Published online May 28, 2022. doi: 10.3748/wjg.v28.i20.2227
Peer-review started: January 3, 2022
First decision: January 27, 2022
Revised: February 14, 2022
Accepted: April 27, 2022
Article in press: April 27, 2022
Published online: May 28, 2022
Processing time: 143 Days and 18.7 Hours
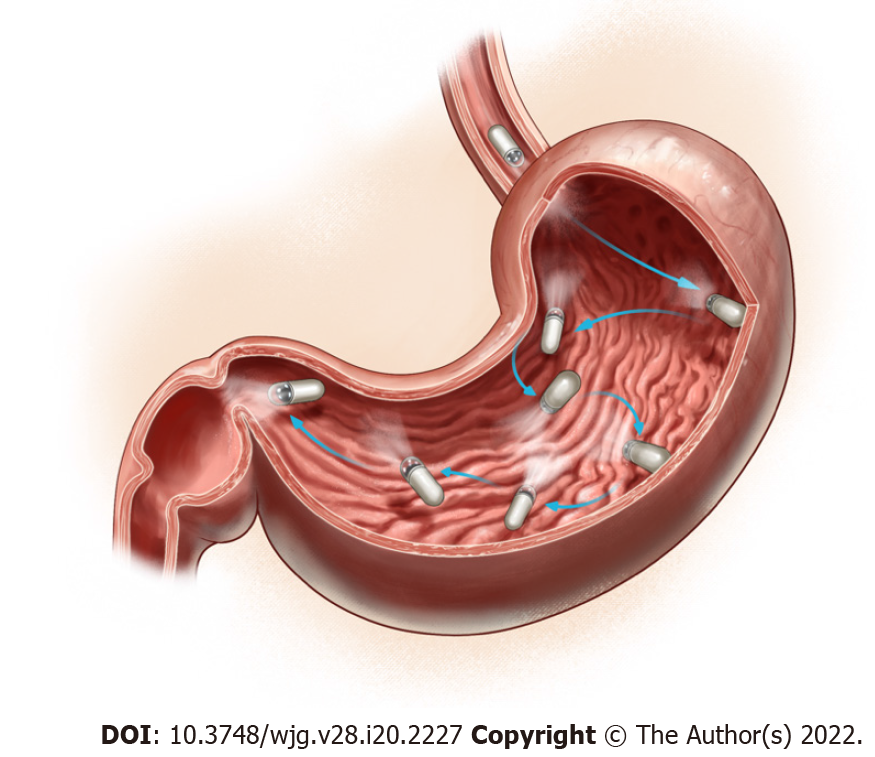
While capsule endoscopy (CE) is the gold standard diagnostic method of detecting small bowel (SB) diseases and disorders, a novel magnetically controlled capsule endoscopy (MCCE) system provides non-invasive evaluation of the gastric mucosal surface, which can be performed without sedation or discomfort. During standard SBCE, passive movement of the CE may cause areas of the complex anatomy of the gastric mucosa to remain unexplored, whereas the precision of MCCE capsule movements inside the stomach promises better visualization of the entire mucosa.
To evaluate the Ankon MCCE system’s feasibility, safety, and diagnostic yield in patients with gastric or SB disorders.
Of outpatients who were referred for SBCE, 284 (male/female: 149/135) were prospectively enrolled and evaluated by MCCE. The stomach was examined in the supine, left, and right lateral decubitus positions without sedation. Next, all patients underwent a complete SBCE study protocol. The gastric mucosa was explored with the Ankon MCCE system with active magnetic control of the capsule endoscope in the stomach, applying three standardized pre-programmed computerized algorithms in combination with manual control of the magnetic movements.
The urea breath test revealed Helicobacter pylori positivity in 32.7% of patients. The mean gastric and SB transit times with MCCE were 0 h 47 min 40 s and 3 h 46 min 22 s, respectively. The average total time of upper gastrointestinal MCCE examination was 5 h 48 min 35 s. Active magnetic movement of the Ankon capsule through the pylorus was successful in 41.9% of patients. Overall diagnostic yield for detecting abnormalities in the stomach and SB was 81.9% (68.6% minor; 13.3% major pathologies); 25.8% of abnormalities were in the SB; 74.2% were in the stomach. The diagnostic yield for stomach/SB was 55.9%/12.7% for minor and 4.9%/8.4% for major pathologies.
MCCE is a feasible, safe diagnostic method for evaluating gastric mucosal lesions and is a promising non-invasive screening tool to decrease morbidity and mortality in upper gastro-intestinal diseases.
Core Tip: We evaluate the feasibility, safety, and diagnostic yield of magnetically controlled capsule endoscopy (CE) system developed by Ankon and AnX robotics that provides non-invasive evaluation of the gastric mucosal surface, which can be performed without sedation or patient discomfort. During gold-standard small bowel CE, passive movement may cause areas of the complex gastric mucosa to remain unexplored, whereas the precision of magnetically controlled capsule endoscopy (MCCE) capsule movements inside the gastrointestinal tract achieves better visualization of the entire mucosa, including the stomach. The Ankon MCCE system is a promising, non-invasive screening tool that achieved ~90% diagnostic yield of mucosal abnormalities in this study.
- Citation: Szalai M, Helle K, Lovász BD, Finta Á, Rosztóczy A, Oczella L, Madácsy L. First prospective European study for the feasibility and safety of magnetically controlled capsule endoscopy in gastric mucosal abnormalities. World J Gastroenterol 2022; 28(20): 2227-2242
- URL: https://www.wjgnet.com/1007-9327/full/v28/i20/2227.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i20.2227
Capsule endoscopy (CE) is a non-invasive, painless, patient-friendly, and safe diagnostic method. It is currently the gold standard examination for small bowel (SB) diseases, including inflammatory bowel disease, suspected polyposis syndromes, unexplained abdominal pain, celiac disease, and obscure gastrointestinal bleeding (OGIB). Small bowel capsule endoscopy (SBCE) provides excellent diagnostic yield, which has been proven in many clinical trials since its introduction in the late 1990s. Currently, it has a fundamental role in gastrointestinal (GI) endoscopy, especially in patients with suspected SB diseases[1]. Attempts to expand the diagnostic role of CE to areas that are traditionally explored with standard endoscopic procedures (gastroscopy and colonoscopy), e.g., the esophagus, stomach, and colon. These aims have been challenged by significant drawbacks in the past due to a lack of optimal preparation, standardized procedural design, and ability to visualize the entire mucosal surface[2]. A common perception is that the anatomical structures of the stomach and colon are too complex for adequate visualization with a passive capsule endoscope[3-5].
Although the SB seems to be a simple pipe, the more complex gastric anatomy with passive movements may cause unexplored areas of the gastric mucosa when utilizing standard CE. Magnetically controlled capsule endoscopy (MCCE) systems were developed over the past ten years to precisely control capsule movements inside the GI tract and to achieve better visualization of the entire mucosa, especially in the stomach. Different approaches have been developed and studied over time, which have enabled manual steering or active movement of the capsule systems. The external magnetic field seems to be the most adequate and energetically effective method for steering and holding the capsule within the stomach[6,7].
According to recent, prospective studies that were mainly conducted in China, a methodology developed by Ankon and AnX robotics could be an optimal method for non-invasive screening and early diagnosis of gastric diseases such as gastric cancer and intramucosal high-grade dysplasia. It has the potential to become a valuable and accepted screening method in a population with high gastric cancer risk[8].
The aim of this study was to evaluate the feasibility, safety, and diagnostic yield of the Ankon MCCE system in patients with gastric and SB disorders who were referred to our endoscopy unit for SBCE examination between September 2017 and December 2020. Our secondary aim was to evaluate the overall gastric examinations, SB transit times (TTs) and efficacy of MCCE to achieve complete gastric and SBCE investigation by utilizing the same upper GI capsule.
Our present study prospectively enrolled outpatients who were seen at the Endo-Kapszula Endoscopy Unit, Székesfehérvár, Hungary, between September 2017 and December 2020. A combined investigation of the stomach and SB of patients referred to for SBCE was performed with a robotically MCCE system (NaviCam, Ankon Technologies Co, Ltd, Shanghai, China).
The primary endpoint of the recent study was to investigate the diagnostic yield of MCCE in the evaluation of gastric and SB abnormalities. The secondary endpoints were to evaluate the feasibility of performing complete gastric and SB investigation by utilizing the same diagnostic procedure; to address safety parameters, including adverse and severe adverse events, and to calculate the capsule TTs, such as esophageal, gastric, and SB TTs, and the overall procedure time.
All patients who were referred to our endoscopy unit for SBCE and who agreed to our gastric study protocol were included in the current study. During the study period, we enrolled 284 consecutive, eligible patients, of which there were 149 males (52.5%) and 135 females (47.5 %), with a mean age of 44 years. Detailed patient characteristics are shown in Table 1. The indications of MCCE were iron deficiency anemia, OGIB, suspected/established SB Crohn’s disease, suspected/confirmed celiac disease, suspected SB neoplastic disease, carcinoid syndrome, and SB polyposis syndromes.
| All cases | Male | Female | |
| Patient characteristics | |||
| n | 284 | 149 | 135 |
| Age (mean ± SD) | 44.0 ± 13.3 | 44.0 ± 13.3 | 44.0 ± 13.3 |
| BMI | 26.5 | 27.1 | 25.5 |
| Indications | |||
| OGiV | 61 (21.5%) | 30 (20.1%) | 31 (22.9%) |
| Celiac disease | 80 (28.2%) | 40 (26.8%) | 40 (29.7%) |
| Crohn’s or susp. Crohn’s | 47 (16.5%) | 31 (20.9%) | 16 (11.9%) |
| Unexplained abdominal pain | 92 (32.4%) | 47 (31.5%) | 45 (33.3%) |
| Susp. SB tumor | 4 (1.4%) | 1 (0.7%) | 3 (2.2%) |
| 13C urea breath test | |||
| No. of performed tests | 110(38.7%) | 56 (50.1%) | 54 (49.1%) |
| Positive | 36 (32.7%) | 16 (44.4%) | 20 (55.6%) |
| Negative | 74 (67.3%) | 40 (54%) | 34 (46%) |
This study was approved by the Ethical Committee of the University of Szeged (Registry No. 5/17.04.26) and registered in the ClinicalTrials.gov trial registry (Identifier: NCT03234725). The present study was conducted according to the World Medical Association's Declaration of Helsinki provisions in 1995. Patients agreed to undergo capsule endoscopies and Helicobacter pylori (H. pylori) urea breath tests (UBTs) by written informed consent.
According to the SBCE guidelines, we applied the following patient preparation and study protocol: Patients followed a liquid diet and consumed 2 L of water with two sacks of polyethylene glycol (PEG) the day before the study. First, H. pylori C13 UBT was performed on the morning of the study, while the patient was in a fasting condition. The UBT requires an average of 30 min, during which time the patient should not consume any fluids or food. During that visit, the patient's medical history was recorded, and a physical examination was performed. After the UBT, the patient ingested 2 dL water with simethicone suspension (80 mg) to reduce mucus in the stomach. Then, to distend the stomach properly, approximately 8-10 dL of clean water consumed by all patients within 10 min. Water ingestion may be repeated as needed to enhance gastric distension during examination. After complete visualization of the total gastric mucosal surface, active pyloric propulsion of the capsule was attempted in all patients with the external magnetic field. If it was unsuccessful, 10 mg intravenous metoclopramide was applied 60 min after the capsule was swallowed.
Contraindications of MCCE were the same as of those of SBCE and magnetic resonance imaging (MRI) examination. In our study, absolute contraindications were the patients with previous abdominal surgery and/or a previous capsule retention; with implanted MRI-incompatible electronic devices (e.g., implantable cardioverter-defibrillator and pacemakers), or magnetic, metal foreign bodies; and who were not competent or refused the informed consent form; who were under age 18 or above age 70; and those who were pregnant. A patency capsule test was initially performed in all patients to determine those with relative contraindications, including known or suspected GI obstruction due to SB Crohn’s disease, neoplasia, or SB surgery.
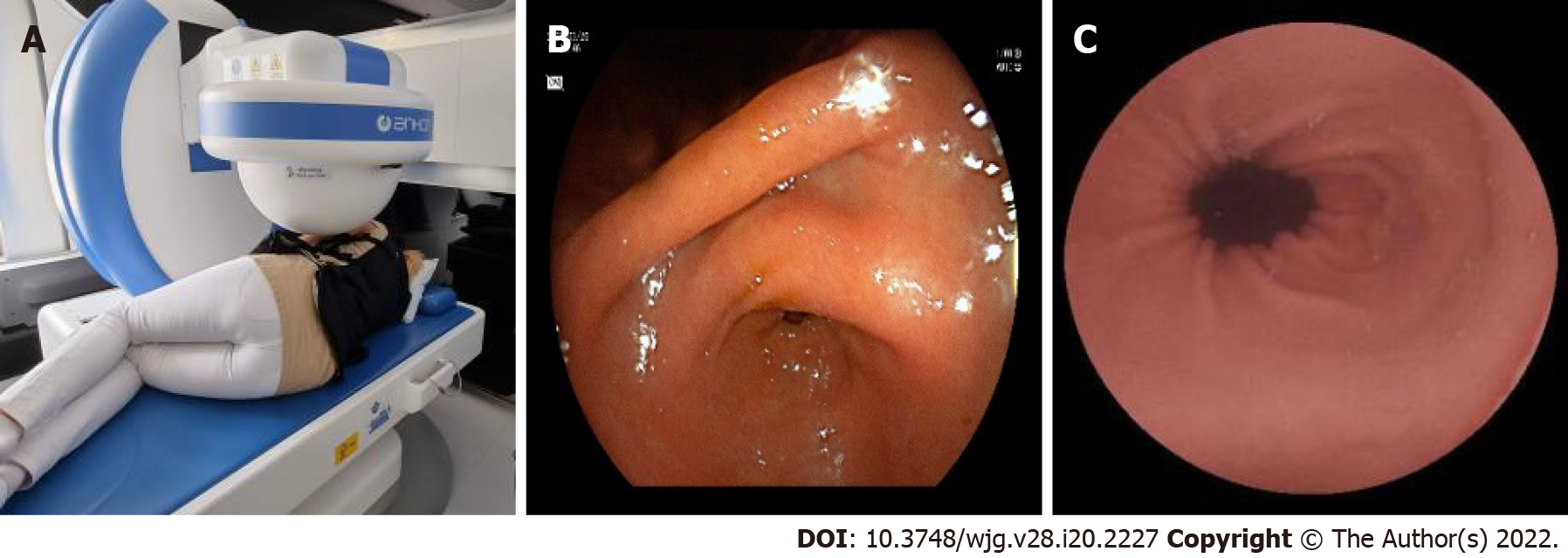
Our system involves a special static magnet with robotic and manual guidance, computer workstation with ESNavi software (Ankon Technologies Co, Ltd) (Figure 1), magnetic capsule endoscope, capsule locator, and data recorder. The capsule endoscope sizes 26.8 × 11.6 mm, weighs 4.8 g, and has a permanent spherical magnet inside (Figure 2). The operator can adjust the frequency of captured pictures from 0.5 to 6 frames per second (fps). Capsule functioning can be stopped temporarily and restarted by the operator remotely from the workstation. The picture resolution is 480 × 480 pixels, and the field of view is 140°. The illumination can be automatically adjusted by an automatic picture focusing mechanism, which enables the view depth to shift from 0 mm to 60 mm. Depending on the fps, the battery life can be over 10 h, which allows combined gastric and SB capsule investigations with the same capsule.
The magnetic robotic C-arm generates an adjustable magnetic field outside of the body with a maximum strength of 0.2 T, which allows precise controlled movements in three spatial directions. During the process, the physician guides the magnetic capsule by two joysticks in any chosen, spatial direction or along its axis, and therefore, he can rotate or tilt it. Moreover, the capsule can advance forward 360° by a rotational automatism in the direction of the capsule visual axis, which is helpful for transposition of the capsule from the long axis of the stomach.
The capsule locator is a unique device that activates the capsule by infrared light prior to the patient swallowing it. The presence of the capsule can be detected in the case of suspected capsule retention (Figure 3).
The system is capable of real-time digital transmission of images to the operating system. At the same time, it is continuously obtaining information about the gyroscope, which is built into the capsule [three-dimensional (3D) motion detector]. It also receives data from the actual spatial location of the camera and localizes the capsule inside the body at any time. The data recorder (Figure 4) may receive all motion information and high-quality pictures via wireless transmission from the capsule endoscope. In contrast, the connection between the data recorder and the workstation via a USB wire makes visible the real-time pictures and gyroscope information.
Magnetic CE can be transferred along five different axes with the controlling magnet: two rotational and three 3D spaces. To achieve this, there are two joysticks on the workstation desktop: the left one controls the capsule in two rotational axes (horizontal and vertical directions) and the right joystick directs it in the three translational axes (forward/backwards, up/down, left/right). Precise magnetic control is achieved by positioning the examining table, modifying the position of the spherical magnet axis along the 3D space, and dynamically adjusting the strength and direction of the vectorial magnetic fields perpendicular to each other. The above-mentioned robotic systems can also automatically run the magnetic field vector and position it by robotically controlled computer-based software (scripts) in default mode, which can achieve a standardized mucosal scanning during the MCCE procedure without the direct intervention of a physician.
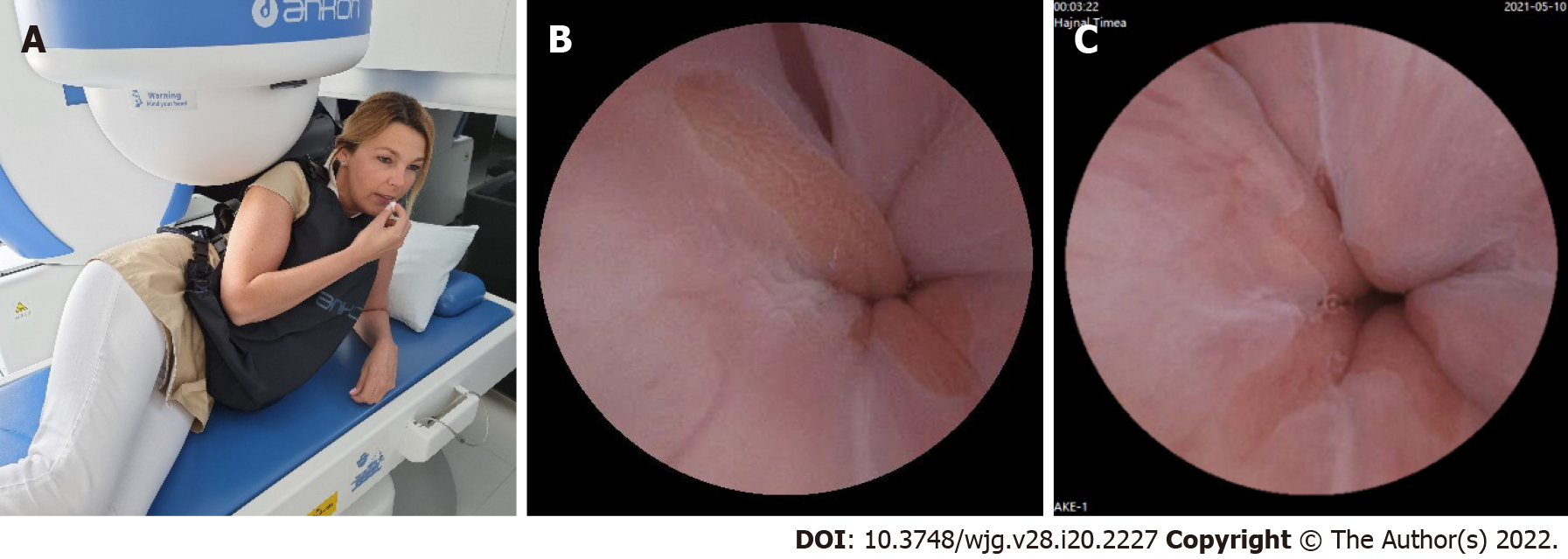
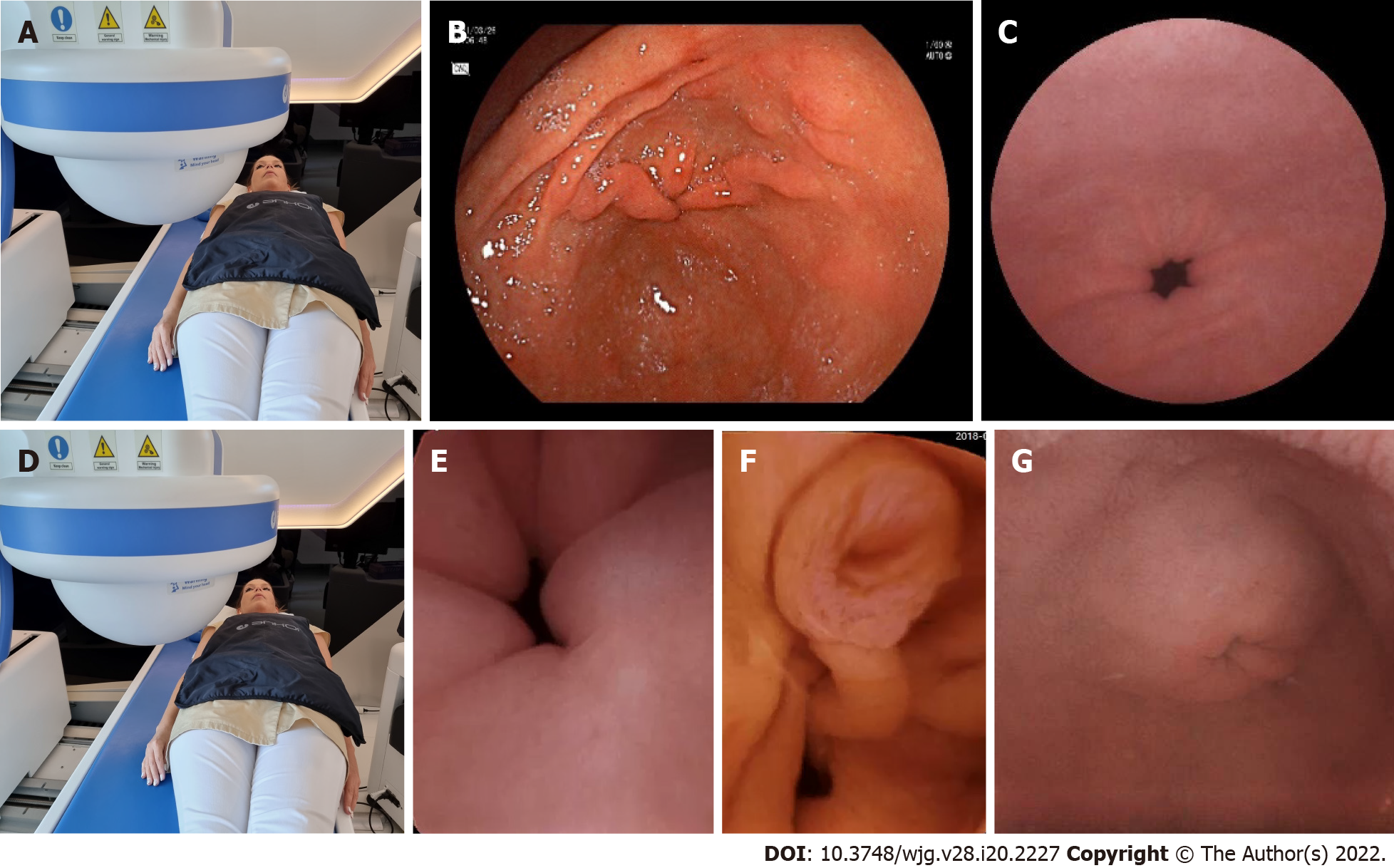
To achieve optimal gastric mucosal visualization and standardization of the MCCE protocol in the stomach, we defined eight different stations with different patient positions to visualize the entire inner gastric surface as during upper GI endoscopy. Changing the patient position from the left lateral decubitus to the supine and right lateral position is necessary to combine gravity and magnetic force, which improve capsule maneuvering (Figure 5). The capsule swallowed by the patients in the left lateral decubitus position (Figure 6).
Stations at the left lateral decubitus position: Station I. Visualization of the gastric fundus and subcar-dial region with the cardia (posterior J type retroflexion).
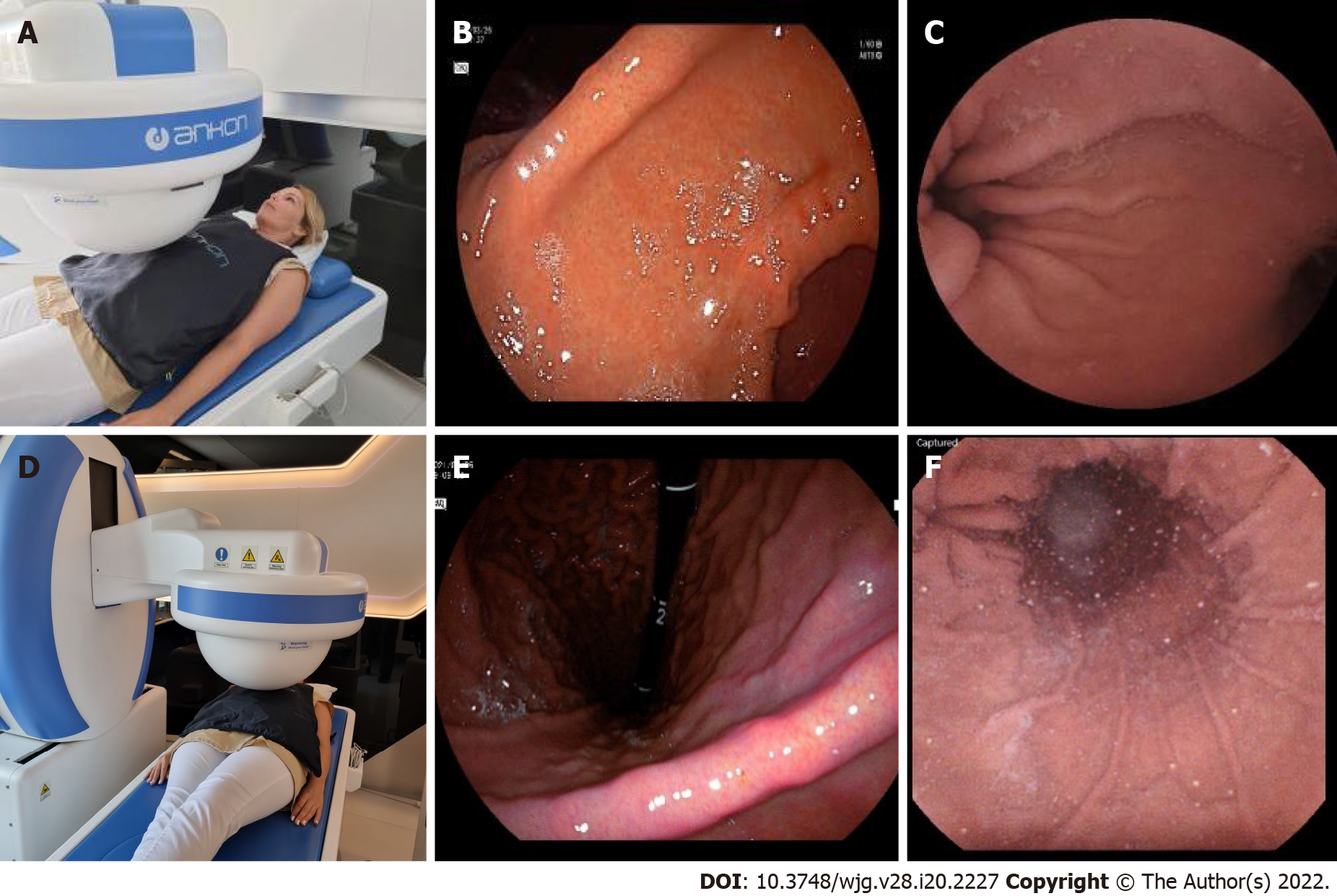
After entering the stomach, the capsule was lowered into the large curvature at the body of the stomach. The magnetic ball was held high up at the level of the patient's right shoulder. The capsule camera was maintained in an obliquely upward orientation of 45° and then horizontally rotated to observe the gastric fundus and the cardia (Figure 7A-C).
Station II. Moving closer to the cardia and the fundus (anterior J type retroflexion).
While using the right joystick, the ball magnet was lowered and fitted closely to the patient's right arm. Due to the proximity of the magnetic field, the capsule rose to the anterior wall and small curvature of the stomach. The capsule was then rotated with the camera oriented vertically upward to observe the cardia up close, with the fundus at a distance (Figure 7D-F).
Station III. Visualization of the gastric body from the fundus.
With the ball magnet in the same position, using the left joystick, the capsule camera was rotated and tilted downwards, enabling visualization of the proximal body of the stomach (corpus) and the gastric folds at the large curvature with a longitudinal view (Figure 7G-I).
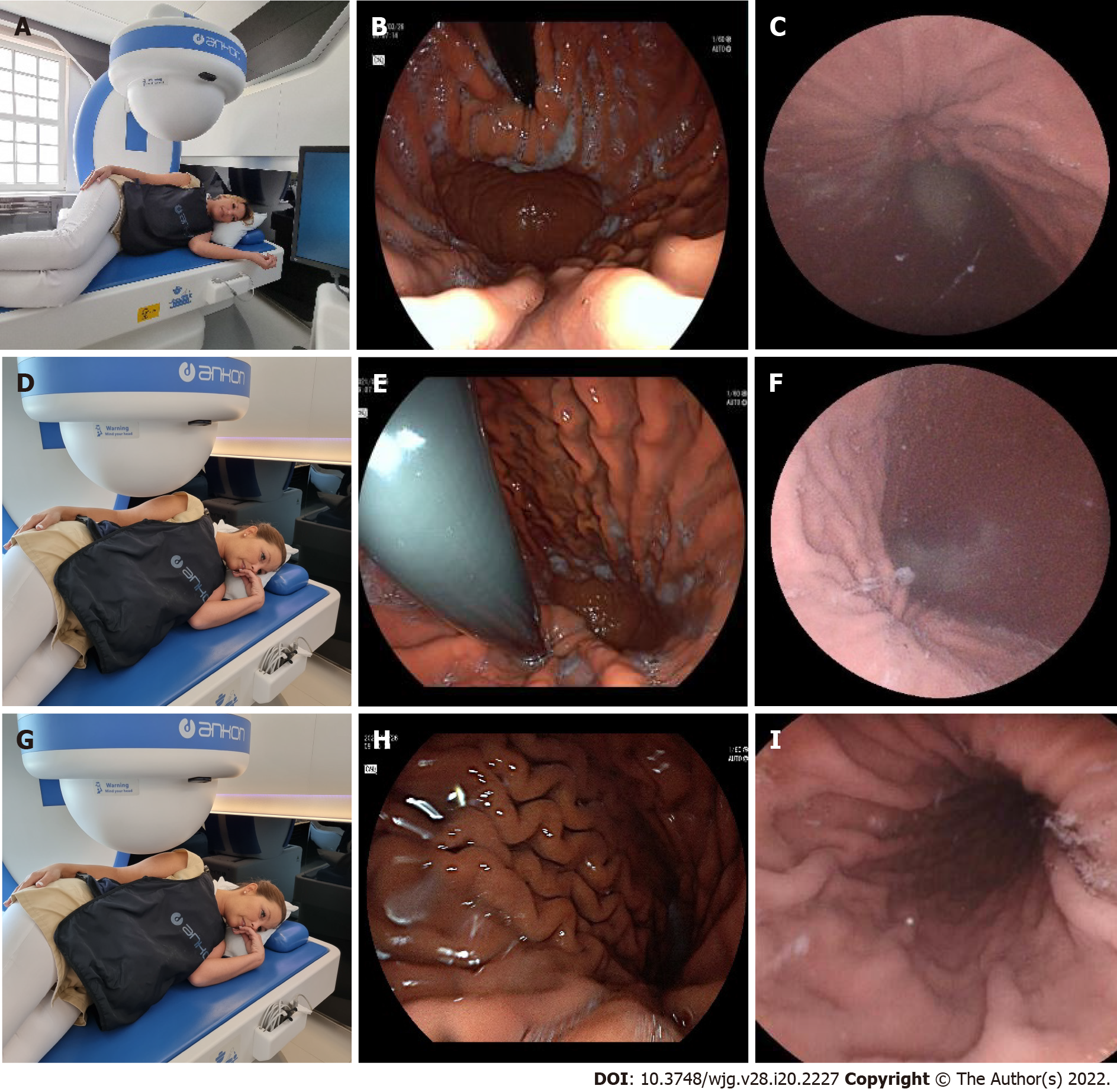
Stations in the supine position: Station IV. Visualization of the angular incisure.
Patients were asked to lie on their back, in a supine position. The capsule was moved and located to the distal body into the angular region due to a change of gravity and stepwise magnetic movements. The magnetic ball was moved to the left upper abdomen (hypochondrium) and then lowered, close to the patient abdomen. In this case, the capsule was raised to the anterior wall of the stomach; therefore, the small curvature and the angular incisure were examined as well (Figure 8A-C).
Station V. Visualization of the larger curvature of the distal body from the angular incisure (U type of retroversion).
The magnetic ball was moved to the epigastric area, close to the abdomen. Then the capsule camera was oriented upward to observe the anterior wall of the gastric body. In this position, the capsule can be turned and rotated (e.g., towards the cardia), enabling visualization of the distal body of the stomach longitudinally, as in the endoscopic view of U-type retroversion (Figure 8D-F).
Stations at the right lateral decubitus patient position: Station VI. Visualization of the antral canal.
In the next phase, patients were asked to turn to the right lateral decubitus position. Due to the gravity force, the capsule sank and moved spontaneously into the antral region. Then, the ball magnet was positioned over the left kidney. The capsule was brought closer to the large curvature with the camera oriented obliquely downward at 45°, which enabled observation of the antrum. Then, the antral canal could be examined with the pylorus, and the angular incisure became visible from the antrum (Figure 9A-C).
Stations in the supine position: Station VII. Prepyloric view and visualization of the pylorus.
After that, each patients were asked to lie on his or her back in the supine position. The ball magnet was positioned close to the body on the right upper abdomen (hypochondrium). The capsule camera was oriented horizontally and laterally toward the pylorus for closer observation. The magnet position ensured that the capsule remained in the antrum. Using both the right and left joysticks, we moved the capsule closer to the pylorus (Figure 10A-C).
Station VIII: Magnetically controlled transpyloric passage of the capsule.
The magnetic ball was placed at the patient's right side at the level of the duodenal bulb. The capsule was then rotated until the camera faced the pylorus. The capsule was dragged close to the pylorus under the guidance of the magnet robot. As the pylorus was opened, peristalsis assisted the capsule into the duodenum. After transpyloric passage, first we depicted the duodenal bulb, then from the descending and inferior horizontal part of the duodenum, we visualized the ampulla of the Vater by tilting the capsule camera upwards to facilitate the retrograde view (Figure 10D-G).
If the capsule entered via the small intestine, patients were asked to drink 1 L of PEG solution, and from then on, they were requested to drink clear fluids (i.e., water). The movement of the capsule in the small intestine was then monitored hourly in real-time visualization mode. The examination ended when the capsule arrives at the colon or stops functioning due to the low battery.
An UBT test revealed H. pylori positivity in 32.7% of cases. (Table 2). No significant association with HP status and the type (proximal or distal), distribution (diffuse or focal) and severity (minimal or active, erosive) of the gastritis described on MCCE results were depicted.
| n | H. pylori positive | % | H. pylori negative | % | χ2 | P value | ||
| Normal | 30 | 7 | 23% | 23 | 77% | 0.9775 | 0.3228 | NS |
| Minor proximalgastritis | 19 | 9 | 47% | 10 | 53% | 1.529 | 0.2163 | NS |
| Minor antralgastritis | 19 | 4 | 21% | 15 | 79% | 1.0322 | 0.3096 | NS |
| Active, erosive antralgastritis | 15 | 6 | 40% | 9 | 60% | 0.3129 | 0.5759 | NS |
| Proximal erosivegastritis | 22 | 7 | 32% | 15 | 68% | 0.0069 | 0.9338 | NS |
| Pangastritis (proximal and antral) | 4 | 3 | 75% | 1 | 25% | 0.5 | 0.4795 | NS |
| Total HP tested patients | 110 | 36 | 33% | 74 | 67% | - | - | - |
The mean gastric, SB, and colon TT with MCCE was: 47 h 40 min (M/F: 44 h 15 min/51 h 14 min), 3 h 46 min 22 s (3 h 52 min 44 s/3 h 38 min 21 s) and 1 h 4 min 34 s (1 h 1 min 16 s/1 h 8 min 53 s), respectively. Average total time of MCCE examination: 5 h 48 min 35 s (5 h 46 min 37 s/5 h 50 min 18 s) (Table 3).
| Transit time | All cases | SD | Male | Female |
| Stomach | 0 h 47 min 40 s | 0 h 43 min 29 s | 0 h 44 min 15 s | 0 h 51 min 14 s |
| Small bowel | 3 h 46 min 22 s | 2 h 1 min 24 s | 3 h 52 min 46 s | 3 h 38 min 21 s |
| Total | 5 h 48 min 35 s | 1 h 50 min 49 s | 5 h 46 min 37 s | 5 h 50 min 18 s |
The diagnostic yields for detecting any abnormalities in the stomach and SB with MCCE were 81.9%: 68.6% for minor pathologies and 13.3% for major pathologies. 25.8% of the abnormalities were found in the SB, and 74.2% were in the stomach. The diagnostic yield for stomach/SB was 4.9%/8.4% for major pathologies and 55.9%/12.7% for minor pathologies (Table 4). In the stomach, ulcers and polyps were considered major, while signs of gastritis were minor pathologies. In the SB signs of Crohn’s and celiac disease were the major and non-specific inflammation, diverticula and angiodysplasia the minor pathologies. Based on findings from MCCE examinations, the distribution of pathologies is depicted in Table 5.
| Diagnostic yield | Major | Minor | Total |
| Total | 38 (13.3%) | 195 (68.6%) | 233 (81.9%) |
| Gastric | 14 (4.9%) | 159 (55.9%) | 173 (60.8%) |
| SB | 24 (8.4%) | 36 (12.7%) | 60 (21.1%) |
| Polyp ventriculi | Ulcus ventriculi | Coeliakia | Crohn | Gastritis | Small intestinal diverticula | Angiodysplasia | Aspecific infalmmation in small bowel | |
| Major pathologies | 5 | 9 | 1 | 23 | 159 | 1 | 26 | 9 |
The capsule's active magnetic movement through the pylorus was successful using the magnet in 41.9% of all patients (automatized protocol in 56 patients and active manually controlled magnetic activity in 63 patients) (Table 6).
| Cases | Mean total transit time | Mean gastric transit time | Mean SB transit time | Transpyloric transit | |||
| With magnet by automatic protocol | With magnet manually | Without magnet | |||||
| Total study population | 284 | 5 h 48 min 35 s | 0 h 47 min 40 s | 3 h 46 min 22 s | 56 (19.7%) | 63 (22.2%) | 165 (58.1%) |
| Incomplete studies | 18 (6.3%) | 7 h 13 min 41 s | 0 h 52 min 35 s | 6 h 19 min 51 s | 2 | 5 | 11 |
| The capsule depleted (> 5 h) | 10 (3.5%) | 9 h 12 min 9 s | 0 h 46 min 5 s | 8 h 26 min 4 s | 0 | 3 | 7 |
| The capsule depleted (< 5 h) | 3 (1%) | 2 h 23 min 25 s | 0 h 24 min 9 s | 1 h 51 min 22 s | 1 | 0 | 2 |
| The patient requested to terminate | 3 (1%) | 4 h 45 min 9 s | 0 h 50 min 3 s | 3 h 55 min 6 s | 1 | 1 | 1 |
| The capsule stopped because of a disease | 2 (0.7%) | 8 h 19 min 36 s | 2 h 11 min 33 s | 6 h 8 min 33 s | 0 | 1 | 1 |
In 18 (M/F: 6/12) patients (6.3%), SB visualization with MCCE was incomplete. There were 13 occurrences of incomplete examinations as a result of capsule depletion. In 3 of these 13 cases, the capsule was depleted within 5 h of operation, indicating a manufacturing flaw. The average total study time in the remaining 10 cases was 9 h 12 min 9 s. From the pylorus to the last image, the average transit time was 8 h 26 min 4 s. The examination was discontinued sooner than planned in 3 cases due to the patient's request. In 2 cases, there was capsule retention due to Crohn's-like ulceration, accompanied by a narrow bowel lumen (Table 6). These problems were resolved spontaneously without surgery, but with medical treatment. There were no serious adverse events or capsule retention during the study period.
There are two main directions in developing capsule endoscopes with active movements: internally and externally guided techniques. Internal guidance means that the locomotive system is integrated into the capsule, but these systems require longer-lasting batteries for full functionality. Its main advantage is a more precise navigation with the help of the internalized locomotive system. External guidance refers to outwardly conducted capsule movements, e.g., with a magnetic control unit or device/moving arm generating the magnetic field. The exceptional advantage of this method is that the magnetic field (and its adjustment) built by the external unit controls the locomotive mechanism, thus making this an energy-saving solution. However, a disadvantage of this technique is that it is limited to more passive, less flexible locomotion. The examining physician controls the capsule outside the body with an external control unit, which decreases the accuracy of the spatial and temporal movements. Locomotion of the capsule endoscope would result in numerous long-term advantages in the diagnostics and therapy of GI diseases (focused biopsies, treatment options, e.g., laser tumor ablation, local targeted release of certain medications, and endoscopic ultrasound applications)[1].
Magnetic assisted capsule endoscopy (MACE) systems have been developed over the past 5-10 years, primarily for research goals. Experiences drawn from previously conducted in vitro studies showed that locomotion and precision of the capsule are significantly influenced by the physical characteristics of the generated magnetic field created by the external magnetic unit while travelling in the human body[1].
In 2011, Olympus was the first to introduce a modified MRI machine prototype that moved a MACE system which allowed the operator to successfully guide the capsule in a chosen spatial direction inside the stomach after drinking water. However, the adoption of this diagnostic procedure did not spread worldwide and get medical acceptance[6].
In 2014, IntroMedic developed a navigation (NAVI) magnetic capsule system that could be moved externally with a small, hammer-shaped, external static magnet device. The technology involving a magnet that assisted the physician in manually moving the capsule endoscope appeared viable and valuable in many experimental settings. However, it did not achieve any breakthroughs, as it only allowed sudden and harsh position changes of the capsule. In clinical trials studying the application of the NAVI capsule system using a small cohort, 65%-86% of the mucosal layer of the stomach was visualized accurately using external magnetically controlled locomotion after the ingestion of water. Its diagnostic accuracy was similar to that of traditional gastroscopy[9]. Using a similar type of NAVI capsule system, CE was performed on the large intestines, where magnetic locomotion directed the capsule from the cecum to the sigmoid colon. At the same time, a colonoscopy probe was inserted to monitor capsule movements to provide dilatation[10]. In iron deficiency anemia, after routine colonoscopy of 49 patients, the performance of a MACE system was compared with the IntroMedic Navi system in providing a diagnostic examination of the stomach and small intestine in one sitting. According to the results, MACE detected more pathologies than by gastroscopy alone, and no significant difference in diagnostic sensitivity was found between the methods when examining the upper GI tract[11].
Precise locomotion of the magnetic capsule inside the GI tract by manual control is not possible due to the variable density of tissues and variable distance of the capsule, e.g., in the stomach from the external magnet. Moreover, exact spatial location of the capsule, its relation to the surrounding organs, or the ante-/retrograde orientation cannot be judged accurately. Therefore, a control mechanism is needed alongside the magnetic capsule, which is based on external robotics that enable the physician's input to be executed (i.e., joystick: in forward, backward, upward, downward, and sideways directions). By pre-programming instruction sequences (script) in the computing control unit to examine the stomach from the fundus to the antrum, we created a reproducible examination of the complete inner mucosal lining of the stomach, which lowered the variability among investigations. If the examining physician notices significant pathology, he/she can intervene and move the examination to manual control and revisit the alteration; doing so increases the number of images taken of the lesion and optimizes the diagnostic accuracy of the study.
NaviCam was the first magnetically controlled capsule system that enables bidirectional data transmission and robotic control. The capsule functions at 1-6 fps and its spatial orientation with continuous monitoring of energy levels are transmitted via the recording vest to control the data and screen of the computing unit. At the console, we can control the precise locomotion of the capsule, image capturing speed, and brightness, as well as turn it on or off.
We combined manual and automatic, robotic capsule control to optimize the gastric procedure. As we previously published in an in vitro setting, complete 100% visualization of the inner surface of a plastic stomach model with the medium and large stomach autoscan program module and with the freehand controlling method could be successfully achieved in all cases. With the small and medium stomach mode, we could observe only 97.5% of the inner surface, because of the incomplete visualization of the prepyloric region. With freehand method, we needed nearly twice as much average time (749 s) to make a complete examination, compared to the robotic maneuvering with autoscan program (390 s). In an everyday praxis, in each patient position, the following stations and capsule positioning were performed after running the 3 different autoscan programs[12,13].
CE is a potentially patient-friendly and noncontact/infection-free diagnostic modality of screening for gastric diseases, which may result in complete and excellent examinations of the stomach. During the coronavirus disease 2019 pandemic, direct contact with the patients in the practice is a potential risk factor for infections. Non-contact medicine is a possibility for further examinations, such as remote control of CE[14]. The prevalence of focal gastric lesions (polyps, gastric ulcers, submucosal tumors, etc.) increases with age, and they may be detected with MCCE as effectively as with gastroscopy[8]. The sensitivity is between 90% and 92%, and the specificity is between 94% and 96.7%[15]. Several studies proved that, altogether, reliable preparation (gastric cleanliness), careful examination, and MCCE are pivotal for adequate gastric visualization and, therefore, for the detection of superficial gastric neoplasms[16]. In a large multicenter gastric cancer screening trial conducted in China seven asymptomatic patients out of 3182 were diagnosed with advanced cancer by MCCE. Cancer prevalence was highest in the gastric body (3 cases), followed by 2 cases in the cardia and 2 in the antrum, while 1-1 cases were detected in the region of the angulus, in the fundus and in the esophagus. All were confirmed to be adenocarcinoma pathologically. These results indicate that screening with MCCE may be useful in high family risk populations or people aged over 50 years old as a gastric cancer screening modality[8].
Magnetic steering may also impact TTs, as delayed gastric transit of the CE (especially in patients with gastroparesis) may lead to an incomplete SB exam. This method may increase the papilla detection rate as well. In addition to magnetic steering, there are several other independent factors, such as male gender and higher BMI, which increase gastric TTs and decrease SB TTs, thus impacting the time required for CE completion[10]. Despite the fact that delayed gastric TT may cause incomplete SBCE, we should avoid esophago-gastro-duodenoscopy to resolve temporary gastric capsule retention and instead use MCCE[17].
The visual diagnosis of the presence of H. pylori with standard white light endoscopy (WLI) has a relatively low accuracy, especially in population with a low pretest probability. Moreover, WLI endoscopy correlates poorly with histopathological findings of H. pylori induced gastritis too. Recently, low quality retrospective studies proposed the theory, that with the application of a special electronic chromoendoscopy, linked color imaging, diffuse reddish appearance of the mucosa in the gastric body and fundic glands correlates with the presence of HP[18].
In our study we found no correlation between the HP status and the activity and type of gastritis observed on MCCE as follows, but not included this into the Table 2, since it would be relevant in another publication, focusing HP status and gastritis on MCCE.
MCCE is an excellent method to visualize the entire gastric mucosal surface up to 100% precision in healthy volunteers[5]. In a previous study of 75 consecutive patients, we demonstrated that with our combined method with automatic and manual control, a complete visualization of the cardia, fundus, corpus, angulus, antrum, and duodenal bulb was 95.4%, 100%, 100%, 100%, 100% and 100%, respectively. The ampulla of Vater was detected and observed in 20 patients (26%). Moreover, the visualization of the distal esophagus and the Z-line was possible in 67/75 patients (89%), which was assessed as complete in 23, and partially complete in 44 patients. The mean stationary time for MCCE in the distal esophagus was 1 min and 32 s[19].
The use of MCCE needs to be considered in western and eastern countries, as there are two different scenarios, depending on the different levels of prevalence of UGI diseases and gastric cancer in each country. Nowadays, MCCE is mostly used for the detection of malignant and premalignant gastric lesions, which are more prevalent in eastern countries. However, the present technology opens the door to further, new technologies; subsequent developments would allow MCCE technology to be extended to other regions of the GI tract, e.g., the esophagus, which may facilitate the detection of more relevant lesions, such as Barrett metaplasia in western countries as well. With second-generation MCCE, visualization of the esophagus, Z-line, and duodenal papilla would be improved, according to a study by Jiang et al[20] Furthermore, the gastric TT could shorten by one-third, which was nearly achieved by conventional endoscopy with an examination of approximately 10 min.
It is known that the current gold standard gastroscopy has some clear disadvantages, as it is commonly uncomfortable for patients, and therefore it is mostly performed under sedation, which carries definite procedure related risks. MCCE, as a patient-friendly, non-invasive test, might be an alternative for patients who refuse to undergo gastroscopy and increase patients’ adherence for screening. Furthermore, MCCE of the stomach was recently approved by the Chinese Food and Drug Association for the following diagnostic indications: (1) As an alternative diagnostic tool for patients who refuse to undergo gastroscopy; (2) Screening of gastric diseases as a part of physical examination; (3) Screening for gastric cancer; (4) To diagnose various causes of GI inflammation; (5) To perform follow-up for diseases like gastric varices, gastric ulcer, atrophic gastritis, and polyps after surgical or endoscopic removal[21].
There is no similar study in the literature, as we performed a complete upper GI capsule examination, including the stomach and the SB with the same capsule endoscope during MCCE. Denzer et al[22] published a blinded, prospective trial from two French centers with the Intromedic manually controlled MCCE. A total of 189 patients were enrolled into this multicenter study. Lesions were defined as major (requiring biopsy or removal) or minor ones. The final gold-standard was unblinded conventional gastroscopy with biopsy, under sedation with propofol. Twenty-three major lesions were found in 21 patients and in this population, the capsule accuracy was 90.5% as compared to gastroscopy. Of the remaining 168 patients, 94% had minor and mostly multiple lesions; the capsule accuracy was 88.1%. All patients preferred MCCE over gastroscopy[22].
One of the risk factors of incomplete SB capsule endoscopy is a prolonged gastric transit time, which could be considered as a limitation of our combined gastric and SB study protocol. In our patient population, the average gastric transit time with magnetic transpyloric, manual control was 26 min. In contrast, in those cases where the magnetic transpyloric control failed, after examining the stomach, we left the capsule to propel through the pylorus by spontaneous peristaltic activity. In these patient groups, the average gastric transit time took 1 h and 9 min. In 10 cases out of 18 incomplete SB studies caused by battery low energy, this event occurred in 3 patients with manual magnetic passage and in 7 patients with spontaneous transpyloric passage[23].
Visibility and identification of landmarks are important factors to consider in accurate examination of stomach using MCCE. Gastric landmarks and typical stations described in the methods were always forced to achieve during combined automatic and manual maneuvering. For improving the learning curve of our gastroenterologist, we started to train the examinations in a plastic stomach model. In our previously published abstract, we described the improvement of the learning curves with manual magnetic controls both in experts and in trainees[1]. In this study, we find significant differences in the examination time of the complete inner surface mapping between trainees and experts, and moreover automatic protocols were faster and equally accurate as experts to achieve a complete inner surface mapping.
The problem how to minimalize bubbles and mucoid secretions is an existing problem in real life studies. To improve visibility, we established a unique preparation process with a combination of bicarbonate, Pronase B, and simethicone combined with a patient body rotation technique[2]. Moreover, in our described stations, we rotate our patients from left lateral to supine, then from supine top right lateral, and finally from right lateral to supine position during MCCE study. During this protocol, the gastric mucoid secretions also moving into different parts of the stomach due to the gravity making visible all the landmarks and the majority of the mucosal abnormalities. Application of prokinetics or motilin agonist erythromycin might also be an option in future studies to improve the visibility and reduce gastric lake content[24,25].
An inherent limitation of our present study that we performed gastroscopy only in a few patients with major gastric pathologies to accomplish final diagnosis and biopsy; and therefore, we could not assess the accuracy of MCCE in all patients compared to gastroscopy. However, several previous studies demonstrated excellent diagnostic value and high accuracy. In a recent meta-analysis of Zhang et al[26] four studies with 612 patients were included, in which the results of blinded MCCE and gastroscopy were compared. MCCE demonstrated a pooled sensitivity and specificity of 91% (95%CI: 0.87-0.93) and 90% (95%CI: 0.75-0.96), respectively. The diagnostic accuracy of MCCE was 91% (95%CI: 0.88-0.94) for assessing gastric diseases.
MCCE is an effective and safe diagnostic method for evaluating gastric and SB mucosal lesions while being utilized as a single upper GI capsule endoscope in patients referred for SBCE evaluation. MCCE using the novel Ankon capsule endoscopic technique is a proper diagnostic method for evaluating the gastric mucosa. It is a promising non-invasive screening tool that may be applied in future monitoring of patients with high gastric cancer family risk to decrease morbidity and mortality of benign and malignant upper GI disorders.
While capsule endoscopy (CE) is the gold standard diagnostic method of detecting small bowel diseases and disorders, a novel magnetically controlled capsule endoscopy (MCCE) system provides non-invasive evaluation of the gastric mucosal surface, which can be performed without sedation or discomfort.
During standard small bowel capsule endoscopy (SBCE), passive movement of the CE may cause areas of the complex anatomy of the gastric mucosa to remain unexplored, whereas the precision of MCCE capsule movements inside the stomach promises better visualization of the entire mucosa.
To evaluate the Ankon MCCE system’s feasibility, safety and diagnostic yield in patients with gastric or small bowel disorders.
Of outpatients who were referred for SBCE, 284 (male/female: 149/135) were prospectively enrolled and evaluated by MCCE. The stomach was examined in the supine, left, and right lateral decubitus positions without sedation. Next, all patients underwent a complete small bowel CE study protocol. The gastric mucosa was explored with the Ankon MCCE system with active magnetic control of the capsule endoscope in the stomach, applying three standardized pre-programmed computerized algorithms in combination with manual control of the magnetic movements.
The urea breath test revealed Helicobacter pylori positivity in 32.7% of patients. The mean gastric and small bowel transit times with MCCE were 47 min 40 s and 3 h 46 min 22 s, respectively. The average total time of upper GI MCCE examination was 5 h 48 min 35 s min. Active magnetic movement of the Ankon capsule through the pylorus was successful in 41.9% of patients. Overall diagnostic yield for detecting abnormalities in the stomach and small bowel was 81.9% (68.6% minor; 13.3% major pathologies); 25.8% of abnormalities were in the small bowel; 74.2% were in the stomach. The diagnostic yield for stomach/small bowel was 55.9%/12.7% for minor and 4.9%/8.4% for major pathologies.
MCCE is a feasible, safe diagnostic method for evaluating gastric mucosal lesions and is a promising non-invasive screening tool to decrease morbidity and mortality in upper gastrointestinal diseases.
MCCE is promising as a non-invasive screening tool that may be applied in future monitoring of patients for evaluating gastric mucosal lesions and decreasing morbidity and mortality of benign and malignant upper GI disorders.
Acknowledgements: special thanks for Zoltán Tóbiás MD for the graphical work and visual explanations of different gastric stations and Kata Tőgl our nurse manager for assistance and modelling the patients positioning during magnetically controlled capsule endoscopy.
| 1. | Adler SN. The history of time for capsule endoscopy. Ann Transl Med. 2017;5:194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Eliakim R, Yassin K, Shlomi I, Suissa A, Eisen GM. A novel diagnostic tool for detecting oesophageal pathology: the PillCam oesophageal video capsule. Aliment Pharmacol Ther. 2004;20:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1994] [Cited by in RCA: 1410] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 4. | Keller J, Fibbe C, Volke F, Gerber J, Mosse AC, Reimann-Zawadzki M, Rabinovitz E, Layer P, Schmitt D, Andresen V, Rosien U, Swain P. Inspection of the human stomach using remote-controlled capsule endoscopy: a feasibility study in healthy volunteers (with videos). Gastrointest Endosc. 2011;73:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Liao Z, Duan XD, Xin L, Bo LM, Wang XH, Xiao GH, Hu LH, Zhuang SL, Li ZS. Feasibility and safety of magnetic-controlled capsule endoscopy system in examination of human stomach: a pilot study in healthy volunteers. J Interv Gastroenterol. 2012;2:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, Aihara H, Pangtay I, Hibi T, Kudo S, Tajiri H. Feasibility of stomach exploration with a guided capsule endoscope. Endoscopy. 2010;42:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, Aihara H, Pangtay I, Hibi T, Kudo SE, Tajiri H. Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest Endosc. 2012;75:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Zhao AJ, Qian YY, Sun H, Hou X, Pan J, Liu X, Zhou W, Chen YZ, Jiang X, Li ZS, Liao Z. Screening for gastric cancer with magnetically controlled capsule gastroscopy in asymptomatic individuals. Gastrointest Endosc. 2018;88:466-474.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Nam SJ, Lee HS, Lim YJ. Evaluation of Gastric Disease with Capsule Endoscopy. Clin Endosc. 2018;51:323-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Gu H, Zheng H, Cui X, Huang Y, Jiang B. Maneuverability and safety of a magnetic-controlled capsule endoscopy system to examine the human colon under real-time monitoring by colonoscopy: a pilot study (with video). Gastrointest Endosc. 2017;85:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Ching HL, Hale MF, Kurien M, Campbell JA, Chetcuti Zammit S, Healy A, Thurston V, Hebden JM, Sidhu R, McAlindon ME. Diagnostic yield of magnetically assisted capsule endoscopy versus gastroscopy in recurrent and refractory iron deficiency anemia. Endoscopy. 2019;51:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 12. | Szalai M, Oczella L, Lovasz BD, Madacsy L. Surface mapping in plasctic gastric model assisted by a robotic autoscan program with new magnetically controlled gastroc capsule endoscopy system compared to manual controlling. United European Gastroenterol J. 2018;865. |
| 13. | Jiang X, Qian YY, Liu X, Pan J, Zou WB, Zhou W, Luo YY, Chen YZ, Li ZS, Liao Z. Impact of magnetic steering on gastric transit time of a capsule endoscopy (with video). Gastrointest Endosc. 2018;88:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Pan J, Li Z, Liao Z. Noncontact endoscopy for infection-free gastric examination during the COVID-19 pandemic. VideoGIE. 2020;5:402-403.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Liao Z, Hou X, Lin-Hu EQ, Sheng JQ, Ge ZZ, Jiang B, Hou XH, Liu JY, Li Z, Huang QY, Zhao XJ, Li N, Gao YJ, Zhang Y, Zhou JQ, Wang XY, Liu J, Xie XP, Yang CM, Liu HL, Sun XT, Zou WB, Li ZS. Accuracy of Magnetically Controlled Capsule Endoscopy, Compared With Conventional Gastroscopy, in Detection of Gastric Diseases. Clin Gastroenterol Hepatol. 2016;14:1266-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 16. | Qian YY, Zhu SG, Hou X, Zhou W, An W, Su XJ, McAlindon ME, Li ZS, Liao Z. Preliminary study of magnetically controlled capsule gastroscopy for diagnosing superficial gastric neoplasia. Dig Liver Dis. 2018;50:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Luo YY, Pan J, Chen YZ, Jiang X, Zou WB, Qian YY, Zhou W, Liu X, Li ZS, Liao Z. Magnetic Steering of Capsule Endoscopy Improves Small Bowel Capsule Endoscopy Completion Rate. Dig Dis Sci. 2019;64:1908-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Madacsy L, Lovasz BD, Zsobrak K, Szalai M, Oczella L. Feasibility and safety of robotocally controlled magnetic capsule endoscopy in the visualization of the upper gastrointestinal tract. United European Gastroenterol J. 2018;1484. |
| 19. | Dohi O, Yagi N, Onozawa Y, Kimura-Tsuchiya R, Majima A, Kitaichi T, Horii Y, Suzuki K, Tomie A, Okayama T, Yoshida N, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O, Konishi H, Naito Y, Itoh Y. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc Int Open. 2016;4:E800-E805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Jiang B, Qian YY, Pan J, Jiang X, Wang YC, Zhu JH, Zou WB, Zhou W, Li ZS, Liao Z. Second-generation magnetically controlled capsule gastroscopy with improved image resolution and frame rate: a randomized controlled clinical trial (with video). Gastrointest Endosc. 2020;91:1379-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. |
|
| 22. | Denzer UW, Rösch T, Hoytat B, Abdel-Hamid M, Hebuterne X, Vanbiervielt G, Filippi J, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, Aihara H, Kudo SE, Tajiri H, Treszl A, Wegscheider K, Greff M, Rey JF. Magnetically guided capsule versus conventional gastroscopy for upper abdominal complaints: a prospective blinded study. J Clin Gastroenterol. 2015;49:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc. 2009;69:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Szalai M, Oczella L, Lovasz BD, Madacsy L. Surface mapping in plastic gastric model assisted by a robotic autoscan program with a new magnetically controlled gastric capsule endoscopy system compared to manual controlling. United European Gastroenterol J. 2018;415-A415. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Hungarian Society of Gastroenterology, 45326.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kawano S, Japan; Nam SJ, South Korea; Nishida T, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H