liu Y. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chiu CC, Taiwan; Kao JT, Taiwan; Senousy M, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY