Published online Jul 7, 2022. doi: 10.3748/wjg.v28.i25.2968
Peer-review started: January 4, 2022
First decision: March 9, 2022
Revised: April 1, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: July 7, 2022
Processing time: 181 Days and 10 Hours
Laparoscopic liver resection (LLR) has become a safe surgical procedure that needs additional summarization.
To review 4 years of total LLR surgeries, exceeding 1000 cases, which were performed at a single center.
Patients who underwent LLR at West China Hospital of Sichuan University between January 2015 and December 2018 were identified. Surgical details, including the interventional year, category of liver disease, and malignant liver tumors prognosis, were evaluated. The learning curve for LLR was evaluated using the cumulative sum method. The Kaplan-Meier method was used to perform survival analysis.
Ultimately, 1098 patients were identified. Hepatocellular carcinoma (HCC) was the most common disease that led to the need for LLR at the center (n = 462, 42.08%). The average operation time was 216.94 ± 98.51 min. The conversion rate was 1.82% (20/1098). The complication rate was 9.20% (from grade II to V). The 1-year and 3-year overall survival rates of HCC patients were 89.7% and 81.9%, respectively. The learning curve was grouped into two phases for local resection (cases 1-106 and 107-373), three phases for anatomical segmentectomy (cases 1-44, 45-74 and 75-120), and three phases for hemihepatectomy (cases 1-17, 18-48 and 49-88).
LLR may be considered a first-line surgical intervention for liver resection that can be performed safely for a variety of primary, secondary, and recurrent liver tumors and for benign diseases once technical competence is proficiently attained.
Core Tip: West China Hospital of Sichuan University is the biggest and most advanced one in the western region of China. About 1500-2500 cases of liver resection have been performed in our center every year. Since the first laparoscopic liver resection (LLR) was performed in 2015, we have accumulated more than 1000 cases rapidly, including hemi-hepatectomy, mesohepatectomy, anatomical segmentectomy from segment I to VIII and even the first case of laparoscopic donor hepatectomy in Mainland China. In this study, we want to share our experiences and introduce technical innovation of LLR.
- Citation: Lan X, Zhang HL, Zhang H, Peng YF, Liu F, Li B, Wei YG. Four-year experience with more than 1000 cases of total laparoscopic liver resection in a single center. World J Gastroenterol 2022; 28(25): 2968-2980
- URL: https://www.wjgnet.com/1007-9327/full/v28/i25/2968.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i25.2968
Laparoscopic liver resection (LLR) has become a safe approach for liver resection, following advances in surgical techniques, anesthesiology, and perioperative care. Since its first use was reported in 1996 by Azagra et al[1], LLR has evolved to become a primary choice of intervention for many patients with liver tumors[2-6]. Experience with laparoscopic procedures has allowed for the development of complex liver resection techniques using laparoscopy, including mesohepatectomy, caudate lobectomy, and even combined resection of several hepatic segments[7-9].
Total LLR has been performed since 2015 at the West China Hospital of Sichuan University. Although total LLR has been performed for a shorter period of time at the West China Hospital of Sichuan University compared to other centers, a large number of clinical patients in the western region of China have been treated with LLR at the West China Hospital of Sichuan University, conveying a rich experience in performing open liver resection to surgeons at the center. Over the past 4 years, LLR, including hemihepatectomy, mesohepatectomy, anatomical segmentectomy from segment I to VIII, and even the first case of laparoscopic donor hepatectomy in mainland China, has been performed successfully at the center[10]. The present study reviews the single center’s 4-year experience with LLR in more than 1000 patients and discusses its safety and feasibility, the learning curve for performing laparoscopic surgery, LLR technical innovations, and patient outcomes.
Patients who underwent LLR between January 2015 and December 2018 at the West China Hospital of Sichuan University were studied retrospectively. Inclusion criteria were as follows: patients who had undergone LLR due to liver disease; patients with hemangioma who met the following criteria: (1) Symptomatic hemangioma; (2) Increasing tumor size; (3) Heavy psychological burden and anxiety symptoms that affect daily life and require surgical treatment; (4) Tumor located in special segments (e.g., the caudate lobe or the porta hepatis) that are difficult to treat as tumor size increases; and (5) Unclear diagnosis in which the possibility of a malignant tumor cannot be completely ruled out; and patients with hepatocellular carcinoma (HCC) who met the following criteria: Barcelona Clinic Liver Cancer 0-B stage HCC[11]; A3 stage with normal liver function after conservative treatment; and A4 or B stage with a tumor located in the same hemiliver. Exclusion criteria were as follows: patients who only received laparoscopic surgery without hepatectomy.
Patient data (age, sex, liver function before and after the operation, complications, hospital stay after the operation, operation time, hemorrhage, ascites, and perioperative mortality) were collected. All patients provided written informed consent for surgery, and this study was approved by the Ethical Review Board of West China Hospital of Sichuan University.
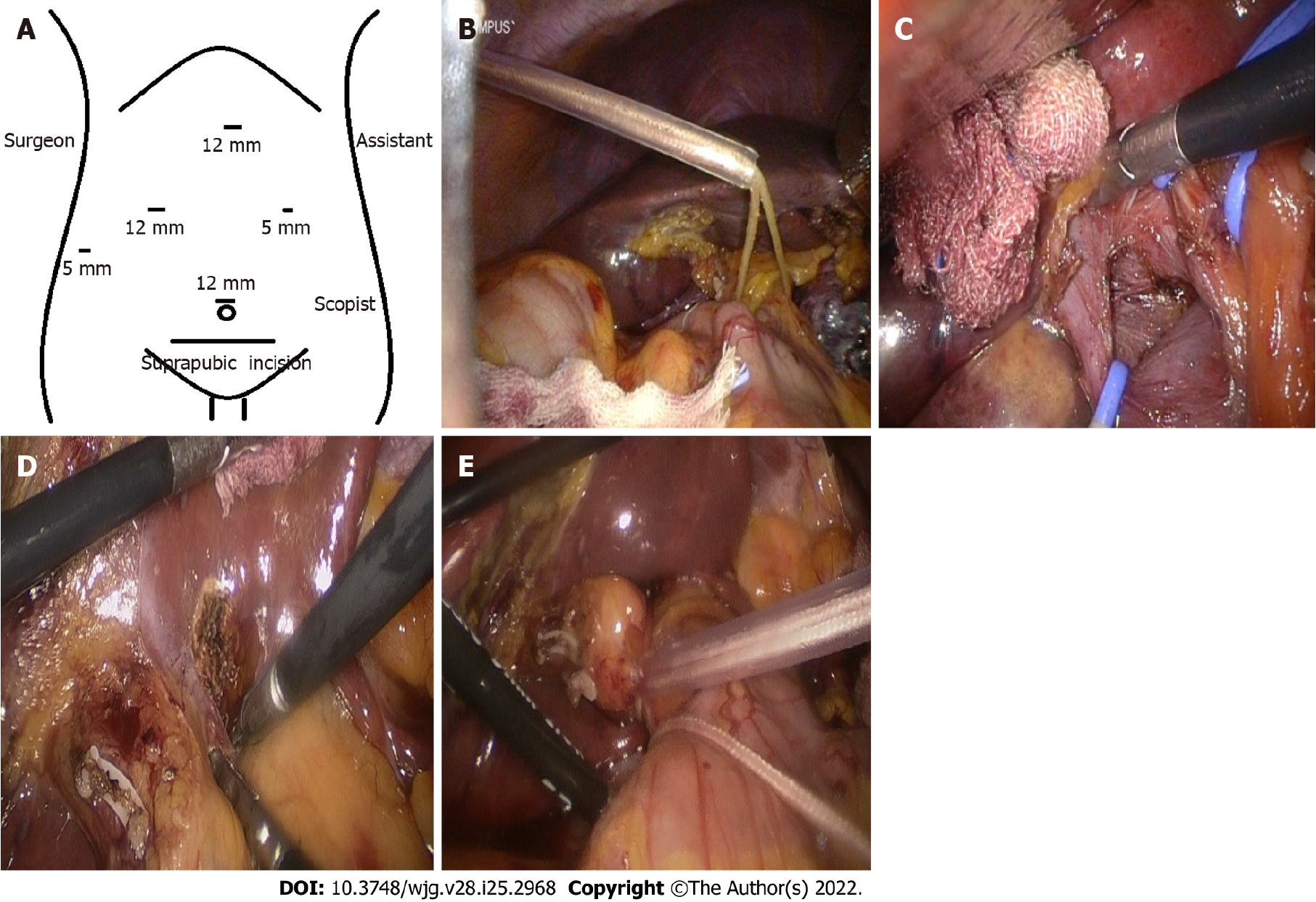
Surgical procedures were described in our previous study[12]. A five-trocar approach was used at the tumor location (Figure 1A). Two hepatic inflow occlusion methods were adopted at the center[12]. Intermittent pringle (IP): During the operation, hepatic inflow was blocked for 15 min and released for 5 min (Figure 1B) and continuous hemihepatic vascular inflow occlusion (CHVIO) (Figure 1C-E).
Liver capsule invasion and tumor location: Liver capsule invasion was determined by the histopathological report. In this study, “central tumor” was defined as a tumor within the main hepatic body, located in segments I/IV/V/VIII; otherwise, the tumor was considered a “marginal tumor”. If the tumor spanned across regions I/IV/V/VIII and another region, it was considered a “central tumor”.
Diagnosis of cirrhosis: Liver cirrhosis was diagnosed by histological examination of hepatic tissues by two experienced pathologists. The histopathology diagnostic standard used to diagnose chronic hepatitis was previously described by Desmet et al[13]. G0-4/S1-3 was not considered liver cirrhosis, whereas G0-4/S4 was diagnosed as liver cirrhosis.
HCC differentiation: HCC differentiation was classified according to the World Health Organization Classification of Tumors of the Digestive System[14], with grade 1 being well differentiated, grade 2 moderately differentiated, and grade 3 poorly differentiated. At the center, pathological reports sometimes describe grade 1-2 or grade 2-3 HCC in a single patient. Patients with grades 1, 2 and 1-2 were allocated to the “well-to-moderately differentiated” group, and patients with grades 2-3 and 3 were allocated to the “poorly differentiated” group in this study.
Classification of complications: The Clavien–Dindo classification of surgical complications was adopted[15]. Grade I was regarded as the absence of complications, while Grades II-V were regarded as the presence of complications.
The learning curve of LLR was evaluated using the cumulative sum (CUSUM) method. The CUSUM procedure is a well-established method that detects data changes and monitors surgical performance. In this study, the operation time of each case was ordered chronologically (date of surgery). The formulation of the CUSUM was defined as: Where was an individual operation time, and was the mean of the overall operation time[16]. The learning curve is presented as a broken line graph according to the above formulation.
Patients were followed up 1, 3, 6, 12, 24 and 36 mo postoperatively and assessed by computerized tomography or magnetic resonance imaging, liver function testing, and serum tumor marker measurement. All patients were followed until confirmation of death. If a patient lost contact during follow-up, the survival time was divided into the former interval of follow-up and was included in the censored data.
Measurement data between 2 comparative intervals were evaluated by independent-samples t test. Pearson’s chi-square test and Fisher’s exact test (when expected cell frequencies were less than 5) were used to determine significant differences in categorical parameters. The Kaplan-Meier method was used to perform survival analysis. Multiple regression analysis was used to assess risk factors determining the patient’s disease prognosis. A P value < 0.05 was considered to represent a statistically significant difference.
A total of 1148 consecutive patients who received laparoscopic surgery (except laparoscopic cholecystectomy) between January 2015 and December 2018 were identified. Fifty patients did not meet the inclusion criteria. The remaining 1098 patients underwent LLR, and their demographic data were collected (Table 1).
| Demographic data | Frequencies | Range/percentage |
| Sex | ||
| Male | 655 | 59.65% |
| Female | 443 | 40.35% |
| Age (yr) | 50.28 ± 13.01 | 15-86 |
| Disease | ||
| HCC | 462 | 42.08% |
| ICC | 58 | 5.28% |
| HCC and ICC mixed carcinoma | 5 | 0.46% |
| Metastasis | 92 | 8.38% |
| Hepatolithiasis | 59 | 5.37% |
| Hemangioma | 213 | 19.40% |
| FNH and hyperplastic disease | 88 | 8.01% |
| Parasitic disease | 30 | 2.73% |
| Living donors | 15 | 1.37% |
| Liver abscess | 11 | 1.00% |
| Caroli disease | 5 | 0.46% |
| Hepatic cyst | 6 | 0.55% |
| Trauma | 2 | 0.18% |
| Other benign liver occupancy | 52 | 4.73% |
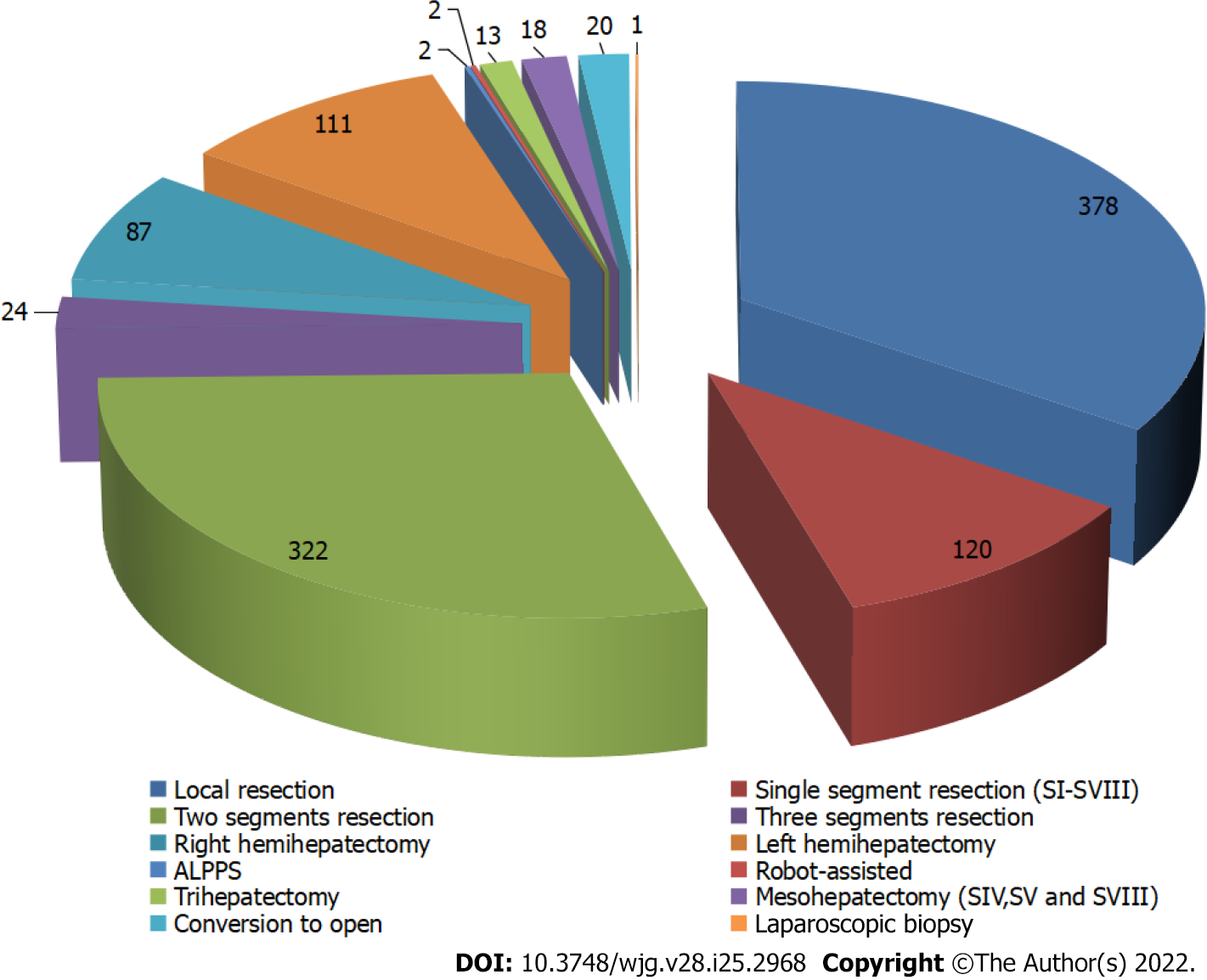
The average operation time was 216.95 ± 98.51 min (range, 31-650 min). The longest operation time was observed in LLR in which resection of a tumor in segments I, IV, V and VIII was combined with resection in the caudate lobe invading the middle hepatic vein[9]. The average blood loss was 242.51 ± 143.23 mL (range, 5-4000 mL). The greatest blood loss, 4000 mL was observed in a patient who had liver paragonimiasis and that underwent conversion to open left hemihepatectomy with a splenectomy. A total of 22 patients (2.00%) underwent blood transfusion. The rate of conversion to open surgery was 1.82% (20/1098). With regard to methods of hepatic inflow occlusion, the IP maneuver was employed in 77.14% of patients (847/1098), and CHVIO was used in 21.58% of patients (237/1098). Local resection, 2 segmental resections and anatomical hepatectomy of a single segment represented the top 3 categories of LLR at the center (Figure 2).
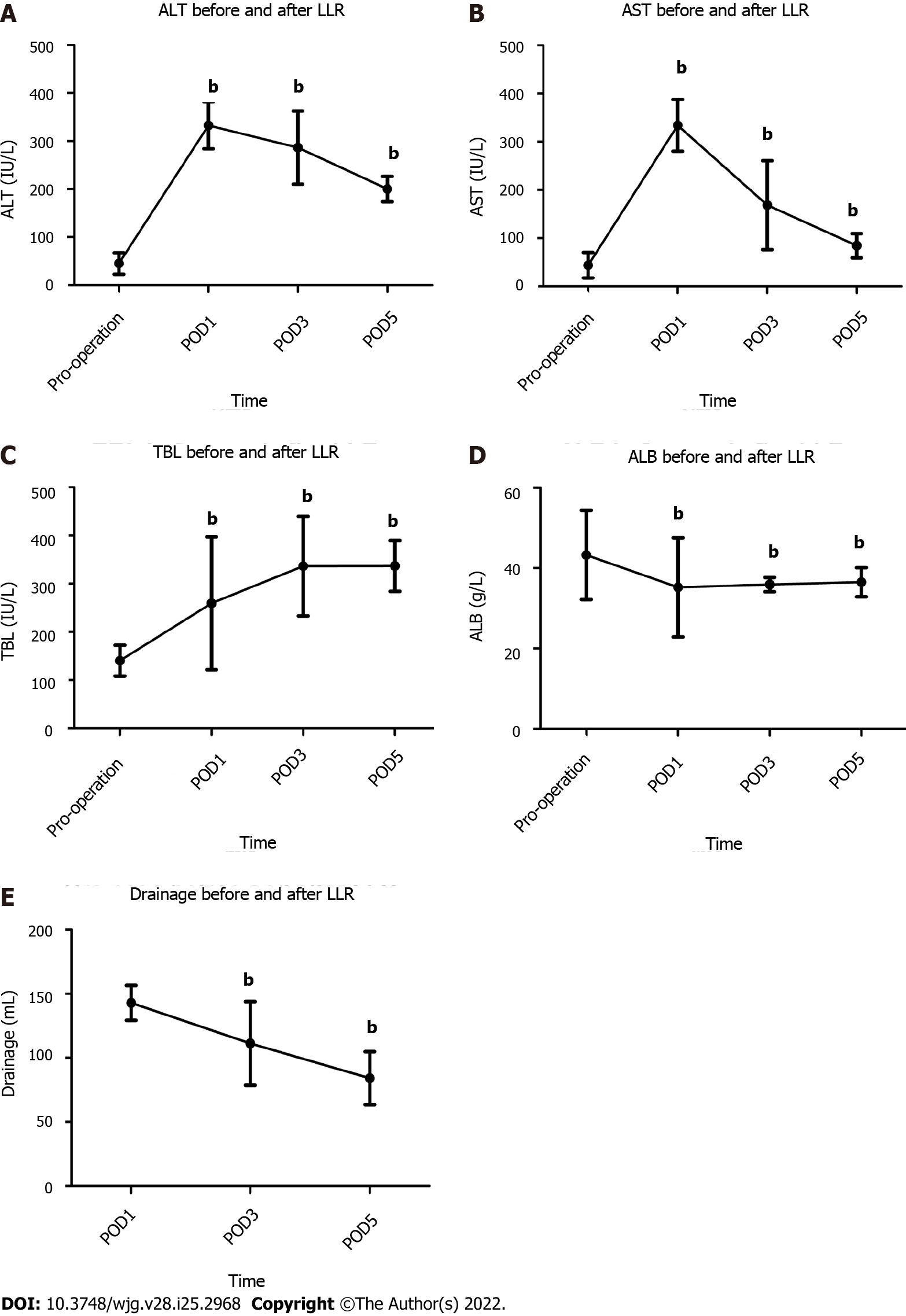
Compared with preoperative data, alanine transaminase and aspartate aminotransferase levels peaked at postoperative day (POD) 1 and then gradually decreased (P < 0.01, Figure 3A and B). However, the average total bilirubin level tended to increase postoperatively and peaked at POD 5 (P < 0.01, Figure 3C). Albumin (ALB) levels and drainage decreased quickly following operation, and ALB levels were maintained at a lower level for a longer period of time (P < 0.01, Figure 3D and E).
Since the first LLR case in 2015, surgical procedures at the center have been continuously improved according to accumulated experiences. For example, the method of hepatic inflow occlusion was changed from CHVIO to IP, as the latter resulted in less blood loss and a clearer view of the operation site[12].
To further examine technique progression, surgical outcomes, including intraoperative data, mortality on POD 90, and surgical complications, were analyzed by year (Table 2). The inpatient time, postoperative inpatient time, operative time, postoperative liver function, mortality, complications, and drainage significantly decreased from year to year. Although some data (hospitalization expenses and blood loss) did not change significantly, the data trended toward decreasing over time. These results imply that surgeons at the center conquered the LLR learning curve, resulting in the LLR technique advancements.
| Index/year | 2015 (n = 88) | 2016 (n = 279) | 2017 (n = 351) | 2018 (n = 380) | F/x2 | P value |
| Age (yr) | 51.97 ± 11.35 | 49.34 ± 12.60 | 49.88 ± 13.05 | 50.95 ± 13.58 | 1.415 | - |
| Sex (M/F) | 55/34 | 159/120 | 203/148 | 239/141 | 3.134 | - |
| LLR types | ||||||
| Local resection | 2.27% (2/88) | 35.13% (98/279) | 39.60% (139/351) | 36.58% (139/380) | 45.306 | < 0.01b |
| Anatomical resection | 23.86% (21/88) | 8.24% (23/279) | 11.40% (40/351) | 9.47% (36/380) | 18.096 | < 0.01b |
| Right hemi-hepatectomy | 14.77% (13/88) | 9.32% (26/279) | 7.41% (26/351) | 5.79% (22/380) | 8.903 | < 0.05a |
| Pre-operative ALT (U/L) | 36.82 ± 28.17 | 36.67 ± 20.05 | 35.71 ± 25.37 | 43.58 ± 33.39 | 1.667 | - |
| Pre-operative TBL (umol/L) | 14.76 ± 5.81 | 13.55 ± 5.83 | 13.51 ± 6.21 | 14.63 ± 7.78 | 2.519 | - |
| Inpatient time (d) | 12.48 ± 6.23 | 11.77 ± 6.67 | 9.53± 5.04 | 8.03 ± 3.20 | 37.583 | < 0.01b |
| Post operative inpatient time (d) | 7.98 ± 5.00 | 6.87 ± 4.82 | 5.39 ± 3.70 | 4.70 ± 2.22 | 29.781 | < 0.01b |
| Hospitalization expense (RMB, Yuan) | 48708.55 ± 20939.76 | 47280.53 ± 17249.26 | 46468.97 ± 35172.25 | 43016.31 ± 14509.30 | 2.448 | - |
| Operation time (min) | 257.98 ± 114.25 | 236.85 ± 100.40 | 203.81 ± 85.83 | 205.61 ± 99.54 | 12.596 | < 0.01b |
| Blood loss (mL) | 275.65 ± 39.19 | 245.53 ± 19.10 | 232.54 ± 12.34 | 241.84 ± 20.32 | 0.413 | - |
| Post operative ALT (U/L) | 355.19 ± 44.44 | 288.22 ± 19.17 | 237.16 ± 10.66 | 251.93 ± 16.03 | 5.262 | < 0.01b |
| Post operative TBL (umol/L) | 36.07 ± 3.49 | 27.86 ± 1.01 | 28.31 ± 0.92 | 24.61 ± 1.02 | 7.443 | < 0.01b |
| Mortality of 90 d | 2.27% (2/88) | 0.36% (1/279) | 0 (0/351) | 0 (0/380) | 14.989 | < 0.01b |
| Complications | 13.64% (12/88) | 13.98% (39/279) | 7.98% (28/351) | 5.79% (22/380) | 15.621 | < 0.01b |
| Drainage (mL) | 133.12 ± 20.44 | 111.88 ± 7.62 | 103.10 ± 6.63 | 82.44 ± 8.27 | 3.433 | < 0.05a |
| Conversion rate | 0 (0/88) | 2.15% (6/279) | 2.56% (9/351) | 1.32% (5/380) | 3.427 | - |
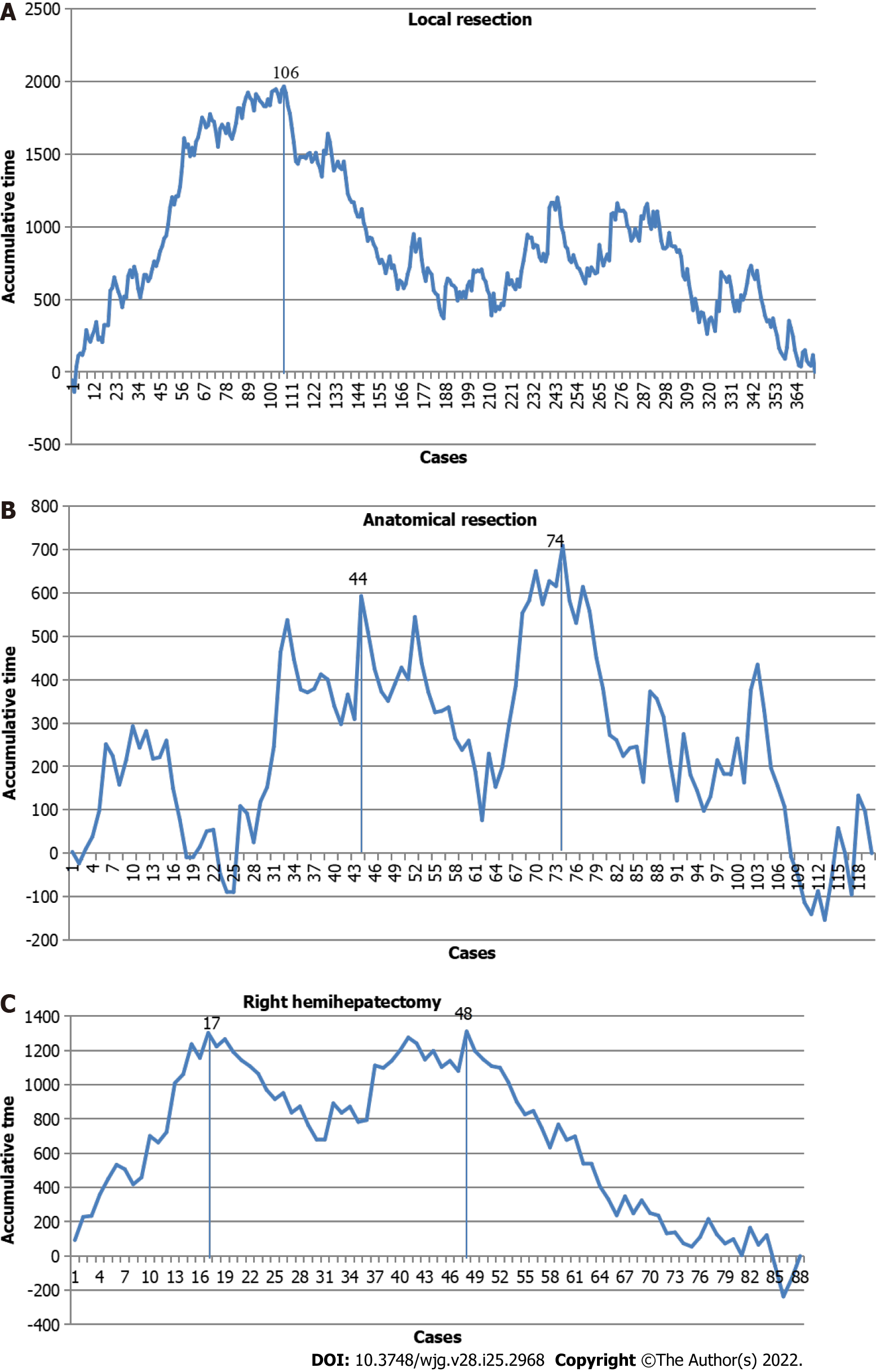
Considering that the surgical difficulty of different types of LLR is distinct, 3 representative LLR techniques were selected for investigation of their learning curves: local resection, anatomical resection, and right hemihepatectomy.
For local resection, there was one peak point observed in the 106th case. Therefore, 2 phases were initially differentiated on the graph: Phase I, cases 1-106, and phase II, cases 107-373 (Figure 4A). For anatomical resection, 2 peak points were observed at the 44th and 74th cases. Therefore, 3 phases were initially differentiated on the graph: Phase I, cases 1-44; phase II, cases 45-74; and phase III, cases 75-120 (Figure 4B). For right hemihepatectomy, there were 2 peak points observed at the 17th and 48th cases. Therefore, 3 phases were initially differentiated on the graph: Phase I, cases 1-17; phase II, cases 18-48; and phase III, cases 49-88 (Figure 4C).
These results indicate that the learning processes of anatomical resection and right hemihepatectomy were more complicated than the learning process of local resection and involved an initial period, increased competence in LLR, and then mastery and the challenging period.
Among the 1098 patients enrolled in the study, 462 patients suffered from HCC, and 5 patients suffered from mixed carcinoma (Table 1). The details of all 467 patients with HCC-related disease are shown in Table 3.
| Frequencies | Range/percentage | |
| Incision margin (cm) | 1.18 ± 1.0 | 0-20 |
| Liver cirrhosis | ||
| Cirrhosis | 201 | 43.04% |
| Non | 266 | 56.96% |
| Tumor location | ||
| Central | 229 | 49.03% |
| Marginal | 238 | 50.97% |
| BCLC stage | ||
| 0 | 81 | 17.34% |
| A1 | 245 | 52.46% |
| A2 | 40 | 8.57% |
| A3 | 22 | 4.71% |
| A4 | 14 | 3.00% |
| B | 48 | 10.27% |
| C | 17 | 3.64% |
| D | 0 | 0 |
| TNM stage | ||
| Ia | 302 | 64.66% |
| Ib | 99 | 21.20% |
| IIa | 43 | 9.21% |
| IIb | 15 | 3.21% |
| IIIa | 7 | 1.50% |
| IIIb | 1 | 0.21% |
| IV | 0 | 0 |
| Liver capsule invasion | ||
| Positive | 156 | 33.40% |
| Differentiation | 311 | 66.60% |
| Well-moderate | 275 | 58.87% |
| Poor | 192 | 41.13% |
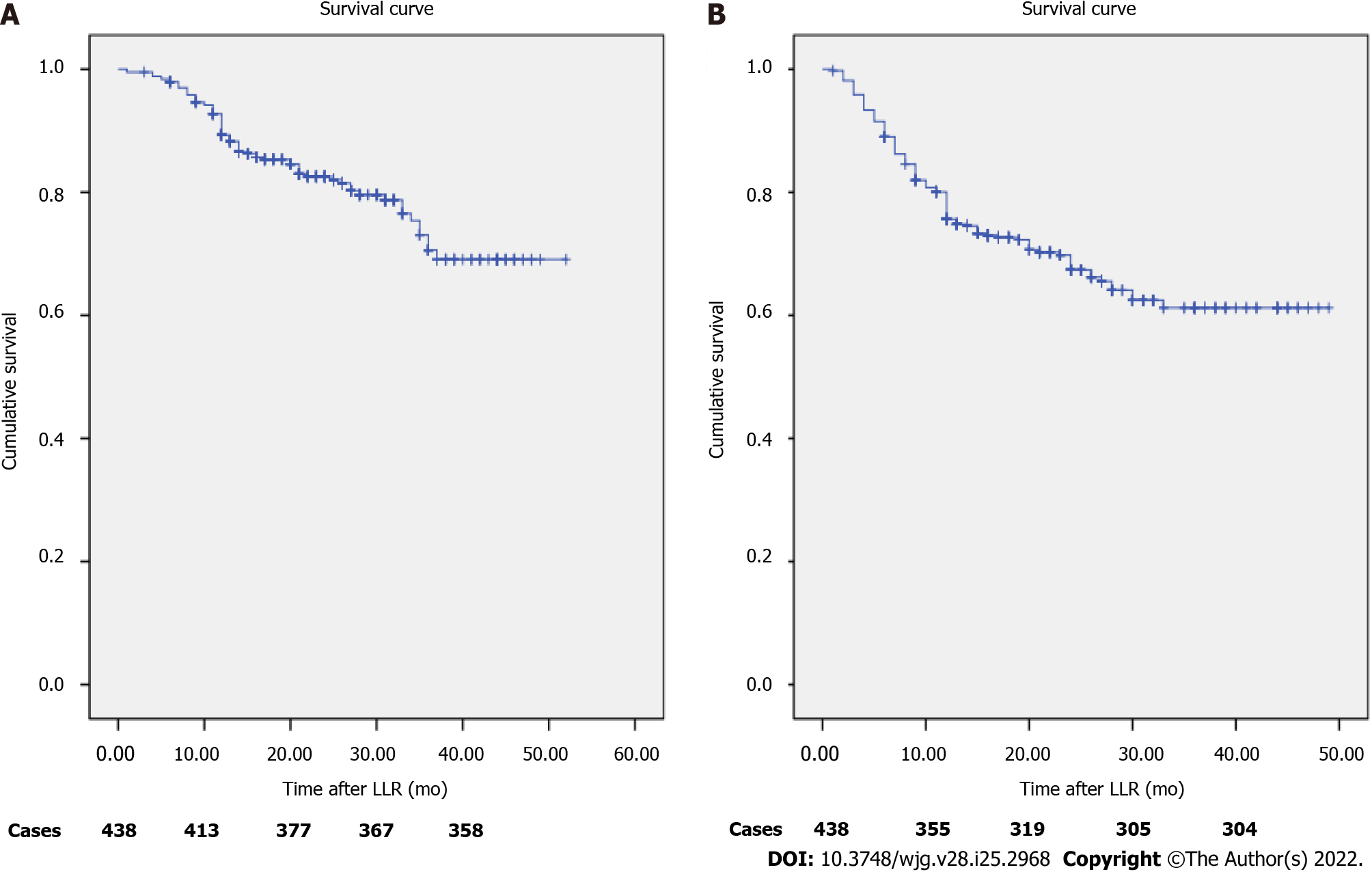
Follow-up data were obtained for 438 patients, and disease-free survival (DFS) and overall survival (OS) were assessed. The 3-year DFS and OS rates were 69.4% and 81.9%, respectively. The 1-year DFS and OS rates were 76.3% and 89.7%, respectively (Figure 5).
The present study shows that LLR is a safe and efficient treatment for a variety of primary, secondary, and recurrent liver tumors and for benign diseases. LLR has several advantages, including minimal damage to the abdominal wall, faster postoperative recovery, and fewer patient complaints[17,18]. Currently, LLR is widely performed in many liver surgery centers. The College of Medicine, Zhejiang University, was one of the first medical centers to perform LLR in China. They reported a 14-year, single-center experience with 365 cases and claimed that LLR has several advantages: lower economic burden, time saving, less blood loss, minimal harm, and improved safety[19]. Researchers also found that surgeons who use LLR must have extensive experience in performing open hepatectomy and that these surgeons will experience a learning curve when performing LLR. The need for a stepwise progression through the learning curve to minimize morbidity and mortality has been highlighted by many centers[20-22]. Furthermore, using laparoscopic surgery for major liver resections and liver resections for lesions adjacent to major vessels has been confirmed to be both feasible and safe[23-25]. Previous researchers have claimed that the use of LLR for major liver resection has similar morbidity and mortality rates as open surgery. Moreover, tumor recurrence rates following LLR for lesions adjacent to major vessels was not increased[24]. Recent studies support the consensus that LLR is feasible, safe, and minimally invasive. Once surgeons progress through the learning curve, this procedure can offer benefits to patients. Halls et al[26] and Ban et al[27] established a difficulty score model to predict intraoperative complications during LLR. Patient characteristics such as neoadjuvant chemotherapy, lesion type and size, classification of resection, and previous open liver resection were associated with a higher risk of surgery-related complications after LLR when compared to surgery-related complications after open liver resection. These findings may provide insight for surgeons when making treatment decisions to obtain better patient outcomes following LLR.
The large Chinese population, especially in Sichuan Province, allowed the center in the present study to accumulate over 1000 LLR cases in a 4-year period. These cases involved all types of LLR, including laparoscopic living donor resection. As reported, the increase in the volume of LLRs performed in 2009-2012 vs 2000-2008 may be partially attributed to the Louisville 2009 Consensus[28]. Therefore, approximately 22 out of the total of 160 cases were necessary to overcome the learning curve[22,29,30]. A decrease in blood loss during LLR was observed after a minimum of performing 50 cases[29]; and at least 25 cases were needed to master laparoscopic living donor resection[31]. At the center, 106 cases were needed to optimize local resection via LLR. However, for complex LLR, 2 peak points were observed in the learning curve. Repetitive training (an initial period, increased competence in LLR, and then mastery and the challenging period) was necessary for surgeons to master hemihepatectomy and anatomical resection via LLR. Furthermore, an increase in the conversion rate in 2016/2017 was noted. This time period corresponded to the second and third years of performing LLR at the center when surgeons were overcoming the second peak of the LLR learning curve and at which time there was a huge increase in the number of LLR cases requiring more complex surgeries. After the LLR technology was mastered, the conversion rate at the center decreased significantly in 2018.
Some studies have claimed that continuous hemi-hepatic vascular inflow occlusion is associated with similar outcomes as IP[32,33]. However, at the center in the present study, the IP method was adopted instead of hemihepatic vascular inflow occlusion once the LLR learning curve was overcome, as the IP method resulted in less blood loss than the hemihepatic vascular inflow occlusion method[12]. Additionally, the intrahepatic Glissonian approach was adopted to replace the extra-Glissonian approach.
The morbidity and mortality rates at the center are similar to those in other centers. One study reviewed 2804 patients who received LLR and found a cumulative mortality rate of 0.3% and a morbidity rate of 10.5%. Liver-specific complications included bile leaks (1.5%), transient liver ascites (1%), and abscesses (2%)[34]. At the center in the present study, in addition to observing these common complications, 4 patients who underwent right anterior lobectomy suffered from right posterior branch injury. One of these 4 patients suffered from liver failure and ultimately died. Due to this complication the LLR technique was improved so that a longer right anterior pedicle is now exposed prior to transection. Similarly, for patients who undergo right hemihepatectomy, the right anterior and posterior pedicle must be transected to prevent left pedicle injury. For patients who undergo left hemihepatectomy, the presence of ischemia in the right lobe following ligation of the left Glissonian sheath must be determined. The cause of death in the other 2 patients was refractory hypernatremia and ascites with liver failure. The most common postoperative complications at the center were pneumonia, hypohepatia, ascites, and bile leakage. Postoperative bleeding requiring a second operation was rare in these cases. Once the LLR learning curve was conquered and the LLR technique advanced and was optimized, postoperative complications significantly decreased from year to year.
In conclusion, LLR may be performed safely for a variety of primary, secondary and recurrent liver tumors and for benign diseases. Once the learning curve is overcome, LLR can offer short-term benefits for patients.
Laparoscopic liver resection (LLR) has become a safe approach but still need to be further summarized.
The present study reviews the 4-year experience of total LLR in a single center, which exceeded 1000 cases.
Summarize the past, in order to obtain better progress in this technology in the future.
Patients who underwent LLR at West China Hospital of Sichuan University between January 2015 and December 2018 were identified. Surgical details in different years, categories of liver disease and prognosis of malignant liver tumors were evaluated. The learning curve for LLR was evaluated using the cumulative sum method. The Kaplan-Meier method was used to perform survival analysis.
Ultimately, 1098 patients were identified. Hepatocellular carcinoma (HCC) was the most common disease that led to the need for LLR in our center (n = 462, 42.08%). The average operation time was 216.94 min ± 98.51 min. The conversion rate was 1.82% (20/1098). The complication rate was 9.20% (from grade II to V). The 1-year and 3-year overall survival rates of HCC patients were 89.7% and 81.9%, respectively. The learning curve was grouped into two phases for local resection (cases 1-106 and 107-373), three phases for anatomical segmentectomy (cases 1-44, 45-74 and 75-120) and three phases for hemi-hepatectomy (cases 1-17, 18-48 and 49-88).
LLR may be considered a first-line surgical intervention for liver resection that can be performed safely for a variety of primary, secondary, and recurrent liver tumors and for benign diseases once technical competence is proficiently attained.
It is a very promising surgical procedure that can give patients a faster recovery time.
We thank Dr. Chen YF (Department of Liver Surgery & Liver Transplantation Center, West China Hospital of Sichuan University) for the assistance of data collection.
| 1. | Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10:758-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 216] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Feldbrügge L, Wabitsch S, Benzing C, Krenzien F, Kästner A, Haber PK, Atanasov G, Andreou A, Öllinger R, Pratschke J, Schmelzle M. Safety and feasibility of laparoscopic liver resection in patients with a history of abdominal surgeries. HPB (Oxford). 2020;22:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Schmelzle M, Krenzien F, Schöning W, Pratschke J. Laparoscopic liver resection: indications, limitations, and economic aspects. Langenbecks Arch Surg. 2020;405:725-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Kaibori M, Ichihara N, Miyata H, Kakeji Y, Nanashima A, Kitagawa Y, Yamaue H, Yamamoto M, Endo I. Surgical outcomes of laparoscopic versus open repeat liver resection for liver cancers: A report from a nationwide surgical database in Japan. J Hepatobiliary Pancreat Sci. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Cai LX, Tong YF, Yu H, Liang X, Liang YL, Cai XJ. Is Laparoscopic Hepatectomy a Safe, Feasible Procedure in Patients with a Previous Upper Abdominal Surgery? Chin Med J (Engl). 2016;129:399-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Monden K, Sadamori H, Hioki M, Ohno S, Takakura N. Short-term outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma in older patients: a propensity score matching analysis. BMC Surg. 2022;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Coelho FF, Kruger JA, Fonseca GM, Araújo RL, Jeismann VB, Perini MV, Lupinacci RM, Cecconello I, Herman P. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg. 2016;8:5-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (2)] |
| 8. | Ho KM, Han HS, Yoon YS, Cho JY, Choi YR, Jang JS, Kwon SU, Kim S, Choi JK. Laparoscopic Total Caudate Lobectomy for Hepatocellular Carcinoma. J Laparoendosc Adv Surg Tech A. 2017;27:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Li H, Wei Y, Li B, Peng B. Laparoscopic hepatectomy for segments I, IV, V and VIII. Surg Endosc. 2017;31:3028-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Li H, Wei Y, Li B, Peng B. The First Case of Total Laparoscopic Living Donor Right Hemihepatectomy in Mainland China and Literature Review. Surg Laparosc Endosc Percutan Tech. 2016;26:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist. 2010;15 Suppl 4:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 12. | Lan X, Li H, Liu F, Li B, Wei Y, Zhang H, Xu H. Does liver cirrhosis have an impact on the results of different hepatic inflow occlusion methods in laparoscopic liver resection? HPB (Oxford). 2019;21:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [PubMed] |
| 14. | Li ZS, Li Q. [The latest 2010 WHO classification of tumors of digestive system]. Zhonghua Bing Li Xue Za Zhi. 2011;40:351-354. [PubMed] |
| 15. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26161] [Article Influence: 1189.1] [Reference Citation Analysis (2)] |
| 16. | Steiner SH, Cook RJ, Farewell VT, Treasure T. Monitoring surgical performance using risk-adjusted cumulative sum charts. Biostatistics. 2000;1:441-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Chen J, Li H, Liu F, Li B, Wei Y. Surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma for various resection extent. Medicine (Baltimore). 2017;96:e6460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Morise Z, Ciria R, Cherqui D, Chen KH, Belli G, Wakabayashi G. Can we expand the indications for laparoscopic liver resection? J Hepatobiliary Pancreat Sci. 2015;22:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Cai X, Li Z, Zhang Y, Yu H, Liang X, Jin R, Luo F. Laparoscopic liver resection and the learning curve: a 14-year, single-center experience. Surg Endosc. 2014;28:1334-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Ibuki S, Hibi T, Tanabe M, Geller DA, Cherqui D, Wakabayashi G; INSTALL-2 Collaborative Study Group. Short-term Outcomes of "Difficult" Laparoscopic Liver Resection at Specialized Centers: Report From INSTALL (International Survey on Technical Aspects of Laparoscopic Liver Resection)-2 on 4478 Patients. Ann Surg. 2022;275:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Sultana A, Nightingale P, Marudanayagam R, Sutcliffe RP. Evaluating the learning curve for laparoscopic liver resection: a comparative study between standard and learning curve CUSUM. HPB (Oxford). 2019;21:1505-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Shanti H, Raman R, Chakravartty S, Belgaumkar AP, Patel AG. Effect of the learning curve on survival after laparoscopic liver resection for colorectal metastases. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Wang ZY, Chen QL, Sun LL, He SP, Luo XF, Huang LS, Huang JH, Xiong CM, Zhong C. Laparoscopic versus open major liver resection for hepatocellular carcinoma: systematic review and meta-analysis of comparative cohort studies. BMC Cancer. 2019;19:1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Abu Hilal M, van der Poel MJ, Samim M, Besselink MG, Flowers D, Stedman B, Pearce NW. Laparoscopic liver resection for lesions adjacent to major vasculature: feasibility, safety and oncological efficiency. J Gastrointest Surg. 2015;19:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Isetani M. How Far Can We Go with Laparoscopic Liver Resection for Hepatocellular Carcinoma? Biomed Res Int. 2015;2015:960752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Halls MC, Berardi G, Cipriani F, Barkhatov L, Lainas P, Harris S, D'Hondt M, Rotellar F, Dagher I, Aldrighetti L, Troisi RI, Edwin B, Abu Hilal M. Development and validation of a difficulty score to predict intraoperative complications during laparoscopic liver resection. Br J Surg. 2018;105:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 27. | Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, Kaneko H, Wakabayashi G. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 431] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 28. | Chen TH, Yang HR, Jeng LB, Hsu SC, Hsu CH, Yeh CC, Yang MD, Chen WT. Laparoscopic Liver Resection: Experience of 436 Cases in One Center. J Gastrointest Surg. 2019;23:1949-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Tomassini F, Scuderi V, Colman R, Vivarelli M, Montalti R, Troisi RI. The single surgeon learning curve of laparoscopic liver resection: A continuous evolving process through stepwise difficulties. Medicine (Baltimore). 2016;95:e5138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Efanov M, Alikhanov R, Tsvirkun V, Kazakov I, Melekhina O, Kim P, Vankovich A, Grendal K, Berelavichus S, Khatkov I. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB (Oxford). 2017;19:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Broering DC, Berardi G, El Sheikh Y, Spagnoli A, Troisi RI. Learning Curve Under Proctorship of Pure Laparoscopic Living Donor Left Lateral Sectionectomy for Pediatric Transplantation. Ann Surg. 2020;271:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Ni JS, Lau WY, Yang Y, Pan ZY, Wang ZG, Liu H, Wu MC, Zhou WP. A prospective randomized controlled trial to compare pringle manoeuvre with hemi-hepatic vascular inflow occlusion in liver resection for hepatocellular carcinoma with cirrhosis. J Gastrointest Surg. 2013;17:1414-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Fu SY, Lau WY, Li GG, Tang QH, Li AJ, Pan ZY, Huang G, Yin L, Wu MC, Lai EC, Zhou WP. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 892] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Labori KJ, Norway; Morise Z, Japan; Rakić M, Croatia; Tsoulfas G, Greece A-Editor: Soldera J, Brazil S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY