Published online Jun 21, 2022. doi: 10.3748/wjg.v28.i23.2625
Peer-review started: December 18, 2021
First decision: January 23, 2022
Revised: January 29, 2022
Accepted: May 5, 2022
Article in press: May 5, 2022
Published online: June 21, 2022
Processing time: 180 Days and 9.4 Hours
Liposarcoma is one of the most common adult mesenchymal tumors but is uncommon in the gastrointestinal tract and extremely rare in the stomach. Furthermore, the histological subtypes of liposarcoma usually reported in the stomach are well-differentiated or myxoid, and few reports have been issued on small-sized gastric liposarcomas resected endoscopically and followed up. Herein, we report a case of primary gastric dedifferentiated liposarcoma (DL) that was resected endoscopically.
A 67-year-old female Korean patient was referred to our institution for further evaluation of a gastric submucosal tumor (SMT) located in the lesser curvature of the gastric body by esophagogastroduodenoscopy. Endoscopic ultrasound revealed a well-circumscribed, slightly heterogeneous, isoechoic, 17 mm × 10 mm sized mass originating from the third sonographic layer. Computed tomography showed no evidence of significant lymph node enlargement or distant metastasis. Endoscopic resection was undertaken using the snare resection technique after mucosal precutting to provide a definitive histopathologic diagnosis, which proved to be consistent with DL, based on its morphology and the immunoexpressions of MDM2 and CDK4. The patient was planned for surgery because the deep resection margin was positive for malignancy. After declining any invasive procedure or adjuvant treatment, the patient was placed under close follow-up, and at one year after endoscopic resection, remained disease free.
This is the first reported case of a small primary gastric DL resected endoscopically and followed up. This report demonstrates that when diagnosis of a SMT is uncertain, the use of invasive techniques, including endoscopic resection, should be considered.
Core Tip: Liposarcoma is uncommon in the gastrointestinal tract and rarely encountered in the stomach. Furthermore, the dedifferentiated histologic subtype has not been previously reported in the stomach in the English literature. We experienced a case of a small (1.7 cm) primary gastric dedifferentiated liposarcoma, which was resected endoscopically. This report cautions that if a diagnosis of submucosal tumor is uncertain, the use of aggressive techniques, including endoscopic resection, should be considered.
- Citation: Cho JH, Byeon JH, Lee SH. Primary gastric dedifferentiated liposarcoma resected endoscopically: A case report. World J Gastroenterol 2022; 28(23): 2625-2632
- URL: https://www.wjgnet.com/1007-9327/full/v28/i23/2625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i23.2625
Liposarcoma is one of the most common adult soft tissue sarcomas and has a peak incidence between the ages 50 and 65 and a prevalence of 15%-20% among all sarcoma patients[1]. There are four histological subtypes of liposarcomas[2]. Atypical lipomatous tumor/well-differentiated liposarcoma (WDL) is the most common subtype followed by dedifferentiated liposarcoma (DL), which frequently occurs in retroperitoneum[3,4]. The myxoid and pleomorphic types usually present in the extremities[5]. Thus, liposarcoma usually arises in deep soft tissues of the proximal extremities, retroperitoneum, or trunk[6], and is encountered in the gastrointestinal tract in only 2% of cases[7].
The majority of liposarcomas of the alimentary tract arise in the esophagus[8], and liposarcomas originating at more distal sites, such as stomach, small intestine, and large intestine, are rare[9]. Less than 40 cases of primary liposarcoma of the stomach have been described in the medical literature, and the most reported histological subtypes of gastric liposarcoma are WDL and myxoid liposarcoma[10]. In fact, no report on DL of the stomach has been published in the English literature, though one case report on DL of the gastroesophageal junction was issued in 2018[11]. Here, we describe the first case of a small primary gastric DL resected endoscopically and provide a review of the literature.
A 67-year-old female Korean patient was referred to our institution for further evaluation and treatment of a gastric submucosal tumor (SMT) incidentally discovered by esophagogastroduodenoscopy (EGD) during a routine medical check-up.
The patient had experienced no abdominal pain or discomfort.
She had a history of breast conserving surgery due to breast cancer 6 years previously.
The patient had diabetes and was being treated with oral hypoglycemic agents. She was a non-smoker and non-alcohol drinker and had no significant family history.
Physical examination was unremarkable, and her abdomen was soft, nontender, and nondistended with no palpable mass.
Laboratory tests, which included common serum tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9), were normal.
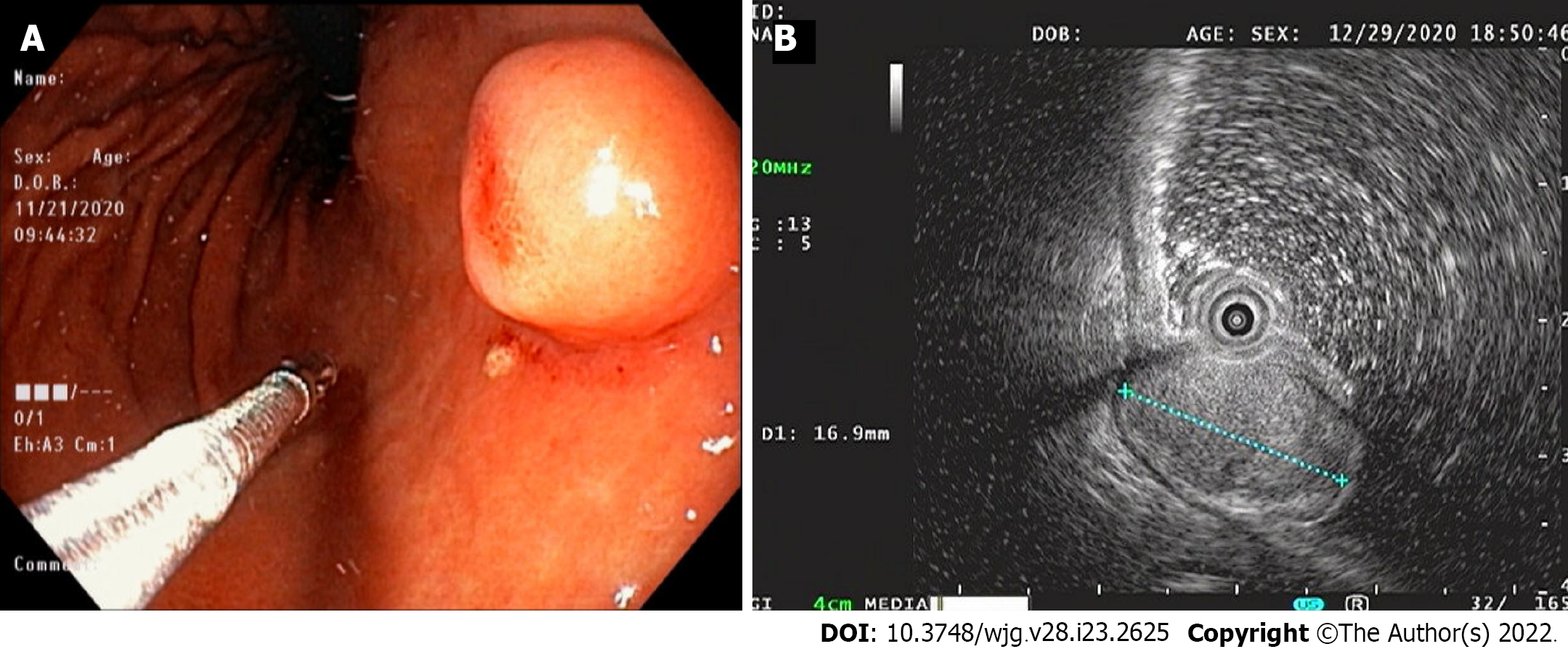
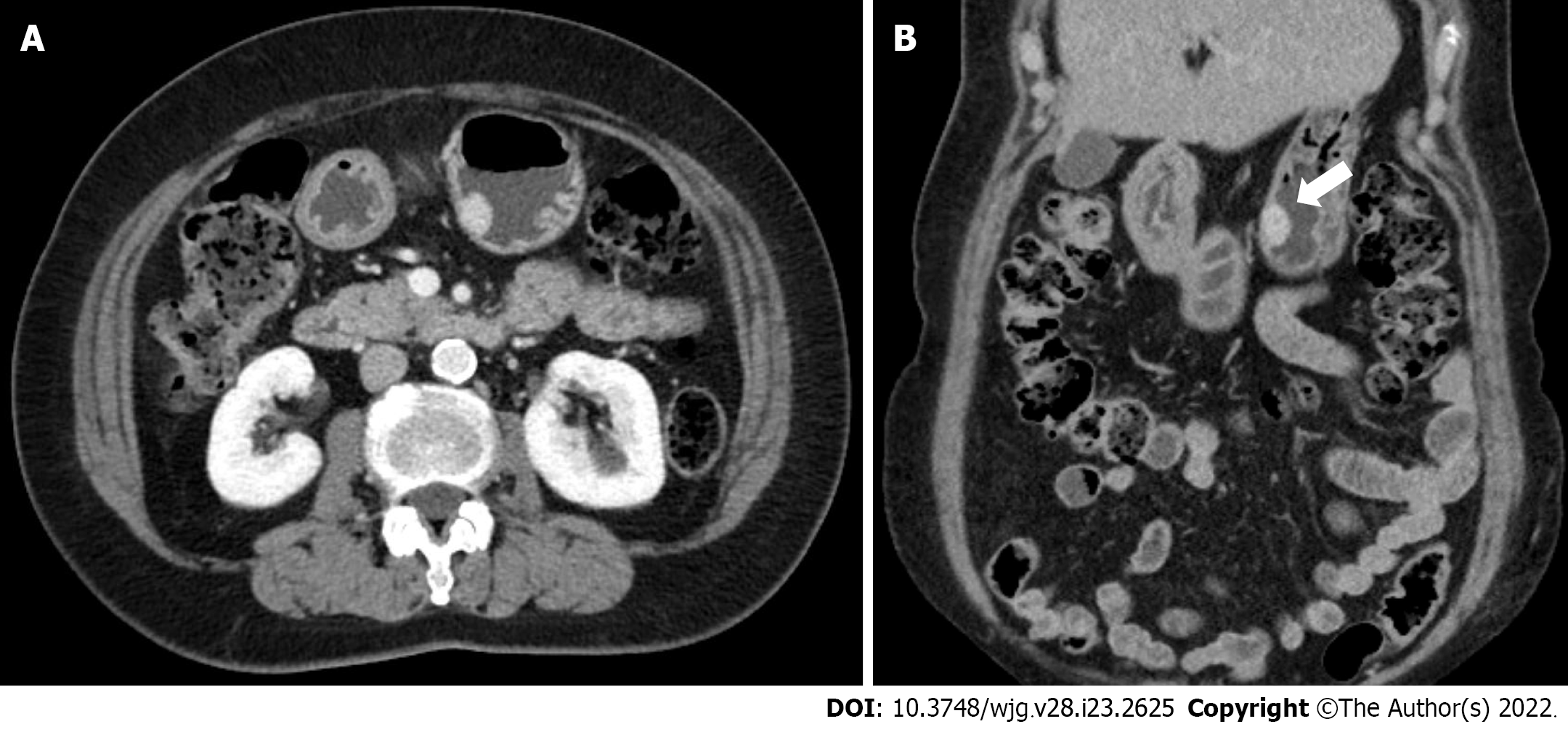
EGD revealed a SMT-like protruding lesion of approximately 15 mm diameter located in the posterior wall of the lesser curvature of the distal part of the gastric body (Figure 1A). The lesion was covered with normally appearing mucosa except for focal mucosal erythema and depression over the lesion. Gastric endoscopic ultrasonography (EUS) examination demonstrated a well-demarcated mass, which measured 17 mm × 10 mm, located in the third layer of the gastric wall. The echo pattern of the mass was slightly heterogeneous and isoechoic (Figure 1B), but its EUS appearance was insufficient for diagnosis. The differential diagnosis included lipoma, neuroendocrine tumor, and ectopic pancreas. Initial biopsy specimens obtained at the site of the focal mucosal erythema and depression were negative for a neoplasm. Computed tomography (CT) of the abdomen showed a well-enhanced, intraluminal protruding polypoid lesion arising from the gastric body but no evidence of lymph node enlargement or distant metastasis (Figure 2).
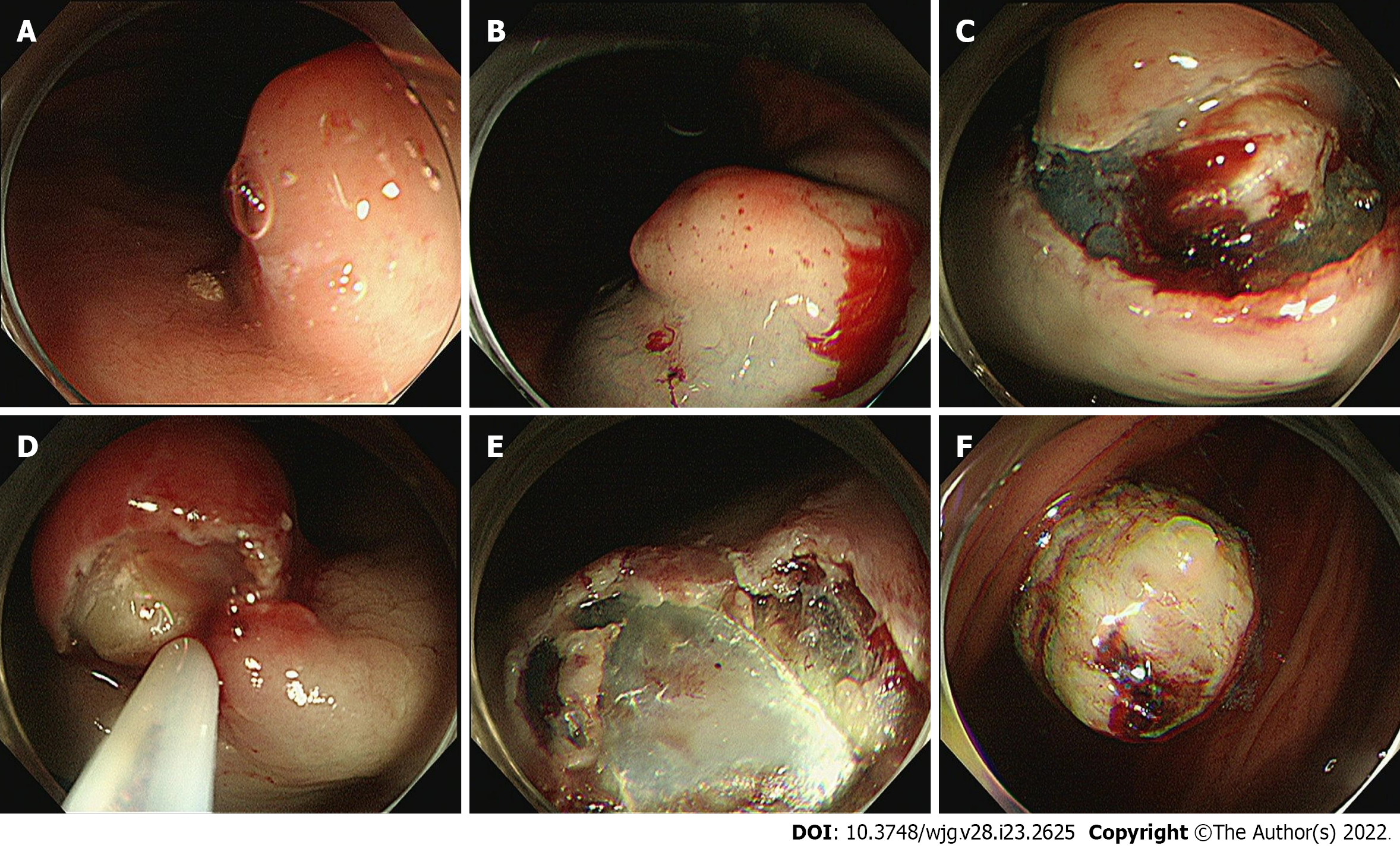
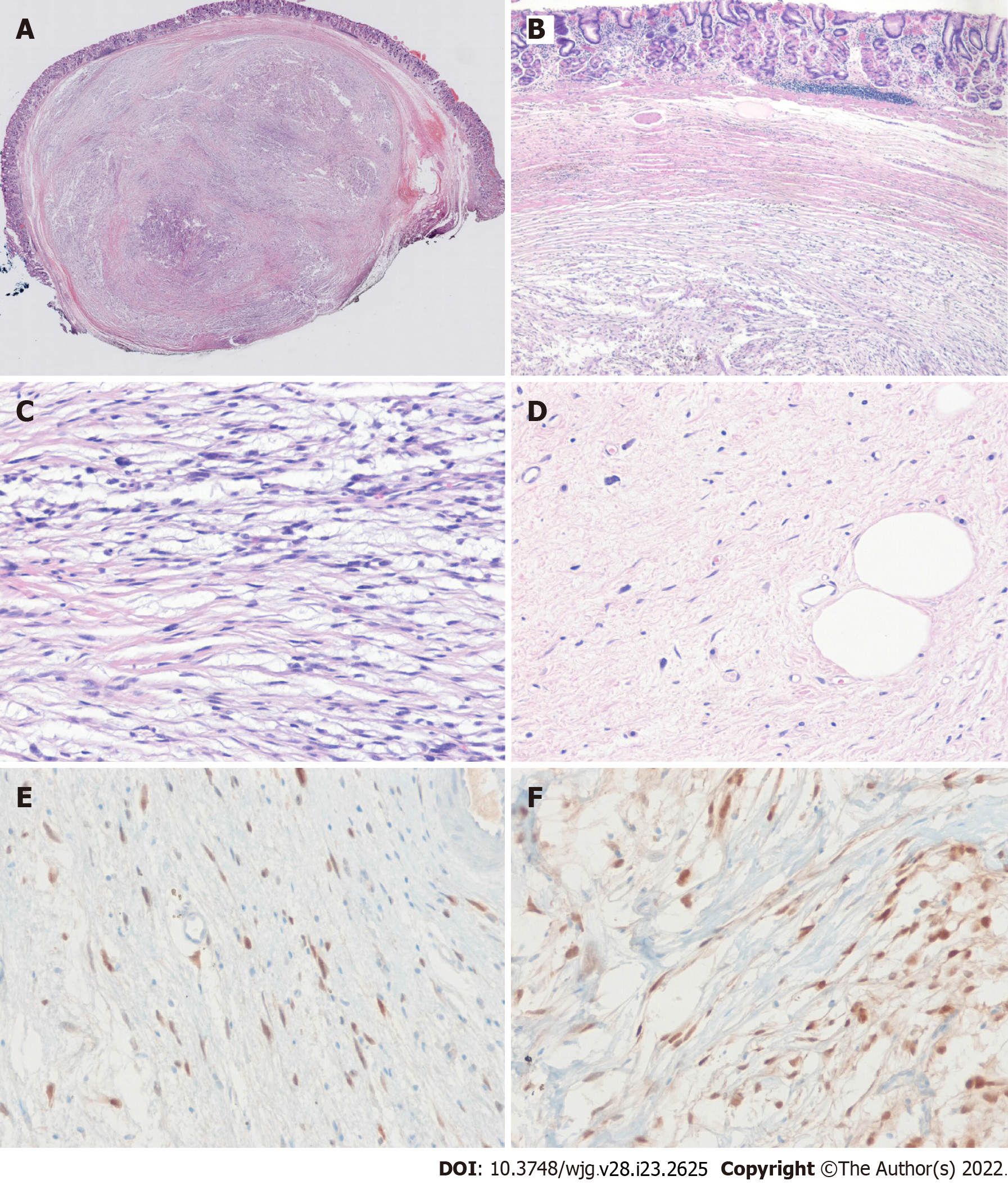
After initial work-up, endoscopic resection was conducted using the snare resection technique after mucosal precutting for a definitive histopathologic result (Figure 3). At gross examination, the tumor was whitish yellow, well-shaped, and solid. Histopathologic examination showed the tumor was located submucosally and primarily composed of spindle-shaped atypical cells with pleomorphic and hyperchromatic nuclei and indistinct pale cytoplasm (Figure 4A-D). Deep resection margins were positive for tumor. Immunohistochemical staining performed for the differential diagnosis of gastrointestinal stromal tumor, schwannoma, leiomyosarcoma, malignant melanoma, and any poorly or undifferentiated sarcoma, revealed tumor cells were negative for CD117, DOG1, CD34, SMA, S-100, Desmin, HMB45, and SOX10, but positive for MDM2 and CDK4 nuclear staining (Figure 4E and F). Based on these findings, the histopathological diagnosis was consistent with DL.
The patient was planned for surgery because the deep resection margin was positive for DL, but declined any invasive procedure or adjuvant treatment.
She remains under close follow-up, which includes biannual CT scanning and EGD, and at one year after endoscopic resection remained disease free.
Although they are the most common mesenchymal neoplasms, liposarcomas are rarely found in the gastrointestinal tract. Since the disease was first described by Abrams et al[12] in 1941, fewer than 40 cases of gastric liposarcoma have been reported in the literature worldwide[10]. The etiology of gastric liposarcoma remains uncertain, though some patients have a family history of soft tissue neoplasms, which suggests the involvement of genetic factors[13]. Gastric liposarcomas originate due to the proliferation of undifferentiated mesenchymal cells within submucosa and the tunica muscularis layer of stomach, and an exophytic growth is typical[10,14]. Most gastric liposarcomas are located in the antrum or lesser curvature.
As is the case for other soft tissue sarcomas, there are no characteristic clinical findings[15], and patients may remain asymptomatic for years. Symptoms depend primarily on tumor location and size and the presence or absence of ulceration. The most common symptoms are a palpable mass, mechanical obstruction, and gastrointestinal bleeding. In general, nonspecific symptoms are the main cause of delayed diagnoses, and thus, most liposarcomas are large at diagnosis. However, our patient underwent regular biennial EGD under a national cancer screening program, and the small SMT was detected early. Furthermore, endoscopic resection performed for a definite diagnosis resulted in the diagnosis of a small, asymptomatic gastric liposarcoma.
The characteristic histological features of liposarcoma are the presence of immature fat cells and lipoblasts[14]. Liposarcomas are classified histologically as WDL, DL, myxoid/round cell liposarcoma, or pleomorphic liposarcoma[2], and considerations of histological subtype are important during the disease course. While DL, round-cell, and pleomorphic liposarcomas are high-grade aggressive tumors with the ability to metastasize, WDL and myxoid liposarcomas are low-grade tumors that progress more slowly[3,5]. Most of the gastric liposarcomas reported have been of the well-differentiated or myxoid histologic subtypes[10], and DL arising in the stomach has not been previously reported in the English literature; although a case of DL of heart and stomach was reported in 2017[16]. Notably, in this report, histopathological examination failed to determine that the primary lesion was located in the stomach. In addition, one case of primary DL of the gastroesophageal junction has been reported[11]. Thus, to the best of our knowledge, this is the first reported case of primary gastric DL.
DL is defined as a combination of WDL and high-grade sarcoma that displays evidence of non-lipogenic differentiation like undifferentiated high-grade pleomorphic sarcoma, fibrosarcoma, or myxofibrosarcoma[17,18]. DL can occur de novo (90% of cases) or as recurrence from a preexisting WDL (10% of cases)[19]. If DL develops from the transformation of a preexisting WDL into non-lipogenic sarcoma, dedifferentiation develops in 20% of the first and 44% of the second local recurrences and has been shown to be associated with poor progression and metastasis[5]. The histologic diagnosis of DL is generally based on the identification of WDL areas, which were scarce in our patient. In these cases, the differential diagnosis of any poorly-differentiated or undifferentiated sarcoma is important, and MDM2 and CDK4 immunohistochemical staining or FISH testing for amplification of the MDM2 and CDK4 genes is diagnostically helpful[20]. In our patient, DL was confirmed by CDK4 and MDM2 immunostaining.
DL often exhibits abdominal cavity involvement usually of retroperitoneum, and an intraperitoneal origin is extremely rare[3,21,22]. When DL occurs inside the abdominal cavity it presents as a space-occupying mass lesion. The pathognomonic findings of DL in CT and magnetic resonance images are a heterogeneous, non-lipogenic, encapsulated mass[23], which are sufficient for diagnosis and obviate the need for needle biopsy. In our patient, because DL arising from the gastric wall presented as a small gastric SMT, CT was not diagnostically useful. Although EUS is considered the most useful modality in terms of defining the presumptive nature of SMT, our case demonstrates the difficulty of differentiating benign and malignant SMT by EUS alone, particularly when a lesion is small. Lesion echogenicity is also worth mentioning. Unlike the hyperechogenic EUS feature of other pathologic types[10,24,25], our case showed isoechoic EUS features, which appeared to be related to a sparsity of immature lipocytes or lipoblasts as the WDL area was extremely limited in our patient. DL seems to have a better prognosis than other high-grade sarcomas, especially in terms of its metastatic potential. Yet, careful long-term follow-up is essential because approximately 40% of DLs recur locally, 17% metastasize, and 28% of patients eventually die of the disease[1]. Surgery remains the treatment of choice for DL, and it is crucial that the tumor be completely removed[4], though the efficacies of targeted chemotherapy and radiotherapy are being investigated[26].
Liposarcoma is uncommon in the gastrointestinal tract and rare in the stomach, and primary DL in the stomach is extremely rare but should be considered in the differential diagnosis of any poorly-differentiated or undifferentiated sarcoma. The reasons why this case report is valuable are: (1) It presents the first reported case of a small primary gastric DL, resected endoscopically, and followed up; and (2) it cautions that if a diagnosis of SMT is uncertain after initial examination by EGD, EUS, and/or CT, the use of aggressive techniques, including endoscopic resection, should be considered.
| 1. | Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia: Elsevier, 2014. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Fletcher CDM, Bridge JA, Hogendoom PCW. World Health Organization classification of tumours. In: Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2013: 10-238. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 3. | Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358-70; discussion 370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 468] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Choi YY, Kim YJ, Jin SY. Primary liposarcoma of the ascending colon: a rare case of mixed type presenting as hemoperitoneum combined with other type of retroperitoneal liposarcoma. BMC Cancer. 2010;10:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 6. | Lahat G, Lazar A, Lev D. Sarcoma epidemiology and etiology: potential environmental and genetic factors. Surg Clin North Am. 2008;88:451-481, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Enzinger FM, Weiss SW. Liposarcoma. In: Soft Tissue Tumors. St Louis: Mosby Year Book, 1995: 431-466. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Graham RP, Yasir S, Fritchie KJ, Reid MD, Greipp PT, Folpe AL. Polypoid fibroadipose tumors of the esophagus: 'giant fibrovascular polyp' or liposarcoma? Mod Pathol. 2018;31:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Gajzer DC, Fletcher CD, Agaimy A, Brcic I, Khanlari M, Rosenberg AE. Primary gastrointestinal liposarcoma—a clinico-pathological study of 8 cases of a rare entity. Human Pathol. 2020;97:80-93. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Kang WZ, Xue LY, Wang GQ, Ma FH, Feng XL, Guo L, Li Y, Li WK, Tian YT. Liposarcoma of the stomach: Report of two cases and review of the literature. World J Gastroenterol. 2018;24:2776-2784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Aşkan G, Bağci P, Hameed M, Baştürk O. Dedifferentiated Liposarcoma of the Gastroesophageal Junction. Turk Patoloji Derg. 2018;34:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Abrams MJ, Truberville JS. Liposarcoma of the stomach. South Surg. 1941;10:891-896. |
| 13. | Matone J, Okazaki S, Maccapani GN, Amancio TT, Filippi RZ, Macedo AL. Giant gastric lipossarcoma: case report and review of the literature. Einstein (Sao Paulo). 2016;14:557-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Tepetes K, Christodoulidis G, Spyridakis ME, Nakou M, Koukoulis G, Hatzitheofilou K. Liposarcoma of the stomach: a rare case report. World J Gastroenterol. 2007;13:4154-4155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | López-Negrete L, Luyando L, Sala J, López C, Menéndez de Llano R, Gomez JL. Liposarcoma of the stomach. Abdom Imaging. 1997;22:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Hisata Y, Tasaki Y, Kozaki S, Yamada T. A case of dedifferentiated liposarcoma of the heart and stomach. Int J Surg Case Rep. 2017;41:36-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 378] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Dei Tos AP. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol. 2000;4:252-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol. 1997;21:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 441] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 20. | Binh MB, Sastre-Garau X, Guillou L, de Pinieux G, Terrier P, Lagacé R, Aurias A, Hostein I, Coindre JM. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: a comparative analysis of 559 soft tissue neoplasms with genetic data. Am J Surg Pathol. 2005;29:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 433] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | D'Annibale M, Cosimelli M, Covello R, Stasi E. Liposarcoma of the colon presenting as an endoluminal mass. World J Surg Oncol. 2009;7:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Goel AK, Sinha S, Kumar A, Karak AK, Chattopadhyay TK. Liposarcoma of the mesocolon--case report of a rare lesion. Surg Today. 1994;24:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25:1371-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Yamamoto K, Teramae N, Uehira H, Wakabayashi N, Fukuda S, Kodama T, Kashina K, Tsuchihashi Y. Primary liposarcoma of the stomach resected endoscopically. Endoscopy. 1995;27:711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Cure HW, Gómez D, Pedraza M, Bulicie HC, Cabrera LF, Gil LPG, Acevedo D, Cabrera L, Moreno V, Mendoza A. Laparoscopic management of gastric liposarcoma: A case report and review of the literature. Int J Surg Case Rep. 2020;73:268-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 26. | Radaelli S, Stacchiotti S, Casali PG, Gronchi A. Emerging therapies for adult soft tissue sarcoma. Expert Rev Anticancer Ther. 2014;14:689-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang X, United States; Zhao JW, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL