Published online Jul 21, 2022. doi: 10.3748/wjg.v28.i27.3476
Peer-review started: February 11, 2022
First decision: April 10, 2022
Revised: April 19, 2022
Accepted: June 19, 2022
Article in press: June 19, 2022
Published online: July 21, 2022
Processing time: 157 Days and 4.9 Hours
The combined index of hemoglobin, albumin, lymphocyte, and platelet (HALP) can reflect systemic inflammation and nutritional status simultaneously, with some evidence revealing its prognostic value for some tumors. However, the effect of HALP on recurrence-free survival (RFS) in patients with gastrointestinal stromal tumors (GISTs) has not been reported.
To investigate the prognostic value of HALP in GIST patients.
Data from 591 untreated patients who underwent R0 resection for primary and localized GISTs at West China Hospital between December 2008 and December 2016 were included. Clinicopathological data, preoperative albumin, blood routine information, postoperative treatment, and recurrence status were recorded. To eliminate baseline inequivalence, the propensity scores matching (PSM) method was introduced. Ultimately, the relationship between RFS and preoperative HALP was investigated.
The optimal cutoff value for HALP was determined to be 31.5 by X-tile analysis. HALP was significantly associated with tumor site, tumor size, mitosis, Ki67, National Institutes of Health (NIH) risk category, and adjuvant therapy (all P < 0.001). Before PSM, GIST patients with an increased HALP had a significantly poor RFS (P < 0.001), and low HALP was an independent risk factor for poor RFS [hazard ratio (HR): 0.506, 95% confidence interval (95%CI): 0.291-0.879, P = 0.016]. In NIH high-risk GIST patients, GIST patients with low HALP had a worse RFS than patients with high HALP (P < 0.05). After PSM, 458 GIST patients were identified; those with an increased HALP still had significantly poor RFS after PSM (P < 0.001) and low HALP was still an independent risk factor for poor RFS (HR: 0.558, 95%CI: 0.319-0.976, P = 0.041).
HALP was significantly correlated with postoperative pathology and postoperative treatment. Furthermore, HALP showed a strong ability to predict RFS in GIST patients who underwent radical resection.
Core Tip: The combined index of hemoglobin, albumin, lymphocyte, and platelet (HALP) can reflect systemic inflammation and nutritional status simultaneously. We demonstrated that HALP has a statistically significant correlation with postoperative pathology and postoperative treatment in patients with gastrointestinal stromal tumors (GISTs). Furthermore, we revealed that a low level of HALP was an independent risk factor for poor recurrence-free survival in GIST patients following radical resection before and after propensity scores matching.
- Citation: Zhao Z, Yin XN, Wang J, Chen X, Cai ZL, Zhang B. Prognostic significance of hemoglobin, albumin, lymphocyte, platelet in gastrointestinal stromal tumors: A propensity matched retrospective cohort study. World J Gastroenterol 2022; 28(27): 3476-3487
- URL: https://www.wjgnet.com/1007-9327/full/v28/i27/3476.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i27.3476
Gastrointestinal stromal tumors (GISTs), a rare type of tumor, are the most frequent mesenchymal tumors arising from the gastrointestinal tract[1]. GISTs may occur anywhere in the digestive tract and even occasionally outside the gastrointestinal tract, with the stomach accounting for 60% and the small intestine 30% of all GISTs[2]. The morphology, immunohistochemistry, and molecular markers are helpful to the diagnosis of GISTs. Surgical resection is the standard treatment for resectable GISTs[3]. Nowadays, novel small molecular tyrosine kinase inhibitors, such as imatinib and sunitinib, have revolutionized the integrated treatment of GISTs and greatly improved the long-term prognosis of patients[4].
Some GIST-specific parameters based on postoperative pathologies, such as tumor size, primary tumor location, mitotic index, and tumor rupture, have been used to stratify the risk of recurrence for GISTs[2,5-7]. Meanwhile, a recent effort has shed light on the role of preoperative cancer-related inflammation and nutrition status in progression of various cancers, such as those of gastric[8], colorectal[9], non-small lung[10], and GIST[11-15]. Several preoperative immuno-inflammatory-based prognostic scores, such as the preoperative neutrophil-to-lymphocyte ratio (NLR), the lymphocyte-to-monocyte ratio (LMR), and the platelet-to-lymphocyte ratio (PLR), reflect the systematic inflammatory response, with some evidence supporting their prognostic ability for GISTs[13-17]. Furthermore, nutritional status, such as measured by the prognostic nutritional index (PNI), has also been shown to play an important role in GIST progression[10,11].
Recent studies have proposed a new combined index of hemoglobin, albumin, lymphocyte, and platelet (HALP) which can reflect systemic inflammation and nutritional status simultaneously[18]. It has already been reported as related to the prognosis of patients with pancreatic cancer[19], renal cancer[20], gastric cancer[18], prostate cancer[21], bladder cancer[22], esophageal cancer[23], and small cell lung cancer[24]. However, there are no studies on the relationship between HALP and recurrence in GIST patients who undergo radical resection. Therefore, this study aimed to investigate the prognostic value of preoperative HALP in resected GIST patients.
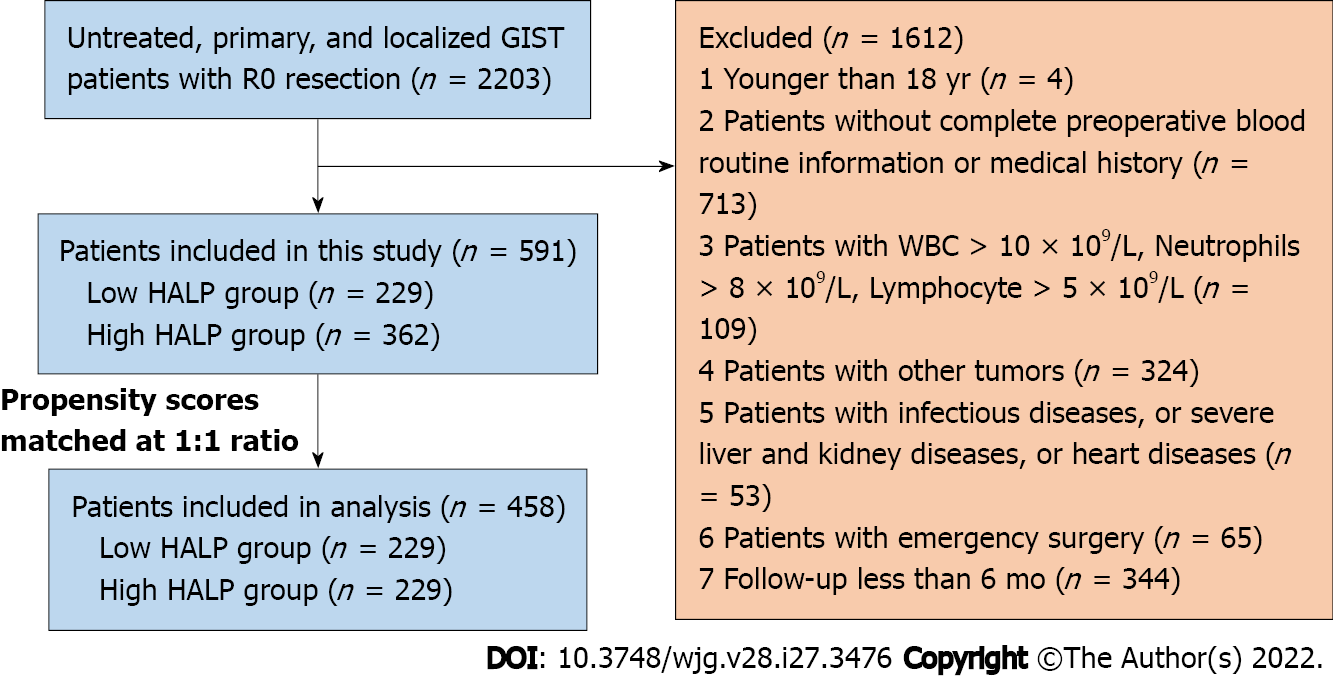
A flow diagram of the patient selection process is shown in Figure 1. Data from consecutive, previously untreated patients who underwent R0 resection for primary, localized GISTs at West China Hospital between December 2008 and December 2016 were included in this study. Patients who were younger than 18 years in age, without complete preoperative blood routine information or medical history, or with infectious diseases, blood counts with white blood cells (WBCs) > 10 × 109/L, neutrophils > 8 × 109/L, or lymphocytes > 5 × 109/L, other tumors, severe liver, kidney or heart diseases, emergency surgery, or follow-up less than 6 mo were excluded. In total, 591 GIST patients were enrolled for the current analysis.
This study was reviewed and approved by the Ethics Committee of the West China Hospital of Sichuan University, No. 1135(2019) and adhered to the tenets of the Declaration of Helsinki. All patients provided written informed consent.
Recurrence-free survival (RFS) was defined as the time interval between the time of surgery and the time of the first documented appearance of tumor after complete resection. The HALP, PNI, NLR, PLR, and LMR were calculated using the following formulas: HALP = hemoglobin level (g/L) × albumin level (g/L) × lymphocyte count (/L)/platelet count (/L)[19]; PNI = albumin level (g/L) + 5 × lymphocyte count (n/mm3)[25]; NLR = neutrophil count (n/mm3)/lymphocyte count (n/mm3)[15,16]; PLR = platelet count (n/mm3)/lymphocyte count (n/mm3)[14]; LMR = lymphocyte count (n/mm3)/monocyte count (n/mm3)[26].
Clinicopathological data, postoperative treatment, and recurrence status were recorded. The following data of each patient were retrieved from the self-built GISTs database: Demographic characteristics, tumor sites, tumor size, mitotic index [mitosis/50 high-power field (HPF) or mitosis/50 mm2], morphology, immunohistochemistry, molecular markers, preoperative hemoglobin, albumin, WBC count, absolute neutrophil count, monocyte count, platelet count, and lymphocyte count. Tumor risk stratification was determined based on the modified National Institutes of Health (NIH) classification[27].
For all patients, the laboratory tests were evaluated within 1 wk before operation. Preoperative blood routine and blood biochemical examination were performed by the Laboratory Department of Sichuan University West China Hospital. The parameters included complete blood cell count and serum albumin. Histopathological diagnosis was performed by the Department of Pathology of Sichuan University West China Hospital; the postoperative pathological findings included data on gross appearance, tumor size, tumor site, resection margin status, tumor cell morphology, lymph node metastasis status, and immunohistochemical staining, etc.
Abdominal/pelvic computed tomography was performed every 3-6 mo in the first 3 years after operation, and then every 6-12 mo, until 5 years after the operation, and then once a year until recurrence. Recurrence status was ascertained up to December 2020.
The optimal cutoff values for the HALP, PNI, NLR, PLR, and LMR were determined to be 31.5, 48.6, 2.60, 134.8, and 4.0, respectively, by X-tile analysis[28]. Propensity scores matching (PSM) was performed as 1:1 matching and a 0.02 caliper based on the patient's age, tumor size, tumor site, mitosis, and adjuvant targeted therapy using nearest neighbor matching with the MatchIt R package (https://cran.r-project.org/web/packages/MatchIt/MatchIt.pdf). The categorical variables are reported as n (%) and quantitative variables are reported as mean ± SD or median (range). Statistical significance of group comparisons was analyzed via parametric and nonparametric tests for continuous variables and via chi-square analysis or Fisher’s test for categorical variables. Survival curves of the RFS were calculated by the Kaplan-Meier methods and compared by log-rank tests. Hazard ratio (HR) for recurrence was calculated by Cox regression analysis. Sensitivity and specificity of HALP, PNI, NLR, LMR, and PLR were defined using time-dependent receiver operating characteristic (ROC) curves, and areas under the curve (AUCs) were detected utilizing survival ROC R package[29]. All statistical analyses were performed using SPSS Statistics version 21 (SPSS 21.0; IBM Corp., Armonk, NY, United States) and GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA, United States). Statistical significance was set at P < 0.05 as two-sided.
The demographic and clinicopathological characteristics of the 591 GIST patients are listed in Table 1 and Supplementary Table 1. The study population consisted of 280 (46.8%) male and 311 (53.2%) female patients. The median age was 57 (range: 21-86) years. The median follow-up time was 56 (range: 4-138) mo. The mean ± SD findings for the HALP, PNI, NLR, PLR, and LMR values were 45.81 ± 33.73, 49.04 ± 5.43, 2.64 ± 1.74, 152.8 ± 84.6 and 5.13 ± 3.00, respectively. The mean ± SD of tumor size was 6.16 ± 4.87 cm. One hundred ninety-one tumors (32.3%) had a mitotic index of > 5/50 HPF. A total of 34.0% (201/691) of the GIST patients received adjuvant therapy with imatinib or sunitinib. According to NIH risk classification, 72 (12.2%) patients were classified as very low risk, 178 (30.1%) patients as low risk, 114 (19.3%) patients as intermediate risk, and 227 (38.4%) patients as high risk. Recurrence occurred in 62 GIST patients.
| Characteristics | Before PSM1 | After PSM | ||||||
| All | Low HALP, < 31.5 | High HALP, ≥ 31.5 | P value | All | Low HALP, < 31.5 | High HALP, ≥ 31.5 | P value | |
| n (%) | 591 | 229 (38.7) | 362 (61.3) | - | 458 | 229 (50) | 229 (50) | - |
| Age in yr | 56.3 ± 12.0 | 56.7 ± 12.2 | 56.1 ± 11.8 | 56.8 ± 12.1 | 56.7 ± 12.2 | 57.0 ± 12.1 | ||
| < 60 | 337 (57.0) | 129 | 208 | 256 (55.9) | 129 | 127 | ||
| ≥ 60 | 254 (43.0) | 100 | 154 | 0.788 | 202 (44.1) | 100 | 102 | 0.851 |
| Sex | ||||||||
| Male | 280 (47.4) | 98 | 182 | 233 (50.9) | 131 | 102 | ||
| Female | 311 (52.6) | 131 | 180 | 0.076 | 225 (49.1) | 98 | 127 | 0.007a |
| Tumor site | ||||||||
| Stomach | 424 (71.7) | 143 | 281 | 299 (65.3) | 143 | 156 | ||
| Non-stomach | 167 (28.3) | 86 | 81 | < 0.001a | 159 (34.7) | 86 | 73 | 0.202 |
| Tumor size in cm | 6.16 ± 4.87 | 7.69 ± 5.65 | 5.18 ± 4.02 | 7.13 ± 5.08 | 7.69 ± 5.65 | 6.57 ± 4.38 | ||
| ≤ 2 | 86 (14.6) | 10 | 76 | 27 (5.9) | 10 | 17 | ||
| 2.1-5.0 | 251 (42.5) | 87 | 164 | 177 (38.6) | 87 | 90 | ||
| 5.1-10.0 | 184 (31.1) | 95 | 89 | 184 (40.2) | 95 | 89 | ||
| > 10.0 | 70 (11.8) | 37 | 33 | < 0.001a | 70 (15.3) | 37 | 33 | 0.514 |
| Mitotic index/50 HPF | ||||||||
| ≤ 5 | 332 (56.2) | 107 | 225 | 220 (48.0) | 107 | 113 | ||
| 6-10 | 100 (16.9) | 45 | 55 | 91 (19.9) | 45 | 46 | ||
| > 10 | 91 (15.4) | 49 | 42 | 89 (19.4) | 49 | 40 | ||
| Unknown | 68 (11.5) | 28 | 40 | 0.001a | 58 (12.7) | 28 | 30 | 0.764 |
| Ki67 | ||||||||
| ≤ 10 | 417 (70.6) | 140 | 277 | 308 (67.3) | 140 | 168 | ||
| > 10 | 98 (16.6) | 61 | 37 | 94 (20.5) | 61 | 33 | ||
| Unknown | 76 (12.9) | 28 | 48 | < 0.001a | 26 (12.2) | 28 | 28 | 0.004a |
| NIH risk category | ||||||||
| Very low risk | 72 (12.2) | 9 | 63 | 21 (4.6) | 9 | 12 | ||
| Low risk | 178 (30.1) | 52 | 126 | 113 (24.7) | 52 | 61 | ||
| Intermediate risk | 114 (19.3) | 43 | 71 | 100 (21.8) | 43 | 57 | ||
| High risk | 227 (38.4) | 125 | 102 | < 0.001a | 224 (48.9) | 125 | 99 | 0.106 |
| Adjuvant therapy | ||||||||
| Yes | 201 (34.0) | 99 | 102 | 193 (42.1) | 99 | 94 | ||
| No | 390 (66.0) | 130 | 260 | < 0.001a | 265 (57.9) | 130 | 135 | 0.636 |
| Recurrence | ||||||||
| Yes | 62 (10.5) | 42 | 20 | 61 (13.3) | 42 | 19 | ||
| No | 529 (89.5) | 187 | 342 | < 0.001a | 397 (86.7) | 187 | 210 | 0.002a |
The clinicopathological characteristics between the high and low groups of HALP were categorized and analyzed as shown in Table 1 and Supplementary Table 1. Together, 229 patients were assigned to the low HALP group and 362 patients to the high HALP group. The results demonstrated that tumor site, tumor size, mitotic index, Ki67, NIH risk category, and adjuvant therapy were significantly associated with HALP (all P < 0.05).
PSM analysis was further carried out to avoid confounding variables that might interfere with the association between RFS and HALP level. After 1:1 matching, PSM analysis identified 229 pairs of GIST patients. After PSM, HALP was still associated with sex, Ki67, and recurrence but not with any other clinicopathological characteristics (Table 1 and Supplementary Table 1).
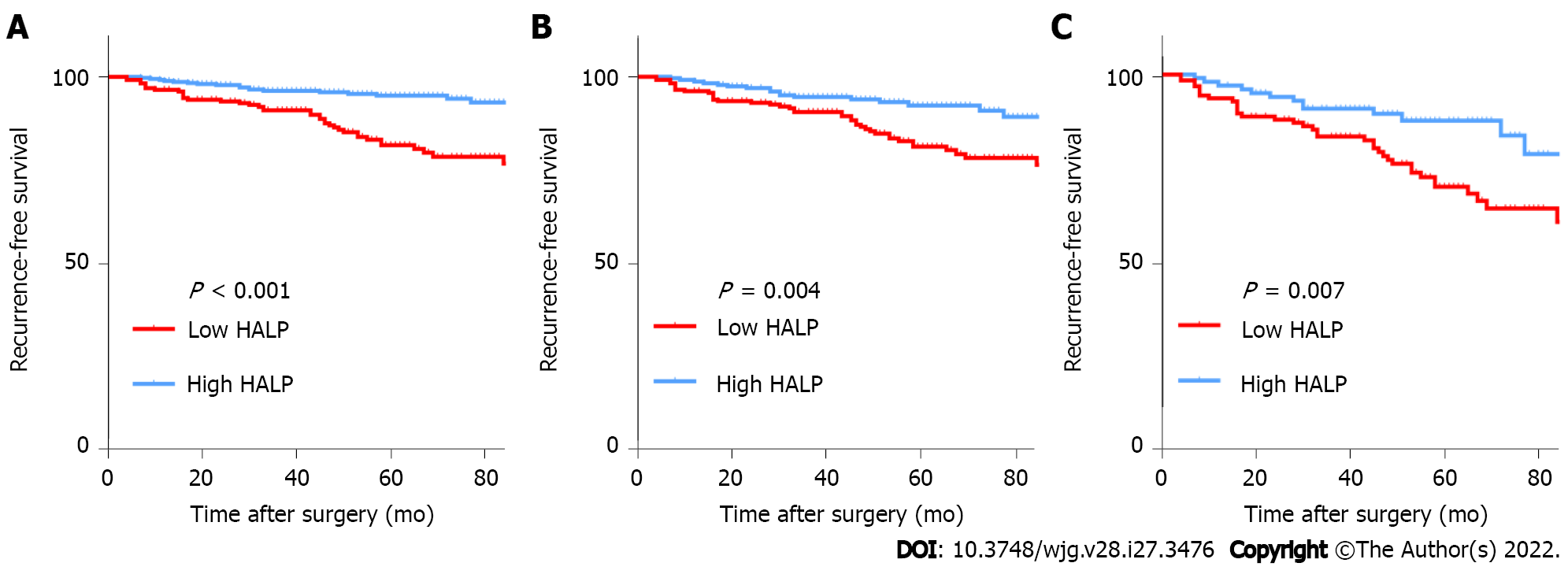
Before PSM, tumor site, tumor size, mitotic index, Ki67, NIH risk category, NLR, PLR, PNI, and HALP were associated with RFS (all P < 0.05) (Table 2). RFS in GIST patients with low HALP was significantly worse than in those with high HALP (Figure 2). Cox multiple regression analysis showed that HALP was an independent prognostic factor for RFS in GIST patients before PSM [HR: 0.506, 95% confidence interval (CI): 0.291-0.879, P = 0.016].
| Risk factors | Before PSM | After PSM | ||||||
| Univariate analysis, HR (95%CI) | Univariate analysis, P value | Multivariate analysis, HR (95%CI) | Multivariate analysis, P value | Univariate analysis, HR (95%CI) | Univariate analysis, P value | Multivariate analysis, HR (95%CI) | Multivariate analysis, P value | |
| Age | 1.009 (0.987-1.030) | 0.431 | NS | 1.006 (0.984-1.027) | 0.607 | NS | ||
| Sex: Male vs female | 0.639 (0.386-1.056) | 0.081 | NS | 0.711 (0.429-1.179) | 0.186 | NS | ||
| Tumor site: Stomach vs non-stomach | 2.273 (1.377-3.752) | 0.001a | 2.979 (1.716-5.171) | < 0.001a | 1.702 (1.028-2.818) | 0.039a | 2.865 (1.631-5.032) | < 0.001a |
| Tumor size in cm: ≤ 2/2.1-5.0/5.1-10.0/> 10.0 | 2.629 (1.948-3.548) | < 0.001a | 1.070 (1.032-1.109) | 0.001a | 1.086 (1.056-1.116) | < 0.001a | 1.068 (1.029-1.107) | < 0.001a |
| Mitotic index as/50 HPF: ≤ 5/6-10/> 10/unknown | 2.071 (1.686-2.545) | < 0.001a | < 0.001a | < 0.001a | 0.001a | |||
| ≤ 5 vs 6-10 | 5.659 (2.151-14.887) | 0.002a | 5.442 (2.067-14.323) | 0.001a | 5.444 (1.955-15.162) | 0.001a | ||
| ≤ 5 vs > 10 | 8.259 (3.140-21.720) | < 0.001a | 14.722 (6.037-35.904) | < 0.001a | 7.675 (2.759-21.348) | < 0.001a | ||
| ≤ 5 vs unknown | 5.299 (2.041-13.757) | < 0.001a | 9.851 (3.843-25.251) | < 0.001a | 5.107 (1.873-13.923) | 0.001a | ||
| CD117: +/- | 1.231 (0.300-5.059) | 0.773 | NA | 1.291 (0.314-5.313) | 0.723 | - | NA | |
| DOG1: +/-/unknown | 1.464 (0.773-2.774) | 0.242 | NA | 1.626 (0.853-3.102) | 0.140 | - | NA | |
| Ki67: ≤ 10/> 10/unknown | 1.919 (1.453-2.533) | < 0.001a | 0.001a | < 0.001a | 0.001a | |||
| < 10 vs ≤ 10 | 3.579 (1.771-7.233) | < 0.001a | 8.625 (4.750-15.660) | < 0.001a | 3.710 (1.811-7.599) | < 0.001a | ||
| Unknown vs ≤ 10 | 2.844 (1.290-6.270) | 0.024a | 3.310 (1.528-7.169) | 0.002a | 3.050 (1.365-6.816) | 0.007 | ||
| Histologic subtypes: Spindle/epithelioid/mixed | 1.361 (0.981-1.889) | 0.065 | NS | 1.236 (0.891-1.715) | 0.204 | - | NA | |
| NIH risk category: Very low/low/intermediate/high | 3.218 (2.180-4.751) | < 0.001a | NS | 2.892 (1.865-4.484) | < 0.001a | - | NS | |
| Adjuvant therapy: Yes/no | 1.289 (0.768-2.162) | 0.336 | 0.445 (0.257-0.769) | 0.004a | 0.923 (0.549-1.551) | 0.761 | 0.003a | |
| NLR: < 2.60/≥ 2.60 | 2.025 (1.229-3.337) | 0.006a | NS | 1.746 (1.055-2.890) | 0.030a | NS | ||
| PLR: < 134.8/≥ 134.8 | 2.925 (1.673-5.112) | < 0.001a | NS | 1.991 (1.137-3.486) | 0.016a | NS | ||
| LMR: < 4.0/≥ 4.0 | 1.296 (0.777-2.163) | 0.321 | NA | 1.088 (0.650-1.821) | 0.749 | - | NA | |
| PNI: < 48.6/≥ 48.6 | 0.291 (0.171-0.496) | < 0.001a | NS | 1.991 (1.137-3.486) | 0.016a | NS | ||
| HALP: < 31.5/≥ 31.5 | 0.341 (0.197-0.590) | < 0.001a | 0.506 (0.291-0.879) | 0.016a | 0.457 (0.265-0.785) | 0.005a | 0.558 (0.319-0.976) | 0.041a |
After PSM, tumor site, tumor size, mitotic index, Ki67, NIH risk category, PNI, NLR, PLR, and HALP were still related to RFS (all P < 0.05) (Table 2). RFS was also significantly worse in GIST patients with low HALP than in those with high HALP (Figure 2). Furthermore, Cox multiple regression analysis showed that HALP was an independent prognostic factor for RFS in GIST patients (HR: 0.558, 95%CI: 0.319-0.976, P = 0.041).
The clinicopathological characteristics of high-risk GIST patients between the high and low groups of HALP were categorized in Supplementary Table 1. Together, 125 patients were assigned to the low HALP group and 102 patients to the high HALP group. The results demonstrated that sex and Ki67 were associated with HALP (both P < 0.05). Not surprisingly, patients in the low HALP group had significantly worse survival than patients in the high HALP group (Figure 2). Furthermore, Cox multiple regression analysis indicated that HALP was an independent prognostic factor for RFS in GIST patients (HR: 0.469, 95%CI: 0.245-0.896, P = 0.022) (Supplementary Table 2).
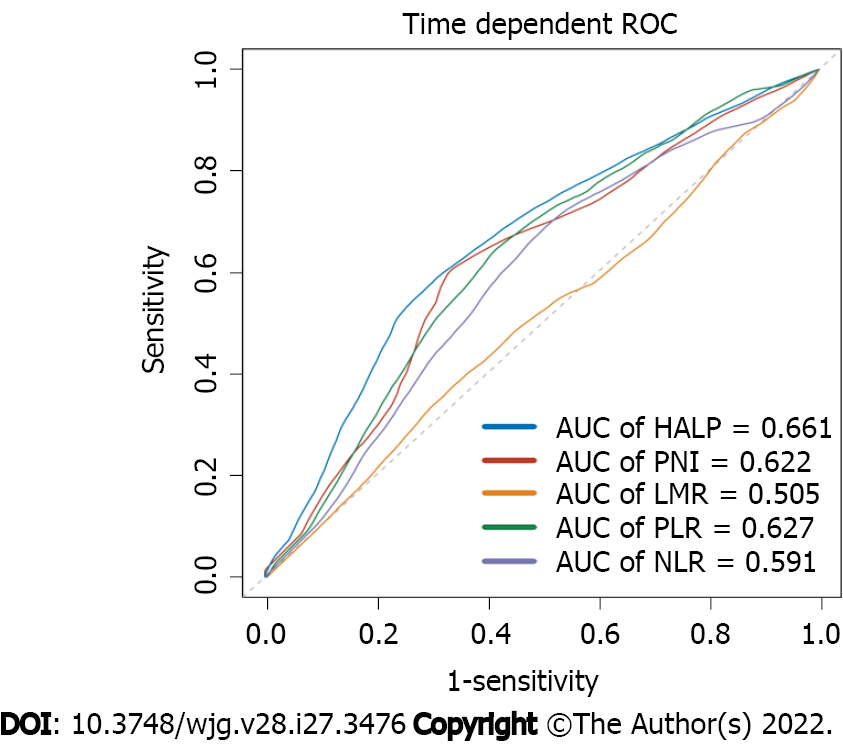
Time-dependent ROCs were generated for HALP, PNI, NLR, LMR, and PLR to predict 5-year RFS. According to the results, the 5-year AUC reached 0.661 in the HALP group, while PNI, NLR, LMR, and PLR reached 0.622, 0.591, 0.505, and 0.627, respectively (Figure 3).
There is growing evidence that preoperative nutritional status and inflammatory response may be a potentially powerful predictor of the prognosis of cancer patients. Consistent with previous research, the present study found that preoperative inflammation scores, such as NLR and PLR, were associated with the prognosis of GIST patients, both before and after PSM[14,16,30,31] (Supplementary Figure 1). However, LMR seemed to have no effect on the RFS of GIST patients (Supplementary Figure 1), which differs from findings of previous studies[14]. In addition, the PNI, a nutritional score based on albumin levels and lymphocytes, was also related to RFS of GIST patients, both before and after PSM in the present study[11,12] (Supplementary Figure 1).
In this study, we also found that preoperative HALP was significantly correlated with tumor site, tumor size, mitosis, Ki67, NIH risk category, and adjuvant therapy (Table 1). To balance the patient characteristics and standard prognostic factors between groups, we utilized the PSM method to balance patient's age, tumor size, tumor site, mitosis, and adjuvant targeted therapy. After PSM, sex, Ki67, PNI, NLR, LMR, and PLR were still associated with HALP (Supplementary Table 1). Notably, there was no difference in standard prognostic factors (i.e. tumor site, tumor size, mitosis, NIH risk category, and adjuvant therapy) between the low and high HALP groups (Table 1). Given that HALP shared several parameters with PNI, NLR, LMR, and PLR, their statistically significant correlation is unsurprising. The correlation between HALP and sex may be due to the fact that the male and female patients had significantly different hemoglobin levels (123.22 ± 2.08 g/L for males and 105.46 ± 1.84 g/L for females, P < 0.001). Remarkably, recurrence was not associated with either sex or histologic subtype (Supplementary Table 1). Subgroup analysis by sex revealed that a low level of HALP was associated with recurrence in both male and female patients (P = 0.048 and P = 0.018, respectively) (Supplementary Figure 2).
Finally, consistent with previous research on HALP in other tumors[18,19], our findings revealed prognostic value of HALP in GIST[20-24]. HALP was an independent risk factor for GIST patients before PSM, after PSM, and in high-risk subgroups (Table 2 and Supplementary Table 3). Thus, HALP can be used to not only evaluate GIST patients' postoperative risk prior to surgery but also to assess their prognosis. Notably, the HALP index can be utilized to predict the prognosis of patients in a convenient and cost-effective manner.
Although the underlying mechanism of systemic inflammation in tumorigenesis, progression and metastasis remains obscure, some theories suggest that it stimulates angiogenesis, immunosuppression, and formation of the supporting microenvironment. Lymphocytes are well known to play a critical role in tumor growth inhibition[32-34]. A higher lymphocyte signature is associated with improved prognosis in a variety of tumors[34], whereas platelets can infiltrate the tumor microenvironment and interact directly with cancer cells[35,36], assisting circulating tumor cells in adhering to endothelial cells and establishing a niche environment prior to metastasis[37-41].
Anemia is one of the most common symptoms of GIST, which can be caused by both gastrointestinal bleeding and intratumoral bleeding[42]. Yang et al[43] identified GIST with gastrointestinal bleeding as an independent prognostic predictor of poor RFS. Several studies have demonstrated that low hemoglobin levels can result in tumor hypoxia, which is associated with an increased risk of local failure and distant metastasis[31,44]. Furthermore, a hypoxic tumor environment may result in limited drug accumulation and hinder drug efficacy[45]. Most importantly, anemia is a common adverse effect of imatinib[46], which may require the prescribing physician to stop the drug or reduce the dose. High levels of preoperative hemoglobin may help to prevent this adverse effect.
Low levels of serum albumin are also associated with poor long-term survival in GIST patients[44,45], which is consistent with our findings. Serum albumin is generally considered as associated with nutritional status and liver or renal function, both of which may affect the prescribing physician's decision-making, similar to hemoglobin. Additionally, tumor tissues have abnormal vascular endothelial gaps and lack effective lymphatic drainage, allowing macromolecules, such as albumin, to accumulate more readily in tumor tissue than in normal tissue[47,48]. Consequently, serum albumin is suspected of being a possible nutritional source for tumor growth, due to its elevated accumulation in tumors[49-51]. This effect is referred to as the ‘enhanced permeability and retention effect’. Moreover, about 95% of imatinib is bound to serum proteins, mainly albumin and 1-acid glycoprotein, which may facilitate drug accumulation in tumors and improve therapeutic effect[52,53]. Subsequently, serum albumin levels have been shown to be an independent prognostic factor of survival in a variety of cancers, including those of colorectal[54], gastric[55], pancreatic[56], and breast[57]. As a result, it is unsurprising that HALP, which reflects systemic inflammation and nutritional status simultaneously, is associated with the risk and prognosis of GIST.
There are some limitations to this study. First, because this is a retrospective study, biases in the data collection process are possible. Second, our cases were collected between 2008 and 2016, the period during which imatinib was used for adjuvant treatment of GIST in China. Despite the adverse reaction and high costs, 201/591 (34.0%) of GIST patients still received adjuvant imatinib therapy. As an important treatment after GIST, adjuvant imatinib therapy can significantly improve the prognosis of GIST patients[58], and its benefits are also shown in the present study (Supplementary Figure 3). However, there was no adequate collection and analysis of the time, dose, and adverse reactions of patients with imatinib or sunitinib therapy, which may also be related to HALP. Moreover, this study did not evaluate other clinicopathological factors related to prognosis, especially gene mutation status. Furthermore, the effect of preoperative or postoperative improvement of nutritional status or inflammation response on the prognosis of GIST remains obscure, and will require further confirmation in clinical studies.
HALP was associated with postoperative pathological data (i.e. tumor site, tumor size, mitosis, Ki67, NIH risk category) and adjuvant therapy. Furthermore, HALP was an independent risk factor for RFS in GIST patients who underwent radical resection.
The combination index of hemoglobin, albumin, lymphocyte, and platelet (HALP) has been reported as associated with prognosis in many cancers but not yet in gastrointestinal stromal tumors (GISTs). Therefore, this study aimed to investigate the prognostic value of preoperative HALP in resected GIST patients.
At present, the risk of GIST is mainly based on postoperative pathological indicators. The motivation for this article involved the need to find a convenient, non-invasive, preoperative indicator that will assist in prognostic prediction of GIST.
To investigate the prognostic value of HALP in GIST patients.
This retrospective cohort study enrolled patients with GIST using propensity scores matching to explore the relationship between HALP, postoperative clinicopathological data, and the prognostic significance of HALP.
HALP can be conveniently used preoperatively to assess risk and prognosis of GIST patients. However, the effect of improving nutritional status or immune-inflammatory status on the prognosis of GIST is still unclear and requires further confirmation through clinical studies.
HALP was associated with postoperative pathological data (i.e. tumor site, tumor size, mitosis, Ki67, National Institutes of Health risk category) and adjuvant therapy. Furthermore, HALP was an independent risk factor for recurrence-free survival in GIST patients who underwent radical resection. This study is the first to report the prognostic significance of HALP in GIST. In this study, HALP was found to be an independent risk factor for GIST patients with R0 resection. Consistent with reports of HALP in other tumors, HALP is also associated with prognosis in GIST. HALP was also found to be an independent risk factor for GIST patients with R0 resection. In clinical practice, convenient and non-invasive preoperative HALP may be used to assist in the prediction of risk and prognosis for GIST patients.
Through this retrospective cohort study, we found the prognostic significance of HALP in GIST. This study did not evaluate other clinicopathological factors related to prognosis, especially gene mutation status. Subsequent studies should employ a prospective cohort method and incorporate additional factors to further explore the prognostic significance of HALP in GIST patients.
| 1. | Ma GL, Murphy JD, Martinez ME, Sicklick JK. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 344] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 3. | Barrios CH, Blackstein ME, Blay JY, Casali PG, Chacon M, Gu J, Kang YK, Nishida T, Purkayastha D, Woodman RC, Reichardt P. The GOLD ReGISTry: a Global, Prospective, Observational Registry Collecting Longitudinal Data on Patients with Advanced and Localised Gastrointestinal Stromal Tumours. Eur J Cancer. 2015;51:2423-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | DeMatteo RP, Ballman KV, Antonescu CR, Corless C, Kolesnikova V, von Mehren M, McCarter MD, Norton J, Maki RG, Pisters PW, Demetri GD, Brennan MF, Owzar K; American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team for the Alliance for Clinical Trials in Oncology. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, Sundar HM, Trent JC, Wayne JD. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 832] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 6. | Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY; ESMO Guidelines Committee and EURACAN. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv68-iv78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (5)] |
| 7. | Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, Nishida T, Shen L, Chen LT, Kang YK. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat. 2016;48:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 9. | Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Sun J, Mei Y, Zhu Q, Shou C, Tjhoi WEH, Yang W, Yu H, Zhang Q, Liu X, Yu J. Relationship of prognostic nutritional index with prognosis of gastrointestinal stromal tumors. J Cancer. 2019;10:2679-2686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Shi WK, Zhang XH, Zhang J, Yu M, Yuan YJ, Xiong W, Zhang CH, He YL, Wei ZW. Predictive ability of prognostic nutritional index in surgically resected gastrointestinal stromal tumors: a propensity score matching analysis. Jpn J Clin Oncol. 2019;49:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Yin XN, Tang SM, Yin Y, Shen CY, Zhang B, Chen ZX. [Associations of Preoperative Platelet-to-lymphocyte Ratio and Derived Neutrophil-to-lymphocyte Ratio with thePrognosis of Gastrointestinal Stromal Tumor]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:239-243. [PubMed] |
| 14. | Feng F, Tian Y, Liu S, Zheng G, Liu Z, Xu G, Guo M, Lian X, Fan D, Zhang H. Combination of PLR, MLR, MWR, and Tumor Size Could Significantly Increase the Prognostic Value for Gastrointestinal Stromal Tumors. Medicine (Baltimore). 2016;95:e3248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Yin Z, Gao J, Liu W, Huang C, Shuai X, Wang G, Tao K, Zhang P. Clinicopathological and Prognostic Analysis of Primary Gastrointestinal Stromal Tumor Presenting with Gastrointestinal Bleeding: a 10-Year Retrospective Study. J Gastrointest Surg. 2017;21:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Racz JM, Cleghorn MC, Jimenez MC, Atenafu EG, Jackson TD, Okrainec A, Venkat Raghavan L, Quereshy FA. Predictive Ability of Blood Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios in Gastrointestinal Stromal Tumors. Ann Surg Oncol. 2015;22:2343-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Luo XF, Zhou LH. Prognostic significance of neutrophil to lymphocyte ratio in patients with gastrointestinal stromal tumors: A meta-analysis. Clin Chim Acta. 2018;477:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6:41370-41382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, Gao HL, Jiang W, Zhang WH, Li TJ, Ni QX, Liu L, Yu XJ. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol. 2020;26:828-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 20. | Peng D, Zhang CJ, Tang Q, Zhang L, Yang KW, Yu XT, Gong Y, Li XS, He ZS, Zhou LQ. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol. 2018;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, Zhang Z, Jin L, Yang B, Ye L, Yao X. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score is a Novel Significant Prognostic Factor for Patients with Metastatic Prostate Cancer Undergoing Cytoreductive Radical Prostatectomy. J Cancer. 2019;10:81-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Peng D, Zhang CJ, Gong YQ, Hao H, Guan B, Li XS, Zhou LQ. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. 2018;8:794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2017;46:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Shen XB, Zhang YX, Wang W, Pan YY. The Hemoglobin, Albumin, Lymphocyte, and Platelet (HALP) Score in Patients with Small Cell Lung Cancer Before First-Line Treatment with Etoposide and Progression-Free Survival. Med Sci Monit. 2019;25:5630-5639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Ichikawa K, Mizuno S, Hayasaki A, Kishiwada M, Fujii T, Iizawa Y, Kato H, Tanemura A, Murata Y, Azumi Y, Kuriyama N, Usui M, Sakurai H, Isaji S. Prognostic Nutritional Index After Chemoradiotherapy Was the Strongest Prognostic Predictor Among Biological and Conditional Factors in Localized Pancreatic Ductal Adenocarcinoma Patients. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Hsu JT, Wang CC, Le PH, Chen TH, Kuo CJ, Lin CJ, Chou WC, Yeh TS. Lymphocyte-to-monocyte ratios predict gastric cancer surgical outcomes. J Surg Res. 2016;202:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 899] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 28. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 3084] [Article Influence: 146.9] [Reference Citation Analysis (9)] |
| 29. | Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1735] [Cited by in RCA: 2117] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 30. | Goh BK, Chok AY, Allen JC Jr, Quek R, Teo MC, Chow PK, Chung AY, Ong HS, Wong WK. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery. 2016;159:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Rutkowski P, Teterycz P, Klimczak A, Bylina E, Szamotulska K, Lugowska I. Blood neutrophil-to-lymphocyte ratio is associated with prognosis in advanced gastrointestinal stromal tumors treated with imatinib. Tumori. 2018;104:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Ostroumov D, Fekete-Drimusz N, Saborowski M, Kühnel F, Woller N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell Mol Life Sci. 2018;75:689-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 397] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 33. | Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2028] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 34. | Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Mokrab Y, Newman AM, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CS; Cancer Genome Atlas Research Network, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich I. The Immune Landscape of Cancer. Immunity. 2018;48:812-830.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4007] [Cited by in RCA: 4122] [Article Influence: 515.3] [Reference Citation Analysis (5)] |
| 35. | Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol. 2002;3:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 279] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 36. | Haemmerle M, Stone RL, Menter DG, Afshar-Kharghan V, Sood AK. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell. 2018;33:965-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 479] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 37. | Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1519] [Cited by in RCA: 1601] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 38. | Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 434] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 39. | Olsson AK, Cedervall J. The pro-inflammatory role of platelets in cancer. Platelets. 2018;29:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 40. | Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 498] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 41. | Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36:249-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 42. | Sorour MA, Kassem MI, Ghazal Ael-H, El-Riwini MT, Abu Nasr A. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg. 2014;12:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Yang Z, Wang F, Liu S, Guan W. Comparative clinical features and short-term outcomes of gastric and small intestinal gastrointestinal stromal tumours: a retrospective study. Sci Rep. 2019;9:10033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Italiano A, Cioffi A, Coco P, Maki RG, Schöffski P, Rutkowski P, Le Cesne A, Duffaud F, Adenis A, Isambert N, Bompas E, Blay JY, Casali P, Keohan ML, Toulmonde M, Antonescu CR, Debiec-Rychter M, Coindre JM, Bui B. Patterns of care, prognosis, and survival in patients with metastatic gastrointestinal stromal tumors (GIST) refractory to first-line imatinib and second-line sunitinib. Ann Surg Oncol. 2012;19:1551-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Hompland I, Bruland ØS, Hølmebakk T, Poulsen JP, Stoldt S, Hall KS, Boye K. Prediction of long-term survival in patients with metastatic gastrointestinal stromal tumor: analysis of a large, single-institution cohort. Acta Oncol. 2017;56:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Xia Y, Chen S, Luo M, Wu J, Cai S, He Y, Chen X, Zhang X. Correlations between imatinib plasma trough concentration and adverse reactions in Chinese patients with gastrointestinal stromal tumors. Cancer. 2020;126 Suppl 9:2054-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3286] [Cited by in RCA: 3040] [Article Influence: 138.2] [Reference Citation Analysis (0)] |
| 48. | Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 582] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 49. | Babson AL, Winnick T. Protein transfer in tumor-bearing rats. Cancer Res. 1954;14:606-611. [PubMed] |
| 50. | Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1512] [Cited by in RCA: 1652] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 51. | Kratz F, Beyer U. Serum proteins as drug carriers of anticancer agents: a review. Drug Deliv. 1998;5:281-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Peng B, Lloyd P, Schran H. Clinical pharmacokinetics of imatinib. Clin Pharmacokinet. 2005;44:879-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 487] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 53. | Kim B, Seo B, Park S, Lee C, Kim JO, Oh KT, Lee ES, Choi HG, Youn YS. Albumin nanoparticles with synergistic antitumor efficacy against metastatic lung cancers. Colloids Surf B Biointerfaces. 2017;158:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Boonpipattanapong T, Chewatanakornkul S. Preoperative carcinoembryonic antigen and albumin in predicting survival in patients with colon and rectal carcinomas. J Clin Gastroenterol. 2006;40:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Oñate-Ocaña LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, Cervera E, Mohar-Betancourt A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 56. | Siddiqui A, Heinzerling J, Livingston EH, Huerta S. Predictors of early mortality in veteran patients with pancreatic cancer. Am J Surg. 2007;194:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003;27:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Cohen MH, Cortazar P, Justice R, Pazdur R. Approval summary: imatinib mesylate in the adjuvant treatment of malignant gastrointestinal stromal tumors. Oncologist. 2010;15:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elpek GO, Turkey; Fusaroli P, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR