Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3503
Peer-review started: February 14, 2022
First decision: March 9, 2022
Revised: March 23, 2022
Accepted: June 18, 2022
Article in press: June 18, 2022
Published online: July 21, 2022
Processing time: 153 Days and 21 Hours
Noninvasive, practical, and convenient means of detection for the prediction of liver fibrosis and cirrhosis in China are greatly needed.
To develop a precise noninvasive test to stage liver fibrosis and cirrhosis.
With liver biopsy as the gold standard, we established a new index, [alkaline phosphatase (U/L) + gamma-glutamyl transpeptidase (U/L)/platelet (109/L) (AGPR)], to predict liver fibrosis and cirrhosis. In addition, we compared the area under the receiver operating characteristic curve (AUROC) of AGPR, gamma-glutamyl transpeptidase to platelet ratio, aspartate transaminase to platelet ratio index, and FIB-4 and evaluated the accuracy of these routine laboratory indices in predicting liver fibrosis and cirrhosis.
Correlation analysis revealed a significant positive correlation between AGPR and liver fibrosis stage (P < 0.001). In the training cohort, the AUROC of AGPR was 0.83 (95%CI: 0.78-0.87) for predicting fibrosis (≥ F2), 0.84 (95%CI: 0.79-0.88) for predicting extensive fibrosis (≥ F3), and 0.87 (95%CI: 0.83-0.91) for predicting cirrhosis (F4). In the validation cohort, the AUROCs of AGPR to predict ≥ F2, ≥ F3 and F4 were 0.83 (95%CI: 0.77-0.88), 0.83 (95%CI: 0.77-0.89), and 0.84 (95%CI: 0.78-0.89), respectively.
The AGPR index should become a new, simple, accurate, and noninvasive marker to predict liver fibrosis and cirrhosis in chronic hepatitis B patients.
Core Tip: Chronic hepatitis B virus (HBV) infection is highly endemic in China, and routine assessment of chronic hepatitis B patients is greatly needed to guide management and indicate the need for treatment. In this study, we established a new index to stage liver fibrosis and cirrhosis in patients with chronic HBV infection in China. In addition, the study compared the predictive performance between the new index and other noninvasive indices. The new index is suitable for regular monitoring and is crucial for the management of patients with liver fibrosis/cirrhosis.
- Citation: Liao MJ, Li J, Dang W, Chen DB, Qin WY, Chen P, Zhao BG, Ren LY, Xu TF, Chen HS, Liao WJ. Novel index for the prediction of significant liver fibrosis and cirrhosis in chronic hepatitis B patients in China. World J Gastroenterol 2022; 28(27): 3503-3513
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3503.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3503
Chronic hepatitis B virus (HBV) infection is a public issue that affects human health. Patients who are chronically infected with HBV have a tendency to develop liver fibrosis, liver cirrhosis and even more serious conditions, such as hepatocellular carcinoma (HCC). It has been reported that liver fibrosis can be reversed in patients with varying degrees of fibrosis by removing pathogenic factors, including HBV infection and alcohol[1,2]. It would benefit patients to obtain early diagnosis and effective treatment of liver fibrosis before the disease worsens. Hence, measures should be taken to achieve early diagnosis of liver fibrosis to avoid disease progression caused by HBV infection.
Liver biopsy has always been regarded as the gold standard to evaluate liver histology and assess the degree of fibrosis[3]. However, liver biopsy has shortcomings. Like any surgery, liver biopsy carries some risks, such as puncture of the lung or gallbladder, infection, bleeding, and pain, although these complications are rare[4]. In addition, a major problem is the sampling error and significant variability of fibrosis assessment by liver biopsy[5]. Transient elastography performed with FibroScan is a new technique to measure liver stiffness, and it has the advantages of noninvasiveness, good reproducibility and higher objectivity[6-8]. However, the FibroScan device and its maintenance are expensive, limiting the use of transient elastography in low- and middle-income countries and making it unsuitable for routine monitoring of liver fibrosis/cirrhosis in patients with chronic liver disease in economically poor areas. In recent years, researchers have been interested in finding a potential marker of liver fibro
In this study, we tested a novel noninvasive index, the alkaline phosphatase (ALP) and γ-GT to PLT ratio (AGPR), for the assessment of liver fibrosis and cirrhosis through statistical analysis of clinical data. Moreover, we compared the diagnostic values of the AGPR, GPR, APRI and FIB-4 indices.
The patients who participated in this study were divided into a training set and a validation set. Patients in the training set received treatment at the Affiliated Hospital of Guilin Medical University (Guilin, People’s Republic of China). Patients in the validation set received treatments at the Peking University People’s Hospital (Beijing, Guangxi Zhuang Autonomous Region, China). All patients were under treatment from May 2005 to October 2016, and all underwent systematic examinations, including abdominal ultrasound, routine laboratory tests and liver histological examination tests. The clinicopathologic characteristics of all patients, including age, gender, alcohol consumption, smoking status, hepatitis B e-antigen (HbeAg) status, fibrosis and cirrhosis stage, activity grade, white blood cell (WBC) count, neutrophil count (NEUT), lymphocyte count (LYMPH), PLT count, albumin, globulin, total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), AST, ALP, γ-GT and AGPR were collected and are detailed in Table 1. All patients in the study were chronically infected with HBV and were positive for hepatitis B surface antigen.
| Parameter | Training cohort (n = 296)1 | Validation cohort (n = 211)1 | P value |
| Age (yr) | 42.47 ± 11.98 | 41.21 ± 11.36 | 0.167 |
| Gender: Female/male (n) | 75/221 | 40/171 | 0.091 |
| Drinking: Yes/no (n) | 127/169 | 101/110 | 0.268 |
| Smoking: Yes/no (n) | 92/204 | 68/143 | 0.784 |
| HbeAg: Negative/positive | 16/280 | 15/196 | 0.430 |
| Fibrosis stage: F0/F1/F2/F3/F4 | 55/58/75/64/44 | 47/47/37/54/26 | 0.194 |
| Activity grade: A0/A1/A2/A3/A4 | 16/92/113/69/6 | 15/72/69/52/3 | 0.446 |
| WBC (× 109/L) | 5.89 ± 2.55 | 6.34 ± 4.32 | 0.150 |
| NEUT × 109/L) | 3.55 ± 2.44 | 3.66 ± 2.61 | 0.611 |
| LYMPH (× 109/L) | 1.72 ± 0.62 | 1.80 ± 0.63 | 0.063 |
| PLT (× 109/L) | 171.61 ± 66.87 | 174.48 ± 61.62 | 0.636 |
| Albumin (g/L) | 41.12 ± 19.09 | 39.94 ± 6.07 | 0.380 |
| Globulin (g/L) | 29.67 ± 5.45 | 29.14 ± 5.80 | 0.298 |
| TBIL (μmol/L) | 16.51 ± 8.44 | 16.73 ± 7.63 | 0.768 |
| DBIL (μmol/L) | 6.91 ± 5.03 | 7.07 ± 4.33 | 0.699 |
| ALT (U/L) | 73.31 ± 65.41 | 96.85 ± 83.62 | 0.002 |
| AST (U/L) | 72.59 ± 63.29 | 77.44 ± 67.34 | 0.411 |
| ALP (U/L) | 97.70 ± 41.98 | 104.32 ± 49.25 | 0.104 |
| γ-GT (U/L) | 85.64 ± 69.68 | 97.98 ± 75.36 | 0.058 |
| AGPR | 1.26 ± 0.84 | 1.32 ± 0.89 | 0.431 |
Some basic clinical examinations that ensure that patients are relatively safe when performing liver puncture surgery should be performed. In addition, physicians should obtain informed consent from the patients prior to surgery. Ultrasound localization was performed on consenting and suitable patients for liver biopsy procedures. Qualified liver tissue samples were formalin-fixed and paraffin-embedded for pathological analysis. Liver fibrosis and cirrhosis were graded as follows according to the METAVIR system: F0, no fibrosis; F1, fibrosis in the portal vein zone but no fibrous septa; F2, a small amount of fibrous septa; F3, many fibrous septa but no cirrhosis; F4, cirrhosis[15]. All biopsy samples were assessed separately by two liver pathologists who were blinded to the clinical information. If the results of their evaluation were discordant, a third highly experienced hepatopathologist blinded to patient information reviewed the contested samples. Samples were excluded from the study population if the pathologists failed to reach consensus.
AGPR was calculated as [ALP (U/L) + γ-GT (U/L)]/PLT count (109/L). APRI was calculated as (AST/ULN)/PLT count (109/L) × 100[10]. FIB-4 was calculated by the formula: Age (years) × AST (U/L)/[PLT (109/L) × ALT (U/L)1/2][11]. The formula for GPR was γ-GT/ULN of γ-GT/PLT count (109/L) × 100[12].
Student’s t test was used for continuous variables, and Pearson’s χ2 test or Fisher’s exact test was used for categorical variables to compare baseline characteristics. Data are presented as the mean ± SD or proportions. Univariable logistic regression was used for the variables of age, gender, WBC, NEUT, LYMPH, PLT, albumin, globulin, TBIL, DBIL, ALT, AST, ALP, γ-GT and AGPR. The receiver operating characteristic (ROC) curves were drawn to evaluate the accuracy rate of diagnosis for the AGPR, GPR, APRI, and FIB-4. The sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratio, hazard ratio and the area under the ROC curve (AUROC) of the four non-invasive markers for fibrosis and cirrhosis staging were obtained through comparison and analysis of F0-1 vs F2-4, F0-2 vs F3-4, and F0-3 vs F4, respectively. Data analysis was performed using SPSS software (version 24.0). A P value < 0.05 was regarded as statistically significant.
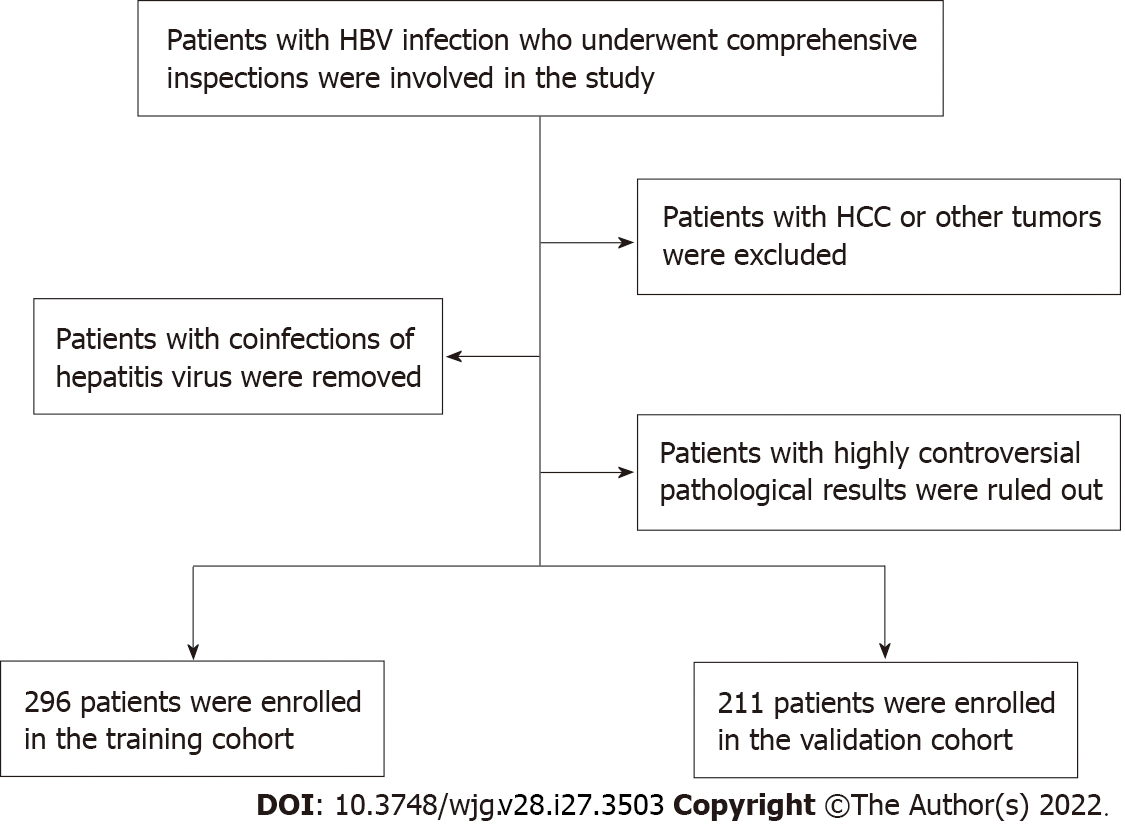
Patients involved in this study needed to meet certain criteria. Those criteria were as follows: (1) Study patients were infected with HBV and underwent systematic examinations, including abdominal ultrasound, routine laboratory tests and liver biopsies; (2) Patients with HCC or other tumors were excluded; (3) Patients with coinfection of HCV, HIV or HDV were excluded; and (4) Patients with highly controversial results on liver fibrosis pathological grading were excluded. Figure 1 displays the selection principles.
Clinical data related to this study are summarized in Table 1. There were no statistically significant differences between the training and validation cohorts in terms of age, gender, drinking status, smoking status, HbeAg, fibrosis stage, activity grade, WBC, NEUT, LYMPH, PLT, albumin, globulin, TBIL, DBIL, ALT, AST, ALP, γ-GT or AGPR (P > 0.05). Nonsignificant differences between the two study groups revealed that the selection of training and validation cohorts was reasonable.
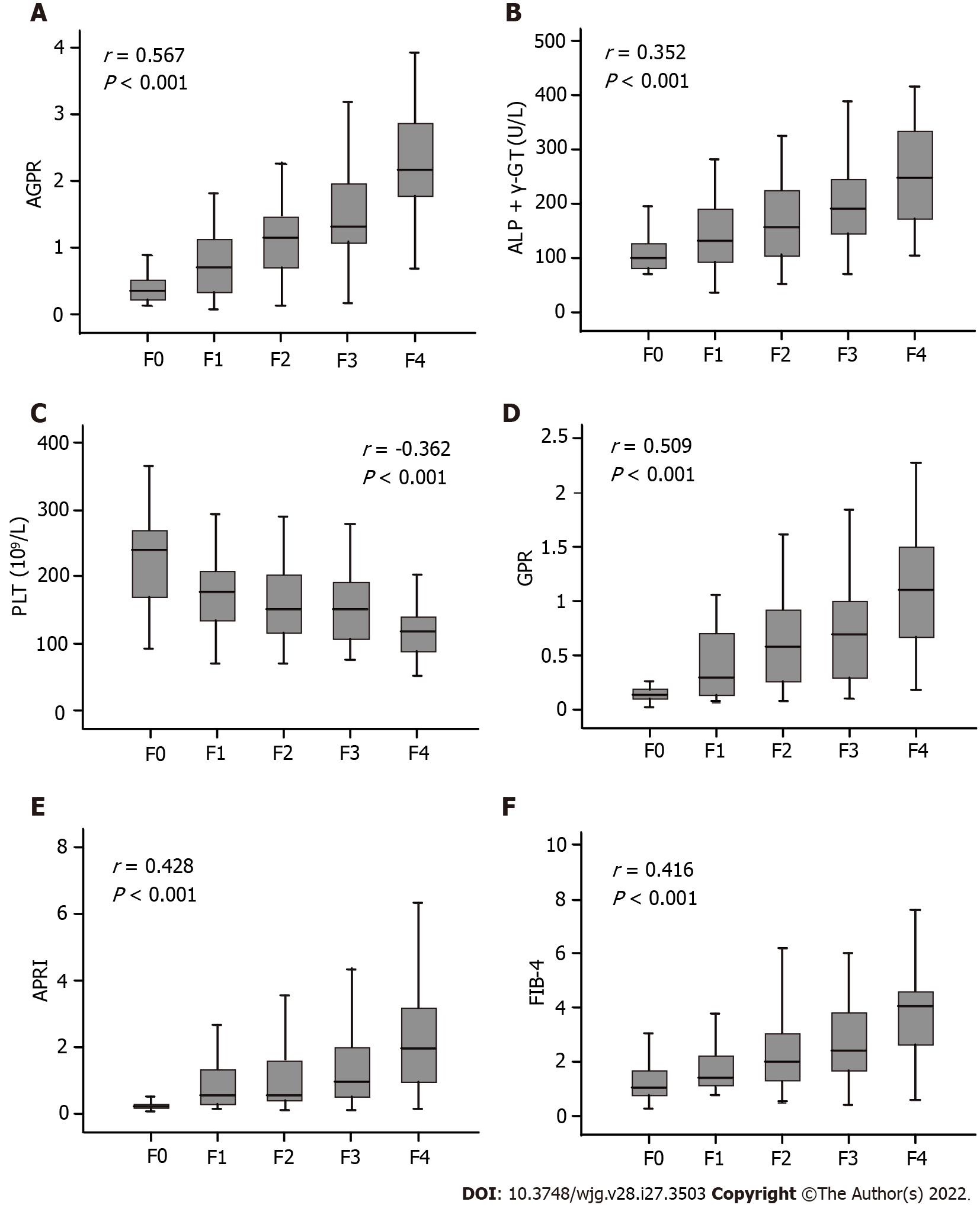
Univariable analyses showed that the presence of significant liver fibrosis (≥ F2) was related to gender, WBC, NEUT, PLT, albumin, globulin, TBIL, DBIL, AST, ALP, γ-GT and AGPR (Table 2). In the training cohort, correlation analysis revealed a significant positive correlation between AGPR and liver fibrosis stage (r = 0.567, P < 0.001) (Figure 2A). In the validation cohort, there was also a positive correlation between AGPR and liver fibrosis stage (r = 0.524, P < 0.001), as shown in Supplementary Table 1 and Supplementary Figure 1A. Box plots showed that liver fibrosis stage positively correlated with ALP + γ-GT (r = 0.352, P < 0.001), GPR (r = 0.509, P < 0.001), APRI (r = 0.428, P < 0.001) and FIB-4 (r = 0.416, P < 0.001) in the training cohort (Figure 2B and D-F). The severity of liver fibrosis stage correlated significantly with a gradual increase in the levels of these indicators. There was a negative correlation between PLT count and liver fibrosis stage (r = -0.362, P < 0.001) in the training cohort (Figure 2C). The severity of liver fibrosis decreased with increasing PLT count. Similar results were obtained in the validation cohort, and the specific data related to these results are shown in Supplementary Figure 1B-F and Supplementary Table 2.
| Parameter | Fibrosis (F0-1) (n = 113)1 | Fibrosis (F2-4) (n = 183)1 | P value |
| Age (yr) | 45.15 ± 14.97 | 43.63 ± 13.32 | 0.363 |
| Gender: Female/male (n) | 37/76 | 38/145 | 0.021 |
| WBC (× 109/L) | 6.45 ± 3.02 | 5.55 ± 2.14 | 0.003 |
| NEUT (× 109/L) | 4.14 ± 3.0 | 3.18 ± 1.95 | 0.001 |
| LYMPH (× 109/L) | 1.67 ± 0.60 | 1.68 ± 0.63 | 0.828 |
| PLT (× 109/L) | 206.0 ± 68.39 | 150.55 ± 56.49 | < 0.001 |
| Albumin (g/L) | 41.26 ± 5.93 | 39.29 ± 5.66 | 0.005 |
| Globulin (g/L) | 27.46 ± 4.46 | 31.04 ± 5.56 | < 0.001 |
| TBIL (μmol/L) | 13.82 ± 7.06 | 17.12 ± 8.8 | < 0.001 |
| DBIL (μmol/L) | 6.43 ± 3.72 | 7.81 ± 4.49 | < 0.001 |
| ALT (U/L) | 65.01 ± 70.72 | 78.42 ± 66.62 | 0.101 |
| AST (U/L) | 56.61 ± 55.11 | 82.45 ± 66.09 | 0.001 |
| ALP (U/L) | 79.06 ± 34.16 | 109.2 ± 42.31 | < 0.001 |
| γ-GT (U/L) | 51.84 ± 47.06 | 106.5 ± 73.21 | < 0.001 |
| AGPR | 0.72 ± 0.49 | 1.61 ± 0.83 | < 0.001 |
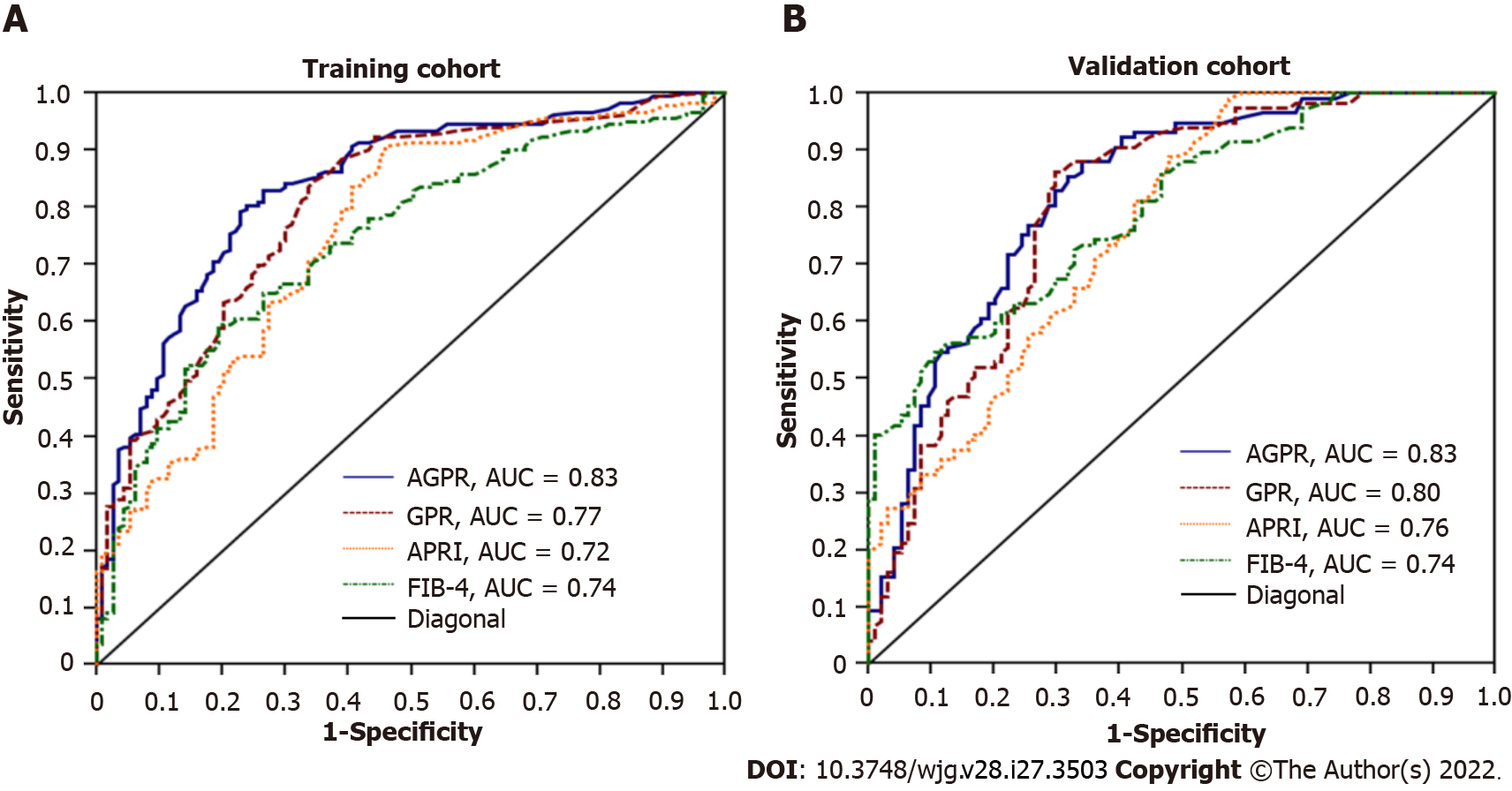
The summary AUROC, sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, and hazard ratios for the detection of fibrosis and cirrhosis for AGPR, GPR, APRI and FIB-4 are displayed in Table 3. In the training cohort, the AUROC of AGPR (0.83, 95%CI: 0.78-0.87) was higher than that of GPR (0.77, 95%CI: 0.72-0.82; P = 0.008), APRI (0.72, 95%CI: 0.67-0.77; P < 0.0001) and FIB-4 (0.74, 95%CI: 0.69-0.79; P = 0.0004) for the prediction of significant fibrosis (≥ F2). For the assessment of extensive fibrosis (≥ F3), the AUROC of AGPR (0.84, 95%CI: 0.79-0.88) was also higher than that of GPR (0.81, 95%CI: 0.76-0.85; P < 0.0001), APRI (0.70, 95%CI: 0.64-0.75; P < 0.0001) and FIB-4 (0.75, 95%CI: 0.69-0.80; P = 0.0005). For the diagnosis of cirrhosis (F4), the AUROC of AGPR was 0.87 (95%CI: 0.83-0.91), which was higher than that of GPR (0.80, 95%CI: 0.75-0.84; P = 0.0001), APRI (0.76, 95%CI: 0.70-0.8; P = 0.0002) and FIB-4 (0.80, 95%CI: 0.75-0.84; P = 0.022) (Figure 3A and Table 3). For identifying patients with significant fibrosis and cirrhosis, the summary sensitivities of AGPR were 83.1% and 88.6%, respectively, while the summary specificities of AGPR were 73.4% and 75.4%, respectively. The sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios, and hazard ratios of the other noninvasive indices are detailed in Table 3. Our results revealed that AGPR had the best overall performance among these noninvasive indices.
| Training cohort (n = 296) | Validation cohort (n = 211) | |||||||||||
| F0-1 vs F2-4 | F0-2 vs F3-4 | F0-3 vs F4 | F0-1 vs F2-4 | F0-2 vs F3-4 | F0-3 vs F4 | |||||||
| AGPR | ||||||||||||
| AUROC (95%CI) | 0.83 (0.78-0.87) | 0.84 (0.79-0.88) | 0.87 (0.83-0.91) | 0.83 (0.77-0.88) | 0.83 (0.77-0.89) | 0.84 (0.78-0.89) | ||||||
| Cut-off values | 0.87 | 1.20 | 1.40 | 0.87 | 1.20 | 1.40 | ||||||
| Se/Sp (%) | 83.1/73.4 | 72.22/80.3 | 88.6/75.4 | 85.5/68.1 | 78.7/70.2 | 92.3/67.0 | ||||||
| PPV/NPV (%) | 83.5/72.8 | 67.8/83.4 | 38.6/97.4 | 76.9/79.0 | 61.8/84.4 | 28.2/98.4 | ||||||
| Positive/negative LR | 3.13/0.23 | 3.67/0.35 | 3.60/0.15 | 2.68/0.21 | 2.65/0.30 | 2.80/0.11 | ||||||
| HR (95%CI) | 8.48 (5.22-14.33) | 7.56 (4.76-9.83) | 8.10 (5.11-11.63) | 8.02 (4.95-15.62) | 7.06 (4.51-12.35) | 5.06 (3.89-7.72) | ||||||
| GPR | ||||||||||||
| AUROC (95%CI) | 0.77 (0.72-0.82) | 0.81 (0.76-0.85) | 0.80 (0.75-0.84) | 0.80 (0.74-0.85) | 0.81 (0.74-0.87) | 0.78 (0.71-0.83) | ||||||
| Cut-off values | 0.32 | 0.32 | 0.56 | 0.32 | 0.32 | 0.56 | ||||||
| Se/Sp (%) | 73.7/70.8 | 76.8/54.8 | 79.5/65.9 | 86.3/67.0 | 91.2/56.5 | 75.0/62.3 | ||||||
| PPV/NPV (%) | 80.4/62.5 | 49.4/80.5 | 28.9/94.9 | 76.5/79.7 | 56.2/91.4 | 26.5/86.9 | ||||||
| Positive/negative LR | 2.53/0.37 | 1.70/0.42 | 2.33/0.31 | 2.62/0.20 | 2.10/0.15 | 1.95/0.41 | ||||||
| HR (95%CI) | 6.53 (4.81-9.62) | 6.72 (3.96-9.63) | 6.47 (4.08-9.83) | 6.45 (3.58-11.61) | 7.30 (3.87-14.62) | 4.84 (3.28-7.95) | ||||||
| APRI | ||||||||||||
| AUROC (95%CI) | 0.72 (0.67-0.77) | 0.70 (0.64-0.75) | 0.76 (0.70-0.81) | 0.76 (0.70-0.82) | 0.74 (0.67-0.82) | 0.77 (0.70-0.83) | ||||||
| Cut-off values | 0.5 | 1.5 | - | 1.0 | 2.0 | 0.5 | 1.5 | - | 1.0 | 2.0 | ||
| Se/Sp (%) | 80.9/58.4 | 36.1/79.7 | - | 77.3/64.7 | 43.2/83.3 | 83.8/54.3 | 34.1/76.6 | - | 70.1/68.6 | 50.1/78.3 | ||
| PPV/NPV (%) | 75.9/65.3 | 74.2/43.5 | - | 27.6/94.2 | 31.1/89.4 | 69.5/72.9 | 64.5/48.3 | - | 26.3/92.7 | 30.8/91.9 | ||
| Positive/negative LR | 1.94/0.33 | 1.77/0.80 | - | 2.19/0.35 | 2.59/0.68 | 1.83/0.30 | 1.46/0.86 | - | 2.12/0.44 | 3.16/0.63 | ||
| HR (95%CI) | 2.61 (1.85-3.67) | 1.85 (1.41-2.42) | 2.09 (1.63-2.55) | 1.59 (1.23-2.07) | 2.64 (1.76-4.32) | 2.13 (1.71-2.58) | ||||||
| FIB-4 | ||||||||||||
| AUROC (95%CI) | 0.74 (0.69-0.79) | 0.75 (0.69-0.80) | 0.80 (0.75-0.84) | 0.74 (0.67-0.80) | 0.79 (0.73-0.84) | 0.77 (0.71-0.83) | ||||||
| Cut-off values | - | 1.45 | 3.25 | - | - | 1.45 | 3.25 | - | ||||
| Se/Sp (%) | - | 87.9/47.3 | 42.6/87.2 | - | - | 77.5/58.1 | 37.5/93.1 | - | ||||
| PPV/NPV (%) | - | 49.0/87.3 | 65.7/72.6 | - | - | 53.0/80.9 | 76.9/70.9 | - | ||||
| Positive/negative LR | - | 1.67/0.25 | 3.34/0.66 | - | - | 1.85/0.39 | 5.46/0.67 | - | ||||
| HR (95%CI) | 1.86 (1.52-2.53) | 1.94 (1.55-2.42) | 2.17 (1.77-2.56) | 1.98 (1.50-2.62) | 2.11 (1.63-2.74) | 1.87 (1.68-2.36) | ||||||
| Comparison of AUROC | ||||||||||||
| AGPR and GPR | P = 0.008 | P < 0.0001 | P = 0.0001 | P = 0.028 | P = 0.005 | P = 0.0008 | ||||||
| AGPR and APRI | P < 0.0001 | P < 0.0001 | P = 0.0002 | P = 0.0001 | P = 0.0001 | P = 0.134 | ||||||
| AGPR and FIB-4 | P = 0.0004 | P = 0.0005 | P = 0.022 | P = 0.007 | P = 0.174 | P = 0.100 | ||||||
| GPR and APRI | P = 0.0007 | P = 0.0028 | P = 0.284 | P = 0.003 | P = 0.016 | P = 0.831 | ||||||
| GPR and FIB-4 | P = 0.026 | P = 0.331 | P = 0.922 | P = 0.093 | P = 0.929 | P = 0.591 | ||||||
| APRI and FIB-4 | P = 0.455 | P = 0.028 | P = 0.061 | P = 0.248 | P = 0.008 | P = 0.795 | ||||||
We further evaluated the diagnostic accuracy and performance of these noninvasive indices in the validation cohort. Similar to the results from the training cohort, the AUROC of AGPR was better than that of GPR (0.78, 95%CI: 0.71-0.83), APRI (0.77, 95%CI: 0.70-0.83), and FIB-4 (0.77, 95%CI: 0.71-0.83) (Figure 3B and Table 3).
Cirrhosis is one of the top 20 causes of disability-adjusted life years and life lost years, accounting for 1.6% and 2.1% of the global burden, respectively[16]. Cirrhosis is also the 11th most common cause of death worldwide[16]. Recently, Xu et al[17] provided updated guidelines for the management of liver cirrhosis in China. However, better screening for early fibrosis or cirrhosis remains a challenge. Finding an inexpensive, noninvasive, convenient, feasible and precise parameter to stage liver fibrosis is the expectation of all medical staff in China. Although liver biopsy is a good measure for the assessment of liver fibrosis grade, many patients do not accept it, or they are not suitable for the test[18,19]. FibroScan is a noninvasive diagnostic technique and its diagnostic accuracy is high. However, its application is limited because of its high cost[20]. Consequently, the development of noninvasive assessment methods for liver fibrosis in patients with chronic HBV infection appears especially important in clinical practice.
In the present study, we developed a new simple, convenient and noninvasive index (AGPR) to predict significant liver fibrosis in chronic HBV-infected patients in China. The correlation coefficients between liver fibrosis stage and AGPR suggest that AGPR is a good test for the assessment of significant liver fibrosis and cirrhosis.
The AGPR index was established on the basis of ALP, γ-GT and PLT. These three indicators are clinical evaluations of features of fibrosis/cirrhosis and evidence of decompensation. Hepatitis is related to ALP[21,22] and γ-GT[23,24]. ALP is useful in the diagnosis of chronic liver diseases[25]. A study showed that serum ALP level was significantly different in patients with or without liver cirrhosis[26]. In a report, γ-GT was demonstrated to be an independent predictor of hepatic fibrosis[27]. The circulating PLT count has been recommended as a biomarker of hepatic fibrosis and cirrhosis[28]. Based on these findings, each of the three variables is related to the degree of liver fibrosis. The antiviral treatment, anti-inflammatory and hepatoprotective treatment have a greater impact on serum levels of aminotransferases, such as AST and ALT. However, according to clinical observation, ALP and γ-GT are regarded as less specific for liver injury than AST and ALT. The effects of antiviral therapy, anti-inflammatory therapy and hepatoprotective therapy on ALP and γ-GT are not as obvious as on AST and ALT. As a matter of fact, elevated serum γ-GT levels are strongly associated with alcohol consumption. However, the researchers have reported that the high-risk liver disease mortality due to elevated γ-GT was not affected by alcohol consumption[29].
A routine assessment of liver fibrosis stage for patients with chronic HBV infection is needed to guide management and to indicate the need for treatment. However, the diagnosis of liver fibrosis and compensated cirrhosis cannot be based on clinically obvious features. Noninvasive fibrosis tests are now increasingly used for evaluating liver fibrosis, reducing the need for liver biopsy. AGPR may be a new promising noninvasive fibrosis test to assist in the selection of optimal candidates for antiviral therapy. The AGPR test is inexpensive, routinely available at health-care facilities, and can be performed by untrained staff. It is suitable for conventional monitoring of hepatic fibrosis and cirrhosis. The Guidelines Development Group prioritized urgent initiation of antiviral therapy for patients with cirrhosis based on APRI score > 2 in adults, regardless of ALT or HBV DNA levels[13]. However, when applying an APRI score > 2 in this study, the sensitivity for the diagnosis of cirrhosis was only 43.2% and 50.1% in the training and validation cohorts, respectively. This suggested that more than 50% of patients with cirrhosis would be incorrectly classified as not having cirrhosis, which may lead to delayed initiation of treatment. In contrast, the sensitivity of AGPR for the diagnosis of cirrhosis was high at 88.6% and 92.3% in the training and validation cohorts, respectively. Therefore, our data suggested that AGPR may be a preferred noninvasive test to detect the presence of significant fibrosis and cirrhosis. It may serve as a simple index to make treatment decisions in patients without evidence of cirrhosis in China and other resource-limited settings where HBV infection is endemic.
Our study has some limitations. First, the selection of samples was limited to a population with chronic HBV infection in China. Whether AGPR can be generalized to different geographical areas (the infection of hepatitis C virus is endemic) remains to be determined. Second, the time span of over 10 years for our selected samples was too long (from May 2005 to October 2016). Over this time period, substantial changes have taken place in terms of medical equipment and physical examination technologies, which may reduce the accuracy of our study results. Finally, there are many factors involved in liver fibrosis. The impact of various interference factors on the diagnostic accuracy of the AGPR index has not been fully evaluated. Some interference may affect the precision of our index.
In summary, the AGPR index may be an accurate noninvasive test for predicting significant liver fibrosis and cirrhosis in patients with chronic HBV infection in China. In addition, it is suitable for conventional monitoring. Therefore, for the prediction of liver fibrosis and cirrhosis, the AGPR index is a promising noninvasive marker that is worthy of further attention and research.
Patients infected with hepatitis B virus (HBV) tend to develop liver fibrosis and liver cirrhosis. Those with cirrhosis have a high risk of hepatic decompensation and hepatitis B- related hepatocellular carcinoma.
Liver biopsy was used to ascertain the degree of fibrosis/cirrhosis. However, as an invasive procedure, liver biopsy has many disadvantages. The Guidelines Development Group recommended the use of noninvasive tests to assist in the assessment of liver disease stage and the diagnosis of fibrosis/cirrhosis. The use of a noninvasive test can reduce the need for liver biopsy.
The present study aimed to develop a precise noninvasive test to stage liver fibrosis/cirrhosis and compare the diagnostic values between different noninvasive methods.
Univariable logistic regression was used to identify significant predictive factors. Correlation analysis was performed to reveal the correlation between clinical parameters and liver stage. Receiver operating characteristic (ROC) curves were drawn to evaluate the diagnostic accuracy of different noninvasive methods.
The presence of liver fibrosis was significantly related to alkaline phosphatase and the gamma-glutamyl transpeptidase to platelet ratio (AGPR). There was a significant positive correlation between AGPR and liver fibrosis stage. The area under the ROC curve values of AGPR were 0.83, 0.84, and 0.87 for the prediction of significant fibrosis, extensive fibrosis, and cirrhosis, respectively. The AGPR index had a better overall performance than other noninvasive indices.
AGPR can be used to detect the presence of significant fibrosis, extensive fibrosis, and cirrhosis with high diagnostic accuracy, sensitivity, and specificity in patients with chronic HBV infection.
The AGPR index is a promising noninvasive marker for assessing liver disease stage. The use of AGPR can help with the routine monitoring of hepatic fibrosis and cirrhosis.
| 1. | Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 2015;39 Suppl 1:S60-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 204] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Rockey DC. Liver Fibrosis Reversion After Suppression of Hepatitis B Virus. Clin Liver Dis. 2016;20:667-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 3. | Park YE, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Han S, Jeon MY, Heo JY, Song K, Kim SU. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol. 2017;32:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Li Y, Chen Y, Zhao Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PLoS One. 2014;9:e105728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Friedman LS. Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep. 2004;6:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Meng F, Zheng Y, Zhang Q, Mu X, Xu X, Zhang H, Ding L. Noninvasive evaluation of liver fibrosis using real-time tissue elastography and transient elastography (FibroScan). J Ultrasound Med. 2015;34:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Dong DR, Hao MN, Li C, Peng Z, Liu X, Wang GP, Ma AL. Acoustic radiation force impulse elastography, FibroScan®, Forns' index and their combination in the assessment of liver fibrosis in patients with chronic hepatitis B, and the impact of inflammatory activity and steatosis on these diagnostic methods. Mol Med Rep. 2015;11:4174-4182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Jia J, Hou J, Ding H, Chen G, Xie Q, Wang Y, Zeng M, Zhao J, Wang T, Hu X, Schuppan D. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Liao Y, Wei R, Yao R, Qin L, Li J, Yu J, Liao W. AGLR is a novel index for the prognosis of hepatocellular carcinoma patients: a retrospective study. BMC Surg. 2021;21:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3343] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 11. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3812] [Article Influence: 190.6] [Reference Citation Analysis (0)] |
| 12. | Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M, Cooke G, D'Alessandro U, Vray M, Mbaye PS, Njie R, Mallet V, Thursz M. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (1)] |
| 13. | World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with chronic Hepatitis B infection. 2015. [Cited 13 Mar 2018]. Available from: http://whoint/hiv/pub/hepatitis/hepatitis-b-guidelines/en/. |
| 14. | Li Q, Song J, Huang Y, Li X, Zhuo Q, Li W, Chen C, Lu C, Qi X, Chen L. The Gamma-Glutamyl-Transpeptidase to Platelet Ratio Does not Show Advantages than APRI and Fib-4 in Diagnosing Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis B: A Retrospective Cohort Study in China. Medicine (Baltimore). 2016;95:e3372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3129] [Article Influence: 104.3] [Reference Citation Analysis (1)] |
| 16. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2467] [Article Influence: 352.4] [Reference Citation Analysis (1)] |
| 17. | Xu XY, Ding HG, Li WG, Xu JH, Han Y, Jia JD, Wei L, Duan ZP, Ling-Hu EQ, Zhuang H. Chinese guidelines on the management of liver cirrhosis (abbreviated version). World J Gastroenterol. 2020;26:7088-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 427] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Nalbantoglu IL, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:9026-9037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 97] [Reference Citation Analysis (1)] |
| 20. | Sun W, Cui H, Li N, Wei Y, Lai S, Yang Y, Yin X, Chen DF. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: A meta-analysis study. Hepatol Res. 2016;46:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 21. | Aggarwal SK, Radhakrishnan S. Syphilitic hepatitis: Look for raised alkaline phosphatase level. Med J Armed Forces India. 2016;72:192-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kasztelan-Szczerbinska B, Slomka M, Celinski K, Szczerbinski M. Alkaline phosphatase: the next independent predictor of the poor 90-day outcome in alcoholic hepatitis. Biomed Res Int. 2013;2013:614081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Huang CF, Chuang WL, Yu ML. Interference of diabetes on the association of γ-glutamyl transpeptidase to platelet ratio with liver fibrosis in chronic hepatitis C. Kaohsiung J Med Sci. 2016;32:334-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Benini F, Pigozzi MG, Baisini O, Romanini L, Ahmed H, Pozzi A, Ricci C, Lanzini A. Increased serum gamma-glutamyl-transpeptidase concentration is associated with nonalcoholic steatosis and not with cholestasis in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2007;22:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Barreto AV, Alecrim VM, Medeiros TB, Domingues AL, Lopes EP, Martins JR, Nader HB, Diniz GT, Montenegro SM, Morais CN. New index for the diagnosis of liver fibrosis in Schistosomiasis mansoni. Arq Gastroenterol. 2017;54:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Cho HJ, Kim SS, Ahn SJ, Park JH, Kim DJ, Kim YB, Cho SW, Cheong JY. Serum transferrin as a liver fibrosis biomarker in patients with chronic hepatitis B. Clin Mol Hepatol. 2014;20:347-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Nishikawa H, Hasegawa K, Ishii A, Takata R, Enomoto H, Yoh K, Kishino K, Shimono Y, Iwata Y, Nakano C, Nishimura T, Aizawa N, Sakai Y, Ikeda N, Takashima T, Iijima H, Nishiguchi S. A proposed predictive model for advanced fibrosis in patients with chronic hepatitis B and its validation. Medicine (Baltimore). 2016;95:e4679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Pradella P, Bonetto S, Turchetto S, Uxa L, Comar C, Zorat F, De Angelis V, Pozzato G. Platelet production and destruction in liver cirrhosis. J Hepatol. 2011;54:894-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477-85.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang KP, China; Leowattana W, Thailand S-Editor: Yan JP L-Editor: A P-Editor: Yan JP