Published online Apr 21, 2022. doi: 10.3748/wjg.v28.i15.1548
Peer-review started: December 2, 2021
First decision: January 23, 2022
Revised: February 5, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: April 21, 2022
Processing time: 134 Days and 3.7 Hours
Although the criteria for the indication of endoscopic submucosal dissection (ESD) for undifferentiated early gastric cancer (UD-EGC) have been recently proposed, accumulating reports on the non-negligible rate of lymph node metastasis (LNM) after indicated ESD raise questions on the reliability of the current criteria.
To investigate the prevalence and risk factors of LNM in UD-EGC cases meeting the expanded indication for ESD.
We retrospectively reviewed 4780 UD-EGC cases that underwent surgical resection between January 2008 and February 2019 at Asan Medical Center, a tertiary university hospital in Korea. To identify the risk factors of LNM of UD-EGC meeting the expanded criteria for ESD, we performed a case-control study by matching the cases with LNM to those without at a ratio of 1:4. We reviewed the clinical, endoscopic, and histologic features of the cases to identify features with a significant difference according to the presence of LNM. Univariate and multivariate logistic regression analyses were performed to estimate the odds ratios (ORs).
Of the 4780 UD-EGC cases, 1240 (25.9%) were identified to meet the expanded indication for ESD. Of the 1240 cases, 14 (1.1%) cases had LNM. Among the various clinical, endoscopic, and histopathological features that were evaluated, mixed histology (tumors consisting of 10%-90% of signet ring cells) had a marginally significant association (P = 0.059) with the risk of LNM. Moreover, diffuse blurring of the muscularis mucosae (MM) underneath the tumorous epithelium, a previously unrecognized histologic feature, had a significant association with the absence of LNM (P = 0.028). Multivariate logistic regression analysis showed that the blurring of MM was the only explanatory variable significantly associated with a reduced risk of LNM (OR: 0.12, 95%CI: 0.02-0.95; P = 0.045).
The risk of LNM is higher than expected when using the current expanded indication for UD-EGC. Histological evaluation could provide useful clues for reducing the risk of LNM.
Core Tip: This was a retrospective study investigating the prevalence and risk factors of lymph node metastasis (LNM) in cases with undifferentiated early gastric cancer meeting the expanded indication for endoscopic submucosal dissection (ESD). We found that the incidence rate of LNM was 1.1% (14/1240), which was higher than expected for indicated ESD. A subsequent case-control study revealed that two histological features-histologic purity of tumors and blurring of the muscularis mucosae underneath the tumorous epithelium-are promising factors for predicting the risk of LNM. Combining these histologic features could improve the current expanded indication criteria for ESD.
- Citation: Yoon J, Yoo SY, Park YS, Choi KD, Kim BS, Yoo MW, Lee IS, Yook JH, Kim GH, Na HK, Ahn JY, Lee JH, Jung KW, Kim DH, Song HJ, Lee GH, Jung HY. Reevaluation of the expanded indications in undifferentiated early gastric cancer for endoscopic submucosal dissection. World J Gastroenterol 2022; 28(15): 1548-1562
- URL: https://www.wjgnet.com/1007-9327/full/v28/i15/1548.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i15.1548
Endoscopic submucosal dissection (ESD) has gained popularity in the treatment of early gastric cancer (EGC) due to the benefits of organ preservation and maintenance of the quality of life. However, ESD cannot dissect lymph nodes around the stomach and diagnostic modalities such as endoscopic ultrasonography, computed tomography, and positron emission tomography cannot provide adequate data for detecting lymph node metastasis (LNM); as such, the indication for ESD for EGCs has been suggested based on the analysis of the risk of LNM in a large number of surgically resected specimens[1], and ESD is usually considered for tumors with a very low risk of LNM.
Undifferentiated EGC (UD-EGC) accounts for 40%-50% of EGCs, and has been reported to show a higher incidence of LNM than EGCs with differentiated histology[2,3]. Thus, gastrectomy with lymphadenectomy has been used as the standard treatment for UD-EGCs, and ESD for UD-EGC remains an investigational treatment[3,4]. In an attempt to expand the indication of ESD in UD-EGC, some researchers have reported that a select group of UD-EGCs had a very low possibility of LNM[1]. Accordingly, it has been suggested that ESD could be considered for UD-EGC cases when the tumor is an intramucosal lesion with a size of less than 2 cm and no sign of ulcer, and no further surgery is indicated when pathologic evaluation of the ESD specimen does not reveal lymphovascular invasion (LVI) or positive vertical and horizontal margin[1,4]. However, there have been several reports of lymph node or distant metastases arising after curative ESD in UD-EGC cases meeting the expanded criteria[5,6]. These conflicting data raise a question on the reliability of the current expanded criteria for UD-EGC, and necessitates further efforts to identify more clinicopathologic features associated with the risk of LNM in UD-EGC.
Because LNM-negative patients can be curatively treated with minimally invasive ESD, evaluating the risk factor of LNM is crucial for determining the appropriate treatment. Although many studies have been performed to identify the clinicopathological factors associated with LNM in UD-EGC[7,8], the only risk factors that were identified include tumor size, depth of invasion, presence of LVI, and ulcer. Therefore, to obtain clarity regarding the treatment of UD-EGC, we investigated the risk of LNM of UD-EGCs meeting the criteria for the expanded indication for ESD, and performed a case-control study to identify the clinical, endoscopic, and histopathologic features related to the risk of LNM.
We retrospectively reviewed the medical records of all patients who underwent curative gastrectomy with extended lymphadenectomy for UD-EGC at Asan Medical Center between January 2008 and February 2019. To focus on the histologic types that are most frequently encountered in clinical practice, we only included tumors diagnosed as “adenocarcinoma, poorly differentiated (with or without signet ring cell component),” “poorly cohesive carcinoma,” and “signet ring cell carcinoma (SRCC),” and excluded rare variants such as mucinous adenocarcinoma and gastric carcinoma with lymphoid stroma. We also excluded patients with multiple tumors, tumors in the remnant stomach, any synchronous malignancy in other organs, a history of preoperative treatment such as ESD, and those who received neoadjuvant chemotherapy. Cases with less than 15 lymph nodes harvested were also excluded. Based on the original pathology reports of the remaining cases, we included those meeting all of the following criteria for the expanded indication of ESD: (1) confinement to the mucosal layer (pT1a); (2) size ≤ 2 cm; (3) absence of ulcer; and (4) absence of LVI[3,4].
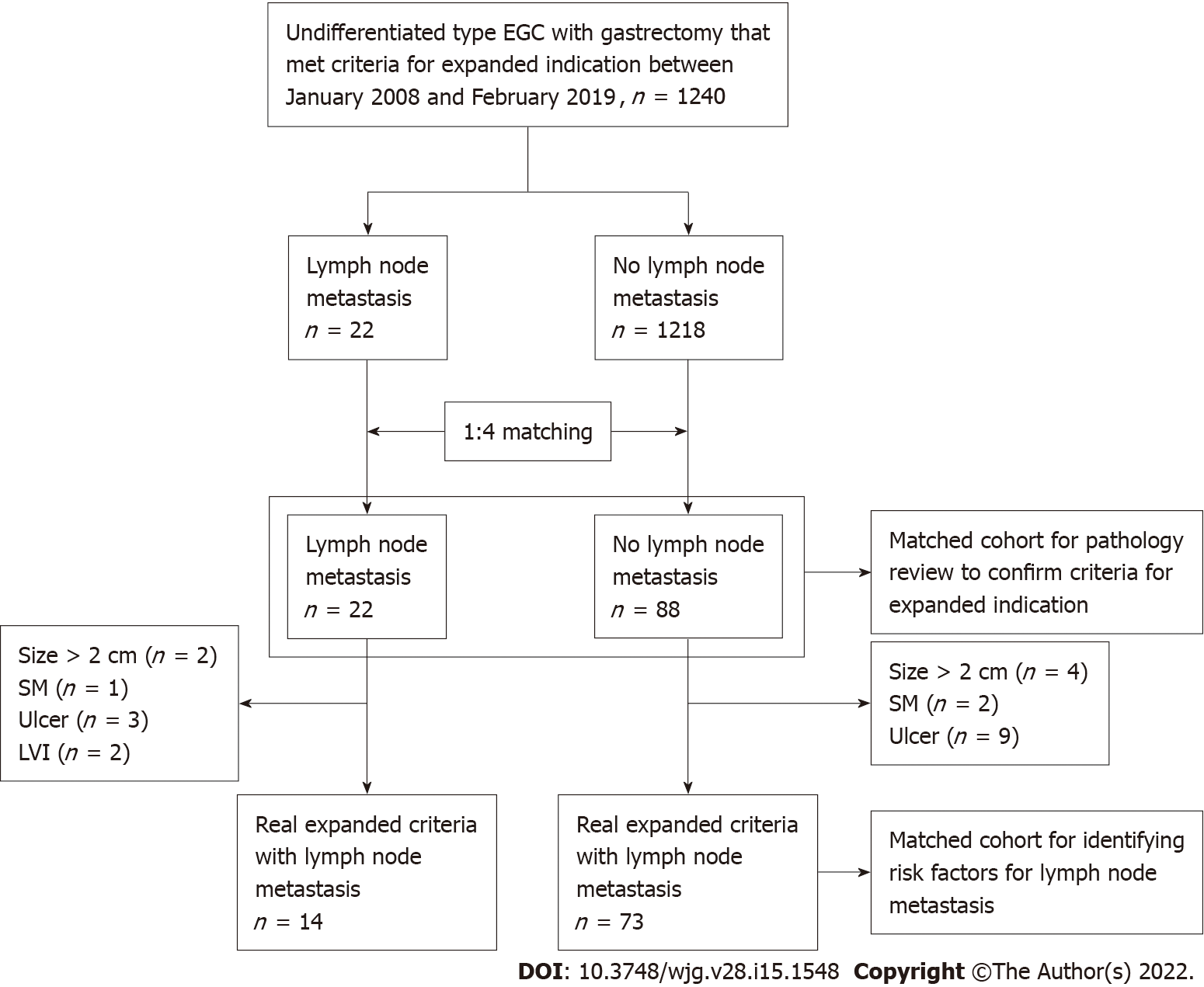
To identify the clinical, endoscopic, and pathologic findings associated with the risk of LNM, we performed a case-control study by matching the patients with LNM to those without at a ratio of 1:4 in terms of sex, age at gastrectomy (± 2 years), and tumor size. Histologic review was conducted to confirm whether the cases indeed satisfy the expanded criteria. The selection process for our study population is shown as a flowchart (Figure 1).
Clinical data, endoscopic features, and pathological characteristics of the study patients were evaluated. Endoscopic characteristics (e.g., tumor location, macroscopic type of the lesion, endoscopic presence of ulcer, converging folds, exudates, and tumor island) were evaluated by two endoscopists (JY and KDC); the endoscopists independently reviewed the initial endoscopic images obtained before biopsy, and discussed with each other until a consensus was reached. The tumor locations were specified both longitudinally (upper vs middle vs lower third) and cross-sectionally (lesser curvature vs posterior wall vs greater curvature vs anterior wall). By referring to the classification system of the Japanese Research Society for Gastric Cancer[3], the macroscopic type of the lesion was evaluated based on the predominant type into three categories as follows: elevated type (including the protruded type and superficial elevated type), flat type (the superficial flat type), and depressed type (the superficial depressed type and excavated type)[3]. Endoscopic ulcer was defined as the presence of a mucosal defect of ≥ 3 mm. Converging folds were indicated by the presence of any centripetal folds in the EGC lesions. The representative endoscopic appearance is depicted in Supplementary Figure 1.
Hematoxylin and eosin (HE)-stained glass slides produced at the time of initial diagnosis were evaluated to double-check the size of tumors in the greatest dimension, depth of invasion, and the status of lymph node metastasis. Entire tumors and adjacent non-tumor areas were serially sectioned at 3-to-4-millimeter intervals and made into paraffin blocks, which were then used to generate slides for histologic evaluation. Histologic mapping was performed to explicitly measure the size of a tumor, and the entirely embedded tumor and adjacent normal area were examined for the percentage of signet ring cells (SRCs), and status of background gastric mucosa. The percentage of SRCs was evaluated by examining the entire tumor sections. Definition of SRCs was established by the agreement between two pathologists (SYY and YSP) based on a recent consensus guideline on poorly cohesive gastric carcinoma[9]; according to the guideline, tumors almost exclusively (≥ 90%) consisting of SRCs were designated as SRCC, and those with < 10% of SRC components as poorly differentiated carcinomas (PDs), which encompass both poorly differentiated adenocarcinomas and non-SRC type of poorly cohesive carcinomas. The remaining tumors in which SRCs comprise 10%-90% of the components were designated as mixed tumors (e.g., adenocarcinoma, poorly differentiated with SRC component and adenocarcinoma, moderately differentiated with SRCC). The status of background gastric mucosa was evaluated based on the likelihood of Helicobacter pylori (H. pylori) colonization as follows: less likely [minimal to mild chronic gastritis (CG) with no intestinal metaplasia (IM)], indeterminate (moderate CG or presence of IM), possible [chronic active gastritis (CAG)], and definite (CAG with clearly visible H. pylori). For each case, the section that seemed most likely to harbor H. pylori was selected for immunohistochemical studies.
Immunohistochemical staining was performed on 4 μm-thick serial tissue sections of formalin-fixed paraffin-embedded (FFPE) blocks. For H. pylori evaluation, tissue sections were stained using the antibody against H. pylori (1:500, rabbit polyclonal, catalog No. 215A-76, Cell Marque, Rocklin, CA) using the OptiView DAB IHC Detection Kit on the BenchMark XT automatic immunostaining device (Ventana Medical Systems, Tucson, AZ, United States) according to the manufacturer's instructions. The abundance of immunohistochemically highlighted H. pylori was evaluated based on the Sydney system[10] as follows: 0 (absent), 1+ (H. pylori occupies < 1/3 of mucosa), 2+ (H. pylori occupies 1/3–2/3 of mucosa) and 3+ (H. pylori occupies > 2/3 of mucosa). H. pylori stain could not be performed in two cases due to the unavailability of FFPE blocks. TP53 staining was performed on representative sections at the time of initial diagnosis following the same protocol described above (1:1000, mouse monoclonal, clone DO-7, catalog No. M7001, Dako, Glostrup, Denmark). The degree of TP53 nuclear immunoreactivity was graded as 0 (no positive cells), 1+ (focal, faint positivity), 2+ (focal, moderate positivity), and 3+ (unanimously strong positivity); 0 was interpreted as the loss of expression, 1+ and 2+ as wildtype pattern, and 3+ as overexpression. TP53 status could not be evaluated in four cases due to the loss of stained slides.
The abundance of tumor-infiltrating lymphocytes (TILs) was evaluated in HE slides in a semi-quantitative manner according to the proposed standardized methodology described in a recent consensus guideline[11].
The degree of peritumoral fibrosis was evaluated by Masson’s trichrome (MT) staining. For 85 cases with accessible FFPE blocks, two sections per case-the deepest section and the edge of the tumor-were selected, and MT stain was performed on 4 μm-thick serial tissue sections of FFPE blocks using Trichome III Blue Staining Kit (Ventana Medical Systems) at BenchMark Special Stains platform (Ventana Medical Systems). The degree of fibrosis was visually graded as mild, moderate, or marked (Supplementary Figure 2C). For computer-aided image analysis, slides were scanned by the Pannoramic 250 Flash slide scanner (3D HISTECH, Budapest, Hungary) at 20× magnification with a resolution of 0.22 μm per pixel.
The degree of MT staining was quantified by pixel classification functionality of QuPath, an open-source software for analyzing digital pathology images (Supplementary Figure 2D)[12]. Briefly, the interface between MM and submucosa underneath the tumorous epithelium was manually annotated as the region of interest (ROI). Two different types of pixel classifiers were trained and sequentially applied. The first classifier was trained to classify the ROI into empty (empty space and fat vacuoles) and non-empty areas. Non-empty areas were placed into the second classifier that graded the intensity of MT as 0 (vessel and MM), 1+ (mild), 2+ (moderate), or 3+ (marked). To express the intensity and extent of MT staining, a metric named “fibrosis score” was defined as follows:
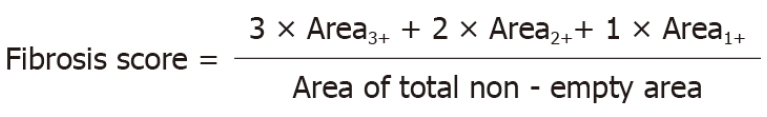
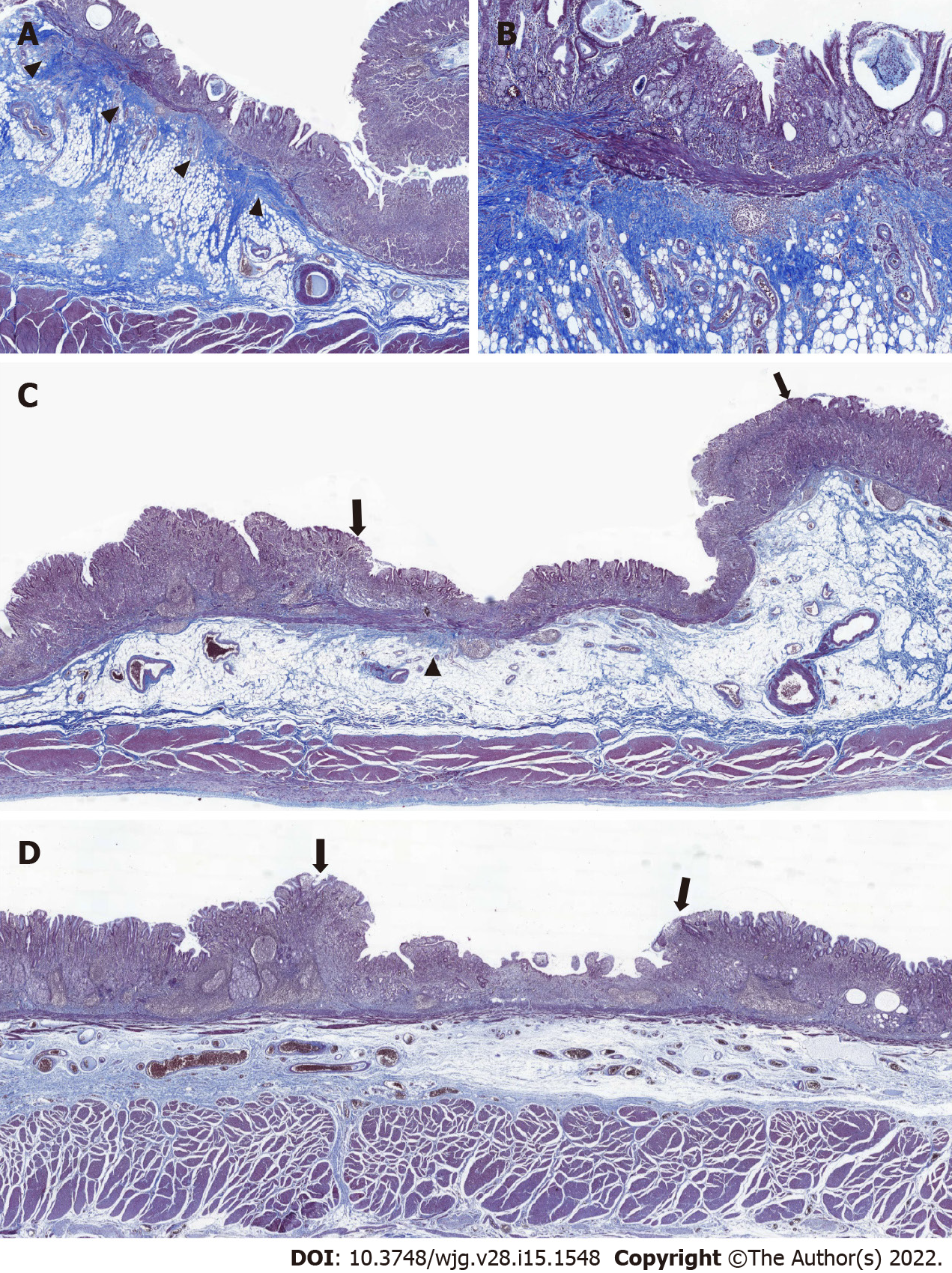
Blurring of MM underneath tumorous epithelium was primarily evaluated at scanning magnification. A case was judged to have blurred MM when any of the two MT-stained slides showed a focus of blurring or disruption of MM by fibrosis that caused loss of integrity relative to adjacent MM underneath the non-tumorous epithelium. If a case had more than one of such foci or the disruption was prominent enough to localize the tumor at scanning magnification, the case was judged to show diffuse blurring of MM (Figure 2A and B). At the foci of MM blurring, the distance from the invasive front to MM was measured. The value of 0 was assigned for tumors touching or invading into the MM. The interobserver reproducibility of MM blurring (non-diffuse or diffuse) was assessed by independent assessment of two pathologists (SYY and YSP). MM blurring assessed by SYY was used for subsequent statistical analysis.
Categorical variables are expressed as numbers with percentages and continuous variables are expressed as medians with interquartile ranges (IQRs). The Chi-squared test or Fisher’s exact test was used to compare categorical variables as appropriate, and the t-test or Wilcoxon rank-sum test was used to compare continuous variables depending on the result of the Shapiro–Wilk normality test. Univariable and multivariable logistic regression analyses were performed to identify the risk factors by estimating the ORs and 95%CIs. Cohen’s kappa was computed as a metric of interobserver reproducibility of MM blurring. P values < 0.05 were considered statistically significant. Statistical evaluations were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, United States) and R version 3.6.2 for Windows (R Foundation for Statistical Computing, Vienna, Austria). The statistical methods of this study were reviewed by Kim HJ from the Department of Clinical Epidemiology and Biostatistics at Asan Medical Center, University of Ulsan College of Medicine.
During the study period, 4780 patients underwent curative gastrectomy with extended lymphadenectomy for EGCs whose histology showed SRCs, PD, or a mixed type of both tumors. Of the 4780 patients, 1240 satisfied the criteria for the expanded indication for ESD. Among them, 22 patients had LNM and the remaining 1218 patients did not.
To identify the risk factors of LNM in patients with UD-EGC satisfying the expanded indication, a 1:4 case-control study was designed. According to the matching conditions, 22 patients with LNM were matched to 88 patients without. Subsequent pathology review was conducted for case and control patients to ensure that they satisfied the criteria for the expanded indication; as a result, 8 cases in the case group revealed histologic features inconsistent with the original pathology report and did not meet the criteria for the expanded indication as follows: size > 2 cm (n = 2), presence of submucosal invasion (n = 1), ulcer (n = 3), and LVI (n = 2). Similarly, 15 cases in the control group were excluded from the study owing to the following discrepancies from the original pathology report: size > 2 cm (n = 4), presence of submucosal invasion (n = 2), and ulcer (n = 9). Consequently, 14 (1.1%) patients among 1240 patients with UD-EGC were designated to have LNM and were included in the case group (LNM+), and 73 patients without LNM were included in the control group (LNM-).
Clinical and endoscopic features of the 87 UD-EGC cases are summarized in Table 1. The median tumor size of the LNM+ group was 1.5 cm (IQR 1.3-1.8 cm), and the size of 11 (78.6%) LNM+ lesions exceeded 1 cm. Ten (71.4%) patients showed macroscopically depressed morphology, and the median number of harvested lymph nodes in the LNM+ group was 30.5. There were no significant differences between the LNM- and LNM+ groups in terms of the tumor location, gross type, and the number of retrieved LNs. Also, no significant differences were noted between the two groups in the preoperative endoscopic findings such as exudate, endoscopic ulcer, converging fold, and tumor island.
| Variables | LNM- (n = 73) | LNM+ (n = 14) | P value |
| Age at diagnosis, yr (median, IQR) | 47.0 (41.0-52.0) | 43.5 (37.0-51.0) | 0.276 |
| Lesion size, cm (median, IQR) | 1.5 (1.2-1.7) | 1.5 (1.3-1.8) | 0.485 |
| Male, n (%) | 25 (34.2) | 4 (28.6) | 0.918 |
| Longitudinal location, n (%) | 0.269 | ||
| Upper | 4 (5.5) | 0 (0.0) | |
| Middle | 22 (30.1) | 2 (14.3) | |
| Lower | 47 (64.4) | 12 (85.7) | |
| Cross-sectional circumference, n (%) | 0.421 | ||
| Anterior wall | 16 (21.9) | 6 (42.9) | |
| Great curvature | 16 (21.9) | 2 (14.3) | |
| Posterior wall | 23 (31.5) | 3 (21.4) | |
| Lesser curvature | 18 (24.7) | 3 (21.4) | |
| Gross type, n (%) | 0.440 | ||
| Depressed | 42 (57.5) | 10 (71.4) | |
| Flat | 25 (34.2) | 4 (28.6) | |
| Elevated | 6 (8.2) | 0 (0.0) | |
| Number of retrieved LNs (median, IQR) | 30.0 (25.0-37.0) | 30.5 (25.5-37.0) | 0.862 |
| Endoscopic appearances, n (%) | |||
| Exudate | 6 (8.2) | 1 (7.1) | > 0.999 |
| Endoscopic ulcer | 30 (41.1) | 7 (50.0) | 0.747 |
| Converging fold | 11 (15.1) | 1 (7.1) | 0.715 |
| Tumor island | 14 (19.2) | 3 (21.4) | > 0.999 |
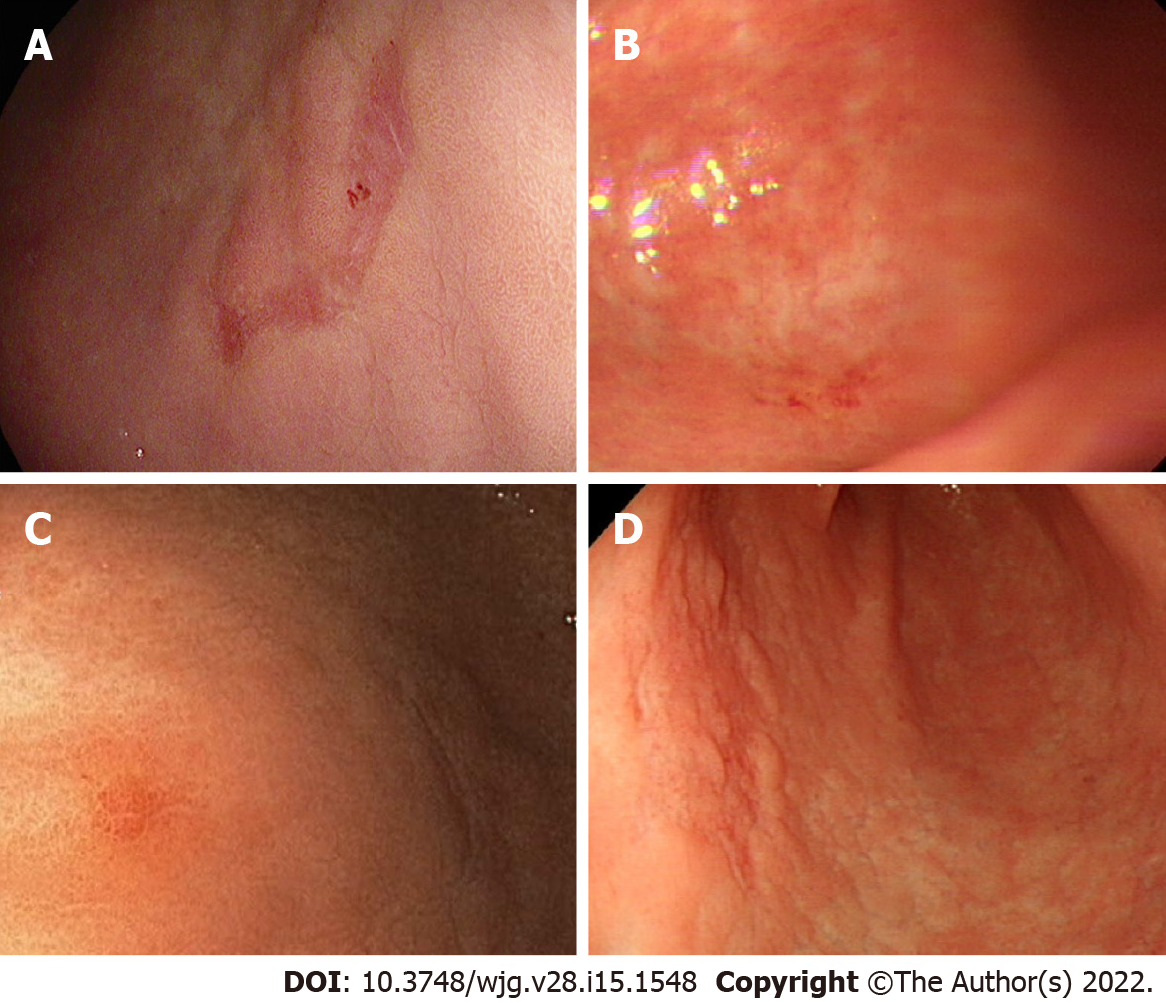
Detailed information on the 14 LNM+ cases is presented in Table 2; of those, cases 1, 3, 5, and 9 did not show endoscopic findings such as exudate, mucosal break, converging fold, and tumor island (Figure 3). Case 5 had six lymph node metastasis, but the endoscopic findings showed only a flat lesion with hyperemic mucosa (Figure 3C).
| Case No. | Age (yr) | Sex | Type | Size (cm) | Location | Histology | Depth of invasion | Total number of dissected LNs | Number of metastatic LNs |
| 1 | 59 | Female | IIb | 1.5 | Middle | PD with SRC | LP | 31 | 3 |
| 2 | 41 | Female | IIc | 1.3 | Lower | PD with SRC | LP | 18 | 3 |
| 3 | 57 | Male | IIc | 1.3 | Lower | SRC | LP | 21 | 1 |
| 4 | 47 | Female | IIc | 1.8 | Middle | PD with SRC | LP | 21 | 3 |
| 5 | 46 | Female | IIb | 2.0 | Lower | PD with SRC | LP | 25 | 6 |
| 6 | 37 | Female | IIc | 1.5 | Lower | PD with SRC | MM | 31 | 3 |
| 7 | 48 | Female | IIc | 1.5 | Lower | PD with SRC | LP | 37 | 1 |
| 8 | 35 | Male | IIc | 0.9 | Lower | PD with SRC | LP | 38 | 1 |
| 9 | 35 | Female | IIc | 1.5 | Lower | PD with SRC | LP | 27 | 1 |
| 10 | 52 | Female | IIb | 0.7 | Lower | PD with SRC | LP | 29 | 1 |
| 11 | 60 | Male | III | 0.6 | Lower | PD with SRC | MM | 30 | 1 |
| 12 | 39 | Female | IIc | 2.0 | Lower | PD with SRC | LP | 37 | 1 |
| 13 | 37 | Female | IIb | 1.8 | Lower | PD with SRC | LP | 42 | 1 |
| 14 | 33 | Male | IIc | 2.0 | Lower | PD with SRC | LP | 48 | 3 |
Histopathologic features of the study patients and their background gastric mucosa were evaluated from HE stains and immunohistochemical stains for H. pylori and TP53 (Table 3). Except for the diagnostic category according to the proportion of SRCs, none of the histologic features showed significant differences between the LNM- and LNM+ groups. Although no significant difference was noted in the average percentage of SRCs, the LNM+ group tended to have more patients with mixed histology (consisting of 10–90% of SRCs) than pure SRCC or PD carcinoma (P = 0.059).
| Variables | LNM- (n = 73) | LNM+ (n = 14) | P value |
| Depth of invasion | 0.503 | ||
| LP | 53 (72.6) | 12 (85.7) | |
| MM | 20 (27.4) | 2 (14.3) | |
| Size, cm | 1.5 (1.2-1.7) | 1.5 (1.3-1.8) | 0.642 |
| % of SRCs | 0.157 | ||
| < 10% | 17 (23.3) | 0 (0) | |
| ≥ 10% and < 50% | 35 (47.9) | 10 (71.4) | |
| ≥ 50% and < 90% | 14 (19.2) | 3 (21.4) | |
| ≥ 90% | 7 (9.6) | 1 (7.1) | |
| Diagnostic category according to the proportion of SRCs | 0.059 | ||
| Non-mixed (SRCC and PD) | 24 (32.9) | 1 (7.1) | |
| Mixed (PD with SRC component) | 49 (67.1) | 13 (92.9) | |
| Background stomach | 0.278 | ||
| Mild CG | 8 (11.0) | 0 (0) | |
| Moderate CG or IM | 24 (32.9) | 3 (21.4) | |
| CAG | 22 (30.1) | 4 (28.6) | |
| CAG with visible H. pylori | 19 (26.0) | 7 (50.0) | |
| H. pylori abundance, n/total n | 0.263 | ||
| 0 | 23/71 (32.4) | 2/14 (14.3) | |
| 1+ | 14/71 (19.7) | 3/14 (21.4) | |
| 2+ | 14/71 (19.7) | 6/14 (42.9) | |
| 3+ | 20/71 (28.2) | 3/14 (21.4) | |
| TP53 expression, n/total | > 0.999 | ||
| Loss (0) | 3/70 (4.3) | 0/14 (0) | |
| Wildtype pattern (1+/2+) | 63/70 (90.0) | 13/14 (92.9) | |
| Overexpression (3+) | 4/70 (5.7) | 1/14 (7.1) |
We further evaluated histologic features of the tumor microenvironment, abundance of TILs, and degree of peritumoral fibrosis (Table 4). We specifically focused on fibrosis because while evaluating histologic features of tumors and the background stomach, we observed that some tumors showed marked peritumoral fibrosis (Supplementary Figure 2A and B). We performed MT staining and analyzed the slides visually and computationally to investigate the degree, extent, and pattern of peritumoral fibrosis (Supplementary Figure 2C-E). However, neither TIL abundance nor the pattern and degree of peritumoral fibrosis showed a statistically significant association with the risk of LNM.
| Variables | LNM- (n = 73) | LNM+ (n = 14) | P value |
| TIL abundance | 0.438 | ||
| < 10% | 25 (34.2) | 7 (50.0) | |
| 10%-20% | 33 (45.2) | 6 (42.9) | |
| ≥ 20% | 15 (20.5) | 1 (7.1) | |
| Degree of central fibrosis | 0.522 | ||
| Mild | 6 (8.5) | 2 (14.3) | |
| Moderate | 35 (49.3) | 8 (57.1) | |
| Marked | 30 (42.3) | 4 (28.6) | |
| Degree of peripheral fibrosis | |||
| Mild | 11 (15.5) | 2 (14.3) | 0.495 |
| Moderate | 53 (74.6) | 9 (64.3) | |
| Marked | 7 (9.9) | 3 (21.4) | |
| Distribution of fibrosis | |||
| Central = peripheral | 32 (45.1) | 7 (50.0) | 0.204 |
| Central > peripheral | 33 (46.5) | 4 (28.6) | |
| Central < peripheral | 6 (8.5) | 3 (21.4) | |
| Fibrosis score | 0.55 (0.38-0.83) | 0.53 (0.31-0.77) | 0.273 |
| Blurring of MM, n/total | 0.028 | ||
| Non-diffuse (absent/focal) | 43/71 (60.6) | 13/14 (92.9) | |
| Diffuse | 28/71 (39.4) | 1/14 (7.1) |
During the evaluation of MT stain, we noticed a peculiar pattern of fibrosis disrupting the MM, which could be categorized into diffuse and non-diffuse (focal plus no disruption) types (Figure 2). Importantly, the diffuse blurring of MM (Figure 2A and B) was significantly associated with the invasion of MM, shorter distance between the invasive front and MM, and higher fibrosis score (all P < 0.001, Supplementary Table 1). Some cases that lacked diffuse MM blurring had substantial peritumoral fibrosis (Supplementary Figure 3A) or invading MM (Supplementary Figure 3B-C), and other cases showed diffuse blurring of MM while being confined to the lamina propria or devoid of peritumoral fibrosis (Supplementary Figures 3D-E). Most importantly, we found a significant association between diffuse MM blurring and the absence of regional LNM (P = 0.028, Table 4). Multivariate logistic regression analysis with backward variable selection revealed that of the multiple clinical and histological variables, diffuse blurring of MM was the only statistically significant explanatory variable associated with the risk of LNM (OR: 0.12, 95%CI: 0.02–0.95; P = 0.045, Table 5).
| Variable | OR | 95%CI | P value |
| Depth of invasion | |||
| LP | 1 | ||
| MM | 0.44 | 0.09-2.15 | 0.312 |
| Diagnostic category according to % of SRCs | |||
| Non-mixed (SRC and PD) | 1 | ||
| Mixed (PD with SRC component) | 6.37 | 0.79-51.6 | 0.083 |
| Background stomach | |||
| CG | 1 | ||
| CAG | 2.86 | 0.74-11.1 | 0.129 |
| Presence of H. pylori | |||
| Absent (0) | 1 | ||
| Present (≥ 1+) | 2.88 | 0.59–13.9 | 0.190 |
| TIL abundance | 0.409 | ||
| < 10% | 1 | ||
| 10%-20% | 0.65 | 0.19-2.17 | 0.484 |
| ≥ 20% | 0.24 | 0.03-2.13 | 0.199 |
| Fibrosis score | 0.34 | 0.05-2.49 | 0.285 |
| Blurring of MM | |||
| Non-diffuse (absent/focal) | 1 | ||
| Diffuse | 0.12 | 0.02-0.95 | 0.045 |
Independent assessment of MM blurring by a second pathologist revealed strong interobserver reproducibility with a Cohen’s Kappa coefficient of 0.90 (95%CI: 0.80–0.96, Supplementary Table 2). Furthermore, MT staining on the ESD specimens of UD-EGC cases demonstrated that the presence of MM blurring could be readily evaluated in ESD specimens (Supplementary Figure 4). Collectively, these results suggest that MM blurring could serve as a feasible histologic marker that aids the decision on the follow-up strategy after ESD for UD-EGC cases meeting the expanded indication criteria.
In this study, we investigated the risk factors for LNM in patients with UD-EGC meeting the criteria for the expanded indication for ESD by using surgically resected cases. Our results demonstrated that the incidence rate of LNM in cases of UD-EGC meeting the criteria for the expanded indication for ESD was 1.1% (14/1240). By reviewing the clinical, endoscopic features, and pathologic results, we found that the LNM- and LNM+ groups did not show significant differences in terms of preoperative clinical and endoscopic features. On the other hand, histologic features such as mixed histology (P = 0.059) and blurring of MM (P = 0.028) showed a notable difference according to the presence of LNM, suggesting that histologic evaluation could be useful for improving patient stratification.
To date, ESD for UD-EGC has required an expanded indication, and surgery has been the standard treatment because the risk of LNM has been shown to be relatively higher in UD-EGCs than in differentiated EGCs, thus raising concern about the long-term outcomes. However, in the recent guidelines in Japan, UD-EGC lesions have been integrated into the absolute indication for ESD[13]. Li et al[14] reported that there were no cases of LNM in patients with UD-EGC meeting the expanded indications of ESD. Another study revealed that LNM was not found in intramucosal cancer when the lesion was 20 mm or less without LVI and ulcerative findings[1]. However, these studies have the limitations of small sample sizes and retrospective study design. In a recent multicenter clinical trial, Takizawa et al[15]. reported that patients who were followed after undergoing curative ESD for UD-EGC showed neither local/distant recurrence nor deaths due to gastric cancer, thereby suggesting favorable clinical outcomes of ESD for UD-EGC; however, this study had a single-arm design and the outcomes after ESD were not compared with those after surgery. In addition, other studies reported contrasting results in that up to 2.3% of patients with UD-EGC meeting the criteria of expanded indications (intramucosal cancer, size of ≤ 20 mm without LVI and ulcerative findings) were found to have LNM[16]. Our study also showed that 1.1% of 1240 patients meeting the criteria for the expanded indication for ESD showed LNM. Considering these conflicting results, further targeted investigations are required regarding the risk of LNM in UD-EGC cases meeting the expanded criteria.
LNM is the most important factor for deciding the treatment strategy in cases of UD-EGC. For the appropriate usage of ESD in UD-EGC, the characteristics of UD-EGCs with LNM should be clarified so that such cases should be excluded from consideration for ESD. Hence, we reviewed the original pathologic reports of 4781 surgically resected UD-EGC and found that 1240 cases met the criteria for the expanded indication for ESD, 22 of whom exhibited regional LNM. However, a subsequent histologic review revealed that 8 of the 22 cases with LNM and 15 out of the matched 88 control cases did not satisfy the criteria for expanded indications due to deviation in size from the original pathology reports (n = 2 in the case group, n = 4 in the control group) and the presence of ulcer (n = 3 in the case group, n = 9 in the control group). The discrepancy likely occurred because unlike mucosectomy specimens, gastrectomy specimens do not mandatorily undergo systematic evaluation for the tumor size and the presence of an ulcer[3,17]. In our study, three cases with submucosal invasion that had been misdiagnosed as mucosal cancer showed deceptive histologic features that called for careful examination. In one case, it seemed that the site of submucosal invasion had been missed because the tumor was located in an extensively thick and undulating mucosa. The tumor in another case was accompanied by a massively lymphoid stroma so that the tumor cells in the submucosa were barely visible. Tumor cells of the remaining case were almost indistinguishable from macrophages. TP53 immunostaining, which is routinely performed for all stomach cancer cases at our institution, was useful in highlighting the tumor cells in the submucosa in the latter two cases. Careful attention is needed to diagnose ESD cases showing features of discrepant cases.
This discrepancy after the second histologic review implies a more serious message. Considering that the number of patients in the LNM+ group decreased from 22 to 14 after the second histologic review, the total number of UD-EGC patients satisfying the expanded criteria could decrease from 1240 if the entire case cohort was reviewed. As a consequence, the actual incidence rate can be actually higher than 1.1%. Therefore, our results suggest that UD-EGC cases meeting the criteria for the expanded indication does have a risk of LNM, which may be too higher to consider endoscopic resection. Therefore, further research is needed to discover clinical, endoscopic, and histologic features of UD-EGC that can aptly supplement the current expanded criteria.
For this purpose, the tumor characteristics of 14 patients with LNM were evaluated. The tumor characteristics including tumor location, gross type, the number of retrieved LNs, and endoscopic appearances did not have significant associations with LNM. According to histologic analysis, among various histologic features, mixed histology (consisting of 10%-90% SRCs) in comparison with non-mixed histology (i.e., pure SRCC, poorly cohesive carcinoma, or poorly differentiated adenocarcinoma) was the only variable with a marginal statistical significance (P = 0.059). This is consistent with previous studies suggesting more aggressive biology of EGC by using mixed histology rather than pure adenocarcinoma and SRCC[18,19]. Therefore, it is likely that we would have reached statistical significance if a larger number of cases were analyzed.
Inspired by the recent interest in the role of the tumor microenvironment on metastasis[20], we further investigated the histologic features of the tumor microenvironment, especially the pattern and degree of peritumoral fibrosis. Previous studies on submucosal fibrosis of EGCs have mostly focused on its negative effect on successful ESD[21-25]. Conversely, we focused on whether the extent or pattern of submucosal fibrosis had an impact on LNM. While the degree of submucosal fibrosis did not show a significant association with LNM, we unexpectedly discovered a significant association between MM blurring and LNM. A structural study on the distribution of lymphatic and blood capillaries of human gastric mucosa showed that lymphatic capillaries were present in the deep lamina propria adjacent to and within the MM[26]. As such, we hypothesize that the blurring of MM is a consequence of an exaggerated anti-tumoral reaction against tumor cells trying to invade the lymphatics within the MM. In contrast with the traditional concept of the pro-tumorigenic role of fibrosis, recent studies have suggested that tumor-related fibrosis can also restrain cancer initiation, proliferation, and metastasis[27]. Therefore, it is possible that tumors that managed to elicit anti-tumoral fibrosis against the tumor cells’ attempt to invade lymphatics are seen as having blurred MM, and are hence less likely to metastasize into the regional lymph nodes.
Considering the intimate relationship between lymphatics and the MM, it can be assumed that the tumor’s proximity to the MM would be significantly associated with the risk of LNM. Indeed, it has been reported that tumors invading the MM are more likely to metastasize into regional lymph nodes than those limited to the lamina propria[28]. However, we failed to reach the same conclusion in our current study, and our study might suggest the opposite conclusion considering the significant association between MM invasion and MM blurring. This may be due to the fact that our control group is biased toward tumors invading the MM; however, unlike the two previous studies, we only focused on UD-EGC cases meeting the expanded criteria for ESD.
We hypothesize that the seemingly counterintuitive result of our study might be explained by the differences in the mode of invasion between differentiated and undifferentiated GCs, considering the results of recent studies that elucidated the various modes of cancer cell invasion, ranging from single-cell migration to collective invasion[29]. Because poor differentiation often involves the loss of cellular adhesion molecules[30], undifferentiated GCs might preferentially invade as single cells. For this reason, the main body of poorly differentiated tumors does not necessarily need to be in close proximity to lymphatics to invade them, and desmoplasia is more likely attributable to anti-tumoral microenvironmental responses rather than invading tumor cells. On the other hand, differentiated GCs would invade the adjacent normal structures by forming glands. Glandular structures are likely more destructive than scattered cells, and massive violation of the normal structure itself is sufficient enough to elicit fibrosis; for this reason, desmoplasia in differentiated GCs would more reflect the invasiveness of the tumors than anti-tumoral responses. Further studies in independent cohorts are needed to validate the hypothesis on the differential significance of MM blurring in differentiated and undifferentiated GCs.
There are several limitations to our study. First, because we excluded cases that showed deviation from the original pathology reports (e.g., larger tumor size, ulcer, LVI, submucosal invasion), the study population was reduced and the case-control study could not be performed as originally intended. Second, the validity of MM blurring may benefit from further scrutiny; aside from the four tumors that were small enough to be embedded in single blocks, we examined two representative sections per each case and judged a case as having blurred MM when such foci were identified in any of the two sections. As such, it is possible that the cases classified as clear MM might have disrupted MM in unexamined sections. Finally, this study had limitations inherent to the nature of a retrospective, single-center study. Although the number of patients with LNM was small, this is because the incidence of LNM in patients with UD-EGC meeting the expanded indications of ESD is low. Considering the low incidence, a large-scale, prospective, multicenter study is needed to confirm our findings. Despite these limitations, we believe that our study appropriately highlights the fact that diffuse blurring of MM may be a potential predictive factor for the risk of LNM in cases of UD-EGC meeting the expanded criteria for ESD. Further research is needed to validate our results and to elucidate its mechanistic basis.
In conclusion, our results show that cases of UD-EGC meeting the criteria for the expanded indication have the risk for LNM, albeit low (1.1%), and that routine histological examination has practical limitations for identifying such cases. When ESD is planned for a case of UD-EGC, obtaining detailed informed consent after the disclosure of the risk of LNM is necessary, and careful observation is essential. A model for patient stratification based on histologic evaluation of the proportion of SRCs and MM blurring in ESD specimens could be useful for identifying the patients with a higher risk of LNM.
There have been several reports of lymph node or distant metastases arising after curative endoscopic submucosal dissection (ESD) in undifferentiated early gastric cancer (UD-EGC) cases meeting the expanded criteria.
The clinicopathologic features associated with the risk of lymph node metastasis (LNM) in UD-EGC have not been well-studied.
To investigate the prevalence and risk factors of LNM in UD-EGC cases meeting the criteria for the expanded indication for ESD.
In this retrospective study, we investigated the risk of LNM of UD-EGC meeting the criteria for the expanded indication for ESD, and performed a matched case-control study to identify the clinical, endoscopic, and histopathological features associated with the risk of LNM. Univariate and multivariate logistic regression analyses were performed to identify the risk factors by estimating the odds ratios.
The incidence rate of LNM in UD-EGC cases meeting the criteria for the expanded indication for ESD was 1.1% (14/1240). No significant differences existed between the LNM group and the matched non-LNM group in terms of preoperative clinical endoscopic features and conventional histologic features. In the tumor microenvironment, blurring of muscularis mucosa (MM) underneath the tumorous epithelium was associated with the risk of LNM.
The risk of LNM was higher than expected when using the current expanded indication for UD-EGC. Evaluation of blurring of MM could provide useful clues for reducing the risk of LNM.
Further studies are needed to validate the significance of MM blurring and elucidate its mechanistic basis. Eventually, an improved model for patient stratification based on detailed histologic evaluation of ESD specimens should be established to identify patients with a high risk of LNM.
The authors would like to acknowledge Kim HJ from the Department of Clinical Epidemiology and Biostatistics at Asan Medical Center, University of Ulsan College of Medicine for the statistical review.
| 1. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1350] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 2. | Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, Seo HG; Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res Treat. 2021;53:301-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1954] [Article Influence: 217.1] [Reference Citation Analysis (1)] |
| 4. | Guideline Committee of the Korean Gastric Cancer Association (KGCA); Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer. 2019;19:1-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 319] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 5. | Kang HJ, Kim DH, Jeon TY, Lee SH, Shin N, Chae SH, Kim GH, Song GA, Srivastava A, Park DY, Lauwers GY. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Zhu ZL, Shi HP, Beeharry MK, Feng TN, Yan M, Yuan F, Zheng-Gang Zhu, Zhang BY, Wu W. Expanding the indication of endoscopic submucosal dissection for undifferentiated early gastric cancer is safe or not? Asian J Surg. 2020;43:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Hu Q, Dekusaah R, Cao S, Pang T, Wang Y, Zhang B, Lv Y, Zhang X, Ling T, Zhuge Y, Wang L, Zou X, Zhang W, Huang Q, Xu G. Risk Factors of Lymph Node Metastasis in Patients with Early Pure and Mixed Signet Ring Cell Gastric Carcinomas. J Cancer. 2019;10:1124-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Liu Y, Zhang J, Wu X, Ji X, Fu T, Li Z, Wu Q, Bu Z, Ji J. Construction and external validation of a nomogram that predicts lymph node metastasis in early gastric cancer patients using preoperative parameters. Chin J Cancer Res. 2018;30:623-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 10. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3624] [Article Influence: 120.8] [Reference Citation Analysis (6)] |
| 11. | Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden GGGM, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O'Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol. 2017;24:311-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 596] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 12. | Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5567] [Cited by in RCA: 5931] [Article Influence: 659.0] [Reference Citation Analysis (0)] |
| 13. | Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Fujimoto K. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33:4-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 363] [Article Influence: 72.6] [Reference Citation Analysis (1)] |
| 14. | Li H, Lu P, Lu Y, Liu CG, Xu HM, Wang SB, Chen JQ. Predictive factors for lymph node metastasis in poorly differentiated early gastric cancer and their impact on the surgical strategy. World J Gastroenterol. 2008;14:4222-4226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer 2020: 1-13. |
| 16. | Oh SY, Lee KG, Suh YS, Kim MA, Kong SH, Lee HJ, Kim WH, Yang HK. Lymph Node Metastasis in Mucosal Gastric Cancer: Reappraisal of Expanded Indication of Endoscopic Submucosal Dissection. Ann Surg. 2017;265:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Pathologists CoA. Protocol for the Examination of Specimens from Patients with Carcinoma of the Stomach. College of American Pathologists: Northfield 2014. |
| 18. | Huh CW, Jung DH, Kim JH, Lee YC, Kim H, Yoon SO, Youn YH, Park H, Lee SI, Choi SH, Cheong JH, Noh SH. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol. 2013;107:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Seo HS, Lee GE, Kang MG, Han KH, Jung ES, Song KY. Mixed Histology Is a Risk Factor for Lymph Node Metastasis in Early Gastric Cancer. J Surg Res. 2019;236:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4060] [Cited by in RCA: 6082] [Article Influence: 506.8] [Reference Citation Analysis (0)] |
| 21. | Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH, Jung HY, Kim JH. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM, Lee KJ, Kim JH, Cho SW. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26:2456-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Emura F, Mejía J, Donneys A, Ricaurte O, Sabbagh L, Giraldo-Cadavid L, Oda I, Saito Y, Osorio C. Therapeutic outcomes of endoscopic submucosal dissection of differentiated early gastric cancer in a Western endoscopy setting (with video). Gastrointest Endosc. 2015;82:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Kim JH, Nam HS, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Hwang SH, Lee SH. Risk factors associated with difficult gastric endoscopic submucosal dissection: predicting difficult ESD. Surg Endosc. 2017;31:1617-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Lee GH, Park JW, Roh J, Kim YB, Lee E, Lim SG, Shin SJ, Lee KM, Noh CK. Association Between Waiting Time from Diagnosis to Endoscopic Submucosal Dissection and Non-curative Resection in Gastric Neoplasm. Anticancer Res. 2021;41:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Lehnert T, Erlandson RA, Decosse JJ. Lymph and blood capillaries of the human gastric mucosa. A morphologic basis for metastasis in early gastric carcinoma. Gastroenterology. 1985;89:939-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Chandler C, Liu T, Buckanovich R, Coffman LG. The double edge sword of fibrosis in cancer. Transl Res. 2019;209:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 28. | Song SY, Park S, Kim S, Son HJ, Rhee JC. Characteristics of intramucosal gastric carcinoma with lymph node metastatic disease. Histopathology. 2004;44:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 704] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 30. | Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY, Lai KH, Tseng HH, Chou NH, Huang MS, Tseng CJ, Hsiao M. Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett. 2003;201:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Sun Q, China; Sun Q, China; Tian Y, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H