Published online May 7, 2022. doi: 10.3748/wjg.v28.i17.1725
Peer-review started: October 4, 2021
First decision: December 4, 2021
Revised: December 12, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 7, 2022
Processing time: 206 Days and 10.4 Hours
The integrity of the gastrointestinal mucosa plays a crucial role in gut homeostasis, which depends upon the balance between mucosal injury by destructive factors and healing via protective factors. The persistence of noxious agents such as acid, pepsin, nonsteroidal anti-inflammatory drugs, or Helicobacter pylori breaks down the mucosal barrier and injury occurs. Depending upon the size and site of the wound, it is healed by complex and overlapping processes involving membrane resealing, cell spreading, purse-string contraction, restitution, differentiation, angiogenesis, and vasculogenesis, each modulated by extracellular regulators. Unfortunately, the gut does not always heal, leading to such pathology as peptic ulcers or inflammatory bowel disease. Currently available therapeutics such as proton pump inhibitors, histamine-2 receptor antagonists, sucralfate, 5-aminosalicylate, antibiotics, corticosteroids, and immunosuppressants all attempt to minimize or reduce injury to the gastro
Core Tip: The integrity of the gastrointestinal mucosa is crucial in gut homeostasis, which depends upon the balance between mucosal injury by destructive factors and healing via protective factors. An excess of destructive agents breaks down the mucosal barrier. Upon injury, under physiological conditions, gastrointestinal mucosa heals itself by complex processes. However, the gut may not heal under pathological conditions. Currently available drugs attempt to minimize or reduce injury to the gastrointestinal tract. Recent studies have focused on improving mucosal defense or directly promoting mucosal repair. This article summarizes the pathobiology of gastrointestinal mucosal healing and reviews potential new therapeutic targets.
- Citation: Oncel S, Basson MD. Gut homeostasis, injury, and healing: New therapeutic targets. World J Gastroenterol 2022; 28(17): 1725-1750
- URL: https://www.wjgnet.com/1007-9327/full/v28/i17/1725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i17.1725
Upon injury, gastrointestinal (GI) epithelial tissue is capable of renewing itself within hours to months by replacing damaged or dead cells, depending on the site and size of the wound. In order to appreciate potential new therapeutic targets, this review will first summarize the current understanding of the processes of mucosal healing and defense and describe their major extracellular regulators. Then, the importance of the quality of ulcer healing and novel approaches to promote such healing will be reviewed. This review focuses on mucosal injury and repair. Deeper injuries such as a deep ulcer, trauma, fistula, or surgical transection and anastomotic healing all require a complex interaction among endothelial cells, fibroblasts, and other cell types to reconstitute the submucosal and muscular layers of the bowel wall. This is beyond the scope of the current review but has been previously reviewed[1-5]. Angiogenesis is critical to these efforts, and requires a complex interaction between endothelial cells, the extracellular matrix, growth factors and cytokines, and other cell types[6,7].
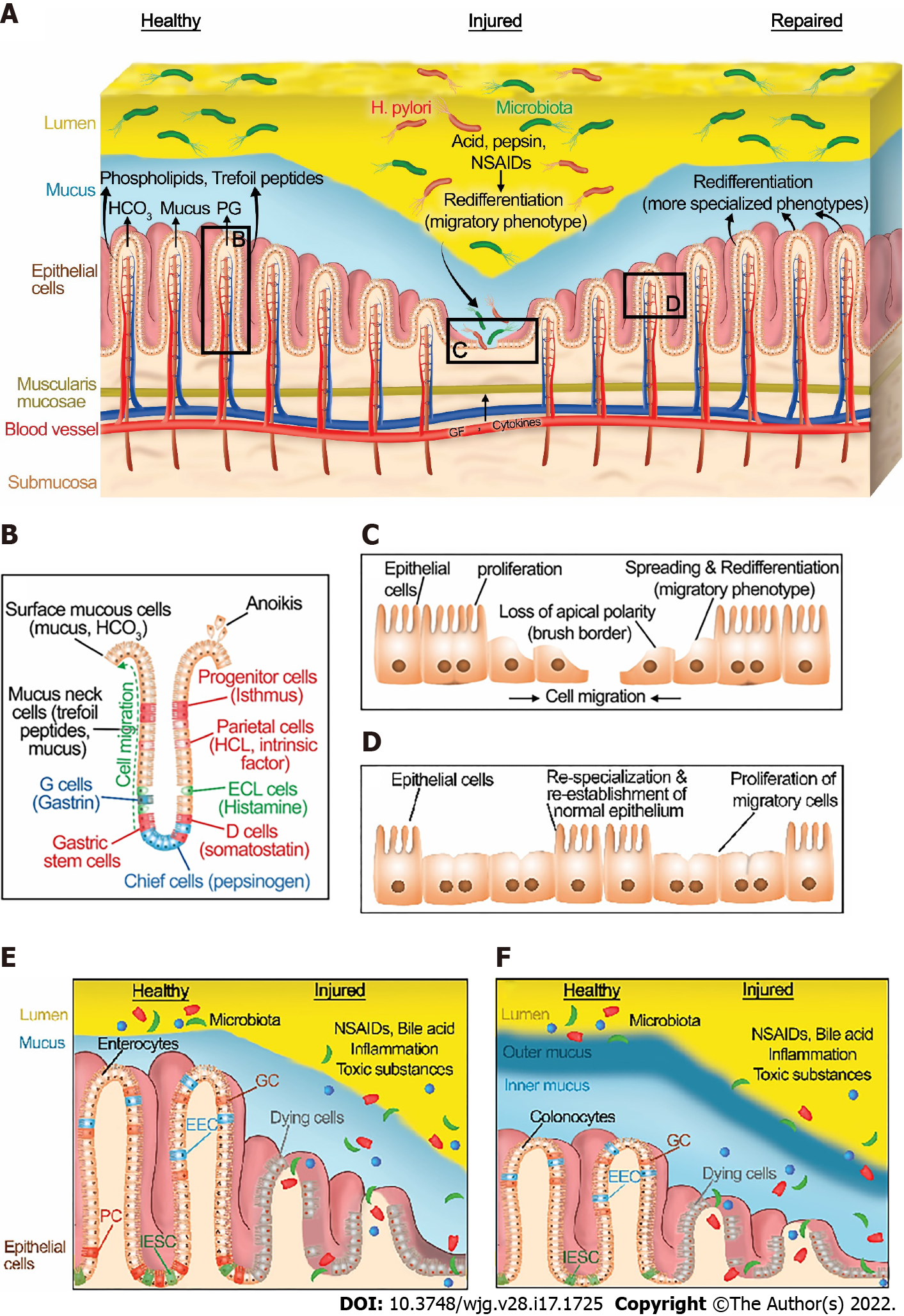
The integrity of the gastrointestinal mucosa is crucial for gut homeostasis. The gut lining is continuously injured during normal gut function[8] by a variety of noxious luminal substances and abrasive interactions with luminal contents (Figure 1A). However, there is normally an equilibrium between gut injury, mucosal healing, and diverse factors that protect the mucosa[5]. This equilibrium favors healing in a healthy state. Under normal physiological conditions, GI epithelial cells migrate from the base of the crypt to the villi, where their interaction with each other and the extracellular matrix (ECM) is disrupted leading to epithelial cell shedding (anoikis)[9] (Figure 1B).
The gastrointestinal mucosa is protected at three levels: pre-epithelial, epithelial, and sub-epithelial defenses. Pre-epithelial protection, the first line of mucosal defense, is provided by the secretion of mucus, bicarbonate, phospholipids, prostaglandins, and trefoil peptides (Figure 1A and B). These factors not only neutralize the acid but also inactivate pepsin at the gastric mucosal surface. In addition, phospholipids secreted into mucus contribute to the hydrophobicity of mucus and prevent back-diffusion of hydrogen ions[10]. Prostaglandins are abundant in gastric juice. They inhibit acid secretion and stimulate mucus and bicarbonate secretion[11]. Bicarbonate-rich mucus is secreted throughout the GI tract, by mucoid cells in the stomach and goblet cells in the intestines, creating a near-neutral pH at the epithelial surfaces in the GI tract, thereby protecting the GI mucosa against autodigestion by the gastric juice and other noxious agents in the lumen[12,13].
Intestinal epithelial cells consist of four important cell types (Figure 1E and F). Enterocytes and colonocytes are most common in the surface epithelium. They are critical for the digestion and absorption of nutrients. Paneth cells are highly specialized cells located in small intestinal crypts. Paneth cells are essential for the secretion of antimicrobial peptides (AMP) such as defensins. These AMPs modulate the composition of the small intestinal microbiota. Goblet cells produce various types of mucin and are found throughout the GI tract. Similarly, enteroendocrine cells are scattered along with the epithelial cells of the GI tract. They produce and secrete more than 20 different hormones in response to nutrients in the lumen that regulate hunger, appetite, and satiety[14]. Intestinal epithelial stem cells (IESCs) are crucial to maintaining intestinal epithelial function and homeostasis in both the small intestine and large intestine. IESCs divide for self-renewal and generate progenitor cells that undergo differentiation into enterocytes, colonocytes, Paneth cells, goblets cells, and enteroendocrine cells[15].
Epithelial protection represents the second line of defense. GI epithelial cells are connected to each other via tight junctions and act as a physical barrier against acid or toxic luminal agents. In addition to stimulating mucus and bicarbonate secretion, prostaglandins reduce the permeability of the epithelium by closing the apical spaces between the epithelial cells, and thus decreasing the exposure of deeper layers along the GI tract to noxious agents by modulating these tight junctions[16]. Tight junction proteins are either transmembrane proteins such as occludin, claudins, and junction adhesion molecule proteins or cytoplasmic plaque proteins such as the zonula occludens proteins. The dysregulation of these proteins via toxin exposure or autoimmune diseases such as celiac disease may lead to disruption of gastrointestinal barrier function[17]. For example, ulcerative colitis may alter the intestinal barrier function via changing the phosphorylation of colonic claudins[18]. The architecture and function of tight junctions are slightly divergent between the different regions of the GI tract and also between different epithelial cells. For example, disruption of occludin alters intestinal barrier function whereas occludin disruption does not cause barrier dysfunction in the stomach[19]. Moreover, the expression of tight junction proteins varies even among the different epithelial cells. For instance, IESCs and Paneth cells have high occludin levels whereas claudin-1, -2, and -7 expression is elevated in Paneth cells, IESCs, and enterocytes, respectively[20].
The final mucosal defense is sub-epithelial protection through augmentation of mucosal blood flow. Vascular flow not only removes acid rather than allowing it to diffuse deeper into the mucosa but also supplies necessary nutrients and oxygen to the epithelial cells for energy-consuming processes such as ion transport and secretion. Gut epithelial cells undergo continuous dynamic self-renewal in response to the damage caused by destructive factors under normal physiological conditions[21,22].
Although the gut epithelium can maintain normal homeostasis in the presence of modest or transient exposure to injurious stimuli, high level or extensive interactions with noxious factors such as excessive secretion of gastric acid and pepsinogen, the substantial inflammation caused by inflammatory bowel disease, or toxic luminal contents including ethanol or medication such as non-steroidal anti-inflammatory drugs (NSAIDs) can unbalance the equilibrium between mucosal injury and healing (Figure 1A).
Gastric juice includes mucus, hydrochloric acid (HCl), bicarbonate, pepsin, and intrinsic factor secreted by mucoid cells, parietal (oxyntic) cells, and chief (zymogenic) cells in the stomach (Figure 1B). Helicobacter pylori (H. pylori) infection, a gram-negative bacterium responsible for 90% of duodenal and gastric ulcers, impairs the bicarbonate secretion and promotes gastric acidity as well[23]. Such hyperacidity may injure the mucosa, causing gastritis, duodenitis, peptic ulcer disease (PUD), or gastroesophageal reflux disease (GERD)[24].
The gastrointestinal mucosa is subjected to numerous physical forces such as strain and pressure during both normal gut function and illness. For instance, luminal chyme, peristalsis contractions, rhythmic villous motility, and some pathological conditions such as inflammatory bowel disease (IBD) may adversely impact GI mucosal healing by increasing luminal pressure[25-29]. Such pressure increases have been shown to inhibit mucosal healing, at least in mice, despite increased mucosal proliferation, and appear to act by inhibiting the cell motility required for restitution[28].
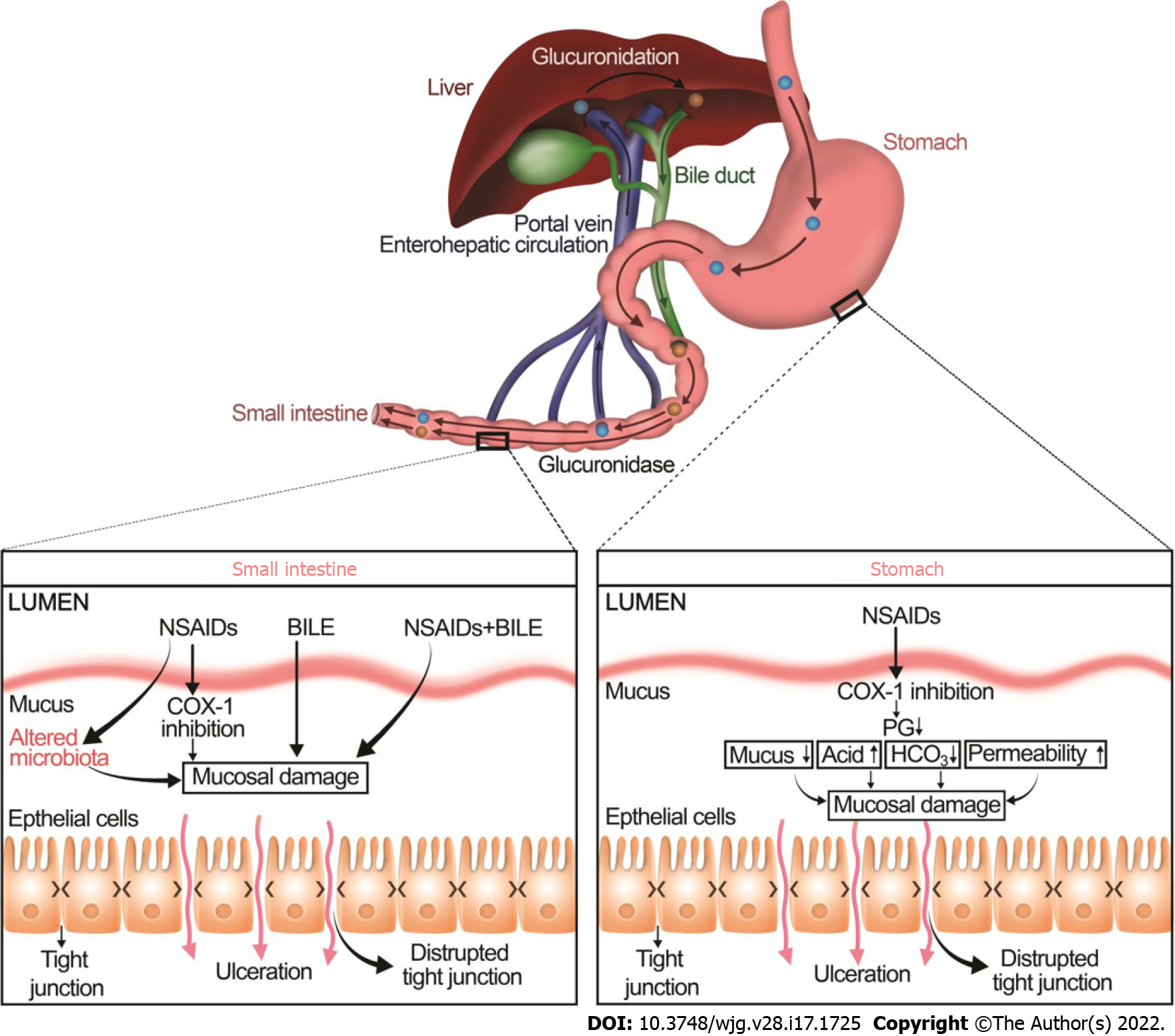
The balance between mucosal injury and healing may also be shifted by drugs such as NSAIDs, corticosteroids, bisphosphonates, potassium chloride, steroids, and fluorouracil[23,30,31]. In particular, many studies have documented that NSAIDs decrease mucus hydrophobicity as measured by contact angle goniometry whereas prostaglandins, gastroprotective compounds, increase the contact angle of gastric mucosa[32,33]. NSAIDs, the most commonly prescribed medications, increase the development of ulcers in the upper and lower GI tract by two distinct mechanisms (Figure 2)[34-37].
NSAIDs injure the upper GI mucosa mainly by cyclooxygenase (COX)-1 inhibition, resulting in a decrease in prostaglandins, mucus, and bicarbonate secretion. Moreover, NSAIDs also alter another important component of mucosal defense, the gastric microcirculatory system. Upon irritation, the gastric mucosa normally increases blood flow to remove any toxins, bacterial products, or back-diffusing acid. Impairment of this hyperemic reaction increases the vulnerability of gastric mucosa to damage[38]. Inhibition of prostaglandins, potent vasodilators, by NSAIDs leads to an increase in vascular tone and thus reduces gastric mucosal blood flow[39], consequently, increases ischemic tissue damage and exacerbating the mucosal injury[40]. NSAIDs may also induce local gastric mucosal injury independent of prostaglandin deficiency[41]. NSAIDs may lyse phospholipids from mucosal epithelial cells and may increase mucosal permeability, which then allows mucosal exposure to luminal aggressive factors such as bacteria and gastric acid[42].
The molecular and cellular mechanisms of NSAID-induced lower GI mucosal injury are clearly distinct from NSAID-induced upper GI injury[42,43]. As in the stomach, NSAIDs may inhibit COX-1 and contribute to mucosal damage. However, unlike gastric injury, the bile acid and intestinal microbiota play a crucial role in the pathophysiology of NSAID-induced intestinal injury[42,44]. NSAIDs and gut microbiota have complex and dynamic interactions. The gut microbiota can alter the efficacy and toxicity of NSAIDs either directly by biotransforming them into metabolites or indirectly by altering the host metabolism (e.g., interfering with hepatic function)[45]. On the other hand, NSAIDs themselves can directly change the composition and function of the gut microbiota or indirectly by altering the physiological functions of the host[45]. For instance, NSAIDs alter the intestinal microbiome by increasing the total number of bacteria and the proportion of gram-negative bacteria, which seems to be linked to the activation of toll-like receptor (TLR) 4 that increases inflammation and contributes to an intestinal injury[46-48].
NSAIDs make complexes with bile acids by glucuronidation in the liver. This interaction alters the stability and structure of bile acids and potentiates bile acid toxicity in the lower GI tract[42]. These NSAID-bile acid complexes are secreted into the duodenum and subsequently reabsorbed back in the ileum via the enterohepatic circulation. Within the intestinal lumen, particularly, in the colon, conjugated primary bile acids are deconjugated into more toxic secondary bile acids, mainly by the gram-positive bacteria[49]. There is crosstalk between the microbiome and the bile acids because bile acids can control the composition of the intestinal microbiome, which in turn regulates the composition and size of the bile acid pool[50,51]. Alteration in the colonic microbiota may cause a shift towards to generation of more toxic secondary bile acids, which eventually increase intestinal permeability, particularly in the colon, bacterial translocation, and mucosal inflammation[52-54].
NSAID-induced ulcers are traditionally treated with proton pump inhibitors (PPIs) or histamine-2 receptor antagonists (H2-antagonists)[55,56], which permit ulcer healing by reducing gastric acid secretion without directly affecting mucosal restitution[5,57,58]. Although PPIs have historically been co-prescribed with NSAIDs to ameliorate gastroduodenal injury and are used to treat NSAID injury, such use may increase the risk of a different problem. There is no evidence that gastric acid plays a key role in the pathogenesis of NSAID-induced lower GI[42,59]. PPIs may worsen NSAID-induced enteropathy by increasing gastric pH and thus changing the enteric microbiome by increasing the number of gram-negative bacteria[35,42,60-62]. Thus, even though PPIs are still recommended to treat upper GI ulcers, their prophylactic use with NSAIDs to prevent upper GI injury is no longer recommended unless the patient has a moderate to high risk of peptic ulcer disease[62,63]. Similar concerns are likely to exist for H-2 blockers.
Inflammatory bowel disease is a broad term to describe disorders including Crohn’s disease (CD) and ulcerative colitis (UC) that are characterized by excessive activation of the mucosal immune system to normal microflora. This causes chronic inflammation and damages the gut mucosa[64,65]. Since the etiology of IBD is still unclear, the primary goal of treatment is centered on the elimination of inflammation with medical therapies such as 5-aminosalicylate, antibiotics, corticosteroids, immunosuppressants, and biological therapy[66-68]. Management of IBD with targeted therapies has been discussed in detail in a recent review[68]. However, none of these therapies is perfect, and even if patients achieve symptomatic remission, maintaining that remission can be challenging[69]. Recent evidence highlights the importance of mucosal healing over and above symptomatic remission in the quality of life and long-term prognosis of IBD patients[70-72].
Once an injury has occurred, diverse processes such as redifferentiation to a migratory phenotype[73-75], migration, proliferation, and eventual redifferentiation back to more specialized cells after healing are all regulated by various factors including growth factors, cytokines, physical forces, and the extracellular matrix itself. These coordinate healing of the injury (Figure 1). At the subcellular level, wounding of the apical plasma membrane is common in the epithelial cells of the intact, normal functioning stomach and intestines in vivo after mechanical and chemical stressors[8,76,77]. Since maintenance of plasma membrane integrity is essential for cell viability, the wounded cell rapidly repairs the injury to restore internal homeostasis and prevent cell death. Plasma repair processes such as tension reduction, budding, patch, endocytosis, and exocytosis may be triggered by the toxic level of Ca2+ influx through the plasma membrane wound to then reseal the injured plasma membrane[78,79].
Relatively small or superficial multicellular mucosal injury undergoes complex wound healing processes that quickly reconstitute the mucosal barrier, depending on the size and depth of the injury. Small wounds, less than eight cells in size, may close by the spreading of neighboring cells and formation of new cell-cell contacts[80,81] or by purse-string wound closure, which involves the formation of a multicellular actin cable purse string around the wound, with actin cables that parallel the wound edge. This then contracts, pulling the adjacent cells together[5,82].
Mucosal injury involving more than eight cells is generally too large for purse-string wound closure. This then requires restitutive epithelial sheet migration to close the injury. Depending on the size and depth of these larger wounds, wound closure will require a longer healing time and may require one or more complex overlapping processes such as differentiation, proliferation, and angiogenesis for wound healing[5,83,84].
Restitution requires a phenotypic redifferentiation. Although some authors describe the initial steps of this process as dedifferentiation, it is the firm opinion of the senior author that this should rather be considered a redifferentiation toward a migratory phenotype. The gut epithelium normally consists of a monostratified layer of differentiated epithelial cells. At the edge of a mucosal wound, epithelial cells change their phenotype from differentiated columnar enterocytes or gastric cells to a migratory phenotype. They lose their typical morphology and (for enterocytes and parietal cells) their microvilli[85], disassemble their apical specialized membrane components[86], flatten out and extend lamellipodia toward the defect. Such migrating cells adopt a squamous morphology with altered integrin[87-89] and cytoskeletal organization[73,85] and specialized cell signaling pathways[90-93] that adapt these cells toward motility (Figure 1C)[73,85,94-96] Moreover, it is worth noting that these signaling events are not only regulated by the activation of signaling proteins but also by the distribution and the amount of the signaling proteins within the migrating cells. For instance, both the actual amount of total focal adhesion kinase (FAK) and the amount of active FAK decrease while the ratio of activated to total FAK increases both in vitro[94] and in vivo[92] as the epithelial cells shift to the migratory phenotype[73]. Similarly, both paxillin protein and tyrosine-phosphorylated paxillin decrease in migrating cells compare to static cells[94]. (Paxillin is an adapter protein critical to focal adhesion complex assembly and disassembly in response to various stimuli.)[97-100]. Total p38, ERK1, and ERK2 proteins do not show differences between migrating and static cells[94]. However, phosphorylated p38 increases, and phosphorylated ERK1 and ERK2 decreases in motile cells compared with nonmigrating cells[94].
Furthermore, the distribution of these signaling proteins also changes in migratory phenotype. In confluent cells, FAK localizes mainly in a perinuclear pattern while FAK appears explicitly at the cell borders contacting other cells in motile cells, with FAK immunoreactivity decreasing toward the migrating lamellipodia that face the wound edge[94]. In contrast to FAK, paxillin is localized at the lamellipodial edges in migrating cells[94]. The difference is more than semantic because considering these migratory cells as a specialized phenotype opens up the possibility for therapy to modulate that phenotype and thereby promote mucosal healing.
The transverse actin cables that drive purse-string closure for smaller wounds line up parallel to the wound edge at the migrating front, connected by cell-cell contacts, and unite the migrating front, so that these redifferentiated cells collectively migrate as a sheet, a.k.a., restitution, to close the wound (Figure 1C)[101-103]. Slightly deeper wounds that injure the basement membrane expose the cells to the interstitial extracellular matrix. While the basement membrane is predominantly laminin and type IV collagen, the deeper interstitial matrix is rich in type I collagen, across which the cells may migrate more rapidly[104,105].
After the closure of the wound by successful restitution, the migrating cells must redifferentiate back to the more specialized phenotypes required for the normal biology of the mucosa (Figure 1D)[102]. Tarnawski et al[106] have demonstrated the critical relationship between defective redifferentiation of these migratory cells and subsequent ulcer recurrence. This will be considered in more detail below.
If the wound surface area is extensive, restitution will likely be insufficient to seal the wound. In this situation, epithelial cell proliferation increases behind the migrating cells to create a larger pool of epithelial cells that can then migrate across and cover the defect (Figure 1C)[107]. However, if the wound extends into deeper layers such as the submucosa and muscularis, these must also be reconstructed for healing by processes beyond the scope of this review. In particular, the reconstitution of nutrient vessels in the submucosa is critical for mucosal wound healing because these provide oxygen and nutrients to the mucosa and remove waste products from the wound site[108,109]. This neovascularization can occur by two distinct processes called angiogenesis and vasculogenesis[110-114]. Angiogenesis refers to the process where new blood vessels are formed from preexisting blood vessels from the wound’s adjacent vasculature by sprouting and forming tube-like structures and networks. Vasculogenesis is the de novo formation of new blood vessels from the differentiation of bone marrow-derived progenitor stem cells.
GI ulcers have traditionally been assessed in clinical settings by a superficial visual endoscopic examination that cannot assess the histological and ultrastructural characteristics of the mucosa or deeper layers. Ulcer recurrence is, unfortunately, common, with rates exceeding 60% if the underlying problem has not been successfully addressed[23]. Recurrence of GI ulcers may be related to many factors including gastric acid secretion, H. pylori, NSAIDs, hormonal complications, size and depth of ulcers, anti-ulcer treatment, age, gender, comorbidity, alcohol consumption, and smoking[115-118]. In 1991, Tarnawski et al[119] drew attention to the relationship between recurrence of ulcers and ultrastructural abnormalities of deeper layers such as poor `redifferentiation, dilation of glands, reduced mucosal height, and disorganized microvascular network after ulcer healing and proposed the concept of the quality of ulcer healing (QOUH)[119-121]. QOUH is defined as ideal ulcer healing, demonstrating flat ulcer scar, high functional restoration, and histological maturity of the regenerated tissue[115,122]. Many patients treated with PPIs for GI ulcers still suffered from a recurrence of ulcers despite continuous anti-ulcer therapy[115,122-124]. It appears that acid inhibition by PPIs or H2-antagonists may be insufficient for successful high-quality gastroduodenal ulcer healing because low levels of prostaglandins and high levels of oxygen free radicals entail poor QOUH and thus potentiate ulcer recurrence[122,125,126].
Overall, cumulative data highlight the necessity of QOUH for successful and permanent ulcer healing and point out that contemporary treatments such as PPIs and H2-antagonists do not always provide such high-quality healing. Therefore, to improve QOUH and decrease the rate of recurrence of GI ulcers, new antiulcer drugs need to be developed to address this. Investigation of the endogenous biologic regulation of mucosal healing, suggests new therapeutic targets, both extracellular and intracellular.
Supplementing available therapeutic modalities that attempt to minimize or reduce injury, investigators have more recently focused on enhancing mucosal defense or promoting mucosal repair. Both mucosal defense and mucosal healing processes such as restitution, proliferation, angiogenesis, and vasculogenesis can be influenced by acid secretagogues, growth factors, trefoil peptides, cytokines, angiogenic factors, luminal nutrients, and the gastrointestinal microbiota[5,25,127]. In addition, physical forces like strain and pressure, engendered by peristalsis, villous motility, and interaction with luminal contents can influence intestinal epithelial migration and proliferation in a complex manner influenced by the deposition of fibronectin at the site of injury[25,128,129].
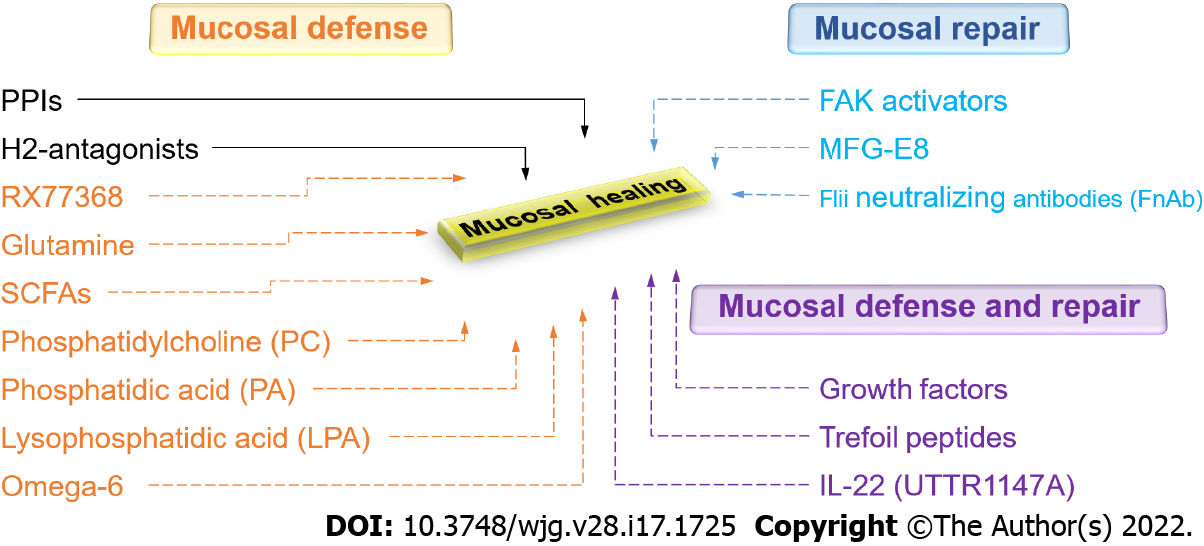
Under physiological conditions, the stomach protects itself against various forms of endogenous and exogenous injury, primarily by gastric acid. Gastroprotective mechanisms could be triggered by acid secretagogues such as gastrin, histamine, and thyrotropin-releasing hormone (TRH)[130-132]. Pentagastrin, synthetic gastrin, stimulates gastroprotection in acidified aspirin-induced gastric injury in rats, likely through the activation of histamine-2 receptors, since this is abolished by ranitidine[133]. However, exogenous gastrin protects the rat gastric mucosa against ethanol-induced lesions but not against aspirin-induced gastric damage in rats[134]. Several studies have shown that exogenous histamine-stimulated acid secretion also exerts a protective effect on the gastric mucosa against erosions induced by exogenous HCl in rabbits and frogs by stimulating a greater alkaline tide[135,136]. The central vagal activation by intracisternal injection of the thyrotropin-releasing hormone analog RX77368 enhances mucosal resistance as well by stimulating mucosal blood flow via prostaglandin-independent manner which eventually results in the removal of diffused acid from the subepithelial interstitial space[137]. In addition, RX77368 increases the thickness of the mucus gel via prostaglandin-dependent manner which slows down the acidification of surface cells[137]. The potential therapeutic adaptation of molecules like RX77368 and other acid secretagogues awaits the further exploration of the disparities between results depending on how the ulcers are induced, as well as challenges with their pharmacologic delivery.
Growth factors have diverse pathophysiologic effects, including cytoprotection against destructive agents, epithelial wound healing in response to injury[102,138-140], and angiogenesis[141-144]. Epidermal growth factor (EGF) and transforming growth factor TGF-α are structurally related but different polypeptide growth factors[145]. They both bind to the same cell-surface EGF/TGF-α -receptor and induce generally similar effects[145].
EGF may act in a cytoprotective fashion against mucosal injury by increasing secretion of mucus and bicarbonate[146-148], enhancing blood flow[149-151], or releasing other cytoprotective agents such as prostaglandins[152]. Pretreatment of the stomach[153,154], small intestine[146,155], and colon[149,150] tissues, both in vivo and in vitro, with EGF decreases mucosal damage by various noxious agents. TGF-α is similarly cytoprotective against gastric injury by ethanol, acetic acid, or aspirin[156,157]. Pretreatment of Caco-2 cells with EGF prevents deoxycholate-induced cellular damage, at least in part, by changes in intracellular calcium content[158], suggesting that EGF exerts direct cytoprotective effects on the epithelium in addition to its effects on blood flow and mucus secretion. Furthermore, because this EGF-induced cytoprotection was observed following only 30 minutes of pretreatment (insufficient for proliferation), these results also suggest that this protection is independent of the mitogenic effects of EGF[158]. Consistent with this idea, adding EGF to the basal surface of rabbit primary gastric epithelial cell monolayers cultured on collagen-coated inserts enhances cytoprotection against apical surface acid by opening the plasma membrane calcium channels and increasing intracellular calcium[159].
In addition to their cytoprotective effects, EGF and TGF-α also promote mucosal healing after injury, stimulating both cell motility and cell proliferation[104,140,154]. Indeed, part of the epithelial mucosal shift to a phenotype adapted to wound healing may be an increase in sensitivity to these growth factors. A recent study demonstrated a 75-fold increase in the number of cells expressing detectable EGF-receptors at the ulcer margin after gastric ulcer induction in rats[160]. Either parenteral or local submucosal intra-ulcer injection of EGF caused a comparable acceleration in the healing of acetic-acid-induced rat gastric ulcers, at least in part by increasing gastric blood flow, decreasing gastric acid secretion, and upregulating COX-2 expression[161]. This is in agreement with previous reports suggesting that COX-2 -influences mucosal healing by regulating both the hyperemic response and epithelial cell proliferation[162,163].
The trefoil peptides may also offer new opportunities for therapy because they are important both for mucosal defense[164-166] (by increasing the viscoelasticity of mucus[167,168]) and mucosal repair[169-171] (by influencing reepithelization[171] and inflammation[172]). The trefoil factor (TFF) family includes TFF1 (also called pS2) expressed in gastric surface mucous cells, TFF2 (also called a spasmolytic polypeptide or SP) produced by mucus-producing gastric mucous neck cells, antral gland cells, and duodenal Brunner’s glands, and TFF3 (also called intestinal trefoil factor or ITF), predominantly produced by goblet cells of the small and large intestine and found abundantly within the mucus. A trefoil domain consists of three loops created by disulfide bonds and all TFFs are comprised of two trefoil domains[173]. Trefoil peptides have been detected in different forms including monomers, dimers, and complexes with other molecules. This influences the strength of their association with mucin[173]. In particular, TFF dimers tightly interact with mucin, increasing the viscosity and elasticity of mucus in comparison to the effect of TFF monomers[167,174].
The TFFs are mostly distributed to the basolateral domain of gastric neck cells and parietal cells in the stomach, the Paneth cells in the small intestine, and the crypt cells in the colon[175]. TFF interactions and specific functions have been discussed in detail in a recent review[176]. A specific TFF receptor has not yet been described. However, some binding and functional studies propose potential TFF receptors that may influence epithelial restitution. TFFs have been reported to bind to transmembrane proteins such as the β1 integrin subunit, CRP-ductin, CXC chemokine receptor (CXCR) 4, CXCR7, proteinase-activated receptor (PAR) 2, PAR4, leucine-rich repeat and Immunoglobin-like domain-containing protein (LINGO) 2, LINGO3, and EGFR[177-181]. TFF3 enhances wound healing by activating EGFR and inducing MAPK[182] and PI3K/Akt signaling pathways in vitro[183] whereas TFF2 directly activates CXCR4 and enhances the phosphorylation of ERK1/2 and Akt in gastric epithelial cells[184]. Indeed, the CXCR4 antagonist AMD3100 blocks TFF2-dependent gastric epithelial repair[170]. TFFs, specifically TFF2 and TFF3, regulate epithelial motility via integrin-binding and activating focal adhesion kinase as well[175]. TFF2 also promotes cell migration via PAR4[185], while TFF3 activates PAR2[186]. Furthermore, TFF2 peptide may be required for optimum activity of EGFR and/or EGF signaling in the stomach because heparin-binding EGF and TGF-α do not induce EGFR activation in the stomachs of Tff2 KO mice[177].
Oral administration of trefoil peptides, recombinant human SP, or rat ITF protects the gastric mucosa against ethanol or indomethacin-induced injury in a prostaglandin-independent manner[164]. Similarly, a more recent study has also shown that both parentally and topically applied trefoil peptides reduce ethanol-induced gastric damage, assessed by measurement of gastric mucosal Na+ leakage and area of macroscopic injury in rats[187]. Complementing these results, transgenic mice that overexpress human TFF1 display increased resistance to indomethacin-induced small intestinal damage[188] whereas ITF-deficient mice are more prone to ulceration and hemorrhage after oral administration of dextran sulfate sodium (DSS)[189], suggesting that trefoil peptides play an important role in GI mucosal protection. There are likely to be several mechanisms by which the trefoil peptides promote mucosal healing. For instance, exogenous recombinant TFF2 increases epithelial wound healing by decreasing inflammation by negatively regulating IL-12 production from macrophages and dendritic cells[172] whereas exogenous TFF3 activates epithelial wound healing via the Na/H exchanger-2[171] and accelerates gastric repair via a mechanism that does not require cyclooxygenase activation[170].
TGF-β expression increases in affected mucosa from patients with IBD[190], and at the edge of human gastric and colonic ulcers[92]. Intravenous administration of recombinant bone morphogenetic protein (BMP)-7, a subfamily of TGF-β superfamily, for five days significantly accelerates the healing of trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats by decreasing the expression of pro-inflammatory cytokines (IL-6, TNF-b, ICAM-1)[191]. It should be noted that all of these growth factors and cytokines mentioned in this section interact in a complex fashion, and TGF-β potentiates many of them[127,192,193]. TGF-β also stimulates the synthesis of FAK, a key intracellular signal protein for cell motility and proliferation[92].
Basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF) stimulate the healing of acetic acid-induced gastric lesions in rats similarly when administered intraperitoneally or by local submucosal injection at the ulcer site, suggesting that these growth factors also accelerate mucosal repair[161]. The healing of gastric ulcers by bFGF and HGF may involve enhancement of gastric blood blow around the ulcer, suppression of gastric acid secretion, and upregulation of COX-2 expression[161].
When the mucus barrier fails due to overexposure to the noxious agents, acid-back diffusion occurs. In healthy mucosa, increased blood flow response rapidly increases the circulation of pH neutral or slightly alkaline blood through the mucosa to neutralize the diffused acid[103]. Moreover, new vasculature is needed to perfuse and support the newly forming tissue. Therefore, wounds deeper than the epithelial layer also require the formation of new blood vessels in granulation tissue for mucosal healing. Like restitution and proliferation, neovascularization is also modulated by growth factors. Vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), fibroblast growth factor (FGF), and angiopoietin-1(Ang1) are essential for blood vessel regeneration via angiogenesis and vasculogenesis following mucosal damage[141-144] and can therefore facilitate deep wound healing[112,193-195]. VEGF, the most potent angiogenic factor, is an indi
Cytokines are also involved in the regulation of mucosal barrier function at multiple levels including mucosal homeostasis and inflammation[198]. Proinflammatory cytokines such as tumor necrosis factor (TNF) and IL-13 are upregulated in the inflamed mucosa of IBD patients. Anti-TNF therapy promotes mucosal healing in many patients, but not all patients respond to anti-TNF therapy. Many investigators have therefore focused on other cytokines to improve mucosal barrier function. Unfortunately, many of these attempts have ended disappointingly. For instance, experimental colitis in mice is controlled by using ROR-gamma null Th17 cells, which cannot produce IL17A/F and not induce colitis[199]. However, an anti-IL17A monoclonal antibody not only failed to improve CD in clinical trials but actually aggravated adverse symptoms[200]. IL-13 has seemed similarly promising in pre-clinical studies[201-204], but a trial of IL-13 blockade in UC failed[205]. Such failures of blockade of pro-inflammatory cytokines have recently prompted attempts to use anti-inflammatory IL-10 family cytokines to promote colonic mucosal healing. IL-10 is a major anti-inflammatory cytokine that targets hematopoietic cells in various autoimmune diseases[206]. Gene therapy with a single intravenous injection of an adenoviral vector encoding IL-10 (AdvmuIL-10) diminishes TNBS-induced colitis in mice, decreasing histological injury scores, weight loss, stool markers of inflammation (IL-1β and TNFR-II), and serum amyloid protein in comparison to empty cassette virus (Adv0) or PBS treated mice in TNBS-induced colitis model[207]. Gelatin microspheres containing IL-10 have been developed to increase local bioavailability as a sustained release preparation[208]. Gelatin-microsphere-IL-10 treatment remarkably decreases colonic inflammation in IL-10(-/-) mice compared to treatment with IL-10 alone treatment, at least in part by decreasing IL-12 mRNA expression and down-regulating CD40 expression in macrophage-1 positive cells[208]. However, recombinant IL-10 did not improve clinical symptoms in Crohn’s disease[209]. Such disappointing results raise the possibility that manipulating a single cytokine may have unpredictable results because of its effects on the web of compensatory pro-inflammatory and anti-inflammatory cytokine pathways in the inflamed mucosa[198].
One cytokine that may be promising is IL-22. Even though it belongs to the IL-10 family of cytokines, IL-22 is unlike IL-10 in that it targets non-hematopoietic epithelial cells. IL-22 is produced by, apart from the adaptive T cell, innate cells including innate lymphoid cells (ILCs), specifically ILC3 cells in the GI tract. IL-22 has dual roles in inflammation. It can act as a protective (anti-inflammatory) cytokine or a pathological (pro-inflammatory) cytokine[210]. IL-22 influences various tissue epithelial functions such as inflammation[211,212], barrier integrity, regeneration, wound healing[213-215], and host defense against pathogens[216,217]. Beneficial effects of IL-22 have been demonstrated in various murine colitis models[210,218]. However, IL-22 actually appears to worsen the anti-CD40-induced colitis model, in that neutralization of IL-22 reduces the weight loss and colitis scores caused by the anti-CD40 injection and administration of IL-22 then recreates the colitis[219]. Thus, although most animal studies raise the possibility that recombinant human (rh) IL-22 might be a promising therapy for IBD, it remains unclear which effect will be seen in human disease. However, as for other cytokines, the short half-life of rhIL-22 (less than 2 h) limits its clinical applications. Several groups have sought to overcome this obstacle by engineering recombinant fusion proteins with a half-life of 1-2 wk to improve the cytokine’s pharmacokinetic properties. Currently, seven IL-22 clinical trials have been investigated for different indications. UTTR1147A is a human IL-22 fusion protein that links the human IL-22 with the Fc portion of human immunoglobulin (Ig) G4, which is prepared for IBD studies[220]. Extensive in vitro and in vivo studies suggest that UTTR1147A decreases histologic colitis severity by a pathway involving STAT3 activation[220]. These pre-clinical studies demonstrate that UTTR1147A is well tolerated and is not associated with increased inflammatory cytokines in mouse, rat, and monkey studies[220]. A randomized phase-I healthy volunteer study of UTTR1147A demonstrated satisfactory safety and pharmacokinetic profile[221]. A phase-II open-label extension study to evaluate the long-term safety and tolerability of UTTR1147A in patients with moderate to severe UC and CD continues with an estimated completion date in 2025[222].
Therapeutic use of growth factors (except BMP-7) may be limited by their low protein stability[144]. In addition, despite their beneficial effects on the GI tract, long-term or systemic use of any growth factors, trefoil peptides, or cytokines that stimulate cell proliferation, either for cytoprotection or for mucosal healing, may raise concerns about inducing hyperproliferative or dysplastic lesions and potential tumorigenesis. This remains an open issue for such mitogens.
Luminal nutrients and microbiota are also crucial for the maintenance and repair of the gut mucosa. Short-chain fatty acids (SCFAs) are produced by commensal microbiota, mostly by gram-positive anaerobic bacteria, and are essential for perpetuating intestinal health[223,224]. These SCFAs, especially butyrate, are a major energy source for enterocytes and support gut homeostasis[225-227]. SCFAs may stimulate the differentiation of epithelial cells and their proliferation in vivo[228-230], whereas they promote only differentiation in cell culture models but inhibit proliferation and migration[231-235]. Long known as an energy supply for colonocytes and enterocytes, SCFAs have attracted may also enhance gut barrier function. SCFAs decrease acid-back diffusion by dilating arterial walls and increasing blood flow in gut mucosa[236,237]. In addition, several studies have documented improved intestinal barrier function after SCFA supplementation[238-240]. The SCFAs activate 5’ adenosine monophosphate (AMP) kinase and therefore promote tight junction assembly, which in turn enhances intestinal barrier function[241,242]. However, a recent clinical study found no evidence that butyrate monotherapy or a combination of three SCFAs offered any advantage over placebo in improving the disease activity index in ulcerative colitis patients receiving maintenance oral anti-inflammatory medication[243].
Amino acids such as arginine, histidine, and glutamine promote enterocyte proliferation and decrease mucosal permeability by regulating tight junction proteins[244-247]. A recent study proposed that histidine and arginine play an important role in stimulating intestinal restitution, probably stimulating FAK via the TGF-β/Smad2 signaling pathway[248]. Glutamine modulates the phenotype of gut epithelial cells by stimulating proliferation and decreasing differentiation in vitro[249]. Similarly, many studies have been shown that glutamine also promotes cell proliferation of intestinal epithelial cells in weanling mice[250] and weaning piglets[251], prevents mucosal injury, and regulates enterocyte restitution following acetic acid-induced intestinal injury in rats[252].
Biologically active phospholipids in milk, phosphatidylcholine (PC) and phosphatidic acid (PA), and their metabolites such as lysophosphatidic acid (LPA), all act to increase the barrier function of GI mucosa by increasing the hydrophobicity of the mucus[253]. This makes the tissue non-wettable[10] and provides mucosal protection against aspirin-induced gastric injury in mice[253]. Dietary essential omega-6 fatty acids can enhance the biosynthesis of prostaglandins and increase the GI mucosal barrier[254]. Milk fat globule-epidermal growth factor 8 (MFG-E8), a glycoprotein found in mammary epithelial cells but also produced by lamina propria macrophages, also plays a vital role in modulating enterocyte migration along the crypt-villus axis[255].
Epithelial sheet migration during gut-healing requires crosstalk between focal adhesion (FA) complexes in the lamellipodium and the ECM. The extracellular matrix is an extremely dynamic meshwork comprised of proteins, glycosaminoglycans, and glycoconjugates. Its composition and organization differ between tissue types and with physiological and pathological conditions[256,257]. Besides its structural support, the ECM has a direct role in gastrointestinal wound healing by inducing extensive signaling cascades[258-260]. Plasma and tissue fibronectin accumulating in deeper wounds also help to shift the cells to a phenotype that responds to repetitive deformation by increased motility rather than by classical differentiation[129,261]. ECM remodeling is performed by matrix proteinases such as matrix metalloproteinases (MMPs), lysyl oxidases, and heparanases[262]. The gelatinases, a subgroup of MMPs, consist of two proteinases gelatinase A (MMP-2) and gelatinase B (MMP-9). In particular, MMP-9 is upregulated in the inflamed intestinal mucosa of IBD patients[263-267]. Furthermore, anti-gelatinase neutralizing antibodies have been reported effective in murine DSS-induced colitis[268]. However, a phase II, randomized, placebo-controlled study found that the MMP-9 inhibitor andecaliximab did not induce a significant symptomatic or endoscopic response in patients with active Crohn’s disease[269]. This lack of efficacy in Crohn’s disease prompted the termination of another clinical trial of the same agent in active ulcerative colitis[270]. Thus, while modulation of matrix metalloproteinases remains an attractive target in IBD, further exploration of the science involved and the reasons for the failure of the clinical trial are needed.
Epithelial restitution begins at the edge of the wound with the redifferentiation of epithelial cells. Reorganization of the actin cytoskeleton is controlled by the Rho family of GTPases including RhoA, Rac1, and Cdc42 (Figure 1C)[93,271-273]. Epithelial cells then form protrusions called lamellipodia with new focal adhesions (FAs) at the leading edge of the motile cells. The migrating cell increases its contractile forces and disassembles focal adhesions at the rear edge allowing the entire cell to move forward[274-276]. Cell-cell linkages[276] transmit this force to other cells behind the migrating front and stretch the epithelial layer across the wound as a sheet. This sheet migration is characteristic of epithelial cells and differs from the individual cell motility displayed by other cell types.
The cytoskeleton, a complex and dynamic network of actin filaments, microtubules, and intermediate filaments, is also an important factor in wound healing[277]. Epithelial restitution relies on the coordination of forward protrusions and retraction forces at the rear edge, which is orchestrated by the actin and microtubule cytoskeleton[278]. In the lamellipodium, the elongating actin filaments produce the driving forces for the protrusion while microtubules form a polarized network that permits organelle and protein transport throughout the cell during cell migration[279,280]. Intermediate filaments, however, are generally considered for the maintenance of the overall cell shape[280]. Alternatively, or in combination with therapy to reduce ongoing injury by improving mucus barrier function and promoting angiogenesis, one could consider attempting to directly stimulate restitution in order to accelerate barrier reconstitution. Thus, proteins that modulate cytoskeleton dynamics might be targeted for optimal wound repair. Fidgetin-like 2 (FL2), a microtubule-severing enzyme, regulates the organization of the microtubule cytoskeleton for faster and successful repair of murine wounds[281]. Actin remodeling proteins such as talin[282], Ehm2[283], filamin-a[284], gelsolin[285], and flightless I (Flii)[286] have also been identified as potential new targets for improved wound healing. Unlike other members of the gelsolin family, Flii inhibits actin polymerization and FA turnover, thus decreasing migration[286,287]. Flii neutralizing antibodies (FnAb) decreased wound area with a quicker rate of healing in porcine and murine models of wound healing, respectively[288,289].
Cell migration, and consequently wound healing, depend critically on the dynamics of assembly and disassembly of FAs. The subunit composition of integrin receptors and the downstream signaling pathways may vary in different scenarios[290-293]. Nevertheless, integrin binding to ECM triggers focal adhesion formation by recruiting many structural and signaling proteins including FAK, a non-receptor tyrosine kinase[294-298]. FAK regulates FA dynamics both by recruiting other FA proteins such as Src to FA sites and by phosphorylating other signaling and adapter FA proteins such as paxillin and p130Cas[299-301]. FAK also influences the cytoskeletal remodeling essential for cell migration by regulating the Rho family of small GTPases such as Cdc42, Rac1, and RhoA[302-305]. Inhibition of FAK inhibits cell migration[94,298].
Although FAK appears to activate cell motility and promote restitution, and FAK is indeed activated during cell motility, levels of both activated FAK and total FAK protein (including both active and inactive FAK) actually decrease in migrating GI epithelial cells in vitro and at the edge of human gastric and colonic ulcers in vivo even though the proportion of activated FAK increases (at least in vitro)[92,94]. This reflects decreased FAK synthesis in cells that have adopted the migratory phenotype[306]. This apparently paradoxical reduction in this important protein makes FAK an attractive target for possible therapeutic intervention to promote mucosal healing.
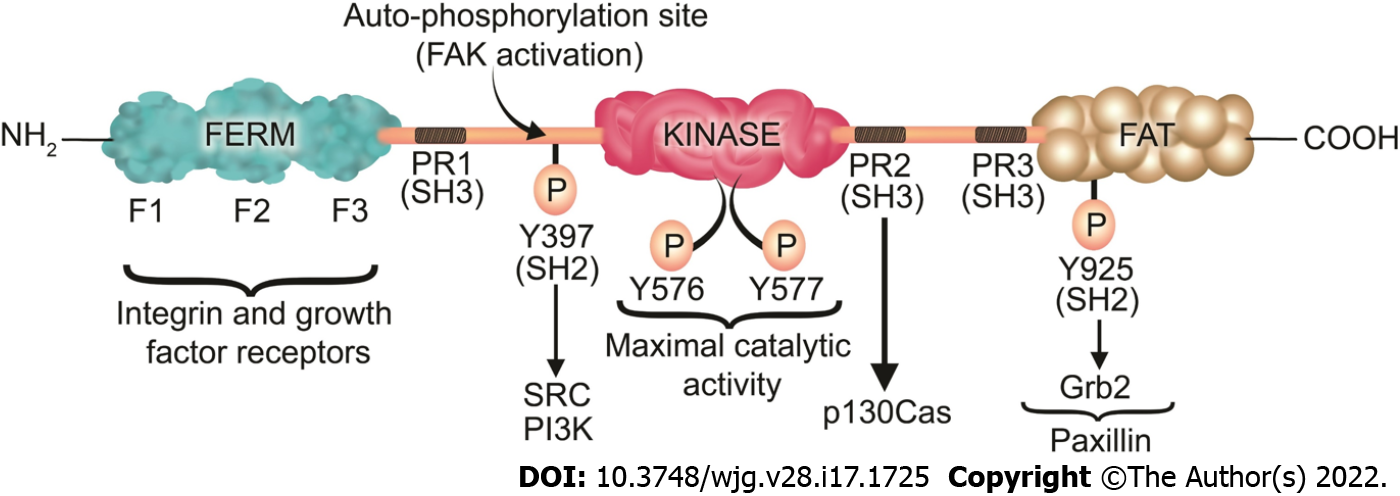
FAK is a 125 kDa protein comprised of an N-terminal FERM (band 4.1-ezrin-radixin-moesin) domain, a central kinase domain, three proline-rich regions that are binding sites for Src homology 3 (SH3) domain-containing proteins, and a C-terminal focal adhesion targeting (FAT) domain (Figure 3).
The FAT domain consists of a four-helix bundle[307] and is critical for targeting FAK to FAs via binding to paxillin[308]. In an inactive (autoinhibited) state there is an interaction between the FERM and kinase domains which prevents FAK autophosphorylation at Y397[309]. Upon competitive binding of candidate activating proteins such as the cytoplasmic regions of β-integrins or growth factor receptors on the F2 domain of FERM, the autoinhibited conformation of FAK is disassembled[310]. This conformational change allows Y397 phosphorylation, a key event in FAK activation[311]. In a subsequent step, Src is recruited and activated via SH2 binding to pY397 and SH3 binding to the PxxP sequence in the linker region, an essential step in promoting cell migration[311]. Then, Src phosphorylates the activation loop residues Y576 and Y577 of FAK and it acquires full catalytic activity after phosphorylation of the activation loop[312]. Phosphorylation of FAK at tyrosine 925 residue creates an SH2 binding site for the growth factor receptor-bound protein 2 (Grb2), adaptor protein[313]. The Grb2 binding site at FAK-Y-925 overlaps with one of the paxillin binding sites in the FAT domain of FAK[313]. The binding of Grb2 disassociates paxillin from FAK and potentiates the release of FAK from FAs[313]. On the other hand, paxillin acts as a scaffold protein for ERK signaling[305]. Subsequently, ERK may modulate FA turnover by further phosphorylating paxillin[305]. Therefore, Paxillin and Grb2 are critical FA proteins that interact with FAK and play an important role in FA turnover[97,313].
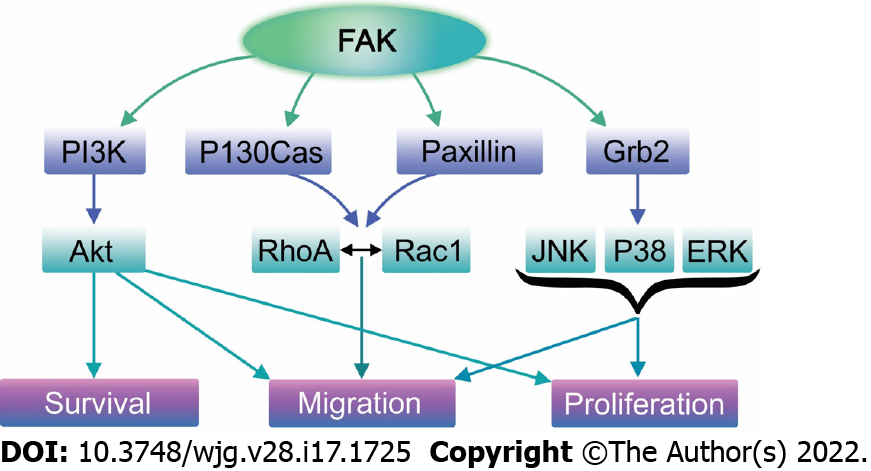
FAK has both a structural role as a scaffold for protein-protein interactions and a kinase function that phosphorylates many substrates in diverse signaling events[314,315]. Its non-kinase scaffolding function allows several different proteins to bind its N-terminal FERM domain and C-terminal FAT domain, tethering them into complexes (Figure 3). For instance, FAK may regulate cell migration serving as a scaffold for Src phosphorylation of p130Cas[316] in FAs. Similarly, nuclear FAK may promote cell survival functioning as a scaffold to stabilize p53-Mdm2 complexes, promoting p53 ubiquitination and proteasomal degradation[317]. On the other hand, in its kinase signaling capacity, FAK triggers many downstream signals including the Ras/Raf/MAPK[97,296,318-320], p130Cas-Crk[321-324], and phosphatidylinositol 3-kinase (PI3K)-Akt pathways[317], which in turn coordinate to regulate cell proliferation, migration, and survival (Figure 4)[313,325].
Recent evidence suggests that direct modulation of FAK activity is possible, practical, and effective via small molecule FAK activators[326]. A novel small molecule with drug-like properties, ZINC40099027 (ZN27), that mimics the FERM domain of FAK has been identified from the ZINC database and activates FAK in human intestinal epithelial cells without activating Pyk2, the closest paralogue of FAK, or Src, another canonical nonreceptor tyrosine kinase within focal adhesions[327]. Indeed, ZN27 directly activates both full-length 125 kDa and its 35 kDa kinase domain, increasing the maximal activity (Vmax) of FAK, suggesting that ZN27 is a highly potent and selective activator acting allosterically on the 35 kDa FAK kinase domain[328]. ZN27 not only activates FAK but also stimulates intestinal epithelial migration in vitro and mucosal healing in mice after ischemic injury or injury by indomethacin[327]. ZN27 also activates FAK in gastric epithelial cells and promotes gastric mucosal healing in mice subjected to chronic ongoing injury by aspirin[58]. Structure-activity-relationship studies have developed a library of novel FAK activators based on ZN27, that have drug-like properties, activate FAK, and stimulate epithelial sheet migration in vitro[329]. At least one such molecule (dubbed compound 3) demonstrates reasonable drug-like properties based on in vitro, in vivo, and in silico results with no obvious toxicity[329]. Further development of this lead molecule may offer the potential for a new therapeutic approach to actually stimulate mucosal healing by activating FAK.
Given the enormous impact of GI mucosal healing on human health, there is certainly a need to expand therapeutic options in this regard. A new understanding of the biology of mucosal healing suggests several different possibilities (Figure 5). These include FAK activators, UTTR1147A, endoscopic gene therapy for angiogenic growth factors, mucus barrier enhancement via the thyrotropin-releasing hormone analog RX77368 or trefoil peptides, enhanced energy for the mucosa with butyrate, and attempts to increase the regenerative ability of the epithelium with growth factors, cytokines, or trefoil peptides. Future work will determine which of these potentially promising avenues will prove successful and will need to balance their effects against potential risks and issues, including bioavailability, mitogenicity, and tumorigenesis.
| 1. | Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:1250-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Aviello G, Knaus UG. ROS in gastrointestinal inflammation: Rescue Or Sabotage? Br J Pharmacol. 2017;174:1704-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 218] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 3. | Lam A, Fleischer B, Alverdy J. The Biology of Anastomotic Healing-the Unknown Overwhelms the Known. J Gastrointest Surg. 2020;24:2160-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Mortensen JH, Lindholm M, Langholm LL, Kjeldsen J, Bay-Jensen AC, Karsdal MA, Manon-Jensen T. The intestinal tissue homeostasis - the role of extracellular matrix remodeling in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2019;13:977-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Basson MD. Hierarchies of healing in gut mucosal injury. J Physiol Pharmacol. 2017;68:789-795. [PubMed] |
| 6. | Powell RJ, Bhargava J, Basson MD, Sumpio BE. Coculture conditions alter endothelial modulation of TGF-beta 1 activation and smooth muscle growth morphology. Am J Physiol. 1998;274:H642-H649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Ahluwalia A, Patel K, Hoa N, Brzozowska I, Jones MK, Tarnawski AS. Melatonin ameliorates aging-related impaired angiogenesis in gastric endothelial cells via local actions on mitochondria and VEGF-survivin signaling. Am J Physiol Gastrointest Liver Physiol. 2021;321:G682-G689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 8. | McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology. 1989;96:1238-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 9. | Liu X, Xiao L, Wang JY. Posttranscriptional control of intestinal epithelium homeostasis by RNA-binding protein HuR. Sheng Li Xue Bao. 2020;72:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 222] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 11. | Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol Rev. 2008;88:1547-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 444] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Matúz J. Role of mucus in mucosal protection through ethanol and pepsin damage models. Acta Physiol Hung. 1992;80:189-194. [PubMed] |
| 13. | Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922-G929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 718] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 14. | Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 443] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 15. | Moorefield EC, Andres SF, Blue RE, Van Landeghem L, Mah AT, Santoro MA, Ding S. Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging (Albany NY). 2017;9:1898-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Takezono Y, Joh T, Oshima T, Suzuki H, Seno K, Yokoyama Y, Alexander JS, Itoh M. Role of prostaglandins in maintaining gastric mucus-cell permeability against acid exposure. J Lab Clin Med. 2004;143:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Slifer ZM, Blikslager AT. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 18. | Li J, Li YX, Chen MH, Li J, Du J, Shen B, Xia XM. Changes in the phosphorylation of claudins during the course of experimental colitis. Int J Clin Exp Pathol. 2015;8:12225-12233. [PubMed] |
| 19. | Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131-4142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 909] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 20. | Pearce SC, Al-Jawadi A, Kishida K, Yu S, Hu M, Fritzky LF, Edelblum KL, Gao N, Ferraris RP. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018;16:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Liu Y, Chen YG. Intestinal epithelial plasticity and regeneration via cell dedifferentiation. Cell Regen. 2020;9:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Xiao S, Zhou L. Gastric Stem Cells: Physiological and Pathological Perspectives. Front Cell Dev Biol. 2020;8:571536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Malik TF, Gnanapandithan K, Singh K. Peptic Ulcer Disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021: 170-179. |
| 24. | Osefo N, Ito T, Jensen RT. Gastric acid hypersecretory states: recent insights and advances. Curr Gastroenterol Rep. 2009;11:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Basson MD. Paradigms for mechanical signal transduction in the intestinal epithelium. Category: molecular, cell, and developmental biology. Digestion. 2003;68:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Gayer CP, Basson MD. The effects of mechanical forces on intestinal physiology and pathology. Cell Signal. 2009;21:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 27. | Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 309] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Flanigan TL, Owen CR, Gayer C, Basson MD. Supraphysiologic extracellular pressure inhibits intestinal epithelial wound healing independently of luminal nutrient flow. Am J Surg. 2008;196:683-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Andersson P, Olaison G, Hallböök O, Boeryd B, Sjödahl R. Increased anal resting pressure and rectal sensitivity in Crohn's disease. Dis Colon Rectum. 2003;46:1685-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Yamamoto K, Kishino M, Nakamura S, Tokushige K. Symptoms and Upper Gastrointestinal Mucosal Injury Associated with Bisphosphonate Therapy. Intern Med. 2019;58:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Scarpignato C, Bjarnason I. Drug-Induced Small Bowel Injury: a Challenging and Often Forgotten Clinical Condition. Curr Gastroenterol Rep. 2019;21:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Lugea A, Antolín M, Mourelle M, Guarner F, Malagelada JR. Deranged hydrophobic barrier of the rat gastroduodenal mucosa after parenteral nonsteroidal anti-inflammatory drugs. Gastroenterology. 1997;112:1931-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Kaunitz JD. Barrier function of gastric mucus. Keio J Med. 1999;48:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Davis JS, Lee HY, Kim J, Advani SM, Peng HL, Banfield E, Hawk ET, Chang S, Frazier-Wood AC. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4:e000550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Gwee KA, Goh V, Lima G, Setia S. Coprescribing proton-pump inhibitors with nonsteroidal anti-inflammatory drugs: risks versus benefits. J Pain Res. 2018;11:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 36. | Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Yamagata M, Hiraishi H. [Prevalence and incidence of NSAID-induced gastrointestinal ulcers and bleeding]. Nihon Rinsho. 2007;65:1749-1753. [PubMed] |
| 38. | Holzer P, Livingston EH, Guth PH. Sensory neurons signal for an increase in rat gastric mucosal blood flow in the face of pending acid injury. Gastroenterology. 1991;101:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 122] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15:691-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Maricic N, Ehrlich K, Gretzer B, Schuligoi R, Respondek M, Peskar BM. Selective cyclo-oxygenase-2 inhibitors aggravate ischaemia-reperfusion injury in the rat stomach. Br J Pharmacol. 1999;128:1659-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr. 2011;48:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 42. | Wallace JL. NSAID gastropathy and enteropathy: distinct pathogenesis likely necessitates distinct prevention strategies. Br J Pharmacol. 2012;165:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Whittle BJ. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol. 2004;500:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Otani K, Tanigawa T, Watanabe T, Shimada S, Nadatani Y, Nagami Y, Tanaka F, Kamata N, Yamagami H, Shiba M, Tominaga K, Fujiwara Y, Arakawa T. Microbiota Plays a Key Role in Non-Steroidal Anti-Inflammatory Drug-Induced Small Intestinal Damage. Digestion. 2017;95:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Maseda D, Ricciotti E. NSAID-Gut Microbiota Interactions. Front Pharmacol. 2020;11:1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (14)] |
| 46. | Tai FWD, McAlindon ME. NSAIDs and the small bowel. Curr Opin Gastroenterol. 2018;34:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 47. | Zhou Y, Dial EJ, Doyen R, Lichtenberger LM. Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G722-G731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, Nomoto K, Takeuchi K, Arakawa T. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 49. | Hegyi P, Maléth J, Walters JR, Hofmann AF, Keely SJ. Guts and Gall: Bile Acids in Regulation of Intestinal Epithelial Function in Health and Disease. Physiol Rev. 2018;98:1983-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 50. | Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. Intestinal Absorption of Bile Acids in Health and Disease. Compr Physiol. 2019;10:21-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 51. | Urdaneta V, Casadesús J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front Med (Lausanne). 2017;4:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 52. | Murakami Y, Tanabe S, Suzuki T. High-fat Diet-induced Intestinal Hyperpermeability is Associated with Increased Bile Acids in the Large Intestine of Mice. J Food Sci. 2016;81:H216-H222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 53. | Higashimura Y, Naito Y, Takagi T, Uchiyama K, Mizushima K, Ushiroda C, Ohnogi H, Kudo Y, Yasui M, Inui S, Hisada T, Honda A, Matsuzaki Y, Yoshikawa T. Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G367-G375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 54. | Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. 2014;12:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 55. | Tuskey A, Peura D. The use of H2 antagonists in treating and preventing NSAID-induced mucosal damage. Arthritis Res Ther. 2013;15 Suppl 3:S6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Sturkenboom MC, Burke TA, Tangelder MJ, Dieleman JP, Walton S, Goldstein JL. Adherence to proton pump inhibitors or H2-receptor antagonists during the use of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2003;18:1137-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Physician. 2002;66:273-280. [PubMed] |
| 58. | Oncel S, Gupta R, Wang Q, Basson MD. ZINC40099027 Promotes Gastric Mucosal Repair in Ongoing Aspirin-Associated Gastric Injury by Activating Focal Adhesion Kinase. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Hunt RH, Lanas A, Stichtenoth DO, Scarpignato C. Myths and facts in the use of anti-inflammatory drugs. Ann Med. 2009;41:423-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Mahmoud YI, Abd El-Ghffar EA. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed Pharmacother. 2019;109:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 61. | Handa O, Naito Y, Fukui A, Omatsu T, Yoshikawa T. The impact of non-steroidal anti-inflammatory drugs on the small intestinal epithelium. J Clin Biochem Nutr. 2014;54:2-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Marlicz W, Loniewski I, Grimes DS, Quigley EM. Nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and gastrointestinal injury: contrasting interactions in the stomach and small intestine. Mayo Clin Proc. 2014;89:1699-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Szeto CC, Sugano K, Wang JG, Fujimoto K, Whittle S, Modi GK, Chen CH, Park JB, Tam LS, Vareesangthip K, Tsoi KKF, Chan FKL. Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: joint APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut. 2020;69:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 64. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1571] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 65. | Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 949] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 66. | Klenske E, Bojarski C, Waldner M, Rath T, Neurath MF, Atreya R. Targeting mucosal healing in Crohn's disease: what the clinician needs to know. Therap Adv Gastroenterol. 2019;12:1756284819856865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 67. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1021] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 68. | Baumgart DC, Le Berre C. Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. N Engl J Med. 2021;385:1302-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 251] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 69. | Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, Kato J, Kobayashi K, Koganei K, Kunisaki R, Motoya S, Nagahori M, Nakase H, Omata F, Saruta M, Watanabe T, Tanaka T, Kanai T, Noguchi Y, Takahashi KI, Watanabe K, Hibi T, Suzuki Y, Watanabe M, Sugano K, Shimosegawa T. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 394] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 70. | Atreya R, Neurath MF. Current and Future Targets for Mucosal Healing in Inflammatory Bowel Disease. Visc Med. 2017;33:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 359] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 72. | Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 73. | Basson MD, Rashid Z, Turowski GA, West AB, Emenaker NJ, Sgambati SA, Hong F, Perdikis DM, Datta S, Madri JA. Restitution at the cellular level: regulation of the migrating phenotype. Yale J Biol Med. 1996;69:119-129. [PubMed] |
| 74. | Modlin IM, Basson MD, Soroka CJ, Wright N, Poulsom R, Chinnery R, Hanby A, Patel K, Rogers L, Alison M. Ulcer-induced alterations in cell phenotype and growth factor expression. Eur J Gastroenterol. 1993;93:S59-S67. |
| 75. | Basson MD, Modlin IM, Turowski G, Madri JA. Enterocyte-matrix interactions in the healing of mucosal injury. Eur J Gastroenterol. 1993;5:S21-S28. |
| 76. | McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nat Cell Biol. 2001;3:E124-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Cooper ST, McNeil PL. Membrane Repair: Mechanisms and Pathophysiology. Physiol Rev. 2015;95:1205-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 78. | Andrews NW, Corrotte M. Plasma membrane repair. Curr Biol. 2018;28:R392-R397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 79. | Blazek AD, Paleo BJ, Weisleder N. Plasma Membrane Repair: A Central Process for Maintaining Cellular Homeostasis. Physiology (Bethesda). 2015;30:438-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 80. | Walsh MF, Thamilselvan V, Grotelueschen R, Farhana L, Basson M. Absence of adhesion triggers differential FAK and SAPKp38 signals in SW620 human colon cancer cells that may inhibit adhesiveness and lead to cell death. Cell Physiol Biochem. 2003;13:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Williams JM, Duckworth CA, Burkitt MD, Watson AJ, Campbell BJ, Pritchard DM. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet Pathol. 2015;52:445-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 82. | Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 242] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 83. | Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861-3885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 1206] [Article Influence: 120.6] [Reference Citation Analysis (4)] |
| 84. | Zundler S, Tauschek V, Neurath MF. Immune Cell Circuits in Mucosal Wound Healing: Clinical Implications. Visc Med. 2020;36:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Ubelmann F, Chamaillard M, El-Marjou F, Simon A, Netter J, Vignjevic D, Nichols BL, Quezada-Calvillo R, Grandjean T, Louvard D, Revenu C, Robine S. Enterocyte loss of polarity and gut wound healing rely upon the F-actin-severing function of villin. Proc Natl Acad Sci U S A. 2013;110:E1380-E1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Albers TM, Lomakina I, Moore RP. Fate of polarized membrane components and evidence for microvillus disassembly on migrating enterocytes during repair of native intestinal epithelium. Lab Invest. 1995;73:139-148. [PubMed] |
| 87. | Goggins BJ, Chaney C, Radford-Smith GL, Horvat JC, Keely S. Hypoxia and Integrin-Mediated Epithelial Restitution during Mucosal Inflammation. Front Immunol. 2013;4:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Keely S, Glover LE, MacManus CF, Campbell EL, Scully MM, Furuta GT, Colgan SP. Selective induction of integrin beta1 by hypoxia-inducible factor: implications for wound healing. FASEB J. 2009;23:1338-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Tétreault MP, Chailler P, Beaulieu JF, Rivard N, Ménard D. Specific signaling cascades involved in cell spreading during healing of micro-wounded gastric epithelial monolayers. J Cell Biochem. 2008;105:1240-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via alpha1beta1- and alpha2beta1-integrin-dependent Src kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G547-G557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Walsh MF, Ampasala DR, Hatfield J, Vander Heide R, Suer S, Rishi AK, Basson MD. Transforming growth factor-beta stimulates intestinal epithelial focal adhesion kinase synthesis via Smad- and p38-dependent mechanisms. Am J Pathol. 2008;173:385-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Sanders MA, Ampasala D, Basson MD. DOCK5 and DOCK1 regulate Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J Biol Chem. 2009;284:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Yu CF, Sanders MA, Basson MD. Human caco-2 motility redistributes FAK and paxillin and activates p38 MAPK in a matrix-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2000;278:G952-G966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 95. | Olson AD. Contraction of collagen gels by intestinal epithelial cells depends on microfilament function. Dig Dis Sci. 1993;38:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 96. | Yu CF, Basson MD. Matrix-specific FAK and MAPK reorganization during Caco-2 cell motility. Microsc Res Tech. 2000;51:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, López E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 286] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 98. | Conway WC, Van der Voort van Zyp J, Thamilselvan V, Walsh MF, Crowe DL, Basson MD. Paxillin modulates squamous cancer cell adhesion and is important in pressure-augmented adhesion. J Cell Biochem. 2006;98:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Ibata N, Terentjev EM. Development of Nascent Focal Adhesions in Spreading Cells. Biophys J. 2020;119:2063-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Legerstee K, Houtsmuller AB. A Layered View on Focal Adhesions. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 101. | Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 433] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 102. | Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 237] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 103. | Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 496] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 104. | Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest. 1992;90:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 153] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 105. | Basson MD, Modlin IM, Flynn SD, Jena BP, Madri JA. Independent modulation of enterocyte migration and proliferation by growth factors, matrix proteins, and pharmacologic agents in an in vitro model of mucosal healing. Surgery. 1992;112:299-307; discussion 307. [PubMed] |
| 106. | Tarnawski A, Tanoue K, Santos AM, Sarfeh IJ. Cellular and molecular mechanisms of gastric ulcer healing. Is the quality of mucosal scar affected by treatment? Scand J Gastroenterol Suppl. 1995;210:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Gao JH, Guo LJ, Huang ZY, Rao JN, Tang CW. Roles of cellular polyamines in mucosal healing in the gastrointestinal tract. J Physiol Pharmacol. 2013;64:681-693. [PubMed] |
| 108. | Basson MD. Gut mucosal healing: is the science relevant? Am J Pathol. 2002;161:1101-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 109. | Baatar D, Jones MK, Tsugawa K, Pai R, Moon WS, Koh GY, Kim I, Kitano S, Tarnawski AS. Esophageal ulceration triggers expression of hypoxia-inducible factor-1 alpha and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am J Pathol. 2002;161:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 110. | Dudar GK, D'Andrea LD, Di Stasi R, Pedone C, Wallace JL. A vascular endothelial growth factor mimetic accelerates gastric ulcer healing in an iNOS-dependent manner. Am J Physiol Gastrointest Liver Physiol. 2008;295:G374-G381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Bauer SM, Bauer RJ, Velazquez OC. Angiogenesis, vasculogenesis, and induction of healing in chronic wounds. Vasc Endovascular Surg. 2005;39:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 112. | Tarnawski AS, Ahluwalia A, Jones MK. Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J Gastroenterol Hepatol. 2014;29 Suppl 4:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 113. | Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond). 2011;120:263-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 114. | Sato T, Amano H, Ito Y, Eshima K, Minamino T, Ae T, Katada C, Ohno T, Hosono K, Suzuki T, Shibuya M, Koizumi W, Majima M. Vascular endothelial growth factor receptor 1 signaling facilitates gastric ulcer healing and angiogenesis through the upregulation of epidermal growth factor expression on VEGFR1+CXCR4 + cells recruited from bone marrow. J Gastroenterol. 2014;49:455-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Arakawa T, Kobayashi K. Quality of ulcer healing--a new concept to rank healed peptic ulcers. Gastroenterol Jpn. 1993;28 Suppl 5:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 116. | Seo JH, Hong SJ, Kim JH, Kim BW, Jee SR, Chung WC, Shim KN, Baik GH, Kim SS, Kim SG, Kim JI. Long-Term Recurrence Rates of Peptic Ulcers without Helicobacter pylori. Gut Liver. 2016;10:719-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Pantea M, Negovan A, Banescu C, Bataga S, Neagoe R, Mocan S, Iancu M. Factors Associated with Recurrent Ulcers in Patients with Gastric Surgery after More Than 15 Years: A Cross-Sectional Single-Center Study. Gastroenterol Res Pract. 2018;2018:8319481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Ramakrishnan K, Salinas RC. Peptic ulcer disease. Am Fam Physician. 2007;76:1005-1012. [PubMed] |
| 119. | Tarnawski A, Douglass TG, Stachura J, Krause WJ. Quality of gastric ulcer healing: histological and ultrastructural assessment. Aliment Pharmacol Ther. 1991;5 Suppl 1:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | Tarnawski A, Stachura J, Krause WJ, Douglass TG, Gergely H. Quality of gastric ulcer healing: a new, emerging concept. J Clin Gastroenterol. 1991;13 Suppl 1:S42-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Arakawa T, Watanabe T, Tanigawa T, Tominaga K, Fujiwara Y, Morimoto K. Quality of ulcer healing in gastrointestinal tract: its pathophysiology and clinical relevance. World J Gastroenterol. 2012;18:4811-4822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 122. | Young Oh T, Ok Ahn B, Jung Jang E, Sang Park J, Jong Park S, Wook Baik H, Hahm KB. Accelerated Ulcer Healing and Resistance to Ulcer Recurrence with Gastroprotectants in Rat Model of Acetic Acid-induced Gastric Ulcer. J Clin Biochem Nutr. 2008;42:204-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Kangwan N, Park JM, Kim EH, Hahm KB. Quality of healing of gastric ulcers: Natural products beyond acid suppression. World J Gastrointest Pathophysiol. 2014;5:40-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 124. | Nebiki H, Arakawa T, Higuchi K, Kobayashi K. Quality of ulcer healing influences the relapse of gastric ulcers in humans. J Gastroenterol Hepatol. 1997;12:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Niv Y. H pylori recurrence after successful eradication. World J Gastroenterol. 2008;14:1477-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 126. | Tarnawski A, Hollander D, Krause WJ, Dabros W, Stachura J, Gergely H. "Healed" experimental gastric ulcers remain histologically and ultrastructurally abnormal. J Clin Gastroenterol. 1990;12 Suppl 1:S139-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. 2011;17:2161-2171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (2)] |
| 128. | Kovalenko PL, Flanigan TL, Chaturvedi L, Basson MD. Influence of defunctionalization and mechanical forces on intestinal epithelial wound healing. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1134-G1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 129. | Zhang J, Owen CR, Sanders MA, Turner JR, Basson MD. The motogenic effects of cyclic mechanical strain on intestinal epithelial monolayer wound closure are matrix dependent. Gastroenterology. 2006;131:1179-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Komuro Y, Ishihara K, Ohara S, Saigenji K, Hotta K. Effects of tetragastrin on mucus glycoprotein in rat gastric mucosal protection. Gastroenterol Jpn. 1992;27:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 131. | Yoneda M, Taché Y. Central thyrotropin-releasing factor analog prevents ethanol-induced gastric damage through prostaglandins in rats. Gastroenterology. 1992;102:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Takeuchi K, Nishiwaki H, Okada M, Okabe S. Mucosal protective action of histamine against gastric lesions induced by HCl in rats: importance of antigastric motor activity mediated by H2-receptors. J Pharmacol Exp Ther. 1988;245:632-638. [PubMed] |
| 133. | Tanaka S, Akiba Y, Kaunitz JD. Pentagastrin gastroprotection against acid is related to H2 receptor activation but not acid secretion. Gut. 1998;43:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Konturek SJ, Brzozowski T, Bielanski W, Schally AV. Role of endogenous gastrin in gastroprotection. Eur J Pharmacol. 1995;278:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 135. | Kivilaakso E, Fromm D, Silen W. Effect of the acid secretory state on intramural pH of rabbit gastric mucosa. Gastroenterology. 1978;75:641-648. [PubMed] [DOI] [Full Text] |
| 136. | Smith P, O'Brien P, Fromm D, Silen W. Secretory state of gastric mucosa and resistance to injury by exogenous acid. Am J Surg. 1977;133:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 137. | Tanaka S, Taché Y, Kaneko H, Guth PH, Kaunitz JD. Central vagal activation increases mucus gel thickness and surface cell intracellular pH in rat stomach. Gastroenterology. 1997;112:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Ciacci C, Lind SE, Podolsky DK. Transforming growth factor beta regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology. 1993;105:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 170] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 139. | Dignass AU, Podolsky DK. Interleukin 2 modulates intestinal epithelial cell function in vitro. Exp Cell Res. 1996;225:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 140. | Dise RS, Frey MR, Whitehead RH, Polk DB. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2008;294:G276-G285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 141. | Jones MK, Kawanaka H, Baatar D, Szabó IL, Tsugawa K, Pai R, Koh GY, Kim I, Sarfeh IJ, Tarnawski AS. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001;121:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 142. | Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;209-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 143. | Szabo S, Folkman J, Vattay P, Morales RE, Pinkus GS, Kato K. Accelerated healing of duodenal ulcers by oral administration of a mutein of basic fibroblast growth factor in rats. Gastroenterology. 1994;106:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 144. | Tarnawski AS, Ahluwalia A. The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 145. | Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 193] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 146. | Ishikawa S, Cepinskas G, Specian RD, Itoh M, Kvietys PR. Epidermal growth factor attenuates jejunal mucosal injury induced by oleic acid: role of mucus. Am J Physiol. 1994;267:G1067-G1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 147. | Sarosiek J, Bilski J, Murty VL, Slomiany A, Slomiany BL. Role of salivary epidermal growth factor in the maintenance of physicochemical characteristics of oral and gastric mucosal mucus coat. Biochem Biophys Res Commun. 1988;152:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 148. | Kelly SM, Hunter JO. Epidermal growth factor stimulates synthesis and secretion of mucus glycoproteins in human gastric mucosa. Clin Sci (Lond). 1990;79:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Procaccino F, Reinshagen M, Hoffmann P, Zeeh JM, Lakshmanan J, McRoberts JA, Patel A, French S, Eysselein VE. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994;107:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 150. | Riegler M, Sedivy R, Sogukoglu T, Castagliuolo I, Pothoulakis C, Cosentini E, Bischof G, Hamilton G, Teleky B, Feil W, Lamont JT, Wenzl E. Epidermal growth factor attenuates Clostridium difficile toxin A- and B-induced damage of human colonic mucosa. Am J Physiol. 1997;273:G1014-G1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 151. | Hui WM, Chen BW, Kung AW, Cho CH, Luk CT, Lam SK. Effect of epidermal growth factor on gastric blood flow in rats: possible role in mucosal protection. Gastroenterology. 1993;104:1605-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 152. | Konturek PK, Brzozowski T, Konturek SJ, Dembiński A. Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions. Gastroenterology. 1990;99:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 153. | Sakamoto T, Swierczek JS, Ogden WD, Thompson JC. Cytoprotective effect of pentagastrin and epidermal growth factor on stress ulcer formation. Possible role of somatostatin. Ann Surg. 1985;201:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 154. | Tarnawski AS, Jones MK. The role of epidermal growth factor (EGF) and its receptor in mucosal protection, adaptation to injury, and ulcer healing: involvement of EGF-R signal transduction pathways. J Clin Gastroenterol. 1998;27 Suppl 1:S12-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 155. | Kirkegaard P, Olsen PS, Poulsen SS, Nexø E. Epidermal growth factor inhibits cysteamine-induced duodenal ulcers. Gastroenterology. 1983;85:1277-1283. [PubMed] [DOI] [Full Text] |
| 156. | Kosone T, Takagi H, Kakizaki S, Sohara N, Horiguchi N, Sato K, Yoneda M, Takeuchi T, Mori M. Integrative roles of transforming growth factor-alpha in the cytoprotection mechanisms of gastric mucosal injury. BMC Gastroenterol. 2006;6:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 157. | Konturek SJ, Brzozowski T, Majka J, Dembinski A, Slomiany A, Slomiany BL. Transforming growth factor alpha and epidermal growth factor in protection and healing of gastric mucosal injury. Scand J Gastroenterol. 1992;27:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 158. | Kokoska ER, Wolff AB, Smith GS, Miller TA. Epidermal growth factor-induced cytoprotection in human intestinal cells involves intracellular calcium signaling. J Surg Res. 2000;88:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 159. | Nylander-Koski O, Mustonen H, Puolakkainen P, Kiviluoto T, Kivilaakso E. Epidermal growth factor enhances intracellular pH regulation via calcium signaling in acid-exposed primary cultured rabbit gastric epithelial cells. Dig Dis Sci. 2006;51:1322-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 160. | Tarnawski AS, Jones MK, Ahluwalia A. Dedifferentiation and reprogramming of epithelial cells during gastric ulcer healing is triggered by hypoxia and a well-coordinated, sequential activation of EGFR signaling: cross talk with IGF1 and COX2. Gastroenterology. 2021;160:77-78. [DOI] [Full Text] |
| 161. | Brzozowski T, Konturek PC, Konturek SJ, Schuppan D, Drozdowicz D, Kwiecień S, Majka J, Nakamura T, Hahn E. Effect of local application of growth factors on gastric ulcer healing and mucosal expression of cyclooxygenase-1 and -2. Digestion. 2001;64:15-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 162. | Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109:285-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 272] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 163. | Jones MK, Sasaki E, Halter F, Pai R, Nakamura T, Arakawa T, Kuroki T, Tarnawski AS. HGF triggers activation of the COX-2 gene in rat gastric epithelial cells: action mediated through the ERK2 signaling pathway. FASEB J. 1999;13:2186-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 164. | Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 188] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 165. | FitzGerald AJ, Pu M, Marchbank T, Westley BR, May FE, Boyle J, Yadollahi-Farsani M, Ghosh S, Playford RJ. Synergistic effects of systemic trefoil factor family 1 (TFF1) peptide and epidermal growth factor in a rat model of colitis. Peptides. 2004;25:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 166. | Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, Rottiers P, Steidler L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 167. | Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest. 2002;32:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 168. | Bastholm SK, Samson MH, Becher N, Hansen LK, Stubbe PR, Chronakis IS, Nexo E, Uldbjerg N. Trefoil factor peptide 3 is positively correlated with the viscoelastic properties of the cervical mucus plug. Acta Obstet Gynecol Scand. 2017;96:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 169. | Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut. 1999;44:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 170. | Xue L, Aihara E, Podolsky DK, Wang TC, Montrose MH. In vivo action of trefoil factor 2 (TFF2) to speed gastric repair is independent of cyclooxygenase. Gut. 2010;59:1184-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Xue L, Aihara E, Wang TC, Montrose MH. Trefoil factor 2 requires Na/H exchanger 2 activity to enhance mouse gastric epithelial repair. J Biol Chem. 2011;286:38375-38382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 172. | McBerry C, Egan CE, Rani R, Yang Y, Wu D, Boespflug N, Boon L, Butcher B, Mirpuri J, Hogan SP, Denkers EY, Aliberti J, Herbert DR. Trefoil factor 2 negatively regulates type 1 immunity against Toxoplasma gondii. J Immunol. 2012;189:3078-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 173. | Järvå MA, Lingford JP, John A, Soler NM, Scott NE, Goddard-Borger ED. Trefoil factors share a lectin activity that defines their role in mucus. Nat Commun. 2020;11:2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 174. | Newton JL, Allen A, Westley BR, May FE. The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach. Gut. 2000;46:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 175. | Aihara E, Engevik KA, Montrose MH. Trefoil Factor Peptides and Gastrointestinal Function. Annu Rev Physiol. 2017;79:357-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 176. | Braga Emidio N, Brierley SM, Schroeder CI, Muttenthaler M. Structure, Function, and Therapeutic Potential of the Trefoil Factor Family in the Gastrointestinal Tract. ACS Pharmacol Transl Sci. 2020;3:583-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 177. | Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, Wang TC. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 178. | Belle NM, Ji Y, Herbine K, Wei Y, Park J, Zullo K, Hung LY, Srivatsa S, Young T, Oniskey T, Pastore C, Nieves W, Somsouk M, Herbert DR. TFF3 interacts with LINGO2 to regulate EGFR activation for protection against colitis and gastrointestinal helminths. Nat Commun. 2019;10:4408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 179. | Hoffmann W. Trefoil Factor Family (TFF) Peptides and Their Links to Inflammation: A Re-evaluation and New Medical Perspectives. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 180. | Dieckow J, Brandt W, Hattermann K, Schob S, Schulze U, Mentlein R, Ackermann P, Sel S, Paulsen FP. CXCR4 and CXCR7 Mediate TFF3-Induced Cell Migration Independently From the ERK1/2 Signaling Pathway. Invest Ophthalmol Vis Sci. 2016;57:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 181. | Orime K, Shirakawa J, Togashi Y, Tajima K, Inoue H, Ito Y, Sato K, Nakamura A, Aoki K, Goshima Y, Terauchi Y. Trefoil factor 2 promotes cell proliferation in pancreatic β-cells through CXCR-4-mediated ERK1/2 phosphorylation. Endocrinology. 2013;154:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 182. | Taupin D, Wu DC, Jeon WK, Devaney K, Wang TC, Podolsky DK. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J Clin Invest. 1999;103:R31-R38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 183. | Sun Z, Liu H, Yang Z, Shao D, Zhang W, Ren Y, Sun B, Lin J, Xu M, Nie S. Intestinal trefoil factor activates the PI3K/Akt signaling pathway to protect gastric mucosal epithelium from damage. Int J Oncol. 2014;45:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 184. | Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009;284:3650-3662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 185. | Zhang Y, Yu G, Wang Y, Xiang Y, Gao Q, Jiang P, Zhang J, Lee W, Zhang Y. Activation of protease-activated receptor (PAR) 1 by frog trefoil factor (TFF) 2 and PAR4 by human TFF2. Cell Mol Life Sci. 2011;68:3771-3780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 186. | Barrera GJ, Tortolero GS. Trefoil factor 3 (TFF3) from human breast milk activates PAR-2 receptors, of the intestinal epithelial cells HT-29, regulating cytokines and defensins. Bratisl Lek Listy. 2016;117:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 187. | McKenzie C, Thim L, Parsons ME. Topical and intravenous administration of trefoil factors protect the gastric mucosa from ethanol-induced injury in the rat. Aliment Pharmacol Ther. 2000;14:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 188. | Playford RJ, Marchbank T, Goodlad RA, Chinery RA, Poulsom R, Hanby AM. Transgenic mice that overexpress the human trefoil peptide pS2 have an increased resistance to intestinal damage. Proc Natl Acad Sci USA. 1996;93:2137-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 189. | Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 525] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 190. | Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 286] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 191. | Maric I, Poljak L, Zoricic S, Bobinac D, Bosukonda D, Sampath KT, Vukicevic S. Bone morphogenetic protein-7 reduces the severity of colon tissue damage and accelerates the healing of inflammatory bowel disease in rats. J Cell Physiol. 2003;196:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 192. | Sommer K, Wiendl M, Müller TM, Heidbreder K, Voskens C, Neurath MF, Zundler S. Intestinal Mucosal Wound Healing and Barrier Integrity in IBD-Crosstalk and Trafficking of Cellular Players. Front Med (Lausanne). 2021;8:643973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 193. | Bulut K, Pennartz C, Felderbauer P, Ansorge N, Banasch M, Schmitz F, Schmidt WE, Hoffmann P. Vascular endothelial growth factor (VEGF164) ameliorates intestinal epithelial injury in vitro in IEC-18 and Caco-2 monolayers via induction of TGF-beta release from epithelial cells. Scand J Gastroenterol. 2006;41:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 194. | Ahluwalia A, Jones MK, Brzozowski T, Tarnawski AS. Nerve growth factor is critical requirement for in vitro angiogenesis in gastric endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2016;311:G981-G987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 195. | Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45 Suppl A:A39-A47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 196. | Chai J, Baatar D, Tarnawski A. Serum response factor promotes re-epithelialization and muscular structure restoration during gastric ulcer healing. Gastroenterology. 2004;126:1809-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 197. | Ahluwalia A, Jones MK, Brzozowska I, Tarnawski AS. In vitro model of vasculo-angiogenesis: demonstration that bone marrow derived endothelial progenitor cells form new hybrid capillary blood vessels jointly with gastric endothelial cells. J Physiol Pharmacol. 2017;68:841-846. [PubMed] |
| 198. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 2084] [Article Influence: 173.7] [Reference Citation Analysis (1)] |
| 199. | Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, Becher B, Littman DR, Neurath MF. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 387] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 200. | Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP; Secukinumab in Crohn's Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1238] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 201. | Rosen MJ, Frey MR, Washington MK, Chaturvedi R, Kuhnhein LA, Matta P, Revetta FL, Wilson KT, Polk DB. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis. 2011;17:2224-2234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 202. | Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 508] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 203. | Weigmann B, Lehr HA, Yancopoulos G, Valenzuela D, Murphy A, Stevens S, Schmidt J, Galle PR, Rose-John S, Neurath MF. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. J Exp Med. 2008;205:2099-2110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 204. | Hoving JC. Targeting IL-13 as a Host-Directed Therapy Against Ulcerative Colitis. Front Cell Infect Microbiol. 2018;8:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 205. | Tilg H, Kaser A. Failure of interleukin 13 blockade in ulcerative colitis. Gut. 2015;64:857-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 206. | Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1477] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 207. | Lindsay J, Van Montfrans C, Brennan F, Van Deventer S, Drillenburg P, Hodgson H, Te Velde A, Sol Rodriguez Pena M. IL-10 gene therapy prevents TNBS-induced colitis. Gene Ther. 2002;9:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 208. | Li MC, He SH. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J Gastroenterol. 2004;10:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 209. | Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A, Feagan B. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 353] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 210. | Wei HX, Wang B, Li B. IL-10 and IL-22 in Mucosal Immunity: Driving Protection and Pathology. Front Immunol. 2020;11:1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 211. | Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1525] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 212. | Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol. 2007;178:5973-5981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 213. | Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 791] [Cited by in RCA: 885] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 214. | Gao B, Xiang X. Interleukin-22 from bench to bedside: a promising drug for epithelial repair. Cell Mol Immunol. 2019;16:666-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 215. | Lee DW, Zhong S, Pai R, Rae J, Sukumaran S, Stefanich EG, Lutman J, Doudement E, Wang X, Harder B, Lekkerkerker A, Herman A, Ouyang W, Danilenko DM. Nonclinical safety assessment of a human interleukin-22FC IG fusion protein demonstrates in vitro to in vivo and cross-species translatability. Pharmacol Res Perspect. 2018;6:e00434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 216. | Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1584] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 217. | Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 960] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 218. | Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 574] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 219. | Eken A, Singh AK, Treuting PM, Oukka M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 2014;7:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 220. | Stefanich EG, Rae J, Sukumaran S, Lutman J, Lekkerkerker A, Ouyang W, Wang X, Lee D, Danilenko DM, Diehl L, Loyet KM, Herman A. Pre-clinical and translational pharmacology of a human interleukin-22 IgG fusion protein for potential treatment of infectious or inflammatory diseases. Biochem Pharmacol. 2018;152:224-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 221. | Rothenberg ME, Wang Y, Lekkerkerker A, Danilenko DM, Maciuca R, Erickson R, Herman A, Stefanich E, Lu TT. Randomized Phase I Healthy Volunteer Study of UTTR1147A (IL-22Fc): A Potential Therapy for Epithelial Injury. Clin Pharmacol Ther. 2019;105:177-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 222. | Genentech, Inc. An Extension Study to Evaluate the Long-Term Safety and Tolerability of UTTR1147A in Participants With Moderate to Severe Ulcerative Colitis or Crohn’s Disease. ClinicalTrials.gov Identifier: NCT03650413 Available from: https://clinicaltrials.gov/ct2/show/NCT03650413. |
| 223. | Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 802] [Cited by in RCA: 961] [Article Influence: 64.1] [Reference Citation Analysis (22)] |
| 224. | Rao JN, Wang JY. Regulation of Gastrointestinal Mucosal Growth. In: Integrated Systems Physiology: from Molecule to Function to Disease. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 225. | Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1919] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 226. | Bedford A, Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. 2018;4:151-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 227. | Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017;49:e338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 228. | Park JH, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S, Usui Y, Hatano N, Shinohara M, Saito Y, Murata Y, Matozaki T. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS One. 2016;11:e0156334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 229. | Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 970] [Cited by in RCA: 2457] [Article Influence: 351.0] [Reference Citation Analysis (0)] |
| 230. | Bilotta AJ, Ma C, Yang W, Yu Y, Zhao X, Zhou Z, Yao S, Dann SM, Cong Y. Propionate Enhances Cell Speed and Persistence to Promote Intestinal Epithelial Turnover and Repair. Cell Mol Gastroenterol Hepatol. 2021;11:1023-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 231. | Basson MD, Liu YW, Hanly AM, Emenaker NJ, Shenoy SG, Gould Rothberg BE. Identification and comparative analysis of human colonocyte short-chain fatty acid response genes. J Gastrointest Surg. 2000;4:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 232. | Basson MD, Turowski GA, Rashid Z, Hong F, Madri JA. Regulation of human colonic cell line proliferation and phenotype by sodium butyrate. Dig Dis Sci. 1996;41:1989-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 233. | Basson MD, Emenaker NJ, Hong F. Differential modulation of human (Caco-2) colon cancer cell line phenotype by short chain fatty acids. Proc Soc Exp Biol Med. 1998;217:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 234. | Gamet L, Daviaud D, Denis-Pouxviel C, Remesy C, Murat JC. Effects of short-chain fatty acids on growth and differentiation of the human colon-cancer cell line HT29. Int J Cancer. 1992;52:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 235. | Dyson JE, Daniel J, Surrey CR. The effect of sodium butyrate on the growth characteristics of human cervix tumour cells. Br J Cancer. 1992;65:803-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 236. | Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut. 1990;31:1391-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 156] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 237. | Mortensen FV, Hessov I, Birke H, Korsgaard N, Nielsen H. Microcirculatory and trophic effects of short chain fatty acids in the human rectum after Hartmann's procedure. Br J Surg. 1991;78:1208-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 238. | Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 398] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 239. | Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 240. | Terzi C, Sevinç AI, Koçdor H, Oktay G, Alanyali H, Küpelioğlu A, Ergör G, Füzün M. Improvement of colonic healing by preoperative rectal irrigation with short-chain fatty acids in rats given radiotherapy. Dis Colon Rectum. 2004;47:2184-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 241. | Elamin EE, Masclee AA, Dekker J, Pieters HJ, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr. 2013;143:1872-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 242. | Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139:1619-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1460] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 243. | Scheppach W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig Dis Sci. 1996;41:2254-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 244. | Duggan C, Gannon J, Walker WA. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr. 2002;75:789-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 245. | Robinson EK, Kelly DP, Mercer DW, Kozar RA. Differential effects of luminal arginine and glutamine on metalloproteinase production in the postischemic gut. JPEN J Parenter Enteral Nutr. 2008;32:433-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 246. | Kim MH, Kim H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 247. | Fritz JH. Arginine cools the inflamed gut. Infect Immun. 2013;81:3500-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 248. | Matsui T, Ichikawa H, Fujita T, Takemura S, Takagi T, Osada-Oka M, Minamiyama Y. Histidine and arginine modulate intestinal cell restitution via transforming growth factor-β1. Eur J Pharmacol. 2019;850:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 249. | Turowski GA, Rashid Z, Hong F, Madri JA, Basson MD. Glutamine modulates phenotype and stimulates proliferation in human colon cancer cell lines. Cancer Res. 1994;54:5974-5980. [PubMed] |
| 250. | Chen S, Xia Y, Zhu G, Yan J, Tan C, Deng B, Deng J, Yin Y, Ren W. Glutamine supplementation improves intestinal cell proliferation and stem cell differentiation in weanling mice. Food Nutr Res. 2018;62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 251. | Ji FJ, Wang LX, Yang HS, Hu A, Yin YL. Review: The roles and functions of glutamine on intestinal health and performance of weaning pigs. Animal. 2019;13:2727-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 252. | Swaid F, Sukhotnik I, Matter I, Berkowitz D, Hadjittofi C, Pollak Y, Lavy A. Dietary glutamine supplementation prevents mucosal injury and modulates intestinal epithelial restitution following acetic acid induced intestinal injury in rats. Nutr Metab (Lond). 2013;10:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 253. | Tanaka T, Morito K, Kinoshita M, Ohmoto M, Urikura M, Satouchi K, Tokumura A. Orally administered phosphatidic acids and lysophosphatidic acids ameliorate aspirin-induced stomach mucosal injury in mice. Dig Dis Sci. 2013;58:950-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 254. | Hollander D, Tarnawski A. Is there a role for dietary essential fatty acids in gastroduodenal mucosal protection? J Clin Gastroenterol. 1991;13 Suppl 1:S72-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 255. | Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V, Hsueh W, Raymond AS, Shur BD, Tan XD. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117:3673-3683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 256. | Hussey GS, Keane TJ, Badylak SF. The extracellular matrix of the gastrointestinal tract: a regenerative medicine platform. Nat Rev Gastroenterol Hepatol. 2017;14:540-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 257. | Sonbol HS. Extracellular Matrix Remodeling in Human Disease. J Microsc Ultrastruct. 2018;6:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 258. | Olson AD, Pysher T, Bienkowski RS. Organization of intestinal epithelial cells into multicellular structures requires laminin and functional actin microfilaments. Exp Cell Res. 1991;192:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 259. | Vachon PH, Beaulieu JF. Extracellular heterotrimeric laminin promotes differentiation in human enterocytes. Am J Physiol. 1995;268:G857-G867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 260. | Göke M, Zuk A, Podolsky DK. Regulation and function of extracellular matrix intestinal epithelial restitution in vitro. Am J Physiol. 1996;271:G729-G740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 261. | Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J. 2003;17:926-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 262. | Shimshoni E, Yablecovitch D, Baram L, Dotan I, Sagi I. ECM remodelling in IBD: innocent bystander or partner in crime? Gut. 2015;64:367-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 263. | Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 264. | Kirkegaard T, Hansen A, Bruun E, Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut. 2004;53:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 265. | Efsen E, Saermark T, Hansen A, Bruun E, Brynskov J. Ramiprilate inhibits functional matrix metalloproteinase activity in Crohn's disease fistulas. Basic Clin Pharmacol Toxicol. 2011;109:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 266. | Kofla-Dlubacz A, Matusiewicz M, Krzystek-Korpacka M, Iwanczak B. Correlation of MMP-3 and MMP-9 with Crohn's disease activity in children. Dig Dis Sci. 2012;57:706-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 267. | Faubion WA Jr, Fletcher JG, O'Byrne S, Feagan BG, de Villiers WJ, Salzberg B, Plevy S, Proctor DD, Valentine JF, Higgins PD, Harris JM, Diehl L, Wright L, Tew GW, Luca D, Basu K, Keir ME. EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn's disease activity: role of cross-sectional imaging. Am J Gastroenterol. 2013;108:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 268. | Sela-Passwell N, Kikkeri R, Dym O, Rozenberg H, Margalit R, Arad-Yellin R, Eisenstein M, Brenner O, Shoham T, Danon T, Shanzer A, Sagi I. Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential. Nat Med. 2011;18:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 269. | Schreiber S, Siegel CA, Friedenberg KA, Younes ZH, Seidler U, Bhandari BR, Wang K, Wendt E, McKevitt M, Zhao S, Sundy JS, Lee SD, Loftus EV. A Phase 2, Randomized, Placebo-Controlled Study Evaluating Matrix Metalloproteinase-9 Inhibitor, Andecaliximab, in Patients With Moderately to Severely Active Crohn's Disease. J Crohns Colitis. 2018;12:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 270. | Schreiber S. Lack of Efficacy in Crohn's Disease Prompts Unplanned Interim Analysis and Termination of Study in Ulcerative Colitis. J Crohns Colitis. 2020;14:282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 271. | Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 2011;5:170-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 334] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 272. | Abreu-Blanco MT, Watts JJ, Verboon JM, Parkhurst SM. Cytoskeleton responses in wound repair. Cell Mol Life Sci. 2012;69:2469-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 273. | Chaturvedi LS, Marsh HM, Basson MD. Role of RhoA and its effectors ROCK and mDia1 in the modulation of deformation-induced FAK, ERK, p38, and MLC motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;301:C1224-C1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 274. | Kopecki Z, Cowin AJ. The Role of Actin Remodelling Proteins in Wound Healing and Tissue Regeneration. In: Wound Healing - New insights into Ancient Challenges. Intech Open, 2016. [DOI] [Full Text] |
| 275. | Heath JP. Epithelial cell migration in the intestine. Cell Biol Int. 1996;20:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 276. | Tang DD, Gerlach BD. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res. 2017;18:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 277. | Hohmann T, Dehghani F. The Cytoskeleton-A Complex Interacting Meshwork. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 278. | Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 279. | Rottner K, Schaks M. Assembling actin filaments for protrusion. Curr Opin Cell Biol. 2019;56:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 280. | Garcin C, Straube A. Microtubules in cell migration. Essays Biochem. 2019;63:509-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 281. | Charafeddine RA, Nosanchuk JD, Sharp DJ. Targeting Microtubules for Wound Repair. Adv Wound Care (New Rochelle). 2016;5:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 282. | Campbell ID, Ginsberg MH. The talin-tail interaction places integrin activation on FERM ground. Trends Biochem Sci. 2004;29:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 283. | Bosanquet DC, Ye L, Harding KG, Jiang WG. FERM family proteins and their importance in cellular movements and wound healing (review). Int J Mol Med. 2014;34:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 284. | Gurtner GC, Wong VW. Filamin A Mediates Wound Closure by Promoting Elastic Deformation and Maintenance of Tension in the Collagen Matrix. J Invest Dermatol. 2015;135:2569-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 285. | Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179-33182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 453] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 286. | Kopecki Z, O'Neill GM, Arkell RM, Cowin AJ. Regulation of focal adhesions by flightless i involves inhibition of paxillin phosphorylation via a Rac1-dependent pathway. J Invest Dermatol. 2011;131:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 287. | Mohammad I, Arora PD, Naghibzadeh Y, Wang Y, Li J, Mascarenhas W, Janmey PA, Dawson JF, McCulloch CA. Flightless I is a focal adhesion-associated actin-capping protein that regulates cell migration. FASEB J. 2012;26:3260-3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 288. | Jackson JE, Kopecki Z, Adams DH, Cowin AJ. Flii neutralizing antibodies improve wound healing in porcine preclinical studies. Wound Repair Regen. 2012;20:523-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 289. | Kopecki Z, Ruzehaji N, Turner C, Iwata H, Ludwig RJ, Zillikens D, Murrell DF, Cowin AJ. Topically applied flightless I neutralizing antibodies improve healing of blistered skin in a murine model of epidermolysis bullosa acquisita. J Invest Dermatol. 2013;133:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 290. | Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 291. | Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155:1319-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 287] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 292. | Laukaitis CM, Webb DJ, Donais K, Horwitz AF. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153:1427-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 355] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 293. | Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116:4605-4613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 513] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 294. | Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010;26:315-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 766] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 295. | Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers. 2018;6:1539595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 296. | Owen KA, Abshire MY, Tilghman RW, Casanova JE, Bouton AH. FAK regulates intestinal epithelial cell survival and proliferation during mucosal wound healing. PLoS One. 2011;6:e23123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 297. | Burridge K. Focal adhesions: a personal perspective on a half century of progress. FEBS J. 2017;284:3355-3361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 298. | Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 411] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 299. | Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1085] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 300. | Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 301. | Thomas JW, Cooley MA, Broome JM, Salgia R, Griffin JD, Lombardo CR, Schaller MD. The role of focal adhesion kinase binding in the regulation of tyrosine phosphorylation of paxillin. J Biol Chem. 1999;274:36684-36692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 302. | Hu YL, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC, Chien S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep. 2014;4:6024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 303. | Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1105] [Article Influence: 50.2] [Reference Citation Analysis (2)] |
| 304. | Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1011] [Cited by in RCA: 998] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 305. | Ishibe S, Joly D, Liu ZX, Cantley LG. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol Cell. 2004;16:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 306. | Basson MD, Sanders MA, Gomez R, Hatfield J, Vanderheide R, Thamilselvan V, Zhang J, Walsh MF. Focal adhesion kinase protein levels in gut epithelial motility. Am J Physiol Gastrointest Liver Physiol. 2006;291:G491-G499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 307. | Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure. 2002;10:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 308. | Deramaudt TB, Dujardin D, Noulet F, Martin S, Vauchelles R, Takeda K, Rondé P. Altering FAK-paxillin interactions reduces adhesion, migration and invasion processes. PLoS One. 2014;9:e92059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 309. | Lietha D, Cai X, Ceccarelli DF, Li Y, Schaller MD, Eck MJ. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129:1177-1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 378] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 310. | Mousson A, Sick E, Carl P, Dujardin D, De Mey J, Rondé P. Targeting Focal Adhesion Kinase Using Inhibitors of Protein-Protein Interactions. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 311. | Cary LA, Chang JF, Guan JL. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109 ( Pt 7):1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 437] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 312. | Lawson C, Schlaepfer DD. pHocal adhesion kinase regulation is on a FERM foundation. J Cell Biol. 2013;202:833-836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 313. | Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 2027] [Article Influence: 96.5] [Reference Citation Analysis (1)] |
| 314. | Fan H, Zhao X, Sun S, Luo M, Guan JL. Function of focal adhesion kinase scaffolding to mediate endophilin A2 phosphorylation promotes epithelial-mesenchymal transition and mammary cancer stem cell activities in vivo. J Biol Chem. 2013;288:3322-3333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 315. | McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 825] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 316. | Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev. 2011;63:610-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 640] [Cited by in RCA: 654] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 317. | Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 403] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 318. | Teranishi S, Kimura K, Nishida T. Role of formation of an ERK-FAK-paxillin complex in migration of human corneal epithelial cells during wound closure in vitro. Invest Ophthalmol Vis Sci. 2009;50:5646-5652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 319. | Tanimura S, Takeda K. ERK signalling as a regulator of cell motility. J Biochem. 2017;162:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 320. | Wang Y, Zheng J, Han Y, Zhang Y, Su L, Hu D, Fu X. JAM-A knockdown accelerates the proliferation and migration of human keratinocytes, and improves wound healing in rats via FAK/Erk signaling. Cell Death Dis. 2018;9:848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 321. | Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692:63-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 322. | Sanders MA, Basson MD. p130cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J Biol Chem. 2005;280:23516-23522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 323. | Sharma A, Mayer BJ. Phosphorylation of p130Cas initiates Rac activation and membrane ruffling. BMC Cell Biol. 2008;9:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 324. | Geiger B. A role for p130Cas in mechanotransduction. Cell. 2006;127:879-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 325. | Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006;99:35-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 230] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 326. | Raschka S, More SK, Devadoss D, Zeng B, Kuhn LA, Basson MD. Identification of potential small-molecule protein-protein inhibitors of cancer metastasis by 3D epitope-based computational screening. J Physiol Pharmacol. 2018;69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 327. | Wang Q, More SK, Vomhof-DeKrey EE, Golovko MY, Basson MD. Small molecule FAK activator promotes human intestinal epithelial monolayer wound closure and mouse ulcer healing. Sci Rep. 2019;9:14669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 328. | Rashmi, More SK, Wang Q, Vomhof-DeKrey EE, Porter JE, Basson MD. ZINC40099027 activates human focal adhesion kinase by accelerating the enzymatic activity of the FAK kinase domain. Pharmacol Res Perspect. 2021;9:e00737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 329. | Wang Q, Gallardo-Macias R, Rashmi, Golovko MY, Elsayed AAR, More SK, Oncel S, Gurvich VJ, Basson MD. Discovery of Novel Small-Molecule FAK Activators Promoting Mucosal Healing. ACS Med Chem Lett. 2021;12:356-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barisani D, Italy; Dbar S, Russia; Gassler N, Germany S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM