Published online Apr 28, 2022. doi: 10.3748/wjg.v28.i16.1705
Peer-review started: August 19, 2021
First decision: October 16, 2021
Revised: November 1, 2021
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: April 28, 2022
Processing time: 247 Days and 18.6 Hours
A two- to three-fold increased risk of venous thrombotic events (VTE) has been demonstrated in patients with inflammatory bowel disease (IBD) compared to the general population, but less is known about the risk of VTE in child- and pediatric-onset IBD. In recent years, several studies have reported the rising incidence rate of VTE in juvenile patients with IBD, and the related risk factors have been explored.
To evaluate the risk of VTE in children and adolescents with IBD.
Articles published up to April 2021 were retrieved from PubMed, Embase, Cochrane Library, Web of Science, SinoMed, CNKI, and WANFANG. Data from observational studies and clinical work were extracted. The outcome was the occurrence of VTE according to the type of IBD. The available odds ratio (OR) and the corresponding 95% confidence interval (CI) were extracted to compare the outcomes. Effect size (P), odds ratio (OR), and 95%CI were used to assess the association between VTE risk and IBD disease. Subgroup analyses stratified by subtypes of VTE and IBD were performed.
Twelve studies (7450272 IBD patients) were included in the meta-analysis. Child and adolescent IBD patients showed increased VTE risk (P = 0.02, 95%CI: 0.01-0.03). Subgroup analyses stratified by IBD (ulcerative colitis (UC): P = 0.05, 95%CI: 0.03-0.06; Crohn’s disease (CD): P = 0.02, 95%CI: 0.00-0.04) and VTE subtypes (portal vein thrombosis: P = 0.04, 95%CI: 0.02-0.06; deep vein thrombosis: P = 0.03, 95%CI: 0.01-0.05; central venous catheter-related thrombosis: P = 0.23, 95%CI: 0.00-0.46; thromboembolic events: P = 0.02, 95%CI: 0.01-0.03) revealed a sign
The current meta-analysis showed that the incidence and risk of VTE are significantly increased in pediatric and adolescent IBD patients. Thus, IBD might be a risk factor for VTE in children and young adults. High-quality prospective cohort studies are necessary to confirm these findings.
Core Tip: This study suggested that child and adolescent inflammatory bowel disease (IBD) patients showed an increased risk of venous thrombotic events (VTE) (P = 0.02, 95%CI: 0.01-0.03). Subgroup analysis stratified by IBD (ulcerative colitis: P = 0.04, 95%CI: 0.03-0.06; Crohn’s disease: P = 0.02, 95%CI: 0.00-0.04) and VTE subtypes (portal vein thrombosis: P = 0.04, 95%CI: 0.02-0.06; deep vein thrombosis: P = 0.03, 95%CI: 0.01-0.05; central venous catheter-related thrombosis: P = 0.13, 95%CI: 0.02-0.23; thromboembolic events: P = 0.02, 95%CI: 0.01-0.03) revealed a significant correlation between VTE incidence and IBD.
- Citation: Zhang XY, Dong HC, Wang WF, Zhang Y. Risk of venous thromboembolism in children and adolescents with inflammatory bowel disease: A systematic review and meta-analysis. World J Gastroenterol 2022; 28(16): 1705-1717
- URL: https://www.wjgnet.com/1007-9327/full/v28/i16/1705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i16.1705
Inflammatory bowel disease (IBD) is a digestive system disorder characterized by chronic inflammation of the gastrointestinal (GI) tract[1-5]. The two common manifestations of IBD are ulcerative colitis (UC) and Crohn’s disease (CD)[1-5]. The pathogenesis of IBD involves a complex interaction between the host genetics, external environment, gut microbiota, and immune responses[6-8]. The incidence of pediatric and adult IBD is rising steadily worldwide, especially in developed countries[9-11]. Approximately 25% of patients with IBD are diagnosed before the age of 18 years[12,13]. Furthermore, 4% of pediatric IBD occur before the age of 5 years and 18% before the age of 10 years, peaking in adolescence[7].
Venous thromboembolism (VTE) is a potentially severe medical condition with a high recurrence rate[14,15]. IBD is a high-risk factor for the occurrence and development of VTE in adults[16,17]. Routine VTE prophylaxis is recommended for IBD patients during hospitalization[1,18-20]. Pediatric patients with IBD also have an increased predisposition to develop VTE, including deep venous thrombosis (DVT) and pulmonary embolism (PE), although the available evidence is of lower quality[21,22]. The etiology of VTE in IBD is multifactorial and not well defined. It involves the cross-talk between coagulation and inflammation[23,24]. IBD-associated inflammation causes a hypercoagulable state, leading to systemic thrombotic events and local microthrombi in the vessels of the inflamed intestinal mucosa[25,26]. Pediatric IBD patients exhibit various clinical features, including disease location and severity, endoscopic appearance, histology, comorbidities, complications, and response to treatment options[27]. The reported risk factors for VTE in children with IBD include inherent genetics, ethnicity, gender, infection, parenteral nutrition, surgery, specific therapies, disease history, and increased use of central venous catheters (CVCs; the most common factor)[28-30].
Nonetheless, whether the development of VTE complications in IBD treatment is age-dependent is yet to be clarified. The increased risk of VTE in adult patients with IBD is widely recognized[31]. A two- to three-fold increased risk of VTE has been demonstrated in patients with IBD compared to the general population[26]. However, less is known about the risk of VTE in child- and pediatric-onset IBD. In recent years, several studies have reported the rising incidence rate of VTE in juvenile patients with IBD, and the related risk factors have been explored[32-35]. Therefore, this meta-analysis evaluated the association between postoperative VTE risk and IBD in children and adolescent populations.
According to the Preferred Reporting Items for Systematic Reviews (PRISMA, 2020) guidelines, we conducted a systematic review and meta-analysis. PubMed, Embase, Cochrane Library, Web of Science, SinoMed, CNKI, and WANFANG databases were systematically searched for studies published up to April 2021.
Articles were included if they met the following criteria: (1) Investigating the incidence or risk of VTE in children and adolescents with IBD; (2) IBD and VTE (DVT, pulmonary embolism; CVC-related thrombosis, intracranial venous sinus thrombosis, portal vein thrombosis, thromboembolic events, intra-abdominal thrombus, and cerebrovascular thrombosis) were confirmed medically; (3) language was limited to English or Chinese; and (4) RCT, cohort, or database review design.
Publications were excluded if they were: (1) Letters, review articles, meta-analyses, case-control, case reports, and experimental animal studies; (2) Missing primary data; or (3) Full text unavailable.
PubMed, Embase, Cochrane Library, Web of Science, SinoMed, CNKI, and WANFANG databases were systematically searched for potentially eligible studies published up to April 2021. The MeSH terms used were as follows: “inflammatory bowel disease”, “Crohn Disease”, “Colitis, Ulcerative” and “Children”, “pediatric*” and “Venous Thrombosis Pulmonary”, “Embolism vein thrombosis”, as well as relevant keywords. The search strategy for PubMed, Embase and Cocharane Central is listed in Supplementary Table 1.
The selection and inclusion of studies were performed by two independent reviewers in two stages, which included the analysis of the titles/abstracts, followed by a full-text assessment. Disagreements were resolved by a third reviewer. Data including the authors’ names, publication year, study design, country, sample size, mean age, IBD therapy, patient, VTE type, and male percentage were extracted. The quality assessment was performed in duplicate by two researchers independently (xx and xx). The Newcastle-Ottawa Scale (NOS)[36] was used to assess the methodological quality of eligible observational studies.
Statistical analyses were performed using STATA SE 14.0 (StataCorp, College Station, TX, USA). The risk of VTE in IBD was estimated by ES (p) and the corresponding 95% confidence interval (CI). The available odds ratio (OR) and the corresponding 95%CI were extracted to compare the outcomes. In addition, the event incidence was relatively small (0 < P < 0.2), and the double arcsine method was also used for data conversion.
Cochran’s Q statistic (P < 0.10 indicated evidence of heterogeneity) was used to assess heterogeneity among the included studies[37]. When significant heterogeneity (P < 0.10) was detected, the random-effects model was applied to combine the effect sizes of the included studies; otherwise, the fixed-effects model was adopted[38]. In addition, sensitivity analyses were performed to identify individual study effects on pooled results and test the reliability of the results.
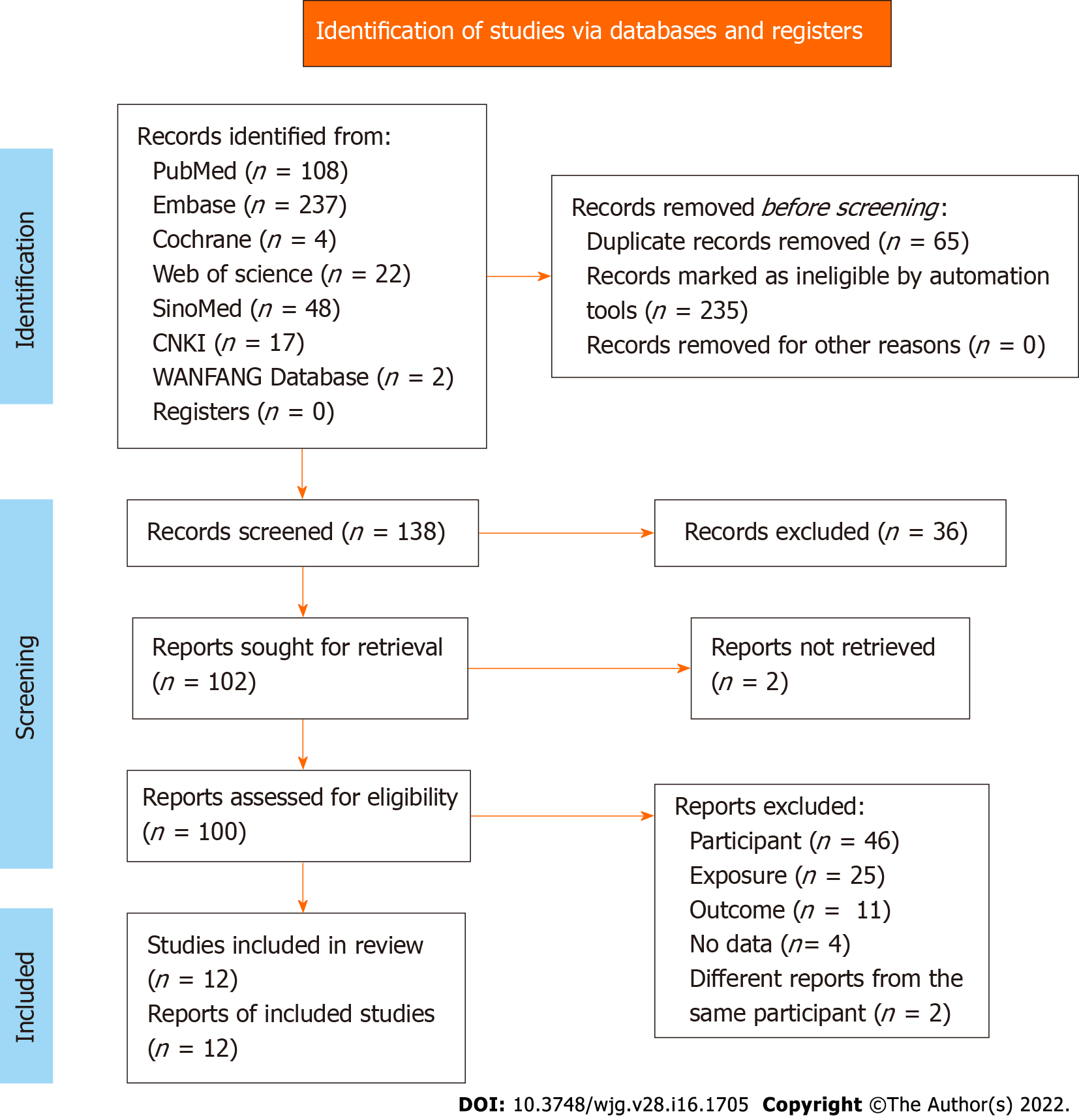
The initial literature database search retrieved 438 studies. The duplicate records and automatically marked ineligible records were filtered out. After screening, 100 reports were assessed for eligibility. Due to insufficient information on participants, exposure, outcomes, and different reports from the same participants, only 12 reports were finally included in this meta-analysis. The study screening flowchart is illustrated in Figure 1.
The characteristics of the 12 eligible studies, including nine cohort studies[21,27,30,32-35,39,40], one RCT[28], and two database reviews[25,41], are listed in Table 1. These studies encompassed 7450272 IBD patients. Eight studies were carried out in the United States, and the others were performed in Canada, Switzerland, Iran, and Korea. The IBD patients had a mean age of < 1-21 years. The proportion of male patients ranged from 42.9% to 71.2%. The IBD subtypes covered UC, CD, indeterminate colitis (IC), and undefined IBD type (IBD-U). Moreover, various VTE subtypes, including intra-abdominal thrombosis (IAT), DVT, venous thromboembolism (VTE), TE, pulmonary thromboembolism (PTE), intracranial venous sinus thrombosis (IVST), PVT, and cerebrovascular thrombosis (CVT), were investigated. In addition, the confounding factors, including age, sex, hypercoagulable status, CVC, parenteral nutrition, cancer, sickle cell anemia, tobacco use, race/ethnicity, payer status, urban/rural status, hospital region, and length of inpatient stay, were adjusted in various studies. According to the NOS evaluation criteria, the methodological quality score was 6 for 10 studies and 8 and 9 for the other two studies, respectively, indicating low-to-moderate bias (Table 2).
| Ref. | Country | Study design | Period | Sample size N | Age, years (mean or median) | Male, (%) | Patient | IBD therapy | VTE type |
| Antiel et al[31], 2013 | United States | Cohort | 1999.1-2011.12 | 366 | ≤ 21 | No data | UC | Surgery | IAT |
| Bence et al[26], 2020 | United States | Cohort | 2020.1-2016.6 | 276 | 15 (13, 17) | 46 | CD, UC, IC | Corticosteroids, Biologic therapy, Immunologic therapy | DVT |
| Cairo et al[36], 2017 | United States | RCS | 2012-2015 | 410 | < 1 (9.8%); 1-5 (15.7%); 6-10 (30%); 11-15 (34.6%); 16-18 (9.9%) | 59.5 | IBD | No data | VTE |
| Derderian et al[27], 2020 | United States | Cohort | 2008-2018 | 49 | 9.8 ± 4.4 | 65 | UC/IBD-U | Surgery | DVT |
| Diamond et al[32], 2018 | United States | Cohort | 2015-2017 | 47 | 14 | 46.8 | IBD | Systemic steroids; Anti-TNF therapy | CVC-related thrombosis |
| Diamond et al[33], 2010 | Canada | Cohort | 1999.11.1-2008.2.29 | 85 | 14.8 (2.8) | 54.1 | IBD | Surgery | DVT |
| Herzog et al[34], 2018 | Switzerland | Cohort | 2006-2017 | 63 | < 17 | 54 | CD; UC or IBD-U | 5-ASA Systemic CS; Immunomodulators; Calcineurin inhibitors; TNFα inhibitors, Surgery | TE |
| Jarchin et al[29], 2019 | United States | Cohort | 2008.1-2017.12 | 58 | 0-5 (5%); 6-10 (10%); 11-18 (85%) | 66 | UC | Anti-TNF only, Vedolizumab only, Anti-TNF+ vedolizumab, Anti-TNF+ vedolizumab+ ustekinumab, Surgery | PVT (within 30 d, > 30 d) |
| Khosravi et al[28], 2020 | Iran | Cohort | 2019 | 21 | 11.12 ± 5.65 | 42.9 | IBD | Surgery | DVT |
| Lee et al[35], 2016 | Korea | Cohort | 1995.7-2011.6 | 73 | 12.49 ± 2.47 | 71.2 | CD | Systemic corticosteroids 5-ASA only, 5-ASA+; azathioprine; 5-ASA only or 5-ASA+ azathioprine | PTE |
| Nylund et al[19], 20131 | United States | Database review | 1997, 2000, 2003, 2006, and 2009 | 61076/7318/7379898 | 15.72 ± 0.02/15.39 ± 0.06/13.57 ± 0.01 | 49.32/47.55/49.64 | CD, UC | No data | TE, PE, DVT, IVST, PVT |
| Zitomersky et al[37], 2013 | United States | Database review | 2006-2011 | 532 | No data | No data | CD, UC | No data | CVT |
| Ref. | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | Total quality scores |
| Antiel et al[31], 2013 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Bence et al[26], 2020 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Cairo et al[36], 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Derderian et al[27], 2020 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Diamond et al[32], 2018 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Diamond et al[33], 2010 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Herzog et al[34], 2018 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Jarchin et al[29], 2019 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Khosravi et al[28], 2020 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Lee et al[35], 2016 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Nylund et al[19], 2013 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zitomersky et al[37], 2013 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
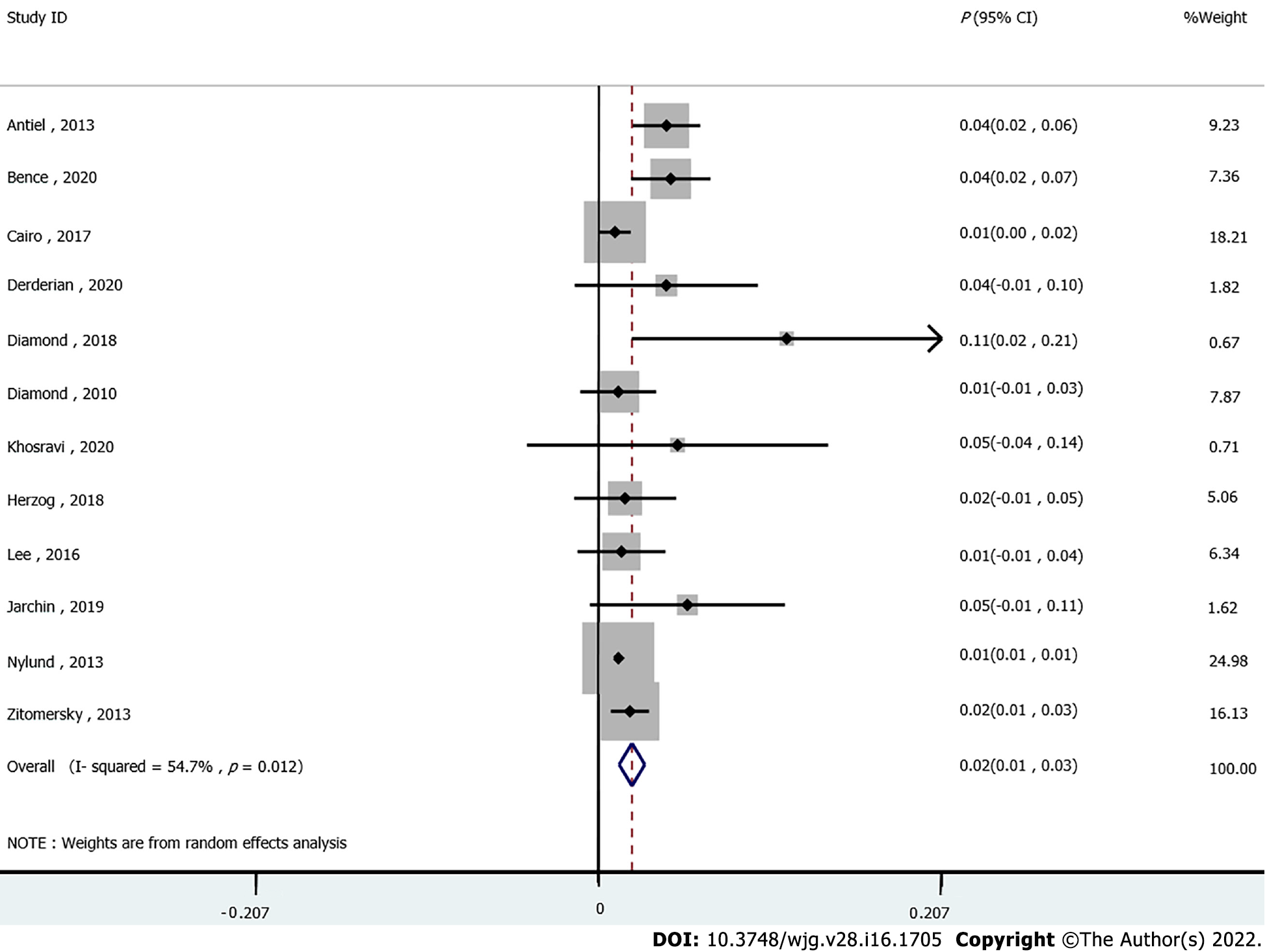
The association between IBD and the incidence of VTE was assessed in 12 studies. The pooled results showed that the risk of VTE was significantly increased in IBD patients compared to those without IBD (P = 0.02, 95%CI: 0.01-0.03). Moderate heterogeneity was detected across the included studies (I2 = 54.7%, Pheterogeneity = 0.012, Figure 2). In addition, double arcsine analysis showed similar results (P = 0.03, 95%CI: 0.02-0.04, Supplementary Figure 1).
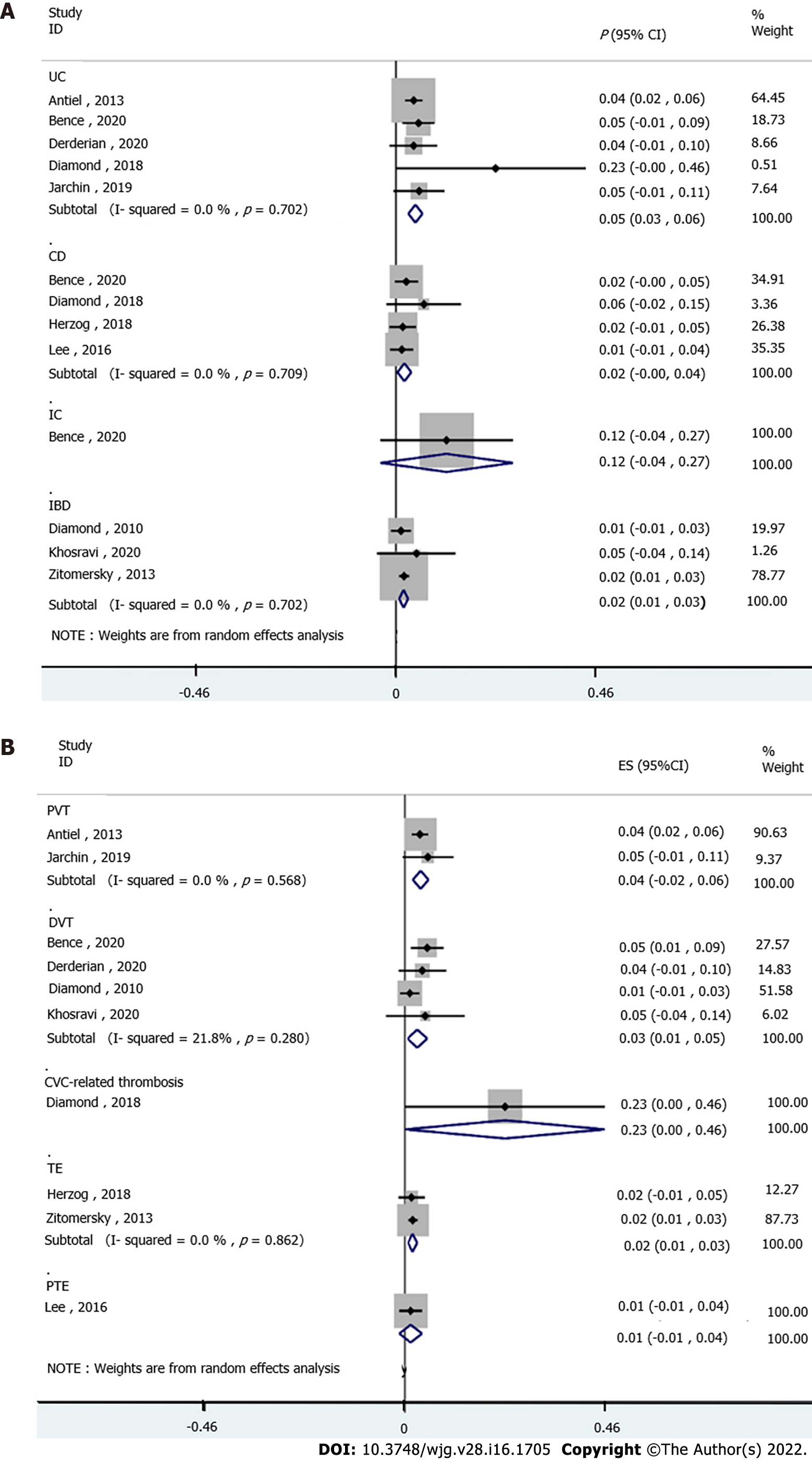
The subgroup analyses stratified by IBD subtype were performed to evaluate the VTE risk in IBD. Five studies analyzed the UC subtype, and the pooled results suggested significantly increased VTE incidence in UC patients (P = 0.05, 95%CI: 0.03-0.06). No heterogeneity was detected among the studies (I2 = 0.0%, pheterogeneity = 0.571, Figure 3A). Pooled analysis of four studies established a correlation between VTE risk and CD (P = 0.02, 95%CI: 0.00-0.04), without heterogeneity (I2 = 0.0%, pheterogeneity = 0.709, Figure 3A). The IBD subtype was not defined in three studies, and a high rate of VTE was also detected in these patients (P = 0.02, 95%CI: 0.01-0.03), without obvious heterogeneity (I2 = 0.0%, pheterogeneity = 0.702, Figure 3A). Based on one study, the VTE incidence was not increased in IC patients (P = 0.12, 95%CI: -0.04 to 0.27, Figure 3A).
Stratified by VTE subtype, two studies showed significantly increased PVT incidence in IBD patients (P = 0.04, 95%CI: 0.02-0.06; I2 = 0.0%, pheterogeneity = 0.568, Figure 3B). The pooled analysis of four studies showed a high risk of DVT in IBD patients (P = 0.03, 95%CI: 0.01-0.05) with low heterogeneity (I2 = 21.8%, pheterogeneity = 0.280, Figure 3B). In addition, one study revealed an increased risk of CVC-related thrombosis in IBD (P = 0.23, 95%CI: 0.00-0.46, Figure 3B). Furthermore, two studies showed a significant association between TE risk and IBD (P = 0.02, 95%CI: 0.01-0.03), without heterogeneity (I2 = 0.0%, pheterogeneity = 0.862, Figure 3B). No association was found between PTE and IBD disease (P = 0.01, 95%CI: -0.01 to 0.04, Figure 3B) based on one study.
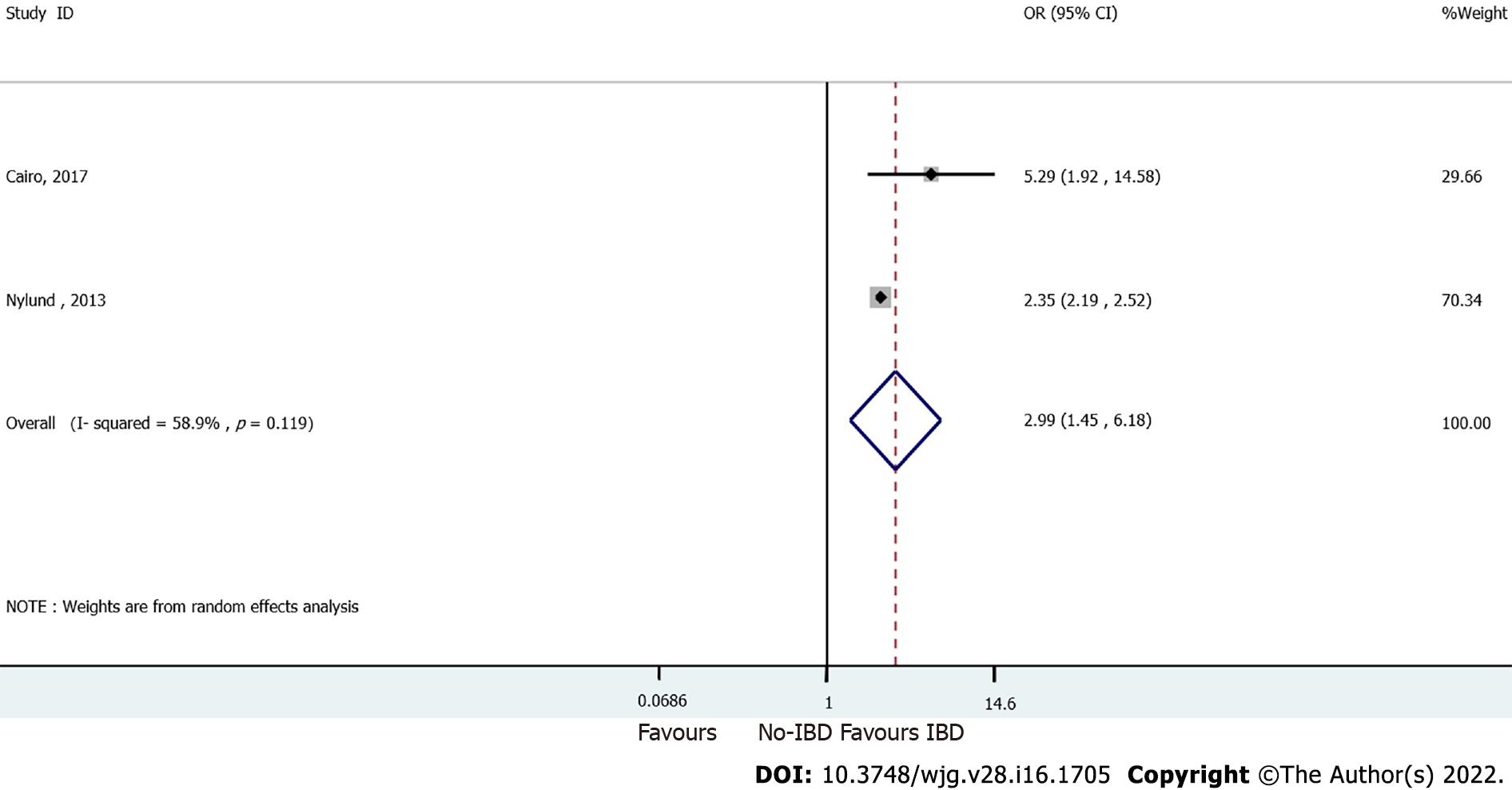
Pooled analysis of two studies comparing IBD and non-IBD patients showed a significant correlation between VTE risk and IBD disease (OR = 2.99, 95%CI: 1.45-6.18). Moderate heterogeneity was observed among these studies (I2 = 58.9%, pheterogeneity = 0.119, Figure 4).
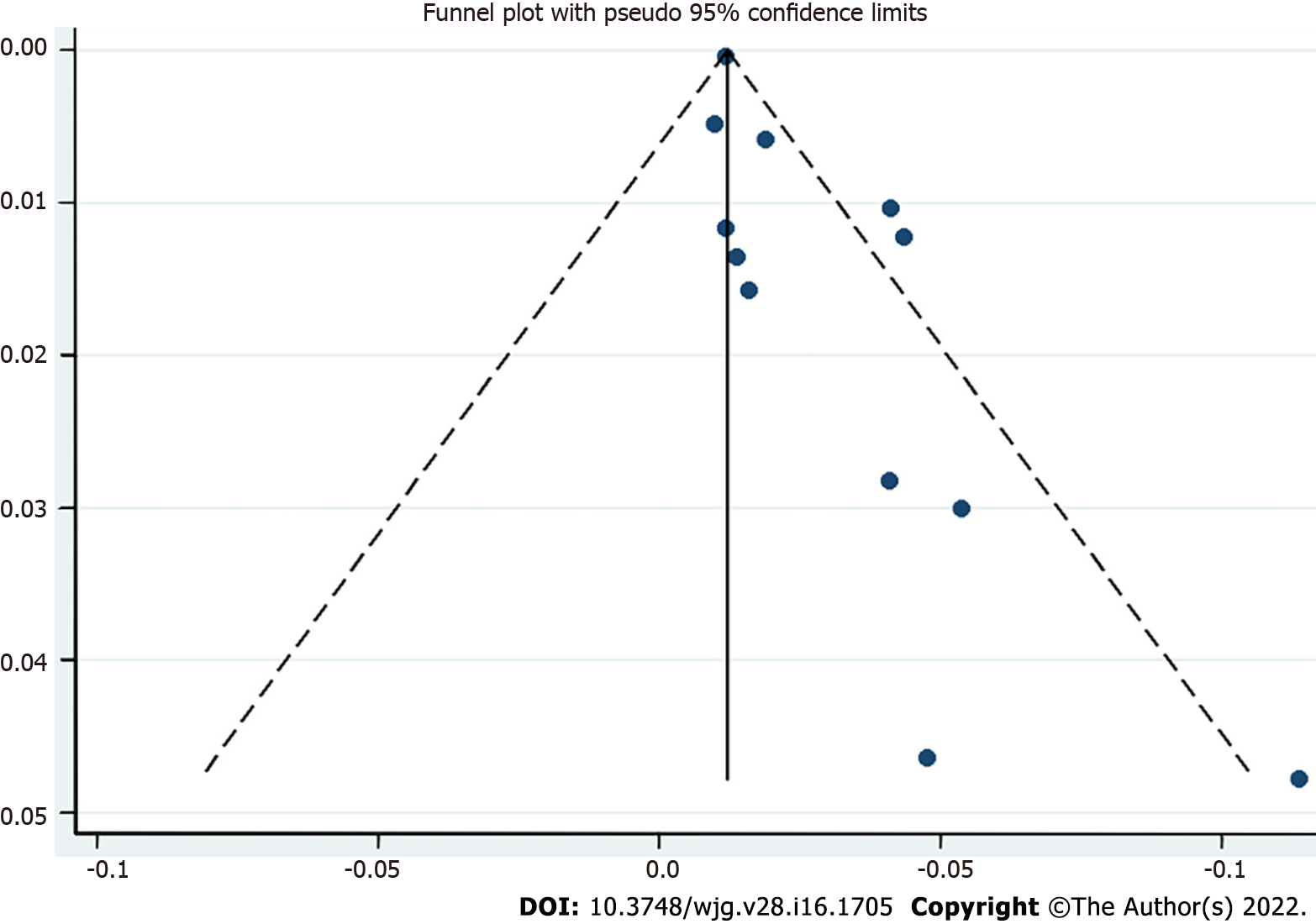
Publication bias was analyzed using a funnel plot. The asymmetrical distribution suggested significant publication bias (Figure 5).
The present meta-analysis found that the VTE risk was significantly increased in children and young adults with IBD. A high incidence of VTE was observed in the different subtypes of IBD, including UC, CD, and other undefined IBD diseases. Moreover, multiple VTE signs related to PVT, DVT, TE, and CVC-related thrombosis might be prone to developing during the progression of pediatric and adolescent IBD.
This study suggested that children and young adult IBD patients were susceptible to VTE risk after treatment, despite a low overall incidence. The increased risk of VTE is specific to IBD. Other chronic inflammatory diseases, such as rheumatoid arthritis, or chronic bowel diseases, such as celiac disease, are not found to be accompanied by a high incidence of VTE[26]. The association between VTE risk and IBD has been demonstrated in various IBD patient populations. Currently, there is a lack of systemic reviews on VTE risk in pediatric and adolescent patients. Female pediatric IBD patients were found to be at a high risk of developing VTE[28]. The meta-analysis by Kim et al[42] focused on pregnant women with IBD. The findings showed a significantly higher incidence of VTE during pregnancy and postpartum in female IBD patients. According to the VTE subtype, the risk of DVT increased significantly in pregnant women, whereas the increase in PE was not significant during pregnancy. In the subgroup analysis based on the location of IBD, patients with UC had significantly higher VTE risk during both pregnancy and the postpartum period, while CD patients exhibited increased VTE incidence only during pregnancy. Therefore, elevated VTE risk was found to be more apparent in UC patients than CD patients. A recent study showed a high postsurgical VTE risk in patients with UC but not in those with CD[43]. Similar results were published earlier[44,45]. However, those studies were performed in the postsurgical setting, which is a state known to be associated with an increased risk of VTE[46,47]. Similarly, the current study showed a higher risk of VTE in UC compared to CD in children and young adult IBD patients, and consistently suggested that children with UC might be more susceptible to VTE. The exact causes for this higher VTE risk in UC than CD are unknown, but severe UC is associated with anemia and reactive thrombocytosis, which is conducive to VTE[48,49].
Another meta-analysis by Yuhara et al[50] demonstrated that adult patients with IBD (> 20-years-old) were at increased risk for both DVT and PE. The study also showed that confounding factors, such as smoking and body mass index (BMI), did not affect the correlation between IBD and DVT/PE. A large-population database study by Nylund et al[25] found that hospitalized children and adolescents with IBD had a high incidence of TE, including DVT and PE. In addition, the study identified older age and abdominal surgery as risk factors for TE in children and young adults with IBD. The Hispanics had a low risk of TE among all children and adolescents. Although tobacco use was a risk factor for TE in adults, it was a protective factor in children with IBD. Interestingly, an increasing trend of TE was observed through the years among children and young adults with IBD, which might be associated with increased hospital admission rates. However, this increasing trend was not clear after multivariable regression analysis. Similarly, the review by Lazzerini et al[51] suggested an increased risk of TE in pediatric IBD patients compared to the general population. Our meta-analysis included several updated publications and showed that the risk of PVT, DVT, TE, and CVC-related thrombosis was increased in IBD child and adolescent patients. Moreover, the underlying mechanism of location-related VTE occurrence and development needs further exploration.
Nevertheless, the present study has several limitations. First, moderate heterogeneity was detected in the overall analysis of the correlation between VTE and IBD. On the other hand, low or no heterogeneity was detected after stratifying the studies by subtypes of VTE and IBD. Patients’ characteristics, diagnosis of VTE, and intervention therapies might contribute to the variations across the studies and the magnitude of association. Second, certain subgroup analyses only involved a small number of studies and patients, and hence, the findings should be interpreted with caution. Third, the included large-size database review study incorporated registry data of medical records with different populations and settings, which might generate a high selection bias. Fourth, VTE was challenging to diagnose, which might have led to the misclassification of VTE.
Pediatric and adolescent IBD patients have an increased risk of VTE. UC and CD patients exhibited a high risk of VTE. The risk of VTE subtypes was increased in IBD patients.
A two- to three-fold increased risk of venous thrombotic events (VTE) has been demonstrated in patients with inflammatory bowel disease (IBD) compared to the general population, but less is known about the risk of VTE in child- and pediatric-onset IBD. In recent years, several studies have reported the rising incidence rate of VTE in juvenile patients with IBD, and the related risk factors have been explored.
To evaluate the risk of VTE in children and adolescents with IBD.
To evaluate the risk of VTE in children and adolescents with IBD.
Articles published up to April 2021 were retrieved from PubMed, Embase, Cochrane Library, Web of Science, SinoMed, CNKI, and WANFANG. Data from observational studies and clinical work were extracted. The outcome was the occurrence of VTE according to the type of IBD. The available odds ratio (OR) and the corresponding 95% confidence interval (CI) were extracted to compare the outcomes. Effect size (P), OR, and 95%CI were used to assess the association between VTE risk and IBD disease. Subgroup analyses stratified by subtypes of VTE and IBD were performed.
Twelve studies (7450272 IBD patients) were included in the meta-analysis. Child and adolescent IBD patients showed an increased risk of VTE (P = 0.02, 95%CI: 0.01-0.03). Subgroup analyses stratified by IBD (ulcerative colitis (UC): P = 0.05, 95%CI: 0.03-0.06; Crohn’s disease (CD): P = 0.02, 95%CI: 0.00-0.04) and VTE subtypes (portal vein thrombosis (PVT): P = 0.04, 95%CI: 0.02-0.06; deep vein thrombosis: P = 0.03, 95%CI: 0.01-0.05; central venous catheter-related thrombosis: P = 0.23, 95%CI: 0.00-0.46; thromboembolic events: P = 0.02, 95%CI: 0.01-0.03) revealed a significant correlation between VTE risk and IBD. Patients with IBD were more susceptible to VTE risk than those without IBD (OR = 2.99, 95%CI: 1.45-6.18). The funnel plot was asymmetric, suggesting the presence of significant publication bias.
Pediatric and adolescent IBD patients have an increased VTE risk. UC and CD patients exhibited a high risk of VTE. The risk of VTE subtypes was increased in IBD patients.
Pediatric and adolescent IBD patients have an increased VTE risk. UC and CD patients exhibited a high risk of VTE. The risk of VTE subtypes was increased in IBD patients.
| 1. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1139] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 2. | Ko CW, Singh S, Feuerstein JD, Falck-Ytter C, Falck-Ytter Y, Cross RK; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology. 2019;156:748-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 3. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2712] [Article Influence: 301.3] [Reference Citation Analysis (2)] |
| 4. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1030] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 5. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1976] [Article Influence: 219.6] [Reference Citation Analysis (113)] |
| 6. | Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 751] [Cited by in RCA: 1133] [Article Influence: 94.4] [Reference Citation Analysis (32)] |
| 7. | Rosen MJ, Dhawan A, Saeed SA. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015;169:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 8. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 709] [Article Influence: 101.3] [Reference Citation Analysis (1)] |
| 9. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 2021] [Article Influence: 183.7] [Reference Citation Analysis (1)] |
| 10. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1626] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 11. | Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741-2763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 271] [Cited by in RCA: 284] [Article Influence: 35.5] [Reference Citation Analysis (6)] |
| 12. | Oliveira SB, Monteiro IM. Diagnosis and management of inflammatory bowel disease in children. BMJ. 2017;357:j2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 13. | Conrad MA, Rosh JR. Pediatric Inflammatory Bowel Disease. Pediatr Clin North Am. 2017;64:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 735] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 15. | Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 643] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 16. | Scoville EA, Konijeti GG, Nguyen DD, Sauk J, Yajnik V, Ananthakrishnan AN. Venous thromboembolism in patients with inflammatory bowel diseases: a case-control study of risk factors. Inflamm Bowel Dis. 2014;20:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Novacek G, Weltermann A, Sobala A, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T, Fuchssteiner H, Knoflach P, Vogelsang H, Miehsler W, Platzer R, Tillinger W, Jaritz B, Schmid A, Blaha B, Dejaco C, Eichinger S. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779-787, 787.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Bitton A, Buie D, Enns R, Feagan BG, Jones JL, Marshall JK, Whittaker S, Griffiths AM, Panaccione R; Canadian Association of Gastroenterology Severe Ulcerative Colitis Consensus Group. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2012;107:179-94; author reply 195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Nguyen GC, Yeo EL. Prophylaxis of venous thromboembolism in IBD. Lancet. 2010;375:616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, Geerts W, Bressler B, Butzner JD, Carrier M, Chande N, Marshall JK, Williams C, Kearon C. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835-848.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 21. | Antiel RM, Hashim Y, Moir CR, Rodriguez V, Elraiyah T, Zarroug AE. Intra-abdominal venous thrombosis after colectomy in pediatric patients with chronic ulcerative colitis: incidence, treatment, and outcomes. J Pediatr Surg. 2014;49:614-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | McKie K, McLoughlin RJ, Hirsh MP, Cleary MA, Aidlen JT. Risk Factors for Venous Thromboembolism in Children and Young Adults With Inflammatory Bowel Disease. J Surg Res. 2019;243:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Branchford BR, Carpenter SL. The Role of Inflammation in Venous Thromboembolism. Front Pediatr. 2018;6:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 276] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 24. | Vazquez-Garza E, Jerjes-Sanchez C, Navarrete A, Joya-Harrison J, Rodriguez D. Venous thromboembolism: thrombosis, inflammation, and immunothrombosis for clinicians. J Thromb Thrombolysis. 2017;44:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Nylund CM, Goudie A, Garza JM, Crouch G, Denson LA. Venous thrombotic events in hospitalized children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Coremans L, Strubbe B, Peeters H. Venous thromboembolism in patients with inflammatory bowel disease: review of literature and practical algorithms. Acta Gastroenterol Belg. 2021;84:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Herzog D, Fournier N, Buehr P, Rueger V, Koller R, Heyland K, Nydegger A, Spalinger J, Schibli S, Petit LM, Braegger CP; Swiss IBD Cohort Study Group. Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications. Eur J Gastroenterol Hepatol. 2018;30:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Cairo SB, Lautz TB, Schaefer BA, Yu G, Naseem HU, Rothstein DH. Risk factors for venous thromboembolic events in pediatric surgical patients: Defining indications for prophylaxis. J Pediatr Surg. 2018;53:1996-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Lassandro G, Palmieri VV, Palladino V, Amoruso A, Faienza MF, Giordano P. Venous Thromboembolism in Children: From Diagnosis to Management. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Diamond CE, Hennessey C, Meldau J, Guelcher CJ, Guerrera MF, Conklin LS, Sharma KV, Diab YA. Catheter-Related Venous Thrombosis in Hospitalized Pediatric Patients with Inflammatory Bowel Disease: Incidence, Characteristics, and Role of Anticoagulant Thromboprophylaxis with Enoxaparin. J Pediatr. 2018;198:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Zitomersky NL, Verhave M, Trenor CC 3rd. Thrombosis and inflammatory bowel disease: a call for improved awareness and prevention. Inflamm Bowel Dis. 2011;17:458-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Bence CM, Traynor MD, Polites SF, Ha D, Muenks P, St. Peter SD, Landman MP, Densmore JC, Potter DD. The incidence of venous thromboembolism in children following colorectal resection for inflammatory bowel disease: A multi-center study. J Pediatric Surg. 2020;. |
| 33. | Derderian SC, Phillips R, Acker SN, Bruny J, Partrick DA. Pediatric ulcerative colitis: three- vs two-stage colectomy with ileal pouch-anal anastomosis. Pediatric Surg International. 2020;36:171-177. |
| 34. | Khosravi F, Ziaeefar P. Early and long-term outcome of surgical intervention in children with inflammatory bowel disease. Arq Bras Cir Dig. 2020;33:e1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Jarchin L, Spencer EA, Khaitov S, Greenstein A, Jossen J, Lai J, Dunkin D, Pittman N, Benkov K, Dubinsky MC. De Novo Crohn’s Disease of the Pouch in Children Undergoing Ileal Pouch-Anal Anastomosis for Ulcerative Colitis. J Pediatric Gastroenterol Nutr. 2019;69:455-460. |
| 36. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1843] [Article Influence: 153.6] [Reference Citation Analysis (0)] |
| 37. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48588] [Article Influence: 2112.5] [Reference Citation Analysis (4)] |
| 38. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. London: Cochrane Collaboration, 2020. |
| 39. | Diamond IR, Gerstle JT, Kim PCW, Langer JC. Outcomes after laparoscopic surgery in children with inflammatory bowel disease. Surgical Endoscopy. 2010;24:2796-2802. |
| 40. | Lee YA, Chun P, Hwang EH, Mun SW, Lee YJ, Park JH. Clinical Features and Extraintestinal Manifestations of Crohn Disease in Children. Pediatr Gastroenterol Hepatol Nutr. 2016;19:236-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Zitomersky NL, Levine AE, Atkinson BJ, Harney KM, Verhave M, Bousvaros A, Lightdale JR, Trenor CC 3rd. Risk factors, morbidity, and treatment of thrombosis in children and young adults with active inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;57:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Kim YH, Pfaller B, Marson A, Yim HW, Huang V, Ito S. The risk of venous thromboembolism in women with inflammatory bowel disease during pregnancy and the postpartum period: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e17309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | McCurdy JD, Ellen Kuenzig M, Spruin S, Fung OW, Mallik R, Williams L, Murthy SK, Carrier M, Nguyen G, Benchimol EI. Surgery and the Subtype of Inflammatory Bowel Disease Impact the Risk of Venous Thromboembolism After Hospital Discharge. Dig Dis Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Faye AS, Wen T, Ananthakrishnan AN, Lichtiger S, Kaplan GG, Friedman AM, Lawlor G, Wright JD, Attenello FJ, Mack WJ, Lebwohl B. Acute Venous Thromboembolism Risk Highest Within 60 Days After Discharge From the Hospital in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1133-1141.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 45. | McKechnie T, Wang J, Springer JE, Gross PL, Forbes S, Eskicioglu C. Extended thromboprophylaxis following colorectal surgery in patients with inflammatory bowel disease: a comprehensive systematic clinical review. Colorectal Dis. 2020;22:663-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Lewis-Lloyd CA, Pettitt EM, Adiamah A, Crooks CJ, Humes DJ. Risk of Postoperative Venous Thromboembolism After Surgery for Colorectal Malignancy: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2021;64:484-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 47. | Toth S, Flohr TR, Schubart J, Knehans A, Castello MC, Aziz F. A meta-analysis and systematic review of venous thromboembolism prophylaxis in patients undergoing vascular surgery procedures. J Vasc Surg Venous Lymphat Disord. 2020;8:869-881.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Papa A, Gerardi V, Marzo M, Felice C, Rapaccini GL, Gasbarrini A. Venous thromboembolism in patients with inflammatory bowel disease: focus on prevention and treatment. World J Gastroenterol. 2014;20:3173-3179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (2)] |
| 49. | Yan SL, Russell J, Harris NR, Senchenkova EY, Yildirim A, Granger DN. Platelet abnormalities during colonic inflammation. Inflamm Bowel Dis. 2013;19:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Yuhara H, Steinmaus C, Corley D, Koike J, Igarashi M, Suzuki T, Mine T. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;37:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 51. | Lazzerini M, Bramuzzo M, Maschio M, Martelossi S, Ventura A. Thromboembolism in pediatric inflammatory bowel disease: systematic review. Inflamm Bowel Dis. 2011;17:2174-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anysz H, Poland; Dabravolski SA, Belarus; Moradi L, Iran S-Editor: Wang LL L-Editor: Webster JR P-Editor: Yu HG