Published online May 21, 2022. doi: 10.3748/wjg.v28.i19.2076
Peer-review started: November 9, 2021
First decision: March 11, 2022
Revised: March 25, 2022
Accepted: April 28, 2022
Article in press: April 28, 2022
Published online: May 21, 2022
Processing time: 189 Days and 8.6 Hours
Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs) are rare mixed tumors containing both neuroendocrine (NE) and non-NE components. Each component must occupy at least 30% of the tumor volume by definition. Recent molecular evidence suggests MiNENs are clonal neoplasms and potentially harbor targetable mutations similar to conventional carcinomas. There have been multiple changes in the nomenclature and classification of MiNENs which has created some confusion among pathologists on how to integrate the contributions of each component in a MiNEN, an issue which in turn has resulted in confusion in communication with front-line treating oncologists. This mini review summarizes our current understanding of MiNENs and outline diagnosis, prognosis, and management of these neoplasms. The authors emphasize the importance of treating the most aggressive component of the tumor regardless of its percentage volume.
Core Tip: Mixed neuroendocrine neoplasms have been referred to by a long list of names. The latest term, mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN), captures a wider spectrum of neoplasms than did previous nomenclature. This mini review summarizes the development of the term MiNEN and reviews current knowledge about the molecular pathogenesis, diagnosis, prognosis, and management of these neoplasms. MiNENs are viewed as clonal neoplasms and their clinical behaviour and management are ultimately determined by the most aggressive component present.
- Citation: Toor D, Loree JM, Gao ZH, Wang G, Zhou C. Mixed neuroendocrine-non-neuroendocrine neoplasms of the digestive system: A mini-review. World J Gastroenterol 2022; 28(19): 2076-2087
- URL: https://www.wjgnet.com/1007-9327/full/v28/i19/2076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i19.2076
Mixed neuroendocrine-non-neuroendocrine neoplasms (MiNENs) are tumors containing two or more histologically distinct components with one component showing neuroendocrine (NE) differentiation[1]. The non-NE component consists of an adenocarcinoma in over 90% of cases, but it can consist of any other epithelial neoplasm, including squamous cell carcinoma or hepatocellular carcinoma[1,2]. By definition each component must individually comprise > 30% of the total tumor volume in order for the tumor to qualify as a MiNEN[3].
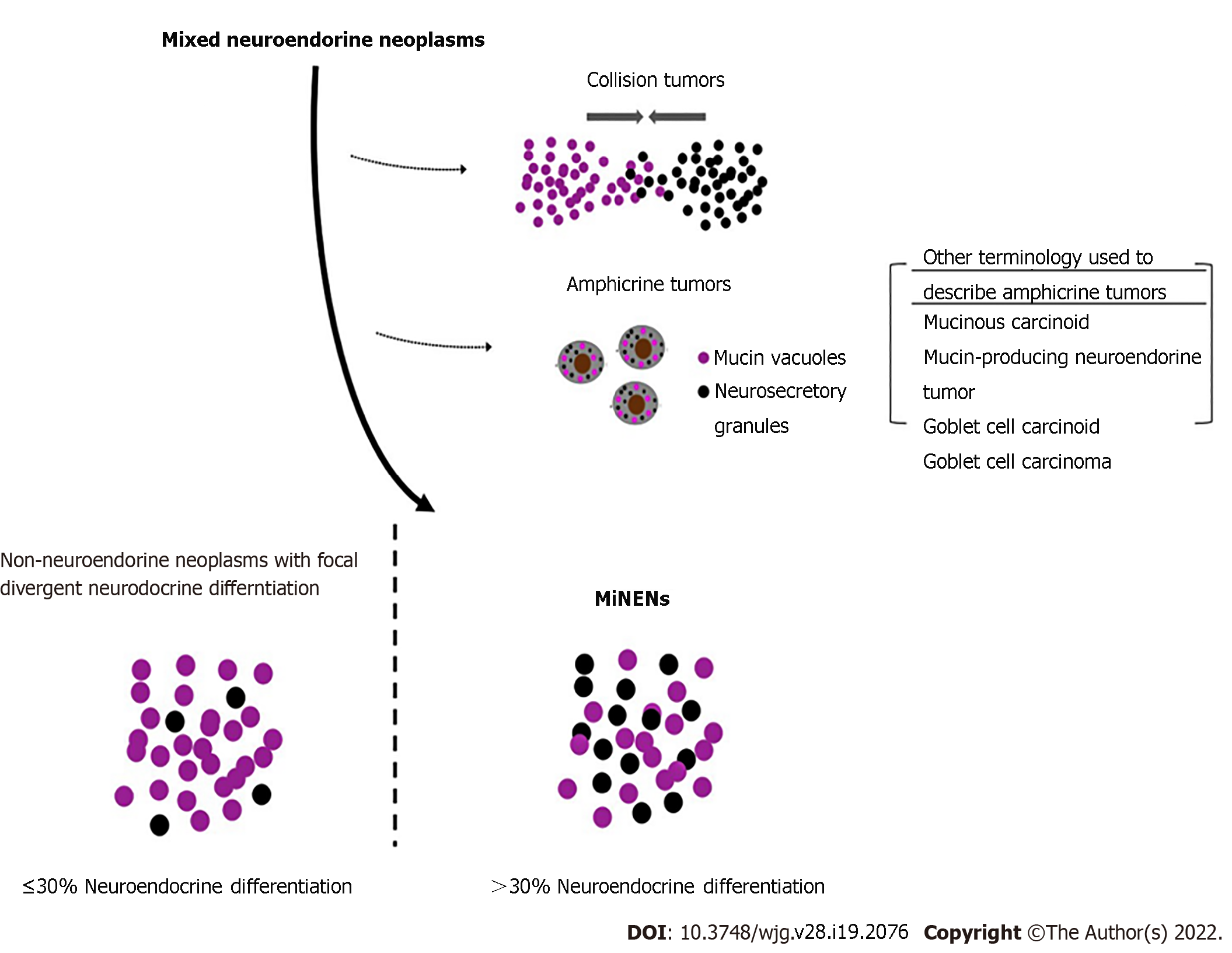
MiNENs are a specific subtype of mixed NE neoplasms (Figure 1)[4]. Traditionally, mixed NE neoplasms include composite tumor, collision tumors, amphicrine tumors and non-NE neoplasms with focal neuroendocrine differentiation (NNE-NEDs). Strictly speaking, in MiNENs the distinct NE and non-NE components are intimately intermingled and both are derived from a common single precursor cell, morphologically expressing usually as composite tumors. Collision tumors consist of two independent primary neoplasms which have grown into the same space by chance. Each neoplasm is derived from a separate precursor cell which has independently undergone its own molecular evolution[5]. Amphicrine neoplasms consist of a single cell type in which each cell displays both NE and non-NE phenotypes simultaneously. For example, electron microscopy may demonstrate both mucous vacuoles and neurosecretory granules in the same cell. These tumors are extremely rare, with approximately only 4 reported in the stomach[6] but are slightly more common in the pancreas. Despite that collision tumors might not come from a common single precursor cell; they have still been referred to as MiNENs by authors based on the traditional practice.
NNE-NEDs are non-NE neoplasms which show NE differentiation but not enough to meet the criteria to be considered MiNENs. The exact percentage threshold of NED required to be considered a MiNEN is artificial and will likely continue to evolve in the future as we learn more about these neoplasms. The current 30% threshold was originally defined arbitrarily in 1987 to recognize and create a new diagnostic entity for truly mixed NE tumors with substantial amounts of both non-NE and NE components, and to exclude non-NE tumors with scattered NE cells of uncertain clinical significance[4,7]. Although it has served a valuable role in the establishment of MiNENs as distinct diagnostic entities, this cut-off does not have a basis in clinical or scientific evidence[1]. Recent studies suggest a lower threshold may be more appropriate, with even 10% NED significantly affecting prognosis, thus raising the possibility the formally defined threshold may change in the future[8,9]. Indeed, poorly differentiated NE or even non-NE components, even when < 5% of the tumor volume, may determine patient outcome. Regardless of what the threshold is, it is important to note neoplasms may show a spectrum of divergent NED, and that only a subset of these neoplasms should be classified as MiNENs.
Other entities which may be confused with MiNENs include usual adenocarcinomas displaying aberrant expression of NE markers (i.e., synaptophysin and chromogranin) but no histologically recognizable NE morphology[7]. These features do not qualify a neoplasm as a MiNEN, and aberrant synaptophysin expression in conventional adenocarcinomas does not affect prognosis[10]. Neoplasms which overgrow and entrap physiologic NE structures, for example acinar adenocarcinoma in the pancreas which can entrap normal physiologic islets of Langerhans, should also not be mistaken for MiNENs despite the pitfall of positive staining for NE markers[7].
The individual components of MiNENs are common entities which pathologists and oncologists encounter routinely. The major challenge in understanding MiNENs is simply an issue of nomenclature. Unfortunately, the nomenclature has undergone numerous rapid and successive changes over the past 20 years. This has been complicated by substantial changes in the nomenclature for simple pure NE neoplasms themselves which have occurred over the same timeframe. The potential clinical confusion which may occur because of these rapid changes is not insignificant. An illustrative example of such potential confusion comes from appendiceal goblet cell neoplasms which have similarly undergone numerous changes in nomenclature. Two cases diagnosed as "goblet cell adenocarcinoma" and "adenocarcinoma ex goblet cell carcinoid" were misinterpreted by oncologists as NE tumors and then considered for inappropriate adjuvant chemotherapy[11].
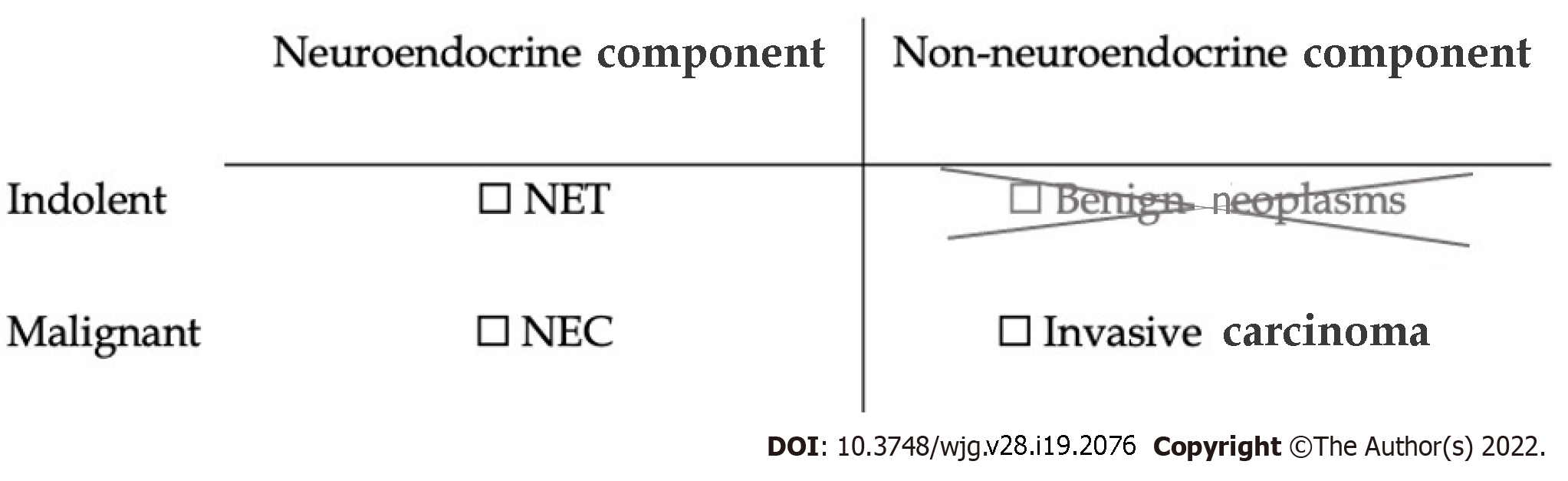
A conceptual framework for understanding MiNENs is shown in Figure 2. In order to constitute a MiNEN a tumor must show at least two lines of differentiation, one of which is NE and the other of which is epithelial but non-NE. In the digestive system only, there is an additional stipulation that each component of the neoplasm is required to be malignant in order for it to qualify as a MiNEN[3]. Low-grade NE tumors, formerly known as carcinoids, satisfy this "malignant" requirement because of their potential for metastasis, despite their usually indolent behavior[1,3]. However indolent non-NE epithelial tumors, such as adenomas or papillomas, do not metastasize and so would not constitute MiNENs in the digestive system[3]. For example, mixed adenomas well-differentiated NE tumors, or "MANETs", consisting of a composite tubular adenoma-carcinoid tumor, are no longer considered MiNENs with these updated criteria[12]. Similarly, in-situ lesions such as intraductal papillary mucinous neoplasms of the pancreas were formerly included as potential non-NE components of digestive system MiNENs, but now have been excluded by the latest WHO classification[1,3]. The non-NE component is required to be an invasive carcinoma.
Although perhaps daunting, becoming familiar with the host of names that has been used to refer to MiNENs in the past may help reduce confusion in clinical practice. We will attempt to briefly summarize the history of the nomenclature of MiNENs towards this end. We begin by describing the changes in nomenclature of pure NE neoplasms, before moving on to the broader category of MiNENs.
The nomenclature of NE neoplasms in the digestive system has undergone a series of changes in the last 20 years. After being first recognized in 1907, NE neoplasms were designated "carcinoid tumors" and referred to as such for almost a century thereafter[8,13]. By 1994 poorly differentiated NE neoplasms were noted to have a drastically worse prognosis than their well-differentiated counterparts[8,14]. The poorly differentiated neoplasms were designated “small cell carcinomas”. The term carcinoid continued to refer to well-differentiated NE neoplasms.
Increasing use of Ki-67 immunohistochemistry revealed a wide spectrum of proliferative rates within carcinoid tumors and small cell carcinomas. A grading system based on Ki-67 index was proposed in 2006 and formally adopted by the WHO classification in 2010[15,16]. NE tumors with low Ki-67 indices (≤ 20%; grades 1 or 2) were called neuroendocrine tumors (NETs) while tumors with high Ki-67 index (> 20%; grade 3) were called neuroendocrine carcinomas (NECs).
Grade 3 tumors were recently recognized to consist of two types of tumors, although both have increased Ki-67 indices. One type of tumors shows well-differentiated morphology while another type shows poorly differentiated morphology[8]. Further studies have revealed tumors showing poorly differentiated morphology have half the survival of their well-differentiated counterparts[8]. Genetic studies further show the two tumor populations actually develop via entirely separate and independent molecular pathways[8,17]. These findings have led to any poorly differentiated tumor being referred to as a NEC, regardless of Ki-67 index. Well-differentiated tumors which are designated NETs and further graded according to their proliferation rate[3].
Any NE neoplasm, either a NET or a NEC, can constitute the NE component of a MiNEN.
Mixed NE neoplasms were first recognized in 1924, only shortly after pure NE neoplasms themselves were first recognized[7,18]. In 1987 Lewin[4] formally proposed a classification system for mixed NE neoplasms in which he termed what we now call MiNENs as “composite glandular-endocrine cell carcinomas”[4]. His classification system distinguished these tumors from collision tumors and amphicrine tumors, as well as from usual adenocarcinomas with < 30% NED.
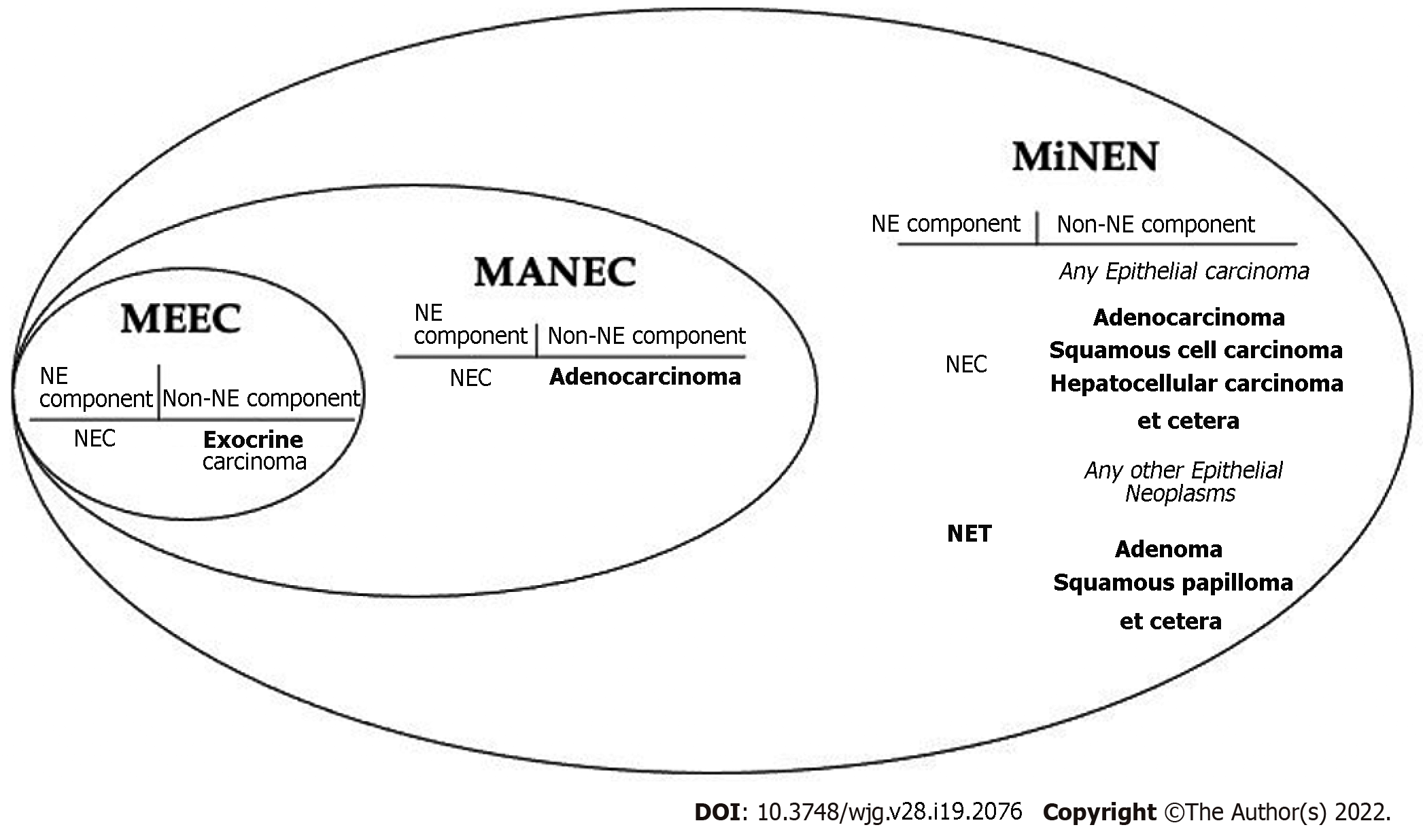
In 2000 these neoplasms were adopted into the WHO Classification of Tumors of the Digestive System as “mixed exocrine-endocrine carcinomas” (MEECs)[7,19]. This terminology was short-lived and changed to “mixed adenoneuroendocrine carcinoma” (MANEC) in the subsequent 2010 edition of the classification[16].
It soon became apparent adenocarcinomas were not the only non-NE neoplasms observed in mixed NE tumors. Other epithelial neoplasms, such as squamous cell carcinomas and hepatocellular carcinomas, were also observed in mixed NE neoplasms[1,7]. This prompted “mixed adenoneuroendocrine carcinomas” (MANECs) to instead be viewed as “mixed neuroendocrine-non-neuroendocrine carcinomas” (Figure 3).
In 2016, La Rosa et al[1] proposed the term "mixed neuroendocrine-non-neuroendocrine neoplasms" (MiNENs)[1]. The intention was to create an umbrella term for mixed NE neoplasms broad enough to encompass all the changes we have discussed so far, while also being generalizable to any mixed NE tumor occurring in any organ in the body. The latter task is difficult considering the heterogeneity in nomenclature for NE neoplasms in different sites such as the lung. It is even more difficult if one anticipates the nomenclature for pure NE neoplasms continuing to evolve in other organs such as it has in the digestive system.
MiNENs are 'neoplasms' instead of 'carcinomas' because benign non-NE components such as adenomas or papillomas can still be a part of MiNENs in sites outside of the digestive system[1]. This had been the case in the digestive system as well until only recently when the latest WHO classification was published in 2019.
The current prevailing view is that MiNENs are clonal neoplasms derived from a single pluripotent stem cell[8]. Multiple studies have compared the mutational landscape in the NE and non-NE components and have demonstrated a common trunk of mutations shared between them, beyond which are further mutations specific to each component[20-22]. Interestingly these shared mutations are very similar to the mutations found in standard pure adenocarcinomas from the same anatomic site[22]. For example, in the colon, MiNENs can show BRAF, KRAS, and APC mutations in both components[22]. The mutated genes are expressed at similar levels in both components, and expression is significantly altered in comparison to normal background tissue[20,21].
Some authors have theorized MiNENs may begin as non-NE tumors and then at some point trans-differentiate to form an aggressive NEC component[2,23-26]. Several reports suggest c-Myc and SMARCA mutations may play a role in trans-differentiation, with one study even finding only a single additional SMARCA mutation in the NE component of a MiNEN[23-25]. Other studies have shown increased chromosomal aberrations and allelic imbalances in the NE component[26].
Definitive diagnosis of a MiNEN usually follows after surgical resection. The presurgical biopsy diagnoses are roughly evenly split between 1/3 adenocarcinoma, 1/3 NEC, and 1/3 suspicious for MiNEN[2]. The NE component tends to lie deeper in the tumor which can influence the diagnosis on biopsy depending on the depth of tumor sampled[27,28].
Diagnosing a MiNEN is equivalent to individually diagnosing the constituent components with a few additional steps. Neither the NE nor non-NE components differ from their pure counterparts, including their immunohistochemical profiles.
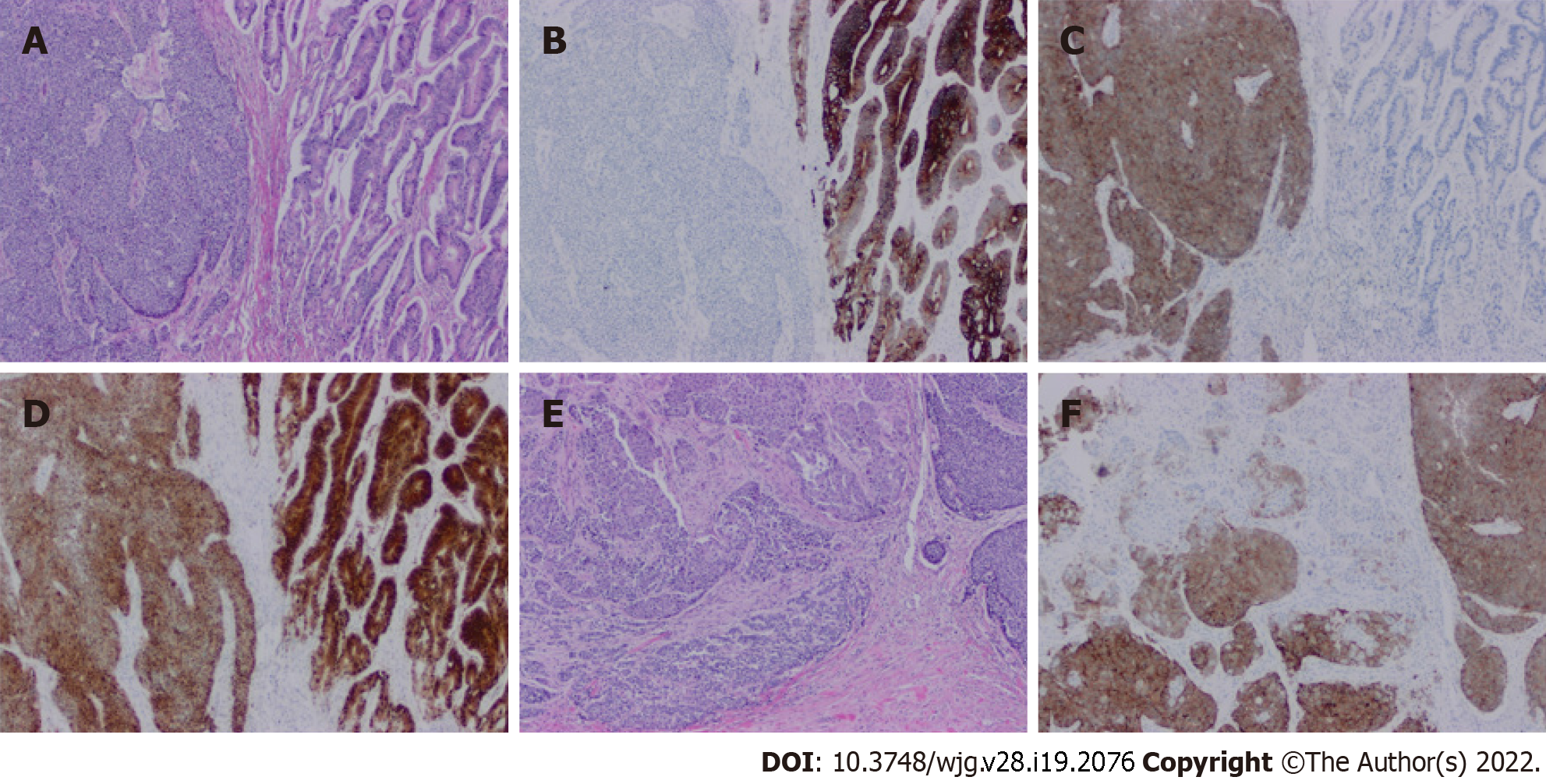
Figure 4 illustrates a typical MiNEN of colon origin. Both morphologic and immunohistochemical evidence of NED is required to diagnose a NE component in a MiNEN[3,7]. Morphologic evidence is required avoid the pitfall of focal aberrant NE marker expression on immunohistochemistry in otherwise standard non-NE tumors, a commonly recognized phenomenon with no current known clinical significance[10,29]. Common markers of NED that are used include synaptophysin, chromogranin, INSM1, CD56, and neuron-specific enolase. Synaptophysin is traditionally be regarded as the most sensitive and chromogranin as the most specific[30].
The NE component may be either a NEC or a NET[3,7]. Ki-67 proliferative index (or equivalently mitotic rate) should be reported for all NE neoplasms, including the NE component present in MiNENs. NETs are graded as usual by Ki-67 index: Grade 1 tumors are < 3%; grade 2 between 3%-20%; and grade 3 > 20%[3]. Grade 3 NETs usually have a Ki-67 index less than 60%, while the indices for NECs are higher, typically more than 50%-60%[8].
Well-differentiated NETs are usually organized in nested, solid, trabecular, ribbon, or insular patterns. Rosette-like formations can be present. Tumors are cohesive without isolated single cells. Granular eosinophilic cytoplasm may be appreciated. Lymphovascular or perineural invasion is allowed in NETs and does not necessitate a diagnosis of NEC. NETs retain immunohistochemical markers from the primary site of origin[8].
The majority of MiNENs do not contain NETs but rather contain poorly differentiated NECs. Tumor cells in NECs show nuclear crowding and pleomorphism. Nuclei are hyperchromatic with increased nuclear membrane irregularity. Single cells are common, and mitoses are obvious. Necrosis can be present, and nuclei display smearing or crush artifact. NE markers remain sensitive, however immunohistochemical markers for the primary site of origin are lost[8,30]. Aberrant expression of TTF-1 and CDX-2 is common, making these markers unreliable and a potential pitfall for mistaking the site of origin[8]. The average Ki-67 proliferative index of NECs in MiNENs is 70%[30].
Morphologically NECs can show either small or large cell patterns. Small cell morphology is similar to traditional small cell carcinoma elsewhere with small uniform round to oval nuclei and evenly distributed finely granular (“salt and pepper”) chromatin. Cytoplasm is scant. Cells are crowded with nuclear moulding and indistinct cell membranes. In contrast, large cell NECs (LCNECs) have a larger cell size than small cell carcinoma and a vesicular nucleus with prominent nucleolus. They can have appreciable eosinophilic cytoplasm. Distinguishing LCNECs from small cell carcinoma can sometimes be a difficult task. Ki-67 index can potentially be helpful as the index of LCNECs ranges from 40%-80% while small cell carcinoma averages 80%[31]. In MiNENs the NEC component is more commonly a LCNECs than a small cell carcinoma[32,33].
The non-NE component of MiNENs is adenocarcinoma 92% of the time[2]. Squamous cell carcinoma, hepatocellular carcinoma, and mixed adenosquamous carcinoma make up the majority of remaining non-NE components in reported cases thus far[2].
The exact threshold of NED required to define a tumor as a MiNEN vs an NNE-NED is an area of current controversy in the literature. As mentioned, the 30% threshold which persists today was originally chosen arbitrarily. A commonly cited and relatively recent study by Park et al[9] provides evidence arguing in favor of lowering the threshold to 10%[9]. The authors examined 88 gastric carcinomas with varying degrees of NED and compared overall survival to 650 gastric carcinomas with no NED. NED of just under 10% was associated with worse survival: Overall 5-year survival in cases with < 10% NED was 85.6% compared to only 53.3% survival in cases with ≥ 10% NED.
Another similar study found 20% NED to be associated with significantly worse prognosis in adenocarcinomas, again suggesting the 30% threshold may be too high[34]. The current formal threshold for diagnosing a MiNEN remains 30% but this is likely to be lowered in the future. In the interim while this does preclude a diagnosis of MiNEN in cases with 10%-30% NED, we recommend always quantifying the percentage of NED in non-NE tumors and informing the oncologist of the possible prognostic impact, for example by making a diagnostic comment citing the studies above. Regardless of how the report is handled, the possible prognostic impact of 10%–30% is an important topic to be raised in multidisciplinary meetings discussing diagnosis and management of these rare tumors.
The significance of divergent differentiation in NECs is a less well explored topic. This may be because even small degrees of NED, beginning at as little as 10% as mentioned above, quickly approach the same dismal prognosis as NECs regardless of the size of the non-NE component (Table 1).
| Classification | |||||
| Neuroendocrine differentiation (%) | 0 | < 10 | 10-30 | 30-70 | > 70 |
| Current classification | Non-NE | Non-NE | NNE-NED | MiNEN | NEC |
| 5-yr overall survival | 85 | 89 | 171 | 54 | 59 |
There is currently no widely accepted system for grading MiNENs. This is somewhat unfortunate because MiNEN is an umbrella term for a wide range of neoplasms whose behaviour can range from indolent (i.e., a MiNEN in which a NET is the most biologically aggressive component) to aggressively malignant, paralleling the prognosis of NECs. Simply designating a tumor as a MiNEN is insufficient and does not convey much useful information. Therefore, it is incumbent upon the pathologist to clearly communicate the nature and aggressiveness of each component present in the tumor to ensure appropriate management.
The original publication by La Rosa et al[1] in which the term MiNEN was coined also proposed a grading scheme in which MiNENs were divided into low-grade, intermediate grade, and high grade categories[1]. Tumors with a NEC component, which constitute the vast majority of MiNENs, would be designated high grade neoplasms. Tumors in which the non-NE component was the most aggressive, such as adenocarcinomas with a NET component, would be intermediate grade. Indolent tumors in which a NET was the most aggressive component would be low-grade. This scheme succinctly classifies MiNENs according to their most aggressive component. It is also clinically useful because the most aggressive component usually drives prognosis and management. While the scheme may not be widely used, it still serves as a useful guide as to how to portray grade in MiNENs.
The fact that MiNENs are considered single clonal neoplasms greatly simplifies staging. The two components do not need to be staged separately as they would in a collision tumor. Standard TNM staging applies as per AJCC guidelines, using the same protocols as for other carcinomas from the same body site. For the rare MiNENs in which a NET is the most aggressive component the protocol for NETs from the same body site is used. There does not appear to be literature yet to consider alternative methods for staging MiNENs.
While lymph node staging proceeds as per staging of the rest of the MiNEN as described above, the composition of lymph node metastases in MiNENs is an interesting issue because of the many different possible scenarios: Only one component may have metastasized, or both may have; the components may metastasize together to the same lymph node, or separately to different lymph nodes. Further it is interesting to consider whether the metastatic component of a MiNEN is a better marker of the biologic behavior of the tumor than the de facto assumption that behaviour is dictated by the worst component found in the primary tumor.
The pattern of metastasis in MiNENs was investigated in a study of 80 patients[35]. In approximately 70% of patients only one component metastasized; this was usually the more aggressive NEC component. In 20% of patients both components metastasized, but none were found together in the same lymph node. In 10% of patients there was lymph node metastasis present containing both components within the same node.
In terms of the components present in metastases, they reflected the composition of the primary tumor; in a tumor composed of 60% NEC and 40% adenocarcinoma, roughly 60% of metastases would be NEC and 40% would be adenocarcinoma[35].
Included in the study were 3 cases of NNE-NEDs. In all 3 of these cases the metastases were composed solely of the NEC component. Notably, by current criteria these tumors would not have qualified as MiNENs, providing further evidence that < 30% NED can be biologically significant.
The question of whether the biologic behaviour of MiNENs is better reflected by the metastatic component or the most aggressive component in the primary tumor is moot since these data show the composition of the metastases simply reflect the composition of the primary tumor; in other words, it is unlikely further useful information will be gained by determining the pattern of lymph node metastasis once the composition of the primary tumor is known. The grading scheme proposed by La Rosa appears to be the most useful gauge of the potential biological behavior of the tumor.
'MiNEN' is not a diagnosis but rather an umbrella term for mixed NE neoplasms[1]. The term in and of itself does not convey useful information to the oncologist. The useful information is knowing the components which make up the tumor, and pathologists must ensure these are communicated clearly to allow oncologists to stratify risk and plan management accordingly.
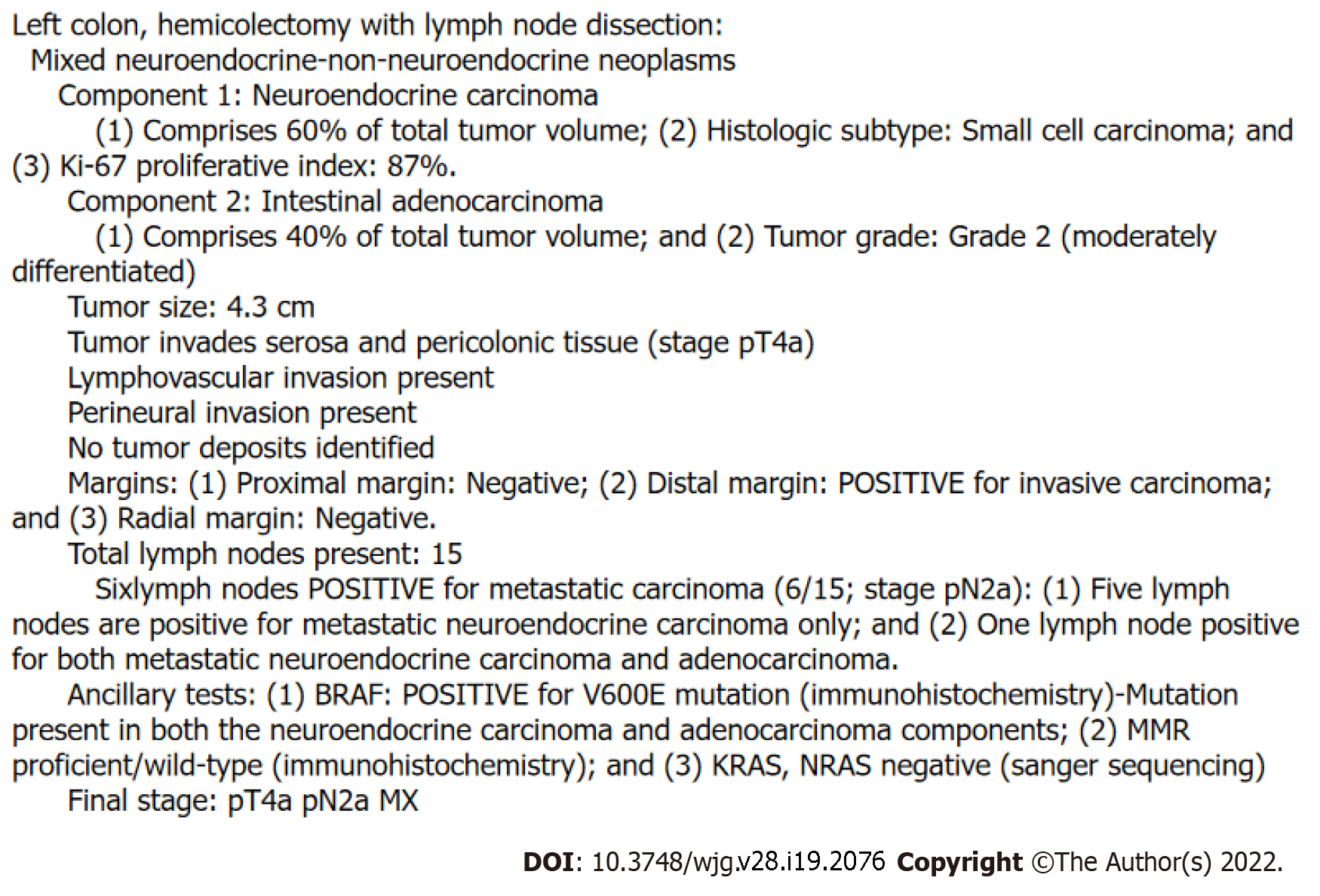
Figure 5 shows the example of the pathology report of a MiNEN. The first line of the report should identify that the tumor is a MiNEN. Because there is currently no widely accepted grading system, grade must be conveyed within the next few lines. The most aggressive or high-grade component should be listed first, and the system by La Rosa et al[1] serves a useful guide here[1]. A NEC component is the highest possible grade neoplasm found in MiNENs and should always be listed first. Next in the hierarchy of grade usually comes a non-NE carcinoma component, usually an adenocarcinoma, which is then followed by a NET component, and then lastly followed by the lowest grade component possible, a benign epithelial neoplasm such as an adenoma or papilloma. Accordingly, La Rosa's scheme describes tumors driven by a NEC component as high-grade, tumors driven by a carcinoma component intermediate grade, and tumors driven by a NET component low-grade or indolent. For each component listed the histologic subtype and grade should be described the same way they would be as if the component were a pure neoplasm. The percentage of total tumor volume occupied by each component should also be listed.
We recommend designating non-NE tumors with 10%-30% NED (NNE-NEDs) as 'mixed neoplasms' and then reporting them in the same manner as a MiNEN. A diagnostic comment citing the Park et al[9] and Jiang et al[34] studies mentioning the possible prognostic impact of NE components occupying < 30% of the tumor volume may help provide important background information for these rare tumors[9,34].
Because of their genetic similarity to adenocarcinomas, we recommend performing the same molecular studies on MiNENs as one would perform for an adenocarcinoma from the same anatomic site. Alterations involving MMR, BRAF V600E, and Her-2 have been demonstrated to exist in both components of MiNENs, inviting the exciting possibility of using the same targeted therapies during treatment[22,25,36]. Although ideally both components in a MiNEN would be tested individually to allow for better prediction of how the entire tumor will respond to a targeted therapy, in practice this may be very difficult or impossible to achieve with the closely intermingled components.
The rarity of MiNENs, combined with their changing terminology over the years, has made it difficult to examine outcomes data. Controversy exists as to whether MiNENs behave better than pure NECs, but ultimately the prognosis appears to be similar[2,30,37,38]. A recently reported survival comparison of 503 NECs, 401 MiNENs, and 2785 adenocarcinomas of the stomach showed that the 5-year disease-free survival (DFS) was 47.5%, 51.1%, and 57.8% respectively[37]. The shorter DFS of NECs and MiNENs compared to adenocarcinoma was statistically significant while the difference between NECs and MiNENs was not.
In the digestive system in general, 82% of MiNENs present with localized disease and 18% present with distant metastases[2]. Patients with localized disease generally proceed to surgical resection. Select patients with advanced disease may undergo palliative resection[38]. The median overall survival for localized MiNENs, including regional lymph node metastases (Stages I-III), is 39 mo. For advanced disease with distant metastasis (Stage IV) median overall survival is 11 mo[38].
MiNENs may be missed in patients with an accessible metastasis that is used for the initial diagnostic biopsy. These cases may be diagnosed as pure NECs or pure adenocarcinomas, if resection is not indicated and further tissue is not sampled, thus may result in under recognition of MiNENs[30]. This leads to bias in outcome data as lower stage tumors are more likely to undergo surgical resection and be diagnosed as MiNENs whereas higher stage tumors are more likely to be missed and labeled pure NECs or pure adenocarcinomas. Thus, MiNENs may appear to have improved prognosis, when in reality they are just more likely to be diagnosed at a lower stage.
Management for these rare tumors is usually discussed at multidisciplinary rounds with the expertise of pathologists and oncologists who have special interest in NE neoplasms. Treatment is tailored towards the most aggressive component of the tumor, which is usually a NEC. MiNENs in which the most aggressive component is an adenocarcinoma (i.e., tumors composed of adenocarcinomas with a NET component) are much rarer, but in these cases treatment is tailored towards the adenocarcinoma component.
No guidelines exist currently for adjuvant or neoadjuvant chemotherapy. NEC driven tumors are usually treated with etoposide and cisplatin-based regimens[2,27,39]. Adenocarcinoma driven cancers are commonly treated with 5-FU based backbones, however treatment options vary based on the site of the primary, with chemotherapies for MiNENs arising from a particular organ mirroring the systemic therapy for adenocarcinomas at that site. The oncologist may attempt a tailored combination of therapy in which the agents employed display efficacy against both components[30]. Radiation therapy has been suggested in tumors from the esophagus, stomach, rectum, and anus[40].
The genetic similarity of MiNENs to adenocarcinomas and reports of KRAS, BRAF V600E, APC, MMR, and HER-2 amplifications occurring in both components of MiNENs invites the possibility of targeted therapies in these tumors[2,22,40,41]. One series of 44 cases found MMR deficiency in 39% of these tumors[40]. While this did not appear to have prognostic impact, it is an exciting finding raising the possibility for potential response to programmed death 1 blockade.
Mixed NE neoplasms have a complex history particularly in regard to nomenclature which has evolved alongside our increasing recognition of these tumors. The term 'MiNEN' was created to encompass a broader range of non-NE epithelial components beyond just adenocarcinomas. The term also incorporates updates in terminology used for NE neoplasms. MiNEN can be used to refer to tumors outside of the digestive system as well, although the digestive system has been the leading-edge area for these tumors. Diagnosis is in fact very familiar to both pathologists and oncologists alike, with no significant differences compared to diagnosis of each individual neoplasms. Staging is simplified because MiNENs are considered single clonal neoplasms. Prognosis and management are based on, and in general equivalent to, that of the most aggressive component.
| 1. | La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol. 2016;27:284-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (2)] |
| 2. | Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (2)] |
| 3. | Lokuhetty D, White VA, Watanabe R, Cree IA, World Health O, International Agency for Research on C. Digestive system tumours. Fifth ed. Lyon: International Agency for Research on Cancer, 2019. |
| 4. | Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11 Suppl 1:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, Capella C. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel). 2012;4:11-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Uccella S, La Rosa S. Looking into digestive mixed neuroendocrine - nonneuroendocrine neoplasms: subtypes, prognosis, and predictive factors. Histopathology. 2020;77:700-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | La Rosa S. Challenges in High-grade Neuroendocrine Neoplasms and Mixed Neuroendocrine/Non-neuroendocrine Neoplasms. Endocr Pathol. 2021;32:245-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Park JY, Ryu MH, Park YS, Park HJ, Ryoo BY, Kim MG, Yook JH, Kim BS, Kang YK. Prognostic significance of neuroendocrine components in gastric carcinomas. Eur J Cancer. 2014;50:2802-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 10. | Konukiewitz B, Kasajima A, Schmitt M, Schwamborn K, Groll T, Schicktanz F, Delbridge C, Schütze LM, Wilhelm D, Lang C, Lange S, Foersch S, Jank P, Steiger K, Werder AV, Denkert C, Weichert W, Klöppel G, Jesinghaus M. Neuroendocrine Differentiation in Conventional Colorectal Adenocarcinomas: Incidental Finding or Prognostic Biomarker? Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Zhang K, Meyerson C, Kassardjian A, Westbrook LM, Zheng W, Wang HL. Goblet Cell Carcinoid/Carcinoma: An Update. Adv Anat Pathol. 2019;26:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | La Rosa S, Uccella S, Molinari F, Savio A, Mete O, Vanoli A, Maragliano R, Frattini M, Mazzucchelli L, Sessa F, Bongiovanni M. Mixed Adenoma Well-differentiated Neuroendocrine Tumor (MANET) of the Digestive System: An Indolent Subtype of Mixed Neuroendocrine-NonNeuroendocrine Neoplasm (MiNEN). Am J Surg Pathol. 2018;42:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Oberndorfer S. Uber die kleinen dunndarmcarcinome. Verh Dtsch Ges Pathol. 1907;11:113-116. |
| 14. | Capella C, Heitz PU, Höfler H, Solcia E, Klöppel G. Revised classification of neuroendocrine tumors of the lung, pancreas and gut. Digestion. 1994;55 Suppl 3:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B; all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 16. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system: World Health Organization, 2010. |
| 17. | Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 774] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 18. | Cordier R. Les cellules argentaffines dans les tumeurs intestinales. Arch Int Med Exp. 1924;1. |
| 19. | Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system: IARC press Lyon: 2000. |
| 20. | Sun L, Zhang J, Wang C, Zhao S, Shao B, Guo Y, Liu Y, Sun Y. Chromosomal and molecular pathway alterations in the neuroendocrine carcinoma and adenocarcinoma components of gastric mixed neuroendocrine-nonneuroendocrine neoplasm. Mod Pathol. 2020;33:2602-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Yuan W, Liu Z, Lei W, Sun L, Yang H, Wang Y, Ramdas S, Dong X, Xu R, Cai H, Li JZ, Ke Y. Mutation landscape and intra-tumor heterogeneity of two MANECs of the esophagus revealed by multi-region sequencing. Oncotarget. 2017;8:69610-69621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 22. | Jesinghaus M, Konukiewitz B, Keller G, Kloor M, Steiger K, Reiche M, Penzel R, Endris V, Arsenic R, Hermann G, Stenzinger A, Weichert W, Pfarr N, Klöppel G. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol. 2017;30:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 23. | Sinha N, Gaston D, Manders D, Goudie M, Matsuoka M, Xie T, Huang WY. Characterization of genome-wide copy number aberrations in colonic mixed adenoneuroendocrine carcinoma and neuroendocrine carcinoma reveals recurrent amplification of PTGER4 and MYC genes. Hum Pathol. 2018;73:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | La Rosa S, Bernasconi B, Vanoli A, Sciarra A, Notohara K, Albarello L, Casnedi S, Billo P, Zhang L, Tibiletti MG, Sessa F. c-MYC amplification and c-myc protein expression in pancreatic acinar cell carcinomas. New insights into the molecular signature of these rare cancers. Virchows Archiv. 2018;473:435-441. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Vanacker L, Smeets D, Hoorens A, Teugels E, Algaba R, Dehou Mf, De Becker A, Lambrechts D, De Greve J. Mixed Adenoneuroendocrine Carcinoma of the Colon: Molecular Pathogenesis and Treatment. Anticancer Res. 2014;34:5517-5521. [PubMed] |
| 26. | Fujita Y, Uesugi N, Sugimoto R, Eizuka M, Matsumoto T, Sugai T. Gastric mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) with pancreatic acinar differentiation: a case report. Diagn Pathol. 2019;14:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Desai GS, Pande P, Shah RC, Jagannath P. Dilemmas in Diagnosis and Management of Gastroenteropancreatic Mixed Neuroendocrine Non-neuroendocrine Neoplasms: First Single-Centre Report from India. J Gastrointest Cancer. 2020;51:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Moritz AW, Schlumbrecht MP, Nadji M, Pinto A. Expression of neuroendocrine markers in non-neuroendocrine endometrial carcinomas. Pathology. 2019;51:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Duan K, Mete O. Algorithmic approach to neuroendocrine tumors in targeted biopsies: Practical applications of immunohistochemical markers. Cancer Cytopathol. 2016;124:871-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Frizziero M, Wang X, Chakrabarty B, Childs A, Luong TV, Walter T, Khan MS, Morgan M, Christian A, Elshafie M, Shah T, Minicozzi A, Mansoor W, Meyer T, Lamarca A, Hubner RA, Valle JW, McNamara MG. Retrospective study on mixed neuroendocrine non-neuroendocrine neoplasms from five European centres. World J Gastroenterol. 2019;25:5991-6005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | World Health Organization. WHO Classification of Tumours Editorial Board. Thoracic tumours. 5th ed. Lyon: IARC Press, 2021. |
| 32. | Olevian DC, Nikiforova MN, Chiosea S, Sun W, Bahary N, Kuan SF, Pai RK. Colorectal poorly differentiated neuroendocrine carcinomas frequently exhibit BRAF mutations and are associated with poor overall survival. Hum Pathol. 2016;49:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Watanabe J, Suwa Y, Ota M, Ishibe A, Masui H, Nagahori K, Tsuura Y, Endo I. Clinicopathological and Prognostic Evaluations of Mixed Adenoneuroendocrine Carcinoma of the Colon and Rectum: A Case-Matched Study. Dis Colon Rectum. 2016;59:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006;30:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Zhang P, Li Z, Li J, Zhang X, Lu Z, Sun Y, Li Y, Zhou J, Wang X, Peng Z, Shen L, Lu M. Clinicopathological features and lymph node and distant metastasis patterns in patients with gastroenteropancreatic mixed neuroendocrine-non-neuroendocrine neoplasm. Cancer Med. 2021;10:4855-4863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Girardi DM, Silva ACB, Rêgo JFM, Coudry RA, Riechelmann RP. Unraveling molecular pathways of poorly differentiated neuroendocrine carcinomas of the gastroenteropancreatic system: A systematic review. Cancer Treat Rev. 2017;56:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Lin J, Zhao Y, Zhou Y, Tian Y, He Q, Lin J, Hao H, Zou B, Jiang L, Zhao G, Lin W, Xu Y, Li Z, Xue F, Li S, Fu W, Li Y, Xu Z, Chen J, Zhou X, Zhu Z, Cai L, Li E, Li H, Zheng C, Li P, Huang C, Xie J. Comparison of Survival and Patterns of Recurrence in Gastric Neuroendocrine Carcinoma, Mixed Adenoneuroendocrine Carcinoma, and Adenocarcinoma. JAMA Netw Open. 2021;4:e2114180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Pommergaard HC, Nielsen K, Sorbye H, Federspiel B, Tabaksblat EM, Vestermark LW, Janson ET, Hansen CP, Ladekarl M, Garresori H, Hjortland GO, Sundlöv A, Galleberg R, Knigge P, Kjaer A, Langer SW, Knigge U. Surgery of the primary tumour in 201 patients with high-grade gastroenteropancreatic neuroendocrine and mixed neuroendocrine-non-neuroendocrine neoplasms. J Neuroendocrinol. 2021;33:e12967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | de Mestier L, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, Bouché O, Cadiot G, Ruszniewski P, Couvelard A, Hammel P. Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology. 2017;105:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 40. | Lou L, Lv F, Wu X, Li Y, Zhang X. Clinical implications of mismatch repair deficiency screening in patients with mixed neuroendocrine non-neuroendocrine neoplasms (MiNEN). Eur J Surg Oncol. 2021;47:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Golombek T, Henker R, Rehak M, Quäschling U, Lordick F, Knödler M. A Rare Case of Mixed Adenoneuroendocrine Carcinoma (MANEC) of the Gastroesophageal Junction with HER2/neu Overexpression and Distinct Orbital and Optic Nerve Toxicity after Intravenous Administration of Cisplatin. Oncol Res Treat. 2019;42:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jesinghaus M, Germany; Yang Y, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR