Published online Apr 14, 2022. doi: 10.3748/wjg.v28.i14.1444
Peer-review started: February 27, 2021
First decision: March 28, 2021
Revised: April 10, 2021
Accepted: September 2, 2021
Article in press: September 2, 2021
Published online: April 14, 2022
Processing time: 403 Days and 6.7 Hours
Liver transplantation is a therapy for irreversible liver failure; however, at present, donor organs are in short supply. Cell transplantation therapy for liver failure is still at the developmental stage and is critically limited by a shortage of human primary hepatocytes.
To investigate the possibility that hepatic progenitor cells (HPCs) prepared from the portal branch-ligated hepatic lobe may be used in regenerative medicine, we attempted to enable the implantation of extracellular matrices containing organoids consisting of HPC-derived hepatocytes and non-parenchymal cells.
In vitro liver organoid tissue has been generated by accumulating collagen fibrils, fibroblasts, and HPCs on a mesh of polylactic acid fabric using a bioreactor; this was subsequently implanted into syngeneic wild-type mice.
The in vitro liver organoid tissues generated transplantable tissues in the condensed collagen fibril matrix and were obtained from the mouse through partial hepatectomy.
Liver organoid tissue was produced from expanded HPCs using an originally designed bioreactor system. This tissue was comparable to liver lobules, and with fibroblasts embedded in the network collagen fibrils of this artificial tissue, it is useful for reconstructing the hepatic interstitial structure.
Core Tip: Liver transplantation is a therapeutic procedure used to recover liver function in patients with irreversible liver failure; however, there is presently a shortage of transplant organs available. Hepatic stem and progenitor cells are expected to allow regenerative medicine to produce a cell source as an alternative to whole organs. The portal branch-ligated, hepatic lobe-derived HPCs multiplied in a bioreactor chamber to form liver organoid tissues comparable to liver lobules. These organoid tissues were implanted into syngeneic mice. This portal branch-ligated-derived HPC line has the potential to proliferate, mature, and form implantable hepatic tissue.
- Citation: Tamai M, Adachi E, Kawase M, Tagawa YI. Syngeneic implantation of mouse hepatic progenitor cell-derived three-dimensional liver tissue with dense collagen fibrils. World J Gastroenterol 2022; 28(14): 1444-1454
- URL: https://www.wjgnet.com/1007-9327/full/v28/i14/1444.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i14.1444
Liver transplantation is a therapy for irreversible liver failure; however, donor organs are currently in short supply[1,2]. Cell transplantation therapy for liver failure is still at the developmental stage and has a critical problem in terms of a shortage of human primary hepatocytes[3]. Human embryonic stem/induced pluripotent stem (ES/iPS) cell-derived hepatocytes are thought to be an alternative to human primary hepatocytes, but ES/iPS cells are difficult to differentiate into mature hepatocytes in culture[4,5]. ES/iPS cell-derived immature hepatocytes successfully developed into mature liver tissue in animals after implantation[6,7]. However, this process requires a great deal of time, effort, and expense in order to obtain a sufficient number of ES/iPS cell-derived hepatocytes in culture to achieve the amount needed for them to continue to proliferate. There have been several reports of rat HPCs, such as small hepatocytes[8] and Lgr5+ rat and mouse liver stem cells[9,10], becoming established in culture. These hepatic stem/progenitor cells have the ability to proliferate and differentiate into hepatocytes and cholangiocytes[11,12]. Recently, we also succeeded in establishing HPC lines prepared from the portal branch-ligated hepatic lobe in mice (PBL-HPCs)[13]. These cells could differentiate into mature hepatocytes in the presence of oncostatin M, or to cholangiocytes in EHS gel.
Besides dissociated hepatocyte implantation, regenerative medicine is also expected to enable the implantation of extracellular matrices containing aggregate, or organoids consisting of hepatocytes and non-parenchymal cells[14-16]. In vitro liver organoid tissue has previously been generated by accumulating collagen fibrils, human fibroblast cell line (HFO cell), and human hepatocarcinoma cell line (Hep G2) on a mesh of polylactic acid (PLA) fabric using a bioreactor[14]. Also, instead of HFO and Hep G2, mouse embryonic fibroblasts and primary hepatocytes were used for this in vitro liver organoid tissue. These in vitro liver organoid tissues generated transplantable liver organoid tissues in the right portal vein branch-ligated nu/nu mouse with a condensed collagen fibril matrix[14]. The fibroblasts are embedded in the network collagen fibrils of this artificial tissue, and it is therefore useful for reconstructing the hepatic interstitial structure.
In this study, the PBL-HPCs were expanded and formed liver organoid tissue, which was comparable to liver lobules using an originally designed bioreactor system, and was also implanted into its syngeneic wild-type mouse.
Pregnant BALB/cA mice at 13.5 d post-coitus (CLEA Japan, Tokyo, Japan) were used for embryonic fibroblast isolation. Six-week-old female and male BALB/cA Jcl and BALB/cA Jcl-nu/nu 3 mice (CLEA Japan, Tokyo, Japan) were used as transplant recipients. The animal protocol was approved by the Animal Experimentation Committee of the Tokyo Institute of Technology.
The PBL-HPCs were established in a previous study with portal vein ligated methods[13]. The cells were cultured in Williams’ E medium (GIBCO Laboratories, Grand Island, NY, United States) supplemented with 5% fetal bovine serum, 10 mmol/L nicotinamide (Sigma-Aldrich, St. Louis, MO, United States), 0.1 μmol/L dexamethasone (Sigma-Aldrich), 1×Insulin-Transferrin-Sodium Selenite Supplement (Roche Diagnostics, Mannheim, Germany), and 20 ng/mL Recombinant Mouse Epidermal Growth Factor (R&D Systems, Minneapolis, MN, United States) in 5% CO2 at 37°C. These cells were passaged by treatment with 0.05% trypsin (Invitrogen) and 20 μmol/L ethylenediaminetetraacetic acid (EDTA; NACALAI TESQUE, Kyoto, Japan).
A pregnant female BALB/c mouse at 13.5 dpc (days post-coitus) was sacrificed by cervical dislocation, and embryos were removed. The limbs of the embryos were minced and treated with 0.25% trypsin (Invitrogen, Tokyo, Japan) + 1 mmol/L EDTA (about 2 mL per embryo) and incubated with gentle stirring at 37 °C for 10-15 min. The cells were subsequently cultured in DMEM containing 10% (v/v) FBS.
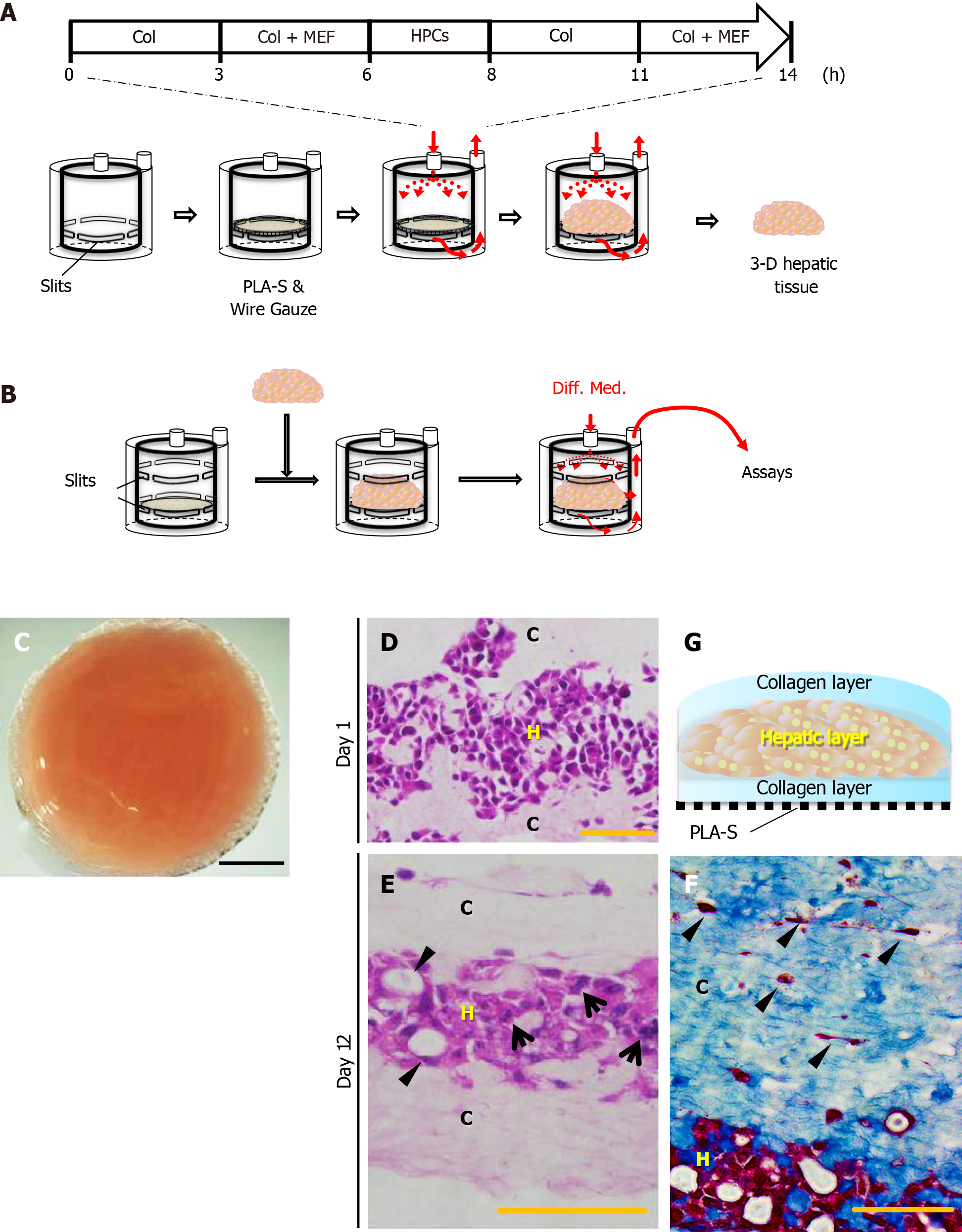
As shown in Figure 1, the three-dimensional (3-D) liver tissue culture model was generated by accumulating collagen fibrils, primary murine embryonic fibroblasts, and PBL-HPCs using a closed-loop system with a bioreactor chamber (diameter 17 mm; thickness 20 mm) developed by our group as previously reported[14,17]. Briefly, primary embryonic fibroblasts (1.0 × 105 cells/mL) in 10% FBS and 7.5 mg/mL type I collagen prepared from calfskin (Koken Collagen, Tokyo, Japan) in Williams’ E medium flowed through the closed-loop system at a predetermined flow rate (1-5 mL/min) for 6 h. Subsequently, the same medium was circulated through the closed-loop system, and PBL-HPCs (5.0 × 106 cells/mL) were injected using a syringe into the system upstream of the bioreactor chamber for 2 h. Finally, a suspension of fibroblasts (1.0 × 105 cells/mL) was circulated for 6 h.
The 3-D liver tissue culture models were fixed with Zamboni’s fixative for light microscopy. The samples were dehydrated with an ethanol series and embedded in paraffin. The sections were stained with hematoxylin and eosin or AZAN and examined with a light microscope.
Urea production in the medium was quantified using a urea assay kit (Bioassay Systems, Hayward, CA, United States) 24 h after the addition of 2 mmol/L NH4Cl. Albumin production was quantified in the medium using an albumin EIA (Albuwell M) mouse kit (Exocell, Philadelphia, PA, United States).
The metabolites of testosterone in the medium were quantified by HPLC analysis[18]. The 3-D liver tissue culture models were incubated with fresh medium containing 0.25 mmol/L testosterone and the medium was collected at 24 h. After sample treatment, HPLC analysis was performed using LC-10ADVP (Shimadzu, Kyoto, Japan) with Cadenza columns (Cadenza CD-C18) (Imtakt, Kyoto, Japan) and SPD-10A VP (Shimadzu, Kyoto, Japan).
Under isoflurane anesthesia, mice were subjected to an upper-abdominal incision, followed by exposure and ligation of the left portal vein branch and subsequent hepatectomy of the left and middle lobes (70%). The 3-D liver tissue culture model was transplanted into the subcutaneous layer of a mouse. Two weeks later, the 3-D liver tissue culture model was removed for histological analysis of the vascular network.
Results of multiple experiments were reported as the mean ± SE. Statistical comparisons were made using a Tukey-Kramer method and a Welch t-test using the IBM SPSS Statistic 27.
Type I collagen solution was circulated through a sheet of PLA into a reverse radial flow-type bioreactor, followed by the suspension of 5.0 × 106 cells of mouse embryonic fibroblasts (MEFs). In the next step, the suspension of 1.0 × 107 PBL-HPCs was circulated without oncostatin M. After these steps, the type I collagen solution was circulated again followed by the suspension of 5.0 × 106 MEFs. Finally a 3-D aggregate was prepared (Figure 1A). The surface of this 3-D aggregate which included PBL-HPCs, primary fibroblasts, and type I collagen was glossy and measured 17 mm in diameter and 1.5 mm in height. This glossy aggregate consisted of a layer of PBL-HPCs, sandwiched between two layers of collagen fibrils with MEFs, and was constructed on a sheet of PLA as shown in Figure 1G. Cross-sectional profiles of the 3-D liver tissue culture models were stained with hematoxylin-eosin and AZAN as shown in Figure 1D-F. The collagen layers were composed of densely packed collagen fibrils running parallel to the plane of the PLA sheet. A layer of PBL-HPCs 200-300 μm thick (Figure 1D), and two layers of collagen fibrils populated with embryonic fibroblasts, approximately 400 μm thick, were observed in the 3-D liver tissue culture model. To culture the 3-D liver tissue model, the cylinder inside the bioreactor was changed from construction to culture (Figure 1B). The 3-D liver tissue culture model was cultured using a differentiation medium containing 20 ng/mL oncostatin M in the bioreactor for 12 d. After this, PBL-HPCs differentiated into mature hepatocyte-like cells, in binuclear populations, and with a bile duct-like structure (Figure 1E). Collagen layers were maintained by the collagen fibers and fibroblasts on day 12.
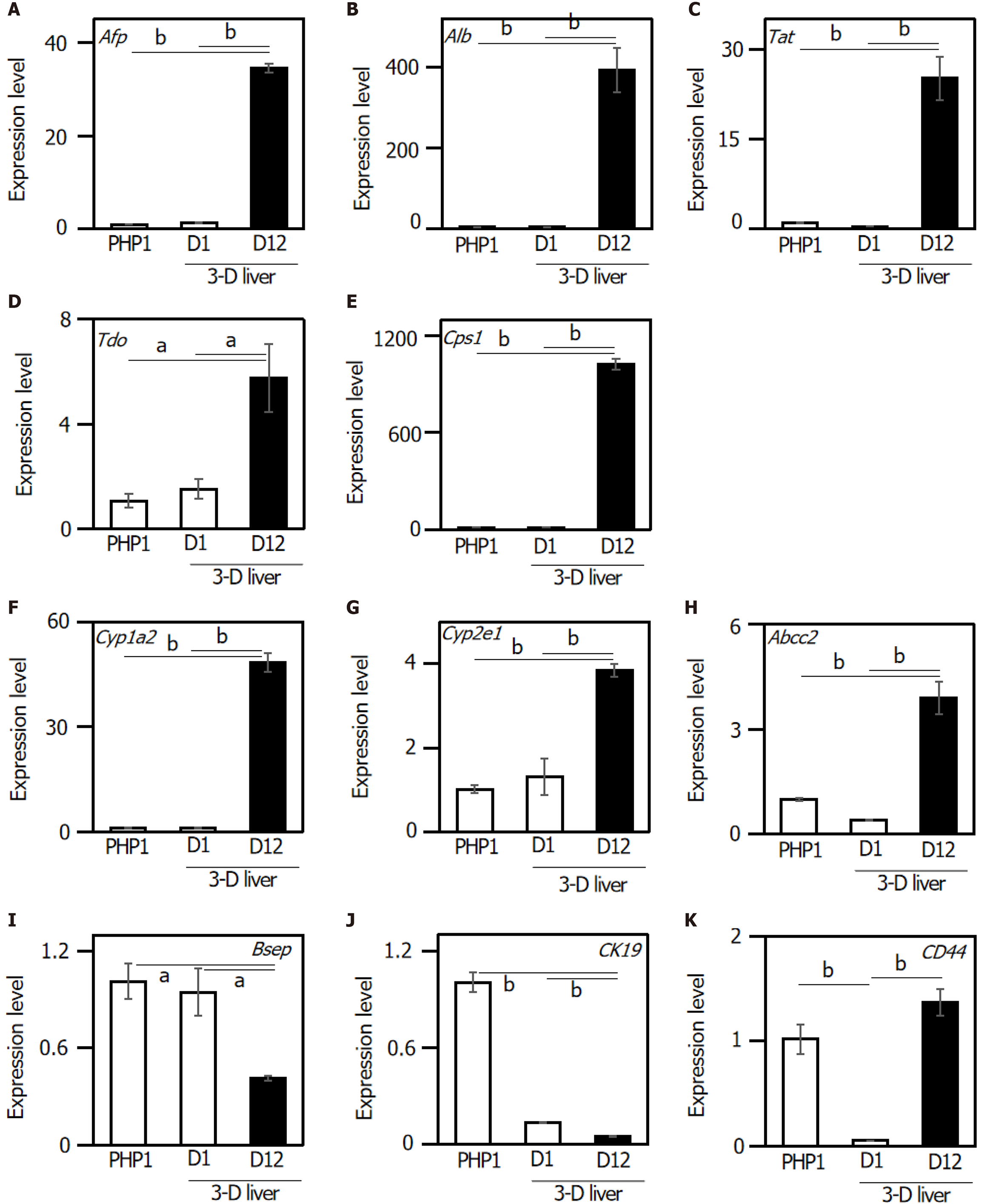
The expression of liver-specific genes was determined in the 3-D liver tissue culture model. To investigate whether the 3-D liver tissue culture models were able to show hepatic lineage differentiation (i.e., to mature into hepatocytes and bile duct cells), the cells were cultured in the presence of oncostatin M. By day 12 of the culture in the hepatic lineage differentiation medium, quantitative real-time polymerase chain reaction analysis revealed that the cells expressed hepatocyte differentiation markers including: Afp (α-fetoprotein); Albumin; Tat (tyrosine aminotransferase); Tdo (tryptophan 2,3-dioxygenase); Cps1 (carbamoyl-phosphate synthetase 1); Cyp1a2 (Cytochrome P450, family 1, sub-family a2); Cyp2e1 (Cytochrome P450, family 2, sub-family e1); and Abcc2 [ATP-binding cassette, sub-family C (CFTR/MRP), member 2] (Figure 2A-H). On the other hand, the gene expression of Bsep (bile salt export pump) and ABCB11 (ATP-binding cassette, sub-family B member 11) decreased in the 3-D liver tissue culture model (Figure 2I). The expression of CK19, a representative marker for HPCs, was confirmed in the PBL-HPCs. The gene expression of CK19 decreased in the 3-D liver tissue culture models (Figure 2J). In addition, PBL-HPCs expressed the CD44 gene, a progenitor cell marker, which decreased in the 3-D liver tissue culture models (Figure 2K). After that, CD44 expression increased in the 3-D liver tissue culture models on day 12 of culture (Figure 2K).
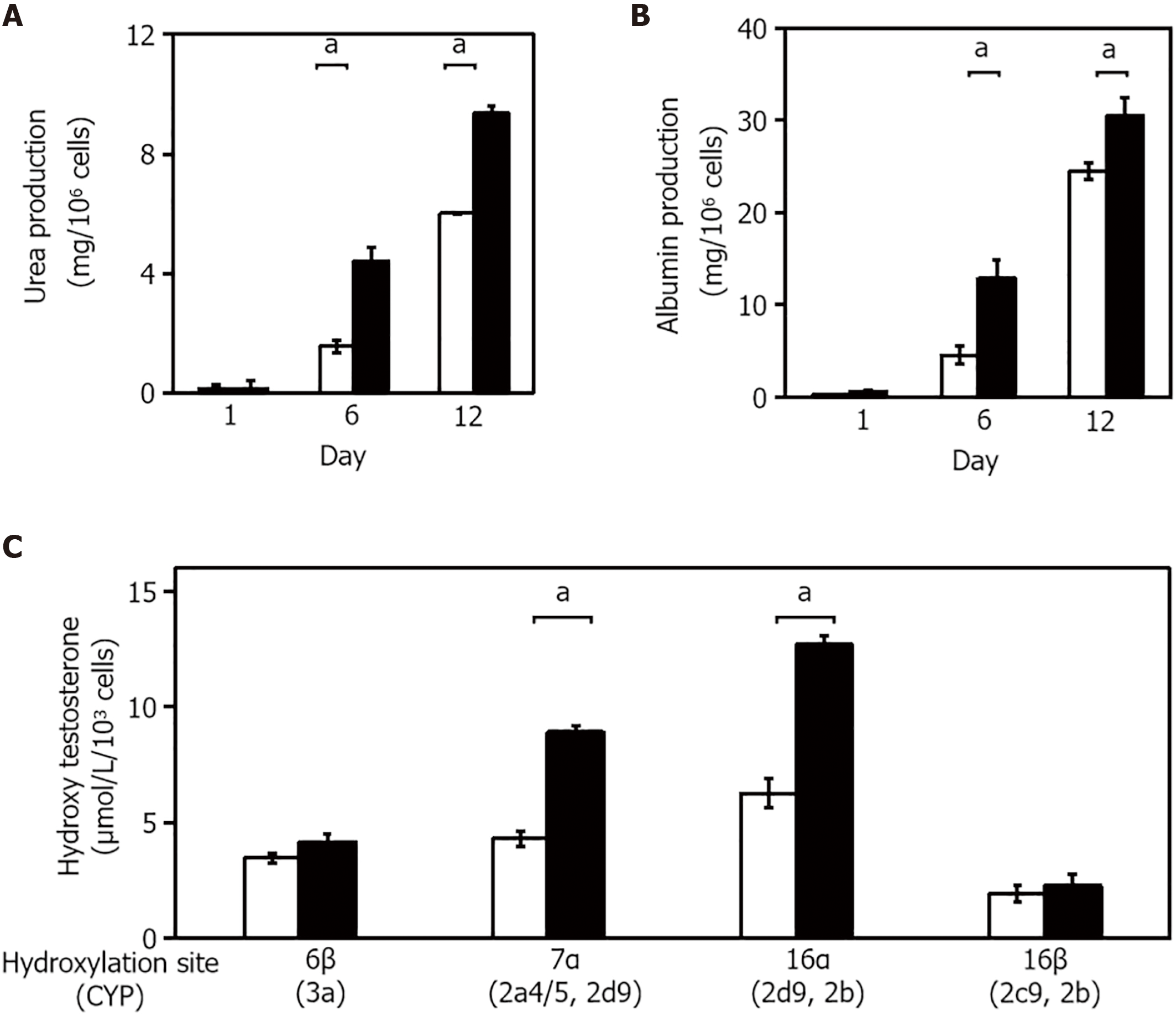
The expression of several liver-specific functions, such as the production of urea and albumin, and drug metabolism, were analyzed in the 3-D liver tissue culture models. Urea production was examined in cultured 3-D liver tissue culture models in a hepatic lineage differentiation medium containing 2 mmol/L NH4+. The level of urea production in the 3-D liver tissue culture model gradually increased and was significantly higher on days 6 and 12 than in the 2-D culture (Figure 3A). The amount of albumin released from the 3-D liver tissue culture model into the medium was measured in each medium on day 1, day 6, and day 12 by enzyme-linked immunosorbent assay. As seen in Figure 3B, the albumin level increased gradually from day 1 to day 12. These results suggest that the 3-D liver tissue culture model with PBL-HPCs had differentiated.
To quantify P450 activities, the hydroxylated pattern of testosterone by cultured 3-D liver tissue culture models in a hepatic lineage differentiation medium, containing 250 μmol/L testosterone, was examined using high-performance liquid chromatography. The concentration of each hydroxylated testosterone (6β-OHT, 7α-OHT, 16α-OHT, and 16β-OHT, respectively, corresponding to oxidation by Cyp3a, Cyp2a4/5 and 2d9, Cyp2d9 and 2b, and Cyp2c29 and 2e) was quantified. The concentrations of hydroxylated testosterones, such as 6β-OHT, 7α-OHT, 16α-OHT, and 16β-OHT, in the media of the 3-D liver tissue culture models after 12 days culture were measured. As compared with the hydroxylation levels of 6b-OHT, 7a-OHT, and 16a-OHT in the 2-D culture, the hydroxylation levels were significantly increased in the 3-D liver tissue models (Figure 3C).
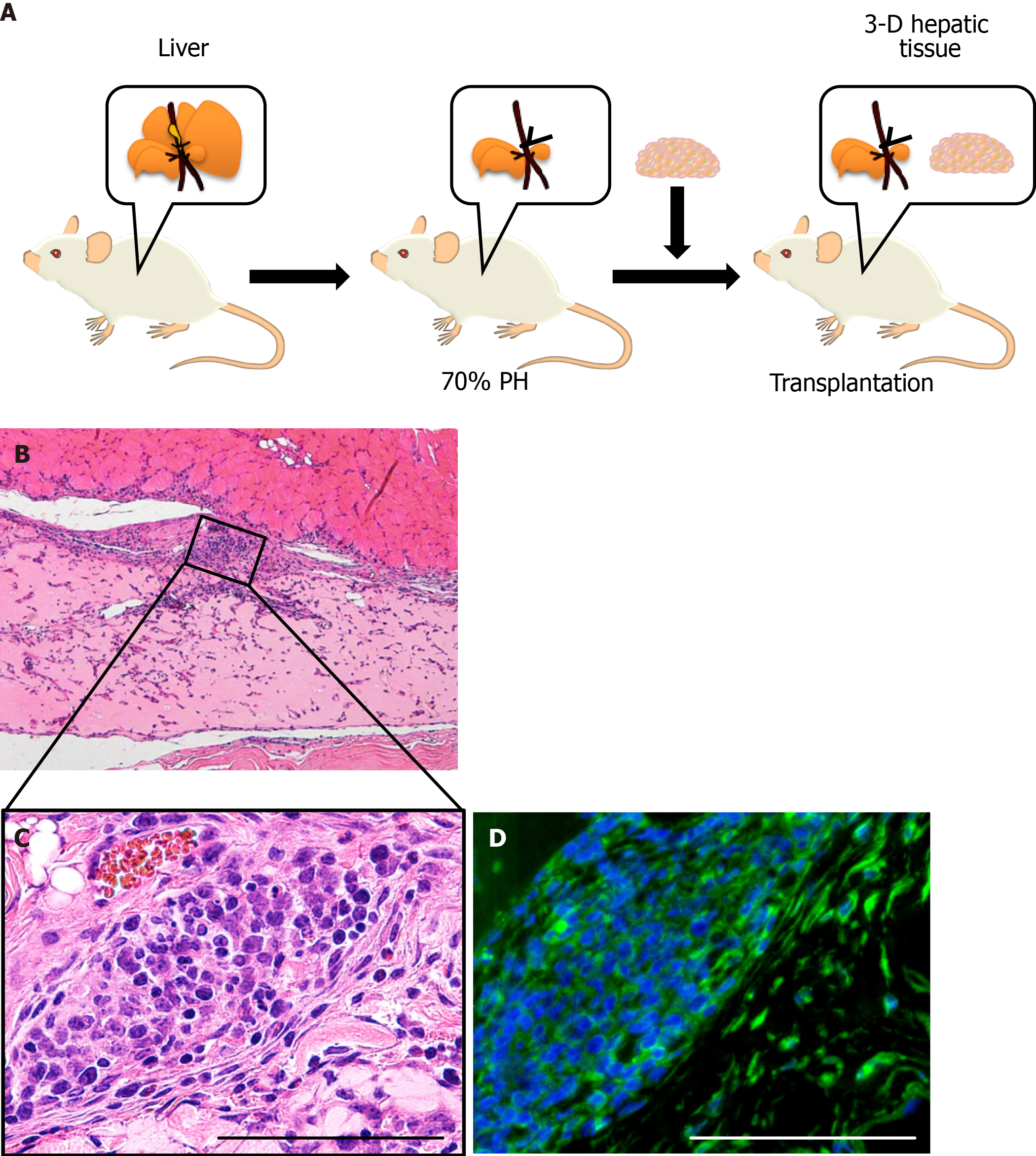
The 3-D liver tissue culture model of PBL-HPCs was syngeneically transplanted into the subcutaneous layer of a BALB/cA mouse which had received a 70% partial hepatectomy as shown in Figure 4A. The graft could be observed in the subcutaneous layer two weeks after transplantation. Microvascular networks were seen throughout this engrafted tissue. As shown in Figure 4B and C, the hematoxylin-eosin staining of this specimen in the graft area showed that collagen remained rich in the graft, fibroblasts existed in the collagen area, and vessel-like tube formation was observed. To investigate whether the cells in these areas were hepatocytes, an immunohistochemical examination using staining with anti-albumin antibodies was carried out (Figure 4D). This confirmed that the PBL-HPCs were accepted as albumin-positive cells after transplantation. These results indicate that the 3-D liver tissue culture model was successfully grafted with angiogenesis in the partially hepatectomized mouse.
It is crucial to develop a technology that enables transplantable engineered tissues to be functionally engrafted and long-lasting, in order to maximize the therapeutic effects of this procedure[19-21]. There are reports of transplanted hepatocytes at several different extrahepatic sites such as the small intestine[22,23]. Tissue engineering has been a promising procedure for providing transplantable tissues mimicking liver ex vivo[24,25]. The attachment of hepatocytes to extracellular matrix scaffolds can help in their engraftment in extrahepatic sites[26,27]. It is important to provide scaffold materials for hepatocytes to enable significantly greater hepatocyte survival in heterotypic transplantation[28]. The liver is encased mainly with collagen fibrils. By fabricating graded structures specific for target tissues and organs, one can obtain suitable scaffolds for tissue regeneration[29]. Taking into account the architecture of the liver, we have generated a 3-D liver tissue culture model of HPCs with a collagen fibril matrix using a bioreactor. Furthermore, defining and validating new sources is mandatory for ensuring functional hepatic cell supply[30]. Hepatic stem/progenitor cells have many advantages compared to adult hepatocytes as they are bipotent cells, so they can differentiate into hepatocytes and cholangiocytes[20]. Moreover, they have high proliferation ability.
In this study, HPCs — PBL-HPCs — were used in an original procedure to generate a 3-D liver tissue culture model. The histological structure of this model resembled that of the liver, with respect to its capillary network and surrounding cell clusters. As PBL-HPCs have the potential to reproduce themselves, it is easy to prepare the necessary numbers of cells (1 × 107 cells order). The PBL-HPC-derived 3-D liver tissue culture model reconstructed in a bioreactor produced cells that differentiated into hepatic-like cells, binuclear populations, and bile duct–like structures (Figure 1F). These cells expressed hepatocyte differentiation markers (Figure 2) after 12 days of culture. The PBL-HPCs differentiated not only into hepatic cells but also bile duct-like cells in a reconstituted collagen fibril matrix. In the 3-D liver tissue culture model derived from PBL-HPCs, the levels of urea and albumin production were gradually enhanced and were significantly higher than those cultured in dishes on days 6 and 12 (Figure 3A and B). Cyp3a, 2a4/5, 2d9, and 2b activities were significantly increased in the 3-D liver tissue culture models (Figure 3C). These results suggest that the PBL-HPCs differentiated to mature hepatocytes in the 3-D liver tissue culture model.
Since PBL-HPCs differentiated into cells expressing hepatic functions in a 3-D liver tissue culture model, further investigation was carried out to determine whether PBL-HPCs also maintained their acquired functions after transplantation. Following implantation of the PBL-HPC-derived 3-D liver tissue culture model into mice, the 3-D liver tissue culture model was grafted, and vessel-like tube formation was observed (Figure 4B and C).
Also, the 3-D liver tissue culture model was grafted in the healthy mouse which did not undergo partial hepatectomy, and vascularization was observed (Supplementary Figure 1A and B). On the other hand, the Matrigel-embedded PBL-HPCs were transplanted into male healthy mice or nu/nu mice, and vascularization was not observed (Supplementary Figure 1C-F). PBL-HPCs differentiated into albumin positive cells (Figure 4D). This haptic tissue consisted of fibroblasts and HPCs which can be differentiated into only hepatocytes and bile duct cells, not immune cells. As some blood vessels were observed after implantation, lymphocytes and monocytes may have been circulating. However, we did not detect Kupffer cells in the grafts. The 3-D liver tissue culture model was investigated for efficiency of transplantation in extrahepatic sites. These findings demonstrated that a reconstituted collagen fibril matrix can provide an extracellular microenvironment to promote the maturation of progenitor cells into hepatic cells. A local vascular network would allow nutrient and gas transport to the graft. These findings could make a significant contribution to the problem of liver graft shortage.
In conclusion, a 3-D liver tissue culture model was developed using HPCs. The advantage of our system is that it consists of proliferative HPCs. The 3-D liver tissue culture models can be generated in an originally designed bioreactor within 24 h. By mimicking the structure of the natural liver, our system was effective in constructing a functional liver tissue model.
Liver transplantation is a therapeutic procedure to recover liver function in patients with irreversible liver failure; however, there is currently a shortage of available transplant organs, which limits the availability of this treatment.
Portal branch-ligated (PBL) HPCs are expected to allow regenerative medicine to produce a cell source to provide an alternate source for transplantation.
We aimed to development a liver model using HPCs.
Hepatic stem/progenitor cells have the ability to multiply ex vivo and differentiate into hepatocytes and cholangiocytes. We have previously established HPC lines derived from the hepatic tissues of mice after ligation of venous drainage. In this study, the PBL hepatic lobe-derived HPCs multiplied in a bioreactor chamber to form liver organoid tissues comparable to liver lobules. These organoid tissues were implanted into syngeneic wild-type mice.
In the three-dimensional (3-D) liver tissue culture model, PBL-HPCs differentiated into mature hepatocyte-like cells, in binuclear populations, and with a bile duct–like structure. Quantitative real-time polymerase chain reaction analysis revealed that the cells expressed hepatocyte differentiation markers. In the 3-D liver tissue culture model derived from PBL-HPCs, the levels of urea and albumin production and activities of Cytochrome P450 enzymes were gradually enhanced.
By mimicking the structure of the natural liver, our system was effective for the construction of a functional liver tissue model.
This PBL-derived HPC line has the potential to proliferate, mature, and form implantable hepatic tissue.
The authors would like to acknowledge Sakai H and Miyagawa S (Department of Surgery, Shinshu University School of Medicine) for skillful technical assistance.
| 1. | Dwyer BJ, Macmillan MT, Brennan PN, Forbes SJ. Cell therapy for advanced liver diseases: Repair or rebuild. J Hepatol. 2021;74:185-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Sasaki K, Akagi T, Asaoka T, Eguchi H, Fukuda Y, Iwagami Y, Yamada D, Noda T, Wada H, Gotoh K, Kawamoto K, Doki Y, Mori M, Akashi M. Construction of three-dimensional vascularized functional human liver tissue using a layer-by-layer cell coating technique. Biomaterials. 2017;133:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Iansante V, Chandrashekran A, Dhawan A. Cell-based liver therapies: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2018;373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Tricot T, De Boeck J, Verfaillie C. Alternative Cell Sources for Liver Parenchyma Repopulation: Where Do We Stand? Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Inoue T, Iwazaki N, Araki T, Hitotsumachi H. Human-Induced Pluripotent Stem Cell-Derived Hepatocytes and their Culturing Methods to Maintain Liver Functions for Pharmacokinetics and Safety Evaluation of Pharmaceuticals. Curr Pharm Biotechnol. 2020;21:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, Gambhir SS, Zern MA. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3:82ra39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Mitaka T, Mizuguchi T, Sato F, Mochizuki C, Mochizuki Y. Growth and maturation of small hepatocytes. J Gastroenterol Hepatol. 1998;13 Suppl:S70-S77. [PubMed] |
| 9. | Prior N, Hindley CJ, Rost F, Meléndez E, Lau WWY, Göttgens B, Rulands S, Simons BD, Huch M. Lgr5+ stem and progenitor cells reside at the apex of a heterogeneous embryonic hepatoblast pool. Development. 2019;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Kuijk EW, Rasmussen S, Blokzijl F, Huch M, Gehart H, Toonen P, Begthel H, Clevers H, Geurts AM, Cuppen E. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci Rep. 2016;6:22154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 12. | Miyajima A, Tanaka M, Itoh T. Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 2014;14:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 430] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 13. | Sakai H, Tagawa Y, Tamai M, Motoyama H, Ogawa S, Soeda J, Nakata T, Miyagawa S. Isolation and characterization of portal branch ligation-stimulated Hmga2-positive bipotent hepatic progenitor cells. Biochem Biophys Res Commun. 2010;403:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Tamai M, Adachi E, Tagawa Y. Characterization of a liver organoid tissue composed of hepatocytes and fibroblasts in dense collagen fibrils. Tissue Eng Part A. 2013;19:2527-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Soto-Gutierrez A, Navarro-Alvarez N, Yagi H, Nahmias Y, Yarmush ML, Kobayashi N. Engineering of an hepatic organoid to develop liver assist devices. Cell Transplant. 2010;19:815-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Hosoya S, Watanabe M, Ueno M, Adachi E. Hepatoblastoma-derived cells (HepG2) used to restore polarized hepatocyte morphology by culturing them in 3D collagen gels. Kitasato Med J. 2014;44:139-147. |
| 17. | Iwashiro H, Hosoya S, Hirai K, Mima T, Ohashi S, Aihara T, Ito S, Ohara S, Adachi E. Characterization of dense artificial connective tissues generated in a newly designed bioreactor. Connect Tissue Res. 2011;52:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Tsutsui M, Ogawa S, Inada Y, Tomioka E, Kamiyoshi A, Tanaka S, Kishida T, Nishiyama M, Murakami M, Kuroda J, Hashikura Y, Miyagawa S, Satoh F, Shibata N, Tagawa Y. Characterization of cytochrome P450 expression in murine embryonic stem cell-derived hepatic tissue system. Drug Metab Dispos. 2006;34:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Tatsumi K, Okano T. Hepatocyte Transplantation: Cell Sheet Technology for Liver Cell Transplantation. Curr Transplant Rep. 2017;4:184-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Heydari Z, Najimi M, Mirzaei H, Shpichka A, Ruoss M, Farzaneh Z, Montazeri L, Piryaei A, Timashev P, Gramignoli R, Nussler A, Baharvand H, Vosough M. Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Rana D, Zreiqat H, Benkirane-Jessel N, Ramakrishna S, Ramalingam M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J Tissue Eng Regen Med. 2017;11:942-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 22. | Kita S, Yasuchika K, Ishii T, Katayama H, Yoshitoshi EY, Ogiso S, Kawai T, Yasuda K, Fukumitsu K, Mizumoto M, Uemoto S. The Protective Effect of Transplanting Liver Cells Into the Mesentery on the Rescue of Acute Liver Failure After Massive Hepatectomy. Cell Transplant. 2016;25:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Iwasaki J, Hata T, Uemoto S, Fujimoto Y, Kanazawa H, Teratani T, Hishikawa S, Kobayashi E. Portocaval shunt for hepatocyte package: challenging application of small intestinal graft in animal models. Organogenesis. 2013;9:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8352] [Cited by in RCA: 6699] [Article Influence: 203.0] [Reference Citation Analysis (3)] |
| 25. | Han F, Wang J, Ding L, Hu Y, Li W, Yuan Z, Guo Q, Zhu C, Yu L, Wang H, Zhao Z, Jia L, Li J, Yu Y, Zhang W, Chu G, Chen S, Li B. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front Bioeng Biotechnol. 2020;8:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 26. | Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J Hepatol. 2015;62:S157-S169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 27. | Kaur S, Tripathi DM, Venugopal JR, Ramakrishna S. Advances in biomaterials for hepatic tissue engineering. Curr Opin Biomed Eng. 2020;13:190-196. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Nicolas CT, Hickey RD, Chen HS, Mao SA, Lopera Higuita M, Wang Y, Nyberg SL. Concise Review: Liver Regenerative Medicine: From Hepatocyte Transplantation to Bioartificial Livers and Bioengineered Grafts. Stem Cells. 2017;35:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Salvatore L, Gallo N, Natali ML, Terzi A, Sannino A, Madaghiele M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front Bioeng Biotechnol. 2021;9:644595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 30. | Messina A, Luce E, Hussein M, Dubart-Kupperschmitt A. Pluripotent-Stem-Cell-Derived Hepatic Cells: Hepatocytes and Organoids for Liver Therapy and Regeneration. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao W S-Editor: Gao CC L-Editor: Webster JR P-Editor: Guo X